Impaired LPS Signaling in Macrophages Overexpressing the P2X7 C-Terminal Domain or Anti-P2X7 C-Terminal Domain Intrabody
Abstract
1. Introduction
2. Results
2.1. Establishment of BMDM Cell Lines from Wild-Type, P2X7-CT2 Tg, and Anti-P2X7-scFv Tg Mice

2.2. LPS or Pam3CSK4-Induced Activation of NF-κB in BMDMs
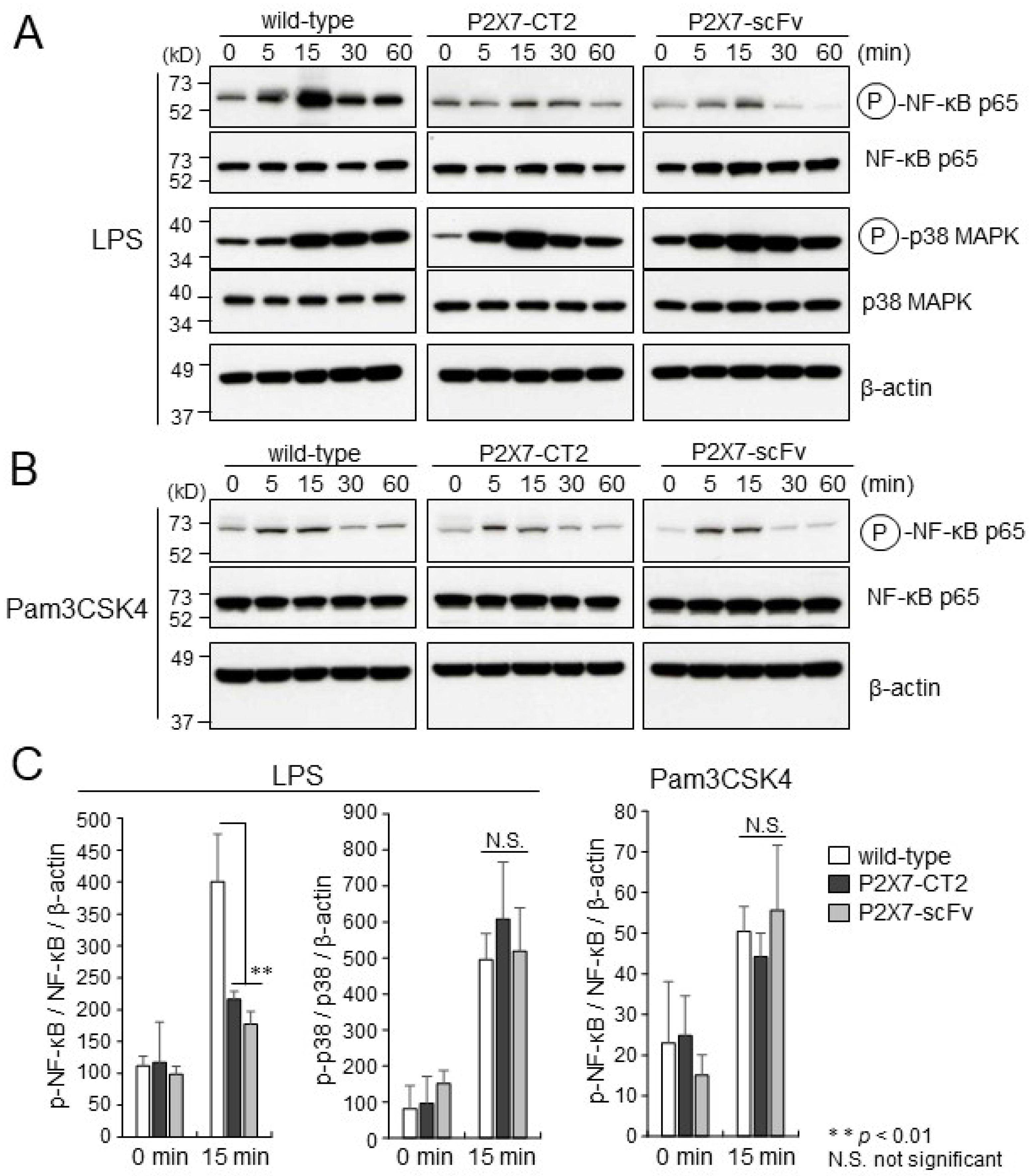
2.3. Impaired Cytokine Production in P2X7-CT2 Tg and Anti-P2X7-scFv Tg BMDMs upon LPS Stimulation
2.4. P2X7 C-Terminal Domain Associates with MyD88 in BMDMs and Interacts with TLR4 C-Terminal Region in in Vitro Binding Assay
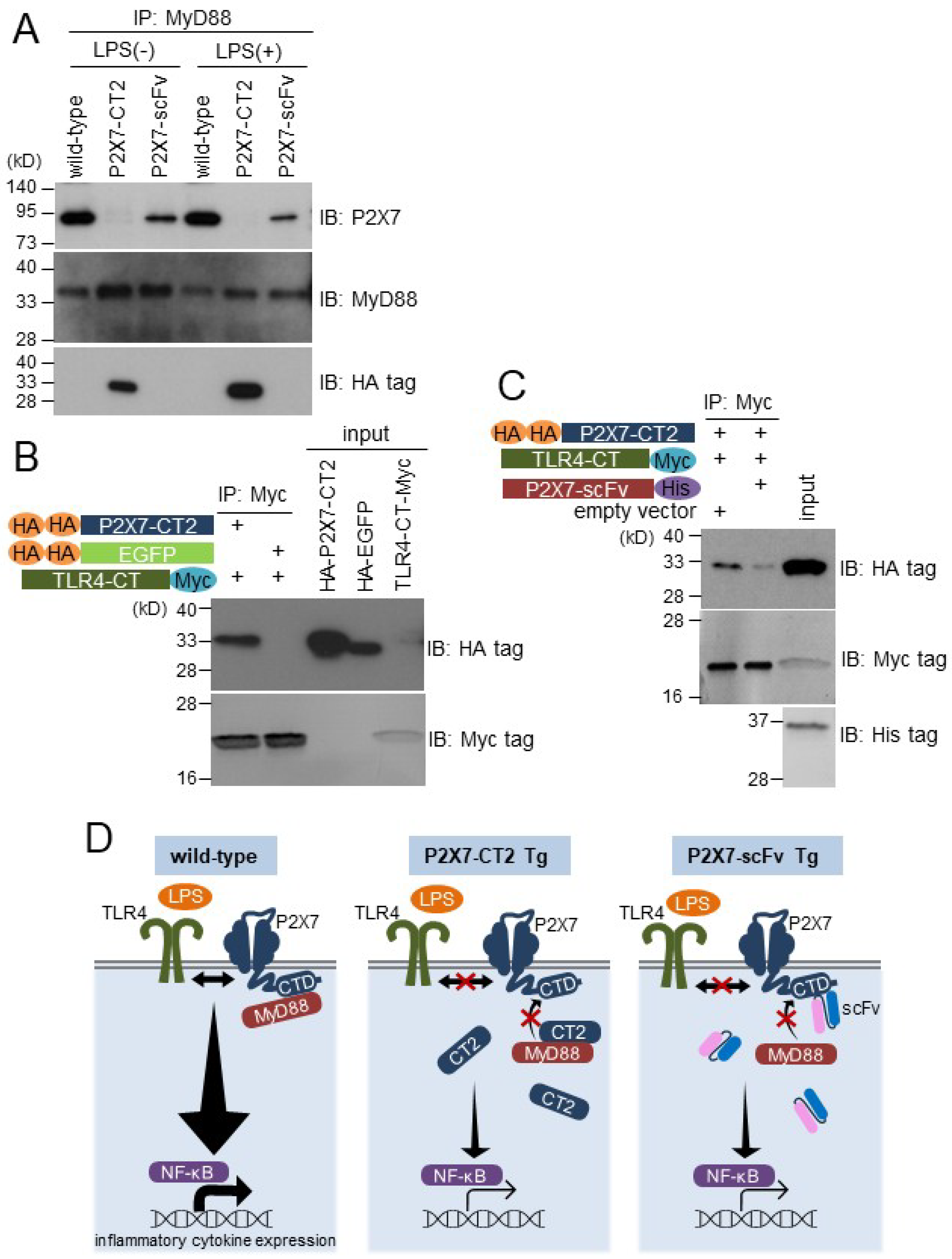
3. Discussion
4. Materials and Methods
4.1. Plasmid Construction
4.2. Cloning and Construction of Anti-P2X7-scFv
4.3. Generation of Transgenic Mice
4.4. Cells and Electroporation
4.5. Establishment of BMDM Cell Lines
4.6. Immunocytochemistry
4.7. FACS Analysis
4.8. Western Blot Analysis
4.9. Quantitative Real-Time PCR
4.10. ELISA
4.11. Immunoprecipitation Assay
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Surprenant, A.; Rassendren, F.; Kawashima, E.; North, R.A.; Buell, G. The cytolytic P2z receptor for extracellular ATP identified as a P2x receptor (P2X7). Science 1996, 272, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Takenouchi, T.; Ogihara, K.; Sato, M.; Kitani, H. Inhibitory effects of U73122 and U73343 on Ca2+ influx and pore formation induced by the activation of P2X7 nucleotide receptors in mouse microglial cell line. Biochim. Biophys. Acta 2005, 1726, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Rotondo, J.C.; Mazziotta, C.; Lanzillotti, C.; Stefani, C.; Badiale, G.; Campione, G.; Martini, F.; Tognon, M. The role of purinergic P2X7 receptor in inflammation and cancer: Novel molecular insights and clinical applications. Cancers 2022, 14, 1116. [Google Scholar] [CrossRef] [PubMed]
- Kopp, R.; Krautloher, A.; Ramírez-Fernández, A.; Nicke, A. P2X7 interactions and signaling—Making head or tail of it. Front. Mol. Neurosci. 2019, 12, 183. [Google Scholar] [CrossRef]
