The Influence of Glucagon-like Peptide-1 Receptor Agonists and Other Incretin Hormone Agonists on Body Composition
Abstract
1. Introduction
2. Physiology of Incretin Hormones
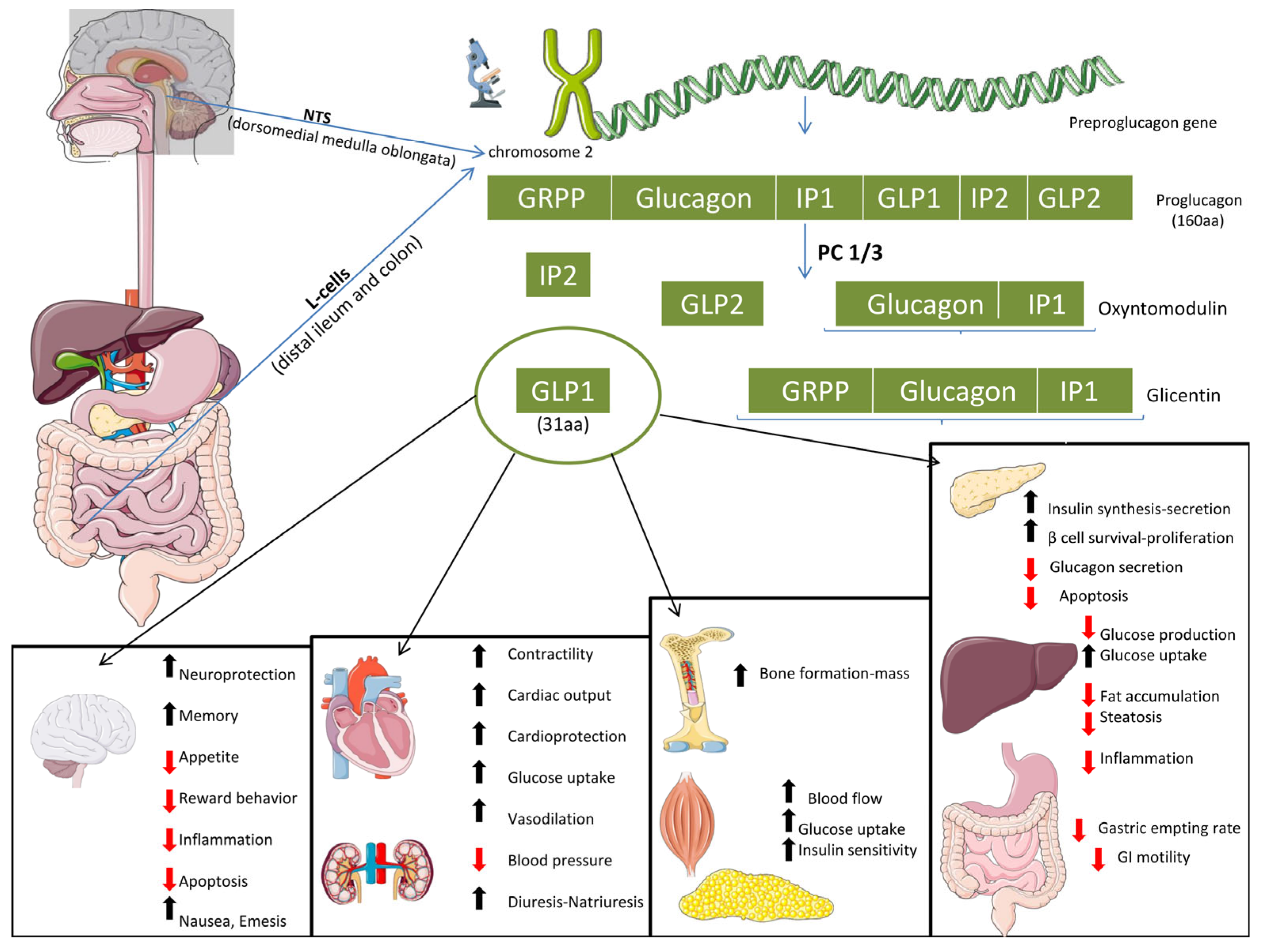
2.1. Glucagon-like Peptide 1 (GLP-1)
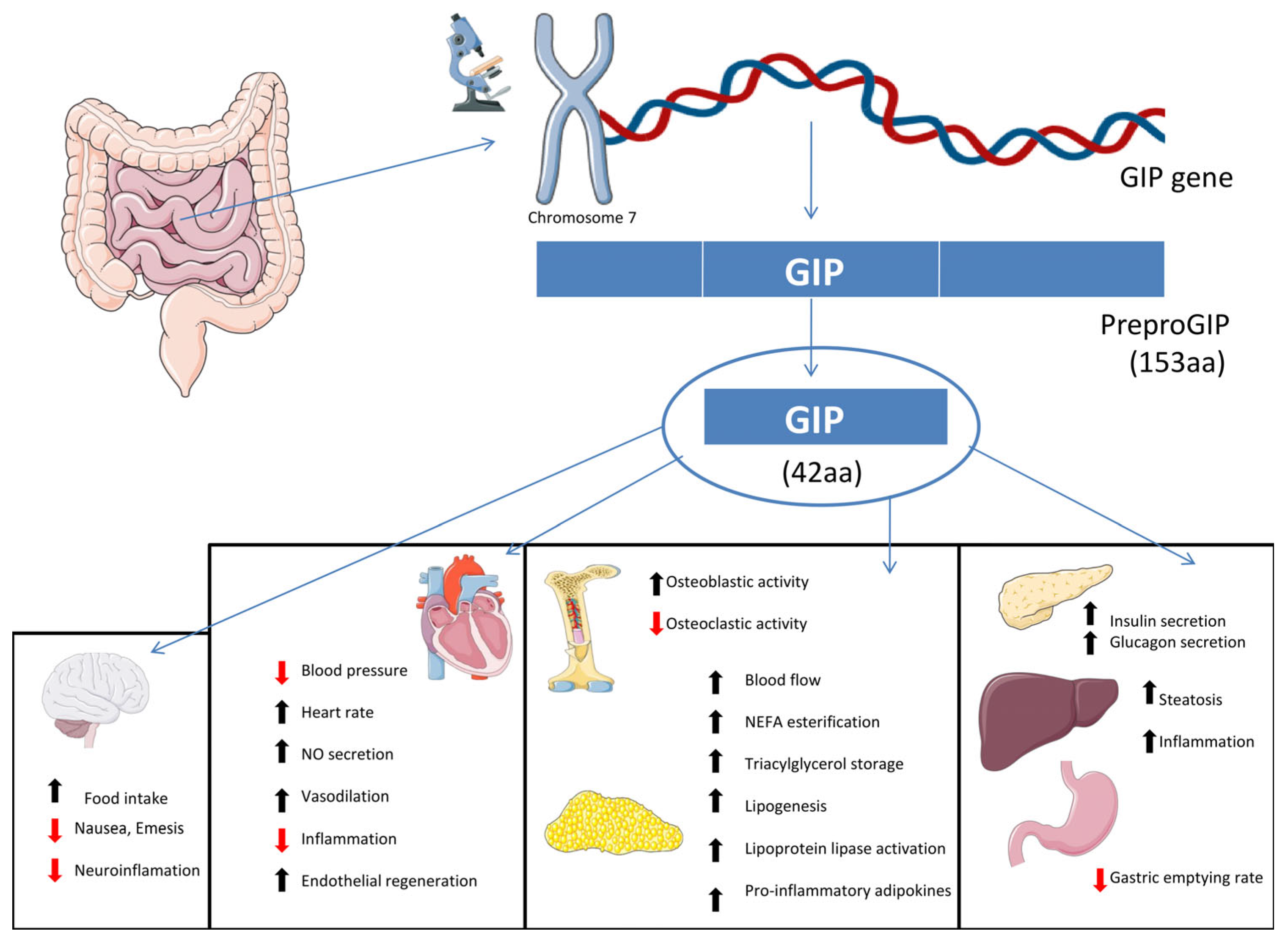
2.2. Glucose-Dependent Insulinotropic Polypeptide (GIP)
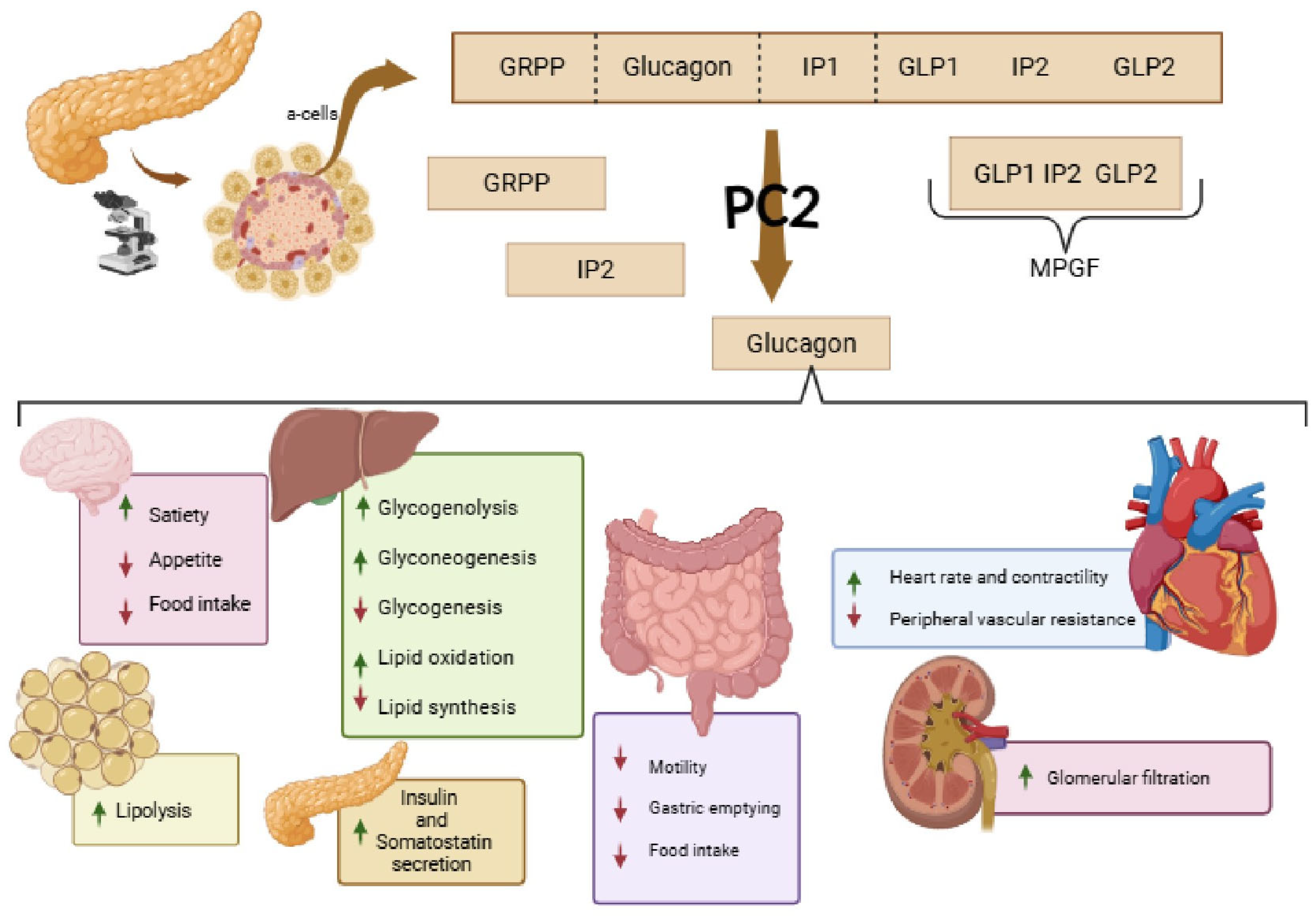
2.3. Glucagon (GCG)
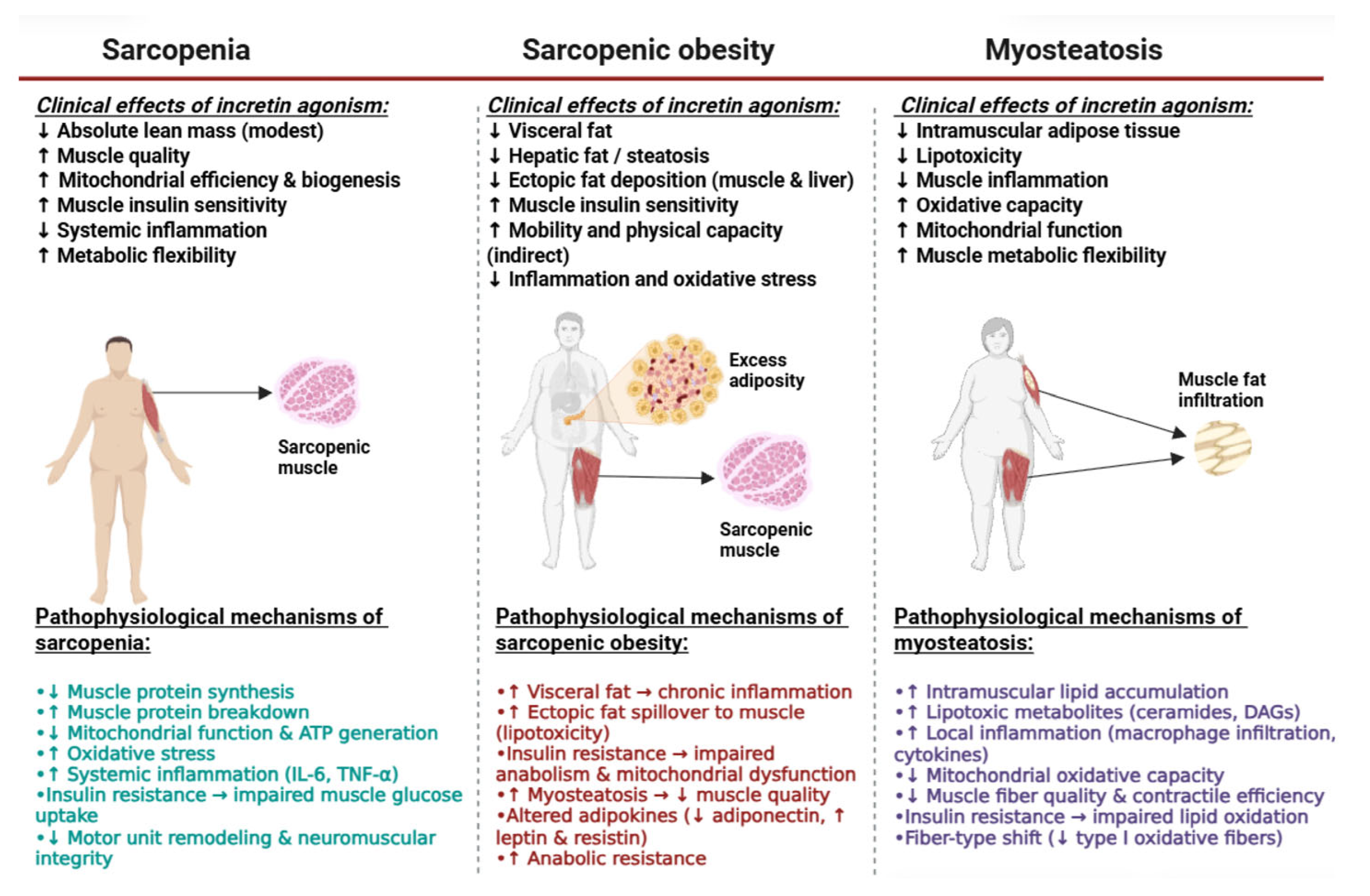
3. Main Dysregulations in Body Composition
3.1. Sarcopenia and Sarcopenic Obesity (SO)
3.2. Myosteatosis
4. Muscle Dysregulations and Their Impact on Metabolic Syndrome and MASLD (Table 1)
5. GLP-1, GIP and GCG Receptor Agonists and Their Impact on Body Composition and Lean Mass
5.1. ActRII Blockade and the Myostatin Pathway
5.2. Dual and Triple Incretin Agonists (GLP-1, GIP, Glucagon)
6. GLP-1 RAs, GIP and GCG Receptor Agonists and Their Efficacy on Body Composition, Skeletal and Lean Mass: Evidence from Randomized Clinical Trials (RCTs)
7. Limitations and Future Perspectives
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Darwish, R.; Abu-Sharia, G.; Butler, A.E. History of glucagon-like peptide-1 receptor agonists. Pharmacol. Res. 2025, 222, 108045. [Google Scholar] [CrossRef] [PubMed]
- Moiz, A.; Filion, K.B.; Tsoukas, M.A.; Yu, O.H.Y.; Peters, T.M.; Eisenberg, M.J. The expanding role of GLP-1 receptor agonists: A narrative review of current evidence and future directions. eClinicalMedicine 2025, 86, 103363. [Google Scholar] [CrossRef] [PubMed]
- Lobstein, T.; Jackson-Leach, R.; Powis, J.; Brinsden, H.; Gray, M. World Obesity Atlas 2023; World Obesity Federation: London, UK, 2023. [Google Scholar]
- Ahmed, S.K.; Mohammed, R.A. Obesity: Prevalence, causes, consequences, management, preventive strategies and future research directions. Metab. Open 2025, 27, 100375. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef]
- Neeland, I.J.; Marso, S.P.; Ayers, C.R.; Lewis, B.; Oslica, R.; Francis, W.; Rodder, S.; Pandey, A.; Joshi, P.H. Effects of liraglutide on visceral and ectopic fat in adults with overweight and obesity at high cardiovascular risk: A randomised, double-blind, placebo-controlled, clinical trial. Lancet Diabetes Endocrinol. 2021, 9, 595–605. [Google Scholar] [CrossRef]
- Ryan, D.H.; Lingvay, I.; Deanfield, J.; Kahn, S.E.; Barros, E.; Burguera, B.; Colhoun, H.M.; Cercato, C.; Dicker, D.; Horn, D.B.; et al. Long-term weight loss effects of semaglutide in obesity without diabetes in the SELECT trial. Nat. Med. 2024, 30, 2049–2057. [Google Scholar] [CrossRef]
- Neeland, I.J.; Linge, J.; Birkenfeld, A.L. Changes in lean body mass with glucagon-like peptide-1-based therapies and mitigation strategies. Diabetes Obes. Metab. 2024, 26 (Suppl. S4), 16–27. [Google Scholar] [CrossRef]
- Jiao, R.; Lin, C.; Cai, X.; Wang, J.; Wang, Y.; Lv, F.; Yang, W.; Ji, L. Characterizing body composition modifying effects of a glucagon-like peptide 1 receptor-based agonist: A meta-analysis. Diabetes Obes. Metab. 2025, 27, 259–267. [Google Scholar] [CrossRef]
- Chrysavgis, L.; Mourelatou, N.G.; Cholongitas, E. Glucagon-like Peptide-1 Receptor Agonists (GLP-1 RAs): A Pan-Steatotic Liver Disease Treatment? Biomedicines 2025, 13, 1516. [Google Scholar] [CrossRef]
- Cannavaro, D.; Leva, F.; Caturano, A.; Berra, C.C.; Bonfrate, L.; Conte, C. Optimizing Body Composition During Weight Loss: The Role of Amino Acid Supplementation. Nutrients 2025, 17, 2000. [Google Scholar] [CrossRef]
- Mechanick, J.I.; Butsch, W.S.; Christensen, S.M.; Hamdy, O.; Li, Z.; Prado, C.M.; Heymsfield, S.B. Strategies for minimizing muscle loss during use of incretin-mimetic drugs for treatment of obesity. Obes. Rev. 2025, 26, e13841. [Google Scholar] [CrossRef] [PubMed]
- Cigrovski Berkovic, M.; Ruzic, L.; Cigrovski, V.; Strollo, F. Saving muscle while losing weight: A vital strategy for sustainable results while on glucagon-like peptide-1 related drugs. World J. Diabetes 2025, 16, 109123. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.C.; Villareal, D.T. The Benefits of Exercise Training in Combination With Weight Loss Therapies. Diabetes 2025, 74, 2199–2206. [Google Scholar] [CrossRef] [PubMed]
- Conte, C.; Hall, K.D.; Klein, S. Is Weight Loss-Induced Muscle Mass Loss Clinically Relevant? JAMA 2024, 332, 9–10. [Google Scholar] [CrossRef]
- Caruso, I.; Cignarelli, A.; Sorice, G.P.; Perrini, S.; Giorgino, F. Incretin-based therapies for the treatment of obesity-related diseases. NPJ Metab. Health Dis. 2024, 2, 31. [Google Scholar] [CrossRef]
- Tricoli, J.V.; Bell, G.I.; Shows, T.B. The human glucagon gene is located on chromosome 2. Diabetes 1984, 33, 200–202. [Google Scholar] [CrossRef]
- Sandoval, D.A.; D’Alessio, D.A. Physiology of proglucagon peptides: Role of glucagon and GLP-1 in health and disease. Physiol. Rev. 2015, 95, 513–548. [Google Scholar] [CrossRef]
- Baggio, L.L.; Drucker, D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef]
- Anini, Y.; Brubaker, P.L. Role of leptin in the regulation of glucagon-like peptide-1 secretion. Diabetes 2003, 52, 252–259. [Google Scholar] [CrossRef]
- Mortensen, K.; Christensen, L.L.; Holst, J.J.; Orskov, C. GLP-1 and GIP are colocalized in a subset of endocrine cells in the small intestine. Regul. Pept. 2003, 114, 189–196. [Google Scholar] [CrossRef]
