Elacridar Reverses P-gp-Mediated Drug Resistance in Ovarian Cancer Cells in 2D and 3D Culture Models
Abstract
1. Introduction
2. Results
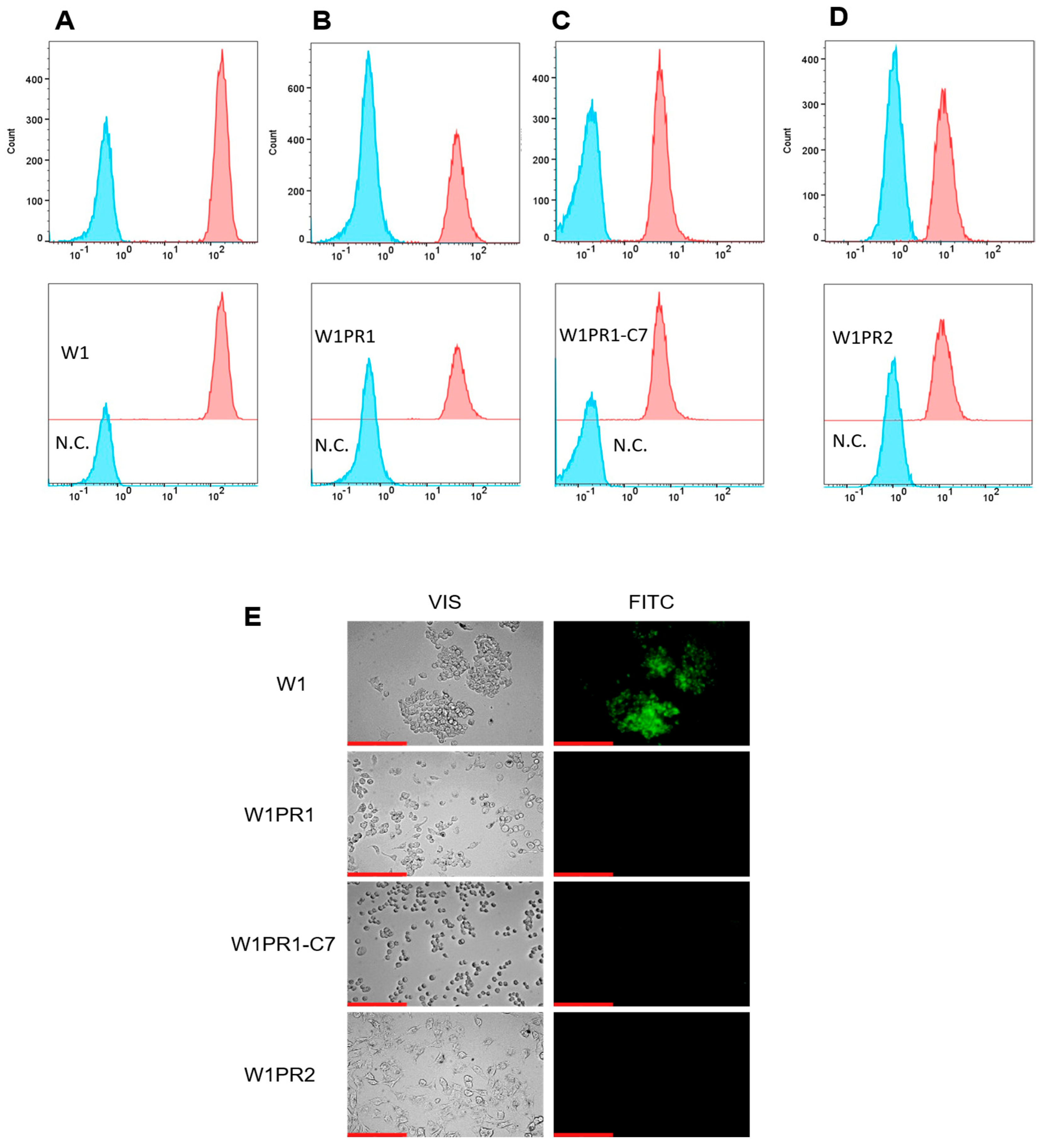
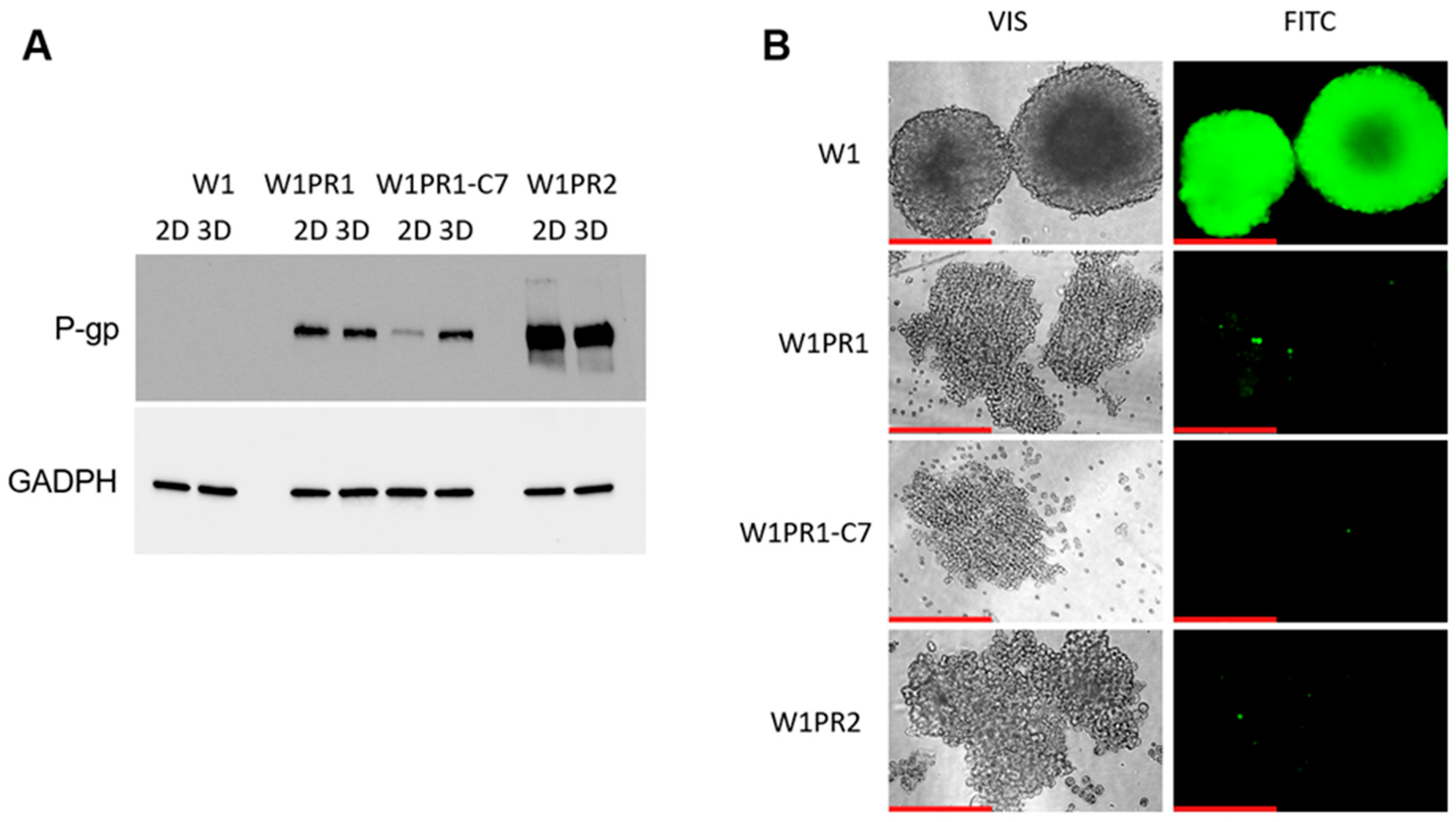
2.1. Characterization of MDR1 Gene and P-gp Protein Expression in W1 Cell Line and PAC-Resistant Cell Lines
2.2. Analysis of P-gp Activity
2.3. Characterization of Drug Resistance in the W1 Cell Line and PAC-Resistant Cell Lines
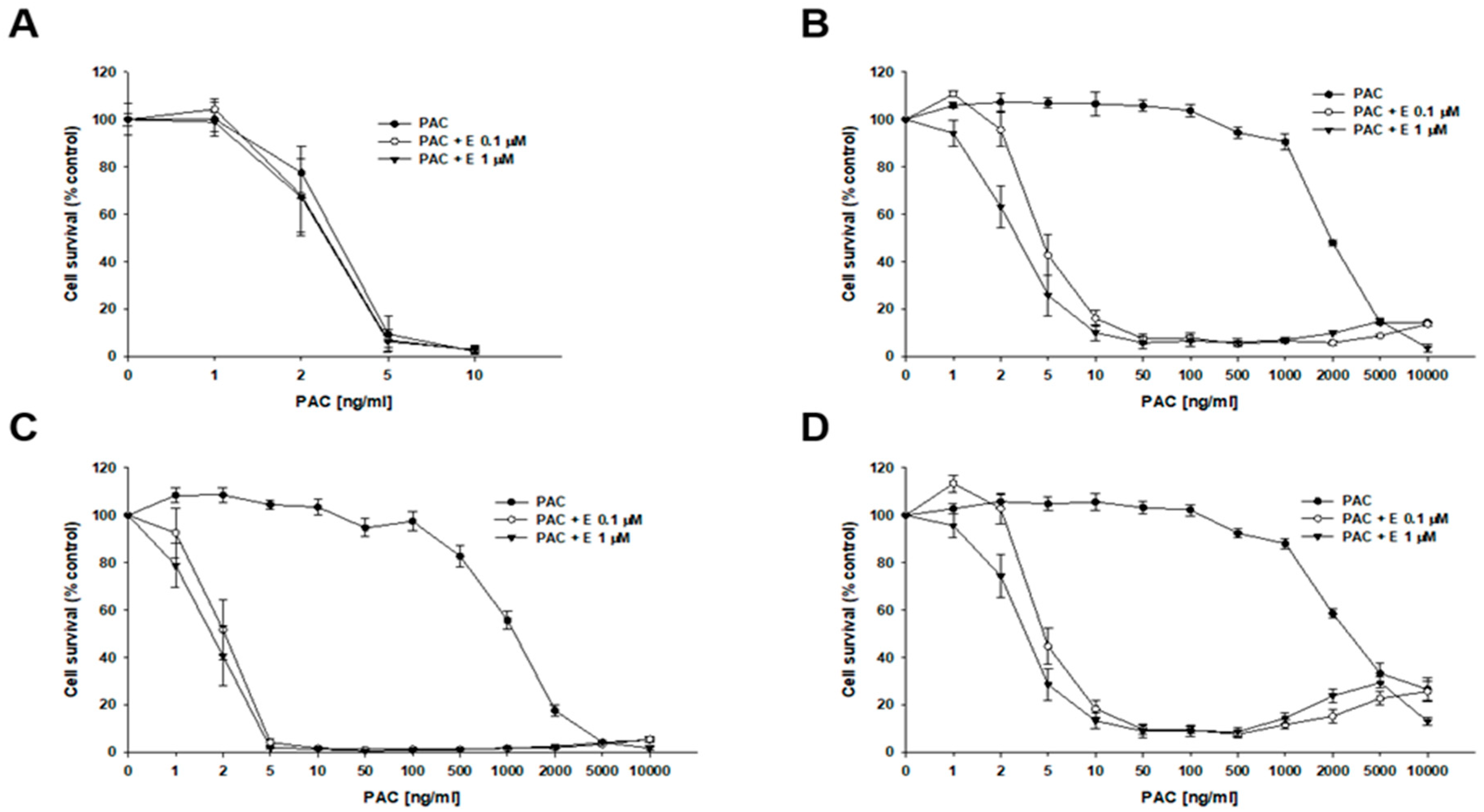
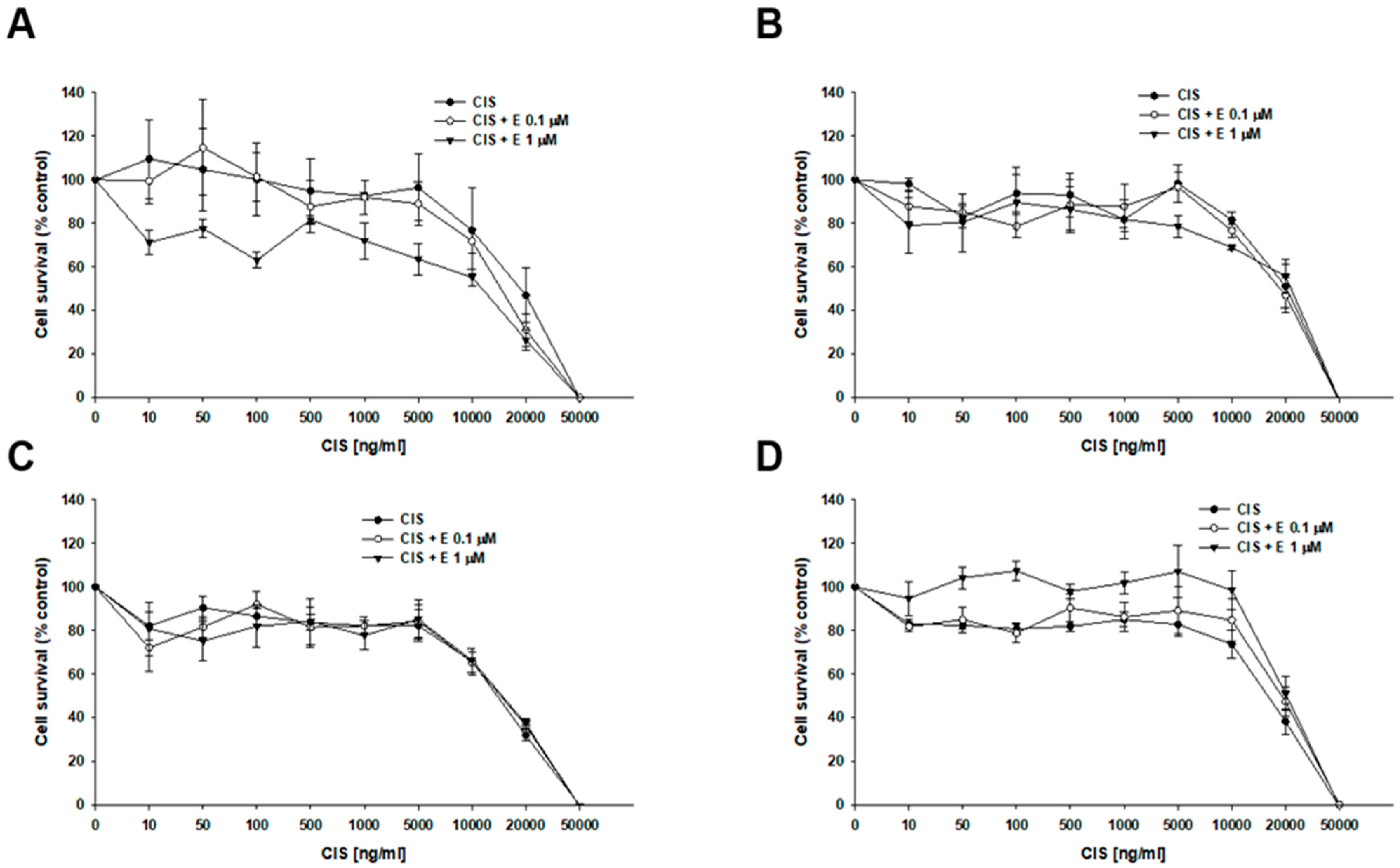
2.4. MTT Analyses of Elacridar Effect on Resistance to Cytotoxic Drugs
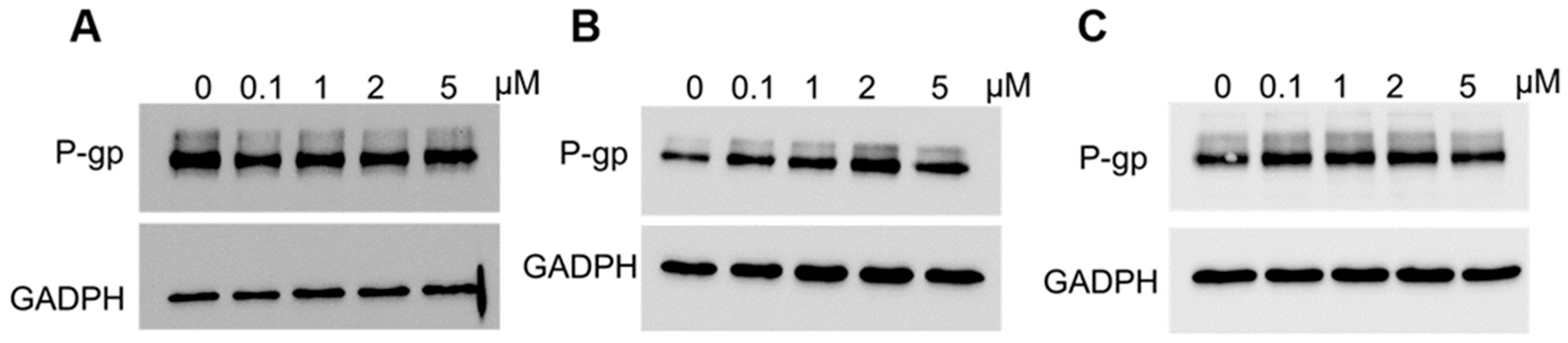
2.5. Analysis of P-gp Expression in Elacridar Treated Cell Lines
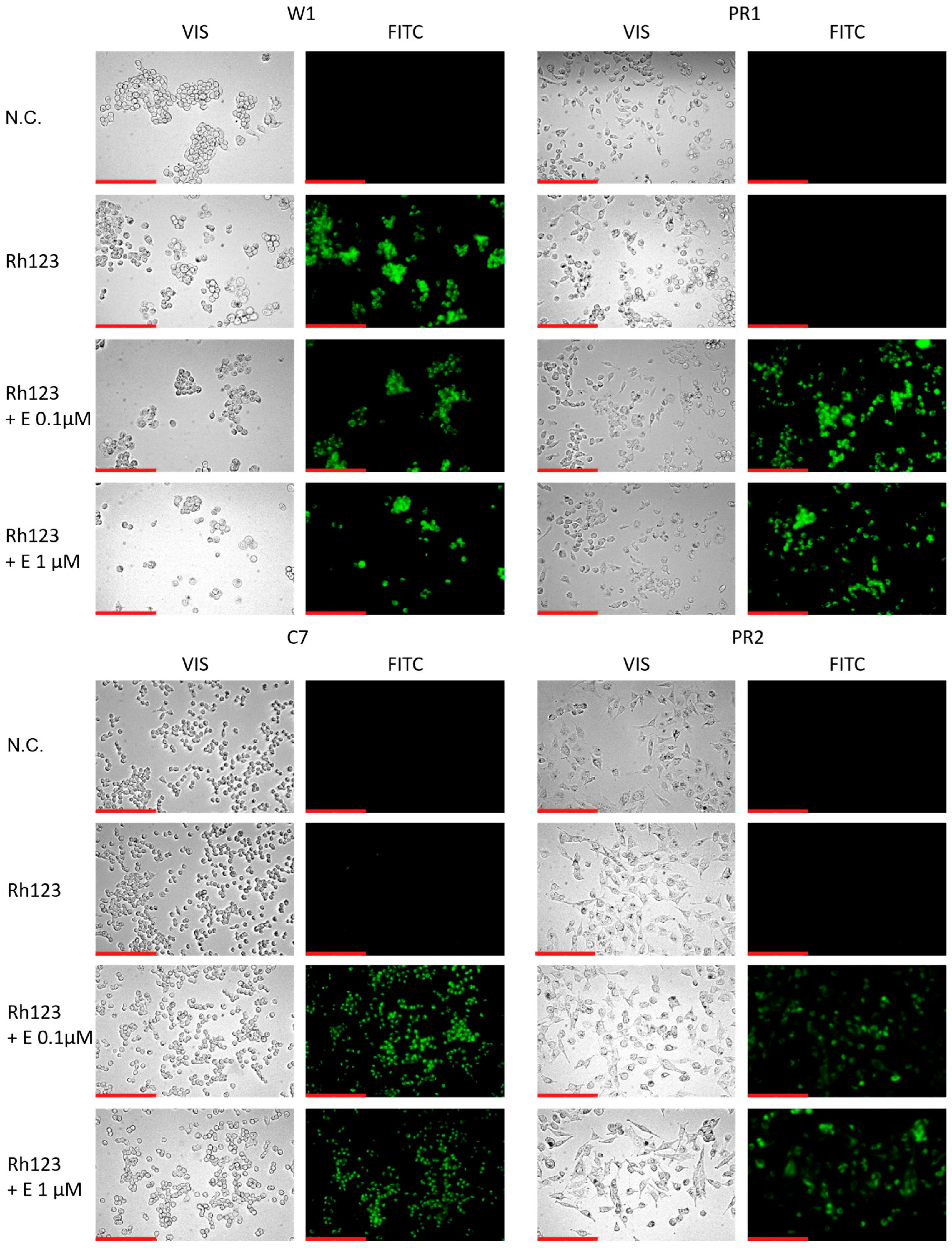
2.6. Analysis of P-gp Activity in Cell Lines Treated with Elacridar
2.7. Expression and Activity of P-gp in Sensitive and PAC-Resistant Cancer Cell Lines in 3D Model
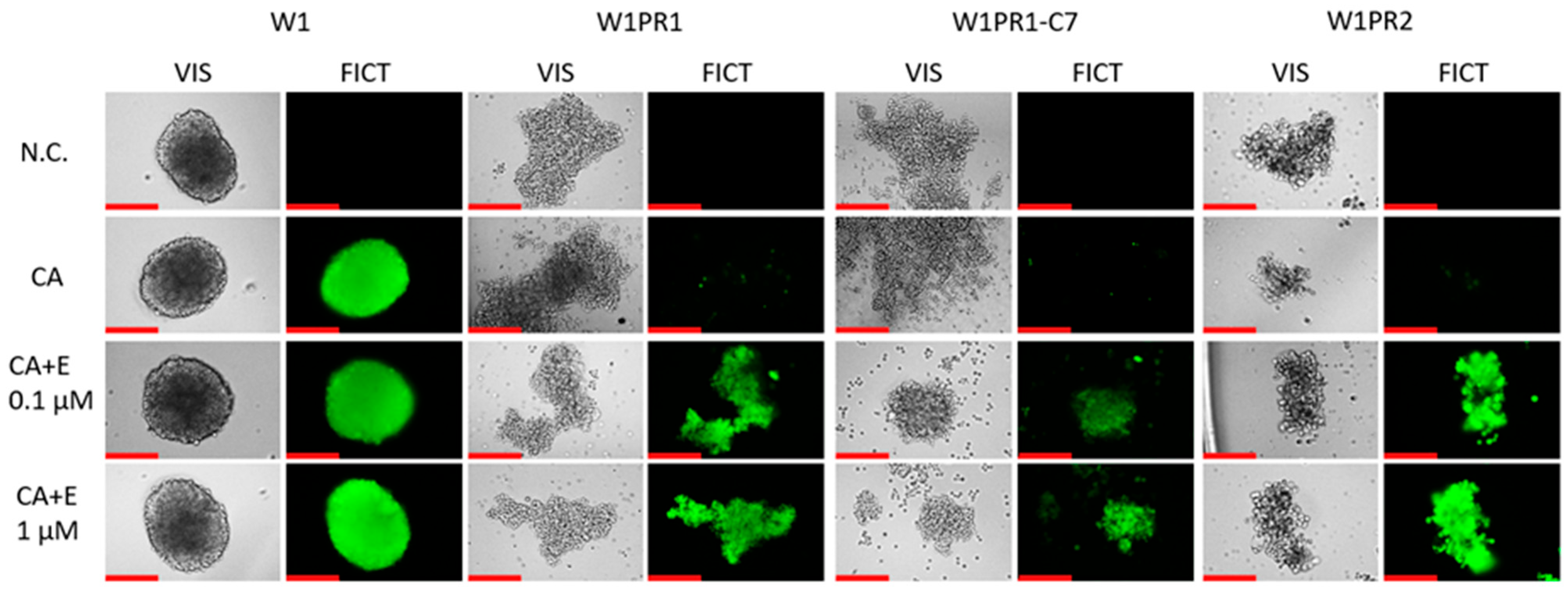
2.8. Analysis of P-gp Activity in Elacridar-Treated Spheroids
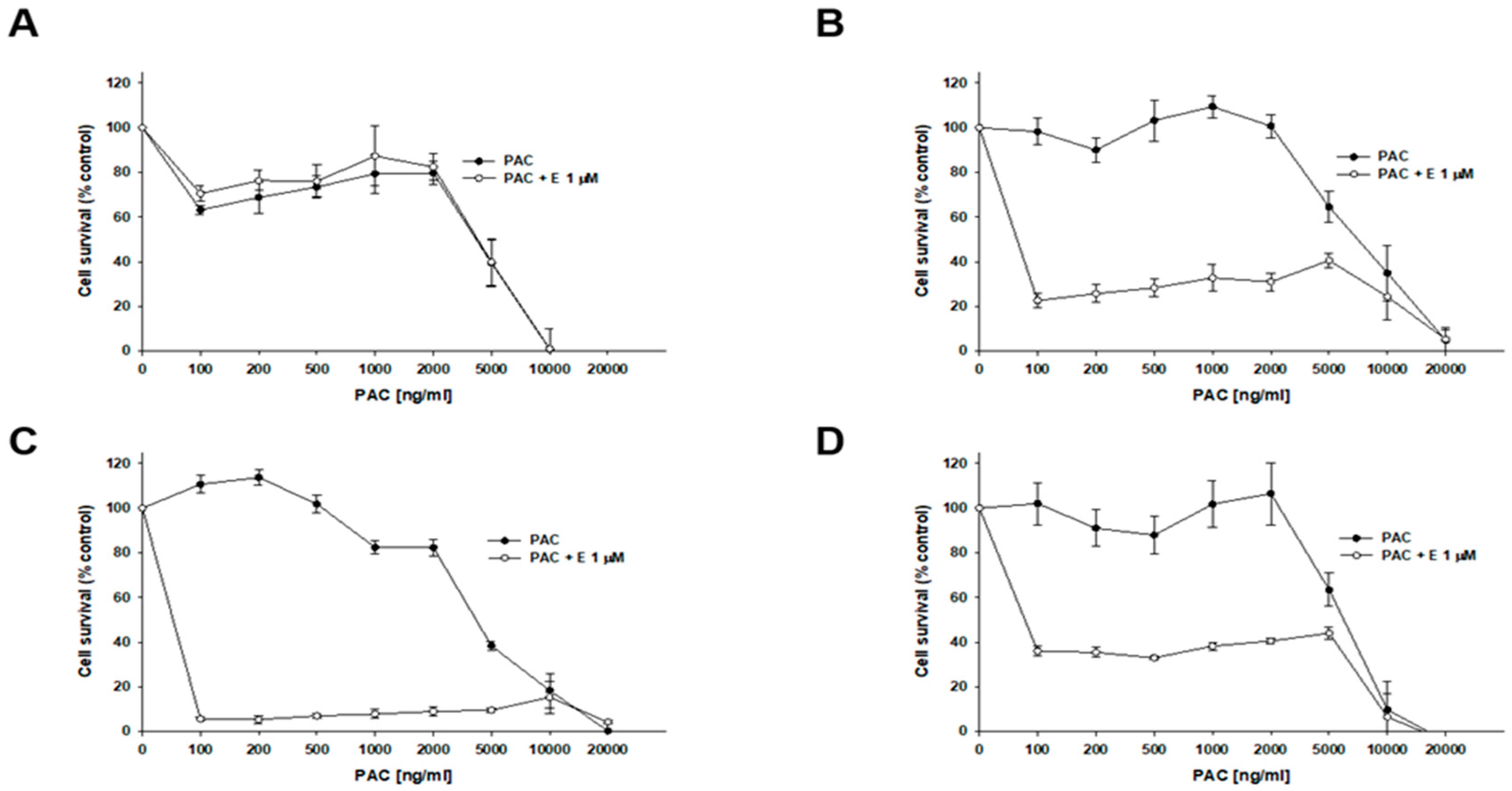
2.9. Analysis of the Effect of Elacridar on Response to Cytotoxic Drug Treatment in a 3D Model
3. Discussion
4. Materials and Methods
4.1. The Reagents and Antibodies
4.2. Cell Culture
4.3. RNA Isolation, cDNA Synthesis, and QPCR
4.4. Two-Dimensional MTT Assay
4.5. Three-Dimensional MTT Assay
4.6. Immunofluorescence
4.7. Life-Cells Fluorescence (Rh123 Accumulation) (2D)
4.8. Live-Cell Fluorescence (CA Accumulation) in 3D
4.9. Protein Isolation and Western Blot Analysis
4.10. Flow Cytometry Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaona-Luviano, P.; Medina-Gaona, L.A.; Magaña-Pérez, K. Epidemiology of Ovarian Cancer. Chin. Clin. Oncol. 2020, 9, 47. [Google Scholar] [CrossRef]
- Matulonis, U.A.; Sood, A.K.; Fallowfield, L.; Howitt, B.E.; Sehouli, J.; Karlan, B.Y. Ovarian Cancer. Nat. Rev. Dis. Primers 2016, 2, 16061. [Google Scholar] [CrossRef]
- Lliberos, C.; Richardson, G.; Papa, A. Oncogenic Pathways and Targeted Therapies in Ovarian Cancer. Biomolecules 2024, 14, 585. [Google Scholar] [CrossRef]
- Kurman, R.J.; Shih, I.-M. Molecular Pathogenesis and Extraovarian Origin of Epithelial Ovarian Cancer--Shifting the Paradigm. Hum. Pathol. 2011, 42, 918–931. [Google Scholar] [CrossRef] [PubMed]
- Pokhriyal, R.; Hariprasad, R.; Kumar, L.; Hariprasad, G. Chemotherapy Resistance in Advanced Ovarian Cancer Patients. Biomark. Cancer 2019, 11, 1179299X19860815. [Google Scholar] [CrossRef]
- Rose, P.G. Ovarian Cancer Recurrence: Is the Definition of Platinum Sensitivity Modified by PARPi, Bevacizumab or Other Intervening Treatments?: A Clinical Perspective. Cancer Drug Resist. 2022, 5, 415–423. [Google Scholar] [CrossRef]
- Gordon, A.N.; Fleagle, J.T.; Guthrie, D.; Parkin, D.E.; Gore, M.E.; Lacave, A.J. Recurrent Epithelial Ovarian Carcinoma: A Randomized Phase III Study of Pegylated Liposomal Doxorubicin versus Topotecan. J. Clin. Oncol. 2001, 19, 3312–3322. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Fatima, K.; Aisha, S.; Malik, F. Unveiling the Mechanisms and Challenges of Cancer Drug Resistance. Cell Commun. Signal. 2024, 22, 109. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.; Howell, V.M.; Colvin, E.K. The Extracellular Matrix in Epithelial Ovarian Cancer—A Piece of a Puzzle. Front. Oncol. 2015, 5, 245. [Google Scholar] [CrossRef]
- Henke, E.; Nandigama, R.; Ergün, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2019, 6, 160. [Google Scholar] [CrossRef]
