The Role of Molecular Profiling in De-Escalation of Toxic Therapy in Breast Cancer
Abstract
1. Introduction
2. Progression of Evidence
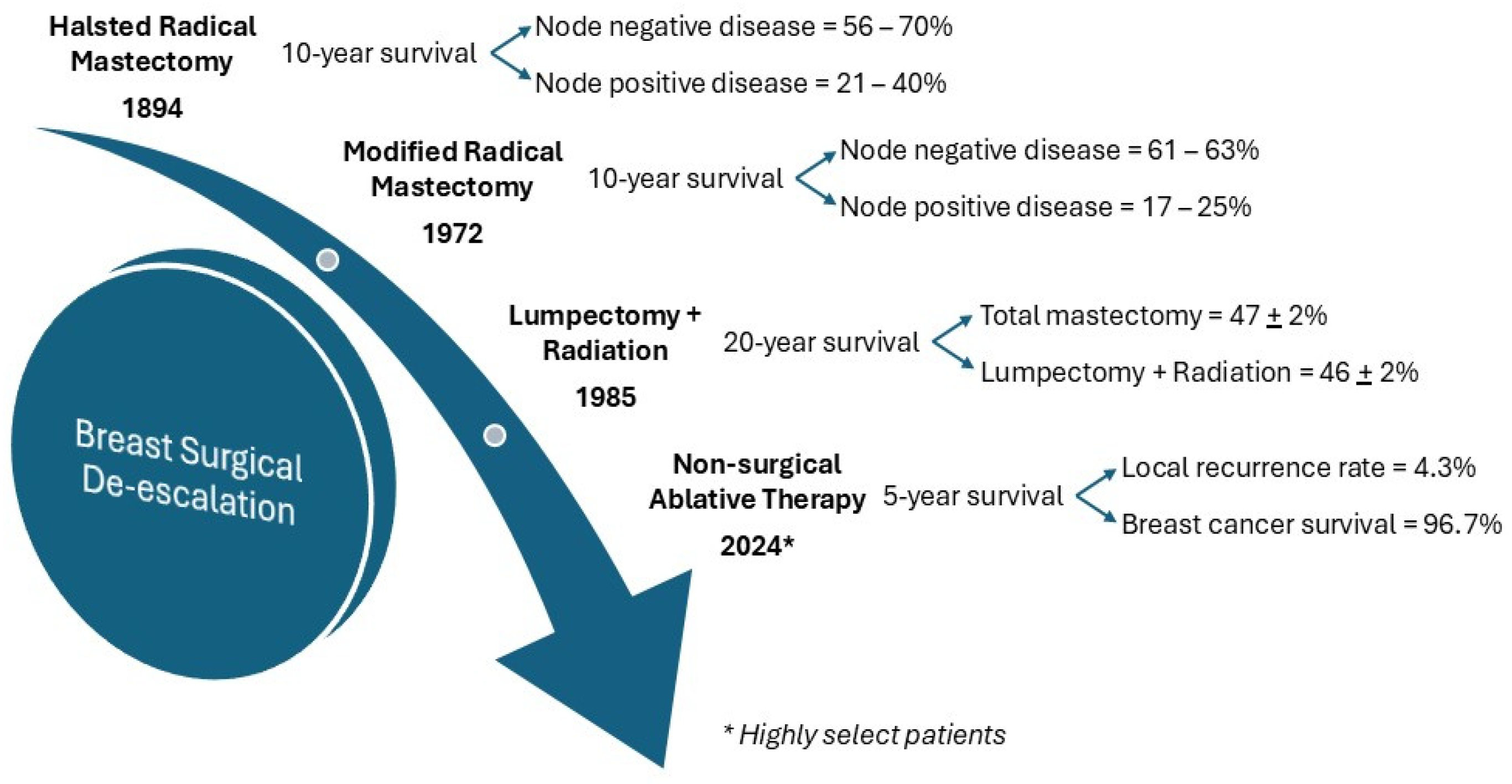
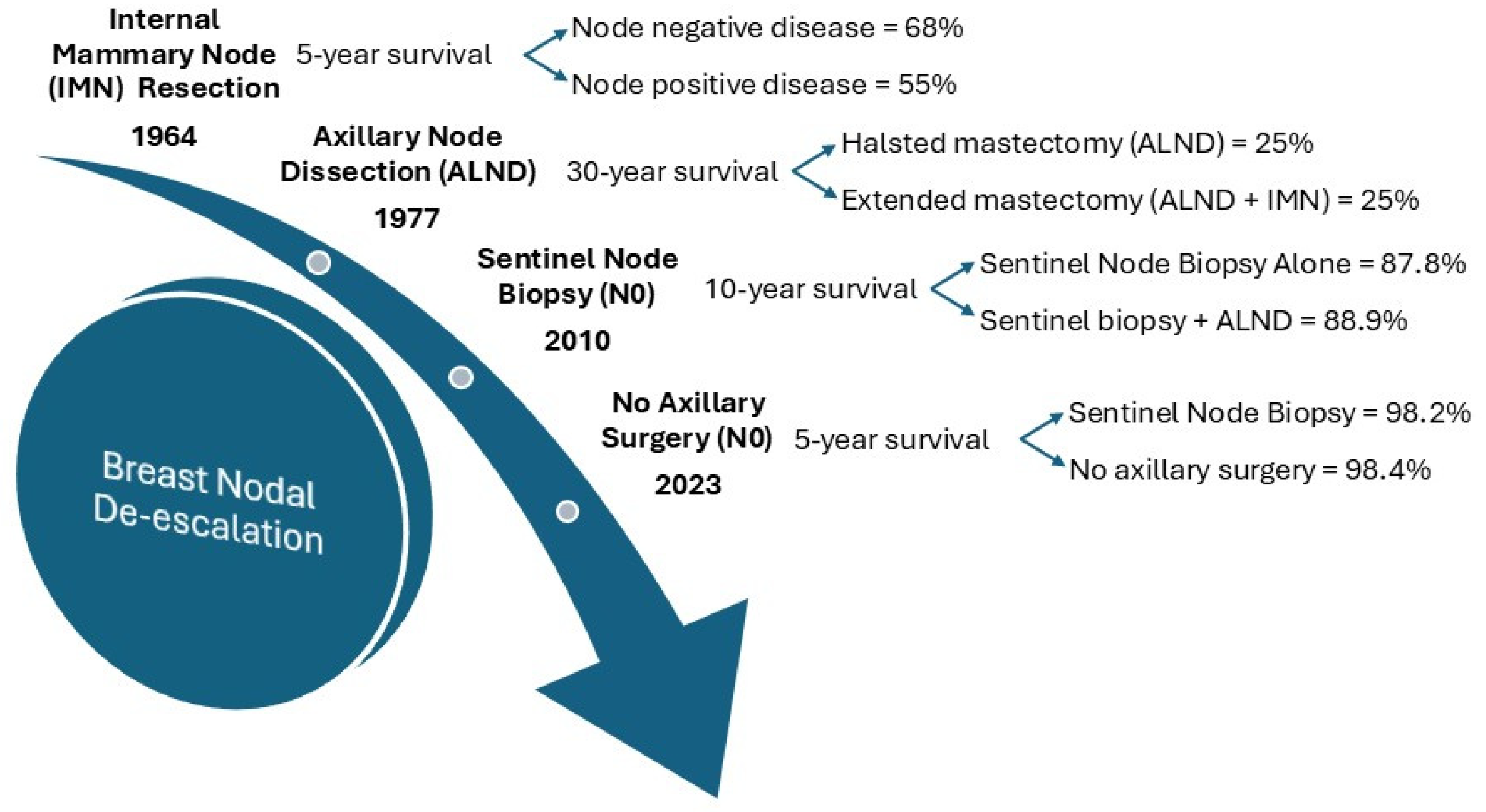
2.1. De-Escalation of Surgery
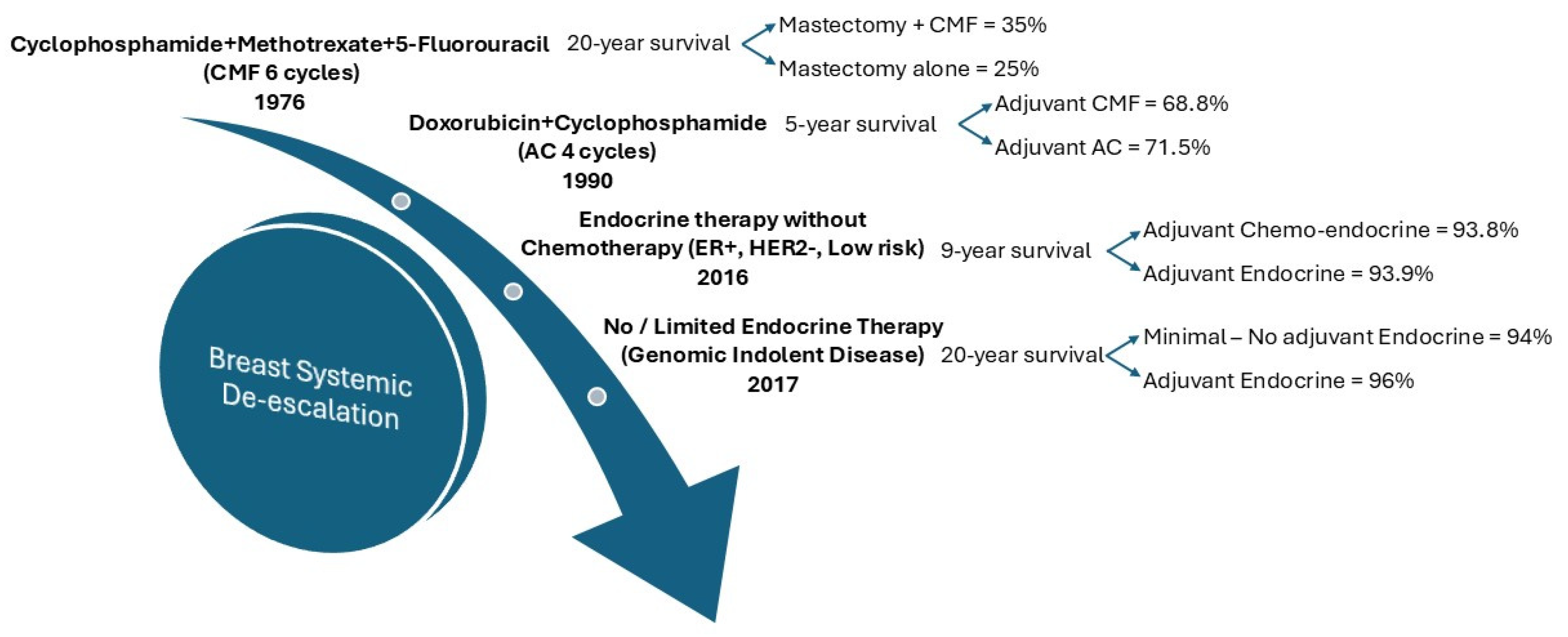
2.2. De-Escalation of Chemo-Endocrine Therapy
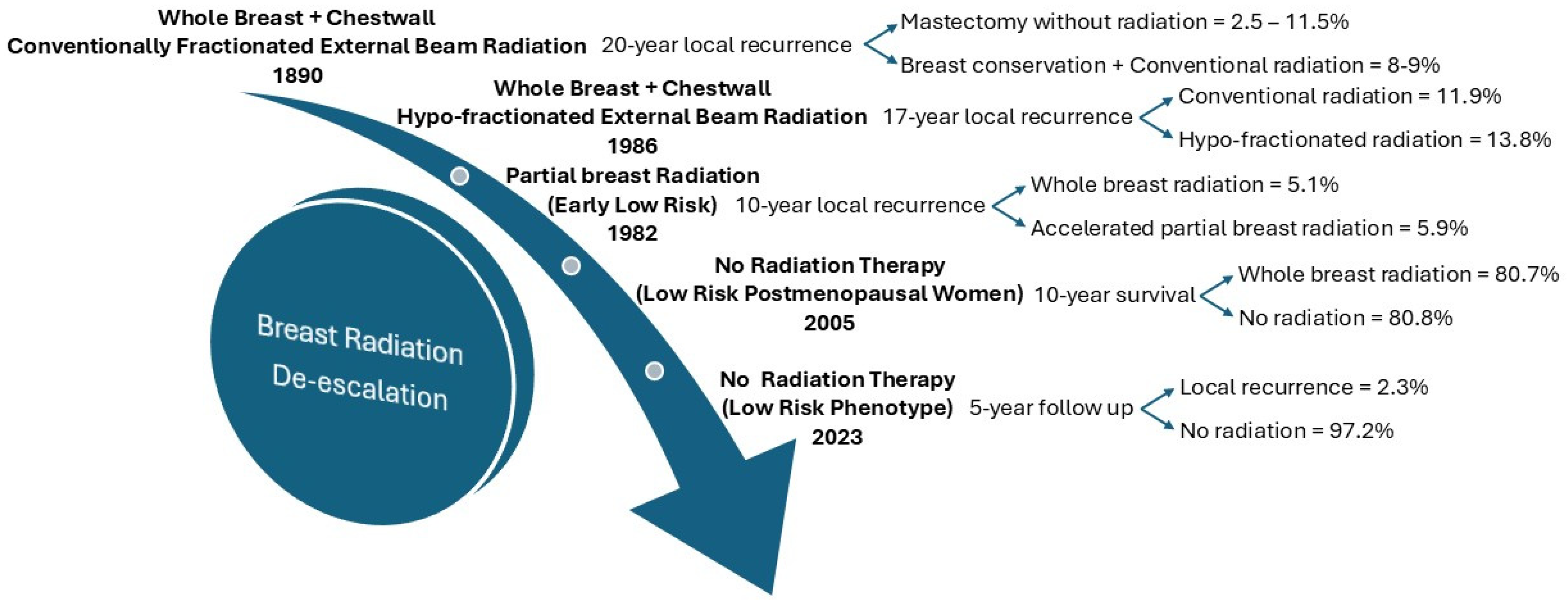
2.3. De-Escalation of Radiation Therapy
3. Molecular Profiling Options
4. Trials of the Future
4.1. Design
4.2. Implementation
4.3. Limitations of Genomic Profiling
4.4. Future Directions
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Fisher, B.; Anderson, S.; Bryant, J.; Margolese, R.G.; Deutsch, M.; Fisher, E.R.; Jeong, J.H.; Wolmark, N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N. Engl. J. Med. 2002, 347, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.; Anderson, S. Conservative surgery for the management of invasive and noninvasive carcinoma of the breast: NSABP trials. National Surgical Adjuvant Breast and Bowel Project. World J. Surg. 1994, 18, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Halsted, W.S.I. The Results of Operations for the Cure of Cancer of the Breast Performed at the Johns Hopkins Hospital from June, 1889, to January, 1894. Ann. Surg. 1894, 20, 497–555. [Google Scholar] [CrossRef] [PubMed]
- Madden, J.L.; Kandalaft, S.; Bourque, R.A. Modified radical mastectomy. Ann. Surg. 1972, 175, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B. United States trials of conservative surgery. World J. Surg. 1977, 1, 327–330. [Google Scholar] [CrossRef]
- Fisher, B.; Bauer, M.; Margolese, R.; Poisson, R.; Pilch, Y.; Redmond, C.; Fisher, E.; Wolmark, N.; Deutsch, M.; Montague, E.; et al. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N. Engl. J. Med. 1985, 312, 665–673. [Google Scholar] [CrossRef]
- Carriero, S.; Lanza, C.; Pellegrino, G.; Ascenti, V.; Sattin, C.; Pizzi, C.; Angileri, S.A.; Biondetti, P.; Ianniello, A.A.; Piacentino, F.; et al. Ablative Therapies for Breast Cancer: State of Art. Technol. Cancer Res. Treat. 2023, 22, 15330338231157193. [Google Scholar] [CrossRef]
- Fine, R.E.; Gilmore, R.C.; Tomkovich, K.R.; Dietz, J.R.; Berry, M.P.; Hernandez, L.E.; Columbus, K.S.; Seedman, S.A.; Fisher, C.S.; Han, L.K.; et al. Cryoablation Without Excision for Early-Stage Breast Cancer: ICE3 Trial 5-Year Follow-Up on Ipsilateral Breast Tumor Recurrence. Ann. Surg. Oncol. 2024, 31, 7273–7283. [Google Scholar] [CrossRef]
- Khan, S.Y.; Snitman, A.; Habrawi, Z.; Crawford, S.; Melkus, M.W.; Layeequr Rahman, R. The Role of Cryoablation in Breast Cancer Beyond the Oncologic Control: COST and Breast-Q Patient-Reported Outcomes. Ann. Surg. Oncol. 2023, 30, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.Y.; Cole, J.; Habrawi, Z.; Melkus, M.W.; Layeequr Rahman, R. Cryoablation Allows the Ultimate De-escalation of Surgical Therapy for Select Breast Cancer Patients. Ann. Surg. Oncol. 2023, 30, 8398–8403. [Google Scholar] [CrossRef]
