Experimental and Computational Investigation of the Target and Mechanisms of Gelsemium Alkaloids in the Central Nervous System
Abstract
1. Introduction
2. Results
2.1. Target Confirmation of Gelsemium Alkaloids
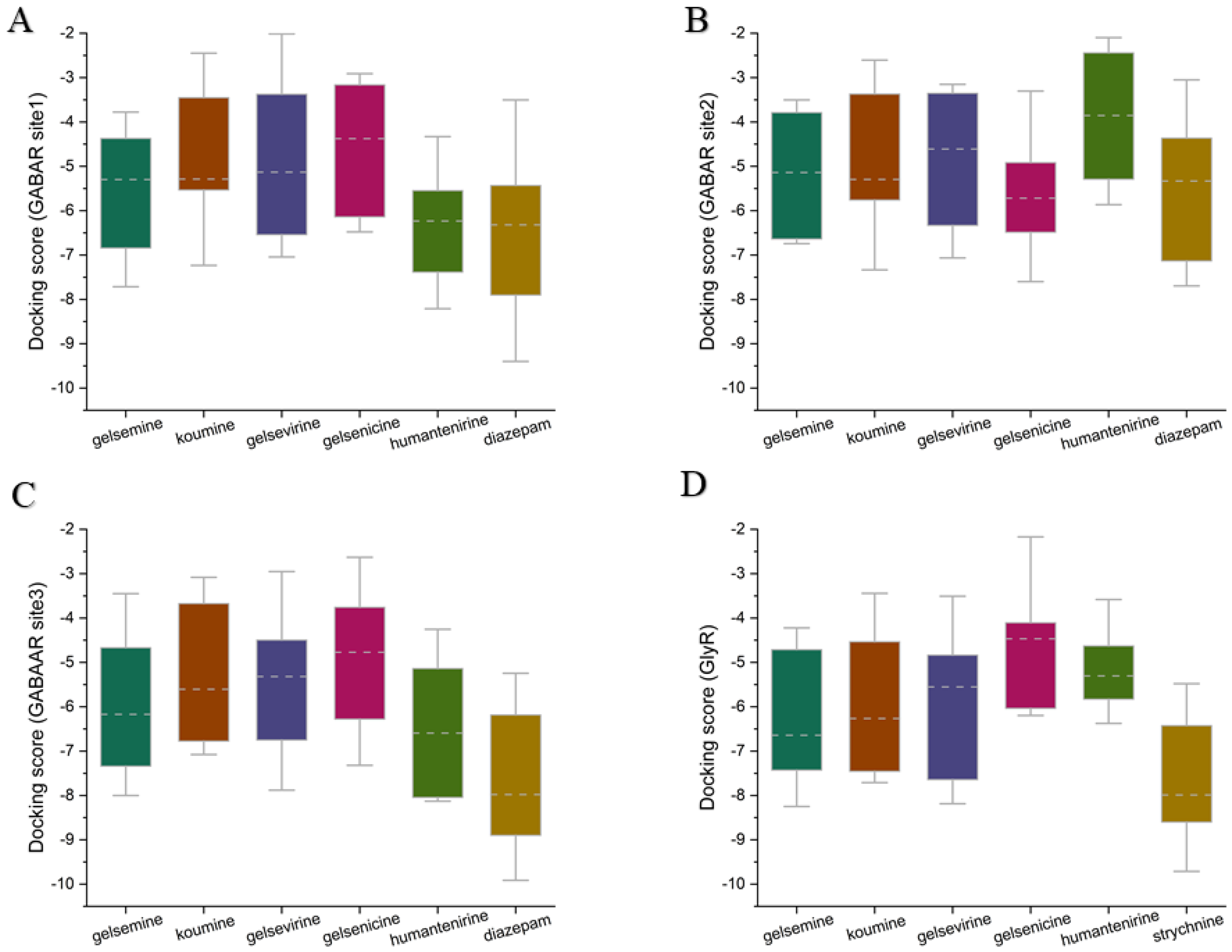
2.2. Target Site Analysis of Gelsemium Alkaloids
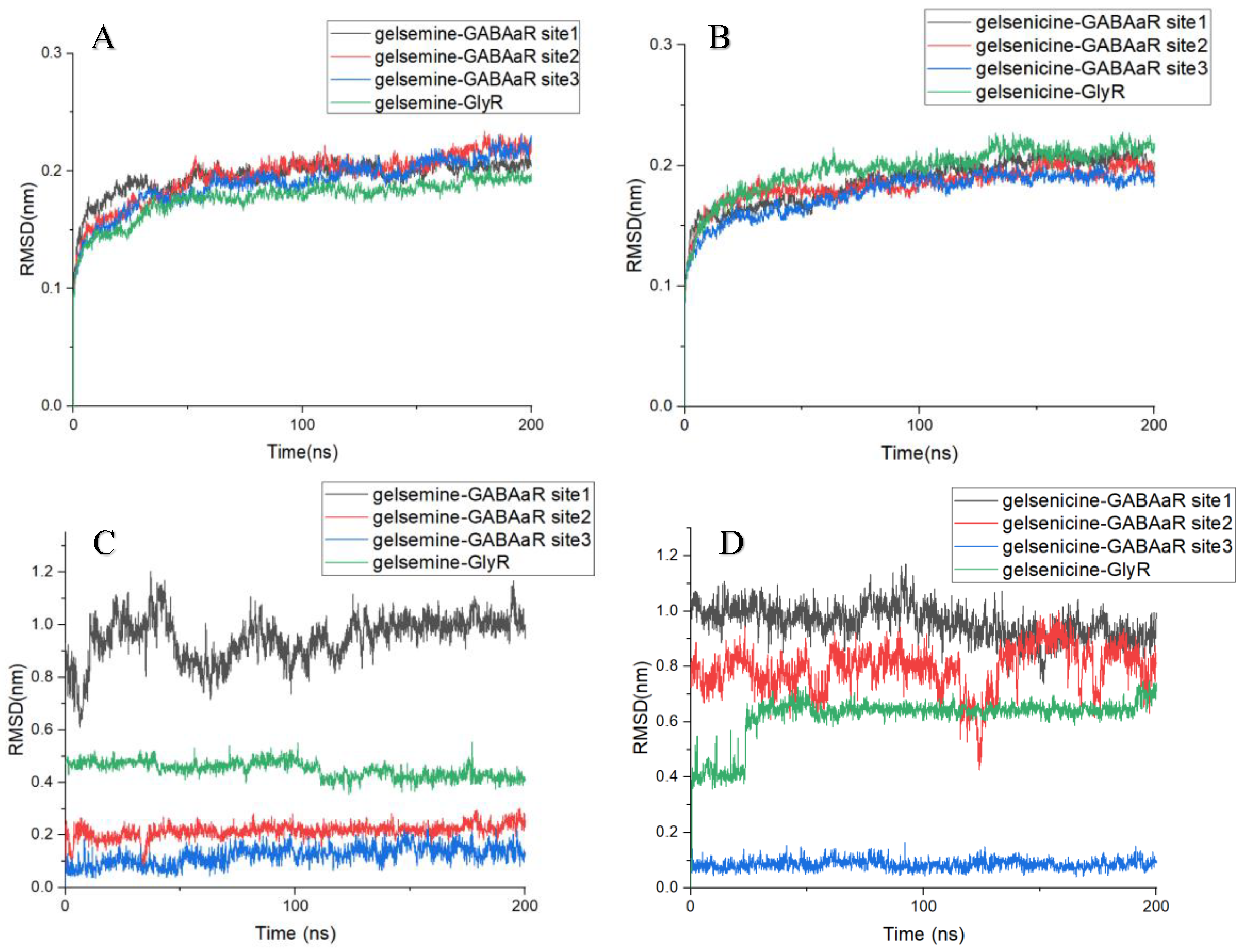
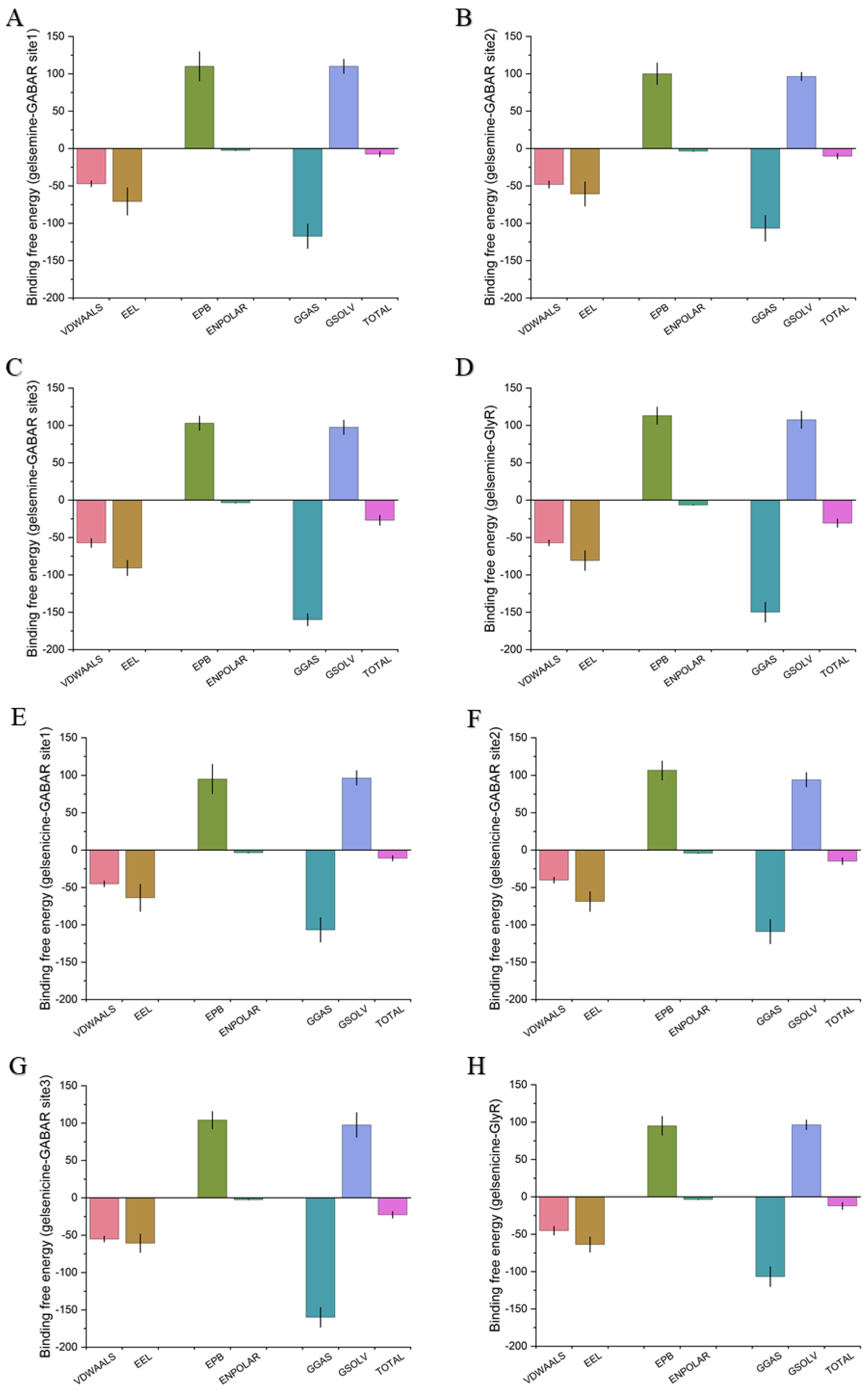
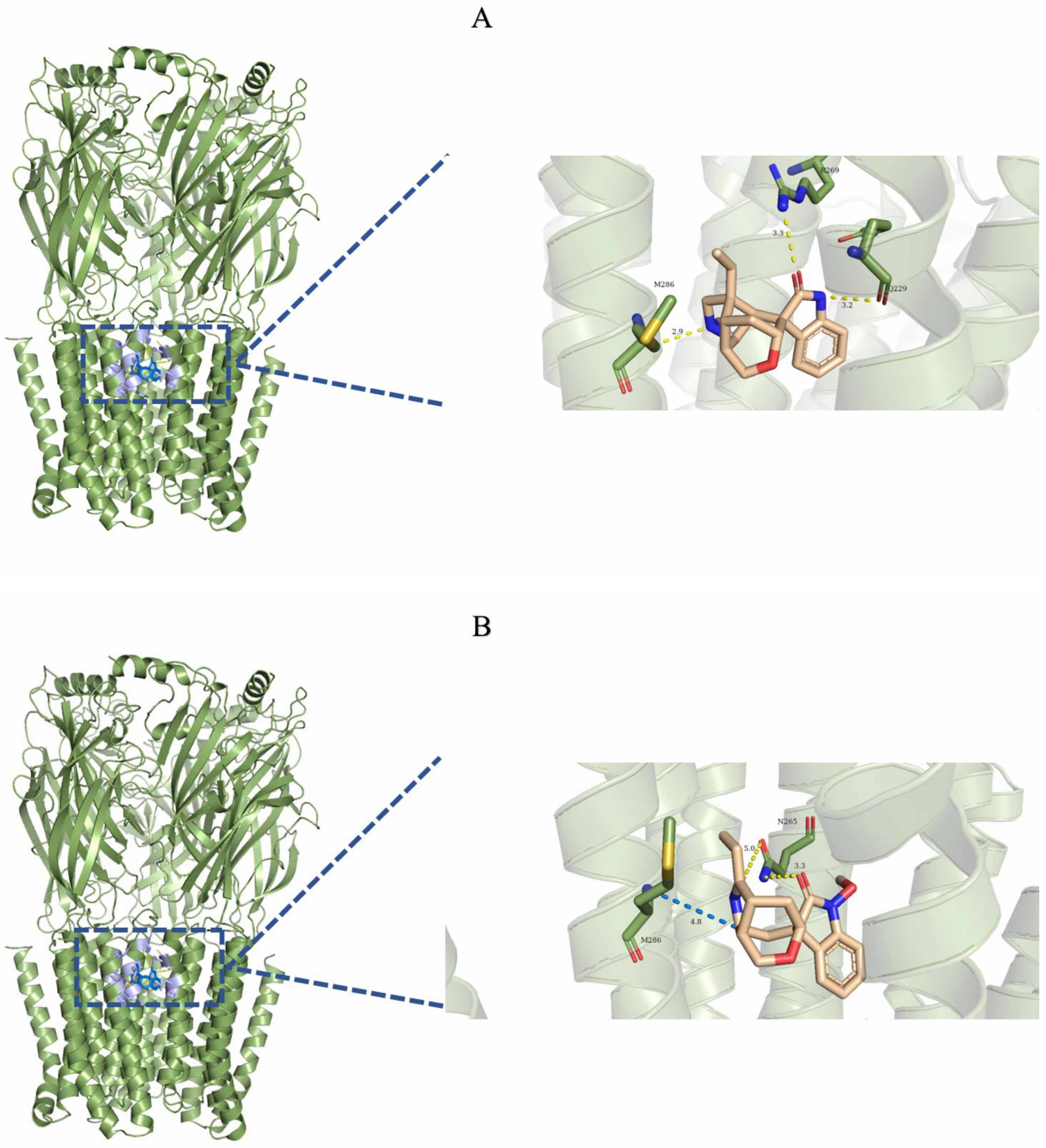
2.3. Analysis of the Binding Mode of Gelsemium Alkaloids to Targets
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture, Plasmids, and Transfection
4.3. Electrophysiology
4.4. Molecular Docking and Molecular Dynamics Simulations
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peng, Y.-L.; Liang, J.-J.; Xue, Y.; Khan, A.; Zhang, P.-P.; Feng, T.-T.; Song, D.; Zhou, Y.; Wei, X. Genus Gelsemium and Its Endophytic Fungi-Comprehensive Review of Their Traditional Uses, Phytochemistry, Pharmacology, and Toxicology. Curr. Top Med. Chem. 2023, 23, 2452–2487. [Google Scholar] [CrossRef]
- Dutt, V.; Dhar, V.J.; Sharma, A. Antianxiety Activity of Gelsemium sempervirens. Pharm. Biol. 2010, 48, 1091–1096. [Google Scholar] [CrossRef]
- Zuo, M.-T.; Gong, M.-D.; Ma, X.; Xu, W.-B.; Wang, Z.-Y.; Tang, M.-H.; Wu, Y.; Liu, Z.-Y. Sex Differences in the In Vivo Exposure Process of Multiple Components of Gelsemium elegans in Rats. Metabolites 2022, 13, 33. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Qiu, H.; Cheng, Y.; Liu, M.; Chen, M.; Que, Y.; Que, W. Gelsemium elegans Benth: Chemical Components, Pharmacological Effects, and Toxicity Mechanisms. Molecules 2021, 26, 7145. [Google Scholar] [CrossRef] [PubMed]
- Rujjanawate, C.; Kanjanapothi, D.; Panthong, A. Pharmacological Effect and Toxicity of Alkaloids from Gelsemium elegans Benth. J. Ethnopharmacol. 2003, 89, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Yang, K.; Cao, J.-J.; Wang, Z.-Y.; Wu, Y.; Sun, Z.-L.; Liu, Z.-Y. Integration of Metabolomics and Transcriptomicsto Comprehensively Evaluate the Metabolic Effects of Gelsemium elegans on Pigs. Animals 2021, 11, 1192. [Google Scholar] [CrossRef]
