Selective STAT3 Allosteric Inhibitors HCB-5300 and HCB-5400 Alleviate Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice
Abstract
1. Introduction
2. Results
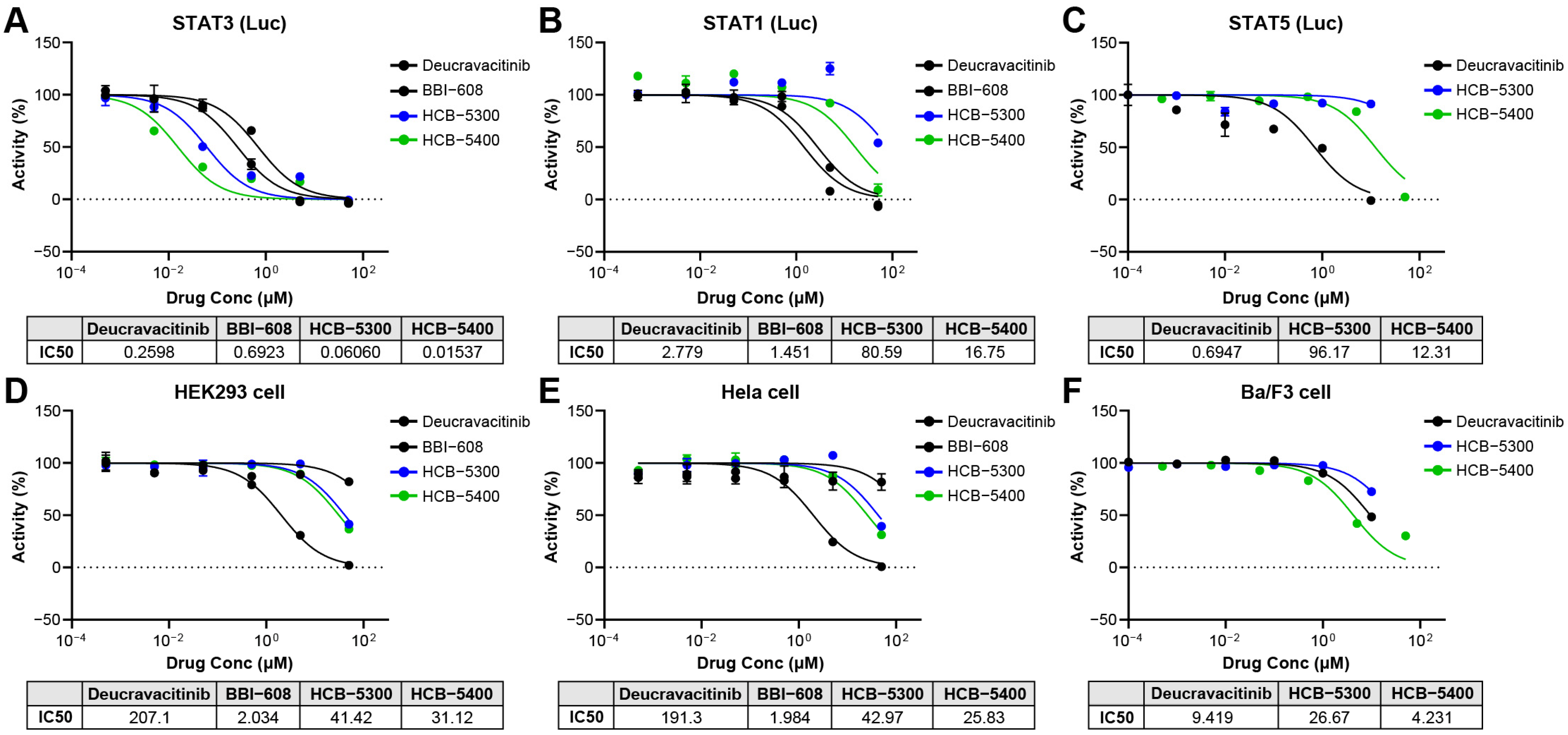
2.1. HCB Compounds Selectively Inhibit STAT3 Transcriptional Activity Without Affecting Cell Viability
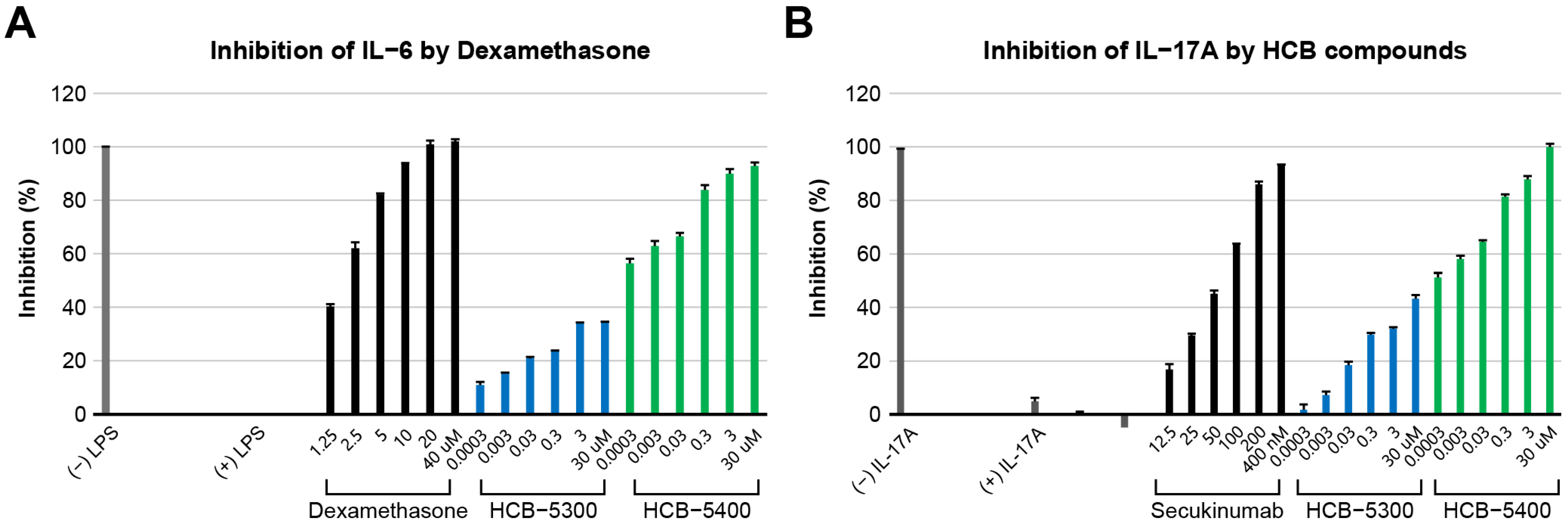
2.2. Evaluation of Anti-Inflammatory Activity (IL-6 and IL-17A Inhibitory Activity)
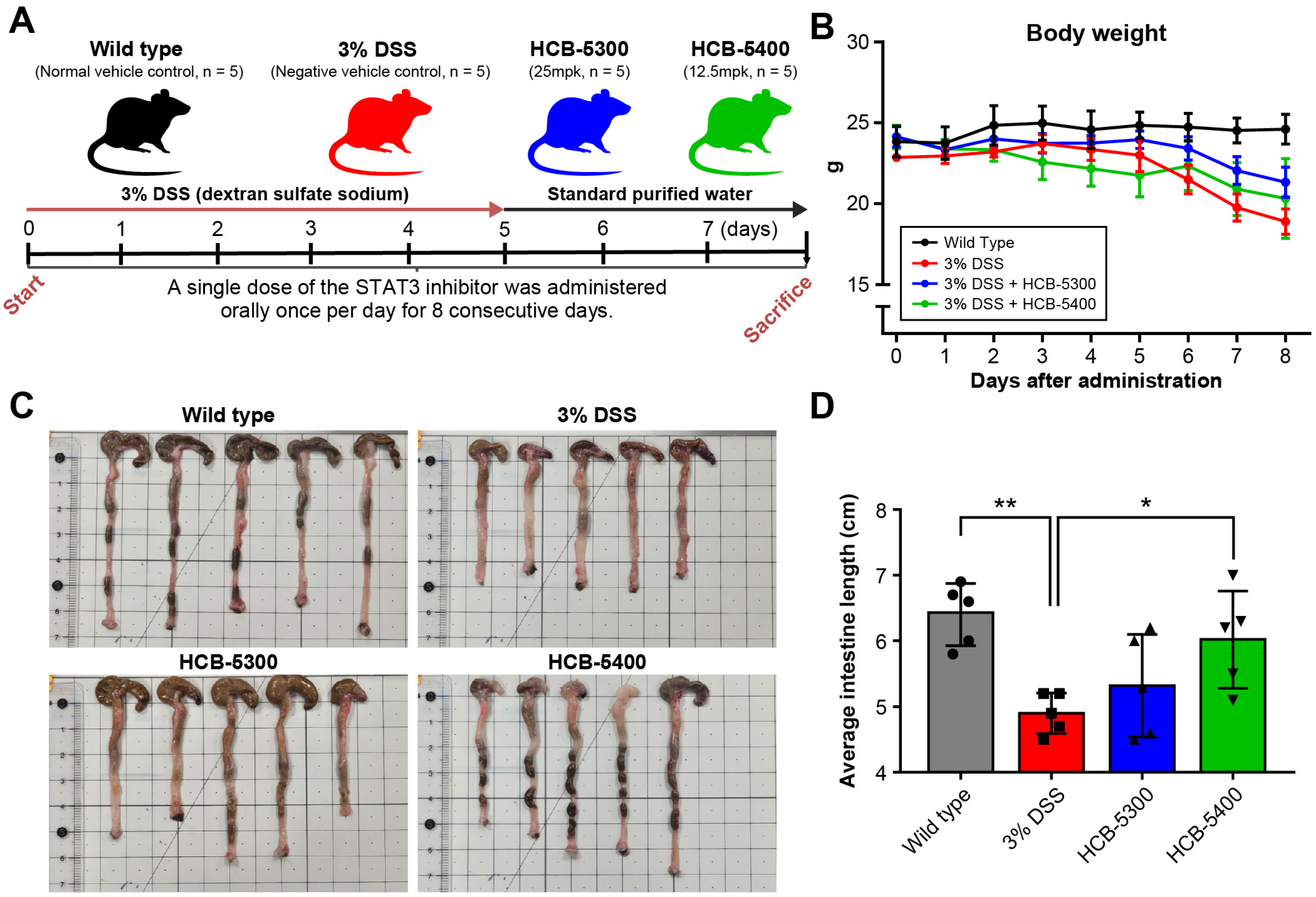
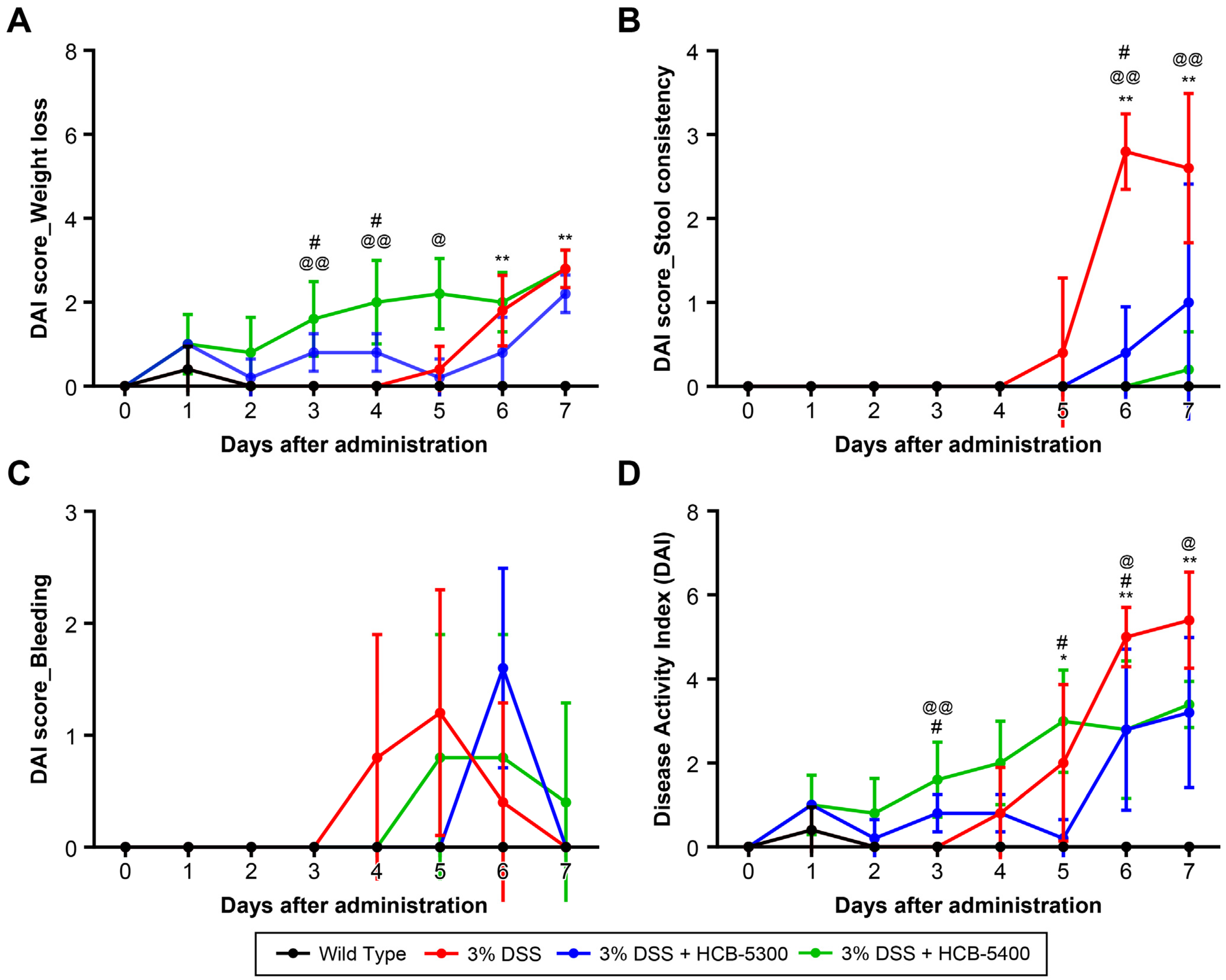
2.3. Therapeutic Effects of HCB-5300 and HCB-5400 on DSS-Induced Colitis
2.4. HCB Treatment Ameliorates Histological Damage in DSS-Induced Colitis
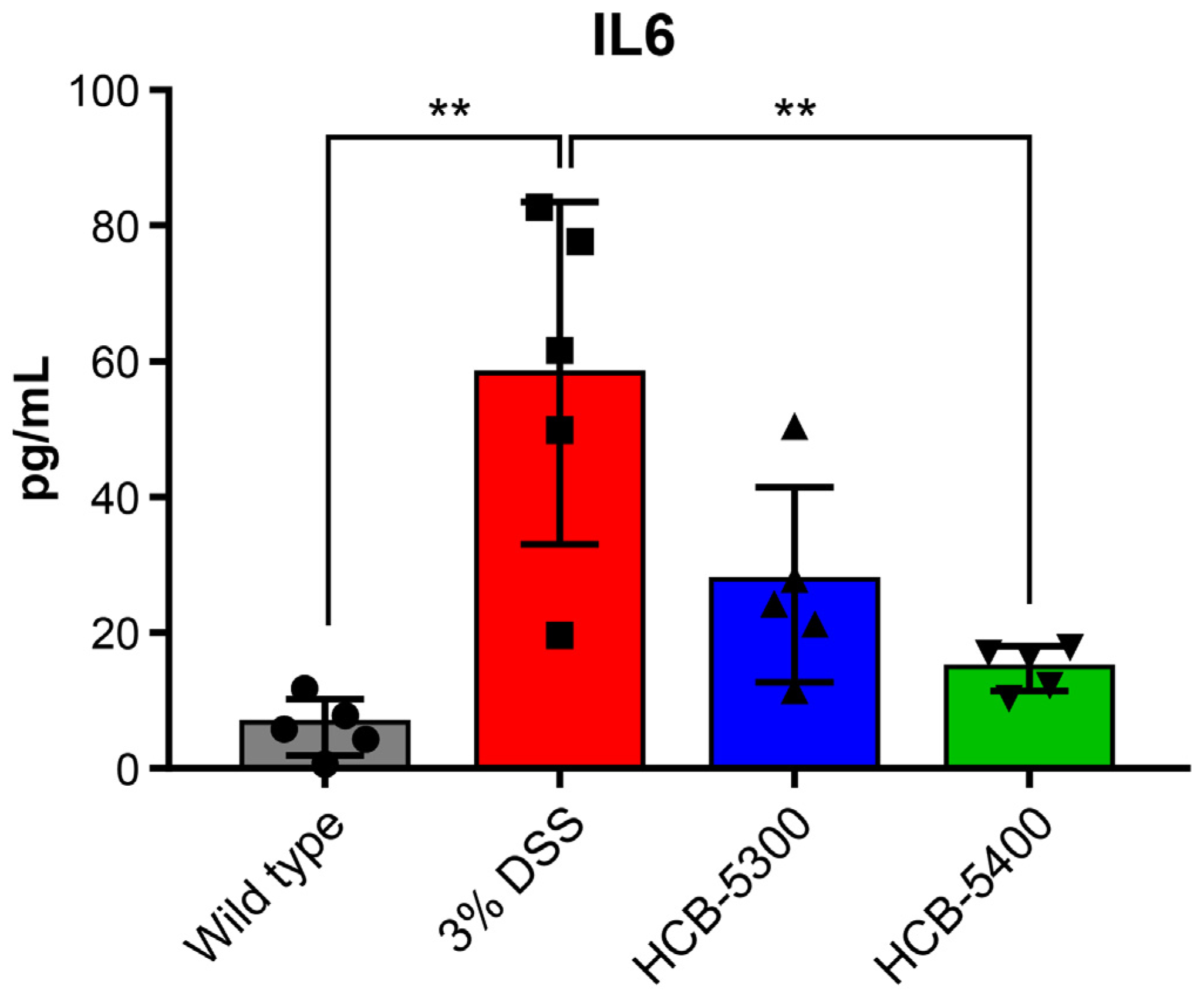
2.5. HCB Treatment Reduces Serum IL-6 Levels in Colitis Mice
2.6. Short-Term HCB Treatment Does Not Alter Bone Microarchitecture
3. Discussion
4. Materials and Methods
4.1. Animal Model and Ethical Approval
4.2. Induction of DSS-Induced Colitis and Treatment Administration
4.3. Sample Collection and Processing
4.4. Reporter Gene Assays for STAT1 and STAT3
