The NbCBP1-NbSAMS1 Module Promotes Ethylene Accumulation to Enhance Nicotiana benthamiana Resistance to Phytophthora parasitica Under High Potassium Status
Abstract
1. Introduction
2. Results
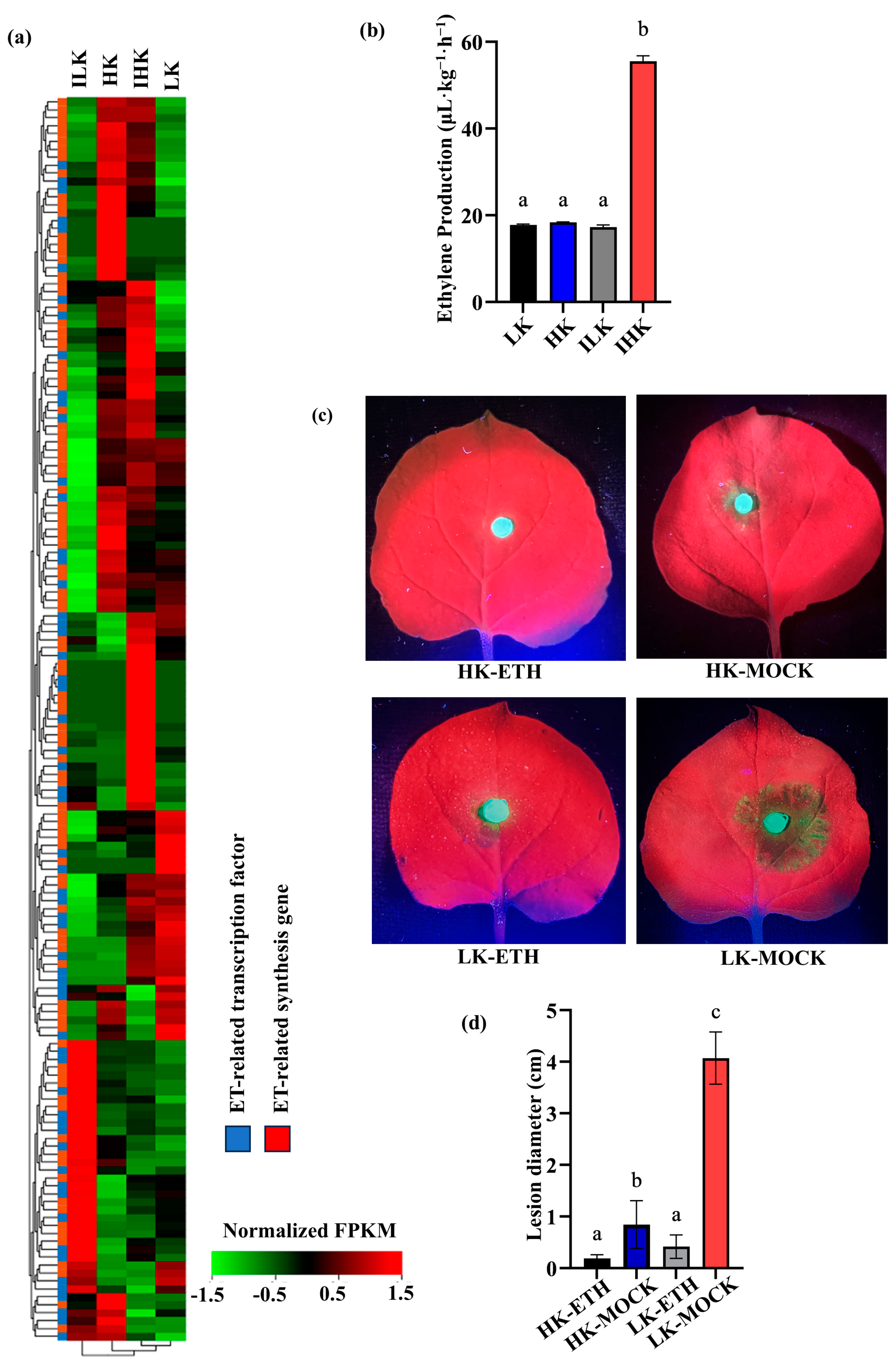
2.1. High Potassium Levels Promote Ethylene Accumulation After Inoculation
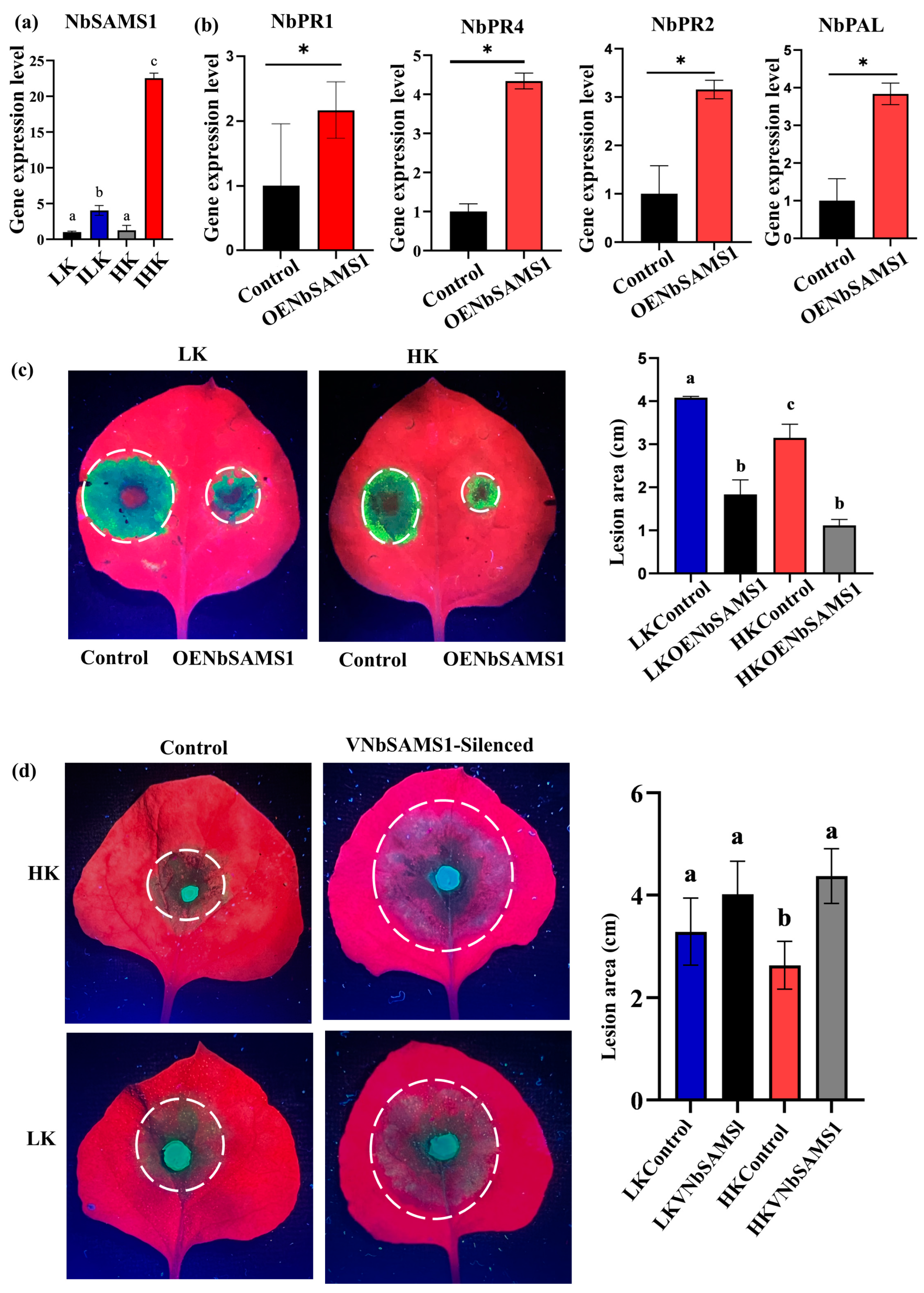
2.2. NbSAMS1 Positively Regulates Nicotiana benthamiana Resistance to Phytophthora parasitica
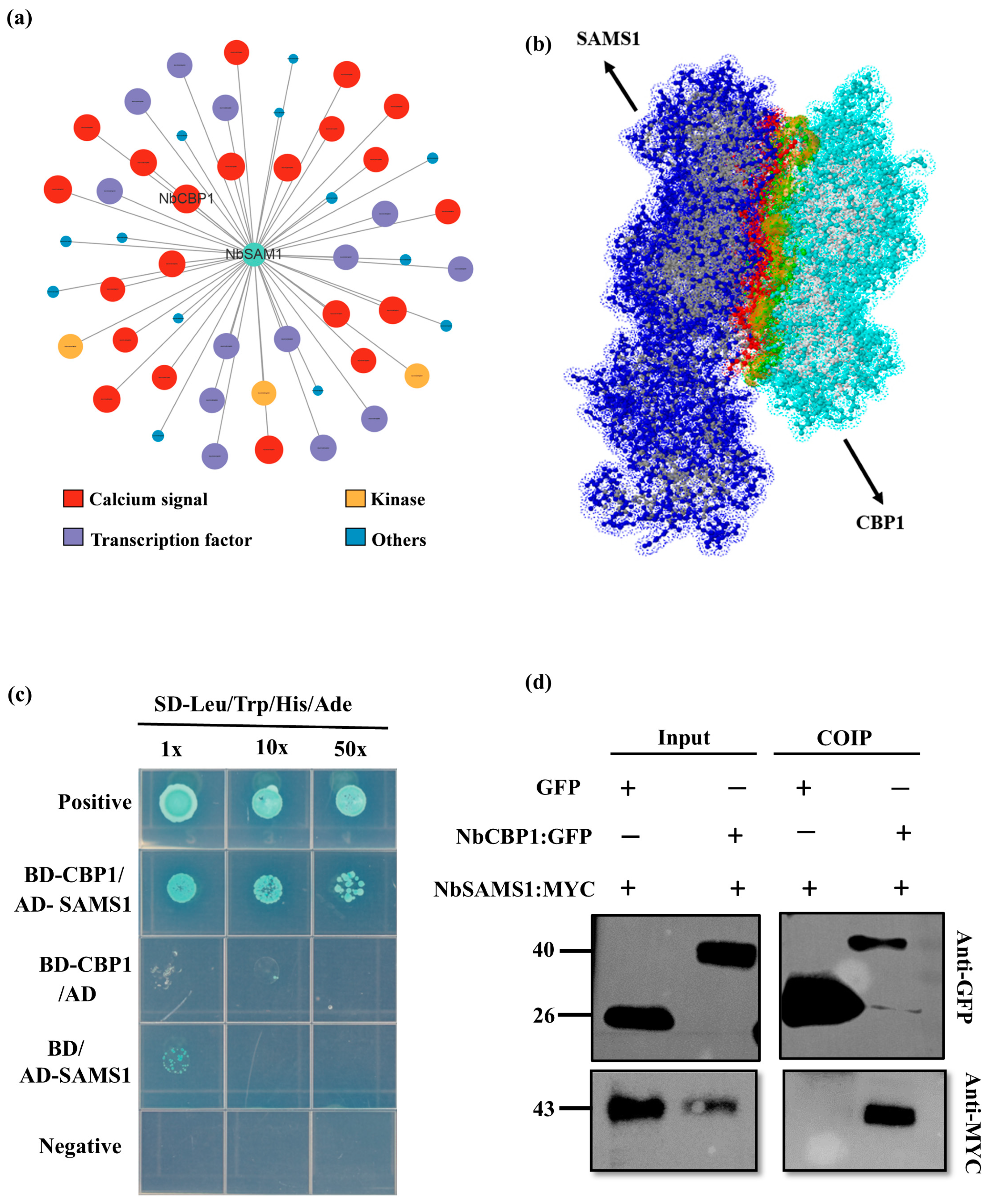
2.3. NbSAMS1 Interacts Directly with NbCBP1
2.4. NbCBP1 Affects Ethylene Accumulation and Positively Regulates Nicotiana benthamiana Resistance
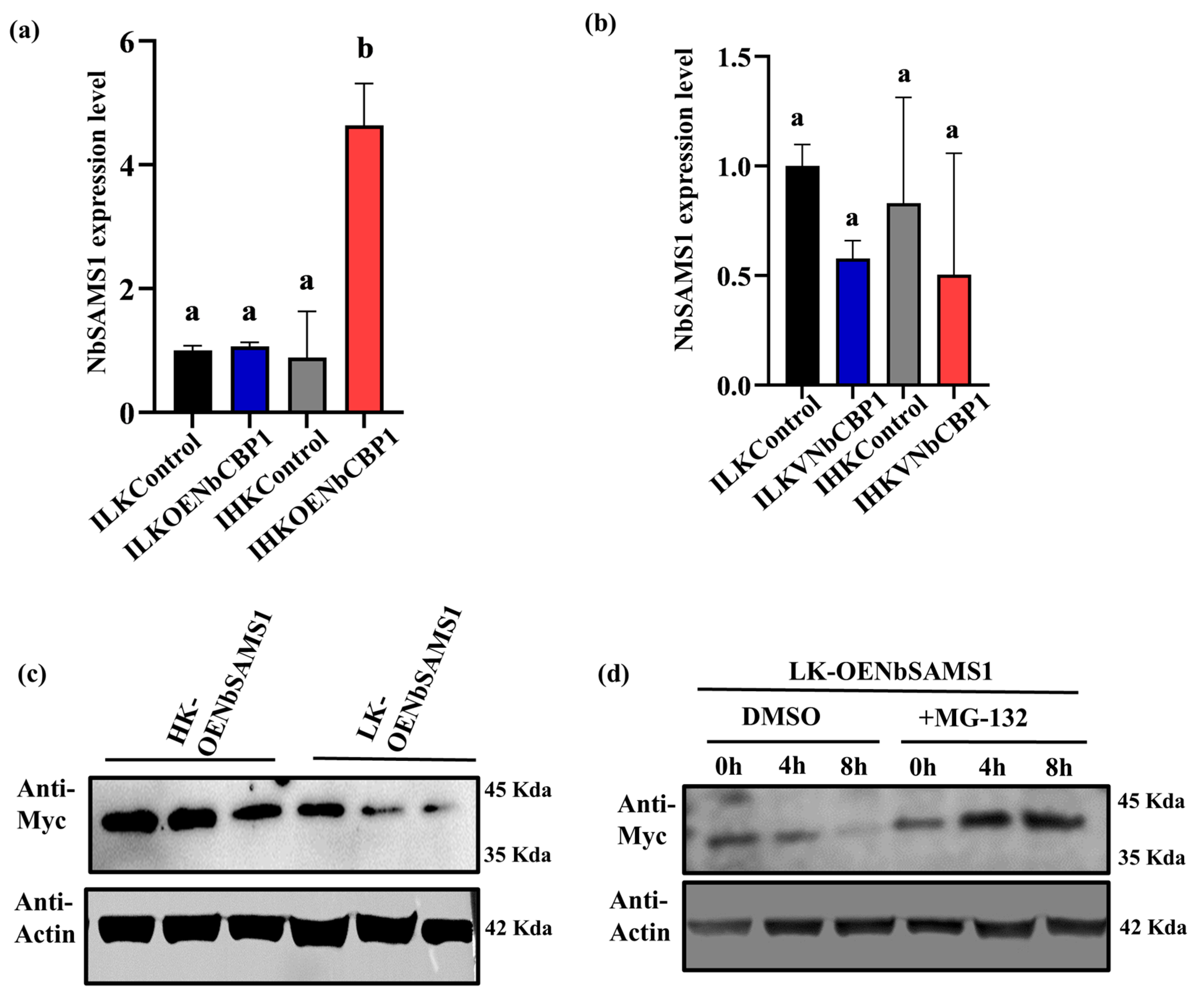
2.5. NbCBP1 Blocks Ubiquitination/Degradation to NbSAMS1 to Promote Ethylene Accumulation in HK N. benthamiana
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Pathogen Inoculation and Disease Severity
4.3. Construction of Vectors and Genetic Transformation in N. benthamiana
4.4. Total RNA Extraction and RT-qPCR Analysis
4.5. Protein Extraction and Western Blotting
4.6. IP-MS Analysis for Target Gene Identification
4.7. Co-IP Assays
4.8. Y2H Assay
4.9. Exogenous Etephon Treatment
4.10. Quantification of Ethylene
4.11. In Vivo Protein Degradation and Western Blotting
4.12. Computational Prediction of Protein–Protein Interactions
4.13. Statistical Analysis and Graphical Presentaion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef]
- Anschütz, U.; Becker, D.; Shabala, S. Going beyond nutrition: Regulation of potassium homoeostasis as a common denominator of plant adaptive responses to environment. J. Plant Physiol. 2014, 171, 670–687. [Google Scholar] [CrossRef]
