Abstract
The interplay between long noncoding RNAs (lncRNAs) and microRNAs (miRNAs) is crucial in the epigenetic regulation of mRNA and protein expression, impacting the development and progression of a plethora of human diseases, such as cancer, cardiovascular disease, inflammatory-associated diseases, and viral infection. Among the many lncRNAs, growth arrest-specific 5 (GAS5) has garnered substantial attention for its evident role in the regulation of significant biological processes such as proliferation, differentiation, senescence, and apoptosis. Through miRNA-mediated signaling pathways, GAS5 modulates disease progression in a cell-type-specific manner, typically by influencing proteins involved in inflammation and cell death. While GAS5 is recognized as a tumor suppressor in cancer, recent reports highlight its broader regulatory capacity in non-cancerous diseases. Its modulation of protein expression through the GAS5/miRNA network has been shown to both mitigate and exacerbate disease, depending on the specific context. Furthermore, the therapeutic potential of GAS5 manipulation, via knockdown or overexpression, offers promising avenues for targeted interventions across human diseases. This review explores the dualistic impacts of the GAS5/miRNA network in conditions such as cancer, cardiovascular disease, viral infections, and inflammatory disorders. Through the evaluation of current evidence, we aim to provide insight into GAS5’s biological functions and its implications for future research and therapeutic development.
1. Introduction
The human genome consists of coding and noncoding sequences. Coding sequences follow the central dogma “DNA makes RNA, and RNA makes protein”; however, coding sequences only account for approximately 2% of the human genome. The vast majority of the human genome is composed of noncoding sequences, which are classified based on their transcript lengths in nucleotides (nt). Transcripts of less than 200 nt are classified as small noncoding RNAs, including microRNAs (miRNAs, typically 22 nt) [1,2], small nucleolar RNAs (snorRNAs), small interfering RNAs (siRNAs), transfer-derived RNAs (tRFs), small nuclear RNAs (snoRNAs), and PIWI-interacting RNAs (piRNAs). Sequences of more than 200 nt are termed long noncoding RNAs (lncRNAs) [3,4]. It is well known that miRNAs play crucial roles in the epigenetic regulation of various cellular biological and pathophysiological pathways [5,6,7,8,9]. While significant advancements have been made in elucidating the roles of lncRNAs in human disease, their functions are highly diverse, varying from transcriptional to post-transcriptional gene regulation, and context-dependent, with their mechanisms of action, particularly lncRNA-miRNA axes, undergoing significant investigation.
Growth arrest-specific 5 (GAS5) is one of the most well-studied lncRNAs and was identified in 1988 [10]. GAS5 is a lncRNA with a transcript length of roughly 630 nt and is encoded by a DNA sequence located on chromosome 1q25 [11]. The gene comprises 12 exons, which are not well conserved because of rapid evolution in higher species. GAS5 introns encode 10 snorRNAs and 2 mature lncRNA isoforms, GAS5a and GAS5b, with their only difference being the length of exon 7 (77 nt and 45 nt, respectively) [12,13]. Given its crucial role in the regulation of cellular proliferation and differentiation, GAS5 is deemed a novel tumor suppressor in various cancers [14,15,16,17,18]. Accumulating evidence suggests that GAS5 may also play a crucial role in the pathophysiological processes occurring in non-cancer diseases, especially inflammation and infectious diseases [19,20,21,22,23].
GAS5 can influence signaling pathways through three modes of action [24]. This review will largely focus on the first mode of action, where GAS5 serves as a decoy, acting as a molecular sponge that can bind directly to its target protein or RNA to disrupt any downstream activities. For example, GAS5 acts as a decoy hormone response element for the glucocorticoid receptor, thus suppressing gene expression [12,25]. Also, GAS5 acts as a biological sponge for miRNAs, suppressing their epigenetic activities and thus directly regulating pathways mediated by miRNAs [26,27]. Moreover, GAS5 acts directly as a signaling molecule, being transcribed in response to specific triggers to facilitate downstream signal transmission. This role has been demonstrated in human colorectal cancer, where, following DNA damage, GAS5 upregulates snoRNAs to subsequently activate the p53 signaling pathway, mitigating DNA damage [24,28]. Furthermore, GAS5 has the ability to function as a transport molecule. This action requires GAS5 to bind to its target protein, which it then guides to a distinct DNA sequence where the protein can regulate transcription. Specifically, GAS5 has been demonstrated to enhance the binding of E2F transcription factor 1 (E2F1) to the promoter of the cyclin-dependent kinase inhibitor 1B (P27kip1), thereby activating P27kip1 [29].
In this review, we summarize the role of GAS5/miRNA axes in cancers, cardiovascular diseases, inflammatory diseases, and viral infections. This is followed by a brief discussion of the impact of GAS5, independent of miRNA-mediated axes, on these conditions. The objective of this article is to provide prospective insight for future studies on the biology of GAS5 and its pathophysiological functions in human diseases.
2. Role of GAS5/miRNA Axes in Cancers
GAS5 is known as a regulator of cellular differentiation and proliferation, thus targeting GAS5 and its regulated pathways has been considered a novel therapeutic treatment in cancer [16,30].
To date, the GAS5/miR-21 axis is one of the most extensively studied lncRNA-miRNA-regulated signaling pathways and has been shown to play a crucial role in the progression of various cancers via downregulated GAS5 and upregulated miR-21 expressions. GAS5 and miR-21 reciprocally regulate each other’s expression, exhibiting oncogenic roles in different human cancer diseases [31]. Specifically, the overexpression of GAS5 decreases the level of miR-21, whereas the overexpression of miR-21 eliminates GAS5-mediated regulatory effects [13,32]. Mechanistically, miR-21 targets a binding site in exon 4 of GAS5, forming a feedback loop that controls their expression via the RNA-induced silencing complex (RISC) [13]. This interaction exemplifies the miRNA-mediated silencing of target RNAs [13]. Thus, the reciprocal regulation between GAS5 and miR-21 emphasizes their critical roles in modulating cellular differentiation and proliferation, which are pivotal processes in cancer progression.
Recently, miR-21 upregulation has been identified as a powerful biomarker in various cancers, since miR-21 is known as a cell death and differentiation modulator, both enhancing cell activation/growth and suppressing cell apoptosis regulatory pathways. For instance, miR-21 has been demonstrated to inhibit the expression of one of the most potent tumor suppressor proteins—Phosphatase and Tensin Homolog (PTEN) [33]. PTEN plays a critical role in tumor suppression through phosphatase-dependent and -independent activities, with one of its more prominent roles being using its phosphatase activity to regulate a crucial pro-survival and cell growth pathway in cancers, i.e., Phosphoinositide 3-Kinase/Phosphorylated-protein Kinase B/Mammalian Target of Rapamycin (PI3KAKT/mTOR) [34]. The miR-21-mediated inhibition of PTEN in the PTEN/Phosphoinositide 3-Kinases (PI3K) pathway results in enhanced cellular growth and differentiation. The repression of PTEN derepresses Phosphorylated-protein Kinase B, also known as AKT, leading to an enhanced expression of growth-promoting protein Mammalian Target of Rapamycin (mTOR) [35]. mTOR signaling, which governs critical processes like cell cycle, proliferation, growth, survival, protein synthesis, and glucose metabolism, is frequently dysregulated in cancers, with studies showing its enhancement in various malignancies and involvement in nearly 30% of solid tumors, making it one of the most commonly affected pathways [36]. MiR-21 also regulates cell growth via regulating the Sprouty RTK signaling antagonist 1 (SPRY1)/rapidly accelerated fibrosarcoma (RAF)/extracellular signal-regulated kinase (ERK) pathway. The repression of SPRY1/2 by miR21 leads to the upregulation of RAF and ERK proteins, thus promoting cellular proliferation and differentiation in many mammalian cells [37]. Additionally, the miR-21-mediated suppression of apoptosis has been linked to the suppression of programmed cell death 4 (PDCD4), resulting in continuous cell cycle progression [38,39]. MiR-21 also directly targets and inhibits SMAD family member 7 (SMAD7), a pro-apoptosis protein, resulting in reduced apoptosis and cell death [40]. Given its role in cell growth and proliferation, miR-21 upregulation serves as a biomarker in cancer diseases.
Due to its inverse correlation with miR-21, GAS5 repression has been characterized as a biomarker in various cancers [41]. A recent study highlighted a significant repression of GAS5 in osteosarcoma patients, as well as a more prominent repression in patients with lung metastatic cancer. Upon further characterization in osteosarcoma cell lines, the GAS5 repression corresponded to upregulated epithelial–mesenchymal transition (EMT) [42], a phenomenon which increases migration and invasion, both integral for metastasis. Interestingly, others have demonstrated a suppression of the EMT process within uveal melanoma (UM) cells that was observed upon the upregulation of GAS5, reducing the overall risk of metastasis [43]. Experiments within xenograft mice models have confirmed the crucial role of GAS5 as a tumor suppressor due to its ability to act as a competing endogenous (ceRNA) for miR-21, sponging the potent pro-cancer regulator. The overexpression of GAS5 resulted in a reduced survival rate of breast cancer cell lines, as well as a decrease in the size of tumors in mice, whereas the inhibition of GAS5 displayed an opposite effect [13]. GAS5 overexpression has also been demonstrated to repress endometrial cancer formation in immunodeficient mice models through PTEN/AKT signaling and directly repressing oncogenic yes-associated protein 1 (YAP1) in tumor-associated macrophages, transforming them into an anti-tumor phenotype [44]. Additional findings support a tumor-promoting function of the aberrant GAS5/miR-21 axis via the PTEN/PIK3 or SPRY1/p21 pathways in hepatocellular carcinoma, oral squamous cell carcinoma, bladder cancer cells, and ovarian cancer [45,46,47,48,49]. Based on these studies, it is believed that the suppression of miR-21 and upregulation of GAS5 have therapeutic potential for the treatment of cancer diseases.
However, GAS5’s tumor-suppressing function extends beyond its inverse correlation with miR-21, as it also modulates cancer progression through various miRNA-mediated signaling pathways. For instance, GAS5 modulates the malignant phenotype, migration, and invasion of glioma cells via binding and suppressing miR-18-a-5p [50]. Deletion analysis detected binding sites for miR-18a-5p on the second exon of GAS5, further confirming their interaction. Another study showed that the natural compound α-Solanine exhibits anti-cancer activity and enhances radio-sensitivity in prostate cancer. Interestingly, enhanced apoptosis and DNA damage were observed, along with an increase in GAS5 level and decreased miR-18a-5p expression, in prostate cancer cells treated with α-Solanine, suggesting that targeting GAS5/miR-18a-5p can be utilized to treat prostate cancer in the presence of α-Solanine [51]. In addition, another study suggested that GAS5, miR-196a-5p, and Forkhead Box Protein O1 (FOXO1) form a positive feedback loop in glioma stem cells [52]. GAS5 increases the level of FOXO1 by inhibiting miR-196a, thus suppressing cancer invasion. Moreover, the GAS5/miR-196 axis increases the level of phosphotyrosine interaction domain containing 1 (PID1) protein, thus inhibiting glioma stem cell tumorigenicity and growth. Interestingly, FOXO1 promotes the transcription of GAS5 as well, thereby forming a positive feedback loop that can be used as an approach to treat glioma cancer. Most recently, the upregulation of GAS5 has been demonstrated to repress miR-135b-5p, resulting in the upregulation of the adenomatous polyposis coli (APC) gene, a known tumor suppressor, through its regulation of the Wnt/β-catenin pathway [53,54]. Furthermore, the tumor-suppressing function of GAS5 was reported in esophageal squamous cell carcinoma [53], in cervical cancer through the miR-196a/miR-205/FOXO1 axis [54], and in ovarian cancer via the miR-196a/Homeobox 5 (HOXA5) axis [55].
The role of GAS5 and its associated miRNAs in the regulation of cellular proliferation and differentiation has been further demonstrated by GAS5 reducing the migration and invasion of pancreatic cancer via miR-221 repression, specifically through increasing the level of suppressor of cytokine signaling 3 (SOCS3) protein [56]. The GAS5-mediated inhibition of miR-221 also results in an increased expression of the aplasia ras homolog member I (ARHI), thereby preventing the EMT process in osteosarcoma cells [57]. The inhibition of miR-221 via GAS5 sponging has also been demonstrated to upregulate tumor protein p63 (TP63), a central tumor suppressor in non-small cell lung cancer (NSCLC) [58]. Additionally, the sponging of miR-221 via GAS5 has been demonstrated to mitigate Adriamycin resistance in breast cancer, specifically through the upregulation of Dickkopf 2 (DDK2) and suppression of P-glycoprotein (ABCB1) expression and the Wnt/β-catenin pathway [59]. The GAS5/miR-222 axis has been suggested to inhibit the BCL-2-modifying factor (BMF) and its downstream BAX protein, thereby delaying the proliferation of glioma cells [60]. Notably, the proliferation of osteosarcoma cells decreased following the activation of the GAS5/miR-23a axis via the PTEN/PI3K/AKT pathway [61]. The GAS5/miR-222 axis also regulates the PTEN/AKT/mTOR pathway to suppress the progression of other cancers, such as papillary thyroid carcinoma [62], human B lymphocytic leukemia [63], and gastric cancer [64]. Additionally, recent evidence suggests that a modulation of GAS5 can alter the proliferation rates of cancer cells, specifically through the use of GAS5-enriched extracellular vesicles (EVs) as a delivery system. A recent study demonstrated the ability of sorafenib-induced EVs, which were enriched with tumor suppressor ncRNAs such as GAS5, to significantly reduce xenograft tumor area, suppress angiogenesis, and reduce the number of micrometastases in the tails of zebra fish [65].
