Deciphering the Role of Mast Cells in HPV-Related Cancers
Abstract
1. Introduction
2. Human Papillomavirus (HPV) Infection and Cancer Establishment
3. Mast Cell Biology and Its Role in HPV-Susceptible Tissues
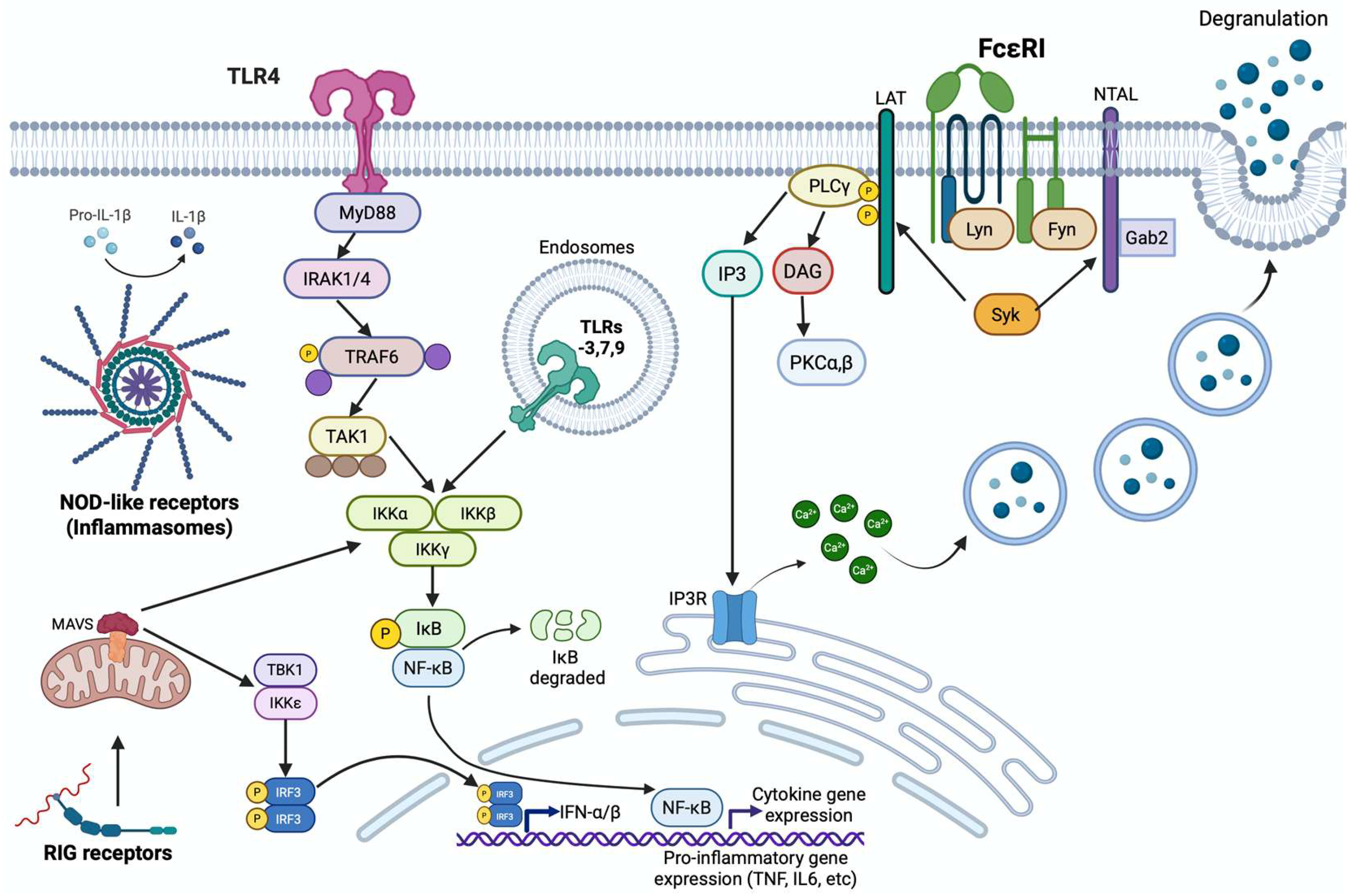
4. Types of Mast Cell Activation
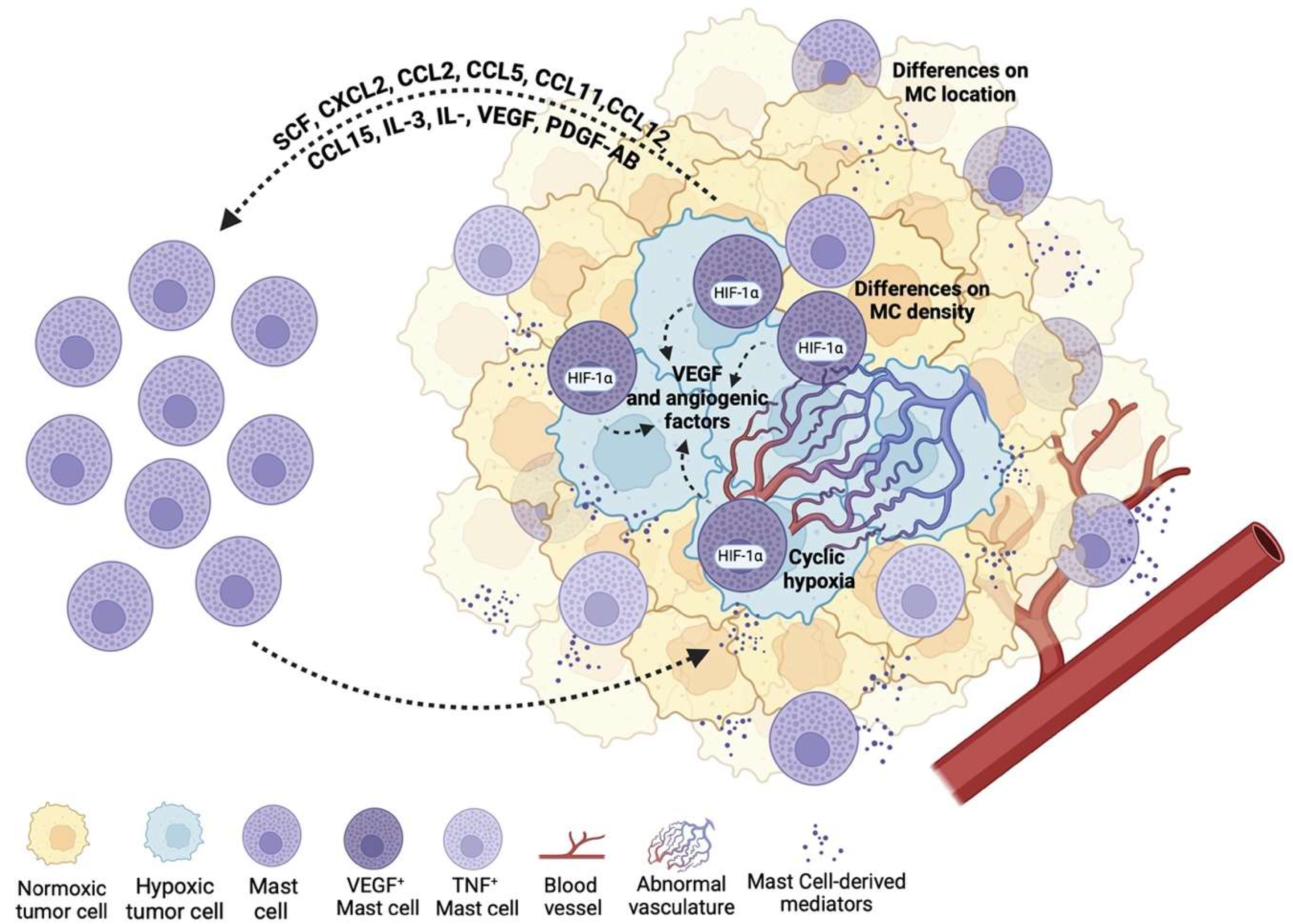
5. Mast Cells in the Tumor Microenvironment (TME)
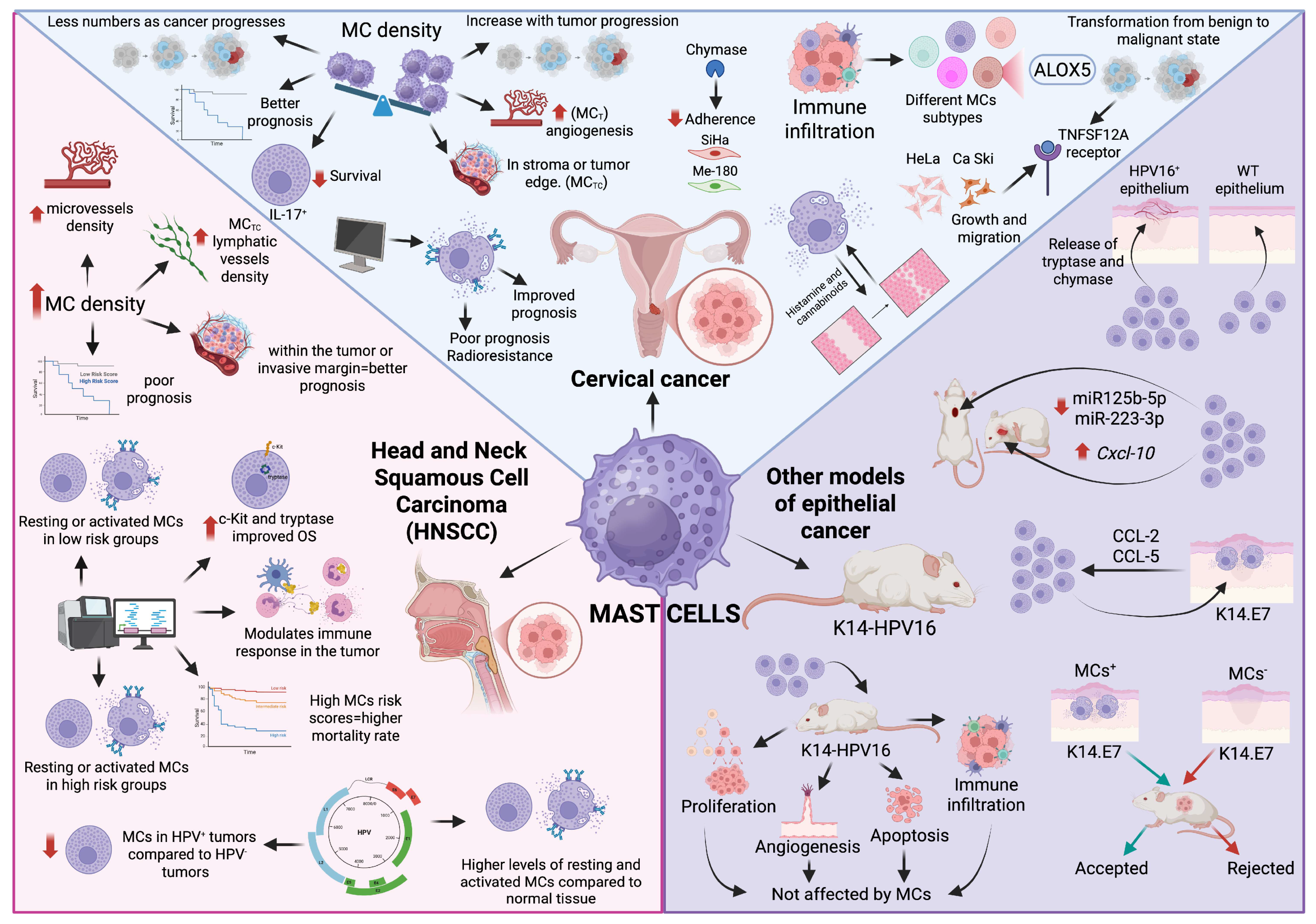
6. Mast Cells in HPV-Induced Cancer
6.1. Mast Cells in Cervical Cancer
6.2. Mast Cells in Head and Neck Squamous Cell Carcinoma
6.3. Mast Cells in Other Models of HPV-Induced Epithelial Cancer
7. Mast Cells as Potential Therapeutic Targets for HPV-Related Cancers
8. Perspectives and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TME | Tumor microenvironment |
| MCs | Mast cells |
| HPV | Human papilloma virus |
| LR-HPV | Low-risk Human papilloma virus |
| HR-HPV | High-risk Human papilloma virus |
| HNCs | Head and neck cancers |
| IAPs | Inhibitory of apoptosis proteins |
| pRb | Retinoblastoma tumor suppressor protein |
| EGFR | Epithelial growth factor receptor |
| EGF | Epithelial growth factor |
| CDK | Cyclin-dependent kinase |
| ISGs | Interferon stimulated genes |
| scRNA-seq | Single cell RNA sequencing |
| CTMCs | Connective tissue mast cells |
| MMCs | Mucosal mast cells |
| MCT | Tryptase positive mast cells |
| MCTC | Tryptase and chymase mast cells |
| ToMCs | Human tonsillar mast cells |
| TNF-α | Tumor necrosis factor alpha |
| VEGF | Vascular endothelial growth factor |
| FGF | Fibroblast growth factor |
| NGF | Nerve growth factor |
| GPCRSs | G protein-coupled receptors |
| PRRs | Pattern-recognition receptors |
| TLRs | Toll-like receptors |
| NLRs | Nucleotide-binding and oligomerization-like receptors |
| RLRs | RIG-I-like receptors |
| CLRs | C-type lectin receptors |
| STINGs | Stimulated interferon genes |
| TIMs | Tumor-infiltrating myeloid cells |
| SCF | Stem cell factor |
| TAMCs | Tumor-associated mast cells |
| BMMCs | Bone marrow derived mast cells |
| CIN1, 2, 3 | Cervical intraepithelial neoplasia 1, 2, or 3 |
| CIS | Carcinoma in situ |
| HNSCC | Head and neck squamous cell carcinoma |
| MCD | Mast cell density |
| MVD | Microvessels density |
| OSCC | Oral squamous cell carcinoma |
| TIME | Tumor immune microenvironment |
| TILs | Tumor infiltrating lymphocytes |
| DFS | Disease-free survival |
| ICB | Immune checkpoint blockade |
| PD-1 | Programmed cell death 1 |
| PD-L1 | Programmed cell death ligand 1 |
References
- Serrano, B.; Brotons, M.; Bosch, F.X.; Bruni, L. Epidemiology and Burden of HPV-Related Disease. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 47, 14–26. [Google Scholar] [CrossRef]
- Bzhalava, D.; Guan, P.; Franceschi, S.; Dillner, J.; Clifford, G. A Systematic Review of the Prevalence of Mucosal and Cutaneous Human Papillomavirus Types. Virology 2013, 445, 224–231. [Google Scholar] [CrossRef] [PubMed]
- de Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide Burden of Cancer Attributable to HPV by Site, Country and HPV Type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Kreimer, A.R.; Clifford, G.M.; Boyle, P.; Franceschi, S. Human Papillomavirus Types in Head and Neck Squamous Cell Carcinomas Worldwide: A Systematic Review. Cancer Epidemiol. Biomark. Prev. 2005, 14, 467–475. [Google Scholar] [CrossRef]
- Carlander, A.F.; Jakobsen, K.K.; Bendtsen, S.K.; Garset-Zamani, M.; Lynggaard, C.D.; Jensen, J.S.; Grønhøj, C.; Buchwald, C.V. A Contemporary Systematic Review on Repartition of HPV-Positivity in Oropharyngeal Cancer Worldwide. Viruses 2021, 13, 1326. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-I.; Francoeur, A.A.; Kapp, D.S.; Caesar, M.A.P.; Huh, W.K.; Chan, J.K. Trends in Human Papillomavirus-Associated Cancers, Demographic Characteristics, and Vaccinations in the US, 2001–2017. JAMA Netw. Open 2022, 5, e222530. [Google Scholar] [CrossRef]
- Li, H.-X.; Wang, S.-Q.; Lian, Z.-X.; Deng, S.-L.; Yu, K. Relationship between Tumor Infiltrating Immune Cells and Tumor Metastasis and Its Prognostic Value in Cancer. Cells 2023, 12, 64. [Google Scholar] [CrossRef]
- Vahidian, F.; Duijf, P.H.G.; Safarzadeh, E.; Derakhshani, A.; Baghbanzadeh, A.; Baradaran, B. Interactions between Cancer Stem Cells, Immune System and Some Environmental Components: Friends or Foes? Immunol. Lett. 2019, 208, 19–29. [Google Scholar] [CrossRef]
- Partlová, S.; Bouček, J.; Kloudová, K.; Lukešová, E.; Zábrodský, M.; Grega, M.; Fučíková, J.; Truxová, I.; Tachezy, R.; Špíšek, R.; et al. Distinct Patterns of Intratumoral Immune Cell Infiltrates in Patients with HPV-Associated Compared to Non-Virally Induced Head and Neck Squamous Cell Carcinoma. Oncoimmunology 2015, 4, e965570. [Google Scholar] [CrossRef]
- Gameiro, S.F.; Evans, A.M.; Mymryk, J.S. The Tumor Immune Microenvironments of HPV+ and HPV− Head and Neck Cancers. WIREs Mech. Dis. 2022, 14, e1539. [Google Scholar] [CrossRef]
