Persistent Metabolic Changes Are Induced by 24 h Low-Dose Lead (Pb) Exposure in Zebrafish Embryos
Abstract
1. Introduction
2. Results
2.1. Metal and Morphological Analysis of Zebrafish Embryos After Pb Exposure
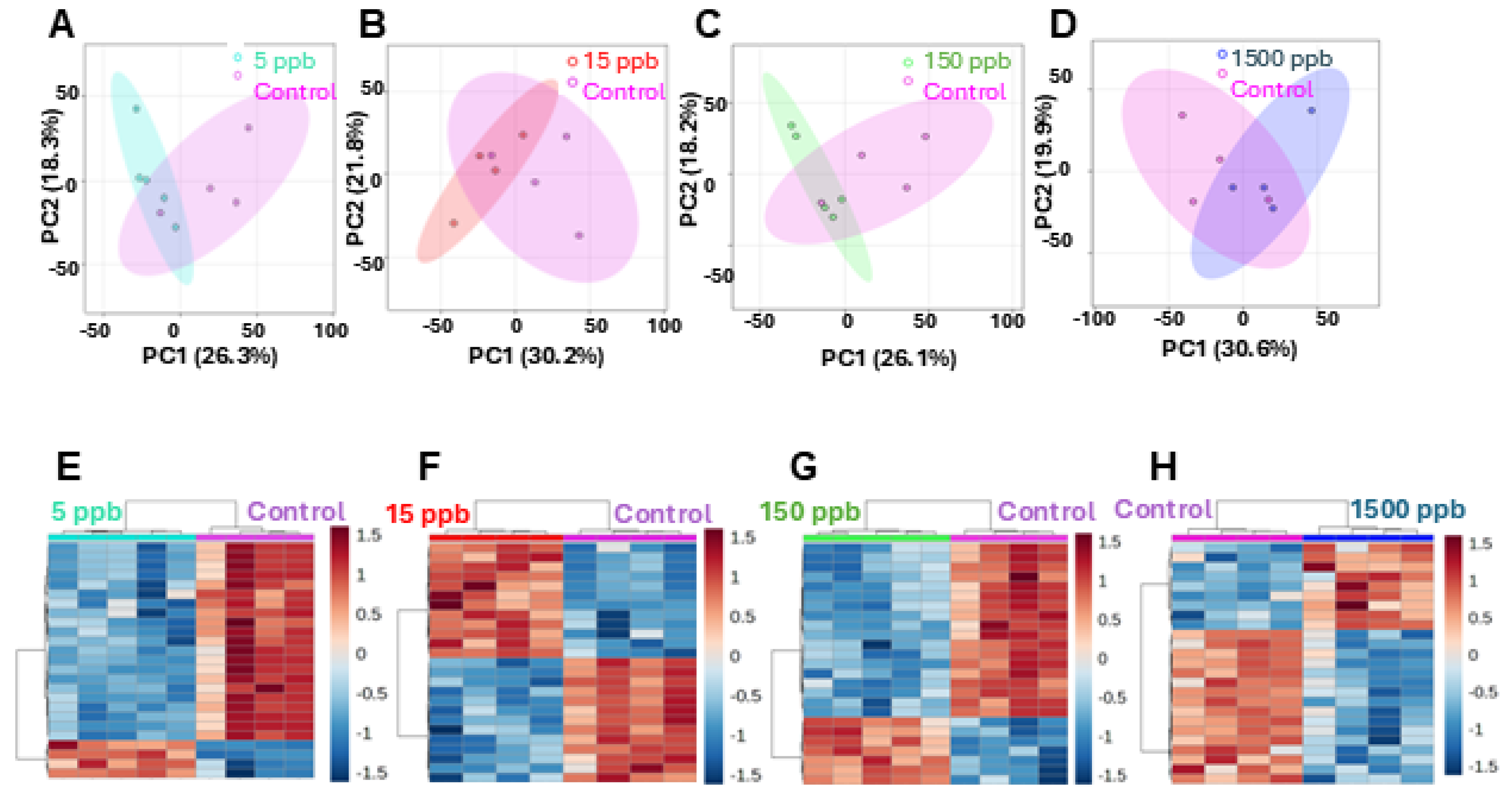
2.2. Metabolic Analysis
2.3. The Metabolomic Shift Compared to Pb Concentration
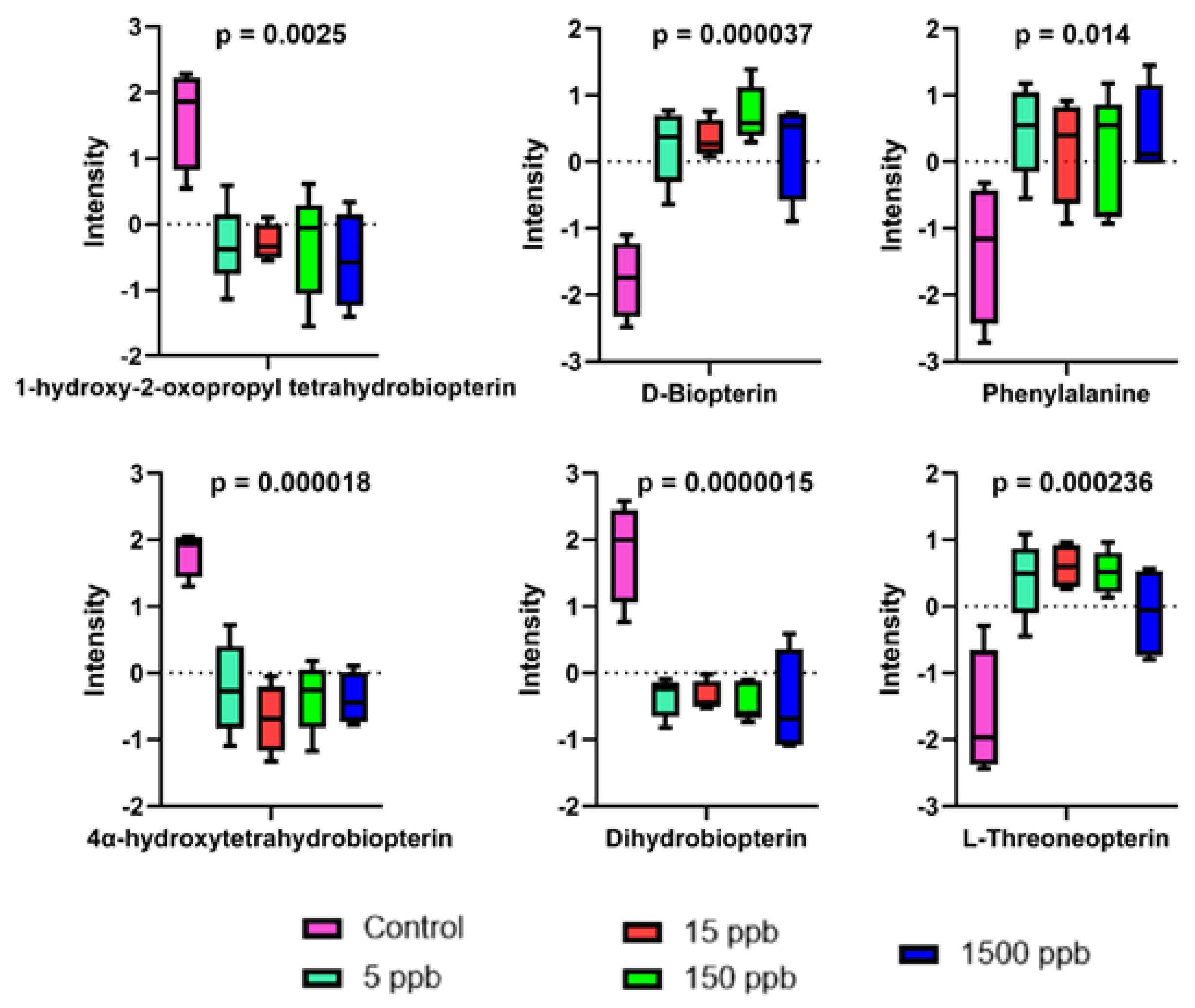
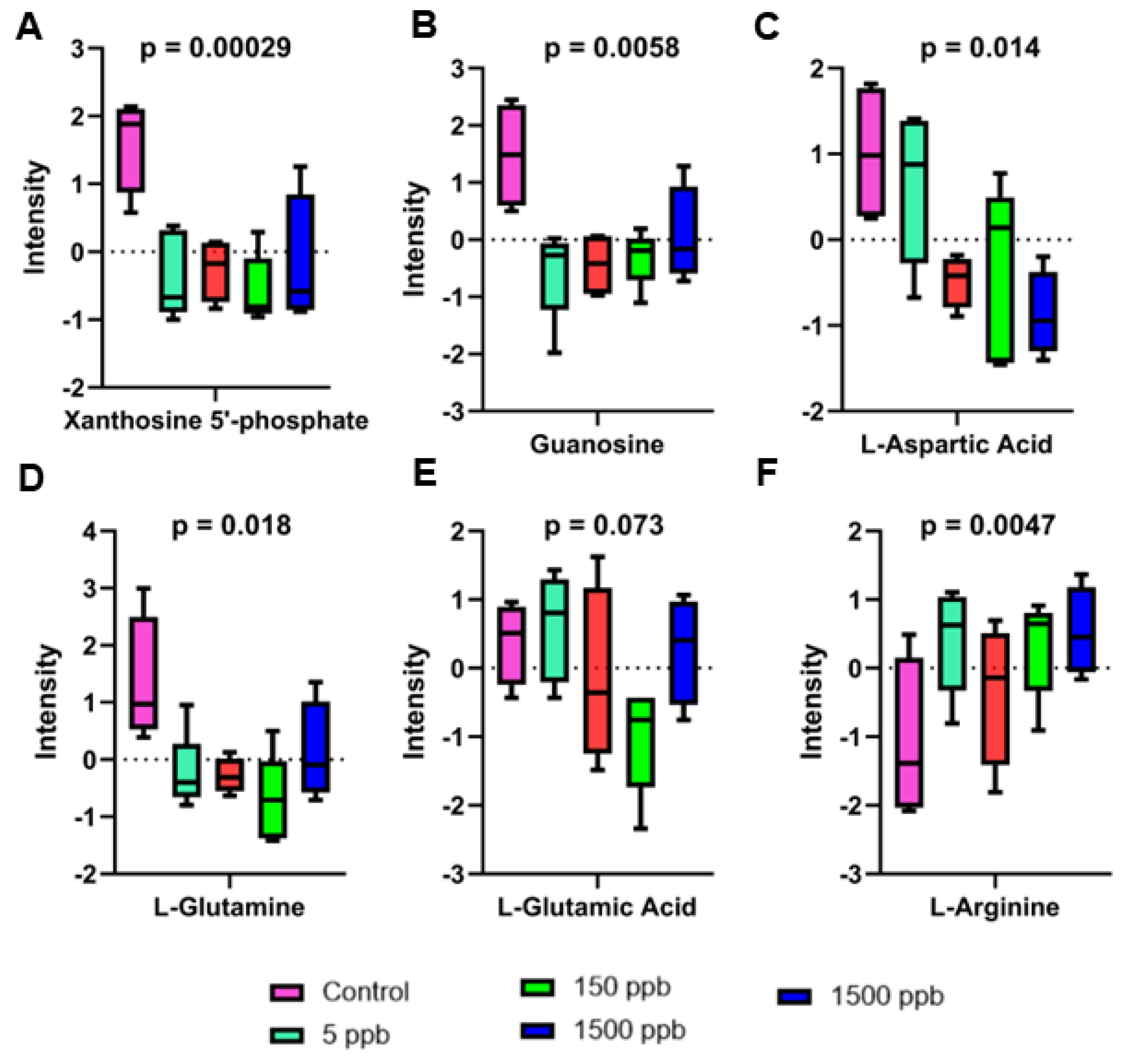
2.4. Altered Metabolic Pathways
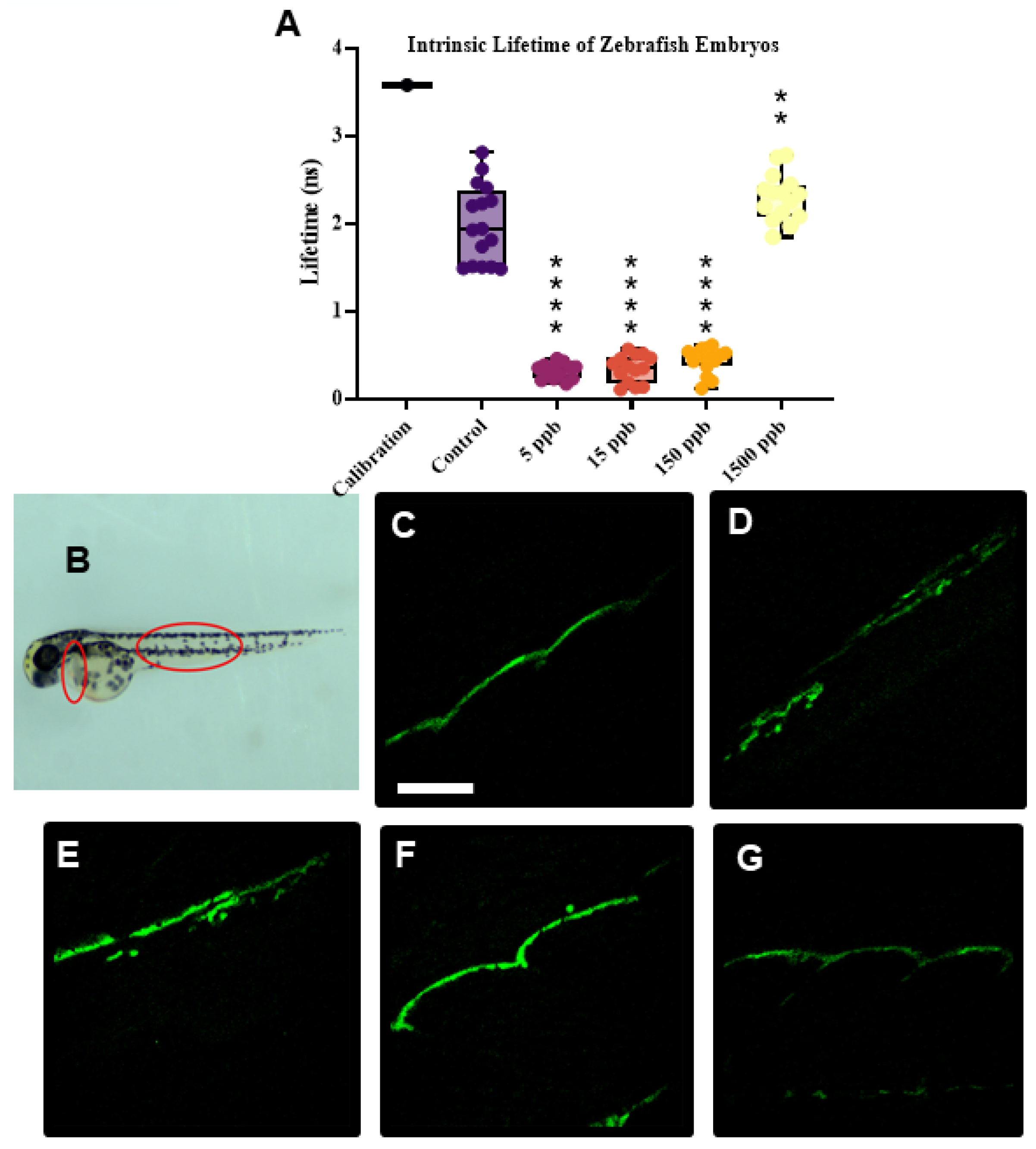
2.5. Intrinsic Fluorescent Lifetime Imaging Measurements (FLIMs)
3. Discussion
3.1. Metabolic Profiles of Pb-Exposed Embryos Are Distinct
