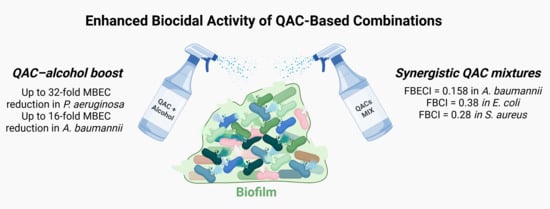
In Pursuit of a Better Biocide Composition: Synergistic and Additive Effects of QAC-Based Formulations Against Planktonic and Biofilm Cultures
Abstract
1. Introduction
2. Results and Discussion
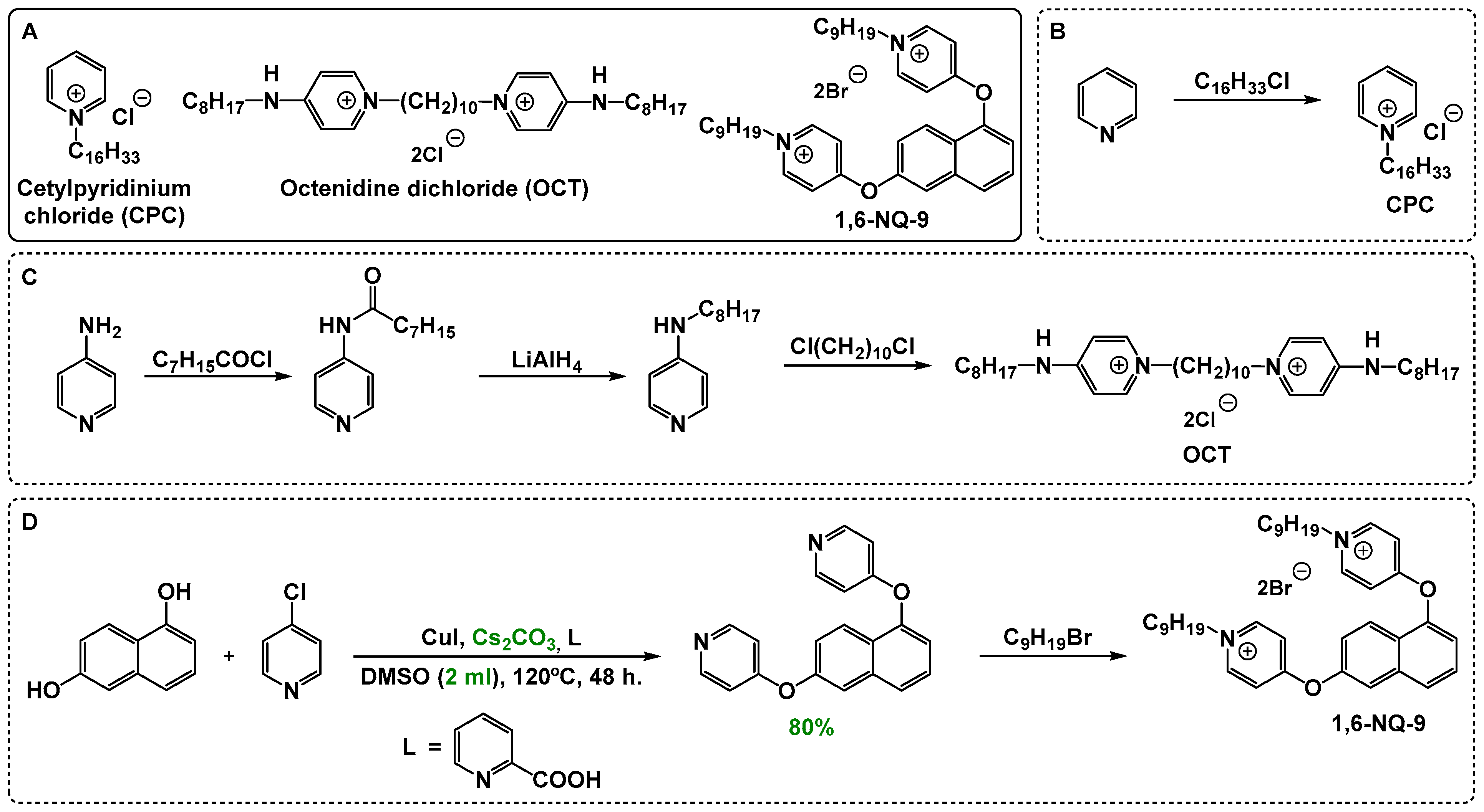
2.1. Basic Concepts of QAC Synthesis and the Biocidal Activity of QAC-Based Compositions
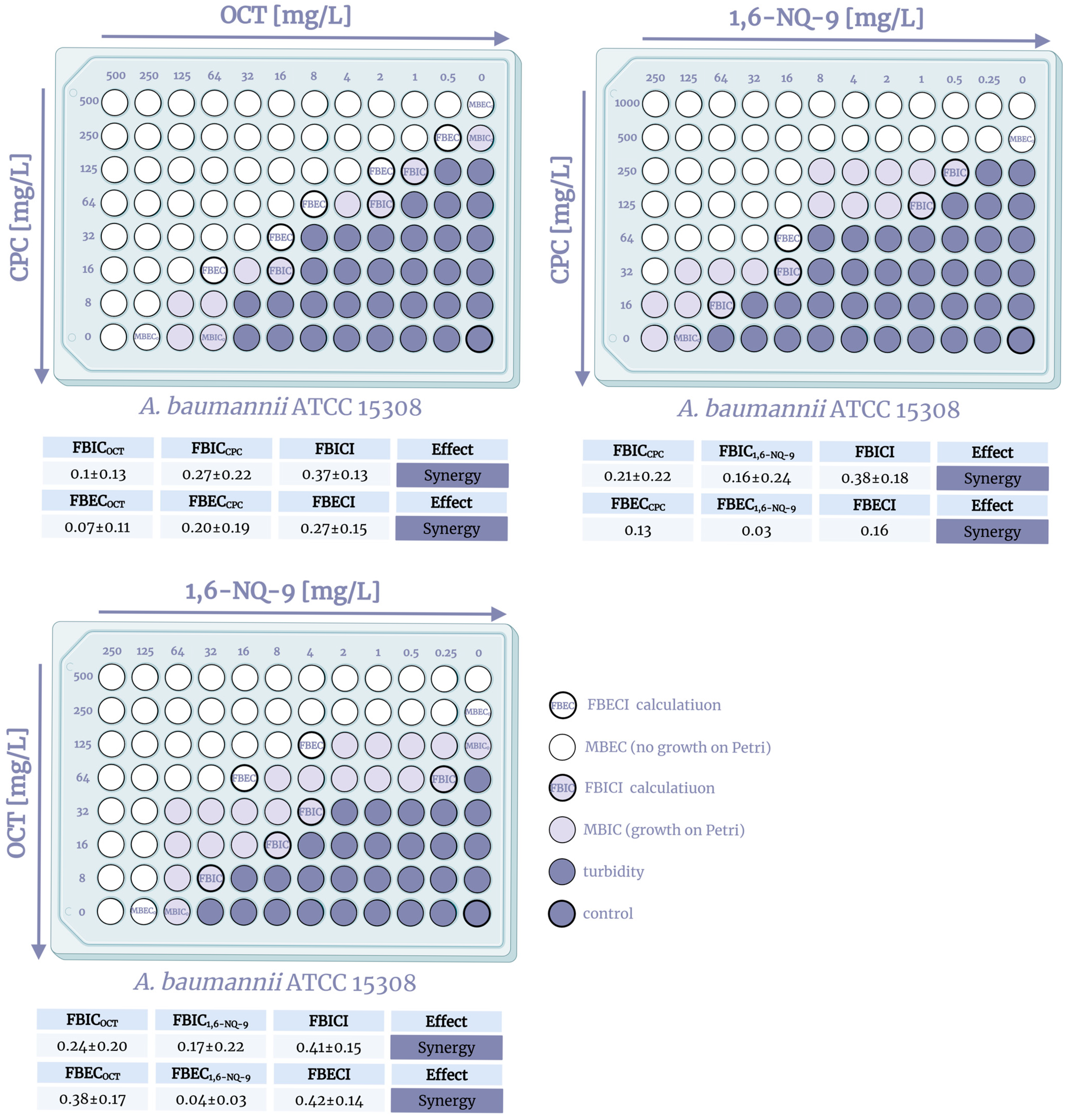
2.2. QAC Combinations Synergy Test
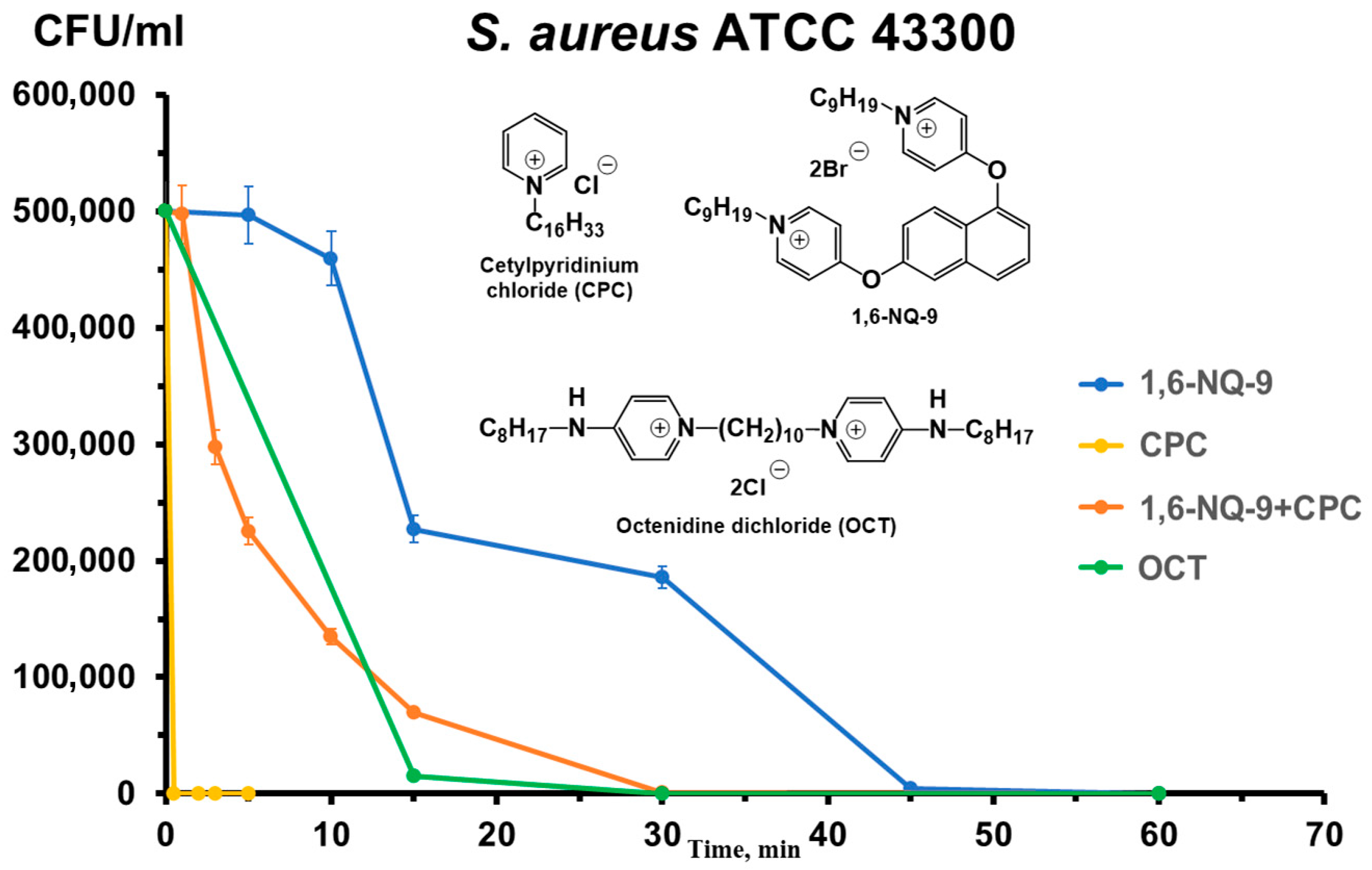
2.3. Time–Kill Kinetics Assay
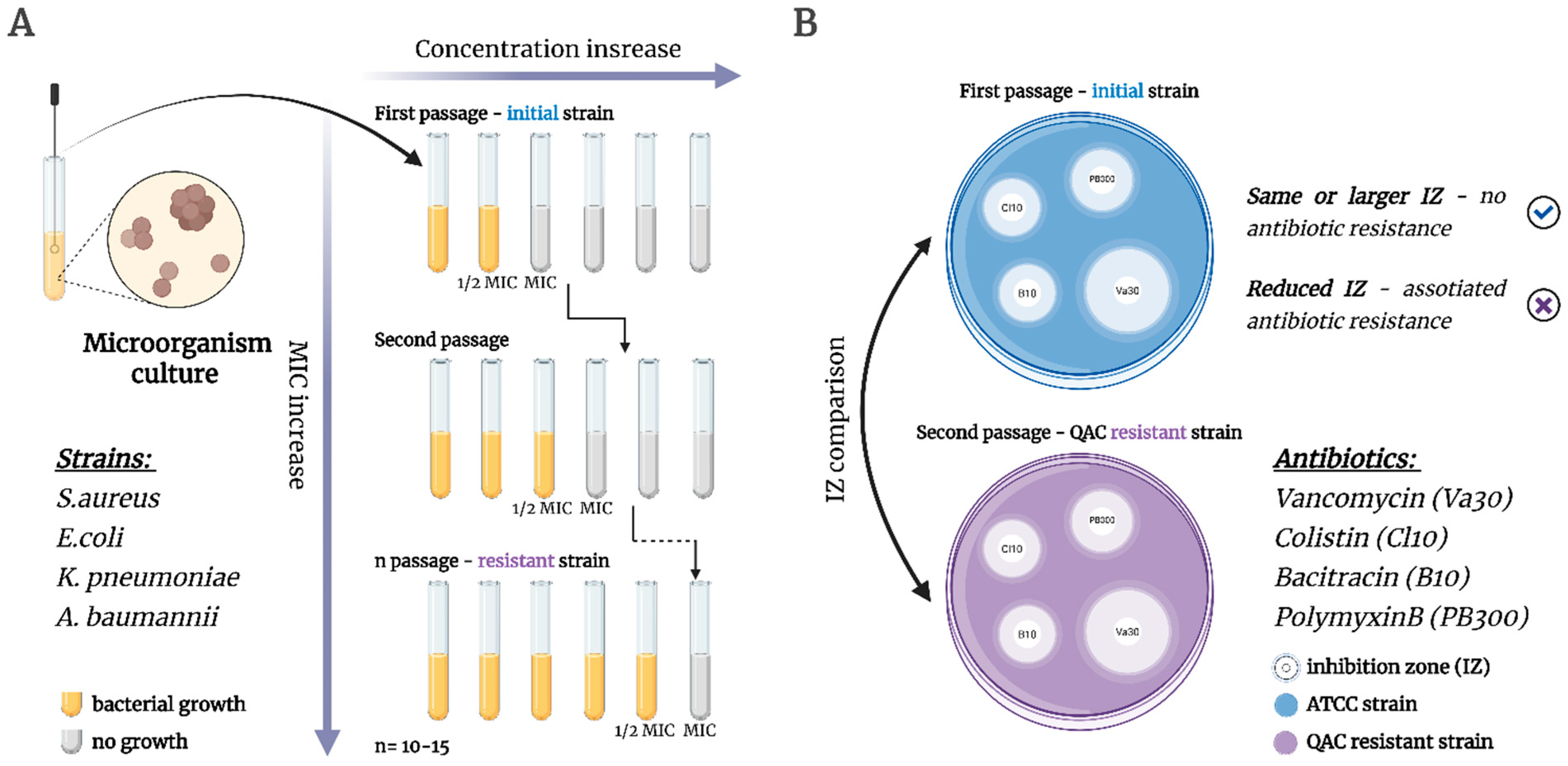
2.4. Bacterial Resistance Assay
3. Materials and Methods
3.1. Chemistry
3.1.1. General Information
3.1.2. Tested Compounds
3.1.3. Synthesis Procedure and Characterization of 1,6-NQ-9 Precursor-4,4′-(naphthalene-1,6-diylbis(oxy))dipyridine
3.2. Microbiological Assay
3.2.1. Bacterial and Fungi Strains
3.2.2. Identification of Microorganisms
3.2.3. Cultivation of Microorganisms
3.2.4. Determination of the Biofilms
3.2.5. Antimicrobial and Antibiofilm Activity
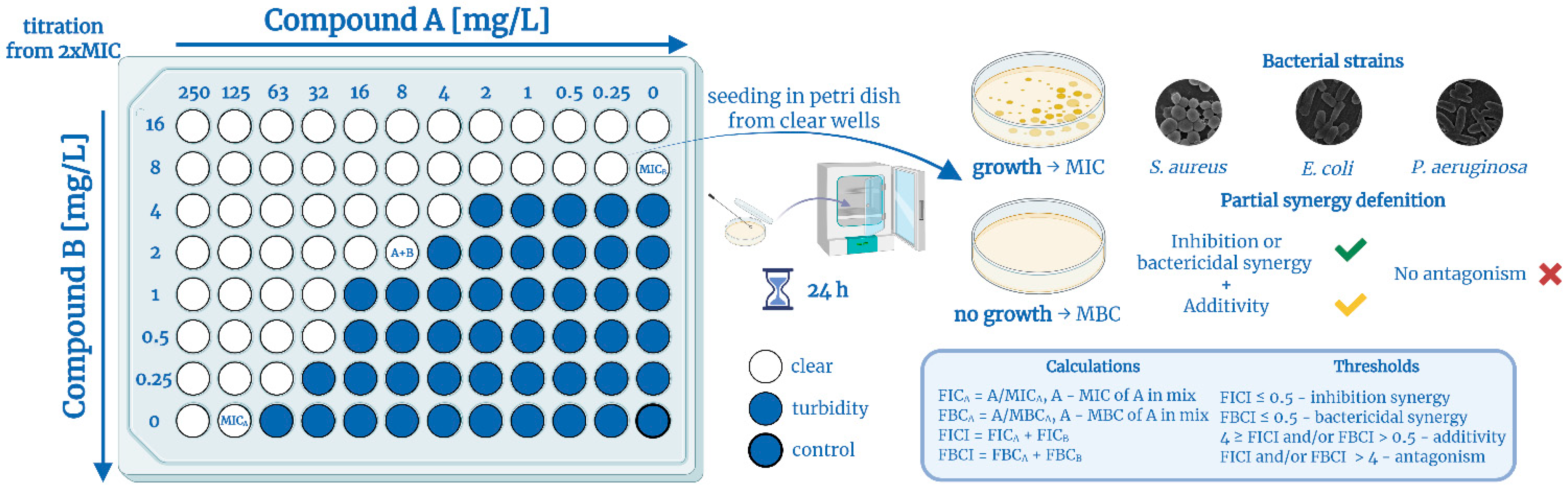
3.2.6. Checkerboard Assay
- MIC (comb.)—MIC value of components in combination
- MIC (alone)—MIC value of individual components
- MBIC (comb.)—MBIC value of components in combination
- MBIC (alone)—MBIC value of individual components
- MBC (comb.)—MBC value of components in combination
- MBC (alone)—MBC value of individual components
- MBEC (comb.)—MBEC value of components in combination
- MBEC (alone)—MBEC value of individual components
3.2.7. Time–Kill Kinetics
