Correlations Between MR Apparent Diffusion Coefficients and PET Standard Uptake Values in Simultaneous MR-PET Imaging of Prostate Cancer
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Patients and Methods
4.2. Study Protocol
4.3. Deep Learning-Based AC μ-Maps
4.4. SUV and ADC Histogram Analyses
4.5. Statistical Testing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | attenuation correction |
| ADC | apparent diffusion coefficient |
| DL | deep learning |
| DWA | diffusion-weighted imaging |
| GAN | generative adversarial network |
| LN | lymph node |
| PET-CT | positron emission tomography—computed tomography |
| PET-MR | positron emission tomography—magnetic resonance imaging |
| PSA | prostate-specific antigen |
| PSMA | prostate-specific membrane antigen |
| PZ | peripheral zone |
| ROI | region of interest |
| SUV | standardized uptake values |
| TZ | transition zone |
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Murthy, V.; Sonni, I.; Jariwala, N.; Juarez, R.; Reiter, R.E.; Raman, S.S.; Hope, T.A. The role of PSMA PET/CT and PET/MRI in the initial staging of prostate cancer. Eur. Urol. Focus 2021, 7, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Peltier, A.; Aoun, F.; Lemort, M.; Kwizera, F.; Paesmans, M.; Van Velthoven, R. MRI-targeted biopsies versus systematic transrectal ultrasound guided biopsies for the diagnosis of localized prostate cancer in biopsy naïve men. Biomed. Res. Int. 2015, 2015, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Jadvar, H. PSMA PET in prostate cancer. J. Nucl. Med. 2015, 56, 1131–1132. [Google Scholar] [CrossRef] [PubMed]
- Nepal, A.; Sharma, P.; Bhattarai, S.; Mahajan, Z.; Sharma, A.; Sapkota, A.; Sharma, A. Extremely Elevated Prostate-Specific Antigen in Acute Prostatitis: A Case Report. Cureus 2023, 15, e43730. [Google Scholar] [CrossRef]
- Brown, L.C.; Ahmed, H.U.; Faria, R.; El-Shater Bosaily, A.; Gabe, R.; Kaplan, R.S.; Parmar, M.; Collaco-Moraes, Y.; Ward, K.; Hindley, R.G.; et al. Multiparametric MRI to improve detection of prostate cancer compared with transrectal ultrasound-guided prostate biopsy alone: The PROMIS study. Health Technol. Assess. 2018, 22, 1–176. [Google Scholar] [CrossRef] [PubMed]
