Enhanced Anti-Lung Cancer Effects of Steamed Panacis Japonici Rhizoma: Insights from Metabolomics, Network Pharmacology and Molecular Dynamics Simulation
Abstract
1. Introduction
2. Results
2.1. Chemical Profiling and Multivariate Statistical Analysis of Distinctive Markers Between Raw and Steamed PJR
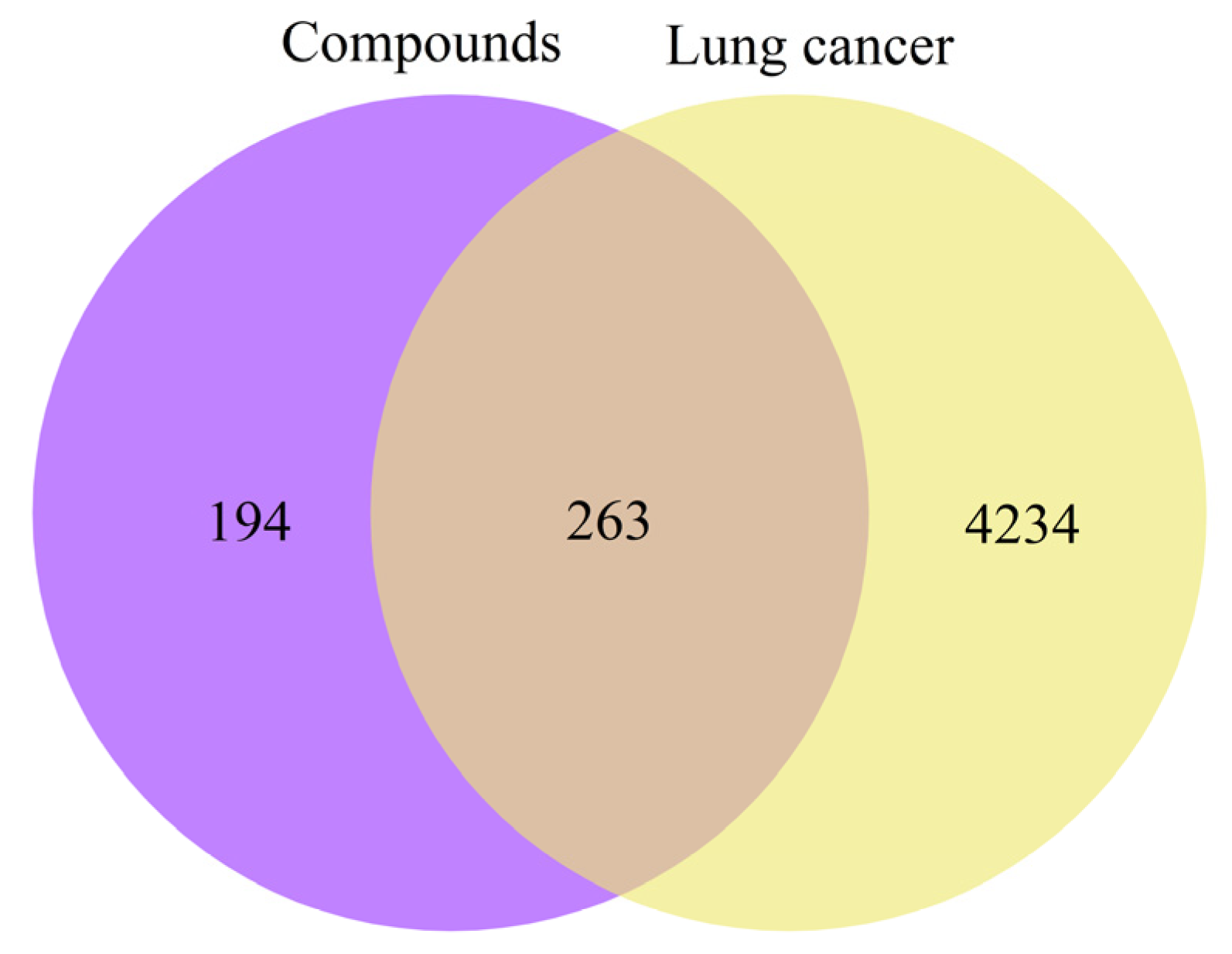
2.2. Network Analysis of the Increased Compounds
2.2.1. Target Prediction
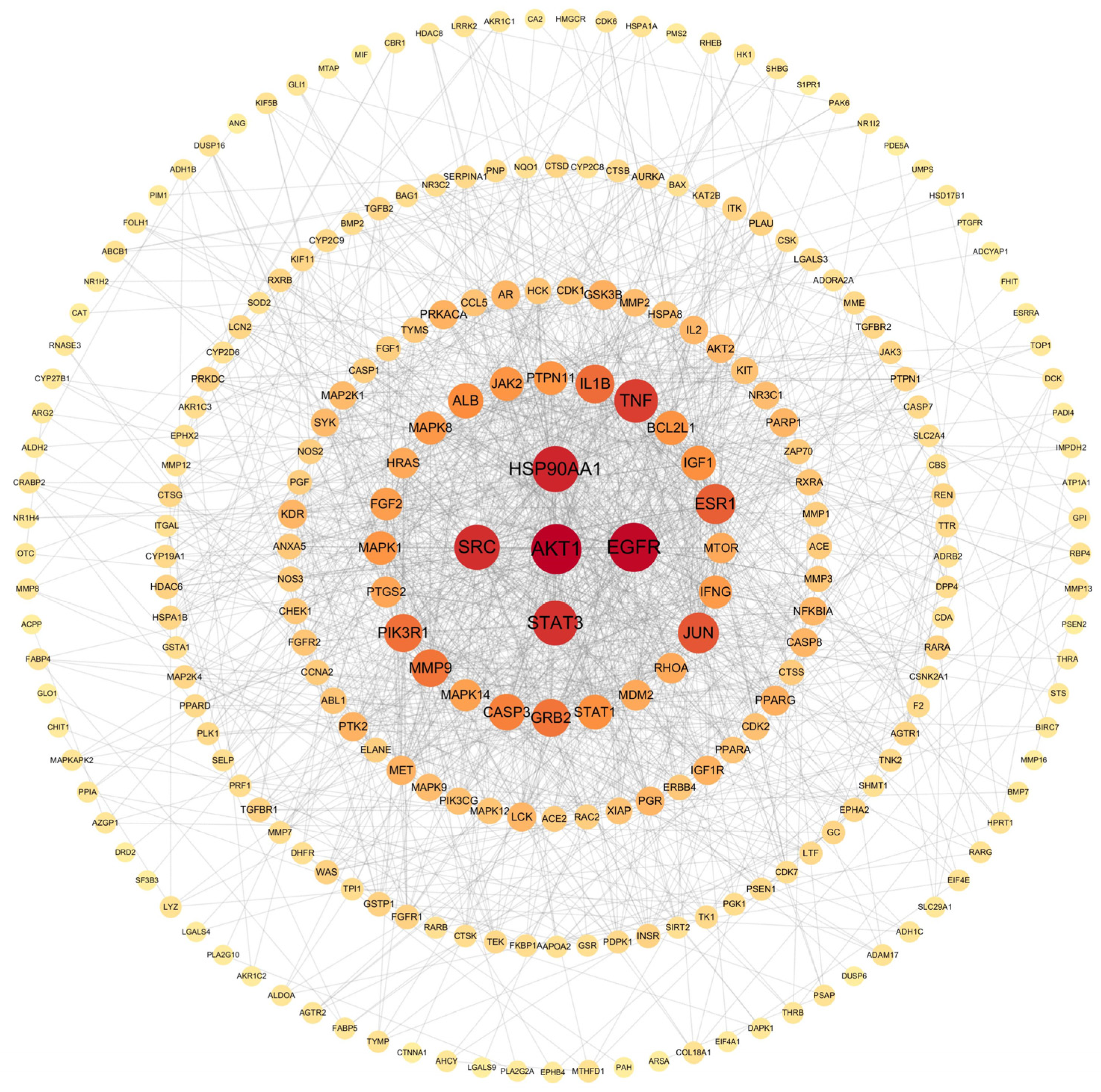
2.2.2. Protein–Protein Interaction (PPI) Network Construction
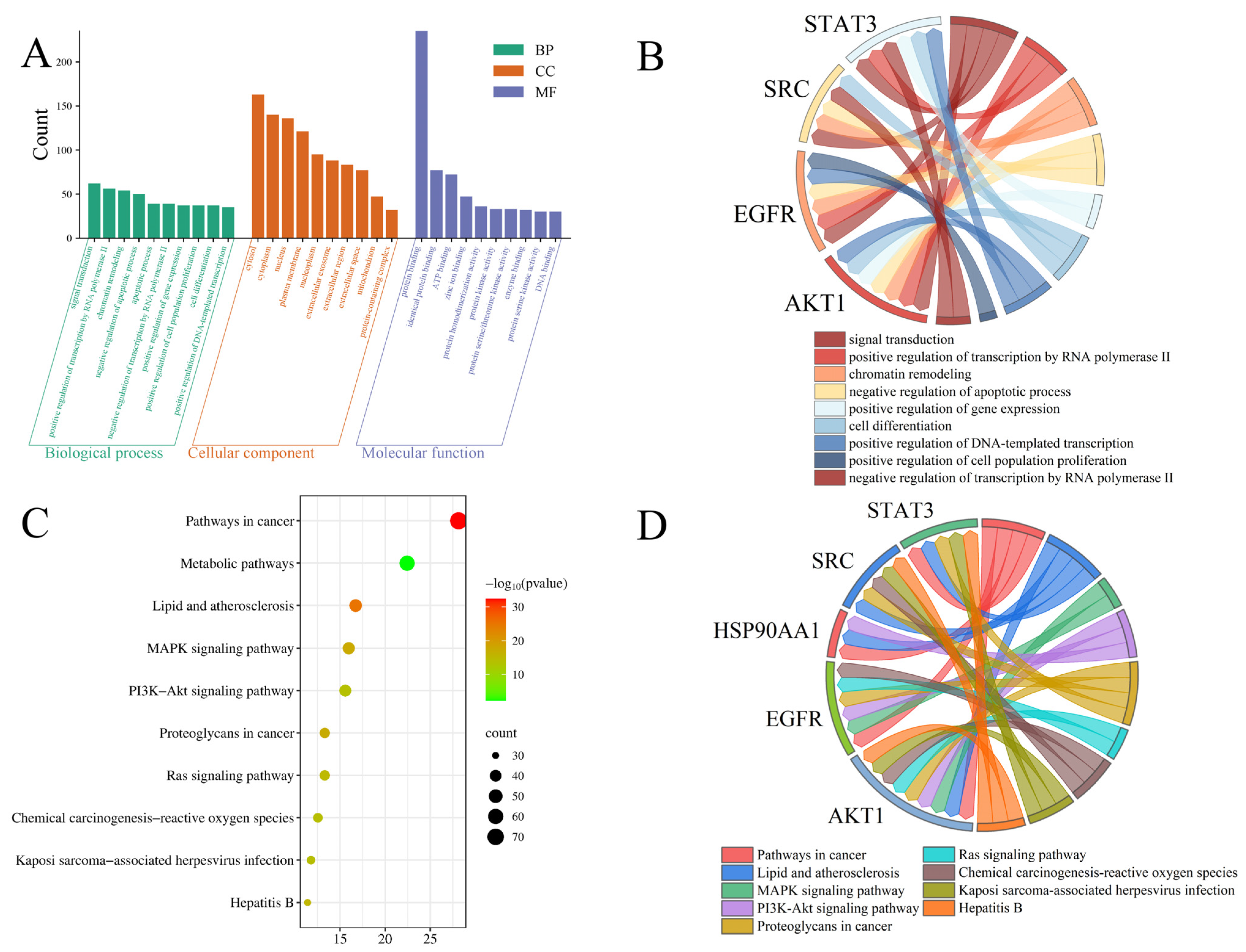
2.2.3. Functional Enrichment Analysis of Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathways
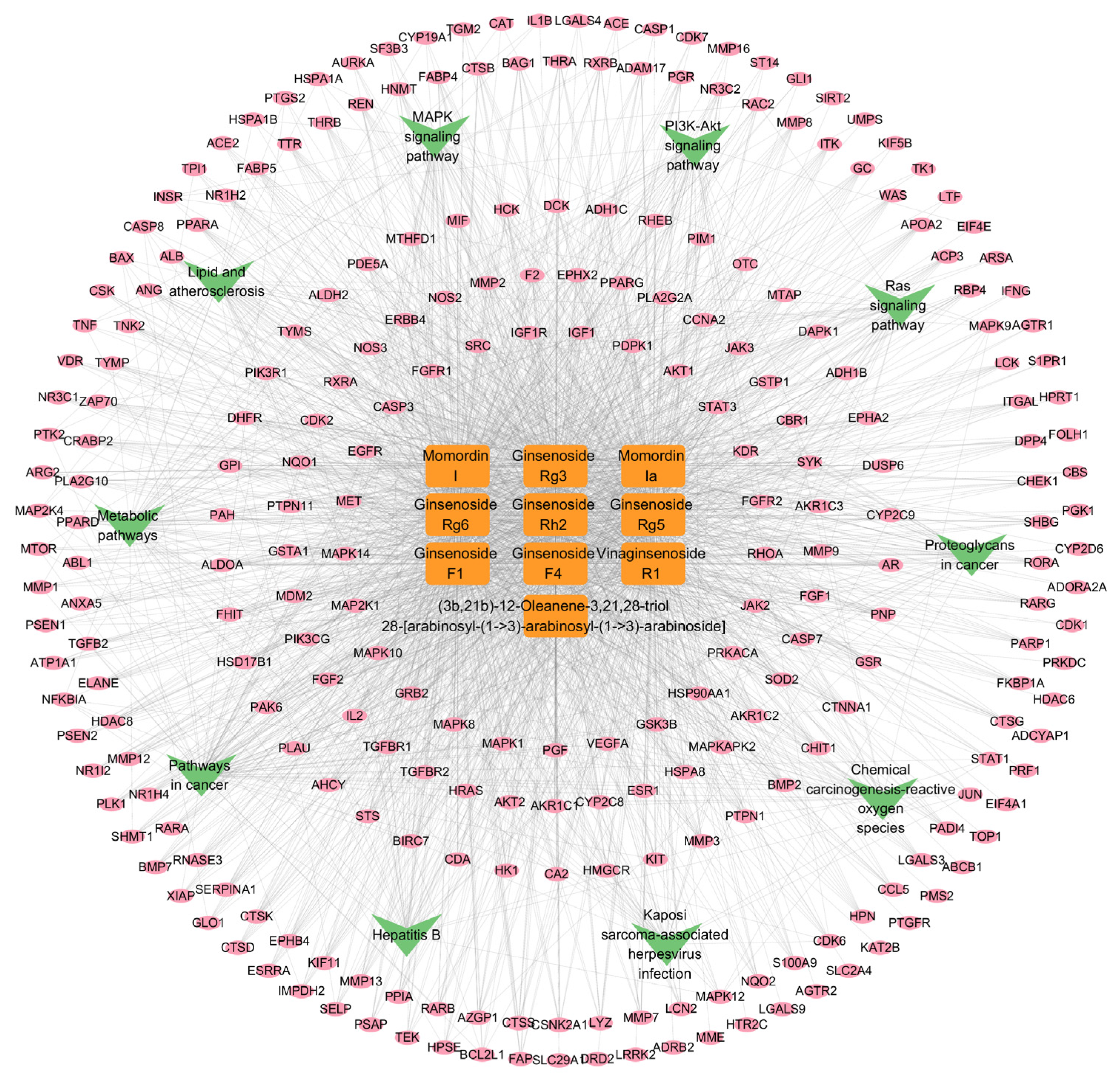
2.2.4. The “Component-Target-Pathway” Network
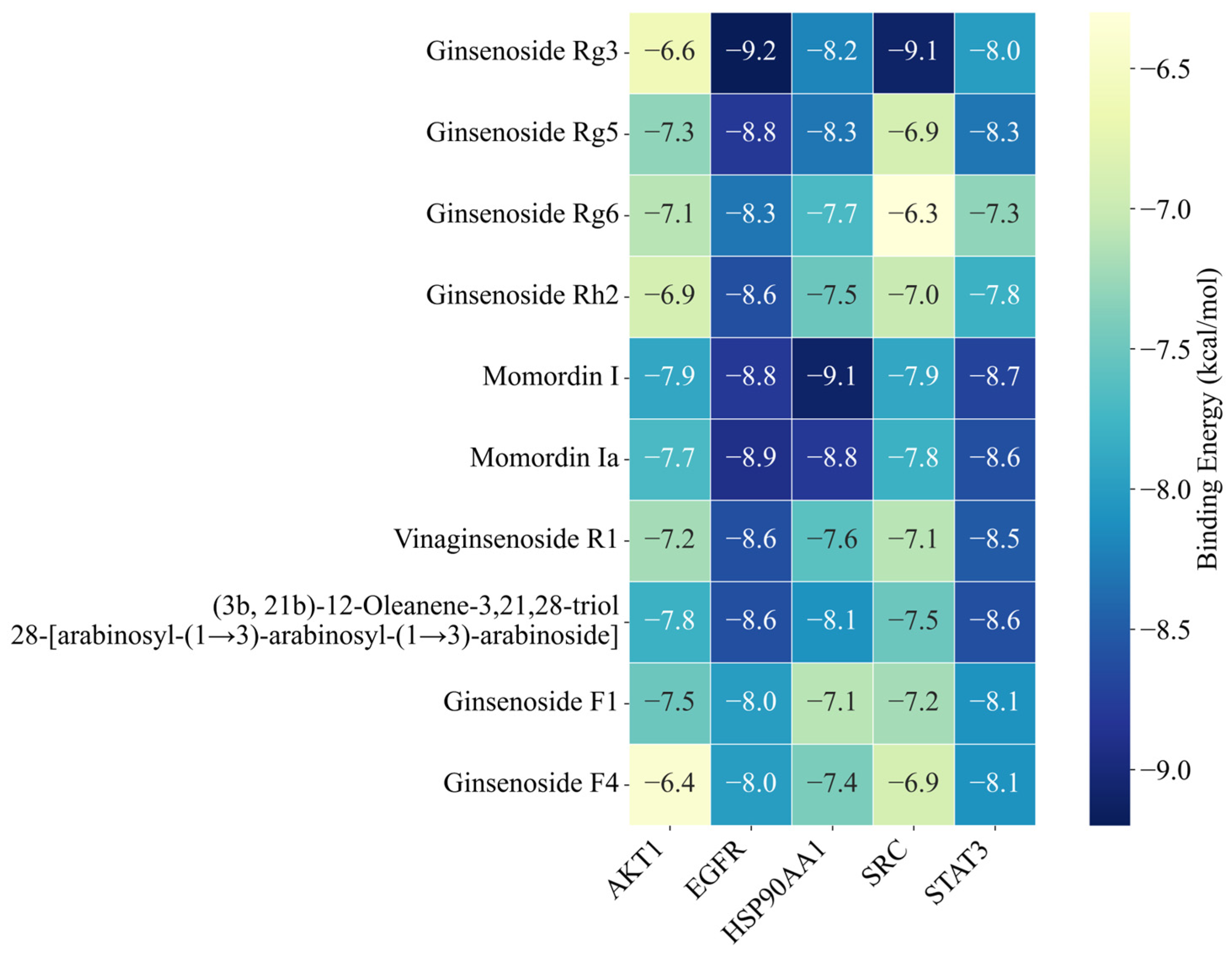
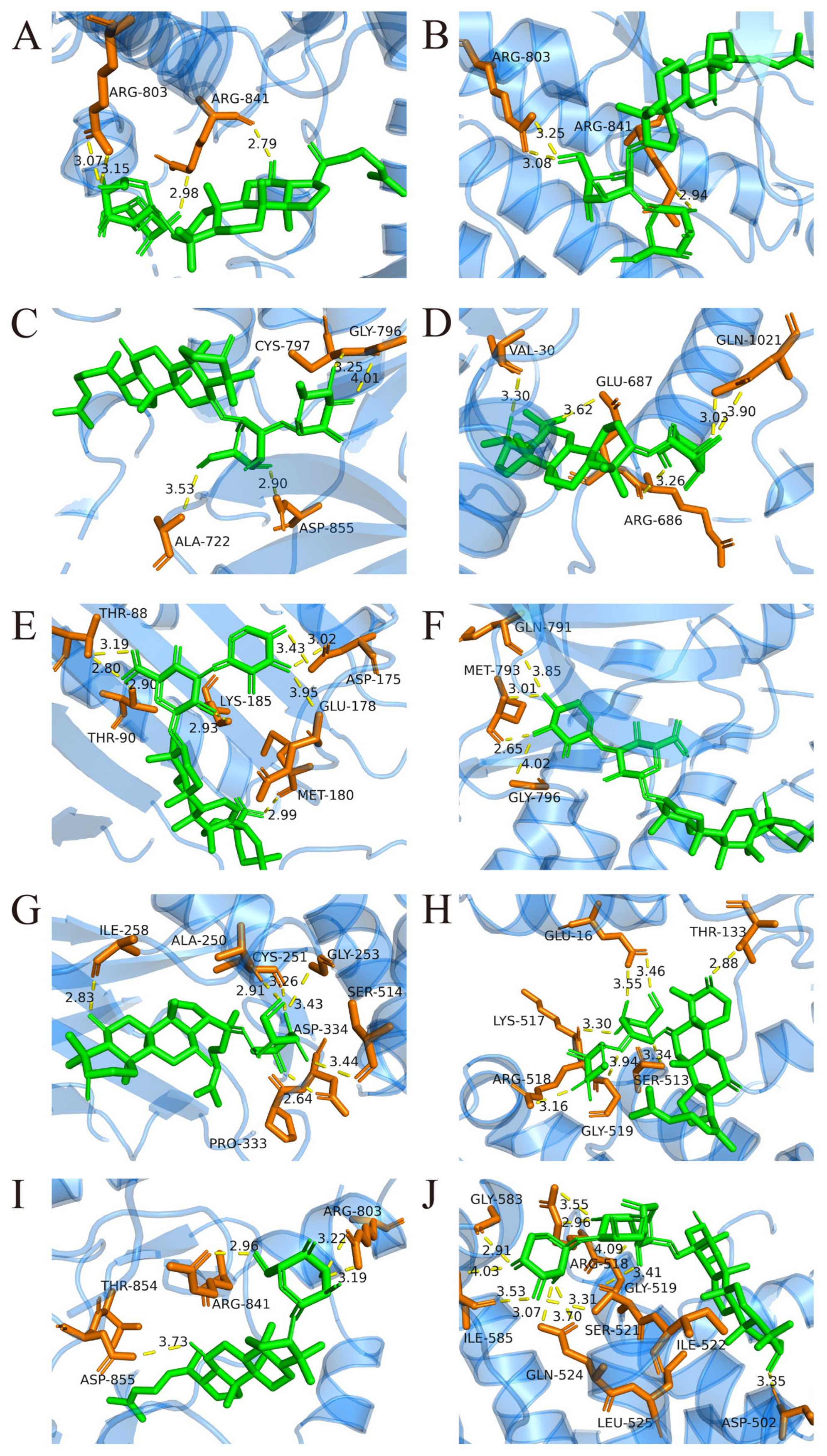
2.3. Molecular Docking Analysis
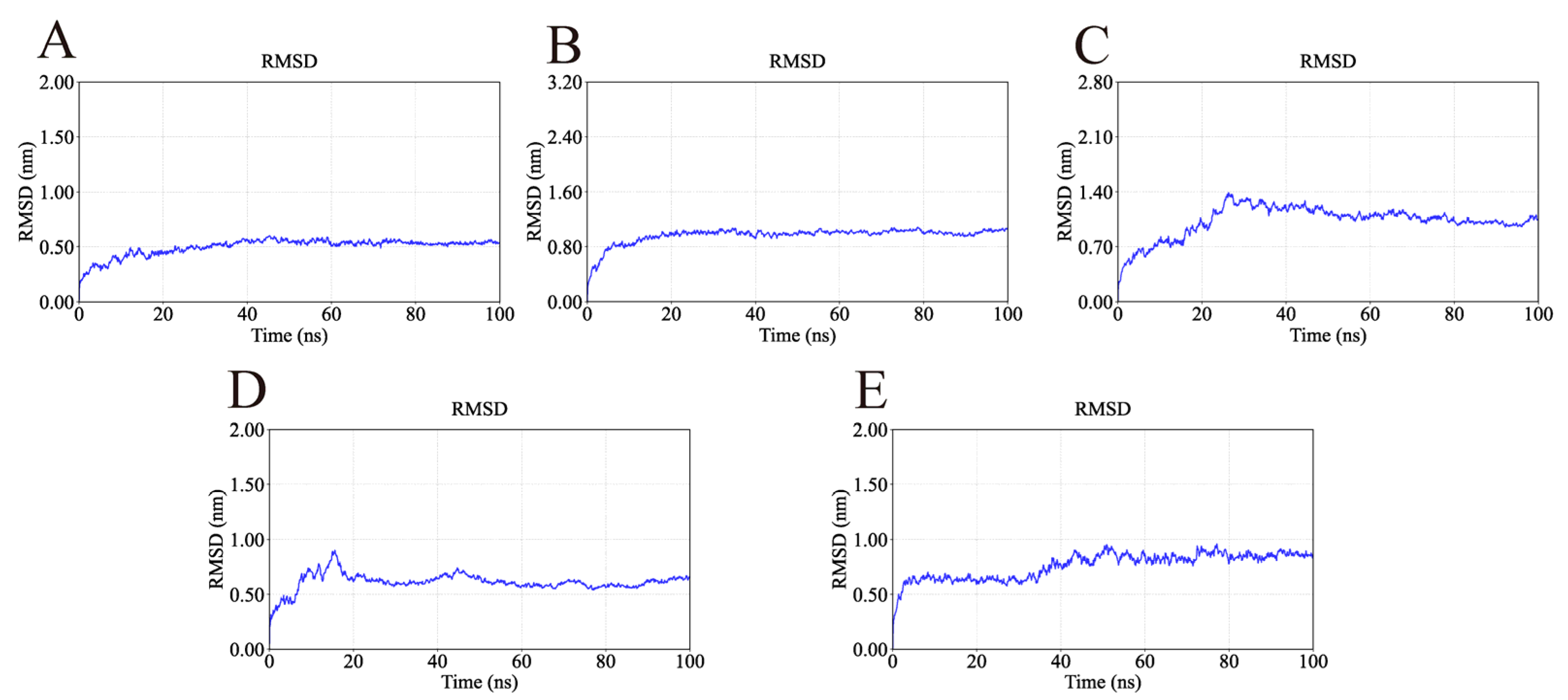
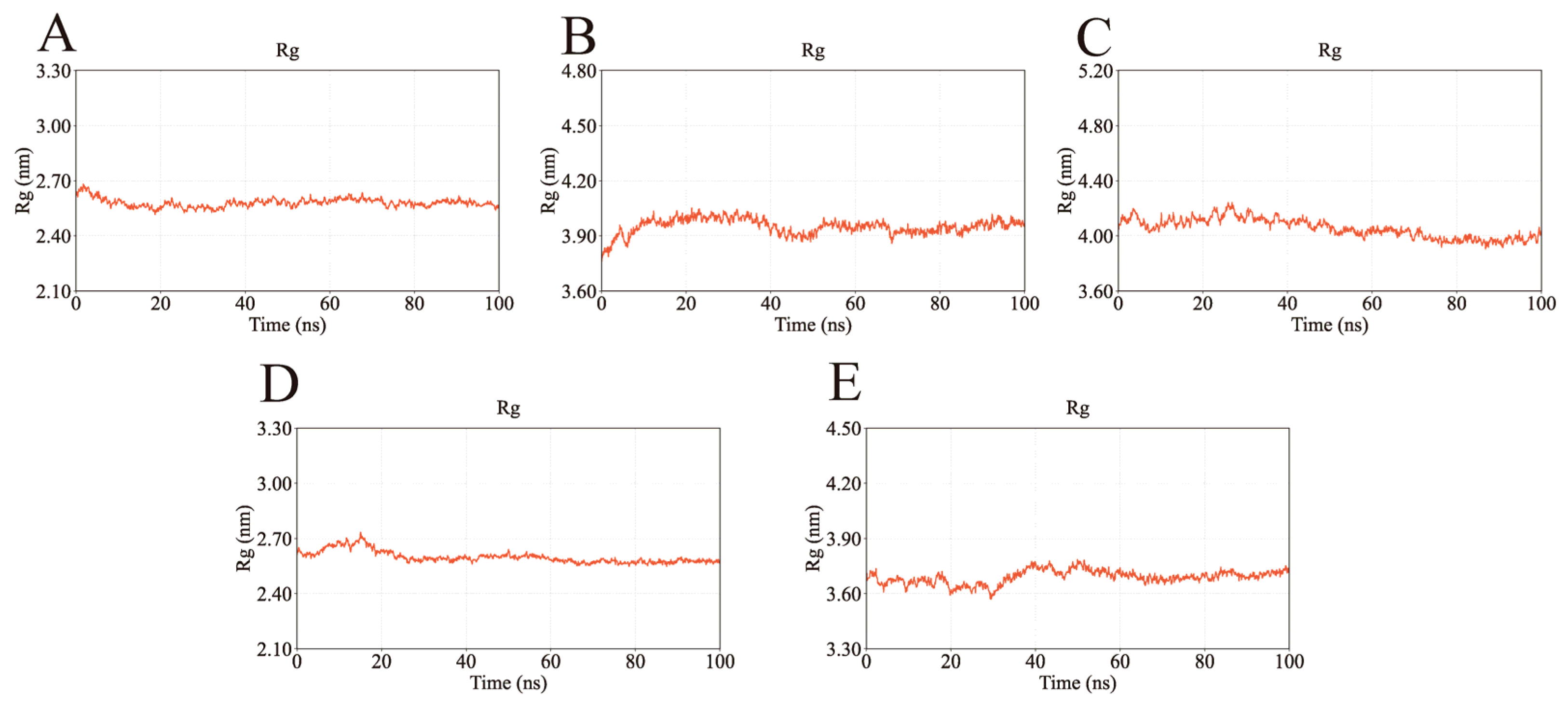
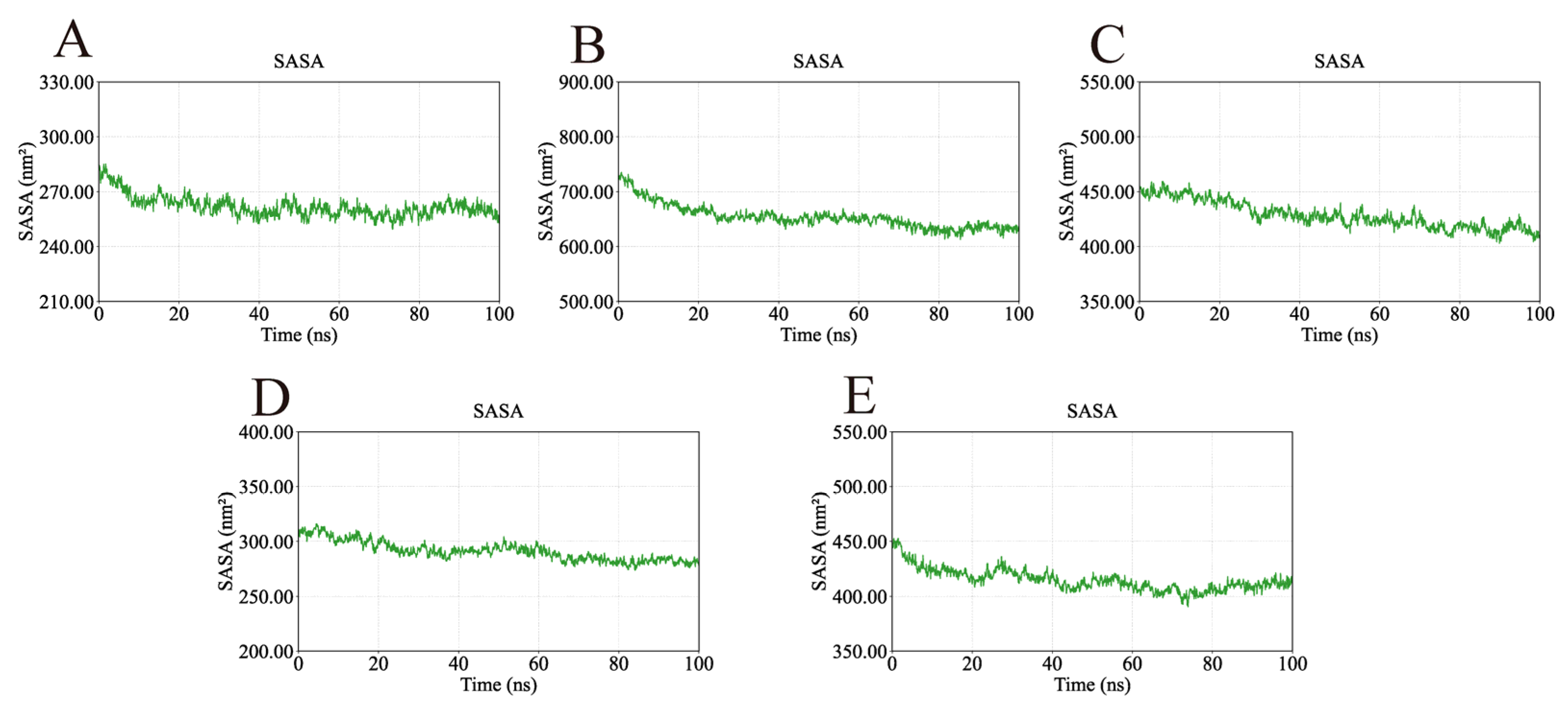
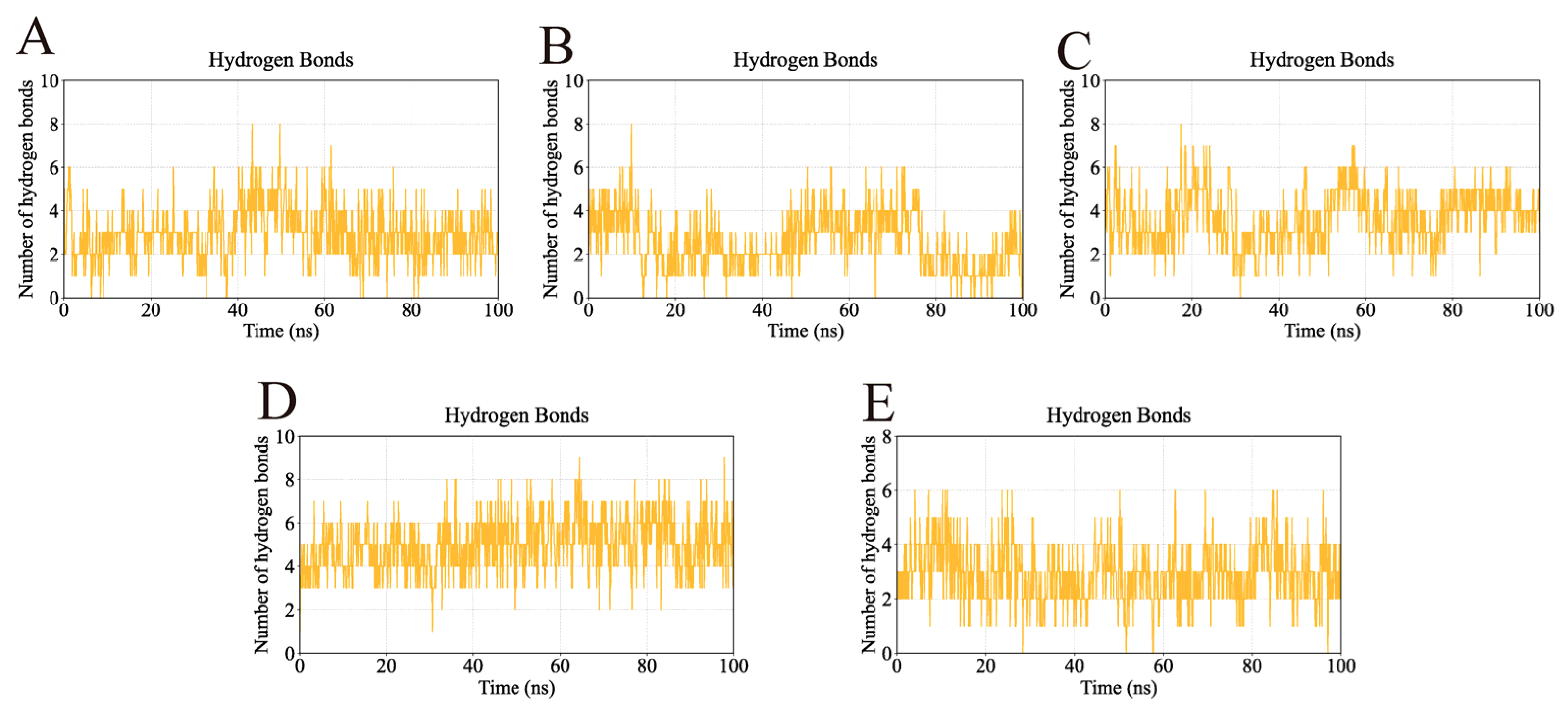
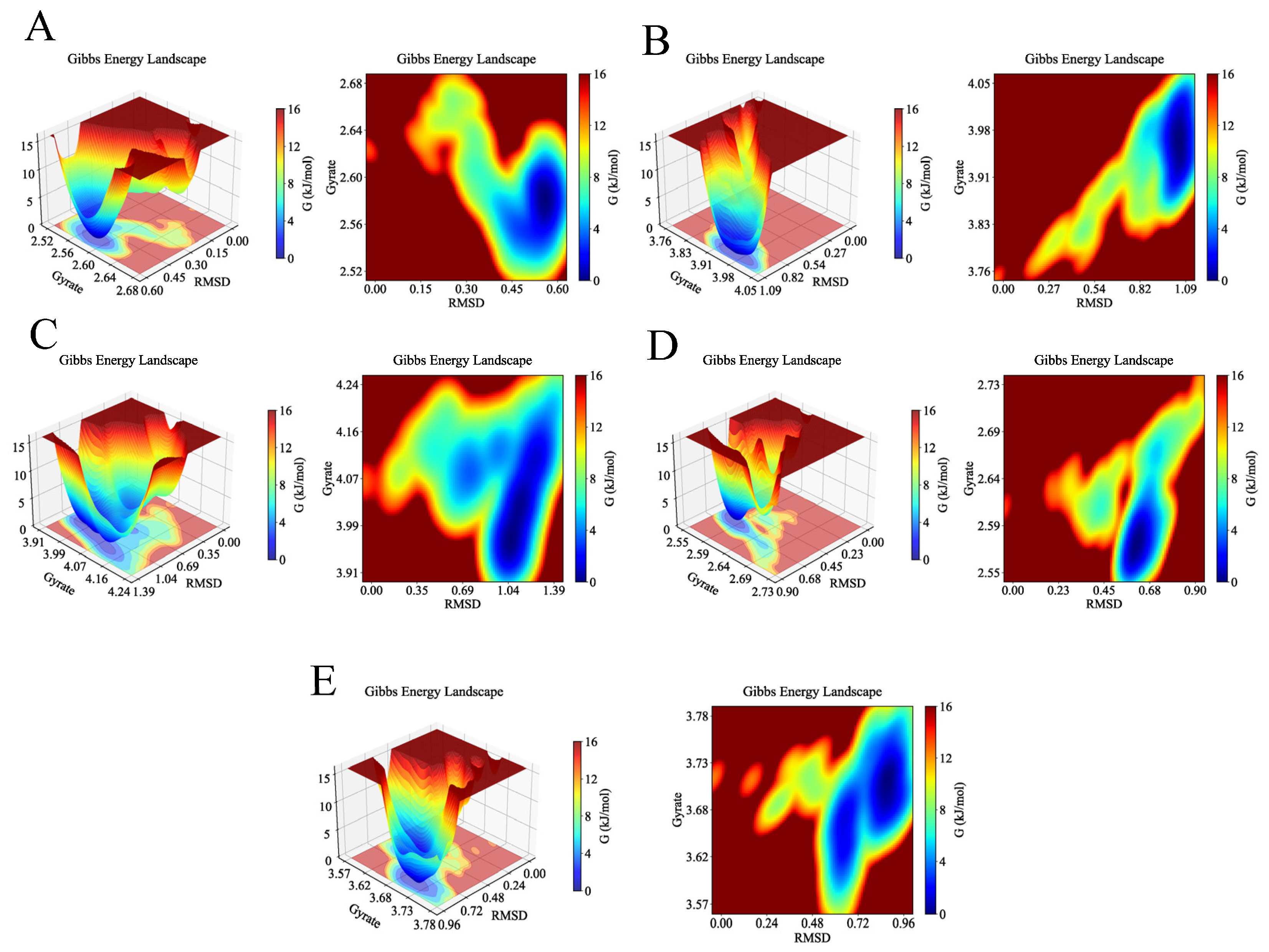
2.4. MD Simulation Analysis
2.5. Gene Expression Omnibus (GEO) Database Validation
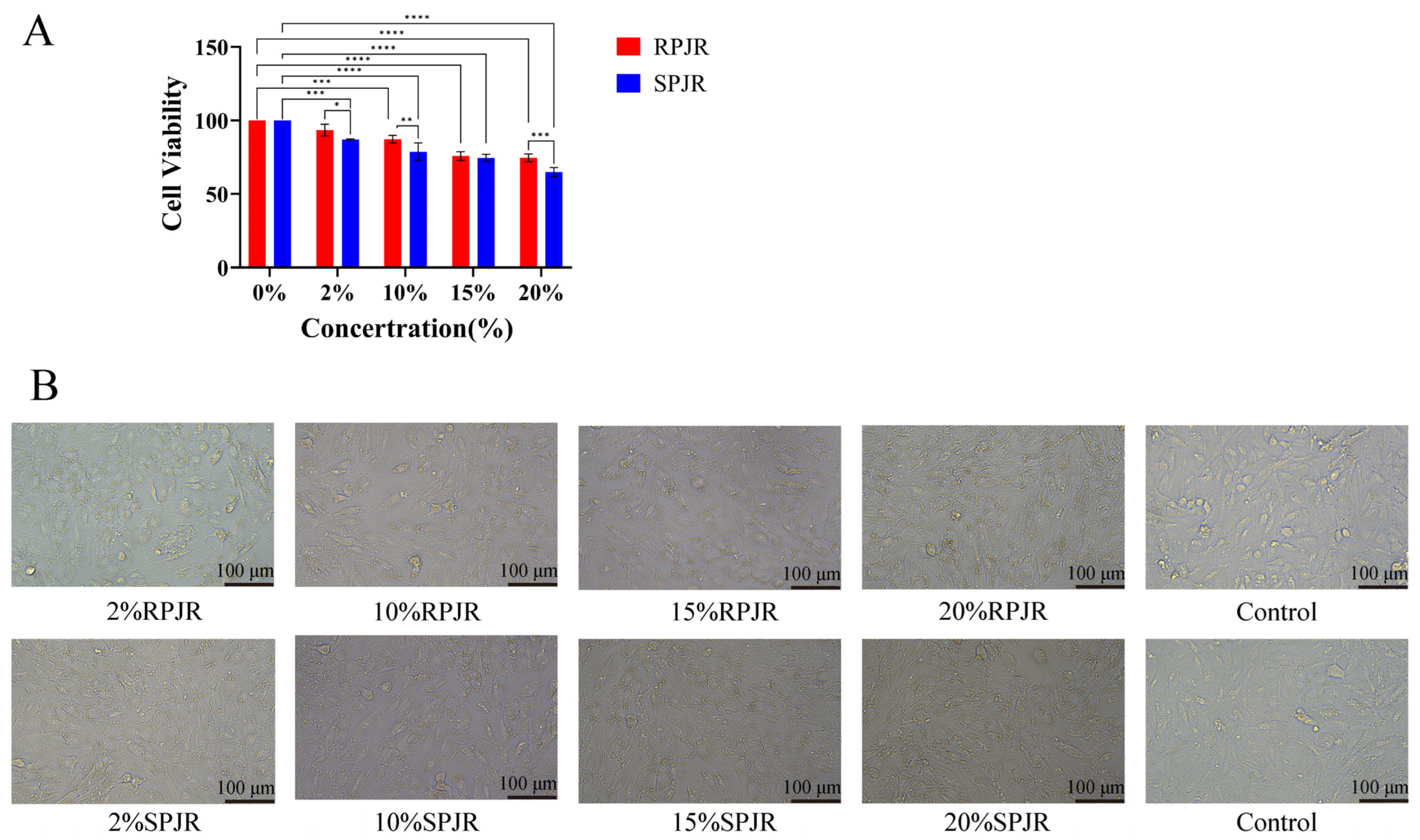
2.6. Cellular Viability Under Drug-Containing Serum Treatment
2.7. Cellular Morphology Alterations Induced by Drug-Containing Serum
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Metabolomic Profiling of Raw and Steamed PJR
4.2.1. Preparation of Standard Solutions
4.2.2. Preparation of Test Products
4.2.3. UPLC-Q-TOF-MS Conditions
4.2.4. Data Processing and Analysis
4.3. Network Pharmacology
4.3.1. Acquisition of Targets for Differential Metabolites
4.3.2. Screening of Disease-Associated Targets
4.3.3. Construction of a Venn Diagram
4.3.4. Construction of the PPI Network
4.3.5. GO Functional Annotation and KEGG Pathway Enrichment Analysis
4.3.6. Construction of the Compound-Target-Pathway Network
4.4. Molecular Docking
4.5. MD Simulation
4.6. GEO Database Validation
4.7. Experimental Verification
4.7.1. Preparation of Experimental Liquid Medicine
4.7.2. Animal Grouping and Drug Administration
4.7.3. Preparation of Drug-Containing Serum
4.7.4. Cell Culture and Passage
4.7.5. Cell Proliferation Assay
4.7.6. Cell Morphology Observation
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PJR | Panacis Japonici Rhizoma |
| UPLC-Q-TOF-MS | Ultra-high performance liquid chromatography-quadrupole/time-of-flight mass spectrometry |
| TCM | Traditional Chinese medicine |
| PCA | Principal component analysis |
| OPLS-DA | Orthogonal partial least squares-discriminant analysis |
| VIP | Variable importance in projection |
