Mechanistic Fingerprints from Chloride to Iodide: Halide vs. Ammonia Release in Platinum Anticancer Complexes
Abstract
1. Introduction
2. Results and Discussion
2.1. Cisplatin vs. cisPtI2: NMR Investigation of Ligand Exchange with Histidine, Cysteine, and Methionine
2.2. Computational Assessment
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.M.P. Cisplatin in Cancer Treatment. Biochem. Pharmacol. 2022, 206, 115323. [Google Scholar] [CrossRef] [PubMed]
- Mariconda, A.; Ceramella, J.; Catalano, A.; Saturnino, C.; Sinicropi, M.S.; Longo, P. Cisplatin, the Timeless Molecule. Inorganics 2025, 13, 246. [Google Scholar] [CrossRef]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The Side Effects of Platinum-Based Chemotherapy Drugs: A Review for Chemists. Dalton Trans. 2018, 47, 6645–6653, Erratum in Dalton Trans. 2018, 47, 7848.. [Google Scholar] [CrossRef]
- Elmorsy, E.A.; Saber, S.; Hamad, R.S.; Abdel-Reheim, M.A.; El-kott, A.F.; AlShehri, M.A.; Morsy, K.; Salama, S.A.; Youssef, M.E. Advances in Understanding Cisplatin-Induced Toxicity: Molecular Mechanisms and Protective Strategies. Eur. J. Pharm. Sci. 2024, 203, 106939. [Google Scholar] [CrossRef]
- Fu, R.; Zhao, B.; Chen, M.; Fu, X.; Zhang, Q.; Cui, Y.; Hu, X.; Zhou, W. Moving beyond Cisplatin Resistance: Mechanisms, Challenges, and Prospects for Overcoming Recurrence in Clinical Cancer Therapy. Med. Oncol. 2023, 41, 9. [Google Scholar] [CrossRef]
- Sooriyaarachchi, M.; George, G.N.; Pickering, I.J.; Narendran, A.; Gailer, J. Tuning the Metabolism of the Anticancer Drug Cisplatin with Chemoprotective Agents to Improve Its Safety and Efficacy. Metallomics 2016, 8, 1170–1176. [Google Scholar] [CrossRef]
- Mitchell, E.; Pham, M.H.; Clay, A.; Sanghvi, R.; Williams, N.; Pietsch, S.; Hsu, J.I.; Øbro, N.F.; Jung, H.; Vedi, A.; et al. The Long-Term Effects of Chemotherapy on Normal Blood Cells. Nat. Genet. 2025, 57, 1684–1694, Erratum in Nat. Genet. 2025, 57, 2075.. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, H.; Tan, L.; Siu, K.T.H.; Guan, X.-Y. Exploring Treatment Options in Cancer: Tumor Treatment Strategies. Signal Transduct. Target. Ther. 2024, 9, 175. [Google Scholar] [CrossRef]
