The Lower Concentration of Plasma Acetyl-Carnitine in Epicardial Artery Disease—A Preliminary Report
Abstract
1. Introduction
2. Results
3. Discussion
4. Study Limitations
5. Methods
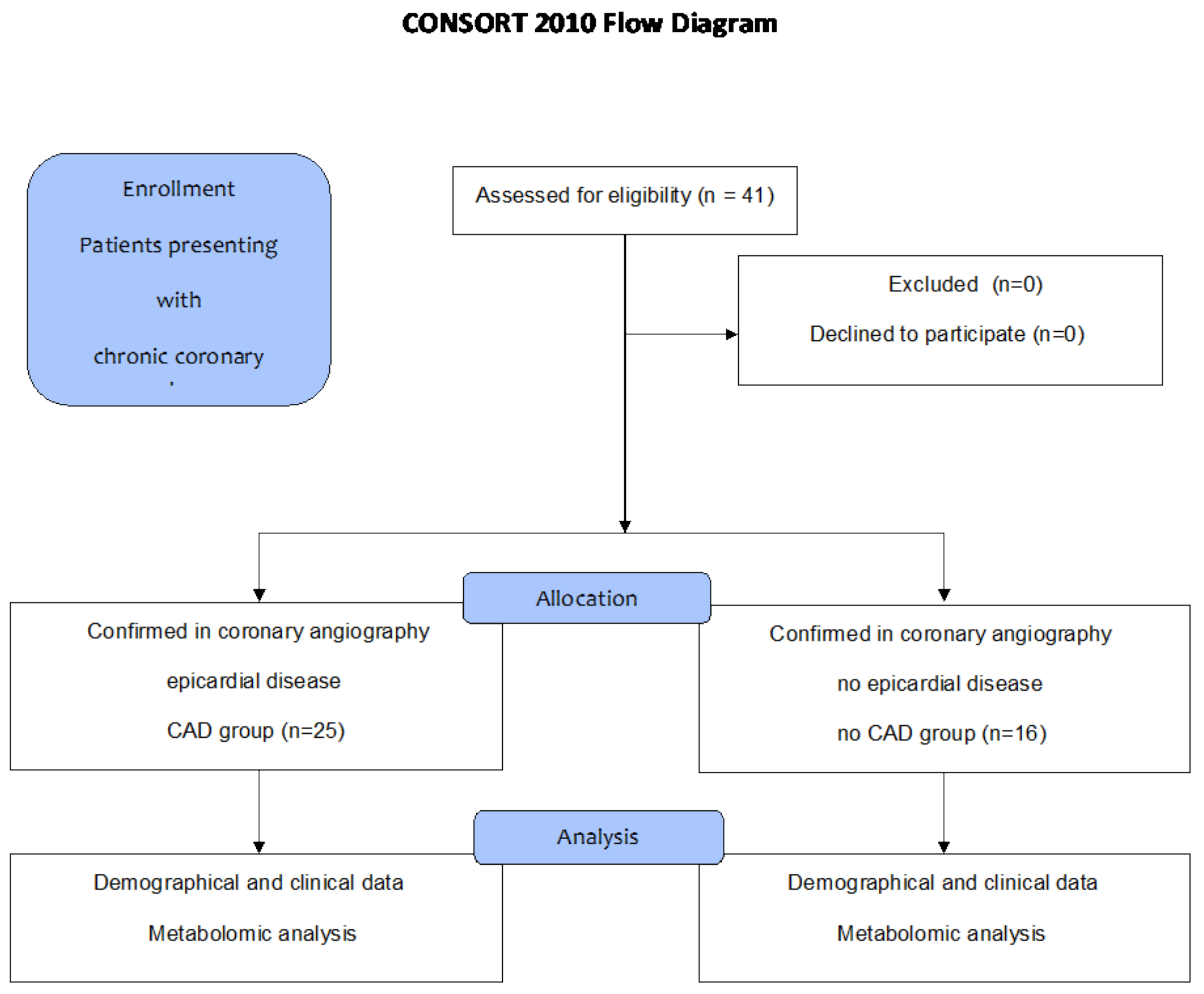
5.1. Patients
5.2. Methods
5.3. Statistical Analysis
5.4. Bioethics Committee
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Lamee, R.K. Agina pectoris 2023: With and without obstructive coronary artery disease: Epidemiology, diagnosis, prognosis, and treatment. Vasc. Pharmacol. 2024, 155, 107285. [Google Scholar] [CrossRef]
- Malakar, A.K.; Choudhury, D.; Halder, B.; Paul, P.; Uddin, A.; Chakraborty, S. A review on coronary artery disease, its risk factors, and therapeutics. J. Cell. Physiol. 2019, 234, 16812–16823. [Google Scholar] [CrossRef] [PubMed]
- Saraste, A.; Knuuti, J.; Bax, J. Screening for Coronary Artery Disease in Patients with Diabetes. Curr. Cardiol. Rep. 2023, 25, 1865–1871. [Google Scholar] [CrossRef]
- Goldsborough, E., 3rd; Osuji, N.; Blaha, M.J. Assessment of Cardiovascular Disease Risk: A 2022 Update. Endocrinol. Metab. Clin. N. Am. 2022, 51, 483–509. [Google Scholar] [CrossRef]
- Gusev, E.; Sarapultsev, A. Atherosclerosis and Inflammation: Insights from the Theory of General Pathological Processes. Int. J. Mol. Sci. 2023, 24, 7910. [Google Scholar] [CrossRef]
- Urbanowicz, T.; Olasińska-Wiśniewska, A.; Michalak, M.; Perek, B.; Al-Imam, A.; Rodzki, M.; Witkowska, A.; Straburzyńska-Migaj, E.; Bociański, M.; Misterski, M.; et al. Pre-operative systemic inflammatory response index influences long-term survival rate in off-pump surgical revascularization. PLoS ONE 2022, 17, e0276138. [Google Scholar] [CrossRef] [PubMed]
- Urbanowicz, T.K.; Rodzki, M.; Michalak, M.; Olasińska-Wiśniewska, A.; Witkowska, A.; Krasińska, B.; Bociański, M.; Krasińska-Płachta, A.; Cieśla, A.; Stefaniak, S.; et al. Large unstained cell (LUC) count as a predictor of carotid artery occlusion. Adv. Clin. Exp. Med. 2023, 32, 987–996. [Google Scholar] [CrossRef]
- Ridker, P.M. The Time to Initiate Anti-Inflammatory Therapy for Patients with Chronic Coronary Atherosclerosis Has Arrived. Circulation 2023, 148, 1071–1073. [Google Scholar] [CrossRef] [PubMed]