| PPI | Protein–protein interaction |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| DEGs | Differentially expressed genes |
| CMC-Na | Carboxymethyl cellulose sodium |
| RMSD | Root mean square deviation |
| RMSF | Root mean square fluctuation |
| Rg | Radius of gyration |
| SASA | Solvent accessible surface area |
References
- Wang, X.J.; Xie, Q.; Liu, Y.; Jiang, S.; Li, W.; Li, B.; Wang, W.; Liu, C.X. Panax japonicus and chikusetsusaponins: A review of diverse biological activities and pharmacology mechanism. Chin. Herb. Med. 2020, 13, 64–77. [Google Scholar] [CrossRef]
- Zheng, H.; Qiu, F.; Zhao, H.; Chen, J.; Wang, L.; Zou, H. Simultaneous determination of six bioactive saponins from Rhizoma Panacis Japonici in rat plasma by UHPLC-MS/MS: Application to a pharmacokinetic study. J. Chromatogr. B 2018, 1092, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China, 2020th ed.; China Medical Science Press: Beijing, China, 2020; p. 144. [Google Scholar]
- Liu, J.; Zhang, X.; Yang, S.; Wang, S.; Liu, C.; Yang, B.; Li, Y.; Cai, T. Rapid Identification of Characteristic Chemical Constituents of Panax ginseng, Panax quinquefolius, and Panax japonicus Using UPLC-Q-TOF/MS. J. Anal. Methods Chem. 2022, 2022, 6463770. [Google Scholar] [CrossRef]
- Wan, J.Z.; Li, C.Q.; Li, Y.N.; Li, A.Z.; Yang, Q.L.; Kang, M.; Cao, J.H.; Ran, H.J.; Liu, Q.L.; Wan, Y.; et al. Chikusetsu saponin IVa attenuates aging by improving autophagy and mitophagy. Free Radic. Biol. Med. 2025, 230, 17–32. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Zhang, Y.; Shi, X.; Li, Y.; Pan, Y. The Anti-Osteoporosis Effects of Panax japonicus via Downregulation of Inflammatory Factors: A Network Pharmacology and Ovariectomized Rat Model Study. Endocr. Metab. Immune Disord. Drug Targets, 2025; in press. [Google Scholar] [CrossRef]
- Povydysh, M.N.; Titova, M.V.; Ivkin, D.Y.; Krasnova, M.V.; Vasilevskaya, E.R.; Fedulova, L.V.; Ivanov, I.M.; Klushin, A.G.; Popova, E.V.; Nosov, A.M. The Hypoglycemic and Hypocholesterolemic Activity of Dioscorea deltoidea, Tribulus terrestris and Panax japonicus Cell Culture Biomass in Rats with High-Fat Diet-Induced Obesity. Nutrients 2023, 15, 656. [Google Scholar] [CrossRef]
- Huang, C.; Li, G.; Yan, X.; Disayathanoowat, T.; Inta, A.; Gao, L.; Yang, L. Molecular Mechanisms of Panax japonicus var. major Against Gastric Cancer: Metabolite Analysis, Signaling Pathways, and Protein Targets. Pharmaceuticals 2025, 18, 823. [Google Scholar] [CrossRef] [PubMed]