- Casini, A.; Pöthig, A. Metals in Cancer Research: Beyond Platinum Metallodrugs. ACS Cent. Sci. 2024, 10, 242–250. [Google Scholar] [CrossRef]
- Wani, M.Y.; Malik, M.A. Non-Platinum Anticancer Agents. In Gold and Its Complexes in Anticancer Chemotherapy; Springer: Singapore, 2021; pp. 51–68. ISBN 978-981-336-313-7. [Google Scholar]
- Wang, Y.; Cao, B.; Wang, Q.; Zhong, S.; Fang, X.; Wang, J.; Chan, A.S.C.; Xiong, X.; Zou, T. Ligand Supplementation Restores the Cancer Therapy Efficacy of the Antirheumatic Drug Auranofin from Serum Inactivation. Nat. Commun. 2025, 16, 7347. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Chen, Y.; Zhang, P.; Ma, R.; Zhang, W.; Liu, J.; Li, T.; Niu, H.; Cao, Y.; Hu, B.; et al. The Role of Platinum(IV)-Based Antitumor Drugs and the Anticancer Immune Response in Medicinal Inorganic Chemistry. A Systematic Review from 2017 to 2022. Eur. J. Med. Chem. 2022, 243, 114680. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D. Pt(IV) Anticancer Prodrugs—A Tale of Mice and Men. ChemMedChem 2021, 16, 2188–2191. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D. Platinum(IV) Anticancer Agents; Are We En Route to the Holy Grail or to a Dead End? J. Inorg. Biochem. 2021, 217, 111353. [Google Scholar] [CrossRef]
- Gibson, D. Platinum(IV) Anticancer Prodrugs—Hypotheses and Facts. Dalton Trans. 2016, 45, 12983–12991. [Google Scholar] [CrossRef]
- Chen, S.; Yao, H.; Zhou, Q.; Tse, M.-K.; Gunawan, Y.F.; Zhu, G. Stability, Reduction, and Cytotoxicity of Platinum(IV) Anticancer Prodrugs Bearing Carbamate Axial Ligands: Comparison with Their Carboxylate Analogues. Inorg. Chem. 2020, 59, 11676–11687. [Google Scholar] [CrossRef]
- González-Ballesteros, M.M.; Mejía, C.; Ruiz-Azuara, L. Metallodrugs: An Approach against Invasion and Metastasis in Cancer Treatment. FEBS Open Bio 2022, 12, 880–899. [Google Scholar] [CrossRef]
- Kim, W.K.; An, J.M.; Lim, Y.J.; Kim, K.; Kim, Y.H.; Kim, D. Recent Advances in Metallodrug: Coordination-Induced Synergy between Clinically Approved Drugs and Metal Ions. Mater. Today Adv. 2025, 25, 100569. [Google Scholar] [CrossRef]
