The Dual Nature of Sinoatrial Node Remodelling in Athletes: A Systematic Review of Electrophysiological Adaptations and the Pathological Tipping Point
Abstract
1. Introduction
2. Evidence Synthesis
3. Divergent Mechanisms of Bradycardia
4. Electrophysiological Mechanisms of Exercise-Induced Sinus Node Remodelling
4.1. Membrane Clock Remodelling: Coordinated Downregulation of Pacemaker Currents
4.2. Calcium Clock Remodelling: A Secondary Role
4.3. Structural Remodelling and the Pathological Shift
5. Moderating Factors in SAN Remodelling
5.1. Pronounced Interspecies Disparities
5.2. Training Modality and Cumulative Load
5.3. The Compounding Effect of Age
6. Discussion and Perspectives
6.1. An Ionic Framework: From Adaptation to Pathology
6.2. Pathological Structural Damage: Structural Remodelling and the Disuse Hypothesis
6.3. Resolving Disparities and Defining the “Tipping Point”
6.4. Clinical Implications and Future Directions
7. Limitation
8. Conclusions
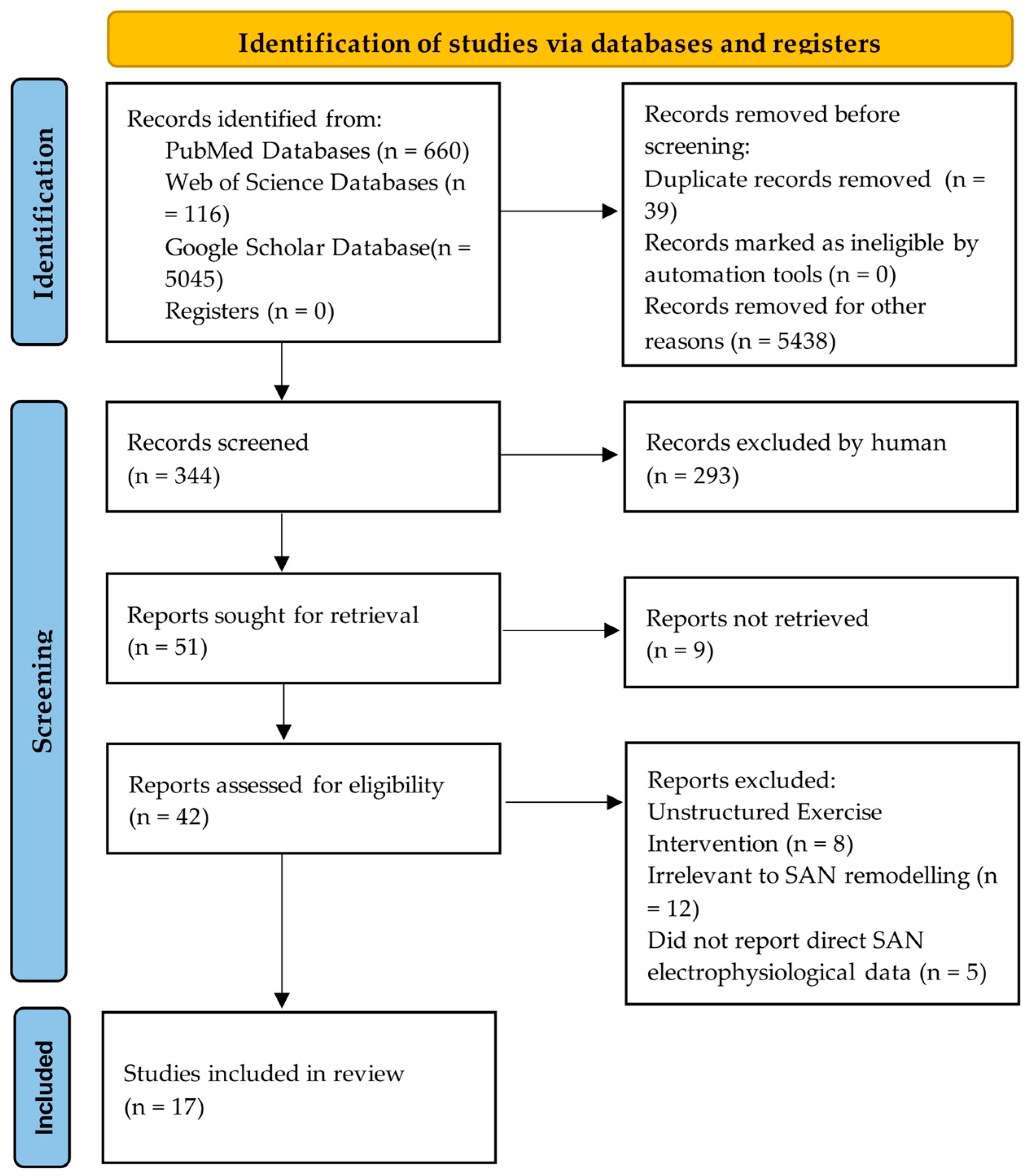
9. Methods
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AV | Atrioventricular Node |
| Cav1.3 | Voltage-Gated L-type Calcium Channel Subunit Alpha-1D |
| Cx43 | Connexin 43 |
| cTnT | Cardiac Troponin T |
| HCN | Hyperpolarization-activated Cyclic Nucleotide-gated Channel |
| HCN4 | Hyperpolarization-activated Cyclic Nucleotide-gated Channel 4 |
| HR | Heart Rate |
| HRV | Heart Rate Variability |
| ICaL | L-type Calcium Current |
| ICaT | T-type Calcium Current |
| If | Hyperpolarization-activated Cyclic Nucleotide-Gated Cation Channel |
| IKACh | Acetylcholine-activated Potassium Current |
| MMP-2 | Matrix Metalloproteinase-2 |
| NRSF | Neuron-Restrictive Silencer Factor |
| NCX | Sodium-Calcium Exchanger |
| PCL | Pacing Cycle Length |
| PM | PaceMaker |
| RyR2 | Ryanodine Receptor Type 2 |
| SAN | Sinoatrial Node |
| SERCA2a | Sarcoplasmic/Endoplasmic Reticulum Calcium ATPase type 2a |
| SCL | Sinus Cycle Length |
| SNRT | Sinus Node Recovery Time |
| Tbx3 | T-box Transcription Factor 3 |
| TGF-β1 | Transforming Growth Factor-β1 |
References
- Fagard, R. Athlete’s heart. Heart (Br. Card. Soc.) 2003, 89, 1455–1461. [Google Scholar] [CrossRef]
- O’Keefe, J.H.; Patil, H.R.; Lavie, C.J.; Magalski, A.; Vogel, R.A.; McCullough, P.A. Potential adverse cardiovascular effects from excessive endurance exercise. Mayo Clin. Proc. 2012, 87, 7, Correction in Mayo Clin. Proc. 2012, 87, 587–595. [Google Scholar] [CrossRef]
- Boyett, M.R.; D’Souza, A.; Zhang, H.; Morris, G.M.; Dobrzynski, H.; Monfredi, O. Viewpoint: Is the resting bradycardia in athletes the result of remodeling of the sinoatrial node rather than high vagal tone? J. Appl. Physiol. 2013, 114, 1351–1355. [Google Scholar] [CrossRef]
