Adeno-Associated Virus-Based Gene Therapy for Lafora Disease in Epm2b-Deficient Mice
Abstract
1. Introduction
2. Results
2.1. ICV Injections of the rAAV-hEPM2B or rAAV-GFP Vectors Enable Efficient Brain Transgene Transcription and Translation
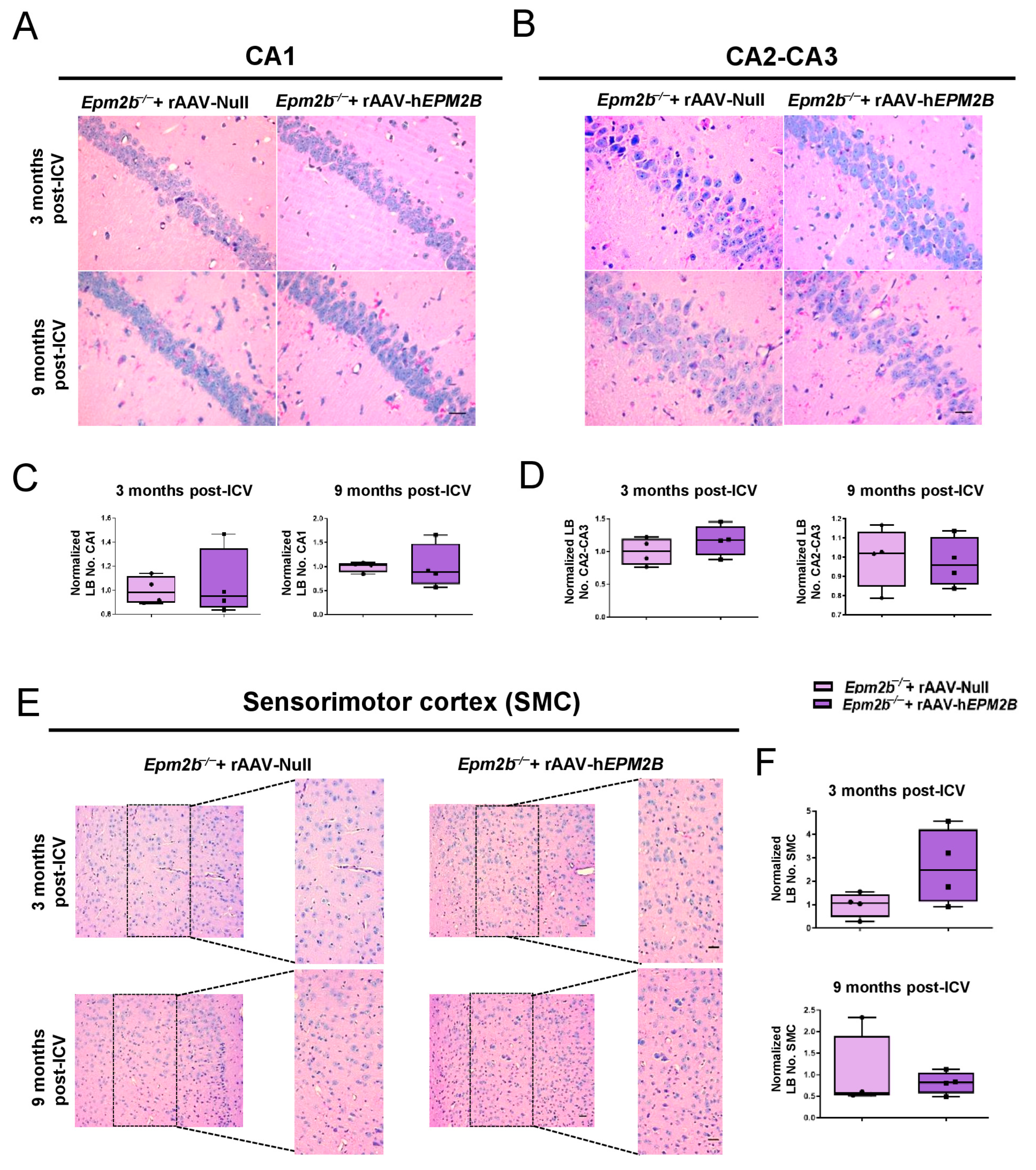
2.2. The rAAV-hEPM2B Vector Does Not Reduce LB Formation in the Brains of Epm2b−/− Mice
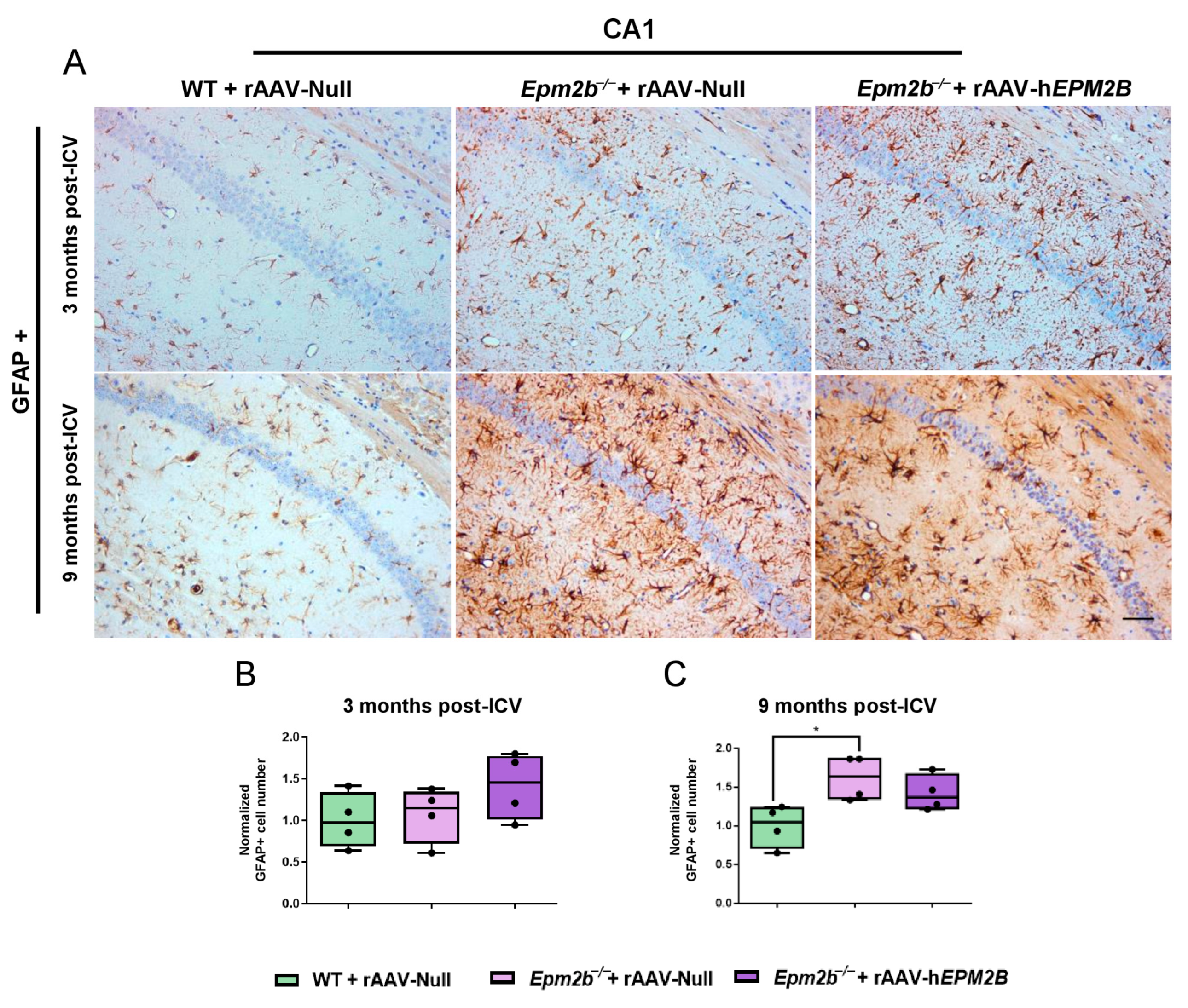
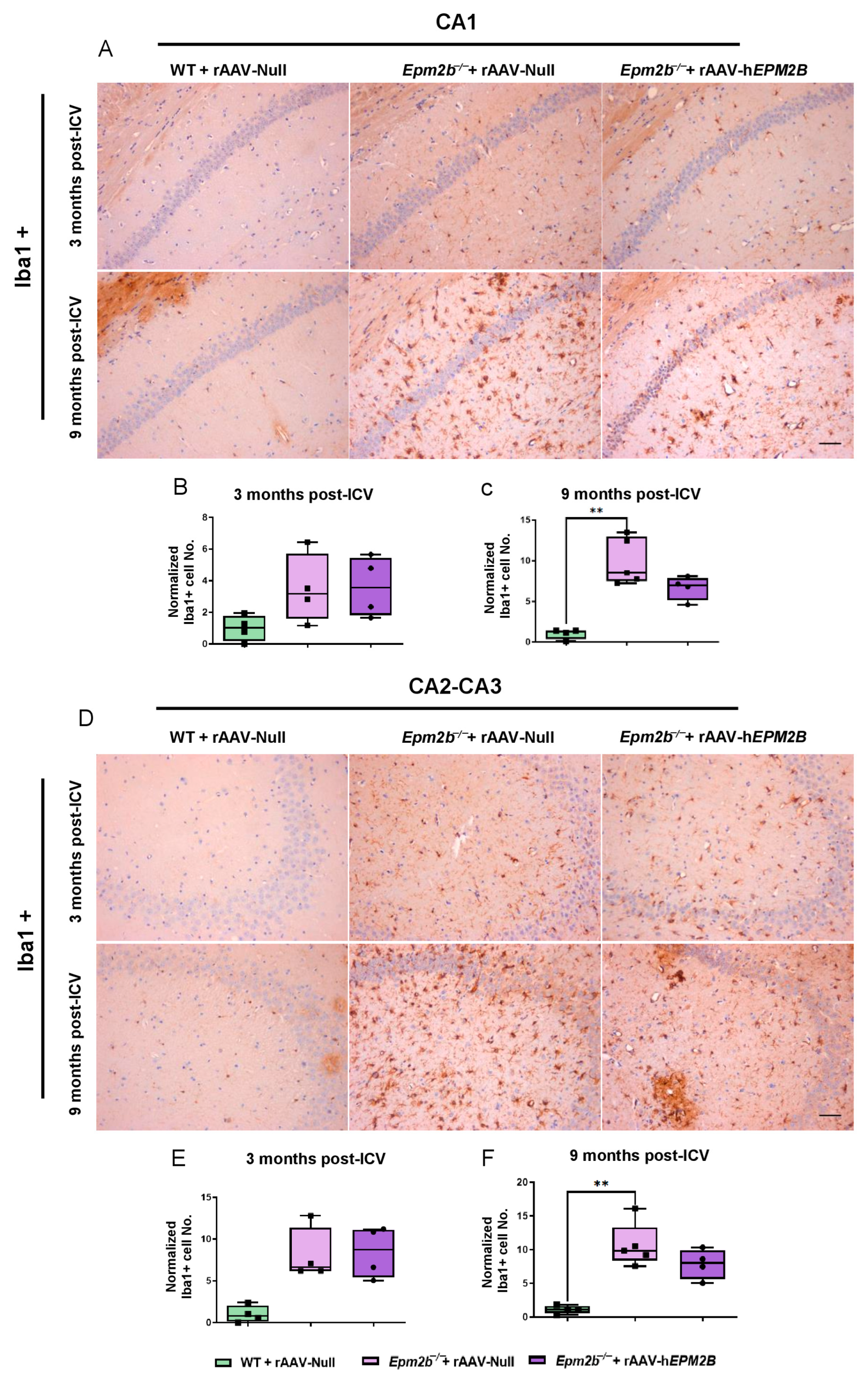
2.3. Treatment with rAAV-hEPM2B Partially Reduces Astrogliosis, Microgliosis, and Neuronal Loss in Epm2b−/− Mice
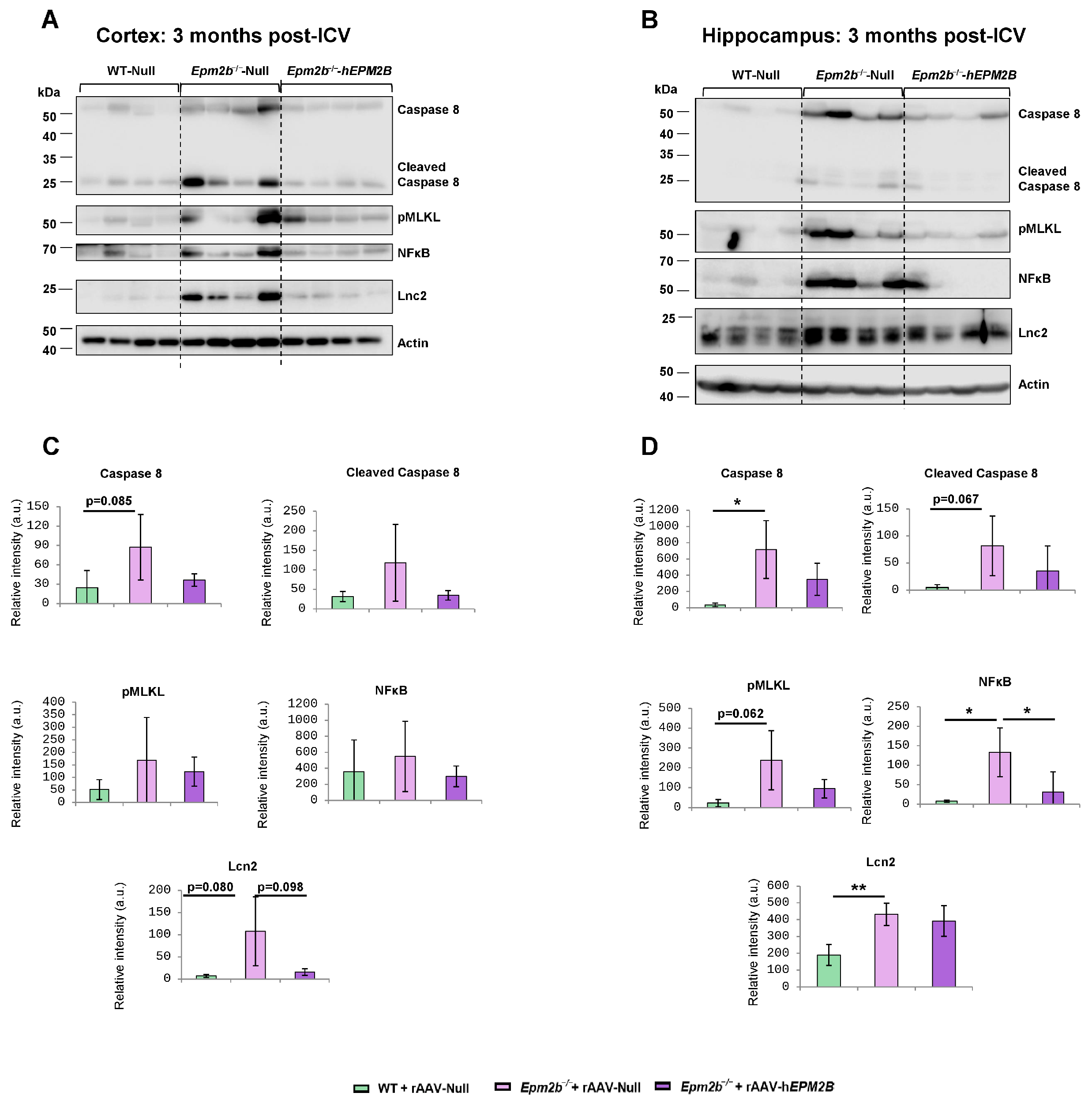
2.4. Treatment with rAAV-hEPM2B Reduces the Levels of Neuroinflammatory and Cell Death Markers in Epm2b−/− Mice
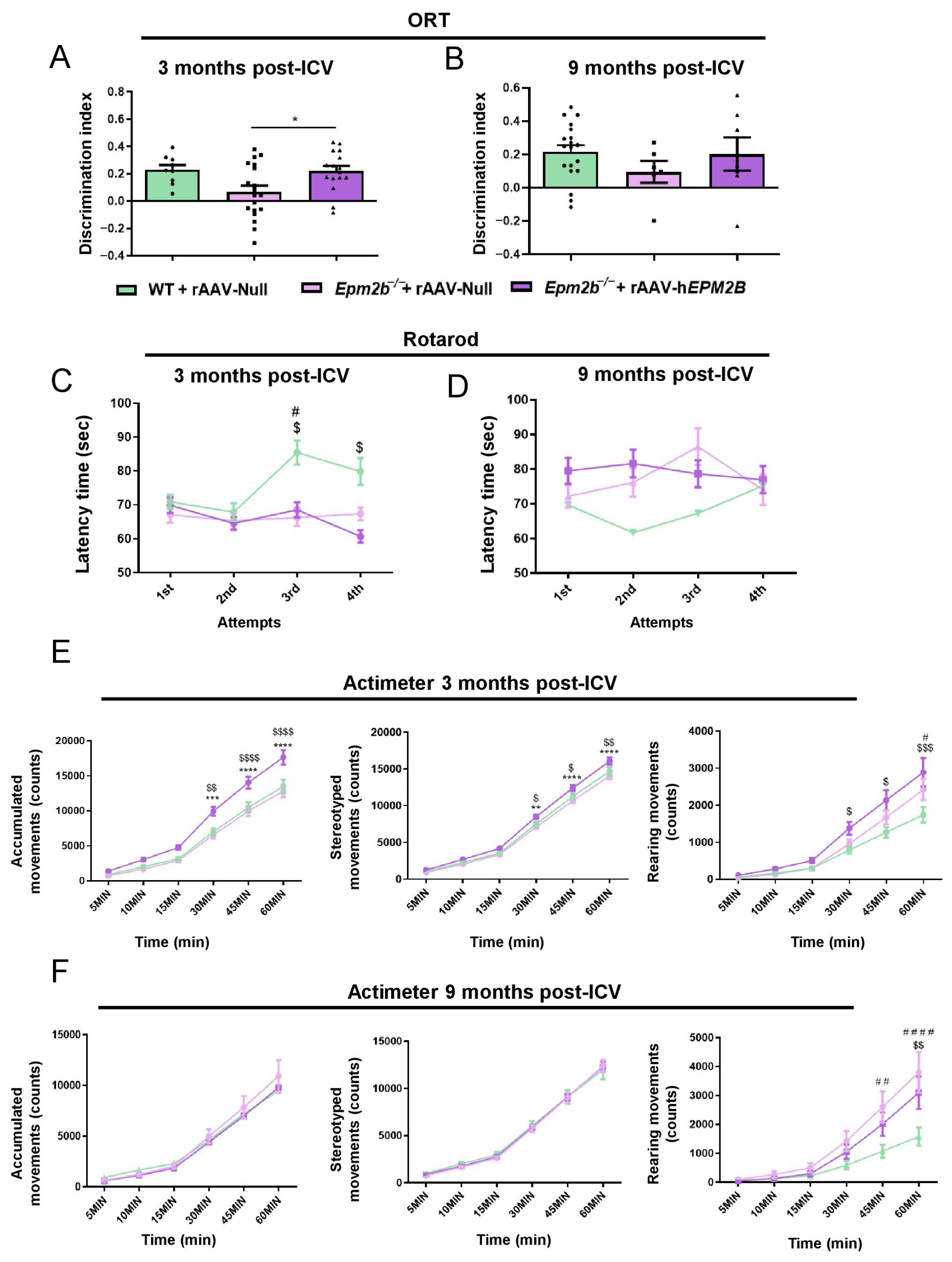
2.5. rAAV-hEPM2B-Based Gene Therapy Reverses Episodic Memory Alterations and Improves Spontaneous Locomotor Activity but Does Not Correct Motor Coordination Impairments in Epm2b−/− Mice
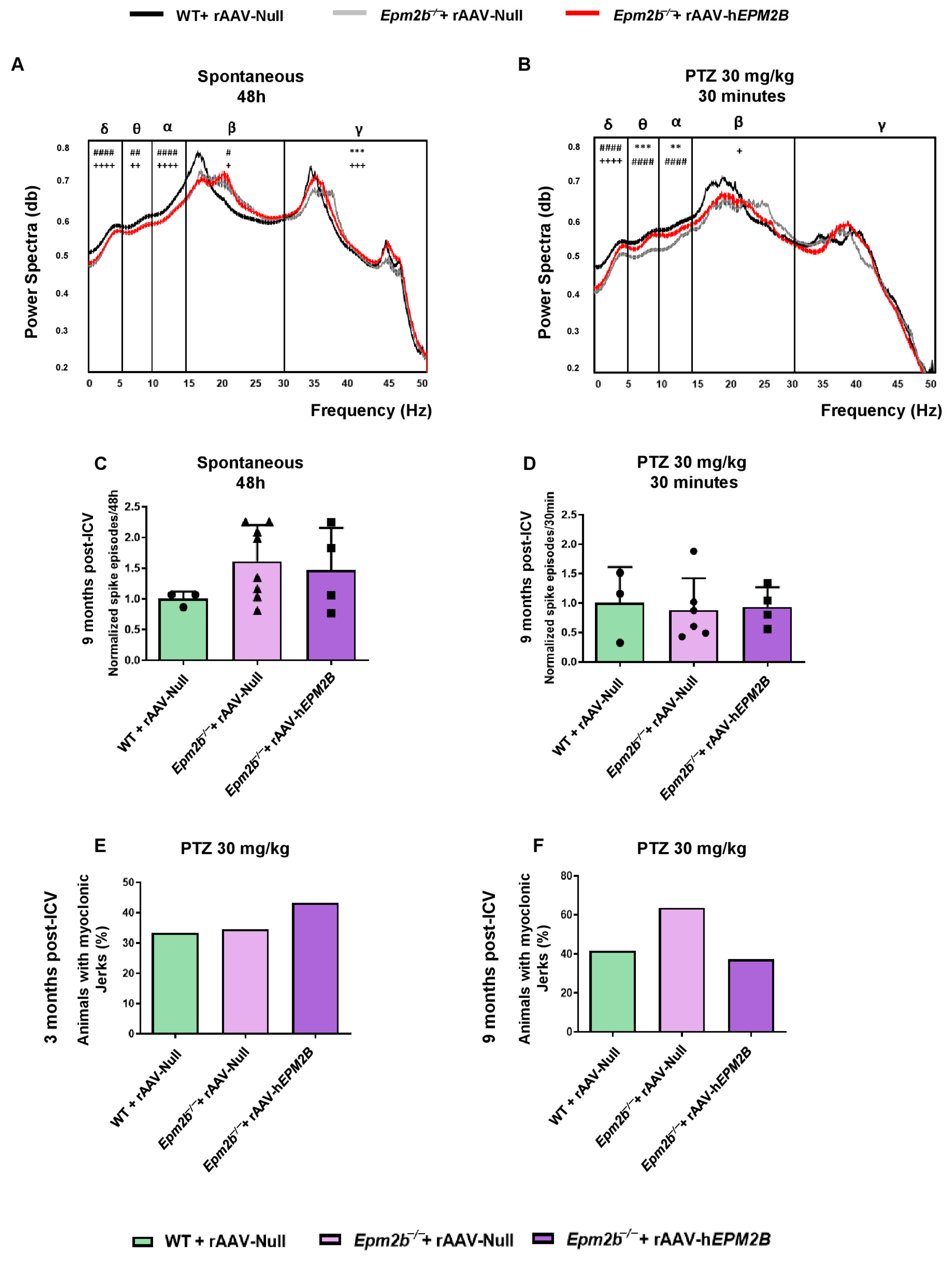
2.6. Treatment with rAAV-hEPM2B in Epm2b−/− Mice Does Not Improve Spontaneous Electrical Brain Activity or Reduce PTZ Sensitivity
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Production of rAAV2/9-CAG-hEPM2B, rAAV2/9-CAG-Null and rAAV2/9-CAG-GFP Vectors
4.3. Stereotaxic ICV Injections
4.4. RNA Extraction and Quantitative Reverse Transcription-Polymerase Chain Reaction
4.5. PAS-Diastase Staining and Immunohistochemistry
4.6. Western Blot Analyses
4.7. Object Recognition Task
4.8. Motor Coordination
4.9. Spontaneous Locomotor Activity
4.10. Video-EEG Recording
4.11. Sensitivity to PTZ
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lafora, G.R.; Glueck, B. Beitrag zur Histopathologie der Myoklonischen Epilepsie. Z. Die Gesamte Neurol. Und Psychiatr. 1911, 6, 1–14. [Google Scholar] [CrossRef]
- Berkovic, S.F.; Andermann, F.; Carpenter, S.; Wolfe, L.S. Progressive myoclonus epilepsies: Specific causes and diagnosis. New Engl. J. Med. 1986, 315, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Heycoptenhamm, V.; Jager, D.J.E. Progressive Myoclonus Epilepsy with Lafora Bodies. Clin. Pathol. Features 1963, 4, 95–119. [Google Scholar]