Not only is GAS5 an effective tumor suppressor through its ability to regulate proliferation and differentiation, but GAS5 has also been demonstrated to inhibit cancer progression by driving cancer cells towards programmed cell death through autophagy. Recent findings in 293T cells emphasize the pivotal role of the GAS5/miR-23a/ATG axis in the modulation of autophagy. GAS5 suppresses the expression of miR-23a, subsequently elevating the levels of key autophagy-related proteins such as LC3II, Beclin1, and the autophagy-related 5 and 12 complex formation (ATG5-ATG12). This cascade facilitates enhanced autophagic activity. Concurrently, GAS5 overexpression leads to the repression of mTOR and p62, critical regulators of cell survival and ubiquitin-binding protein dynamics, respectively [66]. The GAS5/miR-23a axis also acts as a tumor suppressor in non-small cell lung cancer [67], ovarian cancer [68], and glioma [69].
In addition to the miRNAs mentioned, GAS5 also interacts with several other miRNAs involved in the regulation of cellular growth and apoptosis in many other contexts. For instance, by inhibiting the expression of miR-103 and miR-32-5p, GAS5 induces the PTEN/PI3K/AKT pathway, thereby suppressing the tumor progression of endometrial cancer [70] and pancreatic cancer cells [71]. GAS5 also acts as a tumor suppressor in various cancers through functioning as a ceRNA for many miRNAs, which can exacerbate conditions by contributing to the acceleration of cancer. The GAS5-mediated sponging of miRNAs includes targets such as miR-223 in renal cell carcinoma [72]; miR-135b [73] or miR-1323 [74] in hepatocellular carcinoma; miR-106b in cervical cancer [75]; miR-182-5p in colorectal cancer [76]; miR-181c-5p in pancreatic cancer [77]; miR-424 in multiple malignant phenotypes of glioma [78]; miR-145 in prostate cancer [79]; miR-135b in non-small cell lung cancer [80]; and miR-34a in solid tumors [81]. These interactions underscore the role of GAS5 as a versatile tumor suppressor across multiple cancer models. By acting as a ceRNA, GAS5 effectively derepresses either the expression of tumor suppressor genes or critical regulatory pathways that inhibit tumor proliferation, invasion, and resistance to therapy. This highlights the broad therapeutic potential of targeting the GAS5/miRNA axis to modulate cancer progression and improve treatment outcomes.
GAS5/miRNA axes play significant roles in modulating cellular proliferation, differentiation, and apoptosis, allowing them to have a pivotal tumor-suppressive role in cancer progression. The GAS5/miR-21 axis is particularly well-characterized and highlighted by the reciprocal regulation between GAS5 and miR-21 and their impact on prominent oncogenic pathways, such as PTEN/PI3K/AKT or SPRY/RAF/ERK [13,31,32,35,37,38,39,40]. GAS5’s tumor-suppressive ability is not limited to this axis, as it functions as a ceRNA for many other miRNAs such as miR-18a-5p, miR-196a, miR-221, and miR-23a, among others, to inhibit hallmark cancer characteristics such as EMT [42,43,56,57], chemoresistance [46,59,77], and metastasis [63,71] across a multitude of cancer cell types. Altogether, these findings emphasize GAS5’s multifaceted role as a ceRNA, sponging various oncogenic miRNAs, and display the therapeutic potential of targeting GAS5/miRNA axes, through the upregulation of GAS5, to suppress tumorigenesis and metastasis across numerous forms of cancer (see Figure 1 and Table 1).

Figure 1.
The GAS5/miRNA-regulated pathways in cancers. This figure depicts the pathways modulated by the GAS5/miRNA-mediated mechanisms that regulate the progression of cancers. The upregulation of GAS5 results in the downregulation of its target miRNAs, thereby regulating downstream proteins involved in cellular activation, proliferation, differentiation or apoptosis, and cell cycle arrest. Thus, increasing GAS5 levels is considered a promising therapeutic approach for suppressing the progression of various cancers. PTEN, phosphatase and tensin homolog; AKT, protein kinase B; mTOR, mammalian target of rapamycin; SPRY1, Sprouty RTK signaling antagonist 1; RAF, rapidly accelerated fibrosarcoma; ERK, extracellular signal-regulated kinase; PDCD4, programmed cell death 4; P21, cyclin-dependent kinase inhibitor 1; SMAD7, SMAD family member 7; BIM, Bcl-2-like protein 11; BAX, BCL2-associated X; FOXO1, forkhead box protein O1; SOCS3, suppressor of cytokine signaling 3; ARHI, aplasia Ras homolog member I; DKK2, Dickkopf 2; ATG12, autophagy related 12; ATG3, autophagy related 3; ATG8, autophagy related 8; LC3II, LC3-phosphatidylethanolamine conjugate.

Table 1.
Summary of GAS5/miRNA-regulated pathways in human diseases.
3. GAS5/miRNA Axes and Cardiovascular Diseases
As previously discussed, the GAS5/miR-21 axis has been well defined in cancer studies, and unsurprisingly it has also been a significant area of focus in other areas of biomedical research, such as cardiovascular disease. The aberrant expression of GAS5 and miR-21 is associated with cardiovascular disease and dysfunctions such as cardiac fibrosis [87], acute myocardial infarction [85], increased cardiomyocyte damage and apoptosis [86,89], and the aberrant proliferation and migration of vascular smooth muscle cells [90].
Beyond miR-21, GAS5 has been shown to exacerbate various cardiovascular diseases by modulating other miRNAs and their downstream signaling pathways, with studies highlighting the therapeutic potential of GAS5 knockdown or knockout strategies. One study regarding myocardial infarction suggested that by sponging miR-525-5p, GAS5 enhances the expression of calmodulin 2 (CALM2) and induces the apoptosis of myocardial cells, thereby promoting the development and progression of myocardial infarction [121]. In another study, GAS5 acted as a biological sponge for miR-142-5p in cardiomyocyte H9c2 cells, thereby suppressing miR-142-5p activity by targeting the tumor protein P53 inducible nuclear protein 1 (TP53INP1), which resulted in an increase in cell proliferation via the PI3K/AKT and MEK/ERK signaling pathways. Additionally, GAS5 silencing reduces the levels of p53, Bax, and cleaved caspase-3 proteins, while also increasing the expression of cyclinD1, cyclin-dependent Kinase 4 (CDK4), and B-cell lymphoma 2 (BCL-2) proteins, collectively leading to a decrease in cell apoptosis. Thus, GAS5 silencing protects cardiomyocytes against hypoxic injury via sponging miR-142-5p [105]. Importantly, GAS5 also modulates hypoxia/reoxygenation (H/R)-induced cardiomyocytes. GAS5 acts as a ceRNA for miR-335, as knocking down GAS5 leads to the repression of the miR-335 target protein Rho-associated protein kinase 1 (ROCK1). In turn, the repression of ROCK1 activates the PI3K/AKT pathway and inhibits glycogen synthase kinase 3 (GSK-3β) and mitochondrial permeability transition pore (mPTP) from opening proteins. Altogether, these changes shift the balance toward pro-survival pathways and mitochondrial stability, leading to the silencing of GAS5, which limits myocardial infarct size and reduces apoptosis in H/R-induced cardiomyocytes [117]. By targeting miR-135a, GAS5 can impact the progression of atherosclerosis (AS). In a recent study, THP-1 macrophages treated with oxidized low-density lipoprotein (ox-LDL) and apolipoprotein E (apoE)−/− mice on a high-fat diet (HFD) were used as vitro and in vivo models, respectively, to investigate the role of GAS5 in atherosclerosis. The results showed that GAS5 levels increased in LDL-treated macrophages as well as in AS mice. Notably, GAS5 silencing induced miR-135a, decreasing inflammation and AS disease progression [103]. This role of GAS5 in atherosclerosis progression is further supported by a study investigating the role of GAS5 in coronary AS through the miR-194-3p/Thioredoxin-interacting protein (TXNIP) axis. This study demonstrated that elevated GAS5 and TXNIP expression, as well as reduced miR-194-3p expression, corresponded with increased endothelial cell (EC) apoptosis. Upon the inhibition of GAS5 or overexpression of miR-194-3p, significant enhancement of endothelial cell proliferation and a reduction in apoptosis via TXNIP were observed, providing promising potential for reducing the formation of atherosclerotic plaques in AS [110].
While there is an accumulation of evidence suggesting an overall negative role for GAS5 in cardiovascular health, recent reports also suggest a protective role in some contexts. Notably, GAS5 was identified as an anti-senescence lncRNA in endothelial progenitor cells (EPCs). It was reported to function as a ceRNA of miR-223. Upon knocking down GAS5, miR-223 disrupts proliferation and enhances senescence in EPCs through the suppression of the nicotinamide phosphoribosyltransferase (NAMPT) and PI3K/AKT pathways [114]. Recent reports have demonstrated certain protective effects of GAS5 against cardiac fibrosis through the regulation of the miR-217/Sirtuin 1 (SIRT1) pathway. One study utilized isoprenaline-induced myocardial fibrosis in mice to demonstrate that GAS5 overexpression significantly reduced the expression levels of collagen, NOD-like receptor family pyrin domain containing 3 (NLRP3), Caspase-1, and IL-1β, leading to an overall reduction in inflammatory response and pyroptosis. Conversely, the protective effect of GAS5 was ablated following the overexpression of miR-217, which downregulates SIRT1 and promotes NLRP3 inflammasome activation. Altogether, these findings suggest a critical role for the GAS5/miR-217/SIRT1 axis in restraining cardiac fibrosis, leading to the preservation of cardiac function [112]. Furthermore, GAS5 has been shown to play a protective role in myocardial injury in rats with adjuvant arthritis by downregulating the miR-21/ Toll-like-Receptor 4 (TLR4)/NLRP3 axis, resulting in reduced inflammation, pyroptosis, and myocardial damage [91]. These findings underscore GAS5’s broader potential as a regulator of inflammatory and fibrotic processes within the cardiovascular system.
The role of GAS5 in cardiovascular disease highlights its complex and context-dependent interplay with miRNA and downstream pathways. While GAS5 has been demonstrated to function in a manner that exacerbates conditions like myocardial infarction [121], atherosclerosis [103], and hypoxia/reoxygenation injury [117] by promoting apoptosis, inflammation, and fibrosis, it also exhibits protective effects, such as mitigating cardiac fibrosis [112] and countering endothelial progenitor cell senescence [114]. This duality stresses the need for tailored therapeutic approaches targeting GAS5. Leveraging its role as a ceRNA offers significant potential for precision RNA-based therapies to modulate miRNA activity in an effective manner. Future research should aim to clarify GAS5’s mechanisms across a myriad of cardiovascular disease models to advance novel treatments within this field (see Figure 2 and Table 1).
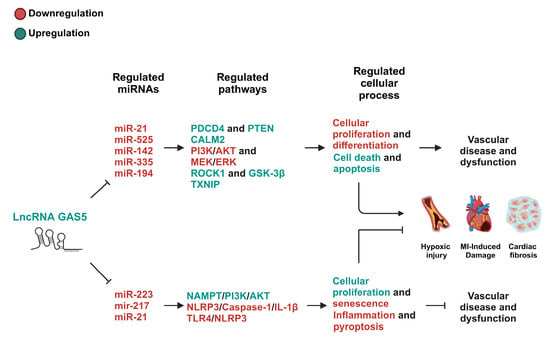
Figure 2.
The GAS5/miRNA-regulated pathways in cardiovascular diseases. This figure depicts the pathways modulated by GAS5/miRNA-mediated mechanisms that have either debilitating or protective effects on cardiovascular diseases. The pathways involved in modulating cell activity in a debilitating manner exert their effects, in general, through the suppression of cellular proliferation and differentiation while promoting cell death and apoptosis. The pathways involved in modulating cell activity in a protective manner exert their effects, in general, through the suppression of senescence, inflammation, or pyroptosis while promoting cellular proliferation. These pathways are largely cell-type- and context-dependent. PDCD4, programmed cell death protein 4; PTEN, phosphatase and tensin homolog; PI3K, phosphoinositide 3-kinase; AKT, protein kinase B; MEK, mitogen-activated protein kinase; ERK, extracellular signal-regulated kinase; ROCK1, rho-associated coiled-coil containing protein kinase 1; GSK-3β, glycogen synthase kinase 3 beta; TXNIP, thioredoxin-interacting protein; NAMPT, nicotinamide phosphoribosyltransferase; NLRP3, NOD-, LRR-, and pyrin domain-containing protein 3; TLR4, toll-like receptor 4; MI, myocardial infarction.
4. Roles of GAS5/miRNA Axes in Inflammation-Associated Diseases
In addition to its roles in cancers and vascular diseases, GAS5 also plays a crucial role in regulating the progression of many inflammation-driven diseases.
