Development of Cell-Assembled Human Endomysial-Type Biomatrix Substrate for the Detection of Celiac Disease Autoantibodies
Abstract
1. Introduction
2. Results
2.1. Development of CD-Antigenic Biomatrix in Cell Culture
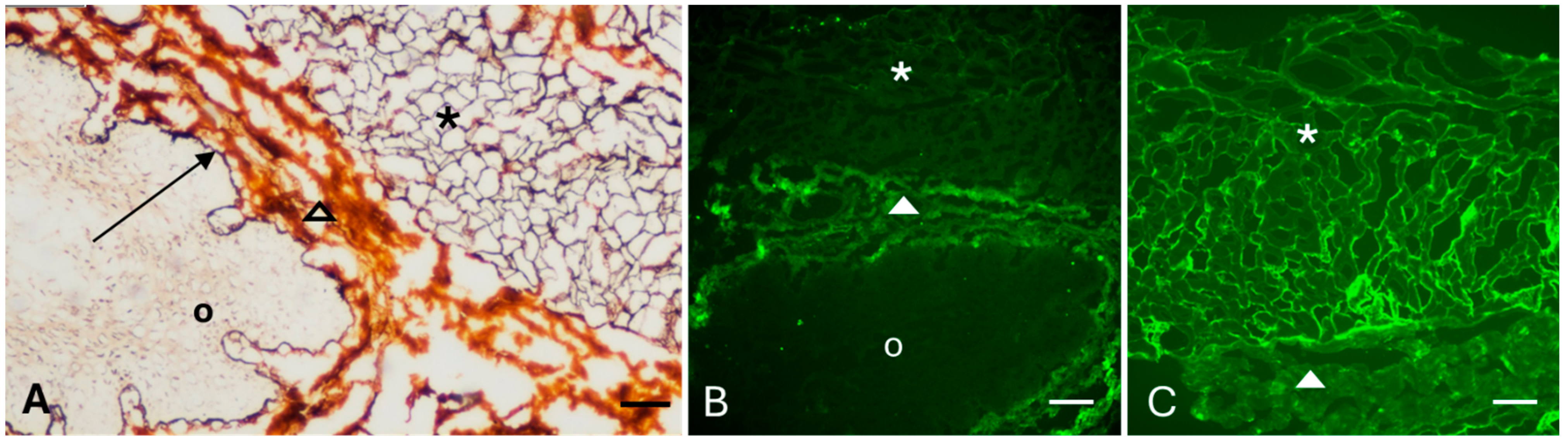
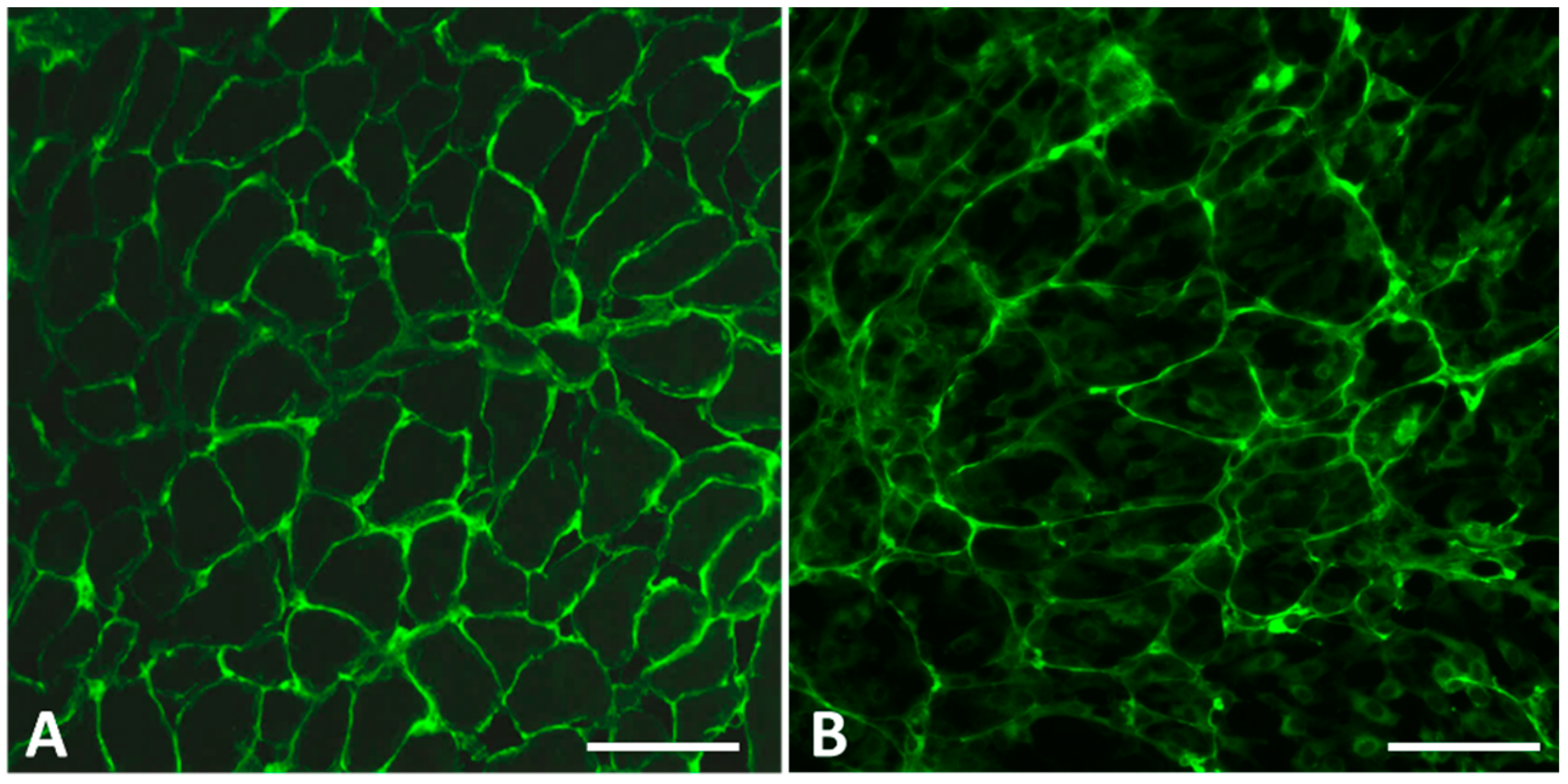
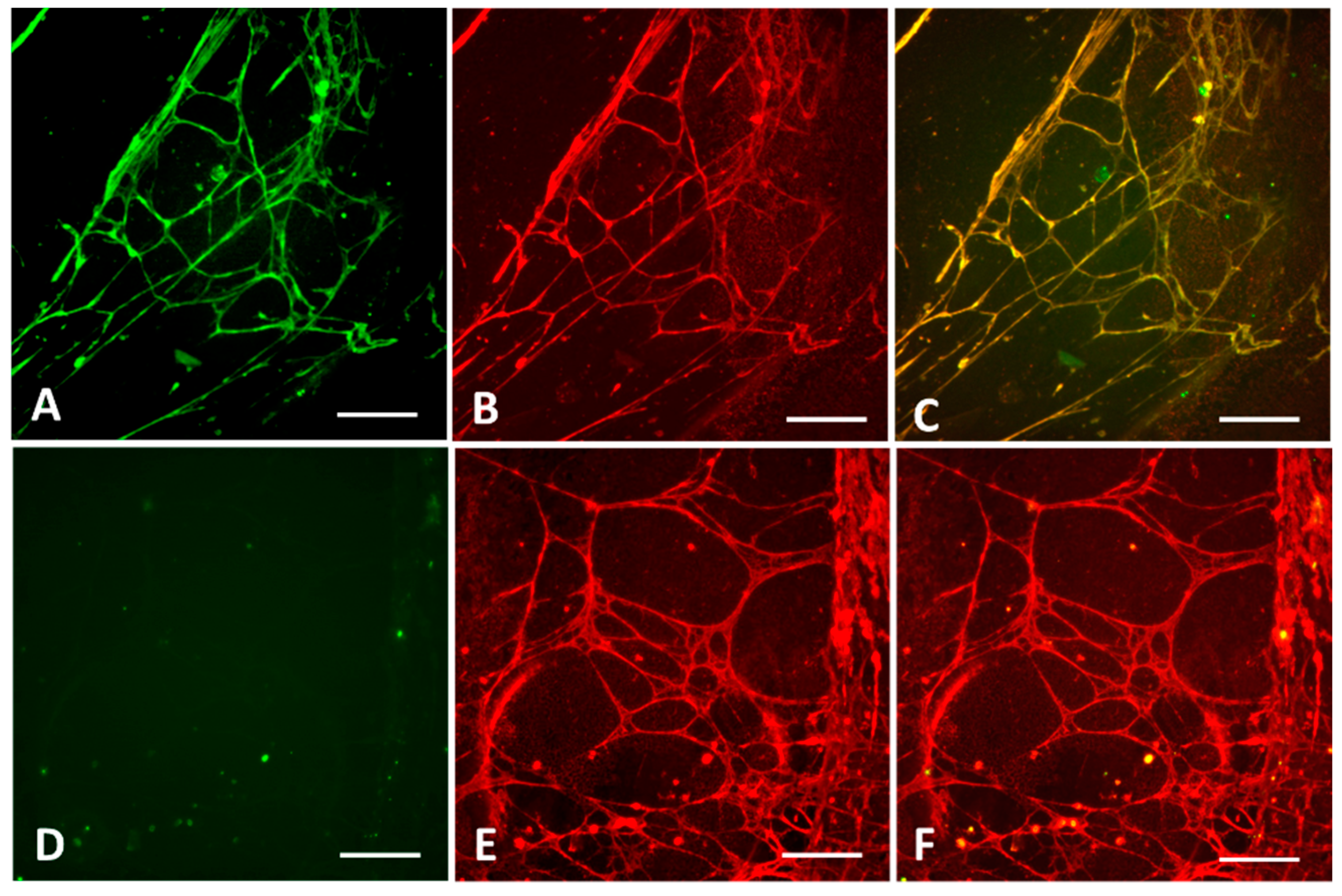
2.1.1. Selection of Cell Lines and Antigen Visualization
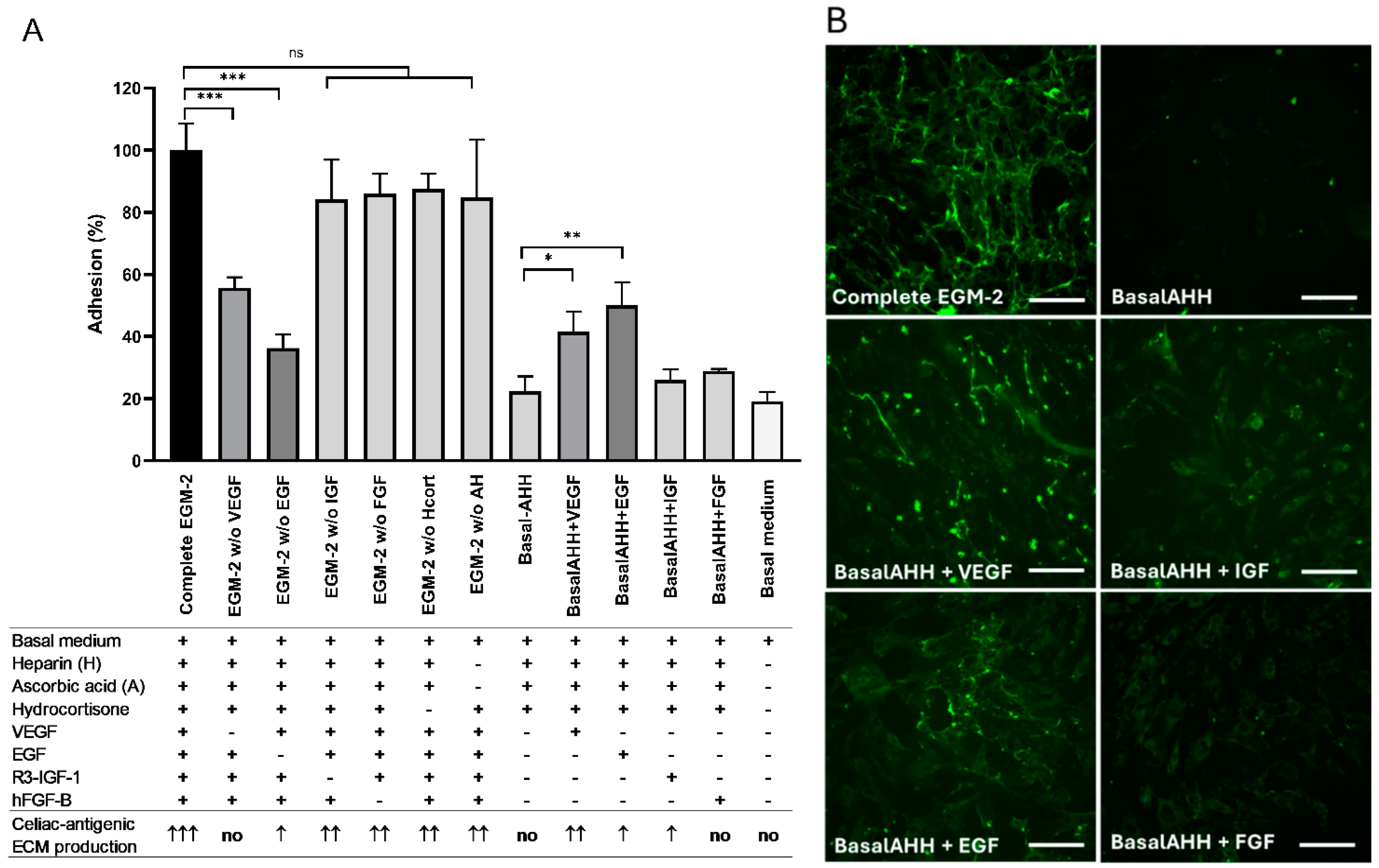
2.1.2. Optimization of the Biomatrix Production
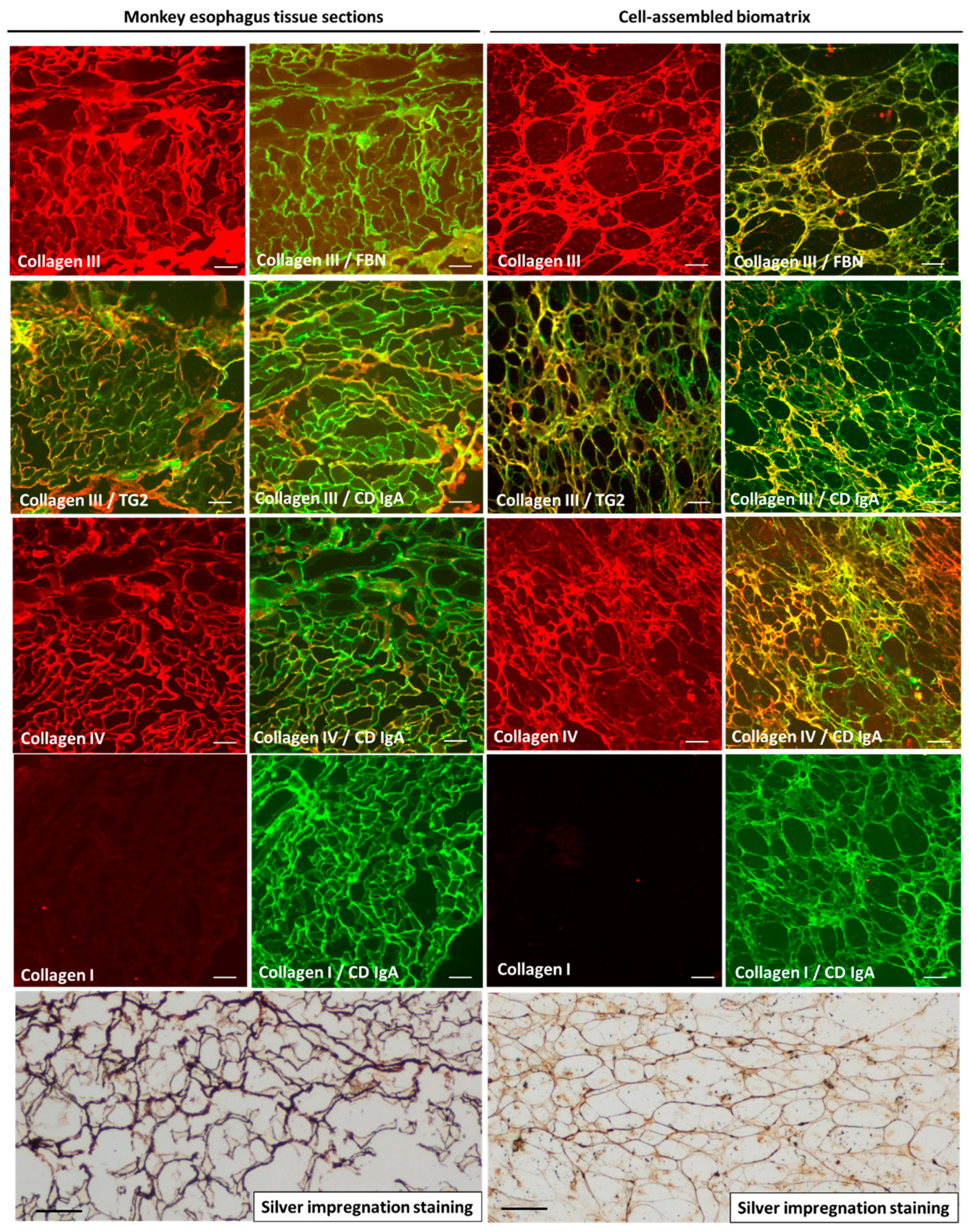
2.2. Molecular Characterization of the Biomatrix
2.3. Diagnostic Performance in Comparison with Established Clinical Assays in Prospectively Tested Patient Cohort
2.4. Decellularization of the Biomatrix Further Improves Sensitivity
2.5. Detection of Both IgA and IgG Antibodies
2.6. Interobserver Variation
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Celiac Antibody Detection on Biomatrix
4.3. Microscopic Analysis of Biomatrix and Tissue Proteins
4.4. Patient Samples
4.5. Clinical Antibody Testing
4.6. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CD | celiac disease |
| DGP | deamidated gliadin peptide antibodies |
| ECM | extracellular matrix |
| EGM-2 | endothelial cell growth medium 2 |
| ELISA | enzyme-linked immunosorbent assay |
| EMA | endomysial antibody |
| FBS | fetal bovine serum |
| FITC | fluorescein isothiocyanate |
| HUVEC | human umbilical vein endothelial cell |
| PBS | phosphate-buffered saline |
| TG2 | type-2 transglutaminase (also called tissue transglutaminase or tTG) |
| TGA | anti-transglutaminase antibody |
Appendix A
References
- Lebwohl, B.; Sanders, D.S.; Green, P.H.R. Coeliac disease. Lancet 2018, 391, 70–81. [Google Scholar] [CrossRef]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.R.; Kurppa, K.; Mearin, M.L.; Ribes-Koninckx, C.; Shamir, R.; Troncone, R.; Auricchio, R.; Castillejo, G.; et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for Diagnosing Coeliac Disease 2020. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Mäki, M. The humoral immune system in coeliac disease. In Baillière’s Clinical Gastoenterology; Baillière Tindall: London, UK, 1995; Volume 9, pp. 231–249. [Google Scholar]