- Zeng, P.Y.F.; Cecchini, M.J.; Barrett, J.W.; Shammas-Toma, M.; De Cecco, L.; Serafini, M.S.; Cavalieri, S.; Licitra, L.; Hoebers, F.; Brakenhoff, R.H.; et al. Immune-Based Classification of HPV-Associated Oropharyngeal Cancer with Implications for Biomarker-Driven Treatment de-Intensification. eBioMedicine 2022, 86, 104373. [Google Scholar] [CrossRef] [PubMed]
- Khazaie, K.; Blatner, N.R.; Khan, M.W.; Gounari, F.; Gounaris, E.; Dennis, K.; Bonertz, A.; Tsai, F.-N.; Strouch, M.J.; Cheon, E.; et al. The Significant Role of Mast Cells in Cancer. Cancer Metastasis Rev. 2011, 30, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Maciel, T.T.; Moura, I.C.; Hermine, O. The Role of Mast Cells in Cancers. F1000Prime Rep. 2015, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Dabiri, S.; Huntsman, D.; Makretsov, N.; Cheang, M.; Gilks, B.; Bajdik, C.; Gelmon, K.; Chia, S.; Hayes, M. The Presence of Stromal Mast Cells Identifies a Subset of Invasive Breast Cancers with a Favorable Prognosis. Mod. Pathol. 2004, 17, 690–695, Erratum in Mod Pathol. 2004, 17, 1025. PMID: 15044916.. [Google Scholar] [CrossRef]
- Rajput, A.B.; Turbin, D.A.; Cheang, M.C.U.; Voduc, D.K.; Leung, S.; Gelmon, K.A.; Gilks, C.B.; Huntsman, D.G. Stromal Mast Cells in Invasive Breast Cancer Are a Marker of Favourable Prognosis: A Study of 4,444 Cases. Breast Cancer Res. Treat. 2008, 107, 249–257. [Google Scholar] [CrossRef]
- Malfettone, A.; Silvestris, N.; Saponaro, C.; Ranieri, G.; Russo, A.; Caruso, S.; Popescu, O.; Simone, G.; Paradiso, A.; Mangia, A. High Density of Tryptase-Positive Mast Cells in Human Colorectal Cancer: A Poor Prognostic Factor Related to Protease-Activated Receptor 2 Expression. J. Cell. Mol. Med. 2013, 17, 1025–1037. [Google Scholar] [CrossRef]
- Guo, F.; Kong, W.-N.; Li, D.-W.; Zhao, G.; Wu, H.-L.; Anwar, M.; Shang, X.-Q.; Sun, Q.-N.; Ma, C.-L.; Ma, X.-M. Low Tumor Infiltrating Mast Cell Density Reveals Prognostic Benefit in Cervical Carcinoma. Technol. Cancer Res. Treat. 2022, 21, 15330338221106530. [Google Scholar] [CrossRef]
- Sabatini, M.E.; Chiocca, S. Human Papillomavirus as a Driver of Head and Neck Cancers. Br. J. Cancer 2020, 122, 306–314. [Google Scholar] [CrossRef]
- White, E.A. Manipulation of Epithelial Differentiation by HPV Oncoproteins. Viruses 2019, 11, 369. [Google Scholar] [CrossRef]
- Lo Cigno, I.; Calati, F.; Girone, C.; Catozzo, M.; Gariglio, M. High-Risk HPV Oncoproteins E6 and E7 and Their Interplay with the Innate Immune Response: Uncovering Mechanisms of Immune Evasion and Therapeutic Prospects. J. Med. Virol. 2024, 96, e29685. [Google Scholar] [CrossRef]
- Moody, C.A. Regulation of the Innate Immune Response during the Human Papillomavirus Life Cycle. Viruses 2022, 14, 1797. [Google Scholar] [CrossRef] [PubMed]
- Vink, M.A.; Bogaards, J.A.; van Kemenade, F.J.; de Melker, H.E.; Meijer, C.J.L.M.; Berkhof, J. Clinical Progression of High-Grade Cervical Intraepithelial Neoplasia: Estimating the Time to Preclinical Cervical Cancer From Doubly Censored National Registry Data. Am. J. Epidemiol. 2013, 178, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Morgan, I.M. The Functions of Papillomavirus E2 Proteins. Virology 2025, 603, 110387. [Google Scholar] [CrossRef] [PubMed]
- Porter, V.L.; Marra, M.A. The Drivers, Mechanisms, and Consequences of Genome Instability in HPV-Driven Cancers. Cancers 2022, 14, 4623. [Google Scholar] [CrossRef]
- Murakami, I.; Egawa, N.; Griffin, H.; Yin, W.; Kranjec, C.; Nakahara, T.; Kiyono, T.; Doorbar, J. Roles for E1-Independent Replication and E6-Mediated P53 Degradation during Low-Risk and High-Risk Human Papillomavirus Genome Maintenance. PLoS Pathog. 2019, 15, e1007755. [Google Scholar] [CrossRef]
- Zhang, Y.; Dakic, A.; Chen, R.; Dai, Y.; Schlegel, R.; Liu, X. Direct HPV E6/Myc Interactions Induce Histone Modifications, Pol II Phosphorylation, and HTERT Promoter Activation. Oncotarget 2017, 8, 96323. [Google Scholar] [CrossRef]
- Moody, C.A.; Laimins, L.A. Human Papillomavirus Oncoproteins: Pathways to Transformation. Nat. Rev. Cancer 2010, 10, 550–560. [Google Scholar] [CrossRef]
- Barrow-Laing, L.; Chen, W.; Roman, A. Low- and High-Risk Human Papillomavirus E7 Proteins Regulate P130 Differently. Virology 2010, 400, 233–239. [Google Scholar] [CrossRef]
- James, C.D.; Saini, S.; Sesay, F.; Ko, K.; Felthousen-Rusbasan, J.; Iness, A.N.; Nulton, T.; Windle, B.; Dozmorov, M.G.; Morgan, I.M.; et al. Restoring the DREAM Complex Inhibits the Proliferation of High-Risk HPV Positive Human Cells. Cancers 2021, 13, 489. [Google Scholar] [CrossRef]
- Shin, M.-K.; Balsitis, S.; Brake, T.; Lambert, P.F. Human Papillomavirus E7 Oncoprotein Overrides the Tumor Suppressor Activity of P21Cip1 in Cervical Carcinogenesis. Cancer Res. 2009, 69, 5656–5663. [Google Scholar] [CrossRef]