- Meyer, L.; Boujedaini, N.; Patte-Mensah, C.; Mensah-Nyagan, A.G. Pharmacological Effect of Gelsemine on Anxiety-like Behavior in Rat. Behav. Brain Res. 2013, 253, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-H.; Zhang, G.-M.; Chen, C.-Y.; He, J.; Chen, C.-J. Prenatal Exposure to Koumine Results in Cognitive Deficits and Increased Anxiety-like Behavior in Mice Offspring. J. Chem. Neuroanat. 2021, 111, 101888. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Tang, M.-H.; Zeng, Z.-Y.; Huang, S.-J.; Zheng, X.-F.; Liu, Z.-Y. Suppressive Effects of Gelsemine on Anxiety-like Behaviors Induced by Chronic Unpredictable Mild Stress in Mice. Brain Sci. 2022, 12, 191. [Google Scholar] [CrossRef]
- Luo, Y.; Xiong, B.; Liu, H.; Chen, Z.; Huang, H.; Yu, C.; Yang, J. Koumine Suppresses IL-1β Secretion and Attenuates Inflammation Associated With Blocking ROS/NF-κB/NLRP3 Axis in Macrophages. Front. Pharmacol. 2021, 11, 622074. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Liang, Z.; Yi, J.; Chen, X.; Li, R.; Wu, Y.; Wu, J.; Sun, Z. Protective Effect of Koumine, an Alkaloid from Gelsemium sempervirens, on Injury Induced by H2O2 in IPEC-J2 Cells. Int. J. Mol. Sci. 2019, 20, 754. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Matias, F.B.; Wu, J.; Liang, Z.; Sun, Z. Koumine Attenuates Lipopolysaccaride-Stimulated Inflammation in RAW264.7 Macrophages, Coincidentally Associated with Inhibition of NF-κB, ERK and P38 Pathways. Int. J. Mol. Sci. 2016, 17, 430. [Google Scholar] [CrossRef] [PubMed]
