Roles, Functions, and Pathological Implications of Exosomes in the Central Nervous System
Abstract
1. Introduction
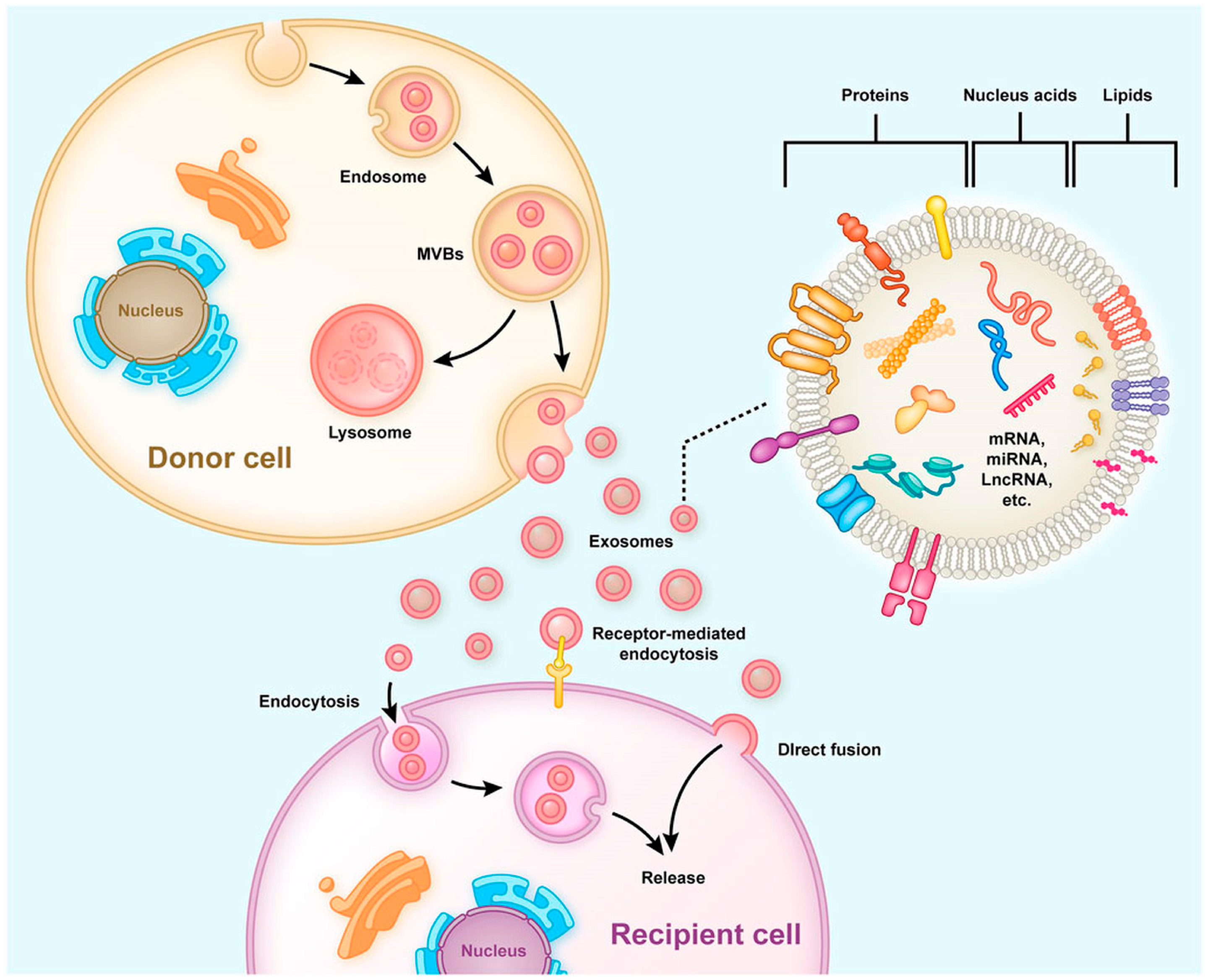
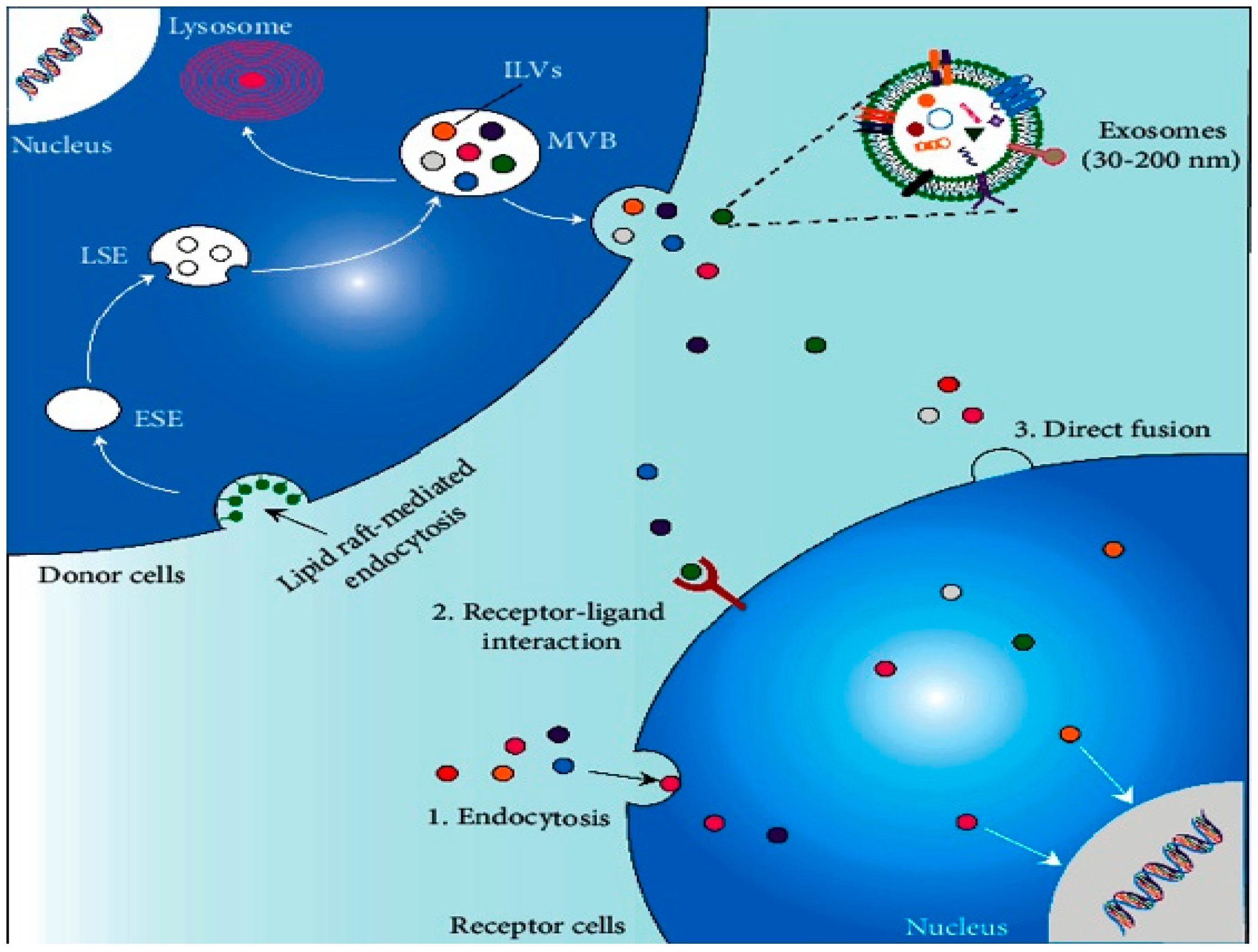
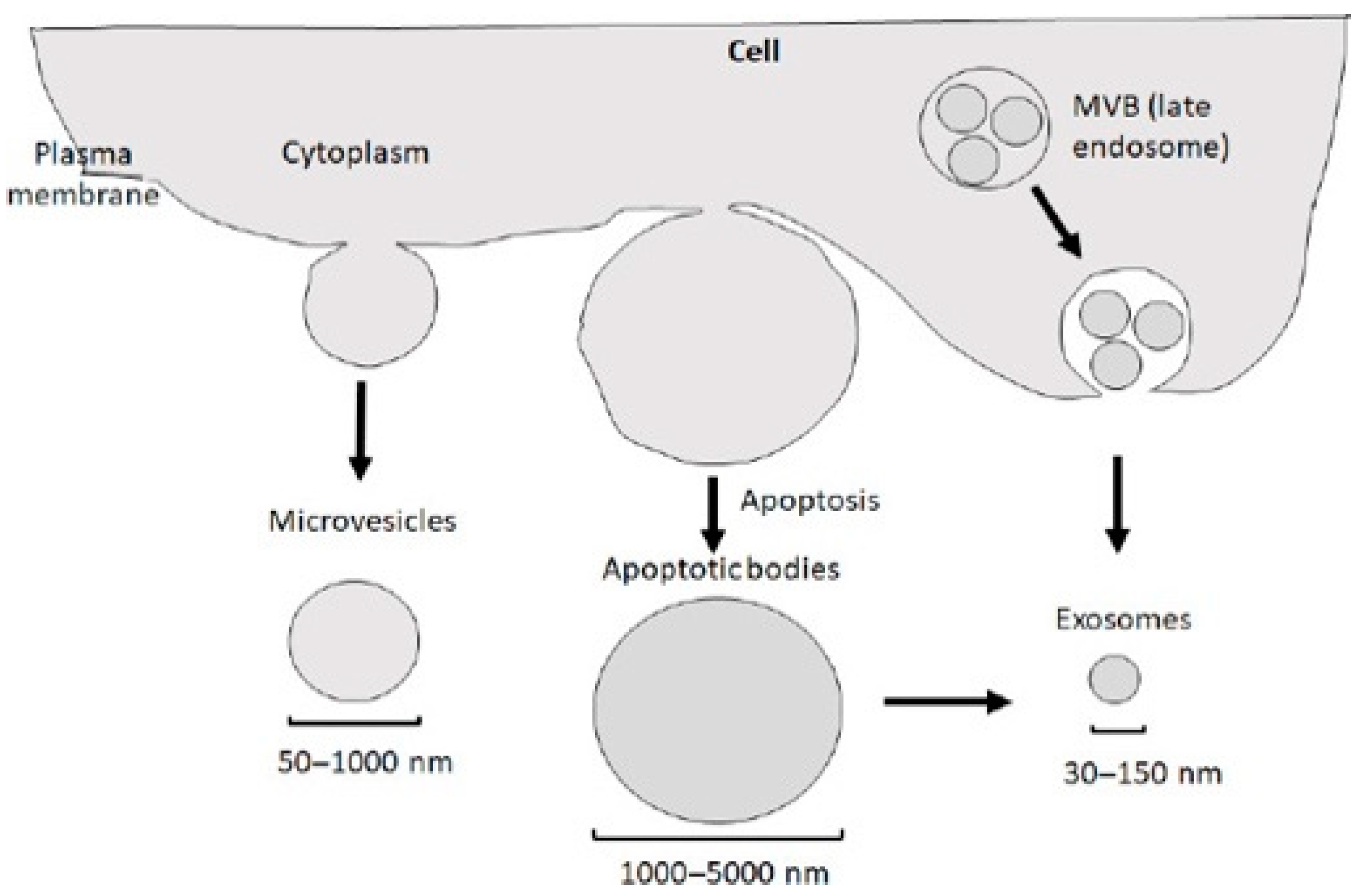
2. Production, Release, Internalization, and Composition of Exosomes
3. Exosome Extraction, Detection, and Characterization
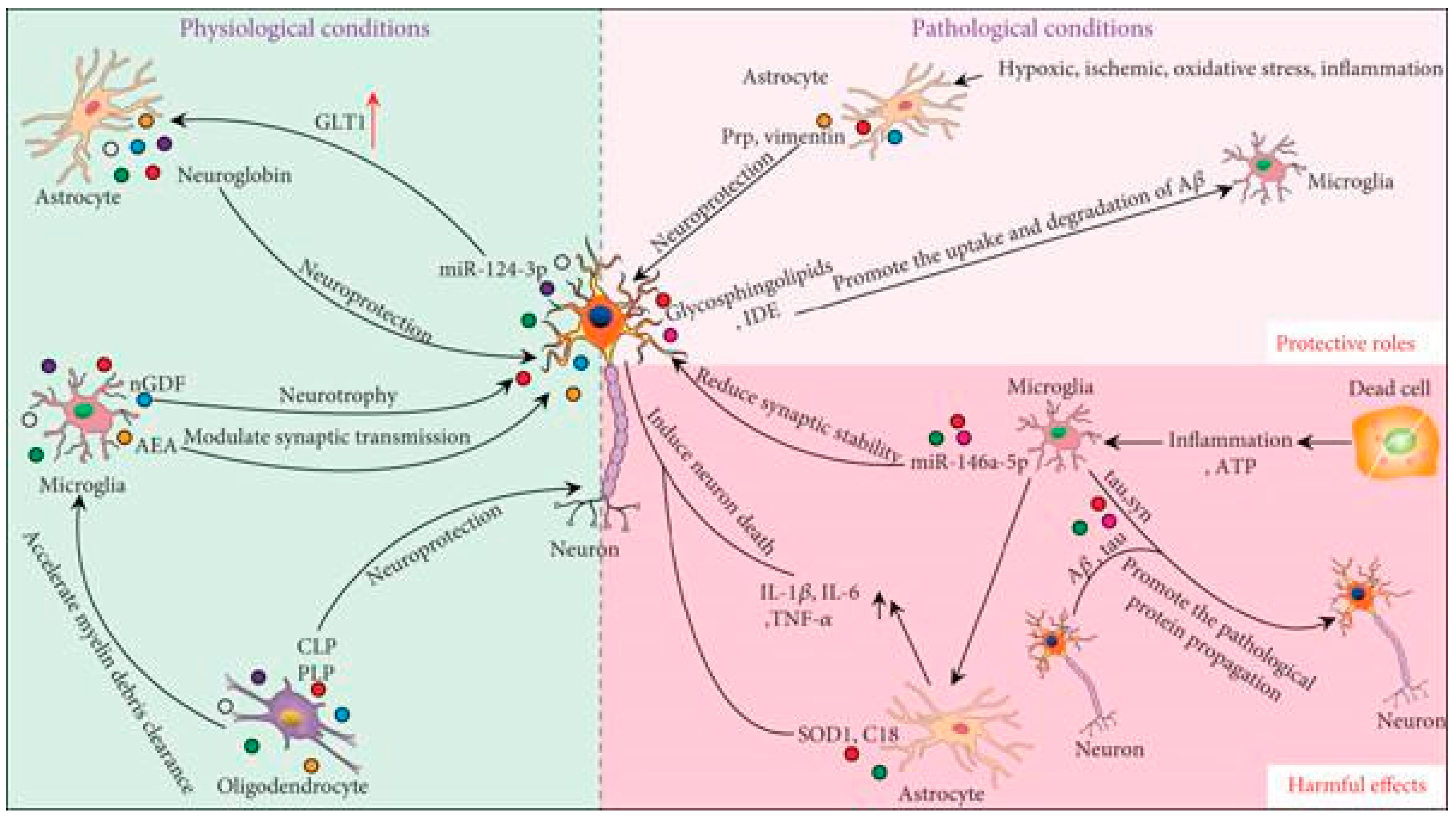
4. Functions of Exosomes as Mediators of Intercellular Communication in Physiological and Pathological Contexts in the CNS
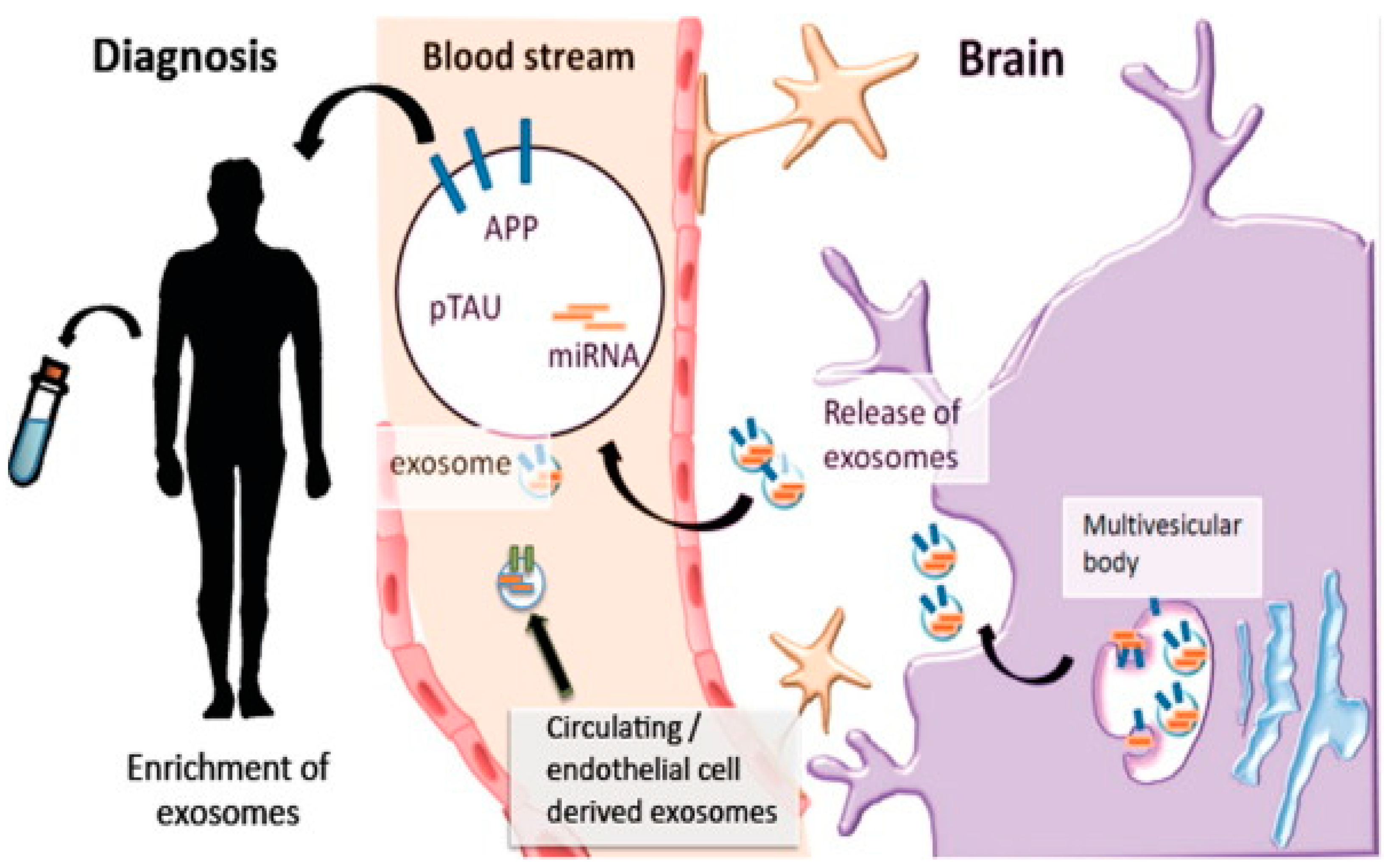
5. Possible Diagnostic Biomarkers of Exosomes in Neurodegenerative Disorders
5.1. Alzheimer’s Disease
5.2. Parkinson’s Disease
5.3. Amyotrophic Lateral Sclerosis
5.4. Pediatric CNS Tumors
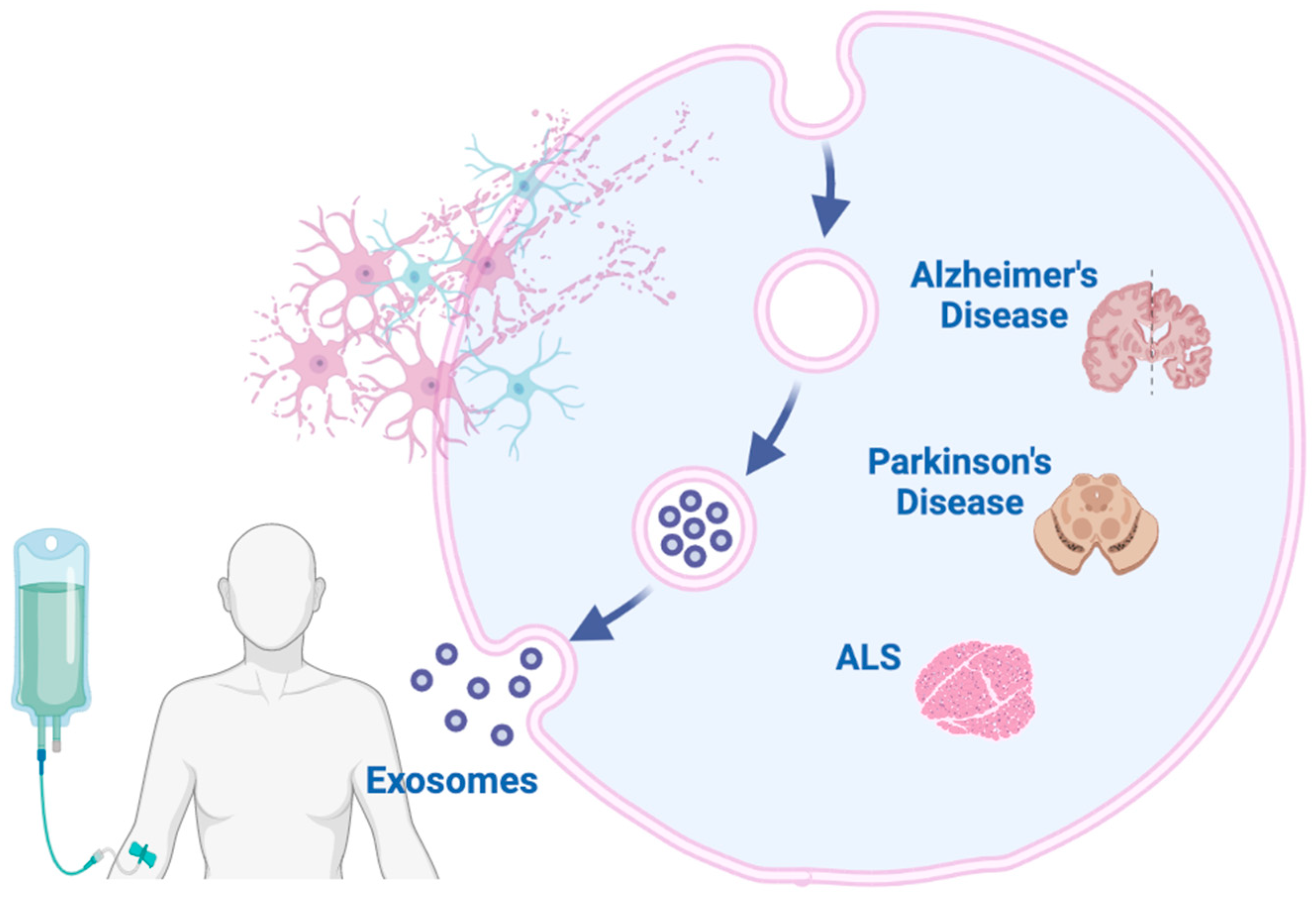
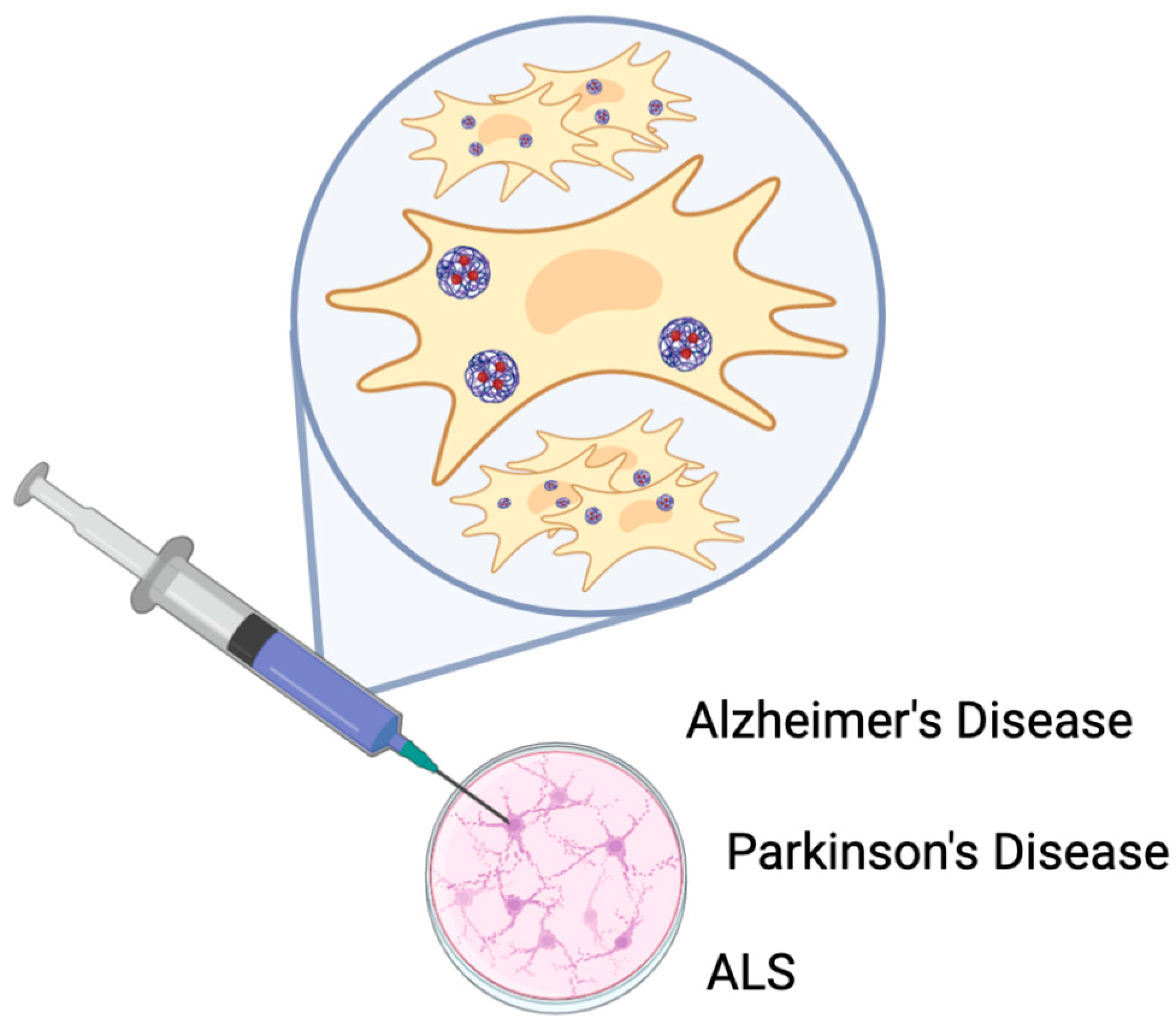
6. The Potential Therapeutic Applications of Exosomes Derived from Stem Cells in the Treatment of Neurodegenerative Diseases
6.1. Stem Cell-Derived Exosomes for Treating Alzheimer’s Disease (AD)
6.2. Stem Cell-Derived Exosomes for Treating Parkinson’s Disease (PD)
6.3. Preclinical Studies on Stem Cell-Derived Exosomes in ALS
| Human Disease | Exosome-Associated Protein | MVB/Endocytic Impairment |
|---|---|---|
| Creutzfeldt–Jakob disease | PrPc, PrPsc | MVB and endosome enlargement |
| Alzheimer’s Disese | Aβ | Endosome enlargement |
| APP | Overexpression of RabGTPases | |
| BACE | CHMP2B high immunoreactivity | |
| Presenilin | PICALM, BIN1 mutation | |
| AD and FTD | Tau | PICALM, BIN1 mutation |
| PD | α-Synuclein | CHMP2B mutation |
| LRRK2 | CHMP2B-positive inclusions | |
| ALS | SOD1 | CHMP2 mutation |
| TDP-43 | Axonal exosome transport deficits | |
| Polyglutamine disease | Heat shock protein | |
| Huntington disease | HSP40, HSP70, HSP90 | |
| Schizophrenia | Dysbindin-1B | Mutations in BLOC-1 subunits, and dysbindin, and muted |
6.4. Clinical Trials Testing Exosomes for Neurodegenerative Disorders
7. Discussion
8. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Rehman, M.U.; Wali, A.F.; Ahmad, A.; Shakeel, S.; Rasool, S.; Ali, R.; Rashid, S.M.; Madkhali, H.; Ganaie, M.A.; Khan, R. Neuroprotective Strategies for Neurological Disorders by Natural Products: An update. Curr. Neuropharmacol. 2019, 17, 247–267. [Google Scholar] [CrossRef] [PubMed]
- Meisel, C.; Schwab, J.M.; Prass, K.; Meisel, A.; Dirnagl, U. Central nervous system injury-induced immune deficiency syndrome. Nat. Rev. Neurosci. 2005, 6, 775–786. [Google Scholar] [CrossRef]
- Qureshi, S.; Dhall, S.S.; A Anderson, P.; Arnold, P.M.; Chi, J.H.; Dailey, A.T.; Eichholz, K.M.; Harrop, J.S.; Hoh, D.J.; Rabb, C.H.; et al. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guidelines on the Evaluation and Treatment of Patients With Thoracolumbar Spine Trauma: Radiological Evaluation. Neurosurgery 2018, 84, E28–E31. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.K.; Winkler, E.A.; Rick, J.W.; Deng, H.; Partow, C.P.; Upadhyayula, P.S.; Birk, H.S.; Chan, A.K.; Dhall, S.S. Update on critical care for acute spinal cord injury in the setting of polytrauma. Neurosurg. Focus 2017, 43, E19. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Qiu, Q.; Zhang, H.; Chu, L.; Du, Y.; Zhang, J.; Zhou, C.; Liang, F.; Shi, S.; Wang, S.; et al. Concordance between the assessment of Aβ42, T-tau, and P-T181-tau in peripheral blood neuronal-derived exosomes and cerebrospinal fluid. Alzheimer’s Dement. 2019, 15, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Nabers, A.; Perna, L.; Lange, J.; Mons, U.; Schartner, J.; Güldenhaupt, J.; Saum, K.; Janelidze, S.; Holleczek, B.; Rujescu, D.; et al. Amyloid blood biomarker detects Alzheimer’s disease. EMBO Mol. Med. 2018, 10, e8763. [Google Scholar] [CrossRef]
- Kwon, M.J.; Kim, S.; Han, M.H.; Lee, S.B. Epigenetic Changes in Neurodegenerative Diseases. Mol. Cells 2016, 39, 783–789. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, B.; Ocansey, D.K.W.; Xu, W.; Qian, H. Extracellular vesicles: A bright star of nanomedicine. Biomaterials 2020, 269, 120467. [Google Scholar] [CrossRef]
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef] [PubMed]
- Hornung, S.; Dutta, S.; Bitan, G. CNS-Derived Blood Exosomes as a Promising Source of Biomarkers: Opportunities and Challenges. Front. Mol. Neurosci. 2020, 13, 38. [Google Scholar] [CrossRef]
- Gorabi, A.M.; Kiaie, N.; Barreto, G.E.; Read, M.I.; Tafti, H.A.; Sahebkar, A. The Therapeutic Potential of Mesenchymal Stem Cell–Derived Exosomes in Treatment of Neurodegenerative Diseases. Mol. Neurobiol. 2019, 56, 8157–8167. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, Y.; Zhao, Z.; Liu, C.; Zhang, L. Study on Transorgan Regulation of Intervertebral Disc and Extra-Skeletal Organs Through Exosomes Derived From Bone Marrow Mesenchymal Stem Cells. Front. Cell Dev. Biol. 2021, 9, 741183. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- De Jong, O.G.; Verhaar, M.C.; Chen, Y.; Vader, P.; Gremmels, H.; Posthuma, G.; Schiffelers, R.M.; Gucek, M.; Van Balkom, B.W.M. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. J. Extracell. Vesicles 2012, 1, 18396. [Google Scholar] [CrossRef] [PubMed]
- Ti, D.; Hao, H.; Fu, X.; Han, W. Mesenchymal stem cells-derived exosomal microRNAs contribute to wound inflammation. Sci. China Life Sci. 2016, 59, 1305–1312. [Google Scholar] [CrossRef]
- Chen, S.; Tang, Y.; Liu, Y.; Zhang, P.; Lv, L.; Zhang, X.; Jia, L.; Zhou, Y. Exosomes derived from miR-375-overexpressing human adipose mesenchymal stem cells promote bone regeneration. Cell Prolif. 2019, 52, e12669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yuan, X.; Shi, H.; Wu, L.; Qian, H.; Xu, W. Exosomes in cancer: Small particle, big player. J. Hematol. Oncol. 2015, 8, 83. [Google Scholar] [CrossRef] [PubMed]