- Yan, X.; Shah, W.; Jing, L.; Chen, H.; Wang, Y. High-Risk Human Papillomavirus Type 18 E7 Caused P27 Elevation and Cytoplasmic Localization. Cancer Biol. Ther. 2010, 9, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Venuti, A.; Paolini, F.; Nasir, L.; Corteggio, A.; Roperto, S.; Campo, M.S.; Borzacchiello, G. Papillomavirus E5: The Smallest Oncoprotein with Many Functions. Mol. Cancer 2011, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Ilahi, N.E.; Bhatti, A. Impact of HPV E5 on Viral Life Cycle via EGFR Signaling. Microb. Pathog. 2020, 139, 103923. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, A.; Riemer, A.B. Immune Evasion Mechanisms of Human Papillomavirus: An Update. Int. J. Cancer 2018, 142, 224–229. [Google Scholar] [CrossRef]
- Kawase, K.; Taguchi, A.; Ishizaka, A.; Lin, J.; Ueno, T.; Yoshimoto, D.; Eguchi, S.; Mori, S.; Sone, K.; Mori, M.; et al. Allelic Loss of HLA Class I Facilitates Evasion from Immune Surveillance in Cervical Intraepithelial Neoplasia. HLA 2024, 103, e15509. [Google Scholar] [CrossRef]
- Miyauchi, S.; Kim, S.S.; Jones, R.N.; Zhang, L.; Guram, K.; Sharma, S.; Schoenberger, S.P.; Cohen, E.E.W.; Califano, J.A.; Sharabi, A.B. Human Papillomavirus E5 Suppresses Immunity via Inhibition of the Immunoproteasome and STING Pathway. Cell Rep. 2023, 42, 112508. [Google Scholar] [CrossRef]
- Barnard, P.; McMillan, N.A. The Human Papillomavirus E7 Oncoprotein Abrogates Signaling Mediated by Interferon-Alpha. Virology 1999, 259, 305–313. [Google Scholar] [CrossRef]
- Lau, L.; Gray, E.E.; Brunette, R.L.; Stetson, D.B. DNA Tumor Virus Oncogenes Antagonize the CGAS-STING DNA-Sensing Pathway. Science 2015, 350, 568–571. [Google Scholar] [CrossRef]
- Li, S.; Labrecque, S.; Gauzzi, M.C.; Cuddihy, A.R.; Wong, A.H.T.; Pellegrini, S.; Matlashewski, G.J.; Koromilas, A.E. The Human Papilloma Virus (HPV)-18 E6 Oncoprotein Physically Associates with Tyk2 and Impairs Jak-STAT Activation by Interferon-α. Oncogene 1999, 18, 5727–5737. [Google Scholar] [CrossRef]
- Hasan, U.A.; Zannetti, C.; Parroche, P.; Goutagny, N.; Malfroy, M.; Roblot, G.; Carreira, C.; Hussain, I.; Müller, M.; Taylor-Papadimitriou, J.; et al. The Human Papillomavirus Type 16 E7 Oncoprotein Induces a Transcriptional Repressor Complex on the Toll-like Receptor 9 Promoter. J. Exp. Med. 2013, 210, 1369–1387. [Google Scholar] [CrossRef]
- Pacini, L.; Savini, C.; Ghittoni, R.; Saidj, D.; Lamartine, J.; Hasan, U.A.; Accardi, R.; Tommasino, M. Downregulation of Toll-Like Receptor 9 Expression by Beta Human Papillomavirus 38 and Implications for Cell Cycle Control. J. Virol. 2015, 89, 11396–11405. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Akin, C.; Hartmann, K.; Nilsson, G.; Reiter, A.; Hermine, O.; Sotlar, K.; Sperr, W.R.; Escribano, L.; George, T.I.; et al. Mast Cells as a Unique Hematopoietic Lineage and Cell System: From Paul Ehrlich’s Visions to Precision Medicine Concepts. Theranostics 2020, 10, 10743–10768. [Google Scholar] [CrossRef] [PubMed]
- Gentek, R.; Ghigo, C.; Hoeffel, G.; Bulle, M.J.; Msallam, R.; Gautier, G.; Launay, P.; Chen, J.; Ginhoux, F.; Bajénoff, M. Hemogenic Endothelial Fate Mapping Reveals Dual Developmental Origin of Mast Cells. Immunity 2018, 1160–1171. [Google Scholar] [CrossRef] [PubMed]
- Suo, C.; Dann, E.; Goh, I.; Jardine, L.; Kleshchevnikov, V.; Park, J.-E.; Botting, R.A.; Stephenson, E.; Engelbert, J.; Tuong, Z.K.; et al. Mapping the Developing Human Immune System across Organs. bioRxiv 2022, 2022.01.17.476665. [Google Scholar] [CrossRef]
- Irani, A.A.; Schechter, N.M.; Craig, S.S.; DeBlois, G.; Schwartz, L.B. Two Types of Human Mast Cells That Have Distinct Neutral Protease Compositions. Proc. Natl. Acad. Sci. USA 1986, 83, 4464–4468. [Google Scholar] [CrossRef]
- Crow, J.; More, L.; Howe, S. The Mast Cells of the Human Uterus. APMIS 1988, 96, 921–926. [Google Scholar] [CrossRef]
- Li, T.; Wang, J.; Guo, X.; Yu, Q.; Ding, S.; Xu, X.; Peng, Y.; Zhu, L.; Zou, G.; Zhang, X. Possible Involvement of Crosstalk between Endometrial Cells and Mast Cells in the Development of Endometriosis via CCL8/CCR1. Biomed. Pharmacother. 2020, 129, 110476. [Google Scholar] [CrossRef]
- McCallion, A.; Nasirzadeh, Y.; Lingegowda, H.; Miller, J.E.; Khalaj, K.; Ahn, S.; Monsanto, S.P.; Bidarimath, M.; Sisnett, D.J.; Craig, A.W.; et al. Estrogen Mediates Inflammatory Role of Mast Cells in Endometriosis Pathophysiology. Front. Immunol. 2022, 13, 961599. [Google Scholar] [CrossRef]
- Menzies, F.M.; Shepherd, M.C.; Nibbs, R.J.; Nelson, S.M. The Role of Mast Cells and Their Mediators in Reproduction, Pregnancy and Labour. Hum. Reprod. Update 2011, 17, 383–396. [Google Scholar] [CrossRef]
- Woidacki, K.; Jensen, F.; Zenclussen, A.C. Mast Cells as Novel Mediators of Reproductive Processes. Front. Immunol. 2013, 4, 29. [Google Scholar] [CrossRef]
- Füreder, W.; Bankl, H.C.; Toth, J.; Walchshofer, S.; Sperr, W.; Agis, H.; Semper, H.; Sillaber, C.; Lechner, K.; Valent, P. Immunophenotypic and Functional Characterization of Human Tonsillar Mast Cells. J. Leukoc. Biol. 1997, 61, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Kadeh, H.; Derakhshanfar, G.; Saravani, S. Comparative Study of Mast Cell Count in Oral Reactive Lesions and Its Association with Inflammation. Turk. J. Pathol. 2016, 32, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Mogoantă, C.A.; Ion, D.A.; Budu, V.; Muţiu, G.; Salplahta, D.; Afrem, E. Evaluation of Microvascular Density in Inflammatory Lesions and Carcinoma of Palatine Tonsil. Rom. J. Morphol. Embryol. 2013, 54, 179–185. [Google Scholar] [PubMed]