- Harriman, D.G.; Millar, J.H.; Stevenson, A.C. Progressive familial myoclonic epilepsy in three families: Its clinical features and pathological basis. Brain A J. Neurol. 1955, 78, 325–349. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, G.A.; Yanoff, M. Lafora’s disease. Distinct clinico-pathologic form of unverricht’s syndrome. Arch. Neurol. 1965, 12, 172–188. [Google Scholar] [CrossRef]
- Desdentado, L.; Espert, R.; Sanz, P.; Tirapu-Ustarroz, J. Lafora disease: A review of the literature. Rev. Neurol. 2019, 68, 66–74. [Google Scholar] [CrossRef]
- Turnbull, J.; Tiberia, E.; Striano, P.; Genton, P.; Carpenter, S.; Ackerley, C.A.; Minassian, B.A. Lafora disease. Epileptic Disord. Int. Epilepsy J. Videotape 2016, 18, 38–62. [Google Scholar] [CrossRef]
- Serratosa, J.M.; Delgado-Escueta, A.V.; Posada, I.; Shih, S.; Drury, I.; Berciano, J.; Zabala, J.A.; Antúnez, M.C.; Sparkes, R.S. The gene for progressive myoclonus epilepsy of the Lafora type maps to chromosome 6q. Hum. Mol. Genet. 1995, 4, 1657–1663. [Google Scholar] [CrossRef]
- Serratosa, J.M.; Gómez-Garre, P.; Gallardo, M.E.; Anta, B.; de Bernabé, D.B.; Lindhout, D.; Augustijn, P.B.; Tassinari, C.A.; Malafosse, R.M.; Topcu, M.; et al. A novel protein tyrosine phosphatase gene is mutated in progressive myoclonus epilepsy of the Lafora type (EPM2). Hum. Mol. Genet. 1999, 8, 345–352. [Google Scholar] [CrossRef]
- Minassian, B.A.; Lee, J.R.; Herbrick, J.A.; Huizenga, J.; Soder, S.; Mungall, A.J.; Dunham, I.; Gardner, R.; Fong, C.Y.; Carpenter, S.; et al. Mutations in a gene encoding a novel protein tyrosine phosphatase cause progressive myoclonus epilepsy. Nat. Genet. 1998, 20, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, S.; Agarwala, K.L.; Ueda, K.; Akagi, T.; Shoda, K.; Usui, T.; Hashikawa, T.; Osada, H.; Delgado-Escueta, A.V.; Yamakawa, K. Laforin, defective in the progressive myoclonus epilepsy of Lafora type, is a dual-specificity phosphatase associated with polyribosomes. Hum. Mol. Genet. 2000, 9, 2251–2261. [Google Scholar] [CrossRef]
- Chan, E.M.; Bulman, D.E.; Paterson, A.D.; Turnbull, J.; Andermann, E.; Andermann, F.; Rouleau, G.A.; Delgado-Escueta, A.V.; Scherer, S.W.; Minassian, B.A. Genetic mapping of a new Lafora progressive myoclonus epilepsy locus (EPM2B) on 6p22. J. Med. Genet. 2003, 40, 671–675. [Google Scholar] [CrossRef]