- Que, W.; Wu, Z.; Chen, M.; Zhang, B.; You, C.; Lin, H.; Zhao, Z.; Liu, M.; Qiu, H.; Cheng, Y. Molecular Mechanism of Gelsemium elegans (Gardner and Champ.) Benth. Against Neuropathic Pain Based on Network Pharmacology and Experimental Evidence. Front. Pharmacol. 2022, 12, 792932. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Liu, Q.; Chen, Z.; Zheng, F.; Huang, H.; Yu, C.; Yang, J. The Immunomodulatory Effect of Koumine on B Cells under Dependent and Independent Responses by T Cells. Eur. J. Pharmacol. 2022, 914, 174690. [Google Scholar] [CrossRef]
- Yuan, Z.; Liang, Z.; Yi, J.; Chen, X.; Li, R.; Wu, J.; Sun, Z. Koumine Promotes ROS Production to Suppress Hepatocellular Carcinoma Cell Proliferation Via NF-κB and ERK/P38 MAPK Signaling. Biomolecules 2019, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhong, Y.; Wang, W.; Jia, X.; Zhu, H.; Jiang, W.; Song, Y.; Xu, W.; Wu, S. Sempervirine Mediates Autophagy and Apoptosis via the Akt/mTOR Signaling Pathways in Glioma Cells. Front. Pharmacol. 2021, 12, 770667. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, X.; Bao, W.; Hong, X.; Li, C.; Lu, J.; Zhang, D.; Zhu, A. Effect of Humantenine on mRNA m6A Modification and Expression in Human Colon Cancer Cell Line HCT116. Genes 2022, 13, 781. [Google Scholar] [CrossRef]
- Wang, D.; Leng, X.; Tian, Y.; Liu, J.; Zou, J.; Xie, S. Toxic Effects of Koumine on the Early-Life Development Stage of Zebrafish. Toxics 2023, 11, 853. [Google Scholar] [CrossRef] [PubMed]
- Fung, H.; Lam, K.; Lam, S.; Wong, O.; Kam, C. Two Cases of Gelsemium elegans Benth. Poisoning. Hong Kong J. Emerg. Med. 2007, 14, 221–224. [Google Scholar] [CrossRef]
- Ma, X.; Zuo, M.-T.; Qi, X.-J.; Wang, Z.-Y.; Liu, Z.-Y. Two-Dimensional Liquid Chromatography Method for the Determination of Gelsemium Alkaloids in Honey. Foods 2022, 11, 2891. [Google Scholar] [CrossRef]
- Wang, L.; Chen, S.; Gao, X.; Liang, X.; Lv, W.; Zhang, D.; Jin, X. Recent Progress in Chemistry and Bioactivity of Monoterpenoid Indole Alkaloids from the Genus Gelsemium: A Comprehensive Review. J. Enzyme Inhib. Med. Chem. 2023, 38, 2155639. [Google Scholar] [CrossRef] [PubMed]
