Network Pharmacology and Bioinformatics Study of Six Medicinal Food Homologous Plants Against Colorectal Cancer
Abstract
1. Introduction
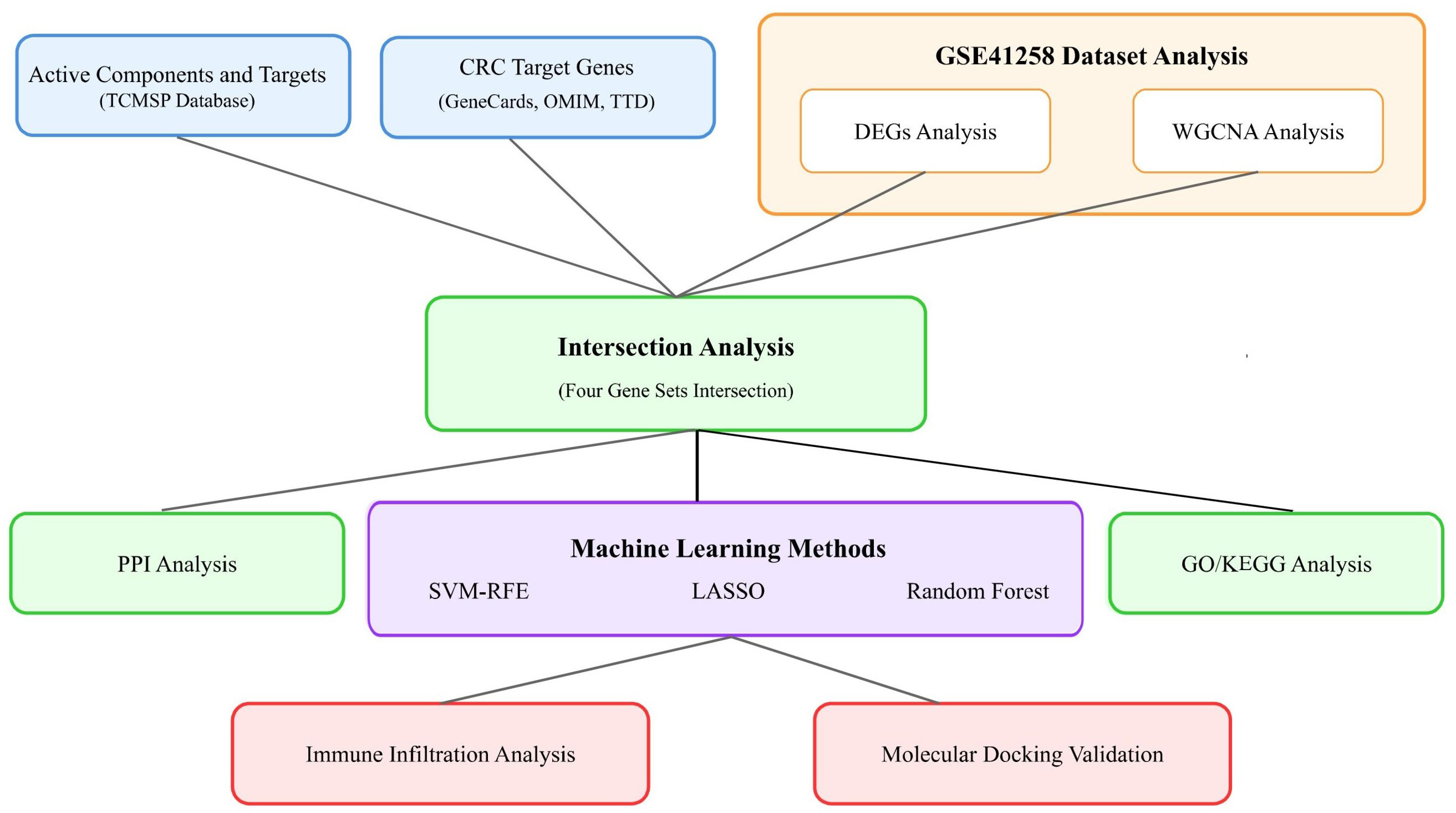
2. Results
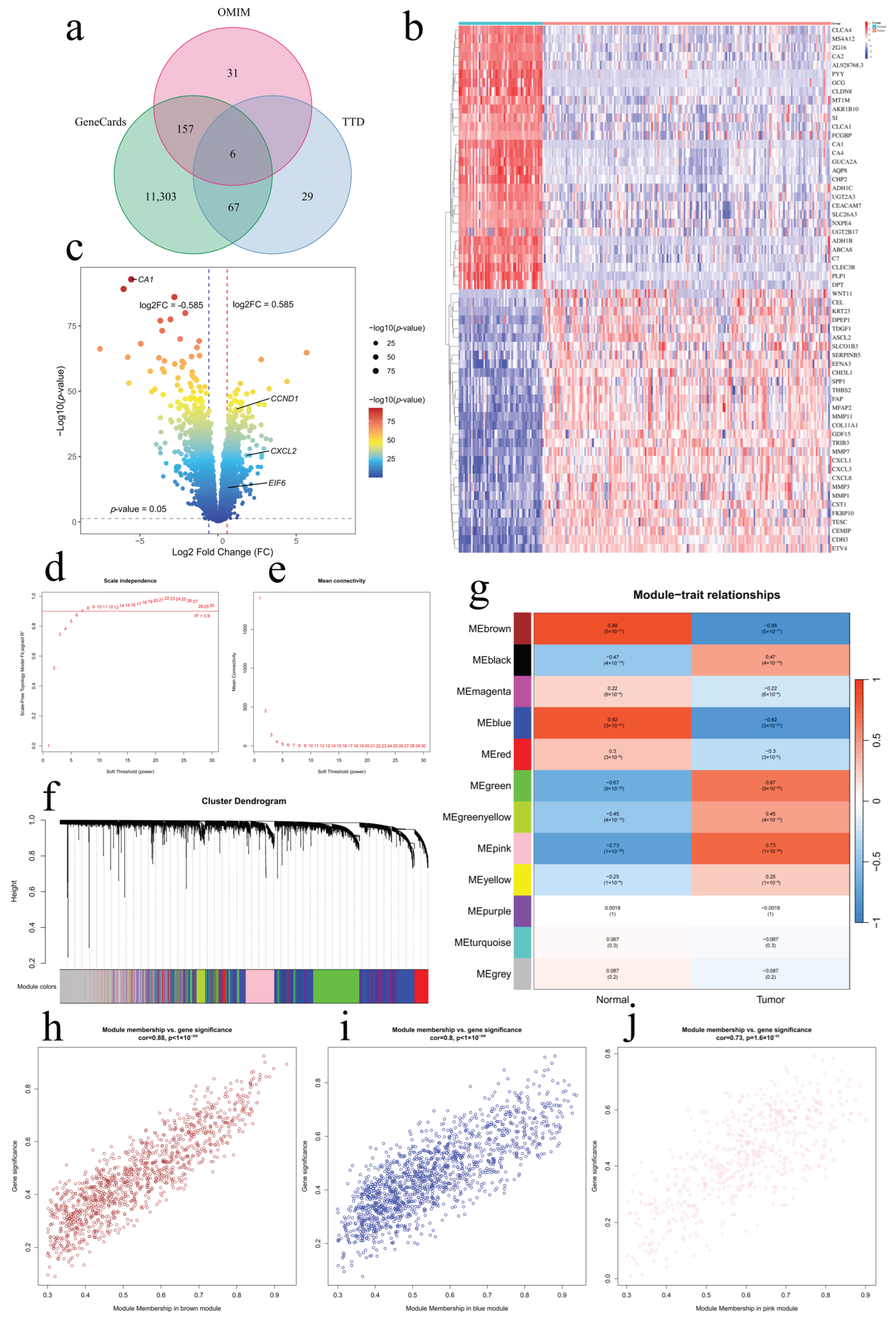
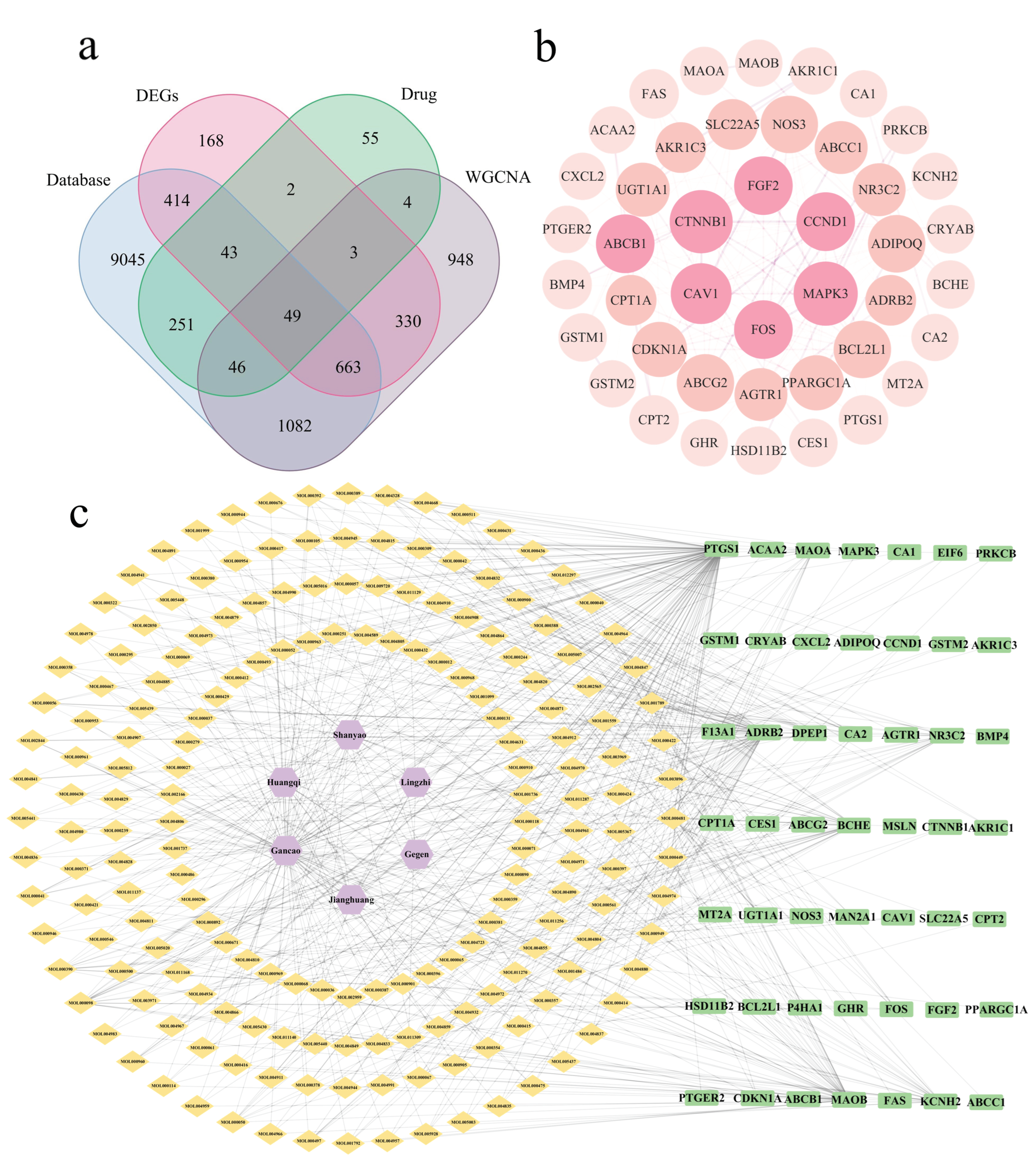
2.1. Screening of Bioactive Compounds and Therapeutic Targets
2.2. Establishment of Protein–Protein Interaction and Drug–Disease Networks
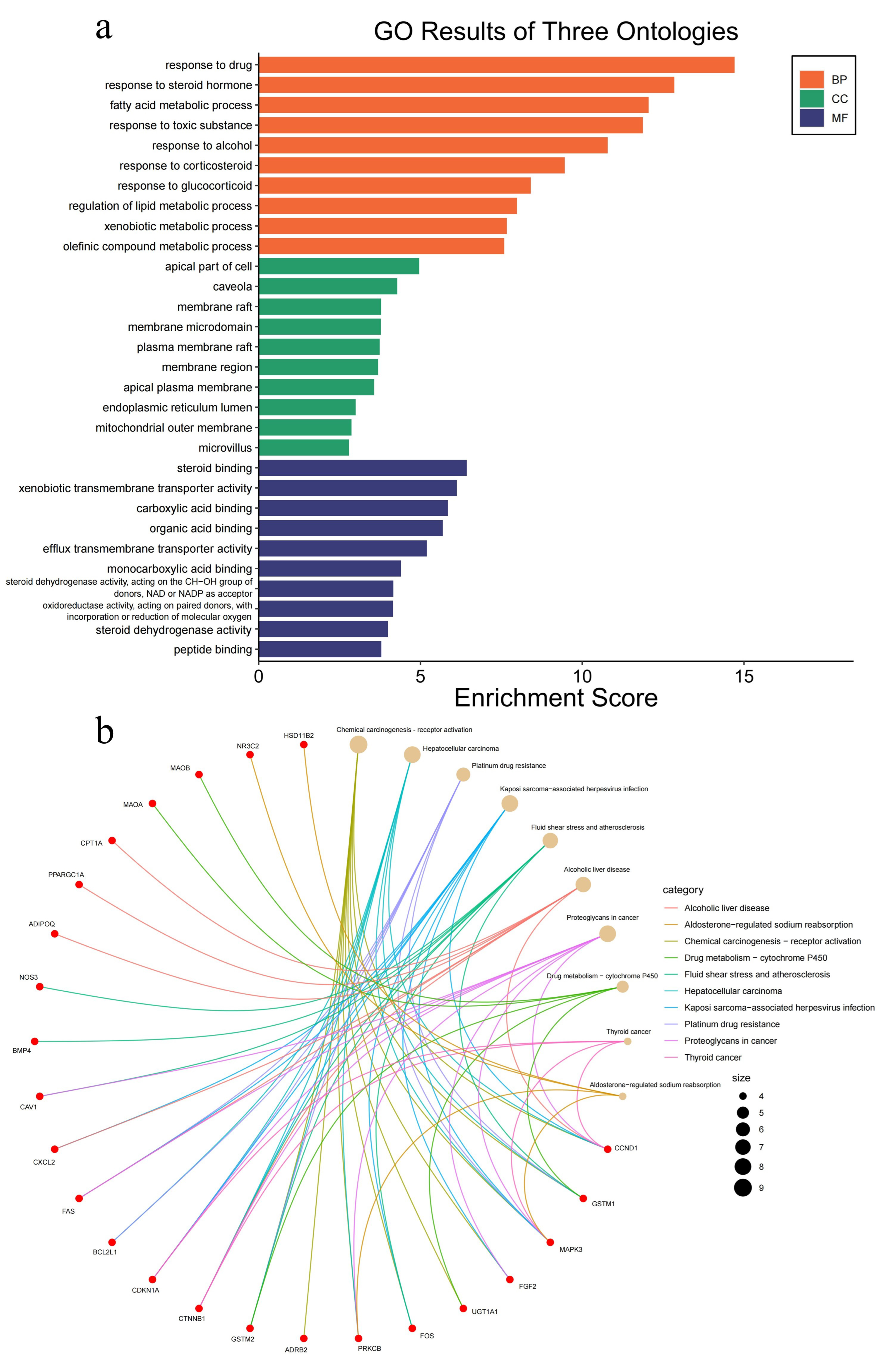
2.3. Gene Ontology and KEGG Pathway Analysis
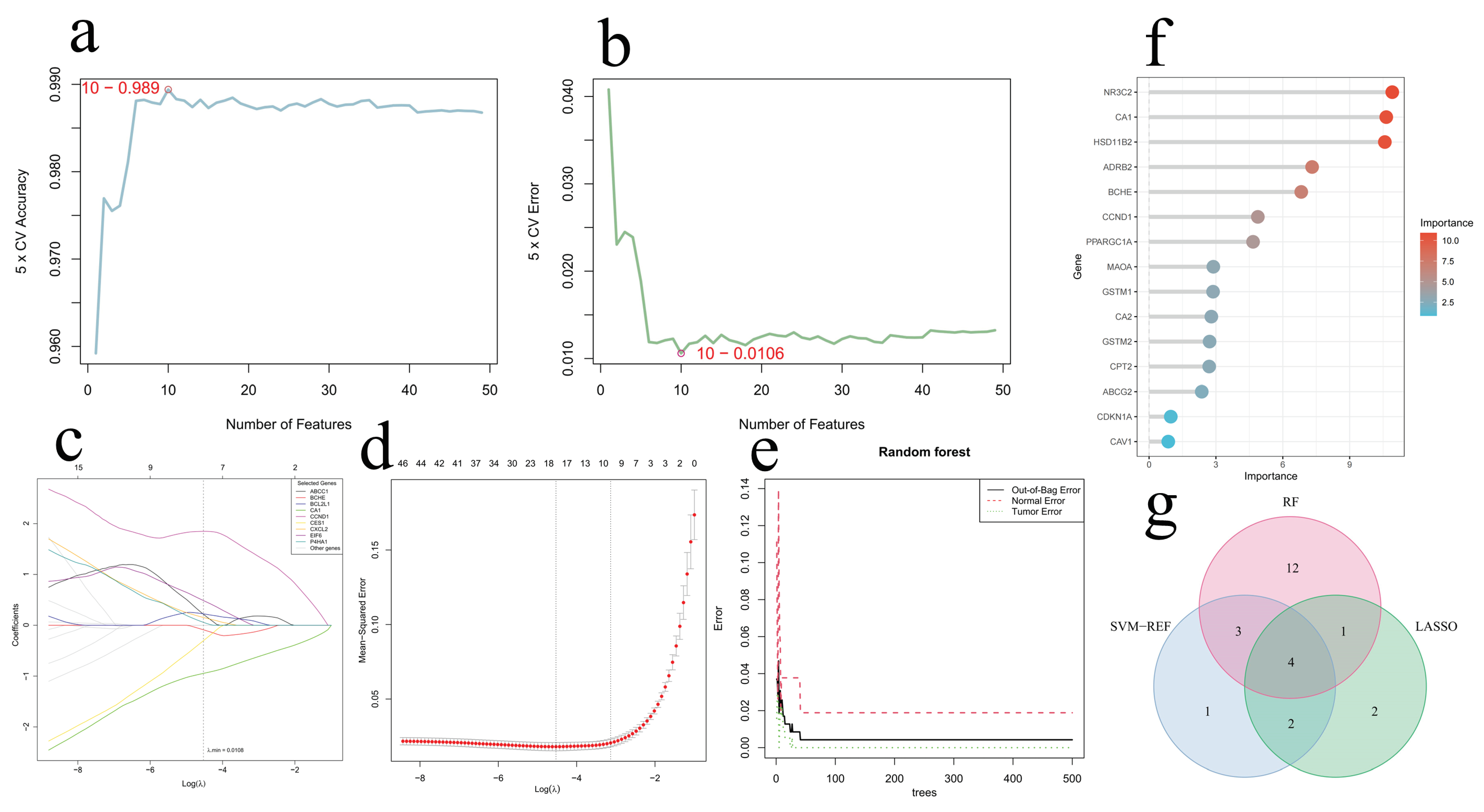
2.4. Identification and Validation of Core Genes
2.5. Immune Cell Infiltration and Core Gene Associations
2.6. Docking Analysis of Core Target Proteins and Their Ligands
3. Discussion
4. Materials and Methods
4.1. Acquisition of Active Components and Drug Targets
4.2. Retrieval of Disease-Related Targets from Public Databases
4.3. Differential Gene Analysis
4.4. Weighted Gene Co-Expression Network Analysis (WGCNA)
4.5. Construction of Protein–Protein Interaction Network
4.6. Functional Enrichment Analysis
4.7. Machine Learning Analysis for Hub Gene Selection
4.8. Immune Infiltration Analysis
4.9. Molecular Docking
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A.; Fuchs, H.E.; Alcaraz, K.I.; Vignat, J.; Bray, F.; Ferlay, J.; Soerjomataram, I. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020, 159, 335–349.e15. [Google Scholar] [CrossRef] [PubMed]
- Fatima, F.; Chourasiya, N.K.; Mishra, M.; Kori, S.; Pathak, S.; Das, R.; Kashaw, V.; Iyer, A.K.; Kashaw, S.K. Curcumin and its derivatives targeting multiple signaling pathways to elicit anticancer activity: A comprehensive perspective. Curr. Med. Chem. 2024, 31, 3668–3714. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.H.; Chen, Y.X.; Fang, J.Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target. Ther. 2020, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Araghi, M.; Soerjomataram, I.; Jenkins, M.; Brierley, J.; Morris, E.; Bray, F.; Arnold, M. Global trends in colorectal cancer mortality: Projections to the year 2035. Int. J. Cancer 2019, 144, 2992–3000. [Google Scholar] [CrossRef]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.K.; Li, Y.H.; Bai, X.S.; Lin, G.L. The Cell Cycle-Associated Protein CDKN2A May Promotes Colorectal Cancer Cell Metastasis by Inducing Epithelial-Mesenchymal Transition. Front. Oncol. 2022, 12, 834235. [Google Scholar] [CrossRef] [PubMed]
- Haggar, F.A.; Boushey, R.P. Colorectal cancer epidemiology: Incidence, mortality, survival, and risk factors. Clin. Colon. Rectal Surg. 2009, 22, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wei, Y.; Wang, J.; Hou, M.; Su, L.; Zheng, C.; Li, Q.; Zhang, Y.; Wu, T.; Zhang, L. Treatment of colorectal cancer by traditional Chinese medicine: Prevention and treatment mechanisms. Front. Pharmacol. 2024, 15, 1377592. [Google Scholar] [CrossRef]
- Zhang, X.; Qiu, H.; Li, C.; Cai, P.; Qi, F. The positive role of traditional Chinese medicine as an adjunctive therapy for cancer. Biosci. Trends 2021, 15, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, Z.; Huang, Y.; O’Barr, S.A.; Wong, R.A.; Yeung, S.; Chow, M.S. Ginseng and anticancer drug combination to improve cancer chemotherapy: A critical review. Evid. Based Complement. Alternat. Med. 2014, 2014, 168940. [Google Scholar] [CrossRef]
- Guo, K.; Wang, Z.; Su, X.; Li, Y.; Xu, J.; Zhang, H.; Wang, T.; Zang, M.; Liu, W.; Yang, G. Chinese medicine in colorectal cancer treatment: From potential targets and mechanisms to clinical application. Chin. J. Integr. Med. 2024. [Google Scholar] [CrossRef]
- Huang, J.; Tang, X.; Ye, F.; He, J.; Kong, X. Clinical Therapeutic Effects of Aspirin in Combination with Fufang Danshen Diwan, a Traditional Chinese Medicine Formula, on Coronary Heart Disease: A Systematic Review and Meta-Analysis. Cell. Physiol. Biochem. 2016, 39, 1955–1963. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, X.; Liu, H.; Sun, B. Medicine and food homology substances: A review of bioactive ingredients, pharmacological effects and applications. Food Chem. 2025, 463 Pt 1, 141111. [Google Scholar] [CrossRef]