- Chan, E.M.; Young, E.J.; Ianzano, L.; Munteanu, I.; Zhao, X.; Christopoulos, C.C.; Avanzini, G.; Elia, M.; Ackerley, C.A.; Jovic, N.J.; et al. Mutations in NHLRC1 cause progressive myoclonus epilepsy. Nat. Genet. 2003, 35, 125–127. [Google Scholar] [CrossRef]
- Gentry, M.S.; Worby, C.A.; Dixon, J.E. Insights into Lafora disease: Malin is an E3 ubiquitin ligase that ubiquitinates and promotes the degradation of laforin. Proc. Natl. Acad. Sci. USA 2005, 102, 8501–8506. [Google Scholar] [CrossRef] [PubMed]
- Vilchez, D.; Ros, S.; Cifuentes, D.; Pujadas, L.; Vallès, J.; García-Fojeda, B.; Criado-García, O.; Fernández-Sánchez, E.; Medraño-Fernández, I.; Domínguez, J.; et al. Mechanism suppressing glycogen synthesis in neurons and its demise in progressive myoclonus epilepsy. Nat. Neurosci. 2007, 10, 1407–1413. [Google Scholar] [CrossRef]
- Skurat, A.V.; Segvich, D.M.; Contreras, C.J.; Hu, Y.C.; Hurley, T.D.; DePaoli-Roach, A.A.; Roach, P.J. Impaired malin expression and interaction with partner proteins in Lafora disease. J. Biol. Chem. 2024, 300, 107271. [Google Scholar] [CrossRef] [PubMed]
- Romá-Mateo, C.; Sanz, P.; Gentry, M.S. Deciphering the role of malin in the lafora progressive myoclonus epilepsy. IUBMB Life 2012, 64, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Solaz-Fuster, M.C.; Gimeno-Alcañiz, J.V.; Ros, S.; Fernandez-Sanchez, M.E.; Garcia-Fojeda, B.; Criado Garcia, O.; Vilchez, D.; Dominguez, J.; Garcia-Rocha, M.; Sanchez-Piris, M.; et al. Regulation of glycogen synthesis by the laforin-malin complex is modulated by the AMP-activated protein kinase pathway. Hum. Mol. Genet. 2008, 17, 667–678. [Google Scholar] [CrossRef]
- DePaoli-Roach, A.A.; Tagliabracci, V.S.; Segvich, D.M.; Meyer, C.M.; Irimia, J.M.; Roach, P.J. Genetic depletion of the malin E3 ubiquitin ligase in mice leads to lafora bodies and the accumulation of insoluble laforin. J. Biol. Chem. 2010, 285, 25372–25381. [Google Scholar] [CrossRef]
- Gentry, M.S.; Guinovart, J.J.; Minassian, B.A.; Roach, P.J.; Serratosa, J.M. Lafora disease offers a unique window into neuronal glycogen metabolism. J. Biol. Chem. 2018, 293, 7117–7125. [Google Scholar] [CrossRef]
- Vernia, S.; Rubio, T.; Heredia, M.; Rodríguez de Córdoba, S.; Sanz, P. Increased endoplasmic reticulum stress and decreased proteasomal function in lafora disease models lacking the phosphatase laforin. PLoS ONE 2009, 4, e5907. [Google Scholar] [CrossRef]
