Graphitic Carbon Nitride-Decorated Cobalt Diselenide Composites for Highly Efficient Hydrogen Evolution Reaction
Abstract
1. Introduction
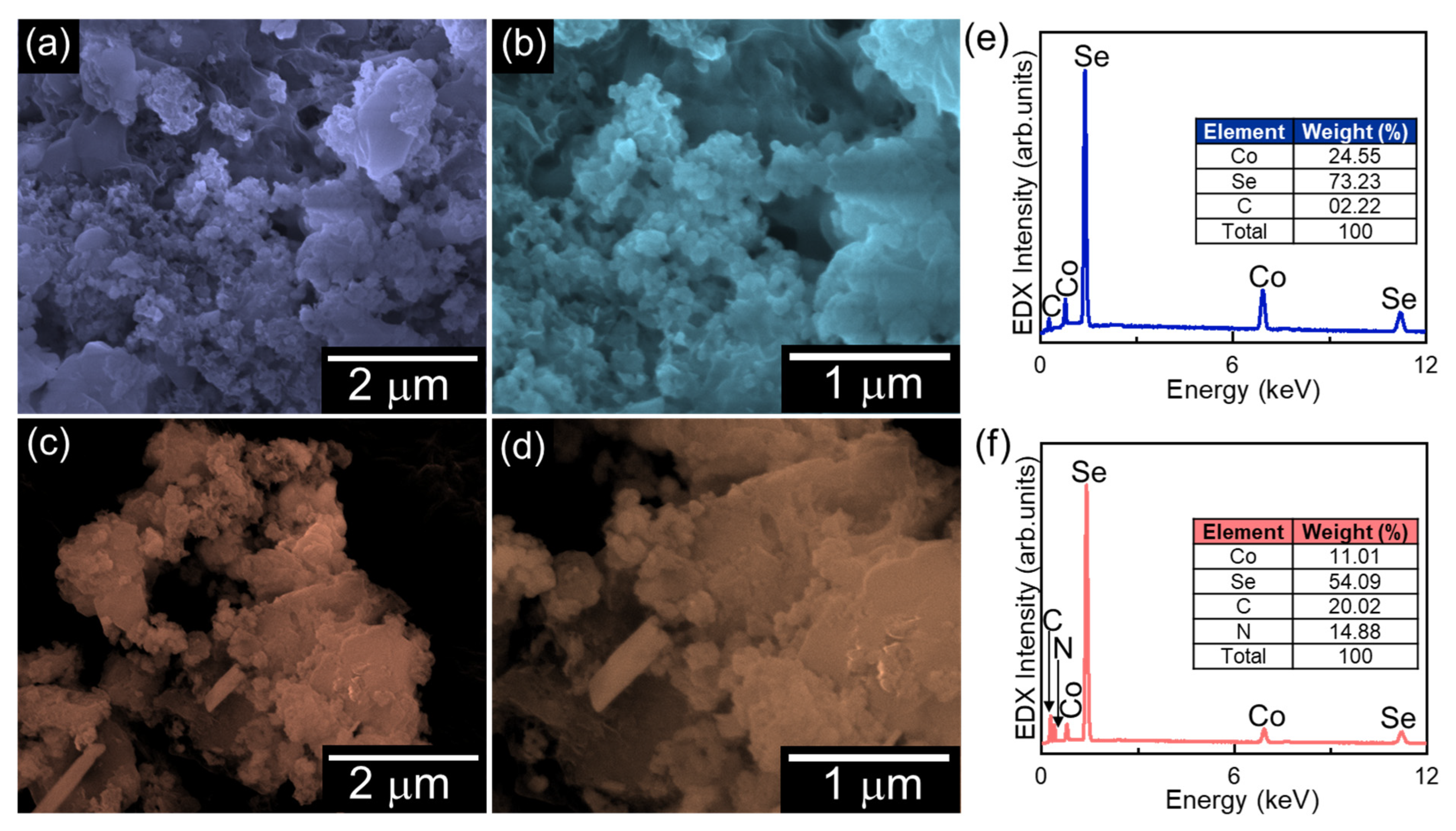
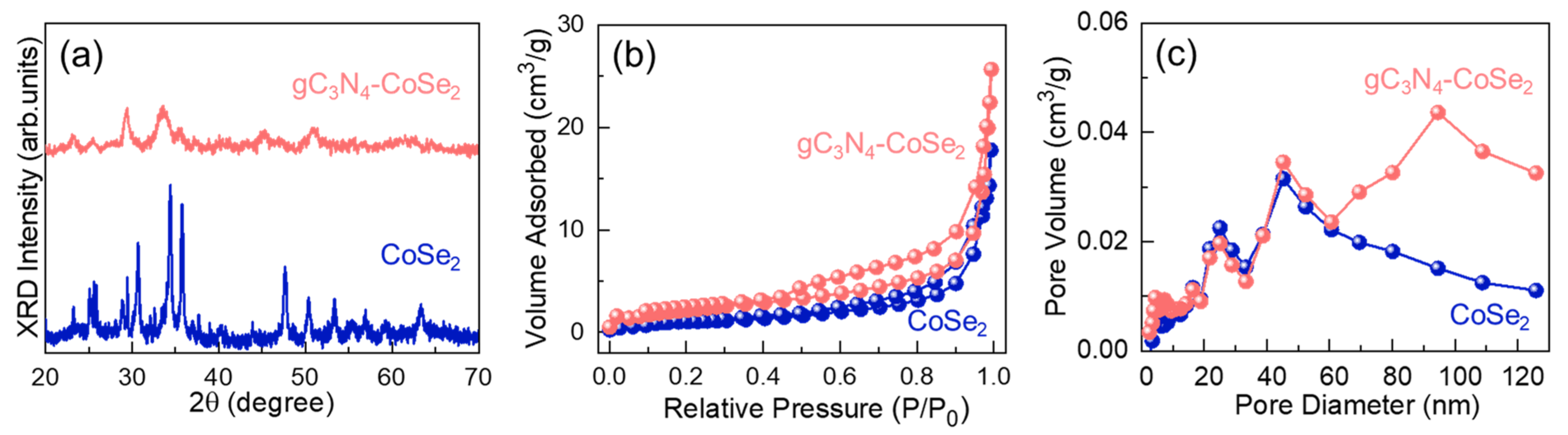
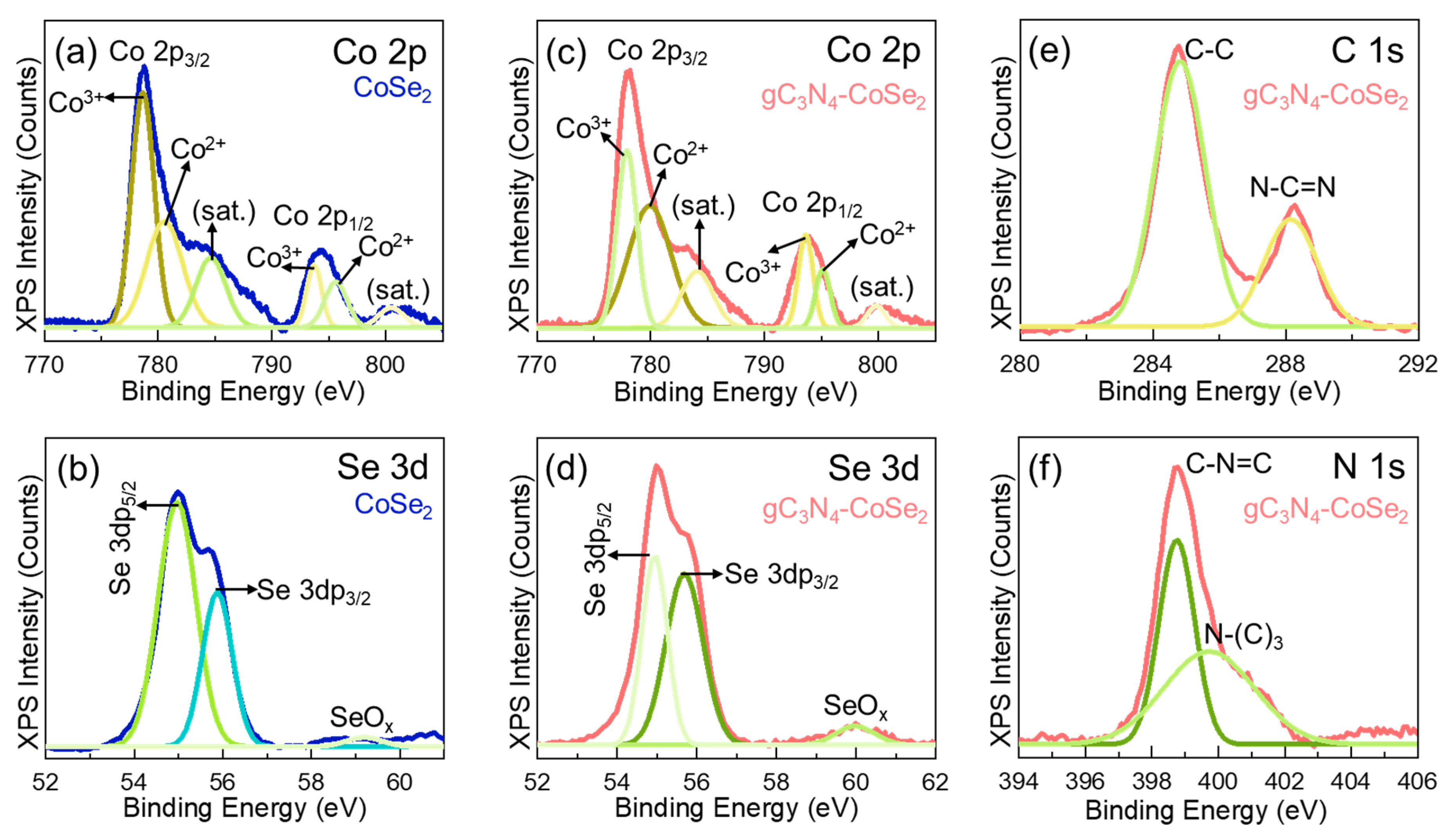
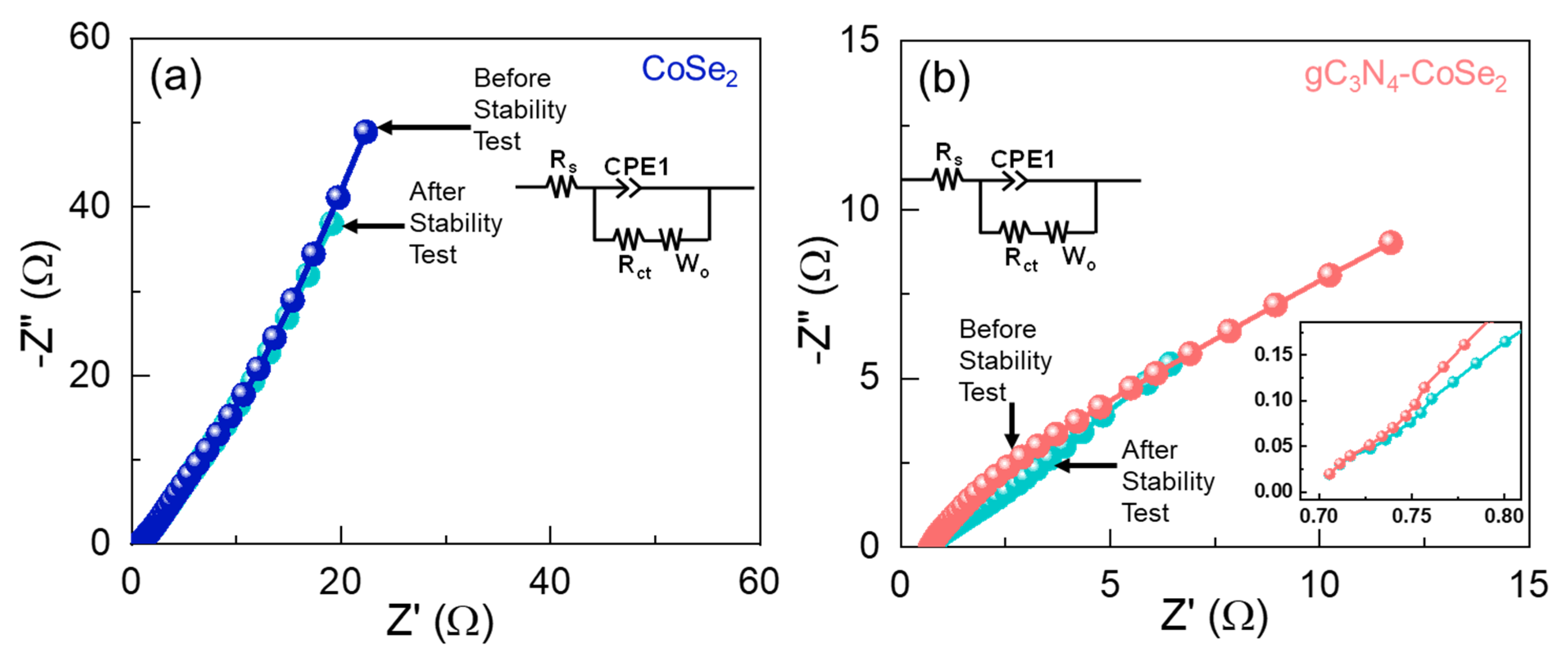
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Synthesis of CoSe2 Nanoparticles
3.3. Synthesis of gC3N4 Nanosheets
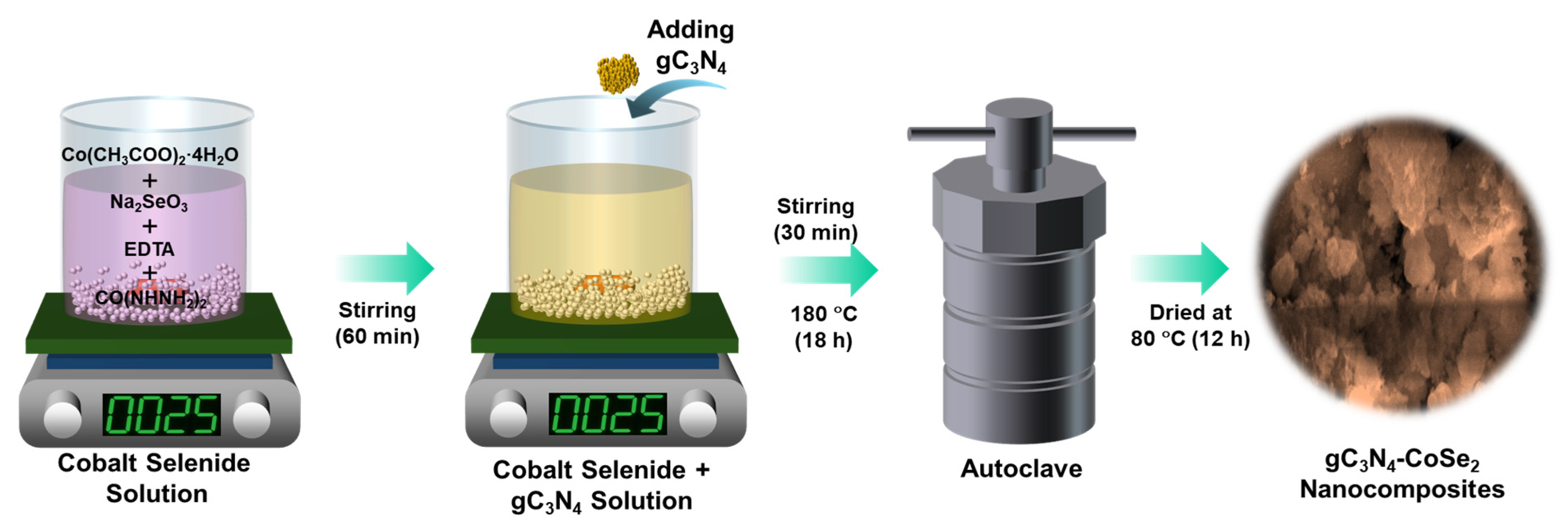
3.4. Synthesis of gC3N4-CoSe2 Nanocomposites
3.5. Material Characterization
3.6. Electrocatalytic HER Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qian, Q.; Zhu, Y.; Ahmad, N.; Feng, Y.; Zhang, H.; Cheng, M.; Liu, H.; Xiao, C.; Zhang, G.; Xie, Y. Recent Advancements in Electrochemical Hydrogen Production via Hybrid Water Splitting. Adv. Mater. 2024, 36, 2306108. [Google Scholar] [CrossRef]
- Bhuiyan, M.M.H.; Siddique, Z. Hydrogen as an alternative fuel: A comprehensive review of challenges and opportunities in production, storage, and transportation. Int. J. Hydrogen Energy 2025, 102, 1026–1044. [Google Scholar] [CrossRef]
- Sekar, S.; Lee, E.; Yun, J.; Arumugasamy, S.K.; Choi, M.-J.; Lee, Y.; Lee, S. High-performance electrocatalyst of activated carbon-decorated molybdenum trioxide nanocomposites for effective production of H2 and H2O2. Sep. Purif. Technol. 2025, 361, 131614. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhong, C.-J. Hydrogen production from water electrolysis: Role of catalysts. Nano Converg. 2021, 8, 4. [Google Scholar] [CrossRef]
- Zhai, W.; Ma, Y.; Chen, D.; Ho, J.C.; Dai, Z.; Qu, Y. Recent progress on the long-term stability of hydrogen evolution reaction electrocatalysts. InfoMat 2022, 4, e12357. [Google Scholar] [CrossRef]
- Kazemi, A.; Manteghi, F.; Tehrani, Z. Metal Electrocatalysts for Hydrogen Production in Water Splitting. ACS Omega 2024, 9, 7310–7335. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, X.; Wang, X.; Zhao, Q.; Shao, M. Some remaining puzzles in hydrogen electrocatalysis mechanisms on platinum surfaces. Joule 2024, 8, 1890–1918. [Google Scholar] [CrossRef]
- Dhanasekaran, B.; Sekar, S.; Veerapandian, M.; Sekar, S.; Lee, S.; Govindaraju, S.; Yun, K. Tailoring active sites on heterostructures through electrocatalytic engineering for efficient bifunctional production of H2 and H2O2. Mater. Today Phys. 2025, 59, 101951. [Google Scholar] [CrossRef]
- Li, J.; Wu, G.; Huang, Z.; Han, X.; Wu, B.; Liu, P.; Hu, H.; Yu, G.; Hong, X. Vertically Stacked Amorphous Ir/Ru/Ir Oxide Nanosheets for Boosted Acidic Water Splitting. JACS Au 2024, 4, 1243–1249. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, P.; Li, L.; Moon, M.-W.; Chung, C.-H.; Li, H.; Lee, J.Y.; Yoo, P.J. Challenges and Emerging Trends in Hydrogen Energy Industrialization: From Hydrogen Evolution Reaction to Storage, Transportation, and Utilization. Small 2025, 21, 2502000. [Google Scholar] [CrossRef]