- Zuo, M.-T.; Wu, Y.; Wang, Z.-Y.; Wang, N.; Huang, S.-J.; Yu, H.; Zhao, X.-J.; Huang, C.-Y.; Liu, Z.-Y. A Comprehensive Toxicity Evaluation in Rats after Long-Term Oral Gelsemium elegans Exposure. Biomed. Pharm. 2021, 137, 111284. [Google Scholar] [CrossRef] [PubMed]
- Ghit, A.; Assal, D.; Al-Shami, A.S.; Hussein, D.E.E. GABAA Receptors: Structure, Function, Pharmacology, and Related Disorders. J. Genet. Eng. Biotechnol. 2021, 19, 123. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.W. Molecular Structure and Function of the Glycine Receptor Chloride Channel. Physiol. Rev. 2004, 84, 1051–1095. [Google Scholar] [CrossRef]
- Shoaib, R.M.; Zhang, J.-Y.; Mao, X.-F.; Wang, Y.-X. Gelsemine and Koumine, Principal Active Ingredients of Gelsemium, Exhibit Mechanical Antiallodynia via Spinal Glycine Receptor Activation-Induced Allopregnanolone Biosynthesis. Biochem. Pharm. 2019, 161, 136–148. [Google Scholar] [CrossRef]
- Marileo, A.M.; Lara, C.O.; Sazo, A.; Contreras, O.V.; González, G.; Castro, P.A.; Aguayo, L.G.; Moraga-Cid, G.; Fuentealba, J.; Burgos, C.F.; et al. Molecular Pharmacology of Gelsemium Alkaloids on Inhibitory Receptors. Int. J. Mol. Sci. 2024, 25, 3390. [Google Scholar] [CrossRef]
- Lara, C.O.; Murath, P.; Muñoz, B.; Marileo, A.M.; Martín, L.S.; San Martín, V.P.; Burgos, C.F.; Mariqueo, T.A.; Aguayo, L.G.; Fuentealba, J.; et al. Functional Modulation of Glycine Receptors by the Alkaloid Gelsemine. Br. J. Pharmacol. 2016, 173, 2263–2277. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liu, Y.; Sun, F.; Zhang, J.; Jin, Y.; Li, Y.; Zhou, J.; Li, Y.; Zhu, K. Gelsedine-Type Alkaloids: Discovery of Natural Neurotoxins Presented in Toxic Honey. J. Hazard. Mater. 2020, 381, 120999. [Google Scholar] [CrossRef]
