Introduction of Mature Mast Cells into Bone Marrow Alters Bone Metabolism in Growing Mice
Abstract
1. Introduction
2. Results
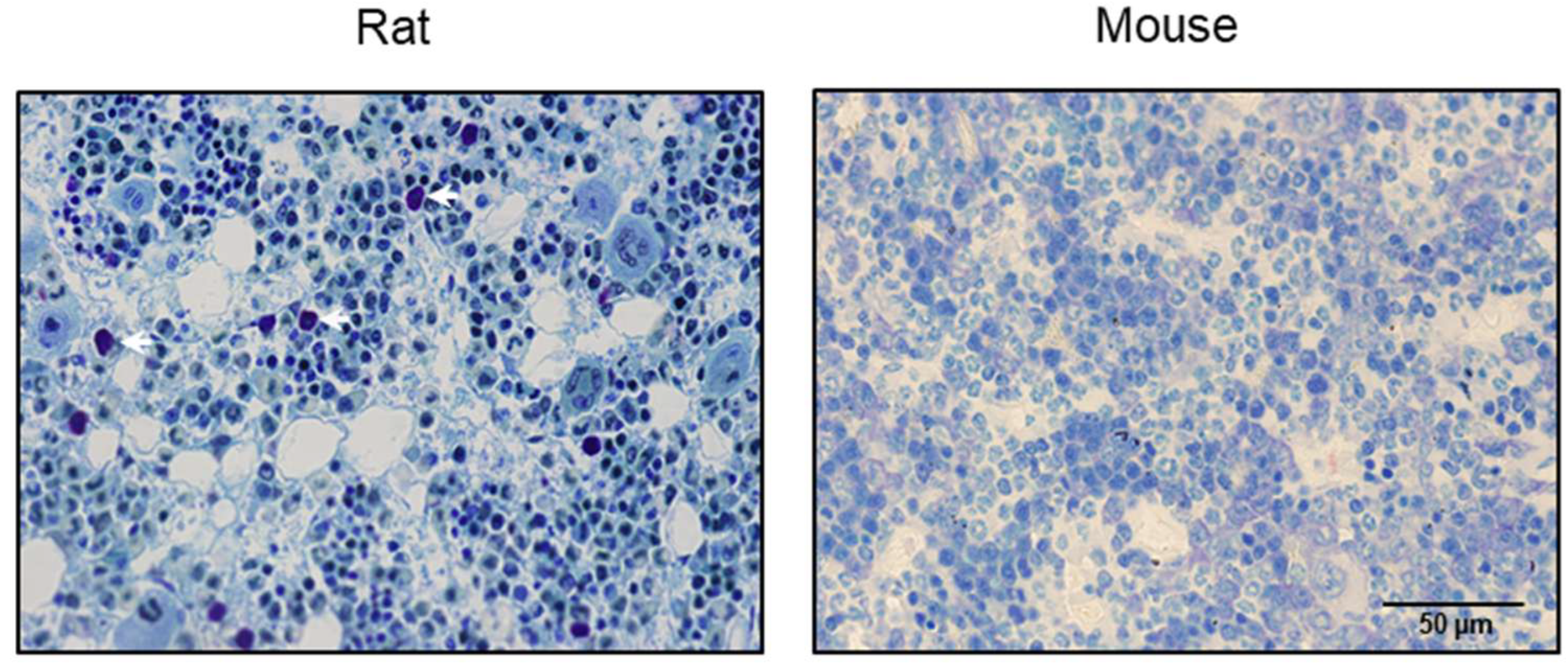
2.1. Evaluation of Bone Marrow in Mice for Presence/Absence of Mast Cells
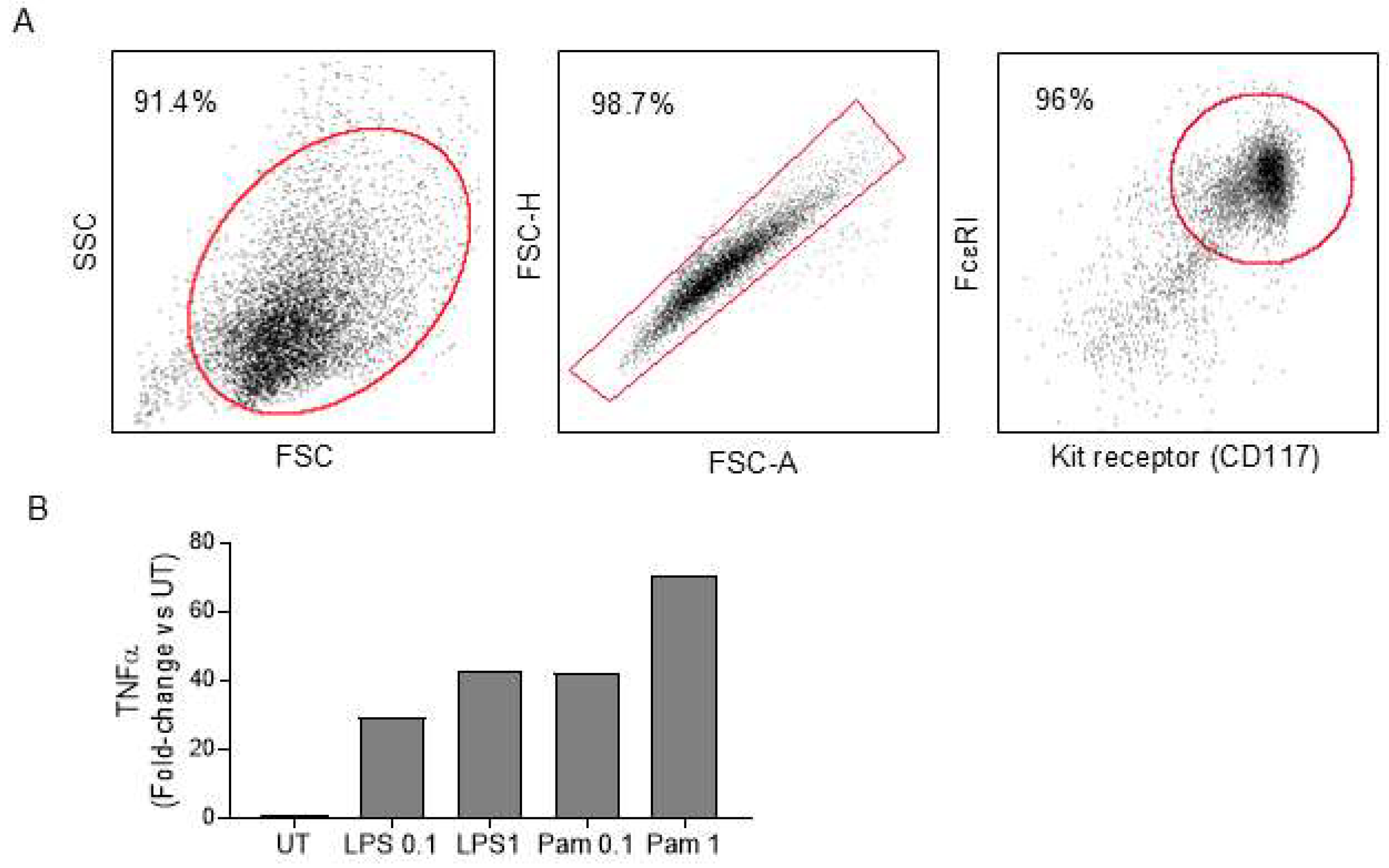
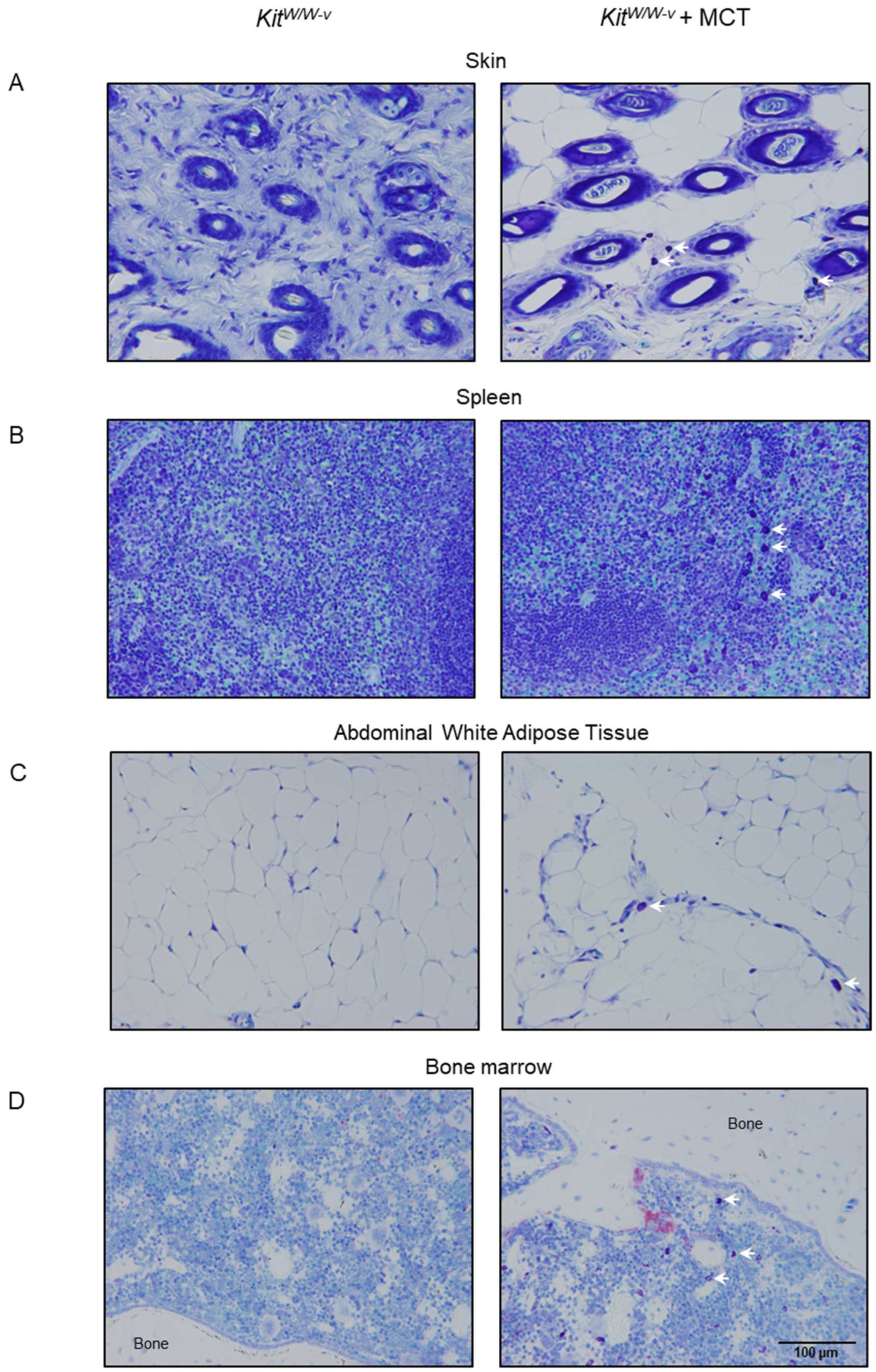
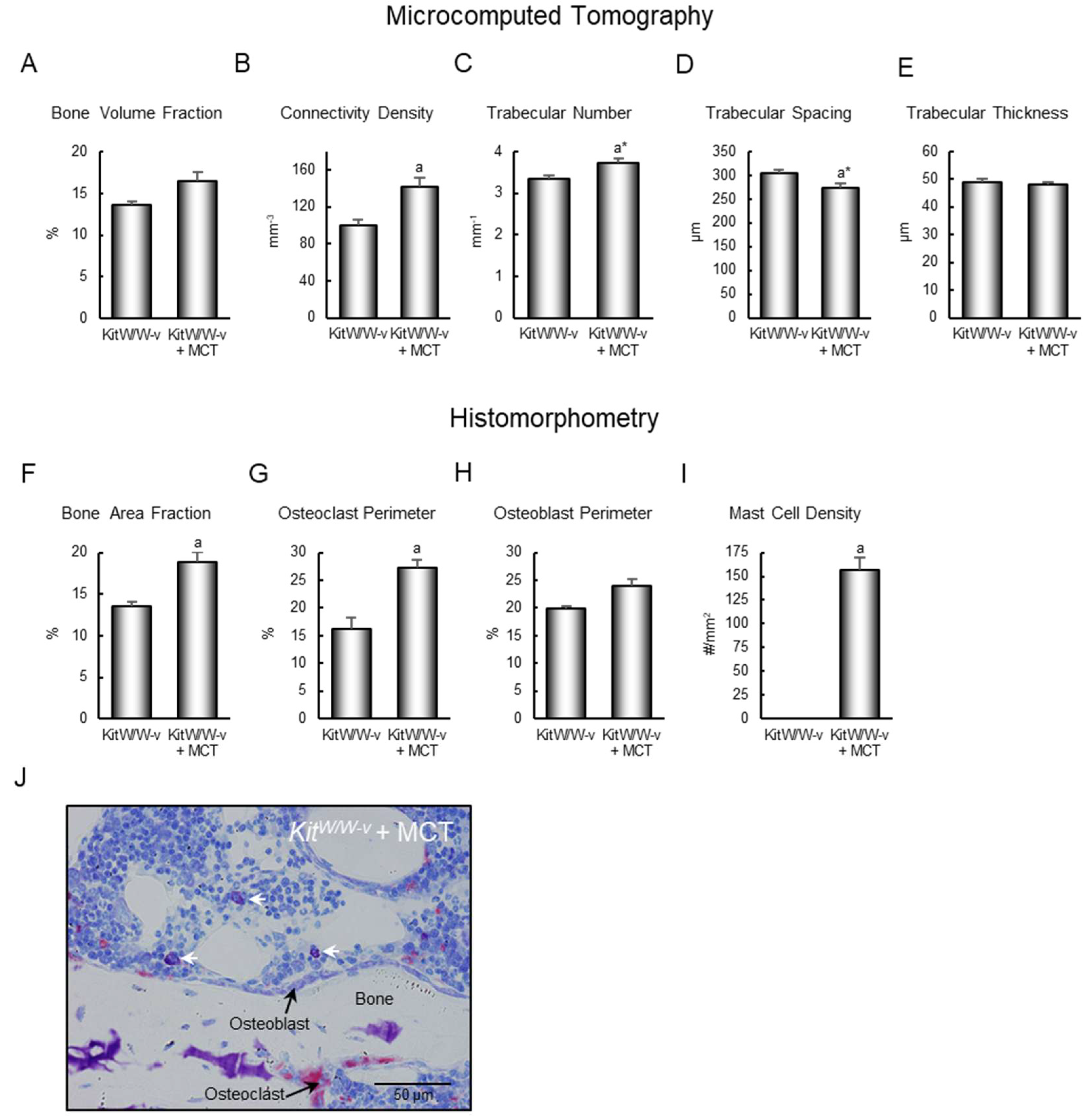
2.2. Homing Mast Cells to Bone Marrow in Mast Cell-Deficient Mice
3. Discussion
4. Methods
4.1. Mast Cell Distribution in Skeletal Tissue of Mice
4.2. Homing Mast Cells to Bone Marrow in Mast Cell-Deficient Mice
4.3. Dual-Energy X-Ray Absorptiometry
4.4. Micro-Computed Tomography
4.5. Histomorphometry
4.6. Gene Expression
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CTX | carboxyterminal telopeptide of type 1 collagen |
| DXA | dual energy X-ray absorptiometry |
| FSC | forward scatter |
| HSC | hematopoietic stem cell |
| KO | knockout |
| LV | lumbar vertebra |
| MCT | mast cell transfer |
| μCT | microcomputed tomography |
| PTH | parathyroid hormone |
| SSC | side scatter |
| TLR2 | toll-like receptor 2 |
| WAT | white adipose tissue |
| WT | wild type |
References
- Krystel-Whittemore, M.; Dileepan, K.N.; Wood, J.G. Mast Cell: A Multi-Functional Master Cell. Front. Immunol. 2015, 6, 620. [Google Scholar] [CrossRef] [PubMed]
- Baccari, G.C.; Pinelli, C.; Santillo, A.; Minucci, S.; Rastogi, R.K. Mast cells in nonmammalian vertebrates: An overview. Int. Rev. Cell Mol. Biol. 2011, 290, 1–53. [Google Scholar] [PubMed]
- Carlson, H.C.; Hacking, M.A. Distribution of mast cells in chicken, turkey, pheasant, and quail, and their differentiation from basophils. Avian Dis. 1972, 16, 574–577. [Google Scholar] [CrossRef]
- Chen, C.C.; Grimbaldeston, M.A.; Tsai, M.; Weissman, I.L.; Galli, S.J. Identification of mast cell progenitors in adult mice. Proc. Natl. Acad. Sci. USA 2005, 102, 11408–11413. [Google Scholar] [CrossRef]
- Reith, A.D.; Rottapel, R.; Giddens, E.; Brady, C.; Forrester, L.; Bernstein, A. W mutant mice with mild or severe developmental defects contain distinct point mutations in the kinase domain of the c-kit receptor. Genes Dev. 1990, 4, 390–400. [Google Scholar] [CrossRef]
- Ragipoglu, D.; Bülow, J.; Hauff, K.; Voss, M.; Haffner-Luntzer, M.; Dudeck, A.; Ignatius, A.; Fischer, V. Mast Cells Drive Systemic Inflammation and Compromised Bone Repair After Trauma. Front. Immunol. 2022, 13, 883707. [Google Scholar] [CrossRef]