- Li, X.; Qu, L.; Dong, Y.; Han, L.; Liu, E.; Fang, S.; Zhang, Y.; Wang, T. A review of recent research progress on the astragalus genus. Molecules 2014, 19, 18850–18880. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Ruiz Beguerie, J.; Sze, D.M.; Chan, G.C. Ganoderma lucidum (Reishi mushroom) for cancer treatment. Cochrane Database Syst. Rev. 2016, 4, Cd007731. [Google Scholar] [CrossRef] [PubMed]
- Sohretoglu, D.; Huang, S. Ganoderma lucidum Polysaccharides as An Anti-cancer Agent. Anti-Cancer Agents Med. Chem. 2018, 18, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, Q.; Zhang, X.; Li, L.; Cao, X.; Zhou, L.; Huang, Y.; Sun, G.; Chen, Y. Purple yam polyphenol extracts exert anticolitis and anticolitis-associated colorectal cancer effects through inactivation of NF-kappaB/p65 and STAT3 signaling pathways. J. Agric. Food Chem. 2023, 71, 12177–12189. [Google Scholar] [CrossRef]
- Vallianou, N.G.; Evangelopoulos, A.; Schizas, N.; Kazazis, C. Potential anticancer properties and mechanisms of action of curcumin. Anticancer Res. 2015, 35, 645–651. [Google Scholar] [PubMed]
- Wang, W.; Li, M.; Wang, L.; Chen, L.; Goh, B.C. Curcumin in cancer therapy: Exploring molecular mechanisms and overcoming clinical challenges. Cancer Lett. 2023, 570, 216332. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, Z.; Meng, R.; Shi, C.; Guo, N. Antioxidative and anticancer properties of Licochalcone A from licorice. J. Ethnopharmacol. 2017, 198, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Deng, N.; Qiao, M.; Li, Y.; Liang, F.; Li, J.; Liu, Y. Anticancer effects of licochalcones: A review of the mechanisms. Front. Pharmacol. 2023, 14, 1074506. [Google Scholar] [CrossRef]
- Van der Eecken, H.; Joniau, S.; Berghen, C.; Rans, K.; De Meerleer, G. The Use of Soy Isoflavones in the Treatment of Prostate Cancer: A Focus on the Cellular Effects. Nutrients 2023, 15, 4856. [Google Scholar] [CrossRef] [PubMed]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Huang, X.X.; Zeng, Y. Puerarin Induces Ferroptosis in Colorectal Cancer Cells via Triggering NCOA4 Upregulation. Nutr. Cancer 2023, 75, 1571–1578. [Google Scholar]