- Riau, A.K.; Ong, H.S.; Yam, G.H.F.; Mehta, J.S. Sustained Delivery System for Stem Cell-Derived Exosomes. Front. Pharmacol. 2019, 10, 1368. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Han, B.; Hai, Y.; Sun, D.; Yin, P. Mechanism of Action of Mesenchymal Stem Cell-Derived Exosomes in the Intervertebral Disc Degeneration Treatment and Bone Repair and Regeneration. Front. Cell Dev. Biol. 2022, 9, 833840. [Google Scholar] [CrossRef]
- He, C.; Zheng, S.; Luo, Y.; Wang, B. Exosome Theranostics: Biology and Translational Medicine. Theranostics 2018, 8, 237–255. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Bebelman, M.P.; Smit, M.J.; Pegtel, D.M.; Baglio, S.R. Biogenesis and function of extracellular vesicles in cancer. Pharmacol. Ther. 2018, 188, 1–11. [Google Scholar] [CrossRef]
- Li, S.-P.; Lin, Z.-X.; Jiang, X.-Y.; Yu, X.-Y. Exosomal cargo-loading and synthetic exosome-mimics as potential therapeutic tools. Acta Pharmacol. Sin. 2018, 39, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Hyenne, V.; Apaydin, A.; Rodriguez, D.; Spiegelhalter, C.; Hoff-Yoessle, S.; Diem, M.; Tak, S.; Lefebvre, O.; Schwab, Y.; Goetz, J.G.; et al. RAL-1 controls multivesicular body biogenesis and exosome secretion. J. Cell Biol. 2015, 211, 27–37. [Google Scholar] [CrossRef]
- Zhou, B.; Xu, K.; Zheng, X.; Chen, T.; Wang, J.; Song, Y.; Shao, Y.; Zheng, S. Application of exosomes as liquid biopsy in clinical diagnosis. Signal Transduct. Target. Ther. 2020, 5, 144. [Google Scholar] [CrossRef]
- Mathivanan, S.; Fahner, C.J.; Reid, G.E.; Simpson, R.J. ExoCarta 2012: Database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2011, 40, D1241–D1244. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Wu, P.; Zhou, X.; Qian, H.; Xu, W. Extracellular Vesicles: Novel Roles in Neurological Disorders. Stem Cells Int. 2021, 2021, 6640836. [Google Scholar] [CrossRef]
- Huber, C.C.; Wang, H. Pathogenic and therapeutic role of exosomes in neurodegenerative disorders. Neural Regen. Res. 2024, 19, 75–79. [Google Scholar] [CrossRef]
- Greening, D.W.; Xu, R.; Ji, H.; Tauro, B.J.; Simpson, R.J. A Protocol for Exosome Isolation and Characterization: Evaluation of Ultracentrifugation, Density-Gradient Separation, and Immunoaffinity Capture Methods. In Proteomic Profiling; Posch, A., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2015; Volume 1295, pp. 179–209. [Google Scholar] [CrossRef]
- Böing, A.N.; van der Pol, E.; Grootemaat, A.E.; Coumans, F.A.W.; Sturk, A.; Nieuwland, R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J. Extracell. Vesicles 2014, 3, 23430. [Google Scholar] [CrossRef]
- Popovic, M.; Mazzega, E.; Toffoletto, B.; de Marco, A. Isolation of anti-extra-cellular vesicle single-domain antibodies by direct panning on vesicle-enriched fractions. Microb. Cell Factories 2018, 17, 6. [Google Scholar] [CrossRef]
- Oeyen, E.; Van Mol, K.; Baggerman, G.; Willems, H.; Boonen, K.; Rolfo, C.; Pauwels, P.; Jacobs, A.; Schildermans, K.; Cho, W.C.; et al. Ultrafiltration and size exclusion chromatography combined with asymmetrical-flow field-flow fractionation for the isolation and characterisation of extracellular vesicles from urine. J. Extracell. Vesicles 2018, 7, 1490143. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Luo, B.; Jiang, P.; Zhou, X.; Lan, F.; Yi, Q.; Wu, Y. Immuno-modified superparamagnetic nanoparticles via host–guest interactions for high-purity capture and mild release of exosomes. Nanoscale 2018, 10, 14280–14289. [Google Scholar] [CrossRef]
- Wu, M.; Ouyang, Y.; Wang, Z.; Zhang, R.; Huang, P.-H.; Chen, C.; Li, H.; Li, P.; Quinn, D.; Dao, M.; et al. Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc. Natl. Acad. Sci. USA 2017, 114, 10584–10589. [Google Scholar] [CrossRef]
- Lobb, R.j.; Becker, M.; Wen, S.W.; Wong, C.S.F.; Wiegmans, A.P.; Leimgruber, A.; Möller, A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J. Extracell. Vesicles 2015, 4, 27031. [Google Scholar] [CrossRef]
- Langevin, S.M.; Kuhnell, D.; Orr-Asman, M.A.; Biesiada, J.; Zhang, X.; Medvedovic, M.; Thomas, H.E. Balancing yield, purity and practicality: A modified differential ultracentrifugation protocol for efficient isolation of small extracellular vesicles from human serum. RNA Biol. 2019, 16, 5–12. [Google Scholar] [CrossRef]
- Sokolova, V.; Ludwig, A.-K.; Hornung, S.; Rotan, O.; Horn, P.A.; Epple, M.; Giebel, B. Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy. Colloids Surf. B Biointerfaces 2011, 87, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Yuana, Y.; Grootemaat, A.E.; van der Pol, E.; Gollwitzer, C.; Krumrey, M.; Nieuwland, R. Towards traceable size determination of extracellular vesicles. J. Extracell. Vesicles 2014, 3, 23298. [Google Scholar] [CrossRef] [PubMed]
- Strack, R. Improved exosome detection. Nat. Methods 2019, 16, 286. [Google Scholar] [CrossRef] [PubMed]
- Notarangelo, M.; Zucal, C.; Modelska, A.; Pesce, I.; Scarduelli, G.; Potrich, C.; Lunelli, L.; Pederzolli, C.; Pavan, P.; la Marca, G.; et al. Ultrasensitive detection of cancer biomarkers by nickel-based isolation of polydisperse extracellular vesicles from blood. EBioMedicine 2019, 43, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Taller, D.; Richards, K.; Slouka, Z.; Senapati, S.; Hill, R.; Go, D.B.; Chang, H.-C. On-chip surface acoustic wave lysis and ion-exchange nanomembrane detection of exosomal RNA for pancreatic cancer study and diagnosis. Lab A Chip 2015, 15, 1656–1666. [Google Scholar] [CrossRef] [PubMed]
- Mustapic, M.; Eitan, E.; Werner Jr, J.K.; Berkowitz, S.T.; Lazaropoulos, M.P.; Tran, J.; Goetzl, E.J.; Kapogiannis, D. Plasma Extracellular Vesicles Enriched for Neuronal Origin: A Potential Window into Brain Pathologic Processes. Front. Neurosci. 2017, 11, 278. [Google Scholar] [CrossRef]
- Greenhalgh, A.D.; David, S.; Bennett, F.C. Immune cell regulation of glia during CNS injury and disease. Nat. Rev. Neurosci. 2020, 21, 139–152. [Google Scholar] [CrossRef]
