Unveiling the Synergistic Effect of Salicylic Acid on Triterpenoid Biosynthesis in Athelia termitophila: Elucidating the Molecular Underpinnings
Abstract
1. Introduction
2. Results
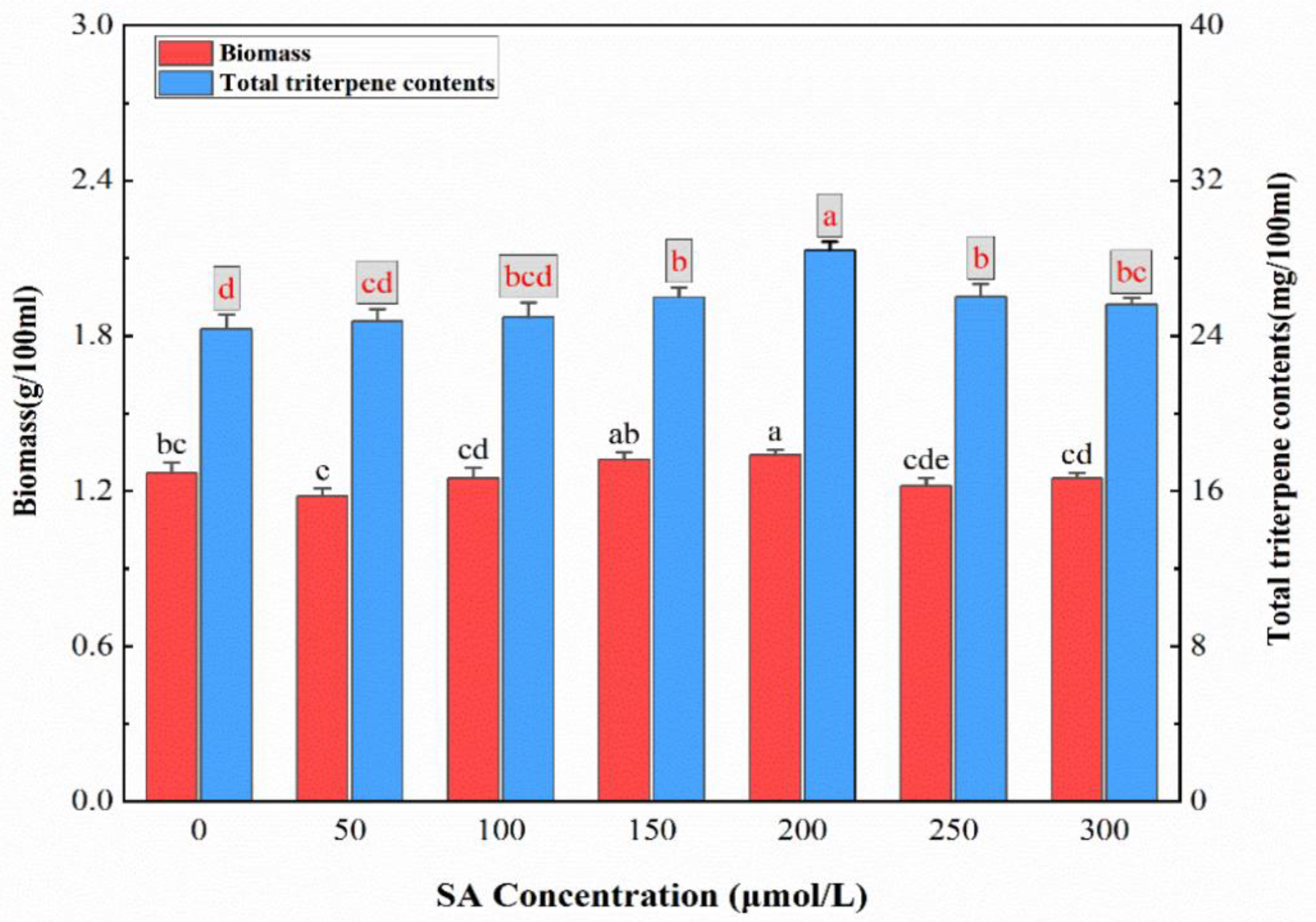
2.1. Evaluation of the Effect of SA Concentration on Mycelial Biomass and Triterpenoid Production in TMB
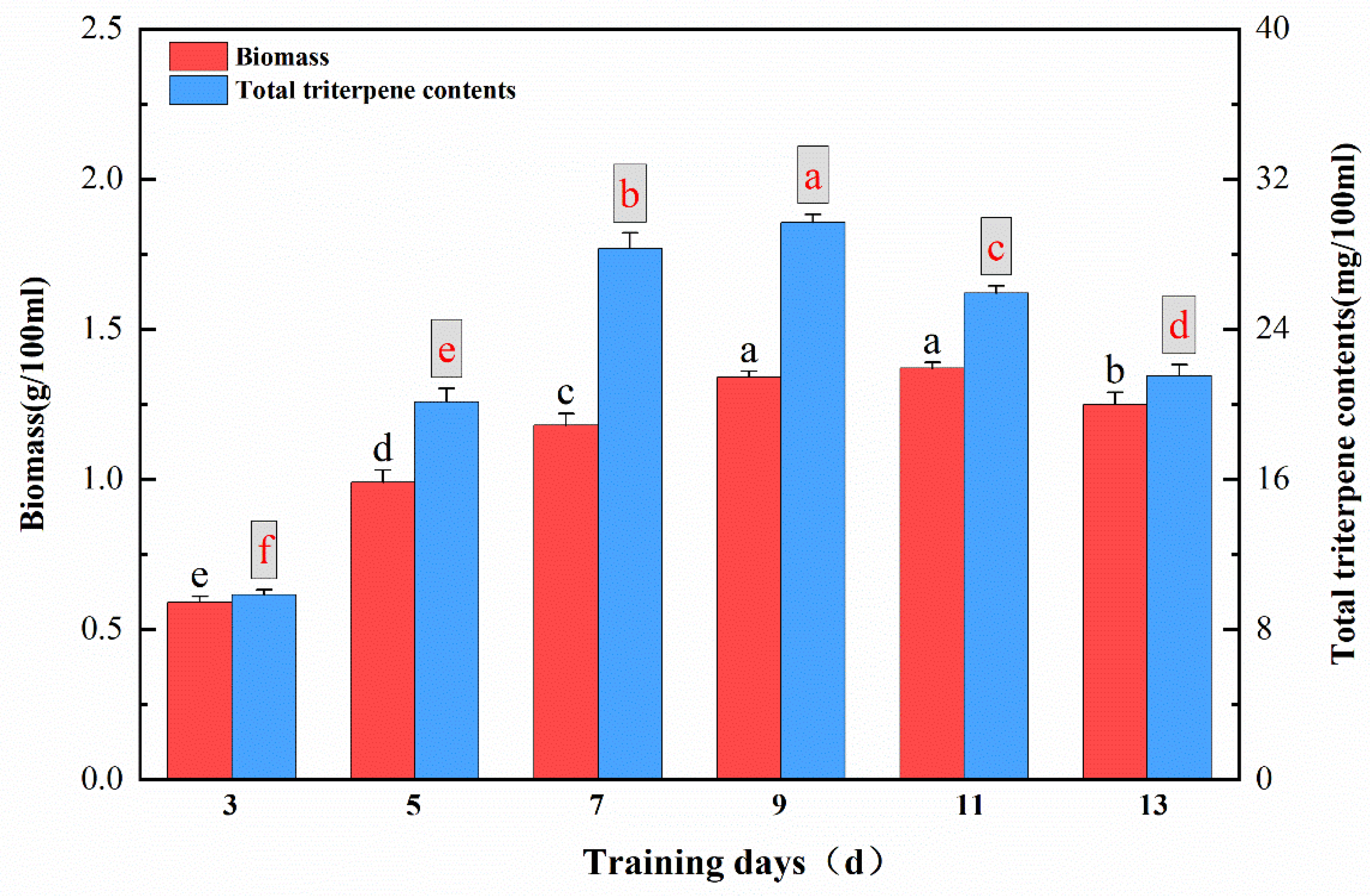
2.2. Influence of Cultivation Duration on Mycelial Biomass and Total Triterpenoid Content of TMB
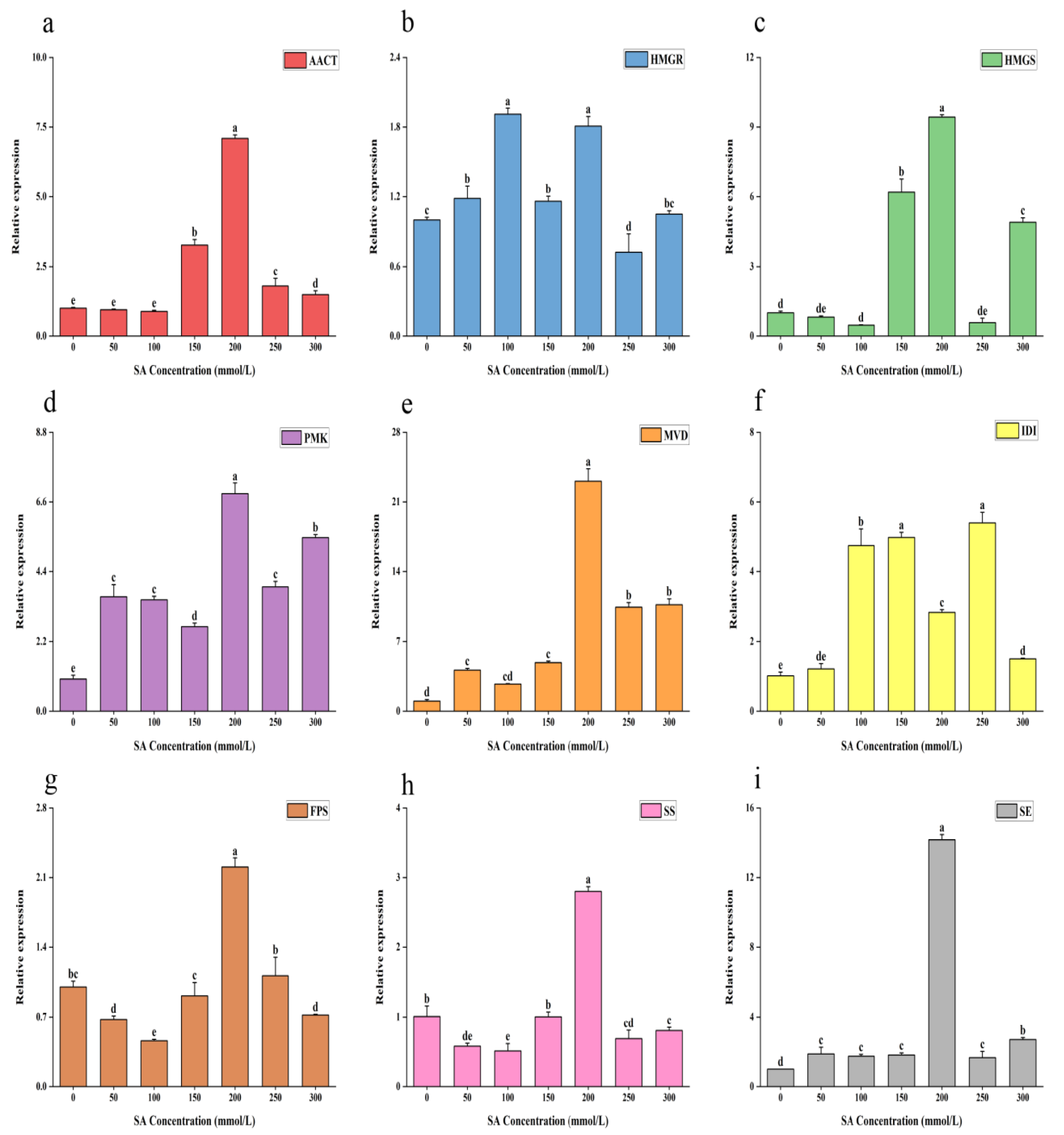
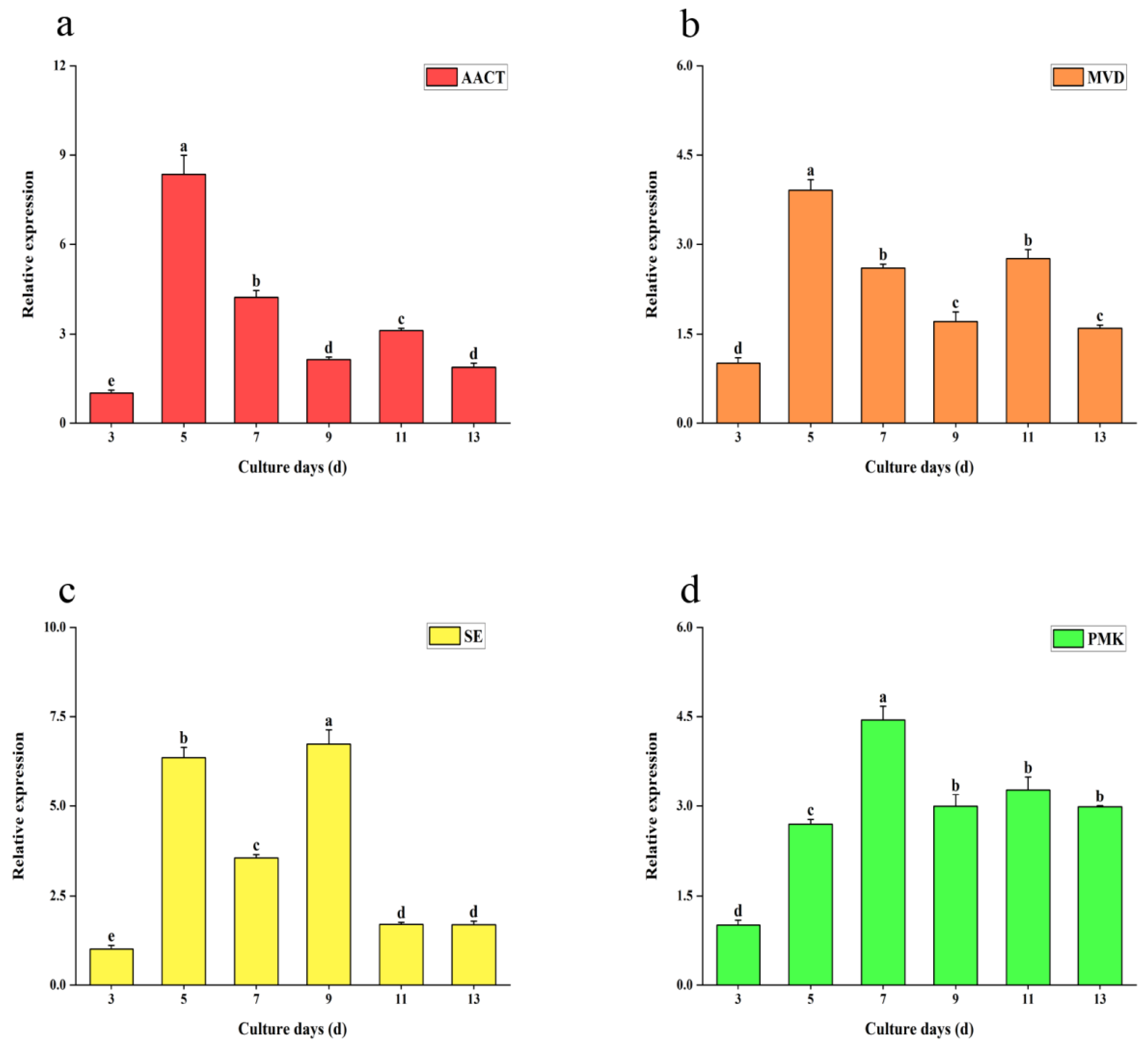
2.3. Expression of Related Genes Under Different Concentrations of Salicylic Acid
2.4. Analysis of Correlation Between Total Triterpenes and Genes Under Salicylic Acid Treatment
2.5. Dynamic Change Trend of Key Genes with Time Under Salicylic Acid Treatment
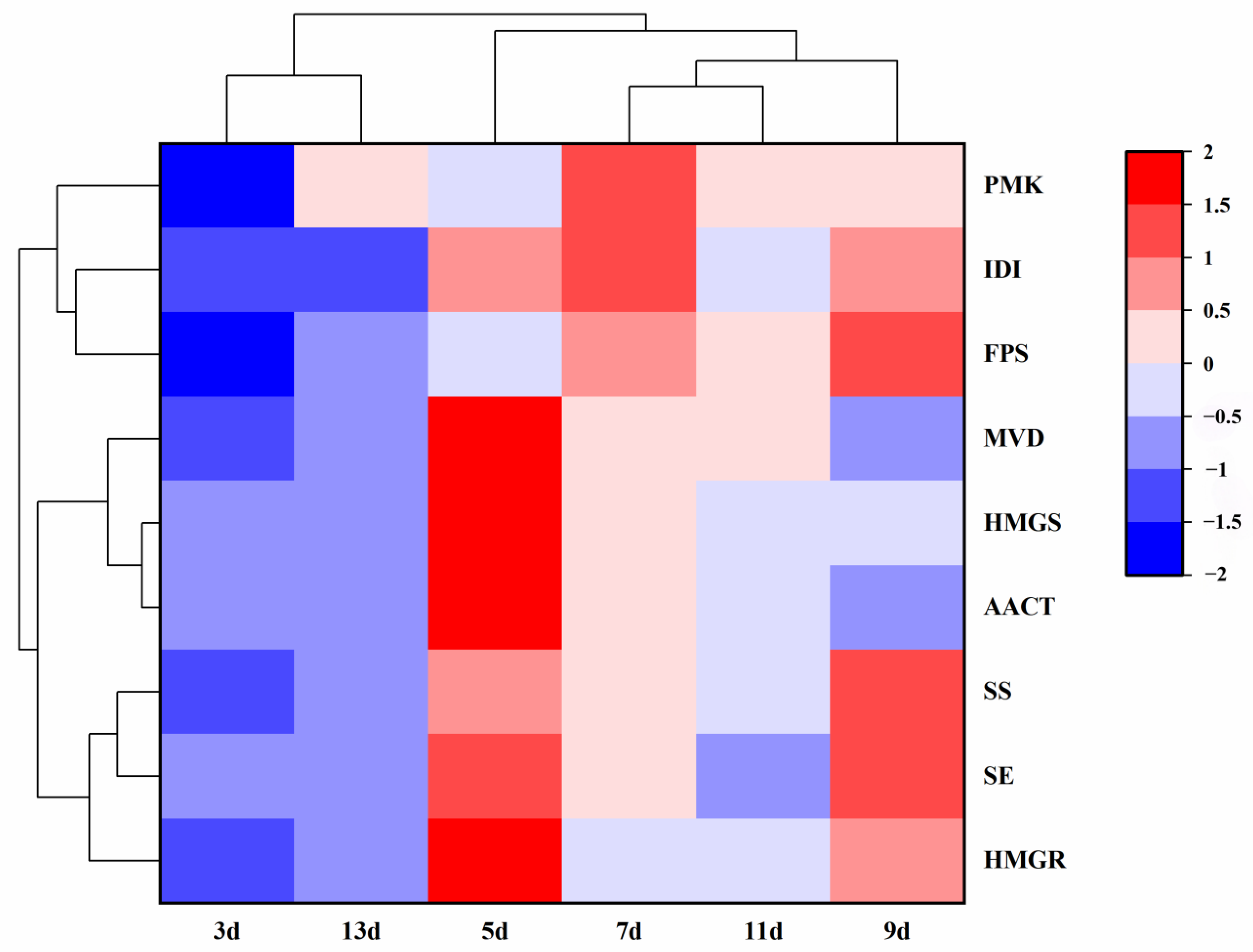
2.6. Cluster Expression Analysis of Related Genes in Response to Salicylic Acid in Different Periods
3. Discussion
4. Materials and Methods
4.1. Strains of Athelia Termitophila and Their Cultural Conditions
4.2. Composition of the Medium
4.3. Method for the Determination of Mycelial Biomass
4.4. Extraction, Detection, and Analysis of Total Triterpenes in a Mycelium
4.5. Optimization of Salicylic Acid Concentration for the Total Triterpenoid Synthesis of TMB
4.6. Effects of Different Incubation Periods on the Yield of Total Triterpenoids in TMB
4.7. Measurement of the Gene Transcription Level by qRT-PCR
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, L.; Ye, Y.; Lin, T.; Li, M.; Bi, Y.; Li, Q.; Liu, H. The evaluation of the nutritional quality of common wild edible fungi in Yunnan Province. J. Food Sci. 2023, 88, 1879–1889. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Ying, T. Isolation, molecular characterization and antioxidant activity of a water-soluble polysaccharide extracted from the fruiting body of Termitornyces albuminosus (Berk.) Heim. Int. J. Biol. Macromol. 2019, 122, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K. Termite-egg mimicry by a sclerotium-forming fungus. Proc. Biol. Sci. 2006, 273, 1203–1209. [Google Scholar] [CrossRef]
- Maekawa, N.; Yokoi, H.; Sotome, K.; Matsuura, K.; Tanaka, C.; Endo, N.; Nakagiri, A.; Ushijima, S. Athelia termitophila sp. nov. is the teleomorph of the termite ball fungus Fibularhizoctonia sp. Mycoscience 2020, 61, 323–330. [Google Scholar] [CrossRef]
- Ye, C.; Li, J.; Ran, Y.; Rasheed, H.; Xing, L.; Su, X. The nest fungus of the lower termite Reticulitermes labralis. Sci. Rep. 2019, 9, 3384. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.A.; Connolly, J.D. Triterpenoids. Nat. Prod. Rep. 2013, 30, 1028–1065. [Google Scholar] [CrossRef]
- Yendo, A.C.; de Costa, F.; Gosmann, G.; Fett-Neto, A.G. Production of plant bioactive triterpenoid saponins: Elicitation strategies and target genes to improve yields. Mol. Biotechnol. 2010, 46, 94–104. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, G.; Sun, Y.; Wu, X.; Zhao, Y. Triterpenes derived from hydrolyzate of total Gynostemma pentaphyllum saponins with anti-hepatic fibrosis and protective activity against H2O2-induced injury. Phytochemistry 2017, 144, 226–232. [Google Scholar] [CrossRef]
- Feng, L.; Yuan, L.; Du, M.; Chen, Y.; Zhang, M.H.; Gu, J.F.; He, J.J.; Wang, Y.; Cao, W. Anti-lung cancer activity through enhancement of immunomodulation and induction of cell apoptosis of total triterpenes extracted from Ganoderma luncidum (Leyss. ex Fr.) Karst. Molecules 2013, 18, 9966–9981. [Google Scholar] [CrossRef]