- Brown, Y.; Hua, S.; Tanwar, P.S. Extracellular Matrix-Mediated Regulation of Cancer Stem Cells and Chemoresistance. Int. J. Biochem. Cell Biol. 2019, 109, 90–104. [Google Scholar] [CrossRef]
- Sterzyńska, K.; Klejewski, A.; Wojtowicz, K.; Świerczewska, M.; Andrzejewska, M.; Rusek, D.; Sobkowski, M.; Kędzia, W.; Brązert, J.; Nowicki, M.; et al. The Role of Matrix Gla Protein (MGP) Expression in Paclitaxel and Topotecan Resistant Ovarian Cancer Cell Lines. Int. J. Mol. Sci. 2018, 19, 2901. [Google Scholar] [CrossRef]
- Erlanson, M.; Daniel-Szolgay, E.; Carlsson, J. Relations between the Penetration, Binding and Average Concentration of Cytostatic Drugs in Human Tumour Spheroids. Cancer Chemother. Pharmacol. 1992, 29, 343–353. [Google Scholar] [CrossRef]
- Holle, A.W.; Young, J.L.; Spatz, J.P. In Vitro Cancer Cell-ECM Interactions Inform in Vivo Cancer Treatment. Adv. Drug Deliv. Rev. 2016, 97, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Freimund, A.E.; Beach, J.A.; Christie, E.L.; Bowtell, D.D.L. Mechanisms of Drug Resistance in High-Grade Serous Ovarian Cancer. Hematol. Oncol. Clin. 2018, 32, 983–996. [Google Scholar] [CrossRef] [PubMed]
- Eke, I.; Cordes, N. Focal Adhesion Signaling and Therapy Resistance in Cancer. Semin. Cancer Biol. 2015, 31, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Sharom, F.J. ABC Multidrug Transporters: Structure, Function and Role in Chemoresistance. Pharmacogenomics 2008, 9, 105–127. [Google Scholar] [CrossRef]
- Leslie, E.M.; Deeley, R.G.; Cole, S.P.C. Multidrug Resistance Proteins: Role of P-Glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in Tissue Defense. Toxicol. Appl. Pharmacol. 2005, 204, 216–237. [Google Scholar] [CrossRef]
- Fletcher, J.I.; Williams, R.T.; Henderson, M.J.; Norris, M.D.; Haber, M. ABC Transporters as Mediators of Drug Resistance and Contributors to Cancer Cell Biology. Drug Resist. Updat. 2016, 26, 1–9. [Google Scholar] [CrossRef]
- Callaghan, R.; Luk, F.; Bebawy, M. Inhibition of the Multidrug Resistance P-Glycoprotein: Time for a Change of Strategy? Drug Metab. Dispos. 2014, 42, 623–631. [Google Scholar] [CrossRef]
- Annese, V.; Valvano, M.; Palmieri, O.; Latiano, A.; Bossa, F.; Andriulli, A. Multidrug Resistance 1 Gene in Inflammatory Bowel Disease: A Meta-Analysis. World J. Gastroenterol. 2006, 12, 3636–3644. [Google Scholar] [CrossRef]
- Loo, T.W.; Clarke, D.M. The Human Multidrug Resistance P-Glycoprotein Is Inactive When Its Maturation Is Inhibited: Potential for a Role in Cancer Chemotherapy. FASEB J. 1999, 13, 1724–1732. [Google Scholar] [CrossRef]
- Wojtowicz, K.; Szaflarski, W.; Januchowski, R.; Zawierucha, P.; Nowicki, M.; Zabel, M. Inhibitors of N-glycosylation as a potential tool for analysis of the mechanism of action and cellular localisation of glycoprotein P. Acta Biochim. Pol. 2012, 59, 445–450. [Google Scholar] [CrossRef]
- Karthika, C.; Sureshkumar, R.; Zehravi, M.; Akter, R.; Ali, F.; Ramproshad, S.; Mondal, B.; Tagde, P.; Ahmed, Z.; Khan, F.S.; et al. Multidrug Resistance of Cancer Cells and the Vital Role of P-Glycoprotein. Life 2022, 12, 897. [Google Scholar] [CrossRef] [PubMed]
- Cordon-Cardo, C.; O’Brien, J.P.; Boccia, J.; Casals, D.; Bertino, J.R.; Melamed, M.R. Expression of the Multidrug Resistance Gene Product (P-Glycoprotein) in Human Normal and Tumor Tissues. J. Histochem. Cytochem. 1990, 38, 1277–1287. [Google Scholar] [CrossRef]