- Nissen, S.D.; Weis, R.; Krag-Andersen, E.K.; Hesselkilde, E.M.; Isaksen, J.L.; Carstensen, H.; Kanters, J.K.; Linz, D.; Sanders, P.; Hopster-Iversen, C.; et al. Electrocardiographic characteristics of trained and untrained standardbred racehorses. J. Vet. Intern. Med. 2022, 36, 1119–1130. [Google Scholar] [CrossRef]
- Hawks, M.K.; Paul, M.L.B.; Malu, O.O. Sinus Node Dysfunction. Am. Fam. Physician 2021, 104, 179–185. [Google Scholar]
- Bashour, T.T. Classification of sinus node dysfunction. Am. Heart J. 1985, 110, 1251–1256. [Google Scholar] [CrossRef]
- Choudhury, M.; Boyett, M.R.; Morris, G.M. Biology of the Sinus Node and its Disease. Arrhythmia Electrophysiol. Rev. 2015, 4, 28–34. [Google Scholar] [CrossRef]
- D’Souza, A.; Bucchi, A.; Johnsen, A.B.; Logantha, S.J.; Monfredi, O.; Yanni, J.; Prehar, S.; Hart, G.; Cartwright, E.; Wisloff, U.; et al. Exercise training reduces resting heart rate via downregulation of the funny channel HCN4. Nat. Commun. 2014, 5, 3775. [Google Scholar] [CrossRef] [PubMed]
- Bidaud, I.; D’Souza, A.; Forte, G.; Torre, E.; Greuet, D.; Thirard, S.; Anderson, C.; Chung You Chong, A.; Torrente, A.G.; Roussel, J.; et al. Genetic Ablation of G Protein-Gated Inwardly Rectifying K+ Channels Prevents Training-Induced Sinus Bradycardia. Front. Physiol. 2020, 11, 519382. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, A.; Pearman, C.M.; Wang, Y.; Nakao, S.; Logantha, S.; Cox, C.; Bennett, H.; Zhang, Y.; Johnsen, A.B.; Linscheid, N.; et al. Targeting miR-423-5p Reverses Exercise Training-Induced HCN4 Channel Remodeling and Sinus Bradycardia. Circ. Res. 2017, 121, 1058–1068. [Google Scholar] [CrossRef] [PubMed]
- Bondarev, S.; Brotto, L.; Graziano, F.; Cipriani, A.; Corrado, D.; Zorzi, A. Does Long-Term Sport Practice Facilitate the Development of Idiopathic Bradycardia Requiring Early Pacemaker Implantation During the Course of Life? J. Cardiovasc. Dev. Dis. 2025, 12, 102. [Google Scholar] [CrossRef]
- Svedberg, N.; Sundström, J.; James, S.; Hållmarker, U.; Hambraeus, K.; Andersen, K. Long-Term Incidence of Bradycardia and Pacemaker Implantations Among Cross-Country Skiers: A Cohort Study. Circulation 2024, 150, 1161–1170. [Google Scholar] [CrossRef]
- Bondarev, S.; Achkasov, E.; Zorzi, A.; Safaryan, A.; Graziano, F.; Sizov, A. Intrinsic Sinus Node/Atrioventricular Node Dysfunction Requiring Pacemaker Implantation: Role of Former Professional Sport Activity. J. Clin. Med. 2023, 13, 203. [Google Scholar] [CrossRef] [PubMed]
- Billman, G.E.; Cagnoli, K.L.; Csepe, T.; Li, N.; Wright, P.; Mohler, P.J.; Fedorov, V.V. Exercise training-induced bradycardia: Evidence for enhanced parasympathetic regulation without changes in intrinsic sinoatrial node function. J. Appl. Physiol. 2015, 118, 1344–1355. [Google Scholar] [CrossRef]