- Incandela, L.; Cesarone, M.R.; Cacchio, M.; De Sanctis, M.T.; Santavenere, C.; D’Auro, M.G.; Bucci, M.; Belcaro, G. Total triterpenic fraction of Centella asiatica in chronic venous insufficiency and in high-perfusion microangiopathy. Angiology 2001, 52 (Suppl. S2), S9–S13. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, Y.; Hu, H.; Zhang, Z. In vitro and in vivo antimammary tumor activities and mechanisms of the apple total triterpenoids. J. Agric. Food Chem. 2012, 60, 9430–9436. [Google Scholar] [CrossRef] [PubMed]
- Sawai, S.; Saito, K. Triterpenoid biosynthesis and engineering in plants. Front. Plant Sci. 2011, 2, 25. [Google Scholar] [CrossRef]
- Wang, X.; Sun, J.; Wang, S.; Sun, T.; Zou, L. Salicylic acid promotes terpenoid synthesis in the fungi Sanghuangporus baumii. Microb. Biotechnol. 2023, 16, 1360–1372. [Google Scholar] [CrossRef]
- Ye, J.B.; Mao, D.; Cheng, S.Y.; Zhang, X.; Tan, J.P.; Zheng, J.R.; Xu, F. Comparative transcriptome analysis reveals the potential stimulatory mechanism of terpene trilactone biosynthesis by exogenous salicylic acid in Ginkgo biloba. Ind. Crop. Prod. 2020, 145, 112104. [Google Scholar] [CrossRef]
- Yin, J.; Sun, L.; Li, Y.; Xiao, J.; Wang, S.; Yang, J.; Qu, Z.; Zhan, Y. Functional identification of BpMYB21 and BpMYB61 transcription factors responding to MeJA and SA in birch triterpenoid synthesis. BMC Plant. Biol. 2020, 20, 374. [Google Scholar] [CrossRef]
- Dinday, S.; Ghosh, S. Recent advances in triterpenoid pathway elucidation and engineering. Biotechnol. Adv. 2023, 68, 108214. [Google Scholar] [CrossRef]