- Kong, L.-L.; Shen, G.-L.; Wang, Z.-Y.; Zhuang, X.-M.; Xiao, W.-B.; Yuan, M.; Gong, Z.-H.; Li, H. Inhibition of P-Glycoprotein and Multidrug Resistance-Associated Protein 2 Regulates the Hepatobiliary Excretion and Plasma Exposure of Thienorphine and Its Glucuronide Conjugate. Front. Pharmacol. 2016, 7, 242. [Google Scholar] [CrossRef]
- Malingré, M.M.; Beijnen, J.H.; Rosing, H.; Koopman, F.J.; Jewell, R.C.; Paul, E.M.; Ten Bokkel Huinink, W.W.; Schellens, J.H. Co-Administration of GF120918 Significantly Increases the Systemic Exposure to Oral Paclitaxel in Cancer Patients. Br. J. Cancer 2001, 84, 42–47. [Google Scholar] [CrossRef]
- Ferry, D.R.; Traunecker, H.; Kerr, D.J. Clinical Trials of P-Glycoprotein Reversal in Solid Tumours. Eur. J. Cancer 1996, 32, 1070–1081. [Google Scholar] [CrossRef]
- Pilotto Heming, C.; Muriithi, W.; Wanjiku Macharia, L.; Niemeyer Filho, P.; Moura-Neto, V.; Aran, V. P-Glycoprotein and Cancer: What Do We Currently Know? Heliyon 2022, 8, e11171. [Google Scholar] [CrossRef] [PubMed]
- Begicevic, R.-R.; Falasca, M. ABC Transporters in Cancer Stem Cells: Beyond Chemoresistance. Int. J. Mol. Sci. 2017, 18, 2362. [Google Scholar] [CrossRef] [PubMed]
- Weaver, B.A. How Taxol/Paclitaxel Kills Cancer Cells. Mol. Biol. Cell 2014, 25, 2677. [Google Scholar] [CrossRef]
- Hilmer, S.N.; Cogger, V.C.; Muller, M.; Couteur, D.G.L. The Hepatic Pharmacokinetics of Doxorubicin and Liposomal Doxorubicin. Drug Metab. Dispos. 2004, 32, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Sane, R.; Mittapalli, R.K.; Elmquist, W.F. Development and Evaluation of a Novel Microemulsion Formulation of Elacridar to Improve Its Bioavailability. J. Pharm. Sci. 2013, 102, 1343–1354. [Google Scholar] [CrossRef]
- Matsson, P.; Pedersen, J.M.; Norinder, U.; Bergström, C.; Artursson, P. Identification of Novel Specific and General Inhibitors of the Three Major Human ATP-Binding Cassette Transporters P-Gp, BCRP and MRP2 Among Registered Drugs. Pharm. Res. 2009, 26, 1816–1831. [Google Scholar] [CrossRef]
- Stasiak, P.; Sopel, J.; Płóciennik, A.; Musielak, O.; Lipowicz, J.M.; Rawłuszko-Wieczorek, A.A.; Sterzyńska, K.; Korbecki, J.; Januchowski, R. Elacridar Inhibits BCRP Protein Activity in 2D and 3D Cell Culture Models of Ovarian Cancer and Re-Sensitizes Cells to Cytotoxic Drugs. Int. J. Mol. Sci. 2025, 26, 5800. [Google Scholar] [CrossRef]
- Dash, R.P.; Jayachandra Babu, R.; Srinivas, N.R. Therapeutic Potential and Utility of Elacridar with Respect to P-Glycoprotein Inhibition: An Insight from the Published In Vitro, Preclinical and Clinical Studies. Eur. J. Drug Metab. Pharmacokinet. 2017, 42, 915–933. [Google Scholar] [CrossRef]
- O’Neill, A.J.; Prencipe, M.; Dowling, C.; Fan, Y.; Mulrane, L.; Gallagher, W.M.; O’Connor, D.; O’Connor, R.; Devery, A.; Corcoran, C.; et al. Characterisation and Manipulation of Docetaxel Resistant Prostate Cancer Cell Lines. Mol. Cancer 2011, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.-C.; Simonin, G.; Faussat, A.-M.; Zittoun, R.; Marie, J.-P. Effect of the Multidrug Inhibitor GG918 on Drug Sensitivity of Human Leukemic Cells. Leukemia 1997, 11, 1516–1522. [Google Scholar] [CrossRef] [PubMed]
- Warmann, S.; Göhring, G.; Teichmann, B.; Geerlings, H.; Fuchs, J. MDR1 Modulators Improve the Chemotherapy Response of Human Hepatoblastoma to Doxorubicin in Vitro. J. Pediatr. Surg. 2002, 37, 1579–1584. [Google Scholar] [CrossRef]
- Schirizzi, A.; Contino, M.; Carrieri, L.; Riganti, C.; De Leonardis, G.; Scavo, M.P.; Perrone, M.G.; Miciaccia, M.; Kopecka, J.; Refolo, M.G.; et al. The Multiple Combination of Paclitaxel, Ramucirumab and Elacridar Reverses the Paclitaxel-Mediated Resistance in Gastric Cancer Cell Lines. Front. Oncol. 2023, 13, 1129832. [Google Scholar] [CrossRef]
