Therapeutic Potential of Beaucarnea recurvata Leaf Extract Against Ulcerative Colitis: Integrating Phytochemical Profiling, Network Pharmacology, and Experimental Validation
Abstract
1. Introduction
2. Results
2.1. Metabolite Characterization via UPLC-ESI-MS/MS
2.1.1. Triterpenoid Compounds and Steroidal Glycosides
2.1.2. Flavonoids
2.1.3. Phenolic Acid Derivatives
2.2. Network Pharmacology-Based Mechanistic Investigation
2.2.1. Assessment of Bioactive Compound Drug-like Properties and Target Identification
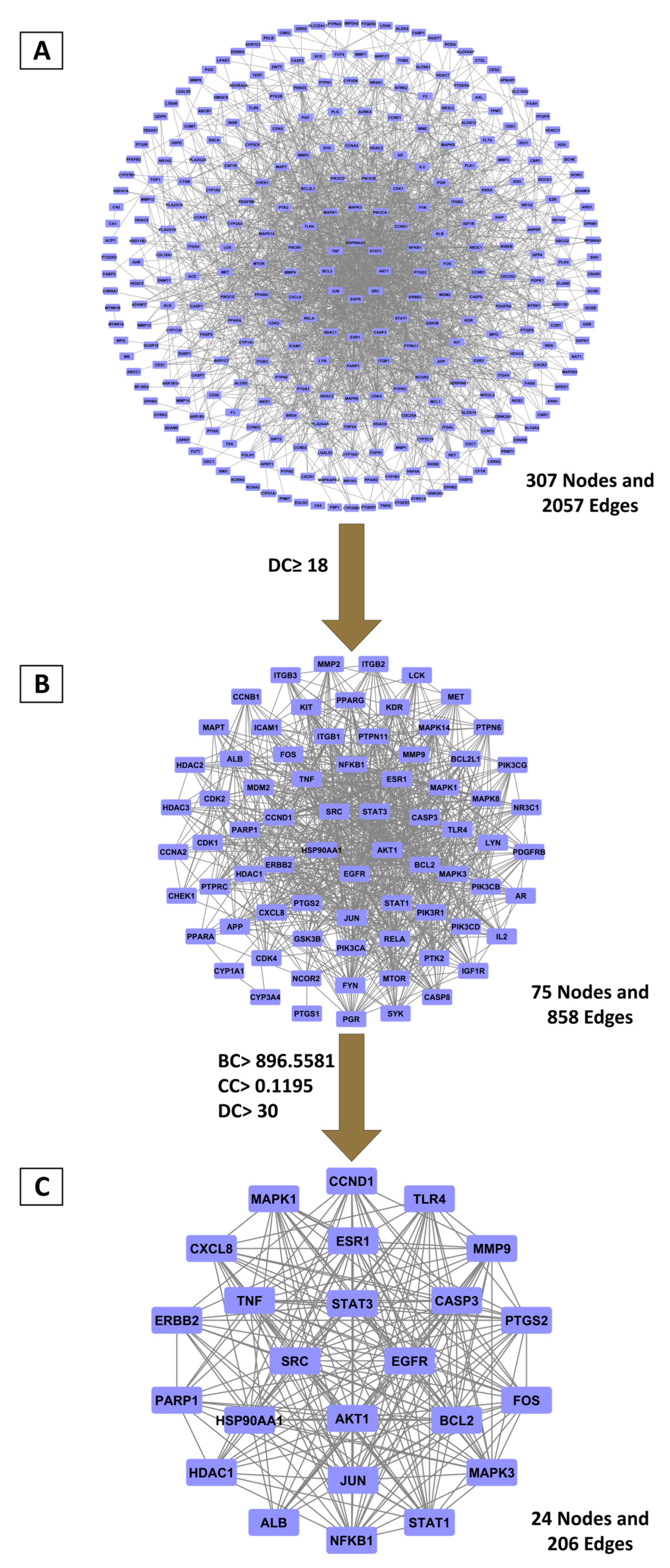
2.2.2. Hub Gene Discovery Through PPI Network Topology Analysis
2.2.3. Primary BRLE Bioactive Components Associated with UC Molecular Targets
2.2.4. Functional Pathway Analysis of BRLE’s Principal Targets in UC Treatment
2.3. Molecular Docking Analysis of BRLE Compounds Against UC-Related Targets
2.4. In Vivo Experimental Validation
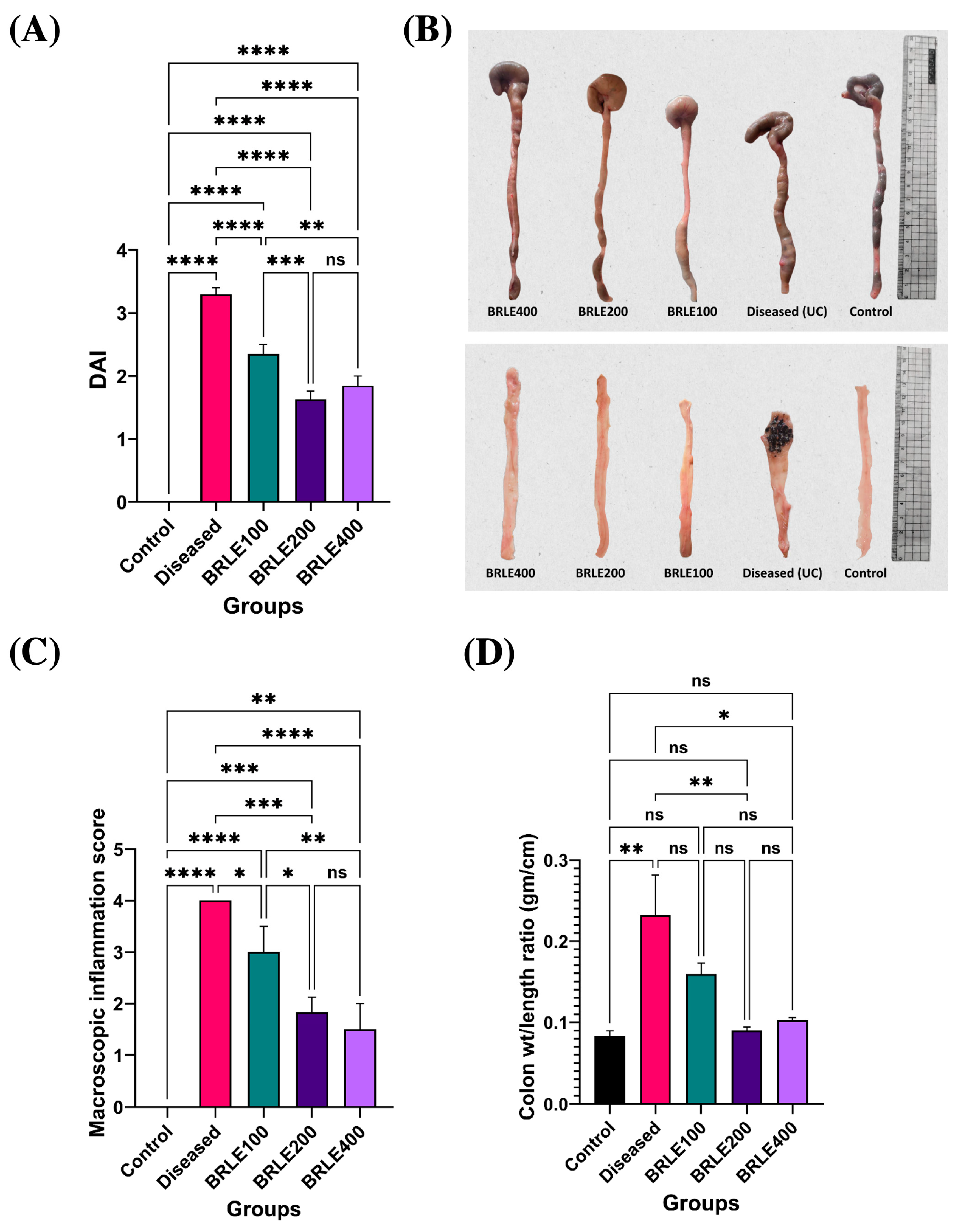
2.4.1. Effect of BRLE on DAI
2.4.2. Macroscopic Assessment of Colonic Inflammation
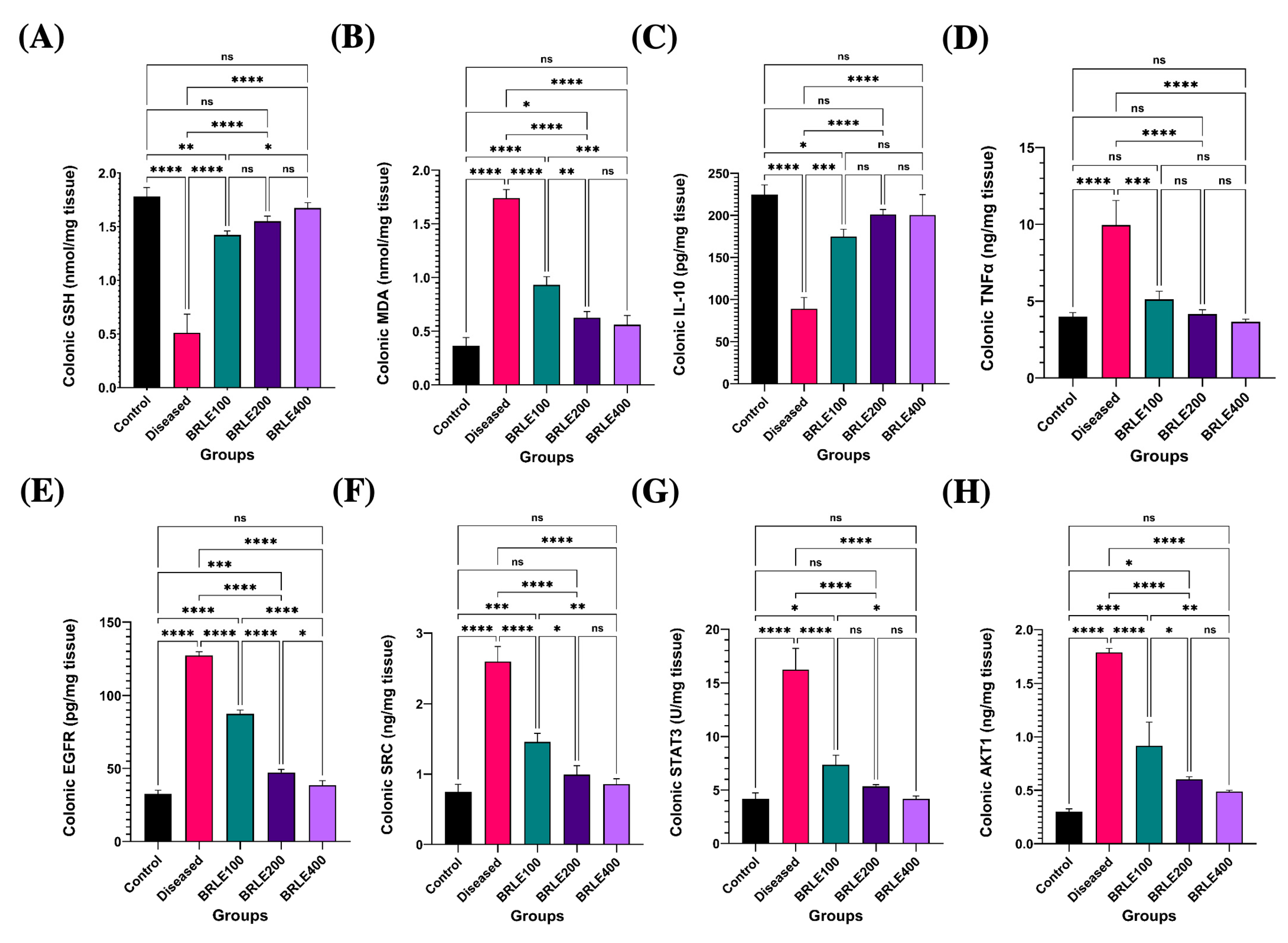
2.4.3. Antioxidant and Immunomodulatory Effects of BRLE
2.4.4. Molecular Target Validation: BRLE-Mediated Modulation of Network Pharmacology-Predicted Pathways
2.4.5. Histopathological Analysis of Colonic Tissue Specimens
3. Discussion
4. Materials and Methods
4.1. Plant Material and Extraction Procedures
4.2. Ultra-Performance Liquid Chromatography-Electrospray Tandem Mass Spectrometry (UPLC-ESI-MS/MS) Analysis of BRLE
4.3. Network Pharmacology
4.3.1. Screening of Bioactive Components in BRLE
4.3.2. Determination of Common Targets Between UC and BRLE Active Components
4.3.3. Construction of Protein–Protein Interaction (PPI) Networks
4.3.4. Development of Compound-Target Interaction Networks
4.3.5. Functional Annotation Through Gene Ontology and KEGG Pathway Analysis
4.4. Molecular Docking Analysis
4.5. In Vivo Experimental Design
4.5.1. Experimental Animals, Ethical Compliance, and Study Design
4.5.2. Animal Welfare and Monitoring
4.5.3. Sample Collection and Preparation
4.5.4. Disease Severity Assessment
4.5.5. Macroscopic Pathological Evaluation
4.5.6. Biochemical and Molecular Marker Analysis
Oxidative Stress Parameters
Inflammatory Cytokines
Network Pharmacology Targets
4.5.7. Histopathological Analysis
4.5.8. Statistical Methodology
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKT1 | RAC-alpha serine/threonine-protein kinase |
| ALA | Alanine |
| ANOVA | Analysis of Variance |
| ARRIVE | Animal Research: Reporting of In Vivo Experiments |
| BC | Betweenness Centrality |
| BP | Biological Processes |
| BRLE | Beaucarnea recurvata Leaf Extract |
| CASTp | Computed Atlas for Surface Topography of Proteins |
| CC | Closeness Centrality |
| CCND1 | Cyclin D1 |
| CD | Cluster of Differentiation (inferred) |
| COX | Cyclooxygenase |
| CytoNCA | Cytoscape Network Centrality Analysis |
| DAI | Disease Activity Index |
| DC | Degree Centrality |
| DNA | Deoxyribonucleic Acid |
| EGFR | Epidermal Growth Factor Receptor |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| ESI | Electrospray Ionization |
| FDR | False Discovery Rate |
| GO | Gene Ontology |
| GSH | Reduced Glutathione |
| H&E | Hematoxylin and Eosin |
| HSP90AA1 | Heat Shock Protein 90 Alpha Family Class A Member 1 |
| IBD | Inflammatory Bowel Disease |
| IL-10 | Interleukin-10 |
| IL-13 | Interleukin-13 |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LC-MS/MS | Liquid Chromatography–Mass Spectrometry/Mass Spectrometry |
| LOX | Lipoxygenase |
| MAPK | Mitogen-Activated Protein Kinase |
| MDA | Malondialdehyde |
| MF | Molecular Function |
| MS/MS | Tandem Mass Spectrometry |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NFKB1 | Nuclear Factor Kappa B Subunit 1 |
| OMIM | Online Mendelian Inheritance in Man |
| PBS | Phosphate-Buffered Saline |
| PDB | Protein Data Bank |
| PPI | Protein–Protein Interaction |
| RDA | Retro-Diels–Alder (fragmentation) |
| RMSD | Root Mean Square Deviation |
| RNA | Ribonucleic Acid |
| SEM | Standard Error of the Mean |
| SRC | Proto-oncogene Tyrosine-Protein Kinase Src |
| STAT1/3 | Signal Transducer and Activator of Transcription 1/3 |
| STRING | Search Tool for the Retrieval of Interacting Genes/Proteins |
| TBARS | Thiobarbituric Acid Reactive Substances |
| TNF-α | Tumor Necrosis Factor-alpha |
| Th2/Th17 | T-helper 2/T-helper 17 Cells |
| UC | Ulcerative Colitis |
| UPLC | Ultra-Performance Liquid Chromatography |
| UniProt | Universal Protein Resource |
| v/v | Volume/Volume (ratio) |
References
- Porter, R.J.; Kalla, R.; Ho, G.T. Ulcerative colitis: Recent advances in the understanding of disease pathogenesis. F1000Research 2020, 9, F1000-Faculty Rev-294. [Google Scholar] [CrossRef] [PubMed]
- Calvez, V.; Puca, P.; Di Vincenzo, F.; Del Gaudio, A.; Bartocci, B.; Murgiano, M.; Iaccarino, J.; Parand, E.; Napolitano, D.; Pugliese, D.; et al. Novel Insights into the Pathogenesis of Inflammatory Bowel Diseases. Biomedicines 2025, 13, 305. [Google Scholar] [CrossRef]
- Magro, F.; Gionchetti, P.; Eliakim, R.; Ardizzone, S.; Armuzzi, A.; Barreiro-de Acosta, M.; Burisch, J.; Gecse, K.B.; Hart, A.L.; Hindryckx, P. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: Definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J. Crohn’s Colitis 2017, 11, 649–670. [Google Scholar] [CrossRef]
- Ungaro, R.; Colombel, J.-F.; Lissoos, T.; Peyrin-Biroulet, L. A treat-to-target update in ulcerative colitis: A systematic review. Off. J. Am. Coll. Gastroenterol.|ACG 2019, 114, 874–883. [Google Scholar] [CrossRef]
- Davila, M.M.; Papada, E. The Role of Plant-Derived Natural Products in the Management of Inflammatory Bowel Disease-What Is the Clinical Evidence So Far? Life 2023, 13, 1703. [Google Scholar] [CrossRef]
- Subudhi, R.N.; Poonia, N.; Singh, D.; Arora, V. Natural approaches for the management of ulcerative colitis: Evidence of preclinical and clinical investigations. Nat. Prod. Bioprospecting 2024, 14, 42. [Google Scholar] [CrossRef]