- Shu, G.; Jiang, S.; Mu, J.; Yu, H.; Duan, H.; Deng, X. Antitumor immunostimulatory activity of polysaccharides from Panax japonicus C. A. Mey: Roles of their effects on CD4+ T cells and tumor associated macrophages. Int. J. Biol. Macromol. 2018, 111, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, J.; Feng, Z.; Ji, J.; Shen, X.; Hou, X.; Mei, Z. The antiangiogenic effect of total saponins of Panax japonicus C.A. Meyer in rheumatoid arthritis is mediated by targeting the HIF-1α/VEGF/ANG-1 axis. J. Ethnopharmacol. 2024, 333, 118422. [Google Scholar] [CrossRef]
- Yang, J.; Jiang, W.; Park, J.H.; Seong, E.S.; Kwon, Y.S.; Kim, M.J. Antioxidant and Pancreatic Lipase Inhibitory Activities of Panax japonicus (T. Nees) C.A. Meyer. Plants 2025, 14, 2003. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, R.; Li, L.; He, Y.; Yuan, D.; Zhang, Y.; Hu, Y.; Wang, S.; Yuan, C. Total saponins from Panax japonicus reduce inflammation in adipocytes through the miR155/SOCS1/NFκB signaling pathway. Phytomedicine 2023, 115, 154827. [Google Scholar] [CrossRef]
- Wang, T.; Huang, Y.; Hu, M.; Huang, X.; Yu, D.; Jia, L.; Zhi, W.; Mu, Y.; Zhou, Z.; Wang, J. Saponins from Panax japonicus Enhance Lipolysis via Acting on FGF21-β-Klotho/FGFR1 in Obese Mice. J. Agric. Food Chem. 2025, 73, 15624–15636. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Gao, G.Z.; Zang, H.R.; Liang, X.H.; Zhang, T.; Wang, X.D. Role of saponins from Panax japonicus inhibiting primary lung cancer through regulating TRL4/NF-κB signaling pathway mediated immunologic function. Shaanxi Med. J. 2020, 49, 1539–1542. (In Chinese) [Google Scholar]
- Gao, G.Z.; Zang, H.R.; Zhang, T.; Wang, X.D.; Liang, X.H. Study on the mechanism of saponins from Panax japonicus on inhibition of A549 cell proliferation and migration through regulating PTEN/PI3K/Akt pathway. Mod. Biomed. Prog. 2020, 20, 242–247. (In Chinese) [Google Scholar]
- Gao, G.Z.; Zang, H.R.; Zhang, T.; Cui, K.; Liang, X.H. Study on the effect and mechanism of saponins from Panax japonicus in inhibiting cisplatin resistant lung cancer cells. Chin. J. Mod. Appl. Pharm. 2020, 37, 2715–2719. (In Chinese) [Google Scholar]
- Tian, Y.; Shi, Y.; Zhu, Y.; Li, H.; Shen, J.; Gao, X.; Cai, B.; Li, W.; Qin, K. The modern scientific mystery of traditional Chinese medicine processing-take some common traditional Chinese medicine as examples. Heliyon 2024, 10, e25091. [Google Scholar] [CrossRef]
- Wang, C.; Tang, X.; Yan, F.; Wang, Y.; Wang, X.; Zhao, D.; Liu, L.; Qi, B. Different processing methods affect the chemical composition and in vitro anti-tumor activity of ginseng. J. Food Sci. 2025, 90, e17639. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, L.; Man, J.; Hu, Y.; Cui, X. Chemical and bioactive comparison of Panax notoginseng root and rhizome in raw and steamed forms. J. Ginseng Res. 2019, 43, 385–393. [Google Scholar] [CrossRef]