3.2. Biopterin Metabolism
3.3. Purine Metabolism
3.4. Alanine and Aspartate Metabolism
3.5. Oxidative Stress
3.6. Hormetic Responses
4. Materials and Methods
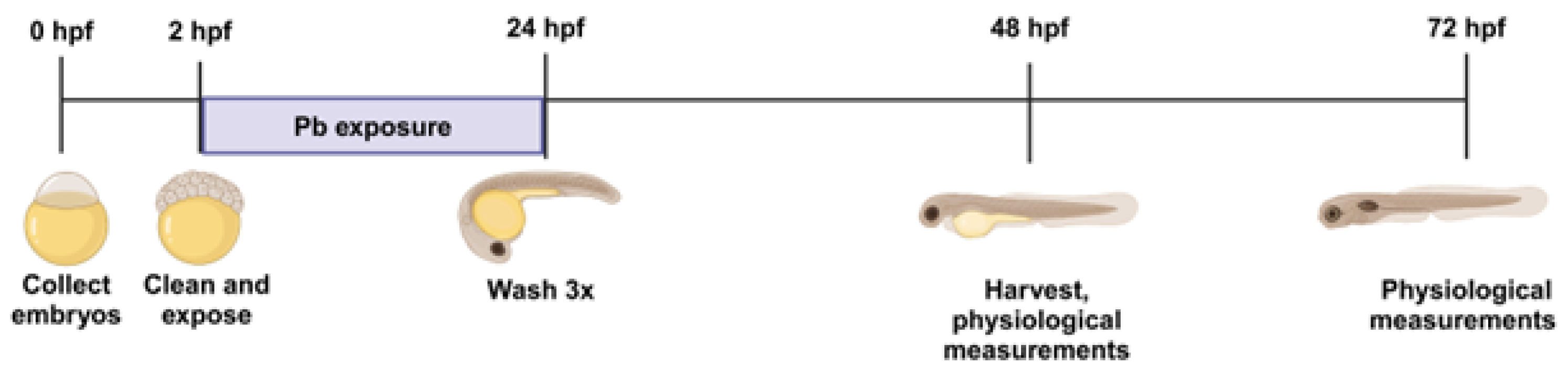
4.1. Zebrafish Maintenance and Exposure
4.2. Embryonic Imaging
4.3. Pb Concentrations Determined by Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
4.4. Sample Preparation for Metabolomic Analysis
4.5. LC-MS/MS Metabolite Analysis
4.6. Global Metabolomic Profiling
4.7. Fluorescence Lifetime Imaging Measurements (FLIMs)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riva, M.A.; Lafranconi, A.; D’orso, M.I.; Cesana, G. Lead Poisoning: Historical Aspects of a Paradigmatic ‘Occupational and Environmental Disease’. Saf. Health Work. 2012, 3, 11–16. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X.-K. Impact of Electronic Wastes Recycling on Environmental Quality. Biomed. Environ. Sci. 2006, 19, 137–142. [Google Scholar]
- Environmental Protection Agency. (2024, Nov 21) Basic Information About Lead in Drinking Water. Available online: https://www.epa.gov/ground-water-and-drinking-water/basic-information-about-lead-drinking-water#:~:text=EPA%20has%20set%20the%20maximum,in%20the%20body%20over%20time (accessed on 20 March 2024).