- Chang, Y.; Yu, T.; Yang, H.; Peng, Z. Exhaustive exercise-induced cardiac conduction system injury and changes of cTnT and Cx43. Int. J. Sports Med. 2015, 36, 1–8. [Google Scholar] [CrossRef]
- Azevedo, L.F.; Perlingeiro, P.S.; Hachul, D.T.; Gomes-Santos, I.L.; Brum, P.C.; Allison, T.G.; Negrão, C.E.; De Matos, L.D. Sport modality affects bradycardia level and its mechanisms of control in professional athletes. Int. J. Sports Med. 2014, 35, 954–959. [Google Scholar] [PubMed]
- Molina, G.E.; Porto, L.G.; Fontana, K.E.; Junqueira, L.F., Jr. Unaltered R-R interval variability and bradycardia in cyclists as compared with non-athletes. Clin. Auton. Res. Off. J. Clin. Auton. Res. Soc. 2013, 23, 141–148. [Google Scholar] [CrossRef]
- Benito, B.; Gay-Jordi, G.; Serrano-Mollar, A.; Guasch, E.; Shi, Y.; Tardif, J.C.; Brugada, J.; Nattel, S.; Mont, L. Cardiac arrhythmogenic remodeling in a rat model of long-term intensive exercise training. Circulation 2011, 123, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Baldesberger, S.; Bauersfeld, U.; Candinas, R.; Seifert, B.; Zuber, M.; Ritter, M.; Jenni, R.; Oechslin, E.; Luthi, P.; Scharf, C.; et al. Sinus node disease and arrhythmias in the long-term follow-up of former professional cyclists. Eur. Heart J. 2008, 29, 71–78. [Google Scholar] [CrossRef]
- De Angelis, K.; Wichi, R.B.; Jesus, W.R.; Moreira, E.D.; Morris, M.; Krieger, E.M.; Irigoyen, M.C. Exercise training changes autonomic cardiovascular balance in mice. J. Appl. Physiol. 2004, 96, 2174–2178. [Google Scholar] [CrossRef]
- Stein, R.; Medeiros, C.M.; Rosito, G.A.; Zimerman, L.I.; Ribeiro, J.P. Intrinsic sinus and atrioventricular node electrophysiologic adaptations in endurance athletes. J. Am. Coll. Cardiol. 2002, 39, 1033–1038. [Google Scholar] [CrossRef]
- Such, L.; Rodriguez, A.; Alberola, A.; Lopez, L.; Ruiz, R.; Artal, L.; Pons, I.; Pons, M.L.; García, C.; Chorro, F.J. Intrinsic changes on automatism, conduction, and refractoriness by exercise in isolated rabbit heart. J. Appl. Physiol. 2002, 92, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Stein, R.; Moraes, R.S.; Cavalcanti, A.V.; Ferlin, E.L.; Zimerman, L.I.; Ribeiro, J.P. Atrial automaticity and atrioventricular conduction in athletes: Contribution of autonomic regulation. Eur. J. Appl. Physiol. 2000, 82, 155–157. [Google Scholar] [CrossRef] [PubMed]
- al-Ani, M.; Munir, S.M.; White, M.; Townend, J.; Coote, J.H. Changes in R-R variability before and after endurance training measured by power spectral analysis and by the effect of isometric muscle contraction. Eur. J. Appl. Physiol. Occup. Physiol. 1996, 74, 397–403. [Google Scholar]