- Yang, L.; Gu, Y.; Zhou, J.; Yuan, P.; Jiang, N.; Wu, Z.; Tan, X. Whole-Genome Identification and Analysis of Multiple Gene Families Reveal Candidate Genes for Theasaponin Biosynthesis in Camellia oleifera. Int. J. Mol. Sci. 2022, 23, 6393. [Google Scholar] [CrossRef]
- Xu, W.; Yao, J.; Liu, L.; Ma, X.; Li, W.; Sun, X.; Wang, Y. Improving squalene production by enhancing the NADPH/NADP+ ratio, modifying the isoprenoid-feeding module and blocking the menaquinone pathway in Escherichia coli. Biotechnol. Biofuels 2019, 12, 68. [Google Scholar] [CrossRef]
- Phan, H.-V.-T.; Nguyen, D.V.; Le, T.-K.-D.; Nguyen, T.-A.-M.; Dong, P.-S.-N.; Tran, T.-N.; Dao, N.-V.-T.; Nguyen, H.C.; Luu, H.T.; Chavasiri, W.; et al. Morusacerane: A new gammacerane triterpenoid from the trunk of Morus Alba linn. with α-glucosidase inhibitory activity. Nat. Prod. Res. 2024, 38, 1–10. [Google Scholar] [CrossRef]
- Matsuura, K.; Tanaka, C.; Nishida, T. Symbiosis of a termite and a sclerotium-forming fungus: Sclerotia mimic termite eggs. Ecol. Res. 2000, 15, 405–414. [Google Scholar] [CrossRef]
- Costa-Leonardo, A.M.; Janei, V.; da Silva, I.B. First Neotropical record of the association between brown sclerotium-forming fungi and termite eggs in a nest of Coptotermes gestroi (Blattaria, Isoptera, Rhinotermitidae). Sci. Nat. 2022, 109, 45. [Google Scholar] [CrossRef] [PubMed]
- Guillamón, E.; García-Lafuente, A.; Lozano, M.; D’Arrigo, M.; Rostagno, M.A.; Villares, A.; Martínez, J.A. Edible mushrooms: Role in the prevention of cardiovascular diseases. Fitoterapia 2010, 81, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.Z. A review of ergostane and cucurbitane triterpenoids of mushroom origin. Nat. Prod. Res. 2014, 28, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Mou, Z. Salicylic acid and its function in plant immunity. J. Integr. Plant Biol. 2011, 53, 412–428. [Google Scholar] [CrossRef]