- Li, Y.J.; Xiao, N.; Jia, W.D.; Li, X.R.; Zheng, X.F.; Sun, Z.L. The Effectiveness of Flumazenil-Epinephrine Combination on Treatment for Acute Gelsemium elegans Poisoning. Chin. J. Anim. Vet. Sci. 2022, 53, 938–946. [Google Scholar] [CrossRef]
- Marileo, A.M.; Gavilán, J.; San Martín, V.P.; Lara, C.O.; Sazo, A.; Muñoz-Montesino, C.; Castro, P.A.; Burgos, C.F.; Leiva-Salcedo, E.; Aguayo, L.G.; et al. Modulation of GABAA Receptors and of GABAergic Synapses by the Natural Alkaloid Gelsemine. Front. Mol. Neurosci. 2022, 15, 1083189. [Google Scholar] [CrossRef]
- McGrath, M.; Hoyt, H.; Pence, A.; Jayakar, S.S.; Zhou, X.; Forman, S.A.; Cohen, J.B.; Miller, K.W.; Raines, D.E. Competitive Antagonism of Etomidate Action by Diazepam. Anesthesiology 2020, 133, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Breitinger, U.; Breitinger, H.-G. Modulators of the Inhibitory Glycine Receptor. ACS Chem. Neurosci. 2020, 11, 1706–1725. [Google Scholar] [CrossRef]
- Kim, J.J.; Gharpure, A.; Teng, J.; Zhuang, Y.; Howard, R.J.; Zhu, S.; Noviello, C.M.; Walsh, R.M.; Lindahl, E.; Hibbs, R.E. Shared Structural Mechanisms of General Anaesthetics and Benzodiazepines. Nature 2020, 585, 303–308. [Google Scholar] [CrossRef]
- Filipe, H.A.L.; Loura, L.M.S. Molecular Dynamics Simulations: Advances and Applications. Molecules 2022, 27, 2105. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Huang, H.-H.; Yang, J.; Su, Y.-P.; Lin, H.-W.; Lin, L.-Q.; Liao, W.-J.; Yu, C.-X. The Active Alkaloids of Gelsemium elegans Benth. Are Potent Anxiolytics. Psychopharmacology 2013, 225, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Tang, M.; Zuo, M.; Xu, W.; Meng, S.; Liu, Z. The Antianxiety Effects of Koumine and Gelsemine, Two Main Active Components in the Traditional Chinese Herbal Medicine Gelsemium: A Comprehensive Review. Brain X 2023, 1, e46. [Google Scholar] [CrossRef]
