An Oxymetazoline-Based Nasal Solution Removes Bacteria–Blood Debris on Dental Surfaces and Has Antimicrobial Activity Toward Streptococcus mutans
Abstract
1. Introduction
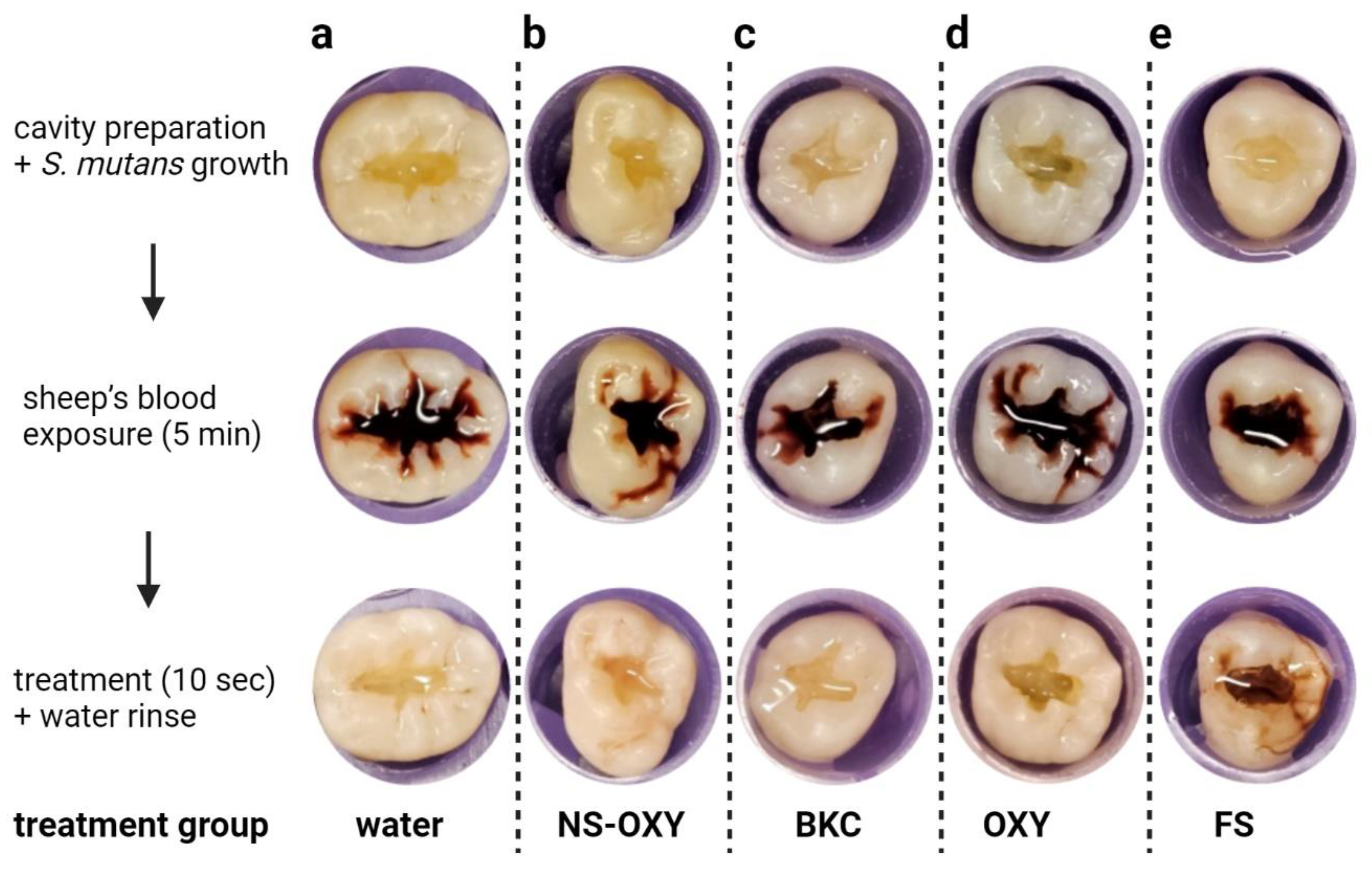
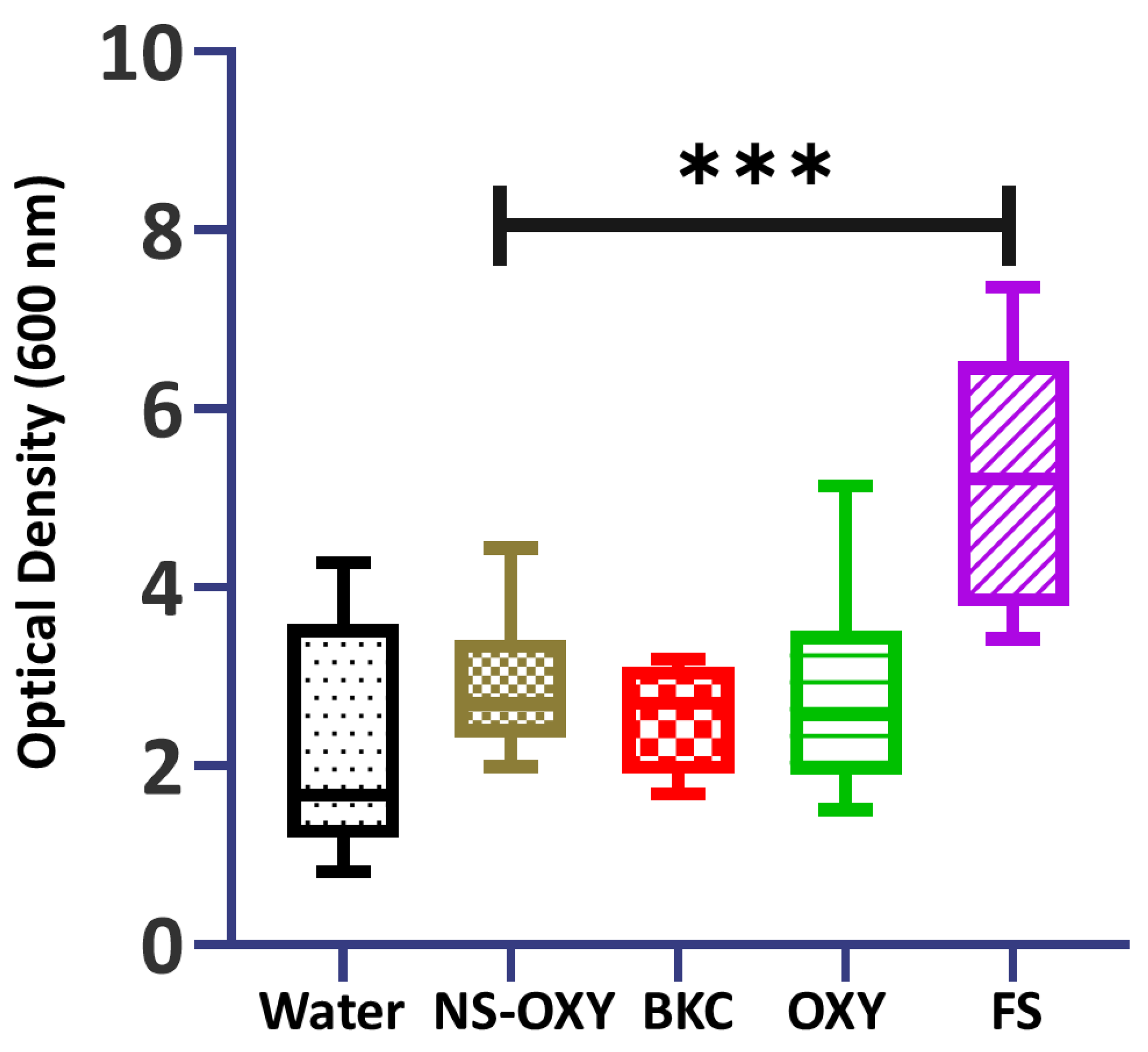
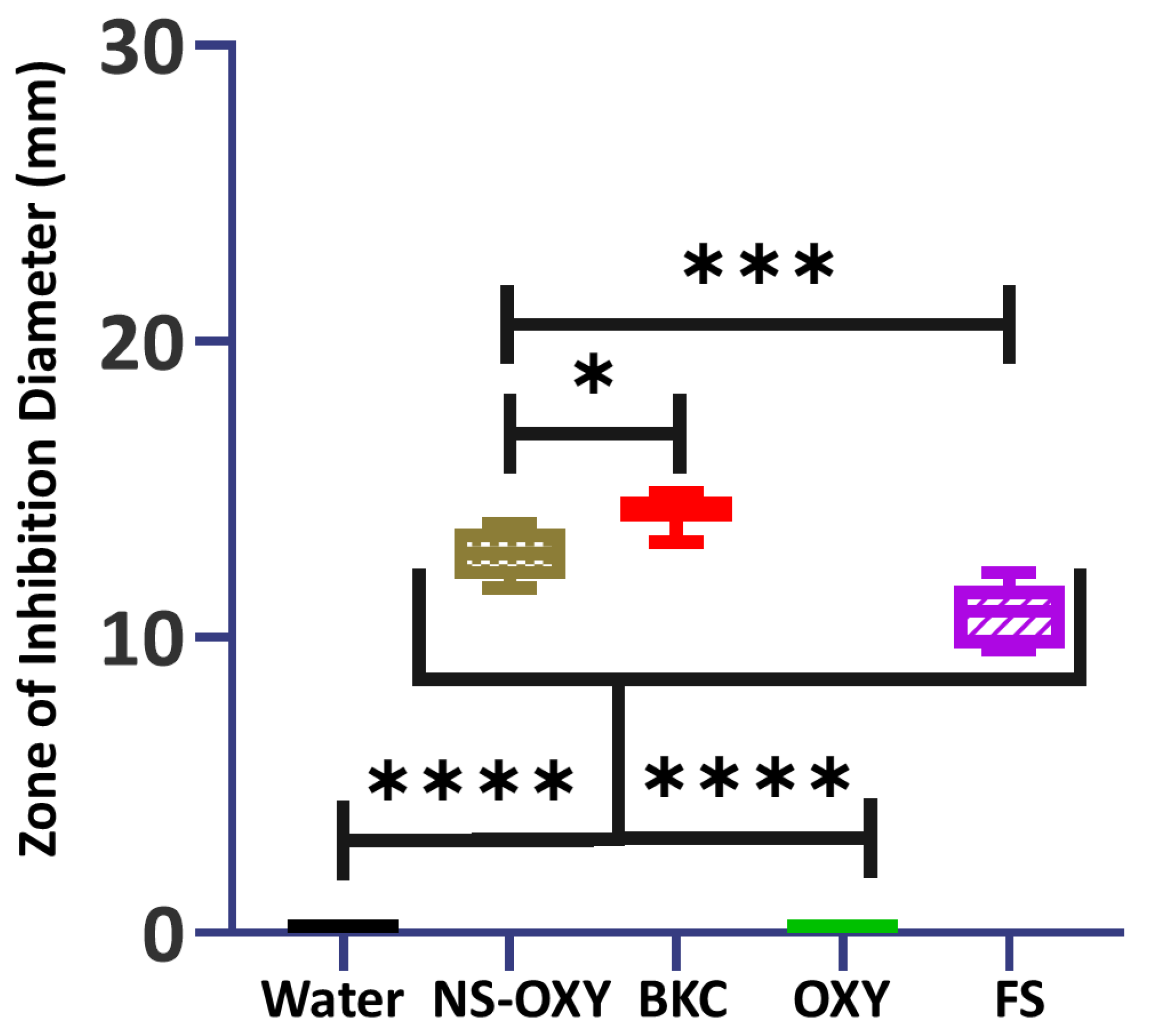
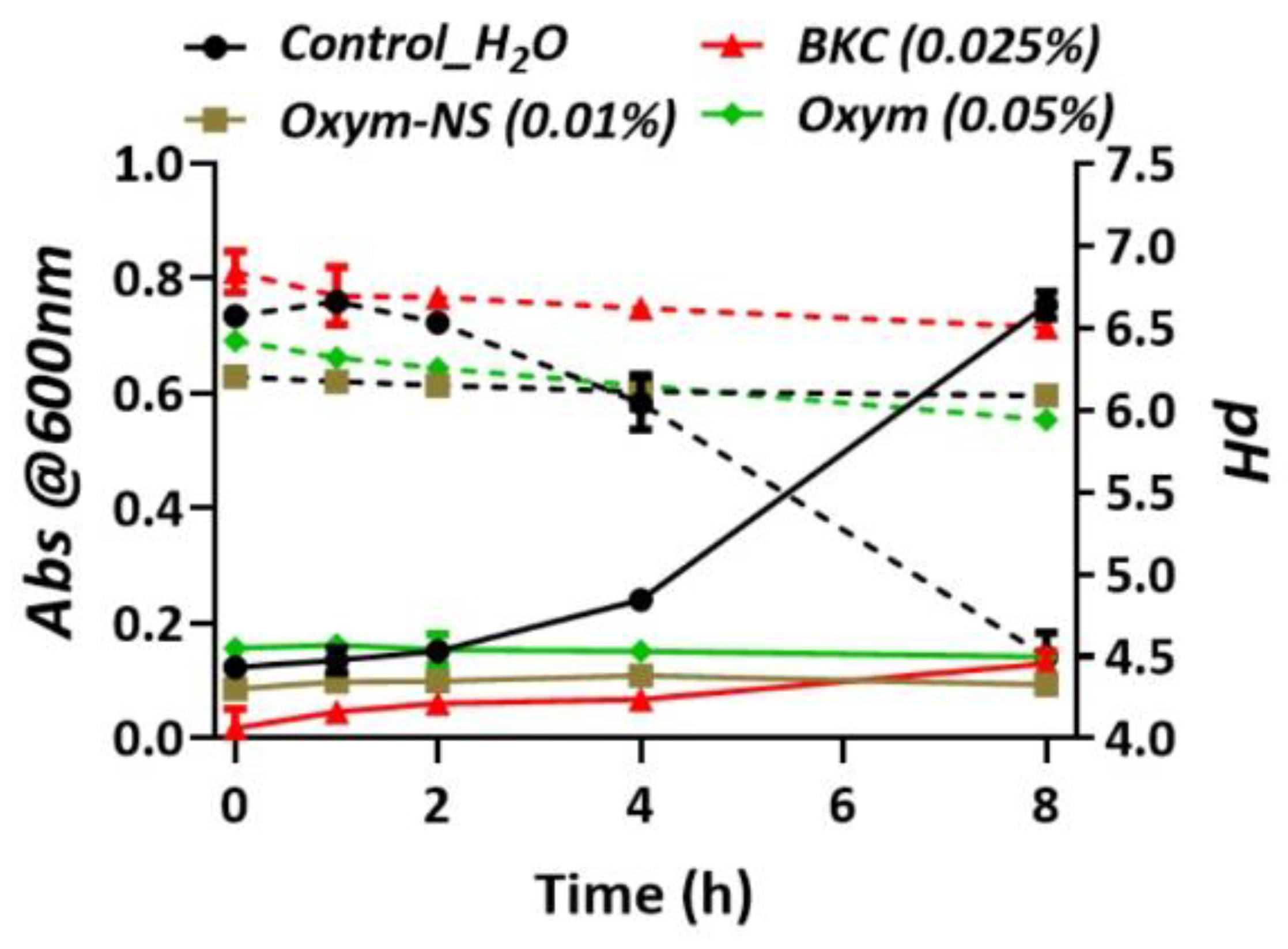
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| α-1 | alpha-1 |
| ABAC | alkylbenzyldimethylammonium chlorides |
| BHI | brain heart infusion |
| BKC | benzalkonium chloride |
| CV | crystal violet |
| DI | Distilled |
| EDTA | edetate disodium |
| FS | ferric sulfate |
| NS-OXY | nasal solution containing OXY |
| OD | optical density |
| OTC | over-the-counter |
| OXY | oxymetazoline (XYL) |
| PCR | polymerase chain reaction |
| SB | sheep’s blood |
| XYL | Xylometazoline |
| ZOI | zone of inhibition |
References
- Higgins, T.S.; Hwang, P.H.; Kingdom, T.T.; Orlandi, R.R.; Stammberger, H.; Han, J.K. Systematic Review of Topical Vasoconstrictors in Endoscopic Sinus Surgery. Laryngoscope 2011, 121, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Deckx, L.; De Sutter, A.I.; Guo, L.; Mir, N.A.; van Driel, M.L. Nasal Decongestants in Monotherapy for the Common Cold. Cochrane. Database. Syst. Rev. 2016, 10, CD009612. [Google Scholar] [CrossRef] [PubMed]
- Cartabuke, R.S.; Anderson, B.J.; Elmaraghy, C.; Rice, J.; Tumin, D.; Tobias, J.D. Hemodynamic and Pharmacokinetic Analysis of Oxymetazoline Use during Nasal Surgery in Children. Laryngoscope 2019, 129, 2775–2781. [Google Scholar] [CrossRef] [PubMed]