- Meng, L.; Bai, X.; Zhang, S.; Zhang, M.; Zhou, S.; Mukhtar, I.; Wang, L.; Li, Z.; Wang, W. Enhanced ganoderic acids accumulation and transcriptional responses of biosynthetic genes in Ganoderma lucidum fruiting bodies by elicitation supplementation. Int. J. Mol. Sci. 2019, 20, 2830. [Google Scholar] [CrossRef]
- Shi, L.; Tan, Y.; Sun, Z.; Ren, A.; Zhu, J.; Zhao, M. Exogenous salicylic acid (SA) promotes the accumulation of biomass and flavonoid content in Phellinus igniarius (Agaricomycetes). Int. J. Med. Mushrooms 2019, 21, 955–963. [Google Scholar] [CrossRef]
- Ong, C.E.; Ahmad, R.; Goh, Y.K.; Azizan, K.A.; Baharum, S.N.; Goh, K.J. Growth modulation and metabolic responses of Ganoderma boninense to salicylic acid stress. PLoS ONE 2021, 16, e0262029. [Google Scholar] [CrossRef]
- Liang, C.X.; Li, Y.B.; Xu, J.W.; Wang, J.L.; Miao, X.L.; Tang, Y.J.; Gu, T.; Zhong, J.J. Enhanced biosynthetic gene expressions and production of ganoderic acids in static liquid culture of Ganoderma lucidum under phenobarbital induction. Appl. Microbiol. Biotechnol. 2010, 86, 1367–1374. [Google Scholar] [CrossRef]
- Ren, A.; Qin, L.; Shi, L.; Dong, X.; da Mu, S.; Li, Y.X.; Zhao, M.W. Methyl jasmonate induces ganoderic acid biosynthesis in the basidiomycetous fungus Ganoderma lucidum. Bioresour. Technol. 2010, 101, 6785–6790. [Google Scholar] [CrossRef]
- Cao, P.F.; Wu, C.G.; Dang, Z.H.; Shi, L.; Jiang, A.L.; Ren, A.; Zhao, M.W. Effects of exogenous salicylic acid on ganoderic acid biosynthesis and the expression of key genes in the ganoderic acid biosynthesis pathway in the LingZhi or Reishi medicinal mushroom, Ganoderma lucidum (Agaricomycetes). Int. J. Med. Mushrooms 2017, 19, 65–73. [Google Scholar] [CrossRef]