- Roberge, J.N.; Brubaker, P.L. Secretion of proglucagon-derived peptides in response to intestinal luminal nutrients. Endocrinology 1991, 128, 3169–3174. [Google Scholar] [CrossRef] [PubMed]
- Deacon, C.F.; Johnsen, A.H.; Holst, J.J. Degradation of glucagon-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide that is a major endogenous metabolite in vivo. J. Clin. Endocrinol. Metab. 1995, 80, 952–957. [Google Scholar] [CrossRef] [PubMed]
- Zander, M.; Madsbad, S.; Deacon, C.F.; Holst, J.J. The metabolite generated by dipeptidyl-peptidase 4 metabolism of glucagon-like peptide-1 has no influence on plasma glucose levels in patients with type 2 diabetes. Diabetologia 2006, 49, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Smits, M.M.; Tonneijck, L.; Muskiet, M.H.; Kramer, M.H.; Cahen, D.L.; van Raalte, D.H. Gastrointestinal actions of glucagon-like peptide-1-based therapies: Glycaemic control beyond the pancreas. Diabetes Obes. Metab. 2016, 18, 224–235. [Google Scholar] [CrossRef]
- Nauck, M.A.; Meier, J.J. The incretin effect in healthy individuals and those with type 2 diabetes: Physiology, pathophysiology, and response to therapeutic interventions. Lancet Diabetes Endocrinol. 2016, 4, 525–536. [Google Scholar] [CrossRef]
- Baggio, L.L.; Drucker, D.J. Therapeutic approaches to preserve islet mass in type 2 diabetes. Annu. Rev. Med. 2006, 57, 265–281. [Google Scholar] [CrossRef]
- Holst, J.J.; Vilsboll, T.; Deacon, C.F. The incretin system and its role in type 2 diabetes mellitus. Mol. Cell. Endocrinol. 2009, 297, 127–136. [Google Scholar] [CrossRef]
- Holst, J.J.; Knop, F.K.; Vilsboll, T.; Krarup, T.; Madsbad, S. Loss of incretin effect is a specific, important, and early characteristic of type 2 diabetes. Diabetes Care 2011, 34 (Suppl. S2), S251–S257. [Google Scholar] [CrossRef]
- Dupre, J.; Ross, S.A.; Watson, D.; Brown, J.C. Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J. Clin. Endocrinol. Metab. 1973, 37, 826–828. [Google Scholar] [CrossRef]
- Ugleholdt, R.; Poulsen, M.L.; Holst, P.J.; Irminger, J.C.; Orskov, C.; Pedersen, J.; Rosenkilde, M.M.; Zhu, X.; Steiner, D.F.; Holst, J.J. Prohormone convertase 1/3 is essential for processing of the glucose-dependent insulinotropic polypeptide precursor. J. Biol. Chem. 2006, 281, 11050–11057. [Google Scholar] [CrossRef]
- El, K.; Campbell, J.E. The role of GIP in alpha-cells and glucagon secretion. Peptides 2020, 125, 170213. [Google Scholar] [CrossRef]
- Maskery, M.; Goulding, E.M.; Gengler, S.; Melchiorsen, J.U.; Rosenkilde, M.M.; Holscher, C. The Dual GLP-1/GIP Receptor Agonist DA4-JC Shows Superior Protective Properties Compared to the GLP-1 Analogue Liraglutide in the APP/PS1 Mouse Model of Alzheimer’s Disease. Am. J. Alzheimers Dis. Other Dementiasr 2020, 35, 1533317520953041. [Google Scholar] [CrossRef]
- Nogi, Y.; Nagashima, M.; Terasaki, M.; Nohtomi, K.; Watanabe, T.; Hirano, T. Glucose-dependent insulinotropic polypeptide prevents the progression of macrophage-driven atherosclerosis in diabetic apolipoprotein E-null mice. PLoS ONE 2012, 7, e35683. [Google Scholar] [CrossRef]
- Nagashima, M.; Watanabe, T.; Terasaki, M.; Tomoyasu, M.; Nohtomi, K.; Kim-Kaneyama, J.; Miyazaki, A.; Hirano, T. Native incretins prevent the development of atherosclerotic lesions in apolipoprotein E knockout mice. Diabetologia 2011, 54, 2649–2659. [Google Scholar] [CrossRef]
- Ding, K.H.; Zhong, Q.; Xu, J.; Isales, C.M. Glucose-dependent insulinotropic peptide: Differential effects on hepatic artery vs. portal vein endothelial cells. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E773–E779. [Google Scholar] [CrossRef] [PubMed]
- Heimburger, S.M.N.; Hoe, B.; Nielsen, C.N.; Bergman, N.C.; Skov-Jeppesen, K.; Hartmann, B.; Holst, J.J.; Dela, F.; Overgaard, J.; Storling, J.; et al. GIP Affects Hepatic Fat and Brown Adipose Tissue Thermogenesis but Not White Adipose Tissue Transcriptome in Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2022, 107, 3261–3274. [Google Scholar] [CrossRef] [PubMed]
- Goralska, J.; Razny, U.; Polus, A.; Dziewonska, A.; Gruca, A.; Zdzienicka, A.; Dembinska-Kiec, A.; Solnica, B.; Micek, A.; Kapusta, M.; et al. Enhanced GIP Secretion in Obesity Is Associated with Biochemical Alteration and miRNA Contribution to the Development of Liver Steatosis. Nutrients 2020, 12, 476. [Google Scholar] [CrossRef] [PubMed]
- Thondam, S.K.; Cuthbertson, D.J.; Wilding, J.P.H. The influence of Glucose-dependent Insulinotropic Polypeptide (GIP) on human adipose tissue and fat metabolism: Implications for obesity, type 2 diabetes and Non-Alcoholic Fatty Liver Disease (NAFLD). Peptides 2020, 125, 170208. [Google Scholar] [CrossRef]
- Donohoe, F.; Wilkinson, M.; Baxter, E.; Brennan, D.J. Mitogen-Activated Protein Kinase (MAPK) and Obesity-Related Cancer. Int. J. Mol. Sci. 2020, 21, 1241. [Google Scholar] [CrossRef]
- Richter, M.M.; Galsgaard, K.D.; Elmelund, E.; Knop, F.K.; Suppli, M.P.; Holst, J.J.; Winther-Sorensen, M.; Kjeldsen, S.A.S.; Wewer Albrechtsen, N.J. The Liver-alpha-Cell Axis in Health and in Disease. Diabetes 2022, 71, 1852–1861. [Google Scholar] [CrossRef]