- Olsen, R.W. GABAA Receptor: Positive and Negative Allosteric Modulators. Neuropharmacology 2018, 136, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Legesse, D.H.; Fan, C.; Teng, J.; Zhuang, Y.; Howard, R.J.; Noviello, C.M.; Lindahl, E.; Hibbs, R.E. Structural Insights into Opposing Actions of Neurosteroids on GABAA Receptors. Nat. Commun. 2023, 14, 5091. [Google Scholar] [CrossRef] [PubMed]
- Marzotto, M.; Olioso, D.; Brizzi, M.; Tononi, P.; Cristofoletti, M.; Bellavite, P. Extreme Sensitivity of Gene Expression in Human SH-SY5Y Neurocytes to Ultra-Low Doses of Gelsemium sempervirens. BMC Complement. Altern. Med. 2014, 14, 104. [Google Scholar] [CrossRef][Green Version]
- Jin, G.-L.; Su, Y.-P.; Liu, M.; Xu, Y.; Yang, J.; Liao, K.-J.; Yu, C.-X. Medicinal Plants of the Genus Gelsemium (Gelsemiaceae, Gentianales)--a Review of Their Phytochemistry, Pharmacology, Toxicology and Traditional Use. J. Ethnopharmacol. 2014, 152, 33–52. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.-J.; Huang, C.-Y.; Zuo, M.-T.; Gong, M.-D.; Huang, S.-J.; Tang, M.-H.; Liu, Z.-Y. Network Pharmacology and Experimental Verification to Unveil the Mechanism of N-Methyl-D-Aspartic Acid Rescue Humantenirine-Induced Excitotoxicity. Metabolites 2023, 13, 195. [Google Scholar] [CrossRef]
- Huang, S.J.; Zuo, M.T.; Qi, X.J.; Huang, C.Y.; Liu, Z.Y. Phosphoproteomics Reveals NMDA Receptor-Mediated Excitotoxicity as a Key Signaling Pathway in the Toxicity of Gelsenicine. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2021, 156, 112507. [Google Scholar] [CrossRef]
- Gong, P.; Hong, H.; Perkins, E.J. Ionotropic GABA Receptor Antagonism-Induced Adverse Outcome Pathways for Potential Neurotoxicity Biomarkers. Biomark. Med. 2015, 9, 1225–1239. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A Web-Based Graphical User Interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef]