- Kumar, N.; Aepuru, R.; Lee, S.-Y.; Park, S.-J. Advances in Catalysts for Hydrogen Production: A Comprehensive Review of Materials and Mechanisms. Nanomaterials 2025, 15, 256. [Google Scholar] [CrossRef]
- Prabhu, P.; Jose, V.; Lee, J.-M. Design Strategies for Development of TMD-Based Heterostructures in Electrochemical Energy Systems. Matter 2020, 2, 526–553. [Google Scholar] [CrossRef]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.-J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef]
- Mohanty, B.; Bhanja, P.; Jena, B.K. An overview on advances in design and development of materials for electrochemical generation of hydrogen and oxygen. Mater. Today Energy 2022, 23, 100902. [Google Scholar] [CrossRef]
- Yu, J.; Peng, G.; Peng, L.; Chen, Q.; Su, C.; Shang, L.; Zhang, T. Recent advancements in two-dimensional transition metal dichalcogenide materials towards hydrogen-evolution electrocatalysis. Green Energy Environ. 2025, 10, 1130–1152. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, J.; Hu, J.; Asiri, A.M.; Luo, Y.; Sun, X. CoSe2 Nanowires Array as a 3D Electrode for Highly Efficient Electrochemical Hydrogen Evolution. ACS Appl. Mater. Interfaces 2015, 7, 3877–3881. [Google Scholar] [CrossRef] [PubMed]
- Mudoi, R.; Saikia, L. Heteroatom Doping, Defect Engineering, and Stability of Transition Metal Diselenides for Electrocatalytic Water Splitting. Chem. Asian J. 2025, 20, e00755. [Google Scholar] [CrossRef]
- Aggarwal, P.; Sarkar, D.; Dwivedi, P.K.; Menezes, P.W.; Awasthi, K. Deciphering the Influence of Morphology and Crystal Structure on Alkaline Hydrogen Evolution Activity in Polymorphic Cobalt Diselenide. ACS Appl. Energy Mater. 2024, 7, 1550–1560. [Google Scholar] [CrossRef]
- Wei, G.; Xu, Z.; Zhao, X.; Wang, S.; Kong, F.; An, C. N-doped CoSe2 nanomeshes as highly-efficient bifunctional electrocatalysts for water splitting. J. Alloys Compd. 2022, 893, 162328. [Google Scholar] [CrossRef]
- Lan, K.; Li, J.; Zhu, Y.; Gong, L.; Li, F.; Jiang, P.; Niu, F.; Li, R. Morphology engineering of CoSe2 as efficient electrocatalyst for water splitting. J. Colloid Interface Sci. 2019, 539, 646–653. [Google Scholar] [CrossRef]
- Jiang, K.; Liu, B.; Luo, M.; Ning, S.; Peng, M.; Zhao, Y.; Lu, Y.-R.; Chan, T.-S.; de Groot, F.M.F.; Tan, Y. Single platinum atoms embedded in nanoporous cobalt selenide as electrocatalyst for accelerating hydrogen evolution reaction. Nat. Commun. 2019, 10, 1743. [Google Scholar] [CrossRef]
- El-marghany, A.; Sillanpää, M.; Manzoor, S.; Abid, A.G.; Nisa, M.U. Solvothermally fabricated cobalt selenide supported on graphitic carbon nitride for enhanced oxygen evolution reaction. Appl. Phys. A 2023, 129, 605. [Google Scholar] [CrossRef]