- de Souza, I.D.; de Andrade, A.S.; Dalmolin, R.J.S. Lead-interacting proteins and their implication in lead poisoning. Crit. Rev. Toxicol. 2018, 48, 375–386. [Google Scholar] [CrossRef]
- ACCLP. Low Level Lead Exposure Harms Children: A Renewed Call for Primary Prevention; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2012. [Google Scholar]
- Masten, S.J.; Davies, S.H.; Mcelmurry, S. Flint Water Crisis: What Happened and Why? J. Am. Water Works Assoc. 2016, 108, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Ruckart, Z.; Ettinger, A.S.; Hanna-Attisha, M.; Jones, N.; Davis, S.I.; Breysse, N. The Flint Water Crisis: A Coordinated Public Health Emergency Response and Recovery Initiative. J. Public. Health Manag. Pract. 2019, 25, S84–S90. [Google Scholar] [CrossRef]
- Montana Department of Environmental Quality. School Lead Results. 2022. Available online: https://mtdeq.equisonline.us/Default.aspx?d=16234658 (accessed on 20 March 2024).
- He, X.; Wu, J.; Yuan, L.; Lin, F.; Yi, J.; Li, J.; Yuan, H.; Shi, J.; Yuan, T.; Zhang, S.; et al. Lead induces apoptosis in mouse TM3 Leydig cells through the Fas/FasL death receptor pathway. Environ. Toxicol. Pharmacol. 2017, 56, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Kasten-Jolly, J.; Lawrence, D.A. Sex-specific effects of developmental lead exposure on the immune-neuroendocrine network. Toxicol. Appl. Pharmacol. 2017, 334, 142–157. [Google Scholar] [CrossRef]
- Xia, J.; Lu, L.; Jin, C.; Wang, S.; Zhou, J.; Ni, Y.; Fu, Z.; Jin, Y. Effects of short term lead exposure on gut microbiota and hepatic metabolism in adult zebrafish. Comp. Biochem. Physiol. Part. C Toxicol. Pharmacol. 2018, 209, 1–8. [Google Scholar] [CrossRef]
- Mason, L.H.; Harp, J.; Han, D.Y. Pb Neurotoxicity: Neuropsychological Effects of Lead Toxicity. Biomed. Res. Int. 2014, 2014, 840547. [Google Scholar] [CrossRef] [PubMed]
- Lidsky, T.I.; Schneider, J.S. Lead neurotoxicity in children: Basic mechanisms and clinical correlates. Brain 2003, 126, 5–19. [Google Scholar] [CrossRef]
- Collin, M.S.; Venkatraman, S.K.; Vijayakumar, N.; Kanimozhi, V.; Arbaaz, S.M.; Stacey, R.G.S.; Anusha, J.; Choudhary, R.; Lvov, V.; Tovar, G.I.; et al. Bioaccumulation of lead (Pb) and its effects on human: A review. J. Hazard. Mater. Adv. 2022, 7, 100094. [Google Scholar] [CrossRef]
- Needleman, H.L.; Gunnoe, C.; Leviton, A.; Reed, R.; Peresie, H.; Maher, C.; Barrett, P. Deficits in Psychologic and Classroom Performance of Children with Elevated Dentine Lead Levels. N. Engl. J. Med. 1979, 300, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Eaves, L.A.; Choi, G.; Hall, E.; Sille, F.C.M.; Fry, R.C.; Buckley, J.P.; Keil, A.P. Prenatal Exposure to Toxic Metals and Neural Tube Defects: A Systematic Review of the Epidemiologic Evidence. Environ. Health Perspect. 2023, 131, 086002. [Google Scholar] [CrossRef] [PubMed]
- Florea, A.-M.; Taban, J.; Varghese, E.; Alost, B.T.; Moreno, S.; Büsselberg, D. Lead (Pb2+) neurotoxicity: Ion-mimicry with calcium (Ca2+) impairs synaptic transmission. A review with animated illustrations of the pre- and post-synaptic effects of lead. J. Local. Glob. Health Sci. 2013, 2013, 4. [Google Scholar] [CrossRef]
- Zhou, J.; Hong, H.; Zhao, J.; Fang, R.; Chen, S.; Tang, C. Metabolome analysis to investigate the effect of heavy metal exposure and chemoprevention agents on toxic injury caused by a multi-heavy metal mixture in rats. Sci. Total Environ. 2024, 906, 167513. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Dou, C.; Zhang, J. Effects of lead on neurogenesis during zebrafish embryonic brain development. J. Hazard. Mater. 2011, 194, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Curcio, V.; Macirella, R.; Sesti, S.; Pellegrino, D.; Ahmed, A.I.M.; Brunelli, E. Morphological and Molecular Alterations Induced by Lead in Embryos and Larvae of Danio rerio. Appl. Sci. 2021, 11, 7464. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, L.; Lin, G.-M.; Lu, J.-G.; Cui, Z.-B. Analysis of Differential Gene Expression under Acute Lead or Mercury Exposure in Larval Zebrafish Using RNA-Seq. Animals 2024, 14, 2877. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, Y.; Liu, W.; Bai, C.; Liu, A.; Liu, K.; Li, R.; Zhu, H.; Huang, C. Developmental lead acetate exposure induces embryonic toxicity and memory deficit in adult zebrafish. Neurotoxicol. Teratol. 2012, 34, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Komoike, Y.; Matsuoka, M. Developmental adverse effects of trace amounts of lead: Evaluation using zebrafish model. Front. Pharmacol. 2022, 13, 1014912. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, C.; He, M.; Yu, L.; Liu, R.; Ma, C.; Zhang, Y.; Jia, J.; Li, B.; Li, L. Lead Exposure Causes Spinal Curvature during Embryonic Development in Zebrafish. Int. J. Mol. Sci. 2022, 23, 9571. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, Q.; Zhang, B.; Xu, T.; Yin, D.; Gu, W.; Bai, J. Developmental exposure to lead at environmentally relevant concentrations impaired neurobehavior and NMDAR-dependent BDNF signaling in zebrafish larvae. Environ. Pollut. 2020, 257, 113627. [Google Scholar] [CrossRef]