- Metwaly, A.M.; Zhu, L.; Huang, L.; Dou, D. Black Ginseng and Its Saponins: Preparation, Phytochemistry and Pharmacological Effects. Molecules 2019, 24, 1856. [Google Scholar] [CrossRef]
- He, M.; Huang, X.; Liu, S.; Guo, C.; Xie, Y.; Meijer, A.H.; Wang, M. The Difference between White and Red Ginseng: Variations in Ginsenosides and Immunomodulation. Planta Med. 2018, 84, 845–854. [Google Scholar] [CrossRef]
- Fan, W.; Yang, Y.; Li, L.; Fan, L.; Wang, Z.; Yang, L. Mass spectrometry-based profiling and imaging strategy, a fit-for-purpose tool for unveiling the transformations of ginsenosides in Panax notoginseng during processing. Phytomedicine 2022, 103, 154223. [Google Scholar] [CrossRef]
- Dai, G.; Sun, B.; Gong, T.; Pan, Z.; Meng, Q.; Ju, W. Ginsenoside Rb2 inhibits epithelial-mesenchymal transition of colorectal cancer cells by suppressing TGF-β/Smad signaling. Phytomedicine 2019, 56, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.C.; Chang, C.Y.; Yar Lee, T.X.; Wu, J.; Saovieng, S.; Hsieh, Y.W.; Zhu, M.; Huang, C.Y.; Kuo, C.H. Longevity, tumor, and physical vitality in rats consuming ginsenoside Rg1. J. Ginseng Res. 2023, 47, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, H.J.; Wu, Y.C. Processing technologies, phytochemistry, bioactivities and applications of black ginseng—A novel manufactured ginseng product: A comprehensive review. Food Chem. 2023, 407, 134714. [Google Scholar] [CrossRef]
- Zhang, W.; Tao, W.W.; Zhou, J.; Wu, C.Y.; Long, F.; Shen, H.; Zhu, H.; Mao, Q.; Xu, J.; Li, S.L.; et al. Structural analogues in herbal medicine ginseng hit a shared target to achieve cumulative bioactivity. Commun. Biol. 2021, 4, 549. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Cao, J.; Liu, L.; Zeng, M.; Yu, H.; Wu, H. Metabolomics-based investigation of the chemical composition changes in Mongolian medicinal plant Euphorbia pekinensis before and after processing with Chebulae Fructus. J. Pharm. Biomed. Anal. 2024, 238, 115838. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xue, Z.; Huang, X.; Ma, W.; Yang, D.; Zhao, L.; Ouyang, H.; Chang, Y.; He, J. Comparison of the chemical profile differences of Aster tataricus between raw and processed products by metabolomics coupled with chemometrics methods. J. Sep. Sci. 2021, 44, 3883–3897. [Google Scholar] [CrossRef]
- Xue, G.; Su, S.; Yan, P.; Shang, J.; Wang, J.; Yan, C.; Li, J.; Wang, Q.; Du, Y.; Cao, L.; et al. Quality control of Zingiberis Rhizoma and its processed products by UHPLC-Q-TOF/MS-based non-targeted metabonomics combining with SIBDV method. Food Res. Int. 2022, 154, 111021. [Google Scholar] [CrossRef]