- Denlinger, L.C.; Fisette, P.L.; Sommer, J.A.; Watters, J.J.; Prabhu, U.; Dubyak, G.R.; Proctor, R.A.; Bertics, P.J. Cutting edge: The nucleotide receptor P2X7 contains multiple protein- and lipid-interaction motifs including a potential binding site for bacterial lipopolysaccharide. J. Immunol. 2001, 167, 1871–1876. [Google Scholar] [CrossRef]
- Costa-Junior, H.M.; Sarmento Vieira, F.; Coutinho-Silva, R. C terminus of the P2X7 receptor: Treasure hunting. Purinergic Signal 2011, 7, 7–19. [Google Scholar] [CrossRef]
- Martínez-Cuesta, M.Á.; Blanch-Ruiz, M.A.; Ortega-Luna, R.; Sánchez-López, A.; Álvarez, Á. Structural and functional basis for understanding the biological significance of P2X7 receptor. Int. J. Mol. Sci. 2020, 21, 8454. [Google Scholar] [CrossRef]
- Rossi, L.; Salvestrini, V.; Ferrari, D.; Di Virgilio, F.; Lemoli, R.M. The sixth sense: Hematopoietic stem cells detect danger through purinergic signaling. Blood 2012, 120, 2365–2375. [Google Scholar] [CrossRef]
- Paik, S.; Kim, J.K.; Silwal, P.; Sasakawa, C.; Jo, E.K. An update on the regulatory mechanisms of NLRP3 inflammasome activation. Cell. Mol. Immunol. 2021, 18, 1141–1160. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, W.; Zhou, R. NLRP3 inflammasome activation and cell death. Cell. Mol. Immunol. 2021, 18, 2114–2127. [Google Scholar] [CrossRef]
- Franceschini, A.; Capece, M.; Chiozzi, P.; Falzoni, S.; Sanz, J.M.; Sarti, A.C.; Bonora, M.; Pinton, P.; Di Virgilio, F. The P2X7 receptor directly interacts with the NLRP3 inflammasome scaffold protein. FASEB J. 2015, 29, 2450–2461. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.A.; Palsson-McDermott, E.M.; Bowie, A.G.; Jefferies, C.A.; Mansell, A.S.; Brady, G.; Brint, E.; Dunne, A.; Gray, P.; Harte, M.T.; et al. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature 2001, 413, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Horng, T.; Barton, G.M.; Medzhitov, R. TIRAP: An adapter molecule in the Toll signaling pathway. Nat. Immunol. 