- Majdi, M.; Abdollahi, M.R.; Maroufi, A. Parthenolide accumulation and expression of genes related to parthenolide biosynthesis affected by exogenous application of methyl jasmonate and salicylic acid in Tanacetum parthenium. Plant Cell Rep. 2015, 34, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Khanom, S.; Jang, J.; Lee, O.R. Overexpression of ginseng cytochrome P450 CYP736A12 alters plant growth and confers phenylurea herbicide tolerance in Arabidopsis. J. Ginseng Res. 2019, 43, 645–653. [Google Scholar] [CrossRef]
- Xie, L.; Zhu, Y.; Gao, M.; Chen, S.; Li, L.; Liu, Y.; Gu, T.; Zhang, J. Mechanisms of the increase triterpenoids content of Morchella eximia induced by salicylic acid and magnetic field. Food Bioprod. Process. 2024, 145, 21–31. [Google Scholar] [CrossRef]
- Noushahi, H.A.; Khan, A.H.; Noushahi, U.F.; Hussain, M.; Javed, T.; Zafar, M.; Batool, M.; Ahmed, U.; Liu, K.; Harrison, M.T.; et al. Biosynthetic pathways of triterpenoids and strategies to improve their biosynthetic efficiency. Plant Growth Regul. 2022, 97, 439–454. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Luo, B.; Yang, Y.; Faruque, M.O.; Zhang, J.; Li, X.; Hu, X. Addition of vegetable oil to improve triterpenoids production in liquid fermentation of medicinal fungus Antrodia cinnamomea. J. Fungi 2021, 7, 926. [Google Scholar] [CrossRef]
- Hao, X.; Shi, M.; Cui, L.; Xu, C.; Zhang, Y.; Kai, G. Effects of methyl jasmonate and salicylic acid on tanshinone production and biosynthetic gene expression in transgenic Salvia miltiorrhiza hairy roots. Biotechnol. Appl. Biochem. 2015, 62, 24–31. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, Z.; Shi, Q.; Yue, R.; Li, X. Metabolite and gene expression analysis underlying temporal and spatial accumulation of pentacyclic triterpenoids in jujube. Genes 2022, 13, 823. [Google Scholar] [CrossRef]