- Boros, E.; Dyson, P.J.; Gasser, G. Classification of Metal-Based Drugs According to Their Mechanisms of Action. Chem 2020, 6, 41–60. [Google Scholar] [CrossRef]
- Cirri, D.; Chiaverini, L.; Pratesi, A.; Marzo, T. Is the Next Cisplatin Already in Our Laboratory? Comments Inorg. Chem. 2023, 43, 465–478. [Google Scholar] [CrossRef]
- Akitsu, T.; Tsvetkova, D.; Yamamoto, Y.; Nakane, D.; Kostova, I. From Basics of Coordination Chemistry to Understanding Cisplatin-Analogue Pt Drugs. Curr. Pharm. Des. 2023, 29, 1747–1774. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Huang, Y.; Wang, Y.; Lu, D.; Sun, Q. Research Progress of Platinum-Based Complexes in Lung Cancer Treatment: Mechanisms, Applications, and Challenges. Int. J. Mol. Sci. 2025, 26, 7958. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, D.; Platella, C.; Riccardi, C.; Merlino, A.; Marzo, T.; Massai, L.; Messori, L.; Montesarchio, D. A First-in-Class and a Fished out Anticancer Platinum Compound: Cis-[PtCl2(NH3)2] and Cis-[PtI2(NH3)2] Compared for Their Reactivity towards DNA Model Systems. Dalton Trans. 2016, 45, 8587–8600. [Google Scholar] [CrossRef] [PubMed]
- Marzo, T.; Pillozzi, S.; Hrabina, O.; Kasparkova, J.; Brabec, V.; Arcangeli, A.; Bartoli, G.; Severi, M.; Lunghi, A.; Totti, F.; et al. Cis-Pt I2(NH3)2: A Reappraisal. Dalton Trans. 2015, 44, 14896–14905. [Google Scholar] [CrossRef]
- Quiroga, A.G.; Cama, M.; Pajuelo-Lozano, N.; Álvarez-Valdés, A.; Perez, I.S. New Findings in the Signaling Pathways of Cis and Trans Platinum Iodido Complexes’ Interaction with DNA of Cancer Cells. ACS Omega 2019, 4, 21855–21861. [Google Scholar] [CrossRef]
- Tolbatov, I.; Marzo, T.; Cirri, D.; Gabbiani, C.; Coletti, C.; Marrone, A.; Paciotti, R.; Messori, L.; Re, N. Reactions of Cisplatin and Cis-[PtI2(NH3)2] with Molecular Models of Relevant Protein Sidechains: A Comparative Analysis. J. Inorg. Biochem. 2020, 209, 111096. [Google Scholar] [CrossRef]
- Pucci, R.; Angilella, G.G.N. Density Functional Theory, Chemical Reactivity, and the Fukui Functions. Found. Chem. 2022, 24, 59–71. [Google Scholar] [CrossRef]
