Analysis of Anxiety Disorders and Post-Traumatic Stress Disorders for Screening Anxiolytic Drugs and Linking Preclinical and Clinical Research
Abstract
1. Introduction
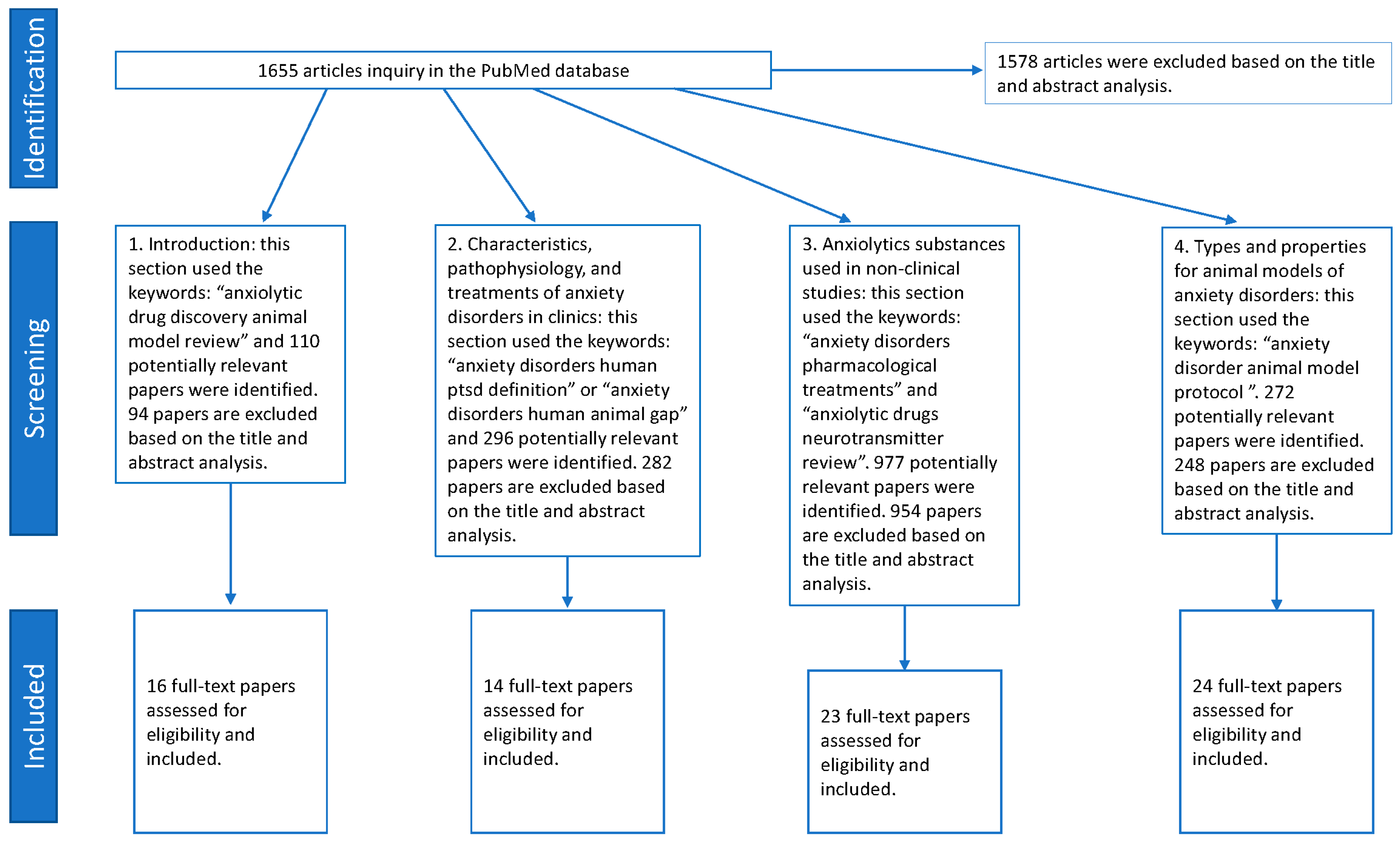
2. Previous Literature Selection Methods
3. Characteristics, Pathophysiology, and Treatments of Anxiety Disorders in the Clinic
3.1. Prevalence and Symptoms of Various Anxiety Disorders in the Clinic
3.2. Pathophysiology of Anxiety Disorders in the Clinic
3.3. Pharmacological Treatments of Anxiety Disorders in the Clinic
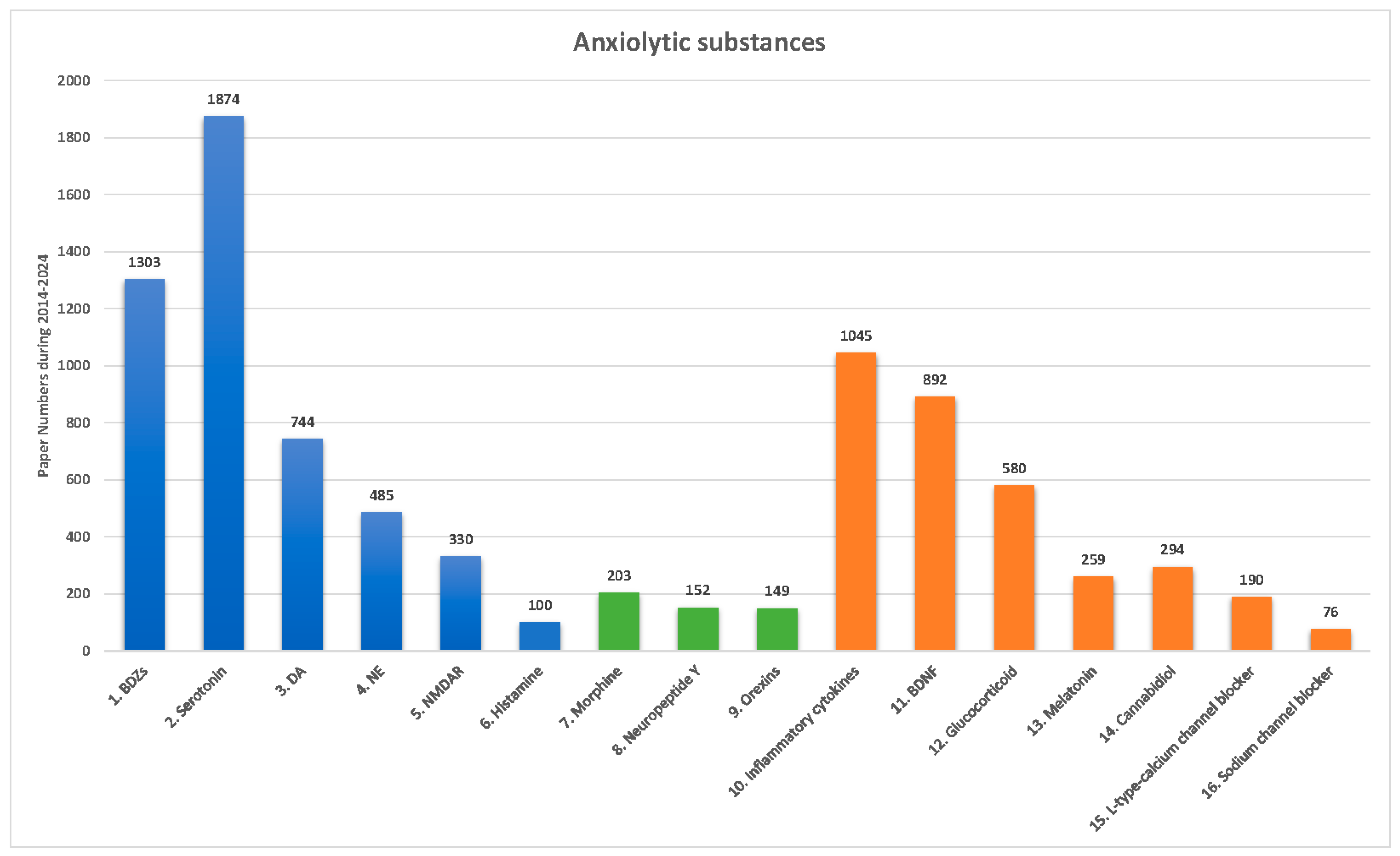
4. Anxiolytic Substances Used in Non-Clinical Studies: Pharmacological Treatments and Neural Mechanisms
4.1. Conventional Anxiolytic Substances
| Mechanism of Action | Mental Illness | Animal Models | Neural Mechanisms and Effects | References |
|---|---|---|---|---|
| Classical Neurotransmitters: | ||||
| 1. Agonism of GABAa receptor | Anxiety disorders and PTSD | Conditioned fear learning | 1. BDZ drugs affiliate with the GABAa receptor 2. Cause anxiolytic effects | [31,32] |
| 2. Inhibition of serotonin reuptake | Anxiety-related disorders (e.g., panic disorder, generalized anxiety disorders, PTSD) | Conditioned fear learning (contextual or cue) or PTSD animal models | 1. SSRIs drugs act on the inhibition of serotonin reuptake 2. Lead to anxiolytic effects | [14,33] |
| 3. Agonism of dopamine receptor | PTSD | PTSD animal model (single prolonged stress) | 1. D2/D3 receptor agonism 2. Lead to anxiolytic effects | [34] |
| 4. Antagonism of norepinephrine receptor | PTSD | Conditioned fear learning | 1. Antagonism of alpha-1 adrenergic receptor 2. Disrupt anxiety- and PTSD-associated symptoms | [35,36] |
| 5. Antagonism of NMDA receptor | Anxiety disorders and PTSD | Conditioned fear learning animal model | 1. Antagonism of NMDA receptor 2. Attenuate fear symptoms | [37] |
| 6. Agonism of histamine receptor | Anxiety disorders | Isolation-induced aggressive behavior; conditioned fear learning | 1. H3 receptor agonism 2. Reduce anxiety disorders | [38] |
| Neuropeptides: | ||||
| 1. Agonism of opiates | PTSD | Conditioned fear learning | 1. Opioid receptor agonism 2. Result in anxiolytic effects | [41,42] |
| 2. Activation of neuropeptide Y | PTSD | PTSD animal model (single prolonged stress) | 1. Neuropeptide Y receptor agonism 2. Reduce anxiety behaviors and PTSD symptoms | [40] |
| 3. Antagonism of orexins receptor | Anxiety disorders (e.g., phobia, panic, and PTSD) | Conditioned fear learning animal models | 1. Orexin receptor antagonism 2. Impair fear behaviors | [39] |
| Nonclassical neurotransmitters: | ||||
| 1. Activation of inflammatory cytokines | Anxiety disorders and PTSD | Multiple anxiety and PTSD animal models | 1. Activation of inflammation cytokines 2. Cause anxiety disorders and PTSD symptoms. | [45] |
| 2. Activation of BDNF | Anxiety disorders and PTSD | PTSD animal model (single prolonged stress) | 1. Activation of BDNF via TrkB receptor 2. Attenuate anxiety disorders | [46,47] |
| 3. Activation of glucocorticoid | PTSD | PTSD animal models | 1. Activation of glucocorticoid receptor 2. Block anxiety disorders | [48] |
| 4. Activation of melatonin | PTSD | Conditioned fear learning animal models | 1. Activation of melatonin receptor 2. Impairs contextual fear conditioning | [16] |
| 5. Activation of cannabidiol | Anxiety disorders (e.g., generalized anxiety disorder, panic disorder, social anxiety disorder, PTSD) | Multiple anxiety disorder animal models | 1. Agonism of the CB1 receptor 2. Impair multiple anxiety disorders (including generalized anxiety disorder, panic disorder, social anxiety disorder, and PTSD) | [15] |
| 6. Action of L-type calcium channel blocker | Anxiety disorders | Caffeine-induced anxiety symptoms | 1. Antagonism of calcium channels 2. Cause anxiolytic effects | [49] |
| 7. Activation of sodium channel blocker | PTSD | Conditioned fear learning (i.e., cue) | 1. Antagonism of sodium channels 2. Lead to anxiolytic effects | [50] |
4.2. Current Anxiolytic Substances: Classical Neurotransmitters, Neuropeptides, and Nonclassical Neurotransmitters
5. Types and Properties for Animal Models of Anxiety Disorders
5.1. Shaping an Animal Model of Anxiety Disorders and PTSD
5.2. Testing Anxiety and PTSD Behaviors
6. Opinion from Preclinical Studies to Clinical Research
7. Limitations