3.2.8. Microbial Resistance Assay
3.2.9. Disk Diffusion Method (Co-Resistance to Antibiotics)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| QACs | quaternary ammonium compounds |
| MIC | minimum inhibitory concentration |
| MBC | minimum bactericidal concentration |
| BAC | benzalkonium chloride |
| CPC | cetylpyridinium chloride |
| OCT | octenidine dichloride |
| CHX | chlorohexidine |
| PA | propyl alcohol, propanol-1 |
| IPA | isopropyl alcohol, propanol-2 |
| PE | phenoxyethanol |
| MBIC | minimum biofilm inhibition concentration |
| MBEC | minimum biofilm eradication concentration |
| FIC | fractional inhibitory concentration |
| FBC | fractional bactericidal concentration |
| DMSO | dimethyl sulfoxide |
| ESKAPEE | Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, Enterobacter species, and Escherichia coli |
| ATCC | American type culture collection |
| FICI | fractional inhibitory concentration index |
| FBCI | fractional bactericidal concentration index |
| FBICI | fractional biofilm inhibition concentration index |
| FBECI | fractional biofilm eradication concentration index |
| IZ | inhibition zone |
| Va30 | Vancomycin |
| Cl10 | Colistin |
| B10 | Bacitracin |
| PB300 | Polymyxin B |
| NMR | nuclear magnetic resonance |
| MRSA | methicillin-resistant Staphylococcus aureus |
| MFC | minimum fungicidal concentration |
| CFU | colony-forming unit |
| UV | ultraviolet |
| MALDI-TOF | matrix-assisted laser desorption ionization mass spectrometry |
| PBS | phosphate-buffered saline |
| OD | optical density |