- Urban, J.A. Surgical Excision of Internal Mammary Nodes for Breast Cancer. Br. J. Surg. 1964, 51, 209–212. [Google Scholar] [CrossRef]
- Veronesi, U.; Marubini, E.; Mariani, L.; Valagussa, P.; Zucali, R. The dissection of internal mammary nodes does not improve the survival of breast cancer patients. 30-year results of a randomised trial. Eur. J. Cancer 1999, 35, 1320–1325. [Google Scholar] [CrossRef]
- Fisher, B.; Jeong, J.H.; Anderson, S.; Bryant, J.; Fisher, E.R.; Wolmark, N. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N. Engl. J. Med. 2002, 347, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.; Montague, E.; Redmond, C.; Barton, B.; Borland, D.; Fisher, E.R.; Deutsch, M.; Schwarz, G.; Margolese, R.; Donegan, W.; et al. Comparison of radical mastectomy with alternative treatments for primary breast cancer. A first report of results from a prospective randomized clinical trial. Cancer 1977, 39, 2827–2839. [Google Scholar] [CrossRef] [PubMed]
- Ashikaga, T.; Krag, D.N.; Land, S.R.; Julian, T.B.; Anderson, S.J.; Brown, A.M.; Skelly, J.M.; Harlow, S.P.; Weaver, D.L.; Mamounas, E.P.; et al. Morbidity results from the NSABP B-32 trial comparing sentinel lymph node dissection versus axillary dissection. J. Surg. Oncol. 2010, 102, 111–118. [Google Scholar] [CrossRef]
- Rahman, R.L. Axilla Management Trials in Breast Cancer-A Requiem for Clinical Equipoise. Adv. Cancer Res. Clin. Imaging 2024, 4, 1–3. [Google Scholar] [CrossRef]
- Giuliano, A.E.; Ballman, K.V.; McCall, L.; Beitsch, P.D.; Brennan, M.B.; Kelemen, P.R.; Ollila, D.W.; Hansen, N.M.; Whitworth, P.W.; Blumencranz, P.W.; et al. Effect of Axillary Dissection vs No Axillary Dissection on 10-Year Overall Survival Among Women With Invasive Breast Cancer and Sentinel Node Metastasis: The ACOSOG Z0011 (Alliance) Randomized Clinical Trial. JAMA 2017, 318, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, V.; Cole, B.F.; Viale, G.; Veronesi, P.; Vicini, E.; Intra, M.; Mazzarol, G.; Massarut, S.; Zgajnar, J.; Taffurelli, M.; et al. Axillary dissection versus no axillary dissection in patients with breast cancer and sentinel-node micrometastases (IBCSG 23-01): 10-year follow-up of a randomised, controlled phase 3 trial. Lancet Oncol. 2018, 19, 1385–1393. [Google Scholar] [CrossRef]
- Donker, M.; van Tienhoven, G.; Straver, M.E.; Meijnen, P.; van de Velde, C.J.; Mansel, R.E.; Cataliotti, L.; Westenberg, A.H.; Klinkenbijl, J.H.; Orzalesi, L.; et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): A randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014, 15, 1303–1310. [Google Scholar] [CrossRef]