- Brockmeyer, P.; Kling, A.; Schulz, X.; Perske, C.; Schliephake, H.; Hemmerlein, B. High Mast Cell Density Indicates a Longer Overall Survival in Oral Squamous Cell Carcinoma. Sci. Rep. 2017, 7, 14677. [Google Scholar] [CrossRef]
- Ishikawa, K.; Yagi-Nakanishi, S.; Nakanishi, Y.; Kondo, S.; Tsuji, A.; Endo, K.; Wakisaka, N.; Murono, S.; Yoshizaki, T. Expression of Interleukin-33 Is Correlated with Poor Prognosis of Patients with Squamous Cell Carcinoma of the Tongue. Auris Nasus Larynx 2014, 41, 552–557. [Google Scholar] [CrossRef]
- Molderings, G.J.; Afrin, L.B. A Survey of the Currently Known Mast Cell Mediators with Potential Relevance for Therapy of Mast Cell-Induced Symptoms. Naunyn. Schmiedebergs. Arch. Pharmacol. 2023, 396, 2881–2891. [Google Scholar] [CrossRef]
- Komi, D.E.A.; Redegeld, F.A. Role of Mast Cells in Shaping the Tumor Microenvironment. Clin. Rev. Allergy Immunol. 2020, 58, 313–325. [Google Scholar] [CrossRef]
- Shi, S.; Ye, L.; Yu, X.; Jin, K.; Wu, W. Focus on Mast Cells in the Tumor Microenvironment: Current Knowledge and Future Directions. Biochim. Biophys. Acta—Rev. Cancer 2023, 1878, 188845. [Google Scholar] [CrossRef]
- Morita, H.; Arae, K.; Unno, H.; Miyauchi, K.; Toyama, S.; Nambu, A.; Oboki, K.; Ohno, T.; Motomura, K.; Matsuda, A.; et al. An Interleukin-33-Mast Cell-Interleukin-2 Axis Suppresses Papain-Induced Allergic Inflammation by Promoting Regulatory T Cell Numbers. Immunity 2015, 43, 175–186. [Google Scholar] [CrossRef]
- Lv, Y.; Tian, W.; Teng, Y.; Wang, P.; Zhao, Y.; Li, Z.; Tang, S.; Chen, W.; Xie, R.; Lü, M.; et al. Tumor-Infiltrating Mast Cells Stimulate ICOS+ Regulatory T Cells through an IL-33 and IL-2 Axis to Promote Gastric Cancer Progression. J. Adv. Res. 2024, 57, 149–162. [Google Scholar] [CrossRef]
- Segura-Villalobos, D.; Ramírez-Moreno, I.G.; Martínez-Aguilar, M.; Ibarra-Sánchez, A.; Muñoz-Bello, J.O.; Anaya-Rubio, I.; Padilla, A.; Macías-Silva, M.; Lizano, M.; González-Espinosa, C. Mast Cell–Tumor Interactions: Molecular Mechanisms of Recruitment, Intratumoral Communication and Potential Therapeutic Targets for Tumor Growth. Cells 2022, 11, 349. [Google Scholar] [CrossRef] [PubMed]
- Ménasché, G.; Longé, C.; Bratti, M.; Blank, U. Cytoskeletal Transport, Reorganization, and Fusion Regulation in Mast Cell-Stimulus Secretion Coupling. Front. Cell Dev. Biol. 2021, 9, 652077. [Google Scholar] [CrossRef] [PubMed]
- Gangwar, R.S.; Landolina, N.; Arpinati, L.; Levi-Schaffer, F. Mast Cell and Eosinophil Surface Receptors as Targets for Anti-Allergic Therapy. Pharmacol. Ther. 2017, 170, 37–63. [Google Scholar] [CrossRef] [PubMed]
- Tauber, M.; Basso, L.; Martin, J.; Bostan, L.; Pinto, M.M.; Thierry, G.R.; Houmadi, R.; Serhan, N.; Loste, A.; Blériot, C.; et al. Landscape of Mast Cell Populations across Organs in Mice and Humans. J. Exp. Med. 2023, 220, e20230570, Erratum in J. Exp Med. 2024, 221, e2023057001172024c. https://doi.org/10.1084/jem.2023057001172024c. PMID: 37462672; PMCID: PMC10354537.. [Google Scholar] [CrossRef]
- St. John, A.L.; Abraham, S.N. Innate Immunity and Its Regulation by Mast Cells. J. Immunol. 2013, 190, 4458–4463. [Google Scholar] [CrossRef]
- Jiménez, M.; Cervantes-García, D.; Córdova-Dávalos, L.E.; Pérez-Rodríguez, M.J.; Gonzalez-Espinosa, C.; Salinas, E. Responses of Mast Cells to Pathogens: Beneficial and Detrimental Roles. Front. Immunol. 2021, 12, 685865. [Google Scholar] [CrossRef]
- Blank, U.; Huang, H.; Kawakami, T. The High Affinity IgE Receptor: A Signaling Update. Curr. Opin. Immunol. 2021, 72, 51–58. [Google Scholar] [CrossRef]
- Gomez, G.; Gonzalez-Espinosa, C.; Odom, S.; Baez, G.; Cid, M.E.; Ryan, J.J.; Rivera, J. Impaired FcεRI-Dependent Gene Expression and Defective Eicosanoid and Cytokine Production as a Consequence of Fyn Deficiency in Mast Cells. J. Immunol. 2005, 175, 7602–7610. [Google Scholar] [CrossRef]
- Klemm, S.; Gutermuth, J.; Hültner, L.; Sparwasser, T.; Behrendt, H.; Peschel, C.; Mak, T.W.; Jakob, T.; Ruland, J. The Bcl10–Malt1 Complex Segregates FcεRI-Mediated Nuclear Factor ΚB Activation and Cytokine Production from Mast Cell Degranulation. J. Exp. Med. 2006, 203, 337–347. [Google Scholar] [CrossRef]
