ETS1 Protein Expression May Be Altered by the Complementarity of ETS1 mRNA Sequences with miR-203a-3p and miR-204-3p in Papillary Thyroid Carcinoma
Abstract
1. Introduction
2. Results
2.1. ETS1 Expression in PTC and Matched NMT
2.2. Bioinformatic Analysis and Model Prediction
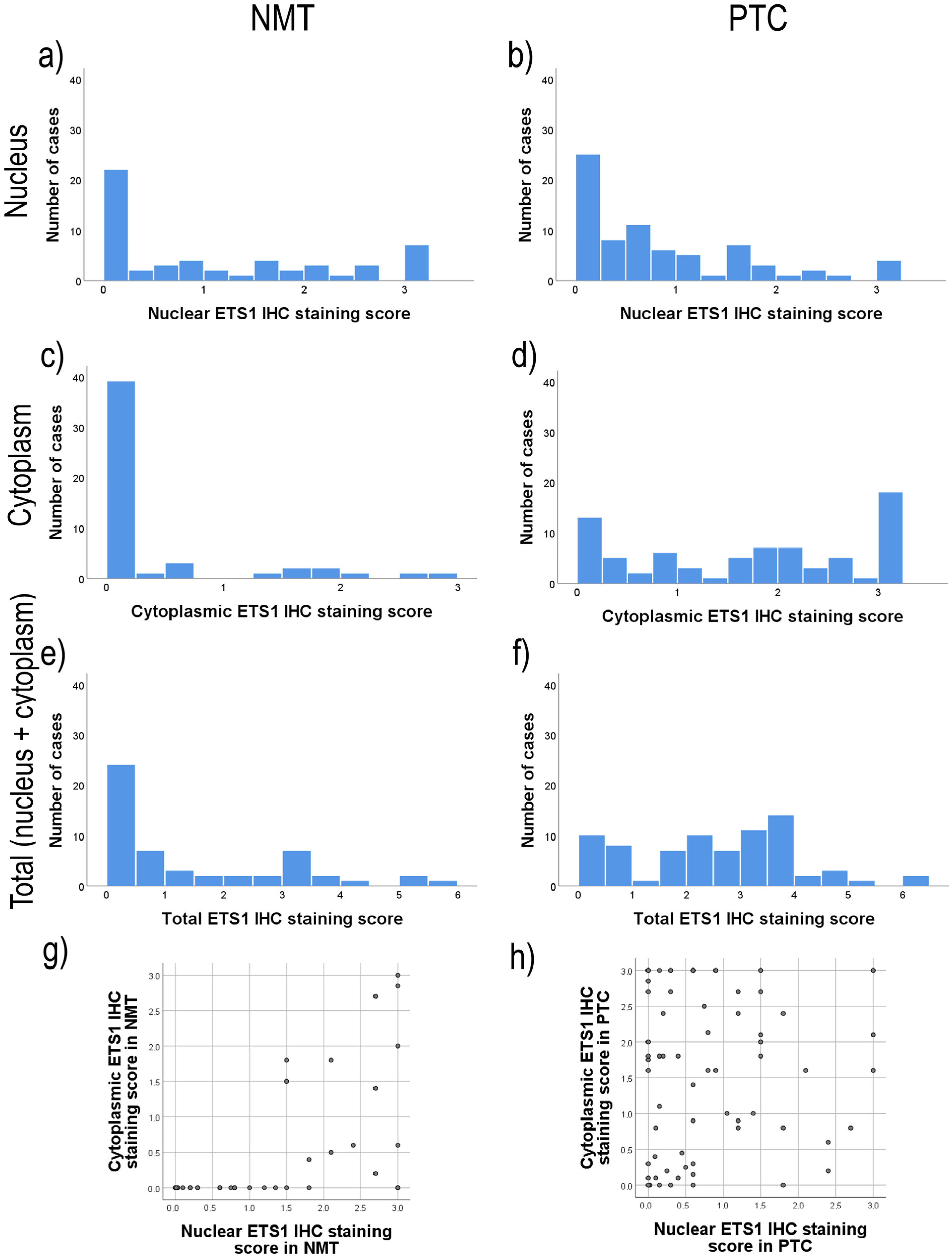
2.3. The Level of Expression of the ETS1 Protein by Cell Compartments
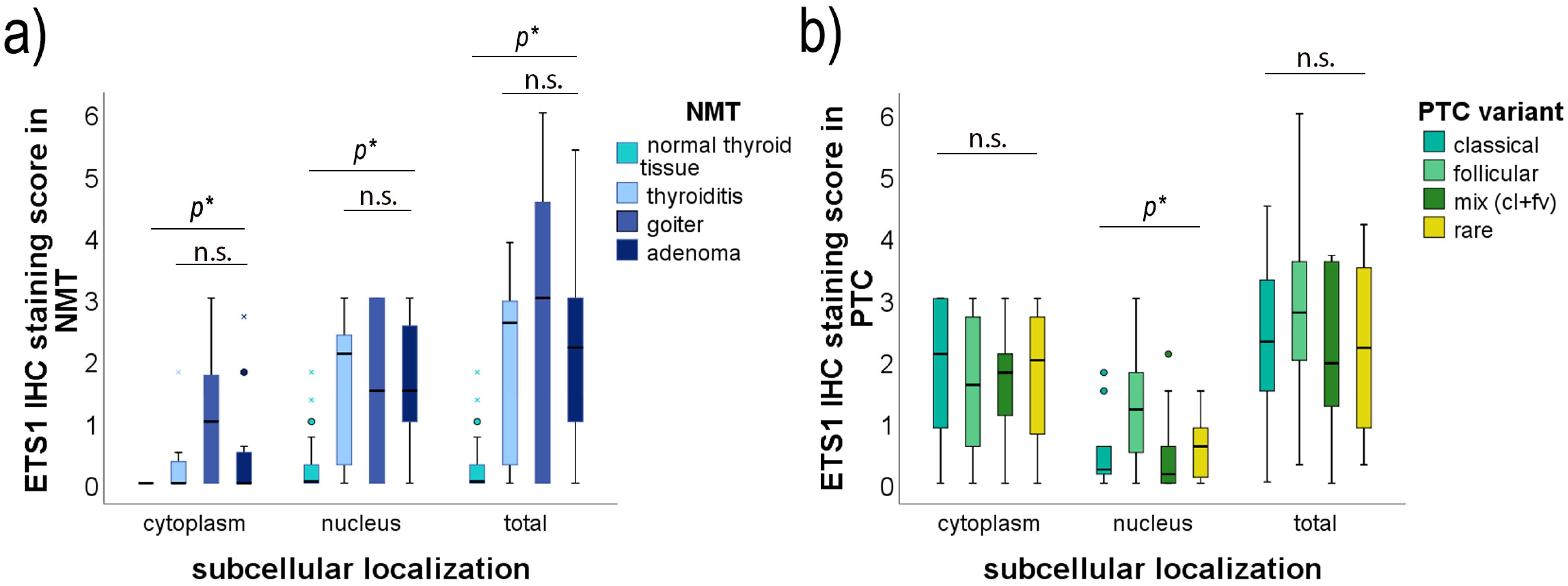
2.4. Association of ETS1 IHC Staining and Clinicopathological Data of PTC Patients
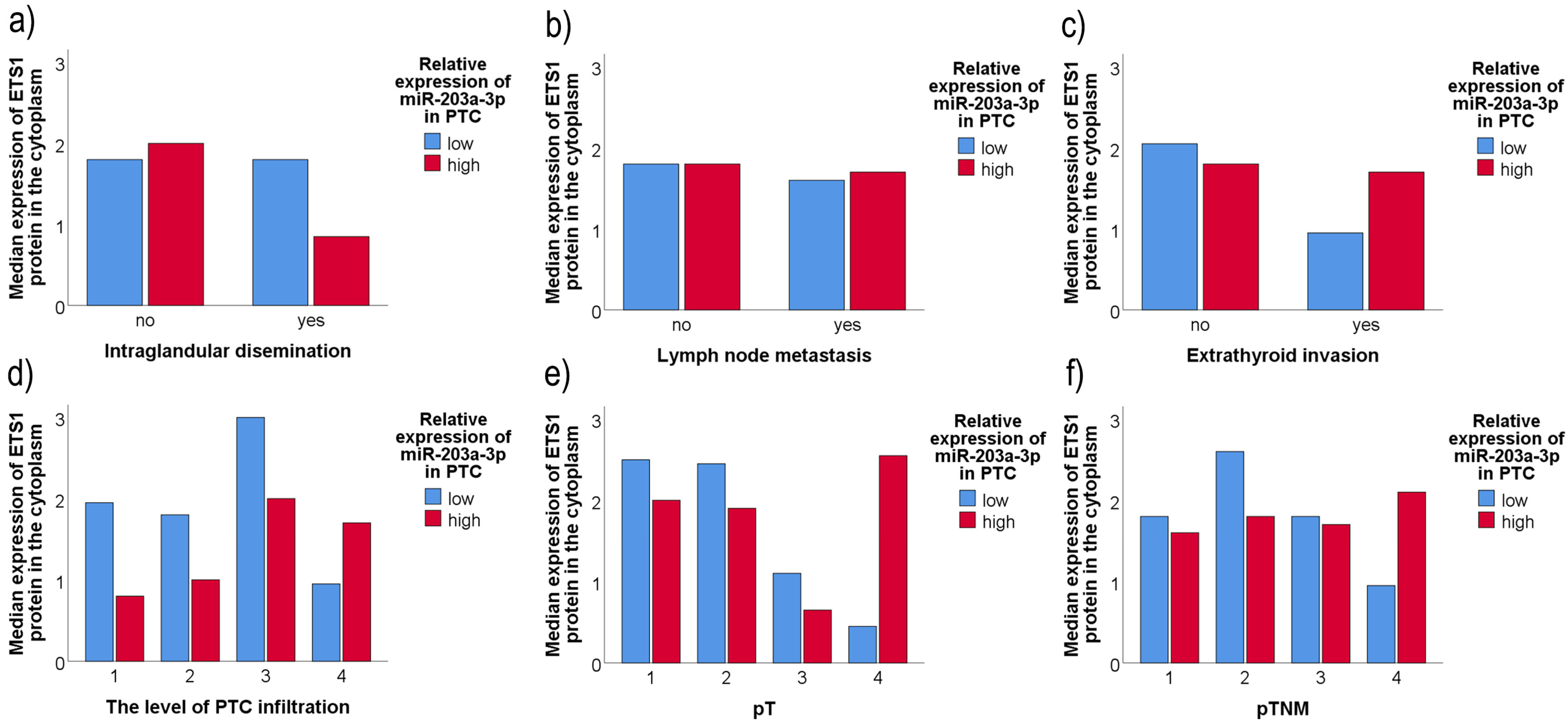
2.5. Association of ETS1 IHC Staining, miR-203a-3p/miR-204-3p Level of Expression, and the Clinicopathological Data of PTC Patients
3. Discussion
4. Materials and Methods
4.1. Patients and Tissue Samples
4.2. RNA Isolation
4.3. Reverse Transcription and Quantitative PCR Analysis for miR-203a-3p and miR-204-3p
- miR-203a-3p-RT: 5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCTAGTG-3′
- miR-204-3p-RT: 5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACACGTCC-3′
- miR-u6-RT: 5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAAAAATATGG-3′
- miR-U6-Fw: 5′-GCGGTCGCAAGGATGACACG-3′
- miR-203a-3p-Fw: 5′-CGGCGGTGTGAAATGTTTAGGAC-3′
- miR-204-3p-Fw: 5′-GCGGTGCUGGGAAGGCAAAG-3′
- universal miR-Rv: 5′-CCAGTGCAGGGTCCGAGGTAT-3′
4.4. Reverse Transcription and Quantitative PCR for ETS1
- GAPDH-Fw: 5′-GAAGGTGAAGGTCGGAGT-3′
- GAPDH-Rv: 5′-GAA GATGGTGATGGGATTTC-3′
- ETS1-Fw: 5′-GCCCAGCTTCATCACAGAGT-3′
- ETS1-Rv: 5′-CCCCGAGTTTACCACGACTG-3′
4.5. Protein Extraction and Western Immunoblotting
4.6. Immunohistochemistry
4.7. Bioinformatic Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Miyauchi, A.; Kihara, M.; Fukushima, M.; Higashiyama, T.; Miya, A. Overall Survival of Papillary Thyroid Carcinoma Patients: A Single-Institution Long-Term Follow-Up of 5897 Patients. World J. Surg. 2018, 42, 615–622. [Google Scholar] [CrossRef]