- Mineo, I.; Matsumura, T.; Shingu, R.; Namba, M.; Kuwajima, M.; Matsuzawa, Y. The role of prohormone convertases PC1 (PC3) and PC2 in the cell-specific processing of proglucagon. Biochem. Biophys. Res. Commun. 1995, 207, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Zhang, B.B. Glucagon and regulation of glucose metabolism. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E671–E678. [Google Scholar] [CrossRef] [PubMed]
- Janah, L.; Kjeldsen, S.; Galsgaard, K.D.; Winther-Sorensen, M.; Stojanovska, E.; Pedersen, J.; Knop, F.K.; Holst, J.J.; Wewer Albrechtsen, N.J. Glucagon Receptor Signaling and Glucagon Resistance. Int. J. Mol. Sci. 2019, 20, 3314. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Pydi, S.P.; Pham, J.; Tanaka, N. Metabolic Functions of G Protein-Coupled Receptors in Hepatocytes-Potential Applications for Diabetes and NAFLD. Biomolecules 2020, 10, 1445. [Google Scholar] [CrossRef]
- Kleinert, M.; Sachs, S.; Habegger, K.M.; Hofmann, S.M.; Muller, T.D. Glucagon Regulation of Energy Expenditure. Int. J. Mol. Sci. 2019, 20, 5407. [Google Scholar] [CrossRef]
- Martinez, M.S.; Manzano, A.; Olivar, L.C.; Nava, M.; Salazar, J.; D’Marco, L.; Ortiz, R.; Chacin, M.; Guerrero-Wyss, M.; Cabrera de Bravo, M.; et al. The Role of the alpha Cell in the Pathogenesis of Diabetes: A World beyond the Mirror. Int. J. Mol. Sci. 2021, 22, 9504. [Google Scholar] [CrossRef]
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997, 127 (Suppl. 5), 990s–991s. [Google Scholar] [CrossRef]
- Janssen, I. Evolution of sarcopenia research. Appl. Physiol. Nutr. Metab. 2010, 35, 707–712. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646, Erratum in Lancet 2019, 393, 2590. [Google Scholar] [CrossRef]
- Yeung, S.S.Y.; Reijnierse, E.M.; Pham, V.K.; Trappenburg, M.C.; Lim, W.K.; Meskers, C.G.M.; Maier, A.B. Sarcopenia and its association with falls and fractures in older adults: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2019, 10, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Larsson, S.C. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metabolism 2023, 144, 155533. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.N.; Jung, C.H.; Hwang, Y.C. Sarcopenia in youth. Metabolism 2023, 144, 155557. [Google Scholar] [CrossRef] [PubMed]
- Chrysavgis, L.; Cholongitas, E. From NAFLD to MASLD: What does it mean? Expert Rev. Gastroenterol. Hepatol. 2024, 18, 217–221. [Google Scholar] [CrossRef]
- Apovian, C.M. Obesity: Definition, comorbidities, causes, and burden. Am. J. Manag. Care 2016, 22 (Suppl. S7), s176–s185. [Google Scholar]
- Donini, L.M.; Busetto, L.; Bischoff, S.C.; Cederholm, T.; Ballesteros-Pomar, M.D.; Batsis, J.A.; Bauer, J.M.; Boirie, Y.; Cruz-Jentoft, A.J.; Dicker, D.; et al. Definition and Diagnostic Criteria for Sarcopenic Obesity: ESPEN and EASO Consensus Statement. Obes. Facts 2022, 15, 321–335. [Google Scholar] [CrossRef]
- Gao, Q.; Mei, F.; Shang, Y.; Hu, K.; Chen, F.; Zhao, L.; Ma, B. Global prevalence of sarcopenic obesity in older adults: A systematic review and meta-analysis. Clin. Nutr. 2021, 40, 4633–4641. [Google Scholar] [CrossRef]
- Lee, D.C.; Shook, R.P.; Drenowatz, C.; Blair, S.N. Physical activity and sarcopenic obesity: Definition, assessment, prevalence and mechanism. Future Sci. OA 2016, 2, Fso127. [Google Scholar] [CrossRef]
- Baumgartner, R.N. Body composition in healthy aging. Ann. NY Acad. Sci. 2000, 904, 437–448. [Google Scholar] [CrossRef]
- Wang, M.; Tan, Y.; Shi, Y.; Wang, X.; Liao, Z.; Wei, P. Diabetes and Sarcopenic Obesity: Pathogenesis, Diagnosis, and Treatments. Front. Endocrinol. 2020, 11, 568. [Google Scholar] [CrossRef]
- Lim, S.; Kim, J.H.; Yoon, J.W.; Kang, S.M.; Choi, S.H.; Park, Y.J.; Kim, K.W.; Lim, J.Y.; Park, K.S.; Jang, H.C. Sarcopenic obesity: Prevalence and association with metabolic syndrome in the Korean Longitudinal Study on Health and Aging (KLoSHA). Diabetes Care 2010, 33, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Ebadi, M.; Tsien, C.; Bhanji, R.A.; Dunichand-Hoedl, A.R.; Rider, E.; Motamedrad, M.; Mazurak, V.C.; Baracos, V.; Montano-Loza, A.J. Myosteatosis in Cirrhosis: A Review of Diagnosis, Pathophysiological Mechanisms and Potential Interventions. Cells 2022, 11, 1216. [Google Scholar] [CrossRef] [PubMed]
- Miljkovic, I.; Zmuda, J.M. Epidemiology of myosteatosis. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Miljkovic, I.; Vella, C.A.; Allison, M. Computed Tomography-Derived Myosteatosis and Metabolic Disorders. Diabetes Metab. J. 2021, 45, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Gumucio, J.P.; Qasawa, A.H.; Ferrara, P.J.; Malik, A.N.; Funai, K.; McDonagh, B.; Mendias, C.L. Reduced mitochondrial lipid oxidation leads to fat accumulation in myosteatosis. FASEB J. 2019, 33, 7863–7881. [Google Scholar] [CrossRef]
- Kim, G.; Kim, J.H. Impact of Skeletal Muscle Mass on Metabolic Health. Endocrinol. Metab. 2020, 35, 32207258. [Google Scholar] [CrossRef]
- Izzo, A.; Massimino, E.; Riccardi, G.; Della Pepa, G. A Narrative Review on Sarcopenia in Type 2 Diabetes Mellitus: Prevalence and Associated Factors. Nutrients 2021, 13, 183. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Wang, J.F.; Yao, Q.; Jian, Q.F.; Luo, Z.P. Prevalence of sarcopenic obesity in patients with diabetes and adverse outcomes: A systematic review and meta-analysis. Clin. Nutr. ESPEN 2023, 58, 128–135. [Google Scholar] [CrossRef]