- Cao, X.; Shi, K.; Xu, Y.; Zhang, P.; Zhang, H.; Pan, S. Integrated metabolomics and network pharmacology to reveal antioxidant mechanisms and potential pharmacological ingredients of citrus herbs. Food Res. Int. 2023, 174, 113514. [Google Scholar] [CrossRef]
- Shah, A.; Apple, J.; Belli, A.J.; Barcellos, A.; Hansen, E.; Fernandes, L.L.; Zettler, C.M.; Wang, C.K. Real-world study of disease-free survival & patient characteristics associated with disease-free survival in early-stage non-small cell lung cancer: A retrospective observational study. Cancer Treat. Res. Commun. 2023, 36, 100742. [Google Scholar] [CrossRef]
- Lamy, D.; Mouillot, P.; Mariet, A.; Barnestein, R.; Quilot, F.; Fraisse, C.; Ghiringhelli, F.; Bonniaud, P.; Zouak, A.; Foucher, P. Real-world comparison of chemo-immunotherapy and chemotherapy alone in the treatment of extensive-stage small-cell lung cancer. Respir. Med. Res. 2024, 86, 101125. [Google Scholar] [CrossRef]
- Wang, M.; Chen, W.; Chen, J.; Yuan, S.; Hu, J.; Han, B.; Huang, Y.; Zhou, W. Abnormal saccharides affecting cancer multi-drug resistance (MDR) and the reversal strategies. Eur. J. Med. Chem. 2021, 220, 113487. [Google Scholar] [CrossRef]
- Roy, S.; Salerno, K.E.; Citrin, D.E. Biology of Radiation-Induced Lung Injury. Semin. Radiat. Oncol. 2021, 31, 155–161. [Google Scholar] [CrossRef]
- Banfill, K.; Giuliani, M.; Aznar, M.; Franks, K.; McWilliam, A.; Schmitt, M.; Sun, F.; Vozenin, M.C.; Faivre Finn, C. IASLC Advanced Radiation Technology committee. Cardiac Toxicity of Thoracic Radiotherapy: Existing Evidence and Future Directions. J. Thorac. Oncol. 2021, 16, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lin, Z. Non-Small Cell Lung Cancer Targeted Therapy: Drugs and Mechanisms of Drug Resistance. Int. J. Mol. Sci. 2022, 23, 15056. [Google Scholar] [CrossRef] [PubMed]
- Valastyan, S.; Weinberg, R.A. Tumor metastasis: Molecular insights and evolving paradigms. Cell 2011, 147, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Qin, C.; Dewanjee, S.; Bhattacharya, H.; Chakraborty, P.; Jha, N.K.; Gangopadhyay, M.; Jha, S.K.; Liu, Q. Tumor-derived small extracellular vesicles in cancer invasion and metastasis: Molecular mechanisms, and clinical significance. Mol. Cancer 2024, 23, 18. [Google Scholar] [CrossRef]
- Loibl, S.; Poortmans, P.; Morrow, M.; Denkert, C.; Curigliano, G. Breast cancer. Lancet 2021, 397, 1750–1769, Erratum in Lancet 2021, 397, 1710. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, M.; Wen, J.; Yang, Z.; Li, G.; Cao, Y.; Sun, L.; Ren, X. Panax japonicus C.A. Meyer: A comprehensive review on botany, phytochemistry, pharmacology, pharmacokinetics and authentication. Chin. Med. 2023, 18, 148. [Google Scholar] [CrossRef]