- Pelizzo, M.R.; Boschin, I.M.; Toniato, A.; Piotto, A.; Pagetta, C.; Gross, M.D.; Al-Nahhas, A.F.; Rubello, D. Papillary Thyroid Carcinoma: 35-Year Outcome and Prognostic Factors in 1858 Patients. Clin. Nucl. Med. 2007, 32, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Dittmer, J. The Biology of the Ets1 Proto-Oncogene. Mol. Cancer 2003, 2, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, Z.; Sun, M.; Huang, W.; Xia, L. ETS transcription factors: Multifaceted players from cancer progression to tumor immunity. Biochim. et Biophys. Acta (BBA)-Rev. Cancer 2023, 1878, 188872. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Ito, M.; Ohtsuru, A.; Naito, S.; Nakashima, M.; A Fagin, J.; Yamashita, S.; Sekine, I. Expression of the Ets-1 proto-oncogene in human gastric carcinoma: Correlation with tumor invasion. Am. J. Pathol. 1996, 149, 1931–1939. [Google Scholar] [PubMed]
- Ito, T.; Nakayama, T.; Ito, M.; Naito, S.; Kanematsu, T.; Sekine, I. Expression of the ets-1 proto-oncogene in human pancreatic carcinoma. Mod. Pathol. 1998, 11, 209–215. [Google Scholar] [PubMed]
- Kar, A.; Gutierrez-Hartmann, A. Molecular mechanisms of ETS transcription factor-mediated tumorigenesis. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 522–543. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Ito, M.; Ohtsuru, A.; Naito, S.; Sekine, I. Expression of the ets-1 Proto-Oncogene in Human Colorectal Carcinoma. Mod. Pathol. 2001, 14, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Takeda, T.; Okada, M.; Matsuura, N. Expression of ets-1 and ets-2 in colonic neoplasms. Anticancer Res. 2002, 22, 1581–1584. [Google Scholar]
- Lincoln, D.W.; Bove, K. The transcription factor Ets-1 in breast cancer. Front. Biosci. 2005, 10, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Alipov, G.; Nakayama, T.; Ito, M.; Kawai, K.; Naito, S.; Nakashima, M.; Niino, D.; Sekine, I. Overexpression of Ets-1 proto-oncogene in latent and clinical prostatic carcinomas. Histopathology 2005, 46, 202–208. [Google Scholar] [CrossRef]
- Fuhrer, D.; Eszlinger, M.; Karger, S.; Krause, K.; Engelhardt, C.; Hasenclever, D.; Dralle, H.; Paschke, R. Evaluation of insulin-like growth factor II, cyclooxygenase-2, ets-1 and thyroid-specific thyroglobulin mRNA expression in benign and malignant thyroid tumours. Eur. J. Endocrinol. 2005, 152, 785–790. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakayama, T.; Ito, M.; Ohtsuru, A.; Naito, S.; Nakashima, M.; Sekine, I. Expression of the ets-1 proto-oncogene in human thyroid tumor. Mod. Pathol. 1999, 12, 61–68. [Google Scholar]