- Munson, R.D.; Sims, J. Potassium Nutrition of Tobacco. In Potassium in Agriculture; ASA-CSSA-SSA: Madison, WI, USA, 1985. [Google Scholar]
- An, G.; Shi, L.; Du, Z.; Yu, J.; Den, F.; Shi, L. Studies on the standard range of apple leaf nutritional elements in Shaanxi Province. Acta Hortic. Sin. 2004, 31, 81–83. [Google Scholar]
- Liu, H.; Zhang, H.; Guo, D.; Wang, L.; Wang, X.; Sun, L.; Kou, T. Foliar nutrition diagnosis of Red Fuji apple in western Henan Province. J. Nutr. Fert. 2009, 15, 457–462. [Google Scholar]
- Peng, F.; Jiang, Y. Characteristics of N, P, and K nutrition in different yield level apple orchards. Sci. Agric. Sin. 2006, 39, 361–367. [Google Scholar]
- Messaoudi, A.; Labdelli, F.; Rebouh, N.Y.; Djerbaoui, M.; Kucher, D.E.; Hadjout, S.; Ouaret, W.; Zakharova, O.A.; Latati, M. Investigating the potassium fertilization effect on morphological and agrophysiological indicators of durum wheat under Mediterranean rain-fed conditions. Agriculture 2023, 13, 1142. [Google Scholar] [CrossRef]
- Peng, H.; Wei, X.; Xiao, Y.; Sun, Y.; Biggs, A.R.; Gleason, M.L.; Shang, S.; Zhu, M.; Guo, Y.; Sun, G. Management of Valsa Canker on apple with adjustments to potassium nutrition. Plant Dis. 2016, 100, 884–889. [Google Scholar] [CrossRef]
- van Loon, L.C.; Geraats, B.P.; Linthorst, H.J. Ethylene as a modulator of disease resistance in plants. Trends Plant Sci. 2006, 11, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Berrocal-Lobo, M.; Molina, A.; Solano, R. Constitutive expression of ETHYLENE-RESPONSE-FACTOR1 in Arabidopsis confers resistance to several necrotrophic fungi. Plant J. 2002, 29, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, P.S.; Sanchez, L.; Li, D.; Enyi, D.; Van de Poel, B.; Chang, C. The plant hormone ethylene promotes abiotic stress tolerance in the liverwort Marchantia polymorpha. Front. Plant Sci. 2022, 13, 998267. [Google Scholar] [CrossRef] [PubMed]
- Zapata, P.J.; Serrano, M.; Pretel, M.T.; Amorós, A.; Botella, M.Á. Changes in ethylene evolution and polyamine profiles of seedlings of nine cultivars of Lactuca sativa L. in response to salt stress during germination. Plant Sci. 2003, 164, 557–563. [Google Scholar] [CrossRef]
- Amtmann, A.; Troufflard, S.; Armengaud, P. The effect of potassium nutrition on pest and disease resistance in plants. Physiol. Plant. 2008, 133, 682–691. [Google Scholar] [CrossRef]
- Ismayil, A.; Haxim, Y.; Wang, Y.; Li, H.; Qian, L.; Han, T.; Chen, T.; Jia, Q.; Liu, A.Y.; Zhu, S.; et al. Cotton Leaf Curl Multan virus C4 protein suppresses both transcriptional and post-transcriptional gene silencing by interacting with SAM synthetase. PLoS Pathog. 2018, 14, e1007282. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Hong, W.; Wu, J.; Wang, Y.; Ji, S.; Zhu, S.; Wei, C.; Zhang, J.; Li, Y. A viral protein promotes host SAMS1 activity and ethylene production for the benefit of virus infection. Elife 2017, 6, e27529. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, W.; Zhou, X.; Situ, J.; Xie, L.; Xi, P.; Yang, B.; Kong, G.; Jiang, Z. Peronophythora litchii RXLR effector P. litchii avirulence homolog 202 destabilizes a host ethylene biosynthesis enzyme. Plant Physiol. 2023, 193, 756–774. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, C. Early molecular events in PAMP-triggered immunity. Curr. Opin. Plant Biol. 2009, 12, 414–420. [Google Scholar] [CrossRef]
