Rationale for Testing TP53 Mutations in Thyroid Cancer—Original Data and Meta-Analysis
Abstract
1. Introduction
2. Results
2.1. Original Study
2.2. Meta-Analysis
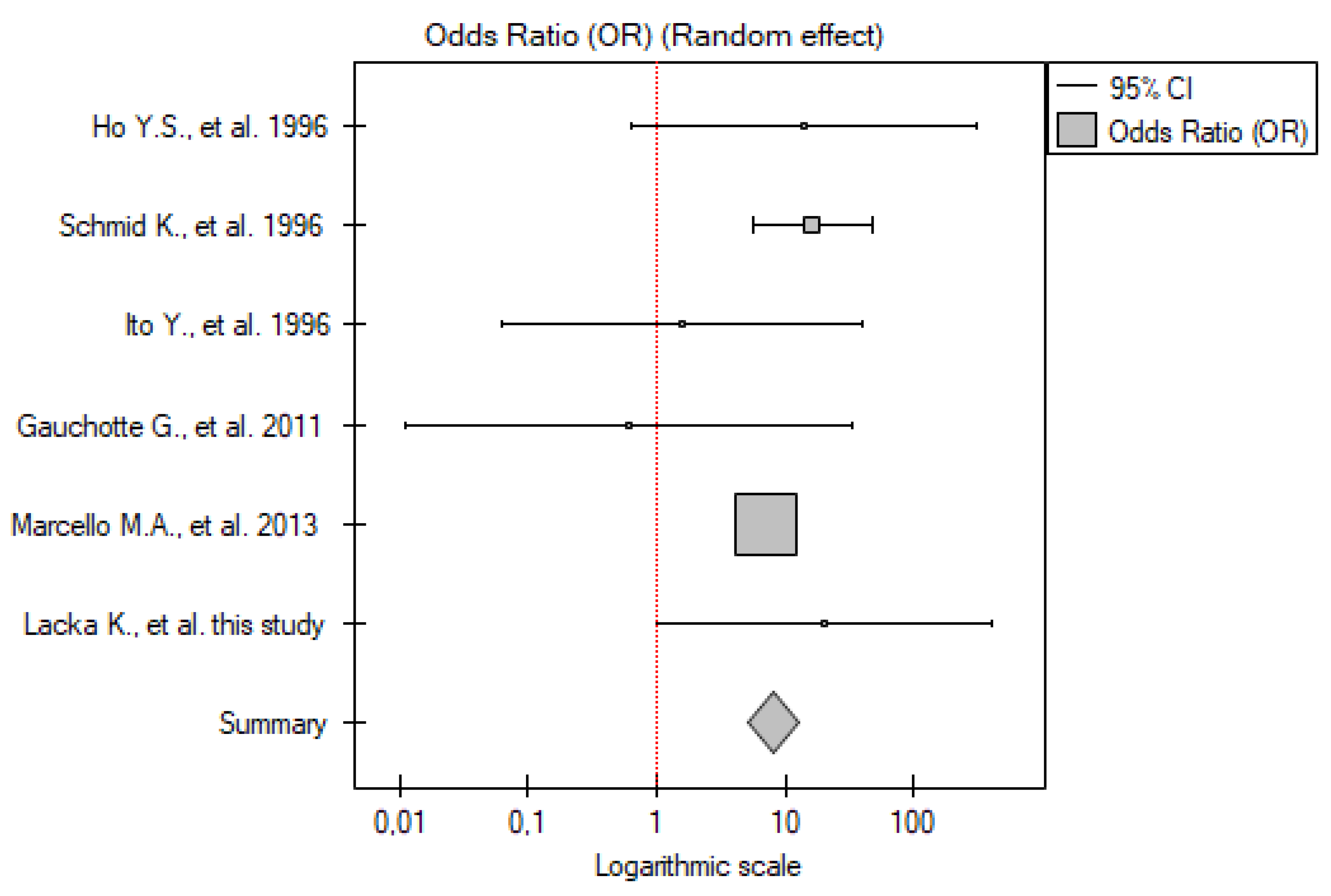
2.3. TP53 Mutations
2.3.1. ATC vs. Control Group
2.3.2. PDTC vs. Control Group
2.3.3. DTC vs. Control Group
2.3.4. PTC vs. Control Group
2.3.5. FTC vs. Control Group
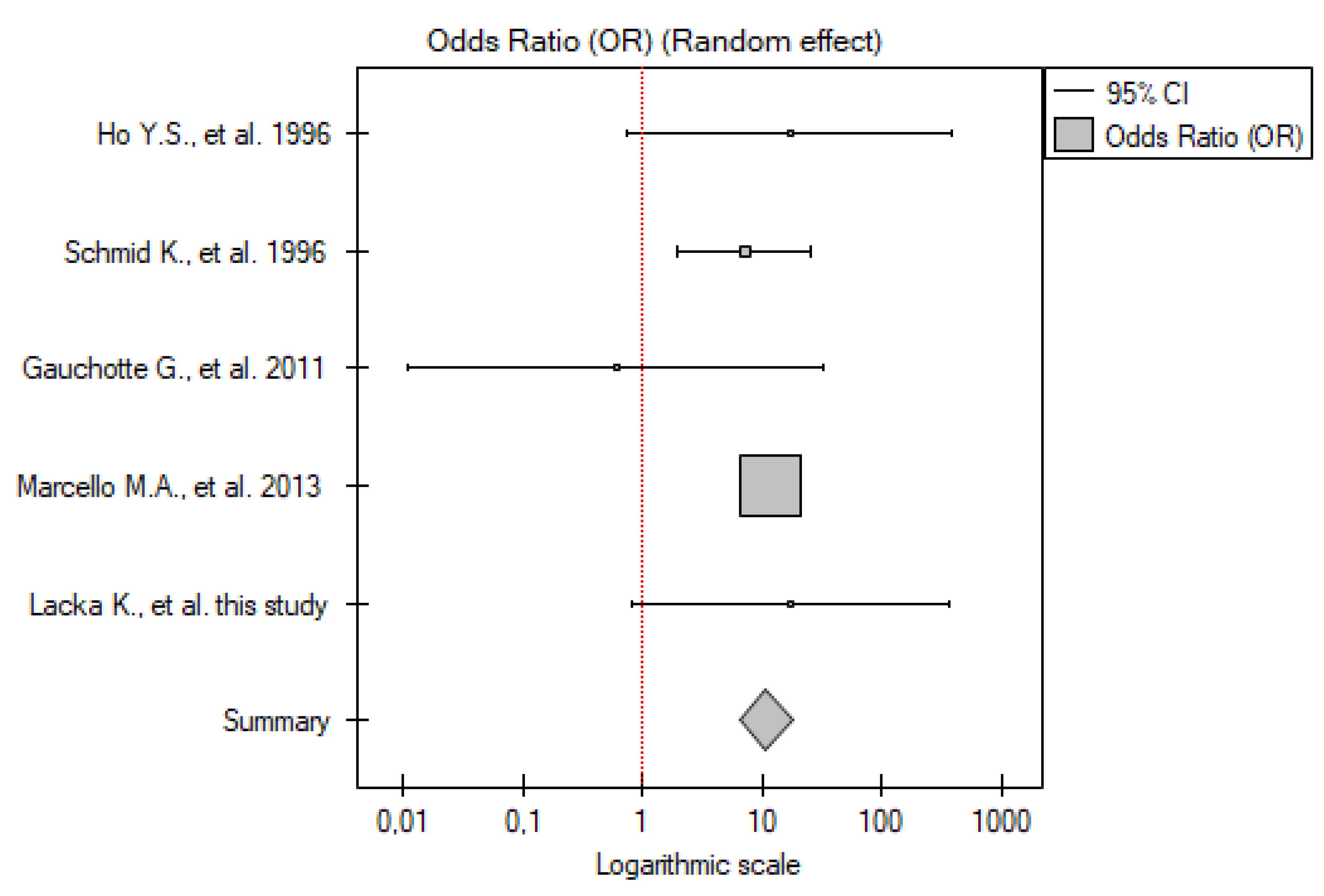
2.4. IHC
2.4.1. ATC vs. Control Group
2.4.2. PDTC vs. Control Group
2.4.3. DTC vs. Control Group
2.4.4. PTC vs. Control Group
2.4.5. FTC vs. Control Group
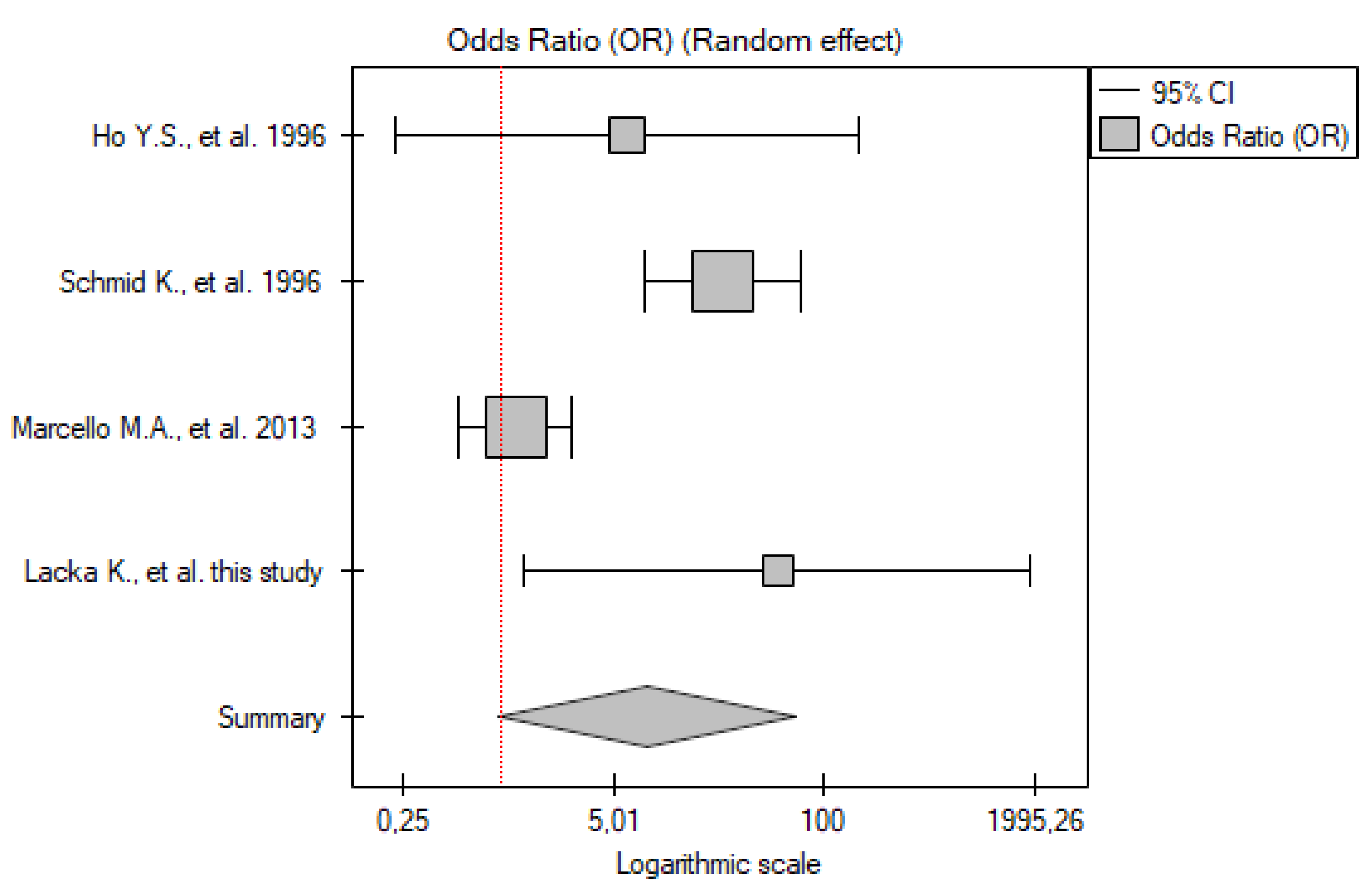
2.5. P53 Ab
PTC vs. Control Group
3. Discussion
4. Material and Methods
4.1. Original Study
4.1.1. Patients
4.1.2. Mutation Detection
- -
- Exon 5
- -
- Exon 6
- -
- Exon 7
- -
- Exon 8
4.1.3. Immunohistochemistry
4.2. Meta-Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATC | Anaplastic thyroid carcinoma |
| CI | Confidence Interval |
| DTC | Differentiated thyroid cancer |
| FTC | Follicular thyroid carcinoma |
| IHC | Immunohistochemistry |
| MTC | Medullary thyroid carcinoma |
| NGS | Next-Generation Sequencing |
| OR | Odds Ratio |
| OTC | Oncocytic carcinoma of the thyroid |
| PDTC | Poorly differentiated thyroid carcinoma |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PTC | Papillary thyroid carcinoma |
| p53-Abs | p53 antibodies |
| SSCP | Single-Strand Conformation Polymorphism |
| TNM | Tumor, Nodes, Metastasis (staging system) |
| TP53 | Tumor protein p53 (gene) |
References
- Lim, D.V.; Woo, W.H.; Lim, J.X.; Loh, X.Y.; Soh, H.T.; Lim, S.Y.A.; Lee, Z.Y.; Yow, H.Y.; Hamzah, S.B.; Sellappans, R.; et al. Targeting Mutant-p53 for Cancer Treatment: Are We There Yet? Curr. Mol. Pharmacol. 2024, 17, e140923221042. [Google Scholar] [CrossRef] [PubMed]
- Donehower, L.A.; Soussi, T.; Korkut, A.; Liu, Y.; Schultz, A.; Cardenas, M.; Li, X.; Babur, O.; Hsu, T.K.; Lichtarge, O.; et al. Integrated Analysis of TP53 Gene and Pathway Alterations in The Cancer Genome Atlas. Cell Rep. 2019, 28, 1370–1384. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.A.; Vousden, K.H. p53 Mutations in Cancer. Nat. Cell Biol. 2013, 15, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.J. The Many Faces of p53: Something for Everyone. J. Mol. Cell Biol. 2019, 11, 524–530. [Google Scholar] [CrossRef]