- Grajek, S.; Michalak, M.; Urbanowicz, T.; Olasińska-Wiśniewska, A. A Meta-Analysis Evaluating the Colchicine Therapy in Patients with Coronary Artery Disease. Front. Cardiovasc. Med. 2021, 8, 740896. [Google Scholar] [CrossRef]
- Lv, J.; Pan, C.; Cai, Y.; Han, X.; Wang, C.; Ma, J.; Pang, J.; Xu, F.; Wu, S.; Kou, T.; et al. Plasma metabolomics reveals the shared and distinct metabolic disturbances associated with cardiovascular events in coronary artery disease. Nat. Commun. 2024, 15, 5729–5742. [Google Scholar] [CrossRef]
- Rastgoo, S.; Fateh, S.T.; Nikbaf-Shandiz, M.; Rasaei, N.; Aali, Y.; Zamani, M.; Shiraseb, F.; Asbaghi, O. The effects of L-carnitine supplementation on inflammatory and anti-inflammatory markers in adults: A systematic review and dose-response meta-analysis. Inflammopharmacology 2023, 31, 2173–2199. [Google Scholar] [CrossRef] [PubMed]
- Gander, J.; Carrard, J.; Gallart-Ayala, H.; Borreggine, R.; Teav, T.; Infanger, D.; Colledge, F.; Streese, L.; Wagner, J.; Klenk, C.; et al. Metabolic Impairment in Coronary Artery Disease: Elevated Serum Acylcarnitines Under the Spotlights. Front. Cardiovasc. Med. 2021, 8, 792350. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Selvaraj, S.; Fu, Z.; Jones, P.; Kwee, L.C.; Windsor, S.L.; Ilkayeva, O.; Newgard, C.B.; Margulies, K.B.; Husain, M.; Inzucchi, S.E.; et al. Metabolomic Profiling of the Effects of Dapagliflozin in Heart Failure With Reduced Ejection Fraction: DEFINE-HF. Circulation 2022, 146, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.D.; Morfino, P.; Aimo, A. Plasma acylcarnitine, risk for heart failure or atrial fibrillation, and effects of the Mediterranean diet or obesity. Rev. Esp. Cardiol. (Engl. Ed.) 2022, 75, 621–622. [Google Scholar] [CrossRef]
- Shah, P.; Sawhney, A.; Anamika, F.; Kanagala, S.G.; Parikh, K.; Mendpara, V.; Garg, N.; Jain, R. Understanding the Relationship between the Ketogenic Diet and the Heart: A Novel Therapeutic Potential for Cardiovascular Health. Cardiovasc. Hematol. Agents Med. Chem. 2024, 22, 407–412. [Google Scholar] [CrossRef]
- Li, X.; Wu, F.; Günther, S.; Looso, M.; Kuenne, C.; Zhang, T.; Wiesnet, M.; Klatt, S.; Zukunft, S.; Fleming, I.; et al. Inhibition of fatty acid oxidation enables heart regeneration in adult mice. Nature 2023, 622, 619–626. [Google Scholar] [CrossRef]