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ren, L.; Fan, Y.; Wu, W.; Qian, Y.; He, M.; Li, X.; Wang, Y.; Yang, Y.; Wen, X.; Zhang, R.; et al. Anxiety disorders: Treatments, models, and circuitry mechanisms. Eur. J. Pharmacol. 2024, 983, 176994. [Google Scholar] [CrossRef]
- Witkin, J.M.; Barrett, J.E. ANXIOLYTICS: Introduction to a special issue celebrating 50 years of Pharmacology, Biochemistry and Behavior. Pharmacol. Biochem. Behav. 2024, 245, 173905. [Google Scholar] [CrossRef] [PubMed]
- Association, A.P. (Ed.) Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Griebel, G.; Holmes, A. 50 years of hurdles and hope in anxiolytic drug discovery. Nat. Rev. Drug Discov. 2013, 12, 667–687. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.M.; Kalueff, A.V. Anxiolytic drug discovery: What are the novel approaches and how can we improve them? Expert Opin. Drug Discov. 2014, 9, 15–26. [Google Scholar] [CrossRef]
- O’Leary, K.B.; Khan, J.S. Pharmacotherapy for Anxiety Disorders. Psychiatr. Clin. N. Am. 2024, 47, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J. An assessment of anxiolytic drug screening tests: Hormetic dose responses predominate. Crit. Rev. Toxicol. 2008, 38, 489–542. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Sweeney, F.F. The age of anxiety: Role of animal models of anxiolytic action in drug discovery. Br. J. Pharmacol. 2011, 164, 1129–1161. [Google Scholar] [CrossRef] [PubMed]
- Bourin, M. Animal models for screening anxiolytic-like drugs: A perspective. Dialogues Clin. Neurosci. 2015, 17, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.C.; Bergner, C.L.; Smolinsky, A.N.; Dufour, B.D.; Egan, R.J.; LaPorte, J.L.; Kalueff, A.V. Experimental Models of Anxiety for Drug Discovery and Brain Research. Methods Mol. Biol. 2016, 1438, 271–291. [Google Scholar] [CrossRef]
- Park, S.C.; Kim, Y.K. Anxiety Disorders in the DSM-5: Changes, Controversies, and Future Directions. Adv. Exp. Med. Biol. 2020, 1191, 187–196. [Google Scholar] [CrossRef]
- Rabow, L.E.; Russek, S.J.; Farb, D.H. From ion currents to genomic analysis: Recent advances in GABAA receptor research. Synapse 1995, 21, 189–274. [Google Scholar] [CrossRef] [PubMed]
- Aspesi, D.; Pinna, G. Animal models of post-traumatic stress disorder and novel treatment targets. Behav. Pharmacol. 2019, 30, 130–150. [Google Scholar] [CrossRef]
- Heesbeen, E.J.; Bijlsma, E.Y.; Verdouw, P.M.; van Lissa, C.; Hooijmans, C.; Groenink, L. The effect of SSRIs on fear learning: A systematic review and meta-analysis. Psychopharmacology 2023, 240, 2335–2359. [Google Scholar] [CrossRef]
- Blessing, E.M.; Steenkamp, M.M.; Manzanares, J.; Marmar, C.R. Cannabidiol as a Potential Treatment for Anxiety Disorders. Neurotherapeutics 2015, 12, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Yang, Z.; Li, C.Q. The Melatonergic System in Anxiety Disorders and the Role of Melatonin in Conditional Fear. Vitam. Horm. 2017, 103, 281–294. [Google Scholar] [CrossRef]
- World Health, O. Depression and Other Common Mental Disorders: Global Health Estimates; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- LeardMann, C.A.; McMaster, H.S.; Warner, S.; Esquivel, A.P.; Porter, B.; Powell, T.M.; Tu, X.M.; Lee, W.W.; Rull, R.P.; Hoge, C.W.; et al. Comparison of Posttraumatic Stress Disorder Checklist Instruments From Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition vs Fifth Edition in a Large Cohort of US Military Service Members and Veterans. JAMA Netw. Open 2021, 4, e218072. [Google Scholar] [CrossRef]
- Murphy, D.L.; Moya, P.R.; Fox, M.A.; Rubenstein, L.M.; Wendland, J.R.; Timpano, K.R. Anxiety and affective disorder comorbidity related to serotonin and other neurotransmitter systems: Obsessive-compulsive disorder as an example of overlapping clinical and genetic heterogeneity. Philos. Trans. R. Soc. Lond B Biol. Sci. 2013, 368, 20120435. [Google Scholar] [CrossRef]
- Kirk, P.A.; Holmes, A.J.; Robinson, O.J. Threat vigilance and intrinsic amygdala connectivity. Hum. Brain Mapp. 2022, 43, 3283–3292. [Google Scholar] [CrossRef]
- Robinson, O.J.; Krimsky, M.; Lieberman, L.; Allen, P.; Vytal, K.; Grillon, C. Towards a mechanistic understanding of pathological anxiety: The dorsal medial prefrontal-amygdala ‘aversive amplification’ circuit in unmedicated generalized and social anxiety disorders. Lancet Psychiatry 2014, 1, 294–302. [Google Scholar] [CrossRef]