- Sharma, P.; Mesci, P.; Carromeu, C.; McClatchy, D.R.; Schiapparelli, L.; Yates, J.R.; Muotri, A.R.; Cline, H.T. Exosomes regulate neurogenesis and circuit assembly. Proc. Natl. Acad. Sci. USA 2019, 116, 16086–16094. [Google Scholar] [CrossRef]
- Fauré, J.; Lachenal, G.; Court, M.; Hirrlinger, J.; Chatellard-Causse, C.; Blot, B.; Grange, J.; Schoehn, G.; Goldberg, Y.; Boyer, V.; et al. Exosomes are released by cultured cortical neurones. Mol. Cell. Neurosci. 2006, 31, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Men, Y.; Yelick, J.; Jin, S.; Tian, Y.; Chiang, M.S.R.; Higashimori, H.; Brown, E.; Jarvis, R.; Yang, Y. Exosome reporter mice reveal the involvement of exosomes in mediating neuron to astroglia communication in the CNS. Nat. Commun. 2019, 10, 4136. [Google Scholar] [CrossRef] [PubMed]
- Venturini, A.; Passalacqua, M.; Pelassa, S.; Pastorino, F.; Tedesco, M.; Cortese, K.; Gagliani, M.C.; Leo, G.; Maura, G.; Guidolin, D.; et al. Exosomes From Astrocyte Processes: Signaling to Neurons. Front. Pharmacol. 2019, 10, 1452. [Google Scholar] [CrossRef]
- Raffo-Romero, A.; Arab, T.; Al-Amri, I.S.; Le Marrec-Croq, F.; Van Camp, C.; Lemaire, Q.; Salzet, M.; Vizioli, J.; Sautiere, P.-E.; Lefebvre, C. Medicinal Leech CNS as a Model for Exosome Studies in the Crosstalk between Microglia and Neurons. Int. J. Mol. Sci. 2018, 19, 4124. [Google Scholar] [CrossRef]
- Gabrielli, M.; Battista, N.; Riganti, L.; Prada, I.; Antonucci, F.; Cantone, L.; Matteoli, M.; Maccarrone, M.; Verderio, C. Active endocannabinoids are secreted on extracellular membrane vesicles. Embo Rep. 2015, 16, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Fitzner, D.; Schnaars, M.; van Rossum, D.; Krishnamoorthy, G.; Dibaj, P.; Bakhti, M.; Regen, T.; Hanisch, U.-K.; Simons, M. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J. Cell Sci. 2011, 124, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Krämer-Albers, E.-M.; Bretz, N.; Tenzer, S.; Winterstein, C.; Möbius, W.; Berger, H.; Nave, K.-A.; Schild, H.; Trotter, J. Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: Trophic support for axons? Proteom. Clin. Appl. 2007, 1, 1446–1461. [Google Scholar] [CrossRef] [PubMed]
- Vanherle, S.; Haidar, M.; Irobi, J.; Bogie, J.F.; Hendriks, J.J. Extracellular vesicle-associated lipids in central nervous system disorders. Adv. Drug Deliv. Rev. 2020, 159, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Guitart, K.; Loers, G.; Buck, F.; Bork, U.; Schachner, M.; Kleene, R. Improvement of neuronal cell survival by astrocyte-derived exosomes under hypoxic and ischemic conditions depends on prion protein. Glia 2016, 64, 896–910. [Google Scholar] [CrossRef]
- Adolf, A.; Rohrbeck, A.; Münster-Wandowski, A.; Johansson, M.; Kuhn, H.; Kopp, M.A.; Brommer, B.; Schwab, J.M.; Just, I.; Ahnert-Hilger, G.; et al. Release of astroglial vimentin by extracellular vesicles: Modulation of binding and internalization of C3 transferase in astrocytes and neurons. Glia 2018, 67, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Yuyama, K.; Sun, H.; Usuki, S.; Sakai, S.; Hanamatsu, H.; Mioka, T.; Kimura, N.; Okada, M.; Tahara, H.; Furukawa, J.-I.; et al. A potential function for neuronal exosomes: Sequestering intracerebral amyloid-β peptide. FEBS Lett. 2014, 589, 84–88. [Google Scholar] [CrossRef]
- Yuyama, K.; Sun, H.; Mitsutake, S.; Igarashi, Y. Sphingolipid-modulated Exosome Secretion Promotes Clearance of Amyloid-β by Microglia. J. Biol. Chem. 2012, 287, 10977–10989. [Google Scholar] [CrossRef] [PubMed]
- Tamboli, I.Y.; Barth, E.; Christian, L.; Siepmann, M.; Kumar, S.; Singh, S.; Tolksdorf, K.; Heneka, M.T.; Lütjohann, D.; Wunderlich, P.; et al. Statins Promote the Degradation of Extracellular Amyloid β-Peptide by Microglia via Stimulation of Exosome-associated Insulin-degrading Enzyme (IDE) Secretion. J. Biol. Chem. 2010, 285, 37405–37414. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, M.; Parolisi, R.; Scaroni, F.; Bonfanti, E.; Gualerzi, A.; Gabrielli, M.; de Rosbo, N.K.; Uccelli, A.; Giussani, P.; Viani, P.; et al. Detrimental and protective action of microglial extracellular vesicles on myelin lesions: Astrocyte involvement in remyelination failure. Acta Neuropathol. 2019, 138, 987–1012. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Drago, F.; Lombardi, M.; Prada, I.; Gabrielli, M.; Joshi, P.; Cojoc, D.; Franck, J.; Fournier, I.; Vizioli, J.; Verderio, C. ATP Modifies the Proteome of Extracellular Vesicles Released by Microglia and Influences Their Action on Astrocytes. Front. Pharmacol. 2017, 8, 910. [Google Scholar] [CrossRef]
- Basso, M.; Pozzi, S.; Tortarolo, M.; Fiordaliso, F.; Bisighini, C.; Pasetto, L.; Spaltro, G.; Lidonnici, D.; Gensano, F.; Battaglia, E.; et al. Mutant Copper-Zinc Superoxide Dismutase (SOD1) Induces Protein Secretion Pathway Alterations and Exosome Release in Astrocytes. J. Biol. Chem. 2013, 288, 15699–15711. [Google Scholar] [CrossRef]
- Wang, G.; Dinkins, M.; He, Q.; Zhu, G.; Poirier, C.; Campbell, A.; Mayer-Proschel, M.; Bieberich, E. Astrocytes Secrete Exosomes Enriched with Proapoptotic Ceramide and Prostate Apoptosis Response 4 (PAR-4). J. Biol. Chem. 2012, 287, 21384–21395. [Google Scholar] [CrossRef]
- Goetzl, E.J.; Schwartz, J.B.; Abner, E.L.; Jicha, G.A.; Kapogiannis, D. High complement levels in astrocyte-derived exosomes of Alzheimer disease. Ann. Neurol. 2018, 83, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Sinha, M.S.; Ansell-Schultz, A.; Civitelli, L.; Hildesjö, C.; Larsson, M.; Lannfelt, L.; Ingelsson, M.; Hallbeck, M. Alzheimer’s disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol. 2018, 136, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Guix, F.X.; Corbett, G.T.; Cha, D.J.; Mustapic, M.; Liu, W.; Mengel, D.; Chen, Z.; Aikawa, E.; Young-Pearse, T.; Kapogiannis, D.; et al. Detection of Aggregation-Competent Tau in Neuron-Derived Extracellular Vesicles. Int. J. Mol. Sci. 2018, 19, 663. [Google Scholar] [CrossRef]