- Marchetti, S.; Oostendorp, R.L.; Pluim, D.; van Eijndhoven, M.; van Tellingen, O.; Schinkel, A.H.; Versace, R.; Beijnen, J.H.; Mazzanti, R.; Schellens, J.H. In Vitro Transport of Gimatecan (7-t-Butoxyiminomethylcamptothecin) by Breast Cancer Resistance Protein, P-Glycoprotein, and Multidrug Resistance Protein 2. Mol. Cancer Ther. 2007, 6, 3307–3313. [Google Scholar] [CrossRef] [PubMed]
- Ward, K.W.; Azzarano, L.M. Preclinical Pharmacokinetic Properties of the P-Glycoprotein Inhibitor GF120918A (HCl Salt of GF120918, 9,10-Dihydro-5-Methoxy-9-Oxo-N-[4-[2-(1,2,3,4-Tetrahydro-6,7-Dimethoxy-2-Isoquinolinyl)Ethyl]Phenyl]-4-Acridine-Carboxamide) in the Mouse, Rat, Dog, and Monkey. J. Pharmacol. Exp. Ther. 2004, 310, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Hendrikx, J.J.M.A.; Lagas, J.S.; Wagenaar, E.; Rosing, H.; Schellens, J.H.M.; Beijnen, J.H.; Schinkel, A.H. Oral Co-Administration of Elacridar and Ritonavir Enhances Plasma Levels of Oral Paclitaxel and Docetaxel without Affecting Relative Brain Accumulation. Br. J. Cancer 2014, 110, 2669–2676. [Google Scholar] [CrossRef] [PubMed]
- Jonker, J.W. Role of Breast Cancer Resistance Protein in the Bioavailability and Fetal Penetration of Topotecan. J. Natl. Cancer Inst. 2000, 92, 1651–1656. [Google Scholar] [CrossRef]
- Hyafil, F.; Vergely, C.; Du Vignaud, P.; Grand-Perret, T. In Vitro and in Vivo Reversal of Multidrug Resistance by GF120918, an Acridonecarboxamide Derivative. Cancer Res. 1993, 53, 4595–4602. [Google Scholar]
- Sun, D.; Liu, J.; Wang, Y.; Dong, J. Co-Administration of MDR1 and BCRP or EGFR/PI3K Inhibitors Overcomes Lenvatinib Resistance in Hepatocellular Carcinoma. Front. Oncol. 2022, 12, 944537. [Google Scholar] [CrossRef]
- Omori, M.; Noro, R.; Seike, M.; Matsuda, K.; Hirao, M.; Fukuizumi, A.; Takano, N.; Miyanaga, A.; Gemma, A. Inhibitors of ABCB1 and ABCG2 Overcame Resistance to Topoisomerase Inhibitors in Small Cell Lung Cancer. Thorac. Cancer 2022, 13, 2142–2151. [Google Scholar] [CrossRef]
- D’Costa, N.M.; Lowerison, M.R.; Raven, P.A.; Tan, Z.; Roberts, M.E.; Shrestha, R.; Urban, M.W.; Monjaras-Avila, C.U.; Oo, H.Z.; Hurtado-Coll, A.; et al. Y-Box Binding Protein-1 Is Crucial in Acquired Drug Resistance Development in Metastatic Clear-Cell Renal Cell Carcinoma. J. Exp. Clin. Cancer Res. 2020, 39, 33. [Google Scholar] [CrossRef]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D Cell Cultures—A Comparison of Different Types of Cancer Cell Cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef]
- Januchowski, R.; Wojtowicz, K.; Sterzyſska, K.; Sosiſska, P.; Andrzejewska, M.; Zawierucha, P.; Nowicki, M.; Zabel, M. Inhibition of ALDH1A1 Activity Decreases Expression of Drug Transporters and Reduces Chemotherapy Resistance in Ovarian Cancer Cell Lines. Int. J. Biochem. Cell Biol. 2016, 78, 248–259. [Google Scholar] [CrossRef]
- Januchowski, R.; Wojtowicz, K.; Sujka-Kordowska, P.; Andrzejewska, M.; Zabel, M. MDR Gene Expression Analysis of Six Drug-Resistant Ovarian Cancer Cell Lines. Biomed. Res. Int. 2013, 2013, 241763. [Google Scholar] [CrossRef]
- Nowacka, M.; Ginter-Matuszewska, B.; Świerczewska, M.; Sterzyńska, K.; Nowicki, M.; Januchowski, R. Effect of ALDH1A1 Gene Knockout on Drug Resistance in Paclitaxel and Topotecan Resistant Human Ovarian Cancer Cell Lines in 2D and 3D Model. Int. J. Mol. Sci. 2022, 23, 3036. [Google Scholar] [CrossRef]