| MHA | mueller–hinton nutrient agar |
| MHB | Mueller–hinton broth |
References
- Levy, S.B.; Marshall, B. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004, 10, S122–S129. [Google Scholar] [CrossRef]
- Ahmed, S.K.; Hussein, S.; Qurbani, K.; Ibrahim, R.H.; Fareeq, A.; Mahmood, K.A.; Mohamed, M.G. Antimicrobial resistance: Impacts, challenges, and future prospects. J. Med. Surg. Public Health 2024, 2, 100081. [Google Scholar] [CrossRef]
- Karnwal, A.; Jassim, A.Y.; Mohammed, A.A.; Al-Tawaha, A.R.M.S.; Selvaraj, M.; Malik, T. Addressing the global challenge of bacterial drug resistance: Insights, strategies, and future directions. Front. Microbiol. 2025, 16, 1517772. [Google Scholar] [CrossRef]
- Chinemerem Nwobodo, D.; Ugwu, M.C.; Oliseloke Anie, C.; Al-Ouqaili, M.T.S.; Chinedu Ikem, J.; Victor Chigozie, U.; Saki, M. Antibiotic resistance: The challenges and some emerging strategies for tackling a global menace. J. Clin. Lab. Anal. 2022, 36, e24655. [Google Scholar] [CrossRef]
- Coque, T.M.; Cantón, R.; Pérez-Cobas, A.E.; Fernández-de-Bobadilla, M.D.; Baquero, F. Antimicrobial Resistance in the Global Health Network: Known Unknowns and Challenges for Efficient Responses in the 21st Century. Microorganisms 2023, 11, 1050. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control; European Medicines Agency. The Bacterial Challenge: Time to React: A Call to Narrow the Gap Between Multidrug-Resistant Bacteria in the EU and the Development of New Antibacterial Agents; EUR-OP: Luxembourg, 2009.