- Shi, Y.; Li, S.; Xie, X.; Feng, Y. Association between Metabolic Syndrome and Musculoskeletal Status: A Cross-Sectional Study of NHANES. Int. J. Endocrinol. 2024, 2024, 7330133. [Google Scholar] [CrossRef]
- Yoon, J.W.; Ha, Y.C.; Kim, K.M.; Moon, J.H.; Choi, S.H.; Lim, S.; Park, Y.J.; Lim, J.Y.; Kim, K.W.; Park, K.S.; et al. Hyperglycemia Is Associated with Impaired Muscle Quality in Older Men with Diabetes: The Korean Longitudinal Study on Health and Aging. Diabetes Metab. J. 2016, 40, 140–146. [Google Scholar] [CrossRef]
- Kalyani, R.R.; Metter, E.J.; Egan, J.; Golden, S.H.; Ferrucci, L. Hyperglycemia predicts persistently lower muscle strength with aging. Diabetes Care 2015, 38, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Mesinovic, J.; Zengin, A.; De Courten, B.; Ebeling, P.R.; Scott, D. Sarcopenia and type 2 diabetes mellitus: A bidirectional relationship. Diabetes Metab. Syndr. Obes. 2019, 12, 1057–1072. [Google Scholar] [CrossRef] [PubMed]
- Bali, T.; Chrysavgis, L.; Cholongitas, E. Metabolic-Associated Fatty Liver Disease and Sarcopenia. Endocrinol. Metab. Clin. North Am. 2023, 52, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Karakousis, N.D.; Chrysavgis, L.; Chatzigeorgiou, A.; Papatheodoridis, G.; Cholongitas, E. Frailty in metabolic syndrome, focusing on nonalcoholic fatty liver disease. Ann. Gastroenterol. 2022, 35, 234–242. [Google Scholar] [CrossRef]
- Murai, J.; Nishizawa, H.; Otsuka, A.; Fukuda, S.; Tanaka, Y.; Nagao, H.; Sakai, Y.; Suzuki, M.; Yokota, S.; Tada, H.; et al. Low muscle quality in Japanese type 2 diabetic patients with visceral fat accumulation. Cardiovasc. Diabetol. 2018, 17, 112. [Google Scholar] [CrossRef]
- Miljkovic, I.; Cauley, J.A.; Wang, P.Y.; Holton, K.F.; Lee, C.G.; Sheu, Y.; Barrett-Connor, E.; Hoffman, A.R.; Lewis, C.B.; Orwoll, E.S.; et al. Abdominal myosteatosis is independently associated with hyperinsulinemia and insulin resistance among older men without diabetes. Obesity 2013, 21, 2118–2125. [Google Scholar] [CrossRef]
- Kim, E.H.; Kim, H.K.; Lee, M.J.; Bae, S.J.; Kim, K.W.; Choe, J. Association between type 2 diabetes and skeletal muscle quality assessed by abdominal computed tomography scan. Diabetes Metab. Res. Rev. 2022, 38, e3513. [Google Scholar] [CrossRef]
- Wakamiya, T.; Fujimoto, T.; Endo, T.; Nishioka, S.; Yokoyama, N.; Yamashita, S.; Kikkawa, K.; Hyodo, Y.; Ishimura, T.; Kohjimoto, Y.; et al. Myosteatosis as a novel predictor of new-onset diabetes mellitus after kidney transplantation. Int. J. Urol. 2024, 31, 39–44. [Google Scholar] [CrossRef]
- Tian, S.; Xu, Y. Association of sarcopenic obesity with the risk of all-cause mortality: A meta-analysis of prospective cohort studies. Geriatr. Gerontol. Int. 2016, 16, 155–166. [Google Scholar] [CrossRef]
- Han, E.; Kim, M.K.; Lee, H.W.; Ryu, S.; Kim, H.S.; Jang, B.K.; Suh, Y. Myosteatosis predicts bariatric surgery response: A longitudinal study in patients with morbid obesity. J. Clin. Endocrinol. Metab. 2024, 110, e1385–e1394. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef]
- Henin, G.; Loumaye, A.; Leclercq, I.A.; Lanthier, N. Myosteatosis: Diagnosis, pathophysiology and consequences in metabolic dysfunction-associated steatotic liver disease. JHEP Rep. 2024, 6, 100963. [Google Scholar] [CrossRef] [PubMed]
- Kamiliou, A.; Lekakis, V.; Chrysavgis, L.; Cholongitas, E. Prevalence and impact on the outcome of myosteatosis in patients with cirrhosis: A systematic review and meta-analysis. Hepatol. Int. 2024, 18, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, J.; Sun, Q. The prevalence and effects of sarcopenia in patients with metabolic dysfunction-associated steatotic liver disease (MASLD): A systematic review and meta-analysis. Clin. Nutr. 2024, 43, 2005–2016. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.C.; Joo, S.K.; Koo, B.K.; Lin, H.C.; Lee, D.H.; Chang, M.S.; Park, J.H.; So, Y.H.; Kim, W. Myosteatosis, but not Sarcopenia, Predisposes NAFLD Subjects to Early Steatohepatitis and Fibrosis Progression. Clin. Gastroenterol. Hepatol. 2023, 21, 388–397.e310. [Google Scholar] [CrossRef]
- Kouvari, M.; Polyzos, S.A.; Chrysohoou, C.; Skoumas, J.; Pitsavos, C.S.; Panagiotakos, D.B.; Mantzoros, C.S. Skeletal muscle mass and abdominal obesity are independent predictors of hepatic steatosis and interact to predict ten-year cardiovascular disease incidence: Data from the ATTICA cohort study. Clin. Nutr. 2022, 41, 1281–1289. [Google Scholar] [CrossRef]
- Chrysavgis, L.; Adamantou, M.; Angelousi, A.; Cholongitas, E. The association of testosterone with sarcopenia and frailty in chronic liver disease. Eur. J. Clin. Investig. 2024, 54, e14108. [Google Scholar] [CrossRef]
- Jeromson, S.; Baranowski, B.; Akcan, M.; Waters, B.D.; Eisner, K.; Bellucci, A.; Trang, S.; Abolhassani, A.; Tello-Palencia, M.A.; Schweitzer, A.; et al. Semaglutide impacts skeletal muscle to a similar extent as caloric restriction in mice with diet-induced obesity. J. Physiol. 2025, 40982727. [Google Scholar] [CrossRef]
- Karasawa, T.; Choi, R.H.; Meza, C.A.; Rout, S.; Drummond, M.J.; Chaix, A.; Funai, K. Unexpected effects of semaglutide on skeletal muscle mass and force-generating capacity in mice. Cell Metab. 2025, 37, 1619–1620. [Google Scholar] [CrossRef]