- Xu, Z.; Chang, L.; Xu, Z.; Chang, L. Asparagaceae. In Identification and Control of Common Weeds: Volume 3; Springer: Berlin/Heidelberg, Germany, 2017; pp. 835–859. [Google Scholar]
- Jaramillo-Carmona, S.; Rodriguez-Arcos, R.; Jiménez-Araujo, A.; López, S.; Gil, J.; Moreno, R.; Guillén-Bejarano, R. Saponin profile of wild asparagus species. J. Food Sci. 2017, 82, 638–646. [Google Scholar] [CrossRef]
- Simmons-Boyce, J.L.; Tinto, W.F. Steroidal saponins and sapogenins from the Agavaceae family. Nat. Prod. Commun. 2007, 2, 1934578X0700200120. [Google Scholar] [CrossRef]
- Marzouk, M.M.; Elkhateeb, A.; Latif, R.R.A.; Abdel-Hameed, E.-S.S.; Kawashty, S.A.; Hussein, S.R. C-glycosyl flavonoids-rich extract of Dipcadi erythraeum Webb & Berthel. bulbs: Phytochemical and anticancer evaluations. J. Appl. Pharm. Sci. 2019, 9, 094–098. [Google Scholar]
- Zhang, Q.; Yang, Z.; Su, W. Review of studies on polysaccharides, lignins and small molecular compounds from three Polygonatum Mill. (Asparagaceae) spp. in crude and processed states. Int. J. Biol. Macromol. 2024, 260, 129511. [Google Scholar] [CrossRef] [PubMed]
- Babu, K.; Prabhu, D.S. Dracaena trifasciata (Prain) Mabb.—Traditional use, pharmacognosy, phytochemistry and pharmacology: A comprehensive review. J. Phytopharm. 2024, 13, 235–241. [Google Scholar] [CrossRef]
- El Sayed, A.M.; Basam, S.M.; El-Naggar, E.-M.B.A.; Marzouk, H.S.; El-Hawary, S. LC–MS/MS and GC–MS profiling as well as the antimicrobial effect of leaves of selected Yucca species introduced to Egypt. Sci. Rep. 2020, 10, 17778. [Google Scholar] [CrossRef]
- Raslan, M.; Abdel Rahman, R.; Fayed, H.; Ogaly, H.; Fikry, R. Metabolomic profiling of Sansevieria trifasciata hort ex. Prain leaves and roots by HPLC-PAD-ESI/MS and its hepatoprotective effect via activation of the NRF2/ARE signaling pathway in an experimentally induced liver fibrosis rat model. Egypt. J. Chem. 2021, 64, 6647–6671. [Google Scholar] [CrossRef]
- Ali, D.E.; Bassam, S.M.; Elatrebi, S.; Habiba, E.S.; Allam, E.A.; Omar, E.M.; Ghareeb, D.A.; Abdulmalek, S.A.; Abdel-Sattar, E. HR LC-MS/MS metabolomic profiling of Yucca aloifolia fruit and the potential neuroprotective effect on rotenone-induced Parkinson’s disease in rats. PLoS ONE 2023, 18, e0282246. [Google Scholar] [CrossRef]
- Yolbaş, İ. Phytochemical profiling and antioxidant activity assessment of Bellevalia pseudolongipes via liquid chromatography-high-resolution mass spectrometry. PeerJ 2024, 12, e18046. [Google Scholar] [CrossRef] [PubMed]
- Walker, C. Beaucarnea updated. CactusWorld 2015, 33, 267–272. [Google Scholar]
- Walker, C. Beaucarnea Ruscaceae. In Monocotyledons; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1319–1328. [Google Scholar]
- Eskander, J.; Lavaud, C.; Harakat, D. Steroidal saponins from the leaves of Beaucarnea recurvata. Phytochemistry 2011, 72, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Mimaki, Y.; Takaashi, Y.; Kuroda, M.; Sashida, Y.; Nikaido, T. Steroidal saponins from Nolina recurvata stems and their inhibitory activity on cyclic AMP phosphodiesterase. Phytochemistry 1996, 42, 1609–1615. [Google Scholar] [CrossRef]
- Takaashi, Y.; Mimaki, Y.; Kameyama, A.; Kuroda, M.; Sashida, Y.; Nikaido, T.; Koike, K.; Ohmoto, T. Three new cholestane bisdesmosides from Nolina recurvata stems and their inhibitory activity on cAMP phosphodiesterase and Na+/K+ ATPase. Chem. Pharm. Bull. 1995, 43, 1180–1185. [Google Scholar] [CrossRef][Green Version]
- Takaashi, Y.; Mimaki, Y.; Kuroda, M.; Sashida, Y.; Nikaido, T.; Ohmoto, T. Recurvosides A-E, new polyhydroxylated steroidal saponins from Nolina recurvata stems. Tetrahedron 1995, 51, 2281–2292. [Google Scholar] [CrossRef]
- Şen, A.; Miranda, I.; Ferreira, J.; Lourenco, A.; Pereira, H. Chemical composition and cellular structure of ponytail palm (Beaucarnea recurvata) cork. Ind. Crops Prod. 2018, 124, 845–855. [Google Scholar] [CrossRef]
- Ajayi, I.I.; Fatoki, T.H.; Alonge, A.S.; Saliu, I.O.; Odesanmi, O.E.; Akinyelu, J.; Oke, O.E. ADME, Molecular Targets, Docking, and Dynamic Simulation Studies of Phytoconstituents of Cymbopogon citratus (DC.). Medinformatics 2024, 1, 152–163. [Google Scholar] [CrossRef]
- Tessema, F.B.; Asfaw, T.B.; Tadesse, M.G.; Gonfa, Y.H.; Bachheti, R.K. In Silico Studies as Support for Natural Products Research. Medinformatics 2024, 2, 11–21. [Google Scholar] [CrossRef]
- Wang, L.; Stanfill, S.; Valentin-Blasini, L.; Watson, C.H.; Cardenas, R.B. LC-MS/MS analysis of sugars, alditols, and humectants in smokeless tobacco products. Beitr. Tab. Int. 2019, 28, 203–213. [Google Scholar] [CrossRef]
- Campos, C.G.; Veras, H.C.T.; de Aquino Ribeiro, J.A.; Costa, P.P.K.G.; Araújo, K.P.; Rodrigues, C.M.; de Almeida, J.R.M.; Abdelnur, P.V. New protocol based on UHPLC-MS/MS for quantitation of metabolites in xylose-fermenting yeasts. J. Am. Soc. Mass. Spectrom. 2017, 28, 2646–2657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Adam, K.P. LC–MS/MS method with chemical derivatization for quantitation of L-threonate in human plasma. Biomed. Chromatogr. 2019, 33, e4636. [Google Scholar] [CrossRef]
- Li, Y.; He, Z.; Zou, P.; Ning, Y.; Zhu, X. Determination of seventeen sugars and sugar alcohols in fruit juice samples using hydrophilic interaction liquid chromatography-tandem mass spectrometry combining response surface methodology design. Microchem. J. 2023, 193, 109136. [Google Scholar] [CrossRef]
- Šofranko, J.; Mitro, P.; Lazúrová, Z.; Péč, M.J.; Bolek, T.; Péčová, R.; Dohál, M.; Samoš, M.; Murín, R. Application of Liquid Chromatography Coupled to Mass Spectrometry for Direct Estimation of the Total Levels of Adenosine and Its Catabolites in Human Blood. Pharmaceuticals 2024, 17, 345. [Google Scholar] [CrossRef]