- Bartels, S.A.L.; Donker, M.; Poncet, C.; Sauve, N.; Straver, M.E.; van de Velde, C.J.H.; Mansel, R.E.; Blanken, C.; Orzalesi, L.; Klinkenbijl, J.H.G.; et al. Radiotherapy or Surgery of the Axilla After a Positive Sentinel Node in Breast Cancer: 10-Year Results of the Randomized Controlled EORTC 10981-22023 AMAROS Trial. J. Clin. Oncol. 2023, 41, 2159–2165. [Google Scholar] [CrossRef]
- McKevitt, E.; Cheifetz, R.; DeVries, K.; Laws, A.; Warburton, R.; Gondara, L.; Lohrisch, C.; Nichol, A. Sentinel Node Biopsy Should Not be Routine in Older Patients with ER-Positive HER2-Negative Breast Cancer Who Are Willing and Able to Take Hormone Therapy. Ann. Surg. Oncol. 2021, 28, 5950–5957. [Google Scholar] [CrossRef]
- Blumencranz, P.; Habibi, M.; Shivers, S.; Acs, G.; Blumencranz, L.E.; Yoder, E.B.; van der Baan, B.; Menicucci, A.R.; Dauer, P.; Audeh, W.; et al. The Predictive Utility of MammaPrint and BluePrint in Identifying Patients with Locally Advanced Breast Cancer Who are Most Likely to Have Nodal Downstaging and a Pathologic Complete Response after Neoadjuvant Chemotherapy. Ann. Surg. Oncol. 2023, 30, 8353–8361. [Google Scholar] [CrossRef] [PubMed]
- Bonadonna, G.; Brusamolino, E.; Valagussa, P.; Rossi, A.; Brugnatelli, L.; Brambilla, C.; De Lena, M.; Tancini, G.; Bajetta, E.; Musumeci, R.; et al. Combination chemotherapy as an adjuvant treatment in operable breast cancer. N. Engl. J. Med. 1976, 294, 405–410. [Google Scholar] [CrossRef]
- Fisher, B.; Brown, A.M.; Dimitrov, N.V.; Poisson, R.; Redmond, C.; Margolese, R.G.; Bowman, D.; Wolmark, N.; Wickerham, D.L.; Kardinal, C.G.; et al. Two months of doxorubicin-cyclophosphamide with and without interval reinduction therapy compared with 6 months of cyclophosphamide, methotrexate, and fluorouracil in positive-node breast cancer patients with tamoxifen-nonresponsive tumors: Results from the National Surgical Adjuvant Breast and Bowel Project B-15. J. Clin. Oncol. 1990, 8, 1483–1496. [Google Scholar] [CrossRef] [PubMed]
- Verrill, M. Chemotherapy for early-stage breast cancer: A brief history. Br. J. Cancer 2009, 101 (Suppl. 1), S2–S5. [Google Scholar] [CrossRef]
- Beatson, G.T. On the Treatment of Inoperable Cases of Carcinoma of the Mamma: Suggestions for a New Method of Treatment, with Illustrative Cases. Trans. Med. Chir. Soc. Edinb. 1896, 15, 153–179. [Google Scholar] [PubMed]
- Nabieva, N.; Fasching, P.A. Endocrine Treatment for Breast Cancer Patients Revisited-History, Standard of Care, and Possibilities of Improvement. Cancers 2021, 13, 5643. [Google Scholar] [CrossRef] [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer: An overview of the randomised trials. Lancet 1998, 351, 1451–1467. [Google Scholar] [CrossRef]