- Tuteja, N.; Mahajan, S. Calcium signaling network in plants: An overview. Plant Signal. Behav. 2007, 2, 79–85. [Google Scholar] [CrossRef]
- Grabarek, Z. Insights into modulation of calcium signaling by magnesium in calmodulin, troponin C and related EF-hand proteins. Mol. Cell Res. 2011, 1813, 913–921. [Google Scholar] [CrossRef]
- Zhou, J.-M.; Zhang, Y. Plant immunity: Danger perception and signaling. Cell 2020, 181, 978–989. [Google Scholar] [CrossRef]
- Ragel, P.; Raddatz, N.; Leidi, E.O.; Quintero, F.J.; Pardo, J.M. Regulation of K+ nutrition in plants. Front. Plant Sci. 2019, 10, 281. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hao, L.; Zhu, B.; Jiang, Z. Plant calcium signaling in response to potassium deficiency. Int. J. Mol. Sci. 2018, 19, 3456. [Google Scholar] [CrossRef] [PubMed]
- Jeworutzki, E.; Roelfsema, M.R.; Anschütz, U.; Krol, E.; Elzenga, J.T.; Felix, G.; Boller, T.; Hedrich, R.; Becker, D. Early signaling through the Arabidopsis pattern recognition receptors FLS2 and EFR involves Ca2+-associated opening of plasma membrane anion channels. Plant J. 2010, 62, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Liu, G.; Jia, H.; Liu, Y.; Tan, Y.; Wang, S.; Mu, J.; Yu, J.; Xue, K.; Zhang, R.; et al. Changes in planta K nutrient content altered the interaction pattern between Nicotiana benthamiana and Alternaria longipes. Plant Cell Environ. 2024, 47, 3619–3637. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Y.; Luo, W.; Li, W.; Chen, N.; Zhang, D.; Chong, K. The F-box protein OsFBK12 targets OsSAMS1 for degradation and affects pleiotropic phenotypes, including leaf senescence, in rice. Plant Physiol. 2013, 163, 1673–1685. [Google Scholar] [CrossRef]