- Korponay-Szabó, I.R.; Dahlbom, I.; Laurila, K.; Koskinen, S.; Woolley, N.; Partanen, J.; Kovács, J.B.; Mäki, M.; Hansson, T. Elevation of IgG antibodies against tissue transglutaminase as a diagnostic tool for coeliac disease in selective IgA deficiency. Gut 2003, 52, 1567–1571. [Google Scholar] [CrossRef] [PubMed]
- Diós, Á.; Srinivasan, B.; Gyimesi, J.; Werkstetter, K.; Valenta, R.; Koletzko, S.; Korponay-Szabó, I.R. Changes in non-deamidated versus deamidated epitope targeting and disease prediction during the antibody response to gliadin and transglutaminase of infants at risk for celiac disease. Int. J. Mol. Sci. 2022, 23, 2498. [Google Scholar] [CrossRef] [PubMed]
- Duncan, A.; Hill, P.G. A review of the quality of gastrointestinal investigations performed in UK laboratories. Ann. Clin. Biochem. 2007, 44, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Giersiepen, K.; Lelgemann, M.; Stuhldreher, N.; Ronfani, L.; Husby, S.; Koletzko, S.; Korponay-Szabó, I.R.; ESPGHAN Working Group on Coeliac Disease Diagnosis. Accuracy of diagnostic antibody tests for coeliac disease in children: Summary of an evidence report. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Korponay-Szabó, I.R.; Sulkanen, S.; Halttunen, T.; Maurano, F.; Rossi, M.; Mazzarella, G.; Laurila, K.; Troncone, R.; Mäki, M. Tissue transglutaminase is the target in both rodent and primate tissues for celiac disease-specific autoantibodies. J. Pediatr. Gastroenterol. Nutr. 2000, 31, 520–527. [Google Scholar] [CrossRef]
- Cardoso, I.; Stamnaes, J.; Andersen, J.T.; Melino, G.; Iversen, R.; Sollid, L.M. Transglutaminase 2 interactions with extracellular matrix proteins as probed with celiac disease autoantibodies. FEBS J. 2015, 282, 2063–2075. [Google Scholar] [CrossRef] [PubMed]