- Wang, J.; Tao, J.; Jia, S.; Wang, M.; Jiang, H.; Du, Z. The Protein-Binding Behavior of Platinum Anticancer Drugs in Blood Revealed by Mass Spectrometry. Pharmaceuticals 2021, 14, 104. [Google Scholar] [CrossRef]
- Zimmermann, T.; Chval, Z.; Burda, J.V. Cisplatin Interaction with Cysteine and Methionine in Aqueous Solution: Computational DFT/PCM Study. J. Phys. Chem. B 2009, 113, 3139–3150. [Google Scholar] [CrossRef]
- Corinti, D.; Paciotti, R.; Coletti, C.; Re, N.; Chiavarino, B.; Crestoni, M.E.; Fornarini, S. Elusive Intermediates in Cisplatin Reaction with Target Amino Acids: Platinum(II)-Cysteine Complexes Assayed by IR Ion Spectroscopy and DFT Calculations. J. Inorg. Biochem. 2022, 237, 112017. [Google Scholar] [CrossRef]
- Scoditti, S.; Vigna, V.; Dabbish, E.; Sicilia, E. Iodido Equatorial Ligands Influence on the Mechanism of Action of Pt(IV) and Pt(II) Anti-Cancer Complexes: A DFT Computational Study. J. Comput. Chem. 2021, 42, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Jansen, B.A.J.; Brouwer, J.; Reedijk, J. Glutathione Induces Cellular Resistance against Cationic Dinuclear Platinum Anticancer Drugs. J. Inorg. Biochem. 2002, 89, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Messori, L.; Marzo, T.; Gabbiani, C.; Valdes, A.A.; Quiroga, A.G.; Merlino, A. Peculiar Features in the Crystal Structure of the Adduct Formed between Cis-PtI2 (NH3)2 and Hen Egg White Lysozyme. Inorg. Chem. 2013, 52, 13827–13829. [Google Scholar] [CrossRef] [PubMed]
- Tolbatov, I.; Marzo, T.; Umari, P.; Mendola, D.L.; Marrone, A. Detailed Mechanism of a DNA/RNA Nucleobase Substituting Bridging Ligand in Diruthenium(II,III) and Dirhodium(II,II) Tetraacetato Paddlewheel Complexes: Protonation of the Leaving Acetate Is Crucial. Dalton Trans. 2025, 54, 662–673. [Google Scholar] [CrossRef]
- Tolbatov, I.; Umari, P.; Marrone, A. The Binding of Diruthenium (II,III) and Dirhodium (II,II) Paddlewheel Complexes at DNA/RNA Nucleobases: Computational Evidences of an Appreciable Selectivity toward the AU Base Pairs. J. Mol. Graph. Model. 2024, 131, 108806. [Google Scholar] [CrossRef]
- Tolbatov, I.; Marrone, A. Reactivity of N-Heterocyclic Carbene Half-Sandwich Ru-, Os-, Rh-, and Ir-Based Complexes with Cysteine and Selenocysteine: A Computational Study. Inorg. Chem. 2022, 61, 746–754. [Google Scholar] [CrossRef]