- Cuzick, J.; Sestak, I.; Baum, M.; Buzdar, A.; Howell, A.; Dowsett, M.; Forbes, J.F.; ATAC/LATTE Investigators. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010, 11, 1135–1141. [Google Scholar] [CrossRef]
- Ruhstaller, T.; Giobbie-Hurder, A.; Colleoni, M.; Jensen, M.B.; Ejlertsen, B.; de Azambuja, E.; Neven, P.; Lang, I.; Jakobsen, E.H.; Gladieff, L.; et al. Adjuvant Letrozole and Tamoxifen Alone or Sequentially for Postmenopausal Women with Hormone Receptor-Positive Breast Cancer: Long-Term Follow-Up of the BIG 1-98 Trial. J. Clin. Oncol. 2019, 37, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Higgins, M.J.; Liedke, P.E.; Goss, P.E. Extended adjuvant endocrine therapy in hormone dependent breast cancer: The paradigm of the NCIC-CTG MA.17/BIG 1-97 trial. Crit. Rev. Oncol. Hematol. 2013, 86, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Zurrida, S.; Veronesi, U. Milestones in breast cancer treatment. Breast J. 2015, 21, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Pivot, X.; Romieu, G.; Debled, M.; Pierga, J.Y.; Kerbrat, P.; Bachelot, T.; Lortholary, A.; Espie, M.; Fumoleau, P.; Serin, D.; et al. 6 months versus 12 months of adjuvant trastuzumab in early breast cancer (PHARE): Final analysis of a multicentre, open-label, phase 3 randomised trial. Lancet 2019, 393, 2591–2598. [Google Scholar] [CrossRef] [PubMed]
- Andre, F.; Ismaila, N.; Henry, N.L.; Somerfield, M.R.; Bast, R.C.; Barlow, W.; Collyar, D.E.; Hammond, M.E.; Kuderer, N.M.; Liu, M.C.; et al. Use of Biomarkers to Guide Decisions on Adjuvant Systemic Therapy for Women with Early-Stage Invasive Breast Cancer: ASCO Clinical Practice Guideline Update-Integration of Results from TAILORx. J. Clin. Oncol. 2019, 37, 1956–1964. [Google Scholar] [CrossRef]
- Piccart, M.; van ’t Veer, L.J.; Poncet, C.; Lopes Cardozo, J.M.N.; Delaloge, S.; Pierga, J.Y.; Vuylsteke, P.; Brain, E.; Vrijaldenhoven, S.; Neijenhuis, P.A.; et al. 70-gene signature as an aid for treatment decisions in early breast cancer: Updated results of the phase 3 randomised MINDACT trial with an exploratory analysis by age. Lancet Oncol. 2021, 22, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.M.S.; Sgroi, D.C.; Treuner, K.; Zhang, Y.; Piper, T.; Salunga, R.C.; Ahmed, I.; Doos, L.; Thornber, S.; Taylor, K.J.; et al. Breast Cancer Index Is a Predictive Biomarker of Treatment Benefit and Outcome from Extended Tamoxifen Therapy: Final Analysis of the Trans-aTTom Study. Clin. Cancer Res. 2022, 28, 1871–1880. [Google Scholar] [CrossRef]
