Design, Synthesis, and Tribological Behavior of an Eco-Friendly Methylbenzotriazole-Amide Derivative
Abstract
1. Introduction
2. Results
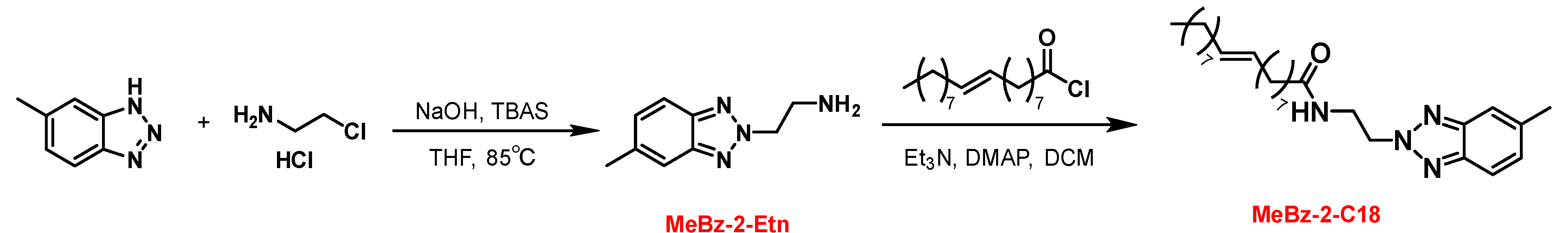
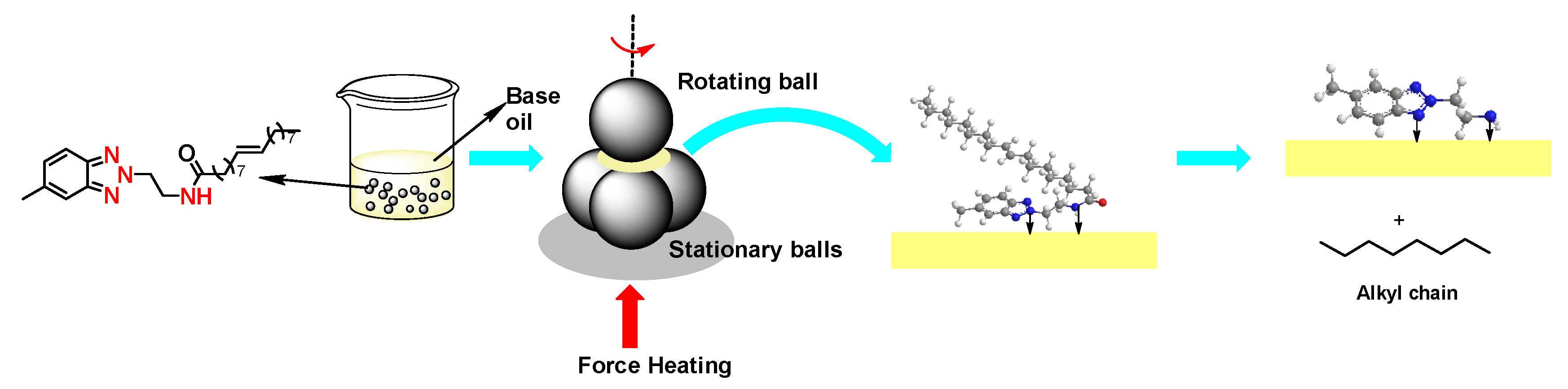
2.1. Synthesis
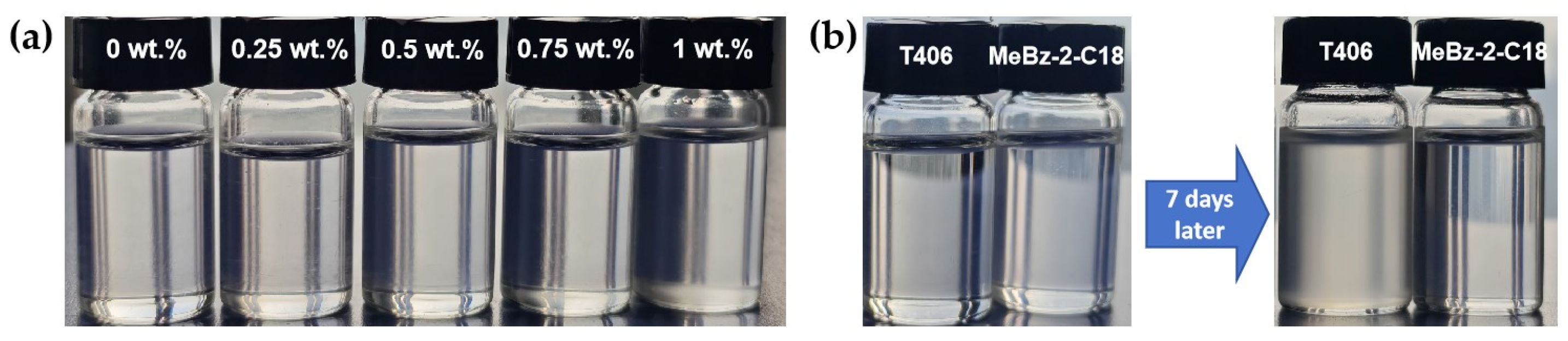
2.2. Stability Characterization