- Vytal, K.E.; Overstreet, C.; Charney, D.R.; Robinson, O.J.; Grillon, C. Sustained anxiety increases amygdala-dorsomedial prefrontal coupling: A mechanism for maintaining an anxious state in healthy adults. J. Psychiatry Neurosci. 2014, 39, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.M. Defining biotypes for depression and anxiety based on large-scale circuit dysfunction: A theoretical review of the evidence and future directions for clinical translation. Depress. Anxiety 2017, 34, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Giacobbe, P.; Flint, A. Diagnosis and Management of Anxiety Disorders. Contin. Lifelong Learn. Neurol. 2018, 24, 893–919. [Google Scholar] [CrossRef] [PubMed]
- Bandelow, B. Current and Novel Psychopharmacological Drugs for Anxiety Disorders. Adv. Exp. Med. Biol. 2020, 1191, 347–365. [Google Scholar] [CrossRef] [PubMed]
- McCabe, C.; Mishor, Z.; Filippini, N.; Cowen, P.J.; Taylor, M.J.; Harmer, C.J. SSRI administration reduces resting state functional connectivity in dorso-medial prefrontal cortex. Mol. Psychiatry 2011, 16, 592–594. [Google Scholar] [CrossRef]
- Murrough, J.W.; Yaqubi, S.; Sayed, S.; Charney, D.S. Emerging drugs for the treatment of anxiety. Expert Opin. Emerg. Drugs 2015, 20, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Muscatello, M.R.; Spina, E.; Bandelow, B.; Baldwin, D.S. Clinically relevant drug interactions in anxiety disorders. Hum. Psychopharmacol. 2012, 27, 239–253. [Google Scholar] [CrossRef] [PubMed]
- DeVeaugh-Geiss, J. Pharmacologic therapy of obsessive compulsive disorder. Adv. Pharmacol. 1994, 30, 35–52. [Google Scholar] [CrossRef]
- Sheehan, D.V. Delineation of anxiety and phobic disorders responsive to monoamine oxidase inhibitors: Implications for classification. J. Clin. Psychiatry 1984, 45, 29–36. [Google Scholar]
- Lu, C.Y.; Liu, X.; Jiang, H.; Pan, F.; Ho, C.S.; Ho, R.C. Effects of Traumatic Stress Induced in the Juvenile Period on the Expression of Gamma-Aminobutyric Acid Receptor Type A Subunits in Adult Rat Brain. Neural Plast. 2017, 2017, 5715816. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.C.; Pollack, M.H. Benzodiazepines in clinical practice: Consideration of their long-term use and alternative agents. J. Clin. Psychiatry 2005, 66 (Suppl. S2), 21–27. [Google Scholar]
- Hendriksen, H.; Olivier, B.; Oosting, R.S. From non-pharmacological treatments for post-traumatic stress disorder to novel therapeutic targets. Eur. J. Pharmacol. 2014, 732, 139–158. [Google Scholar] [CrossRef]
- Malikowska-Racia, N.; Salat, K.; Nowaczyk, A.; Fijalkowski, L.; Popik, P. Dopamine D2/D3 receptor agonists attenuate PTSD-like symptoms in mice exposed to single prolonged stress. Neuropharmacology 2019, 155, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Laitman, B.M.; Gajewski, N.D.; Mann, G.L.; Kubin, L.; Morrison, A.R.; Ross, R.J. The alpha1 adrenoceptor antagonist prazosin enhances sleep continuity in fear-conditioned Wistar-Kyoto rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 49, 7–15. [Google Scholar] [CrossRef][Green Version]
- O’Daniel, M.P.; Petrunich-Rutherford, M.L. Effects of chronic prazosin, an alpha-1 adrenergic antagonist, on anxiety-like behavior and cortisol levels in a chronic unpredictable stress model in zebrafish (Danio rerio). PeerJ 2020, 8, e8472. [Google Scholar] [CrossRef] [PubMed]
- Radulovic, J.; Ren, L.Y.; Gao, C. N-Methyl D-aspartate receptor subunit signaling in fear extinction. Psychopharmacology 2019, 236, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, F.; Yamauchi, M.; Oyama, M.; Okuma, K.; Onozawa, K.; Nagayama, T.; Shinei, R.; Ishikawa, M.; Sato, Y.; Kakui, N. Anxiolytic-like profiles of histamine H3 receptor agonists in animal models of anxiety: A comparative study with antidepressants and benzodiazepine anxiolytic. Psychopharmacology 2009, 205, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Flores, A.; Saravia, R.; Maldonado, R.; Berrendero, F. Orexins and fear: Implications for the treatment of anxiety disorders. Trends Neurosci. 2015, 38, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Serova, L.I.; Laukova, M.; Alaluf, L.G.; Pucillo, L.; Sabban, E.L. Intranasal neuropeptide Y reverses anxiety and depressive-like behavior impaired by single prolonged stress PTSD model. Eur. Neuropsychopharmacol. 2014, 24, 142–147. [Google Scholar] [CrossRef]