- Monfredi, O.; Lyashkov, A.E.; Johnsen, A.B.; Inada, S.; Schneider, H.; Wang, R.; Nirmalan, M.; Wisloff, U.; Maltsev, V.A.; Lakatta, E.G.; et al. Biophysical characterization of the underappreciated and important relationship between heart rate variability and heart rate. Hypertension 2014, 64, 1334–1343. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Wang, K.; Yuan, Y.; Li, Q.; Dobrzynski, H.; Boyett, M.R.; Hancox, J.C.; Zhang, H. Mechanism underlying impaired cardiac pacemaking rhythm during ischemia: A simulation study. Chaos 2017, 27, 093934. [Google Scholar] [CrossRef]
- Ludwig, A.; Budde, T.; Stieber, J.; Moosmang, S.; Wahl, C.; Holthoff, K.; Langebartels, A.; Wotjak, C.; Munsch, T.; Zong, X.; et al. Absence epilepsy and sinus dysrhythmia in mice lacking the pacemaker channel HCN2. EMBO J. 2003, 22, 216–224. [Google Scholar] [CrossRef]
- Yamamoto, M.; Dobrzynski, H.; Tellez, J.; Niwa, R.; Billeter, R.; Honjo, H.; Kodama, I.; Boyett, M.R. Extended atrial conduction system characterised by the expression of the HCN4 channel and connexin45. Cardiovasc. Res. 2006, 72, 271–281. [Google Scholar] [CrossRef]
- Shi, W.; Wymore, R.; Yu, H.; Wu, J.; Wymore, R.T.; Pan, Z.; Robinson, R.B.; Dixon, J.E.; McKinnon, D.; Cohen, I.S. Distribution and prevalence of hyperpolarization-activated cation channel (HCN) mRNA expression in cardiac tissues. Circ. Res. 1999, 85, e1–e6. [Google Scholar] [CrossRef]
- Nikmaram, M.R.; Boyett, M.R.; Kodama, I.; Suzuki, R.; Honjo, H. Variation in effects of Cs+, UL-FS-49, and ZD-7288 within sinoatrial node. Am. J. Physiol. 1997, 272, H2782–H2792. [Google Scholar] [CrossRef]
- Kodama, I.; Nikmaram, M.R.; Boyett, M.R.; Suzuki, R.; Honjo, H.; Owen, J.M. Regional differences in the role of the Ca2+ and Na+ currents in pacemaker activity in the sinoatrial node. Am. J. Physiol. 1997, 272, H2793–H2806. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, N.; Irisawa, H.; Kameyama, M. Contribution of two types of calcium currents to the pacemaker potentials of rabbit sino-atrial node cells. J. Physiol. 1988, 395, 233–253. [Google Scholar] [CrossRef] [PubMed]
- Tellez, J.O.; Dobrzynski, H.; Greener, I.D.; Graham, G.M.; Laing, E.; Honjo, H.; Hubbard, S.J.; Boyett, M.R.; Billeter, R. Differential expression of ion channel transcripts in atrial muscle and sinoatrial node in rabbit. Circ. Res. 2006, 99, 1384–1393. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, K.Y.; Vinogradova, T.M.; Lakatta, E.G. Sinoatrial nodal cell ryanodine receptor and Na+-Ca2+ exchanger: Molecular partners in pacemaker regulation. Circ. Res. 2001, 88, 1254–1258. [Google Scholar] [CrossRef]
- Kohl, P.; Hunter, P.; Noble, D. Stretch-induced changes in heart rate and rhythm: Clinical observations, experiments and mathematical models. Prog. Biophys. Mol. Biol. 1999, 71, 91–138. [Google Scholar] [CrossRef]