- Sundin, G.W.; Castiblanco, L.F.; Yuan, X.; Zeng, Q.; Yang, C.H. Bacterial disease management: Challenges, experience, innovation and future prospects: Challenges in Bacterial Molecular Plant Pathology. Mol. Plant Pathol. 2016, 17, 1506–1518. [Google Scholar] [CrossRef]
- McDonnell, G.; Russell, A.D. Antiseptics and Disinfectants: Activity, Action, and Resistance. Clin. Microbiol. Rev. 1999, 12, 147–179, Erratum in Clin. Microbiol. Rev. 2001, 14, 227. [Google Scholar] [CrossRef]
- Maillard, J.-Y.; Pascoe, M. Disinfectants and antiseptics: Mechanisms of action and resistance. Nat. Rev. Microbiol. 2024, 22, 4–17. [Google Scholar] [CrossRef]
- Jones, S.; Reagan, K.; Saunders, N. Antiseptics, Disinfectants, and Sterilization. In Advanced Monitoring and Procedures for Small Animal Emergency and Critical Care; Wiley Online Library: Hoboken, NJ, USA, 2023; pp. 837–844. [Google Scholar]
- Aranke, M.; Moheimani, R.; Phuphanich, M.; Kaye, A.D.; Ngo, A.L.; Viswanath, O.; Herman, J. Disinfectants In Interventional Practices. Curr. Pain Headache Rep. 2021, 25, 21. [Google Scholar] [CrossRef]
- Boyce, J.M. Alcohols as Surface Disinfectants in Healthcare Settings. Infect. Control. Hosp. Epidemiol. 2018, 39, 323–328. [Google Scholar] [CrossRef]
- Chang, S.C.; Li, W.C.; Huang, K.Y.; Huang, Y.C.; Chiu, C.H.; Chen, C.J.; Hsieh, Y.C.; Kuo, C.Y.; Shih, S.R.; Lin, T.Y. Efficacy of alcohols and alcohol-based hand disinfectants against human enterovirus 71. J. Hosp. Infect. 2013, 83, 288–293. [Google Scholar] [CrossRef]
- Alajlan, A.A.; Mukhtar, L.E.; Almussallam, A.S.; Alnuqaydan, A.M.; Albakiri, N.S.; Almutari, T.F.; Bin Shehail, K.M.; Aldawsari, F.S.; Alajel, S.M. Assessment of disinfectant efficacy in reducing microbial growth. PLoS ONE 2022, 17, e0269850. [Google Scholar] [CrossRef]
- Kampf, G. Antiseptic Stewardship for Alcohol-Based Hand Rubs. In Antiseptic Stewardship: Biocide Resistance and Clinical Implications; Kampf, G., Ed.; Springer International Publishing: Cham, Switzerland, 2024; pp. 965–973. [Google Scholar]
- Kramer, A.; Arvand, M.; Christiansen, B.; Dancer, S.; Eggers, M.; Exner, M.; Müller, D.; Mutters, N.T.; Schwebke, I.; Pittet, D. Ethanol is indispensable for virucidal hand antisepsis: Memorandum from the alcohol-based hand rub (ABHR) Task Force, WHO Collaborating Centre on Patient Safety, and the Commission for Hospital Hygiene and Infection Prevention (KRINKO), Robert Koch Institute, Berlin, Germany. Antimicrob. Resist. Infect. Control 2022, 11, 93. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Frolov, N.A.; Egorova, K.S.; Seitkalieva, M.M.; Ananikov, V.P. Quaternary ammonium compounds (QACs) and ionic liquids (ILs) as biocides: From simple antiseptics to tunable antimicrobials. Int. J. Mol. Sci. 2021, 22, 6793. [Google Scholar] [CrossRef]
- Bezold, E.L.; Minbiole, K.P.C.; Wuest, W.M. Not all disinfectants are created equal: The importance of mechanistic understanding to drive research forward. Future Microbiol. 2025, 20, 445–447. [Google Scholar] [CrossRef]
- Wang, G.; Yang, L.; Jiang, L.; Chen, J.; Jing, Q.; Mai, Y.; Deng, L.; Lin, Y.; Chen, L.; Chen, Z.; et al. A new class of quaternary ammonium compounds as potent and environmental friendly disinfectants. J. Clean. Prod. 2022, 379, 134632. [Google Scholar] [CrossRef]
- Jones, I.A.; Joshi, L.T. Biocide Use in the Antimicrobial Era: A Review. Molecules 2021, 26, 2276. [Google Scholar] [CrossRef]
- Frolov, N.; Detusheva, E.; Fursova, N.; Ostashevskaya, I.; Vereshchagin, A. Microbiological Evaluation of Novel Bis-Quaternary Ammonium Compounds: Clinical Strains, Biofilms, and Resistance Study. Pharmaceuticals 2022, 15, 514. [Google Scholar] [CrossRef]
- Gilbert, P.; Pemberton, D.; Wilkinson, D.E. Synergism within polyhexamethylene biguanide biocide formulations. J. Appl. Bacteriol. 1990, 69, 593–598. [Google Scholar] [CrossRef]
- Shang, D.; Liu, Y.; Jiang, F.; Ji, F.; Wang, H.; Han, X. Synergistic Antibacterial Activity of Designed Trp-Containing Antibacterial Peptides in Combination With Antibiotics Against Multidrug-Resistant Staphylococcus epidermidis. Front. Microbiol. 2019, 10, 2719. [Google Scholar] [CrossRef]
- Kamble, E.; Sanghvi, P.; Pardesi, K. Synergistic effect of antibiotic combinations on Staphylococcus aureus biofilms and their persister cell populations. Biofilm 2022, 4, 100068. [Google Scholar] [CrossRef]
- Kasthuri, T.; Swetha, T.K.; Bhaskar, J.P.; Pandian, S.K. Rapid-killing efficacy substantiates the antiseptic property of the synergistic combination of carvacrol and nerol against nosocomial pathogens. Arch. Microbiol. 2022, 204, 590. [Google Scholar] [CrossRef]
- Taheri-Araghi, S. Synergistic action of antimicrobial peptides and antibiotics: Current understanding and future directions. Front. Microbiol. 2024, 15, 1390765. [Google Scholar] [CrossRef]
- Yi, L.; Cao, M.; Chen, X.; Bai, Y.; Wang, W.; Wei, X.; Shi, Y.; Zhang, Y.; Ma, T.; Zhu, Z.; et al. In Vitro Antimicrobial Synergistic Activity and the Mechanism of the Combination of Naringenin and Amikacin Against Antibiotic-Resistant Escherichia coli. Microorganisms 2024, 12, 1871. [Google Scholar] [CrossRef]
- Lehmann, R.H. Synergism in Disinfectant Formulation; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001. [Google Scholar]