2.2.1. Thermal Stability
2.2.2. Storage Stability in Base Oil
2.3. Tribological Behavior
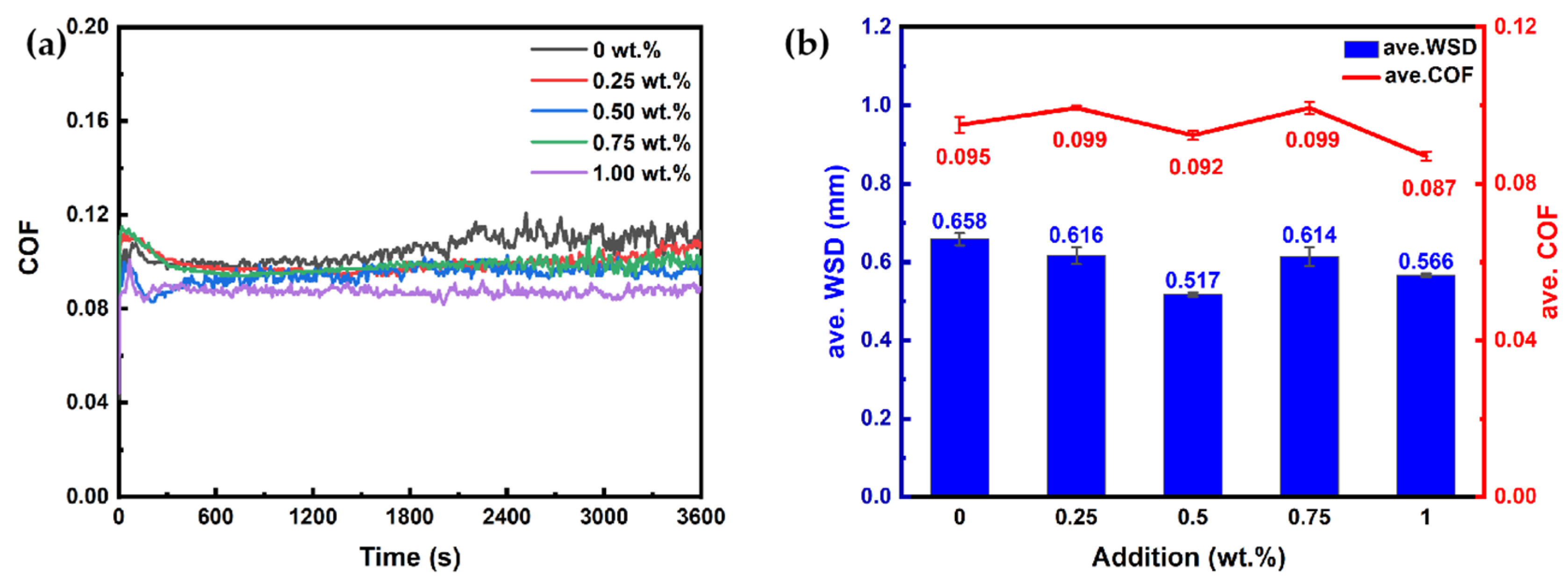
2.3.1. Different MeBz-2-C18 Additions
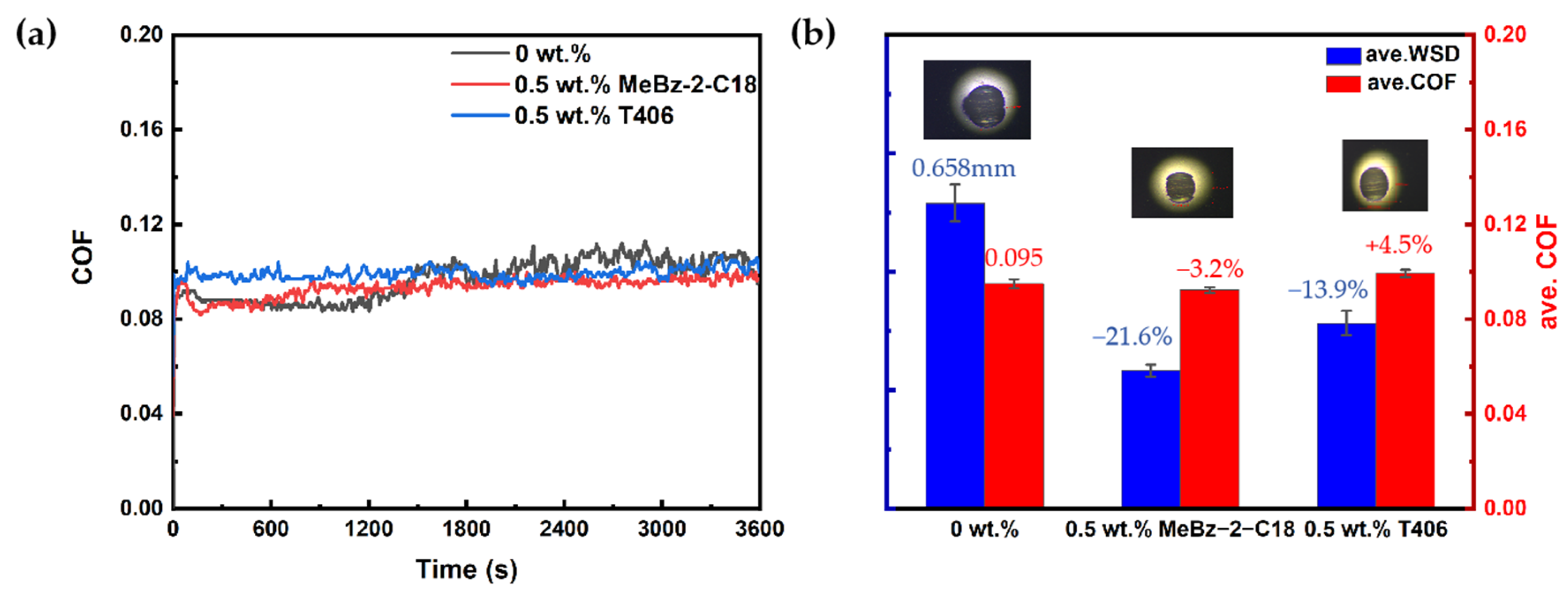
2.3.2. Comparison with the Commercial Additive
2.4. Lubrication Mechanism
2.4.1. Worn Surface Analysis