- Cui, W.; Sun, X.; Xu, S.; Li, C.; Bai, J. CoS2-CoSe2 hybrid nanoparticles grown on carbon nanofibers as electrode for supercapacitor and hydrogen evolution reaction. J. Alloys Compd. 2024, 990, 174366. [Google Scholar] [CrossRef]
- Nithya, V.D. Recent advances in CoSe2 electrocatalysts for hydrogen evolution reaction. Int. J. Hydrogen Energy 2021, 46, 36080–36102. [Google Scholar] [CrossRef]
- Zhou, W.; Lu, J.; Zhou, K.; Yang, L.; Ke, Y.; Tang, Z.; Chen, S. CoSe2 nanoparticles embedded defective carbon nanotubes derived from MOFs as efficient electrocatalyst for hydrogen evolution reaction. Nano Energy 2016, 28, 143–150. [Google Scholar] [CrossRef]
- Taherinia, D.; Moazzeni, M.; Moravej, S. Comparison of hydrothermal and electrodeposition methods for the synthesis of CoSe2/CeO2 nanocomposites as electrocatalysts toward oxygen evolution reaction. Int. J. Hydrogen Energy 2022, 47, 17650–17661. [Google Scholar] [CrossRef]
- Dileepkumar, V.G.; Balaji, K.R.; Vishwanatha, R.; Basavaraja, B.M.; Ashoka, S.; Al-Akraa, I.M.; Santosh, M.S.; Rtimi, S. CoSe2 grafted on 2D gC3N4: A promising material for wastewater treatment, electrocatalysis and energy storage. Chem. Eng. J. 2022, 446, 137023. [Google Scholar] [CrossRef]
- Zulqarnain, M.; Shah, A.; Khan, M.A.; Iftikhar, F.J.; Nisar, J. FeCoSe2 Nanoparticles Embedded in g-C3N4: A Highly Active and Stable bifunctional electrocatalyst for overall water splitting. Sci. Rep. 2020, 10, 6328. [Google Scholar] [CrossRef]
- Kong, D.; Wang, H.; Lu, Z.; Cui, Y. CoSe2 Nanoparticles Grown on Carbon Fiber Paper: An Efficient and Stable Electrocatalyst for Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2014, 136, 4897–4900. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer to Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Pan, F.; Khan, M.; Lei, T.; Kamli, M.R.; Sabir, J.S.M.; Khan, I.; Ansari, M.Z. Visible light driven hydrogen generation and pollutant degradation with Au loaded 2D/2D heterojunctional nanocomposite of MoS2 and g-C3N4. Int. J. Hydrogen Energy 2024, 51, 1141–1153. [Google Scholar] [CrossRef]
- Li, C.; Harikrishnan, L.; Ding, L.; Pitcheri, R.; Qi, K. Graphitic carbon nitride-based photocatalysts and sonocatalysts for energy and environment. Renew. Sustain. Energy Rev. 2026, 226, 116435. [Google Scholar] [CrossRef]
- Sekar, S.; Shanmugam, A.; Lee, Y.; Lee, S. Highly Efficient Electrocatalyst of 2D–2D gC3N4–MoS2 Composites for Enhanced Overall Water Electrolysis. Materials 2025, 18, 3775. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Dai, A.; Vijayalakshmi, M.; Jang, W.Y.; Kakarla, R.R.; Shim, J.; Aminabhavi, T.M.; Reddy, C.V. Synthesis of novel 2D g-C3N4/3D CoSe2 hierarchical microflower-like hybrids for high-performance energy-storage applications. J. Energy Storage 2024, 104, 114577. [Google Scholar] [CrossRef]