- Ciancio, S.G.; Hutcheson, M.C.; Ayoub, F.; Pantera, E.A.J.; Pantera, C.T.; Garlapo, D.A.; Sobieraj, B.D.; Almubarak, S.A. Safety and Efficacy of a Novel Nasal Spray for Maxillary Dental Anesthesia. J. Dent. Res. 2013, 92, 43S–48S. [Google Scholar] [CrossRef]
- Nowakowska, D.; Saczko, J.; Szewczyk, A.; Michel, O.; Ziętek, M.; Weżgowiec, J.; Więckiewicz, W.; Kulbacka, M. In Vitro Effects of Vasoconstrictive Retraction Agents on Primary Human Gingival Fibroblasts. Exp. Ther. Med. 2020, 19, 2037–2044. [Google Scholar] [CrossRef]
- Jones, R.S. Conceptual Model for Using Imidazoline Derivative Solutions in Pulpal Management. J. Clin. Med. 2021, 10, 1212. [Google Scholar] [CrossRef]
- Chou, A.-K.; Chiu, C.-C.; Zhu, G.-C.; Wang, J.-J.; Chen, Y.-W.; Hung, C.-H. Naphazoline and Oxymetazoline Are Superior to Epinephrine in Enhancing the Cutaneous Analgesia of Lidocaine in Rats. Fundam. Clin. Pharmacol. 2023, 37, 296–304. [Google Scholar] [CrossRef]
- Olgart, L. Neural Control of Pulpal Blood Flow. Crit. Rev. Oral. Biol. Med. 1996, 7, 159–171. [Google Scholar] [CrossRef]
- Edwall, L.; Kindlová, M. The Effect of Sympathetic Nerve Stimulation on the Rate of Disappearance of Tracers from Various Oral Tissues. Acta. Odontol. Scand. 1971, 29, 387–400. [Google Scholar] [CrossRef]
- Graf, P.; Juto, J.E. Decongestion Effect and Rebound Swelling of the Nasal Mucosa during 4-Week Use of Oxymetazoline. ORL J. Otorhinolaryngol. Relat. Spec. 1994, 56, 157–160. [Google Scholar] [CrossRef]
- Minyan, W.; Dunn, W.R.; Blaylock, N.A.; Chan, S.L.; Wilson, V.G. Evidence for a Non-Adrenoceptor, Imidazoline-Mediated Contractile Response to Oxymetazoline in the Porcine Isolated Rectal Artery. Br. J. Pharmacol. 2001, 132, 1359–1363. [Google Scholar] [CrossRef] [PubMed]