- Romá-Mateo, C.; Aguado, C.; García-Giménez, J.L.; Ibáñez-Cabellos, J.S.; Seco-Cervera, M.; Pallardó, F.V.; Knecht, E.; Sanz, P. Increased oxidative stress and impaired antioxidant response in Lafora disease. Mol. Neurobiol. 2015, 51, 932–946. [Google Scholar] [CrossRef]
- Romá-Mateo, C.; Aguado, C.; García-Giménez, J.L.; Knecht, E.; Sanz, P.; Pallardó, F.V. Oxidative stress, a new hallmark in the pathophysiology of Lafora progressive myoclonus epilepsy. Free Radic. Biol. Med. 2015, 88, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Lahuerta, M.; Aguado, C.; Sánchez-Martín, P.; Sanz, P.; Knecht, E. Degradation of altered mitochondria by autophagy is impaired in Lafora disease. FEBS J. 2018, 285, 2071–2090. [Google Scholar] [CrossRef]
- Duran, J.; Gruart, A.; García-Rocha, M.; Delgado-García, J.M.; Guinovart, J.J. Glycogen accumulation underlies neurodegeneration and autophagy impairment in Lafora disease. Hum. Mol. Genet. 2014, 23, 3147–3156. [Google Scholar] [CrossRef]
- Lafora, G.R. Über das Vorkommen amyloider Körperchen im Innern der Ganglienzellen. Virchows Arch. Für Pathol. Anat. Und Physiol. Und Für Klin. Med. 1911, 205, 295–303. [Google Scholar] [CrossRef]
- Yokoi, S.; Austin, J.; Witmer, F.; Sakai, M. Studies in myoclonus epilepsy (Lafora body form). I. Isolation and preliminary characterization of Lafora bodies in two cases. Arch. Neurol. 1968, 19, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Sakai, M.; Austin, J.; Witmer, F.; Trueb, L. Studies in myoclonus epilepsy (Lafora body form) II. Polyglucosans in the systemic deposits of myoclonus epilepsy and in corpora amylacea. Neurology 1970, 20, 160–176. [Google Scholar] [CrossRef]
- Sullivan, M.A.; Nitschke, S.; Steup, M.; Minassian, B.A.; Nitschke, F. Pathogenesis of Lafora Disease: Transition of Soluble Glycogen to Insoluble Polyglucosan. Int. J. Mol. Sci. 2017, 18, 1743. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, S.; Delgado-Escueta, A.V.; Sakamoto, T.; Avila, M.R.; Machado-Salas, J.; Hoshii, Y.; Akagi, T.; Gomi, H.; Suzuki, T.; Amano, K.; et al. Targeted disruption of the Epm2a gene causes formation of Lafora inclusion bodies, neurodegeneration, ataxia, myoclonus epilepsy and impaired behavioral response in mice. Hum. Mol. Genet. 2002, 11, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Criado, O.; Aguado, C.; Gayarre, J.; Duran-Trio, L.; Garcia-Cabrero, A.M.; Vernia, S.; San Millán, B.; Heredia, M.; Romá-Mateo, C.; Mouron, S.; et al. Lafora bodies and neurological defects in malin-deficient mice correlate with impaired autophagy. Hum. Mol. Genet. 2012, 21, 1521–1533. [Google Scholar] [CrossRef]