- Xu, Y.; Gao, J.; Wang, N.; Zedenius, J.; Nilsson, I.-L.; Lui, W.-O.; Xu, D.; Juhlin, C.C.; Larsson, C.; Mu, N. BRAF-induced EHF Expression Affects TERT in Aggressive Papillary Thyroid Cancer. J. Clin. Endocrinol. Metab. 2024, dgae589. [Google Scholar] [CrossRef]
- De Nigris, F.; Mega, T.; Berger, N.; Barone, M.V.; Santoro, M.; Viglietto, G.; Verde, P.; Fusco, A. Induction of ETS-1 and ETS-2 transcription factors is required for thyroid cell transformation. Cancer Res. 2001, 61, 2267–2275. [Google Scholar] [PubMed]
- Peyret, V.; Nazar, M.; Martín, M.; Quintar, A.A.; Fernandez, E.A.; Geysels, R.C.; Fuziwara, C.S.; Montesinos, M.M.; Maldonado, C.A.; Santisteban, P.; et al. Functional Toll-like Receptor 4 Overexpression in Papillary Thyroid Cancer by MAPK/ERK–Induced ETS1 Transcriptional Activity. Mol. Cancer Res. 2018, 16, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Choi, Y.W.; Han, J.H.; Lee, J.; Soh, E.Y.; Park, S.H.; Kim, J.-H.; Park, T.J. TSH Signaling Overcomes B-RafV600E–Induced Senescence in Papillary Thyroid Carcinogenesis through Regulation of DUSP6. Neoplasia 2014, 16, 1107–1120. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Song, Y.S.; Yoo, S.-K.; Kim, H.H.; Jung, G.; Oh, A.-R.; Cha, J.-Y.; Kim, S.-J.; Cho, S.W.; Lee, K.E.; Seo, J.-S.; et al. Interaction of BRAF-induced ETS factors with mutant TERT promoter in papillary thyroid cancer. Endocr. Relat. Cancer 2019, 26, 629–641. [Google Scholar] [CrossRef]
- Plotnik, J.P.; Budka, J.A.; Ferris, M.W.; Hollenhorst, P.C. ETS1 is a genome-wide effector of RAS/ERK signaling in epithelial cells. Nucleic Acids Res. 2014, 42, 11928–11940. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Li, W.; Zhang, B.; Liu, X. Overexpression of lncRNA SLC26A4-AS1 inhibits papillary thyroid carcinoma progression through recruiting ETS1 to promote ITPR1-mediated autophagy. J. Cell. Mol. Med. 2021, 25, 8148–8158. [Google Scholar] [CrossRef] [PubMed]