- Ragipoglu, D.; Dudeck, A.; Haffner-Luntzer, M.; Voss, M.; Kroner, J.; Ignatius, A.; Fischer, V. The Role of Mast Cells in Bone Metabolism and Bone Disorders. Front. Immunol. 2020, 11, 163. [Google Scholar] [CrossRef]
- Kroner, J.; Kovtun, A.; Kemmler, J.; Messmann, J.J.; Strauss, G.; Seitz, S.; Schinke, T.; Amling, M.; Kotrba, J.; Froebel, J.; et al. Mast Cells Are Critical Regulators of Bone Fracture-Induced Inflammation and Osteoclast Formation and Activity. J. Bone Miner. Res. 2017, 32, 2431–2444. [Google Scholar] [CrossRef]
- Lotinun, S.; Evans, G.L.; Turner, R.T.; Oursler, M.J. Deletion of membrane-bound steel factor results in osteopenia in mice. J. Bone Miner. Res. 2005, 20, 644–652. [Google Scholar] [CrossRef]
- Keune, J.A.; Wong, C.P.; Branscum, A.J.; Iwaniec, U.T.; Turner, R.T. Bone Marrow Adipose Tissue Deficiency Increases Disuse-Induced Bone Loss in Male Mice. Sci. Rep. 2017, 7, 46325. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.T.; Wong, C.P.; Iwaniec, U.T. Effect of reduced c-Kit signaling on bone marrow adiposity. Anat. Rec. 2011, 294, 1126–1134. [Google Scholar] [CrossRef]
- Keune, J.A.; Wong, C.P.; Branscum, A.J.; Menn, S.A.; Iwaniec, U.T.; Turner, R.T. Bone Marrow Adipose Tissue Is Not Required for Reconstitution of the Immune System Following Irradiation in Male Mice. Int. J. Mol. Sci. 2024, 25, 1980. [Google Scholar] [CrossRef] [PubMed]
- Deyhle, R.T., Jr.; Wong, C.P.; Martin, S.A.; McDougall, M.Q.; Olson, D.A.; Branscum, A.J.; Menn, S.A.; Iwaniec, U.T.; Hamby, D.M.; Turner, R.T. Maintenance of Near Normal Bone Mass and Architecture in Lethally Irradiated Female Mice following Adoptive Transfer with as few as 750 Purified Hematopoietic Stem Cells. Radiat. Res. 2019, 191, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Lotinun, S.; Krishnamra, N. Disruption of c-Kit Signaling in Kit(W-sh/W-sh) Growing Mice Increases Bone Turnover. Sci. Rep. 2016, 6, 31515. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.H.; Ji, K.; Alderson, N.; He, Z.; Li, S.; Liu, W.; Zhang, D.E.; Li, L.; Feng, G.S. Kit-Shp2-Kit signaling acts to maintain a functional hematopoietic stem and progenitor cell pool. Blood 2011, 117, 5350–5361. [Google Scholar] [CrossRef]
- Tsai, M.; Valent, P.; Galli, S.J. KIT as a master regulator of the mast cell lineage. J. Allergy Clin. Immunol. 2022, 149, 1845–1854. [Google Scholar] [CrossRef]