- Bai, B.; An, X.; Qu, Q.; Liu, X.; Liu, Y.; Wei, L. The Clinical Features and Prognostic Implications of the Co-Mutated TP53 Gene in Advanced Non-Small Cell Lung Cancer. Clin. Transl. Oncol. 2024, 26, 3236–3245. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, T.; Su, W.; Dou, Z.; Zhao, D.; Jin, X.; Lei, H.; Wang, J.; Xie, X.; Cheng, B.; et al. Mutant p53 in Cancer: From Molecular Mechanism to Therapeutic Modulation. Cell Death Dis. 2022, 13, 974. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, J.; Xu, D.; Zhang, T.; Hu, W.; Feng, Z. Gain-of-Function Mutant p53 in Cancer Progression and Therapy. J. Mol. Cell Biol. 2020, 12, 674–687. [Google Scholar] [CrossRef]
- Lacka, K.; Maciejewski, A. Rola procesu apoptozy w etiopatogenezie autoimmunologicznego zapalenia tarczycy [The Role of Apoptosis in the Etiopathogenesis of Autoimmune Thyroiditis]. Pol. Merkur Lekarski. 2012, 32, 87–92. [Google Scholar]
- Kitahara, C.M.; Schneider, A.B. Epidemiology of Thyroid Cancer. Cancer Epidemiol. Biomark. Prev. 2022, 31, 1284–1297. [Google Scholar] [CrossRef]
- Quiros, R.M.; Ding, H.G.; Gattuso, P.; Prinz, R.A.; Xu, X. Evidence That One Subset of Anaplastic Thyroid Carcinomas Are Derived from Papillary Carcinomas Due to BRAF and p53 Mutations. Cancer 2005, 103, 2261–2268. [Google Scholar] [CrossRef]
- Romei, C.; Elisei, R. A Narrative Review of Genetic Alterations in Primary Thyroid Epithelial Cancer. Int. J. Mol. Sci. 2021, 22, 1726. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wei, L.; Yang, H. p53-Associated miRNAs Repress lncRNA ZFAS1 to Retard the Proliferation of Papillary Thyroid Carcinoma. Endokrynol. Pol. 2024, 75, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Z.; Liu, X.; Duan, H.; Xiang, T.; He, Q.; Su, Z.; Wu, H.; Liang, Z. DNA Methylation Haplotype Block Markers Efficiently Discriminate Follicular Thyroid Carcinoma from Follicular Adenoma. J. Clin. Endocrinol. Metab. 2021, 106, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Prodosmo, A.; Giglio, S.; Moretti, S.; Mancini, F.; Barbi, F.; Avenia, N.; Di Conza, G.; Schünemann, H.J.; Pistola, L.; Ludovini, V.; et al. Analysis of Human MDM4 Variants in Papillary Thyroid Carcinomas Reveals New Potential Markers of Cancer Properties. J. Mol. Med. 2008, 86, 585–596. [Google Scholar] [CrossRef]