- Nunn, E.; Jaiswal, N.; Gavin, M.; Uehara, K.; Stefkovich, M.; Drareni, K.; Calhoun, R.; Lee, M.; Holman, C.D.; Baur, J.A.; et al. Antibody blockade of activin type II receptors preserves skeletal muscle mass and enhances fat loss during GLP-1 receptor agonism. Mol. Metab. 2024, 80, 101880. [Google Scholar] [CrossRef]
- Xiang, J.; Qin, L.; Zhong, J.; Xia, N.; Liang, Y. GLP-1RA Liraglutide and Semaglutide Improves Obesity-Induced Muscle Atrophy via SIRT1 Pathway. Diabetes Metab. Syndr. Obes. 2023, 16, 2433–2446. [Google Scholar] [CrossRef] [PubMed]
- Sjoberg, K.A.; Holst, J.J.; Rattigan, S.; Richter, E.A.; Kiens, B. GLP-1 increases microvascular recruitment but not glucose uptake in human and rat skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E355–E362. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, C.R.; Blonde, G.D.; Nisi, A.V.; Bloomston, H.M.; Krubitski, B.; le Roux, C.W.; Spector, A.C. Chronic Semaglutide Treatment in Rats Leads to Daily Excessive Concentration-Dependent Sucrose Intake. J. Endocr. Soc. 2023, 7, bvad074. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Oh, S.; Kim, E.K. Glucagon-like peptide-1 analog liraglutide leads to multiple metabolic alterations in diet-induced obese mice. J. Biol. Chem. 2022, 298, 102682. [Google Scholar] [CrossRef]
- Old, V.J.; Davies, M.J.; Papamargaritis, D.; Choudhary, P.; Watson, E.L. The Effects of Glucagon-Like Peptide-1 Receptor Agonists on Mitochondrial Function Within Skeletal Muscle: A Systematic Review. J. Cachexia Sarcopenia Muscle 2025, 16, e13677. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, M.; Li, T.; Dong, N.; Yi, L.; Zhang, Q.; Mi, M. GLP-1 regulates exercise endurance and skeletal muscle remodeling via GLP-1R/AMPK pathway. Biochim. Biophys. Acta Mol. Cell. Res. 2022, 1869, 119300. [Google Scholar] [CrossRef]
- Gurjar, A.A.; Kushwaha, S.; Chattopadhyay, S.; Das, N.; Pal, S.; China, S.P.; Kumar, H.; Trivedi, A.K.; Guha, R.; Chattopadhyay, N.; et al. Long acting GLP-1 analog liraglutide ameliorates skeletal muscle atrophy in rodents. Metabolism 2020, 103, 154044. [Google Scholar] [CrossRef]
- Hong, Y.; Lee, J.H.; Jeong, K.W.; Choi, C.S.; Jun, H.S. Amelioration of muscle wasting by glucagon-like peptide-1 receptor agonist in muscle atrophy. J. Cachexia Sarcopenia Muscle 2019, 10, 903–918. [Google Scholar] [CrossRef]
- Schuelke, M.; Wagner, K.R.; Stolz, L.E.; Hübner, C.; Riebel, T.; Kömen, W.; Braun, T.; Tobin, J.F.; Lee, S.J. Myostatin mutation associated with gross muscle hypertrophy in a child. N. Engl. J. Med. 2004, 350, 2682–2688. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Coleman, L.A.; Miller, R.; Rooks, D.S.; Laurent, D.; Petricoul, O.; Praestgaard, J.; Swan, T.; Wade, T.; Perry, R.G.; et al. Effect of Bimagrumab vs Placebo on Body Fat Mass Among Adults With Type 2 Diabetes and Obesity: A Phase 2 Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e2033457. [Google Scholar] [CrossRef]
- Chrysavgis, L.G.; Kazanas, S.; Bafa, K.; Rozani, S.; Koloutsou, M.E.; Cholongitas, E. Glucagon-like Peptide 1, Glucose-Dependent Insulinotropic Polypeptide, and Glucagon Receptor Agonists in Metabolic Dysfunction-Associated Steatotic Liver Disease: Novel Medication in New Liver Disease Nomenclature. Int. J. Mol. Sci. 2024, 25, 3832. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, S.; Alruwaili, F.; Mavratsas, V.; Serna, M.K.; Murthy, V.L.; Raji, M. Glucagon-Like Peptide-1 Receptor Agonists in the Treatment of Idiopathic Inflammatory Myopathy: From Mechanisms of Action to Clinical Applications. Cureus 2023, 15, e51352. [Google Scholar] [CrossRef] [PubMed]
- Knerr, P.J.; Mowery, S.A.; Douros, J.D.; Premdjee, B.; Hjøllund, K.R.; He, Y.; Kruse Hansen, A.M.; Olsen, A.K.; Perez-Tilve, D.; DiMarchi, R.D.; et al. Next generation GLP-1/GIP/glucagon triple agonists normalize body weight in obese mice. Mol. Metab. 2022, 63, 101533. [Google Scholar] [CrossRef] [PubMed]
- Astrup, A.; Carraro, R.; Finer, N.; Harper, A.; Kunesova, M.; Lean, M.E.J.; Niskanen, L.; Rasmussen, M.F.; Rissanen, A.; Rössner, S.; et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analogue, liraglutide. Int. J. Obes. 2012, 36, 890, Erratum in Int. J. Obes. 2012, 36, 890; Erratum in Int. J. Obes. 2013, 37, 322. [Google Scholar] [CrossRef]
- Gibbons, C.; Blundell, J.; Tetens Hoff, S.; Dahl, K.; Bauer, R.; Baekdal, T. Effects of oral semaglutide on energy intake, food preference, appetite, control of eating and body weight in subjects with type 2 diabetes. Diabetes Obes. Metab. 2021, 23, 581–588. [Google Scholar] [CrossRef]
- Harder, H.; Nielsen, L.; Tu, D.T.; Astrup, A. The effect of liraglutide, a long-acting glucagon-like peptide 1 derivative, on glycemic control, body composition, and 24-h energy expenditure in patients with type 2 diabetes. Diabetes Care 2004, 27, 1915–1921. [Google Scholar] [CrossRef]
- Ghanim, H.; Batra, M.; Green, K.; Abuaysheh, S.; Hejna, J.; Makdissi, A.; Borowski, R.; Kuhadiya, N.D.; Chaudhuri, A.; Dandona, P. Liraglutide treatment in overweight and obese patients with type 1 diabetes: A 26-week randomized controlled trial; mechanisms of weight loss. Diabetes Obes. Metab. 2020, 22, 1742–1752. [Google Scholar] [CrossRef]
- Ishoy, P.L.; Fagerlund, B.; Broberg, B.V.; Bak, N.; Knop, F.K.; Glenthoj, B.Y.; Ebdrup, B.H. No cognitive-enhancing effect of GLP-1 receptor agonism in antipsychotic-treated, obese patients with schizophrenia. Acta Psychiatr. Scand. 2017, 136, 52–62. [Google Scholar] [CrossRef]