- Świerczewska, M.; Nowacka, M.; Stasiak, P.; Iżycki, D.; Sterzyńska, K.; Płóciennik, A.; Nowicki, M.; Januchowski, R. Doxorubicin and Topotecan Resistance in Ovarian Cancer: Gene Expression and Microenvironment Analysis in 2D and 3D Models. Biomed. Pharmacother. 2025, 183, 117804. [Google Scholar] [CrossRef]
- Świerczewska, M.; Sterzyńska, K.; Ruciński, M.; Andrzejewska, M.; Nowicki, M.; Januchowski, R. The Response and Resistance to Drugs in Ovarian Cancer Cell Lines in 2D Monolayers and 3D Spheroids. Biomed. Pharmacother. 2023, 165, 115152. [Google Scholar] [CrossRef]
- Ortiz, M.; Wabel, E.; Mitchell, K.; Horibata, S. Mechanisms of Chemotherapy Resistance in Ovarian Cancer. Cancer Drug Resist. 2022, 5, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Momenimovahed, Z.; Tiznobaik, A.; Taheri, S.; Salehiniya, H. Ovarian Cancer in the World: Epidemiology and Risk Factors. Int. J. Womens Health 2019, 11, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Pote, M.S.; Gacche, R.N. ATP-Binding Cassette Efflux Transporters and MDR in Cancer. Drug Discov. Today 2023, 28, 103537. [Google Scholar] [CrossRef]
- Wojtowicz, K.; Sterzyńska, K.; Świerczewska, M.; Nowicki, M.; Zabel, M.; Januchowski, R. Piperine Targets Different Drug Resistance Mechanisms in Human Ovarian Cancer Cell Lines Leading to Increased Sensitivity to Cytotoxic Drugs. Int. J. Mol. Sci. 2021, 22, 4243. [Google Scholar] [CrossRef]
- Forster, S.; Thumser, A.E.; Hood, S.R.; Plant, N. Characterization of Rhodamine-123 as a Tracer Dye for Use In In Vitro Drug Transport Assays. PLoS ONE 2012, 7, e33253. [Google Scholar] [CrossRef] [PubMed]
- Stasiak, P.; Sopel, J.; Lipowicz, J.M.; Rawłuszko-Wieczorek, A.A.; Korbecki, J.; Januchowski, R. The Role of Elacridar, a P-Gp Inhibitor, in the Re-Sensitization of PAC-Resistant Ovarian Cancer Cell Lines to Cytotoxic Drugs in 2D and 3D Cell Culture Models. Int. J. Mol. Sci. 2025, 26, 1124. [Google Scholar] [CrossRef]
- Sauveur, J.; Conilh, L.; Beaumel, S.; Chettab, K.; Jordheim, L.-P.; Matera, E.-L.; Dumontet, C. Characterization of T-DM1-Resistant Breast Cancer Cells. Pharmacol. Res. Perspect. 2020, 8, e00617. [Google Scholar] [CrossRef]
- Alves, R.; Gonçalves, A.C.; Jorge, J.; Almeida, A.M.; Sarmento-Ribeiro, A.B. Combination of Elacridar with Imatinib Modulates Resistance Associated with Drug Efflux Transporters in Chronic Myeloid Leukemia. Biomedicines 2022, 10, 1158. [Google Scholar] [CrossRef]
- Chen, H.; Shien, K.; Suzawa, K.; Tsukuda, K.; Tomida, S.; Sato, H.; Torigoe, H.; Watanabe, M.; Namba, K.; Yamamoto, H.; et al. Elacridar, a Third-Generation ABCB1 Inhibitor, Overcomes Resistance to Docetaxel in Non-Small Cell Lung Cancer. Oncol. Lett. 2017, 14, 4349–4354. [Google Scholar] [CrossRef]
- Stordal, B.; Hamon, M.; McEneaney, V.; Roche, S.; Gillet, J.-P.; O’Leary, J.J.; Gottesman, M.; Clynes, M. Resistance to Paclitaxel in a Cisplatin-Resistant Ovarian Cancer Cell Line Is Mediated by P-Glycoprotein. PLoS ONE 2012, 7, e40717. [Google Scholar] [CrossRef]
- Sato, H.; Siddig, S.; Uzu, M.; Suzuki, S.; Nomura, Y.; Kashiba, T.; Gushimiyagi, K.; Sekine, Y.; Uehara, T.; Arano, Y.; et al. Elacridar Enhances the Cytotoxic Effects of Sunitinib and Prevents Multidrug Resistance in Renal Carcinoma Cells. Eur. J. Pharmacol. 2015, 746, 258–266. [Google Scholar] [CrossRef]