- Shang, R.; Sun, Z.; Li, H. Effective dosing of L-carnitine in the secondary prevention of cardiovascular disease: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2014, 14, 88–95. [Google Scholar] [CrossRef]
- Lee, B.J.; Lin, J.S.; Lin, Y.C.; Lin, P.T. Effects of L-carnitine supplementation on oxidative stress and antioxidant enzymes activities in patients with coronary artery disease: A randomized, placebo-controlled trial. Nutr. J. 2014, 13, 79–96. [Google Scholar] [CrossRef]
- Dambrova, M.; Makrecka-Kuka, M.; Kuka, J.; Vilskersts, R.; Nordberg, D.; Attwood, M.M.; Smesny, S.; Sen, Z.D.; Guo, A.C.; Oler, E.; et al. Acylcarnitines: Nomenclature, Biomarkers, Therapeutic Potential, Drug Targets, and Clinical Trials. Pharmacol. Rev. 2022, 74, 506–551. [Google Scholar] [CrossRef] [PubMed]
- Izzo, L.T.; Trefely, S.; Demetriadou, C.; Drummond, J.M.; Mizukami, T.; Kuprasertkul, N.; Farria, A.T.; Nguyen, P.T.T.; Murali, N.; Reich, L.; et al. Acetylcarnitine shuttling links mitochondrial metabolism to histone acetylation and lipogenesis. Sci. Adv. 2023, 9, eadf0115. [Google Scholar] [CrossRef] [PubMed]
- Sassi, S.; Bianchi, E.; Diamanti, L.; Tornabene, D.; Sette, E.; Medici, D.; Matà, S.; Leccese, D.; Sperti, M.; Martinelli, I.; et al. Retrospective observational study on the use of acetyl-L-carnitine in ALS. J. Neurol. 2023, 270, 5344–5357. [Google Scholar] [CrossRef] [PubMed]
- Ait Tayeb, A.E.K.; Colle, R.; El-Asmar, K.; Chappell, K.; Acquaviva-Bourdain, C.; David, D.J.; Trabado, S.; Chanson, P.; Feve, B.; Becquemont, L.; et al. Plasma acetyl-l-carnitine and l-carnitine in major depressive episodes: A case-control study before and after treatment. Psychol. Med. 2023, 53, 2307–2316. [Google Scholar] [CrossRef]
- Zhao, J.V.; Burgess, S.; Fan, B.; Schooling, C.M. L-carnitine, a friend or foe for cardiovascular disease? A Mendelian randomization study. BMC Med. 2022, 20, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, L.; Chen, W.; Chen, B.; Li, H.; Wang, S.; Wang, M.; Luo, Y. Application of Metabolomics to Identify Potential Biomarkers for the Early Diagnosis of Coronary Heart Disease. Front Physiol. 2021, 12, 775135. [Google Scholar] [CrossRef]