- Yu, P.; Xu, W.; Li, Y.; Xie, Z.; Shao, S.; Liu, J.; Wang, Y.; Wang, L.; Yang, H. Ginsenosides 20R-Rg3 and Rg5 enriched black ginseng inhibits colorectal cancer tumor growth by activating the Akt/Bax/caspase-3 pathway and modulating gut microbiota in mice. Curr. Res. Food Sci. 2025, 10, 100978. [Google Scholar] [CrossRef]
- Li, Z.M.; Shao, Z.J.; Qu, D.; Huo, X.H.; Hua, M.; Chen, J.B.; Lu, Y.S.; Sha, J.Y.; Li, S.S.; Sun, Y.S. Transformation Mechanism of Rare Ginsenosides in American Ginseng by Different Processing Methods and Antitumour Effects. Front. Nutr. 2022, 9, 833859. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tu, Y.; Zhao, J.; Chen, K.; Wu, C. Reversion-induced LIM interaction with Src reveals a novel Src inactivation cycle. J. Cell Biol. 2009, 184, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha Santos, G.; Shepherd, F.A.; Tsao, M.S. EGFR mutations and lung cancer. Annu. Rev. Pathol. 2011, 6, 49–69. [Google Scholar] [CrossRef] [PubMed]
- Jimeno, A.; Hidalgo, M. Pharmacogenomics of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors. Biochim. Biophys. Acta 2006, 1766, 217–229. [Google Scholar] [CrossRef]
- Mosesson, Y.; Yarden, Y. Oncogenic growth factor receptors: Implications for signal transduction therapy. Semin. Cancer Biol. 2004, 14, 262–270. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, Z.; Chen, H.; Bao, W.; Kuang, X.; Zhou, P.; Gao, Z.; Li, D.; Xie, X.; Yang, C.; et al. SBSN drives bladder cancer metastasis via EGFR/SRC/STAT3 signalling. Br. J. Cancer 2022, 127, 211–222. [Google Scholar] [CrossRef]
- Liu, Y.X.; Xu, B.W.; Niu, X.D.; Chen, Y.J.; Fu, X.Q.; Wang, X.Q.; Yin, C.L.; Chou, J.Y.; Li, J.K.; Wu, J.Y.; et al. Inhibition of Src/STAT3 signaling-mediated angiogenesis is involved in the anti-melanoma effects of dioscin. Pharmacol. Res. 2022, 175, 105983. [Google Scholar] [CrossRef]
- Hers, I.; Vincent, E.E.; Tavaré, J.M. Akt signalling in health and disease. Cell Signal 2011, 23, 1515–1527. [Google Scholar] [CrossRef]
- Jiang, H.; Yu, J.; Guo, H.; Song, H.; Chen, S. Upregulation of survivin by leptin/STAT3 signaling in MCF-7 cells. Biochem. Biophys. Res. Commun. 2008, 368, 1–5. [Google Scholar] [CrossRef]
- Woodford, M.R.; Dunn, D.M.; Blanden, A.R.; Capriotti, D.; Loiselle, D.; Prodromou, C.; Panaretou, B.; Hughes, P.F.; Smith, A.; Ackerman, W.; et al. The FNIP co-chaperones decelerate the Hsp90 chaperone cycle and enhance drug binding. Nat. Commun. 2016, 7, 12037. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, B.; Chen, X.; Zhang, N.; Li, G.; Zhang, L.H.; Tan, L.Y. Naringenin Ameliorates Behavioral Dysfunction and Neurological Deficits in a d-Galactose-Induced Aging Mouse Model Through Activation of PI3K/Akt/Nrf2 Pathway. Rejuvenation Res. 2017, 20, 462–472. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Sun, M.M.; Zhang, G.G.; Yang, J.; Chen, K.S.; Xu, W.W.; Li, B. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct. Target. Ther. 2021, 6, 425. [Google Scholar] [CrossRef] [PubMed]