GAS5 appears to have a distinctly detrimental role in the context of multiple inflammation-related neurological diseases. A previous study has shown that the inhibition of miR-221 by GAS5 induces the transcription of p53-upregulated modulator of apoptosis (PUMA) and its downstream proteins such as c-Jun N-terminal kinase (JNK)-H2A histone family member X (H2AX), thereby promoting neuronal apoptosis under hypoxic conditions [27]. Another study demonstrated that GAS5 levels are upregulated in mice with cerebral ischemic stroke (CIS), a leading cause of neurological disability worldwide. The knockdown of GAS5 was effective at attenuating the cerebral infarct, neurological injury, apoptosis, and inflammatory response within the middle cerebral artery occlusion (MCAO) model. Specifically, GAS5 was found to directly bind to miR-9, which, when overexpressed, displayed similar protective effects to those seen in the GAS5 knockdown. Furthermore, FOXO3 was determined to be directly repressed by miR-9, and when restored, FOXO3 was shown to reverse the protective effects that miR-9 previously demonstrated. Altogether, these observations suggest GAS5 promotes ischemic stroke injury through sponging miR-9 to derepress FOXO3 [82]. Interestingly, others have examined a similar debilitating role of GAS5 in CIS, where the knockdown of GAS5 reduces the progression of CIS by regulating the miR-26b-5p/SMAD1 axis [99]. Others demonstrated that in mice undergoing MCAO surgery and oxygen–glucose deprivation (OGD) treatment, GAS5 negatively correlated with miR-137 levels. Silencing GAS5 led to an upregulation of miR-137 levels, along with a decrease in its endogenous target notch homolog 1 (Notch-1) protein. This miR-137 upregulation increased cell viability and decreased caspase-3 activation and cell apoptosis in neurons under OGD conditions. These findings indicate that GAS5 promotes the progression of CIS by acting as a ceRNA for regulating the miR-137-mediated notch-1 signaling pathway, which may provide a novel therapeutic approach for CIS [104]. Moreover, by regulating miR-21/PTEN/PI3K/AKT signaling, GAS5 promotes neural apoptosis, thereby contributing to the progression of CIS [88].
A link between GAS5 and Parkinson’s disease (PD) has been investigated using an in vitro cell model with lipopolysaccharide-induced microglia and an in vivo model established in C57BL/6 mice [115]. GAS5 has been shown to accelerate Parkinson’s disease (PD) progression by promoting inflammation in microglia through the miR-223-3p/NLRP3 axis. Specifically, GAS5 acts as a competitive sponge for miR-223-3p, thereby increasing NLRP3 expression and activating the NLRP3 inflammasome, which triggers the secretion of pro-inflammatory cytokines like interleukin-1β (IL-1β) and IL-18, further exacerbating the inflammatory response and PD progression in both in vitro and in vivo models [115]. Another study displayed an alternate pathway through which GAS5 promotes PD progression. Here, GAS5 binds and sequesters miR-150, which normally targets and represses FOSs like antigen 1 (Fosl1), thereby activating the PTEN/AKT/mTOR pathway, promoting apoptosis and inhibiting overall neuronal activity [108]. Together, these studies highlight the detrimental role of GAS5 in PD progression, which it enacts primarily through its regulation of inflammatory and apoptotic pathways.
In addition to its detrimental effects in CIS and PD, GAS5 has also been implicated to be involved in the development of other inflammation-related neurological disorders. Most recently, in Alzheimer’s disease (AD) studies, GAS5 has been found to be significantly upregulated in disease models and patient samples, where it exacerbates pathogenesis by acting as a ceRNA, sponging miR-23b-3p. This repression allows for the increased expression of GSK-3β and PTEN, critical mediators of tau hyperphosphorylation, amyloid-beta (Aβ) accumulation, and neuronal apoptosis. The GAS5/miR-23b-3p axis activates a PTEN/PI3K/Akt/GSK-3β signaling pathway, which amplifies AD-associated neurodegeneration and cognitive decline [98]. In the context of epilepsy, GAS5 interacts with miR-135a-5p to regulate KCNQ3, a potassium channel subunit vital for neuronal excitability. The upregulation of GAS5 reduces miR-135a-5p levels, decreasing KCNQ3 expression, which may increase a person’s susceptibility to seizures by destabilizing their membrane potential regulation. The knockdown of GAS5 in epileptic models mitigates seizure frequency and prolongs latency [102]. Additionally, GAS5 has been demonstrated to exacerbate the progress of neonatal hydrocephalus through its ceRNA activity on miR-325-3p, which regulates the expression of chaperonin containing T-complex protein 1, subunit 8 (CCT8), a complex that has been demonstrated to play a crucial role in protein quality control as it relates to neurological disease [116]. Consistently, the knockdown of GAS5 in the context of the inflammation-related neurological diseases appears to have substantial therapeutic potential.
GAS5 has been demonstrated to exacerbate and promote dysfunction in the context of a few other inflammation-associated diseases. For example, GAS5 is reported to induce the progression of childhood pneumonia. Elevated levels of GAS5 promote macrophage differentiation toward the M1 phenotype, which secretes a high level of pro-inflammatory cytokines, instead of the M2 phenotype, which produces anti-inflammatory cytokines such as IL-10 and transforming growth factor β (TGF-β). GAS5 promotes macrophage polarization to the M1 phenotype by regulating the miR-455-5p/SOCS3 axis. By acting as a sponge for miR-455-5p, GAS5 enhances SOCS3 expression, thereby suppressing the Janus kinase 2-signal transducer (JAK2) and activator of transcription 3 (STAT3) pathway, promoting macrophage polarization toward the M1 phenotype and thus leading to more severe cases of pneumonia in children [120]. GAS5 also promotes asthma progression. The upregulation of GAS5 levels in asthmatic airways results in a decrease in miR-10a, which upregulates brain-derived neurotrophic factor (BDNF). As a result, the proliferation rate of the airway smooth muscle cells increases, thereby thickening the airway wall. Thus, targeting the GAS5/miR-10a/BDNF regulatory axis could significantly decrease airway hyperresponsiveness and reduce the progression of asthma [83]. Additionally, GAS5 plays a crucial role in renal ischemia/reperfusion (I/R), which contributes to acute kidney injury. By inhibiting miR-21, GAS5 upregulates the miR-21 target proteins PTEN, PDCD4, or Thrombospondin-1 (TSP-1), leading to the increased apoptosis and cell death of renal epithelial cells [93].
Although much of the literature presents GAS5 as a detrimental factor that promotes the progression of various diseases, its role in other conditions is more nuanced and context-dependent. GAS5 has recently been demonstrated, in varying models, to have a multifaceted role in its involvement in inflammatory responses and mechanisms in sepsis. Recent reports have demonstrated the occurrence of GAS5 upregulation in endothelial cells through oxidative stress-activated MiT-TFE transcription factors, a phenomenon that helps protect mitochondrial function and reduce inflammation. This protective mechanism involved the GAS5-mediated sponging of miR-23a-3p, which stabilized and enhanced MITF activity and Nrf2 to preserve mitochondrial integrity and reduce lung injury in instances of sepsis [97]. Another study implicates GAS5 as a key suppressor of inflammatory responses in sepsis through the modulation of the miR-155-5p/SIRT1/HMGB1 axis [109]. While previous studies highlight a protective role for GAS5 in mitigating inflammation and preserving cellular integrity, others demonstrate the potential of GAS5 to exacerbate inflammatory damage in a different cellular context. One study found GAS5 levels to be elevated in lipopolysaccharide (LPS)-treated THP-1 cells. That study reveals that GAS5 acts as a sponge for miR-23a-3p, increasing the expression of TLR4 and thereby promoting inflammation and apoptosis in sepsis. Specifically, upon GAS5 knockdown, TLR4 overexpression mitigates the reduction in inflammatory cytokines and subsequent cell death, an indication that GAS5 enhances sepsis-related inflammatory responses through altering the miR-23a-3p/TLR4 axis [96]. Recently, others have hypothesized that GAS5 may have a dual role in sepsis, promoting inflammation in early stages and inhibiting excessive inflammatory damage in later stages through different mechanisms [97,123]. Altogether, the context-dependent nature of GAS5 and its interaction with miRNA axes in sepsis highlights its complex role, exhibiting protective effects in some cellular environments while exacerbating inflammation and apoptosis in others, potentially influenced by timing and cell-specific factors.
GAS5 has been cited to have distinct protective effects in the context of a few other inflammation-driven diseases through miRNA-mediated pathways that suppress inflammation, fibrosis, and apoptosis. For instance, in nonalcoholic fatty liver disease (NAFLD), GAS5 acts as a competing endogenous RNA (ceRNA) for miR-28a-5p, which has been demonstrated to promote pyroptosis through MARCH7-mediated NLRP3 activation, thereby exerting a protective effect [100]. In a similar fashion, GAS5 reduces the severity of rheumatoid arthritis (RA) through sponging miR-361-5p, a process which upregulates PDK4, ultimately limiting synovial hyperplasia and cell proliferation [118]. In osteoarthritis (OA), GAS5 attenuates inflammation-driven chondrocyte apoptosis through the upregulation of SMAD4 via miR-146a sponging [107]. This protective function is further exemplified by the ability of GAS5 to reduce renal fibrosis in diabetic nephropathy (DN) through sponging miR-221, which elevates SIRT1 expression, highlighting its potential role in mitigating fibrosis and promoting cellular homeostasis in the kidney [113]. The GAS5/miR-452-5p axis has also been demonstrated to have a prominent role in reducing the extent of diabetic nephropathy-related inflammation, oxidative stress, and pyroptosis in renal tubular cells [119]. Finally, in renal fibrosis independent of diabetes, GAS5 has been demonstrated to mitigate TGF-β1-induced fibrosis by modulating miR-142-5p through the Smad3 pathway [106].
GAS5 plays a dual role in various diseases, displaying protective effects in some contexts and detrimental effects in others. Its promotion of inflammation and apoptosis in the context of ischemic stroke [82,88,99,104], Parkinson’s disease [108,115], and Alzheimer’s disease [98], among many other previously discussed diseases, highlights the potential of GAS5 inhibition to be used as a therapeutic strategy to mitigate inflammation, neuronal apoptosis, and disease progression. However, its protective function in diseases such as NAFLD [100], RA [118], and DN [113,119], among others, highlights the therapeutic potential of GAS5 upregulation. The case of sepsis adds complexity, where GAS5’s dual role, suppressing or encouraging inflammation in what appears to be a timing- and cell-dependent manner [96,97,109,123], offers potential opportunities for finely tuned therapeutic interventions. Overall, these findings emphasize that the therapeutic potential of GAS5 is distinctly cellular- and disease-context-dependent, suggesting that the targeted manipulation of GAS5’s expression and activity could lead to novel therapeutic strategies for a broad spectrum of inflammation-associated diseases (see Figure 3 and Figure 4 and Table 1).
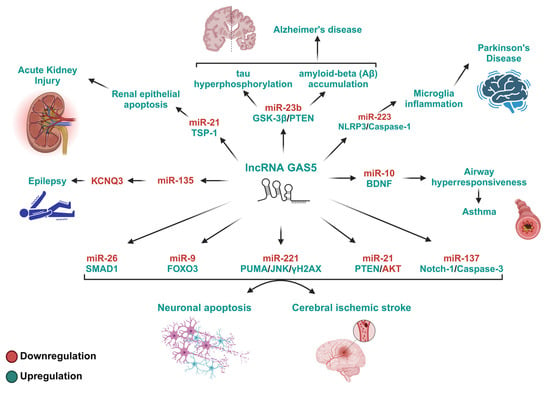
Figure 3.
The GAS5/miRNA-regulated pathways with debilitating roles in inflammation-associated diseases. This figure depicts the pathways modulated by GAS5/miRNA-mediated mechanisms that exert debilitating effects on inflammation-associated diseases. The proteins involved in these pathways are known for their roles in cell activation, proliferation, differentiation, apoptosis, and cell cycle arrest. By inhibiting specific miRNAs and their target proteins, elevated GAS5 levels promote apoptosis and cell death, thereby promoting inflammation and apoptosis, increasing the severity of inflammatory diseases. TSP-1, thrombospondin-1; GSK-3β, glycogen synthase kinase 3; PTEN, phosphatase and tensin homolog; NLRP3, NOD-like receptor family pyrin domain containing 3; BDNF, brain-derived neurotrophic factor; SMAD1, SMAD family member 1; FOXO3, forkhead box protein O3; PUMA, p53-upregulated modulator of apoptosis; JNK, c-Jun N-terminal kinase; γH2AX, phosphorylated histone H2AX; AKT, protein kinase B; Notch-1, neurogenic locus notch homolog protein 1; KCNQ3, potassium voltage-gated channel subfamily Q member 3.
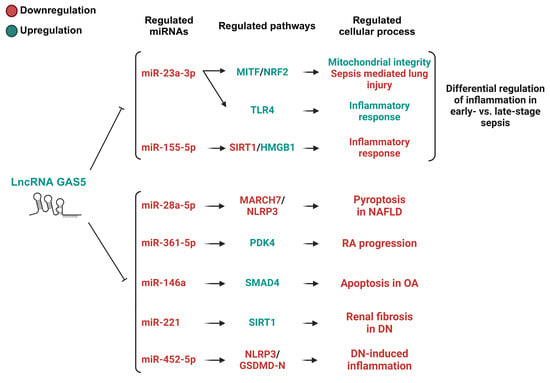
Figure 4.
The GAS5/miRNA-regulated pathways with protective roles in inflammation-associated diseases. This figure depicts the pathways modulated by GAS5/miRNA-mediated mechanisms that exert protective effects and differential effects (sepsis) on inflammation-associated diseases. For sepsis, miRNAs appear to have a stage-dependent role in inflammation, where early sepsis relies on mitochondrial autophagy for antioxidant defense and intracellular stability, while GAS5 depletion in late-stage sepsis exacerbates inflammation, increasing tissue damage. Regarding the other disease models displayed, the overexpression of GAS5 leads to the repression of miRNAs displaying roles in inflammation, pyroptosis, proliferation, and apoptosis. This suppression of inflammation, apoptosis, and pyroptosis is significant in reducing the progression of these inflammation-associated diseases. MITF, microphthalmia-associated transcription factor; NRF2, nuclear factor erythroid 2–related factor 2; TLR4, toll-like receptor 4; SIRT1, Sirtuin 1; HMGB1, high mobility group box 1; MARCH7, membrane-associated ring-CH-type finger 7; NLRP3, NOD-like receptor family pyrin domain containing 3; PDK4, pyruvate dehydrogenase kinase 4; SMAD4, SMAD family member 4; GSDMD-N, gasdermin D N-terminal fragment; NAFLD, nonalcoholic fatty liver disease; OA, osteoarthritis; DN; diabetic nephropathy.