4.5. Histological Analysis and Immunohistochemical Staining
4.6. Enzyme-Linked Immunosorbent Assay
4.7. Evaluation of Anti-Inflammatory Activity (IL-6 and IL-17 Inhibitory Assays)
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMD | bone mineral density |
| BV/TV | bone volume/total volume |
| CD | Crohn’s disease |
| DAI | disease activity index |
| DSS | dextran sulfate sodium |
| ELISA | enzyme-linked immunosorbent assay |
| H&E | hematoxylin and eosin |
| IBD | inflammatory bowel disease |
| IL | Interleukin |
| JAK | Janus kinase |
| STAT3 | signal transducer and activator of transcription 3 |
| TNF-α | tumor necrosis factor-alpha |
| UC | ulcerative colitis |
References
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778, Correction in Lancet 2017, 396, e56. [Google Scholar] [CrossRef]
- Khor, B.; Gardet, A.; Xavier, R.J. Genetics and pathogenesis of inflammatory bowel disease. Nature 2011, 474, 307–317. [Google Scholar] [CrossRef]
- Kaplan, G.G.; Windsor, J.W. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Fiocchi, C. Ulcerative colitis. N. Engl. J. Med. 2011, 365, 1713–1725. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Wang, S.; Li, J. Treatment of inflammatory bowel disease: A comprehensive review. Front. Med. 2021, 8, 765474. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Murad, M.H.; Fumery, M.; Sedano, R.; Jairath, V.; Panaccione, R.; Sandborn, W.J.; Ma, C. Comparative efficacy and safety of biologic therapies for moderate-to-severe Crohn’s disease: A systematic review and network meta-analysis. Lancet Gastroenterol. Hepatol. 2021, 6, 1002–1014. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Panés, J. Loss of response and requirement of infliximab dose intensification in Crohn’s disease: A review. Am. J. Gastroenterol. 2009, 104, 760–767. [Google Scholar] [CrossRef]
- Honap, S.; Agorogianni, A.; Colwill, M.J.; Mehta, S.K.; Donovan, F.; Pollok, R.; Poullis, A.; Patel, K. JAK inhibitors for inflammatory bowel disease: Recent advances. Frontline Gastroenterol. 2024, 15, 59–69. [Google Scholar] [CrossRef]