- Liu, X. Overexpression of lncRNA SLC26A4-AS1 inhibits papillary thyroid carcinoma progression through recruiting ETS1 to promote ITPR1-mediated autophagy. J. Cell. Mol. Med. 2022, 26, 2750–2751. [Google Scholar] [CrossRef]
- Kloosterman, W.P.; Plasterk, R.H. The Diverse Functions of MicroRNAs in Animal Development and Disease. Dev. Cell 2006, 11, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Farazi, T.A.; Hoell, J.I.; Morozov, P.; Tuschl, T. MicroRNAs in human cancer. Adv. Exp. Med. Biol. 2013, 774, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ali Syeda, Z.; Langden, S.S.S.; Munkhzul, C.; Lee, M.; Song, S.J. Regulatory Mechanism of MicroRNA Expression in Cancer. Int. J. Mol. Sci. 2020, 21, 1723. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, S.; Chen, Z.; Huv, S.; Huang, K.; Huang, B.; Du, J.; Huang, C.; Peng, L.; Jian, Z.; et al. MiR-204 regulates HMGA2 expression and inhibits cell proliferation in human thyroid cancer. Cancer Biomarkers 2015, 15, 535–542. [Google Scholar] [CrossRef]
- Liu, L.; Wang, J.; Li, X.; Ma, J.; Shi, C.; Zhu, H.; Xi, Q.; Zhang, J.; Zhao, X.; Gu, M. miR-204-5p suppresses cell proliferation by inhibiting IGFBP5 in papillary thyroid carcinoma. Biochem. Biophys. Res. Commun. 2015, 457, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Stojanović, S.; Dobrijević, Z.; Šelemetjev, S.; Đorić, I.; Miljuš, J.J.; Živaljević, V.; Denčić, T.I. MiR-203a-3p, miR-204-3p, miR-222-3p as useful diagnostic and prognostic tool for thyroid neoplasia spectrum. Endocrine 2022, 79, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Stojanović, S.; Šelemetjev, S.; Đorić, I.; Miljuš, J.J.; Tatić, S.; Živaljević, V.; Denčić, T.I. BRAFV600E, BANCR, miR-203a-3p and miR-204-3p in Risk Stratification of PTC Patients. Biomedicines 2023, 11, 3338. [Google Scholar] [CrossRef]
- Zhu, J.; Zheng, X.; Yang, X. Diagnostic and mechanistic values of microRNA-130a and microRNA-203 in patients with papillary thyroid carcinoma. J. Cell. Biochem. 2019, 121, 3657–3666. [Google Scholar] [CrossRef] [PubMed]
- Le, F.; Luo, P.; Ouyang, Q.; Zhong, X. LncRNA WT1-AS Downregulates Survivin by Upregulating miR-203 in Papillary Thyroid Carcinoma. Cancer Manag. Res. 2020, 12, 443–449. [Google Scholar] [CrossRef]