- Gandaglia, G.; Abdollah, F.; Schiffmann, J.; Trudeau, V.; Shariat, S.F.; Kim, S.P.; Perrotte, P.; Montorsi, F.; Briganti, A.; Trinh, Q.; et al. Distribution of metastatic sites in patients with prostate cancer: A population-based analysis. Prostate 2014, 74, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Lenzo, N.P.; Meyrick, D.; Turner, J.H. Review of gallium-68 PSMA PET/CT imaging in the management of prostate cancer. Diagnostics 2018, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xiang, F.; Lin, X.; Grajo, J.R.; Yang, L.; Xu, Y.; Duan, Y.; Vyas, U.; Harisinghani, M.; Mahmood, U.; et al. The role of imaging in prostate cancer care pathway: Novel approaches to urologic management challenges along 10 imaging touch points. Urology 2018, 119, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Rhee, H.; Blazak, J.; Tham, C.M.; Ng, K.L.; Shepherd, B.; Lawson, M.; Preston, J.; Vela, I.; Thomas, P.; Wood, S. Pilot study: Use of gallium-68 PSMA PET for detection of metastatic lesions in patients with renal tumour. EJNMMI Res. 2016, 6, 76. [Google Scholar] [CrossRef]
- Jung, S.I.; Donati, O.F.; Vargas, H.A.; Goldman, D.; Hricak, H.; Akin, O. Transition zone prostate cancer: Incremental value of diffusion-weighted endorectal MR imaging in tumor detection and assessment of aggressiveness. Radiology 2013, 269, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Surov, A.; Meyer, H.J.; Wienke, A. Correlation between apparent diffusion coefficient (ADC) and cellularity is different in several tumors: A meta-analysis. Oncotarget 2017, 8, 59492. [Google Scholar] [CrossRef] [PubMed]
- Herneth, A.M.; Guccione, S.; Bednarski, M. Apparent diffusion coefficient: A quantitative parameter for in vivo tumor characterization. Eur. J. Radiol. 2003, 45, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Surov, A.; Meyer, H.J.; Wienke, A. Associations between apparent diffusion coefficient (ADC) and KI 67 in different tumors: A meta-analysis. Part 1: ADCmean. Oncotarget 2017, 8, 75434. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-M.; Xu, J.-R.; Gu, H.-Y.; Hua, J.; Chen, J.; Zhang, W.; Zhu, J.; Ye, Y.-Q.; Hu, J. Usefulness of diffusion-weighted magnetic resonance imaging in the diagnosis of prostate cancer. Acad. Radiol. 2012, 19, 1215–1224. [Google Scholar] [CrossRef]
- Bains, L.J.; Zweifel, M.; Thoeny, H.C. Therapy response with diffusion MRI: An update. Cancer Imaging 2012, 12, 395. [Google Scholar] [CrossRef]
- Patterson, D.M.; Padhani, A.R.; Collins, D.J. Technology insight: Water diffusion MRI—A potential new biomarker of response to cancer therapy. Nat. Clin. Pract. Oncol. 2008, 5, 220–233. [Google Scholar] [CrossRef]