- Wirbisky, S.E.; Weber, G.J.; Lee, J.-W.; Cannon, J.R.; Freeman, J.L. Novel dose-dependent alterations in excitatory GABA during embryonic development associated with lead (Pb) neurotoxicity. Toxicol. Lett. 2014, 229, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Martín, F.J.; Fan, Y.; Lindquist, D.M.; Xia, Y.; Puga, A. Lead Induces Similar Gene Expression Changes in Brains of Gestationally Exposed Adult Mice and in Neurons Differentiated from Mouse Embryonic Stem Cells. PLoS ONE 2013, 8, e80558. [Google Scholar] [CrossRef][Green Version]
- Kopp, R.S.; Kumbartski, M.; Harth, V.; Brüning, T.; Käfferlein, H.U. Partition of metals in the maternal/fetal unit and lead-associated decreases of fetal iron and manganese: An observational biomonitoring approach. Arch. Toxicol. 2012, 86, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.-H.; Zheng, G.; Wang, T.; Du, J.; Luo, W.; Shen, Z.; Chen, J. Low-level Gestational Lead Exposure Alters Dendritic Spine Plasticity in the Hippocampus and Reduces Learning and Memory in Rats. Sci. Rep. 2018, 8, 3533. [Google Scholar] [CrossRef] [PubMed]
- Mousa, A.M.; Al-Fadhli, A.S.; Rao, M.S.; Kilarkaje, N. Gestational lead exposure induces developmental abnormalities and up-regulates apoptosis of fetal cerebellar cells in rats. Drug Chem. Toxicol. 2015, 38, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Xu, L.; Liu, Z.-H.; Ge, M.-M.; Ruan, D.-Y.; Wang, H.-L. Developmental Lead Exposure Alters Synaptogenesis through Inhibiting Canonical Wnt Pathway In Vivo and In Vitro. PLoS ONE 2014, 9, e101894. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wu, Q.; Miao, X.; Fan, T.; Meng, Z.; Chen, X.; Zhu, W. Study on toxicity effects of environmental pollutants based on metabolomics: A review. Chemosphere 2022, 286, 131815. [Google Scholar] [CrossRef]
- Zhang, M.; Buckley, J.P.; Liang, L.; Hang, X.; Wang, G.; Wang, M.; Wills-Karp, M.; Wang, X.; Mueller, N.T. A metabolome-wide association study of in utero metal and trace element exposures with cord blood metabolome profile: Findings from the Boston Birth Cohort. Environ. Int. 2022, 158, 106976. [Google Scholar] [CrossRef]
- Kelly, R.S.; Bayne, H.; Spiro II, A.; Vokonas, P.; Sparrow, D.; Weiss, S.T.; Schwartz, J.; Nassan, F.L.; Lee-Sarwar, K.; Huang, M.; et al. Metabolomic signatures of lead exposure in the VA Normative Aging Study. Environ. Res. 2020, 190, 110022. [Google Scholar] [CrossRef]
- Zeng, X.; Zeng, Z.; Wang, Q.; Liang, W.; Guo, Y.; Huo, X. Alterations of the gut microbiota and metabolomics in children with e-waste lead exposure. J. Hazard. Mater. 2022, 434, 128842. [Google Scholar] [CrossRef]
- Gao, B.; Chi, L.; Mahbub, R.; Bian, X.; Tu, P.; Ru, H.; Lu, K. Multi-Omics Reveals that Lead Exposure Disturbs Gut Microbiome Development, Key Metabolites, and Metabolic Pathways. Chem. Res. Toxicol. 2017, 30, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Liu, Y.; Li, M.; Xu, H.; Sheng, J.; Zhang, L.; Wang, J. Integrated 1H NMR-based metabolomics analysis of earthworm responses to sub-lethal Pb exposure. Environ. Chem. 2016, 13, 792. [Google Scholar] [CrossRef]
- Cheng, S.; Shah, S.H.; Corwin, E.J.; Fiehn, O.; Fitzgerald, R.L.; Gerszten, R.E.; Illig, T.; Rhee, E.P.; Srinivas, P.R.; Wang, T.J.; et al. Potential Impact and Study Considerations of Metabolomics in Cardiovascular Health and Disease: A Scientific Statement From the American Heart Association. Circ. Cardiovasc. Genet. 2017, 10, e000032. [Google Scholar] [CrossRef]
- Qiu, S.; Cai, Y.; Yao, H.; Lin, C.; Xie, Y.; Tang, S.; Zhang, A. Small molecule metabolites: Discovery of biomarkers and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Le Gal, K.; Schmidt, E.E.; Sayin, V.I. Cellular Redox Homeostasis. Antioxidants 2021, 10, 1377. [Google Scholar] [CrossRef] [PubMed]
- Vacchi-Suzzi, C.; Viens, L.; Karimi, R.; Meliker, J. Lead exposure and glutathione markers of redox status in human blood. ISEE Conf. Abstr. 2016, 2016, P2-090. [Google Scholar] [CrossRef]