- Borthakur, P.; Boruah, P.K.; Das, M.R.; Ibrahim, M.M.; Altalhi, T.; El-Sheshtawy, H.S.; Szunerits, S.; Boukherroub, R.; Amin, M.A. CoS2 Nanoparticles Supported on rGO, g-C3N4, BCN, MoS2, and WS2 Two-Dimensional Nanosheets with Excellent Electrocatalytic Performance for Overall Water Splitting: Electrochemical Studies and DFT Calculations. ACS Appl. Energy Mater. 2021, 4, 1269–1285. [Google Scholar] [CrossRef]
- Dileepkumar, V.G.; Sirichandana, B.; Sanaulla, P.F.; Hegde, G.; Basavaraja, B.M.; Santosh, M.S. Synergistic effects of NiSe2 on S-doped g-C3N4 for efficient caffeine degradation and electrocatalysis. Inorg. Chem. Commun. 2025, 178, 114657. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Maiyalagan, T. Sea urchin-like nickel-doped cobalt selenide decorated on graphitic carbon nitride nanosheets as efficient electrocatalysts for oxygen evolution reaction in alkaline medium. Int. J. Hydrogen Energy 2025, 133, 491–501. [Google Scholar] [CrossRef]
- Do, H.H.; Le, Q.V.; Cho, J.H.; Ahn, S.H.; Kim, S.Y. Comparative Study on Hydrogen Evolution Reaction of Various Cobalt-Selenide-Based Electrocatalysts. Int. J. Energy Res. 2023, 2023, 6016603. [Google Scholar] [CrossRef]
- Pi, Z.-G.; Ye, H.; Han, Z.; Yu, P.; Yin, Z.; Ma, X. Promising CoSe2-CNT composite catalyst for efficient photoelectrochemical hydrogen evolution reaction. Front. Mater. 2022, 9, 1005221. [Google Scholar] [CrossRef]
- Yue, H.; Yang, D.; Yu, B.; Lu, Y.; Zhang, W.; Chen, Y. Porous interwoven CoSe2/C microsphere: A highly efficient and stable nonprecious electrocatalyst for hydrogen evolution reaction. J. Mater. Sci. 2019, 54, 14123–14133. [Google Scholar] [CrossRef]
- Lu, W.; Xue, M.; Chen, X.; Chen, C. CoSe2 Nanoparticles as Anode for Lithium Ion Battery. Int. J. Electrochem. Sci. 2017, 12, 1118–1129. [Google Scholar] [CrossRef]
- Kale, V.N.; Jayasurya, B.; Bhavani, R.; Maiyalagan, T. Heterostructured FeSe2-CoSe2 Nanorods Supported on Nitrogen and Sulfur co-doped Reduced Graphene Oxide as High-Performance Electrocatalyst for Oxygen Evolution Reaction. J. Alloys Compd. 2024, 978, 173313. [Google Scholar] [CrossRef]
- Zheng, H.; Xu, H.-S.; Hu, J.; Liu, H.; Wei, L.; Wu, S.; Li, J.; Huang, Y.; Tang, K. Electrochemical performance of CoSe2 with mixed phases decorated with N-doped rGO in potassium-ion batteries. RSC Adv. 2022, 12, 21374–21384. [Google Scholar] [CrossRef]
- Che, S.; Ta, N.; Yang, F.; Yan, X.; Liu, H.; Chen, N.; Sun, S.; Wang, C.; Jiang, B.; Sun, Y.; et al. One-pot construction of CoSe nanoparticles anchored on single-atomic-Co doped carbon for pH-universal hydrogen evolution. Mater. Chem. Front. 2022, 6, 3577–3588. [Google Scholar] [CrossRef]
- Wei, W.; Wang, L.; Liang, C.; Liu, W.; Li, C.; An, Y.; Zhang, L.; Sun, X.; Wang, K.; Zhang, H.; et al. Interface engineering of CoSe2/N-doped graphene heterostructure with ultrafast pseudocapacitive kinetics for high-performance lithium-ion capacitors. Chem. Eng. J. 2023, 474, 145788. [Google Scholar] [CrossRef]