- Violante, S.; Achetib, N.; van Roermund, C.W.T.; Hagen, J.; Dodatko, T.; Vaz, F.M.; Waterham, H.R.; Chen, H.; Baes, M.; Yu, C.; et al. Peroxisomes can oxidize medium- and long-chain fatty acids through a pathway involving ABCD3 and HSD17B4. FASEB J. 2019, 33, 4355–4364. [Google Scholar] [CrossRef]
- Cho, K.; Moon, J.S.; Kang, J.H.; Jang, H.B.; Lee, H.J.; Park, S.I.; Yu, K.S.; Cho, J.Y. Combined untargeted and targeted metabolomic profiling reveals urinary biomarkers for discriminating obese from normal-weight adolescents. Pediatr. Obes. 2017, 12, 93–101. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, X.; Liu, X.; He, L.; Zhang, W.; Wang, Y.; Sun, W.; Ji, Z. Investigation of the urinary metabolic variations and the application in bladder cancer biomarker discovery. Int. J. Cancer 2018, 143, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Maerz, T.; Sherman, E.; Newton, M.; Yilmaz, A.; Kumar, P.; Graham, S.F.; Baker, K.C. Metabolomic serum profiling after ACL injury in rats: A pilot study implicating inflammation and immune dysregulation in post-traumatic osteoarthritis. J. Orthop. Res. 2018, 36, 1969–1979. [Google Scholar] [CrossRef]
- Kang, M.; Yoo, H.J.; Kim, M.; Kim, M.; Lee, J.H. Metabolomics identifies increases in the acylcarnitine profiles in the plasma of overweight subjects in response to mild weight loss: A randomized, controlled design study. Lipids Health Dis. 2018, 17, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.E.; Evans, A.M. Carnitine and acylcarnitines: Pharmacokinetic, pharmacological and clinical aspects. Clin. Pharmacokinet. 2012, 51, 553–572. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.J.; Matern, D.; Millington, D.S.; Hillman, S.; Feezor, M.D.; Bennett, M.J.; Qumsiyeh, M.; Kahler, S.G.; Chen, Y.T.; Van Hove, J.L. Acylcarnitines in fibroblasts of patients with long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency and other fatty acid oxidation disorders. J. Inherit. Metab. Dis. 2000, 23, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Pierre, G.; Macdonald, A.; Gray, G.; Hendriksz, C.; Preece, M.A.; Chakrapani, A. Prospective treatment in carnitine-acylcarnitine translocase deficiency. J. Inherit. Metab. Dis. 2007, 30, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Harada, H.; Nishiyama, Y.; Katoh, A.; Niiyama, H.; Iwaki, A.; Ohchi, Y.; Sasaki, M.; Aoki, Y.; Okina, N.; Kai, H. Heart failure treatment and serum carnitines in HFpEF and HFrEF with or without hypertension—A pilot study. Hypertens. Res. 2025. epub ahead of print. [Google Scholar] [CrossRef]