- Scoditti, S.; Dabbish, E.; Russo, N.; Mazzone, G.; Sicilia, E. Anticancer Activity, DNA Binding, and Photodynamic Properties of a N∧C∧N-Coordinated Pt(II) Complex. Inorg. Chem. 2021, 60, 10350–10360. [Google Scholar] [CrossRef]
- Tolbatov, I.; Cirri, D.; Tarchi, M.; Marzo, T.; Coletti, C.; Marrone, A.; Messori, L.; Re, N.; Massai, L. Reactions of Arsenoplatin-1 with Protein Targets: A Combined Experimental and Theoretical Study. Inorg. Chem. 2022, 61, 3240–3248. [Google Scholar] [CrossRef]
- Dunning, T.H. Gaussian Basis Sets Tor Molecular Calculations; Springer: Berlin/Heidelberg, Germany, 2012; Volume 3. [Google Scholar]
- Chai, J.-D.; Head-Gordon, M. Long-Range Corrected Hybrid Density Functionals with Damped Atom–Atom Dispersion Corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef]
- Remya, K.; Suresh, C.H. Which Density Functional Is Close to CCSD Accuracy to Describe Geometry and Interaction Energy of Small Noncovalent Dimers? A Benchmark Study Using Gaussian09. J. Comput. Chem. 2013, 34, 1341–1353. [Google Scholar] [CrossRef]
- Andrae, D.; Häußermann, U.; Dolg, M.; Stoll, H.; Preuß, H. Energy-Adjustedab Initio Pseudopotentials for the Second and Third Row Transition Elements. Theor. Chim. Acta 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M.; Tomasi, J. A New Definition of Cavities for the Computation of Solvation Free Energies by the Polarizable Continuum Model. J. Chem. Phys. 1997, 107, 3210–3221. [Google Scholar] [CrossRef]
- Klamt, A.; Moya, C.; Palomar, J. A Comprehensive Comparison of the IEFPCM and SS(V)PE Continuum Solvation Methods with the COSMO Approach. J. Chem. Theory Comput. 2015, 11, 4220–4225. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.P.; Cramer, C.J.; Truhlar, D.G. Single-Ion Solvation Free Energies and the Normal Hydrogen Electrode Potential in Methanol, Acetonitrile, and Dimethyl Sulfoxide. J. Phys. Chem. B 2007, 111, 408–422. [Google Scholar] [CrossRef] [PubMed]
- Isaev, Y.I.; Makarov, D.M.; Khodov, I.A. Machine Learning Prediction of NMR Shifts for Rare and Transition Metal Complexes (45Sc, 49Ti, 89Y, 91Zr, 139La). J. Mol. Liq. 2025, 437, 128417. [Google Scholar] [CrossRef]