- Easterling, M.; Rossi, S.; Mazzella, A.J.; Bressan, M. Assembly of the Cardiac Pacemaking Complex: Electrogenic Principles of Sinoatrial Node Morphogenesis. J. Cardiovasc. Dev. Dis. 2021, 8, 40. [Google Scholar] [CrossRef]
- Lev, M. Aging changes in the human sinoatrial node. J. Gerontol. 1954, 9, 1–9. [Google Scholar] [CrossRef]
- James, T.N. Anatomy of the human sinus node. Anat. Rec. 1961, 141, 109–139. [Google Scholar] [CrossRef]
- Glukhov, A.V.; Kalyanasundaram, A.; Lou, Q.; Hage, L.T.; Hansen, B.J.; Belevych, A.E.; Mohler, P.J.; Knollmann, B.C.; Periasamy, M.; Györke, S.; et al. Calsequestrin 2 deletion causes sinoatrial node dysfunction and atrial arrhythmias associated with altered sarcoplasmic reticulum calcium cycling and degenerative fibrosis within the mouse atrial pacemaker complex1. Eur. Heart J. 2015, 36, 686–697. [Google Scholar] [CrossRef]
- Allessie, M.; Ausma, J.; Schotten, U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc. Res. 2002, 54, 230–246. [Google Scholar] [CrossRef] [PubMed]
- Brorson, L.; Conradson, T.B.; Olsson, B.; Varnauskas, E. Right atrial monophasic action potential and effective refractory periods in relation to physical training and maximal heart rate. Cardiovasc. Res. 1976, 10, 160–168. [Google Scholar] [CrossRef]
- Kodama, I.; Boyett, M.R. Regional differences in the electrical activity of the rabbit sinus node. Pflug. Arch. Eur. J. Physiol. 1985, 404, 214–226. [Google Scholar] [CrossRef]
- Jones, S.A.; Boyett, M.R.; Lancaster, M.K. Declining into failure: The age-dependent loss of the L-type calcium channel within the sinoatrial node. Circulation 2007, 115, 1183–1190. [Google Scholar] [CrossRef]
- Larson, E.D.; St Clair, J.R.; Sumner, W.A.; Bannister, R.A.; Proenza, C. Depressed pacemaker activity of sinoatrial node myocytes contributes to the age-dependent decline in maximum heart rate. Proc. Natl. Acad. Sci. USA 2013, 110, 18011–18016. [Google Scholar] [CrossRef] [PubMed]
- Boyett, M.R.; Yanni, J.; Tellez, J.; Bucchi, A.; Mesirca, P.; Cai, X.; Logantha, S.; Wilson, C.; Anderson, C.; Ariyaratnam, J.; et al. Regulation of sinus node pacemaking and atrioventricular node conduction by HCN channels in health and disease. Prog. Biophys. Mol. Biol. 2021, 166, 61–85. [Google Scholar] [CrossRef] [PubMed]
- Northcote, R.J.; Canning, G.P.; Ballantyne, D. Electrocardiographic findings in male veteran endurance athletes. Br. Heart J. 1989, 61, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Dobrzynski, H.; Boyett, M.R.; Anderson, R.H. New insights into pacemaker activity: Promoting understanding of sick sinus syndrome. Circulation 2007, 115, 1921–1932. [Google Scholar] [CrossRef]