- Zhang, R.; Zhu, X.; Bai, H.; Ning, K. Network Pharmacology Databases for Traditional Chinese Medicine: Review and Assessment. Front. Pharmacol. 2019, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Guo, F.; Wang, Y.; Li, C.; Zhang, X.; Li, H.; Diao, L.; Gu, J.; Wang, W.; Li, D.; et al. BATMAN-TCM: A Bioinformatics Analysis Tool for Molecular mechANism of Traditional Chinese Medicine. Sci. Rep. 2016, 6, 21146. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, B. Traditional Chinese medicine network pharmacology: Theory, methodology and application. Chin. J. Nat. Med. 2013, 11, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, H.; Li, N.; Chen, J.; Xu, H.; Wang, Y.; Liang, Q. Network pharmacology, a promising approach to reveal the pharmacology mechanism of Chinese medicine formula. J. Ethnopharmacol. 2023, 309, 116306. [Google Scholar] [CrossRef] [PubMed]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for functional genomics data sets—update. Nucleic Acids Res. 2013, 41, D991–D995. [Google Scholar] [CrossRef]
- Tibshirani, R. Regression Shrinkage and Selection via the Lasso. J. R. Stat. Soc. Ser. B-Stat. Methodol. 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Huang, M.L.; Hung, Y.H.; Lee, W.M.; Li, R.K.; Jiang, B.R. SVM-RFE based feature selection and Taguchi parameters optimization for multiclass SVM classifier. Sci. World J. 2014, 2014, 795624. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Hazafa, A.; Rehman, K.U.; Jahan, N.; Jabeen, Z. The Role of Polyphenol (Flavonoids) Compounds in the Treatment of Cancer Cells. Nutr. Cancer 2020, 72, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, A.; Zirak, M.R.; Sahebkar, A. Ellagic acid: A logical lead for drug development? Curr. Pharm. Des. 2018, 24, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Xu, J.; Yan, B. The anti-metastatic effect of baicalein on colorectal cancer. Oncol. Rep. 2017, 37, 2317–2323. [Google Scholar] [CrossRef]