- Iqbal, N.; Trivellini, A.; Masood, A.; Ferrante, A.; Khan, N.A. Current understanding on ethylene signaling in plants: The influence of nutrient availability. Plant Physiol. Biochem. 2013, 73, 128–138. [Google Scholar] [CrossRef]
- Keunen, E.; Schellingen, K.; Vangronsveld, J.; Cuypers, A. Ethylene and metal stress: Small molecule, big impact. Front. Plant Sci. 2016, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Lau, O.-L.; Yang, S.F. Stimulation of ethylene production in the mung bean hypocotyls by cupric ion, calcium ion, and kinetin. Plant Physiol. 1976, 57, 88–92. [Google Scholar] [CrossRef]
- Wi, S.J.; Ji, N.R.; Park, K.Y. Synergistic biosynthesis of biphasic ethylene and reactive oxygen species in response to hemibiotrophic Phytophthora parasitica in tobacco plants. Plant Physiol. 2012, 159, 251–265. [Google Scholar] [CrossRef]
- Yang, C.; Li, W.; Cao, J.; Meng, F.; Yu, Y.; Huang, J.; Jiang, L.; Liu, M.; Zhang, Z.; Chen, X.; et al. Activation of ethylene signaling pathways enhances disease resistance by regulating ROS and phytoalexin production in rice. Plant J. 2017, 89, 338–353. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.; Roebroeck, E.; Heale, J.B. Effects of ethylene biosynthesis in carrot root slices on 6-methoxymellein accumulation and resistance to Botrytis cinerea. Physiol. Plant. 1988, 73, 71–76. [Google Scholar] [CrossRef]