- Li, B.; Liu, Y.; Jin, X.; Jiao, S.; Wang, G.; Peng, B.; Zeng, S.; Shi, L.; Li, J.; Zhang, G. Designed Formation of Hybrid Nanobox Composed of Carbon Sheathed CoSe2 Anchored on Nitrogen-Doped Carbon Skeleton as Ultrastable Anode for Sodium-Ion Batteries. Small 2019, 15, 1902881. [Google Scholar] [CrossRef]
- Cao, H.; Li, H.; Liu, L.; Xue, K.; Niu, X.; Hou, J.; Chen, L. Salt-Templated Nanoarchitectonics of CoSe2-NC Nanosheets as an Efficient Bifunctional Oxygen Electrocatalyst for Water Splitting. Int. J. Mol. Sci. 2022, 23, 5239. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Wang, J.; Zhang, Z.; Lu, D.; Yu, Y.; Ji, Y.; Qiao, H.; Qi, X.; Liu, Y. In situ derived nanocomposites electrocatalysts from cobalt molybdates for hydrogen evolution reaction. J. Mater. Sci. Mater. Electron. 2020, 31, 14977–14985. [Google Scholar] [CrossRef]
- Cao, Y.; Gao, Q.; Li, Q.; Jing, X.; Wang, S.; Wang, W. Synthesis of 3D porous MoS2/g-C3N4 heterojunction as a high efficiency photocatalyst for boosting H2 evolution activity. RSC Adv. 2017, 7, 40727–40733. [Google Scholar] [CrossRef]
- Ye, L.; Wang, D.; Chen, S. Fabrication and Enhanced Photoelectrochemical Performance of MoS2/S-Doped g-C3N4 Heterojunction Film. ACS Appl. Mater. Interfaces 2016, 8, 5280–5289. [Google Scholar] [CrossRef]
- Tan, L.; Xu, J.; Zhang, X.; Hang, Z.; Jia, Y.; Wang, S. Synthesis of g-C3N4/CeO2 nanocomposites with improved catalytic activity on the thermal decomposition of ammonium perchlorate. Appl. Surf. Sci. 2015, 356, 447–453. [Google Scholar] [CrossRef]
- Hao, Q.; Liu, Y.; Zou, R.; Shi, G.; Yang, S.; Zhong, L.; Yang, W.; Chi, X.; Liu, Y.; Admassie, S.; et al. g-C3N4 nanosheets coupled with CoSe2 as co-catalyst for efficient photooxidation of xylose to xylonic acid. Green Energy Environ. 2025, 10, 231–238. [Google Scholar] [CrossRef]
- Zheng, Y.-R.; Gao, M.-R.; Yu, Z.-Y.; Gao, Q.; Gao, H.-L.; Yu, S.-H. Cobalt diselenide nanobelts grafted on carbon fiber felt: An efficient and robust 3D cathode for hydrogen production. Chem. Sci. 2015, 6, 4594–4598. [Google Scholar] [CrossRef]
- Fageria, P.; Sudharshan, K.Y.; Nazir, R.; Basu, M.; Pande, S. Decoration of MoS2 on g-C3N4 surface for efficient hydrogen evolution reaction. Electrochim. Acta 2017, 258, 1273–1283. [Google Scholar] [CrossRef]
- Zhang, X.; Ding, P.; Sun, Y.; Wang, Y.; Li, J.; Guo, J. MoS2 nanosheets on C3N4 realizing improved electrochemical hydrogen evolution. Mater. Lett. 2017, 197, 41–44. [Google Scholar] [CrossRef]
- Bai, X.; Cao, T.; Xia, T.; Wu, C.; Feng, M.; Li, X.; Mei, Z.; Gao, H.; Huo, D.; Ren, X.; et al. MoS2/NiSe2/rGO Multiple-Interfaced Sandwich-like Nanostructures as Efficient Electrocatalysts for Overall Water Splitting. Nanomaterials 2023, 13, 752. [Google Scholar] [CrossRef]
- Sekar, S.; Yun, J.-S.; Park, S.; Kim, D.Y.; Lee, Y.; Lee, S. Excellent Bifunctional Water Electrolysis Activities of α-MoO3/AC Nanocomposites. Int. J. Energy Res. 2024, 2024, 3167699. [Google Scholar] [CrossRef]