2001, 2, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.A.; Rowe, D.C.; Barnes, B.J.; Caffrey, D.R.; Visintin, A.; Latz, E.; Monks, B.; Pitha, P.M.; Golenbock, D.T. LPS-TLR4 signaling to IRF-3/7 and NF-kappaB involves the toll adapters TRAM and TRIF. J. Exp. Med. 2003, 198, 1043–1055. [Google Scholar] [CrossRef]
- Yamamoto, M.; Sato, S.; Hemmi, H.; Uematsu, S.; Hoshino, K.; Kaisho, T.; Takeuchi, O.; Takeda, K.; Akira, S. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat. Immunol. 2003, 4, 1144–1150. [Google Scholar] [CrossRef]
- Li, S.; Strelow, A.; Fontana, E.J.; Wesche, H. IRAK-4: A novel member of the IRAK family with the properties of an IRAK-kinase. Proc. Natl. Acad. Sci. USA 2002, 99, 5567–5572. [Google Scholar] [CrossRef]
- Lomaga, M.A.; Yeh, W.C.; Sarosi, I.; Duncan, G.S.; Furlonger, C.; Ho, A.; Morony, S.; Capparelli, C.; Van, G.; Kaufman, S.; et al. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 1999, 13, 1015–1024. [Google Scholar] [CrossRef]
- Emmerich, C.H.; Ordureau, A.; Strickson, S.; Arthur, J.S.; Pedrioli, P.G.; Komander, D.; Cohen, P. Activation of the canonical IKK complex by K63/M1-linked hybrid ubiquitin chains. Proc. Natl. Acad. Sci. USA 2013, 110, 15247–15252. [Google Scholar] [CrossRef]
- Wang, C.; Deng, L.; Hong, M.; Akkaraju, G.R.; Inoue, J.; Chen, Z.J. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 2001, 412, 346–351. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, Y.; Li, Z. P2X7 receptor positively regulates MyD88-dependent NF-κB activation. Cytokine 2011, 55, 229–236. [Google Scholar] [CrossRef]
- Dagvadorj, J.; Shimada, K.; Chen, S.; Jones, H.D.; Tumurkhuu, G.; Zhang, W.; Wawrowsky, K.A.; Crother, T.R.; Arditi, M. Lipopolysaccharide induces alveolar macrophage necrosis via CD14 and the P2X7 receptor leading to interleukin-1a release. Immunity 2015, 42, 640–653. [Google Scholar] [CrossRef] [PubMed]