- Mohamed, M.E.; El-Shafae, A.M.; Fikry, E.; Elbaramawi, S.S.; Elbatreek, M.H.; Tawfeek, N. Casuarina glauca branchlets’ extract as a potential treatment for ulcerative colitis: Chemical composition, in silico and in vivo studies. Front. Pharmacol. 2023, 14, 1322181. [Google Scholar] [CrossRef]
- Xie, B.; Liu, Y.; Zou, H.; Son, Y.; Wang, H.; Wang, H.; Shao, J. Determination of D-glucaric acid and/or D-glucaro-1, 4-lacton in different apple varieties through hydrophilic interaction chromatography. Food Chem. 2016, 203, 1–7. [Google Scholar] [CrossRef]
- Lin, Q.; Ou, S.; Wen, Q. In vitro antioxidant activity of feruloyl arabinose isolated from maize bran by acid hydrolysis. J. Food Sci. Technol. 2014, 51, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.E.; Tawfeek, N.; Elbaramawi, S.S.; Elbatreek, M.H.; Fikry, E. Agathis robusta bark extract protects from renal ischemia-reperfusion injury: Phytochemical, in silico and in vivo studies. Pharmaceuticals 2022, 15, 1270. [Google Scholar] [CrossRef]
- Shigematsu, Y.; Yuasa, M.; Ishige, N.; Nakajima, H.; Tajima, G. Development of second-tier liquid chromatography-tandem mass spectrometry analysis for expanded newborn screening in Japan. Int. J. Neonatal Screen. 2021, 7, 44. [Google Scholar] [CrossRef]
- Tawfeek, N.; Sobeh, M.; Hamdan, D.I.; Farrag, N.; Roxo, M.; El-Shazly, A.M.; Wink, M. Phenolic compounds from Populus alba L. and Salix subserrata Willd. (Salicaceae) counteract oxidative stress in Caenorhabditis elegans. Molecules 2019, 24, 1999. [Google Scholar] [CrossRef] [PubMed]
- Imam Pasha, S.; Varanasi, M.; Mohammed, I. Bioanalysis of monomethyl fumarate in human plasma by a sensitive and rapid LC-MS/MS method and its pharmacokinetic application LC-MS/MS determination of monomethyl fumarate in human plasma. J. Pharm. Biomed. Anal. 2017, 146, 109–116. [Google Scholar]
- Moradi-Afrapoli, F.; Oufir, M.; Walter, F.R.; Deli, M.A.; Smiesko, M.; Zabela, V.; Butterweck, V.; Hamburger, M. Validation of UHPLC–MS/MS methods for the determination of kaempferol and its metabolite 4-hydroxyphenyl acetic acid, and application to in vitro blood-brain barrier and intestinal drug permeability studies. J. Pharm. Biomed. Anal. 2016, 128, 264–274. [Google Scholar] [CrossRef]
- Ricciutelli, M.; Moretti, S.; Galarini, R.; Sagratini, G.; Mari, M.; Lucarini, S.; Vittori, S.; Caprioli, G. Identification and quantification of new isomers of isopropyl-malic acid in wine by LC-IT and LC-Q-Orbitrap. Food Chem. 2019, 294, 390–396. [Google Scholar] [CrossRef]
- Wu, S.; Gao, J.; Bai, Y.; Bai, Z.; Bai, C. Urinary metabolomic analysis of three fundamental traditional Mongolian Medicine Constitution types using UHPLC-QTOF-MS and nuclear magnetic resonance techniques. J. Radiat. Res. Appl. Sci. 2024, 17, 100915. [Google Scholar] [CrossRef]
- Mahmoud, H.N.; Elshazly, M.A.; Saad, A.M.; Refahy, L.A.-G.; Ghareeb, M.A.; RIZK, S.A. UPLC-QTOF/MS-assisted chemical profiling of Daucus carota leaf extract and evaluation of its antioxidant, antimicrobial and antibiofilm activities: Evidence from in vitro and in silico studies. Egypt. J. Chem. 2023, 66, 2175–2190. [Google Scholar]
- Merghany, R.M.; Salem, M.A.; Ezzat, S.M.; Moustafa, S.F.; El-Sawi, S.A.; Meselhy, M.R. A comparative UPLC-orbitrap-MS-based metabolite profiling of three Pelargonium species cultivated in Egypt. Sci. Rep. 2024, 14, 22765. [Google Scholar] [CrossRef]
- Dimkić, I.; Ristivojević, P.; Janakiev, T.; Berić, T.; Trifković, J.; Milojković-Opsenica, D.; Stanković, S. Phenolic profiles and antimicrobial activity of various plant resins as potential botanical sources of Serbian propolis. Ind. Crops Prod. 2016, 94, 856–871. [Google Scholar] [CrossRef]
- Rafi, M.; Karomah, A.H.; Septaningsih, D.A.; Rahminiwati, M.; Putri, S.P.; Iswantini, D. LC-MS/MS based metabolite profiling and lipase enzyme inhibitory activity of Kaempferia angustifolia Rosc. with different extracting solvents. Arab. J. Chem. 2022, 15, 104232. [Google Scholar] [CrossRef]
- Markowska, P.; Procajło, Z.; Wolska, J.; Jaroszewski, J.J.; Ziółkowski, H. Development, Validation, and Application of the LC-MS/MS Method for Determination of 4-Acetamidobenzoic Acid in Pharmacokinetic Pilot Studies in Pigs. Molecules 2021, 26, 4437. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, S.; Bajpai, V.; Reddy, T.J.; Rameshkumar, K.; Kumar, B. Structural characterization of flavonoid C-and O-glycosides in an extract of Adhatoda vasica leaves by liquid chromatography with quadrupole time-of-flight mass spectrometry. Rapid Commun. Mass. Spectrom. 2015, 29, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Bonte, R.; Bongaerts, M.; Demirdas, S.; Langendonk, J.G.; Huidekoper, H.H.; Williams, M.; Onkenhout, W.; Jacobs, E.H.; Blom, H.J.; Ruijter, G.J. Untargeted metabolomics-based screening method for inborn errors of metabolism using semi-automatic sample preparation with an UHPLC-orbitrap-MS platform. Metabolites 2019, 9, 289. [Google Scholar] [CrossRef]
- Grossert, J.S.; Fancy, P.D.; White, R.L. Fragmentation pathways of negative ions produced by electrospray ionization of acyclic dicarboxylic acids and derivatives. Can. J. Chem. 2005, 83, 1878–1890. [Google Scholar] [CrossRef]
- Schwarz, M.; Weber, F.; Durán-Guerrero, E.; Castro, R.; Rodríguez-Dodero, M.d.C.; García-Moreno, M.V.; Winterhalter, P.; Guillén-Sánchez, D. HPLC-DAD-MS and antioxidant profile of fractions from amontillado sherry wine obtained using high-speed counter-current chromatography. Foods 2021, 10, 131. [Google Scholar] [CrossRef]
- Tine, Y.; Renucci, F.; Costa, J.; Wélé, A.; Paolini, J. A method for LC-MS/MS profiling of coumarins in Zanthoxylum zanthoxyloides (Lam.) B. Zepernich and Timler extracts and essential oils. Molecules 2017, 22, 174. [Google Scholar] [CrossRef]