- Sandig, H.; Bulfone-Paus, S. TLR Signaling in Mast Cells: Common and Unique Features. Front. Immunol. 2012, 3, 185. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern Recognition Receptors and Inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Sundaram, B.; Tweedell, R.E.; Prasanth Kumar, S.; Kanneganti, T.-D. The NLR Family of Innate Immune and Cell Death Sensors. Immunity 2024, 57, 674–699. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, M.; Kato, H.; Fujita, T. Physiological Functions of RIG-I-like Receptors. Immunity 2024, 57, 731–751. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Li, Z.; Gao, R.; Xing, B.; Gao, Y.; Yang, Y.; Qin, S.; Zhang, L.; Ouyang, H.; Du, P.; et al. A Pan-Cancer Single-Cell Transcriptional Atlas of Tumor Infiltrating Myeloid Cells. Cell 2021, 184, 792–809.e23. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Sun, M.; Yang, P.; Meng, X.; Liu, R. Role of Mast Cells Activation in the Tumor Immune Microenvironment and Immunotherapy of Cancers. Eur. J. Pharmacol. 2023, 960, 176103. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, J.; Wang, Z.; Yang, C.; Chen, W.; Jiang, J.; Zheng, Z.; Jia, F.; Zhang, Y.; Jiang, J.; et al. Multi-Omics Profiling Suggesting Intratumoral Mast Cells as Predictive Index of Breast Cancer Lung Metastasis. Front. Oncol. 2022, 11, 788778. [Google Scholar] [CrossRef]
- Mao, Y.; Feng, Q.; Zheng, P.; Yang, L.; Zhu, D.; Chang, W.; Ji, M.; He, G.; Xu, J. Low Tumor Infiltrating Mast Cell Density Confers Prognostic Benefit and Reflects Immunoactivation in Colorectal Cancer. Int. J. Cancer 2018, 143, 2271–2280. [Google Scholar] [CrossRef]
- Yin, H.; Wang, X.; Jin, N.; Ling, X.; Leng, X.; Wang, Y.; Ma, K.; Jiang, X.; Zhu, J.; Ma, J. Integrated Analysis of Immune Infiltration in Esophageal Carcinoma as Prognostic Biomarkers. Ann. Transl. Med. 2021, 9, 1697. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, Y.; Xu, L.; Zhang, J.; Xie, H.; Fu, H.; Zhou, Q.; Chang, Y.; Dai, B.; Xu, J. Tumor Stroma-Infiltrating Mast Cells Predict Prognosis and Adjuvant Chemotherapeutic Benefits in Patients with Muscle Invasive Bladder Cancer. Oncoimmunology 2018, 7, e1474317. [Google Scholar] [CrossRef]
- Hempel, H.A.; Cuka, N.S.; Kulac, I.; Barber, J.R.; Cornish, T.C.; Platz, E.A.; De Marzo, A.M.; Sfanos, K.S. Low Intratumoral Mast Cells Are Associated With a Higher Risk of Prostate Cancer Recurrence. Prostate 2017, 77, 412–424. [Google Scholar] [CrossRef]
- Hempel Sullivan, H.; Heaphy, C.M.; Kulac, I.; Cuka, N.; Lu, J.; Barber, J.R.; De Marzo, A.M.; Lotan, T.L.; Joshu, C.E.; Sfanos, K.S. High Extratumoral Mast Cell Counts Are Associated with a Higher Risk of Adverse Prostate Cancer Outcomes. Cancer Epidemiol. Biomark. Prev. 2020, 29, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.; Rudolfsson, S.; Hammarsten, P.; Halin, S.; Pietras, K.; Jones, J.; Stattin, P.; Egevad, L.; Granfors, T.; Wikström, P.; et al. Mast Cells Are Novel Independent Prognostic Markers in Prostate Cancer and Represent a Target for Therapy. Am. J. Pathol. 2010, 177, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Moreno, I.G.; Ibarra-Sánchez, A.; Castillo-Arellano, J.I.; Blank, U.; González-Espinosa, C. Mast Cells Localize in Hypoxic Zones of Tumors and Secrete CCL-2 under Hypoxia through Activation of L-Type Calcium Channels. J. Immunol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Maltby, S.; Khazaie, K.; McNagny, K.M. Mast Cells in Tumor Growth: Angiogenesis, Tissue Remodelling and Immune-Modulation. Biochim. Biophys. Acta—Rev. Cancer 2009, 1796, 19–26. [Google Scholar] [CrossRef]
- Lichterman, J.N.; Reddy, S.M. Mast Cells: A New Frontier for Cancer Immunotherapy. Cells 2021, 10, 1270. [Google Scholar] [CrossRef]
- Solimando, A.G.; Desantis, V.; Ribatti, D. Mast Cells and Interleukins. Int. J. Mol. Sci. 2022, 23, 14004. [Google Scholar] [CrossRef]
- Derakhshani, A.; Vahidian, F.; Alihasanzadeh, M.; Mokhtarzadeh, A.; Lotfi Nezhad, P.; Baradaran, B. Mast Cells: A Double-Edged Sword in Cancer. Immunol. Lett. 2019, 209, 28–35. [Google Scholar] [CrossRef]
- Kalyani, R.; Rajeshwari, G. Significance of Mast Cells in Non-Neoplastic and Neoplastic Lesions of Uterine Cervix. Biomed. Res. Ther. 2016, 3, 3. [Google Scholar] [CrossRef]
- Naik, R.; Pai, M.R.; Poornima Baliga, B.; Nayak, K.S.; Shankarnarayana; Dighe, P. Mast Cell Profile in Uterine Cervix. Indian J. Pathol. Microbiol. 2004, 47, 178–180. [Google Scholar]
- Punt, S.; Fleuren, G.J.; Kritikou, E.; Lubberts, E.; Trimbos, J.B.; Jordanova, E.S.; Gorter, A. Angels and Demons: Th17 Cells Represent a Beneficial Response, While Neutrophil IL-17 Is Associated with Poor Prognosis in Squamous Cervical Cancer. Oncoimmunology 2015, 4, e984539. [Google Scholar] [CrossRef]
- Benítez-Bribiesca, L.; Wong, A.; Utrera, D.; Castellanos, E. The Role of Mast Cell Tryptase in Neoangiogenesis of Premalignant and Malignant Lesions of the Uterine Cervix. J. Histochem. Cytochem. 2001, 49, 1061–1062. [Google Scholar] [CrossRef] [PubMed]
- Utrera-Barillas, D.; Castro-Manrreza, M.; Castellanos, E.; Gutiérrez-Rodríguez, M.; Arciniega-Ruíz de Esparza, O.; García-Cebada, J.; Velazquez, J.R.; Flores-Reséndiz, D.; Hernández-Hernández, D.; Benítez-Bribiesca, L. The Role of Macrophages and Mast Cells in Lymphangiogenesis and Angiogenesis in Cervical Carcinogenesis. Exp. Mol. Pathol. 2010, 89, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Dasgupta, S.; Mandal, P.; Chatterjee, S.; Chakraborty, D. Is There Any Role of Mast Cell Density and Microvessel Density in Cervical Squamous Cell Carcinoma? A Histologic Study with Special Reference to CD-34 Immunomarker Staining. Indian J. Med. Paediatr. Oncol. 2014, 35, 165. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas-Saez, A.; Schalper, J.A.; Nicovani, S.M.; Rudolph, M.I. Characterization of Mast Cells According to Their Content of Tryptase and Chymase in Normal and Neoplastic Human Uterine Cervix. Int. J. Gynecol. Cancer 2002, 12, 92–98. [Google Scholar] [CrossRef]