- Flanagan, J.G.; Leder, P. The kit ligand: A cell surface molecule altered in steel mutant fibroblasts. Cell 1990, 63, 185–194. [Google Scholar] [CrossRef]
- Turner, R.T.; Martin, S.A.; Iwaniec, U.T. Metabolic Coupling Between Bone Marrow Adipose Tissue and Hematopoiesis. Curr. Osteoporos. Rep. 2018, 16, 95–104. [Google Scholar] [CrossRef]
- Grimbaldeston, M.A.; Chen, C.C.; Piliponsky, A.M.; Tsai, M.; Tam, S.Y.; Galli, S.J. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am. J. Pathol. 2005, 167, 835–848. [Google Scholar] [CrossRef]
- Kissel, H.; Timokhina, I.; Hardy, M.P.; Rothschild, G.; Tajima, Y.; Soares, V.; Angeles, M.; Whitlow, S.R.; Manova, K.; Besmer, P. Point mutation in kit receptor tyrosine kinase reveals essential roles for kit signaling in spermatogenesis and oogenesis without affecting other kit responses. EMBO J. 2000, 19, 1312–1326. [Google Scholar] [CrossRef]
- Turner, R.T.; Iwaniec, U.T.; Marley, K.; Sibonga, J.D. The role of mast cells in parathyroid bone disease. J. Bone Miner. Res. 2010, 25, 1637–1649. [Google Scholar] [CrossRef]
- Bi, L.; Sarkar, R.; Naas, T.; Lawler, A.M.; Pain, J.; Shumaker, S.; Bedian, V.; Kazazian, H.H. Further characteriza-tion of factor VIII-deficient mice created by gene targeting: RNA and protein studies. Blood 1996, 88, 3446–3450. [Google Scholar] [CrossRef]
- Ho, P.C.; Tsui, Y.C.; Feng, X.; Greaves, D.R.; Wei, L.N. NF-κB-mediated degradation of the coactivator RIP140 regulates inflammatory responses and contributes to endotoxin tolerance. Nat. Immunol. 2012, 13, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Bensamoun, S.F.; Hawse, J.R.; Subramaniam, M.; Ilharreborde, B.; Bassillais, A.; Benhamou, C.L.; Fraser, D.G.; Oursler, M.J.; Amadio, P.C.; An, K.N.; et al. TGFbeta inducible early gene-1 knockout mice display defects in bone strength and microarchi-tecture. Bone 2006, 39, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Vasu, V.T.; Ott, S.; Hobson, B.; Rashidi, V.; Oommen, S.; Cross, C.E.; Gohil, K. Sarcolipin and ubiquitin carboxy-terminal hydrolase 1 mRNAs are over-expressed in skeletal muscles of alpha-tocopherol deficient mice. Free. Radic. Res. 2009, 43, 106–116. [Google Scholar] [CrossRef]