- Delahaye, L.; Drukker, C.A.; Dreezen, C.; Witteveen, A.; Chan, B.; Snel, M.; Beumer, I.J.; Bernards, R.; Audeh, M.W.; Van’t Veer, L.J.; et al. A breast cancer gene signature for indolent disease. Breast Cancer Res. Treat. 2017, 164, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Opdam, M.; van der Noort, V.; Kleijn, M.; Glas, A.; Mandjes, I.; Kleiterp, S.; Hilbers, F.S.; Kruger, D.T.; Bins, A.D.; de Jong, P.C.; et al. Limiting systemic endocrine overtreatment in postmenopausal breast cancer patients with an ultralow classification of the 70-gene signature. Breast Cancer Res. Treat. 2022, 194, 265–278. [Google Scholar] [CrossRef]
- Mansfield, C.M.; Krishnan, L.; Komarnicky, L.T.; Ayyangar, K.M.; Kramer, C.A. A review of the role of radiation therapy in the treatment of patients with breast cancer. Semin. Oncol. 1991, 18, 525–535. [Google Scholar]
- Ohri, N.; Haffty, B.G. The evolution of adjuvant radiation therapy for early-stage and locally advanced breast cancer. Breast J. 2020, 26, 59–64. [Google Scholar] [CrossRef]
- Prionas, N.D.; Stephens, S.J.; Blitzblau, R.C. Early-stage Breast Cancer: Tailored External Beam Fractionation Approaches for Treatment of the Whole or Partial Breast. Semin. Radiat. Oncol. 2022, 32, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.A.; Mendenhall, N.P. Novel Radiotherapy Techniques for Breast Cancer. Annu. Rev. Med. 2018, 69, 277–288. [Google Scholar] [CrossRef]
- Powell, S. Radiotherapy for breast cancer in the 21st Century. Breast J. 2010, 16 (Suppl. 1), S34–S38. [Google Scholar] [CrossRef] [PubMed]
- Haviland, J.S.; Owen, J.R.; Dewar, J.A.; Agrawal, R.K.; Barrett, J.; Barrett-Lee, P.J.; Dobbs, H.J.; Hopwood, P.; Lawton, P.A.; Magee, B.J.; et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013, 14, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, J.S.; Bulsara, M.; Baum, M.; Wenz, F.; Massarut, S.; Pigorsch, S.; Alvarado, M.; Douek, M.; Saunders, C.; Flyger, H.L.; et al. Long term survival and local control outcomes from single dose targeted intraoperative radiotherapy during lumpectomy (TARGIT-IORT) for early breast cancer: TARGIT-A randomised clinical trial. BMJ 2020, 370, m2836. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.S.; Schnaper, L.A.; Bellon, J.R.; Cirrincione, C.T.; Berry, D.A.; McCormick, B.; Muss, H.B.; Smith, B.L.; Hudis, C.A.; Winer, E.P.; et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: Long-term follow-up of CALGB 9343. J. Clin. Oncol. 2013, 31, 2382–2387. [Google Scholar] [CrossRef] [PubMed]
- Kunkler, I.H.; Williams, L.J.; Jack, W.J.; Cameron, D.A.; Dixon, J.M.; PRIME II Investigators. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): A randomised controlled trial. Lancet Oncol. 2015, 16, 266–273. [Google Scholar] [CrossRef]
- Whelan, T.J.; Smith, S.; Parpia, S.; Fyles, A.W.; Bane, A.; Liu, F.F.; Rakovitch, E.; Chang, L.; Stevens, C.; Bowen, J.; et al. Omitting Radiotherapy after Breast-Conserving Surgery in Luminal A Breast Cancer. N. Engl. J. Med. 2023, 389, 612–619. [Google Scholar] [CrossRef]