- Asai, H.; Ikezu, S.; Tsunoda, S.; Medalla, M.; Luebke, J.; Haydar, T.; Wolozin, B.; Butovsky, O.; Kügler, S.; Ikezu, T. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat. Neurosci. 2015, 18, 1584–1593. [Google Scholar] [CrossRef]
- Grey, M.; Dunning, C.J.; Gaspar, R.; Grey, C.; Brundin, P.; Sparr, E.; Linse, S. Acceleration of α-Synuclein Aggregation by Exosomes. J. Biol. Chem. 2015, 290, 2969–2982. [Google Scholar] [CrossRef]
- Guo, M.; Wang, J.; Zhao, Y.; Feng, Y.; Han, S.; Dong, Q.; Cui, M.; Tieu, K. Microglial exosomes facilitate α-synuclein transmission in Parkinson’s disease. Brain 2020, 143, 1476–1497. [Google Scholar] [CrossRef] [PubMed]
- Emmanouilidou, E.; Melachroinou, K.; Roumeliotis, T.; Garbis, S.D.; Ntzouni, M.; Margaritis, L.H.; Stefanis, L.; Vekrellis, K. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 6838–6851. [Google Scholar] [CrossRef]
- Xia, X.; Wang, Y.; Huang, Y.; Zhang, H.; Lu, H.; Zheng, J.C. Exosomal miRNAs in central nervous system diseases: Biomarkers, pathological mediators, protective factors and therapeutic agents. Prog. Neurobiol. 2019, 183, 101694. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, J.; Chen, L.; Jin, Y.; Zhang, G.; Lin, Z.; Du, S.; Fu, Z.; Chen, T.; Qin, Y.; et al. Serum secreted miR-137-containing exosomes affects oxidative stress of neurons by regulating OXR1 in Parkinson’s disease. Brain Res. 2019, 1722, 146331. [Google Scholar] [CrossRef]
- Prada, I.; Gabrielli, M.; Turola, E.; Iorio, A.; D’arrigo, G.; Parolisi, R.; De Luca, M.; Pacifici, M.; Bastoni, M.; Lombardi, M.; et al. Glia-to-neuron transfer of miRNAs via extracellular vesicles: A new mechanism underlying inflammation-induced synaptic alterations. Acta Neuropathol. 2018, 135, 529–550. [Google Scholar] [CrossRef]
- Illes, S. More than a drainage fluid: The role of CSF in signaling in the brain and other effects on brain tissue. Handb. Clin. Neurol. 2018, 146, 33–46. [Google Scholar] [CrossRef]
- Teuber-Hanselmann, S.; Rekowski, J.; Vogelgsang, J.; von Arnim, C.; Reetz, K.; Stang, A.; Jöckel, K.-H.; Wiltfang, J.; Esselmann, H.; Otto, M.; et al. CSF and blood Kallikrein-8: A promising early biomarker for Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2019, 91, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zlokovic, B.V. Remote control of BBB: A tale of exosomes and microRNA. Cell Res. 2017, 27, 849–850. [Google Scholar] [CrossRef]
- Subra, C.; Laulagnier, K.; Perret, B.; Record, M. Exosome lipidomics unravels lipid sorting at the level of multivesicular bodies. Biochimie 2006, 89, 205–212. [Google Scholar] [CrossRef]
- Zhao, Z.-H.; Chen, Z.-T.; Zhou, R.-L.; Zhang, X.; Ye, Q.-Y.; Wang, Y.-Z. Increased DJ-1 and α-Synuclein in Plasma Neural-Derived Exosomes as Potential Markers for Parkinson’s Disease. Front. Aging Neurosci. 2019, 10, 438. [Google Scholar] [CrossRef]
- Eren, E.; Hunt, J.F.V.; Shardell, M.; Chawla, S.; Tran, J.; Gu, J.; Vogt, N.M.; Johnson, S.C.; Bendlin, B.B.; Kapogiannis, D. Extracellular vesicle biomarkers of Alzheimer’s disease associated with sub-clinical cognitive decline in late middle age. Alzheimer’s Dement. 2020, 16, 1293–1304. [Google Scholar] [CrossRef]
- Devitt, G.; Howard, K.; Mudher, A.; Mahajan, S. Raman Spectroscopy: An Emerging Tool in Neurodegenerative Disease Research and Diagnosis. ACS Chem. Neurosci. 2018, 9, 404–420. [Google Scholar] [CrossRef]
- Fevrier, B.; Vilette, D.; Archer, F.; Loew, D.; Faigle, W.; Vidal, M.; Laude, H.; Raposo, G. Cells release prions in association with exosomes. Proc. Natl. Acad. Sci. USA 2004, 101, 9683–9688. [Google Scholar] [CrossRef]
- Rajendran, L.; Honsho, M.; Zahn, T.R.; Keller, P.; Geiger, K.D.; Verkade, P.; Simons, K. Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc. Natl. Acad. Sci. USA 2006, 103, 11172–11177. [Google Scholar] [CrossRef]
- Perez-Gonzalez, R.; Gauthier, S.A.; Kumar, A.; Levy, E. The Exosome Secretory Pathway Transports Amyloid Precursor Protein Carboxyl-terminal Fragments from the Cell into the Brain Extracellular Space. J. Biol. Chem. 2012, 287, 43108–43115. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.; Turola, E.; Ruiz, A.; Bergami, A.; Libera, D.D.; Benussi, L.; Giussani, P.; Magnani, G.; Comi, G.; Legname, G.; et al. Microglia convert aggregated amyloid-β into neurotoxic forms through the shedding of microvesicles. Cell Death Differ. 2013, 21, 582–593. [Google Scholar] [CrossRef]
- Jia, L.; Zhu, M.; Kong, C.; Pang, Y.; Zhang, H.; Qiu, Q.; Wei, C.; Tang, Y.; Wang, Q.; Li, Y.; et al. Blood neuro-exosomal synaptic proteins predict Alzheimer’s disease at the asymptomatic stage. Alzheimer’s Dement. 2020, 17, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Goetzl, E.J.; Kapogiannis, D.; Schwartz, J.B.; Lobach, I.V.; Goetzl, L.; Abner, E.L.; Jicha, G.A.; Karydas, A.M.; Boxer, A.; Miller, B.L. Decreased synaptic proteins in neuronal exosomes of frontotemporal dementia and Alzheimer’s disease. FASEB J. 2016, 30, 4141–4148. [Google Scholar] [CrossRef] [PubMed]
- Goetzl, E.J.; Mustapic, M.; Kapogiannis, D.; Eitan, E.; Lobach, I.V.; Goetzl, L.; Schwartz, J.B.; Miller, B.L. Cargo proteins of plasma astrocyte-derived exosomes in Alzheimer’s disease. FASEB J. 2016, 30, 3853–3859. [Google Scholar] [CrossRef] [PubMed]
- Winston, C.N.; Goetzl, E.J.; Akers, J.C.; Carter, B.S.; Rockenstein, E.M.; Galasko, D.; Masliah, E.; Rissman, R.A. Prediction of conversion from mild cognitive impairment to dementia with neuronally derived blood exosome protein profile. Alzheimer’s Dement. 2016, 3, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Z.; Ruan, Z.; Pathak, D.; Pathak, D.; Kalavai, S.V.; Kalavai, S.V.; Yoshii-Kitahara, A.; Yoshii-Kitahara, A.; Muraoka, S.; Muraoka, S.; et al. Alzheimer’s disease brain-derived extracellular vesicles spread tau pathology in interneurons. Brain 2020, 144, 288–309. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Balaji, V.; Kaniyappan, S.; Krüger, L.; Irsen, S.; Tepper, K.; Chandupatla, R.; Maetzler, W.; Schneider, A.; Mandelkow, E.; et al. The release and trans-synaptic transmission of Tau via exosomes. Mol. Neurodegener. 2017, 12, 5. [Google Scholar] [CrossRef]