- García-Cabrero, A.M.; Marinas, A.; Guerrero, R.; de Córdoba, S.R.; Serratosa, J.M.; Sánchez, M.P. Laforin and malin deletions in mice produce similar neurologic impairments. J. Neuropathol. Exp. Neurol. 2012, 71, 413–421. [Google Scholar] [CrossRef] [PubMed]
- García-Cabrero, A.M.; Sánchez-Elexpuru, G.; Serratosa, J.M.; Sánchez, M.P. Enhanced sensitivity of laforin- and malin-deficient mice to the convulsant agent pentylenetetrazole. Front. Neurosci. 2014, 8, 291. [Google Scholar] [CrossRef]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef]
- Berthier, A.; Payá, M.; García-Cabrero, A.M.; Ballester, M.I.; Heredia, M.; Serratosa, J.M.; Sánchez, M.P.; Sanz, P. Pharmacological Interventions to Ameliorate Neuropathological Symptoms in a Mouse Model of Lafora Disease. Mol. Neurobiol. 2016, 53, 1296–1309. [Google Scholar] [CrossRef] [PubMed]
- Burgos, D.F.; Machío-Castello, M.; Iglesias-Cabeza, N.; Giráldez, B.G.; González-Fernández, J.; Sánchez-Martín, G.; Sánchez, M.P.; Serratosa, J.M. Early Treatment with Metformin Improves Neurological Outcomes in Lafora Disease. Neurotherapeutics 2023, 20, 230–244. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Elexpuru, G.; Serratosa, J.M.; Sanz, P.; Sánchez, M.P. 4-Phenylbutyric acid and metformin decrease sensitivity to pentylenetetrazol-induced seizures in a malin knockout model of Lafora disease. Neuroreport 2017, 28, 268–271. [Google Scholar] [CrossRef]
- Bisulli, F.; Muccioli, L.; d’Orsi, G.; Canafoglia, L.; Freri, E.; Licchetta, L.; Mostacci, B.; Riguzzi, P.; Pondrelli, F.; Avolio, C.; et al. Treatment with metformin in twelve patients with Lafora disease. Orphanet J. Rare Dis. 2019, 14, 149. [Google Scholar] [CrossRef]
- Sánchez-Elexpuru, G.; Serratosa, J.M.; Sánchez, M.P. Sodium selenate treatment improves symptoms and seizure susceptibility in a malin-deficient mouse model of Lafora disease. Epilepsia 2017, 58, 467–475. [Google Scholar] [CrossRef]