- Cartabuke, R.; Tobias, J.D.; Jatana, K.R. Topical Nasal Decongestant Oxymetazoline: Safety Considerations for Perioperative Pediatric Use. Pediatrics 2021, 148, e2021054271. [Google Scholar] [CrossRef] [PubMed]
- Schröder, U. Effect of an Extra-Pulpal Blood Clot on Healing Following Experimental Pulpotomy and Capping with Calcium Hydroxide. Odontol. Revy 1973, 24, 257–268. [Google Scholar] [PubMed]
- Hørsted, P.; El Attar, K.; Langeland, K. Capping of Monkey Pulps with Dycal and a Ca-Eugenol Cement. Oral. Surg. Oral. Med. Oral. Pathol. 1981, 52, 531–553. [Google Scholar] [CrossRef]
- Sonmez, D.; Duruturk, L. Success Rate of Calcium Hydroxide Pulpotomy in Primary Molars Restored with Amalgam and Stainless Steel Crowns. Br. Dent. J. 2010, 208, E18. [Google Scholar] [CrossRef]
- Caicedo, R.; Abbott, P.; Alongi, D.; Alarcon, M. Clinical, Radiographic and Histological Analysis of the Effects of Mineral Trioxide Aggregate Used in Direct Pulp Capping and Pulpotomies of Primary Teeth. Aust. Dent. J. 2006, 51, 297–305. [Google Scholar] [CrossRef]
- Pinto, K.P.; Barbosa, A.F.A.; Silva, E.J.N.L.; Santos, A.P.P.; Sassone, L.M. What Is the Microbial Profile in Persistent Endodontic Infections? A Scoping Review. J. Endod. 2023, 49, 786–798.e7. [Google Scholar] [CrossRef]
- Lima, A.R.; Herrera, D.R.; Francisco, P.A.; Pereira, A.C.; Lemos, J.; Abranches, J.; Gomes, B.P.F.A. Detection of Streptococcus Mutans in Symptomatic and Asymptomatic Infected Root Canals. Clin. Oral. Investig. 2021, 25, 3535–3542. [Google Scholar] [CrossRef]