- Ahmad, T.; Kelly, J.P.; McGarrah, R.W.; Hellkamp, A.S.; Fiuzat, M.; Testani, J.M.; Wang, T.S.; Verma, A.; Samsky, M.D.; Donahue, M.P.; et al. Prognostic Implications of Long-Chain Acylcarnitines in Heart Failure and Reversibility with Mechanical Circulatory Support. J. Am. Coll. Cardiol. 2016, 67, 291–299. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Whole Group n = 41 | Group 1 CAD Group n = 25 | Group 2 Non-CAD Group n = 16 | p Group 1 vs. 2 |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) (median (Q1–Q3)) | 69 (63–73) | 69 (63–73) | 70 (64–74) | 0.556 |
| Height (cm) (median (Q1–Q3)) | 165 (163–176) | 167 (164–176) | 164 (162–176) | 0.628 |
| Weight (kg) (median (Q1–Q3)) | 80 (70–91) | 80 (71–92) | 80 (67–88) | 0.645 |
| BMI (median (Q1–Q3)) | 28.6 (25.4–31.8) | 29.1 (25.4–30.9) | 28.2 (25.5–32.3) | 0.822 |
| BMI > 30 | 17 (41) | 10 (40) | 7 (44) | 1.000 |
| Clinical status: | ||||
| CCS class | ||||
| class I (n (%)) | 1 (2) | 0 (0) | 1 (6) | |
| class II (n (%)) | 34 (83) | 19 (76) | 15 (94) | 0.056 |
| class III (n (%)) | 6 15 | 6 (24) | 0 (0) | |
| NYHA class | ||||
| class I (n (%)) | 23 (56) | 13 (52) | 10 (63) | |
| class II (n (%)) | 17 (42) | 11 (44) | 6 (37) | 0.628 |
| class III (n (%)) | 1 (2) | 1 (4) | 0 (0) | |
| Co-morbidities: | ||||
| Arterial hypertension (n (%)) | 35 (85) | 22 (88) | 13 (81) | 0.662 |
| Diabetes mellitus (n (%)) | 12 (29) | 7 (28) | 5 (31) | 1.000 |
| Dyslipidemia (n (%)) | 33 (83) | 21 (84) | 13 (81) | 1.000 |
| COPD (n (%)) | 7 (17) | 5 (20) | 2 (13) | 0.685 |
| Kidney dysfunction * (n (%)) | 5 (12) | 3 (12) | 2 (13) | 1.000 |
| PAD (n (%)) | 4 (10) | 3 (12) | 1 (6) | 1.000 |
| Thyroid disease (n (%)) | 6 (15) | 2 (8) | 4 (25) | 0.187 |
| Atrial fibrillation (n (%)) | 5 (12) | 2 (8) | 3 (19) | 0.362 |
| Parameters | Whole Group n = 41 | Group 1 CAD Group n = 25 | Group 2 Non-CAD Group n = 16 | p Group 1 vs. 2 |
|---|---|---|---|---|