- RaiseAbdullahi, P.; Vafaei, A.A.; Ghanbari, A.; Dadkhah, M.; Rashidy-Pour, A. Time-dependent protective effects of morphine against behavioral and morphological deficits in an animal model of posttraumatic stress disorder. Behav. Brain Res. 2019, 364, 19–28. [Google Scholar] [CrossRef]
- Szczytkowski-Thomson, J.L.; Lebonville, C.L.; Lysle, D.T. Morphine prevents the development of stress-enhanced fear learning. Pharmacol. Biochem. Behav. 2013, 103, 672–677. [Google Scholar] [CrossRef]
- Inutsuka, A.; Yamanaka, A. The physiological role of orexin/hypocretin neurons in the regulation of sleep/wakefulness and neuroendocrine functions. Front. Endocrinol. 2013, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Zarrabian, S.; Riahi, E.; Karimi, S.; Razavi, Y.; Haghparast, A. The potential role of the orexin reward system in future treatments for opioid drug abuse. Brain Res. 2020, 1731, 146028. [Google Scholar] [CrossRef] [PubMed]
- Felger, J.C. Imaging the Role of Inflammation in Mood and Anxiety-related Disorders. Curr. Neuropharmacol. 2018, 16, 533–558. [Google Scholar] [CrossRef] [PubMed]
- Andero, R.; Ressler, K.J. Fear extinction and BDNF: Translating animal models of PTSD to the clinic. Genes Brain Behav. 2012, 11, 503–512. [Google Scholar] [CrossRef]
- Yin, J.B.; Liu, H.X.; Shi, W.; Ding, T.; Hu, H.Q.; Guo, H.W.; Jin, S.; Wang, X.L.; Zhang, T.; Lu, Y.C.; et al. Various BDNF administrations attenuate SPS-induced anxiety-like behaviors. Neurosci. Lett. 2022, 788, 136851. [Google Scholar] [CrossRef]
- Florido, A.; Velasco, E.R.; Monari, S.; Cano, M.; Cardoner, N.; Sandi, C.; Andero, R.; Perez-Caballero, L. Glucocorticoid-based pharmacotherapies preventing PTSD. Neuropharmacology 2023, 224, 109344. [Google Scholar] [CrossRef] [PubMed]
- Khurana, K.; Bansal, N. Lacidipine attenuates caffeine-induced anxiety-like symptoms in mice: Role of calcium-induced oxido-nitrosative stress. Pharmacol. Rep. 2019, 71, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Mirza, N.R.; Bright, J.L.; Stanhope, K.J.; Wyatt, A.; Harrington, N.R. Lamotrigine has an anxiolytic-like profile in the rat conditioned emotional response test of anxiety: A potential role for sodium channels? Psychopharmacology 2005, 180, 159–168. [Google Scholar] [CrossRef]
- Ragen, B.J.; Seidel, J.; Chollak, C.; Pietrzak, R.H.; Neumeister, A. Investigational drugs under development for the treatment of PTSD. Expert Opin. Investig. Drugs 2015, 24, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Sartori, S.B.; Singewald, N. Novel pharmacological targets in drug development for the treatment of anxiety and anxiety-related disorders. Pharmacol. Ther. 2019, 204, 107402. [Google Scholar] [CrossRef]
- Tiller, J.W. Depression and anxiety. Med. J. Aust. 2013, 199, S28–S31. [Google Scholar] [CrossRef]
- Fuchs, E.; Fliugge, G. Experimental animal models for the simulation of depression and anxiety. Dialogues Clin. Neurosci. 2006, 8, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Kiess, K.O. Statistical Concepts for the Behavioral Sciences; Allyn and Bacon, Inc.: Boston, MA, USA, 1989. [Google Scholar]
- Flandreau, E.I.; Toth, M. Animal Models of PTSD: A Critical Review. Curr. Top. Behav. Neurosci. 2018, 38, 47–68. [Google Scholar] [CrossRef] [PubMed]
- Gencturk, S.; Unal, G. Rodent tests of depression and anxiety: Construct validity and translational relevance. Cogn. Affect. Behav. Neurosci. 2024, 24, 191–224. [Google Scholar] [CrossRef]
- Morel, C.; Paoli, J.; Camonin, C.; Marchal, N.; Grova, N.; Schroeder, H. Comparison of predictive validity of two autism spectrum disorder rat models: Behavioural investigations. Neurotoxicology 2024, 103, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Lissek, S.; Kaczkurkin, A.N.; Rabin, S.; Geraci, M.; Pine, D.S.; Grillon, C. Generalized anxiety disorder is associated with overgeneralization of classically conditioned fear. Biol. Psychiatry 2014, 75, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.H.; Lim, Y.S.; Ou, C.Y.; Chang, K.C.; Tsai, A.C.; Chang, F.C.; Huang, A.C.W. The Medial Prefrontal Cortex, Nucleus Accumbens, Basolateral Amygdala, and Hippocampus Regulate the Amelioration of Environmental Enrichment and Cue in Fear Behavior in the Animal Model of PTSD. Behav. Neurol. 2022, 2022, 7331714. [Google Scholar] [CrossRef]