- Chevalier, J.; Corre, J.; Crémieux, A. Evaluation of synergistic effects of three bactericidal agents associated in an antiseptic formulation. Pharm. Acta Helv. 1995, 70, 155–159. [Google Scholar] [CrossRef]
- Araújo, P.A.; Lemos, M.; Mergulhão, F.; Melo, L.; Simões, M. The Influence of Interfering Substances on the Antimicrobial Activity of Selected Quaternary Ammonium Compounds. Int. J. Food Sci. 2013, 2013, 237581. [Google Scholar] [CrossRef]
- Leclercq, L.; Nardello-Rataj, V. A New Synergistic Strategy for Virus and Bacteria Eradication: Towards Universal Disinfectants. Pharmaceutics 2022, 14, 2791. [Google Scholar] [CrossRef]
- Gao, S.; Sun, Y.; Lu, Z.; Jiang, N.; Yao, H. Synergistic antibacterial and biofilm eradication activity of quaternary-ammonium compound with copper ion. J. Inorg. Biochem. 2023, 243, 112190. [Google Scholar] [CrossRef]
- Pedreira, A.; Fernandes, S.; Simões, M.; García, M.R.; Vázquez, J.A. Synergistic Bactericidal Effects of Quaternary Ammonium Compounds with Essential Oil Constituents. Foods 2024, 13, 1831. [Google Scholar] [CrossRef]
- Jain, D.; Gupta, R.; Mehta, R.; Prabhakaran, P.N.; Kumari, D.; Bhui, K.; Murali, D. Revisiting the Synergistic In Vitro Antimicrobial and Antibiofilm Potential of Chlorhexidine Gluconate and Cetrimide in Combination as an Antiseptic and Disinfectant Agent. Microbiol. Res. 2025, 16, 16. [Google Scholar] [CrossRef]
- Liao, M.; Shen, K.; Ma, K.; Chen, Y.; Li, P.; Gutfreund, P.; Hu, X.; Petkov, J.T.; Lu, J.R. Unveiling the multifaceted mechanisms of action in nonionic and cationic biocide combinations against Gram-negative bacteria. J. Colloid Interface Sci. 2025, 696, 137891. [Google Scholar] [CrossRef]
- Noel Daniel, J.; Keevil, C.W.; Wilks Sandra, A. Synergism versus Additivity: Defining the Interactions between Common Disinfectants. mBio 2021, 12, 10-1128. [Google Scholar] [CrossRef]
- Sanchez, C.A.; Vargas-Cuebas, G.G.; Michaud, M.E.; Allen, R.A.; Morrison-Lewis, K.R.; Siddiqui, S.; Minbiole, K.P.C.; Wuest, W.M. Highly Effective Biocides against Pseudomonas aeruginosa Reveal New Mechanistic Insights Across Gram-Negative Bacteria. ACS Infect. Dis. 2024, 10, 3868–3879. [Google Scholar] [CrossRef]
- Saverina, E.A.; Frolov, N.A.; Kamanina, O.A.; Arlyapov, V.A.; Vereshchagin, A.N.; Ananikov, V.P. From Antibacterial to Antibiofilm Targeting: An Emerging Paradigm Shift in the Development of Quaternary Ammonium Compounds (QACs). ACS Infect. Dis. 2023, 9, 394–422. [Google Scholar] [CrossRef]
- Bailey, D.M.; DeGrazia, C.G.; Hoff, S.J.; Schulenberg, P.L.; O’Connor, J.R.; Paris, D.A.; Slee, A.M. Bispyridinamines: A new class of topical antimicrobial agents as inhibitors of dental plaque. J. Med. Chem. 1984, 27, 1457–1464. [Google Scholar] [CrossRef]
- Frolov, N.A.; Seferyan, M.A.; Detusheva, E.V.; Son, E.; Kolmakov, I.G.; Kartseva, A.S.; Firstova, V.V.; Vereshchagin, A.N.; Elinson, M.N. Development of Naphthalene-Derivative Bis-QACs as Potent Antimicrobials: Unraveling Structure–Activity Relationship and Microbiological Properties. Molecules 2024, 29, 5526. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, D.; Wang, X.; Ding, K. (2-Pyridyl)acetone-Promoted Cu-Catalyzed O-Arylation of Phenols with Aryl Iodides, Bromides, and Chlorides. J. Org. Chem. 2009, 74, 7187–7190. [Google Scholar] [CrossRef]
- Kampf, G. Propan-2-ol. In Antiseptic Stewardship: Biocide Resistance and Clinical Implications; Kampf, G., Ed.; Springer International Publishing: Cham, Switzerland, 2024; pp. 89–115. [Google Scholar]
- Kampf, G. Propan-1-ol. In Antiseptic Stewardship: Biocide Resistance and Clinical Implications; Kampf, G., Ed.; Springer International Publishing: Cham, Switzerland, 2024; pp. 75–88. [Google Scholar]
- Vila, T.; Montelongo-Jauregui, D.; Ahmed, H.; Puthran, T.; Sultan Ahmed, S.; Jabra-Rizk Mary, A. Comparative Evaluations of the Pathogenesis of Candida auris Phenotypes and Candida albicans Using Clinically Relevant Murine Models of Infections. mSphere 2020, 5, e00760-20. [Google Scholar] [CrossRef]
- Satala, D.; Juszczak, M.; Wronowska, E.; Surowiec, M.; Kulig, K.; Kozik, A.; Rapala-Kozik, M.; Karkowska-Kuleta, J. Similarities and Differences among Species Closely Related to Candida albicans: C. tropicalis, C. dubliniensis, and C. auris. Cell. Microbiol. 2022, 2022, 2599136. [Google Scholar] [CrossRef]
- Rutala, W.A.; Weber, D.J. Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008; Centers for Disease Control and Prevention: Atlanta, GR, USA, 2019.
- Luther, M.K.; Bilida, S.; Mermel, L.A.; LaPlante, K.L. Ethanol and Isopropyl Alcohol Exposure Increases Biofilm Formation in Staphylococcus aureus and Staphylococcus epidermidis. Infect. Dis. Ther. 2015, 4, 219–226. [Google Scholar] [CrossRef]
- Thi, M.T.; Wibowo, D.; Rehm, B.H.A. Pseudomonas aeruginosa Biofilms. Int. J. Mol. Sci. 2020, 21, 8671. [Google Scholar] [CrossRef]
- Kundu, S.; Aswal, V.K.; Kohlbrecher, J. Effect of ethanol on structures and interactions among globular proteins. Chem. Phys. Lett. 2017, 670, 71–76. [Google Scholar] [CrossRef]
- Martinez-Irujo, J.J.; Villahermosa, M.L.; Alberdi, E.; Santiago, E. A checkerboard method to evaluate interactions between drugs. Biochem. Pharmacol. 1996, 51, 635–644. [Google Scholar] [CrossRef]