5. GAS5/miRNA Axes in Viral Infections
While extensive research shows that GAS5 and miRNAs play important roles in viral infections, the literature exploring their interactions and the implications of these interactions in viral pathogenesis remains limited. GAS5 has been demonstrated to be downregulated in HIV infection [124]. The significance of GAS5 expression in HIV infection has been further elucidated through its role as a sponge for miR-873, which has been demonstrated to significantly alter HIV-1 replication. The upregulation of GAS5 led to a substantial repression of miR-873, resulting in decreased HIV replication. Conversely, the siRNA-mediated knockdown of GAS5 increased miR-873 levels, which correspondingly enhanced HIV infection. Additionally, the overexpression of GAS5 was found to reduce the levels of HIV-1 Gag and Pol proteins, while its knockdown resulted in elevated levels of HIV-1 mRNA. Similar results were observed for the HIV-1 p24 and Tat proteins [122]. In addition to HIV-1 proteins, GAS5 has also been shown to suppress the replication of hepatitis C virus (HCV) infection. By acting as a decoy for the viral NS3 protein, GAS5 directly suppresses HCV replication, thus decreasing the progression of HCV disease [21]. Recent reports also suggest GAS5 deficiency could be a potential biomarker for discriminating between moderate and severe SARS-CoV-2 cases. The downregulation of GAS5 allows for increased miR-200, yielding a reduction in ACE-2 which is linked to increased inflammation and cytokine storms [111]. We have recently reported that GAS5 regulates the function of CD4 T cells derived from people living with HIV (PLWH). Our data showed that increasing GAS5 in CD4 T cells restored T cell functions via repressing miR-21, which acts as an accelerator of apoptosis and senescence in CD4 T cells. Importantly, our study also suggested that in addition to the effect of miR-21-mediated signaling, other miRNAs may play a role in the regulation of CD4 T cell functions [94].
These studies demonstrate that GAS5 plays a crucial role in regulating the immune dysregulation associated with HIV, HCV, and SARS-CoV-2. By modulating miRNA activity, GAS5 effectively suppresses viral replication, reduces inflammation, and restores immune function, specifically through functioning as a ceRNA of miR-873, miR-21, and miR-200 [21,94,111,122,124]. The ability of GAS5 to attenuate HIV- and HCV-induced immune dysfunction, as well as its potential as a biomarker of the severity of SARS-CoV-2 infections, demonstrates its potential as a target for lncRNA-based therapeutic interventions. Modulating GAS5 levels could serve as a novel strategy to mitigate disease progression and improve patient outcomes in viral infections (see Figure 5 and Table 1).
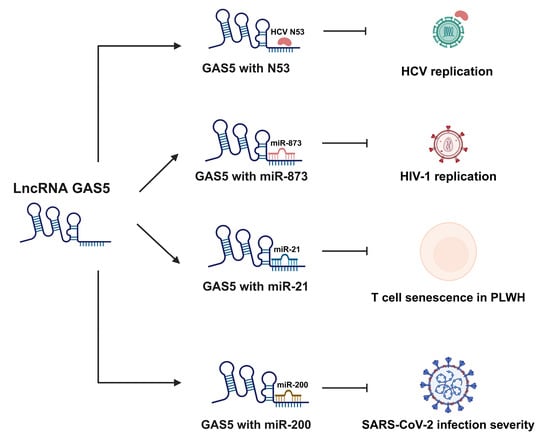
Figure 5.
The GAS5/miRNA-mediated regulation of viral infections. This figure depicts the mechanisms by which GAS5 regulates the progression of infectious diseases caused by viral infections. GAS5 plays a critical role in regulating infectious diseases associated with HCV and HIV infections by directly binding to viral proteins or miRNAs that promote viral replication. GAS5 also demonstrates therapeutic potential in PLWH, displaying an ability to improve the activity and longevity of CD4 T cells in ART-treated PLWH. Finally, GAS5 serves as an important biomarker of severe SARS-CoV-2 infection through its ability to function as a ceRNA for miR-200. Unbound miR-200 can inhibit ACE-2 expression, a phenomenon which is linked to increased inflammation and cytokine storms in SARS-CoV-2 infection. HCV, hepatitis C virus; HIV, human immunodeficiency virus; PLWH, patients living with HIV; ART, antiretroviral therapy; ACE-2, angiotensin-converting enzyme 2.
6. GAS5 in Human Diseases Independent of miRNA Axes
While much of this review focuses on the impact of miRNA-mediated GAS5 regulatory functions, it is crucial to understand that GAS5 itself has regulatory functions apart from serving as a ceRNA for miRNAs. Whether directly participating in the regulation of the p53 signaling pathway as a signaling molecule [28], acting as a decoy for glucocorticoid receptors (GRs) [12,25,125], or acting as a guide to promote gene transcription [29], GAS5 has been demonstrated to be involved in a myriad of biological processes at the epigenetic, transcriptional, and post-transcriptional levels [93,126,127]. This impact can be seen across a wide variety of disease presentations and is applicable in the context of cancers, cardiovascular diseases, inflammatory diseases, and viral infections, all of which have been discussed in detail throughout this review.
In general, GAS5 has been demonstrated to play significant roles in the suppression of cancers through the regulation of oncogenic signaling pathways, cell cycle progression, and cellular apoptosis [128]. Regarding oncogenic signaling pathways, GAS5 serves a critical regulator of either the PI3K/AKT/mTOR pathway or the PTEN/AKT pathway through sponging miRNAs such as miR-32 [71], miR-23a [61,95,98], miR-21 [44], and miR-222 [62,63,64]. However, the discussion of the GAS5-mediated regulation of important cell cycle mediators is not limited to the ability of GAS5 to sponge miRNAs. Indeed, GAS5 has been demonstrated to suppress c-Myc, which is required for the activation of CDKs during cell cycle progression, through cooperation with eIF4E during the translation initiation phase. This interaction prevents c-Myc mRNA from entering the polysome, subsequently blocking the translation of c-Myc. This repression of c-Myc can potentially induce cell growth arrest through reduced cyclin E/CDK2 function and the subsequent upregulation of p27 levels, a potent inducer of growth arrest [129,130]. GAS5 has also been demonstrated to have a regulatory role, seemingly independent of miRNA activity, on crucial negative regulators of cell cycle progression such as p21. One study focusing on stomach cancer identified a mechanism wherein the depletion of GAS5 increased the turnover of transcriptional activator Y-box-binding protein 1 (YBX1) through direct interaction, thereby reducing p21 expression and subsequent G1 phase arrest [131]. This novel mechanism of GAS5 suppressing stomach carcinogenesis via the YBX1/p21 pathway serves as a potential target for the development of lncRNA-based therapeutics for the treatment of stomach cancer. Additionally, GAS5 suppresses the oncogenic activity of enhancer of zeste homolog 2 (EZH2), an epigenetic modifier overexpressed in cancers like melanoma [132] and bladder cancer [18], by recruiting the transcriptional repressor E2F4 to the EZH2 promoter, thereby downregulating EZH2 expression and restoring tumor suppressor genes such as cyclin dependent kinase inhibitor 1C (CDKN1C) [133]. Finally, GAS5 displays significant regulatory functions on cellular apoptosis, specifically through its ability to function as a decoy hormone response element for the glucocorticoid receptor [12,25]. GAS5 acts as a riborepressor of the glucocorticoid receptor (GR), suppressing GR-induced transcriptional activity through its double-stranded glucocorticoid response element (GRE) mimic sequence, encoded in exon 12, which binds to the DNA binding domain of GR [12]. Through this mechanism, GAS5 overexpression has been shown to promote apoptosis in breast cancer cells [134] and increase basal and drug-induced apoptosis in prostate cancer cells, while GAS5 downregulation attenuates apoptosis [135]. Interestingly, one current clinical trial is aimed at further identifying and validating the role of GAS5 in prostate cancer through the detection and characterization of GAS5 gene polymorphisms (clinicaltrials.gov; accessed on 29 January 2025 ID: NCT06505356).
Several research groups have demonstrated the ability of GAS5 to significantly regulate cardiovascular function through mechanisms independent of miRNA axes, with GAS5 binding directly to the target protein in this case. In the context of myocardial infarction, GAS5 was demonstrated to ameliorate cardiomyocyte apoptosis and reduce infarct size in vivo by negatively regulating semaphorin 3a (sema3a) [136]. Sema3a plays a critical role in cardiac remodeling and vascular biology, as it potently inhibits angiogenesis and promotes apoptosis [137,138], both of which can exacerbate cardiac damage following infarction. GAS5 was demonstrated to directly bind to and interact with sema3a in RNA immunoprecipitation (RIP) and RNA pull-down assays [136]. Additionally, the RNA pull-down assay demonstrated GAS5’s direct interaction with a target protein. In primary varicose great saphenous veins (GSVs), GAS5 can directly bind to Annexin A2 to promote the pathogenesis of varicosities through the regulation of smooth muscle cell (SMC) proliferation, migration, and apoptosis [139]. This interaction suppresses the pro-proliferative and pro-migratory effects of Annexin A2, thereby inhibiting the excessive SMC activity that drives vascular remodeling, ultimately supporting vascular homeostasis. Additionally, GAS5 has been demonstrated to be a potent suppressor of TGF-β/Smad3 signaling in SMC differentiation, binding to Smad3 via RNA Smad-binding elements (rSBEs) and thereby preventing Smad3 from associating with DNA and inhibiting the transcription of TGF-β-responsive genes [140]. This example is noteworthy, because it demonstrates the ability of GAS5 to function as a molecular brake in terms of Smad3 activity in the basal state to maintain cellular homeostasis and refine Smad3 signaling during TGF-β induction, further highlighting the role of GAS5 as a critical regulator of SMC differentiation in various physiological contexts.
GAS5 has long been recognized for its ability to regulate inflammatory pathways through its interaction with glucocorticoid receptors (GRs) [12], making its activity independent of miRNA-mediated axes in the context of inflammation. GRs are critical in treating inflammatory conditions as they mediate the effects of glucocorticoid drugs by directly suppressing the activity of NF-κB [141], a central transcription factor in the immune and inflammatory responses. Importantly, GAS5 directly interacts with both GR and the NF-κB subunit p65, as verified by RIP assays [142]. This interaction inhibits NF-κB activity by modulating its DNA binding capacity and reducing the expression of pro-inflammatory target genes, such as TNF-α. Notably, GAS5 is dysregulated in prominent inflammatory disorders, such as rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis, and sarcoidosis [22]. Currently, there is a clinical trial aimed at investigating GAS5 expression elevation in peripheral blood mononuclear cells (PBMCs), which has been associated with steroid resistance in idiopathic nephrotic syndrome (INS), particularly in steroid-resistant nephrotic syndrome (SRNS), where GAS5 may impede GR activity and contribute to glucocorticoid insensitivity (clinicaltrials.gov; ID: NCT06325137). Given that glucocorticoids remain the pre-eminent therapeutics for the treatment of inflammatory diseases [143], the ability of GAS5 to modulate glucocorticoid sensitivity has significant therapeutic implications. The increasing prevalence of glucocorticoid resistance has significant clinical relevancy [144], and GAS5’s established role as a competitive inhibitor of GR-DNA binding suggests it may be critical in the development of steroid insensitivity. Understanding this mechanism could be particularly valuable as cases of glucocorticoid resistance continue to rise, potentially offering new therapeutic strategies to restore steroid sensitivity in resistant patients.
Finally, while the role of GAS5 in a variety of different viral infections is widely recognized, most of the mechanisms described rely on GAS5/miRNA-mediated axes [94,111,122]. However, in the context of HCV infection, this review has discussed how GAS5 can act as a decoy for the viral NS3 protein through direct RNA-to-protein interactions [21]. Similarly, in HBV-related hepatocellular carcinoma, GAS5 has been shown to function through direct RNA–protein interactions with YBX1, where its downregulation via HBx-induced methylation promotes cancer progression, demonstrating another example of GAS5’s miRNA-independent regulatory mechanisms in viral pathogenesis [145].
The above studies are indicative of the ability of GAS5 to exert a broad range of regulatory functions beyond its role as a ceRNA for miRNAs, influencing cellular processes at epigenetic, transcriptional, and post-transcriptional levels. In cancer, GAS5 functions through mechanisms such as c-Myc translation repression [129,130], YBX1/p21 pathway regulation [131], and EZH2 suppression [133], providing cellular protection in each context. In cardiovascular disease, GAS5 exhibits three key protein interactions: binding to sema3a to reduce cardiac damage and apoptosis following myocardial infarction [136], interacting with Annexin A2 to regulate smooth muscle cell activity and maintain vascular homeostasis in varicose veins [139], and binding to Smad3 to act as a molecular brake on TGF-β signaling in smooth muscle cell differentiation [140]. Furthermore, GAS5’s role in inflammatory conditions is quite complex, involving a direct interaction with either NF-κB or glucocorticoid receptors [12,141], with particular relevance in instances of steroid resistance. Lastly, GAS5 displays unique RNA–protein interactions in viral pathogenesis, such as acting as a decoy for viral NS3 [21] or regulating YBX1 in HBV-associated cancers [145]. These diverse functions position GAS5 as a crucial molecular regulator and a promising target for the development of novel therapeutic strategies against cancer, cardiovascular diseases, inflammatory disorders, and viral infections.