- Olivares-Vicente, M.; Sánchez-Marzo, N.; Herranz-López, M.; Micol, V. Analysis of Lemon Verbena Polyphenol Metabolome and Its Correlation with Oxidative Stress under Glucotoxic Conditions in Adipocyte. J. Agric. Food Chem. 2024, 72, 9768–9781. [Google Scholar] [CrossRef]
- Huang, L.; Teng, H.; Wang, M.; Fang, J.; Yuan, Y.; Ma, M.; Luo, Z.; Chen, B.; Guo, B. Isotope-coded derivatization with designed Girard-type reagent as charged isobaric mass tags for non-targeted profiling and discovery of natural aldehydes by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2023, 1702, 464084. [Google Scholar] [CrossRef]
- Yehia, S.M.; Ayoub, I.M.; Watanabe, M.; Devkota, H.P.; Singab, A.N.B. Metabolic profiling, antioxidant, and enzyme inhibition potential of Iris pseudacorus L. from Egypt and Japan: A comparative study. Sci. Rep. 2023, 13, 5233. [Google Scholar] [CrossRef]
- Ali, A.; Cottrell, J.J.; Dunshea, F.R. Lc-ms/ms characterization of phenolic metabolites and their antioxidant activities from australian native plants. Metabolites 2022, 12, 1016. [Google Scholar] [CrossRef]
- Falcão, S.I.; Vilas-Boas, M.; Estevinho, L.M.; Barros, C.; Domingues, M.R.; Cardoso, S.M. Phenolic characterization of Northeast Portuguese propolis: Usual and unusual compounds. Anal. Bioanal. Chem. 2010, 396, 887–897. [Google Scholar] [CrossRef]
- Mateos, R.; Baeza, G.; Martínez-López, S.; Sarriá, B.; Bravo, L. LC–MSn characterization of saponins in mate (Ilex paraguariens, St. Hil) and their quantification by HPLC-DAD. J. Food Compos. Anal. 2017, 63, 164–170. [Google Scholar] [CrossRef]
- Wu, F.; Lai, S.; Feng, H.; Liu, J.; Fu, D.; Wang, C.; Wang, C.; Liu, J.; Li, Z.; Li, P. Protective effects of protopanaxatriol saponins on ulcerative colitis in mouse based on UPLC-Q/TOF-MS serum and colon metabolomics. Molecules 2022, 27, 8346. [Google Scholar] [CrossRef] [PubMed]
- Passos, F.R.S.; Araújo-Filho, H.G.; Monteiro, B.S.; Shanmugam, S.; de Souza Araújo, A.A.; da Silva Almeida, J.R.G.; Thangaraj, P.; Júnior, L.J.Q.; Quintans, J.d.S.S. Anti-inflammatory and modulatory effects of steroidal saponins and sapogenins on cytokines: A review of pre-clinical research. Phytomedicine 2022, 96, 153842. [Google Scholar] [CrossRef]
- Culhuac, E.B.; Maggiolino, A.; Elghandour, M.M.; De Palo, P.; Salem, A.Z. Antioxidant and anti-inflammatory properties of phytochemicals found in the yucca genus. Antioxidants 2023, 12, 574. [Google Scholar] [CrossRef] [PubMed]
- Bouzier, A.; Rojas, J.; Ibinga, S.K.K.; Lamarti, A.; Martin, P.; Morillo, M. The impact of saponins on health-review. Biointerface Res. Appl. Chem. 2023, 13, 362. [Google Scholar]
- Liu, B.; Piao, X.; Guo, L.; Liu, S.; Chai, F.; Gao, L. Ursolic acid protects against ulcerative colitis via anti-inflammatory and antioxidant effects in mice. Mol. Med. Rep. 2016, 13, 4779–4785. [Google Scholar] [CrossRef]
- Shi, Y.; Leng, Y.; Liu, D.; Liu, X.; Ren, Y.; Zhang, J.; Chen, F. Research advances in protective effects of ursolic acid and oleanolic acid against gastrointestinal diseases. Am. J. Chin. Med. 2021, 49, 413–435. [Google Scholar] [CrossRef]
- Niu, Y.; Zhang, J.; Shi, D.; Zang, W.; Niu, J. Glycosides as Potential Medicinal Components for Ulcerative Colitis: A Review. Molecules 2023, 28, 5210. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Cheng, Y.; Yan, H.; Wei, X.; Dong, X.; Chi, L. Anti-inflammatory effect of luteolin-7-O-glucoside via the JAK1/STAT6/SOCS1 pathway in ulcerative colitis treatment. Pharmacogn. Mag. 2022, 18, 627–634. [Google Scholar]
- Li, C.; Tang, Y.; Ye, Y.; Zuo, M.; Lu, Q. Potential of natural flavonols and flavanones in the treatment of ulcerative colitis. Front. Pharmacol. 2023, 14, 1120616. [Google Scholar] [CrossRef]
- Ginsberg, A. 4-Amino Salicylic Acid in the Treatment of Ulcerative Colitis: Preliminary Report of a Randomized) Double-Blind Placebo Controlled Trial of Oral 4-ASA. In Inflammatory Bowel Diseases 1990: Proceedings of the Third International Symposium on Inflammatory Bowel Diseases, Jerusalem, September 10–13, 1989; Springer: Dordrecht, The Netherlands, 2012; pp. 213–218. [Google Scholar]
- Van Assche, G.; Baert, F.; De Reuck, M.; De Vos, M.; De Wit, O.; Hoang, P.; Louis, E.; Mana, F.; Pelckmans, P.; Rutgeerts, P. The role of aminosalicylates in the treatment of ulcerative colitis. Acta Gastro-Enterol. Belg. 2002, 65, 196–199. [Google Scholar]
- Xiu, M.; Li, B.; He, L.; Shi, Y.; Zhang, Y.; Zhou, S.; Liu, Y.; Wang, N.; He, J. Caffeic Acid Protects Against Ulcerative Colitis via Inhibiting Mitochondrial Apoptosis and Immune Overactivation in Drosophila. Drug Des. Dev. Ther. 2025, 19, 2157–2172. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, D.; Zieliński, H.; Laparra-Llopis, J.M.; Szawara-Nowak, D.; Honke, J.; Giménez-Bastida, J.A. Caffeic acid modulates processes associated with intestinal inflammation. Nutrients 2021, 13, 554. [Google Scholar] [CrossRef] [PubMed]
- Kishore, N.; Balakumar, S.; Raj, C.D.; Sivakumar, N.; Thirumalaivasan, R.; Mahesh, N.; Selvankumar, T. Implications of Asparagus racemosus and Terminalia chebula extracts on oxazolone induced inflammatory bowel disease in Danio rerio (zebrafish). Biocatal. Agric. Biotechnol. 2023, 51, 102790. [Google Scholar] [CrossRef]
- Bhatnagar, M.; Sisodia, S. Antisecretory and antiulcer activity of Asparagus racemosus Willd. against indomethacin plus pyloric ligation-induced gastric ulcer in rats. J. Herb. Pharmacother. 2006, 6, 13–20. [Google Scholar] [CrossRef]
- Qiao, M.; Xue, T.; Zhu, Y.; Yang, J.; Hu, J. Polysaccharides from Cistanche deserticola mitigate inflammatory bowel disease via modulating intestinal microbiota and SRC/EGFR/PI3K/AKT signaling pathways. Int. J. Biol. Macromol. 2025, 308, 142452. [Google Scholar] [CrossRef] [PubMed]
- López-Cabrera, S.D.; Calles-Arriaga, C.A.; Rocha-Rangel, E.; Maldonado-Sada, M.T.; López-Hernández, J.; Castillo-Robles, J.A.; Pech-Rodríguez, W.J. ZnO mesoscale nanoparticles photoluminescence obtained by green synthesis based on Beaucarnea gracilis. Appl. Nanosci. 2024, 14, 1015–1020. [Google Scholar] [CrossRef]