- Bellio, P.; Fagnani, L.; Nazzicone, L.; Celenza, G. New and simplified method for drug combination studies by checkerboard assay. MethodsX 2021, 8, 101543. [Google Scholar] [CrossRef]
- Fatsis-Kavalopoulos, N.; Sánchez-Hevia, D.L.; Andersson, D.I. Beyond the FIC index: The extended information from fractional inhibitory concentrations (FICs). J. Antimicrob. Chemother. 2024, 79, 2394–2396. [Google Scholar] [CrossRef] [PubMed]
- Meletiadis, J.; Pournaras, S.; Roilides, E.; Walsh Thomas, J. Defining Fractional Inhibitory Concentration Index Cutoffs for Additive Interactions Based on Self-Drug Additive Combinations, Monte Carlo Simulation Analysis, and In Vitro-In Vivo Correlation Data for Antifungal Drug Combinations against Aspergillus fumigatus. Antimicrob. Agents Chemother. 2010, 54, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Verderosa, A.D.; Totsika, M.; Fairfull-Smith, K.E. Bacterial Biofilm Eradication Agents: A Current Review. Front Chem 2019, 7, 824. [Google Scholar] [CrossRef]
- Paluch, E.; Szperlik, J.; Lamch, Ł.; Wilk, K.A.; Obłąk, E. Biofilm eradication and antifungal mechanism of action against Candida albicans of cationic dicephalic surfactants with a labile linker. Sci. Rep. 2021, 11, 8896. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, P.; Wang, Y.; Hao, Y. Mechanisms and Control Measures of Mature Biofilm Resistance to Antimicrobial Agents in the Clinical Context. ACS Omega 2020, 5, 22684–22690. [Google Scholar] [CrossRef]
- Crnčević, D.; Krce, L.; Brkljača, Z.; Cvitković, M.; Babić Brčić, S.; Čož-Rakovac, R.; Odžak, R.; Šprung, M. A dual antibacterial action of soft quaternary ammonium compounds: Bacteriostatic effects, membrane integrity, and reduced in vitro and in vivo toxicity. RSC Adv. 2025, 15, 1490–1506. [Google Scholar] [CrossRef]
- Lu, Z.; Mahony, A.K.; Arnold, W.A.; Marshall, C.W.; McNamara, P.J. Quaternary ammonia compounds in disinfectant products: Evaluating the potential for promoting antibiotic resistance and disrupting wastewater treatment plant performance. Environ. Sci. Adv. 2024, 3, 208–226. [Google Scholar] [CrossRef]
- Knauf Gregory, A.; Cunningham Ashley, L.; Kazi Misha, I.; Riddington Ian, M.; Crofts Alexander, A.; Cattoir, V.; Trent, M.S.; Davies Bryan, W. Exploring the Antimicrobial Action of Quaternary Amines against Acinetobacter baumannii. mBio 2018, 9, 10-1128. [Google Scholar] [CrossRef]
- Hossain, T.J. Methods for screening and evaluation of antimicrobial activity: A review of protocols, advantages, and limitations. Eur. J. Microbiol. Immunol. 2024, 14, 97–115. [Google Scholar] [CrossRef]
- Asín-Prieto, E.; Rodríguez-Gascón, A.; Isla, A. Applications of the pharmacokinetic/pharmacodynamic (PK/PD) analysis of antimicrobial agents. J. Infect. Chemother. 2015, 21, 319–329. [Google Scholar] [CrossRef]
- Frolov, N.A.; Seferyan, M.A.; Detusheva, E.V.; Saverina, E.A.; Son, E.; Akchurin, R.N.; Kartseva, A.S.; Firstova, V.V.; Vereshchagin, A.N. Exploring the correlation of linker structure and antimicrobial activities of pyridinium-based cationic biocides: Aromatic versus aliphatic architectures. Eur. J. Med. Chem. 2025, 292, 117673. [Google Scholar] [CrossRef] [PubMed]
- Hora, P.I.; Pati, S.G.; McNamara, P.J.; Arnold, W.A. Increased Use of Quaternary Ammonium Compounds during the SARS-CoV-2 Pandemic and Beyond: Consideration of Environmental Implications. Environ. Sci. Technol. Lett. 2020, 7, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Mahony, A.K.; McNamara, P.J.; Arnold, W.A. Quaternary Ammonium Compounds (QACs) in Wastewater Influent and Effluent Throughout the COVID-19 Pandemic. Environ. Sci. Technol. 2023, 57, 20148–20158. [Google Scholar] [CrossRef]
- Boyce, J.M. Quaternary ammonium disinfectants and antiseptics: Tolerance, resistance and potential impact on antibiotic resistance. Antimicrob. Resist. Infect. Control 2023, 12, 32. [Google Scholar] [CrossRef]
- Murray, L.M.; Hayes, A.; Snape, J.; Kasprzyk-Hordern, B.; Gaze, W.H.; Murray, A.K. Co-selection for antibiotic resistance by environmental contaminants. npj Antimicrob. Resist. 2024, 2, 9. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, L.; Li, H.; Chen, X.; Zhang, L.; Shan, T.; Wang, J.; Chen, D.; Shen, J.; Zhou, X.; et al. Challenges of quaternary ammonium antimicrobial agents: Mechanisms, resistance, persistence and impacts on the microecology. Sci. Total Environ. 2025, 958, 178020. [Google Scholar] [CrossRef] [PubMed]
- Bland, R.; Waite-Cusic, J.; Weisberg, A.J.; Riutta, E.R.; Chang, J.H.; Kovacevic, J. Adaptation to a Commercial Quaternary Ammonium Compound Sanitizer Leads to Cross-Resistance to Select Antibiotics in Listeria monocytogenes Isolated From Fresh Produce Environments. Front. Microbiol. 2022, 12, 782920. [Google Scholar] [CrossRef]
- Bialvaei, A.Z.; Samadi Kafil, H. Colistin, mechanisms and prevalence of resistance. Curr. Med. Res. Opin. 2015, 31, 707–721. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.; Yang, S.; Rao, X. Vancomycin resistant Staphylococcus aureus infections: A review of case updating and clinical features. J. Adv. Res. 2020, 21, 169–176. [Google Scholar] [CrossRef]
- Yin, J.; Meng, Q.; Cheng, D.; Fu, J.; Luo, Q.; Liu, Y.; Yu, Z. Mechanisms of bactericidal action and resistance of polymyxins for Gram-positive bacteria. Appl. Microbiol. Biotechnol. 2020, 104, 3771–3780. [Google Scholar] [CrossRef]