- Yang, S.; Liu, T.; Sun, Y.; Liang, X. The long noncoding RNA LINC00483 promotes lung adenocarcinoma progression by sponging miR-204-3p. Cell. Mol. Biol. Lett. 2019, 24, 1–14. [Google Scholar] [CrossRef]
- Chmielik, E.; Rusinek, D.; Oczko-Wojciechowska, M.; Jarzab, M.; Krajewska, J.; Czarniecka, A.; Jarzab, B. Heterogeneity of Thyroid Cancer. Pathobiology 2018, 85, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, W.; Zhang, X.; Hu, L.; Tang, G.; Kong, J.; Wang, Z. E26 transformation (ETS)-specific related transcription factor-3 (ELF3) orchestrates a positive feedback loop that constitutively activates the MAPK/Erk pathway to drive thyroid cancer. Oncol. Rep. 2018, 41, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Bories, J.-C.; Willerford, D.M.; Grévin, D.; Davidson, L.; Camus, A.; Martin, P.; Stéhelin, D.; Alt, F.W. Increased T-cell apoptosis and terminal B-cell differentiation induced by inactivation of the Ets-1 proto-oncogene. Nature 1995, 377, 635–638. [Google Scholar] [CrossRef]
- Huang, C.-C.; Papas, T.S.; Bhat, N.K. A variant form of ETS1 induces apoptosis in human colon cancer cells. Oncogene 1997, 15, 851–856. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, R.; Pei, H.; Papas, T. The p42 variant of ETS1 protein rescues defective Fas-induced apoptosis in colon carcinoma cells. Proc. Natl. Acad. Sci. USA 1999, 96, 3876–3881. [Google Scholar] [CrossRef] [PubMed]
- Behrens, P.; Rothe, M.; Wellmann, A.; Krischler, J.; Wernert, N. The Ets-1 transcription factor is up-regulated together with MMP 1 and MMP 9 in the stroma of pre-invasive breast cancer. J. Pathol. 2001, 194, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Kitange, G.; Tsunoda, K.; Anda, T.; Nakamura, S.; Yasunaga, A.; Naito, S.; Shibata, S. Immunohistochemical expression of Ets-1 transcription factor and the urokinase-type plasminogen activator is correlated with the malignant and invasive potential in meningiomas. Cancer 2000, 89, 2292–2300. [Google Scholar] [CrossRef] [PubMed]