- Yakar, S.; Bouxsein, M.L.; Canalis, E.; Sun, H.; Glatt, V.; Gundberg, C.; Cohen, P.; Hwang, D.; Boisclair, Y.; Leroith, D.; et al. The ternary IGF complex influences postnatal bone acquisition and the skeletal response to intermittent parathyroid hormone. J. Endocrinol. 2006, 189, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Wiren, K.M.; Semirale, A.A.; Zhang, X.W.; Woo, A.; Tommasini, S.M.; Price, C.; Schaffler, M.B.; Jepsen, K.J. Targeting of androgen receptor in bone reveals a lack of androgen anabolic action and inhibition of osteogenesis: A model for compartment-specific androgen action in the skeleton. Bone 2008, 43, 440–451. [Google Scholar] [CrossRef]
- Lydon, J.P.; DeMayo, F.J.; Conneely, O.M.; O’Malley, B.W. Reproductive phenotpes of the progesterone receptor null mutant mouse. J. Steroid Biochem. Mol. Biol. 1996, 56, 67–77. [Google Scholar] [CrossRef]
- Martin, S.A.; Riordan, R.T.; Wang, R.; Yu, Z.; Aguirre-Burk, A.M.; Wong, C.P.; Olson, D.A.; Branscum, A.J.; Turner, R.T.; Iwaniec, U.T.; et al. Rapamycin impairs bone accrual in young adult mice independent of Nrf2. Exp. Gerontol. 2021, 154, 111516. [Google Scholar] [CrossRef]
- Sobacchi, C.; Schulz, A.; Coxon, F.P.; Villa, A.; Helfrich, M.H. Osteopetrosis: Genetics, treatment and new insights into osteoclast function. Nat. Rev. Endocrinol. 2013, 9, 522–536. [Google Scholar] [CrossRef]
- Mendoza, R.P.; Fudge, D.H.; Brown, J.M. Cellular Energetics of Mast Cell Development and Activation. Cells 2021, 10, 524. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shi, G.P. Mast cell stabilization: Novel medication for obesity and diabetes. Diabetes/Metab. Res. Rev. 2011, 27, 919–924. [Google Scholar] [CrossRef]
- Elieh Ali Komi, D.; Shafaghat, F.; Christian, M. Crosstalk Between Mast Cells and Adipocytes in Physiologic and Pathologic Conditions. Clin. Rev. Allergy Immunol. 2020, 58, 388–400. [Google Scholar] [CrossRef]
- Iwaniec, U.T.; Turner, R.T. Failure to generate bone marrow adipocytes does not protect mice from ovariectomy-induced osteopenia. Bone 2013, 53, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Akin, C.; Hartmann, K.; Nilsson, G.; Reiter, A.; Hermine, O.; Sotlar, K.; Sperr, W.R.; Escribano, L.; George, T.I.; et al. Advances in the Classification and Treatment of Mastocytosis: Current Status and Outlook toward the Future. Cancer Res. 2017, 77, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Degboé, Y.; Severino-Freire, M.; Couture, G.; Apoil, P.A.; Gaudenzio, N.; Hermine, O.; Ruyssen-Witrand, A.; Paul, C.; Laroche, M.; Constantin, A.; et al. The Prevalence Of Osteoporosis Is Low in Adult Cutaneous Mastocytosis Patients. J. Allergy Clin. Immunol. Pract. 2024, 12, 1306–1312. [Google Scholar] [CrossRef]