- Chance, B.; Schoener, B.; Oshino, R.; Itshak, F.; Nakase, Y. Oxidation-reduction ratio studies of mitochondria in freeze-trapped samples. NADH and flavoprotein fluorescence signals. J. Biol. Chem. 1979, 254, 4764–4771. [Google Scholar] [CrossRef] [PubMed]
- Stringari, C.; Cinquin, A.; Cinquin, O.; Digman, M.A.; Donovan, J.; Gratton, E. Phasor approach to fluorescence lifetime microscopy distinguishes different metabolic states of germ cells in a live tissue. Proc. Natl. Acad. Sci. 2011, 108, 13582–13587. [Google Scholar] [CrossRef]
- Bartolomé, F.; Abramov, A.Y. Measurement of Mitochondrial NADH and FAD Autofluorescence in Live Cells. Mitochondrial Med. 2015, 1264, 263–270. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Buijtendijk, M.F.J.; Barnett, P.; van den Hoff, M.J.B. Development of the human heart. Am. J. Med. Genet. C Semin. Med. Genet. 2020, 184, 7–22. [Google Scholar] [CrossRef]
- Nguyen, T.K.; Petrikas, M.; Chambers, B.E.; Wingert, R.A. Principles of Zebrafish Nephron Segment Development. J. Dev. Biol. 2023, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Verbueken, E.; Bars, C.; Ball, J.S.; Periz-Stanacev, J.; Marei, W.F.A.; Tochwin, A.; Babriels, I.J.; Michiels, E.D.G.; Stinckens, E.; Vergauwen, L.; et al. From mRNA Expression of Drug Disposition Genes to In Vivo Assessment of CYP-Mediated Biotransformation during Zebrafish Embryonic and Larval Development. Int. J. Mol. Sci. 2018, 19, 3976. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J. The Maturing of Hormesis as a Credible Dose-Response Model. Nonlinearity Biol. Toxicol. Med. 2003, 1, 15401420390249907. [Google Scholar] [CrossRef]
- Steward, K.F.; Refai, M.; Dyer, W.E.; Copié, V.; Lachowiec, J.; Bothner, B. Acute stress reduces population-level metabolic and proteomic variation. BMC Bioinform. 2023, 24, 87. [Google Scholar] [CrossRef] [PubMed]
- Seeman, P.; Kapur, S. Schizophrenia: More dopamine, more D2 receptors. Proc. Natl. Acad. Sci. USA 2000, 97, 7673–7675. [Google Scholar] [CrossRef]
- Kim, H.-L.; Park, Y.-S. Maintenance of cellular tetrahydrobiopterin homeostasis. BMB Rep. 2010, 43, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Maximilian, B. Characterizing the Function and Role of Three Dihydropteridine Reductase Homologs, Qdpra, Qdprb1 and Qdprb2 in the Embryonic Development of Danio Rerio. Ph.D. Thesis, Ruprecht-Karls-University of Heidelberg, Heidelberg, Germany, 2018. [Google Scholar]
- Ziegler, I. The Pteridine Pathway in Zebrafish: Regulation and Specification during the Determination of Neural Crest Cell-Fate. Pigment. Cell Res. 2003, 16, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, I.; McDonaldo, T.; Hesslinger, C.; Pelletier, I.; Boyle, P. Development of the Pteridine Pathway in the Zebrafish, Danio rerio. J. Biol. Chem. 2000, 275, 18926–18932. [Google Scholar] [CrossRef] [PubMed]
- Engin, A.B.; Tuzun, D.; Sahin, G. Evaluation of pteridine metabolism in battery workers chronically exposed to lead. Hum. Exp. Toxicol. 2006, 25, 353–359. [Google Scholar] [CrossRef]
- Peterson, S.M.; Zhang, J.; Weber, G.; Freeman, J.L. Global Gene Expression Analysis Reveals Dynamic and Developmental Stage–Dependent Enrichment of Lead-Induced Neurological Gene Alterations. Environ. Health Perspect. 2011, 119, 615–621. [Google Scholar] [CrossRef]
- Akinyemi, A.J.; Miah, M.R.; Ijomone, O.M.; Tsatsakis, A.; Soares, F.A.A.; Tinkov, A.A.; Skalny, A.V.; Venkataramani, V.; Aschner, M. Lead (Pb) exposure induces dopaminergic neurotoxicity in Caenorhabditis elegans: Involvement of the dopamine transporter. Toxicol. Rep. 2019, 6, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Taki, Y.; Nouchi, R.; Yokoyama, R.; Kotozaki, Y.; Nakagawa, Y.; Sekiguchi, A.; Iizuka, K.; Hanawa, S.; Araki, T.; et al. Lead exposure is associated with functional and microstructural changes in the healthy human brain. Commun. Biol. 2021, 4, 912. [Google Scholar] [CrossRef] [PubMed]
- Mitra, M.L.; Goyal, T.; Sharma, S.; Purohit, P.; Sharma, P. Association of blood lead levels with neurobehavior and BDNF expression in school going children. J. Trace Elem. Med. Biol. 2021, 66, 126749. [Google Scholar] [CrossRef]
- Tamegart, L.; Abbaoui, A.; El khiat, A.; Bouyatas, M.M.; Gamrani, H. Lead (Pb) exposure induces physiological alterations in the serotoninergic and vasopressin systems causing anxiogenic-like behavior in Meriones shawi: Assessment of BDMC as a neuroprotective compound for Pb-neurotoxicity and kidney damages. J. Trace Elem. Med. Biol. 2021, 65, 126722. [Google Scholar] [CrossRef]