- Diaconu, N.-C.; Rummukainen, J.; Naukkarinen, A.; Mättö, M.; Harvima, R.J.; Pelkonen, J.; Harvima, I.T. Mast Cell Chymase Is Present in Uterine Cervical Carcinoma and It Detaches Viable and Growing Cervical Squamous Carcinoma Cells from Substratum in Vitro. Arch. Dermatol. Res. 2011, 303, 499–512. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Gao, A.; Wen, Q.; Sun, Y. The Prognostic Landscape of Tumor-Infiltrating Immune Cells in Cervical Cancer. Biomed. Pharmacother. 2019, 120, 109444. [Google Scholar] [CrossRef]
- Wu, Z.; Lin, Q.; Sheng, L.; Chen, W.; Liang, M.; Wu, D.; Ke, Y. A Novel Immune-Related Risk-Scoring System Associated with the Prognosis and Response of Cervical Cancer Patients Treated with Radiation Therapy. Front. Mol. Biosci. 2023, 10, 1297774. [Google Scholar] [CrossRef]
- Huang, B.; Zheng, J.; Chen, B.; Wu, M.; Xiao, L. Analysis of the Correlation between RFC4 Expression and Tumor Immune Microenvironment and Prognosis in Patients with Cervical Cancer. Front. Genet. 2025, 16, 1514383. [Google Scholar] [CrossRef]
- Wang, L.; Liu, H.; Feng, Y.; Liu, X.; Wang, Y.; Liu, Y.; Li, H.; Zhang, Y. Decoding the Immune Landscape: A Comprehensive Analysis of Immune-Associated Biomarkers in Cervical Carcinoma and Their Implications for Immunotherapy Strategies. Front. Genet. 2024, 15, 1340569. [Google Scholar] [CrossRef]
- Mo, X.; Wang, N.; He, Z.; Kang, W.; Wang, L.; Han, X.; Yang, L. The Sub-Molecular Characterization Identification for Cervical Cancer. Heliyon 2023, 9, e16873. [Google Scholar] [CrossRef]
- Zhao, F.; Hong, J.; Zhou, G.; Huang, T.; Lin, Z.; Zhang, Y.; Liang, L.; Tang, H. Elucidating the Role of Tumor-Associated ALOX5+ Mast Cells with Transformative Function in Cervical Cancer Progression via Single-Cell RNA Sequencing. Front. Immunol. 2024, 15, 1434450. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Gao, L.; Zhou, S.; Yan, C.; Zhang, X.; Lin, W.; Li, H.; Shen, Y.; Wang, X. Single-Cell RNA Sequencing Revealed Changes in the Tumor Microenvironment Induced by Radiotherapy for Cervical Cancer and the Molecular Mechanism of Mast Cells in Immunosuppression. Funct. Integr. Genom. 2025, 25, 63. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, M.I.; Boza, Y.; Yefi, R.; Luza, S.; Andrews, E.; Penissi, A.; Garrido, P.; Rojas, I.G. The Influence of Mast Cell Mediators on Migration of SW756 Cervical Carcinoma Cells. J. Pharmacol. Sci. 2008, 106, 208–218. [Google Scholar] [CrossRef]
- Muñoz-Bello, J.O.; Romero-Córdoba, S.L.; García-Chávez, J.N.; González-Espinosa, C.; Langley, E.; Lizano, M. Potential Transcript-Based Biomarkers Predicting Clinical Outcomes of HPV-Positive Head and Neck Squamous Cell Carcinoma Patients. Cells 2024, 13, 1107. [Google Scholar] [CrossRef]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and Neck Squamous Cell Carcinoma. Nat. Rev. Dis. Prim. 2020, 6, 92. [Google Scholar] [CrossRef]
- Laishram, D.; Rao, K.; Devi, H.S.U.; Priya, N.S.; Smitha, T.; Sheethal, H.S. Mast Cells and Angiogenesis in Malignant and Premalignant Oral Lesions: An Immunohistochemical Study. J. Oral Maxillofac. Pathol. 2017, 21, 229–238. [Google Scholar] [CrossRef]
- Kurihara-Shimomura, M.; Sasahira, T.; Shimomura, H.; Bosserhoff Katrin, A.; Kirita, T. Mast Cell Chymase Promotes Angiogenesis and Lymphangiogenesis Mediated by Activation of Melanoma Inhibitory Activity Gene Family Members in Oral Squamous Cell Carcinoma. Int. J. Oncol. 2020, 56, 1093–1100. [Google Scholar] [CrossRef]
- Tzorakoleftheraki, S.-E.; Koletsa, T. The Complex Role of Mast Cells in Head and Neck Squamous Cell Carcinoma: A Systematic Review. Medicina 2024, 60, 1173. [Google Scholar] [CrossRef]
- Newman, A.M.; Liu, C.L.; Green, M.R.; Gentles, A.J.; Feng, W.; Xu, Y.; Hoang, C.D.; Diehn, M.; Alizadeh, A.A. Robust Enumeration of Cell Subsets from Tissue Expression Profiles. Nat. Methods 2015, 12, 453–457. [Google Scholar] [CrossRef]
- Cai, Z.; Tang, B.; Chen, L.; Lei, W. Mast Cell Marker Gene Signature in Head and Neck Squamous Cell Carcinoma. BMC Cancer 2022, 22, 577. [Google Scholar] [CrossRef]
- Ding, Y.; Chu, L.; Cao, Q.; Lei, H.; Li, X.; Zhuang, Q. A Meta-Validated Immune Infiltration-Related Gene Model Predicts Prognosis and Immunotherapy Sensitivity in HNSCC. BMC Cancer 2023, 23, 45. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wu, F.; Huang, X.; Zhang, Z.; Liu, C.; Lin, Y.; Xu, Y.; Guo, H.; Hong, C. A Novel Mast Cell Marker Gene-Related Prognostic Signature to Predict Prognosis and Reveal the Immune Landscape in Head and Neck Squamous Cell Carcinoma. Front. Immunol. 2025, 16, 1538641. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Gong, X.; Xia, M.; Yu, F.; Wu, J.; Yu, C.; Li, J. The Aging-Related Prognostic Signature Reveals the Landscape of the Tumor Immune Microenvironment in Head and Neck Squamous Cell Carcinoma. Front. Oncol. 2022, 12, 857994. [Google Scholar] [CrossRef] [PubMed]