- Maisonneuve, E.; Chevrier, J.; Dubus, M.; Varin, J.; Sergheraert, J.; Gangloff, S.C.; Reffuveille, F.; Mauprivez, C.; Kerdjoudj, H. Infection of Human Dental Pulp Stromal Cells by Streptococcus Mutans: Shedding Light on Bacteria Pathogenicity and Pulp Inflammation. Front. Cell Dev. Biol. 2020, 8, 785. [Google Scholar] [CrossRef]
- Inenaga, C.; Hokamura, K.; Nakano, K.; Nomura, R.; Naka, S.; Ohashi, T.; Ooshima, T.; Kuriyama, N.; Hamasaki, T.; Wada, K.; et al. A Potential New Risk Factor for Stroke: Streptococcus Mutans With Collagen-Binding Protein. World Neurosurg. 2018, 113, e77–e81. [Google Scholar] [CrossRef]
- Nomura, R.; Matayoshi, S.; Otsugu, M.; Kitamura, T.; Teramoto, N.; Nakano, K. Contribution of Severe Dental Caries Induced by Streptococcus Mutans to the Pathogenicity of Infective Endocarditis. Infect. Immun. 2020, 88, e00897-19. [Google Scholar] [CrossRef] [PubMed]
- Richards, R.M.; Cavill, R.H. Electron Microscope Study of Effect of Benzalkonium Chloride and Edetate Disodium on Cell Envelope of Pseudomonas Aeruginosa. J. Pharm. Sci. 1976, 65, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Tezvergil-Mutluay, A.; Mutluay, M.M.; Gu, L.; Zhang, K.; Agee, K.A.; Carvalho, R.M.; Manso, A.; Carrilho, M.; Tay, F.R.; Breschi, L.; et al. The Anti-MMP Activity of Benzalkonium Chloride. J. Dent. 2011, 39, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Alovisi, M.; Pasqualini, D.; Mandras, N.; Roana, J.; Costamagna, P.; Comba, A.; Cavalli, R.; Luganini, A.; Iandolo, A.; Cavallo, L.; et al. Confocal Laser Scanner Evaluation of Bactericidal Effect of Chitosan Nanodroplets Loaded with Benzalkonium Chloride. J. Clin. Med. 2022, 11, 1650. [Google Scholar] [CrossRef]