- Engelman, J.A.; Luo, J.; Cantley, L.C. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 2006, 7, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Ji, S.H.; Choi, B.R.; Choi, D.J.; Lee, Y.G.; Kim, H.G.; Kim, G.S.; Kim, K.; Lee, Y.H.; Baek, N.I.; et al. UPLC-QTOF/MS-Based Metabolomics Applied for the Quality Evaluation of Four Processed Panax ginseng Products. Molecules 2018, 23, 2062. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Song, Z.; Liu, Q.; Fan, D.; Song, X. Ginsenosides: A potential natural medicine to protect the lungs from lung cancer and inflammatory lung disease. Food Funct. 2023, 14, 9137–9166. [Google Scholar] [CrossRef]
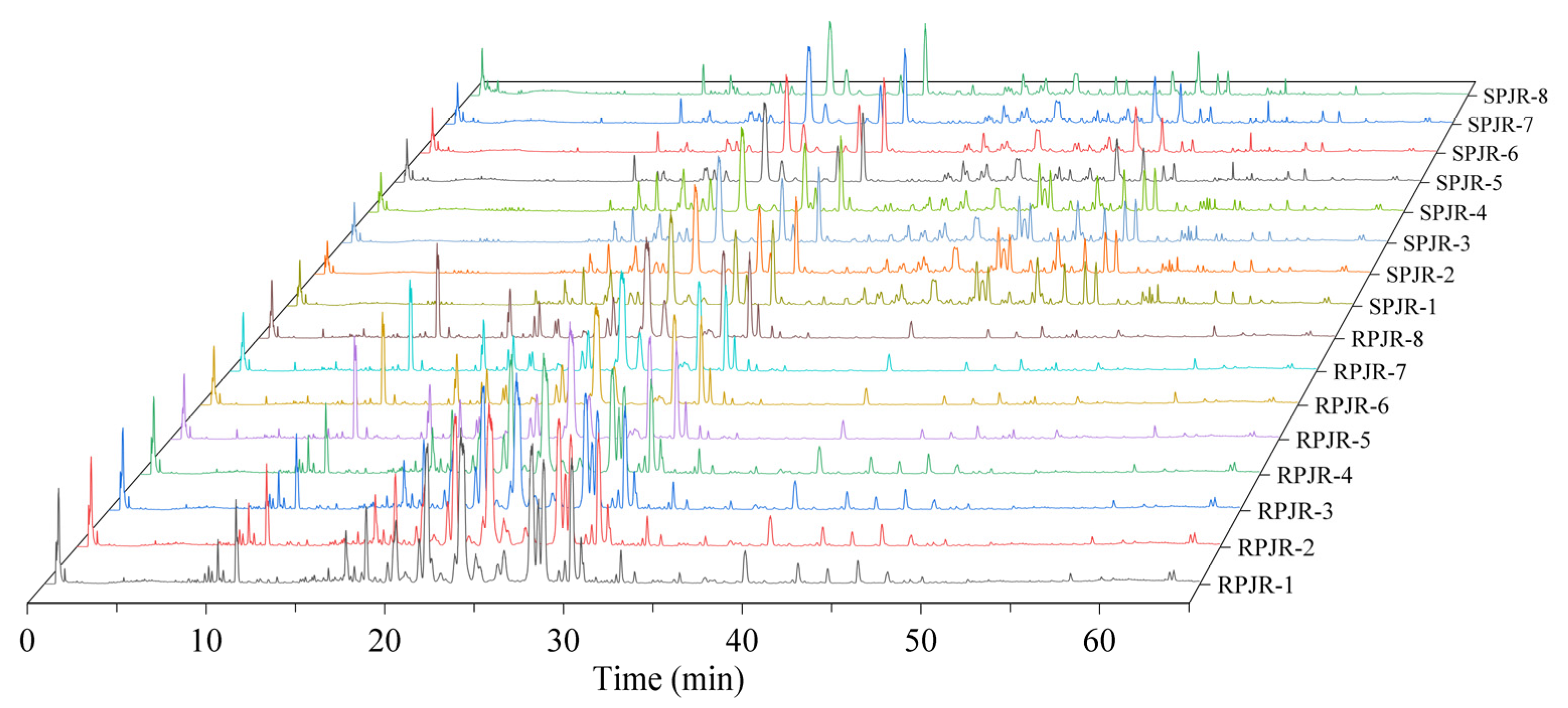
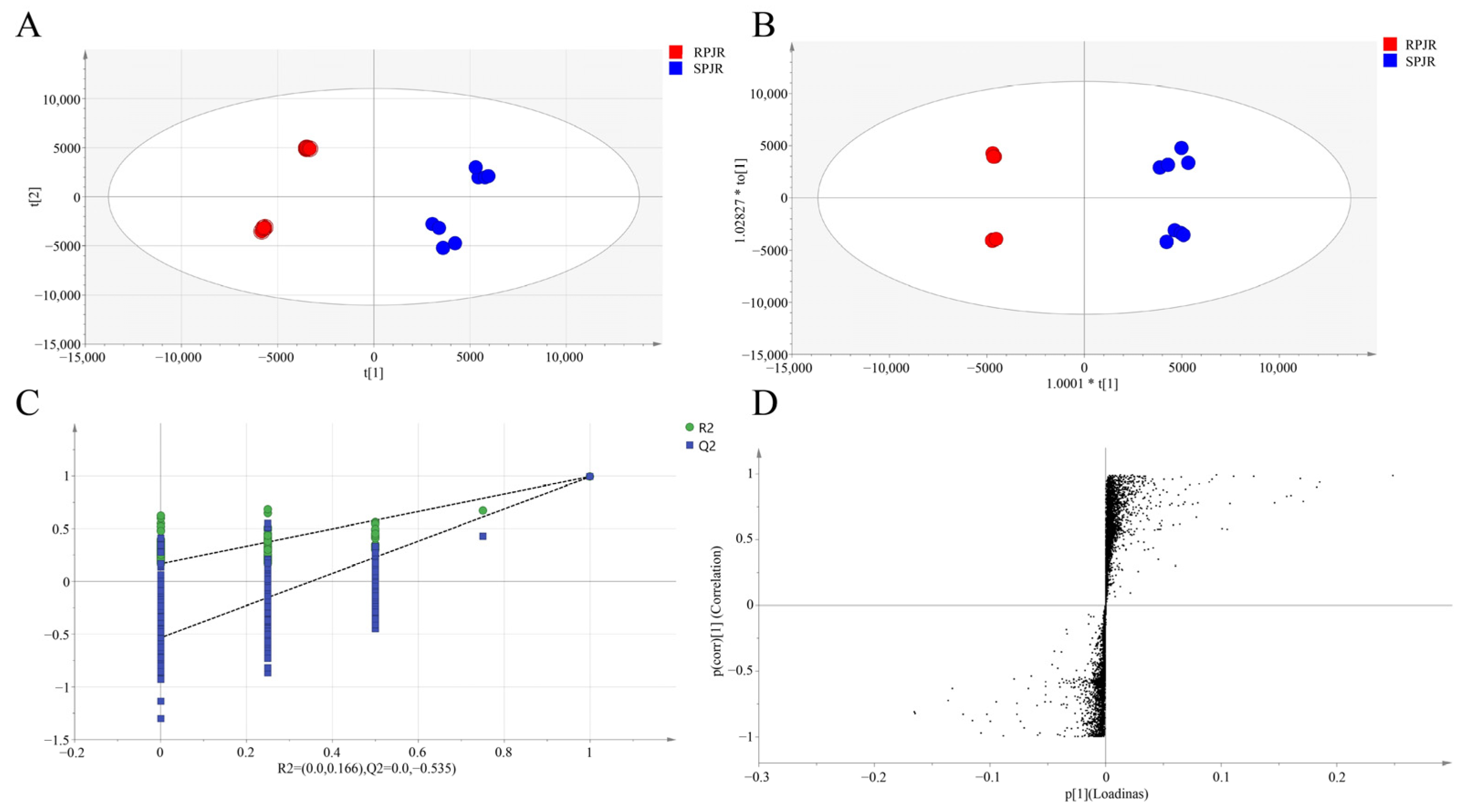
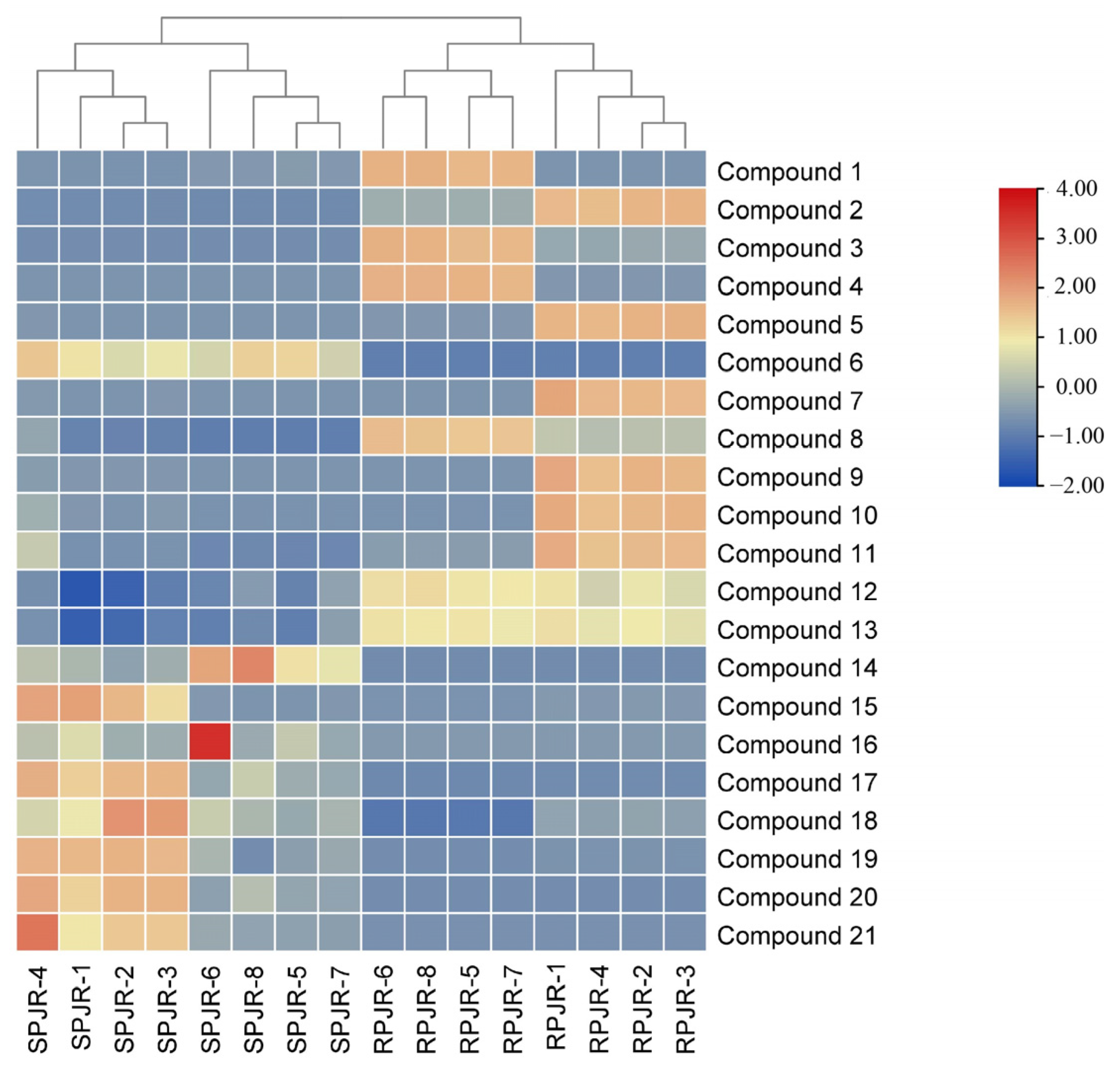
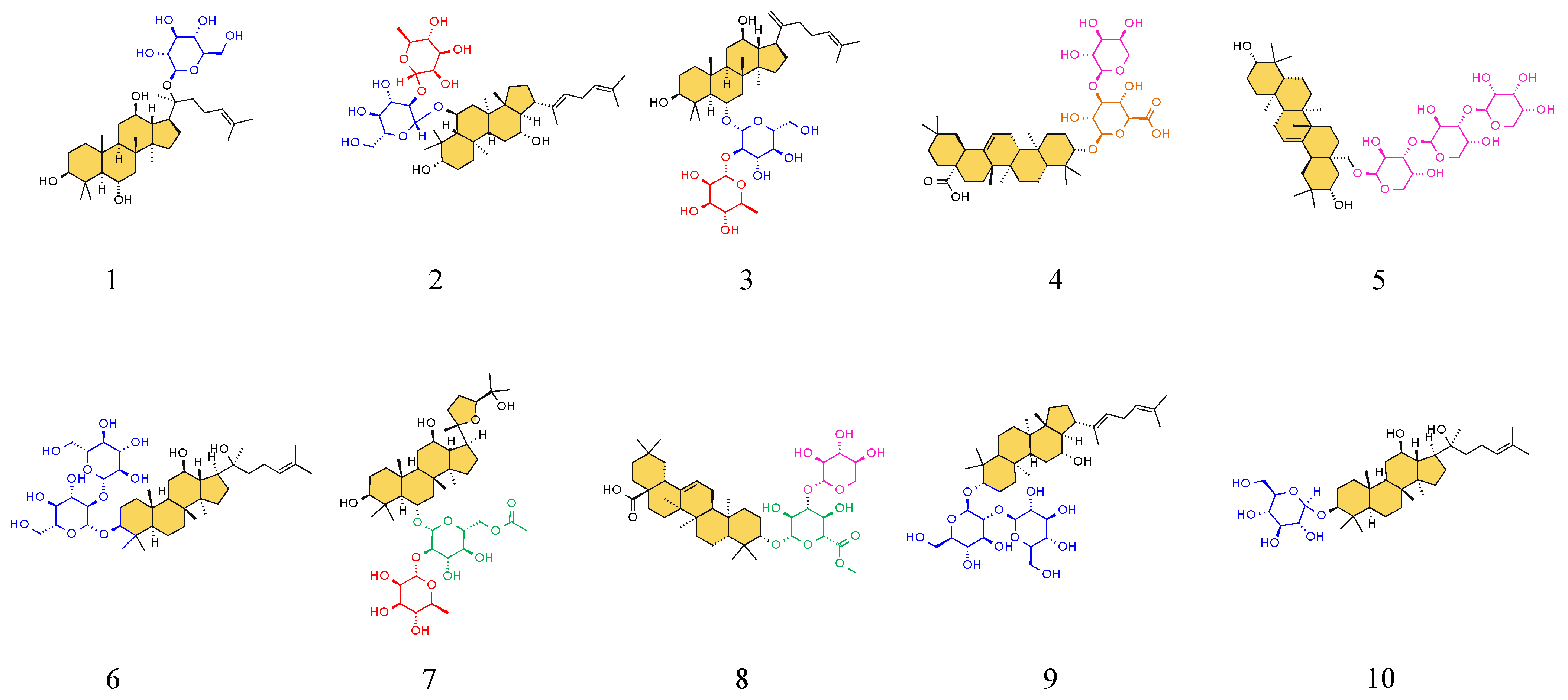


| Peak ID | RT (min) | Identification | Molecular Formula | Ions | Exact Mass | Error (ppm) | MS/MS Fragment | VIP | p | Trend |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 6.37 | Notoginsenoside J | C42H74O16 | [M−H]− | 833.4899 | 1.47 | 671.4501, 509.4091, 453.1986 | 1.61 | 2.69 × 10−2 | ↓ |
| 2 | 9.79 | Notoginsenoside R1 | C47H80O18 | [M−H]− | 931.5267 | 2.80 | 799.5081, 637.45000, 475.3922 | 4.61 | 4.04 × 10−5 | ↓ |
| 3 | 10.9 | Ginsenoside B2 | C48H82O18 | [M−H]− | 945.5400 | 3.10 | 783.5142, 637.4500, 475.3922 | 1.33 | 4.91 × 10−10 | ↓ |
| 4 | 17.97 | Notoginsenoside R4 | C59H100O27 | [M−H]− | 1239.6345 | 3.25 | 807.4406, 645.3884, 569.3983 | 2.85 | 3.39 × 10−8 | ↓ |
| 5 | 19.31 | Notoginsenoside Fa | C59H100O27 | [M−H]− | 1239.6345 | 2.68 | 879.5244, 623.3054, 593.2994 | 2.56 | 1.61 × 10−4 | ↓ |
| 6 | 20.99 | Ginsenoside F1 | C36H62O9 | [M−H]− | 637.4299 | 0.49 | 597.2381, 461.2545, 345.1388 | 7.21 | 1.89 × 10−15 | ↑ |
| 7 | 23.78 | Vinaginsenoside R7 | C53H90O22 | [M−H]− | 1077.5820 | 3.38 | 955.5255, 793.4662, 569.4081 | 1.35 | 1.90 × 10−3 | ↓ |
| 8 | 30.21 | Ginsenoside Rd | C48H82O18 | [M+HCOO]− | 991.5454 | 3.16 | 793.4662, 725.4743, 419.2361 | 8.94 | 4.13 × 10−6 | ↓ |
| 9 | 30.34 | Ginsenoside Rs2 | C55H92O23 | [M+HCOO]− | 1165.5979 | 3.56 | 991.5887, 793.4662, 605.3209 | 1.8 | 1.90 × 10−2 | ↓ |
| 10 | 32.18 | Ginsenoside Rs1 | C55H92O23 | [M+HCOO]− | 1165.5979 | 3.16 | 605.3209, 592.2969 | 1.39 | 1.30 × 10−2 | ↓ |
| 11 | 32.45 | Gypenoside XVII | C48H82O18 | [M+HCOO]− | 991.5454 | 2.72 | 945.5776, 869.5281, 518.2928 | 3.98 | 5.47 × 10−4 | ↓ |