- Attramadal, C.G.; Kumar, S.; Gao, J.; Boysen, M.E.; Halstensen, T.S.; Bryne, M. Low Mast Cell Density Predicts Poor Prognosis in Oral Squamous Cell Carcinoma and Reduces Survival in Head and Neck Squamous Cell Carcinoma. Anticancer Res. 2016, 36, 5499–5506. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.-Q.; Chen, J.-H.; Feng, J.; Liao, Y.-Z.; Zou, Y.; Liu, R. Tumor-Infiltrating Immune Cells and Survival in Head and Neck Squamous Cell Carcinoma: A Retrospective Computational Study. Sci. Rep. 2024, 14, 6390. [Google Scholar] [CrossRef]
- Zhou, D.; Wang, J.; Wang, J.; Liu, X. Profiles of Immune Cell Infiltration and Immune-Related Genes in the Tumor Microenvironment of HNSCC with or without HPV Infection. Am. J. Transl. Res. 2021, 13, 2163–2180. [Google Scholar]
- Tosi, A.; Parisatto, B.; Menegaldo, A.; Spinato, G.; Guido, M.; Del Mistro, A.; Bussani, R.; Zanconati, F.; Tofanelli, M.; Tirelli, G.; et al. The Immune Microenvironment of HPV-Positive and HPV-Negative Oropharyngeal Squamous Cell Carcinoma: A Multiparametric Quantitative and Spatial Analysis Unveils a Rationale to Target Treatment-Naïve Tumors with Immune Checkpoint Inhibitors. J. Exp. Clin. Cancer Res. 2022, 41, 279. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Q.; Tao, Y.; Chen, J.; Yuan, Z.; Wang, P. Characterization of Immune Microenvironment in Patients with HPV-Positive and Negative Head and Neck Cancer. Sci. Data 2023, 10, 694. [Google Scholar] [CrossRef]
- Coussens, L.M.; Hanahan, D.; Arbeit, J.M. Genetic Predisposition and Parameters of Malignant Progression in K14-HPV16 Transgenic Mice. Am. J. Pathol. 1996, 149, 1899–1917. [Google Scholar]
- Coussens, L.M.; Raymond, W.W.; Bergers, G.; Laig-Webster, M.; Behrendtsen, O.; Werb, Z.; Caughey, G.H.; Hanahan, D. Inflammatory Mast Cells Up-Regulate Angiogenesis during Squamous Epithelial Carcinogenesis. Genes Dev. 1999, 13, 1382–1397. [Google Scholar] [CrossRef]
- Bergot, A.-S.; Ford, N.; Leggatt, G.R.; Wells, J.W.; Frazer, I.H.; Grimbaldeston, M.A. HPV16-E7 Expression in Squamous Epithelium Creates a Local Immune Suppressive Environment via CCL2- and CCL5- Mediated Recruitment of Mast Cells. PLoS Pathog. 2014, 10, e1004466. [Google Scholar] [CrossRef] [PubMed]
- Ghouse, S.M.; Polikarpova, A.; Muhandes, L.; Dudeck, J.; Tantcheva-Poór, I.; Hartmann, K.; Lesche, M.; Dahl, A.; Eming, S.; Müller, W.; et al. Although Abundant in Tumor Tissue, Mast Cells Have No Effect on Immunological Micro-Milieu or Growth of HPV-Induced or Transplanted Tumors. Cell Rep. 2018, 22, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.C.; Santos, J.M.O.; Medeiros-Fonseca, B.; Oliveira, P.A.; Bastos, M.M.S.M.; Brito, H.O.; Gil da Costa, R.M.; Medeiros, R. Characterizing the Inflammatory Microenvironment in K14-HPV16 Transgenic Mice: Mast Cell Infiltration and MicroRNA Expression. Cancers 2022, 14, 2216. [Google Scholar] [CrossRef] [PubMed]
- Lyons, J.J.; Metcalfe, D.D. Targeting Mast Cells with Biologics. Immunol. Allergy Clin. N. Am. 2020, 40, 667–685. [Google Scholar] [CrossRef]
- Ribatti, D. New Insights into the Role of Mast Cells as a Therapeutic Target in Cancer through the Blockade of Immune Checkpoint Inhibitors. Front. Med. 2024, 11, 1373230. [Google Scholar] [CrossRef]
- Siebenhaar, F.; Metz, M.; Maurer, M. Mast Cells Protect from Skin Tumor Development and Limit Tumor Growth during Cutaneous de Novo Carcinogenesis in a Kit-Dependent Mouse Model. Exp. Dermatol. 2014, 23, 159–164. [Google Scholar] [CrossRef]
- Bodduluri, S.R.; Mathis, S.; Maturu, P.; Krishnan, E.; Satpathy, S.R.; Chilton, P.M.; Mitchell, T.C.; Lira, S.; Locati, M.; Mantovani, A.; et al. Mast Cell–Dependent CD8+ T-Cell Recruitment Mediates Immune Surveillance of Intestinal Tumors in ApcMin/+ Mice. Cancer Immunol. Res. 2018, 6, 332–347. [Google Scholar] [CrossRef]
- Shibata, T.; Lieblong, B.J.; Sasagawa, T.; Nakagawa, M. The Promise of Combining Cancer Vaccine and Checkpoint Blockade for Treating HPV-Related Cancer. Cancer Treat. Rev. 2019, 78, 8–16. [Google Scholar] [CrossRef]
- Han, X.; Chang, W.; Xia, X. Immune Checkpoint Inhibitors in Advanced and Recurrent/Metastatic Cervical Cancer. Front. Oncol. 2022, 12, 996495. [Google Scholar] [CrossRef]
- Cao, K.; Zhang, G.; Zhang, X.; Yang, M.; Wang, Y.; He, M.; Lu, J.; Liu, H. Stromal Infiltrating Mast Cells Identify Immunoevasive Subtype High-Grade Serous Ovarian Cancer with Poor Prognosis and Inferior Immunotherapeutic Response. Oncoimmunology 2021, 10, 1969075. [Google Scholar] [CrossRef]
- Somasundaram, R.; Connelly, T.; Choi, R.; Choi, H.; Samarkina, A.; Li, L.; Gregorio, E.; Chen, Y.; Thakur, R.; Abdel-Mohsen, M.; et al. Tumor-Infiltrating Mast Cells Are Associated with Resistance to Anti-PD-1 Therapy. Nat. Commun. 2021, 12, 346. [Google Scholar] [CrossRef]