- Núñez, P.; Quera, R.; Yarur, A.J. Safety of Janus kinase inhibitors in inflammatory bowel diseases. Drugs 2023, 83, 299–314. [Google Scholar] [CrossRef]
- Xu, Q.; He, L.; Yin, Y. Risk of herpes zoster associated with JAK inhibitors in immune-mediated inflammatory diseases: A systematic review and network meta-analysis. Front. Pharmacol. 2023, 14, 1241954. [Google Scholar] [CrossRef]
- Sugimoto, K. Role of STAT3 in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 5110–5114. [Google Scholar] [CrossRef]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15.25.1–15.25.14. [Google Scholar] [CrossRef]
- Wirtz, S.; Neufert, C.; Weigmann, B.; Neurath, M.F. Chemically induced mouse models of intestinal inflammation. Nat. Protoc. 2007, 2, 541–546. [Google Scholar] [CrossRef]
- Deenick, E.K.; Pelham, S.J.; Kane, A.; Ma, C.S. Signal transducer and activator of transcription 3 control of human T and B cell responses. Front. Immunol. 2018, 9, 168. [Google Scholar] [CrossRef]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Pietschner, R.; Rath, T.; Neurath, M.F.; Atreya, R. Current and emerging targeted therapies for ulcerative colitis. Visc. Med. 2023, 39, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Roda, G.; Jharap, B.; Neeraj, N.; Colombel, J.-F. Loss of response to anti-TNFs: Definition, epidemiology, and management. Clin. Transl. Gastroenterol. 2016, 7, e135. [Google Scholar] [CrossRef] [PubMed]
- Huynh, J.; Chand, A.; Gough, D.; Ernst, M. Therapeutically exploiting STAT3 activity in cancer—Using tissue repair as a road map. Nat. Rev. Cancer 2019, 19, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Current and emerging therapeutic targets for IBD. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 269–278, Erratum in Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 688. [Google Scholar] [CrossRef]
- Cullen, G.; Donnellan, F.; Long, S.; Forry, M.; Murray, F.E. Perceptions of medication safety among patients with inflammatory bowel disease. Scand. J. Gastroenterol. 2010, 45, 1076–1083. [Google Scholar] [CrossRef]