- Sblattero, D.; Florian, F.; Azzoni, E.; Zyla, T.; Park, M.; Baldas, V.; Not, T.; Ventura, A.; Bradbury, A.; Marzari, R. The analysis of the fine specificity of celiac disease antibodies using tissue transglutaminase fragments. Eur. J. Biochem. 2002, 269, 5175–5181. [Google Scholar] [CrossRef] [PubMed]
- Simon-Vecsei, Z.; Király, R.; Bagossi, P.; Tóth, B.; Dahlbom, I.; Caja, S.; Csosz, É.; Lindfors, K.; Sblattero, D.; Nemes, É.; et al. A single conformational transglutaminase 2 epitope contributed by three domains is critical for celiac antibody binding and effects. Proc. Natl. Acad. Sci. USA 2012, 109, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Werkstetter, K.J.; Korponay-Szabó, I.R.; Popp, A.; Villanacci, V.; Salemme, M.; Heilig, G.; Lillevang, S.T.; Mearin, M.L.; Ribes-Koninckx, C.; Thomas, A.; et al. Accuracy in diagnosis of celiac disease without biopsies in clinical practice. Gastroenterology 2017, 153, 924–935. [Google Scholar] [CrossRef]
- Sulkanen, S.; Halttunen, T.; Laurila, K.; Kolho, K.L.; Korponay-Szabó, I.R.; Sarnesto, A.; Savilahti, E.; Collin, P.; Mäki, M. Tissue transglutaminase autoantibody enzyme-linked immunosorbent assay in detecting celiac disease. Gastroenterology 1998, 115, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Kanchan, K.; Ergülen, E.; Király, R.; Simon-Vecsei, Z.; Fuxreiter, M.; Fésüs, L. Identification of a specific one amino acid change in recombinant human transglutaminase 2 that regulates its activity and calcium sensitivity. Biochem. J. 2013, 455, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Korponay-Szabó, I.R.; Kovács, J.B.; Lőrincz, M.; Török, É.; Gorácz, G.; Csitáry, F. The human appendix: A composite substrate for anti-endomysium, anti-reticulin and anti-bowel antibody testing in coeliac disease. Med. Sci. Monit. 1997, 3, 285–289. [Google Scholar]