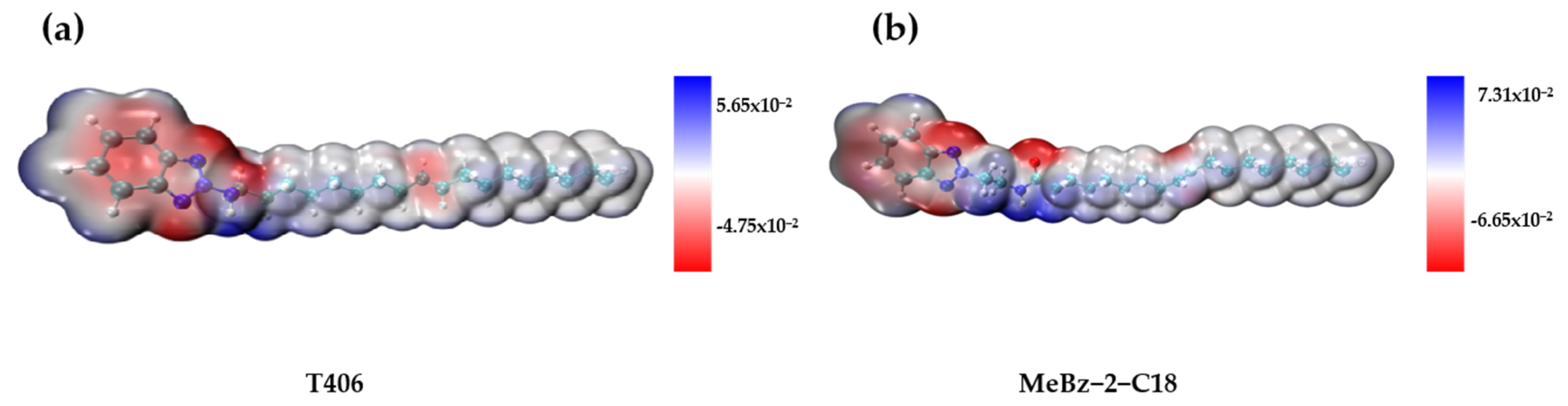
2.4.2. DFT Calculations
3. Materials and Methods
3.1. Materials
3.2. Synthesis of MeBz-2-Etn and MeBz-2-C18
3.2.1. Synthesis of MeBz-2-Etn
3.2.2. Synthesis of MeBz-2-C18
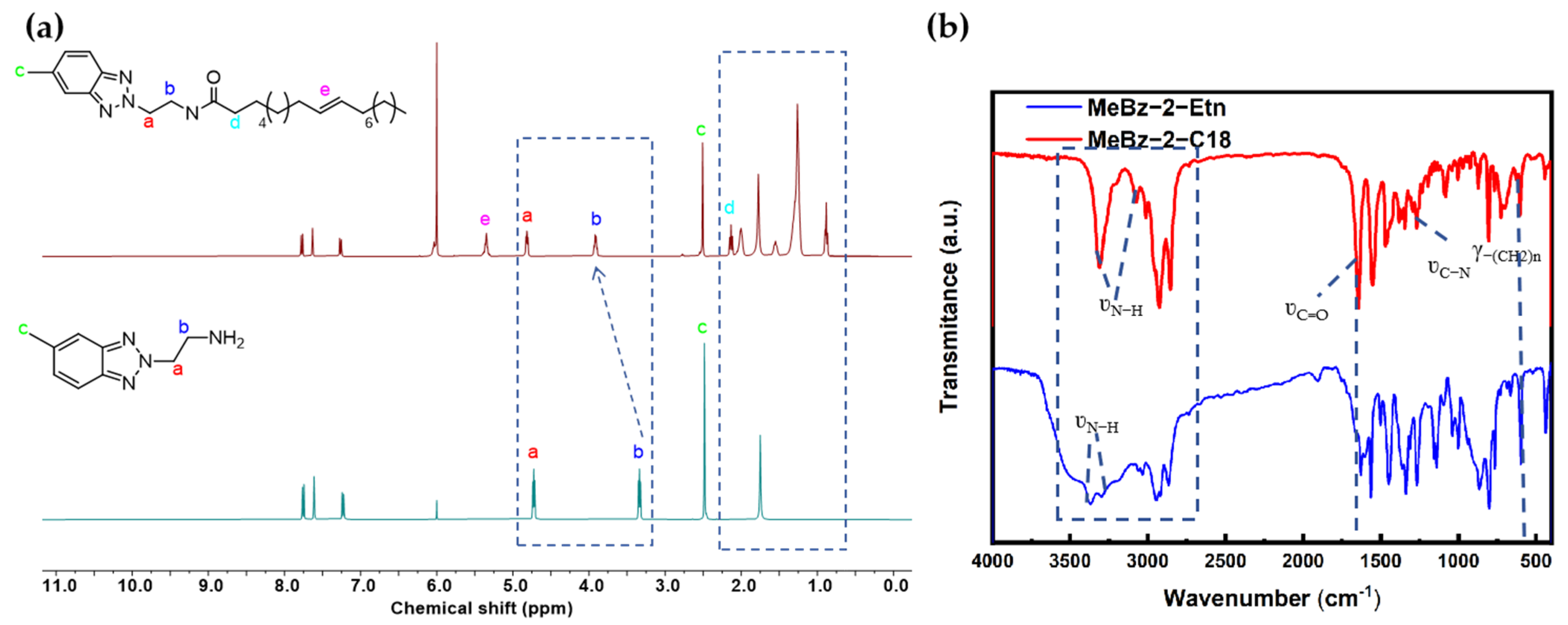
3.3. Characterizations
3.4. Preparation of Oil Samples
3.5. Tribological Testing
4. Conclusions
- (1)
- The covalently-bonded amide bond endows MeBz-2-C18 with superior thermal stability and better storage stability in synthetic base oil compared with T406. The residual mass of MeBz-2-C18 at 300 °C is 89.3%, while that of T406 is merely 11.0%.