- Shah, C.; Bremer, T.; Cox, C.; Whitworth, P.; Patel, R.; Patel, A.; Brown, E.; Gold, L.; Rock, D.; Riley, L.; et al. The Clinical Utility of DCISionRT((R)) on Radiation Therapy Decision Making in Patients with Ductal Carcinoma In Situ Following Breast-Conserving Surgery. Ann. Surg. Oncol. 2021, 28, 5974–5984. [Google Scholar] [CrossRef]
- White, J.R.; Cecchini, R.S.; Harris, E.E.; Mamounas, E.P.; Stover, D.G.; Ganz, P.A.; Jagsi, R.; Anderson, S.J.; Bergom, C.; Theberge, V.; et al. NRG-BR007: A phase III trial evaluating de-escalation of breast radiation (DEBRA) following breast-conserving surgery (BCS) of stage 1, HR+, HER2−, RS ≤ 18 breast cancer. J. Clin. Oncol. 2023, 41 (Suppl. 16), TPS623. [Google Scholar] [CrossRef]
- Rakovitch, E.; Nofech-Mozes, S.; Hanna, W.; Baehner, F.L.; Saskin, R.; Butler, S.M.; Tuck, A.; Sengupta, S.; Elavathil, L.; Jani, P.A.; et al. A population-based validation study of the DCIS Score predicting recurrence risk in individuals treated by breast-conserving surgery alone. Breast Cancer Res. Treat. 2015, 152, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Shah, C.; Whitworth, P.; Vicini, F.A.; Narod, S.; Gerber, N.; Jhawar, S.R.; King, T.A.; Mittendorf, E.A.; Willey, S.C.; Rabinovich, R.; et al. The Clinical Utility of a 7-Gene Biosignature on Radiation Therapy Decision Making in Patients with Ductal Carcinoma In Situ Following Breast-Conserving Surgery: An Updated Analysis of the DCISionRT((R)) PREDICT Study. Ann. Surg. Oncol. 2024, 31, 5919–5928. [Google Scholar] [CrossRef] [PubMed]
- Machiels, M.; Oulkadi, R.; Tramm, T.; Stecklein, S.R.; Somaiah, N.; De Caluwe, A.; Klein, J.; Tran, W.T.; Salgado, R. Individualising radiation therapy decisions in breast cancer patients based on tumour infiltrating lymphocytes and genomic biomarkers. Breast 2023, 71, 13–21. [Google Scholar] [CrossRef]
- Varga, Z.; Sinn, P.; Fritzsche, F.; von Hochstetter, A.; Noske, A.; Schraml, P.; Tausch, C.; Trojan, A.; Moch, H. Comparison of EndoPredict and Oncotype DX test results in hormone receptor positive invasive breast cancer. PLoS ONE 2013, 8, e58483. [Google Scholar] [CrossRef]
- Curigliano, G.; Dent, R.; Llombart-Cussac, A.; Pegram, M.; Pusztai, L.; Turner, N.; Viale, G. Incorporating clinicopathological and molecular risk prediction tools to improve outcomes in early HR+/HER2- breast cancer. NPJ Breast Cancer 2023, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Andre, F.; Ismaila, N.; Allison, K.H.; Barlow, W.E.; Collyar, D.E.; Damodaran, S.; Henry, N.L.; Jhaveri, K.; Kalinsky, K.; Kuderer, N.M.; et al. Biomarkers for Adjuvant Endocrine and Chemotherapy in Early-Stage Breast Cancer: ASCO Guideline Update. J. Clin. Oncol. 2022, 40, 1816–1837. [Google Scholar] [CrossRef] [PubMed]