- Hayashida, Y.; Hirai, T.; Morishita, S.; Kitajima, M.; Murakami, R.; Korogi, Y.; Makino, K.; Nakamura, H.; Ikushima, I.; Yamura, M.; et al. Diffusion-weighted imaging of metastatic brain tumors: Comparison with histologic type and tumor cellularity. AJNR Am. J. Neuroradiol. 2006, 27, 1419–1425. [Google Scholar]
- Kitajima, K.; Takahashi, S.; Ueno, Y.; Miyake, H.; Fujisawa, M.; Kawakami, F.; Sugimura, K. Do apparent diffusion coefficient (ADC) values obtained using high b-values with a 3-T MRI correlate better than a transrectal ultrasound (TRUS)-guided biopsy with true Gleason scores obtained from radical prostatectomy specimens for patients with prostate cancer? Eur. J. Radiol. 2013, 82, 1219–1226. [Google Scholar] [PubMed]
- Wetter, A.; Lipponer, C.; Nensa, F.; Beiderwellen, K.; Olbricht, T.; Rübben, H.; Bockisch, A.; Schlosser, T.; Heusner, T.A.; Lauenstein, T.C. Simultaneous 18F choline positron emission tomography/magnetic resonance imaging of the prostate: Initial results. Investig. Radiol. 2013, 48, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Hicks, R.M.; Simko, J.P.; Westphalen, A.C.; Nguyen, H.G.; Greene, K.L.; Zhang, L.; Carroll, P.R.; Hope, T.A. Diagnostic accuracy of 68Ga-PSMA-11 PET/MRI compared with multiparametric MRI in the detection of prostate cancer. Radiology 2018, 289, 730–737. [Google Scholar] [CrossRef]
- Just, N. Improving tumour heterogeneity MRI assessment with histograms. Br. J. Cancer 2014, 111, 2205–2213. [Google Scholar] [CrossRef] [PubMed]
- Uslu-Beşli, L.; Bakır, B.; Asa, S.; Güner, E.; Demirdağ, Ç.; Şahin, O.E.; Karayel, E.; Sağer, M.S.; Sayman, H.B.; Sönmezoğlu, K. Correlation of SUVmax and apparent diffusion coefficient values detected by Ga-68 PSMA PET/MRI in primary prostate lesions and their significance in lymph node metastasis: Preliminary results of an on-going study. Mol. Imaging Radionucl. Ther. 2019, 28, 104. [Google Scholar] [CrossRef]
- Vag, T.; Heck, M.M.; Beer, A.J.; Souvatzoglou, M.; Weirich, G.; Holzapfel, K.; Krause, B.J.; Schwaiger, M.; Eiber, M.; Rummeny, E.J. Preoperative lymph node staging in patients with primary prostate cancer: Comparison and correlation of quantitative imaging parameters in diffusion-weighted imaging and 11C-choline PET/CT. Eur. Radiol. 2014, 24, 1821–1826. [Google Scholar] [CrossRef] [PubMed]
- Wetter, A.; Nensa, F.; Schenck, M.; Heusch, P.; Pöppel, T.; Bockisch, A.; Forsting, M.; Schlosser, T.W.; Lauenstein, T.C.; Nagarajah, J. Combined PET imaging and diffusion-weighted imaging of intermediate and high-risk primary prostate carcinomas with simultaneous [18F] choline PET/MRI. PLoS ONE 2014, 9, e101571. [Google Scholar] [CrossRef] [PubMed]