- Winston, C.N.; Aulston, B.; Rockenstein, E.M.; Adame, A.; Prikhodko, O.; Dave, K.N.; Mishra, P.; Rissman, R.A.; Yuan, S.H. Neuronal Exosome-Derived Human Tau is Toxic to Recipient Mouse Neurons in vivo. J. Alzheimer’s Dis. 2019, 67, 541–553. [Google Scholar] [CrossRef]
- Fiandaca, M.S.; Kapogiannis, D.; Mapstone, M.; Boxer, A.; Eitan, E.; Schwartz, J.B.; Abner, E.L.; Petersen, R.C.; Federoff, H.J.; Miller, B.L.; et al. Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimer’s Dement. 2015, 11, 600–607.e1. [Google Scholar] [CrossRef] [PubMed]
- Goetzl, E.J.; Boxer, A.; Schwartz, J.B.; Abner, E.L.; Petersen, R.C.; Miller, B.L.; Carlson, O.D.; Mustapic, M.; Kapogiannis, D. Low neural exosomal levels of cellular survival factors in Alzheimer’s disease. Ann. Clin. Transl. Neurol. 2015, 2, 769–773. [Google Scholar] [CrossRef]
- Goetzl, E.J.; Boxer, A.; Schwartz, J.B.; Abner, E.L.; Petersen, R.C.; Miller, B.L.; Kapogiannis, D. Altered lysosomal proteins in neural-derived plasma exosomes in preclinical Alzheimer disease. Neurology 2015, 85, 40–47. [Google Scholar] [CrossRef]
- Lim, W.Q.; Luk, K.H.M.; Lee, K.Y.; Nurul, N.; Loh, S.J.; Yeow, Z.X.; Wong, Q.X.; Looi, Q.H.D.; Chong, P.P.; How, C.W.; et al. Small Extracellular Vesicles’ miRNAs: Biomarkers and Therapeutics for Neurodegenerative Diseases. Pharmaceutics 2023, 15, 1216. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Doecke, J.D.; A Sharples, R.; Villemagne, V.L.; Fowler, C.J.; Rembach, A.; Martins, R.N.; Rowe, C.C.; Macaulay, S.L.; Masters, C.L.; et al. Prognostic serum miRNA biomarkers associated with Alzheimer’s disease shows concordance with neuropsychological and neuroimaging assessment. Mol. Psychiatry 2014, 20, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Ting, T.T.; Geng, C.G.; Chao, S.C.; Zhang, Y.; Wang, P.C. The Serum Exosome Derived MicroRNA-135a,-193b, and-384 Were Potential Alzheimer’s Disease Biomarkers. Biomed. Environ. Sci. 2018, 31, 87–96. [Google Scholar] [CrossRef]
- Cone, A.S.; Yuan, X.; Sun, L.; Duke, L.C.; Vreones, M.P.; Carrier, A.N.; Kenyon, S.M.; Carver, S.R.; Benthem, S.D.; Stimmell, A.C.; et al. Mesenchymal stem cell-derived extracellular vesicles ameliorate Alzheimer’s disease-like phenotypes in a preclinical mouse model. Theranostics 2021, 11, 8129–8142. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, Y.; Eid, L.; Parent, M.; Soucy, G.; Bareil, C.; Riku, Y.; Kawai, K.; Takagi, S.; Yoshida, M.; Katsuno, M.; et al. Exosome secretion is a key pathway for clearance of pathological TDP-43. Brain 2016, 139, 3187–3201. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloem, B.R. The Emerging Evidence of the Parkinson Pandemic. J. Park. Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef]
- Tysnes, O.-B.; Storstein, A. Epidemiology of Parkinson’s disease. J. Neural Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Hopfner, F.; Katsikoudi, A.; Hein, R.; Catli, C.; Evetts, S.; Huang, Y.; Wang, H.; Ryder, J.W.; Kuhlenbaeumer, G.; et al. Serum neuronal exosomes predict and differentiate Parkinson’s disease from atypical parkinsonism. J. Neurol. Neurosurg. Psychiatry 2020, 91, 720–729. [Google Scholar] [CrossRef]
- Zheng, H.; Xie, Z.; Zhang, X.; Mao, J.; Wang, M.; Wei, S.; Fu, Y.; Zheng, H.; He, Y.; Chen, H.; et al. Investigation of α-Synuclein Species in Plasma Exosomes and the Oligomeric and Phosphorylated α-Synuclein as Potential Peripheral Biomarker of Parkinson’s Disease. Neuroscience 2021, 469, 79–90. [Google Scholar] [CrossRef]
- Niu, M.; Li, Y.; Li, G.; Zhou, L.; Luo, N.; Yao, M.; Kang, W.; Liu, J. A longitudinal study on α-synuclein in plasma neuronal exosomes as a biomarker for Parkinson’s disease development and progression. Eur. J. Neurol. 2020, 27, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Xiong, N.; Guo, X.; Huang, J.; Ma, K.; Liu, L.; Xia, Y.; Shen, Y.; Li, J.; Jiang, H.; et al. Exosomes from patients with Parkinson’s disease are pathological in mice. J. Mol. Med. 2019, 97, 1329–1344. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-Y.; Yang, C.-C.; Chang, C.-W.; Wu, Y.-R. Plasma and Serum Alpha-Synuclein as a Biomarker of Diagnosis in Patients With Parkinson’s Disease. Front. Neurol. 2020, 10, 1388. [Google Scholar] [CrossRef]
- Xu, L.; Liu, T.; Liu, L.; Yao, X.; Chen, L.; Fan, D.; Zhan, S.; Wang, S. Global variation in prevalence and incidence of amyotrophic lateral sclerosis: A systematic review and meta-analysis. J. Neurol. 2019, 267, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.P.; Brown, R.H., Jr.; Cleveland, D.W. Decoding ALS: From genes to mechanism. Nature 2016, 539, 197–206. [Google Scholar] [CrossRef]
- Silverman, J.M.; Christy, D.; Shyu, C.C.; Moon, K.-M.; Fernando, S.; Gidden, Z.; Cowan, C.M.; Ban, Y.; Stacey, R.G.; Grad, L.I.; et al. CNS-derived extracellular vesicles from superoxide dismutase 1 (SOD1)G93A ALS mice originate from astrocytes and neurons and carry misfolded SOD1. J. Biol. Chem. 2019, 294, 3744–3759. [Google Scholar] [CrossRef]
- Chen, P.-C.; Wu, D.; Hu, C.-J.; Chen, H.-Y.; Hsieh, Y.-C.; Huang, C.-C. Exosomal TAR DNA-binding protein-43 and neurofilaments in plasma of amyotrophic lateral sclerosis patients: A longitudinal follow-up study. J. Neurol. Sci. 2020, 418, 117070. [Google Scholar] [CrossRef] [PubMed]
- Damodharan, S.; Puccetti, D. Pediatric Central Nervous System Tumor Overview and Emerging Treatment Considerations. Brain Sci. 2023, 13, 1106. [Google Scholar] [CrossRef]
- Ganz, J.C. Low grade gliomas. Prog. Brain Res. 2022, 268, 271–277. [Google Scholar]