- Kittaneh, M.; Montero, A.J.; Gluck, S. Molecular profiling for breast cancer: A comprehensive review. Biomark. Cancer 2013, 5, 61–70. [Google Scholar] [CrossRef]
- Van ’t Veer, L.J.; Dai, H.; van de Vijver, M.J.; He, Y.D.; Hart, A.A.; Mao, M.; Peterse, H.L.; van der Kooy, K.; Marton, M.J.; Witteveen, A.T.; et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature 2002, 415, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M.; Sorlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Wallden, B.; Storhoff, J.; Nielsen, T.; Dowidar, N.; Schaper, C.; Ferree, S.; Liu, S.; Leung, S.; Geiss, G.; Snider, J.; et al. Development and verification of the PAM50-based Prosigna breast cancer gene signature assay. BMC Med. Genom. 2015, 8, 54. [Google Scholar] [CrossRef]
- Sanft, T.B.; Wong, J.; O’Neal, B.; Siuliukina, N.; Jankowitz, R.C.; Pegram, M.D.; Fox, J.R.; Zhang, Y.; Treuner, K.; O’Shaughnessy, J.A. Impact of the Breast Cancer Index for Extended Endocrine Decision-Making: First Results of the Prospective BCI Registry Study. J. Natl. Compr. Cancer Netw. 2024, 22, 99–107. [Google Scholar] [CrossRef]
- Filipits, M.; Rudas, M.; Jakesz, R.; Dubsky, P.; Fitzal, F.; Singer, C.F.; Dietze, O.; Greil, R.; Jelen, A.; Sevelda, P.; et al. A new molecular predictor of distant recurrence in ER-positive, HER2-negative breast cancer adds independent information to conventional clinical risk factors. Clin. Cancer Res. 2011, 17, 6012–6020. [Google Scholar] [CrossRef] [PubMed]
- Fountzilas, E.; Tsimberidou, A.M.; Vo, H.H.; Kurzrock, R. Clinical trial design in the era of precision medicine. Genome Med. 2022, 14, 101. [Google Scholar] [CrossRef]
- Hobbs, B.P.; Pestana, R.C.; Zabor, E.C.; Kaizer, A.M.; Hong, D.S. Basket Trials: Review of Current Practice and Innovations for Future Trials. J. Clin. Oncol. 2022, 40, 3520–3528. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Burris, H.A., 3rd; Kurzrock, R. Revolutionizing cancer drug development: Harnessing the potential of basket trials. Cancer 2024, 130, 186–200. [Google Scholar] [CrossRef] [PubMed]
- Ouma, L.O.; Wason, J.M.S.; Zheng, H.; Wilson, N.; Grayling, M. Design and analysis of umbrella trials: Where do we stand? Front. Med. 2022, 9, 1037439. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.H.; Detry, M.A.; Murthy, S.; Guyatt, G.; Mills, E.J. How to Use and Interpret the Results of a Platform Trial: Users’ Guide to the Medical Literature. JAMA 2022, 327, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Selker, H.P.; Cohen, T.; D’Agostino, R.B.; Dere, W.H.; Ghaemi, S.N.; Honig, P.K.; Kaitin, K.I.; Kaplan, H.C.; Kravitz, R.L.; Larholt, K.; et al. A Useful and Sustainable Role for N-of-1 Trials in the Healthcare Ecosystem. Clin. Pharmacol. Ther. 2022, 112, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Zardavas, D.; Maetens, M.; Irrthum, A.; Goulioti, T.; Engelen, K.; Fumagalli, D.; Salgado, R.; Aftimos, P.; Saini, K.S.; Sotiriou, C.; et al. The AURORA initiative for metastatic breast cancer. Br. J. Cancer 2014, 111, 1881–1887. [Google Scholar] [CrossRef] [PubMed]