- Rui, X.; Yan, X.; Zhang, K. Baicalein inhibits the migration and invasion of colorectal cancer cells via suppression of the AKT signaling pathway. Oncol. Lett. 2016, 11, 685–688. [Google Scholar] [CrossRef]
- Feng, Y.; Lu, J.; Jiang, J.; Wang, M.; Guo, K.; Lin, S. Berberine: Potential preventive and therapeutic strategies for human colorectal cancer. Cell Biochem. Funct. 2024, 42, e4033. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ye, C.; Wu, C.; Zhang, J.; Xu, L.; Wang, X.; Xu, C.; Zhang, J.; Guo, Y.; Yao, Q. Berberine inhibits high fat diet-associated colorectal cancer through modulation of the gut microbiota-mediated lysophosphatidylcholine. Int. J. Biol. Sci. 2023, 19, 2097–2113. [Google Scholar] [CrossRef] [PubMed]
- Gamet-Payrastre, L.; Li, P.; Lumeau, S.; Cassar, G.; Dupont, M.A.; Chevolleau, S.; Gasc, N.; Tulliez, J.; Tercé, F. Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res. 2000, 60, 1426–1433. [Google Scholar]
- Unlu, A.; Nayir, E.; Kirca, O.; Ozdogan, M. Ganoderma lucidum (Reishi mushroom) and cancer. J. BUON. 2016, 21, 792–798. [Google Scholar] [PubMed]
- Guo, C.; Guo, D.; Fang, L.; Sang, T.; Wu, J.; Guo, C.; Wang, Y.; Wang, Y.; Chen, C.; Chen, J.; et al. Ganoderma lucidum polysaccharide modulates gut microbiota and immune cell function to inhibit inflammation and tumorigenesis in colon. Carbohydr. Polym. 2021, 267, 118231. [Google Scholar] [CrossRef]
- Komal; Nanda, B.P.; Singh, L.; Bhatia, R.; Singh, A. Paclitaxel in colon cancer management: From conventional chemotherapy to advanced nanocarrier delivery systems. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 9449–9474. [Google Scholar] [CrossRef]
- Shah, M.A.; Abuzar, S.M.; Ilyas, K.; Qadees, I.; Bilal, M.; Yousaf, R.; Kassim, R.M.T.; Rasul, A.; Saleem, U.; Alves, M.S.; et al. Ginsenosides in cancer: Targeting cell cycle arrest and apoptosis. Chem. Biol. Interact. 2023, 382, 110634. [Google Scholar] [CrossRef]