- Yamamoto, S.; Morinobu, S.; Takei, S.; Fuchikami, M.; Matsuki, A.; Yamawaki, S.; Liberzon, I. Single prolonged stress: Toward an animal model of posttraumatic stress disorder. Depress. Anxiety 2009, 26, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, B.N.; Fleshner, M. Exercise, learned helplessness, and the stress-resistant brain. Neuromol. Med. 2008, 10, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Maier, S.F.; Watkins, L.R. Stressor controllability and learned helplessness: The roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci. Biobehav. Rev. 2005, 29, 829–841. [Google Scholar] [CrossRef]
- Banagozar Mohammadi, A.; Torbati, M.; Farajdokht, F.; Sadigh-Eteghad, S.; Fazljou, S.M.B.; Vatandoust, S.M.; Golzari, S.E.J.; Mahmoudi, J. Sericin alleviates restraint stress induced depressive- and anxiety-like behaviors via modulation of oxidative stress, neuroinflammation and apoptosis in the prefrontal cortex and hippocampus. Brain Res. 2019, 1715, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Donner, N.C.; Kubala, K.H.; Hassell, J.E., Jr.; Lieb, M.W.; Nguyen, K.T.; Heinze, J.D.; Drugan, R.C.; Maier, S.F.; Lowry, C.A. Two models of inescapable stress increase tph2 mRNA expression in the anxiety-related dorsomedial part of the dorsal raphe nucleus. Neurobiol. Stress 2018, 8, 68–81. [Google Scholar] [CrossRef]
- Park, H.; Rhee, J.; Park, K.; Han, J.S.; Malinow, R.; Chung, C. Exposure to Stressors Facilitates Long-Term Synaptic Potentiation in the Lateral Habenula. J. Neurosci. 2017, 37, 6021–6030. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, X.Z.; Li, H.; Li, X.; Yu, T.; Dohl, J.; Ursano, R.J. Updates in PTSD Animal Models Characterization. Methods Mol. Biol. 2019, 2011, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.C.; Fogaca, M.V.; Aguiar, D.C.; Guimaraes, F.S. Animal models of anxiety disorders and stress. Braz J. Psychiatry 2013, 35 (Suppl. S2), S101–S111. [Google Scholar] [CrossRef] [PubMed]
- Karl, T.; Duffy, L.; Herzog, H. Behavioural profile of a new mouse model for NPY deficiency. Eur. J. Neurosci. 2008, 28, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Kraeuter, A.K.; Guest, P.C.; Sarnyai, Z. The Open Field Test for Measuring Locomotor Activity and Anxiety-Like Behavior. Methods Mol. Biol. 2019, 1916, 99–103. [Google Scholar] [CrossRef]
- Critchley, M.A.; Handley, S.L. Effects in the X-maze anxiety model of agents acting at 5-HT1 and 5-HT2 receptors. Psychopharmacology 1987, 93, 502–506. [Google Scholar] [CrossRef]
- Handley, S.L.; McBlane, J.W. An assessment of the elevated X-maze for studying anxiety and anxiety-modulating drugs. J. Pharmacol. Toxicol. Methods 1993, 29, 129–138. [Google Scholar] [CrossRef]
- Kedia, S.; Chattarji, S. Marble burying as a test of the delayed anxiogenic effects of acute immobilisation stress in mice. J. Neurosci. Methods 2014, 233, 150–154. [Google Scholar] [CrossRef]
- Yang, M.; Augustsson, H.; Markham, C.M.; Hubbard, D.T.; Webster, D.; Wall, P.M.; Blanchard, R.J.; Blanchard, D.C. The rat exposure test: A model of mouse defensive behaviors. Physiol. Behav. 2004, 81, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Tuohimaa, P. The Suok (“ropewalking”) murine test of anxiety. Brain Res. Brain Res. Protoc. 2005, 14, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Kliethermes, C.L.; Crabbe, J.C. Pharmacological and genetic influences on hole-board behaviors in mice. Pharmacol. Biochem. Behav. 2006, 85, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Powell, S.B.; Geyer, M.A.; Gallagher, D.; Paulus, M.P. The balance between approach and avoidance behaviors in a novel object exploration paradigm in mice. Behav. Brain Res. 2004, 152, 341–349. [Google Scholar] [CrossRef] [PubMed]
| Anxiety Disorders | Prevalence | Symptoms |
|---|---|---|
| 1. Generalized anxiety disorder (GAD) | Approximately 0.9% and 2.9% prevalence rates for adolescents and adults in the United States. | 1. Persistent and excessive anxiety. 2. Worry about school and work performance. |
| 2. Panic disorder | Approximately 2–3% for adolescents and adults in the United States. | 1. Recurrent unexpected panic attacks. 2. Persistently concerned or worried about further panic attacks. |
| 3. Agoraphobia | Approximately 1.7% for adolescents and adults in the United States. | 1. Significant and intense fear or anxiety induced by an extendable range of surroundings in real or anticipated exposure. |