- Ryall, S.; Tabori, U.; Hawkins, C. Pediatric low-grade glioma in the era of molecular diagnostics. Acta Neuropathol. Commun. 2020, 8, 30. [Google Scholar] [CrossRef]
- Salles, D.; Laviola, G.; Malinverni, A.C.M.; Stavale, J.N. Pilocytic Astrocytoma: A Review of General, Clinical, and Molecular Characteristics. J. Child. Neurol. 2020, 35, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Bauman, M.M.J.; Harrison, D.J.; Giesken, M.B.; Daniels, D.J. The evolving landscape of pilocytic astrocytoma: A bibliometric analysis of the top-100 most cited publications. Childs Nerv. Syst. 2022, 38, 1271–1280. [Google Scholar] [CrossRef]
- Milde, T.; Rodriguez, F.J.; Barnholtz-Sloan, J.S.; Patil, N.; Eberhart, C.G.; Gutmann, D.H. Reimagining pilocytic astrocytomas in the context of pediatric low-grade gliomas. Neuro Oncol. 2021, 23, 1634–1646. [Google Scholar] [CrossRef] [PubMed]
- Mustansir, F.; Mushtaq, N.; Darbar, A. Dabrafenib in BRAFV600E mutant pilocytic astro-cytoma in a pediatric patient. Childs Nerv. Syst. 2020, 36, 203–207. [Google Scholar] [CrossRef]
- Nicolaides, T.; Nazemi, K.J.; Crawford, J.; Kilburn, L.; Minturn, J.; Gajjar, A.; Gauvain, K.; Leary, S.; Dhall, G.; Aboian, M.; et al. Phase I study of vemurafenib in children with recurrent or progressive BRAF(V600E) mutant brain tumors: Pacific Pediatric Neuro-Oncology Consortium study (PNOC-002). Oncotarget 2020, 11, 1942–1952. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Phi, J.H.; Kim, S.K.; Lee, J.H.; Park, S.H.; Won, J.K.; Choi, J.Y.; Kang, H.J.; Park, C.K. Comparison of the clinical features and treatment outcomes of pilocytic astrocytoma in pediatric and adult patients. Childs Nerv. Syst. 2023, 39, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, A.; Stichel, D.; Schrimpf, D.; Sahm, F.; Korshunov, A.; Reuss, D.E.; Koelsche, C.; Huang, K.; Wefers, A.K.; Hovestadt, V.; et al. Anaplastic astrocytoma with piloid features, a novel molecular class of IDH wildtype glioma with recurrent MAPK pathway, CDKN2A/B and ATRX alterations. Acta Neuropathol. 2018, 136, 273–291. [Google Scholar] [CrossRef]
- Ali, R.H.; Almanabri, M.; Ali, N.Y.; Alsaber, A.R.; Khalifa, N.M.; Hussein, R.; Alateeqi, M.; Mohammed, E.M.A.; Jama, H.; Almarzooq, A.; et al. Clinicopathological analysis of BRAF and non-BRAF MAPK pathway-altered gliomas in paediatric and adult patients: A single-institution study of 40 patients. J. Clin. Pathol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, N.; Singh, A.; Kumar, P.; Kaushik, M. Protein kinases: Role of their dysregulation in carcinogenesis, identification and inhibition. Drug Res. 2023, 73, 189–199. [Google Scholar] [CrossRef]
- Migliozzi, S.; Oh, Y.T.; Hasanain, M.; Garofano, L.; D’Angelo, F.; Najac, R.D.; Picca, A.; Biel-le, F.; Di Stefano, A.L.; Lerond, J.; et al. Integrative multi-omics networks identify PKCdelta and DNA-PK as master kinases of glioblastoma subtypes and guide targeted cancer therapy. Nat. Cancer 2023, 4, 181–202. [Google Scholar] [CrossRef]
- Parker, M.I.; Nikonova, A.S.; Sun, D.; Golemis, E.A. Proliferative signaling by ERBB proteins and RAF/MEK/ERK effectors in polycystic kidney disease. Cell Signal 2020, 67, 109497. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, A.; John, V.; Wadden, J.; Kong, S.; Sharba, S.; Koschmann, C. Liquid biopsy in pediatric brain tumors. Front. Genet. 2022, 13, 1114762. [Google Scholar] [CrossRef]
- Wu, X.; Chen, W.; Lin, F.; Huang, Q.; Zhong, J.; Gao, H.; Song, Y.; Liang, H. DNA methyla-tion profile is a quantitative measure of biological aging in children. Aging 2019, 11, 10031–10051. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.D.; Young, A.M.; Karri, S.K. Biomarkers of pediatric brain tumors. Front. Pediatr. 2013, 1, 7. [Google Scholar] [CrossRef]
- Mirian, C.; Thastrup, M.; Mathiasen, R.; Schmiegelow, K.; Olsen, J.V.; Ostergaard, O. Mass spectrometry-based proteomics of cerebrospinal fluid in pediatric central nervous system malignancies: A systematic review with meta-analysis of individual patient data. Fluids Barriers CNS 2024, 21, 14. [Google Scholar] [CrossRef]
- Bruschi, M.; Petretto, A.; Cama, A.; Pavanello, M.; Bartolucci, M.; Morana, G.; Ramenghi, L.A.; Garré, M.L.; Ghiggeri, G.M.; Panfoli, I.; et al. Potential biomarkers of childhood brain tumor identified by proteomics of cerebrospinal fluid from extraventricular drainage (EVD). Sci. Rep. 2021, 11, 1818. [Google Scholar] [CrossRef]
- Bruschi, M.; Kajana, X.; Petretto, A.; Bartolucci, M.; Pavanello, M.; Ghiggeri, G.M.; Panfoli, I.; Candiano, G. Weighted Gene Co-Expression Network Analysis and Support Vector Machine Learning in the Proteomic Profiling of Cerebrospinal Fluid from Extraventricular Drainage in Child Medulloblastoma. Metabolites 2022, 12, 724. [Google Scholar] [CrossRef]
- Kajana, X.; Spinelli, S.; Garbarino, A.; Balagura, G.; Bartolucci, M.; Petretto, A.; Pavanello, M.; Candiano, G.; Panfoli, I.; Bruschi, M. Identification of Central Nervous System Oncologic Disease Biomarkers in EVs from Cerebrospinal Fluid (CSF) of Pediatric Patients: A Pilot Neuro-Proteomic Study. Biomolecules 2023, 13, 1730. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, S.; Kajana, X.; Garbarino, A.; Bartolucci, M.; Petretto, A.; Pavanello, M.; Verrina, E.; Candiano, G.; Panfoli, I.; Bruschi, M. Proteomic Profiling of Cerebrospinal Fluid and Its Extracel-lular Vesicles from Extraventricular Drainage in Pediatric Pilocytic Astrocytoma, towards Precision Oncology. Cancers 2024, 16, 1223. [Google Scholar] [CrossRef] [PubMed]
- Lamptey, R.N.L.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A Review of the Common Neurodegenerative Disorders: Current Therapeutic Approaches and the Potential Role of Nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Wu, J.; Wang, J.; Li, Y.; Hu, X.; Luo, S.; Xiang, D. Extracellular vesicles derived from different sources of mesenchymal stem cells: Therapeutic effects and translational potential. Cell Biosci. 2020, 10, 69. [Google Scholar] [CrossRef]