7. Conclusions
Through the evaluation of an extensive amount of research in this review, it is evident that GAS5 displays complex and context-dependent methods for regulating miRNAs and their downstream pathways across various pathological conditions. The ability of GAS5 to function as a ceRNA for multiple miRNAs positions it as a critical regulatory molecule with diverse implications for disease progression and potential therapeutic interventions.
In cancer, GAS5 consistently demonstrates its ability to function as a powerful tumor suppressor through many miRNAs, most notably through the well-characterized GAS5/miR-21 axis [13,31,32,35,37,38,39,40]. By modulating multiple classical oncogenic pathways such as PTEN/PI3K/AKT and SPRY/RAF/ERK, it regulates critical processes central to cancer progression such as EMT [42,43,56,57], chemoresistance [46,59,77], and metastasis [63,71]. This persistent anti-tumor activity of GAS5 across various cancer types suggests that strategies aimed at upregulating GAS5 expression could represent a promising therapeutic approach in oncology.
The role of GAS5 in cardiovascular diseases reveals a more nuanced relationship. While GAS5 can exacerbate conditions such as myocardial infarction [121] and atherosclerosis [103] through the promotion of apoptosis and inflammation, it also yields protective effects in specific contexts, such as mitigating cardiac fibrosis through the miR-217/SIRT1 pathway [112] and preventing EPC senescence via miR-223 regulation [114]. This duality implies the need for a careful consideration of context when developing GAS5-targeted therapies for cardiovascular conditions.
In inflammatory-associated diseases, the effects of GAS5 can typically be defined as having a context-dependent relationship, as described for cardiovascular diseases. Consistently, GAS5 shows detrimental effects in neurological conditions like cerebral ischemic stroke [82,88,99,104], Parkinson’s disease [108,115], and Alzheimer’s disease [98], where its inhibition could offer an improvement in patient outcomes; however, GAS5 also demonstrates protective effects in conditions such as NAFLD [100], rheumatoid arthritis [118], and diabetic nephropathy [113,119], where the suppression of GAS5 could have potentially detrimental effects. In fact, the case of sepsis particularly demonstrates this complexity, with GAS5 potentially playing different roles in inflammation modulation depending on the disease stage and cellular context [96,97,109,123], making GAS5 manipulation in this case particularly complicated. Despite this complexity, with further exploration, GAS5’s therapeutic potential could be elucidated.
In viral infections, GAS5 generally seems to play a protective role, which is particularly evident in HIV infection, where it can suppress viral replication through the sponging of miR-873 [122] and aid in restoring T cell function via miR-21 modulation [94]. Its potential as a biomarker of COVID-19 severity [111] and ability to suppress HCV replication [21] further underscore its significance in viral pathogenesis.
All things considered, the literature highlights GAS5’s conserved role as a ceRNA across varying pathological conditions, with its specific miRNA interactions and therapeutic implications varying by situation. While GAS5 upregulation demonstrates clear therapeutic potential in cancer, its effects in cardiovascular and inflammatory diseases rely heavily on the target cell type and context of the disease, allowing it to have either detrimental or protective functions. Future research should prioritize elucidating GAS5’s context-dependent roles, developing tissue-specific therapies, and exploring its potential as a biomarker. Targeting GAS5/miRNA axes holds significant promise for innovative treatments, but substantial success will require precise tailoring to best serve the disease context.
Author Contributions
Conceptualization, L.N.T.N., J.S.P. and N.L.N.; writing—original draft preparation, L.N.T.N. and J.S.P.; writing—review and editing, L.N.T.N., J.S.P., N.L.N., M.B.S., J.Z., L.W., T.O.L., M.E.G., J.P.M. and Z.Q.Y.; supervision, J.P.M. and Z.Q.Y.; funding acquisition, J.Z. and Z.Q.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National Institutes of Health (NIH) grants R01AI177624, R21AI179794, and R15AG069544 and VA Merit Review Award I01BX002670 to Z.Q.Y and NIH grants R15AG076370 and R15AG076370-01S1 to J. Zhao. This publication is the result of work supported by resources and the use of facilities at the James H. Quillen Veterans Affairs Medical Center. The contents of this publication do not represent the views of the Department of Veterans Affairs or the United States Government.
Acknowledgments
Figures were created with BioRender software (https://biorender.com/ accessed on 29 January 2025).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| miRNAs | MicroRNAs |
| LncRNAs | Long noncoding RNA |
| GAS5 | Growth arrest-specific 5 |
| PTEN | Phosphatase and tensin homolog |
| PIK3 | Phosphoinositide 3-kinases |
| AKT | Protein kinase B |
| mTOR | Mammalian target of rapamycin |
| BAD | BCL2 associated agonist of cell death |
| p21 | Cyclin-dependent kinase inhibitor 1 |
| SPRY1 | Sprouty RTK signaling antagonist 1 |
| RAF | Rapidly accelerated fibrosarcoma |
| ERK | Extracellular-regulated kinase |
| PDCD4 | Programmed cell death 4 |
| SMAD | Sma- and Mad- related protein |
| SMAD7 | SMAD family member 7 |
| BAX | BCL2 associated X |
| BIM | Bcl-2-like protein 11 |
| FGSC | Female germline stem cell |
| ATG3 | Autophagy related 3 |
| ATG8 | Autophagy related 8 |
| p62 | Ubiquitin-binding protein |
| FOXO1 | Forkhead box protein O1 |
| MCF7 | Michigan cancer foundation-7 |
| HOXA5 | Homeobox 5 |
| GR | Glucocorticoid receptor |
| GSC | Glioma stem cell |
| PID1 | Phosphotyrosine interaction domain containing 1 |
| TP63 | Tumor protein p63 |
| SOCS3 | Suppressor of cytokine signaling 3 |
| PUMA | P53-upregulated modulator of apoptosis |
| JNK | c-Jun N-terminal kinase |
| H2AX | H2A histone family member X |
| SIRT1 | Sirtuin 1 |
| ARHI | Aplasia Ras homolog member I |
| ABCB1 | P-glycoprotein |
| DKK2 | Dickkopf 2 |
| BMF | BCL-2-modifying factor |
| NAMPT | Nicotinamide phosphoribosyltransferase |
| JAK2 | Janus kinase 2/signal transducer |
| STAT3 | Activator of transcription 3 |
| TP53INP1 | Tumor protein p53 inducible nuclear protein 1 |
| CDK4 | Cyclin-dependent kinase 4 |
| BCL-2 | B-cell lymphoma 2 |
| ceRNA | Competing endogenous RNA |
| ROCK1 | Rho-associated protein kinase 1 |
| YAP1 | Yes-associated protein |
| GSK-3β | Glycogen synthase kinase 3 |
| mPTP | Mitochondrial permeability transition pore |
| UM | Uveal melanoma |
| CALM2 | Calmodulin 2 |
| CIS | Cerebral ischemic stroke |
| FOXO3 | Forkhead box protein O3 |
| SMAD1 | SMAD family member 1 |
| MCAO | Middle cerebral artery occlusion |
| OGD | Oxygen-glucose deprivation |
| NLRP3 | NOD-like receptor family pyrin domain containing 3 |
| Caspase-1 | Cysteinyl aspartate-specific protease |
| IL-1β | Interleukin-1β |
| IL-18 | Interleukin-18 |
| KCNQ3 | Potassium Voltage-Gated Channel Subfamily Q Member 3 |
| ox-LDL | Oxidized low-density lipoprotein |
| apoE | Apolipoprotein E |
| HFD | High-fat diet |
| AS | Atherosclerosis |
| BMSCs | Bone marrow mesenchymal stem cells |
| BDNF | Brain-derived neurotrophic factor |
| TSP-1 | Thrombospondin-1 |
| EMT | Epithelial to mesenchymal transition |
| H/R | Hypoxia/reoxygenation |
| TXNIP | Thioredoxin-interacting protein |
| EC | Endothelial cell |
| EPC | Endothelial progenitor cell |
| TLR4 | Toll-like receptor 4 |
| Notch-1 | Neurogenic locus notch homolog protein 1 |
| PD | Parkinson’s Disease |
| ASC | Apoptosis-associated speck-like protein containing a CARD |
| Aβ | Amyloid beta |
| CCT8 | Chaperonin containing TCP1 subunit 8 |
| TGF-β | Transforming growth factor β |
| LPS | Lipopolysaccharide |
| MiT/TFE | Microphthalmia/transcription factor E |
| MARCH7 | Membrane-associated ring-CH finger protein 7 |
| HMGB1 | High mobility group box 1 |
| NRF2 | Nuclear factor erythroid 2–related factor 2 |
| NAFLD | Nonalcoholic fatty liver disease |
| RA | Rheumatoid arthritis |
| PDK4 | Pyruvate dehydrogenase kinase 4 |
| OA | Osteoarthritis |
| DN | Diabetic nephropathy |
| HIV | Human immunodeficiency virus |
| Gag | Group-specific antigen |
| HCV | Hepatitis C virus |
| Fosl1 | FOS like antigen 1 |
| E2F1 | E2F transcription factor 1 |
| APC | Adenomatous polyposis coli |
| EVs | Extracellular vesicles |
| CDKN1C | Cyclin dependent kinase inhibitor 1C |
| GRE | Glucocorticoid response element |
| Sema3a | Semaphorin 3a |
| GSVs | Great saphenous veins |
| YBX1 | Y-box-binding protein 1 |
| rSBEs | RNA Smad-binding elements |
| RIP | RNA immunoprecipitation |
| SMC | Smooth muscle cell |
| PBMCs | Peripheral blood mononuclear cells |
| INS | Idiopathic nephrotic syndrome |
| SRNS | Steroid-resistant nephrotic syndrome |
References
- Lambrou, G.I.; Hatziagapiou, K.; Zaravinos, A. The Non-Coding RNA GAS5 and Its Role in Tumor Therapy-Induced Resistance. Int. J. Mol. Sci. 2020, 21, 7633. [Google Scholar] [CrossRef]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef]
- St. Laurent, G.; Wahlestedt, C.; Kapranov, P. The Landscape of long noncoding RNA classification. Trends Genet. 2015, 31, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long non-coding RNAs: Definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Garcia, I.; Miska, E.A. MicroRNA functions in animal development and human disease. Development 2005, 132, 4653–4662. [Google Scholar] [CrossRef] [PubMed]
- Szabo, G.; Bala, S. MicroRNAs in liver disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Shen, X.J.; Zou, Q.; Wang, S.P.; Tang, S.M.; Zhang, G.Z. Biological functions of microRNAs: A review. J. Physiol. Biochem. 2011, 67, 129–139. [Google Scholar] [CrossRef]
- Pauley, K.M.; Cha, S.; Chan, E.K. MicroRNA in autoimmunity and autoimmune diseases. J. Autoimmun. 2009, 32, 189–194. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef]
- Schneider, C.; King, R.M.; Philipson, L. Genes specifically expressed at growth arrest of mammalian cells. Cell 1988, 54, 787–793. [Google Scholar] [CrossRef]
- Fleming, J.; Fontanier, N.; Harries, D.; Rees, W. The Growth Arrest Genes gas5, gas6, and CHOP-10 (gadd153) are expressed in the mouse preimplantation embryo. Mol. Reprod. Dev. 1997, 48, 310–316. [Google Scholar] [CrossRef]
- Kino, T.; Hurt, D.E.; Ichijo, T.; Nader, N.; Chrousos, G.P. Noncoding RNA gas5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Sci. Signal 2010, 3, ra8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhu, Z.; Watabe, K.; Zhang, X.; Bai, C.; Xu, M.; Wu, F.; Mo, Y.Y. Negative regulation of lncRNA GAS5 by miR-21. Cell Death Differ. 2013, 20, 1558–1568. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Shi, X.; Zhu, Q.; Li, Q.; Liu, Y.; Yao, Y.; Song, Y. The growth arrest-specific transcript 5 (GAS5): A pivotal tumor suppressor long noncoding RNA in human cancers. Tumour Biol. 2016, 37, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, Z. Long non-coding RNA growth arrest-specific transcript 5 in tumor biology. Oncol. Lett. 2015, 10, 1953–1958. [Google Scholar] [CrossRef]