- Austin, G.L.; Simmons, Z.R.; Klier, J.E.; Rondon, A.; Hodges, B.L.; Shaffer, R.; Aziz, N.M.; McKnight, T.R.; Pauly, J.R.; Armstrong, D.D.; et al. Central Nervous System Delivery and Biodistribution Analysis of an Antibody-Enzyme Fusion for the Treatment of Lafora Disease. Mol. Pharm. 2019, 16, 3791–3801. [Google Scholar] [CrossRef]
- Brewer, M.K.; Uittenbogaard, A.; Austin, G.L.; Segvich, D.M.; DePaoli-Roach, A.; Roach, P.J.; McCarthy, J.J.; Simmons, Z.R.; Brandon, J.A.; Zhou, Z.; et al. Targeting Pathogenic Lafora Bodies in Lafora Disease Using an Antibody-Enzyme Fusion. Cell Metab. 2019, 30, 689–705.e6. [Google Scholar] [CrossRef]
- Ahonen, S.; Nitschke, S.; Grossman, T.R.; Kordasiewicz, H.; Wang, P.; Zhao, X.; Guisso, D.R.; Kasiri, S.; Nitschke, F.; Minassian, B.A. Gys1 antisense therapy rescues neuropathological bases of murine Lafora disease. Brain 2021, 144, 2985–2993. [Google Scholar] [CrossRef]
- Donohue, K.J.; Fitzsimmons, B.; Bruntz, R.C.; Markussen, K.H.; Young, L.E.A.; Clarke, H.A.; Coburn, P.T.; Griffith, L.E.; Sanders, W.; Klier, J.; et al. Gys1 Antisense Therapy Prevents Disease-Driving Aggregates and Epileptiform Discharges in a Lafora Disease Mouse Model. Neurotherapeutics 2023, 20, 1808–1819. [Google Scholar] [CrossRef] [PubMed]
- Zafra-Puerta, L.; Colpaert, M.; Iglesias-Cabeza, N.; Burgos, D.F.; Sánchez-Martín, G.; Gentry, M.S.; Sánchez, M.P.; Serratosa, J.M. Effect of intracerebroventricular administration of alglucosidase alfa in two mouse models of Lafora disease: Relevance for clinical practice. Epilepsy Res. 2024, 200, 107317. [Google Scholar] [CrossRef]
- Mollá, B.; Heredia, M.; Sanz, P. Modulators of Neuroinflammation Have a Beneficial Effect in a Lafora Disease Mouse Model. Mol. Neurobiol. 2021, 58, 2508–2522. [Google Scholar] [CrossRef]
- Zafra-Puerta, L.; Iglesias-Cabeza, N.; Burgos, D.F.; Sciaccaluga, M.; González-Fernández, J.; Bellingacci, L.; Canonichesi, J.; Sánchez-Martín, G.; Costa, C.; Sánchez, M.P.; et al. Gene therapy for Lafora disease in the Epm2a−/− mouse model. Mol. Ther. 2024, 32, 2130–2149. [Google Scholar] [CrossRef]