- (2)
- The optimal addition of MeBz-2-C18 in the selected synthetic base oil is 0.5 wt.%, which reduces the ave. WSD and ave. COF by 21.6% and 3.2%, which is better than that of the commercial additive T406.
- (3)
- Worn surface analysis and DFT calculations suggest that MeBz-2-C18 has stronger adsorption on the metal surface than that of T406. The amide bond in MeBz-2-C18 breaks preferentially during friction, reducing the interfacial shear force and facilitating the film formation of iron oxides.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Danilov, A.M.; Bartko, R.V.; Antonov, S.A. Current Advances in the Application and Development of Lubricating Oil Additives. Pet. Chem. 2020, 61, 35–42. [Google Scholar] [CrossRef]
- Naletova, A.V.; Davydov, D.V.; Bakunin, V.N. Derivatives of 2,5-Dimercapto-1,3,4-Thiadiazole as Multifunctional Lubricant Additives. Chem. Technol. Fuels Oils 2021, 57, 783–791. [Google Scholar] [CrossRef]
- Xiong, L.; He, Z.; Han, S.; Tang, J.; Wu, Y.; Zeng, X. Tribological Properties Study of N-Containing Heterocyclic Imidazoline Derivatives as Lubricant Additives in Water-Glycol. Tribol. Int. 2016, 104, 98–108. [Google Scholar] [CrossRef]
- Ding, J.; Fang, J.; Chen, B.; Zhang, N.; Fan, X.; Zheng, Z. Improved Biodegradability and Tribological Performances of Mineral Lubricating Oil by Two Synthetic Nitrogenous Heterocyclic Additives. Ind. Lubr. Tribol. 2019, 71, 578–585. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, R.; He, Z.; Xiong, L. Tribological Performance of N-Containing Heterocyclic Triazine Derivative as Lubricant Additive in Ethylene Glycol. Surf. Interface Anal. 2021, 53, 1027–1034. [Google Scholar] [CrossRef]
- Xiong, S.; Wu, H.; Liu, Z. N-Containing Heterocyclic Benzotriazole Derivatives as New Corrosion Inhibitor for Mild Steel Contained in Emulsion. Anti. Corros. Methods Mater. 2022, 69, 183–193. [Google Scholar] [CrossRef]
- Wang, X.; Xu, P. Microbial Degradation of Nitrogen Heterocycles. Int. Biodeterior. Biodegrad. 2019, 142, 170–171. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, Y.; Li, N.; Tang, Y.; Zhang, C.; Wang, W.; Zhou, J.; Chen, F.; Rittmann, B.E. Using Ultrasonic Treated Sludge to Accelerate Pyridine and p-Nitrophenol Biodegradation. Int. Biodeterior. Biodegrad. 2020, 153, 105051. [Google Scholar] [CrossRef]
- Piccioni, M.; Varesano, A.; Tummino, M.L. Behavior of Polypyrrole-Coated Cotton Fabric Undergoing Biodegradation in Compost-Enriched Soil. Environ. Res. Commun. 2024, 6, 065001. [Google Scholar] [CrossRef]
- Adavodi, R.; Dini, G. Benzotriazoium Bis(2-Ethylhexyl) Phosphate Ionic Liquid as a Catalyst and Multifunctional Lubricant Additive: Synthesis, Optimization, Characterization, and Tribological Evaluation. Arab. J. Sci. Eng. 2024, 49, 7995–8010. [Google Scholar] [CrossRef]
- Wang, J.; Hu, W.; Li, J. Lubrication and Anti-Rust Properties of Jeffamine-Triazole Derivative as Water-Based Lubricant Additive. Coatings 2021, 11, 679. [Google Scholar] [CrossRef]