| 4. Post-traumatic stress disorder (PTSD) | Approximately 3.5% for adults in the United States. | 1. Concern intrusions and avoidance of memories associated with the traumatic event itself. 2. The critical features of PTSD vary. 3. Some patients encounter fear-based reexperiencing, emotional, and behavioral symptoms. 4. Others feel anhedonic or dysphoric mood states, and negative cognitions may be most distressing. 5. In some cases, arousal and reactive externalizing symptoms are prominent. 6. Others produce dissociative symptoms that predominate. 7. Some individuals exhibit combinations of these symptom patterns. |
| 5. Social anxiety disorder (SAD; social phobia) | Approximately 7% in the United States. | 1. Social phobia. 2. Fearful or anxious about or avoidant of social interactions and social surroundings that involve the possibility of being scrutinized. |
| 6. Acute stress disorder (ASD) | Less than 20% (do not involve interpersonal assault) in the United States. | 1. Symptoms may vary by individual. 2. Anxiety response for reexperiencing or reactivity to the traumatic event. 3. A dissociative or detached presentation, although these individuals typically will also display strong emotional or physiological reactivity in response to trauma reminders. 4. A strong anger response in which reactivity is characterized by irritable or possibly aggressive responses. 5. The symptoms are development at least lasting from 3 days to 1 month. |
| 7. Separation anxiety disorder | About 0.9–1.9% for adults, 4% for children, and 1.6% for adolescents in the United States. | 1. Excessive fear or anxiety concerning separation from home or attachment figures. |
| 8. Obsessive–compulsive disorder (OCD) | About 1.2% in the United States. | 1. The presence of obsessions and compulsions. 2. Obsessions are repeated, persistent thoughts, images, or urges. 3. Persistent thoughts are voluntarily associated with marked distress or anxiety. 4. Compulsions are repetitive behaviors or mental acts. |
| Anxiety Disorders and Treatments | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Medicines | Drugs | 1. GAD | 2. PD | 3. Agoraphobia | 4. PTSD | 5. SAD | 6. ASD | 7. Separation Anxiety Disorder | 8. OCD |
| 1. BDZs | Alprazolam | V | V | V | V | V | V | V | V |
| Chlordiazepoxide | V | V | V | V | V | V | V | V | |
| Clonazepam | V | ||||||||
| Diazepam | V | V | V | V | V | V | V | V | |
| Lorazepam | V | V | V | V | V | V | V | V | |
| Oxazepam | V | V | V | V | V | V | V | V | |
| 2. SSRIs | Escitalopam | V | V | V | V | ||||
| Fluoxetine | V | V | V | ||||||
| Fluvoxamine | V | V | V | V | |||||
| Paroxetine | V | V | V | V | V | V | |||
| Citalopram | V | V | V | ||||||
| Sertaline | V | V | V | V | V | V | |||
| 3. SNRIs | Duloxetine | V | |||||||
| Venlafaxine | V | V | V | V | |||||
| 4. TCA | Clomipramine | V | V | V | |||||
| Doxepine | V | V | V | V | V | V | V | V | |
| Imipramine | V | ||||||||
| 5. MAOIs | Phenelzine | V | |||||||
| Moclobemide | V | ||||||||
| 6. Calcium modulators | Pregabalin | V | V | ||||||
| 7. Azapirone | Buspirone | V | V | V | V | V | V | V | V |
| 8. Antihistamine | Hydroxyzine | V | V | V | V | V | V | V | V |
| Animal Models | Characteristics | Advantages | Disadvantages | When to Use | Use Frequency | References |
|---|---|---|---|---|---|---|
| A. Shaping anxiety models | ||||||
| 1. Fear conditioning: Cue/footshock | Applying a discrete cue stimulus to pair with footshock-induced stress. | Cue is a clear-cut stimulus with high face, predictive, and constructive validity. | --- | Anxiety disorders; PTSD | *** | [59] |
| 2. Fear conditioning: Context/footshock | Applying a contextual stimulus to pair with footshock-induced stress. | A contextual stimulus mimics the environment: high face, predictive, and constructive validity. | Context is a complex stimulus combining various environmental stimuli. | Anxiety disorders; PTSD | *** | [60] |
| 3. Single prolonged stress | Animals are restrained for 2 h and then forced to swim tests for 20 min. Following recovery for 15 min, animals are exposed to ether until they lose consciousness. | Stable stress; face, predictive, and constructive validity. | Require complex and long-term stress manipulations. The single prolonged stress model is complex compared to the fear conditioning model. | PTSD | *** | [61] |
| 4. Learned helplessness | Animals are exposed to uncontrolled stressors through behavioral responses. | Manipulate footshock to shape the stressor; thus, effective and easy manipulation. | Also used to test depression behaviors. | PTSD; MDD | * | [62,63] |