- Geerlings, P.; De Proft, F.; Langenaeker, W. Conceptual Density Functional Theory. Chem. Rev. 2003, 103, 1793–1874. [Google Scholar] [CrossRef]
- Chakraborty, D.; Chattaraj, P.K. Conceptual Density Functional Theory Based Electronic Structure Principles. Chem. Sci. 2021, 12, 6264–6279. [Google Scholar] [CrossRef]
- Yang, W.; Mortier, W.J. The Use of Global and Local Molecular Parameters for the Analysis of the Gas-Phase Basicity of Amines. J. Am. Chem. Soc. 1986, 108, 5708–5711. [Google Scholar] [CrossRef]
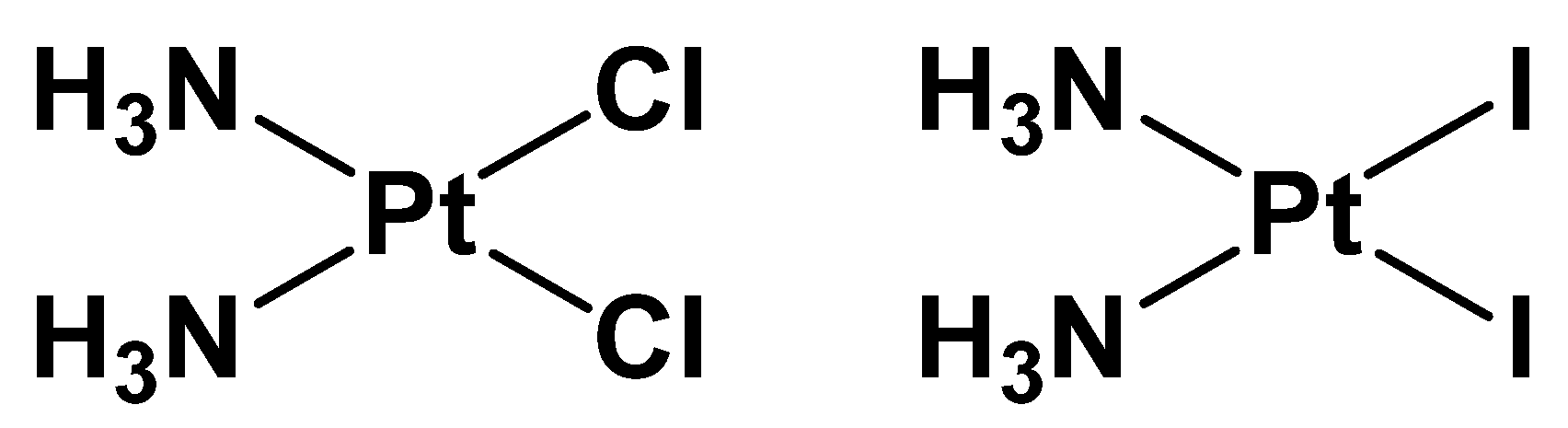
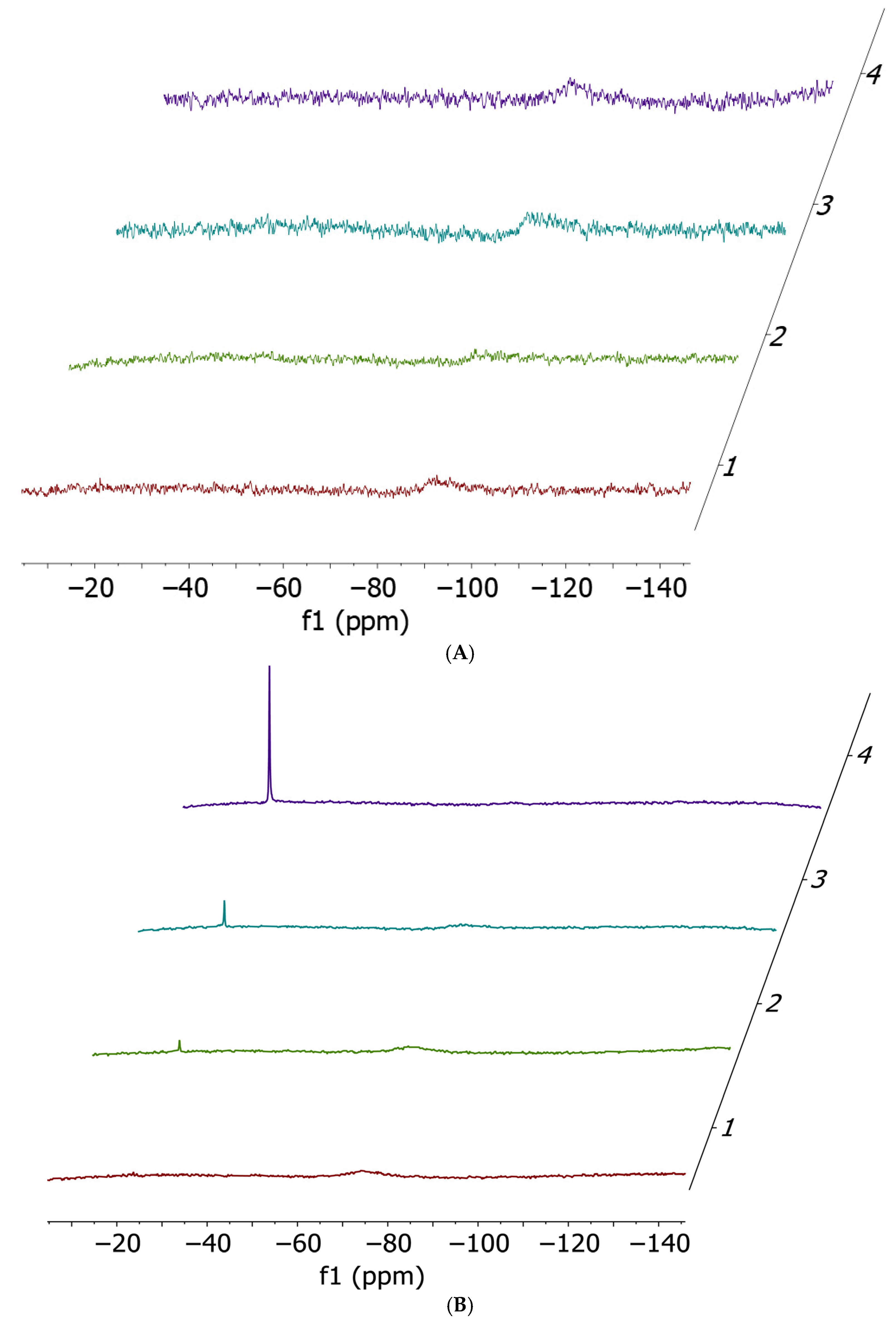

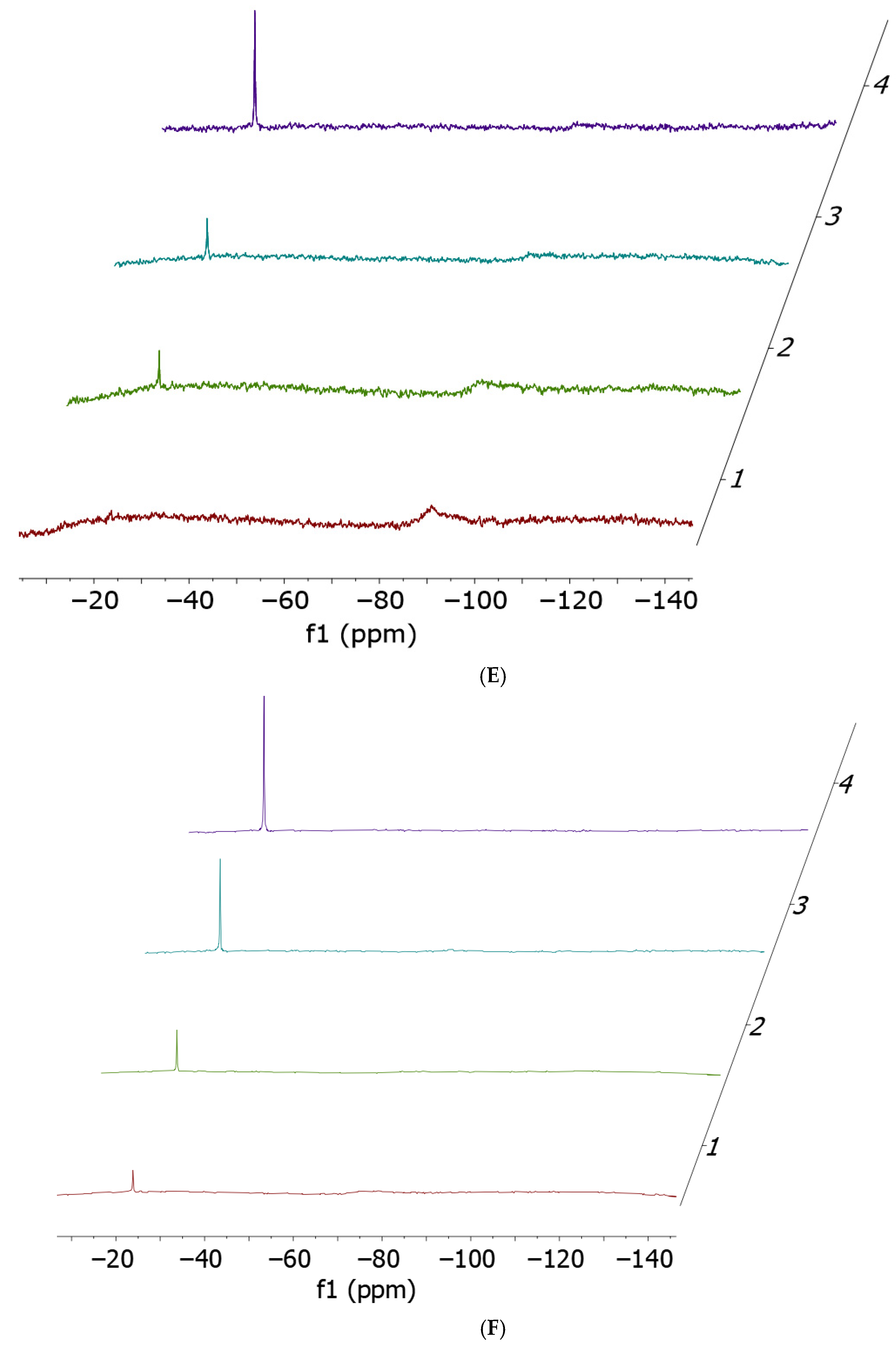
| Cisplatin + His | Cisplatin + Met | Cisplatin + Cys | cis-[PtI2(NH3)2] + His | cis-[PtI2(NH3)2] + Met | cis-[PtI2(NH3)2] + Cys |
|---|---|---|---|---|---|
| No release | 11% | 14% | 54% | 41% | 100% |
| Complex | GFE | ||||
|---|---|---|---|---|---|
| [Pt(NH3)2Cl2] | 0.0 | −0.140 | 0.169 | 2.957 | 0.058 |
| [Pt(NH3)2Cl(CH3SH)] | 7.5 | −0.163 | 0.168 | 2.968 | 0.079 |
| [Pt(NH3)2Cl(CH3S−)] | −18.6 | −0.113 | 0.154 | 3.249 | 0.041 |
| [Pt(NH3)2Cl(CH3SCH3)] | 2.7 | −0.162 | 0.170 | 2.944 | 0.077 |
| [Pt(NH3)2Cl(imi)] | −1.8 | −0.146 | 0.177 | 2.821 | 0.060 |
| [Pt(NH3)Cl2(CH3SH)] | 4.9 | −0.153 | 0.163 | 3.073 | 0.072 |
| [Pt(NH3)Cl2(CH3S−)] | −15.7 | −0.099 | 0.150 | 3.329 | 0.033 |
| [Pt(NH3)Cl2(CH3SCH3)] | 0.6 | −0.153 | 0.164 | 3.056 | 0.071 |
| [Pt(NH3)Cl2(imi)] | −1.7 | −0.137 | 0.169 | 2.956 | 0.056 |
| Complex | GFE | μ | η | S | ω |
|---|---|---|---|---|---|
| [Pt(NH3)2I2] | 0.0 | −0.149 | 0.147 | 3.391 | 0.075 |
| [Pt(NH3)2I(CH3SH)] | 2.1 | −0.163 | 0.152 | 3.285 | 0.087 |
| [Pt(NH3)2I(CH3S−)] | −24.4 | −0.122 | 0.147 | 3.408 | 0.051 |
| [Pt(NH3)2I(CH3SCH3)] | −2.5 | −0.161 | 0.153 | 3.264 | 0.084 |
| [Pt(NH3)2I(imi)] | −7.6 | −0.148 | 0.160 | 3.123 | 0.068 |
| [Pt(NH3)I2(CH3SH)] | 3.7 | −0.159 | 0.142 | 3.533 | 0.089 |
| [Pt(NH3)I2(CH3S−)] | −19.7 | −0.123 | 0.140 | 3.578 | 0.054 |
| [Pt(NH3)I2(CH3SCH3)] | −0.5 | −0.157 | 0.142 | 3.516 | 0.086 |
| [Pt(NH3)I2(imi)] | −3.1 | −0.146 | 0.149 | 3.358 | 0.071 |