- Zhai, K.; Liang, D.I.; Li, H.; Jiao, F.; Yan, B.; Liu, J.; Lei, Z.; Huang, L.; Gong, X.; Wang, X.; et al. NLRs guard metabolism to coordinate pattern- and effector-triggered immunity. Nature 2022, 601, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Boerjan, W.; Bauw, G.; Van Montagu, M.; Inzé, D. Distinct phenotypes generated by overexpression and suppression of S-adenosyl-L-methionine synthetase reveal developmental patterns of gene silencing in tobacco. Plant Cell 1994, 6, 1401–1414. [Google Scholar] [PubMed]
- Kawalleck, P.; Plesch, G.; Hahlbrock, K.; Somssich, I.E. Induction by fungal elicitor of S-adenosyl-L-methionine synthetase and S-adenosyl-L-homocysteine hydrolase mRNAs in cultured cells and leaves of Petroselinum crispum. Proc. Natl. Acad. Sci. USA 1992, 89, 4713–4717. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.-Y.; Shin, R.; Schachtman, D.P. Ethylene mediates response and tolerance to potassium deprivation in Arabidopsis. Plant Cell 2009, 21, 607–621. [Google Scholar] [CrossRef]
- Feng, J.; Barker, A.V. Ethylene evolution and ammonium accumulation by nutrient-stressed tomato plants. J. Plant Nutr. 1992, 15, 137–153. [Google Scholar] [CrossRef]
- Haghpanah, M.; Namdari, A. Multiple Defense Layers in Plant-Pathogen Interactions. J. Plant Mol. Breed. 2024, 12, 1–13. [Google Scholar]
- Yadav, V.; Wang, Z.; Wei, C.; Amo, A.; Ahmed, B.; Yang, X.; Zhang, X. Phenylpropanoid Pathway Engineering: An Emerging Approach Towards Plant Defense. Pathogens 2020, 9, 312. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.-L.; Wu, W.-H.; Wang, Y. Transcriptome analysis of rice roots under potassium deficiency reveals a potential regulatory network. Mol. Plant 2020, 13, 1429–1441. [Google Scholar]