- Mohapatra, S.S.; Dwibedy, S.K.; Padhy, I. Polymyxins, the last-resort antibiotics: Mode of action, resistance emergence, and potential solutions. J. Biosci. 2021, 46, 85. [Google Scholar] [CrossRef] [PubMed]
- Frolov, N.A.; Smirnov, V.A.; Detusheva, E.V.; Vereshchagin, A.N. Novel naphthalene-based bis-pyridinium compounds with pronounced antibacterial activity. Mendeleev Commun. 2022, 32, 606–608. [Google Scholar] [CrossRef]
- O’Toole, G.A.; Kolter, R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: A genetic analysis. Mol. Microbiol. 1998, 28, 449–461. [Google Scholar] [CrossRef]
- StepanoviĆ, S.; VukoviĆ, D.; Hola, V.; Bonaventura, G.D.; DjukiĆ, S.; ĆIrkoviĆ, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by Staphylococci. Apmis 2007, 115, 891–899. [Google Scholar] [CrossRef]
- Weinstein, M.P.; Lewis, J.S., II; Bobenchik, A.M.; Campeau, S.; Cullen, S.K.; Galas, M.F.; Gold, H.; Humphries, R.M.; Kirn, T.J., Jr.; Limbago, B.; et al. Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Kim, M.; Weigand, M.R.; Oh, S.; Hatt, J.K.; Krishnan, R.; Tezel, U.; Pavlostathis, S.G.; Konstantinidis, K.T. Widely used benzalkonium chloride disinfectants can promote antibiotic resistance. Appl. Environ. Microbiol. 2018, 84, e01201-18. [Google Scholar] [CrossRef]
- Braoudaki, M.; Hilton, A. Adaptive resistance to biocides in Salmonella enterica and Escherichia coli O157 and cross-resistance to antimicrobial agents. J. Clin. Microbiol. 2004, 42, 73–78. [Google Scholar] [CrossRef]
- Rensch, U.; Klein, G.; Kehrenberg, C. Analysis of triclosan-selected Salmonella enterica mutants of eight serovars revealed increased aminoglycoside susceptibility and reduced growth rates. PLoS ONE 2013, 8, e78310. [Google Scholar] [CrossRef] [PubMed]




| Compounds | Reference Strains | Clinical Isolates | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sa | Ec | Kp | Ab | Pa | Sa | Ec | Kp | Ab | Pa | |
| Planktonic cells (MIC/MBC [mg/L]) | ||||||||||
| Control | ||||||||||
| CPC | 4 ± 2.3 | 8 ± 4.6 | 63 ± 17.9 | 32 ± 9.2 | 500 | 2 ± 0.6 | 8 ± 4.6 | 8 ± 2.3 | 16 | 32 |
| 16 | 8 ± 2.3 | 250 | 125 | >500 | 16 ± 4.6 | 63 | 16 | 32 ± 9.2 | 125 ± 35.8 | |
| OCT | 0.5 ± 0.3 | 0.5 | 4 ± 1.2 | 32 | 8 ± 4.6 | 0.5 | 2 ± 1.2 | 2 | 32 ± 9.2 | 32 ± 9.2 |
| 2 ± 1.2 | 0.5 ± 0.3 | 8 ± 4.6 | 125 ± 72.2 | 16 | 2 ± 0.6 | 4 ± 1.2 | 4 ± 1.2 | 125 | 63 ± 35.8 | |
| 1,6-NQ-9 | 2 | 8 | 16 ± 9.2 | 63 | 32 ± 18.5 | 2 ± 0.6 | 8 ± 2.3 | 32 ± 9.2 | 125 | 63 |
| 8 ± 4.6 | 8 ± 4.6 | 32 | 63 ± 18.5 | 63 | 4 ± 2.3 | 16 | 63 | 125 ± 95.3 | 63 ± 17.9 | |
| PhE * | 125 ± 35.8 | 32 | 63 ± 18.5 | 16 ± 9.2 | 16 ± 4.6 | 250 ± 72.2 | 63 ± 17.9 | 125 | 16 ± 4.6 | 32 |
| 500 | 32 ± 9.2 | 125 | 125 | 125 | 500 | 63 | 250 | 125 ± 72.2 | 125 | |
| IPA * | 63 | 32 ± 9.2 | 32 | 16 ± 4.6 | 16 ± 4.6 | 63 | 32 ± 18.5 | 32 ± 9.2 | 16 ± 4.6 | 16 ± 4.6 |
| 250 | 32 ± 18.5 | 63 ± 35.8 | 63 | 63 ± 18.5 | 250 | 32 | 125 | 32 | 63 ± 35.8 | |
| IPAP * | 16 ± 9.2 | 32 | 63 ± 18.5 | 16 ± 4.6 | 16 ± 4.6 | 63 ± 17.9 | 63 | 125 | 16 ± 9.2 | 16 ± 9.2 |
| 250 | 32 ± 9.2 | 125 ± 72.2 | 63 ± 17.9 | 63 | 250 ± 72.2 | 63 ± 35.8 | 125 ± 72.2 | 63 | 63 | |
| Compositions | ||||||||||
| PhE CPC | 0.5 ± 0.3 | 16 | 32 | 8 | 32 ± 9.2 | 0.5 | 16 ± 4.6 | 16 | 8 ± 2.3 | 63 |
| 4 | 16 ± 9.2 | 32 | 8 ± 4.6 | 125 | 4 ± 1.2 | 32 | 32 ± 18.5 | 16 ± 4.6 | 125 ± 35.8 | |
| PhE OCT | 1 ± 0.6 | 2 | 1 | 2 ± 0.6 | 2 | 1 | 2 ± 1.2 | 2 ± 0.6 | 4 | 2 ± 1.2 |
| 8 ± 4.6 | 2 ± 1.2 | 1 ± 0.6 | 2 | 2 ± 1.2 | 8 ± 4.6 | 4 | 4 ± 2.3 | 8 ± 2.3 | 8 | |
| PhE 1,6-NQ-9 | 2 ± 0.6 | 8 ± 2.3 | 32 | 32 ± 9.2 | 32 ± 17.9 | 0.5 | 8 ± 4.6 | 32 | 8 | 32 |
| 8 ± 4.6 | 8 ± 4.6 | 32 ± 9.2 | 32 | 63 | 8 ± 4.6 | 8 | 32 ± 9.2 | 32 | 125 ± 35.8 | |
| IPA CPC | 2 | 8 | 32 | 4 ± 1.2 | 32 ± 18.5 | 0.5 | 16 ± 4.6 | 16 | 8 ± 2.3 | 32 ± 18.5 |
| 8 | 16 ± 4.6 | 32 | 8 ± 4.6 | 63 | 2 ± 0.6 | 32 | 16 ± 9.2 | 8 | 63 | |
| IPA OCT | 1 ± 0.3 | 4 | 1 | 2 ± 0.6 | 1 | 0.5 | 4 ± 3.2 | 2 ± 0.6 | 2 | 4 ± 1.2 |
| 2 | 4 ± 2.3 | 1 ± 0.3 | 2 ± 0.6 | 1 ± 0.3 | 2 ± 0.6 | 4 ± 2.3 | 4 | 4 ± 2.3 | 4 | |
| IPA 1,6-NQ-9 | 1 | 4 ± 1.2 | 3 ± 18.52 | 16 | 16 ± 9.2 | 0.5 ± 0.3 | 4 | 32 | 16 ± 9.2 | 32 ± 18.5 |
| 4 | 8 ± 4.6 | 32 | 16 | 63 ± 18.5 | 4 ± 1.2 | 4 ± 3.2 | 63 ± 18.5 | 32 | 63 | |
| IPAP CPC | 1 ± 0.3 | 16 | 16 | 8 ± 2.3 | 16 | 0.5 | 8 ± 4.6 | 16 | 4 ± 1.2 | 16 ± 4.6 |
| 4 | 16 ± 4.6 | 16 ± 9.2 | 8 ± 4.6 | 32 ± 9.2 | 1 | 16 | 16 ± 4.6 | 16 | 63 ± 35.8 | |
| IPAP OCT | 1 ± 0.6 | 1 | 1 ± 0.8 | 2 | 2 ± 0.6 | 0.5 ± 0.3 | 2 | 2 ± 0.6 | 2 | 2 |
| 4 ± 2.3 | 1 ± 0.3 | 1 ± 0.3 | 2 ± 1.2 | 2 ± 1.2 | 4 | 2 ± 0.6 | 8 | 4 ± 2.3 | 4 ± 3.2 | |
| IPAP 1,6-NQ-9 | 0.5 | 4 ± 1.2 | 16 | 16 ± 4.6 | 16 | 0.5 ± 0.3 | 4 | 16 | 4 | 16 |
| 2 ± 1.2 | 4 ± 2.3 | 16 ± 4.6 | 16 ± 9.2 | 63 ± 17.9 | 1 ± 0.6 | 4 ± 2.3 | 16 ± 4.6 | 8 ± 4.6 | 32 ± 9.2 | |
| Compounds | Reference Strains | Clinical Isolates | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sa | Ec | Kp | Ab | Pa | Sa | Ec | Kp | Ab | Pa | |
| Biofilms (MBIC/MBEC [mg/L]) | ||||||||||
| Control | ||||||||||
| CPC | 16 ± 9.2 | 16 | 63 ± 35.8 | 250 ± 144.3 | 500 | 8 ± 4.6 | 125 ± 72.2 | 63 ± 35.8 | 250 ± 144.3 | 500 |
| 63 | 63 ± 35.8 | 250 | 500 | >500 | 32 ± 9.2 | 250 | 500 | 500 | 500 ± 144.3 | |