| Complex | Fukui Index | Pt | N | N | Cl | Cl | N(imi) | S |
|---|---|---|---|---|---|---|---|---|
| [Pt(NH3)2Cl2] | −0.410 | −0.023 | −0.023 | −0.171 | −0.171 | n/a | n/a | |
| −0.655 | −0.015 | −0.015 | −0.104 | −0.104 | n/a | n/a | ||
| [Pt(NH3)2Cl(CH3SH)] | −0.358 | −0.023 | −0.031 | −0.174 | n/a | n/a | −0.137 | |
| −0.452 | −0.001 | −0.009 | −0.292 | n/a | n/a | −0.055 | ||
| [Pt(NH3)2Cl(CH3S−)] | −0.370 | −0.001 | −0.008 | −0.160 | n/a | n/a | −0.210 | |
| −0.200 | 0.018 | −0.010 | −0.057 | n/a | n/a | −0.524 | ||
| [Pt(NH3)2Cl(CH3SCH3)] | −0.348 | −0.019 | −0.035 | −0.242 | n/a | n/a | −0.111 | |
| −0.449 | 0.000 | −0.008 | −0.281 | n/a | n/a | −0.054 | ||
| [Pt(NH3)2Cl(imi)] | −0.421 | −0.010 | −0.040 | −0.191 | n/a | 0.003 | n/a | |
| −0.476 | 0.003 | −0.023 | −0.299 | n/a | 0.016 | n/a | ||
| [Pt(NH3)Cl2(CH3SH)] | −0.354 | −0.025 | n/a | −0.179 | −0.193 | n/a | −0.111 | |
| −0.432 | 0.002 | n/a | −0.279 | −0.105 | n/a | −0.045 | ||
| [Pt(NH3)Cl2(CH3S−)] | −0.333 | 0.002 | n/a | −0.162 | −0.124 | n/a | −0.212 | |
| −0.165 | 0.016 | n/a | −0.053 | −0.080 | n/a | −0.537 | ||
| [Pt(NH3)Cl2(CH3SCH3)] | −0.339 | −0.022 | n/a | −0.155 | −0.157 | n/a | −0.116 | |
| −0.432 | 0.003 | n/a | −0.262 | −0.108 | n/a | −0.043 | ||
| [Pt(NH3)Cl2(imi)] | −0.400 | −0.019 | n/a | −0.175 | −0.169 | 0.007 | n/a | |
| −0.461 | 0.000 | n/a | −0.115 | −0.251 | 0.027 | n/a |
| Complex | Fukui Index | Pt | N | N | I | I | N(imi) | S |
|---|---|---|---|---|---|---|---|---|
| [Pt(NH3)2I2] | −0.300 | −0.022 | −0.022 | −0.244 | −0.244 | n/a | n/a | |
| −0.344 | 0.005 | 0.005 | −0.287 | −0.287 | n/a | n/a | ||
| [Pt(NH3)2I(CH3SH)] | −0.303 | −0.024 | −0.027 | −0.280 | n/a | n/a | −0.117 | |
| −0.220 | −0.024 | 0.003 | −0.637 | n/a | n/a | −0.026 | ||
| [Pt(NH3)2I(CH3S−)] | −0.305 | −0.008 | −0.007 | −0.265 | n/a | n/a | −0.191 | |
| −0.162 | 0.022 | −0.009 | −0.089 | n/a | n/a | −0.528 | ||
| [Pt(NH3)2I(CH3SCH3)] | −0.307 | −0.024 | −0.025 | −0.275 | n/a | n/a | −0.100 | |
| −0.220 | −0.011 | 0.004 | −0.633 | n/a | n/a | −0.024 | ||
| [Pt(NH3)2I(imi)] | −0.340 | −0.016 | −0.035 | −0.307 | n/a | 0.004 | n/a | |