- Wang, Y.; Dai, X.; Xu, G.; Dai, Z.; Chen, P.; Zhang, T.; Zhang, H. The Ca2+-CaM signaling pathway mediates potassium uptake by regulating reactive oxygen species homeostasis in tobacco roots under low-K+ stress. Front. Plant Sci. 2021, 12, 658609. [Google Scholar] [CrossRef]
- Lu, L.; Rong, W.; Zhou, R.; Huo, N.; Zhang, Z. TaCML36, a wheat calmodulin-like protein, positively participates in an immune response to Rhizoctonia cerealis. Crop J. 2019, 7, 608–618. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, Z.; Ren, T.; Cong, R.; Lu, J.; Li, X. Metabolomic and transcriptomic changes induced by potassium deficiency during Sarocladium oryzae infection reveal insights into rice sheath rot disease resistance. Rice 2021, 14, 81. [Google Scholar] [CrossRef]
- Du, Y.; Jia, H.; Yang, Z.; Wang, S.; Liu, Y.; Ma, H.; Liang, X.; Wang, B.; Zhu, M.; Meng, Y.; et al. Sufficient coumarin accumulation improves apple resistance to Cytospora mali under high-potassium status. Plant Physiol. 2023, 192, 1396–1419. [Google Scholar] [CrossRef]
- Guo, H.; Ecker, J.R. The ethylene signaling pathway: New insights. Curr. Opin. Plant Biol. 2004, 7, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Pei, H.; Zhang, S.; Chen, J.; Chen, W.; Yang, R.; Meng, Y.; You, J.; Gao, J.; Ma, N. TRV–GFP: A modified Tobacco rattle virus vector for efficient and visualizable analysis of gene function. J. Exp. Bot. 2014, 65, 311–322. [Google Scholar] [CrossRef]
- Chouteau, J.; Fauconnier, D. Fertilizing for high quality and yield. In Tobacco; IPI Bulletin 19; International Potash Institute: Horgen, Switzerland, 1988. [Google Scholar]