- Yamamoto, M.; Inokoshi, M.; Tamura, M.; Shimizubata, M.; Nozaki, K.; Takahashi, R.; Yoshihara, K.; Minakuchi, S. Development of 4-META/MMA-TBB Resin with Added Benzalkonium Chloride or Cetylpyridinium Chloride as Antimicrobial Restorative Materials for Root Caries. J. Mech. Behav. Biomed. Mater. 2021, 124, 104838. [Google Scholar] [CrossRef]
- Kurt, A.; Tüzüner, T.; Baygın, Ö. Antibacterial Characteristics of Glass Ionomer Cements Containing Antibacterial Agents: An in Vitro Study. Eur. Arch. Paediatr. Dent. 2020, 22, 49–56. [Google Scholar] [CrossRef]
- Torres-Garcia, M.L.; Llavore, L.D.; Bungay, A.; Sarol, J.D.J.; Pineda, R.R.; Peñas, K.D. Benzalkonium Chloride in an Orthodontic Adhesive: Its Effect on Rat Enamel Demineralization Using Color-Based Image Analysis. Am. J. Orthod. Dentofacial. Orthop. 2019, 155, 88–97. [Google Scholar] [CrossRef]
- Botelho, M.G. Fractional Inhibitory Concentration Index of Combinations of Antibacterial Agents against Cariogenic Organisms. J. Dent. 2000, 28, 565–570. [Google Scholar] [CrossRef]
- Aas, J.A.; Griffen, A.L.; Dardis, S.R.; Lee, A.M.; Olsen, I.; Dewhirst, F.E.; Leys, E.J.; Paster, B.J. Bacteria of Dental Caries in Primary and Permanent Teeth in Children and Young Adults. J. Clin. Microbiol. 2008, 46, 1407–1417. [Google Scholar] [CrossRef]
- Bandi, M.; Mallineni, S.K.; Nuvvula, S. Clinical Applications of Ferric Sulfate in Dentistry: A Narrative Review. J. Conserv. Dent. 2017, 20, 278–281. [Google Scholar]