| Cine-angiography results: | ||||
| Normal angiograms (n (%)) | 16 (39) | 0 (0) | 16 (100) | - |
| Epicardial disease | 25 (61) | 25 (100) | 0 (0) | - |
| LMCA (n (%)) | 5 (12) | 5 (20) | 0 (0) | - |
| One-vessel (n (%)) | 2 (5) | 2 (8) | 0 (0) | - |
| Two-vessel (n (%)) | 10 (24) | 10 (40) | 0 (0) | - |
| Three-vessel disease (n (%)) | 8 (20) | 8 (32) | 0 (0) | - |
| Echocardiographic results: | ||||
| LVEDD (mm) (median (Q1–Q3)) | 48 (44–52) | 49 (46–52) | 44 (42–50) | 0.117 |
| RVEDD (mm) (median (Q1–Q3)) | 29 (27–31) | 29 (27–31) | 29 (28–31) | 0.958 |
| LAD (mm) (median (Q1–Q3)) | 36 (33–39) | 37 (34–39) | 34 (30–38) | 0.537 |
| IVS (mm) (median (Q1–Q3)) | 12 (10–13) | 12 (11–13) | 12 (10–13) | 0.494 |
| LVEF (%) (median (Q1–Q3)) | 55 (50–60) | 50 (50–60) | 60 (52–62) | 0.309 |
| LV contractility disturbances (n (%)) | 24 (59) | 15 (60) | 9 (56) | 1.000 |
| IM (grade) mild vs. moderate-severe (n (%)) | 21 (51)/8 (20) | 10 (40)/5 (20) | 11 (69)/3 (19) | 0.682 |
| IT (grade) mild vs. moderate (n (%)) | 30 (73)/5 (12) | 16 (64)/3 (12) | 14 (88)/2 (13) | 1.000 |
| Laboratory tests: | ||||
| Whole blood count analysis | ||||
| WBC (109/L) (median (Q1–Q3)) | 6.98 (6.03–8.02) | 7.51 (6.47–8.86) | 6.79 (5.68–7.41) | 0.073 |
| Hb (mmol/L) (median (Q1–Q3)) | 8.9 (8.20–9.40) | 9.20 (8.40–9.70) | 8.50 (8.00–9.00) | 0.051 |
| Hct (%) (median (Q1–Q3)) | 43 (41–45) | 44 (41–46) | 41 (40.5–43.3) | 0.104 |
| Plt (109/L) (median (Q1–Q3)) | 221 (196–264) | 226 (209–264) | 221 (180–253) | 0.479 |
| NLR | 2.63 (2.09–3.45) | 2.86 (2.46–3.90) | 2.09 (1.81–2.66) | 0.009 |
| MLR | 0.29 (0.24–0.37) | 0.28 (0.23–0.36) | 0.30 (0.26–0.35) | 0.680 |
| C-reactive protein (mg/L) | 8 (7–9) | 8 (7–9) | 8 (7–9) | 0.554 |
| Kidney function tests | ||||
| Creatinine (mmol/L) (median (Q1–Q3)) | 81.0 (73.0–91.0) | 83 (73–101) | 77.5 (71.3–89.5) | 0.454 |
| Liver function tests | ||||
| ALT (IU/L) (median (Q1–Q3)) | 29 (24.5–38.5) | 31 (26–42) | 27.5 (22–33) | 0.241 |
| Lipidogram | ||||
| Total cholesterol (mmol/L) (median (Q1–Q3)) | 4.16 (3.52–4.70) | 3.83 (3.18–4.69) | 4.37 (3.83–4.76) | 0.235 |
| LDL (mmol/L) (median (Q1–Q3)) | 2.43 (1.67–3.10) | 2.45 (1.76–3.17) | 2.16 (1.697–2.92) | 0.652 |