- Ji, J.; Dai, X.; Yeung, S.J.; He, X. The role of long non-coding RNA GAS5 in cancers. Cancer Manag. Res. 2019, 11, 2729–2737. [Google Scholar] [CrossRef]
- Yu, Y.; Hann, S.S. Novel Tumor Suppressor lncRNA Growth Arrest-Specific 5 (GAS5) In Human Cancer. Onco Targets Ther. 2019, 12, 8421–8436. [Google Scholar] [CrossRef]
- Wang, M.; Guo, C.; Wang, L.; Luo, G.; Huang, C.; Li, Y.; Liu, D.; Zeng, F.; Jiang, G.; Xiao, X. Long noncoding RNA GAS5 promotes bladder cancer cells apoptosis through inhibiting EZH2 transcription. Cell Death Dis. 2018, 9, 238. [Google Scholar] [CrossRef]
- Mourtada-Maarabouni, M.; Hedge, V.L.; Kirkham, L.; Farzaneh, F.; Williams, G.T. Growth arrest in human T-cells is controlled by the non-coding RNA growth-arrest-specific transcript 5 (GAS5). J. Cell Sci. 2008, 121, 939–946. [Google Scholar] [CrossRef]
- Williams, G.T.; Mourtada-Maarabouni, M.; Farzaneh, F. A critical role for non-coding RNA GAS5 in growth arrest and rapamycin inhibition in human T-lymphocytes. Biochem. Soc. Trans. 2011, 39, 482–486. [Google Scholar] [CrossRef]
- Qian, X.; Xu, C.; Zhao, P.; Qi, Z. Long non-coding RNA GAS5 inhibited hepatitis C virus replication by binding viral NS3 protein. Virology 2016, 492, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Mayama, T.; Marr, A.K.; Kino, T. Differential Expression of Glucocorticoid Receptor Noncoding RNA Repressor Gas5 in Autoimmune and Inflammatory Diseases. Horm. Metab. Res. 2016, 48, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Wang, N.; Zhu, Y.; You, L.; Wang, L.; De, W.; Tang, W. Downregulation of Long Noncoding RNA Gas5 Affects Cell Cycle and Insulin Secretion in Mouse Pancreatic beta Cells. Cell Physiol. Biochem. 2017, 43, 2062–2073. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, B. GAS5-mediated regulation of cell signaling (Review). Mol. Med. Rep. 2020, 22, 3049–3056. [Google Scholar] [CrossRef]
- Pickard, M.R.; Williams, G.T. Molecular and Cellular Mechanisms of Action of Tumour Suppressor GAS5 LncRNA. Genes 2015, 6, 484–499. [Google Scholar] [CrossRef]
- Ye, J.; Wang, C.; Wang, D.; Yuan, H. LncRBA GSA5, up-regulated by ox-LDL, aggravates inflammatory response and MMP expression in THP-1 macrophages by acting like a sponge for miR-221. Exp. Cell Res. 2018, 369, 348–355. [Google Scholar] [CrossRef]
- Zhou, X.B.; Lai, L.F.; Xie, G.B.; Ding, C.; Xu, X.; Wang, Y. LncRNAGAS5 sponges miRNA-221 to promote neurons apoptosis by up-regulated PUMA under hypoxia condition. Neurol. Res. 2020, 42, 8–16. [Google Scholar] [CrossRef]
- Krell, J.; Frampton, A.E.; Mirnezami, R.; Harding, V.; De Giorgio, A.; Roca Alonso, L.; Cohen, P.; Ottaviani, S.; Colombo, T.; Jacob, J.; et al. Growth arrest-specific transcript 5 associated snoRNA levels are related to p53 expression and DNA damage in colorectal cancer. PLoS ONE 2014, 9, e98561. [Google Scholar] [CrossRef]
- Luo, G.; Liu, D.; Huang, C.; Wang, M.; Xiao, X.; Zeng, F.; Wang, L.; Jiang, G. LncRNA GAS5 Inhibits Cellular Proliferation by Targeting P27(Kip1). Mol. Cancer Res. 2017, 15, 789–799. [Google Scholar] [CrossRef]
- Thangavelu, L.; Moglad, E.; Gupta, G.; Menon, S.V.; Gaur, A.; Sharma, S.; Kaur, M.; Chahar, M.; Sivaprasad, G.V.; Deorari, M. GAS5 lncRNA: A biomarker and therapeutic target in breast cancer. Pathol. Res. Pract. 2024, 260, 155424. [Google Scholar] [CrossRef]
- Giordo, R.; Ahmadi, F.A.M.; Husaini, N.A.; Al-Nuaimi, N.; Ahmad, S.M.S.; Pintus, G.; Zayed, H. microRNA 21 and long non-coding RNAs interplays underlie cancer pathophysiology: A narrative review. Non-Coding RNA Res. 2024, 9, 831–852. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Ye, H.; Huang, G.; Luo, F.; Liu, Y.; Liu, Y.; Yang, X.; Shen, J.; Liu, Q.; Zhang, J. Long noncoding RNA GAS5 suppresses the migration and invasion of hepatocellular carcinoma cells via miR-21. Tumour Biol. 2016, 37, 2691–2702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, C.; Li, H.; Guo, L. Effects of miR-21 on proliferation and apoptosis of WT cells via PTEN/Akt pathway. Exp. Ther. Med. 2020, 19, 2155–2160. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Chen, M.; Pandolfi, P.P. The functions and regulation of the PTEN tumour suppressor: New modes and prospects. Nat. Rev. Mol. Cell Biol. 2018, 19, 547–562. [Google Scholar] [CrossRef]
- Dey, N.; Ghosh-Choudhury, N.; Kasinath, B.S.; Choudhury, G.G. TGFbeta-stimulated microRNA-21 utilizes PTEN to orchestrate AKT/mTORC1 signaling for mesangial cell hypertrophy and matrix expansion. PLoS ONE 2012, 7, e42316. [Google Scholar] [CrossRef]
- Fruman, D.A.; Rommel, C. PI3K and cancer: Lessons, challenges and opportunities. Nat. Rev. Drug Discov. 2014, 13, 140–156. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, A.; Li, X.; Zhang, S.; Liu, S.; Zhao, H.; Wu, S.; Chen, L.; Ma, C.; Zhao, H. MiR-21-5p regulates extracellular matrix degradation and angiogenesis in TMJOA by targeting Spry1. Arthritis Res. Ther. 2020, 22, 99. [Google Scholar] [CrossRef]
- Gaur, A.B.; Holbeck, S.L.; Colburn, N.H.; Israel, M.A. Downregulation of Pdcd4 by mir-21 facilitates glioblastoma proliferation in vivo. Neuro Oncol. 2011, 13, 580–590. [Google Scholar] [CrossRef]
- Fang, X.; Zhong, G.; Wang, Y.; Lin, Z.; Lin, R.; Yao, T. Low GAS5 expression may predict poor survival and cisplatin resistance in cervical cancer. Cell Death Dis. 2020, 11, 531. [Google Scholar] [CrossRef]
- Tang, J.; Li, X.; Cheng, T.; Wu, J. miR-21-5p/SMAD7 axis promotes the progress of lung cancer. Thorac. Cancer 2021, 12, 2307–2313. [Google Scholar] [CrossRef]
- Rhim, J.; Baek, W.; Seo, Y.; Kim, J.H. From Molecular Mechanisms to Therapeutics: Understanding MicroRNA-21 in Cancer. Cells 2022, 11, 2791. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ren, X.; Yuan, Y.; Yuan, B.S. Downregulated lncRNA GAS5 and Upregulated miR-21 Lead to Epithelial-Mesenchymal Transition and Lung Metastasis of Osteosarcomas. Front. Cell Dev. Biol. 2021, 9, 707693. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Cui, Q.; Zhang, W.; Yao, R.; Xu, D.; Zhang, F. Long Non-Coding RNA GAS5 Targeting microRNA-21 to Suppress the Invasion and Epithelial-Mesenchymal Transition of Uveal Melanoma. Cancer Manag. Res. 2020, 12, 12259–12267. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Tan, X.; Chen, Y.; Chen, Y.; Li, Z.; Zhang, Y.; Chen, X.; Yang, H.; Chen, H.; Yu, Z. Growth arrest-specific transcript 5 represses endometrial cancer development by promoting antitumor function of tumor-associated macrophages. Cancer Sci. 2022, 113, 2496–2512. [Google Scholar] [CrossRef]
- Zeng, B.; Li, Y.; Jiang, F.; Wei, C.; Chen, G.; Zhang, W.; Zhao, W.; Yu, D. LncRNA GAS5 suppresses proliferation, migration, invasion, and epithelial-mesenchymal transition in oral squamous cell carcinoma by regulating the miR-21/PTEN axis. Exp. Cell Res. 2019, 374, 365–373. [Google Scholar] [CrossRef]
- Cao, L.; Chen, J.; Ou, B.; Liu, C.; Zou, Y.; Chen, Q. GAS5 knockdown reduces the chemo-sensitivity of non-small cell lung cancer (NSCLC) cell to cisplatin (DDP) through regulating miR-21/PTEN axis. Biomed. Pharmacother. 2017, 93, 570–579. [Google Scholar] [CrossRef]
- Chen, D.; Guo, Y.; Chen, Y.; Guo, Q.; Chen, J.; Li, Y.; Zheng, Q.; Jiang, M.; Xi, M.; Cheng, L. LncRNA growth arrest-specific transcript 5 targets miR-21 gene and regulates bladder cancer cell proliferation and apoptosis through PTEN. Cancer Med. 2020, 9, 2846–2858. [Google Scholar] [CrossRef]
- Lyu, K.; Xu, Y.; Yue, H.; Li, Y.; Zhao, J.; Chen, L.; Wu, J.; Zhu, X.; Chai, L.; Li, C.; et al. Long Noncoding RNA GAS5 Acts As A Tumor Suppressor In Laryngeal Squamous Cell Carcinoma Via miR-21. Cancer Manag. Res. 2019, 11, 8487–8498. [Google Scholar] [CrossRef]
- Ma, N.; Li, S.; Zhang, Q.; Wang, H.; Qin, H.; Wang, S. Long non-coding RNA GAS5 inhibits ovarian cancer cell proliferation via the control of microRNA-21 and SPRY2 expression. Exp. Ther. Med. 2018, 16, 73–82. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, W.; Zhu, S.; Cheng, K.; Xu, H.; Lv, Y.; Long, X.; Ma, L.; Huang, J.; Sun, S.; et al. Long noncoding RNA GAS5 regulates the proliferation, migration, and invasion of glioma cells by negatively regulating miR-18a-5p. J. Cell Physiol. 2018, 234, 757–768. [Google Scholar] [CrossRef]
- Yang, J.; Hao, T.; Sun, J.; Wei, P.; Zhang, H. Long noncoding RNA GAS5 modulates alpha-Solanine-induced radiosensitivity by negatively regulating miR-18a in human prostate cancer cells. Biomed. Pharmacother. 2019, 112, 108656. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, Y.; Zheng, J.; Liu, X.; Chen, J.; Liu, L.; Wang, P.; Xue, Y. GAS5 suppresses malignancy of human glioma stem cells via a miR-196a-5p/FOXO1 feedback loop. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1605–1617. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, J.; Xiong, G.; He, G.; Guan, X.; Yang, K.; Bai, Y. Negative regulation of lncRNA GAS5 by miR-196a inhibits esophageal squamous cell carcinoma growth. Biochem. Biophys. Res. Commun. 2018, 495, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Hong, L.; Xu, X.; Wang, Q.; Huang, J.; Jiang, L. LncRNA GAS5 suppresses the tumorigenesis of cervical cancer by downregulating miR-196a and miR-205. Tumour Biol. 2017, 39, 1010428317711315. [Google Scholar] [CrossRef]
- Zhao, H.; Yu, H.; Zheng, J.; Ning, N.; Tang, F.; Yang, Y.; Wang, Y. Lowly-expressed lncRNA GAS5 facilitates progression of ovarian cancer through targeting miR-196-5p and thereby regulating HOXA5. Gynecol. Oncol. 2018, 151, 345–355. [Google Scholar] [CrossRef]
- Liu, B.; Wu, S.; Ma, J.; Yan, S.; Xiao, Z.; Wan, L.; Zhang, F.; Shang, M.; Mao, A. lncRNA GAS5 Reverses EMT and Tumor Stem Cell-Mediated Gemcitabine Resistance and Metastasis by Targeting miR-221/SOCS3 in Pancreatic Cancer. Mol. Ther. Nucleic Acids 2018, 13, 472–482. [Google Scholar] [CrossRef]
- Ye, K.; Wang, S.; Zhang, H.; Han, H.; Ma, B.; Nan, W. Long Noncoding RNA GAS5 Suppresses Cell Growth and Epithelial-Mesenchymal Transition in Osteosarcoma by Regulating the miR-221/ARHI Pathway. J. Cell Biochem. 2017, 118, 4772–4781. [Google Scholar] [CrossRef]
- Shen, Q.; Wang, H.; Zhang, L. TP63 Functions as a Tumor Suppressor Regulated by GAS5/miR-221-3p Signaling Axis in Human Non-Small Cell Lung Cancer Cells. Cancer Manag. Res. 2023, 15, 217–231. [Google Scholar] [CrossRef]