- Poel, D.; Iyer, K.K.; van Gasteren, B.; Dagniaux, B.B.C.; van den Hombergh, E.; Tauriello, D.V.F.; Verheul, H.M.W.; van Erp, N.P. Elacridar Improves Sunitinib Efficacy in Colorectal Cancer Models. Eur. J. Pharm. Sci. 2025, 212, 107194. [Google Scholar] [CrossRef] [PubMed]
- Nowacka, M.; Sterzynska, K.; Andrzejewska, M.; Nowicki, M.; Januchowski, R. Drug Resistance Evaluation in Novel 3D in Vitro Model. Biomed. Pharmacother. 2021, 138, 111536. [Google Scholar] [CrossRef] [PubMed]
- Di, L.; Whitney-Pickett, C.; Umland, J.P.; Zhang, H.; Zhang, X.; Gebhard, D.F.; Lai, Y.; Federico, J.J., III; Davidson, R.E.; Smith, R.; et al. Development of a New Permeability Assay Using Low-Efflux MDCKII Cells. J. Pharm. Sci. 2011, 100, 4974–4985. [Google Scholar] [CrossRef]
- Homolya, L.; Holló, M.; Müller, M.; Mechetner, E.B.; Sarkadi, B. A New Method for a Quantitative Assessment of P-Glycoprotein-Related Multidrug Resistance in Tumour Cells. Br. J. Cancer 1996, 73, 849–855. [Google Scholar] [CrossRef]
- Nováková, E.; Mikušová, V.; Šupolíková, M. Spheroids as 3D Cell Models for Testing of Drugs. Eur. Pharm. J. 2023, 70, 37–43. [Google Scholar] [CrossRef]
- Januchowski, R.; Świerczewska, M.; Sterzyńska, K.; Wojtowicz, K.; Nowicki, M.; Zabel, M. Increased Expression of Several Collagen Genes Is Associated with Drug Resistance in Ovarian Cancer Cell Lines. J. Cancer 2016, 7, 1295–1310. [Google Scholar] [CrossRef]
- Grantab, R.; Sivananthan, S.; Tannock, I.F. The Penetration of Anticancer Drugs through Tumor Tissue as a Function of Cellular Adhesion and Packing Density of Tumor Cells. Cancer Res. 2006, 66, 1033–1039. [Google Scholar] [CrossRef]
- Wang, J.; Lu, Z.; Gao, Y.; Wientjes, M.G.; Au, J.L.-S. Improving Delivery and Efficacy of Nanomedicines in Solid Tumors: Role of Tumor Priming. Nanomedicine 2011, 6, 1605–1620. [Google Scholar] [CrossRef] [PubMed]
- Kuh, H.-J.; Jang, S.H.; Wientjes, M.G.; Weaver, J.R.; Au, J.L.-S. Determinants of Paclitaxel Penetration and Accumulation in Human Solid Tumor. J. Pharmacol. Exp. Ther. 1999, 290, 871–880. [Google Scholar] [CrossRef]
- Imamura, Y.; Mukohara, T.; Shimono, Y.; Funakoshi, Y.; Chayahara, N.; Toyoda, M.; Kiyota, N.; Takao, S.; Kono, S.; Nakatsura, T.; et al. Comparison of 2D- and 3D-Culture Models as Drug-Testing Platforms in Breast Cancer. Oncol. Rep. 2015, 33, 1837–1843. [Google Scholar] [CrossRef]
- Lam, C.R.I.; Wong, H.K.; Nai, S.; Chua, C.K.; Tan, N.S.; Tan, L.P. A 3D Biomimetic Model of Tissue Stiffness Interface for Cancer Drug Testing. Mol. Pharm. 2014, 11, 2016–2021. [Google Scholar] [CrossRef]
- Işeri, O.D.; Kars, M.D.; Arpaci, F.; Atalay, C.; Pak, I.; Gündüz, U. Drug Resistant MCF-7 Cells Exhibit Epithelial-Mesenchymal Transition Gene Expression Pattern. Biomed. Pharmacother. 2011, 65, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Świerczewska, M.; Klejewski, A.; Wojtowicz, K.; Brązert, M.; Iżycki, D.; Nowicki, M.; Zabel, M.; Januchowski, R. New and Old Genes Associated with Primary and Established Responses to Cisplatin and Topotecan Treatment in Ovarian Cancer Cell Lines. Molecules 2017, 22, 1717. [Google Scholar] [CrossRef]
- Świerczewska, M.; Klejewski, A.; Brązert, M.; Kaźmierczak, D.; Iżycki, D.; Nowicki, M.; Zabel, M.; Januchowski, R. New and Old Genes Associated with Primary and Established Responses to Paclitaxel Treatment in Ovarian Cancer Cell Lines. Molecules 2018, 23, 891. [Google Scholar] [CrossRef] [PubMed]
- Klejewski, A.; Świerczewska, M.; Zaorska, K.; Brązert, M.; Nowicki, M.; Zabel, M.; Januchowski, R. New and Old Genes Associated with Topotecan Resistance Development in Ovarian Cancer Cell Lines. Anticancer Res. 2017, 37, 1625–1636. [Google Scholar] [CrossRef]