- Petkova, M.; Atkinson, A.J.; Yanni, J.; Stuart, L.; Aminu, A.J.; Ivanova, A.D.; Pustovit, K.B.; Geragthy, C.; Feather, A.; Li, N.; et al. Identification of Key Small Non-Coding MicroRNAs Controlling Pacemaker Mechanisms in the Human Sinus Node. J. Am. Heart Assoc. 2020, 9, e016590. [Google Scholar] [CrossRef]
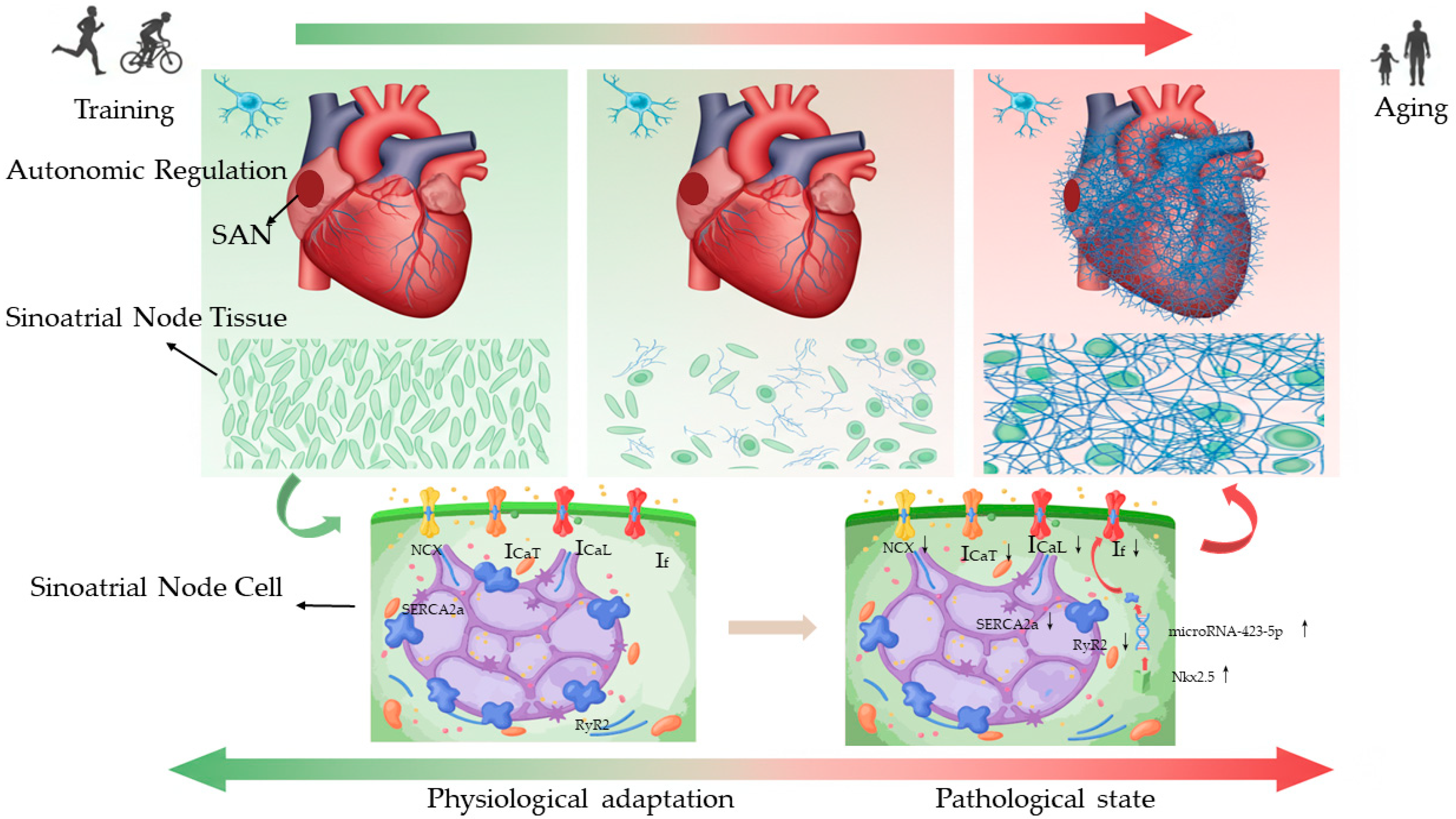
| Study (Author, Year) | Model (Species) | Exercise Protocol | Major Findings Related to SAN | Electrophysiology Conclusion | Pathological Indicators |
|---|---|---|---|---|---|
| Bondarev et al., 2025 [11] | Human | mixed endurance/strength sports | Lifetime endurance sport linked to earlier SAN disease; former athletes required a pacemaker (PM) 5 years earlier (57.9 vs. 64.0 years; p = 0.03); borderline inverse correlation between total training load and implantation age (p = 0.08); no SAN-size echo differences | Long-term intense sport facilitates earlier manifestation of intrinsic SAN/Atrioventricular Node (AV)-node disease, suggesting cumulative exercise accelerates conduction system ageing rather than a reversible vagal effect | pacemaker implantation |
| Svedberg et al., 2024 [12] | Human | Long-term endurance training (Skiing) | Male skiers SSS/pacemaker↑ (Heart Rate (HR) 1.19–1.17), younger implant age (63.5 years); no excess in females. | Male endurance athletes SAN dysfunction↑ and pacemaker need; benign post-implant prognosis (sick sinus syndrome is more common) | pacemaker implantation/sick sinus syndrome |
| Bondarev et al., 2023 [13] | Human | Endurance training, power/mixed training | Endurance athletes received PM ~8 years earlier (65 vs. 73 years, p < 0.01); 78% AV-block vs. 44% in power/mixed | Elite endurance training hastens intrinsic SAN/AV-node ageing and early pacemaker requirement | pacemaker implantation |
| Nissen SD et al., 2022 [4] | Horses | Running | Trained horses had 7% lower resting HR (p = 0.001); Sinus Node Recovery Time (SNRT) trended longer at 800 ms Pacing Cycle Length (PCL) (2286 vs. 1927 ms, p = 0.09), but no training effect was confirmed | Training lowers resting HR in horses, but SAN adaptation (SNRT prolongation) is modest and non-significant compared with human athletes | Not assessed |
| Bidaud et al., 2021 [9] | Mouse (WT vs. Girk4−/−) | Swim training | Training HR↓ 39%, SAN rate 39%; If/ICaT/ICaL↓, HCN4/Cav1.3↓, miR-423-5p↑ (WT only); all blocked in Girk4−/− | Girk4 deletion blocks miR-HCN4/Cav1.3 remodelling, preventing intrinsic bradycardia and athlete’s heart, and offers a therapeutic target for bradycardia | Not assessed |
| D’Souza et al., 2017 [10] | Human/mice | endurance training/swim training | Athletes: intrinsic HR 11%, ivabradine blunted↓; Mice: miR-423-5p↑8× → HCN4/If↓ (R2 = 0.68), SAN rate↓ (R2 = 0.46); anti-miR-423 reverses; Nkx2.5↑ drives miR-423-5p transcription → SAN remodelling; upstream master switch | Nkx2.5–miR-423-5p–HCN4/If axis slows SAN rate; anti-miR-423-5p fully rescues | Not assessed |