- Ren, J.; Yue, B.; Wang, H.; Zhang, B.; Luo, X.; Yu, Z.; Zhang, J.; Ren, Y.; Mani, S.; Wang, Z.; et al. Acacetin Ameliorates Experimental Colitis in Mice via Inhibiting Macrophage Inflammatory Response and Regulating the Composition of Gut Microbiota. Front. Physiol. 2020, 11, 577237. [Google Scholar] [CrossRef]
- Hu, L.; Wu, C.; Zhang, Z.; Liu, M.; Maruthi Prasad, E.; Chen, Y.; Wang, K. Pinocembrin Protects Against Dextran Sulfate Sodium-Induced Rats Colitis by Ameliorating Inflammation, Improving Barrier Function and Modulating Gut Microbiota. Front. Physiol. 2019, 10, 908. [Google Scholar] [CrossRef]
- Yue, B.; Ren, J.; Yu, Z.; Luo, X.; Ren, Y.; Zhang, J.; Mani, S.; Wang, Z.; Dou, W. Pinocembrin alleviates ulcerative colitis in mice via regulating gut microbiota, suppressing TLR4/MD2/NF-kappaB pathway and promoting intestinal barrier. Biosci. Rep. 2020, 40, BSR20200986. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yao, Y.; Yao, T.; Shi, Q.; Zeng, Y.; Li, L. Hesperetin Alleviated Experimental Colitis via Regulating Ferroptosis and Gut Microbiota. Nutrients 2024, 16, 2343. [Google Scholar] [CrossRef] [PubMed]
- Mohanad, M.; El-Awdan, S.A.; Aboulhoda, B.E.; Nossier, A.I.; Elesawy, W.H.; Ahmed, M.A.E. Unraveling the Protective Effect of Hesperetin In Experimentally Induced Colitis: Inhibition of NF-kappaB and NLRP3 Inflammasome Activation. J. Biochem. Mol. Toxicol. 2025, 39, e70229. [Google Scholar] [CrossRef]
- Zhang, J.; Lei, H.; Hu, X.; Dong, W. Hesperetin ameliorates DSS-induced colitis by maintaining the epithelial barrier via blocking RIPK3/MLKL necroptosis signaling. Eur. J. Pharmacol. 2020, 873, 172992. [Google Scholar] [CrossRef]
- Zheng, C.; Rangsinth, P.; Shiu, P.H.T.; Wang, W.; Li, R.; Li, J.; Kwan, Y.W.; Leung, G.P.H. A Review on the Sources, Structures, and Pharmacological Activities of Lucidenic Acids. Molecules 2023, 28, 1756. [Google Scholar] [CrossRef]
- Alrumaihi, F.; Almatroodi, S.A.; Alharbi, H.O.A.; Alwanian, W.M.; Alharbi, F.A.; Almatroudi, A.; Rahmani, A.H. Pharmacological Potential of Kaempferol, a Flavonoid in the Management of Pathogenesis via Modulation of Inflammation and Other Biological Activities. Molecules 2024, 29, 2007. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Khan, M.; Asaf, S.; Lubna; Asif, S.; Kim, K.M. Bioactivity and Therapeutic Potential of Kaempferol and Quercetin: New Insights for Plant and Human Health. Plants 2022, 11, 2623. [Google Scholar] [CrossRef]
- Lin, N.; Sato, T.; Takayama, Y.; Mimaki, Y.; Sashida, Y.; Yano, M.; Ito, A. Novel anti-inflammatory actions of nobiletin, a citrus polymethoxy flavonoid, on human synovial fibroblasts and mouse macrophages. Biochem. Pharmacol. 2003, 65, 2065–2071. [Google Scholar] [CrossRef]
- Ayeleso, T.B.; Matumba, M.G.; Mukwevho, E. Oleanolic Acid and Its Derivatives: Biological Activities and Therapeutic Potential in Chronic Diseases. Molecules 2017, 22, 1915. [Google Scholar] [CrossRef]
- Lin, Y.; Luo, L.; Lin, H.; Li, X.; Huang, R. Potential therapeutic targets and molecular details of anthocyan-treated inflammatory bowel disease: A systematic bioinformatics analysis of network pharmacology. RSC Adv. 2021, 11, 8239–8249. [Google Scholar] [CrossRef] [PubMed]
- Kasembeli, M.M.; Bharadwaj, U.; Robinson, P.; Tweardy, D.J. Contribution of STAT3 to Inflammatory and Fibrotic Diseases and Prospects for its Targeting for Treatment. Int. J. Mol. Sci. 2018, 19, 2299. [Google Scholar] [CrossRef] [PubMed]
- Currie, G.M. Pharmacology, part 1: Introduction to pharmacology and pharmacodynamics. J. Nucl. Med. Technol. 2018, 46, 81–86. [Google Scholar] [CrossRef]
- Salahudeen, M.S.; Nishtala, P.S. An overview of pharmacodynamic modelling, ligand-binding approach and its application in clinical practice. Saudi Pharm. J. 2017, 25, 165–175. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, T.; Ma, X.; Guo, S.; Zhou, Q.; Zahoor, A.; Deng, G. Recent advances in anti-inflammatory active components and action mechanisms of natural medicines. Inflammopharmacology 2023, 31, 2901–2937. [Google Scholar] [CrossRef]
- Jiang, W.-Y.; Seo, G.S.; Kim, Y.-C.; Sohn, D.H.; Lee, S.H. PF2405, standardized fraction of Scutellaria baicalensis, ameliorates colitis in vitro and in vivo. Arch. Pharmacal Res. 2015, 38, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-y.; Wang, X.-j.; Su, Y.-l.; Wang, Q.; Huang, S.-w.; Pan, Z.-f.; Chen, Y.-p.; Liang, J.-j.; Zhang, M.-l.; Xie, X.-q. Baicalein ameliorates ulcerative colitis by improving intestinal epithelial barrier via AhR/IL-22 pathway in ILC3s. Acta Pharmacol. Sin. 2022, 43, 1495–1507. [Google Scholar] [CrossRef]
- Guan, X. Glutathione and glutathione disulfide—Their biomedical and pharmaceutical applications. Med. Chem. Res. 2023, 32, 1972–1994. [Google Scholar] [CrossRef]
- Senthilkumar, M.; Amaresan, N.; Sankaranarayanan, A. Estimation of Malondialdehyde (MDA) by Thiobarbituric Acid (TBA) Assay. In Plant-Microbe Interactions: Laboratory Techniques; Springer: New York, NY, USA, 2021; pp. 103–105. [Google Scholar]
- Adolph, T.E.; Meyer, M.; Schwärzler, J.; Mayr, L.; Grabherr, F.; Tilg, H. The metabolic nature of inflammatory bowel diseases. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 753–767. [Google Scholar] [CrossRef]
- Howes, A.; Stimpson, P.; Redford, P.; Gabrysova, L.; O’Garra, A. Interleukin-10: Cytokines in Anti-inflammation and Tolerance. In Cytokine Frontiers: Regulation of Immune Responses in Health and Disease; Yoshimoto, T., Yoshimoto, T., Eds.; Springer: Japan, Tokyo, 2014; pp. 327–352. [Google Scholar]