- Srividhya, G.; Viswanathan, C.; Ponpandian, N. Interfacing NiV layered double hydroxide with sulphur-doped g-C3N4 as a novel electrocatalyst for enhanced hydrogen evolution reaction through Volmer–Heyrovský mechanism. Energy Adv. 2023, 2, 1464–1475. [Google Scholar] [CrossRef]
- Yue, H.; Yu, B.; Qi, F.; Zhou, J.; Wang, X.; Zheng, B.; Zhang, W.; Li, Y.; Chen, Y. Interwoven CoSe2/CNTs hybrid as a highly efficient and stable electrocatalyst for hydrogen evolution reaction. Electrochim. Acta 2017, 253, 200–207. [Google Scholar] [CrossRef]
- Tong, J.; Li, W.; Wei, B.; Bo, L.; Li, Q.; Li, Y.; Li, T.; Zhang, Q. Facile preparing composite of CoSe2 wrapping N-doped mesoporous carbon with highly electrocatalytic activity for hydrogen evolution reaction. Int. J. Hydrogen Energy 2018, 43, 17021–17029. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Tekalgne, M.; Tran, C.V.; Nguyen, T.P.; Dao, V.; Le, Q.V.; Ahn, S.H.; Kim, S.Y. Synthesis of MoS2/WS2 Nanoflower Heterostructures for Hydrogen Evolution Reaction. Int. J. Energy Res. 2024, 2024, 3192642. [Google Scholar] [CrossRef]
- Zhao, G.; Li, P.; Rui, K.; Chen, Y.; Dou, S.X.; Sun, W. CoSe2/MoSe2 Heterostructures with Enriched Water Adsorption/Dissociation Sites towards Enhanced Alkaline Hydrogen Evolution Reaction. Chem. Eur. J. 2018, 24, 11158–11165. [Google Scholar] [CrossRef]
- Bose, R.; Kim, T.-H.; Koh, B.; Jung, C.-Y.; Yi, S.C. Influence of Phosphidation on CoSe2 Catalyst for Hydrogen Evolution Reaction. ChemistrySelect 2017, 2, 10661–10667. [Google Scholar] [CrossRef]
- Tang, Y.; Li, J.; Lu, Z.; Wang, Y.; Tao, K.; Lin, Y. MOF-Derived CoSe2@NiFeOOH Arrays for Efficient Oxygen Evolution Reaction. Nanomaterials 2023, 13, 2621. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking Heterogeneous Electrocatalysts for the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef]
- Ahmed, A.T.A.; Sekar, S.; Khadtare, S.S.; Rochman, N.T.; Chinna, B.; Ansari, A.S. Anion-exchange synthesis of an MnCo2S4 electrocatalyst towards facilitated ultralong hydrogen evolution reaction in acidic and alkaline media. CrystEngComm 2024, 26, 215–222. [Google Scholar] [CrossRef]
- Pazhamalai, P.; Krishnamoorthy, K.; Natraj, V.; Mohan, V.; Chennakrishnan, J.; Kim, S.J. Unveiling the bi-functional electrocatalytic properties of rhenium di-sulfide nanostructures towards the development of high-rate alkaline water electrolyzer. Chem. Eng. J. 2024, 500, 156356. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Pazhamalai, P.; Swaminathan, R.; Mohan, V.; Kim, S.-J. Unravelling the Bi-Functional Electrocatalytic Properties of {Mo72Fe30} Polyoxometalate Nanostructures for Overall Water Splitting Using Scanning Electrochemical Microscope and Electrochemical Gating Methods. Adv. Sci. 2024, 11, 2401073. [Google Scholar] [CrossRef]