- Smith, N.L.; Seale, N.S.; Nunn, M.E. Ferric Sulfate Pulpotomy in Primary Molars: A Retrospective Study. Pediatr. Dent. 2000, 22, 192–199. [Google Scholar] [PubMed]
- Vargas, K.G.; Packham, B. Radiographic Success of Ferric Sulfate and Formocresol Pulpotomies in Relation to Early Exfoliation. Pediatr. Dent. 2005, 27, 233–237. [Google Scholar] [PubMed]
- Aksoy, B.; Güngör, H.C.; Uysal, S.; Gonzales, C.D.; Ölmez, S. Ferric Sulfate Pulpotomy in Primary Teeth with Different Base Materials: A 2-Year Randomized Controlled Trial. Quintessence Int. 2022, 53, 782–789. [Google Scholar] [PubMed]
- Thrush, D.N. Cardiac Arrest after Oxymetazoline Nasal Spray. J. Clin. Anesth. 1995, 7, 512–514. [Google Scholar] [CrossRef]
- Ramesh, A.S.; Cartabuke, R.; Essig, G.; Tobias, J.D. Oxymetazoline-Induced Postoperative Hypertension. Pediatr. Anesth. Crit. Care. J. 2013, 1, 72–77. [Google Scholar]
- Latham, G.J.; Jardine, D.S. Oxymetazoline and Hypertensive Crisis in a Child: Can We Prevent It? Pediatr. Anesth. 2013, 23, 952–956. [Google Scholar] [CrossRef]
- Latham, G.J. In Reference to Hemodynamic and Pharmacokinetic Analysis of Oxymetazoline Use during Nasal Surgery in Children. Laryngoscope 2019, 129, E347. [Google Scholar] [CrossRef]
- Dokuyucu, R.; Gokce, H.; Sahan, M.; Sefil, F.; Tas, Z.A.; Tutuk, O.; Ozturk, A.; Tumer, C.; Cevik, C. Systemic Side Effects of Locally Used Oxymetazoline. Int. J. Clin. Exp. Med. 2015, 8, 2674–2678. [Google Scholar]
- Nordt, S.P.; Vivero, L.E.; Cantrell, F.L. Not Just a Drop in the Bucket-Inversion of Oxymetazoline Nasal Decongestant Container Increases Potential for Severe Pediatric Poisoning. J. Pediatr. 2016, 168, 240–241. [Google Scholar] [CrossRef]