- Sulkanen, S.; Halttunen, T.; Marttinen, A.; Leivo, E.L.; Laurila, K.; Mäki, M. Autoantibodies in celiac disease: Importance of fibroblasts. J. Pediatr. Gastroenterol. Nutr. 1998, 27, 206–213. [Google Scholar] [CrossRef]
- Whelan, A.; Willoughby, R.; Weir, D. Human umbilical vein endothelial cells: A new easily available source of endomysial antigens. Eur. J. Gastroenterol. Hepatol. 1996, 8, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Roncoroni, L.; Elli, L.; Bardella, M.T.; Perrucci, G.; Ciulla, M.; Lombardo, V.; Tomba, C.; Conte, D.; Doneda, L. Extracellular matrix proteins and displacement of cultured fibroblasts from duodenal biopsies in celiac patients and controls. J. Transl. Med. 2013, 8, 91. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.; Tóth, B.B.; Király, R.; Arianti, R.; Csomós, I.; Póliska, S.; Vámos, A.; Korponay-Szabó, I.R.; Bacso, Z.; Győry, F.; et al. Irisin Stimulates the Release of CXCL1 From Differentiating Human Subcutaneous and Deep-Neck Derived Adipocytes via Upregulation of NFκB Pathway. Front. Cell Dev. Biol. 2021, 9, 737872. [Google Scholar] [CrossRef]
- Sheppard, A.L.; Elwenspoek, M.M.C.; Scott, L.J.; Corfield, V.; Everitt, H.; Gillett, P.M.; Hay, A.D.; Jones, H.E.; Mallett, S.; Watson, J.; et al. Systematic review with meta-analysis: The accuracy of serological tests to support the diagnosis of coeliac disease. Aliment. Pharmacol. Ther. 2022, 55, 514–527. [Google Scholar] [CrossRef] [PubMed]
- Hällström, O. Comparison of IgA-class reticulin and endomysium antibodies in coeliac disease and dermatitis herpetiformis. Gut 1989, 30, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.S.; Silva, J.C.; Cabral, J.M.S.; da Silva, C.L.; Vashishth, D. Cultured cell-derived extracellular matrices to enhance the osteogenic differentiation and angiogenic properties of human mesenchymal stem/stromal cells. J. Tissue Eng. Regen. Med. 2019, 13, 1544–1558. [Google Scholar] [CrossRef] [PubMed]