- Saleem, M.S.M.; Pazhamalai, P.; Ali, N.U.H.L.; Chennakrishnan, J.; Kim, S.J. Exploring the catalytic insights of nanoengineered binder-free cobalt sulfide in industrial-condition alkaline water electrolyzers for sustainable green hydrogen production. Int. J. Hydrogen Energy 2025, 156, 150357. [Google Scholar] [CrossRef]
- Ali, N.U.H.L.; Saleem, M.S.M.; Sathyaseelan, A.; Krishnan, V.; Pazhamalai, P.; Saj, A.A.; Kim, S.-J. Thermo-Electric Powered High Energy-Density Hybrid Supercapattery for Driving Overall Water Splitting: A Novel Trifunctional Builder for Self-Powered Hydrogen Production. Small 2025, 21, 2504667. [Google Scholar] [CrossRef]
- Shinde, K.R.; Mali, S.S.; Patel, T.M.; Sonkawade, R.G.; Pawar, S.M. Recent progress in transition metal based organic framework for electrochemical water splitting and CO2 reduction applications. Int. J. Hydrogen Energy 2026, 197, 152628. [Google Scholar] [CrossRef]
- Vempuluru, N.R.; Yoon, Y.; Das, J.P.; Elumalai, V.; Saj, A.A.; Lee, H.; Kim, T.K.; Kim, K.; Sathyaseelan, A.; Muthukumar, P.; et al. Nanocluster catalyst driving ampere-level current density in direct seawater electrolysis quantum leap towards sustainable energy. Mater. Sci. Eng. R Rep. 2026, 167, 101092. [Google Scholar] [CrossRef]
- Li, W.; Zheng, M.; Tian, Z.; Long, G.; Zhang, S.; Chen, Q.; Zhong, Q. Coral-Like CoSe2-Nitrogen-Doped Porous Carbon as Efficient Counter Electrodes for Quantum Dot Sensitized Solar Cells. ECS J. Solid State Sci. Technol. 2021, 10, 045012. [Google Scholar] [CrossRef]
- Joseph, A.; Kumar, D.S.H.; Ramadoss, M.; Muralidharan, K. Organic-free NiSe2, CoSe2, and NiSe2/CoSe2 for non-enzymatic glucose sensing. J. Solid State Electrochem. 2025, 29, 4461–4471. [Google Scholar] [CrossRef]
- Xinxing, S.; Hongjing, G.; Shuangke, L.; Weiwei, S.; Yujie, L.; Danqin, W.; Qingpeng, G.; Xiaobin, H.; Jing, X.; Chunman, Z. Space-confined synthesis of CoSe2-NC nanoclusters anchored on honeycomb-like carbon framework towards high-performance lithium sulfur battery. Ionics 2023, 29, 4707–4722. [Google Scholar] [CrossRef]
- Gan, R.; Ma, X.; Wang, G.; Jin, Z. CoSe2 Clusters as Efficient Co-Catalyst Modified CdS Nanorod for Enhance Visible Light Photocatalytic H2 Evolution. Catalysts 2019, 9, 616. [Google Scholar] [CrossRef]
- Yan, Z.; Li, J.; Chen, Q.; Chen, S.; Luo, L.; Chen, Y. Synthesis of CoSe2/Mxene composites using as high-performance anode materials for lithium-ion batteries. Adv. Compos. Hybrid Mater. 2022, 5, 2977–2987. [Google Scholar] [CrossRef]