- Sivanantham, A.; Jin, Y. Impact of Storage Conditions on EV Integrity/Surface Markers and Cargos. Life 2022, 12, 697. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Zheng, X.; Jin, H.; Yu, F.; Zhao, W. Exosomes as CNS Drug Delivery Tools and Their Applications. Pharmaceutics 2022, 14, 2252. [Google Scholar] [CrossRef]
- Liu, Y.; Huber, C.C.; Wang, H. Disrupted blood-brain barrier in 5xFAD mouse model of Alzheimer’s disease can be mimicked and repaired in vitro with neural stem cell-derived exosomes. Biochem. Biophys. Res. Commun. 2020, 525, 192–196. [Google Scholar] [CrossRef]
- Zhang, D.; Lee, H.; Zhu, Z.; Minhas, J.K.; Jin, Y. Enrichment of selective miRNAs in exosomes and delivery of exosomal miRNAs in vitro and in vivo. Am. J. Physiol. Cell. Mol. Physiol. 2017, 312, L110–L121. [Google Scholar] [CrossRef]
- Yamamoto, M.; Guo, D.-H.; Hernandez, C.M.; Stranahan, A.M. Endothelial Adora2a Activation Promotes Blood–Brain Barrier Breakdown and Cognitive Impairment in Mice with Diet-Induced Insulin Resistance. J. Neurosci. 2019, 39, 4179–4192. [Google Scholar] [CrossRef] [PubMed]
- Huber, C.C.; Callegari, E.A.; Paez, M.D.; Romanova, S.; Wang, H. Heat Shock-Induced Extracellular Vesicles Derived from Neural Stem Cells Confer Marked Neuroprotection Against Oxidative Stress and Amyloid-β-Caused Neurotoxicity. Mol. Neurobiol. 2022, 59, 7404–7412. [Google Scholar] [CrossRef] [PubMed]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Transl. Res. Clin. Interv. 2018, 4, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; An, N.; Ren, Y.; Yang, C.; Zhang, X.; Li, L. Immunosuppressive Effects of Mesenchymal Stem Cells-derived Exosomes. Stem Cell Rev. Rep. 2020, 17, 411–427. [Google Scholar] [CrossRef]
- Ding, M.; Shen, Y.; Wang, P.; Xie, Z.; Xu, S.; Zhu, Z.; Wang, Y.; Lyu, Y.; Wang, D.; Xu, L.; et al. Exosomes Isolated From Human Umbilical Cord Mesenchymal Stem Cells Alleviate Neuroinflammation and Reduce Amyloid-Beta Deposition by Modulating Microglial Activation in Alzheimer’s Disease. Neurochem. Res. 2018, 43, 2165–2177. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Chen, Z.; Zhang, M. Role of exosomes in the pathogenesis, diagnosis, and treatment of central nervous system diseases. J. Transl. Med. 2022, 20, 291. [Google Scholar] [CrossRef]
- Qu, M.; Lin, Q.; Huang, L.; Fu, Y.; Wang, L.; He, S.; Fu, Y.; Yang, S.; Zhang, Z.; Zhang, L.; et al. Dopamine-loaded blood exosomes targeted to brain for better treatment of Parkinson’s disease. J. Control. Release 2018, 287, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, Z.; Zuo, H.; Chen, H.; Gui, Y. Mesenchymal stem cell-derived exosomes altered neuron cholesterol metabolism via Wnt5a-LRP1 axis and alleviated cognitive impairment in a progressive Parkinson’s disease model. Neurosci. Lett. 2022, 787, 136810. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Zhang, M.; Tan, Z. Bone Marrow Stem Cell-Exo-Derived TSG-6 Attenuates 1-Methyl-4-Phenylpyridinium+-Induced Neurotoxicity via the STAT3/miR-7/NEDD4/LRRK2 Axis. J. Neuropathol. Exp. Neurol. 2022, 81, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.Y.; Kim, D.-Y.; Lee, J.; Shin, Y.J.; Kim, Y.S.; Lee, P.H. Priming mesenchymal stem cells with α-synuclein enhances neuroprotective properties through induction of autophagy in Parkinsonian models. Stem Cell Res. Ther. 2022, 13, 483. [Google Scholar] [CrossRef]
- Bonafede, R.; Turano, E.; Scambi, I.; Busato, A.; Bontempi, P.; Virla, F.; Schiaffino, L.; Marzola, P.; Bonetti, B.; Mariotti, R. ASC-Exosomes Ameliorate the Disease Progression in SOD1(G93A) Murine Model Underlining Their Potential Therapeutic Use in Human ALS. Int. J. Mol. Sci. 2020, 21, 3651. [Google Scholar] [CrossRef]
- Calabria, E.; Scambi, I.; Bonafede, R.; Schiaffino, L.; Peroni, D.; Potrich, V.; Capelli, C.; Schena, F.; Mariotti, R. ASCs-Exosomes Recover Coupling Efficiency and Mitochondrial Membrane Potential in an in vitro Model of ALS. Front. Neurosci. 2019, 13, 1070. [Google Scholar] [CrossRef]
- Yuyama, K.; Igarashi, Y. Physiological and pathological roles of exosomes in the nervous system. Biomol. Concepts 2016, 7, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Mehra, A.; Arora, S.; Gugulothu, D.; Vora, L.K.; Prasad, R.; Khatri, D.K. Exosome-mediated delivery and regulation in neurological disease progression. Int. J. Biol. Macromol. 2024, 264, 130728. [Google Scholar] [CrossRef]
- Kanninen, K.M.; Bister, N.; Koistinaho, J.; Malm, T. Exosomes as new diagnostic tools in CNS diseases. Biochim. Biophys. Acta. 2016, 1862, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Fan, Y.; Zhang, P.; Liu, H.; Han, J.; Yu, Q.; Wang, X.; Wu, S.; Lu, Z. Biomarkers of Synaptic Degeneration in Alzheimer’s Disease. Ageing Res. Rev. 2024, 102642, 104. [Google Scholar] [CrossRef] [PubMed]
- Chandran, D.; Krishnan, S.; Urulangodi, M.; Gopala, S. Exosomal microRNAs in Parkinson’s disease: Insights into biomarker potential and disease pathology. Neurol. Sci. 2024, 8, 3625–3639. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Luo, S.; Li, Q.; Song, G.; Tian, X.; Wang, P.; Zhu, H.; Lv, S.; Zhang, X.; Yang, Z.; et al. The efficacy of plasma exosomal miRNAs as predictive biomarkers for PD-1 blockade plus chemotherapy in gastric cancer. Transl Cancer Res. 2024, 13, 6336–6346. [Google Scholar] [CrossRef]
| ISOLATION TECHNOLOGY | SEPARATION PRINCIPLE | SAMPLE SIZE | ADVANTAGES | DISADVANTAGES |
|---|---|---|---|---|
| Ultracentrifugation | Molecular size, density, and shape | Large | Low risk of pollution, low reagent cost | Expensive equipment, time-consuming, poor biological activity, and integrity of exosomes |