- Sterzyńska, K.; Klejewski, A.; Wojtowicz, K.; Świerczewska, M.; Nowacka, M.; Kaźmierczak, D.; Andrzejewska, M.; Rusek, D.; Brązert, M.; Brązert, J.; et al. Mutual Expression of ALDH1A1, LOX, and Collagens in Ovarian Cancer Cell Lines as Combined CSCs- and ECM-Related Models of Drug Resistance Development. Int. J. Mol. Sci. 2019, 20, 54. [Google Scholar] [CrossRef]
- Di Paolo, A.; Bocci, G. Drug Distribution in Tumors: Mechanisms, Role in Drug Resistance, and Methods for Modification. Curr. Oncol. Rep. 2007, 9, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.P.; Stylianopoulos, T.; Boucher, Y.; Jain, R.K. Delivery of Molecular and Nanoscale Medicine to Tumors: Transport Barriers and Strategies. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Wantoch von Rekowski, K.; König, P.; Henze, S.; Schlesinger, M.; Zawierucha, P.; Januchowski, R.; Bendas, G. The Impact of Integrin-Mediated Matrix Adhesion on Cisplatin Resistance of W1 Ovarian Cancer Cells. Biomolecules 2019, 9, 788. [Google Scholar] [CrossRef] [PubMed]
- Świerczewska, M.; Sterzyńska, K.; Wojtowicz, K.; Kaźmierczak, D.; Iżycki, D.; Nowicki, M.; Zabel, M.; Januchowski, R. PTPRK Expression Is Downregulated in Drug Resistant Ovarian Cancer Cell Lines, and Especially in ALDH1A1 Positive CSCs-Like Populations. Int. J. Mol. Sci. 2019, 20, 2053. [Google Scholar] [CrossRef]
- Tang, C.; Gao, X.; Liu, H.; Jiang, T.; Zhai, X. Decreased Expression of SEMA3A Is Associated with Poor Prognosis in Gastric Carcinoma. Int. J. Clin. Exp. Pathol. 2014, 7, 4782–4794. [Google Scholar]
- Jiang, H.; Qi, L.; Wang, F.; Sun, Z.; Huang, Z.; Xi, Q. Decreased Semaphorin 3A Expression Is Associated with a Poor Prognosis in Patients with Epithelial Ovarian Carcinoma. Int. J. Mol. Med. 2015, 35, 1374–1380. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Huang, J.-H.; Liu, W. Sema3A Drastically Suppresses Tumor Growth in Oral Cancer Xenograft Model of Mice. BMC Pharmacol. Toxicol. 2017, 18, 55. [Google Scholar] [CrossRef]
- Sun, P.-H.; Ye, L.; Mason, M.D.; Jiang, W.G. Protein Tyrosine Phosphatase Kappa (PTPRK) Is a Negative Regulator of Adhesion and Invasion of Breast Cancer Cells, and Associates with Poor Prognosis of Breast Cancer. J. Cancer Res. Clin. Oncol. 2013, 139, 1129–1139. [Google Scholar] [CrossRef]
- Agarwal, S.; Al-Keilani, M.S.; Alqudah, M.A.Y.; Sibenaller, Z.A.; Ryken, T.C.; Assem, M. Tumor Derived Mutations of Protein Tyrosine Phosphatase Receptor Type K Affect Its Function and Alter Sensitivity to Chemotherapeutics in Glioma. PLoS ONE 2013, 8, e62852. [Google Scholar] [CrossRef]
- Sterzyńska, K.; Kaźmierczak, D.; Klejewski, A.; Świerczewska, M.; Wojtowicz, K.; Nowacka, M.; Brązert, J.; Nowicki, M.; Januchowski, R. Expression of Osteoblast-Specific Factor 2 (OSF-2, Periostin) Is Associated with Drug Resistance in Ovarian Cancer Cell Lines. Int. J. Mol. Sci. 2019, 20, 3927. [Google Scholar] [CrossRef] [PubMed]



| Cell Line | PAC IC50 (ng/mL) | DOX IC50 (ng/mL) | CIS IC50 (ng/mL) |
|---|---|---|---|
| W1 | 3.18 (2.77–3.98) 1 | 32.07 (18.3–69.0) 1 | 19,741 (9323–26,667) 1 |
| W1PR1 | 1960 (1871–2062) 616 ↑ *** | 6904 (3303–13,720) 215 ↑ *** | 21,566 (18,033–28,537) 1.09 ↑ |
| W1PR1-C7 | 1199 (934–1433) 377 ↑ *** | 2769 (1139–4446) 86 ↑ ** | 14,118 (9751–16,974) 1.40 ↓ |
| W1PR2 | 3384 (1995–4340) 1064 ↑ *** | 7836 (5674–9938) 244 ↑ ** | 16,779 (13,826–19,986) 1.17 ↓ |