- Korcok, J.; Raimundo, L.N.; Ke, H.Z.; Sims, S.M.; Dixon, S.J. Extracellular nucleotides act through P2X7 receptors to activate NF-κB in osteoclasts. J. Bone Miner. Res. 2004, 19, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Pålsson-McDermott, E.M.; O’Neill, L.A. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology 2004, 113, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R.; Preston-Hurlburt, P.; Kopp, E.; Stadlen, A.; Chen, C.; Ghosh, S.; Janeway, C.A., Jr. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol. Cell 1998, 2, 253–258. [Google Scholar] [CrossRef]
- Kawai, T.; Adachi, O.; Ogawa, T.; Takeda, K.; Akira, S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity 1999, 11, 115–122. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Dal Ben, D.; Sarti, A.C.; Giuliani, A.L.; Falzoni, S. The P2X7 Receptor in Infection and Inflammation. Immunity 2017, 47, 15–31. [Google Scholar] [CrossRef]
- Buss, H.; Dörrie, A.; Schmitz, M.L.; Hoffmann, E.; Resch, K.; Kracht, M. Constitutive and interleukin-1-inducible phosphorylation of p65 NF-κB at serine 536 is mediated by multiple protein kinases including IκB kinase (IKK)-α, IKKβ, IKKε, TRAF family member-associated (TANK)-binding kinase 1 (TBK1), and an unknown kinase and couples p65 to TATA-binding protein-associated factor II31-mediated Interleukin-8 transcription. J. Biol. Chem. 2004, 279, 55633–55643. [Google Scholar] [CrossRef]
- Yang, F.; Tang, E.; Guan, K.; Wang, C.Y. IKKβ plays an essential role in the phosphorylation of RelA/p65 on serine 536 induced by lipopolysaccharide. J. Immunol. 2003, 170, 5630–5635. [Google Scholar] [CrossRef]
- Akira, S.; Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef]
- Shin, C.Y.; Kang, S.J.; Song, M.R.; Park, K.H.; Seo, D.O.; Cheong, J.H.; Ko, K.H. Cross-species reactivity of a monoclonal antibody against glutathione S-transferase fusion protein of human beta 2-adrenergic receptor. Biochem. Mol. Biol. Int. 1998, 45, 215–225. [Google Scholar] [CrossRef]