| 5. Restraint stress | Mice are immobilized by placing them into well-ventilated 50 mL Falcon tubes for 2 h per day over 21 consecutive days. | Restraint mice for immobility to induce the stressor; easy preparation and manipulation. | Also used to test depression behaviors. | Anxiety disorders; PTSD | * | [64] |
| 6. Inescapable tail shock | Animals experience uncontrolled and inescapable tail shock, leading to acute stress. | Easy manipulation for inescapable tail shock to induce stress. | Also used to test depression behaviors. | PTSD | * | [65,66] |
| 7. Underwater trauma | Animals are held underwater for 30 s. | Easy manipulation for holding animals underwater to induce stress. | Doubt in the face, predictive, and constructive validity. | PTSD | * | [67] |
| 8. Social isolation | Animals are raised without any companion or environmental enrichment. | Easy manipulation for animals without any companions. | Long-term conduction. | PTSD | ** | [13] |
| 9. Social defeat | Animals are exposed to a trained aggressor conspecific for 6 h daily for 5 or 10 days. | Easy manipulation for exposing aggressors inducing stress. | --- | PTSD | ** | [68] |
| 10. Early life stress | Maternal separation induces trauma events. | Face, predictive, and constructive validity. | Long-term conduction. | PTSD | ** | [67] |
| 11. Predator-based stress | Predators or predator-related stimuli (such as predator urine) produce trauma induction. | Place the predator and its related stimuli to induce stress; easy manipulation. | --- | PTSD | ** | [67] |
| B. Testing anxiety behaviors | ||||||
| 1. Open field test | Tests time spent on crossing trials in the center area of the open field task for anxiety responses. | Face, predictive, and constructive validity. | Competition between locomotion and anxiety behavior. | Multiple anxiety disorders; PTSD | *** | [69,70] |
| 2. Elevated zero maze test | The test is conducted in the open arm to indicate the strength of the anxiety responses. | No crossing areas, which enforce animals’ decisions. | Conflicts arise from spending time in open arms and closed arms. | Multiple anxiety disorders; PTSD | ** | [68] |
| 3. Elevated plus maze test | The test is conducted in the open arm to indicate the strength of the anxiety responses. | Cross the area to take a rest. | Long-term staying in the cross area between the closed and open arms | Multiple anxiety disorders; PTSD | *** | [69] |
| 4. Elevated x-maze test | Tests the open arm time/total time ratio. | Face, predictive, and constructive validity. | --- | Multiple anxiety disorders | * | [71,72] |
| 5. Light–dark box test | Tests activity and time spent in both brightly lit and dark apparatus compartments using the animal’s innate desire to explore novel areas. | Assessing the activity and time in light and dark boxes; easy manipulation. | --- | Multiple anxiety disorders | ** | [69] |
| 6. Startle response test | Pairing a conditioned stimulus (sound or light) with a footshock induces an anxiogenic “startle” response. | Face, predictive, and constructive validity for anxiety disorders. | Limitations in the style of anxiety behaviors for a cue with footshock. | Multiple anxiety disorders; PTSD | ** | [10] |
| 7. Marble burying test | Animals with previous stress are placed in the test cage and then test amounts of marble burying up to 2/3 of the depth with bedding. | Face, predictive, and constructive validity for anxiety disorders. | A digging activity for a species-typical reaction to stress (e.g., rats and mice). | Multiple anxiety disorders; PTSD | ** | [73] |
| 8. Defensive shock-prod burying test | A familiar test cage or home cage with plentiful bedding and a hole in the wall 2 cm above the bedding. An electrical probe is connected to a shock source. Measuring the depth to which the prod is buried. | Face, predictive, and constructive validity. | Animals do not touch the electrical probe and cannot induce anxiety. | Multiple anxiety disorders | ** | [74] |
| 9. Grooming test | Stressors (e.g., novel environment, predator exposure, bright light) induce grooming. | Test grooming behavior; simple manipulation. | Questionable face, predictive, and constructive validity. | Multiple anxiety disorders; PTSD | * | [10] |
| 10. Social interaction test | Two mice were in the test environment for 5 or 10 min and recorded the duration and frequency of all social interactions, including sniffing, following, chasing, touching, and biting. Higher scores in social interactions indicate lower anxiety behaviors. | More accessible design and manipulation. | Limitations in social anxiety disorders. | Multiple anxiety disorders; PTSD | ** | [10] |