- Isogai, N.; Hashizume, K.; Uegaito, K.; Kamiishi, H. The observation of endothelial cells in vein grafts by the en face silver staining method. Microsurgery 1991, 12, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Korponay-Szabó, I.R.; Halttunen, T.; Szalai, Z.; Laurila, K.; Király, R.; Kovács, J.B.; Fésüs, L.; Mäki, M. In vivo targeting of intestinal and extraintestinal transglutaminase 2 by coeliac autoantibodies. Gut 2004, 53, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Hadjivassiliou, M.; Mäki, M.; Sanders, D.S.; Williamson, C.A.; Grünewald, R.A.; Woodroofe, N.M.; Korponay-Szabó, I.R. Autoantibody targeting of brain and intestinal transglutaminase in gluten ataxia. Neurology 2006, 66, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Myrsky, E.; Syrjänen, M.; Korponay-Szabó, I.R.; Mäki, M.; Kaukinen, K.; Lindfors, K. Altered small-bowel mucosal vascular network in untreated coeliac disease. Scand. J. Gastroenterol. 2009, 44, 162–167. [Google Scholar] [CrossRef]
- Myrsky, E.; Kaukinen, K.; Syrjänen, M.; Korponay-Szabó, I.R.; Mäki, M.; Lindfors, K. Coeliac disease-specific autoantibodies targeted against transglutaminase 2 disturb angiogenesis. Clin. Exp. Immunol. 2008, 152, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Kalliokoski, S.; Sulic, A.M.; Korponay-Szabó, I.R.; Szondy, Z.; Frias, R.; Perez, M.A.; Martucciello, S.; Roivainen, A.; Pelliniemi, L.J.; Esposito, C.; et al. Celiac disease-specific TG2-targeted autoantibodies inhibit angiogenesis ex vivo and in vivo in mice by interfering with endothelial cell dynamics. PLoS ONE 2013, 18, e65887. [Google Scholar] [CrossRef] [PubMed]