- Yu, H.; Guo, W.; Yang, D.; Hou, Z.; Liang, Z. Transcriptional profiles of SMWRKY family genes and their putative roles in the biosynthesis of tanshinone and phenolic acids in Salvia miltiorrhiza. Int. J. Mol. Sci. 2018, 19, 1593. [Google Scholar] [CrossRef]
- Cai, C.; Ma, J.; Han, C.; Jin, Y.; Zhao, G.; He, X. Extraction and antioxidant activity of total triterpenoids in the mycelium of a medicinal fungus, Sanghuangporus sanghuang. Sci. Rep. 2019, 9, 7418. [Google Scholar] [CrossRef]

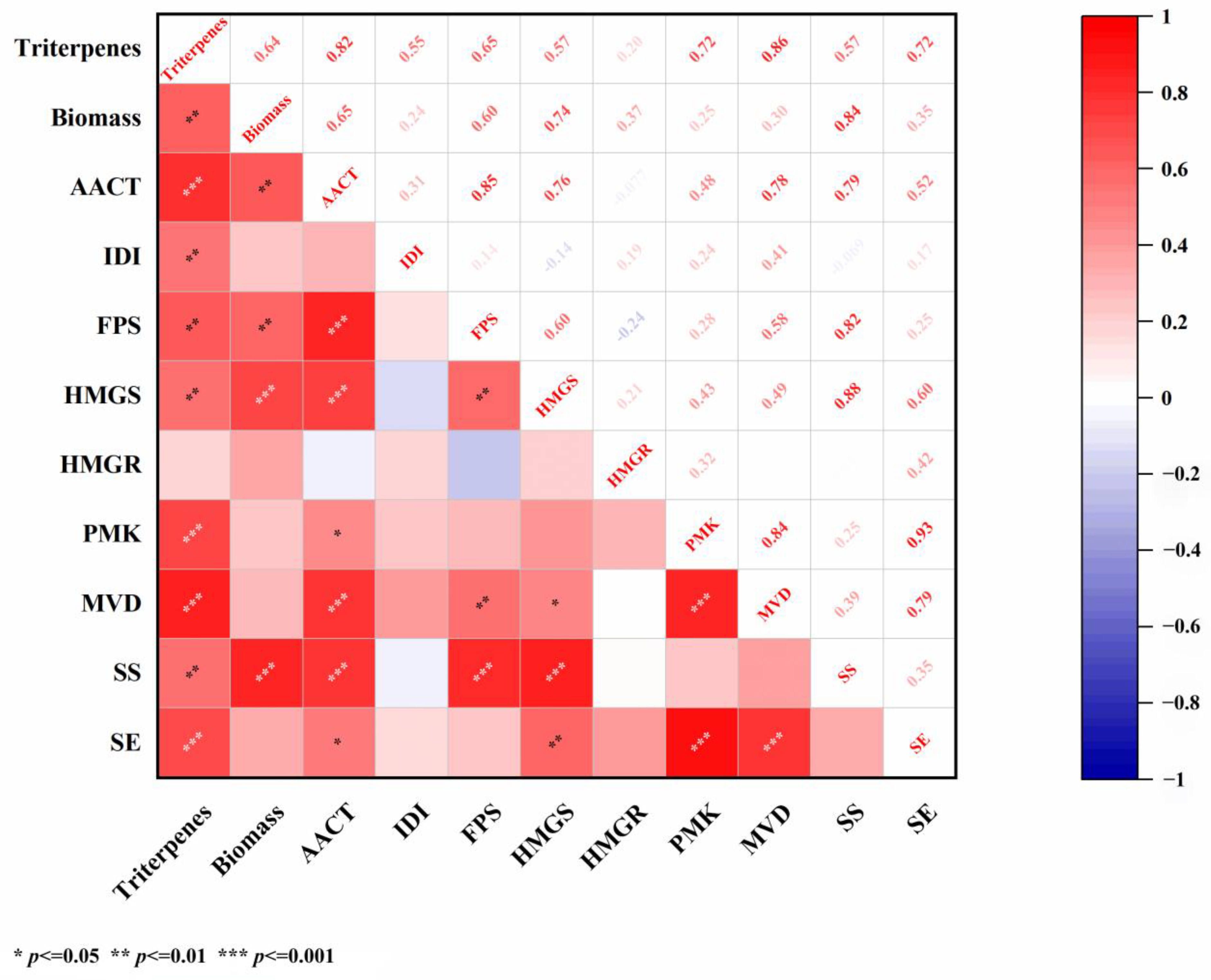
| Project | Correlation Coefficient | ||||
|---|---|---|---|---|---|
| Biomass | AACT | SE | MVD | PMK | |
| Triterpenes | 0.64 ** | 0.82 *** | 0.72 *** | 0.86 *** | 0.72 *** |
| Biomass | 1 | 0.65 ** | 0.35 | 0.3 | 0.25 |
| Gene Abbreviation | Gene Full Name | Primer Name and Size | Sequence (5′-3′) |
|---|---|---|---|
| AACT | Acetyl-Coa Acetyltransferase | AACT-221-F | CTATCAAGGGCAAGAAGGGTG |
| AACT-221-R | GGAGATGACTTTGGCAAGAGGTT | ||
| HMGS | 3-Hydroxy-3-Methylglutaryl-Coa Synthase | HMGS-153-F | GTCTTCGATGGAGTGTCTAAGGG |
| HMGS-153-R | ACCGATAGATTTTGGGTCGATGT | ||
| HMGR | 3-Hydroxy-3-Methylglutaryl-Coa Reductase | HMGR-127-F | ACCAACCAACAACGGCGA |
| HMGR-127-R | GCCCTTCACACCGAGCAT | ||
| PMK | Phosphomevalonate Kinase | PMK-200-F | CGGATGATGCTTGCTGATGT |
| PMK-200-R | GCTGTGAGCGTGTAAGTGCC | ||
| MVD | Mevalonate Diphosphate Decarboxylase | MVD-191-F | TATGGTTGAACGGCAAGGTAGA |
| MVD-191-R | TGATGCGGAAGATGCGAGA | ||