- Lu, T.; Yan, H.; Yang, H. Preparation of highly dispersed silver powder with benzotriazole as dispersant. Electron. Compon. Mater. 2020, 39, 37–41. [Google Scholar]
- Li, J.; Rao, W.; Liu, W. Tribologcal Behavior of an S-P Containing Benzotriazole Derivative as an Additive in Rapeseed Oil. Tribology 2002, 2, 122–125. [Google Scholar]
- Xiong, L.; He, Z.; Han, S.; Hu, J.; Xu, X.; Tang, J.; Wu, Y. Tribological Study of OH- and N-Containing Imidazoline Derivatives as Additives in Water-Glycol. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2019, 233, 466–480. [Google Scholar] [CrossRef]
- Li, S.; Pan, Z.; Yang, Y.; Li, X.; Liu, D. Research Progress of Benzotriazole and Its Derivatives in Lubricating Oil Corrosion Prevention. Lubr. Oil 2023, 38, 23–27. [Google Scholar]
- Li, Q.; Wang, Y.; Yan, F.; Guo, Y. Characterization of Methyl-benzotriazole Amine Salt. Pet. Process. Petrochem. 2016, 47, 82–85. [Google Scholar]
- Wang, J.; Xu, X.M.; Zhou, J.L.; Zhao, Y.N.; Sun, X.L.; Tang, Y.; He, S.F.; Yang, H.M. Synthesis of New Sulfur-free and Phosphorus-free Ether-ester and Study on Its Properties As Ashless Friction Modifier. Acta Chim. Sin. 2023, 81, 461–468. [Google Scholar]
- Xu, X.; Yang, F.; Yang, H.; Zhao, Y.; Sun, X.; Tang, Y. Preparation and tribological behavior of Sulfur- and Phosphorus-Free Organic Friction Modifier of Amide–Ester Type. Lubricants 2024, 12, 196. [Google Scholar] [CrossRef]
- De Barros, M.I.; Bouchet, J.; Raoult, I.; Le Mogne, T.; Martin, J.M.; Kasrai, M.; Yamada, Y. Friction Reduction by Metal Sulfides in Boundary Lubrication Studied by XPS and XANES Analyses. Wear 2003, 254, 863–870. [Google Scholar] [CrossRef]
- Fan, X.; Wang, L. High-Performance Lubricant Additives Based on Modified Graphene Oxide by Ionic Liquids. J. Colloid Interface Sci. 2015, 452, 98–108. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, X.; Guo, Z.; Cai, M.; Shi, L. Effective Sugar-Derived Organic Gelator for Three Different Types of Lubricant Oils to Improve Tribological Performance. Friction 2020, 8, 1025–1038. [Google Scholar] [CrossRef]
- Huang, J.; Li, Y.; Jia, X.; Song, H. Preparation and Tribological Properties of Core-Shell Fe3O4@C Microspheres. Tribol. Int. 2019, 129, 427–435. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, Y.; Yang, G.; Gao, C.; Song, N.; Zhang, S.; Zhang, P. In-Situ Formed Carbon Based Composite Tribo-Film with Ultra-High Load Bearing Capacity. Tribol. Int. 2020, 152, 106577. [Google Scholar] [CrossRef]
- Lee, A.Y.; Blakeslee, D.M.; Powell, C.J.; Rumble, J.R., Jr. Development of the Web-Based NIST X-Ray Photoelectron Spectroscopy (XPS) Database. Data Sci. J. 2006, 1, 1–12. [Google Scholar] [CrossRef]
- Wu, Y.; He, Z.; Zeng, X.; Ren, T.; de Vries, E.; van der Heide, E. Tribological Properties and Tribochemistry Mechanism of Sulfur-Containing Triazine Derivatives in Water-Glycol. Tribol. Int. 2017, 109, 140–151. [Google Scholar] [CrossRef]