- Fagin, J.A.; Matsuo, K.; Karmakar, A.; Chen, D.L.; Tang, S.H.; Koeffler, H.P. High Prevalence of Mutations of the p53 Gene in Poorly Differentiated Human Thyroid Carcinomas. J. Clin. Investig. 1993, 91, 179–184. [Google Scholar] [CrossRef]
- Salvatore, D.; Celetti, A.; Fabien, N.; Paulin, C.; Martelli, M.L.; Battaglia, C.; Califano, D.; Monaco, C.; Viglietto, G.; Santoro, M.; et al. Low Frequency of p53 Mutations in Human Thyroid Tumors; p53 and Ras Mutation in Two Out of Fifty-Six Thyroid Tumors. Eur. J. Endocrinol. 1996, 134, 177–183. [Google Scholar] [CrossRef]
- Zou, M.; Shi, Y.; Farid, N.R. p53 Mutations in All Stages of Thyroid Carcinomas. J. Clin. Endocrinol. Metab. 1993, 77, 1054–1058. [Google Scholar] [CrossRef]
- Marcello, M.A.; Morari, E.C.; Cunha, L.L.; De Nadai Silva, A.C.; Carraro, D.M.; Carvalho, A.L.; Soares, F.A.; Vassallo, J.; Ward, L.S. P53 and Expression of Immunological Markers May Identify Early Stage Thyroid Tumors. Clin. Dev. Immunol. 2013, 2013, 846584. [Google Scholar] [CrossRef]
- Liu, L.; Li, D.; Chen, Z.; Yang, J.; Ma, Y.; Cai, H.; Shan, C.; Lv, Z.; Zhang, X. Wild-Type P53 Induces Sodium/Iodide Symporter Expression Allowing Radioiodide Therapy in Anaplastic Thyroid Cancer. Cell Physiol. Biochem. 2017, 43, 905–914. [Google Scholar] [CrossRef]
- Gauchotte, G.; Philippe, C.; Lacomme, S.; Léotard, B.; Wissler, M.P.; Allou, L.; Toussaint, B.; Klein, M.; Vignaud, J.M.; Bressenot, A. BRAF, p53, and SOX2 in Anaplastic Thyroid Carcinoma: Evidence for Multistep Carcinogenesis. Pathology 2011, 43, 447–452. [Google Scholar] [CrossRef]