- York, A.G.; Skadow, M.H.; Oh, J.; Qu, R.; Zhou, Q.D.; Hsieh, W.-Y.; Mowel, W.K.; Brewer, J.R.; Kaffe, E.; Williams, K.J. IL-10 constrains sphingolipid metabolism to limit inflammation. Nature 2024, 627, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Biberoglu, S.; Ozkan, S. Tumor Necrosis Factor-Alpha (TNF-Alpha) as a Biomarker in Trauma and Critical Care. In Biomarkers in Trauma, Injury and Critical Care; Rajendram, R., Preedy, V.R., Patel, V.B., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 859–874. [Google Scholar]
- Bouwmeester, T.; Bauch, A.; Ruffner, H.; Angrand, P.-O.; Bergamini, G.; Croughton, K.; Cruciat, C.; Eberhard, D.; Gagneur, J.; Ghidelli, S. A physical and functional map of the human TNF-α/NF-κB signal transduction pathway. Nat. Cell Biol. 2004, 6, 97–105, Erratum in Nat. Cell Biol. 2004, 6, 465.. [Google Scholar] [CrossRef]
- Shanmugam, L.; Anuja, A.V.; Rajinikanth, S.K.; Samuel, P.J. Epidermal Growth Factor (EGF) in Wound Repair. In Therapeutic Proteins Against Human Diseases; Zahid Balouch, F.K., Ed.; Springer Nature: Singapore, 2022; pp. 29–49. [Google Scholar]
- Peña, O.A.; Martin, P. Cellular and molecular mechanisms of skin wound healing. Nat. Rev. Mol. Cell Biol. 2024, 25, 599–616. [Google Scholar] [CrossRef]
- Tito, C.; Masciarelli, S.; Colotti, G.; Fazi, F. EGF receptor in organ development, tissue homeostasis and regeneration. J. Biomed. Sci. 2025, 32, 24. [Google Scholar] [CrossRef] [PubMed]
- Byeon, S.E.; Yi, Y.S.; Oh, J.; Yoo, B.C.; Hong, S.; Cho, J.Y. The role of Src kinase in macrophage-mediated inflammatory responses. Mediat. Inflamm. 2012, 2012, 512926. [Google Scholar] [CrossRef] [PubMed]
- Antonia, R.J.; Karelehto, E.; Toriguchi, K.; Matli, M.; Warren, R.S.; Pfeffer, L.M.; Donner, D.B. STAT3 regulates inflammatory cytokine production downstream of TNFR1 by inducing expression of TNFAIP3/A20. J. Cell Mol. Med. 2022, 26, 4591–4601. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Kunnumakkara, A.B.; Harikumar, K.B.; Gupta, S.R.; Tharakan, S.T.; Koca, C.; Dey, S.; Sung, B. Signal transducer and activator of transcription-3, inflammation, and cancer: How intimate is the relationship? Ann. N. Y. Acad. Sci. 2009, 1171, 59–76. [Google Scholar] [CrossRef]
- Duggal, S.; Jailkhani, N.; Midha, M.K.; Agrawal, N.; Rao, K.V.; Kumar, A. Defining the Akt1 interactome and its role in regulating the cell cycle. Sci. Rep. 2018, 8, 1303. [Google Scholar] [CrossRef]
- Kumar, B.H.; Kabekkodu, S.P.; Pai, K.S.R. Structural insights of AKT and its activation mechanism for drug development. Mol. Divers. 2025, 29, 5443–5463. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Fikry, E.; Orfali, R.; Tawfeek, N.; Perveen, S.; Ghafar, S.; El-Domiaty, M.M.; El-Shafae, A.M. Unveiling the Bioactive Efficacy of Cupressus sempervirens ‘Stricta’ Essential Oil: Composition, In Vitro Activities, and In Silico Analyses. Pharmaceuticals 2024, 17, 1019. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef]
- Zaru, R.; Orchard, S.; Consortium, U. UniProt tools: BLAST, align, peptide search, and ID mapping. Curr. Protoc. 2023, 3, e697. [Google Scholar] [CrossRef] [PubMed]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinform. 2016, 54, 1.30.1–1.30.33. [Google Scholar] [CrossRef] [PubMed]
- Piñero, J.; Ramírez-Anguita, J.M.; Saüch-Pitarch, J.; Ronzano, F.; Centeno, E.; Sanz, F.; Furlong, L.I. The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res. 2020, 48, D845–D855. [Google Scholar] [CrossRef]
- Amberger, J.S.; Hamosh, A. Searching Online Mendelian Inheritance in Man (OMIM): A Knowledgebase of Human Genes and Genetic Phenotypes. Curr. Protoc. Bioinform. 2017, 58, 1.2.1–1.2.12. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S. The STRING database in 2023: Protein–protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Tang, Y.; Li, M.; Wang, J.; Pan, Y.; Wu, F.X. CytoNCA: A cytoscape plugin for centrality analysis and evaluation of protein interaction networks. Biosystems 2015, 127, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Fikry, E.; Orfali, R.; Perveen, S.; Ghaffar, S.; El-Shafae, A.M.; El-Domiaty, M.M.; Tawfeek, N. Chemical Composition and Anti-Lung Cancer Activities of Melaleuca quinquenervia Leaf Essential Oil: Integrating Gas Chromatography–Mass Spectrometry (GC/MS) Profiling, Network Pharmacology, and Molecular Docking. Pharmaceuticals 2025, 18, 771. [Google Scholar] [CrossRef]
- Chin, C.-H.; Chen, S.-H.; Wu, H.-H.; Ho, C.-W.; Ko, M.-T.; Lin, C.-Y. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014, 8, S11. [Google Scholar] [CrossRef] [PubMed]
- Dennis, G.; Sherman, B.T.; Hosack, D.A.; Yang, J.; Gao, W.; Lane, H.C.; Lempicki, R.A. DAVID: Database for annotation, visualization, and integrated discovery. Genome Biol. 2003, 4, R60. [Google Scholar] [CrossRef]
- Burley, S.K.; Berman, H.M.; Kleywegt, G.J.; Markley, J.L.; Nakamura, H.; Velankar, S. Protein Data Bank (PDB): The Single Global Macromolecular Structure Archive. In Protein Crystallography: Methods and Protocols; Wlodawer, A., Dauter, Z., Jaskolski, M., Eds.; Springer: New York, NY, USA, 2017; pp. 627–641. [Google Scholar]
- Zhao, Q.; Ouyang, X.; Wan, X.; Gajiwala, K.S.; Kath, J.C.; Jones, L.H.; Burlingame, A.L.; Taunton, J. Broad-Spectrum Kinase Profiling in Live Cells with Lysine-Targeted Sulfonyl Fluoride Probes. J. Am. Chem. Soc. 2017, 139, 680–685. [Google Scholar] [CrossRef]
- Levinson, N.M.; Boxer, S.G. A conserved water-mediated hydrogen bond network defines bosutinib’s kinase selectivity. Nat. Chem. Biol. 2014, 10, 127–132. [Google Scholar] [CrossRef]
- Belo, Y.; Mielko, Z.; Nudelman, H.; Afek, A.; Ben-David, O.; Shahar, A.; Zarivach, R.; Gordan, R.; Arbely, E. Unexpected implications of STAT3 acetylation revealed by genetic encoding of acetyl-lysine. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 1343–1350. [Google Scholar] [CrossRef]