- Garza, A.; Vega, R.; Soto, E. Cellular mechanisms of lead neurotoxicity. Med. Sci. Monit. 2006, 12, RA57-65. [Google Scholar] [PubMed]
- Sanders, T.; Liu, Y.; Buchner, V.; Tchounwou, B. Neurotoxic Effects and Biomarkers of Lead Exposure: A Review. Rev. Environ. Health 2009, 24, 15–46. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Ren, W.; Huang, X.; Deng, J.; Li, T.; Yin, Y. Potential Mechanisms Connecting Purine Metabolism and Cancer Therapy. Front. Immunol. 2018, 9, 1697. [Google Scholar] [CrossRef] [PubMed]
- Marsac, R.; Pinson, B.; Saint-Marc, C.; Olmedo, M.; Artal-Sanz, M.; Daignan-Fornier, B.; Gomes, J. Purine Homeostasis Is Necessary for Developmental Timing, Germline Maintenance and Muscle Integrity in Caenorhabditis elegans. Genetics 2019, 211, 1297–1313. [Google Scholar] [CrossRef]
- Jinnah, H.A.; Sabina, R.L.; Van Den Berghe, G. Metabolic disorders of purine metabolism affecting the nervous system. Handb. Clin. Neurol. 2013, 113, 1827–1836. [Google Scholar] [CrossRef]
- Rathbone, M.; Middlemiss, P.J.; Gysbers, J.W.; Andrew, C.; Herman, M.A.R.; Reed, J.K.; Di Iorio, P.; Caciagli, F. Trophic effects of purines in neurons and glial cells. Prog. Neurobiol. 1999, 59, 663–690. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Zhang, W.; Gup, J.; Zhu, Q.; Chen, H.; Xia, Y.; Zhu, G. Mitochondrion: A sensitive target for Pb exposure. J. Toxicol. Sci. 2021, 46, 345–358. [Google Scholar] [CrossRef] [PubMed]
- De Vitto, H.; Arachchige, D.B.; Richardson, B.C.; French, J.B. The Intersection of Purine and Mitochondrial Metabolism in Cancer. Cells 2021, 10, 2603. [Google Scholar] [CrossRef]
- Pedley, A.M.; Benkovic, S.J. A New View into the Regulation of Purine Metabolism: The Purinosome. Trends Biochem. Sci. 2017, 42, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Bouton, C.M.L.S.; Hossain, M.A.; Frelin, L.; Laterra, J.; Pevsner, J. Microarray Analysis of Differential Gene Expression in Lead-Exposed Astrocytes. Toxicol. Appl. Pharmacol. 2001, 176, 34–53. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Han, H.; Yin, J.; Li, T.; Yin, Y. Role of D-aspartate on biosynthesis, racemization, and potential functions: A mini-review. Anim Nutr. 2018, 4, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Jian, H.; Xu, Q.; Wang, X.; Liu, Y.; Miao, S.; Li, Y.; Mou, T.; Dong, X.; Zou, X. Amino Acid and Fatty Acid Metabolism Disorders Trigger Oxidative Stress and Inflammatory Response in Excessive Dietary Valine-Induced NAFLD of Laying Hens. Front. Nutr. 2022, 9, 849767. [Google Scholar] [CrossRef]
- Eguchi, A.; Nomiyama, K.; Sakurai, K.; Trang, P.T.K.; Viet, P.H.; Takahashi, S.; Iwata, H.; Tanabe, S.; Todaka, E.; Mori, C. Alterations in urinary metabolomic profiles due to lead exposure from a lead–acid battery recycling site. Environ. Pollut. 2018, 242, 98–105. [Google Scholar] [CrossRef]
- Yaqoob, A.; Rehman, K.; Akash, M.S.H.; Alvi, M.; Shoaib, S.M. Biochemical profiling of metabolomics in heavy metal-intoxicated impaired metabolism and its amelioration using plant-based bioactive compound. Front. Mol. Biosci. 2022, 9, 1029729. [Google Scholar] [CrossRef]
- Lasley, S.M. Rat Hippocampal Glutamate and GABA Release Exhibit Biphasic Effects as a Function of Chronic Lead Exposure Level. Toxicol. Sci. 2002, 66, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Christou, A.; Georgiadou, E.C.; Zissimos, A.M.; Christoforou, I.C.; Christofi, W.; Neocleous, D.; Dalias, P.; Torrado, S.O.C.A.; Argyraki, A.; Fotopoulos, V. Hexavalent chromium leads to differential hormetic or damaging effects in alfalfa (Medicago sativa L.) plants in a concentration-dependent manner by regulating nitro-oxidative and proline metabolism. Environ. Pollut. 2020, 267, 115379. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qiu, Y.; Zhao, Q.; Chen, D.; Wu, Z.; Peng, A.; Niazi, N.K.; Trakal, L.; Sakrabani, R.; Gao, B.; et al. Lead and copper-induced hormetic effect and toxicity mechanisms in lettuce (Lactuca sativa L.) grown in a contaminated soil. Sci. Total Environ. 2020, 741, 140440. [Google Scholar] [CrossRef] [PubMed]
- Pfleger, H.; Wolf, H.U. Activation of membrane-bound high-affinity calcium ion-sensitive adenosine triphosphatase of human erythrocytes by bivalent metal ions. Biochem. J. 1975, 147, 359–361. [Google Scholar] [CrossRef]
- Kerper, L.E.; Hinkle, M. Lead Uptake in Brain Capillary Endothelial Cells: Activation by Calcium Store Depletion. Toxicol. Appl. Pharmacol. 1997, 146, 127–133. [Google Scholar] [CrossRef]
- Kerper, L.E.; Hinkle, M. Cellular Uptake of Lead Is Activated by Depletion of Intracellular Calcium Stores. J. Biol. Chem. 1997, 272, 8346–8352. [Google Scholar] [CrossRef] [PubMed]