- Ito, Y.; Kobayashi, T.; Takeda, T.; Komoike, Y.; Wakasugi, E.; Tamaki, Y.; Tsujimoto, M.; Matsuura, N.; Monden, M. Expression of p21 (WAF1/CIP1) Protein in Clinical Thyroid Tissues. Br. J. Cancer. 1996, 74, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Schmid, K.W.; Bankfalvi, A.; Mucke, S.; Ofner, D.; Riehemann, K.; Schroder, S.; Stucker, A.; Totsch, M.; Dockhorn-Dworniczak, B. Possible Relation of p53 and MDM2 Oncoprotein Expression in Thyroid Carcinoma: A Molecular-Pathological and Immunohistochemical Study on Paraffin-Embedded Tissue. Endocr. Pathol. 1996, 7, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; Tseng, S.C.; Chin, T.Y.; Hsieh, L.L.; Lin, J.D. p53 Gene Mutation in Thyroid Carcinoma. Cancer Lett. 1996, 103, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.F.; Pan, P.T.; Zhou, L.; Liu, X.L.; Guo, F.; Wang, L.; Sun, H. Clinical Significance of Preoperative Detection of Serum p53 Antibodies and BRAF(V600E) Mutation in Patients with Papillary Thyroid Carcinoma. Int. J. Clin. Exp. Med. 2015, 8, 21327–21334. [Google Scholar]
- Hasbek, Z.; Turgut, B.; Erselcan, T. p53 Antibody: Is It an Indicator of Dedifferentiated Thyroid Cancer? Ann. Nucl. Med. 2014, 28, 42–46. [Google Scholar] [CrossRef]
- Pan, P.; Han, X.; Li, F.; Fu, Q.; Gao, X.; Sun, H.; Wang, L. Detection of Serum p53 Antibodies from Chinese Patients with Papillary Thyroid Carcinoma Using Phage-SP-ELISA: Correlation with Clinical Parameters. Endocrine 2014, 47, 543–549. [Google Scholar] [CrossRef]
- Chiang, Y.T.; Chien, Y.C.; Lin, Y.H.; Wu, H.H.; Lee, D.F.; Yu, Y.L. The Function of the Mutant p53-R175H in Cancer. Cancers 2021, 13, 4088. [Google Scholar] [CrossRef]
- Maddah, M.M.; Hedayatizadeh-Omran, A.; Moosazadeh, M.; Alizadeh-Navaei, R. Evaluation of the Prognostic Role of TP53 Gene Mutations in Prostate Cancer Outcome: A Systematic Review and Meta-Analysis. Clin. Genitourin. Cancer 2024, 22, 102226. [Google Scholar] [CrossRef]
- Qin, G.; Han, X. The Prognostic Value of TP53 Mutations in Adult Acute Myeloid Leukemia: A Meta-Analysis. Transfus. Med. Hemother. 2022, 50, 234–244. [Google Scholar] [CrossRef]
- Basyuni, S.; Nugent, G.; Ferro, A.; Barker, E.; Reddin, I.; Jones, O.; Lechner, M.; O’Leary, B.; Jones, T.; Masterson, L.; et al. Value of p53 Sequencing in the Prognostication of Head and Neck Cancer: A Systematic Review and Meta-Analysis. Sci. Rep. 2022, 12, 20776. [Google Scholar] [CrossRef]