- Nakada, M.; Yamashita, J.; Okada, Y.; Sato, H. Ets-1 Positively Regulates Expression of Urokinase-type Plasminogen Activator (uPA) and Invasiveness of Astrocytic Tumors. J. Neuropathol. Exp. Neurol. 1999, 58, 329–334. [Google Scholar] [CrossRef][Green Version]
- Naito, S.; Shimizu, K.; Nakashima, M.; Nakayama, T.; Ito, T.; Ito, M.; Yamashita, S.; Sekine, I. Overexpression of Ets-1 Transcription Factor in Angiosarcoma of the Skin. Pathol.-Res. Pr. 2000, 196, 103–109. [Google Scholar] [CrossRef]
- Iwasaka, C.; Tanaka, K.; Abe, M.; Sato, Y. Ets-1 regulates angiogenesis by inducing the expression of urokinase-type plas-minogen activator and matrix metalloproteinase-1 and the migration of vascular endothelial cells. J. Cell Physiol. 1996, 169, 522–531. [Google Scholar] [CrossRef]
- Behrens, P.; Rothe, M.; Florin, A.; Wellmann, A.; Wernert, N. Invasive properties of serous human epithelial ovarian tumors are related to Ets-1, MMP-1 and MMP-9 expression. Int. J. Mol. Med. 2001, 8, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Teruyama, K.; Nakano, T.; Oda, N.; Abe, M.; Tanaka, K.; Iwasaka-Yagi, C. Role of transcription factors in angiogenesis: Ets-1 promotes angiogenesis as well as endothelial apoptosis. Ann. N. Y. Acad. Sci. 2001, 947, 117–123. [Google Scholar] [CrossRef]
- Naito, S.; Shimizuc, S.; Matsuub, M.; Nakashimad, M.; Nakayamab, T.; Yamashitae, S.; Sekineb, I. Ets-1 Upregulates Matrix Metalloproteinase-1 Expression through Extracellular Matrix Adhesion in Vascular Endothelial Cells. Biochem. Biophys. Res. Commun. 2002, 291, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Fisher, R.J.; Riggs, C.W.; Rhim, J.S.; A Lautenberger, J. Inhibition of vascular endothelial growth factor-induced endothelial cell migration by ETS1 antisense oligonucleotides. Cancer Res. 1997, 57, 2013–2019. [Google Scholar]
- Lincoln, D.W.; Phillips, P.G.; Bove, K. Estrogen-Induced Ets-1 Promotes Capillary Formation in an in vitro Tumor Angiogenesis Model. Breast Cancer Res. Treat. 2003, 78, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Santiago, K.; Wongworawat, Y.C.; Khan, S. Differential MicroRNA-Signatures in Thyroid Cancer Subtypes. J. Oncol. 2020, 2020, 1–14. [Google Scholar] [CrossRef]