| Size exclusion chromatography | Molecular size | Medium | Yield, purity, integrity, and biological activity of exosomes can be ensured | Special equipment |
| Immune-affinity capture | Specific binding of antigen and antibody | Small | High purity | High cost, low yield |
| Ultrafiltration | Molecular size and shape | Large | Efficient and convenient | Low purity, exosomes may partially remain on the membrane |
| Microfluidic | Immune affinity, size, and density | Small | Fast, low cost, convenient, and automated | The selectivity and specificity need to be verified |
| Function | Details | Examples |
|---|---|---|
| Intercellular Communication | Facilitate exchange of proteins, lipids, RNA, and other molecules between CNS cells. | Neuron–glia signaling, astrocyte support. |
| CNS Development | Contribute to synapse formation, neuronal differentiation, and neural circuit organization. | Role in neurogenesis during brain development. |
| Homeostasis Maintenance | Regulate cellulular processes like waste clarance and immune response. | Clearing misfolded proteins via microglial exosomes. |
| Pathological Progression | Promote or inhibit disease progression depending on cargo and context. | Spread of toxic proteins in Alzheimer’s and Parkinson’s Diseases. |
| Biomarker Potential | Detectable in peripheral blood, providing non-invasive diagnostic tools for CNS disorders. | Exosomes carrying amyloid-beta or alpha-synuclein. |
| Therapeutic Potential | Deliver neuroprotective molecules and modulate inflammation or repair mechanisms. | MSC-derived exosomes for stroke recovery. |
| Clinical Trial No. (CT) | Phase | Trial Name | Pathological Condition | Intervention |
|---|---|---|---|---|
| NCT05370105 | 1 | EVs as Stroke Biomarkers (EXO4STROKE) | Stroke | Blood withdrawal |
| NCT01811381 | 2 | Curcumin and yoga therapy for those at risk for AD | AD | Drug: Curcumin Behavior: Aerobic yoga Behavior: Non-aerobic yoga |
| NCT05490173 | Not applicable | Long-term Regular Tai Chi Training for Healthy Elderly Circulating EXOs Release and Cognitive Neural Circuits/Networks Activity Characteristics Research | Cognitive | Long-term irregular exercise group |
| ChiCTR2200057303 | Retrospective study | A single-center randomized controlled study of human neural stem cell-derived EXOs in the treatment of ischemic stroke | Ischemic stroke | Treatment group: EXOs Control group: Saline |
| ChiCTR2100048661 | Retrospective study | Differential diagnosis of unipolar depression and bipolar depression based on neurogenic exosome miRNA | Depression | Gold standard: Hamilton Depression Scale—17 items, Young’s Manic Scale, DSM-5, M.I.N.I scale; index test: Methods—Neurogenic EXOs were isolated and miRNA was sequenced; biomarker: Neurogenic exosome miRNA; equipment: Illumina MiSeq |
| ChiCTR2100044323 | 1 | EXOs alterations following electroconvulsive therapy in depression | Depression | Depression cases: Electroconvulsive therapy |
| ChiCTR2000039377 | 1 | EXOs derived from Neural stem cell Induces Osteogenesis and angiogenesis following traumatic brain injury | TBI | Healthy patient group: Nil; patient with limb fracture only: Nil; patient with TBI only: Nil; patient with limb fracture following TBI: Nil |
| ChiCTR2000038262 | Retrospective study | The effects on circRNAs’ expression in the plasma EXOs of patients with Perioperative Neurocognitive Disorders after noncardiac surgery | Cognitive disorders | Control group: After induction of anesthesia, 0.9%NS was injected under load, and then 0.9%NS was continuously pumped into the suture; trial group: After anesthesia induction, 0.25 mg/kg S-ketamine was injected under load, and 0.125 mg/kg/h S-ketamine was continuously pumped until the suture |
| ChiCTR2000032579 | Retrospective study | The Safety and the Efficacy Evaluation of Allogenic Adipose MSC-Exos in Patients with AD | AD | Low-dose group: 5 μg MSC-Exos administrated for nasal drip; mid-dose group: 10 μg MSC-Exos administrated for nasal drip; high-dose group: 20 μg MSC-Exos administrated for nasal drip |
| NCT04202770 | 1 | Focused Ultrasound and EXOs to Treat Depression, Anxiety, and Dementias | Anxiety and dementia | EXOs |
| ChiCTR1900026776 | 1 | Screening for early diagnosis biomarkers of mental disorders in serum EXOs | Mental disorders | N/A |
| NCT05886205 | 1 | Induced Pluripotent Stem Cell Derived EXOs Nasal Drops for the Treatment of Refractory Focal Epilepsy | Epilepsy | Drug: iPSC-Exos |
| ChiCTR2200064447 | Retrospective study | Study on the mechanism of exosome miRNA mediated autophagy in temporal lobe epilepsy | Epilepsy | Oxcarbazepine group: Take oxcarbazepine; oxcarbazepine + CLMD group: Take oxcarbazepine + CLMD; control group: None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spinelli, S.; Tripodi, D.; Corti, N.; Zocchi, E.; Bruschi, M.; Leoni, V.; Dominici, R. Roles, Functions, and Pathological Implications of Exosomes in the Central Nervous System. Int. J. Mol. Sci. 2025, 26, 1345. https://doi.org/10.3390/ijms26031345
Spinelli S, Tripodi D, Corti N, Zocchi E, Bruschi M, Leoni V, Dominici R. Roles, Functions, and Pathological Implications of Exosomes in the Central Nervous System. International Journal of Molecular Sciences. 2025; 26(3):1345. https://doi.org/10.3390/ijms26031345
Chicago/Turabian StyleSpinelli, Sonia, Domenico Tripodi, Nicole Corti, Elena Zocchi, Maurizio Bruschi, Valerio Leoni, and Roberto Dominici. 2025. "Roles, Functions, and Pathological Implications of Exosomes in the Central Nervous System" International Journal of Molecular Sciences 26, no. 3: 1345. https://doi.org/10.3390/ijms26031345
APA StyleSpinelli, S., Tripodi, D., Corti, N., Zocchi, E., Bruschi, M., Leoni, V., & Dominici, R. (2025). Roles, Functions, and Pathological Implications of Exosomes in the Central Nervous System. International Journal of Molecular Sciences, 26(3), 1345. https://doi.org/10.3390/ijms26031345