- Lahuerta, M.; Gonzalez, D.; Aguado, C.; Fathinajafabadi, A.; García-Giménez, J.L.; Moreno-Estellés, M.; Romá-Mateo, C.; Knecht, E.; Pallardó, F.V.; Sanz, P. Reactive Glia-Derived Neuroinflammation: A Novel Hallmark in Lafora Progressive Myoclonus Epilepsy That Progresses with Age. Mol. Neurobiol. 2020, 57, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- Rubio, T.; Viana, R.; Moreno-Estellés, M.; Campos-Rodríguez, Á.; Sanz, P. TNF and IL6/Jak2 signaling pathways are the main contributors of the glia-derived neuroinflammation present in Lafora disease, a fatal form of progressive myoclonus epilepsy. Neurobiol. Dis. 2023, 176, 105964. [Google Scholar] [CrossRef] [PubMed]
- Varea, O.; Guinovart, J.J.; Duran, J. Malin restoration as proof of concept for gene therapy for Lafora disease. Brain Commun. 2022, 4, fcac168. [Google Scholar] [CrossRef]
- Turnbull, J.; Wang, P.; Girard, J.M.; Ruggieri, A.; Wang, T.J.; Draginov, A.G.; Kameka, A.P.; Pencea, N.; Zhao, X.; Ackerley, C.A.; et al. Glycogen hyperphosphorylation underlies lafora body formation. Ann. Neurol. 2010, 68, 925–933. [Google Scholar] [CrossRef]
- Chan, E.M.; Ackerley, C.A.; Lohi, H.; Ianzano, L.; Cortez, M.A.; Shannon, P.; Scherer, S.W.; Minassian, B.A. Laforin preferentially binds the neurotoxic starch-like polyglucosans, which form in its absence in progressive myoclonus epilepsy. Hum. Mol. Genet. 2004, 13, 1117–1129. [Google Scholar] [CrossRef]
- Zafra-Puerta, L.; Iglesias-Cabeza, N.; Sciaccaluga, M.; Bellingacci, L.; Canonichesi, J.; Sánchez-Martín, G.; Costa, C.; Sánchez, M.P.; Serratosa, J.M. Advances in gene therapy for Lafora disease: Intravenous recombinant adeno-associated virus-mediated delivery of EPM2A and EPM2B genes. Clin. Transl. Med. 2025, 15, e70514. [Google Scholar] [CrossRef] [PubMed]
- Duran, J.; Hervera, A.; Markussen, K.H.; Varea, O.; López-Soldado, I.; Sun, R.C.; Del Río, J.A.; Gentry, M.S.; Guinovart, J.J. Astrocytic glycogen accumulation drives the pathophysiology of neurodegeneration in Lafora disease. Brain 2021, 144, 2349–2360. [Google Scholar] [CrossRef]
- López-González, I.; Viana, R.; Sanz, P.; Ferrer, I. Inflammation in Lafora Disease: Evolution with Disease Progression in Laforin and Malin Knock-out Mouse Models. Mol. Neurobiol. 2017, 54, 3119–3130. [Google Scholar] [CrossRef]
- Romá-Mateo, C.; Lorente-Pozo, S.; Márquez-Thibaut, L.; Moreno-Estellés, M.; Garcés, C.; González, D.; Lahuerta, M.; Aguado, C.; García-Giménez, J.L.; Sanz, P.; et al. Age-Related microRNA Overexpression in Lafora Disease Male Mice Provides Links between Neuroinflammation and Oxidative Stress. Int. J. Mol. Sci. 2023, 24, 1089. [Google Scholar] [CrossRef] [PubMed]