| Billman et al., 2015 [14] | Dogs | Running | Resting HR↓ 9%; intrinsic HR/cSNRT unaltered; SAN HCN4↑ | Bradycardia from ↑parasympathetic; intrinsic SAN unchanged | Cardiac Cavity Fibrosis |
| Chang et al., 2015 [15] | Rats | Swim training | SAN: collagen, ischemic morphology↑; Cardiac Troponin T (cTnT)/Connexin 43(Cx43)↓; gap-junction loss; swollen mitochondria, endoplasmic reticulum rupture | Repetitive exhaustive exercise → transient SAN ischemia/fibrosis, cTnT/Cx43↓, structural substrate for bradyarrhythmia without permanent failure | SAN collagen deposition, ischemic morphology, Cx43↓, gap junction loss, mitochondrial swelling |
| D’Souza et al., 2014 [8] | Rats/mice | Running/Swim training | Denervated SAN: cycle↑, If↓ 45%; T-box transcription factor 3 (Tbx3)↓, Neuron-Restrictive Silencer Factor (NRSF)↑, miR-1↑; reversible | Training bradycardia is intrinsic: HCN4/If downregulation via Tbx3↓, NRSF↑, miR-1↑; explains athlete SAN dysfunction | Not assessed |
| Azevedo et al., 2014 [16] | Human | Running/cycling | Runners vs. cyclists: RHR↓ (45 vs.51 b.min−1), vagal↑ (53 vs.41 b.min−1), IHR↑ (91 vs.83 b.min−1), septal/posterior wall thickness (11 vs. 12 and 11 vs. 12 mm); relationship between IHR and wall thickness r ≈ −0.39. | Sport modality determines bradycardia. Runners: vagal tone; cyclists: vagal tone + SAN remodelling | Not assessed |
| Molina et al., 2013 [17] | Human | Bike training | Athletes: resting HR 50 vs. 63 bpm (p = 0.0004); no HRV index differed (p = 0.17–0.97) except trend to lower LF-power | Bradycardia in cyclists is independent of altered autonomic modulation; it likely reflects intrinsic SAN adaptation rather than vagal dominance | Not assessed |
| Benito et al., 2011 [18] | Rats | Running | QRS duration↑ (ventricular conduction delay), atrial fibrosis↑, Transforming Growth Factor-beta 1 (TGF-β1)↑, Matrix Metalloproteinase-2 (MMP-2)↑, collagen-I↑ in Right Atrium/Left Atrium/Right Ventricle, inducible Ventricular Tachycardia in 42% of trained rats vs. 6% in sedentary | Long-term intensive exercise induces atrial and Right ventricular fibrosis, alters conduction, and increases arrhythmia susceptibility; changes are reversible after detraining | Right atrial fibrosis,↑ TGF-β1↑, MMP-2↑, collagen-I↑ |
| Baldesberger et al., 2008 [19] | Human | cycling | Athletes: ventricular tachycardias↑ (15 vs. 3%), HR↓ (66 vs. 70 bpm), Sinus Node Dysfunction (SND)↑ (10 vs. 2%), pacemaker for bradyarrhythmias (3 vs. 0%), maxRR↑ (6 vs. 0%) | Extreme endurance → lifelong SND/brady risk↑; irreversible SAN remodelling | pacemaker implantation/larger right atrial volume (29 ± 12 mL vs. 23 ± 8 mL) |