- Bouvard, B.; Pascaretti-Grizon, F.; Legrand, E.; Lavigne, C.; Audran, M.; Chappard, D. Bone lesions in systemic mastocytosis: Bone histomorphometry and histopathological mechanisms. Morphologie 2020, 104, 97–108. [Google Scholar] [CrossRef]
- Wang, M.; Seibel, M.J. Skin and bones: Systemic mastocytosis and bone. Endocrinol. Diabetes Metab. Case Rep. 2023, 2023, 22-0408. [Google Scholar] [CrossRef]
- Rama, T.A.; Henriques, A.F.; Matito, A.; Jara-Acevedo, M.; Caldas, C.; Mayado, A.; Muñoz-González, J.I.; Moreira, A.; Cavaleiro-Rufo, J.; García-Montero, A.; et al. Bone and Cytokine Markers Associated with Bone Disease in Systemic Mastocytosis. J. Allergy Clin. Immunol. Pract. 2023, 11, 1536–1547, Erratum in J. Allergy Clin. Immunol. Pract. 2024, 12, 269. [Google Scholar] [CrossRef]
- Brown, M.A.; Hatfield, J.K. Mast Cells are Important Modifiers of Autoimmune Disease: With so Much Evidence, Why is There Still Controversy? Front. Immunol. 2012, 3, 147. [Google Scholar] [CrossRef]
- Escribano, L.; Orfao, A.; Villarrubia, J.; Díaz-Agustín, B.; Cerveró, C.; Rios, A.; Velasco, J.L.; Ciudad, J.; Navarro, J.L.; San Miguel, J.F. Immunophenotypic characterization of human bone marrow mast cells. A flow cytometric study of normal and pathological bone marrow samples. Anal. Cell. Pathol. 1998, 16, 151–159. [Google Scholar] [CrossRef]
- Frame, B.; Nixon, R.K. Bone-marrow mast cells in osteoporosis of aging. N. Engl. J. Med. 1968, 279, 626–630. [Google Scholar] [CrossRef]
- Ozdemir, O.; Savasan, S. The role of mast cells in bone marrow diseases. J. Clin. Pathol. 2004, 57, 108–109. [Google Scholar] [CrossRef]
- Collington, S.J.; Williams, T.J.; Weller, C.L. Mechanisms underlying the localisation of mast cells in tissues. Trends Immunol. 2011, 32, 478–485. [Google Scholar] [CrossRef]
- Smith, E.R.; Yeasky, T.; Wei, J.Q.; Miki, R.A.; Cai, K.Q.; Smedberg, J.L.; Yang, W.L.; Xu, X.X. White spotting variant mouse as an experimental model for ovarian aging and menopausal biology. Menopause 2012, 19, 588–596. [Google Scholar] [CrossRef]