- Caja, S.; Myrsky, E.; Korponay-Szabo, I.R.; Nadalutti, C.; Sulic, A.M.; Lavric, M.; Sblattero, D.; Marzari, R.; Collighan, R.; Mongeot, A.; et al. Inhibition of transglutaminase 2 enzymatic activity ameliorates the anti-angiogenic effects of coeliac disease autoantibodies. Scand. J. Gastroenterol. 2010, 45, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Unsworth, D.J.; Scott, D.L.; Almond, T.J.; Beard, H.K.; Holborow, E.J.; Walton, K.W. Studies on reticulin. I: Serological and immunohistological investigations of the occurrence of collagen type III, fibronectin and the non-collagenous glycoprotein of Pras and Glynn in reticulin. Br. J. Exp. Pathol. 1982, 63, 154–166. [Google Scholar] [PubMed]
- Korponay-Szabó, I.R.; Laurila, K.; Szondy, Z.; Halttunen, T.; Szalai, Z.; Dahlbom, I.; Rantala, I.; Kovács, J.B.; Fésüs, L.; Mäki, M. Missing endomysial and reticulin binding of coeliac antibodies in transglutaminase 2 knockout tissues. Gut 2003, 52, 199–204. [Google Scholar] [CrossRef]
- Krutsay, M. Über die Silverimpregnätion des Bindegewebes. Zentralbl. Allg. Pathol. 1988, 134, 518–520. [Google Scholar] [PubMed]
- Kurppa, K.; Collin, P.; Viljamaa, M.; Haimila, K.; Saavalainen, P.; Partanen, J.; Laurila, K.; Huhtala, H.; Paasikivi, K.; Mäki, M.; et al. Diagnosing mild enteropathy celiac disease: A randomized, controlled clinical study. Gastroenterology 2009, 136, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Popp, A.; Jinga, M.; Jurcut, C.; Balaban, V.; Bardas, C.; Laurila, K.; Vasilescu, F.; Ene, A.; Anca, I.; Mäki, M. Fingertip rapid point-of-care test in adult case-finding in coeliac disease. BMC Gastroenterol. 2013, 13, 115. [Google Scholar] [CrossRef] [PubMed]
- Absah, I.; Rishi, A.R.; Gebrail, R.; Snyder, M.R.; Murray, J.A. Lack of utility of anti-tTG IgG to diagnose celiac disease when anti-tTG IgA is negative. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 726–729. [Google Scholar] [CrossRef]