| HDL (mmol/L) (median (Q1–Q3)) | 1.22 (1.01–1.56) | 1.09 (0.91–1.28) | 1.57 (1.33–1.71) | 0.010 |
| Triglycerides (mmol/L) (median (Q1–Q3)) | 1.30 (0.94–1.79) | 1.28 (1.00–1.78) | 1.39 (0.90–1.79) | 0.825 |
| TSH (mIU/L) | 2.5 (1.7–3.2) | 2.5 (1.6–3.2) | 2.6 (1.8–3.1) | 0.911 |
| Parameters Carnitine-Type Serum Concentrations [µM] | Whole Group n = 41 | Group 1 CAD Group n = 25 | Group 2 Non-CAD Group n = 16 | p Group 1 vs. 2 |
|---|---|---|---|---|
| CO-L-Carnitine | 43.60 (38.80–53.90) | 43.40 (36.20–57.50) | 44.65 (39.48–48.90) | 0.873 |
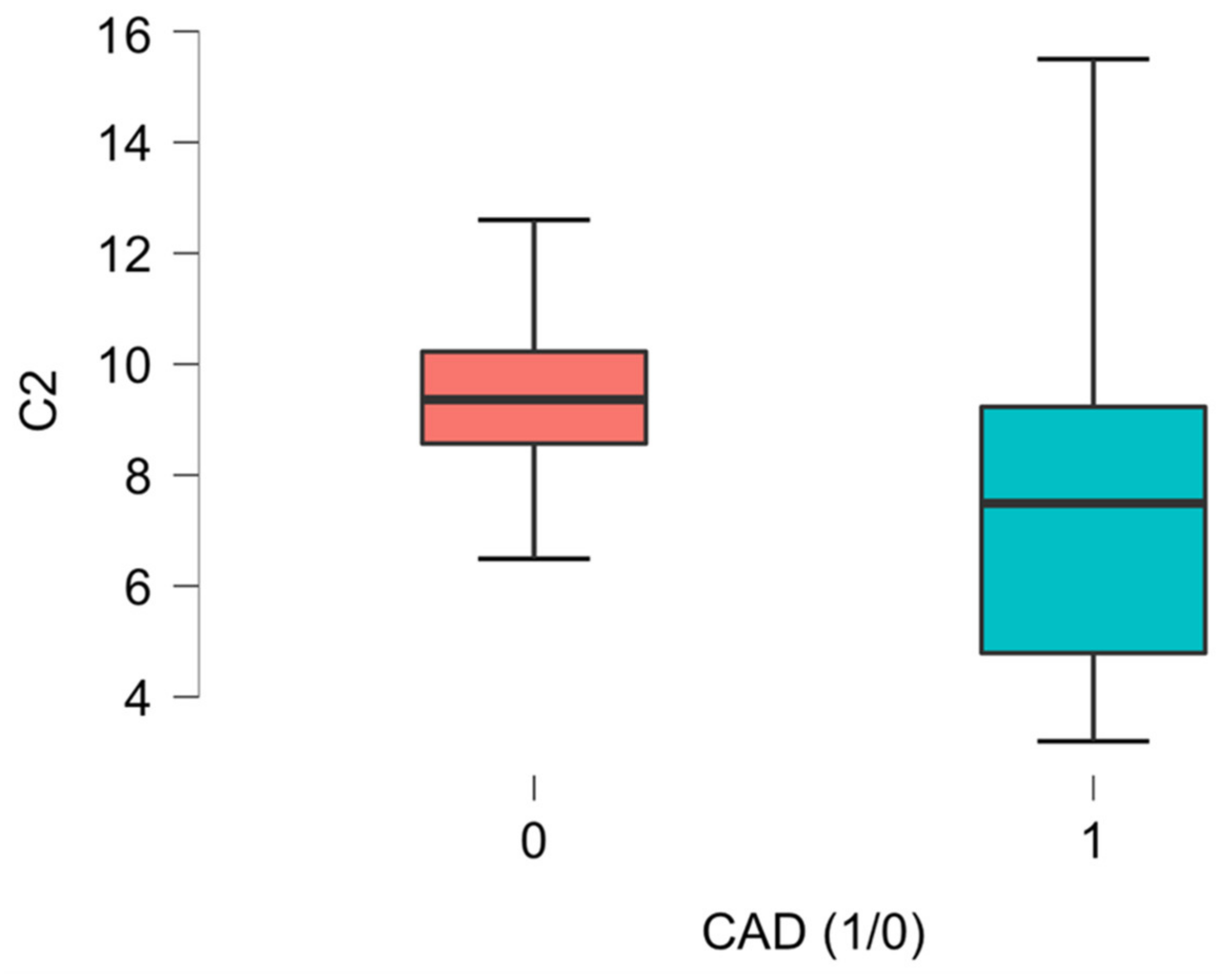
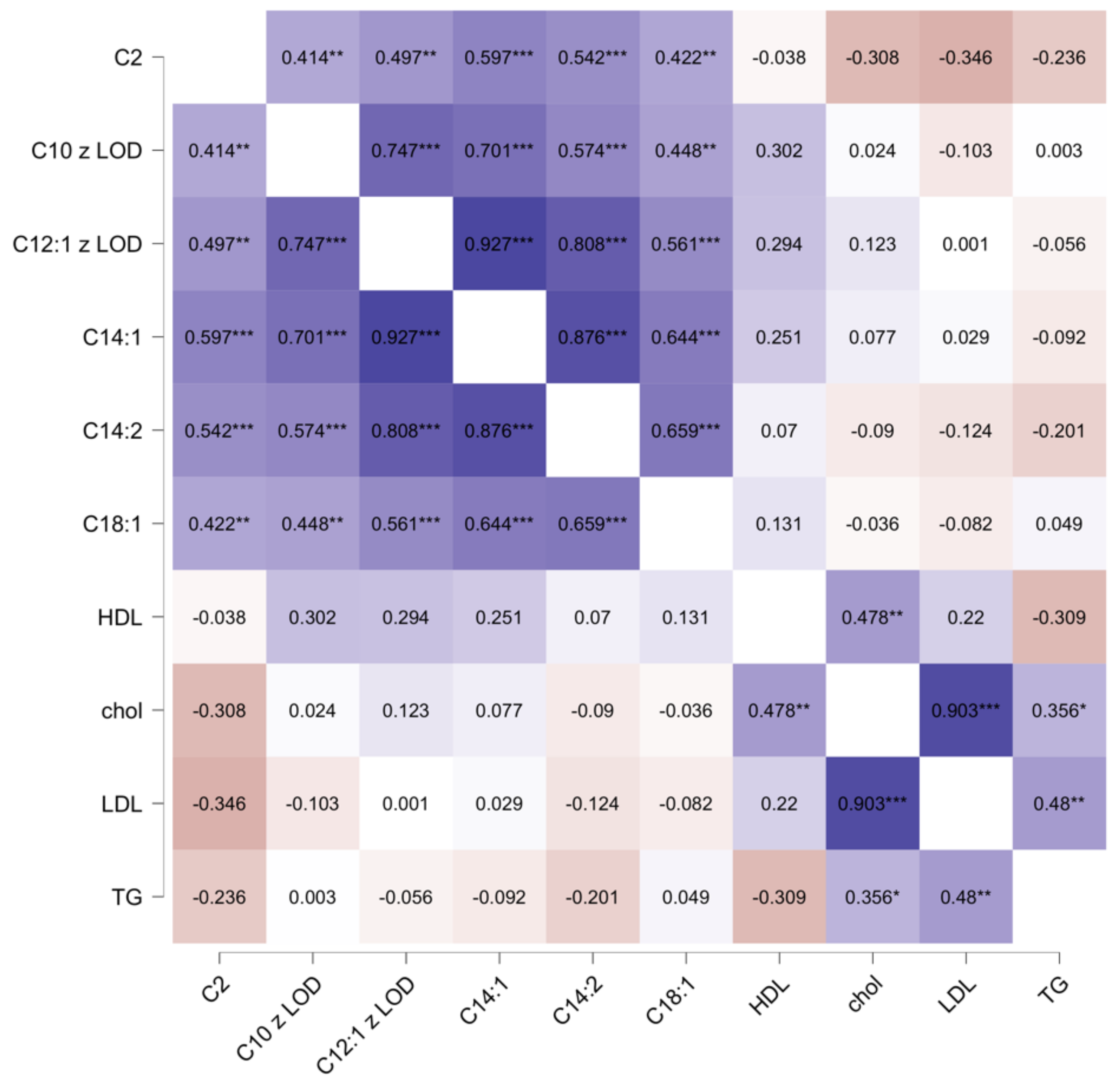
| C2 L-Acetyl-carnitine | 8.56 (6.49–10.10) | 7.49 (4.79–9.23) | 9.36 (8.57–10.23) | 0.009 * |
| C3 ** Propionyl-carnitine | 0.40 (0.31–0.50) | 0.40 (0.00–0.51) | 0.00 (0.00–0.49) | 0.807 |
| C3-DC(C4-OH) Malonyl-carnitine ** | 0.07 (0.00–0.09) | 0.07 (0.00–0.09) | 0.07 (0.00–0.09) | 0.579 |
| C4 Isobutyryl-L-carnitine/Butyryl-carnitine | 0.23 (0.23–0.33) | 0.23 (0.18–0.33) | 0.23 (0.21–0.32) | 0.936 |
| C4:1 Butenyl-carnitine | 0.04 (0.04–0.05) | 0.04 (0.04–0.05) | 0.04 (0.03–0.05) | 0.640 |
| C5 2-Methylbutyroyl-carnitine/Isovaleryl-carnitine/Valeryl-carnitine/Pivaloyl-carnitine | 0.15 (0.13–0.19) | 0.15 (0.12–0.20) | 0.15 (0.13–0.20) | 0.936 |
| C5-DC (C6-OH) Glutaryl-carnitine | 0.04 (0.03–0.05) | 0.04 (0.03–0.04) | 0.05 (0.04–0.06) | 0.164 |
| C5-OH (C3-DC-M) Hydroxyvaleryl-carnitine/Methylmalonyl-carnitine | 0.08 (0.07–0.08) | 0.08 (0.07–0.08) | 0.07 (0.07–0.08) | 0.267 |
| C8 Octanoyl-carnitine ** | 0.00 (0.00–0.25) | 0.00 (0.00–0.21) | 0.22 (0.00–0.27) | 0.086 |
| C10 Decanoyl-carnitine ** | 0.31 (0.00–0.40) | 0.00 (0.00–0.37) | 0.36 (0.19–0.44) | 0.040 * |
| C10:2 2-trans,4-cis-Decadienoyl-carnitine | 0.05 (0.04–0.05) | 0.04 (0.04–0.05) | 0.05 (0.04–0.05) | 0.067 |
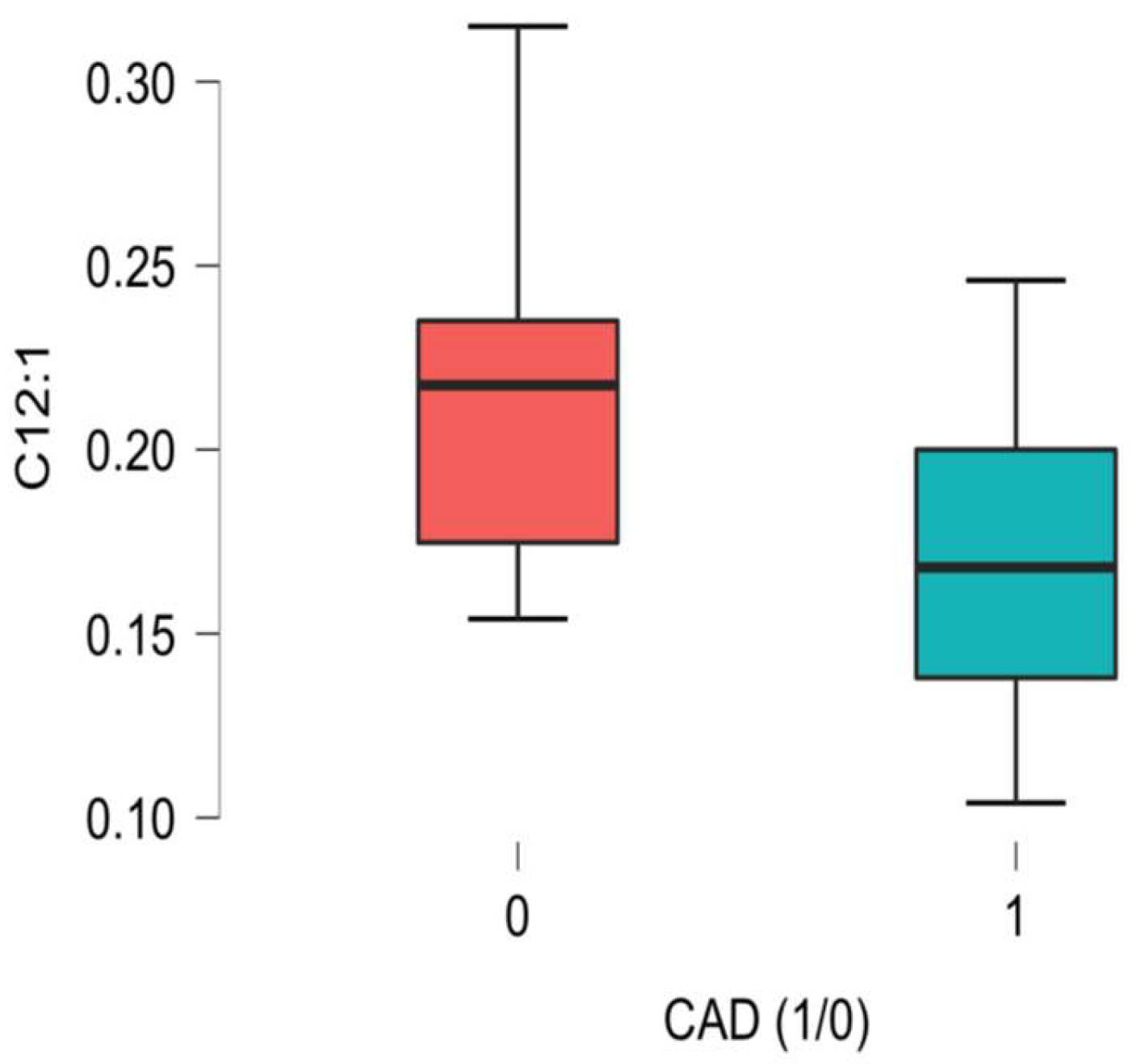
| C12:1 trans-2-Dodecenoyl-carnitine ** | 0.19 (0.15–0.22) | 0.17 (0.13–0.20) | 0.22 (0.18–0.24) | 0.008 * |
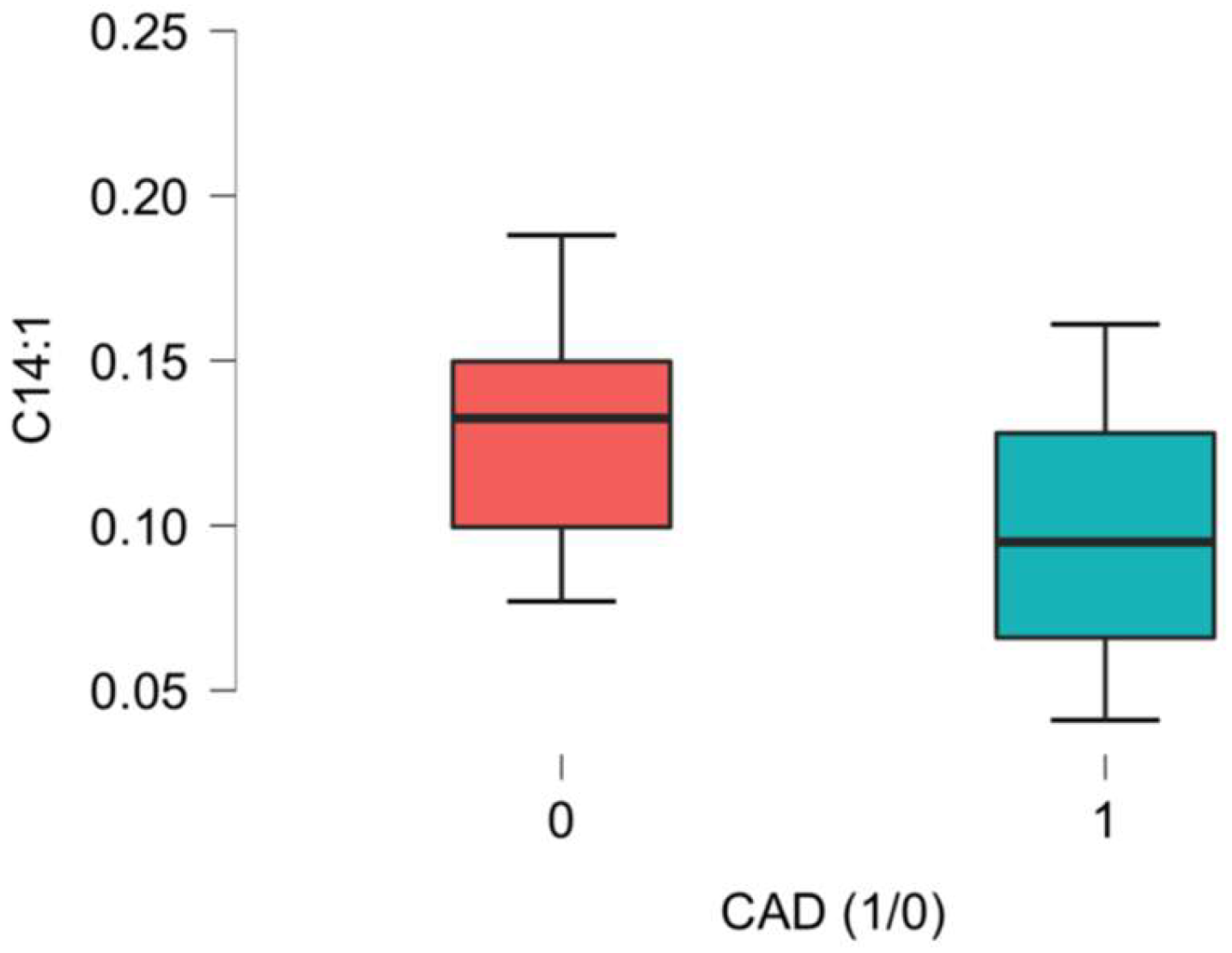
| C14:1 cis-5-Tetradecenoyl-carnitine | 0.10 (0.08–0.14) | 0.10 (0.07–0.13) | 0.13 (0.10–0.15) | 0.002 * |
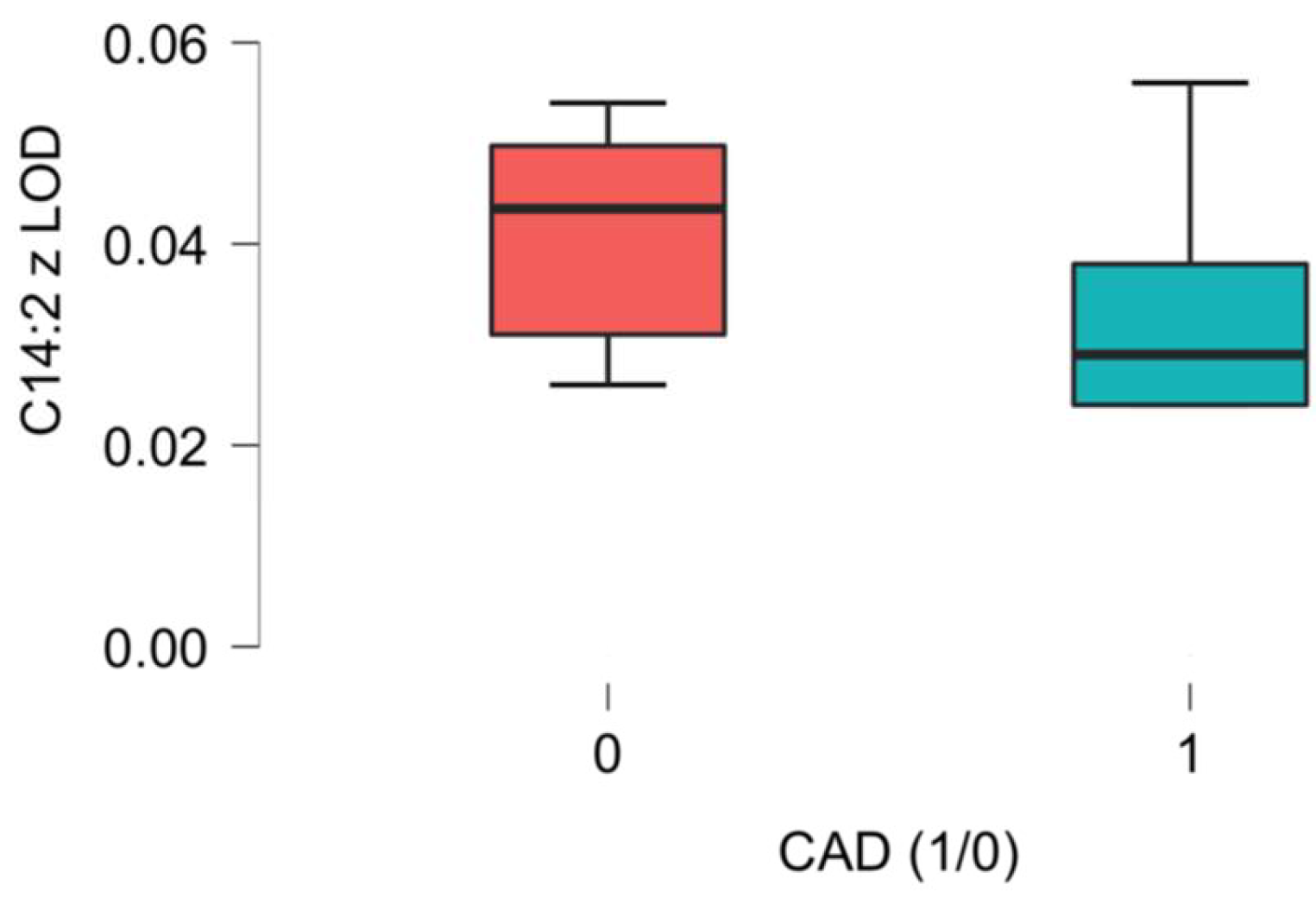
| C14:2 3,5-Tetradecadien-carnitine ** | 0.03 (0.03–0.05) | 0.03 (0.02–0.04) | 0.04 (0.03–0.05) | 0.043 |