- Wetter, A.; Lipponer, C.; Nensa, F.; Heusch, P.; Rübben, H.; Schlosser, T.W.; Pöppel, T.D.; Lauenstein, T.C.; Nagarajah, J. Quantitative evaluation of bone metastases from prostate cancer with simultaneous [18F] choline PET/MRI: Combined SUV and ADC analysis. Ann. Nucl. Med. 2014, 28, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, F.; Yang, L.; Zang, S.; Xue, H.; Yin, X.; Guo, H.; Sun, H.; Wang, F. 68Ga-PSMA-11 PET/CT combining ADC value of MRI in the diagnosis of naive prostate cancer: Perspective of radiologist. Medicine 2020, 99, e20755. [Google Scholar] [CrossRef] [PubMed]
- Bruckmann, N.M.; Rischpler, C.; Kirchner, J.; Umutlu, L.; Herrmann, K.; Ingenwerth, M.; Theurer, S.; Lahner, H.; Antoch, G.; Sawicki, L.M. Correlation between contrast enhancement, standardized uptake value (SUV), and diffusion restriction (ADC) with tumor grading in patients with therapy-naive neuroendocrine neoplasms using hybrid 68Ga-DOTATOC PET/MRI. Eur. J. Radiol. 2021, 137, 109588. [Google Scholar] [CrossRef]
- Meyer, H.J.; Purz, S.; Sabri, O.; Surov, A. Relationships between histogram analysis of ADC values and complex 18F-FDG-PET parameters in head and neck squamous cell carcinoma. PLoS ONE 2018, 13, e0202897. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.; Pichler, B.; Schölkopf, B.; Beyer, T. Towards quantitative PET/MRI: A review of MR-based attenuation correction techniques. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 93–104. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Ay, M.R.; Ahmadian, A.; Riahi Alam, N.; Zaidi, H. MRI-guided attenuation correction in whole-body PET/MR: Assessment of the effect of bone attenuation. Ann. Nucl. Med. 2013, 27, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Torrado-Carvajal, A.; Vera-Olmos, J.; Izquierdo-Garcia, D.; Catalano, O.A.; Morales, M.A.; Margolin, J.; Soricelli, A.; Salvatore, M.; Malpica, N.; Catana, C. Dixon-VIBE deep learning (DIVIDE) pseudo-CT synthesis for pelvis PET/MR attenuation correction. J. Nucl. Med. 2019, 60, 429–435. [Google Scholar] [CrossRef]
- Liu, F.; Jang, H.; Kijowski, R.; Bradshaw, T.; McMillan, A.B. Deep learning MR imaging–based attenuation correction for PET/MR imaging. Radiology 2018, 286, 676–684. [Google Scholar] [CrossRef]