- Dube, M.C.; D’Amours, M.; Weisnagel, S.J. Beyond glycaemic control: A cross-over, double-blinded, 24-week intervention with liraglutide in type 1 diabetes. Diabetes Obes. Metab. 2018, 20, 178–184. [Google Scholar] [CrossRef]
- Mensberg, P.; Nyby, S.; Jorgensen, P.G.; Storgaard, H.; Jensen, M.T.; Sivertsen, J.; Holst, J.J.; Kiens, B.; Richter, E.A.; Knop, F.K.; et al. Near-normalization of glycaemic control with glucagon-like peptide-1 receptor agonist treatment combined with exercise in patients with type 2 diabetes. Diabetes Obes. Metab. 2017, 19, 172–180. [Google Scholar] [CrossRef]
- Silver, H.J.; Olson, D.; Mayfield, D.; Wright, P.; Nian, H.; Mashayekhi, M.; Koethe, J.R.; Niswender, K.D.; Luther, J.M.; Brown, N.J. Effect of the glucagon-like peptide-1 receptor agonist liraglutide, compared to caloric restriction, on appetite, dietary intake, body fat distribution and cardiometabolic biomarkers: A randomized trial in adults with obesity and prediabetes. Diabetes Obes. Metab. 2023, 25, 2340–2350. [Google Scholar] [CrossRef]
- van Eyk, H.J.; Paiman, E.H.M.; Bizino, M.B.; SL, I.J.; Kleiburg, F.; Boers, T.G.W.; Rappel, E.J.; Burakiewicz, J.; Kan, H.E.; Smit, J.W.A.; et al. Liraglutide decreases energy expenditure and does not affect the fat fraction of supraclavicular brown adipose tissue in patients with type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.H.; Bi, Y.; Li, P.; Yin, T.T.; Gao, C.X.; Shen, S.M.; Gao, L.J.; Yang, D.H.; Zhu, D.L. Effects of liraglutide, metformin and gliclazide on body composition in patients with both type 2 diabetes and non-alcoholic fatty liver disease: A randomized trial. J. Diabetes Investig. 2019, 10, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.T.; Bi, Y.; Li, P.; Shen, S.M.; Wang, W.M.; Jiang, C.; Gao, C.X.; Wang, Y.; Gao, L.J.; Zhu, D.L.; et al. Effects of exenatide versus insulin glargine on body composition in overweight and obese T2DM patients: A randomized controlled trial. Nutr. Metab. 2018, 15, 67. [Google Scholar] [CrossRef] [PubMed]
- Heise, T.; DeVries, J.H.; Urva, S.; Li, J.; Pratt, E.J.; Thomas, M.K.; Mather, K.J.; Karanikas, C.A.; Dunn, J.; Haupt, A.; et al. Tirzepatide Reduces Appetite, Energy Intake, and Fat Mass in People With Type 2 Diabetes. Diabetes Care 2023, 46, 998–1004. [Google Scholar] [CrossRef]
- Jendle, J.; Nauck, M.A.; Matthews, D.R.; Frid, A.; Hermansen, K.; During, M.; Zdravkovic, M.; Strauss, B.J.; Garber, A.J. LEAD-2 and LEAD-3 Study Groups. Weight loss with liraglutide, a once-daily human glucagon-like peptide-1 analogue for type 2 diabetes treatment as monotherapy or added to metformin, is primarily as a result of a reduction in fat tissue. Diabetes Obes. Metab. 2009, 11, 1163–1172. [Google Scholar] [CrossRef]
- Kadouh, H.; Chedid, V.; Halawi, H.; Burton, D.D.; Clark, M.M.; Khemani, D.; Vella, A.; Acosta, A.; Camilleri, M. GLP-1 Analog Modulates Appetite, Taste Preference, Gut Hormones, and Regional Body Fat Stores in Adults with Obesity. J. Clin. Endocrinol. Metab. 2020, 105, 1552–1563. [Google Scholar] [CrossRef]
- Lundgren, J.R.; Janus, C.; Jensen, S.B.K.; Juhl, C.R.; Olsen, L.M.; Christensen, R.M.; Svane, M.S.; Bandholm, T.; Bojsen-Moller, K.N.; Blond, M.B.; et al. Healthy Weight Loss Maintenance with Exercise, Liraglutide, or Both Combined. N. Engl. J. Med. 2021, 384, 1719–1730. [Google Scholar] [CrossRef]
- Grannell, A.; Martin, W.P.; Dehestani, B.; Al-Najim, W.; Murphy, J.C.; le Roux, C.W. Liraglutide Does Not Adversely Impact Fat-Free Mass Loss. Obesity 2021, 29, 529–534. [Google Scholar] [CrossRef]
- Blundell, J.; Finlayson, G.; Axelsen, M.; Flint, A.; Gibbons, C.; Kvist, T.; Hjerpsted, J.B. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes. Metab. 2017, 19, 1242–1251. [Google Scholar] [CrossRef]
- McCrimmon, R.J.; Catarig, A.M.; Frias, J.P.; Lausvig, N.L.; le Roux, C.W.; Thielke, D.; Lingvay, I. Effects of once-weekly semaglutide vs once-daily canagliflozin on body composition in type 2 diabetes: A substudy of the SUSTAIN 8 randomised controlled clinical trial. Diabetologia 2020, 63, 473–485. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Bunck, M.C.; Diamant, M.; Eliasson, B.; Corner, A.; Shaginian, R.M.; Heine, R.J.; Taskinen, M.R.; Yki-Jarvinen, H.; Smith, U. Exenatide affects circulating cardiovascular risk biomarkers independently of changes in body composition. Diabetes Care 2010, 33, 1734–1737. [Google Scholar] [CrossRef] [PubMed]
- Jastreboff, A.M.; Aronne, L.J.; Ahmad, N.N.; Wharton, S.; Connery, L.; Alves, B.; Kiyosue, A.; Zhang, S.; Liu, B.; Bunck, M.C.; et al. Tirzepatide Once Weekly for the Treatment of Obesity. N. Engl. J. Med. 2022, 387, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Avcilar, T. 32nd European Congress on Obesity (ECO 2025)—LATE BREAKING ABSTRACTS. Obes. Facts 2025, 18 (Suppl. 1), 657–829. [Google Scholar] [CrossRef]
- Sattar, N.; Neeland, I.J.; Dahlqvist Leinhard, O.; Fernandez Lando, L.; Bray, R.; Linge, J.; Rodriguez, A. Tirzepatide and muscle composition changes in people with type 2 diabetes (SURPASS-3 MRI): A post-hoc analysis of a randomised, open-label, parallel-group, phase 3 trial. Lancet Diabetes Endocrinol. 2025, 13, 482–493. [Google Scholar] [CrossRef]
- Available online: https://clinicaltrials.gov/study/NCT06445075 (accessed on 7 December 2025).