| 11. Suok test | The Suok task simultaneously tests anxiety, vestibular, and neuromuscular deficits by combining an unstable rod with novelty. The threats of height, loss of balance, and novelty are presented to analyze anxiety and assess animal exploration. | Face validity. | Doubt in predictive and constructive validity. Competitions in testing for multiple behaviors. | Multiple anxiety disorders; PTSD | * | [75] |
| 12. Stress-induced hyperthermia test | Based on the evolutionarily important role of hyperthermia, whereby body temperature rises upon encountering stressful stimuli. | Across many species, including humans. | Testing errors from a lot of confounding factors. | Multiple anxiety disorders; PTSD | * | [10] |
| 13. Hole–board test | Tests head-dipping behaviors. More head dips indicate more explorations and lower anxiety. | Assessing animals’ head-dipping behavior; easy preparation and manipulation. | Doubt in the face, predictive, and constructive validity. | Multiple anxiety disorders | * | [76] |
| 14. Rat exposure test | Uses animals’ natural defensive “avoidance” behavioral response to signs of potential danger, such as a natural predator. Defensive behaviors include stretch-attend posture, stretch approach, freezing, burying, and hiding. | Testing nature defensive behavior; thus, it is easy to use and manipulate. | Variations among different species. | Multiple anxiety disorders | * | [10] |
| 15. Novel object test | Testing the approach-avoidance behaviors of mice in response to novel stimuli. Longer time in exploration for a novel object, indicating lower anxiety behaviors. | Face, predictive, and constructive validity. | Confused with recognition tests using the same task. | Multiple anxiety disorders; PTSD | * | [77] |
| Animal Models of Anxiety Disorders | Clinical Anxiolytic Drugs | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anxiety disorders | 1. Fear conditioning (cue) | 2. Fear conditioning (context) | 3. SPS | 4. Learned helplessness | 5. Restraint stress | 6. Inescapable tail shock | 7. Underwater trauma | 8. Social isolation | 9. Social defeat | 10. Early life stress | 11. Predator-based stress | Medicines |
| 1. GAD | V | V | BDZs; SSRIs; SNRIs; TCAs; calcium modulators; azapirones; antihistamines | |||||||||
| 2. PD | V | V | V | BDZs; SSRIs; SNRIs; TCAs; MAOIs; azapirones; antihistamines | ||||||||
| 3. Agoraphobia | V | BDZs; SSRIs; SNRIs; TCAs; azapirones; antihistamines | ||||||||||
| 4. PTSD | V | V | V | V | V | V | V | V | V | V | V | BDZs; SSRIs; SNRIs; TCAs; azapirones; antihistamines |
| 5. SAD | V | V | BDZs; SSRIs; SNRIs; TCAs; MAOIs; calcium modulators; azapirones; antihistamines | |||||||||
| 6. ASD | V | V | V | V | V | BDZs; TCAs; azapirones; antihistamines | ||||||
| 7. Separation anxiety disorder | V | V | BDZs; TCAs; azapirones; antihistamines | |||||||||
| 8. OCD | V | V | V | V | BDZs; SSRIs; TCAs; azapirones; antihistamines | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozłowska, A.; Ma, W.-J.; Shyu, B.-C.; Huang, A.C.W. Analysis of Anxiety Disorders and Post-Traumatic Stress Disorders for Screening Anxiolytic Drugs and Linking Preclinical and Clinical Research. Int. J. Mol. Sci. 2025, 26, 1414. https://doi.org/10.3390/ijms26041414
Kozłowska A, Ma W-J, Shyu B-C, Huang ACW. Analysis of Anxiety Disorders and Post-Traumatic Stress Disorders for Screening Anxiolytic Drugs and Linking Preclinical and Clinical Research. International Journal of Molecular Sciences. 2025; 26(4):1414. https://doi.org/10.3390/ijms26041414
Chicago/Turabian StyleKozłowska, Anna, Wan-Jiun Ma, Bai-Chuang Shyu, and Andrew Chih Wei Huang. 2025. "Analysis of Anxiety Disorders and Post-Traumatic Stress Disorders for Screening Anxiolytic Drugs and Linking Preclinical and Clinical Research" International Journal of Molecular Sciences 26, no. 4: 1414. https://doi.org/10.3390/ijms26041414
APA StyleKozłowska, A., Ma, W.-J., Shyu, B.-C., & Huang, A. C. W. (2025). Analysis of Anxiety Disorders and Post-Traumatic Stress Disorders for Screening Anxiolytic Drugs and Linking Preclinical and Clinical Research. International Journal of Molecular Sciences, 26(4), 1414. https://doi.org/10.3390/ijms26041414