| Cell Line | Control PAC IC50 (ng/mL) | Elacridar 0.1 µM PAC IC50 (ng/mL) | Elacridar 1 µM PAC IC50 (ng/mL) |
|---|---|---|---|
| W1 | 3.18 (2.77–3.98) 1 | 2.70 (1.99–3.76) 1.18 ↓ | 2.65 (1.92–3.69) 1.20 ↓ |
| W1PR1 | 1960 (1871–2062) 1 | 4.86 (3.34–6.34) 404 ↓ *** | 3.23 (1.82–5.02) 607 ↓ *** |
| W1PR1-C7 | 1199 (934–1433) 1 | 2.29 (1.08–3.48) 538 ↓ *** | 1.93 (0.98–3.17) 620 ↓ *** |
| W1PR2 | 3384 (1995–4340) 1 | 5.20 (3.34–7.48) 650 ↓ *** | 3.58 (3.02–5.02) 945 ↓ *** |
| Cell Line | Control DOX IC50 (ng/mL) | Elacridar 0.1 µM DOX IC50 (ng/mL) | Elacridar 1 µM DOX IC50 (ng/mL) |
|---|---|---|---|
| W1 | 32.07 (18.3–69.0) 1 | 25.0 (16.5–48.7) 1.28 ↓ | 21.6 (15.6–32.6) 1.48 ↓ |
| W1PR1 | 6904 (3303–13,720) 1 | 84.6 (36.6–210) 82 ↓ *** | 45.5 (16.2–84.3) 152 ↓ *** |
| W1PR1-C7 | 2769 (1139–4446) 1 | 36.1 (17,6–49.8) 77 ↓ ** | 28.4 (21.8–34.1) 98 ↓ ** |
| W1PR2 | 7836 (5674–9938) 1 | 63.8 (42.5–81.4) 123 ↓ ** | 18.0 (5.67–32.0) 436 ↓ ** |
| Cell Line | Control CIS IC50 (ng/mL) | Elacridar 0.1 µM CIS IC50 (ng/mL) | Elacridar 1 µM CIS IC50 (ng/mL) |
|---|---|---|---|
| W1 | 19,741 (9323–26,667) 1 | 16,355 (13,462–17,921) 1.21 ↓ | 12,752 (8999–16,126) 1.55 ↓ |
| W1PR1 | 21,566 (18,033–28,537) 1 | 20,099 (15,600–25,505) 1.07 ↓ | 22,470 (17,658–28,079) 1.04 ↑ |
| W1PR1-C7 | 14,118 (9751–16,974) 1 | 14,906 (11,404–18,409) 1.06 ↑ | 14,998 (12,429–17,808) 1.06 ↑ |
| W1PR2 | 16,779 (13,826–19,986) 1 | 20541 (14,303–24,763) 1.22 ↑ | 21,291 (16,666–28,983) 1.26 ↑ |
| Cell Line | Control PAC IC50 (ng/mL) | Elacridar 1 µM PAC IC50 (ng/mL) |
|---|---|---|
| W1 | 4539 (3486–6211) 1 | 4597 (3690–6220) 1.01 ↑ |
| W1PR1 | 8405 (4788–13,305) 1 | 65.3 (56.4–74.3) 129 ↓ ** |
| W1PR1-C7 | 4187 (3797–4540) 1 | 53.0 (52.0–54.1) 79.0 ↓ ** |
| W1PR2 | 5894 (4875–6337) 1 | 78.3 (75.1–84.2) 75.3 ↓ ** |
| Transcript | Sequence (5′−3′ Direction) Forward | Sequence (5′−3′ Direction) Reverse | ENST Number http://www.ensembl.org, Accessed on 11 December 2025 | Product Size (bp) |
|---|---|---|---|---|
| MDR1 | TGACAGCTAC- AGCACGGAAG | TCTTCACCTC- CAGGCTCAGT | 00000265724 | 131 |
| GAPDH | GAAGGTGAAG- GTCGGAGTCA | GACAAGCTTC- CCGTTCTCAG | 00000229239 | 199 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stasiak, P.; Sopel, J.; Lipowicz, J.M.; Rawłuszko-Wieczorek, A.A.; Sterzyńska, K.; Korbecki, J.; Januchowski, R. Elacridar Reverses P-gp-Mediated Drug Resistance in Ovarian Cancer Cells in 2D and 3D Culture Models. Int. J. Mol. Sci. 2025, 26, 12105. https://doi.org/10.3390/ijms262412105
Stasiak P, Sopel J, Lipowicz JM, Rawłuszko-Wieczorek AA, Sterzyńska K, Korbecki J, Januchowski R. Elacridar Reverses P-gp-Mediated Drug Resistance in Ovarian Cancer Cells in 2D and 3D Culture Models. International Journal of Molecular Sciences. 2025; 26(24):12105. https://doi.org/10.3390/ijms262412105
Chicago/Turabian StyleStasiak, Piotr, Justyna Sopel, Julia Maria Lipowicz, Agnieszka Anna Rawłuszko-Wieczorek, Karolina Sterzyńska, Jan Korbecki, and Radosław Januchowski. 2025. "Elacridar Reverses P-gp-Mediated Drug Resistance in Ovarian Cancer Cells in 2D and 3D Culture Models" International Journal of Molecular Sciences 26, no. 24: 12105. https://doi.org/10.3390/ijms262412105
APA StyleStasiak, P., Sopel, J., Lipowicz, J. M., Rawłuszko-Wieczorek, A. A., Sterzyńska, K., Korbecki, J., & Januchowski, R. (2025). Elacridar Reverses P-gp-Mediated Drug Resistance in Ovarian Cancer Cells in 2D and 3D Culture Models. International Journal of Molecular Sciences, 26(24), 12105. https://doi.org/10.3390/ijms262412105