- Ottaiano, A.; Santorsola, M.; Capuozzo, M.; Perri, F.; Circelli, L.; Cascella, M.; Ianniello, M.; Sabbatino, F.; Granata, V.; Izzo, F.; et al. The Prognostic Role of p53 Mutations in Metastatic Colorectal Cancer: A Systematic Review and Meta-Analysis. Crit. Rev. Oncol. Hematol. 2023, 186, 104018. [Google Scholar] [CrossRef] [PubMed]
- Landa, I.; Ibrahimpasic, T.; Boucai, L.; Sinha, R.; Knauf, J.A.; Shah, R.H.; Dogan, S.; Ricarte-Filho, J.C.; Krishnamoorthy, G.P.; Xu, B.; et al. Genomic and Transcriptomic Hallmarks of Poorly Differentiated and Anaplastic Thyroid Cancers. J. Clin. Investig. 2016, 126, 1052–1066. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Xia, X.; Kim, G.D.; Liu, Y.; Xue, Z.; Zhang, L.; Shu, Y.; Yang, T.; Chen, Y.; Zhang, S.; et al. Characterizing Dedifferentiation of Thyroid Cancer by Integrated Analysis. Sci. Adv. 2021, 7, eabf3657. [Google Scholar] [CrossRef] [PubMed]
- Koga, T.; Hashimoto, S.; Sugio, K.; Yoshino, I.; Nakagawa, K.; Yonemitsu, Y.; Sugimachi, K.; Sueishi, K. Heterogeneous Distribution of P53 Immunoreactivity in Human Lung Adenocarcinoma Correlates with MDM2 Protein Expression, Rather than with P53 Gene Mutation. Int. J. Cancer 2001, 95, 232–239. [Google Scholar] [CrossRef] [PubMed]
- de Haan, L.M.; de Groen, R.A.L.; de Groot, F.A.; Noordenbos, T.; van Wezel, T.; van Eijk, R.; Ruano, D.; Diepstra, A.; Koens, L.; Nicolae-Cristea, A.; et al. Real-world routine diagnostic molecular analysis for TP53 mutational status is recommended over p53 immunohistochemistry in B-cell lymphomas. Virchows Arch. 2024, 485, 643–654. [Google Scholar] [CrossRef]
- Armbruster, H.; Schotte, T.; Götting, I.; Overkamp, M.; Granai, M.; Volmer, L.L.; Bahlinger, V.; Matovina, S.; Koch, A.; Dannehl, D.; et al. Aberrant p53 Immunostaining Patterns in Breast Carcinoma of No Special Type Strongly Correlate with Presence and Type of TP53 Mutations. Virchows Arch. 2024, 485, 631–642. [Google Scholar] [CrossRef]
- Vermij, L.; Léon-Castillo, A.; Singh, N.; Powell, M.E.; Edmondson, R.J.; Genestie, C.; Khaw, P.; Pyman, J.; McLachlin, C.M.; Ghatage, P.; et al. p53 immunohistochemistry in endometrial cancer: Clinical and molecular correlates in the PORTEC-3 trial. Mod. Pathol. 2022, 35, 1475–1483. [Google Scholar] [CrossRef]