- Eskicorapci, S.Y.; Guliyev, F.; Islamoglu, E.; Ergen, A.; Ozen, H. The effect of prior biopsy scheme on prostate cancer detection for repeat biopsy population: Results of the 14-core prostate biopsy technique. Int. Urol. Nephrol. 2007, 39, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.T.; Sankineni, S.; Xu, S.; Turkbey, B.; Choyke, P.L.; Pinto, P.A.; Moreno, V.; Merino, M.; Wood, B.J. Prostate cancer: A correlative study of multiparametric MR imaging and digital histopathology. Radiology 2017, 285, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Eiber, M.; Weirich, G.; Holzapfel, K.; Souvatzoglou, M.; Haller, B.; Rauscher, I.; Beer, A.J.; Wester, H.-J.; Gschwend, J.; Schwaiger, M.; et al. Simultaneous 68Ga-PSMA HBED-CC PET/MRI improves the localization of primary prostate cancer. Eur. Urol. 2016, 70, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.S.; Gong, N.; Chu, Y.-C.; Anthony, M.-P.; Chan, Q.; Lee, H.F.; Chu, K.M.; Khong, P.-L. Correlation of measurements from diffusion weighted MR imaging and FDG PET/CT in GIST patients: ADC versus SUV. Eur. J. Radiol. 2012, 81, 2122–2126. [Google Scholar] [CrossRef]
- Ergül, N.; Günes, B.Y.; Yücetas, U.; Toktas, M.G.; Çermik, T.F. 68Ga-PSMA-11 PET/CT in newly diagnosed prostate adenocarcinoma. Clin. Nucl. Med. 2018, 43, e422–e427. [Google Scholar] [CrossRef]
- Jena, A.; Taneja, R.; Taneja, S.; Singh, A.; Kumar, V.; Agarwal, A.; Subramanian, N. Improving diagnosis of primary prostate cancer with combined 68Ga–prostate-specific membrane antigen–HBED-CC simultaneous PET and multiparametric MRI and clinical parameters. AJR Am. J. Roentgenol. 2018, 211, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Sokmen, B.K.; Sokmen, D.; Ucar, N.; Ozkurt, H.; Simsek, A. The correlation between biological activity and diffusion-weighted MR imaging and ADC value in cases with prostate cancer. Arch. Ital. Urol. Androl. 2017, 89, 277–281. [Google Scholar] [CrossRef]
- Buchbender, C.; Hartung-Knemeyer, V.; Heusch, P.; Heusner, T.A.; Beiderwellen, K.; Wittsack, H.J.; Kühl, H.; Forsting, M.; Bockisch, A.; Antoch, G.; et al. Does positron emission tomography data acquisition impact simultaneous diffusion-weighted imaging in a whole-body PET/MRI system? Eur. J. Radiol. 