- Tian, C.; Kasavajhala, K.; Belfon, K.A.A.; Raguette, L.; Huang, H.; Migues, A.N.; Bickel, J.; Wang, Y.; Pincay, J.; Wu, Q.; et al. ff19SB: Amino-Acid-Specific Protein Backbone Parameters Trained against Quantum Mechanics Energy Surfaces in Solution. J. Chem. Theory Comput. 2020, 16, 528–552. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and Testing of a General Amber Force Field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Martyna, G.J.; Tobias, D.J.; Klein, M.L. Constant Pressure Molecular Dynamics Algorithms. J. Chem. Phys. 1994, 101, 4177–4189. [Google Scholar] [CrossRef]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical Sampling through Velocity Rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, W.L. Free Energy Calculations: A Breakthrough for Modeling Organic Chemistry in Solution. Acc. Chem. Res. 1989, 22, 184–189. [Google Scholar] [CrossRef]
- Sun, H.; Duan, L.; Chen, F.; Liu, H.; Wang, Z.; Pan, P.; Zhu, F.; Zhang, J.Z.H.; Hou, T. Assessing the Performance of MM/PBSA and MM/GBSA Methods. 7. Entropy Effects on the Performance of End-Point Binding Free Energy Calculation Approaches. Phys. Chem. Chem. Phys. 2018, 20, 14450–14460. [Google Scholar] [CrossRef]
- Valdés-Tresanco, M.S.; Valdés-Tresanco, M.E.; Valiente, P.A.; Moreno, E. gmx_MMPBSA: A New Tool to Perform End-State Free Energy Calculations with GROMACS. J. Chem. Theory Comput. 2021, 17, 6281–6291. [Google Scholar] [CrossRef] [PubMed]

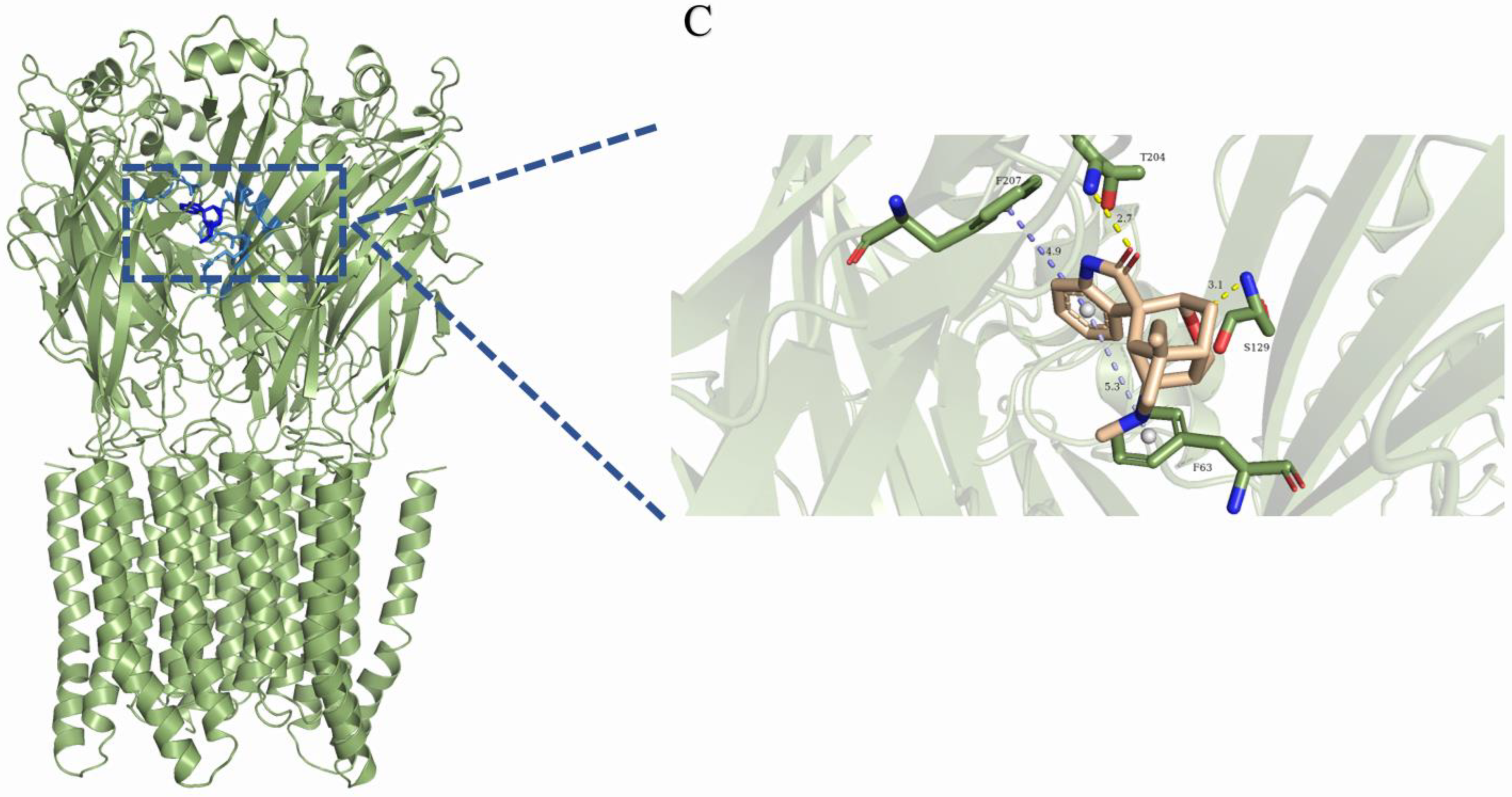
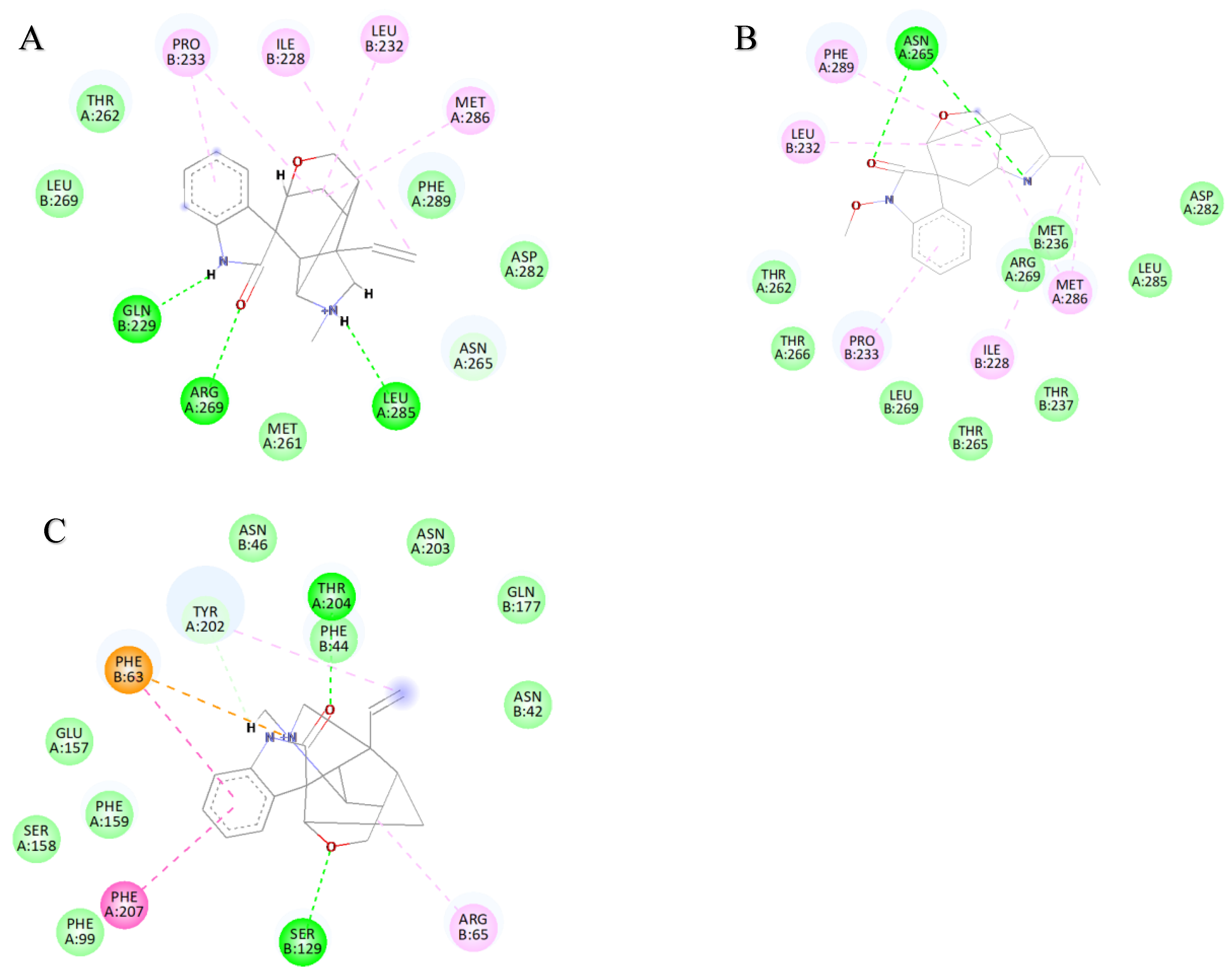

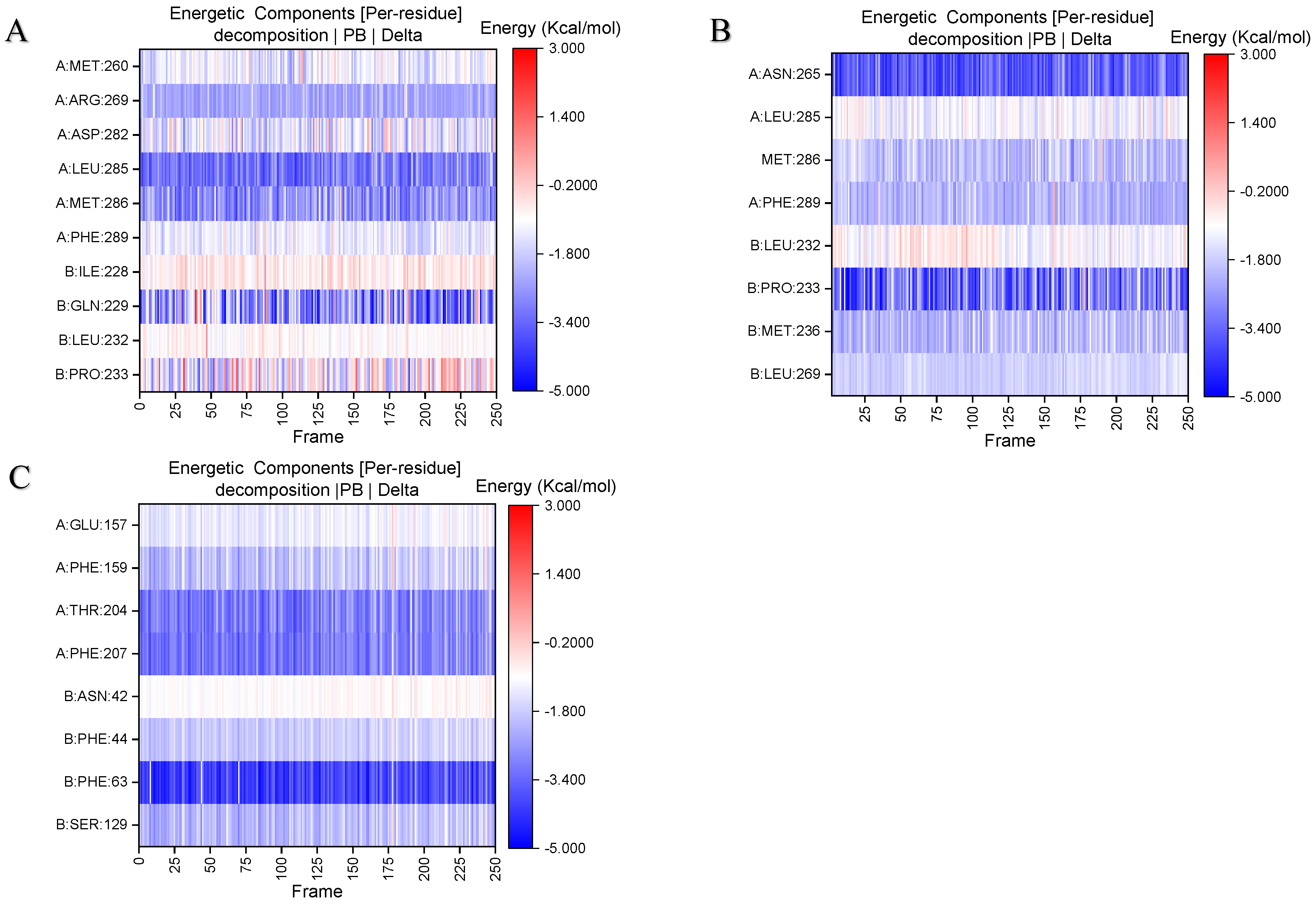
| Koumine | Gelsemine | Gelsevirine | Gelsenicine | Humantenirine | |
|---|---|---|---|---|---|
| GABAAR | −55.54% ± 4.35% | −58.78% ± 1.74% | −55.82% ± 0.35% | 58.63% ± 5.56% | 41.66% ± 0.53% |
| α1GlyR | −95.53% ± 0.70% | −70.39% ± 4.85% | −98.89% ± 0.68% | −13.64% ± 4.34% | −11.75% ± 4.34% |
| α1βGlyR | −87.29% ± 0.52% | −92.18% ± 2.87% | −99.06% ± 0.62% | −10.44% ± 5.99% | −16.82% ± 4.41% |