- Olen, O.; Gudjónsdóttir, A.H.; Browaldh, L.; Hessami, M.; Elvin, K.; Liedberg, A.S.; Neovius, M.; Grahnquist, L. Antibodies against deamidated gliadin peptides and tissue transglutaminase for diagnosis of pediatric celiac disease. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Palatka, K.; Serfozo, Z.; Veréb, Z.; Bátori, R.; Lontay, B.; Hargitay, Z.; Nemes, Z.; Udvardy, M.; Erdodi, F.; Altorjay, I. Effect of IBD sera on expression of inducible and endothelial nitric oxide synthase in human umbilical vein endothelial cells. World J. Gastroenterol. 2006, 12, 1730–1738. [Google Scholar] [CrossRef] [PubMed]
- Counter, C.M.; Hahn, W.C.; Wei, W.; Caddle, S.D.; Beijersbergen, R.L.; Lansdorp, P.M.; Sedivy, J.M.; Weinberg, R.A. Dissociation among in vitro telomerase activity, telomere maintenance, and cellular immortalization. Proc. Natl. Acad. Sci. USA 1998, 95, 14723–14728. [Google Scholar] [CrossRef] [PubMed]
- Csaholczi, B.; Csuth, A.R.; Korponay-Szabó, I.R.; Fésüs, L.; Király, R. Transglutaminase 2 is an RNA-binding protein: Experimental verification and characterisation of a novel transglutaminase feature. FEBS J. 2024; epub ahead of print. [Google Scholar] [CrossRef]
- Korponay-Szabó, I.R.; Mäki, M. Method and Means for Detecting Gluten-Induced Diseases. PCT WO/2002/086509, 31 October 2002. [Google Scholar]


| EMA-IgA+ on HUVEC-ECM | EMA-IgA− on HUVEC- ECM | EMA-IgA+ on Tissues | EMA-IgA− on Tissues | TGA-IgA ELISA+ | TGA-IgA ELISA− | |
|---|---|---|---|---|---|---|
| Celiac disease (n = 68) | 65 | 3 | 62 | 6 | 68 | 0 |
| No celiac disease (n = 6) | 0 | 6 | 0 | 6 | 2 | 4 |
| Total with final diagnosis (n = 74 †) | 65 | 9 | 62 | 12 | 70 | 4 |
| Sensitivity (%) | 95.6 (92.7–98.5) | 91.2 (87.8–94.5) | 100 | |||
| Specificity (%) | 100 | 100 | 66.7 ‡ | |||
| Positive predictive value (%) | 100 | 100 | 97.1 | |||
| Negative predictive value (%) | 66.7 | 50.0 | 100 | |||
| Diagnostic accuracy (%) | 95.9 | 91.9 | 97.3 | |||
| Target Antigen | Antibody Type | Clone | Manufacturer | Catalog Number |
|---|---|---|---|---|
| Collagen I | mouse monoclonal | C11 | Chemicon, Melbourne, Australia | MAB1340 |