- Li, J.; Peng, G.; Zhu, K.; Jie, X.; Xu, Y.; Rao, X.; Xu, Y.; Chen, Y.; Xing, B.; Wu, G.; et al. PD-1+ Mast Cell Enhanced by PD-1 Blocking Therapy Associated with Resistance to Immunotherapy. Cancer Immunol. Immunother. 2023, 72, 633–645. [Google Scholar] [CrossRef]
- Panagi, M.; Mpekris, F.; Voutouri, C.; Hadjigeorgiou, A.G.; Symeonidou, C.; Porfyriou, E.; Michael, C.; Stylianou, A.; Martin, J.D.; Cabral, H.; et al. Stabilizing Tumor-Resident Mast Cells Restores T-Cell Infiltration and Sensitizes Sarcomas to PD-L1 Inhibition. Clin. Cancer Res. 2024, 30, 2582–2597. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Zhao, Y.; Wang, X.; Chen, N.; Mao, F.; Teng, Y.; Wang, T.; Peng, L.; Zhang, J.; Cheng, P.; et al. Increased Intratumoral Mast Cells Foster Immune Suppression and Gastric Cancer Progression through TNF-α-PD-L1 Pathway. J. Immunother. Cancer 2019, 7, 54, Erratum in J. Immunother. Cancer 2020, 8, e0530-3corr1. https://doi.org/10.1136/jitc-2020-0530-3corr1. PMID: 30808413; PMCID: PMC6390584.. [Google Scholar] [CrossRef] [PubMed]
- Soucek, L.; Lawlor, E.R.; Soto, D.; Shchors, K.; Swigart, L.B.; Evan, G.I. Mast Cells Are Required for Angiogenesis and Macroscopic Expansion of Myc-Induced Pancreatic Islet Tumors. Nat. Med. 2007, 13, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Melillo, R.M.; Guarino, V.; Avilla, E.; Galdiero, M.R.; Liotti, F.; Prevete, N.; Rossi, F.W.; Basolo, F.; Ugolini, C.; de Paulis, A.; et al. Mast Cells Have a Protumorigenic Role in Human Thyroid Cancer. Oncogene 2010, 29, 6203–6215. [Google Scholar] [CrossRef]
- Eissmann, M.F.; Dijkstra, C.; Jarnicki, A.; Phesse, T.; Brunnberg, J.; Poh, A.R.; Etemadi, N.; Tsantikos, E.; Thiem, S.; Huntington, N.D.; et al. IL-33-Mediated Mast Cell Activation Promotes Gastric Cancer through Macrophage Mobilization. Nat. Commun. 2019, 10, 2735. [Google Scholar] [CrossRef]
- Aliabadi, A.; Haghshenas, M.R.; Kiani, R.; Panjehshahin, M.R.; Erfani, N. Promising Anticancer Activity of Cromolyn in Colon Cancer: In Vitro and in Vivo Analysis. J. Cancer Res. Clin. Oncol. 2024, 150, 207. [Google Scholar] [CrossRef]
- Jeong, H.J.; Oh, H.A.; Nam, S.Y.; Han, N.R.; Kim, Y.S.; Kim, J.H.; Lee, S.J.; Kim, M.H.; Moon, P.D.; Kim, H.M.; et al. The Critical Role of Mast Cell-Derived Hypoxia-Inducible Factor-1α in Human and Mice Melanoma Growth. Int. J. Cancer 2013, 132, 2492–2501. [Google Scholar] [CrossRef]
- Chen, S.; Luster, A.D. Antihistamines for Cancer Immunotherapy: More than Just Treating Allergies. Cancer Cell 2022, 40, 9–11. [Google Scholar] [CrossRef]
- Ammendola, M.; Leporini, C.; Marech, I.; Gadaleta, C.D.; Scognamillo, G.; Sacco, R.; Sammarco, G.; De Sarro, G.; Russo, E.; Ranieri, G. Targeting Mast Cells Tryptase in Tumor Microenvironment: A Potential Antiangiogenetic Strategy. BioMed Res. Int. 2014, 2014, 154702. [Google Scholar] [CrossRef]
- Oldford, S.A.; Haidl, I.D.; Howatt, M.A.; Leiva, C.A.; Johnston, B.; Marshall, J.S. A Critical Role for Mast Cells and Mast Cell-Derived IL-6 in TLR2-Mediated Inhibition of Tumor Growth. J. Immunol. 2010, 185, 7067–7076. [Google Scholar] [CrossRef]
- Drobits, B.; Holcmann, M.; Amberg, N.; Swiecki, M.; Grundtner, R.; Hammer, M.; Colonna, M.; Sibilia, M. Imiquimod Clears Tumors in Mice Independent of Adaptive Immunity by Converting PDCs into Tumor-Killing Effector Cells. J. Clin. Investig. 2012, 122, 575–585. [Google Scholar] [CrossRef]
- Lyng, H.; Malinen, E. Hypoxia in Cervical Cancer: From Biology to Imaging. Clin. Transl. Imaging 2017, 5, 373–388. [Google Scholar] [CrossRef]
- Li, J.Z.; Gao, W.; Chan, J.Y.-W.; Ho, W.-K.; Wong, T.-S. Hypoxia in Head and Neck Squamous Cell Carcinoma. Int. Sch. Res. Not. 2012, 2012, 708974. [Google Scholar] [CrossRef]
- Ruiz, F.J.; Inkman, M.; Rashmi, R.; Muhammad, N.; Gabriel, N.; Miller, C.A.; McLellan, M.D.; Goldstein, M.; Markovina, S.; Grigsby, P.W.; et al. HPV Transcript Expression Affects Cervical Cancer Response to Chemoradiation. JCI Insight 2021, 6, 138734. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinosa-Riquer, Z.P.; Muñoz-Bello, J.O.; González-Espinosa, C.; Ibarra-Sánchez, A.; Lizano, M. Deciphering the Role of Mast Cells in HPV-Related Cancers. Int. J. Mol. Sci. 2025, 26, 12110. https://doi.org/10.3390/ijms262412110
Espinosa-Riquer ZP, Muñoz-Bello JO, González-Espinosa C, Ibarra-Sánchez A, Lizano M. Deciphering the Role of Mast Cells in HPV-Related Cancers. International Journal of Molecular Sciences. 2025; 26(24):12110. https://doi.org/10.3390/ijms262412110
Chicago/Turabian StyleEspinosa-Riquer, Zyanya P., J. Omar Muñoz-Bello, Claudia González-Espinosa, Alfredo Ibarra-Sánchez, and Marcela Lizano. 2025. "Deciphering the Role of Mast Cells in HPV-Related Cancers" International Journal of Molecular Sciences 26, no. 24: 12110. https://doi.org/10.3390/ijms262412110
APA StyleEspinosa-Riquer, Z. P., Muñoz-Bello, J. O., González-Espinosa, C., Ibarra-Sánchez, A., & Lizano, M. (2025). Deciphering the Role of Mast Cells in HPV-Related Cancers. International Journal of Molecular Sciences, 26(24), 12110. https://doi.org/10.3390/ijms262412110