- Andre, F.; Filleron, T.; Kamal, M.; Mosele, F.; Arnedos, M.; Dalenc, F.; Sablin, M.P.; Campone, M.; Bonnefoi, H.; Lefeuvre-Plesse, C.; et al. Genomics to select treatment for patients with metastatic breast cancer. Nature 2022, 610, 343–348. [Google Scholar] [CrossRef]
- Brown, E.A.; Gold, L.P.; Gray, C.R.; Marks, D.K.; Thomas, V.T.; Santillan, A.A.; Singleton, C.S.; Audeh, M.W.W.; FLEX Investigators Group. FLEX, a real-world evidence full transcriptome study of 30,000 patients with early-stage breast cancer. J. Clin. Oncol. 2023, 41, TPS621. [Google Scholar] [CrossRef]
- Hayes, D.F.; Khoury, M.J.; Ransohoff, D. Why Hasn’t Genomic Testing Changed the Landscape in Clinical Oncology? Am. Soc. Clin. Oncol. Educ. Book 2012, 32, e52–e55. [Google Scholar] [CrossRef]
- Abdou, Y.; Barlow, W.E.; Gralow, J.R.; Meric-Bernstam, F.; Albain, K.S.; Hayes, D.F.; Lin, N.U.; Perez, E.A.; Goldstein, L.J.; Chia, S.K.; et al. Race and clinical outcomes in the RxPONDER Trial (SWOG S1007). In Proceedings of the 2022 San Antonio Breast Cancer Symposium, San Antonio, TX, USA, 6–10 December 2022. [Google Scholar]
- Kim, G.; Karadal-Ferrena, B.; Qin, J.; Sharma, V.P.; Oktay, I.S.; Lin, Y.; Ye, X.; Asiry, S.; Pastoriza, J.M.; Cheng, E.; et al. Racial disparity in tumor microenvironment and distant recurrence in residual breast cancer after neoadjuvant chemotherapy. NPJ Breast Cancer 2023, 9, 52. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, S.Y.; Bah, T.; Layeequr Rahman, R. The Role of Molecular Profiling in De-Escalation of Toxic Therapy in Breast Cancer. Int. J. Mol. Sci. 2025, 26, 1332. https://doi.org/10.3390/ijms26031332
Khan SY, Bah T, Layeequr Rahman R. The Role of Molecular Profiling in De-Escalation of Toxic Therapy in Breast Cancer. International Journal of Molecular Sciences. 2025; 26(3):1332. https://doi.org/10.3390/ijms26031332
Chicago/Turabian StyleKhan, Sonia Y., Tonjeh Bah, and Rakhshanda Layeequr Rahman. 2025. "The Role of Molecular Profiling in De-Escalation of Toxic Therapy in Breast Cancer" International Journal of Molecular Sciences 26, no. 3: 1332. https://doi.org/10.3390/ijms26031332
APA StyleKhan, S. Y., Bah, T., & Layeequr Rahman, R. (2025). The Role of Molecular Profiling in De-Escalation of Toxic Therapy in Breast Cancer. International Journal of Molecular Sciences, 26(3), 1332. https://doi.org/10.3390/ijms26031332