- Shahini, A.; Shahini, A. Role of interleukin-6-mediated inflammation in the pathogenesis of inflammatory bowel disease: Focus on the available therapeutic approaches and gut microbiome. J. Cell Commun. Signal 2023, 17, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Dai, Y.; Yang, J.; Liu, C.; Fan, L.; Feng, L.; Zhao, B.; Zeng, M.; Liu, Z.; Sun, X. Crohn’s disease exacerbated by IL-17 inhibitors in patients with psoriasis: A case report. BMC Gastroenterol. 2020, 20, 340. [Google Scholar] [CrossRef] [PubMed]
- Avila, A.M.; Bebenek, I.; Bonzo, J.A.; Bourcier, T.; Davis Bruno, K.L.; Carlson, D.B.; Dubinion, J.; Elayan, I.; Harrouk, W.; Lee, S.-L.; et al. An FDA/CDER perspective on nonclinical testing strategies: Classical toxicology approaches and new approach methodologies (NAMs). Regul. Toxicol. Pharmacol. 2020, 114, 104662. [Google Scholar] [CrossRef]
- Weisshof, R.; El Jurdi, K.; Zmeter, N.; Rubin, D.T. Emerging therapies for inflammatory bowel disease. Adv. Ther. 2018, 35, 1746–1762. [Google Scholar] [CrossRef]