- Celano, M.; Rosignolo, F.; Maggisano, V.; Pecce, V.; Iannone, M.; Russo, D.; Bulotta, S. MicroRNAs as Biomarkers in Thyroid Carcinoma. Int. J. Genom. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Yang, L.; Liang, H.; Wang, Y.; Gao, S.; Yin, K.; Liu, Z.; Zheng, X.; Lv, Y.; Wang, L.; Zhang, C.-Y.; et al. MiRNA-203 suppresses tumor cell proliferation, migration and invasion by targeting Slug in gastric cancer. Protein Cell 2016, 7, 383–387. [Google Scholar] [CrossRef]
- Wu, X.; Dai, L.; Zhang, Z.; Zheng, J.; Zhao, J. Overexpression of microRNA-203 can downregulate survivin and function as a potential therapeutic target in papillary thyroid cancer. Oncol. Lett. 2019, 19, 61–68. [Google Scholar] [CrossRef] [PubMed]
- LiVolsi, V.A.; Albores-Saavedra, J.; Asa, S.L.; Baloch, Z.W.; Sobrinho-Simões, M.; Wenig, B.; DeLellis, R.A.; Cady, B.; Mazzaferri, E.L.; Hay, I.; et al. Papillary carcinoma Pathology and Genetics of Tumors of Endocrine Organs. In World Health Organization Classification of Tumors; DeLellis, R.A., Lloyd, R., Heitz, P.U., Eng, C., Eds.; IARC Press: Lyon, France, 2004; pp. 50–66. [Google Scholar]
- Edge, S.B.; Byrd, D.R.; Compton, C.C.; Fritz, A.G.; Greene, F.L.; Trotti, A. AJCC Cancer Staging Manual, 7th ed.; Springer: New York, NY, USA, 2010; pp. 87–96. [Google Scholar]
- Basolo, F.; Torregrossa, L.; Giannini, R.; Miccoli, M.; Lupi, C.; Sensi, E.; Berti, P.; Elisei, R.; Vitti, P.; Baggiani, A.; et al. Correlation between the BRAF V600E Mutation and Tumor Invasiveness in Papillary Thyroid Carcinomas Smaller than 20 Millimeters: Analysis of 1060 Cases. J. Clin. Endocrinol. Metab. 2010, 95, 4197–4205. [Google Scholar] [CrossRef]




| NCBI Gene ID | 2113 | GenBank Accession | NM_001143820 |
| Gene Symbol | ETS1 | 3′ UTR Length | 3600 |
| Gene Description | ETS proto-oncogene 1, transcription factor | ||
| miRNA Name | miR-203a-3p | miRNA Sequence | GUGAAAUGUUUAGGACCACUAG |
| Target Score | 63 | Seed Location | 1343, 2350 |
| URL | https://mirdb.org/cgi-bin/target_detail.cgi?targetID=3441945 | ||
| miRNA Name | miR-204-3p | miRNA Sequence | GCUGGGAAGGCAAAGGGACGU |
| Target Score | 98 | Seed Location | 1949, 2802 |