- Garvey, W.T.; Bluher, M.; Osorto Contreras, C.K.; Davies, M.J.; Winning Lehmann, E.; Pietilainen, K.H.; Rubino, D.; Sbraccia, P.; Wadden, T.; Zeuthen, N.; et al. Coadministered Cagrilintide and Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2025, 393, 635–647. [Google Scholar] [CrossRef]
- Ji, L.; Jiang, H.; Bi, Y.; Li, H.; Tian, J.; Liu, D.; Zhao, Y.; Qiu, W.; Huang, C.; Chen, L.; et al. Once-Weekly Mazdutide in Chinese Adults with Obesity or Overweight. N. Engl. J. Med. 2025, 392, 2215–2225. [Google Scholar] [CrossRef]
- Gastaldelli, A.; Cusi, K.; Fernandez Lando, L.; Bray, R.; Brouwers, B.; Rodriguez, A. Effect of tirzepatide versus insulin degludec on liver fat content and abdominal adipose tissue in people with type 2 diabetes (SURPASS-3 MRI): A substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial. Lancet Diabetes Endocrinol. 2022, 10, 393–406. [Google Scholar] [CrossRef]
- Pandey, A.; Patel, K.V.; Segar, M.W.; Ayers, C.; Linge, J.; Leinhard, O.D.; Anker, S.D.; Butler, J.; Verma, S.; Joshi, P.H.; et al. Effect of liraglutide on thigh muscle fat and muscle composition in adults with overweight or obesity: Results from a randomized clinical trial. J. Cachexia Sarcopenia Muscle 2024, 15, 1072–1083. [Google Scholar] [CrossRef]
- Cortes, T.M.; Vasquez, L.; Serra, M.C.; Robbins, R.; Stepanenko, A.; Brown, K.; Barrus, H.; Campos, A.; Espinoza, S.E.; Musi, N. Effect of Semaglutide on Physical Function, Body Composition, and Biomarkers of Aging in Older Adults With Overweight and Insulin Resistance: Protocol for an Open-Labeled Randomized Controlled Trial. JMIR Res. Protoc. 2024, 13, e62667. [Google Scholar] [CrossRef]
- Volpe, S.; Lisco, G.; Racaniello, D.; Fanelli, M.; Colaianni, V.; Vozza, A.; Triggiani, V.; Sabba, C.; Tortorella, C.; De Pergola, G.; et al. Once-Weekly Semaglutide Induces an Early Improvement in Body Composition in Patients with Type 2 Diabetes: A 26-Week Prospective Real-Life Study. Nutrients 2022, 14, 2414. [Google Scholar] [CrossRef]
- Alabadi, B.; Civera, M.; De la Rosa, A.; Martinez-Hervas, S.; Gomez-Cabrera, M.C.; Real, J.T. Low Muscle Mass Is Associated with Poorer Glycemic Control and Higher Oxidative Stress in Older Patients with Type 2 Diabetes. Nutrients 2023, 15, 3167. [Google Scholar] [CrossRef] [PubMed]
- Akoumianakis, I.; Zagaliotis, A.; Konstantaraki, M.; Filippatos, T.D. GLP-1 analogs and regional adiposity: A systematic review and meta-analysis. Obes. Rev. 2023, 24, e13574. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, H.; Phillips, B.; Smith, K.; Wilkinson, D.; Atherton, P.J.; Idris, I. Physiological mechanisms of action of incretin and insulin in regulating skeletal muscle metabolism. Curr. Diabetes. Rev. 2014, 10, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Bagherzadeh-Rahmani, B.; Marzetti, E.; Karami, E.; Campbell, B.I.; Fakourian, A.; Haghighi, A.H.; Mousavi, S.H.; Heinrich, K.M.; Brazzi, L.; Jung, F.; et al. Tirzepatide and exercise training in obesity. Clin. Hemorheol. Microcirc. 2024, 87, 465–480. [Google Scholar] [CrossRef]
- Rubino, D.M.; Greenway, F.L.; Khalid, U.; O’Neil, P.M.; Rosenstock, J.; Sorrig, R.; Wadden, T.A.; Wizert, A.; Garvey, W.T.; Investigators, S. Effect of Weekly Subcutaneous Semaglutide vs Daily Liraglutide on Body Weight in Adults With Overweight or Obesity Without Diabetes: The STEP 8 Randomized Clinical Trial. JAMA 2022, 327, 138–150. [Google Scholar] [CrossRef]
- Mietlicki-Baase, E.G.; Koch-Laskowski, K.; McGrath, L.E.; Krawczyk, J.; Pham, T.; Lhamo, R.; Reiner, D.J.; Hayes, M.R. Daily supplementation of dietary protein improves the metabolic effects of GLP-1-based pharmacotherapy in lean and obese rats. Physiol. Behav. 2017, 177, 122–128. [Google Scholar] [CrossRef]
- Lopes, T.; Hope, D.C.; Ramos-Pittol, J.M.; Curtis, A.; Shrewsbury, J.V.; Davies, I.; Zhou, Z.; Sardini, A.; Minnion, J.S.; Dormann, D.; et al. Dietary protein defends lean mass and maintains the metabolic benefits of glucagon receptor agonism in mice. Mol. Metab. 2024, 89, 102024. [Google Scholar] [CrossRef]
| Condition | Definition/Clinical Features | Key Mechanisms | Metabolic and Hepatic Consequences | Associated Biomarkers/Imaging Findings | Clinical Implications |
|---|---|---|---|---|---|
| Sarcopenia | Progressive loss of muscle mass, strength, and function; prevalent in T2D, obesity, and older adults | ↓ Insulin signalling (Akt/mTOR), mitochondrial dysfunction, ↑ ROS, ↑ TNF-α, IL-6, ↓ myokines (irisin, myostatin imbalance) | ↓ Glucose disposal, ↑ insulin resistance, lipid accumulation in liver and muscle | DXA or CT showing ↓ appendicular lean mass; ↓ grip strength or gait speed | ↑ Risk of MASLD progression, frailty, falls, and mortality |
| Sarcopenic Obesity | Coexistence of sarcopenia and excess adiposity; often central/visceral fat accumulation | Chronic low-grade inflammation, ↑ FFAs, ↑ leptin and resistin, ↓ adiponectin, mitochondrial stress | Synergistic impairment in glucose and lipid metabolism, ↑ oxidative stress, endothelial dysfunction | ↓ skeletal muscle-to-visceral fat ratio (SVR), ↑ fat-to-lean ratio | ↑ Risk of advanced fibrosis, cardiovascular disease, renal dysfunction, and mortality |
| Myosteatosis | Ectopic fat infiltration in muscle (inter- and intramyocellular) | Lipotoxicity, ceramide accumulation, mitochondrial ROS, impaired β-oxidation, hyperinsulinemia | Worsened insulin resistance, hepatic steatosis, and inflammation; precedes sarcopenia | CT or MRI: ↓ muscle attenuation (Hounsfield units); ultrasound fat fraction >5% | Early marker of MASH and fibrosis; predictive of poor post-surgical and long-term metabolic outcomes |
| Hormone/Receptor | Primary Source & Receptor Distribution | Metabolic & Cellular Mechanisms | Effects on Muscle Mass and Lean Tissue | Effects on Adipose Tissue and Body Composition |
|---|---|---|---|---|
| GLP-1 (Glucagon-like peptide-1) | Secreted by intestinal L-cells; GLP-1R expressed in pancreas, skeletal muscle, heart, adipose tissue, CNS |
|