- Katsandegwaza, B.; Horsnell, W.; Smith, K. Inflammatory bowel disease: A review of pre-clinical murine models of human disease. Int. J. Mol. Sci. 2022, 23, 9344. [Google Scholar] [CrossRef]
- Park, S.H.; Park, S.H. Personalized medicine in inflammatory bowel disease: Perspectives on Asia. J. Gastroenterol. Hepatol. 2022, 37, 1434–1445. [Google Scholar] [CrossRef] [PubMed]
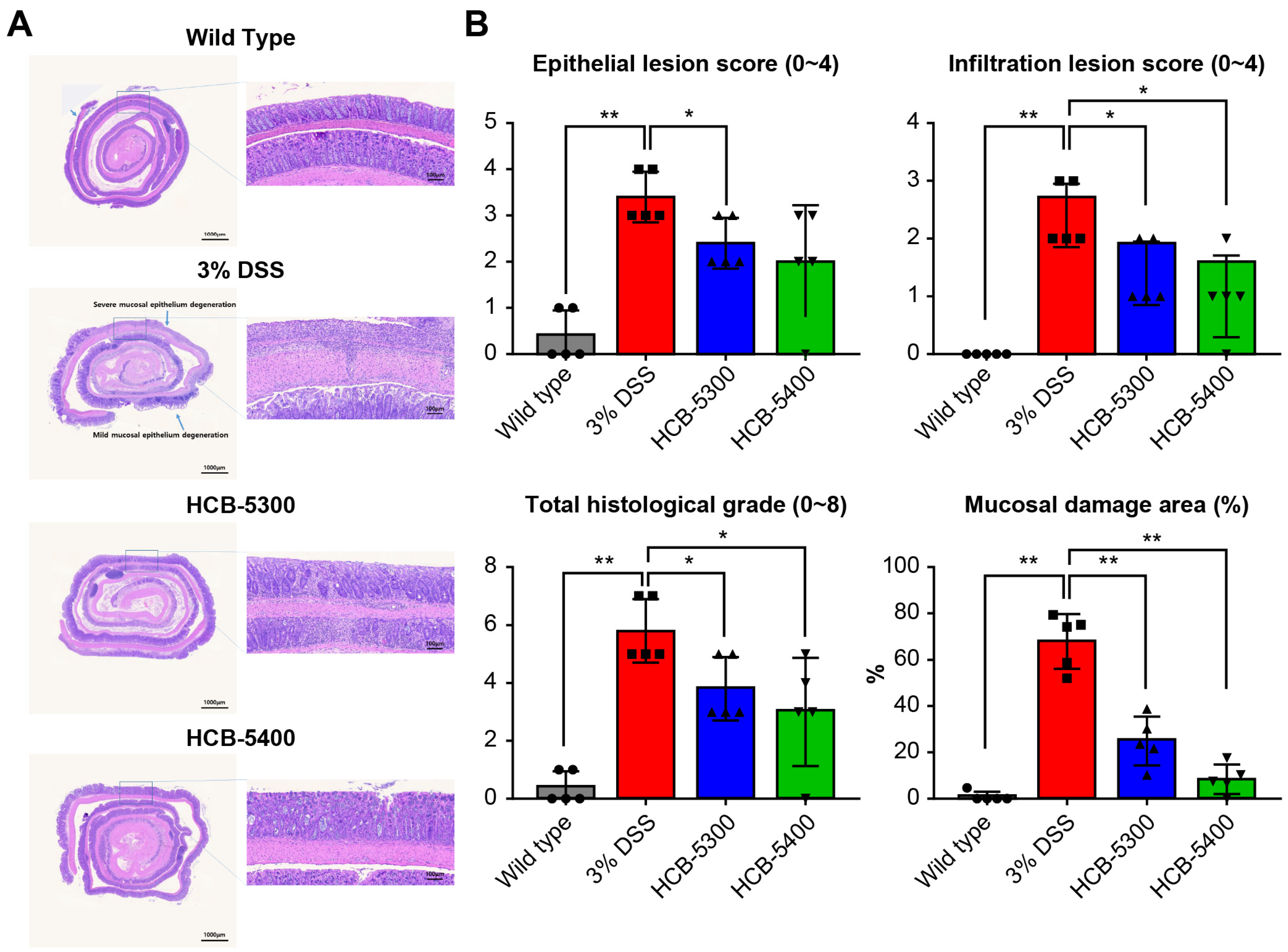

| Epithelial Lesion Score | Infiltration Lesion Score | |
|---|---|---|
| score | Degree of Epithelial damage | Degree of Infiltration |
| 0 | normal | none |
| 1 | loss of goblet cells | infiltration surrounding crypt basis |
| 2 | loss of goblet cells in large areas | infiltration into the mucosa |
| 3 | loss of crypts | widespread infiltration into the mucosa |
| 4 | loss of crypts in large areas | infiltration into the submucosa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, W.-Y.; Kim, J.-W.; Park, S.-W.; Kim, N.; Lim, S.-G.; Suh, C.-H. Selective STAT3 Allosteric Inhibitors HCB-5300 and HCB-5400 Alleviate Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice. Int. J. Mol. Sci. 2025, 26, 11981. https://doi.org/10.3390/ijms262411981
Baek W-Y, Kim J-W, Park S-W, Kim N, Lim S-G, Suh C-H. Selective STAT3 Allosteric Inhibitors HCB-5300 and HCB-5400 Alleviate Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice. International Journal of Molecular Sciences. 2025; 26(24):11981. https://doi.org/10.3390/ijms262411981
Chicago/Turabian StyleBaek, Wook-Young, Ji-Won Kim, So-Won Park, Nan Kim, Sun-Gyo Lim, and Chang-Hee Suh. 2025. "Selective STAT3 Allosteric Inhibitors HCB-5300 and HCB-5400 Alleviate Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice" International Journal of Molecular Sciences 26, no. 24: 11981. https://doi.org/10.3390/ijms262411981
APA StyleBaek, W.-Y., Kim, J.-W., Park, S.-W., Kim, N., Lim, S.-G., & Suh, C.-H. (2025). Selective STAT3 Allosteric Inhibitors HCB-5300 and HCB-5400 Alleviate Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice. International Journal of Molecular Sciences, 26(24), 11981. https://doi.org/10.3390/ijms262411981