- Lin, K.; Lin, J.; Wu, W.I.; Ballard, J.; Lee, B.B.; Gloor, S.L.; Vigers, G.P.; Morales, T.H.; Friedman, L.S.; Skelton, N.; et al. An ATP-site on-off switch that restricts phosphatase accessibility of Akt. Sci. Signal 2012, 5, ra37. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Fikry, E.; Orfali, R.; El-Sayed, S.S.; Perveen, S.; Ghafar, S.; El-Shafae, A.M.; El-Domiaty, M.M.; Tawfeek, N. Potential Hepatoprotective Effects of Chamaecyparis lawsoniana against Methotrexate-Induced Liver Injury: Integrated Phytochemical Profiling, Target Network Analysis, and Experimental Validation. Antioxidants 2023, 12, 2118. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminform 2011, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Chen, C.; Lei, X.; Zhao, J.; Liang, J. CASTp 3.0: Computed atlas of surface topography of proteins. Nucleic Acids Res. 2018, 46, W363–W367. [Google Scholar] [CrossRef] [PubMed]
- Biovia, D.S. Discovery Studio Visualizer, v21. 1.0. 20298; Dassault Systèmes: San Diego, CA, USA, 2021. [Google Scholar]
- Cooper, H.S.; Murthy, S.; Shah, R.; Sedergran, D. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Investig. J. Tech. Methods Pathol. 1993, 69, 238–249. [Google Scholar]
- Mei, Q.; Xu, J.; Xiang, L.; Hu, Y.; Hu, X.; Xu, Z. Change of nitric oxide in experimental colitis and its inhibition by melatonin in vivo and in vitro. Postgrad. Med. J. 2005, 81, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Geboes, K.; Riddell, R.; Ost, A.; Jensfelt, B.; Persson, T.; Lofberg, R. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut 2000, 47, 404–409. [Google Scholar] [CrossRef] [PubMed]



| No. | Rt. | [M-H]− | MS2 Fragments (m/z) | Tentative Identification | Class | Reference |
|---|---|---|---|---|---|---|
| 1. | 1.262 | 181.073 | 163, 145, 127, 101, 89 | Hexitol (D-sorbitol) | Sugar alcohol | [26] |
| 2. | 1.274 | 151.062 | 133, 131, 119, 89, 59 | Pentitol (xylitol) | Sugar alcohol | [27] |
| 3. | 1.361 | 135.031 | 117, 91, 73 | Threonic acid | Sugar acid | [28] |
| 4. | 1.398 | 149.047 | 131, 59 | D-arabinose | Monosaccharide | [29] |
| 5. | 1.493 | 267.075 | 249, 135, 108, 92 | Inosine | Nucleoside | [30] |
| 6. | 1.515 | 133.015 | 115, 89, 71, 59 | Malic acid | Organic acid | [31] |
| 7. | 1.529 | 191.021 | 147, 173 | D-glucaro-1,4-lactone | Sugar acids derivative | [32] |
| 8. | 1.571 | 179.020 | 161, 143, 125, 107, 89, 71 | Glucose | Monosaccharide | [15] |
| 9. | 2.192 | 325.117 | 193, 149 | Ferulic acid pentoside (feruloyl-arabinose) | Phenolic acid dv. | [33] |
| 10. | 2.537 | 421.002 | 191, 111 | Malonylcoumaroylquinic acid | Phenolic acid dv. | [10] |
| 11. | 2.882 | 117.020 | 99, 73 | Succinic acid | Omega-dicarboxylic acid | [34] |
| 12. | 3.946 | 161.047 | 143, 125, 101, 117, 99, 73 | 3-Hydroxy-3-methylglutaric acid [Meglutol] | Dicarboxylic acid | [35] |
| 13. | 4.555 | 164.073 | 147, 119, 103 | D-phenylalanine | Amino acid | [36] |
| 14. | 4.704 | 487.172 | 163, 145 | Coumaric acid dihexoside | Phenolic acid dv. | [10] |
| 15. | 5.828 | 153.021 | 109 | 2,3-Dihydroxybenzoic acid | Phenolic acid | [36] |
| 16. | 5.921 | 195.031 | 177, 151 | Gluconic acid | Gluconic acid | [10] |
| 17. | 5.967 | 529.126 | 349, 193 | Caffeoyl–feruloylquinic acid | Phenolic acid dv. | [14] |
| 18. | 6.125 | 129.020 | 111, 85 | Monomethyl fumarate | Dicarboxylic acid | [37] |
| 19. | 6.444 | 151.041 | 133, 121, 107 | 4-hydroxyphenylacetic acid | Monocarboxylic acid | [38] |
| 20. | 6.674 | 175.063 | 157, 131, 129, 113, 115, 87, 85 | 2-Isopropylmalic acid | Dicarboxylic acid | [39] |
| 21. | 6.680 | 457.178 | 411, 163 | Lucidenic acid A | Triterpenoid | [14] |
| 22. | 6.783 | 145.051 | 127, 101 | Adipic acid | Dicarboxylic acid | [40] |
| 23. | 6.879 | 179.083 | 161, 135 | Caffeic acid | Phenolic acid | [34] |
| 24. | 6.921 | 137.025 | 119, 108 | 3,4-Dihydroxybenzaldehyde (Protocatechuic aldehyde) | Phenolic aldehyde | [41] |
| 25. | 7.007 | 201.115 | 183, 157, 139, 113 | Sebacic acid | Alpha,omega-dicarboxylic acid | [42] |
| 26. | 7.097 | 137.026 | 93 | Salicylic acid | Phenolic acid | [43] |
| 27. | 7.250 | 187.099 | 169, 143, 125, 115 | Azelaic acid | Alpha,omega-dicarboxylic acid | [44] |
| 28. | 7.309 | 593.158 | 503, 473 | Apigenin 6,8-di-C-glucoside | Flavonoids | [10] |
| 29. | 7.525 | 178.053 | 134 | 4-Acetamidobenzoic acid (Acedoben) | 4-Aminobenzoic acid dv. | [45] |
| 30. | 7.692 | 563.146 | 503, 473, 443 | Apigenin 6-C-glucoside 8-C-arabinoside | Flavonoids | [46] |
| 31. | 8.048 | 579.262 | 519, 459 | Luteolin 6-C-β-glucopyranoside-8-C-α-arabinopyranoside (Carlinoside) | Flavonoids | [10] |
| 32. | 8.130 | 159.067 | 141, 115, 71 | 3,3-Dimethylglutaric acid | Alpha,omega-dicarboxylic acid | [47] |
| 33. | 8.133 | 345.099 | 301, 167, 139 | Aucubin | Terpenoid | [14] |
| 34. | 8.497 | 577.164 | 487, 457 | Apigenin 6-C-β-glucopyranoside-8-C-α-rhamnopyranoside (Violanthin) [Vitexin 2″-O-rhamnoside] | Flavonoids | [10] |
| 35. | 8.548 | 299.117 | 178, 150, 122 | Dihydroeucomin | Flavonoids | [15] |
| 36. | 8.671 | 283.124 | 265, 239 | Acacetin | Flavonoids | [14] |
| 37. | 8.786 | 163.041 | 119 | P-coumaric acid | Phenolic acid | [43] |
| 38. | 8.808 | 173.083 | 155, 129, 111, 85 | Suberic acid (Octanedioic acid) | Alpha,omega-dicarboxylic acid | [48] |
| 39. | 9.021 | 167.036 | 152, 108 | Methyl protocatechuate [Protocatechuic acid methyl ester] | Phenolic acid dv. | [49] |
| 40. | 9.280 | 161.025 | 133 | Umbelliferone | Coumarin | [50] |
| 41. | 9.575 | 195.031 | 180, 165, 151 | Homoveratric acid (3,4-Dimethoxyphenylacetic acid) | Phenylacetic acid | [51] |
| 42. | 9.924 | 147.046 | 129, 117 | 2-Hydroxyl cinnamaldehyde | Cinnamaldehyde | [52] |
| 43. | 10.313 | 207.068 | 177, 192, 121 | Sinapaldehyde | Cinnamaldehyde | [52] |
| 44. | 10.589 | 389.166 | 371, 361, 353, 343 | Scaposin | Flavone | [14] |
| 45. | 10.874 | 903.345 | 433[Furostane-triol] | Furostane-triol; rhamnosyldihexoside | Steroidal saponins | [8] |
| 46. | 11.673 | 301.076 | 286, 164, 151 | Hesperetin | Flavonoids | [53] |
| 47. | 11.828 | 431.142 | 387 | 3-Methoxynobiletin | Flavonoids | [54] |