2013, 82, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Metzger, G.J.; Kalavagunta, C.; Spilseth, B.; Bolan, P.J.; Li, X.; Hutter, D.; Nam, J.W.; Johnson, A.D.; Henriksen, J.C.; Moench, L.; et al. Detection of prostate cancer: Quantitative multiparametric MR imaging models developed using registered correlative histopathology. Radiology 2016, 279, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Hambrock, T.; Somford, D.M.; Huisman, H.J.; van Oort, I.M.; Witjes, J.A.; Hulsbergen-van de Kaa, C.A.; Scheenen, T.; Barentsz, J.O. Relationship between apparent diffusion coefficients at 3.0-T MR imaging and Gleason grade in peripheral zone prostate cancer. Radiology 2011, 259, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Reischauer, C.; Froehlich, J.M.; Koh, D.M.; Graf, N.; Padevit, C.; John, H.; Binkert, C.A.; Boesiger, P.; Gutzeit, A. Bone metastases from prostate cancer: Assessing treatment response by using diffusion-weighted imaging and functional diffusion maps—Initial observations. Radiology 2010, 257, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Pozaruk, A.; Pawar, K.; Li, S.; Carey, A.; Cheng, J.; Sudarshan, V.P.; Cholewa, M.; Grummet, J.; Chen, Z.; Egan, G. Augmented deep learning model for improved quantitative accuracy of MR-based PET attenuation correction in PSMA PET-MRI prostate imaging. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Goodfellow, I.; Pouget-Abadie, J.; Mirza, M.; Xu, B.; Warde-Farley, D.; Ozair, S.; Courville, A.; Bengio, Y. Generative adversarial nets. Adv. Neural Inf. Process. Syst. 2014, 27, 1–9. [Google Scholar]
- Ronneberger, O.; Fischer, P.; Brox, T. U-net: Convolutional networks for biomedical image segmentation. In Medical Image Computing and Computer-Assisted Intervention–MICCAI 2015: 18th International Conference, Munich, Germany, 5–9 October 2015; Proceedings, Part III 18; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- Avants, B.B.; Tustison, N.; Song, G. Advanced normalization tools (ANTS). Insight J. 2009, 2, 1–35. [Google Scholar]
- Carney, J.P.; Townsend, D.W.; Rappoport, V.; Bendriem, B. Method for transforming CT images for attenuation correction in PET/CT imaging. Med. Phys. 2006, 33, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Bengio, Y.; Grandvalet, Y. No unbiased estimator of the variance of k-fold cross-validation. Adv. Neural Inf. Process. Syst. 2003, 16, 1089–1105. [Google Scholar]
- Goscinski, W.J. Multi-modal Australian sciences imaging and visualisation environment (MASSIVE). In Proceedings of the ATIP/A* CRC Workshop on Accelerator Technologies for High-Performance Computing: Does Asia Lead the Way? Singapore, 7–10 May 2012; pp. 1–36. [Google Scholar]
- Kinehan, P.; Fletcher, J. PET/CT standardized uptake values (SUVs) in clinical practice and assessing response to therapy. Semin. Ultrasound CT MR 2010, 31, 496–505. [Google Scholar] [CrossRef]