- Otero, I.; López, E.R.; Reichelt, M.; Fernández, J. Tribo-Chemical Reactions of Anion in Pyrrolidinium Salts for Steel–Steel Contact. Tribol. Int. 2014, 77, 160–170. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, T.; Chen, Q. Intermolecular Interaction Characteristics of the All-Carboatomic ring, Cyclo[18]carbon: Focusing on Molecular Adsorption and Stacking. Carbon 2021, 171, 514–523. [Google Scholar] [CrossRef]


| Item | MeBz-2-Etn | MeBz-2-C18 | T406 | |
|---|---|---|---|---|
| Decomposition temperature (°C) | Initial | 19.7 | 185.5 | 76.8 |
| Max. | 217.4 | 374.4 | 221.1 | |
| Terminal | 236.0 | 434.7 | 420.3 | |
| Residual mass (%) | @ Max. decomposition | 15.1 | 22.4 | 39.2 |
| @200 °C | 44.8 | 99.9 | 71.9 | |
| @300 °C | 1.8 | 89.3 | 11.0 | |
| @400 °C | 1.0 | 1.3 | 1.3 | |
| Items | Base Oil | 0.5 wt.% MeBz-2-C18 | ||
| Non-Wear | Wear | Non-Wear | Wear | |
| SEM images (300X) |  |  |  |  |
| Element Distribution (carbon is represented in red, oxgen in green, iron in blue) |  |  |  |  |
| C (%) | 22.54 | 25.57 | 27.57 | 34.24 |
| O (%) | 7.51 | 28.46 | 8.58 | 30.14 |
| Fe (%) | 69.95 | 45.97 | 63.86 | 35.62 |
| Binding Energy (eV) | Corresponding Bond | Content (%) | ||
|---|---|---|---|---|
| Base Oil_Wear | MeBz-2-C18_Wear | |||
| C1s | 283.4 | Metal carbides | - | 3.3 |
| 284.8 | C–C/C=C | 86.9 | 80.6 | |
| 286.0 | C–O/C–N | 13.1 | 14.5 | |
| 288.5 | C=O | - | 1.6 | |
| O1s | 529.6 | Fe–O | 13.1 | 30.0 |
| 531.9 | C=O | 86.9 | 63.3 | |
| 533.1 | C–O | - | 6.7 | |
| Fe2p | 709.6 | Fe (2p3/2) | 51.8 | 48.1 |
| 711.0 | Fe3+ (2p3/2) | 34.3 | 32.2 | |
| 713.5 | Fe2+ (2p3/2) | - | 6.8 | |
| 723.5 | Fe2+ (2p1/2) | 13.9 | 12.9 | |
| N1s | 399.4 | N–O | - | 86.9 |
| 401.7 | C–N | - | 13.1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Li, Z.; Yang, H.; Zhao, Y.; Sun, X.; Tang, Y. Design, Synthesis, and Tribological Behavior of an Eco-Friendly Methylbenzotriazole-Amide Derivative. Int. J. Mol. Sci. 2025, 26, 1112. https://doi.org/10.3390/ijms26031112
Yang F, Li Z, Yang H, Zhao Y, Sun X, Tang Y. Design, Synthesis, and Tribological Behavior of an Eco-Friendly Methylbenzotriazole-Amide Derivative. International Journal of Molecular Sciences. 2025; 26(3):1112. https://doi.org/10.3390/ijms26031112
Chicago/Turabian StyleYang, Fan, Zenghui Li, Hongmei Yang, Yanan Zhao, Xiuli Sun, and Yong Tang. 2025. "Design, Synthesis, and Tribological Behavior of an Eco-Friendly Methylbenzotriazole-Amide Derivative" International Journal of Molecular Sciences 26, no. 3: 1112. https://doi.org/10.3390/ijms26031112
APA StyleYang, F., Li, Z., Yang, H., Zhao, Y., Sun, X., & Tang, Y. (2025). Design, Synthesis, and Tribological Behavior of an Eco-Friendly Methylbenzotriazole-Amide Derivative. International Journal of Molecular Sciences, 26(3), 1112. https://doi.org/10.3390/ijms26031112