| 48. | 11.914 | 255.126 | 211, 151 | Pinocembrine | Flavonoids | [55] |
| 49. | 12.013 | 503.271 | 179, 135 | Caffeic acid dihexoside | Phenolic acid dv. | [10] |
| 50. | 12.235 | 169.087 | 125 | Gallic acid | Phenolic acid | [15] |
| 51. | 12.894 | 769.415 | 329[aglycone-H] | Dihydroxypregna-5,16-dien-20-one deoxyhexoside pentoside hexosid | Pregnane steroidal saponin | [14] |
| 52. | 13.052 | 247.137 | 203 | Pechueloic acid | Terpenoid | [14] |
| 53. | 13.304 | 421.065 | 341, 135 | Caffeic acid hexosidedv. | Phenolic acid dv. | [10] |
| 54. | 13.661 | 285.211 | 267, 257, 241 | Kaempferol | Flavonoids | [36] |
| 55. | 14.922 | 837.439 | 705, 559 | Neoruscogenin deoxyhexoside dipentoside isomer | Spirostane steroidal saponin | [14] |
| 56. | 15.465 | 837.454 | 691, 559 | Neoruscogenin deoxyhexoside dipentoside | Spirostane steroidal saponin | [14] |
| 57. | 15.893 | 503.273 | 153, 109 | Protocatechuic acid dv. | Phenolic acid dv. | [43] |
| 58. | 15.915 | 455.244 | 437, 411 | Oleanolic acid | Pentacyclic triterpenoid | [56] |
| 59. | 16.474 | 455.217 | 437, 411 | Ursolic acid | Pentacyclic triterpenoid | [56] |
| 60. | 16.737 | 901.508 | 323 | Spirostan-3-ol; Glucopyranosyl-glucopyranosyl galactopyranoside | Spirostane steroidal saponin | [15] |
| 61. | 16.919 | 315.128 | 297, 282, 78 | Eucomol | Flavonoids | [15] |
| 62. | 18.144 | 429.303 | 383, 344, 345 | Ruscogenin | Spirostane steroidal saponin | [14] |
| 63. | 19.033 | 311.172 | 183, 119 | Caftaric acid | Cinnamic acid derivative | [14] |
| 64. | 20.205 | 609.281 | 447 | Isoorientin-7-O-β-glucopyranoside | Flavonoids | [10] |
| 65. | 24.466 | 489.364 | 447, 357 | O-acetylisoorientin | Flavonoids | [10] |
| 66. | 28.088 | 919.275 | 433[Furostane-triol] | Furostane-triol; trihexoside | Steroidal saponins | [15] |
| Lesion | Control | Disease | BRLE100 | BRLE200 | BRLE400 |
|---|---|---|---|---|---|
| Structural change | 0, 0, 0, 0, 0 | 1, 3, 2, 3, 3 | 3, 2, 2, 1, 1 | 2, 2, 1, 1, 1 | 2, 1, 0, 0, 0 |
| Chronic inflammatory infiltrate | 0, 0, 0, 0, 0 | 2, 3, 2, 3, 2 | 2, 2, 2, 1, 1 | 2, 1, 1, 1, 0 | 1, 1, 0, 0, 0 |
| Lamina propria neutrophils | 0, 0, 0, 0, 0 | 1, 3, 1, 2, 2 | 2, 3, 1, 1, 1 | 2, 2, 1, 0, 0 | 2, 1, 0, 0, 0 |
| Lamina propria eosinophils | 0, 0, 0, 0, 0 | 1, 3, 2, 2, 2 | 2, 2, 1, 1, 1 | 2, 2, 1, 1, 0 | 1, 1, 0, 0, 0 |
| Neutrophils in epithelium | 0, 0, 0, 0, 0 | 1, 3, 3, 1, 2 | 2, 2, 2, 1, 0 | 1, 1, 1, 1, 0 | 1, 1, 1, 0, 0 |
| Crypt destruction | 0, 0, 0, 0, 0 | 1, 3, 2, 3, 2 | 3, 2, 2, 1, 0 | 2, 2, 1, 1, 0 | 2, 1, 1, 0, 0 |
| Grade 5 Erosion or ulceration | 0, 0, 0, 0, 0 | 2, 2, 3, 4, 4 | 4, 3, 2, 2, 1 | 3, 2, 1, 1, 0 | 3, 1, 1, 0, 0 |
| Parameter | Score 0 | Score 1 | Score 2 | Score 3 | Score 4 |
|---|---|---|---|---|---|
| Weight Loss (%) | 0 | 1–5 | 6–10 | 11–20 | >20 |
| Stool Consistency | Normal | — | Loose | — | Liquid |
| Rectal Bleeding | Absent | Minimal | Evident | — | — |
| Grade 0: Structural change | Subgrades |
| 0.0 No abnormality | |
| 0.1 Mild alteration | |
| 0.2 Mild or moderate diffuse or multifocal alteration | |
| 0.3 Severe diffuse or multifocal alteration | |
| Grade1: Chronic inflammatory infiltrate | Subgrades |
| 1.0 No increase | |
| 1.1 Mild but unequivocal increase | |
| 1.2 Moderate increase | |
| 1.3 Marked increase | |
| Grade 2: Lamina propria neutrophils and eosinophils | 2A Eosinophils 2A. |
| 0 No increase 2A. | |
| 1 Mild but unequivocal increase 2A. | |
| 2 Moderate increase | |
| 3 Marked increase | |
| 2B Neutrophils 2B. | |
| 0 None 2B. | |
| 1 Mild but unequivocal increase 2B. | |
| 2 Moderate increase 2B. | |
| 3 Marked increase | |
| Grade 3: Neutrophils in epithelium | 3.0 None |
| 3.1 <5% crypts involved | |
| 3.2 <50% crypts involved | |
| 3.3 >50% crypts involved | |
| Grade 4: Crypt destruction | 4.0 None |
| 4.1 Probable—local excess of neutrophils in part of crypt | |
| 4.2 Probable—marked attenuation | |
| 4.3 Unequivocal crypt destruction | |
| Grade 5: Erosion or ulceration | 5.0 No erosion, ulceration, or granulation tissue |
| 5.1 Recovering epithelium+adjacent inflammation | |
| 5.2 Probable erosion—focally stripped | |
| 5.3 Unequivocal erosion | |
| 5.4 Ulcer or granulation tissue |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tawfeek, N.; Orfali, R.; Perveen, S.; Ghafar, S.; Fikry, E.; Elbatreek, M.H.; Elbaramawi, S.S.; El-Domiaty, M.M.; El-Shafae, A.M. Therapeutic Potential of Beaucarnea recurvata Leaf Extract Against Ulcerative Colitis: Integrating Phytochemical Profiling, Network Pharmacology, and Experimental Validation. Int. J. Mol. Sci. 2025, 26, 12053. https://doi.org/10.3390/ijms262412053
Tawfeek N, Orfali R, Perveen S, Ghafar S, Fikry E, Elbatreek MH, Elbaramawi SS, El-Domiaty MM, El-Shafae AM. Therapeutic Potential of Beaucarnea recurvata Leaf Extract Against Ulcerative Colitis: Integrating Phytochemical Profiling, Network Pharmacology, and Experimental Validation. International Journal of Molecular Sciences. 2025; 26(24):12053. https://doi.org/10.3390/ijms262412053
Chicago/Turabian StyleTawfeek, Nora, Raha Orfali, Shagufta Perveen, Safina Ghafar, Eman Fikry, Mahmoud H. Elbatreek, Samar S. Elbaramawi, Maher M. El-Domiaty, and Azza M. El-Shafae. 2025. "Therapeutic Potential of Beaucarnea recurvata Leaf Extract Against Ulcerative Colitis: Integrating Phytochemical Profiling, Network Pharmacology, and Experimental Validation" International Journal of Molecular Sciences 26, no. 24: 12053. https://doi.org/10.3390/ijms262412053
APA StyleTawfeek, N., Orfali, R., Perveen, S., Ghafar, S., Fikry, E., Elbatreek, M. H., Elbaramawi, S. S., El-Domiaty, M. M., & El-Shafae, A. M. (2025). Therapeutic Potential of Beaucarnea recurvata Leaf Extract Against Ulcerative Colitis: Integrating Phytochemical Profiling, Network Pharmacology, and Experimental Validation. International Journal of Molecular Sciences, 26(24), 12053. https://doi.org/10.3390/ijms262412053