- Komjarova, I.; Blust, R. Multimetal Interactions between Cd, Cu, Ni, Pb, and Zn Uptake from Water in the Zebrafish Danio rerio. Environ. Sci. Technol. 2009, 43, 7225–7229. [Google Scholar] [CrossRef]
- Hu, S.; Han, J.; Yang, L.; Li, S.; Guo, Y.; Zou, B.; Wu, H. Impact of co-exposure to titanium dioxide nanoparticles and Pb on zebrafish embryos. Chemosphere 2019, 233, 579–589. [Google Scholar] [CrossRef]
- Larson, J.; Tokmina-Lukaszeweska, M.; Fausset, H.; Spurzem, S.; Cox, S.; Cooper, G.; Copie, V.; Bothner, B. Arsenic Exposure Causes Global Changes in the Metalloproteome of Escherichia coli. Microorganisms 2023, 11, 382. [Google Scholar] [CrossRef] [PubMed]
- Shirshin, E.A.; Shirmanova, M.V.; Gayer, A.V.; Lukina, M.M.; Nikonova, E.E.; Yakimov, B.P.; Budylin, G.S.; Dudenkova, V.V.; Ignatova, N.I.; Komarov, D.V.; et al. Label-free sensing of cells with fluorescence lifetime imaging: The quest for metabolic heterogeneity. Proc. Natl. Acad. Sci. USA 2022, 119, e2118241119. [Google Scholar] [CrossRef] [PubMed]
- Kiljańczyk, A.; Matuszczak, M.; Marciniak, W.; Derkacz, R.; Stempa, K.; Baszuk, P.; Bryskiewicz, M.; Lubinski, K.; Cybulski, C.; Debniak, T.; et al. Blood Lead Level as Marker of Increased Risk of Ovarian Cancer in BRCA1 Carriers. Nutrients 2024, 16, 1370. [Google Scholar] [CrossRef]
- Mattson, M. Hormesis defined. Ageing Res. Rev. 2008, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.M.; Cheng, C.N.; Marino, A.; Konsoula, R.; Barile, F.A. Hormesis effect of trace metals on cultural normal and immortal mammary cells. Toxicol. Ind. Health 2004, 20, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Kataba, A.; Botha, T.L.; Nakayama, S.M.; Yohannes, Y.B.; Ikenaka, Y.; Wepener, V.; Ishizuka, M. Environmentally relevant lead (Pb) water concentration induce toxicity in zebrafish (Danio rerio) larvae. Comp. Biochem. Physiol. Part. C Toxicol. Pharmacol. 2022, 252, 109215. [Google Scholar] [CrossRef]
- Pellegrini, O.; Davenas, E.; Morin, L.; Tsangaris, G.T.; Benveniste, J.; Manuel, Y.; Thomas, Y. Modulation of stress proteins by Cd2+ in a human T cell line. Eur. J. Pharmacol. Environ. Toxicol. Pharmacol. 1994, 270, 221–228. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Thomason, R.T.; Pettiglio, M.A.; Herrera, C.; Kao, C.; Gitlin, J.D.; Bartnikas, T.B. Characterization of trace metal content in the developing zebrafish embryo. PLoS ONE 2017, 12, e0179318. [Google Scholar] [CrossRef] [PubMed]


| Pb Concentration | 5 ppb | 15 ppb | 150 ppb | 1500 ppb | |
|---|---|---|---|---|---|
| Biopterin metabolism | Pathway total | 26 | 26 | 26 | 26 |
| Hits total | 9 | 9 | 11 | 9 | |
| Significant hits | 7 | 6 | 11 | 6 | |
| p (gamma) | 0.01 | 0.0073 | 0.0075 | 0.0051 | |
| Purine metabolism | Pathway total | 80 | 80 | 80 | 80 |
| Hits total | 32 | 32 | 27 | 32 | |
| Significant hits | 16 | 15 | 11 | 12 | |
| p (gamma) | 0.012 | 0.0066 | 0.01 | 0.0056 | |
| Alanine and aspartate metabolism | Pathway total | 20 | 20 | 20 | 20 |
| Hits total | 10 | 10 | 9 | 10 | |
| Significant hits | 5 | 7 | 4 | 4 | |
| p (gamma) | 0.036 | 0.0066 | 0.025 | 0.015 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cooper, G.; North, R.; Hunt-Smith, T.; Larson, J.; Rennie, M.; Bailey, M.L.; Scarlata, S.; Merzdorf, C.S.; Bothner, B. Persistent Metabolic Changes Are Induced by 24 h Low-Dose Lead (Pb) Exposure in Zebrafish Embryos. Int. J. Mol. Sci. 2025, 26, 1050. https://doi.org/10.3390/ijms26031050
Cooper G, North R, Hunt-Smith T, Larson J, Rennie M, Bailey ML, Scarlata S, Merzdorf CS, Bothner B. Persistent Metabolic Changes Are Induced by 24 h Low-Dose Lead (Pb) Exposure in Zebrafish Embryos. International Journal of Molecular Sciences. 2025; 26(3):1050. https://doi.org/10.3390/ijms26031050
Chicago/Turabian StyleCooper, Gwendolyn, Ryan North, Tyler Hunt-Smith, James Larson, Madison Rennie, Marguerite L. Bailey, Suzanne Scarlata, Christa S. Merzdorf, and Brian Bothner. 2025. "Persistent Metabolic Changes Are Induced by 24 h Low-Dose Lead (Pb) Exposure in Zebrafish Embryos" International Journal of Molecular Sciences 26, no. 3: 1050. https://doi.org/10.3390/ijms26031050
APA StyleCooper, G., North, R., Hunt-Smith, T., Larson, J., Rennie, M., Bailey, M. L., Scarlata, S., Merzdorf, C. S., & Bothner, B. (2025). Persistent Metabolic Changes Are Induced by 24 h Low-Dose Lead (Pb) Exposure in Zebrafish Embryos. International Journal of Molecular Sciences, 26(3), 1050. https://doi.org/10.3390/ijms26031050