| Collagen III | mouse monoclonal | IE7-D7 | Chemicon, Melbourne, Australia | MAB3392 |
| Collagen IV | mouse monoclonal | 23IIC3 | Chemicon, Melbourne, Australia | MAB1910 |
| Cytokeratin-19 | mouse monoclonal | A-3 | SantaCruz, Heidelberg, Germany | sc378126 |
| Fibronectin | rabbit polyclonal | - | Sigma/Merck, Darmstadt, Germany | F3648 |
| Laminin | rabbit polyclonal | - | Sigma/Merck, Darmstadt, Germany | L9393 |
| Lpp | mouse monoclonal | LPP4 | Sigma/Merck, Darmstadt, Germany | L2920 |
| Transglutaminase-2 | mouse monoclonal | CUB7402 | NeoMarkers, Fremont, CA, USA | CUB7402 |
| VE-cadherin | rabbit monoclonal | 048 | Invitrogen, Carlsbad, CA, USA | MA-29141 |
| Vinculin | rabbit polyclonal | - | Sigma/Merck, Darmstadt, Germany | HPA002131 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korponay-Szabó, I.R.; Király, R.; Gyimesi, J.; Mäki, M. Development of Cell-Assembled Human Endomysial-Type Biomatrix Substrate for the Detection of Celiac Disease Autoantibodies. Int. J. Mol. Sci. 2025, 26, 1012. https://doi.org/10.3390/ijms26031012
Korponay-Szabó IR, Király R, Gyimesi J, Mäki M. Development of Cell-Assembled Human Endomysial-Type Biomatrix Substrate for the Detection of Celiac Disease Autoantibodies. International Journal of Molecular Sciences. 2025; 26(3):1012. https://doi.org/10.3390/ijms26031012
Chicago/Turabian StyleKorponay-Szabó, Ilma R., Róbert Király, Judit Gyimesi, and Markku Mäki. 2025. "Development of Cell-Assembled Human Endomysial-Type Biomatrix Substrate for the Detection of Celiac Disease Autoantibodies" International Journal of Molecular Sciences 26, no. 3: 1012. https://doi.org/10.3390/ijms26031012
APA StyleKorponay-Szabó, I. R., Király, R., Gyimesi, J., & Mäki, M. (2025). Development of Cell-Assembled Human Endomysial-Type Biomatrix Substrate for the Detection of Celiac Disease Autoantibodies. International Journal of Molecular Sciences, 26(3), 1012. https://doi.org/10.3390/ijms26031012