- Lowry, M.B.; Lotinun, S.; Leontovich, A.A.; Zhang, M.; Maran, A.; Shogren, K.L.; Palama, B.K.; Marley, K.; Iwaniec, U.T.; Turner, R.T. Osteitis fibrosa is mediated by Platelet-Derived Growth Factor-A via a phosphoinositide 3-kinase-dependent signaling pathway in a rat model for chronic hyperparathyroidism. Endocrinology 2008, 149, 5735–5746. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.T.; Iwaniec, U.T.; Wong, C.P.; Lindenmaier, L.B.; Wagner, L.A.; Branscum, A.J.; Menn, S.A.; Taylor, J.; Zhang, Y.; Wu, H.; et al. Acute exposure to high dose gamma-radiation results in transient activation of bone lining cells. Bone 2013, 57, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Philbrick, K.A.; Wong, C.P.; Kahler-Quesada, A.M.; Olson, D.A.; Branscum, A.J.; Turner, R.T.; Iwaniec, U.T. Polyethylene particles inserted over calvarium induce cancellous bone loss in femur in female mice. Bone Rep. 2018, 9, 84–92. [Google Scholar] [CrossRef]
- Kang, H.; Dang, A.B.; Joshi, S.K.; Halloran, B.; Nissenson, R.; Zhang, X.; Li, J.; Kim, H.T.; Liu, X. Novel mouse model of spinal cord injury-induced heterotopic ossification. J. Rehabil. Res. Dev. 2014, 51, 1109–1118. [Google Scholar] [CrossRef]
- Dube, M.G.; Torto, R.; Kalra, S.P. Increased leptin expression selectively in the hypothalamus suppresses inflammatory markers CRP and IL-6 in leptin-deficient diabetic obese mice. Peptides 2008, 29, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Coulombe, J.C.; Maridas, D.E.; Chow, J.L.; Bouxsein, M.L. Small animal DXA instrument comparison and validation. Bone 2024, 178, 116923, Erratum in Bone 2025, 190, 117298. [Google Scholar] [CrossRef] [PubMed]
- Grigorev, I.P.; Korzhevskii, D.E. Modern Imaging Technologies of Mast Cells for Biology and Medicine (Review). Sovrem. Tehnol. V. Med. 2021, 13, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
| Genetic Background(s) | Gene Alteration(s) | Sex | Intervention | Age at Sacrifice | Bone(s) Evaluated | Source |
|---|---|---|---|---|---|---|
| B6 | F | Ovariectomy | 14 weeks | LV5 | JL | |
| B6 | F | Ovariectomy | 14 weeks | Femur | JL | |
| B6 | F | Particles | 13 weeks | Femur | JL | |
| B6 | F | Temperature stress, Propranolol | 18 weeks | Femur | JL | |
| B6 | M | High-fat diet | 17 weeks | Femur | JL | |
| B6 | M | Spinal cord injury | 13 weeks | Femur | JL | |
| B6 | M | Temperature stress | 16 weeks | LV5, Femur | JL | |
| B6 | M, F | Irradiation | 10 weeks | LV5 | JL | |