- Osakabe, M.; Yamada, N.; Sugimoto, R.; Uesugi, N.; Nakao, E.; Honda, M.; Yanagawa, N.; Sugai, T. The Pattern-Based Interpretation of p53 Immunohistochemical Expression as a Surrogate Marker for TP53 Mutations in Colorectal Cancer. Virchows Arch. 2024. [Google Scholar] [CrossRef]
- Sung, Y.N.; Kim, D.; Kim, J. p53 Immunostaining Pattern Is a Useful Surrogate Marker for TP53 Gene Mutations. Diagn. Pathol. 2022, 17, 92. [Google Scholar] [CrossRef]
- Köbel, M.; Piskorz, A.M.; Lee, S.; Lui, S.; LePage, C.; Marass, F.; Rosenfeld, N.; Mes Masson, A.M.; Brenton, J.D. Optimized p53 Immunohistochemistry as an Accurate Predictor of TP53 Mutation in Ovarian Carcinoma. J. Pathol. Clin. Res. 2016, 2, 247–258. [Google Scholar] [CrossRef]
- Morita, N.; Ikeda, Y.; Takami, H. Clinical Significance of P53 Protein Expression in Papillary Thyroid Carcinoma. World J. Surg. 2008, 32, 2617–2622. [Google Scholar] [CrossRef] [PubMed]
- Abdelhafez, D.N.; Ayoub, M.M.; Mahmoud, S.A.; El Hanbuli, H.M. YAP1 and P53 Expression in Papillary Thyroid Carcinoma. Iran. J. Pathol. 2023, 18, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.B.; de Assis Batista, F.; Marcello, M.A.; Bufalo, N.E.; Peres, K.C.; Morari, E.C.; Soares, F.A.; Vassallo, J.; Ward, L.S. Clinical Utility of the Immunohistochemical Co-Expression of p53 and MDM2 in Thyroid Follicular Lesions. Ann. Diagn Pathol. 2021, 53, 151766. [Google Scholar] [CrossRef] [PubMed]
- Horie, S.; Maeta, H.; Endo, K.; Ueta, T.; Takashima, K.; Terada, T. Overexpression of p53 Protein and MDM2 in Papillary Carcinomas of the Thyroid: Correlations with Clinicopathologic Features. Pathol. Int. 2001, 51, 11–15. [Google Scholar] [CrossRef]
- Mneimneh, W.S.; Asa, S.L. Divergent Lineage Markers in Anaplastic Thyroid Carcinoma. Am. J. Surg. Pathol. 2024, 48, 230–237. [Google Scholar] [CrossRef]
- Sobhani, N.; Roviello, G.; D’Angelo, A.; Roudi, R.; Neeli, P.K.; Generali, D. p53 Antibodies as a Diagnostic Marker for Cancer: A Meta-Analysis. Molecules 2021, 26, 6215. [Google Scholar] [CrossRef]