- Chen, Z.; Pan, T.; Jiang, D.; Jin, L.; Geng, Y.; Feng, X.; Shen, A.; Zhang, L. The lncRNA-GAS5/miR-221-3p/DKK2 Axis Modulates ABCB1-Mediated Adriamycin Resistance of Breast Cancer via the Wnt/beta-Catenin Signaling Pathway. Mol. Ther. Nucleic Acids 2020, 19, 1434–1448. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, P.; Liu, J.; Zheng, J.; Liu, Y.; Chen, J.; Xue, Y. Gas5 Exerts Tumor-suppressive Functions in Human Glioma Cells by Targeting miR-222. Mol. Ther. 2015, 23, 1899–1911. [Google Scholar] [CrossRef]
- Liu, J.; Chen, M.; Ma, L.; Dang, X.; Du, G. LncRNA GAS5 suppresses the proliferation and invasion of osteosarcoma cells via the miR-23a-3p/PTEN/PI3K/AKT pathway. Cell Transplant. 2020, 29, 0963689720953093. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Ye, Y.; Zhao, S.J. LncRNA Gas5 acts as a ceRNA to regulate PTEN expression by sponging miR-222-3p in papillary thyroid carcinoma. Oncotarget 2017, 9, 3519. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z.; Gao, L.; Wang, H.; Chen, J.; Nie, B.; Hong, Q. Long non-coding RNA GAS5 regulates human B lymphocytic leukaemia tumourigenesis and metastasis by sponging miR-222. Cancer Biomark. 2019, 26, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gu, J.; Lu, H. The GAS5/miR-222 Axis Regulates Proliferation of Gastric Cancer Cells Through the PTEN/Akt/mTOR Pathway. Dig. Dis. Sci. 2017, 62, 3426–3437. [Google Scholar] [CrossRef] [PubMed]
- Pelisenco, I.A.; Zizioli, D.; Guerra, F.; Grossi, I.; Bucci, C.; Mignani, L.; Girolimetti, G.; Di Corato, R.; D’Agostino, V.G.; Marchina, E.; et al. miR-23b-3p, miR-126-3p and GAS5 delivered by extracellular vesicles inhibit breast cancer xenografts in zebrafish. Cell Commun. Signal 2024, 22, 552. [Google Scholar] [CrossRef]
- Li, L.; Huang, C.; He, Y.; Sang, Z.; Liu, G.; Dai, H. Knockdown of Long Non-Coding RNA GAS5 Increases miR-23a by Targeting ATG3 Involved in Autophagy and Cell Viability. Cell Physiol. Biochem. 2018, 48, 1723–1734. [Google Scholar] [CrossRef]
- Mei, Y.; Si, J.; Wang, Y.; Huang, Z.; Zhu, H.; Feng, S.; Wu, X.; Wu, L. Long Noncoding RNA GAS5 Suppresses Tumorigenesis by Inhibiting miR-23a Expression in Non-Small Cell Lung Cancer. Oncol. Res. 2017, 25, 1027–1037. [Google Scholar] [CrossRef]
- Zhou, L.; Jiang, H.; Lin, L.; Li, Y.; Li, J. lncRNA GAS5 suppression of the malignant phenotype of ovarian cancer via the miR-23a-WT1 axis. Ann. Transl. Med. 2023, 11, 119. [Google Scholar] [CrossRef]
- Wang, H.; Wang, D.; Shen, Y.; Sun, C.; Hu, Q.; Jiang, L.; Du, Q. GAS5 attenuates the malignant progression of glioma stem-like cells by promoting E-cadherin. Cancer Gene Ther. 2023, 30, 450–461. [Google Scholar] [CrossRef]
- Guo, C.; Song, W.Q.; Sun, P.; Jin, L.; Dai, H.Y. LncRNA-GAS5 induces PTEN expression through inhibiting miR-103 in endometrial cancer cells. J. Biomed. Sci. 2015, 22, 100. [Google Scholar] [CrossRef]
- Gao, Z.Q.; Wang, J.F.; Chen, D.H.; Ma, X.S.; Wu, Y.; Tang, Z.; Dang, X.W. Long non-coding RNA GAS5 suppresses pancreatic cancer metastasis through modulating miR-32-5p/PTEN axis. Cell Biosci. 2017, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Kong, C.; Liu, X.; Bi, J.; Li, Z.; Li, Z.; Zhu, Y.; Zhang, Z. GAS5 functions as a ceRNA to regulate hZIP1 expression by sponging miR-223 in clear cell renal cell carcinoma. Am. J. Cancer Res. 2018, 8, 1414–1426. [Google Scholar] [PubMed]
- Yang, L.; Jiang, J. GAS5 Regulates RECK Expression and Inhibits Invasion Potential of HCC Cells by Sponging miR-135b. BioMed Res. Int. 2019, 2019, 2973289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Yang, C.; Xing, Z.; Liu, P.; Zhang, B.; Ma, X.; Huang, L.; Zhuang, L. LncRNA GAS5-mediated miR-1323 promotes tumor progression by targeting TP53INP1 in hepatocellular carcinoma. Onco Targets Ther. 2019, 12, 4013–4023. [Google Scholar] [CrossRef]
- Gao, J.; Liu, L.; Li, G.; Cai, M.; Tan, C.; Han, X.; Han, L. LncRNA GAS5 confers the radio sensitivity of cervical cancer cells via regulating miR-106b/IER3 axis. Int. J. Biol. Macromol. 2019, 126, 994–1001. [Google Scholar] [CrossRef]
- Cheng, K.; Zhao, Z.; Wang, G.; Wang, J.; Zhu, W. lncRNA GAS5 inhibits colorectal cancer cell proliferation via the miR1825p/FOXO3a axis. Oncol. Rep. 2018, 40, 2371–2380. [Google Scholar] [CrossRef]
- Gao, Z.Q.; Wang, J.F.; Chen, D.H.; Ma, X.S.; Yang, W.; Zhe, T.; Dang, X.W. Long non-coding RNA GAS5 antagonizes the chemoresistance of pancreatic cancer cells through down-regulation of miR-181c-5p. Biomed. Pharmacother. 2018, 97, 809–817. [Google Scholar] [CrossRef]
- Jin, C.; Zhao, J.; Zhang, Z.P.; Wu, M.; Li, J.; Xiao, G.L.; Liu, B.; Liao, Y.X.; Liu, J.P. Long non-coding RNA GAS5, by up-regulating PRC2 and targeting the promoter methylation of miR-424, suppresses multiple malignant phenotypes of glioma. J. Neurooncol. 2020, 148, 529–543. [Google Scholar] [CrossRef]
- Xie, X.; Dai, J.; Huang, X.; Fang, C.; He, W. MicroRNA-145 inhibits proliferation and induces apoptosis in human prostate carcinoma by upregulating long non-coding RNA GAS5. Oncol. Lett. 2019, 18, 1043–1048. [Google Scholar] [CrossRef]
- Xue, Y.; Ni, T.; Jiang, Y.; Li, Y. Long Noncoding RNA GAS5 Inhibits Tumorigenesis and Enhances Radiosensitivity by Suppressing miR-135b Expression in Non-Small Cell Lung Cancer. Oncol. Res. 2017, 25, 1305–1316. [Google Scholar] [CrossRef]
- Toraih, E.A.; Alghamdi, S.A.; El-Wazir, A.; Hosny, M.M.; Hussein, M.H.; Khashana, M.S.; Fawzy, M.S. Dual biomarkers long non-coding RNA GAS5 and microRNA-34a co-expression signature in common solid tumors. PLoS ONE 2018, 13, e0198231. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Niu, Y.; He, G.; Wang, J. Down-regulation of lncRNA GAS5 attenuates neuronal cell injury through regulating miR-9/FOXO3 axis in cerebral ischemic stroke. RSC Adv. 2019, 9, 16158–16166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Tang, X.Y.; Li, N.; Zhao, L.M.; Guo, Y.L.; Li, X.S.; Tian, C.J.; Cheng, D.J.; Chen, Z.C.; Zhang, L.X. GAS5 promotes airway smooth muscle cell proliferation in asthma via controlling miR-10a/BDNF signaling pathway. Life Sci. 2018, 212, 93–101. [Google Scholar] [CrossRef]
- Li, M.; Xie, Z.; Wang, P.; Li, J.; Liu, W.; Tang, S.; Liu, Z.; Wu, X.; Wu, Y.; Shen, H. The long noncoding RNA GAS5 negatively regulates the adipogenic differentiation of MSCs by modulating the miR-18a/CTGF axis as a ceRNA. Cell Death Dis. 2018, 9, 554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-C.; Xia, L.; Jiang, Y.; Wu, D.-Q.; Liu, S.-C.; Zhou, X.-N.; Zhang, F.-X. Effect of lncRNA GAS5 on rats with acute myocardial infarction through regulating miR-21. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 6. [Google Scholar]
- Zhou, X.H.; Chai, H.X.; Bai, M.; Zhang, Z. LncRNA-GAS5 regulates PDCD4 expression and mediates myocardial infarction-induced cardiomyocytes apoptosis via targeting MiR-21. Cell Cycle 2020, 19, 1363–1377. [Google Scholar] [CrossRef]
- Tao, H.; Zhang, J.G.; Qin, R.H.; Dai, C.; Shi, P.; Yang, J.J.; Deng, Z.Y.; Shi, K.H. LncRNA GAS5 controls cardiac fibroblast activation and fibrosis by targeting miR-21 via PTEN/MMP-2 signaling pathway. Toxicology 2017, 386, 11–18. [Google Scholar] [CrossRef]
- Li, J.; Lv, H.; Che, Y.Q. Long non-coding RNA Gas5 potentiates the effects of microRNA-21 downregulation in response to ischaemic brain injury. Neuroscience 2020, 437, 87–97. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, Z. GAS5 silencing attenuates hypoxia-induced cardiomyocytes injury by targeting miR-21/PTEN. Immun. Inflamm. Dis. 2023, 11, e945. [Google Scholar] [CrossRef]
- Liu, K.; Liu, C.; Zhang, Z. lncRNA GAS5 acts as a ceRNA for miR-21 in suppressing PDGF-bb-induced proliferation and migration in vascular smooth muscle cells. J. Cell Biochem. 2019, 120, 15233–15240. [Google Scholar] [CrossRef]
- Fu, W.; Cao, Y.; Liu, J.; Huang, C.; Shu, K.; Zhu, N. Xinfeng Capsule Inhibits Pyroptosis and Ameliorates Myocardial Injury in Rats with Adjuvant Arthritis via the GAS5/miR-21/TLR4 Axis. Drug Des. Devel Ther. 2024, 18, 2421–2433. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; She, Y.; Wu, H.; Zhong, D.; Zhang, J. Long non-coding RNA Gas5 regulates proliferation and apoptosis in HCS-2/8 cells and growth plate chondrocytes by controlling FGF1 expression via miR-21 regulation. J. Biomed. Sci. 2018, 25, 18. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Song, N.; Zhao, S.; Xu, J.; Liu, Y.; Fang, Y.; Liang, M.; Xu, X.; Ding, X. LncRNA GAS5 promotes apoptosis as a competing endogenous RNA for miR-21 via thrombospondin 1 in ischemic AKI. Cell Death Discov. 2020, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.N.T.; Nguyen, L.N.; Zhao, J.; Schank, M.; Dang, X.; Cao, D.; Khanal, S.; Chand Thakuri, B.K.; Lu, Z.; Zhang, J.; et al. Long Non-coding RNA GAS5 Regulates T Cell Functions via miR21-Mediated Signaling in People Living with HIV. Front. Immunol. 2021, 12, 601298. [Google Scholar] [CrossRef]
- Dong, Z.; Li, S.; Wang, X.; Si, L.; Ma, R.; Bao, L.; Bo, A. lncRNA GAS5 restrains CCl4-induced hepatic fibrosis by targeting miR-23a through the PTEN/PI3K/Akt signaling pathway. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 316, G539–G550. [Google Scholar] [CrossRef]
- Gao, Z.; Huang, D. lncRNA GAS5-mediated miR-23a-3p promotes inflammation and cell apoptosis by targeting TLR4 in a cell model of sepsis. Mol. Med. Rep. 2021, 24, 510. [Google Scholar] [CrossRef]
- Cheng, J.; Ding, C.; Tang, H.; Zhou, H.; Wu, M.; Chen, Y. An Autophagy-Associated MITF-GAS5-miR-23 Loop Attenuates Vascular Oxidative and Inflammatory Damage in Sepsis. Biomedicines 2023, 11, 1811. [Google Scholar] [CrossRef]
- Zeng, L.; Zhao, K.; Liu, J.; Liu, M.; Cai, Z.; Sun, T.; Li, Z.; Liu, R. Long noncoding RNA GAS5 acts as a competitive endogenous RNA to regulate GSK-3beta and PTEN expression by sponging miR-23b-3p in Alzheimer’s disease. Neural Regen. Res. 2024, 10.4103. [Google Scholar] [CrossRef]
- Shangguan, Y.; Han, J.; Su, H. GAS5 knockdown ameliorates apoptosis and inflammatory response by modulating miR-26b-5p/Smad1 axis in cerebral ischaemia/reperfusion injury. Behav. Brain Res. 2020, 379, 112370. [Google Scholar] [CrossRef]
- Chen, T.; Meng, Y.; Zhou, Z.; Li, H.; Wan, L.; Kang, A.; Guo, W.; Ren, K.; Song, X.; Chen, Y.; et al. GAS5 protects against nonalcoholic fatty liver disease via miR-28a-5p/MARCH7/NLRP3 axis-mediated pyroptosis. Cell Death Differ. 2023, 30, 1829–1848. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, D.; Zhu, Y.; Dong, Y.; Liu, Y. Long non-coding RNA GAS5 promotes osteogenic differentiation of bone marrow mesenchymal stem cells by regulating the miR-135a-5p/FOXO1 pathway. Mol. Cell Endocrinol. 2019, 496, 110534. [Google Scholar] [CrossRef] [PubMed]