| B6 | Factor 8 KO, WT | M | None | 20 weeks | Femur | [22] |
| B6 | Leptin-deficient (ob/ob), WT | F | Particles | 8 weeks | Femur | JL |
| B6 | ob/ob, WT | F | None | 8 weeks | Femur | JL |
| B6 | ob/ob, WT | F | Particles | 7 weeks | Femur | JL |
| B6 | ob/ob, WT | M | Leptin gene therapy | 9 months | Femur | JL |
| B6 | ob/ob, WT | M | Leptin gene therapy, high-fat diet | 24, 38 weeks | Femur | JL |
| B6 | ob/ob, WT | M | Hindlimb unloading | 18 weeks | Femur | JL |
| B6 | mϕRIP140KD, WT | M | None | 9 weeks | Femur | [23] |
| B6 | Tieg KO, WT | M, F | None | 8 weeks | Femur | [24] |
| B6 | Tieg KO, WT | F | Ovariectomy | 9 weeks | Tibia | [24] |
| B6 | Tieg KO, WT | F | Sclerostin antibody | 15 weeks | Femur | [24] |
| B6 | TTP KO, WT | M, F | None | 2 years | Femur | [25] |
| B6, C3H/HeJ | F | Temperature stress | 22 weeks | Femur | JL | |
| B6, C3H/HeJ, B6/129 | lit, LID | F | None | 8 weeks | Femur | [26] |
| B6, DBA, WBB6F1/J | M, F | Continuous PTH (DBA only) | 4–26 weeks | Femur | JL | |
| B6, WBB6F1/J | F | Irradiation | 12 &16 weeks | Tibia | JL | |
| B6D2F1 | AR 2.3-TG, AR 3.6-TG | M | Oophorectomy | 25 weeks | LV5 | [27] |
| BALB/cJ | F | Ovariectomy | 8 months | Tibia | JL | |
| BALB/cJ | F | 2-Methoxyestradiol | 9 weeks | Femur | JL | |
| BALB/cJ | Athymic nude | F | Equol, genistein, tumor cells | 30 and 35 weeks | Femur | JL |
| C57BL6/129SvEv | Progesterone receptor KO, WT | F | None | 6, 12, 26 weeks | Tibia | [28] |
| ICR | Nrf2-/-, WT | F | Rapamycin | 28–32 weeks | Femur | [29] |
| Swiss Webster | M | None | 9 months | |||
| WBB6F1/J | F | Ovariectomy | 8 and 14 weeks | LV5, Tibia | JL | |
| WBB6F1/J | M | Hindlimb unloading, irradiation | 18 weeks | Femur | JL |
| KitW/W-v | KitW/W-v + Mast Cell Transfer | |||
|---|---|---|---|---|
| Body composition | ||||
| Body mass (g) | 19.9 ± 0.9 | 20.3 ± 0.9 | ||
| Lean mass(g) | 15.4 ± 0.7 | 16.1 ± 0.6 | ||
| Fat mass (g) | 4.2 ± 0.3 | 4.0 ± 0.3 | ||
| Percent fat | 21.3 ± 1.3 | 19.7 ± 0.8 | ||
| Total bone area (cm2) | 6.98 ± 0.23 | 7.26 ± 0.17 | ||
| Bone mineral content (g) | 0.32 ± 0.01 | 0.33 ± 0.01 | ||
| Bone mineral density (g/cm2) | 0.045 ± 0.001 | 0.046 ± 0.001 | ||
| Abdominal WAT weight (mg) | 606 ± 104 | 588 ± 75 | ||
| Uterus weight (g) | 42 ± 5 | 38 ± 6 | ||
| Blood glucose and serum markers of bone turnover | ||||
| Blood glucose (mg/dL) | 263 ± 29 | 258 ± 16 | ||