- Yu, B.; Tressel, J.; Cui, T.; Pan, D.; DuBois, D.B.; Jones, C.; Hou, B.; Mayford, K.; Liu, Q.; Bridges, F.; et al. Rapid synthesis of carbon-supported CoS2/CoSe2 heterostructures by magnetic induction heating for efficient hydrogen evolution reaction in acidic media. J. Power Sources 2025, 641, 236897. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Li, Y.; Yang, X.; Zhao, L.; Peng, J. Boosting Photocatalytic Performance of ZnO Nanowires via Building Heterojunction with g-C3N4. Molecules 2023, 28, 5563. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, M.; Moradian, S.; Pourhakkak, P.; Zhang, G.; Habibi, M.M.; Madadi, M.; Ghasemi, J.B. Fabrication of S-scheme heterojunction g-C3N4-nanosheet/ZnMoO4 nanocomposite with high efficiency in photocatalytic N2 fixation and Cr(VI) detoxification. J. Mater Sci. 2022, 57, 9145–9163. [Google Scholar] [CrossRef]


| Catalyst | Current Density (mA/cm2) | Overpotential η10 (mV) | Tafel Slope (mV/dec) | Electrolyte | Reference |
|---|---|---|---|---|---|
| gC3N4-CoSe2 | 10 | 141 | 62 | 1 M KOH | This work |
| CoSe2 | 10 | 187 | 76 | 1 M KOH | This work |
| Three-dimensional CoSe2/CFF | 10 | 141 | 68 | 0.5 M H2SO4 | [53] |
| g-C3N4-MoS2 | 10 | 156 | 101 | 1 M KOH | [33] |
| CoSe2/MoO2/MoSe2 | 10 | 230 | 36.8 | 0.5 M H2SO4 | [48] |
| MoS2/g-C3N4 | 10 | 240 | 63 | 1 M KOH | [54] |
| CoSe2-gC3N4/NF | 50 | 210 | 84 | 1 M KOH | [55] |
| CoSe2-g-C3N4/GCE | 20 | 193 | - | 0.5 M H2SO4 | [28] |
| MoS2/NiSe2/rGO | 10 | 127 | 73 | 1 M KOH | [56] |
| MoO3/AC | 10 | 353 | 124 | 1 M KOH | [57] |
| S-gC3N4/NiV LDH | 10 | 560 | 79 | 1 M KOH | [58] |
| CoSe2/C | 10 | 189 | 34 | 1 M KOH | [40] |
| CoSe2/CNT | 10 | 180 | 35 | 0.5 M H2SO4 | [59] |
| CoSe2/NC-170 | 10 | 159 | 83 | 0.5 M H2SO4 | [60] |
| MoS2/WS2 NF | 10 | 251 | 61 | 1 M KOH | [61] |
| CoSe2/MoSe2 | 10 | 218 | 76 | 1 M KOH | [62] |
| CoSe2|CoP/CFP | 10 | 140 | 42 | 0.5 M H2SO4 | [63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, A.T.A.; Sekar, S.; Sadhasivam, S.; Murugan, B.; Cho, S.; Lee, Y.; Lee, S.; Sekar, S. Graphitic Carbon Nitride-Decorated Cobalt Diselenide Composites for Highly Efficient Hydrogen Evolution Reaction. Int. J. Mol. Sci. 2025, 26, 12188. https://doi.org/10.3390/ijms262412188
Ahmed ATA, Sekar S, Sadhasivam S, Murugan B, Cho S, Lee Y, Lee S, Sekar S. Graphitic Carbon Nitride-Decorated Cobalt Diselenide Composites for Highly Efficient Hydrogen Evolution Reaction. International Journal of Molecular Sciences. 2025; 26(24):12188. https://doi.org/10.3390/ijms262412188
Chicago/Turabian StyleAhmed, Abu Talha Aqueel, Saravanan Sekar, Sutha Sadhasivam, Balaji Murugan, Sangeun Cho, Youngmin Lee, Sejoon Lee, and Sankar Sekar. 2025. "Graphitic Carbon Nitride-Decorated Cobalt Diselenide Composites for Highly Efficient Hydrogen Evolution Reaction" International Journal of Molecular Sciences 26, no. 24: 12188. https://doi.org/10.3390/ijms262412188
APA StyleAhmed, A. T. A., Sekar, S., Sadhasivam, S., Murugan, B., Cho, S., Lee, Y., Lee, S., & Sekar, S. (2025). Graphitic Carbon Nitride-Decorated Cobalt Diselenide Composites for Highly Efficient Hydrogen Evolution Reaction. International Journal of Molecular Sciences, 26(24), 12188. https://doi.org/10.3390/ijms262412188