| −0.242 | −0.010 | −0.001 | −0.640 | n/a | 0.029 | n/a | ||
| [Pt(NH3)I2(CH3SH)] | −0.248 | −0.020 | n/a | −0.234 | −0.235 | n/a | −0.107 | |
| −0.205 | −0.005 | n/a | −0.572 | −0.119 | n/a | −0.020 | ||
| [Pt(NH3)I2(CH3S−)] | −0.253 | −0.007 | n/a | −0.220 | −0.179 | n/a | −0.194 | |
| −0.157 | 0.025 | n/a | −0.080 | −0.119 | n/a | −0.484 | ||
| [Pt(NH3)I2(CH3SCH3)] | −0.256 | −0.018 | n/a | −0.230 | −0.233 | n/a | −0.089 | |
| −0.209 | −0.004 | n/a | −0.562 | −0.122 | n/a | −0.019 | ||
| [Pt(NH3)I2(imi)] | −0.284 | −0.020 | n/a | −0.255 | −0.238 | 0.010 | n/a | |
| −0.222 | 0.002 | n/a | −0.250 | −0.447 | 0.017 | n/a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiaverini, L.; Famlonga, L.; Piroddu, D.; Pacini, M.; Di Leo, R.; Baglini, E.; Cirri, D.; Marzo, T.; La Mendola, D.; Pratesi, A.; et al. Mechanistic Fingerprints from Chloride to Iodide: Halide vs. Ammonia Release in Platinum Anticancer Complexes. Int. J. Mol. Sci. 2025, 26, 12138. https://doi.org/10.3390/ijms262412138
Chiaverini L, Famlonga L, Piroddu D, Pacini M, Di Leo R, Baglini E, Cirri D, Marzo T, La Mendola D, Pratesi A, et al. Mechanistic Fingerprints from Chloride to Iodide: Halide vs. Ammonia Release in Platinum Anticancer Complexes. International Journal of Molecular Sciences. 2025; 26(24):12138. https://doi.org/10.3390/ijms262412138
Chicago/Turabian StyleChiaverini, Lorenzo, Luca Famlonga, Davide Piroddu, Matteo Pacini, Riccardo Di Leo, Emma Baglini, Damiano Cirri, Tiziano Marzo, Diego La Mendola, Alessandro Pratesi, and et al. 2025. "Mechanistic Fingerprints from Chloride to Iodide: Halide vs. Ammonia Release in Platinum Anticancer Complexes" International Journal of Molecular Sciences 26, no. 24: 12138. https://doi.org/10.3390/ijms262412138
APA StyleChiaverini, L., Famlonga, L., Piroddu, D., Pacini, M., Di Leo, R., Baglini, E., Cirri, D., Marzo, T., La Mendola, D., Pratesi, A., Ferrari, P., Nicolini, A., Zucchi, A., Marrone, A., & Tolbatov, I. (2025). Mechanistic Fingerprints from Chloride to Iodide: Halide vs. Ammonia Release in Platinum Anticancer Complexes. International Journal of Molecular Sciences, 26(24), 12138. https://doi.org/10.3390/ijms262412138