- Sivasankarapillai, V.S.; Madhu Kumar Nair, R.; Rahdar, A.; Bungau, S.; Zaha, D.C.; Aleya, L.; Tit, D.M. Overview of the anticancer activity of withaferin A, an active constituent of the Indian ginseng Withania somnifera. Environ. Sci. Pollut. Res. Int. 2020, 27, 26025–26035. [Google Scholar] [CrossRef]
- Sharma, V.; Gautam, D.N.S.; Radu, A.F.; Behl, T.; Bungau, S.G.; Vesa, C.M. Reviewing the Traditional/Modern Uses, Phytochemistry, Essential Oils/Extracts and Pharmacology of Embelia ribes Burm. Antioxidants 2022, 11, 1359. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Abo-El-Sooud, K.; Aleya, L.; Bungǎu, S.G.; Najda, A.; Saluja, R. Alleviation of Drugs and Chemicals Toxicity: Biomedical Value of Antioxidants. Oxid. Med. Cell Longev. 2018, 2018, 6276438. [Google Scholar] [CrossRef] [PubMed]
- UniProt Consortium. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef] [PubMed]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinform. 2016, 54, 1.30.1–1.30.33. [Google Scholar] [CrossRef]
- Amberger, J.S.; Bocchini, C.A.; Scott, A.F.; Hamosh, A. OMIM.org: Leveraging knowledge across phenotype-gene relationships. Nucleic Acids Res. 2019, 47, D1038–D1043. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, S.; Li, F.; Zhou, Y.; Zhang, Y.; Wang, Z.; Zhang, R.; Zhu, J.; Ren, Y.; Tan, Y.; et al. Therapeutic target database 2020: Enriched resource for facilitating research and early development of targeted therapeutics. Nucleic Acids Res. 2020, 48, D1031–D1041. [Google Scholar] [CrossRef]
- Dessau, R.B. R: A language and environment for statistical computing. Ugeskr Laeger. 2008, 170, 328–330. [Google Scholar]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Ramalingam, V.; Hwang, I. Identification of Meat Quality Determining Marker Genes in Fibroblasts of Bovine Muscle Using Transcriptomic Profiling. J. Agric. Food Chem. 2021, 69, 3776–3786. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yang, Q.; Zhou, C.; Jiang, H.; Sun, Y.; Wang, H.; Luo, X.; Wang, Z.; Zhang, J.; Wang, K.; et al. Transcriptomic changes in the hypothalamus of ovariectomized mice: Data from RNA-seq analysis. Ann. Anat. 2022, 241, 151886. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Guo, H.; Mo, Y.; Liu, G. Integrating Bioinformatics and Drug Sensitivity Analyses to Identify Molecular Characteristics Associated with Targeting Necroptosis in Breast Cancer and their Clinical Prognostic Significance. Recent. Pat. Anticancer. Drug Discov. 2024, 19, 681–694. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Khodadoust, M.S.; Liu, C.L.; Newman, A.M.; Alizadeh, A.A. Profiling Tumor Infiltrating Immune Cells with CIBERSORT. Methods Mol. Biol. 2018, 1711, 243–259. [Google Scholar]
- Burley, S.K.; Berman, H.M.; Kleywegt, G.J.; Markley, J.L.; Nakamura, H.; Velankar, S. Protein Data Bank (PDB): The Single Global Macromolecular Structure Archive. Methods Mol. Biol. 2017, 1607, 627–641. [Google Scholar]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.S.; Rohane, S.H. Review on Discovery Studio: An important Tool for Molecular Docking. Asian J. Res. Chem. 2021, 14, 1–3. [Google Scholar] [CrossRef]
- Delano, W.L. PyMOL: An Open-Source Molecular Graphics Tool. CCP4 Newsl. Protein Crystallogr. 2002, 4, 82–92. [Google Scholar]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]


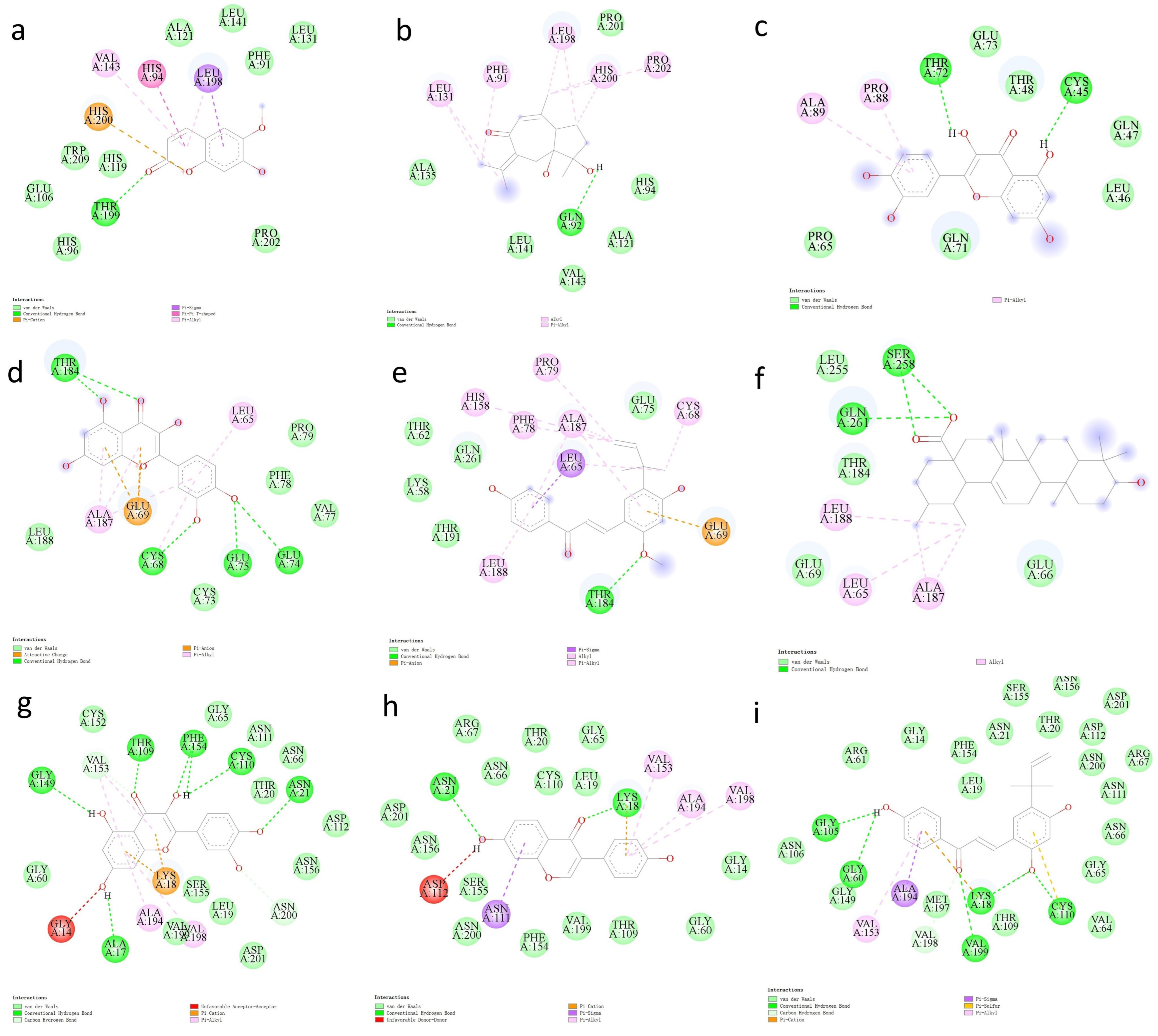
| Receptor | Entry ID | Ligand | Affinity (kcal/mol) |
|---|---|---|---|
| CA1 | P00915 | MOL000040 (Scopoletol) | −6.6 |
| MOL000960 (procurcumadiol) | −6.2 | ||
| CCND1 | P24385 | MOL000497 (licochalcone a) | −7.6 |
| MOL000511 (ursolic acid) | −6.5 | ||
| MOL000098 (quercetin) | −7.5 | ||
| CXCL2 | P56537 | MOL000098 (quercetin) | −5.9 |
| EIF6 | P19875 | MOL000497 (licochalcone a) | −5.4 |
| MOL000098 (quercetin) | −8.0 | ||
| MOL000390 (daidzein) | −9.1 |
| OB (%) | DL | |
|---|---|---|
| MOL000040 (Scopoletol) | 27.77 | 0.08 |
| MOL000960 (procurcumadiol) | 69.82 | 0.13 |
| MOL000497 (licochalcone a) | 40.79 | 0.29 |
| MOL000511 (ursolic acid) | 16.77 | 0.75 |
| MOL000098 (quercetin) | 46.43 | 0.28 |
| MOL000390 (daidzein) | 19.44 | 0.19 |
| Plant Name | Active Compounds | Key Mechanisms | Reference |
|---|---|---|---|