| 12 | 33.22 | Calenduloside H methyl ester | C49H78O19 | [M+HCOO]− | 1015.5091 | 2.92 | 853.4913, 530.2729, 455.3732 | 2.28 | 3.58 × 10−6 | ↓ |
| 13 | 33.23 | Calenduloside G methyl ester | C43H68O14 | [M+HCOO]− | 853.4566 | 1.94 | 609.4003, 455.3688, 153.0221 | 1.22 | 1.84 × 10−7 | ↓ |
| 14 | 34.87 | Ginsenoside F4/Rg6 | C42H70O12 | [M+HCOO]− | 811.4824 | 0.39 | 583.3910, 537.3824, 409.1258 | 4.81 | 2.24 × 10−14 | ↑ |
| 15 | 40.54 | Momordin I | C41H64O13 | [M−H]− | 763.4251 | 0.42 | 703.9182, 654.3607, 339.6309 | 1.46 | 4.98 × 10−3 | ↑ |
| 16 | 41.93 | (3b, 21b)-12-Oleanene-3,21,28-triol 28-[arabinosyl-(1→3)-arabinosyl-(1→3)-arabinoside] | C45H74O15 | [M−H]− | 853.4929 | 1.75 | 793.4778, 731.4792, 455.3732 | 1.12 | 4.11 × 10−12 | ↑ |
| 17 | 42.12 | Ginsenoside Rg3 | C42H72O13 | [M−H]− | 783.4875 | 1.24 | 621.4654, 459.4040, 161.0519 | 2.62 | 4.41 × 10−8 | ↑ |
| 18 | 47.70 | Vinaginsenoside R1 | C44H74O15 | [M−H]− | 841.4929 | 1.84 | 795.5377, 455.3732, 279.2435 | 1.05 | 2.93 × 10−4 | ↑ |
| 19 | 48.41 | Momordin Ia | C42H66O13 | [M−H]− | 777.4407 | 1.48 | 627.4192, 316.8525, 279.2469 | 1.12 | 2.78 × 10−3 | ↑ |
| 20 | 48.73 | Ginsenoside Rg5 | C41H68O10 | [M−H]− | 765.4770 | 0.69 | 603.4623, 501.3495, 113.0265 | 2.29 | 4.13 × 10−6 | ↑ |
| 21 | 50.75 | Ginsenoside Rh2 | C36H62O8 | [M+HCOO]− | 667.4404 | 0.81 | 569.3983393.2220, 146.9706 | 2.89 | 6.67 × 10−6 | ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yang, J.; Qu, B.; Huang, J.; Wang, Y.; Yan, J. Enhanced Anti-Lung Cancer Effects of Steamed Panacis Japonici Rhizoma: Insights from Metabolomics, Network Pharmacology and Molecular Dynamics Simulation. Int. J. Mol. Sci. 2025, 26, 11999. https://doi.org/10.3390/ijms262411999
Zhang Y, Yang J, Qu B, Huang J, Wang Y, Yan J. Enhanced Anti-Lung Cancer Effects of Steamed Panacis Japonici Rhizoma: Insights from Metabolomics, Network Pharmacology and Molecular Dynamics Simulation. International Journal of Molecular Sciences. 2025; 26(24):11999. https://doi.org/10.3390/ijms262411999
Chicago/Turabian StyleZhang, Yijia, Jingxiao Yang, Binqing Qu, Jiacheng Huang, Yuanqing Wang, and Jianye Yan. 2025. "Enhanced Anti-Lung Cancer Effects of Steamed Panacis Japonici Rhizoma: Insights from Metabolomics, Network Pharmacology and Molecular Dynamics Simulation" International Journal of Molecular Sciences 26, no. 24: 11999. https://doi.org/10.3390/ijms262411999
APA StyleZhang, Y., Yang, J., Qu, B., Huang, J., Wang, Y., & Yan, J. (2025). Enhanced Anti-Lung Cancer Effects of Steamed Panacis Japonici Rhizoma: Insights from Metabolomics, Network Pharmacology and Molecular Dynamics Simulation. International Journal of Molecular Sciences, 26(24), 11999. https://doi.org/10.3390/ijms262411999