- Soussi, T. p53 Antibodies in the Sera of Patients with Various Types of Cancer: A Review. Cancer Res. 2000, 60, 1777–1788. [Google Scholar]
- Mudry, P.; Slaby, O.; Neradil, J.; Soukalova, J.; Melichar, B.; Sachlova, M. The Role of the p53 Protein in the Prediction of Clinical Resistance to Cytostatic Treatment in Cancer Patients. Oncol. Rep. 2013, 29, 477–484. [Google Scholar] [CrossRef]
- Yang, B.; Li, X.; Ren, T.; Yin, Y. Autoantibodies as Diagnostic Biomarkers for Lung Cancer: A Systematic Review. Cell Death Discov. 2019, 5, 126. [Google Scholar] [CrossRef]
- Kawada, J.; Saito, T.; Kurokawa, Y.; Kawabata, R.; Takeno, A.; Takeoka, T.; Nose, Y.; Wada, H.; Eguchi, H.; Doki, Y.; et al. Serum NY-ESO-1 and p53 Antibodies as Useful Tumor Markers in Gastric Cancer. Ann. Gastroenterol. Surg. 2023, 8, 243–250. [Google Scholar] [CrossRef]
- Colombo, C.; Pogliaghi, G.; Tosi, D.; Muzza, M.; Bulfamante, G.; Persani, L.; Fugazzola, L.; Cirello, V. Thyroid Cancer Harboring PTEN and TP53 Mutations: A Peculiar Molecular and Clinical Case Report. Front Oncol. 2022, 12, 949098. [Google Scholar] [CrossRef] [PubMed]
- Gąsior-Perczak, D.; Kowalik, A.; Kopczyński, J.; Macek, P.; Niemyska, K.; Walczyk, A.; Gruszczyński, K.; Siołek, M.; Dróżdż, T.; Kosowski, M.; et al. Relationship between the Expression of CHK2 and p53 in Tumor Tissue and the Course of Papillary Thyroid Cancer in Patients with CHEK2 Germline Mutations. Cancers 2024, 16, 815. [Google Scholar] [CrossRef] [PubMed]
- Vrinceanu, D.; Dumitru, M.; Marinescu, A.; Serboiu, C.; Musat, G.; Radulescu, M.; Popa-Cherecheanu, M.; Ciornei, C.; Manole, F. Management of Giant Thyroid Tumors in Patients with Multiple Comorbidities in a Tertiary Head and Neck Surgery Center. Biomedicines 2024, 12, 2204. [Google Scholar] [CrossRef] [PubMed]
- Peuget, S.; Zhou, X.; Selivanova, G. Translating p53-Based Therapies for Cancer into the Clinic. Nat. Rev. Cancer 2024, 24, 192–215. [Google Scholar] [CrossRef] [PubMed]
- Chai, D.; Wang, J.; Fan, C.; Lim, J.M.; Wang, X.; Neeli, P.; Yu, X.; Young, K.H.; Li, Y. Remodeling of Anti-Tumor Immunity with Antibodies Targeting a p53 Mutant. J. Hematol. Oncol. 2024, 17, 45. [Google Scholar] [CrossRef]