| Punica granatum (Pomegranate) | Ellagic Acid | Antioxidant, anti-proliferative, induces tumor cell cycle arrest and apoptosis | [36,37] |
| Scutellaria baicalensis (Chinese Skullcap) | Baicalein | Inhibits tumor cell migration and invasion, suppresses PI3K/Akt signaling pathway | [38,39] |
| Coptis chinensis (Chinese Goldthread) | Berberine | Anti-inflammatory, modulates gut microbiota, inhibits NF-κB signaling pathway | [40,41] |
| Brassica oleracea (Broccoli) | Sulforaphane | Antioxidant, activates Nrf2 pathway, enhances resistance to oxidative stress | [42] |
| Ganoderma lucidum (Reishi Mushroom) | Ganoderma Polysaccharides | Enhances immune function, modulates tumor microenvironment, promotes immune cell infiltration | [43,44] |
| Taxus chinensis (Chinese Yew) | Paclitaxel | Inhibits mitosis, stabilizes microtubules, induces tumor cell apoptosis | [45] |
| Panax ginseng (Ginseng) | Ginsenosides | Anti-proliferative, anti-angiogenesis, modulates immune response, suppresses multiple cancer pathways | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Xiu, J.; Yang, H.; Han, W.; Jin, Y. Network Pharmacology and Bioinformatics Study of Six Medicinal Food Homologous Plants Against Colorectal Cancer. Int. J. Mol. Sci. 2025, 26, 930. https://doi.org/10.3390/ijms26030930
Zhao X, Xiu J, Yang H, Han W, Jin Y. Network Pharmacology and Bioinformatics Study of Six Medicinal Food Homologous Plants Against Colorectal Cancer. International Journal of Molecular Sciences. 2025; 26(3):930. https://doi.org/10.3390/ijms26030930
Chicago/Turabian StyleZhao, Xinyue, Jian Xiu, Hengzheng Yang, Weiwei Han, and Yue Jin. 2025. "Network Pharmacology and Bioinformatics Study of Six Medicinal Food Homologous Plants Against Colorectal Cancer" International Journal of Molecular Sciences 26, no. 3: 930. https://doi.org/10.3390/ijms26030930
APA StyleZhao, X., Xiu, J., Yang, H., Han, W., & Jin, Y. (2025). Network Pharmacology and Bioinformatics Study of Six Medicinal Food Homologous Plants Against Colorectal Cancer. International Journal of Molecular Sciences, 26(3), 930. https://doi.org/10.3390/ijms26030930