- Li, B.G.; Wu, W.J.; Zheng, H.C.; Yang, H.F.; Zuo, Y.X.; Cui, X.P. Long noncoding RNA GAS5 silencing inhibits the expression of KCNQ3 by sponging miR-135a-5p to prevent the progression of epilepsy. Kaohsiung J. Med. Sci. 2019, 35, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Zheng, X.; Zhu, Z.; Zhao, S.; Zhou, Q.; Song, Z.; Wang, G.; Wang, Z. Silencing of GAS5 represses the malignant progression of atherosclerosis through upregulation of miR-135a. Biomed. Pharmacother. 2019, 118, 109302. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhang, L.; Wang, E.; Zhang, C.; Li, X. LncRNA GAS5 regulates ischemic stroke as a competing endogenous RNA for miR-137 to regulate the Notch1 signaling pathway. Biochem. Biophys. Res. Commun. 2018, 496, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Yang, S.T.; Liu, J.; Zhang, K.X.; Leng, J.Y. Silence of LncRNA GAS5 Protects Cardiomyocytes H9c2 against Hypoxic Injury via Sponging miR-142-5p. Mol. Cells 2019, 42, 397–405. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Tan, R.Z.; Yu, Y.; Niu, Y.Y.; Yu, C. LncRNA GAS5 protects against TGF-beta-induced renal fibrosis via the Smad3/miRNA-142-5p axis. Am. J. Physiol. Ren. Physiol. 2021, 321, F517–F526. [Google Scholar] [CrossRef]
- Zhang, D.; Qiu, S. LncRNA GAS5 upregulates Smad4 to suppress the apoptosis of chondrocytes induced by lipopolysaccharide. Arch. Gerontol. Geriatr. 2021, 97, 104478. [Google Scholar] [CrossRef]
- Ma, J.; Sun, W.; Chen, S.; Wang, Z.; Zheng, J.; Shi, X.; Li, M.; Li, D.; Gu, Q. The long noncoding RNA GAS5 potentiates neuronal injury in Parkinson’s disease by binding to microRNA-150 to regulate Fosl1 expression. Exp. Neurol. 2022, 347, 113904. [Google Scholar] [CrossRef]
- Zeng, Z.; Lan, Y.; Chen, Y.; Zuo, F.; Gong, Y.; Luo, G.; Peng, Y.; Yuan, Z. LncRNA GAS5 suppresses inflammatory responses by inhibiting HMGB1 release via miR-155-5p/SIRT1 axis in sepsis. Eur. J. Pharmacol. 2023, 942, 175520. [Google Scholar] [CrossRef]
- Li, Y.; Geng, Y.; Zhou, B.; Wu, X.; Zhang, O.; Guan, X.; Xue, Y.; Li, S.; Zhuang, X.; Zhou, J.; et al. Long Non-coding RNA GAS5 Worsens Coronary Atherosclerosis Through MicroRNA-194-3p/TXNIP Axis. Mol. Neurobiol. 2021, 58, 3198–3207. [Google Scholar] [CrossRef]
- Ayeldeen, G.; Shaker, O.G.; Amer, E.; Zaafan, M.A.; Herzalla, M.R.; Keshk, M.A.; Abdelhamid, A.M. The Impact of lncRNA-GAS5/miRNA-200/ACE2 Molecular Pathway on the Severity of COVID-19. Curr. Med. Chem. 2024, 31, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Sun, T.T.; Liu, Z.H.; Li, X.; Fan, X.F.; Han, L.P. LncRNA GAS5 restrains ISO-induced cardiac fibrosis by modulating mir-217 regulation of SIRT1. Sci. Rep. 2024, 14, 7652. [Google Scholar] [CrossRef]
- Ge, X.; Xu, B.; Xu, W.; Xia, L.; Xu, Z.; Shen, L.; Peng, W.; Huang, S. Long noncoding RNA GAS5 inhibits cell proliferation and fibrosis in diabetic nephropathy by sponging miR-221 and modulating SIRT1 expression. Aging 2019, 11, 8745. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Shi, Z.; Ma, X.; Xu, D.; Ming, G. lncRNA GAS5/miR-223/NAMPT axis modulates the cell proliferation and senescence of endothelial progenitor cells through PI3K/AKT signaling. J. Cell Biochem. 2019, 120, 14518–14530. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhang, L.; Geng, Y.; Liu, Y.; Zhang, N. Long noncoding RNA GAS5 promotes microglial inflammatory response in Parkinson’s disease by regulating NLRP3 pathway through sponging miR-223-3p. Int. Immunopharmacol. 2020, 85, 106614. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.; Zhang, Q.; Gan, H.; Qin, Y.; Zhou, Y.; Zhai, X.; Liang, P. Long Noncoding RNA GAS5-Involved Progression of Neonatal Hydrocephalus and Inflammatory Responses. Mol. Biotechnol. 2024, 66, 2674. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, X.; Bao, Y.; Yu, H.; Jia, D.; Ma, C. Down-regulation of GAS5 ameliorates myocardial ischaemia/reperfusion injury via the miR-335/ROCK1/AKT/GSK-3beta axis. J. Cell Mol. Med. 2019, 23, 8420–8431. [Google Scholar] [CrossRef]
- Zhang, W.; Li, B.; Xia, N.; Zhu, L.; Zhang, Z.; Ren, Z.; Zhang, L.; Xu, P.; Meng, F.; Feng, L.; et al. lncRNA GAS5 suppresses rheumatoid arthritis by inhibiting miR-361-5p and increasing PDK4. Biochem. Biophys. Res. Commun. 2021, 583, 7–13. [Google Scholar] [CrossRef]
- Xie, C.; Wu, W.; Tang, A.; Luo, N.; Tan, Y. lncRNA GAS5/miR-452-5p Reduces Oxidative Stress and Pyroptosis of High-Glucose-Stimulated Renal Tubular Cells. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 2609–2617. [Google Scholar] [CrossRef]
- Chi, X.; Ding, B.; Zhang, L.; Zhang, J.; Wang, J.; Zhang, W. lncRNA GAS5 promotes M1 macrophage polarization via miR-455-5p/SOCS3 pathway in childhood pneumonia. J. Cell Physiol. 2019, 234, 13242–13251. [Google Scholar] [CrossRef]
- Zhang, Y.; Hou, Y.M.; Gao, F.; Xiao, J.W.; Li, C.C.; Tang, Y. lncRNA GAS5 regulates myocardial infarction by targeting the miR-525-5p/CALM2 axis. J. Cell Biochem. 2019, 120, 18678–18688. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, L.; Zuo, L.; Gao, Z.; Shi, Y.; Yuan, P.; Han, S.; Yin, J.; Peng, B.; He, X.; et al. Short Communication: Long Noncoding RNA GAS5 Inhibits HIV-1 Replication Through Interaction with miR-873. AIDS Res. Hum. Retroviruses 2018, 34, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Peng, Y.; Lv, D.; Xue, Y.; Huang, L.; Hu, Y.; Zhu, W.; Luo, S.; Shen, J.; Li, X. LncRNA GAS5 downregulates NLRP3 inflammasome activation-mediated pyroptosis in sepsis-induced myocardial injury by targeting SIRT3/AMPKalpha. Heliyon 2023, 9, e22939. [Google Scholar] [CrossRef] [PubMed]
- Imam, H.; Bano, A.S.; Patel, P.; Holla, P.; Jameel, S. The lncRNA NRON modulates HIV-1 replication in a NFAT-dependent manner and is differentially regulated by early and late viral proteins. Sci. Rep. 2015, 5, 8639. [Google Scholar] [CrossRef]
- Lucafo, M.; De Iudicibus, S.; Di Silvestre, A.; Pelin, M.; Candussio, L.; Martelossi, S.; Tommasini, A.; Piscianz, E.; Ventura, A.; Decorti, G. Long noncoding RNA GAS5: A novel marker involved in glucocorticoid response. Curr. Mol. Med. 2015, 15, 94–99. [Google Scholar] [CrossRef]
- Wang, Y.N.; Shan, K.; Yao, M.D.; Yao, J.; Wang, J.J.; Li, X.; Liu, B.; Zhang, Y.Y.; Ji, Y.; Jiang, Q.; et al. Long Noncoding RNA-GAS5: A Novel Regulator of Hypertension-Induced Vascular Remodeling. Hypertension 2016, 68, 736–748. [Google Scholar] [CrossRef]
- Wang, F.; Li, L.; Xu, H.; Liu, Y.; Yang, C.; Cowley, A.W., Jr.; Wang, N.; Liu, P.; Liang, M. Characteristics of long non-coding RNAs in the Brown Norway rat and alterations in the Dahl salt-sensitive rat. Sci. Rep. 2014, 4, 7146. [Google Scholar] [CrossRef]
- Kaur, J.; Salehen, N.; Norazit, A.; Rahman, A.A.; Murad, N.A.A.; Rahman, N.; Ibrahim, K. Tumor Suppressive Effects of GAS5 in Cancer Cells. Noncoding RNA 2022, 8, 39. [Google Scholar] [CrossRef]
- Vlach, J.; Hennecke, S.; Alevizopoulos, K.; Conti, D.; Amati, B. Growth arrest by the cyclin-dependent kinase inhibitor p27Kip1 is abrogated by c-Myc. EMBO J. 1996, 15, 6595–6604. [Google Scholar] [CrossRef]
- Hu, G.; Lou, Z.; Gupta, M. The long non-coding RNA GAS5 cooperates with the eukaryotic translation initiation factor 4E to regulate c-Myc translation. PLoS ONE 2014, 9, e107016. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; Zhang, W.; Gan, J.; Hu, C.; Huang, G.; Zhang, Y. lncRNA GAS5 enhances G1 cell cycle arrest via binding to YBX1 to regulate p21 expression in stomach cancer. Sci. Rep. 2015, 5, 10159. [Google Scholar] [CrossRef] [PubMed]
- Zingg, D.; Debbache, J.; Schaefer, S.M.; Tuncer, E.; Frommel, S.C.; Cheng, P.; Arenas-Ramirez, N.; Haeusel, J.; Zhang, Y.; Bonalli, M.; et al. The epigenetic modifier EZH2 controls melanoma growth and metastasis through silencing of distinct tumour suppressors. Nat. Commun. 2015, 6, 6051. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yan, Z.; Hu, F.; Wei, W.; Yang, C.; Sun, Z. Long non-coding RNA GAS5 accelerates oxidative stress in melanoma cells by rescuing EZH2-mediated CDKN1C downregulation. Cancer Cell Int. 2020, 20, 116. [Google Scholar] [CrossRef] [PubMed]
- Mourtada-Maarabouni, M.; Pickard, M.R.; Hedge, V.L.; Farzaneh, F.; Williams, G.T. GAS5, a non-protein-coding RNA, controls apoptosis and is downregulated in breast cancer. Oncogene 2009, 28, 195–208. [Google Scholar] [CrossRef]
- Pickard, M.R.; Mourtada-Maarabouni, M.; Williams, G.T. Long non-coding RNA GAS5 regulates apoptosis in prostate cancer cell lines. Biochim. Biophys. Acta 2013, 1832, 1613–1623. [Google Scholar] [CrossRef]
- Hao, S.; Liu, X.; Sui, X.; Pei, Y.; Liang, Z.; Zhou, N. Long non-coding RNA GAS5 reduces cardiomyocyte apoptosis induced by MI through sema3a. Int. J. Biol. Macromol. 2018, 120, 371–377. [Google Scholar] [CrossRef]
- Hu, H.; Xuan, Y.; Xue, M.; Cheng, W.; Wang, Y.; Li, X.; Yin, J.; Li, X.; Yang, N.; Shi, Y.; et al. Semaphorin 3A attenuates cardiac autonomic disorders and reduces inducible ventricular arrhythmias in rats with experimental myocardial infarction. BMC Cardiovasc. Disord. 2016, 16, 16. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, J.; Zhang, M.; Wu, Y. Semaphorin 3A deficiency improves hypoxia-induced myocardial injury via resisting inflammation and cardiomyocytes apoptosis. Cell Mol. Biol. 2016, 62, 8–14. [Google Scholar]
- Li, L.; Li, X.; The, E.; Wang, L.J.; Yuan, T.Y.; Wang, S.Y.; Feng, J.; Wang, J.; Liu, Y.; Wu, Y.H.; et al. Low expression of lncRNA-GAS5 is implicated in human primary varicose great saphenous veins. PLoS ONE 2015, 10, e0120550. [Google Scholar] [CrossRef]
- Tang, R.; Zhang, G.; Wang, Y.C.; Mei, X.; Chen, S.Y. The long non-coding RNA GAS5 regulates transforming growth factor beta (TGF-beta)-induced smooth muscle cell differentiation via RNA Smad-binding elements. J. Biol. Chem. 2017, 292, 14270–14278. [Google Scholar] [CrossRef]
- Hudson, W.H.; Vera, I.M.S.; Nwachukwu, J.C.; Weikum, E.R.; Herbst, A.G.; Yang, Q.; Bain, D.L.; Nettles, K.W.; Kojetin, D.J.; Ortlund, E.A. Cryptic glucocorticoid receptor-binding sites pervade genomic NF-kappaB response elements. Nat. Commun. 2018, 9, 1337. [Google Scholar] [CrossRef] [PubMed]
- Curci, D.; Stankovic, B.; Kotur, N.; Pugnetti, L.; Gasic, V.; Romano, M.; Zukic, B.; Decorti, G.; Stocco, G.; Lucafo, M.; et al. The long non-coding RNA GAS5 contributes to the suppression of inflammatory responses by inhibiting NF-kappaB activity. Front. Pharmacol. 2024, 15, 1448136. [Google Scholar] [CrossRef]
- Martinez, G.J.; Appleton, M.; Kipp, Z.A.; Loria, A.S.; Min, B.; Hinds, T.D., Jr. Glucocorticoids, their uses, sexual dimorphisms, and diseases: New concepts, mechanisms, and discoveries. Physiol. Rev. 2024, 104, 473–532. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.S.; de Castro, M. Generalized and tissue specific glucocorticoid resistance. Mol. Cell Endocrinol. 2021, 530, 111277. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Ye, Z.; Hou, L.; Zhang, X.; Liu, Z.; Wu, R.; Huang, F.; Wang, G.; Geng, X.; Zhao, H. Hepatitis B virus x gene-downregulated growth-arrest specific 5 inhibits the cell viability and invasion of hepatocellular carcinoma cell lines by activating Y-box-binding protein 1/p21 signaling. J. Cell Commun. Signal 2022, 16, 179–190. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).