| OCT | 4 | 8 ± 2.3 | 16 ± 9.2 | 250 | 125 ± 72.2 | 4 ± 1.2 | 16 ± 9.2 | 16 ± 4.6 | 32 ± 17.9 | 250 |
| 8 ± 2.3 | 16 ± 4.6 | 63 | 250 ± 144.3 | 500 | 8 ± 4.6 | 125 ± 35.8 | 125 | 250 | 250 ± 144.3 | |
| 1,6-NQ-9 | 8 | 8 ± 4.6 | 63 ± 35.8 | 63 ± 17.9 | 500 ± 144.3 | 4 ± 1.2 | 8 ± 2.3 | 63 | 63 ± 17.9 | 500 |
| 63 ± 17.9 | 8 | 500 | 250 | 500 ± 144.3 | 8 ± 2.3 | 16 | 125 | 125 ± 35.8 | 500 | |
| PhE * | 32 | 16 ± 4.6 | 63 | 63 ± 17.9 | 125 | 63 ± 17.9 | 32 ± 9.2 | 63 ± 17.9 | 63 | 32 |
| 63 | 32 | 125 ± 72.2 | 250 | 125 ± 35.8 | 125 | 63 ± 35.8 | 125 ± 35.8 | 125 | 125 ± 95.3 | |
| IPA * | 32 ± 9.2 | 16 | 63 | 63 ± 17.9 | 125 | 63 | 32 | 63 ± 17.9 | 63 ± 17.9 | 32 ± 17.9 |
| 63 | 32 ± 17.9 | 63 ± 17.9 | 250 | 125 | 125 ± 72.2 | 63 ± 17.9 | 125 ± 95.3 | 250 | 125 | |
| IPAP * | 32 | 16 ± 4.6 | 63 ± 17.9 | 63 | 125 ± 35.8 | 32 | 32 ± 9.2 | 63 ± 17.9 | 63 | 32 ± 17.9 |
| 63 ± 35.8 | 32 ± 9.2 | 63 ± 17.9 | 12 ± 35.85 | 125 | 125 ± 35.8 | 63 | 125 | 12 ± 72.25 | 125 ± 35.8 | |
| Compositions | ||||||||||
| PhE CPC | 4 | 8 | 32 | 16 ± 4.6 | 32 | 4 ± 2.3 | 16 | 16 ± 9.2 | 16 | 125 ± 35.8 |
| 8 ± 4.6 | 32 ± 17.9 | 32 ± 17.9 | 32 | 63 | 16 | 32 ± 9.2 | 32 | 125 ± 35.8 | 125 ± 35.8 | |
| PhE OCT | 1 ± 0.3 | 4 ± 2.3 | 8 | 16 ± 4.6 | 16 ± 9.2 | 2 ± 1.2 | 4 | 16 ± 4.6 | 16 ± 4.6 | 16 ± 4.6 |
| 4 | 8 | 16 ± 4.6 | 32 | 63 | 4 | 16 ± 4.6 | 32 | 32 | 32 | |
| PhE 1,6-NQ-9 | 8 ± 4.6 | 8 | 32 ± 9.2 | 32 ± 9.2 | 32 | 4 ± 2.3 | 16 ± 4.6 | 32 ± 9.2 | 32 | 32 ± 9.2 |
| 8 | 8 ± 2.3 | 32 ± 9.2 | 63 ± 17.9 | 63 ± 17.9 | 63 | 16 ± 4.6 | 32 ± 9.2 | 63 ± 17.9 | 63 | |
| IPA CPC | 4 ± 1.2 | 8 | 16 | 63 | 63 | 4 ± 1.2 | 8 ± 2.3 | 16 ± 9.2 | 32 ± 9.2 | 32 |
| 4 | 8 ± 2.3 | 32 ± 17.9 | 250 ± 144.3 | 125 ± 35.8 | 4 ± 1.2 | 125 | 32 | 63 ± 17.9 | 63 ± 17.9 | |
| IPA OCT | 2 ± 0.6 | 4 | 4 ± 2.3 | 16 ± 4.6 | 8 | 2 | 4 ± 2.3 | 16 ± 9.2 | 16 | 16 |
| 2 ± 0.6 | 8 ± 4.6 | 8 | 32 ± 9.2 | 32 | 4 ± 2.3 | 4 | 32 | 32 | 32 ± 17.9 | |
| IPA 1,6-NQ-9 | 4 | 8 | 16 | 32 ± 9.2 | 32 ± 17.9 | 4 | 8 ± 4.6 | 16 ± 4.6 | 32 ± 9.2 | 16 |
| 4 | 8 ± 4.6 | 32 ± 9.2 | 32 ± 17.9 | 63 | 8 ± 4.6 | 63 ± 17.9 | 63 | 63 | 32 | |
| IPAP CPC | 8 | 8 | 8 | 32 ± 17.9 | 32 | 8 ± 6.1 | 8 | 16 | 32 | 8 ± 2.3 |
| 8 ± 6.1 | 16 ± 9.2 | 32 ± 17.9 | 32 | 63 ± 17.9 | 8 | 16 ± 4.6 | 32 ± 17.9 | 63 ± 35.8 | 32 | |
| IPAP OCT | 1 | 2 ± 0.6 | 8 | 8 ± 2.3 | 16 | 2 ± 1.2 | 2 ± 0.6 | 16 | 16 | 16 |
| 4 ± 2.3 | 4 | 8 ± 2.3 | 16 | 63 ± 17.9 | 4 | 8 ± 2.3 | 16 ± 4.6 | 32 ± 17.9 | 125 ± 35.8 | |
| IPAP 1,6-NQ-9 | 4 | 4 ± 1.2 | 8 ± 4.6 | 32 ± 9.2 | 32 | 4 | 4 | 16 | 32 ± 9.2 | 8 |
| 8 ± 2.3 | 16 ± 4.6 | 16 | 32 ± 17.9 | 63 ± 35.8 | 8 ± 4.6 | 8 ± 4.6 | 32 ± 9.2 | 32 | 16 ± 4.6 | |
| Combinations | Reference Strains | Clinical Isolates | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sa | Ec | Kp | Ab | Pa | Sa | Ec | Kp | Ab | Pa | |
| Planktonic cells (FICI/FBCI) | ||||||||||
| CPC + OCT | 1.13 | 2.13 | 0.53 | 0.13 | 0.25 | 1.25 | 1.25 | 1.25 | 0.38 | 0.13 |
| 0.28 | 2.13 | 8.06 | 0.03 | 1.03 | 0.56 | 0.53 | 19.53 | 0.31 | 0.38 | |
| CPC + 1,6-NQ-9 | 0.38 | 0.50 | 1.25 | 0.09 | 1.05 | 0.50 | 1.00 | 1.25 | 0.28 | 2.95 |
| 0.19 | 0.50 | 0.56 | 0.10 | 4.47 | 0.31 | 0.63 | 1.25 | 0.31 | 5.97 | |
| OCT + 1,6-NQ-9 | 1.25 | 2.13 | 0.63 | 0.19 | 0.63 | 1.25 | 1.25 | 1.06 | 0.31 | 0.19 |
| 0.31 | 34.00 | 0.31 | 0.10 | 1.25 | 1.50 | 1.25 | 0.53 | 0.26 | 0.25 | |
| Biofilms (FBICI/FBECI) | ||||||||||
| CPC + OCT | 0.63 | 0.38 | 0.63 | 0.06 | 0.16 | 0.75 | 0.14 | 0.63 | 0.56 | 0.10 |
| 0.56 | 0.63 | 0.16 | 0.10 | 0.25 | 0.63 | 0.05 | 0.16 | 0.19 | 1.50 | |
| CPC + 1,6-NQ-9 | 0.38 | 0.75 | 0.51 | 0.16 | 0.25 | 0.75 | 0.53 | 1.00 | 0.32 | 0.13 |
| 0.13 | 4.44 | 0.38 | 0.19 | 1.00 | 1.25 | 2.09 | 1.25 | 0.63 | 1.00 | |
| OCT + 1,6-NQ-9 | 0.75 | 0.50 | 0.63 | 0.16 | 0.16 | 1.00 | 0.38 | 0.63 | 0.75 | 0.38 |
| 0.28 | 3.00 | 0.29 | 0.25 | 1.00 | 1.00 | 0.14 | 0.26 | 0.38 | 1.50 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frolov, N.A.; Seferyan, M.A.; Detusheva, E.V.; Son, E.; Kolmakov, I.G.; Vereshchagin, A.N. In Pursuit of a Better Biocide Composition: Synergistic and Additive Effects of QAC-Based Formulations Against Planktonic and Biofilm Cultures. Int. J. Mol. Sci. 2025, 26, 12098. https://doi.org/10.3390/ijms262412098
Frolov NA, Seferyan MA, Detusheva EV, Son E, Kolmakov IG, Vereshchagin AN. In Pursuit of a Better Biocide Composition: Synergistic and Additive Effects of QAC-Based Formulations Against Planktonic and Biofilm Cultures. International Journal of Molecular Sciences. 2025; 26(24):12098. https://doi.org/10.3390/ijms262412098
Chicago/Turabian StyleFrolov, Nikita A., Mary A. Seferyan, Elena V. Detusheva, Elizabeth Son, Ilya G. Kolmakov, and Anatoly N. Vereshchagin. 2025. "In Pursuit of a Better Biocide Composition: Synergistic and Additive Effects of QAC-Based Formulations Against Planktonic and Biofilm Cultures" International Journal of Molecular Sciences 26, no. 24: 12098. https://doi.org/10.3390/ijms262412098
APA StyleFrolov, N. A., Seferyan, M. A., Detusheva, E. V., Son, E., Kolmakov, I. G., & Vereshchagin, A. N. (2025). In Pursuit of a Better Biocide Composition: Synergistic and Additive Effects of QAC-Based Formulations Against Planktonic and Biofilm Cultures. International Journal of Molecular Sciences, 26(24), 12098. https://doi.org/10.3390/ijms262412098