- Senthil-Kumar, M.; Mysore, K.S. Tobacco rattle virus–based virus-induced gene silencing in Nicotiana benthamiana. Nat. Protoc. 2014, 9, 1549–1562. [Google Scholar] [CrossRef] [PubMed]
- Kurien, B.T.; Scofield, R.H. Western blotting. Methods 2006, 38, 283–293. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noorin, S.; Du, Y.; Liu, Y.; Wang, S.; Wang, Y.; Jia, H.; Hsiang, T.; Zhang, R.; Sun, G. The NbCBP1-NbSAMS1 Module Promotes Ethylene Accumulation to Enhance Nicotiana benthamiana Resistance to Phytophthora parasitica Under High Potassium Status. Int. J. Mol. Sci. 2025, 26, 1384. https://doi.org/10.3390/ijms26031384
Noorin S, Du Y, Liu Y, Wang S, Wang Y, Jia H, Hsiang T, Zhang R, Sun G. The NbCBP1-NbSAMS1 Module Promotes Ethylene Accumulation to Enhance Nicotiana benthamiana Resistance to Phytophthora parasitica Under High Potassium Status. International Journal of Molecular Sciences. 2025; 26(3):1384. https://doi.org/10.3390/ijms26031384
Chicago/Turabian StyleNoorin, Sadia, Youwei Du, Yi Liu, Shuanghong Wang, Yan Wang, Hongchen Jia, Tom Hsiang, Rong Zhang, and Guangyu Sun. 2025. "The NbCBP1-NbSAMS1 Module Promotes Ethylene Accumulation to Enhance Nicotiana benthamiana Resistance to Phytophthora parasitica Under High Potassium Status" International Journal of Molecular Sciences 26, no. 3: 1384. https://doi.org/10.3390/ijms26031384
APA StyleNoorin, S., Du, Y., Liu, Y., Wang, S., Wang, Y., Jia, H., Hsiang, T., Zhang, R., & Sun, G. (2025). The NbCBP1-NbSAMS1 Module Promotes Ethylene Accumulation to Enhance Nicotiana benthamiana Resistance to Phytophthora parasitica Under High Potassium Status. International Journal of Molecular Sciences, 26(3), 1384. https://doi.org/10.3390/ijms26031384