- Pupo, Y.M.; Farago, P.V.; Nadal, J.M.; Esmerino, L.A.; Maluf, D.F.; Zawadzki, S.F.; Michél, M.D.; dos Santos, F.A.; Gomes, O.M.M.; Gomes, J.C. An Innovative Quaternary Ammonium Methacrylate Polymer Can Provide Improved Antimicrobial Properties for a Dental Adhesive System. J. Biomater. Sci. Polym. Ed. 2013, 24, 1443–1458. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, R.S.; Pride, M.A.; Kumar, D. An Oxymetazoline-Based Nasal Solution Removes Bacteria–Blood Debris on Dental Surfaces and Has Antimicrobial Activity Toward Streptococcus mutans. Int. J. Mol. Sci. 2025, 26, 1242. https://doi.org/10.3390/ijms26031242
Jones RS, Pride MA, Kumar D. An Oxymetazoline-Based Nasal Solution Removes Bacteria–Blood Debris on Dental Surfaces and Has Antimicrobial Activity Toward Streptococcus mutans. International Journal of Molecular Sciences. 2025; 26(3):1242. https://doi.org/10.3390/ijms26031242
Chicago/Turabian StyleJones, Robert S., Morgan Annina Pride, and Dhiraj Kumar. 2025. "An Oxymetazoline-Based Nasal Solution Removes Bacteria–Blood Debris on Dental Surfaces and Has Antimicrobial Activity Toward Streptococcus mutans" International Journal of Molecular Sciences 26, no. 3: 1242. https://doi.org/10.3390/ijms26031242
APA StyleJones, R. S., Pride, M. A., & Kumar, D. (2025). An Oxymetazoline-Based Nasal Solution Removes Bacteria–Blood Debris on Dental Surfaces and Has Antimicrobial Activity Toward Streptococcus mutans. International Journal of Molecular Sciences, 26(3), 1242. https://doi.org/10.3390/ijms26031242