| nACHR | 1.42% ± 0.00% | ND | 0.75% ± 0.65% | ND | ND |
| Alkaloids | IC50 (μM) | EC50 (μM) | Hillslope | n | |
|---|---|---|---|---|---|
| GABAAR | Koumine | 142.8 | ND | −0.9283 | 4 |
| Gelsemine | 170.8 | ND | −0.9158 | 4 | |
| Gelsevirine | 251.5 | ND | −1.110 | 4 | |
| Gelsenicine | ND | 192.1 | 0.8002 | 4 | |
| α1GlyR | Koumine | 9.587 | ND | 1.079 | 4 |
| Gelsemine | 10.36 | ND | 1.316 | 4 | |
| Gelsevirine | 82.94 | ND | 1.179 | 4 |
| Gelsemine | Gelsenicine | |
|---|---|---|
| GABAAR Site 1 | −7.44 ± 3.47 | −10.88 ± 3.92 |
| GABAAR Site 2 | −10.25 ± 4.13 | −14.83 ± 5.23 |
| GABAAR Site 3 | −26.89 ± 4.91 | −22.75 ± 4.26 |
| GlyR | −30.7 ± 3.42 | −12.17 ± 3.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Yang, Z.; Huang, T.; Pan, L.; Ding, J.; Liu, Z. Experimental and Computational Investigation of the Target and Mechanisms of Gelsemium Alkaloids in the Central Nervous System. Int. J. Mol. Sci. 2025, 26, 1312. https://doi.org/10.3390/ijms26031312
Wang Y, Yang Z, Huang T, Pan L, Ding J, Liu Z. Experimental and Computational Investigation of the Target and Mechanisms of Gelsemium Alkaloids in the Central Nervous System. International Journal of Molecular Sciences. 2025; 26(3):1312. https://doi.org/10.3390/ijms26031312
Chicago/Turabian StyleWang, Yunfan, Zhijiang Yang, Tengxin Huang, Li Pan, Junjie Ding, and Zhaoying Liu. 2025. "Experimental and Computational Investigation of the Target and Mechanisms of Gelsemium Alkaloids in the Central Nervous System" International Journal of Molecular Sciences 26, no. 3: 1312. https://doi.org/10.3390/ijms26031312
APA StyleWang, Y., Yang, Z., Huang, T., Pan, L., Ding, J., & Liu, Z. (2025). Experimental and Computational Investigation of the Target and Mechanisms of Gelsemium Alkaloids in the Central Nervous System. International Journal of Molecular Sciences, 26(3), 1312. https://doi.org/10.3390/ijms26031312