| URL | https://mirdb.org/cgi-bin/target_detail.cgi?targetID=3259206 | ||
| Clinicopathological Characteristics of PTC Patients | Cytoplasm | Nucleus | ||
|---|---|---|---|---|
| r | p-Value | r | p-Value | |
| Gender | −0.062 | 0.597 | −0.034 | 0.774 |
| Age | −0.077 | 0.508 | 0.175 | 0.137 |
| Tumor size | −0.175 | 0.131 | 0.141 | 0.230 |
| Ei | −0.153 | 0.187 | −0.067 | 0.571 |
| ID | −0.149 | 0.197 | 0.188 | 0.108 |
| LNM | −0.030 | 0.794 | −0.271 | 0.020* |
| DTI | −0.052 | 0.658 | −0.164 | 0.163 |
| pT | −0.267 | 0.020 * | 0.040 | 0.735 |
| pTNM | −0.075 | 0.519 | 0.105 | 0.376 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novković, S.S.; Šelemetjev, S.; Miljuš, J.J.; Živaljević, V.; Dunđerović, D.; Milinković, M.; Denčić, T.I. ETS1 Protein Expression May Be Altered by the Complementarity of ETS1 mRNA Sequences with miR-203a-3p and miR-204-3p in Papillary Thyroid Carcinoma. Int. J. Mol. Sci. 2025, 26, 1253. https://doi.org/10.3390/ijms26031253
Novković SS, Šelemetjev S, Miljuš JJ, Živaljević V, Dunđerović D, Milinković M, Denčić TI. ETS1 Protein Expression May Be Altered by the Complementarity of ETS1 mRNA Sequences with miR-203a-3p and miR-204-3p in Papillary Thyroid Carcinoma. International Journal of Molecular Sciences. 2025; 26(3):1253. https://doi.org/10.3390/ijms26031253
Chicago/Turabian StyleNovković, Stefana Stojanović, Sonja Šelemetjev, Jelena Janković Miljuš, Vladan Živaljević, Duško Dunđerović, Marija Milinković, and Tijana Išić Denčić. 2025. "ETS1 Protein Expression May Be Altered by the Complementarity of ETS1 mRNA Sequences with miR-203a-3p and miR-204-3p in Papillary Thyroid Carcinoma" International Journal of Molecular Sciences 26, no. 3: 1253. https://doi.org/10.3390/ijms26031253
APA StyleNovković, S. S., Šelemetjev, S., Miljuš, J. J., Živaljević, V., Dunđerović, D., Milinković, M., & Denčić, T. I. (2025). ETS1 Protein Expression May Be Altered by the Complementarity of ETS1 mRNA Sequences with miR-203a-3p and miR-204-3p in Papillary Thyroid Carcinoma. International Journal of Molecular Sciences, 26(3), 1253. https://doi.org/10.3390/ijms26031253



_Kim.png)