|
|
| GIP (Glucose-dependent insulinotropic polypeptide) | Secreted by K-cells of duodenum and proximal jejunum; GIPR expressed in pancreatic β-cells, adipocytes, bone, and CNS |
|
|
|
| Glucagon (GCG) | Secreted by pancreatic α-cells; glucagon receptors (GCGR) expressed in liver, adipose tissue, and skeletal muscle |
|
|
|
| Dual/Tri-agonists (GLP-1/GIP ± GCG) | Engineered co-agonists acting at multiple receptors |
|
|
|
| Study, Year | Intervention | Duration (Weeks) | Indication | Sample Size | Lean Mass Change (kg) | Assessment Method | Active Comparator | Total Weight Loss (kg) | Fat Mass Change (kg) | Key Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| Astrup A, 2012 [105] | Liraglutide 1.2–3.0 mg | 20 | Obesity | 371 | −1.5 | DXA | Placebo | ~8.0 | ~6.5 | Lean mass loss ~18% of total; mostly fat loss |
| Gibbons C, 2021 [106] | Oral Semaglutide 14 mg | 12 | T2D | 15 | – | BIA | Placebo | Not reported | Not reported | Short trial; limited lean mass data |
| Harder H, 2004 [107] | Liraglutide 0.6 mg | 8 | T2D | 21 | −0.9 | DXA | Placebo | ~3.2 | ~2.3 | Modest weight loss; lean mass preserved relatively |
| Ghanim H, 2020 [108] | Liraglutide 1.8 mg | 26 | T1D | 37 | −1.1 | BIA | Placebo | ~5.0 | ~3.9 | Consistent lean/fat loss proportion |
| Neeland IJ, 2021 [6] | Liraglutide 3.0 mg | 49 | Obesity | 73 | −2.3 | DXA | Placebo | ~8.5 | ~6.2 | Long duration; lean loss ~27% of total |
| Ishøy PL, 2017 [109] | Exenatide 2.0 mg | 13 | Obesity | 20 | −0.8 | BIA | Placebo | ~2.7 | ~1.9 | Short-term loss; limited impact on lean mass |
| Dubé MC, 2018 [110] | Liraglutide 1.8 mg | 24 | T1D | 15 | −1.4 | DXA | Placebo | ~6.0 | ~4.6 | Liraglutide preserved LBM in T1D |
| Mensberg P, 2017 [111] | Liraglutide 0.6 mg | 16 | T2D | 17 | −0.7 | BIA | Placebo | ~3.5 | ~2.8 | Lean/fat mass loss ratio consistent |
| Silver HJ, 2023 [112] | Liraglutide 1.8 mg | 14 | Obesity/Prediabetes | 44 | −1.2 | DXA | Placebo | ~4.9 | ~3.7 | Lean loss minimized with support |
| van Eyk HJ, 2020 [113] | Liraglutide 1.8 mg | 26 | T2D | 22 | −1.0 | DXA | Placebo | ~4.6 | ~3.6 | Liraglutide modestly reduced lean mass |
| Feng WH, 2019 [114] | Liraglutide 1.8 mg | 24 | T2D/NAFLD | 29 | −1.3 | DXA | Placebo | ~6.0 | ~4.7 | NAFLD patients; body comp improved |
| Yin TT, 2018 [115] | Exenatide 10 μg | 16 | T2D | 19 | −0.9 | BIA | Placebo | ~4.2 | ~3.3 | Exenatide modest weight & lean mass loss |
| Heise T, 2023 [116] | Semaglutide 1 mg/Tirzepatide 15 mg | 28 | T2D | 44 | −1.9 | DXA | Semaglutide | ~7.3 | ~5.4 | Tirzepatide had higher loss than semaglutide |
| Jendle J, 2009 [117] | Liraglutide 0.6–1.8 mg | 26 | T2D | 95 | −1.0 | DXA | Placebo | ~4.5 | ~3.5 | All weight components reduced |
| Kadouh H, 2020 [118] | Liraglutide 3.0 mg | 16 | Obesity | 19 | −1.1 | DXA | Placebo | ~6.5 | ~5.4 | Lifestyle support attenuated lean loss |
| Lundgren JR, 2021 [119] | Liraglutide 3.0 mg | 48 | Obesity | 49 | −2.0 | DXA | Placebo | ~7.9 | ~6.1 | Longer duration = greater absolute LBM loss |
| Grannell A, 2021 [120] | Liraglutide 3.0 mg | 16 | Obesity | 59 | −1.8 | DXA | Placebo | ~7.5 | ~5.7 | Relative lean loss ~24% |
| Blundell J, 2017 [121] | Semaglutide 1.0 mg | 12 | Obesity | 28 | −1.3 | DXA | Placebo | ~5.6 | ~4.3 | Proportional lean/fat mass change |
| McCrimmon RJ, 2020 [122] | Semaglutide 1.0 mg | 52 | T2D | 88 | −1.7 | BIA | Placebo | ~10.0 | ~8.3 | Substantial weight/fat loss; lean preserved |
| Wilding JPH, 2021 [123] | Semaglutide 2.4 mg | 68 | Obesity | 95 | −3.4 | DXA | Placebo | ~13.3 | ~9.9 | Lean mass loss ~25% of total |
| Bunck MC, 2010 [124] | Exenatide 20 μg | 48 | T2D | 29 | −1.6 | DXA | Insulin glargine | ~4.0 | ~2.4 | Insulin glargine vs. Exenatide showed similar LBM |
| Jastreboff AM, 2022 [125] | Tirzepatide 5–15 mg | 72 | Obesity | 255 | −2.8 | DXA | Placebo | ~15.0 | ~12.2 | High % lean mass loss (~26%) |
| Peralta-Reich et al., 2025 [126] | Semaglutide or Tirzepatide + lifestyle | 24 | Obesity | 200 | ~−0.63 (F); ~−1.0 (M) | BIA | Self | ~11.0 | ~10.0 | Exercise/protein support preserved lean mass |
| Glasgow Univ., 2025 [127] | Tirzepatide | 52 | T2D | 246 | Preserved | MRI/CT | Insulin glargine | ~13.0 | ~13.0 | Intramuscular fat ↓; mass preserved |
| Scholar Rock, 2025 (NCT06445075) [128] | Tirzepatide ± Apitegromab | 24 | Obesity | — | −30% (tirz); +1.9 kg w/Apit | — | Tirzepatide | ~15.0 | ~15.0 | Apitegromab preserved 1.9 kg LBM |
| REDEFINE 1/2, 2025 [129] | CagriSema | 68 | Obesity | >3400 | Pending | — | Placebo | ~20.4 | Pending | CagriSema produced highest total WL |
| Mazdutide Phase III, 2025 [130] | Mazdutide | — | Obesity | — | Not reported | — | — | ~15.0 | Not reported | Awaiting lean mass outcomes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrysavgis, L.; Mourelatou, N.G.; Koloutsou, M.-E.; Rozani, S.; Cholongitas, E. The Influence of Glucagon-like Peptide-1 Receptor Agonists and Other Incretin Hormone Agonists on Body Composition. Int. J. Mol. Sci. 2025, 26, 12130. https://doi.org/10.3390/ijms262412130
Chrysavgis L, Mourelatou NG, Koloutsou M-E, Rozani S, Cholongitas E. The Influence of Glucagon-like Peptide-1 Receptor Agonists and Other Incretin Hormone Agonists on Body Composition. International Journal of Molecular Sciences. 2025; 26(24):12130. https://doi.org/10.3390/ijms262412130
Chicago/Turabian StyleChrysavgis, Lampros, Niki Gerasimoula Mourelatou, Maria-Evangelia Koloutsou, Sophia Rozani, and Evangelos Cholongitas. 2025. "The Influence of Glucagon-like Peptide-1 Receptor Agonists and Other Incretin Hormone Agonists on Body Composition" International Journal of Molecular Sciences 26, no. 24: 12130. https://doi.org/10.3390/ijms262412130
APA StyleChrysavgis, L., Mourelatou, N. G., Koloutsou, M.-E., Rozani, S., & Cholongitas, E. (2025). The Influence of Glucagon-like Peptide-1 Receptor Agonists and Other Incretin Hormone Agonists on Body Composition. International Journal of Molecular Sciences, 26(24), 12130. https://doi.org/10.3390/ijms262412130