| IDI | Isopentenyl-Diphosphate Delta Isomerase | IDI-138-F | GTGACACCCAATGAGAACGAA |
| IDI-138-R | CCACCAGCCGAACAGGAA | ||
| FPS | Farnesyl Diphosphate Synthase | FPS-187-F | CATCGGCAAACAAACTGGCA |
| FPS-187-R | GAGAACAAGGAAGGCACCGA | ||
| SS | Squalene Synthase | SS-182-F | TGGATACCATTGAGGACGACA |
| SS-182-R | TGGAGAAAGGCGGTTGACT | ||
| SE | Squalene Epoxidase | SE-132-F | CGGTCGTGCTCGTGAAGGG |
| SE-132-R | GGAGGATGTGCGTCGTTATATGC | ||
| 18S rRNA | 18S ribosomal RNA | 18S rRNA-164-F | ACATCCGCCATCCATTCC |
| 18S rRNA-164-R | TCGCTGCCCATCACCATA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, F.; Fang, Y.; Khan, Z.; Xing, L. Unveiling the Synergistic Effect of Salicylic Acid on Triterpenoid Biosynthesis in Athelia termitophila: Elucidating the Molecular Underpinnings. Int. J. Mol. Sci. 2025, 26, 996. https://doi.org/10.3390/ijms26030996
Hu F, Fang Y, Khan Z, Xing L. Unveiling the Synergistic Effect of Salicylic Acid on Triterpenoid Biosynthesis in Athelia termitophila: Elucidating the Molecular Underpinnings. International Journal of Molecular Sciences. 2025; 26(3):996. https://doi.org/10.3390/ijms26030996
Chicago/Turabian StyleHu, Fangcheng, Yonggang Fang, Zahid Khan, and Lianxi Xing. 2025. "Unveiling the Synergistic Effect of Salicylic Acid on Triterpenoid Biosynthesis in Athelia termitophila: Elucidating the Molecular Underpinnings" International Journal of Molecular Sciences 26, no. 3: 996. https://doi.org/10.3390/ijms26030996
APA StyleHu, F., Fang, Y., Khan, Z., & Xing, L. (2025). Unveiling the Synergistic Effect of Salicylic Acid on Triterpenoid Biosynthesis in Athelia termitophila: Elucidating the Molecular Underpinnings. International Journal of Molecular Sciences, 26(3), 996. https://doi.org/10.3390/ijms26030996