- Meyer, H.J.; Leifels, L.; Schob, S.; Garnov, N.; Surov, A. Histogram analysis parameters identify multiple associations between DWI and DCE MRI in head and neck squamous cell carcinoma. Magn. Reson. Imaging 2018, 45, 72–77. [Google Scholar] [CrossRef] [PubMed]

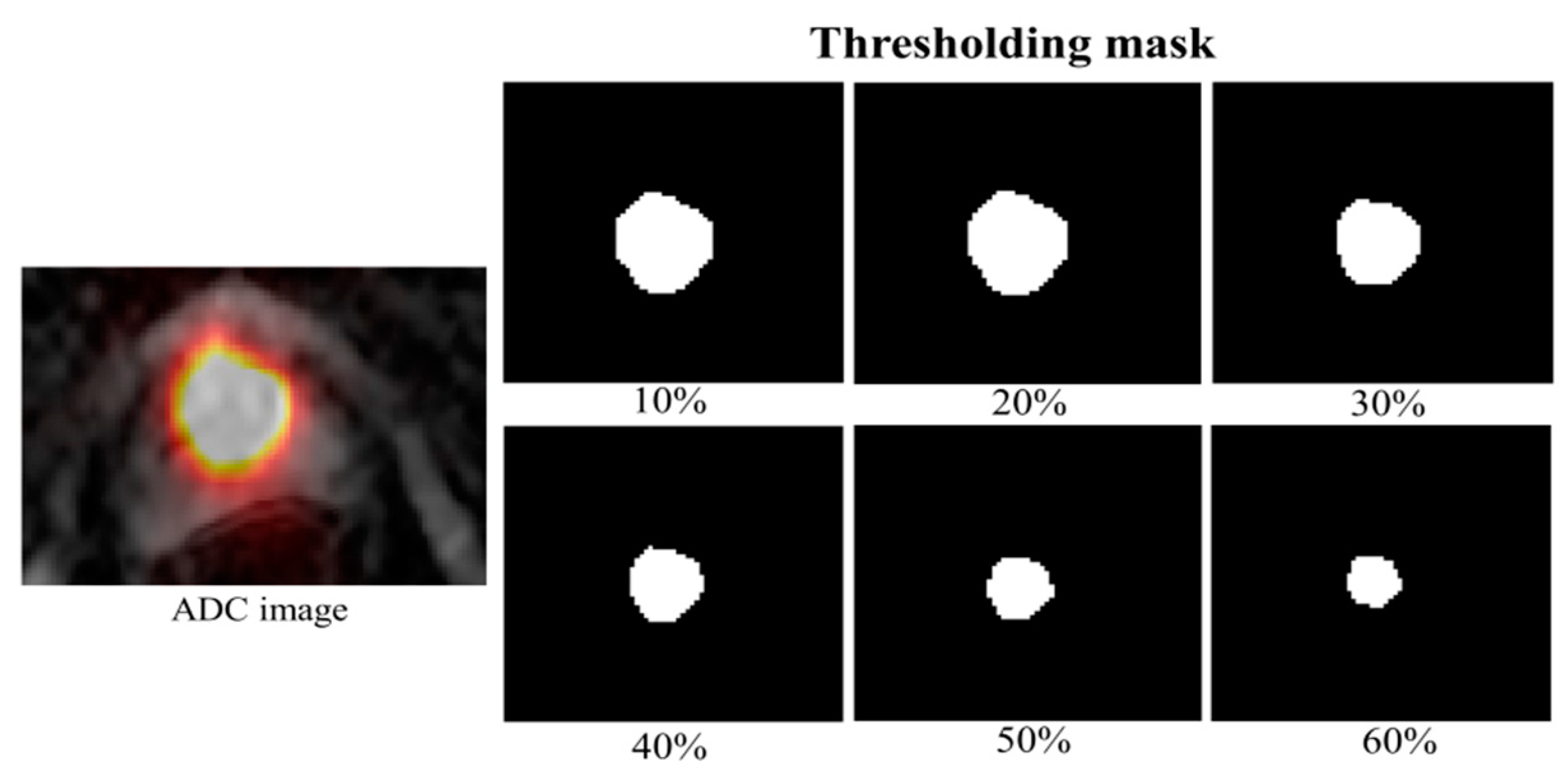

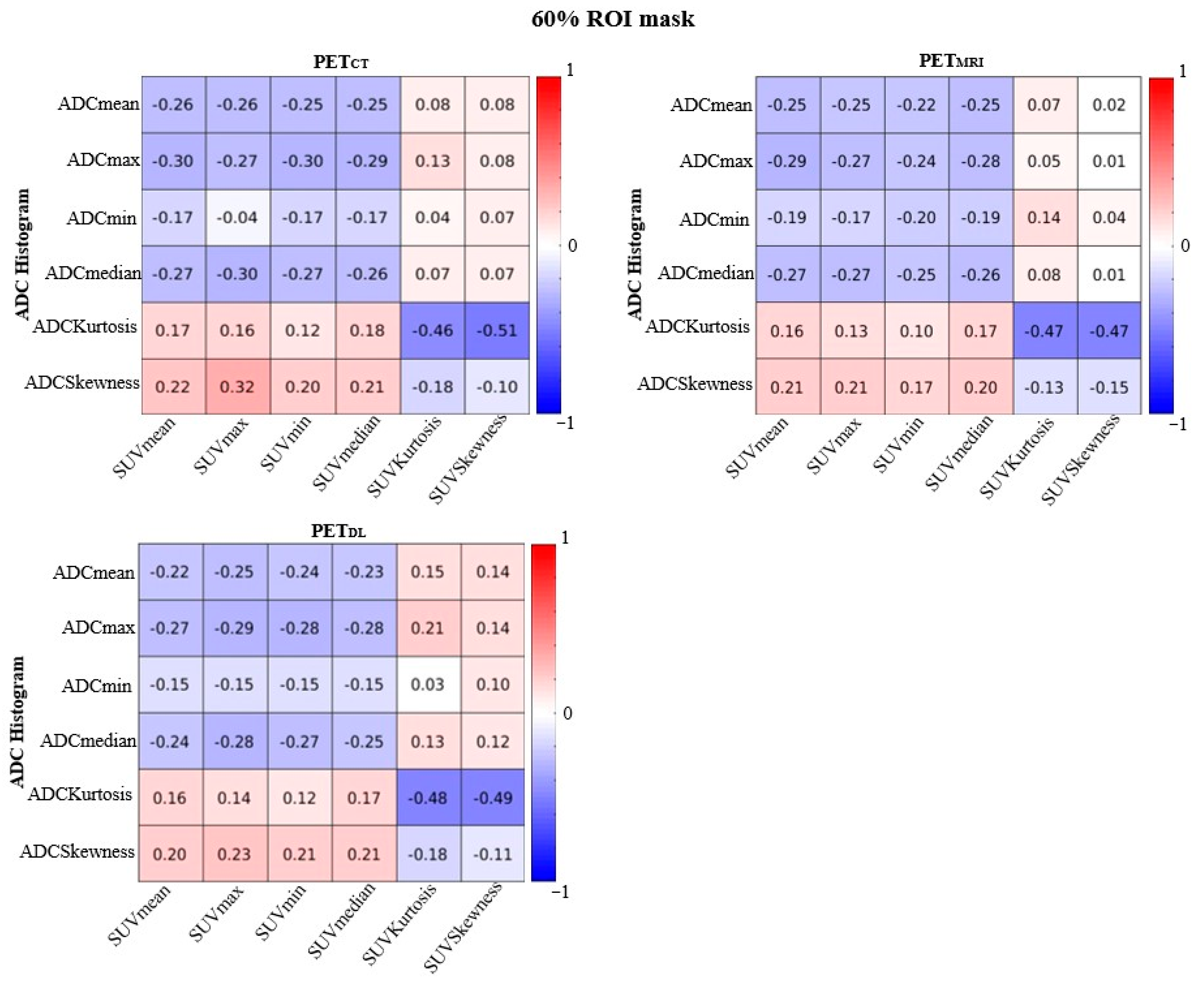
| Parameters | Cancer in PZ and TZ | ||||
|---|---|---|---|---|---|
| ADC Values (×10−3 mm2 s−1) | PETCT (SUV) | Average Relative Absolute Error (%) | |||
| PETMRI | PETDL | t-Test | |||
| Mean | 1.0 ± 0.1 | 9.9 ± 1.5 | 2.7 ± 0.5 | 1.5 ± 0.2 | 0.08 |
| Max | 1.7 ± 0.1 | 15.6 ± 2.7 | 2.6 ± 0.5 | 1.4 ± 0.2 | 0.04 |
| Min | 0.5 ± 0.1 | 6.7 ± 0.9 | 3.6 ± 1.1 | 1.5 ± 0.2 | 0.06 |
| Median | 1.0 ± 0.1 | 9.6 ± 1.4 | 2.2 ± 0.4 | 1.5 ± 0.2 | 0.11 |
| Kurtosis | 0.2 ± 0.4 | 0.2 ± 0.6 | 29.0 ± 7.7 | 9.1 ± 2.7 | 0.01 |
| Skewness | 0.4 ± 0.1 | 0.6 ± 0.1 | 12.6 ± 4.7 | 2.1 ± 0.5 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozaruk, A.; Atamaniuk, V.; Pawar, K.; Carey, A.; Cheng, J.; Cholewa, M.; Grummet, J.; Chen, Z.; Egan, G. Correlations Between MR Apparent Diffusion Coefficients and PET Standard Uptake Values in Simultaneous MR-PET Imaging of Prostate Cancer. Int. J. Mol. Sci. 2025, 26, 905. https://doi.org/10.3390/ijms26030905
Pozaruk A, Atamaniuk V, Pawar K, Carey A, Cheng J, Cholewa M, Grummet J, Chen Z, Egan G. Correlations Between MR Apparent Diffusion Coefficients and PET Standard Uptake Values in Simultaneous MR-PET Imaging of Prostate Cancer. International Journal of Molecular Sciences. 2025; 26(3):905. https://doi.org/10.3390/ijms26030905
Chicago/Turabian StylePozaruk, Andrii, Vitaliy Atamaniuk, Kamlesh Pawar, Alexandra Carey, Jeremy Cheng, Marian Cholewa, Jeremy Grummet, Zhaolin Chen, and Gary Egan. 2025. "Correlations Between MR Apparent Diffusion Coefficients and PET Standard Uptake Values in Simultaneous MR-PET Imaging of Prostate Cancer" International Journal of Molecular Sciences 26, no. 3: 905. https://doi.org/10.3390/ijms26030905
APA StylePozaruk, A., Atamaniuk, V., Pawar, K., Carey, A., Cheng, J., Cholewa, M., Grummet, J., Chen, Z., & Egan, G. (2025). Correlations Between MR Apparent Diffusion Coefficients and PET Standard Uptake Values in Simultaneous MR-PET Imaging of Prostate Cancer. International Journal of Molecular Sciences, 26(3), 905. https://doi.org/10.3390/ijms26030905