| De Angelis et al., 2004 [20] | Mouse | Running | Trained vs. sedentary: HR↓ (485 vs. 612 bpm); vagal effect (methylatropine) effect↑ (139 vs. 40 bpm), sympathetic effect (propranolol) effect (49 vs. 97 bpm); not intrinsic HR change | Training-induced mouse bradycardia is mediated solely by enhanced cardiac vagal tone and reduced sympathetic drive, without intrinsic SAN remodelling | Not assessed |
| Stein et al., 2002 [21] | Human | Running | Athletes vs. nonathletes: Sinus Cycle Length (SCL) (before/after block)↑ (1030 vs. 913 ms/737 vs. 653 43 ms), SNRT/SCL (before/after parasympathetic blockade, after double-autonomic blockade)↑ (1.36 vs. 1.26/0.06 vs. 1.45/0.09 vs. 1.31); indicating intrinsic SAN adaptation. | Athletes: SAN remodelling and AV node conduction changes | Not assessed |
| Such et al., 2002 [22] | Rabbits | Running | Trained vs. untrained: R-R↑28% (365 vs. 286 ms), SNRT↑28% (554 vs. 460 ms); cSNRT/SACT unchanged | Training intrinsically slows SAN rate and recovery, independent of autonomic, structural, or vascular factors—direct electrophysiological remodelling | Not assessed |
| Stein et al., 2000 [23] | Human | Running | Athletes vs. untrained: cSNRT↑ (369 vs. 279 ms, p = 0.09); Wenckebach point↓ (p = 0.01); vagal tone correlates with AV delay (r = 0.48) | Vagal tone/intrinsic adaptations of the conduction system: SAN automaticity slightly reduced; AV delay more marked | Not assessed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, L.; Li, J.; Wang, H.; Li, S.; Zhang, H. The Dual Nature of Sinoatrial Node Remodelling in Athletes: A Systematic Review of Electrophysiological Adaptations and the Pathological Tipping Point. Int. J. Mol. Sci. 2025, 26, 12052. https://doi.org/10.3390/ijms262412052
Yue L, Li J, Wang H, Li S, Zhang H. The Dual Nature of Sinoatrial Node Remodelling in Athletes: A Systematic Review of Electrophysiological Adaptations and the Pathological Tipping Point. International Journal of Molecular Sciences. 2025; 26(24):12052. https://doi.org/10.3390/ijms262412052
Chicago/Turabian StyleYue, Liang, Jiaying Li, Hui Wang, Shuang Li, and Henggui Zhang. 2025. "The Dual Nature of Sinoatrial Node Remodelling in Athletes: A Systematic Review of Electrophysiological Adaptations and the Pathological Tipping Point" International Journal of Molecular Sciences 26, no. 24: 12052. https://doi.org/10.3390/ijms262412052
APA StyleYue, L., Li, J., Wang, H., Li, S., & Zhang, H. (2025). The Dual Nature of Sinoatrial Node Remodelling in Athletes: A Systematic Review of Electrophysiological Adaptations and the Pathological Tipping Point. International Journal of Molecular Sciences, 26(24), 12052. https://doi.org/10.3390/ijms262412052