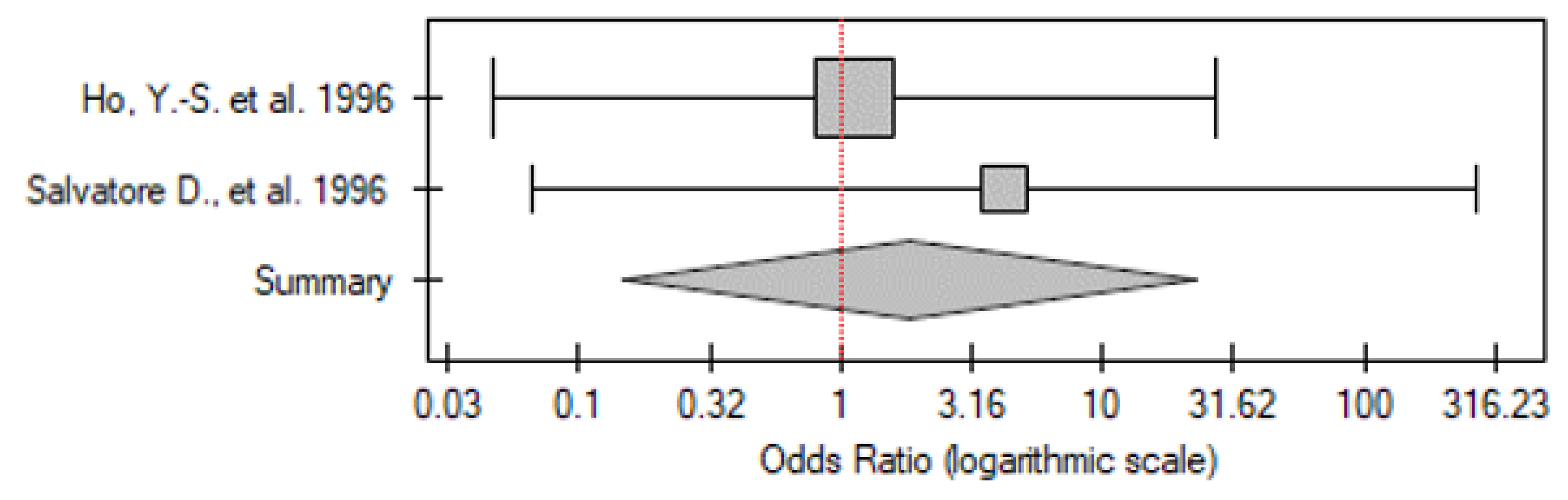

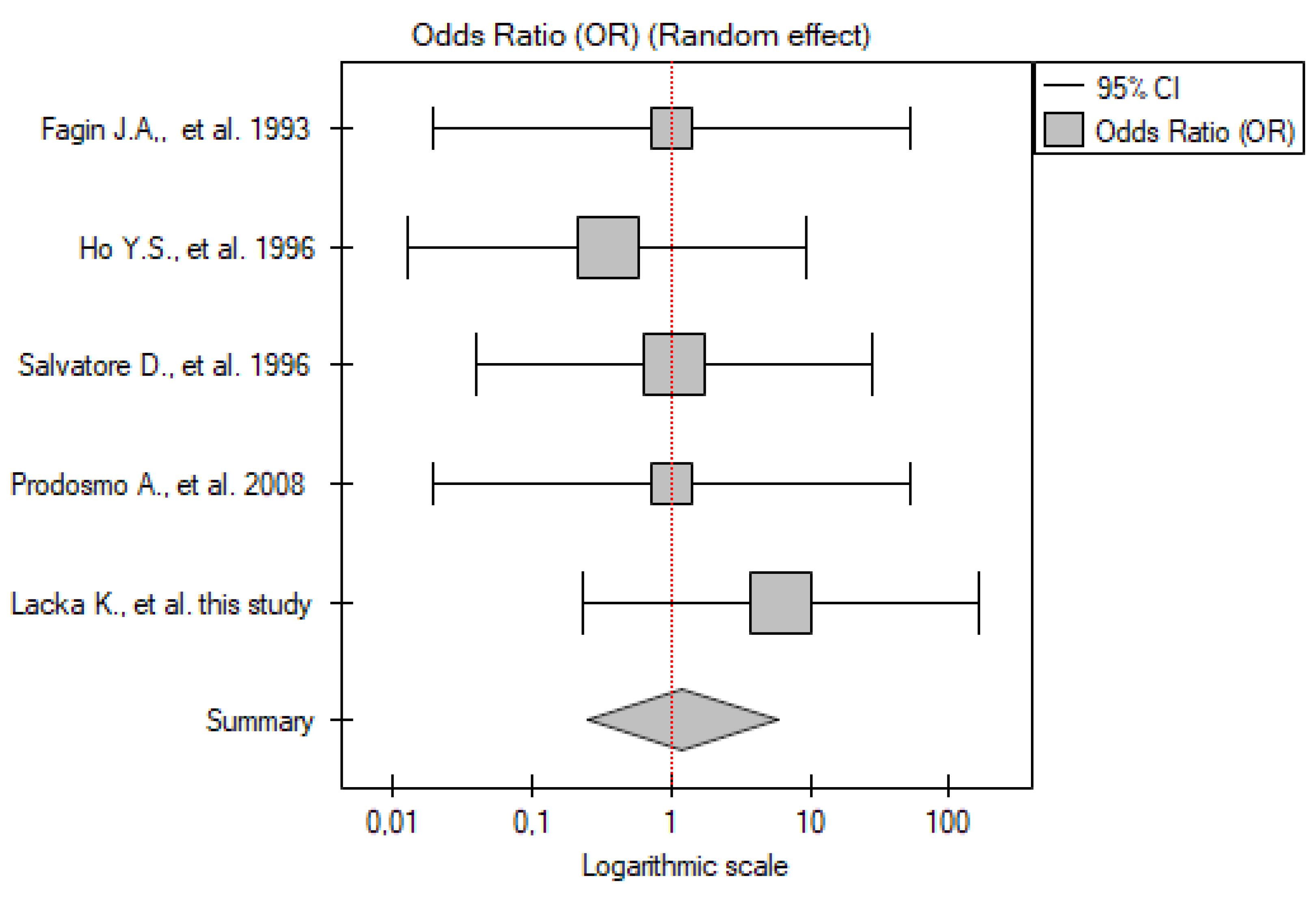
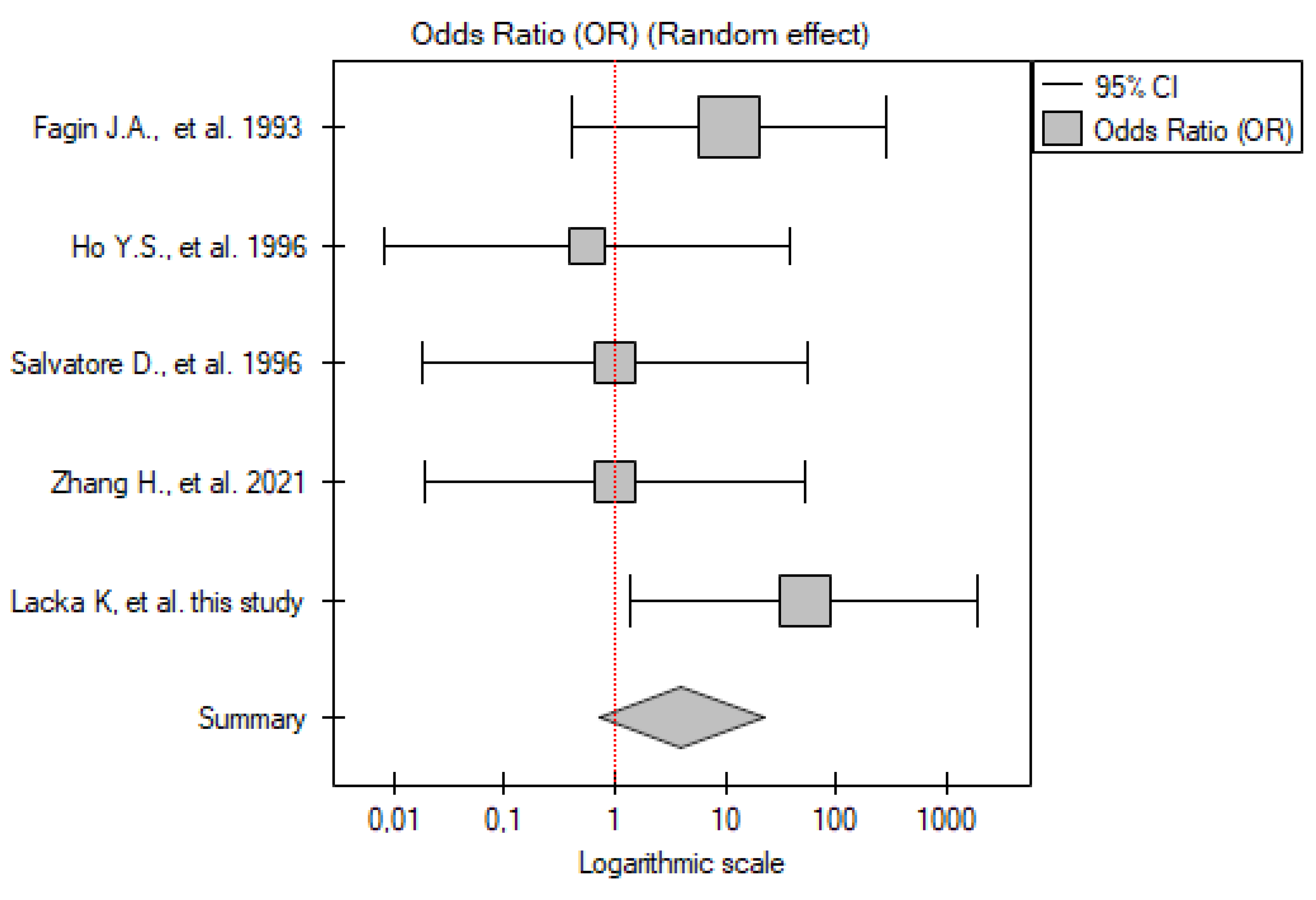

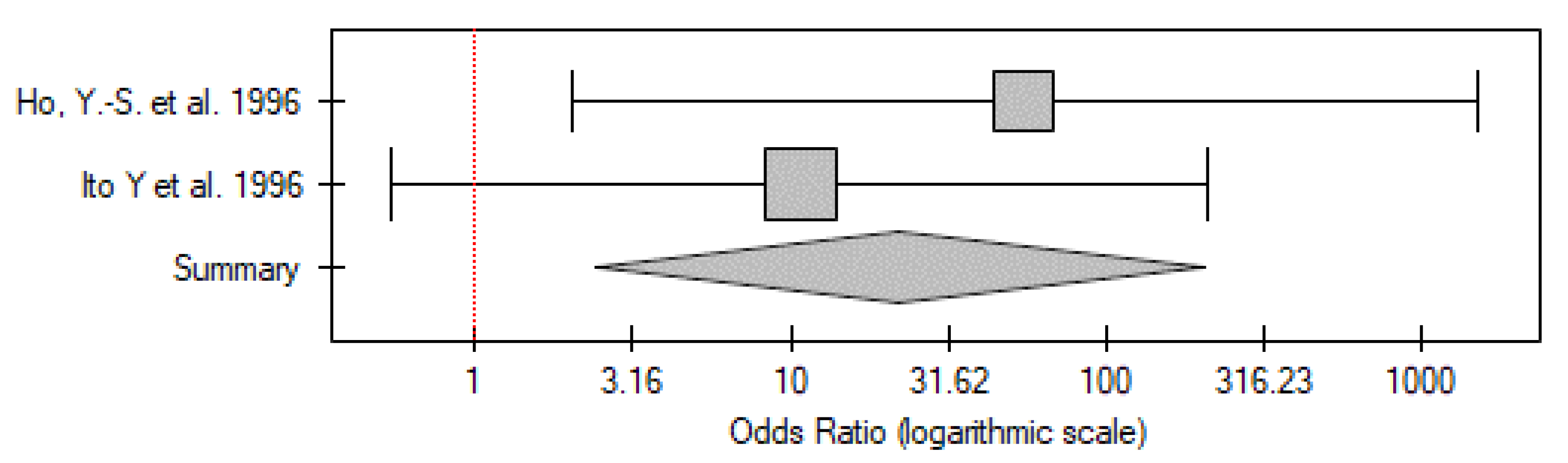


| No. | Gender | Age | TNM | p53 IHC Staining | TP53 Mutation |
|---|---|---|---|---|---|
| PTC | |||||
| 1 | F | 32 | T2N0M0 | + | − |
| 2 | F | 17 | T2N0M0 | − | − |
| 3 | F | 17 | T1N0M0 | − | − |
| 4 | F | 60 | T2N1aM0 | − | − |
| 5 | F | 15 | T2N0M0 | − | − |
| 6 | F | 68 | T4N1bM0 | + | − |
| 7 | F | 50 | T1N0M0 | − | − |
| 8 | F | 75 | T2N0M0 | − | − |
| 9 | F | 40 | T1N0M0 | − | − |
| 10 | F | 16 | T2N0M0 | − | − |
| 11 | M | 40 | T4N1bM0 | + | + exon 5 |
| 12 | F | 33 | T2N0M0 | − | − |
| 13 | M | 15 | T2N1aM1 | − | − |
| FTC | |||||
| 1 | F | 18 | T1N0M0 | − | − |
| 2 | F | 64 | T4N1bM1 | + | + exon 6 |
| ATC | |||||
| 1 | F | 73 | T4N1bM1 | + | + exon 8 |
| 2 | M | 72 | T4N1bM1 | + | + exon 8 |
| 3 | M | 64 | T4N1bM1 | + | + exon 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lacka, K.; Maciejewski, A.; Tyburski, P.; Manuszewska-Jopek, E.; Majewski, P.; Więckowska, B. Rationale for Testing TP53 Mutations in Thyroid Cancer—Original Data and Meta-Analysis. Int. J. Mol. Sci. 2025, 26, 1035. https://doi.org/10.3390/ijms26031035
Lacka K, Maciejewski A, Tyburski P, Manuszewska-Jopek E, Majewski P, Więckowska B. Rationale for Testing TP53 Mutations in Thyroid Cancer—Original Data and Meta-Analysis. International Journal of Molecular Sciences. 2025; 26(3):1035. https://doi.org/10.3390/ijms26031035
Chicago/Turabian StyleLacka, Katarzyna, Adam Maciejewski, Piotr Tyburski, Ewa Manuszewska-Jopek, Przemysław Majewski, and Barbara Więckowska. 2025. "Rationale for Testing TP53 Mutations in Thyroid Cancer—Original Data and Meta-Analysis" International Journal of Molecular Sciences 26, no. 3: 1035. https://doi.org/10.3390/ijms26031035
APA StyleLacka, K., Maciejewski, A., Tyburski, P., Manuszewska-Jopek, E., Majewski, P., & Więckowska, B. (2025). Rationale for Testing TP53 Mutations in Thyroid Cancer—Original Data and Meta-Analysis. International Journal of Molecular Sciences, 26(3), 1035. https://doi.org/10.3390/ijms26031035