- Niwa, H.; Yamamura, K.; Miyazaki, J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 1991, 108, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, Y.; Furuta, M.; Sato, M.; Kubo, M.; Hashimoto, Y. Isolation and characterization of a novel HS1 SH3 domain binding protein, HS1BP3. Int. Immunol. 1999, 11, 1957–1964. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sakuma, C.; Sato, M.; Takenouchi, T.; Chiba, J.; Kitani, H. Critical roles of the WASP N-terminal domain and Btk in LPS-induced inflammatory response in macrophages. PLoS ONE 2012, 7, e30351. [Google Scholar] [CrossRef] [PubMed]
- Takenouchi, T.; Suzuki, S.; Shinkai, H.; Tsukimoto, M.; Sato, M.; Uenishi, H.; Kitani, H. Extracellular ATP does not induce P2X7 receptor-dependent responses in cultured renal- and liver-derived swine macrophages. Results Immunol. 2014, 4, 62–67. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakuma, C.; Takenouchi, T.; Sato, M. Impaired LPS Signaling in Macrophages Overexpressing the P2X7 C-Terminal Domain or Anti-P2X7 C-Terminal Domain Intrabody. Int. J. Mol. Sci. 2025, 26, 1178. https://doi.org/10.3390/ijms26031178
Sakuma C, Takenouchi T, Sato M. Impaired LPS Signaling in Macrophages Overexpressing the P2X7 C-Terminal Domain or Anti-P2X7 C-Terminal Domain Intrabody. International Journal of Molecular Sciences. 2025; 26(3):1178. https://doi.org/10.3390/ijms26031178
Chicago/Turabian StyleSakuma, Chisato, Takato Takenouchi, and Mitsuru Sato. 2025. "Impaired LPS Signaling in Macrophages Overexpressing the P2X7 C-Terminal Domain or Anti-P2X7 C-Terminal Domain Intrabody" International Journal of Molecular Sciences 26, no. 3: 1178. https://doi.org/10.3390/ijms26031178
APA StyleSakuma, C., Takenouchi, T., & Sato, M. (2025). Impaired LPS Signaling in Macrophages Overexpressing the P2X7 C-Terminal Domain or Anti-P2X7 C-Terminal Domain Intrabody. International Journal of Molecular Sciences, 26(3), 1178. https://doi.org/10.3390/ijms26031178