| CTX (ng/mL) | 3.8 ± 1.0 | 5.8 ± 0.9 | ||
| Osteocalcin (ng/mL) | 95 ± 16 | 103 ± 11 | ||
| Data are mean ± SE. n = 7–8/group. | ||||
| Symbol | Fold Change | p-Value |
|---|---|---|
| Ahsg | −2.0 | 0.008 |
| Alpl | −1.6 | 0.015 |
| Bmpr2 | −1.3 | 0.009 |
| Casr | −1.9 | 0.003 |
| Clcn7 | −1.7 | 0.010 |
| Cnr2 | −2.2 | 0.002 |
| Col14a1 | −2.5 | <0.001 |
| Csf2 | −8.5 | <0.001 |
| Dbp | 1.7 | 0.027 |
| Esr1 | −1.3 | 0.008 |
| Esrra | −1.4 | 0.014 |
| Fgfr1 | −1.2 | 0.040 |
| Ihh | −1.6 | 0.011 |
| Itga2 | −1.4 | 0.013 |
| Itgb3 | −2.2 | 0.002 |
| Lrp5 | −1.6 | <0.001 |
| Lrp6 | −1.4 | <0.001 |
| Mab21l2 | −1.7 | <0.001 |
| Mthfr | −2.1 | 0.002 |
| Nog | −2.8 | <0.001 |
| Nos3 | −2.2 | 0.001 |
| Phex | −1.2 | 0.011 |
| P2rx7 | −1.3 | 0.028 |
| Runx2 | −1.5 | 0.001 |
| Serpinh1 | −1.9 | 0.004 |
| Smad3 | −2.0 | 0.001 |
| Smad4 | −1.2 | <0.001 |
| Smad5 | −1.6 | 0.001 |
| Sost | −2.7 | <0.001 |
| Sp7 | −2.5 | <0.001 |
| Spp1 | 1.6 | 0.040 |
| Tgfb3 | −1.7 | 0.002 |
| Tgfbr2 | −1.7 | 0.003 |
| Tgfbr3 | −2.1 | 0.004 |
| Tnfaip3 | −1.7 | <0.001 |
| Tnfrsf11a | −2.1 | 0.001 |
| Tnfrsf1b | −1.7 | 0.001 |
| Vdr | −2.3 | 0.002 |
| Vegfa | −1.2 | 0.008 |
| Vegfb | −1.2 | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, C.P.; Keune, J.A.; Philbrick, K.A.; Branscum, A.J.; Iwaniec, U.T.; Turner, R.T. Introduction of Mature Mast Cells into Bone Marrow Alters Bone Metabolism in Growing Mice. Int. J. Mol. Sci. 2025, 26, 11952. https://doi.org/10.3390/ijms262411952
Wong CP, Keune JA, Philbrick KA, Branscum AJ, Iwaniec UT, Turner RT. Introduction of Mature Mast Cells into Bone Marrow Alters Bone Metabolism in Growing Mice. International Journal of Molecular Sciences. 2025; 26(24):11952. https://doi.org/10.3390/ijms262411952
Chicago/Turabian StyleWong, Carmen P., Jessica A. Keune, Kenneth A. Philbrick, Adam J. Branscum, Urszula T. Iwaniec, and Russell T. Turner. 2025. "Introduction of Mature Mast Cells into Bone Marrow Alters Bone Metabolism in Growing Mice" International Journal of Molecular Sciences 26, no. 24: 11952. https://doi.org/10.3390/ijms262411952
APA StyleWong, C. P., Keune, J. A., Philbrick, K. A., Branscum, A. J., Iwaniec, U. T., & Turner, R. T. (2025). Introduction of Mature Mast Cells into Bone Marrow Alters Bone Metabolism in Growing Mice. International Journal of Molecular Sciences, 26(24), 11952. https://doi.org/10.3390/ijms262411952