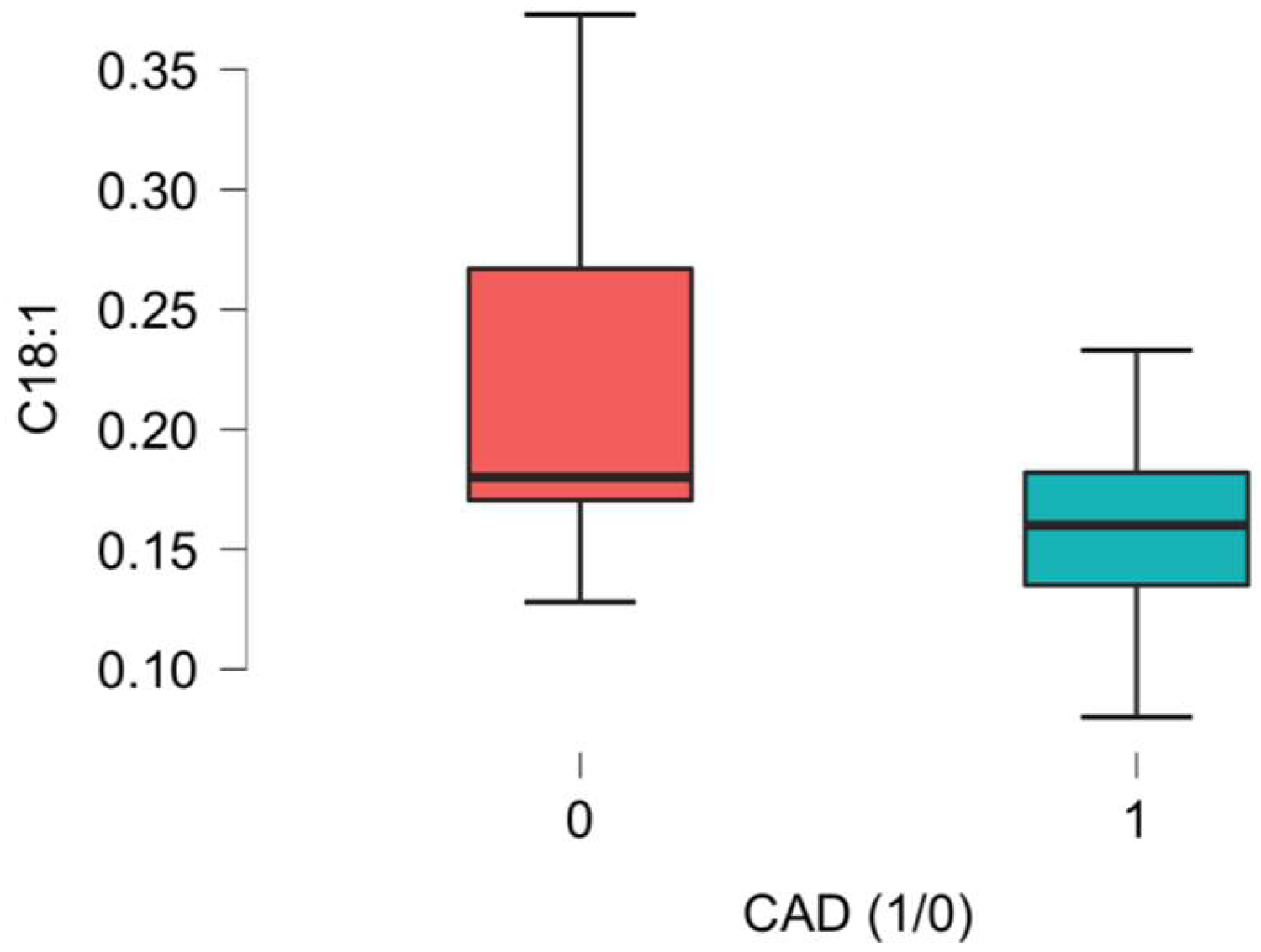
| C18:1 Oleoyl-carnitine/Vaccenyl-carnitine/Elaidic-carnitine | 0.17 (0.15–0.19) | 0.16 (0.14–0.18) | 0.18 (0.17–0.27) | 0.007 * |
| C18:2 Linoelaidyl-carnitine/Linoleyl-carnitine | 0.05 (0.04–0.06) | 0.05 (0.04–0.06) | 0.05 (0.05–0.07) | 0.698 |
| Acetylcarnitines | Male CAD Group Rank-Biserial Correlation | 95% CI | p |
|---|---|---|---|
| C2 | 0.385 | 0.047–0.644 | 0.036 * |
| C10 | 0.524 | 0.219–0.735 | 0.003 * |
| C12.1 | 0.474 | 0.155–0.703 | 0.010 * |
| C14.1 | 0.467 | 0.146–0.698 | 0.011 * |
| C14.2 | 0.285 | (−) 0.066–0.573 | 0.123 |
| C18.1 | 0.388 | 0.050–0.646 | 0.35 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbanowicz, T.; Gutaj, P.; Plewa, S.; Olasińska-Wiśniewska, A.; Spasenenko, I.; Krasińska, B.; Tykarski, A.; Filipiak, K.J.; Pakuła-Iwańska, M.; Krasiński, Z.; et al. The Lower Concentration of Plasma Acetyl-Carnitine in Epicardial Artery Disease—A Preliminary Report. Int. J. Mol. Sci. 2025, 26, 1318. https://doi.org/10.3390/ijms26031318
Urbanowicz T, Gutaj P, Plewa S, Olasińska-Wiśniewska A, Spasenenko I, Krasińska B, Tykarski A, Filipiak KJ, Pakuła-Iwańska M, Krasiński Z, et al. The Lower Concentration of Plasma Acetyl-Carnitine in Epicardial Artery Disease—A Preliminary Report. International Journal of Molecular Sciences. 2025; 26(3):1318. https://doi.org/10.3390/ijms26031318
Chicago/Turabian StyleUrbanowicz, Tomasz, Paweł Gutaj, Szymon Plewa, Anna Olasińska-Wiśniewska, Ievgen Spasenenko, Beata Krasińska, Andrzej Tykarski, Krzysztof J. Filipiak, Martyna Pakuła-Iwańska, Zbigniew Krasiński, and et al. 2025. "The Lower Concentration of Plasma Acetyl-Carnitine in Epicardial Artery Disease—A Preliminary Report" International Journal of Molecular Sciences 26, no. 3: 1318. https://doi.org/10.3390/ijms26031318
APA StyleUrbanowicz, T., Gutaj, P., Plewa, S., Olasińska-Wiśniewska, A., Spasenenko, I., Krasińska, B., Tykarski, A., Filipiak, K. J., Pakuła-Iwańska, M., Krasiński, Z., Grywalska, E., Wender-Ożegowska, E., Jemielity, M., & Matysiak, J. (2025). The Lower Concentration of Plasma Acetyl-Carnitine in Epicardial Artery Disease—A Preliminary Report. International Journal of Molecular Sciences, 26(3), 1318. https://doi.org/10.3390/ijms26031318