- García-Gimeno, M.A.; Knecht, E.; Sanz, P. Lafora Disease: A Ubiquitination-Related Pathology. Cells 2018, 7, 87. [Google Scholar] [CrossRef]
- Xu, J.; Song, D.; Xue, Z.; Gu, L.; Hertz, L.; Peng, L. Requirement of glycogenolysis for uptake of increased extracellular K+ in astrocytes: Potential implications for K+ homeostasis and glycogen usage in brain. Neurochem. Res. 2013, 38, 472–485. [Google Scholar] [CrossRef] [PubMed]
- DiNuzzo, M.; Mangia, S.; Maraviglia, B.; Giove, F. Does abnormal glycogen structure contribute to increased susceptibility to seizures in epilepsy? Metab. Brain Dis. 2015, 30, 307–316. [Google Scholar] [CrossRef]
- Turnbull, J.; DePaoli-Roach, A.A.; Zhao, X.; Cortez, M.A.; Pencea, N.; Tiberia, E.; Piliguian, M.; Roach, P.J.; Wang, P.; Ackerley, C.A.; et al. PTG depletion removes Lafora bodies and rescues the fatal epilepsy of Lafora disease. PLoS Genet. 2011, 7, e1002037. [Google Scholar] [CrossRef]
- Turnbull, J.; Epp, J.R.; Goldsmith, D.; Zhao, X.; Pencea, N.; Wang, P.; Frankland, P.W.; Ackerley, C.A.; Minassian, B.A. PTG protein depletion rescues malin-deficient Lafora disease in mouse. Ann. Neurol. 2014, 75, 442–446. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Piedra, J.; Ontiveros, M.; Miravet, S.; Penalva, C.; Monfar, M.; Chillon, M. Development of a rapid, robust, and universal picogreen-based method to titer adeno-associated vectors. Hum. Gene Ther. Methods 2015, 26, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Burgos, D.F.; Sciaccaluga, M.; Worby, C.A.; Zafra-Puerta, L.; Iglesias-Cabeza, N.; Sánchez-Martín, G.; Prontera, P.; Costa, C.; Serratosa, J.M.; Sánchez, M.P. Epm2aR240X knock-in mice present earlier cognitive decline and more epileptic activity than Epm2a−/− mice. Neurobiol. Dis. 2023, 181, 106119. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zafra-Puerta, L.; Iglesias-Cabeza, N.; Sanz, P.; García-Gimeno, M.A.; Sánchez-Martín, G.; Sánchez, M.P.; Serratosa, J.M. Adeno-Associated Virus-Based Gene Therapy for Lafora Disease in Epm2b-Deficient Mice. Int. J. Mol. Sci. 2025, 26, 11930. https://doi.org/10.3390/ijms262411930
Zafra-Puerta L, Iglesias-Cabeza N, Sanz P, García-Gimeno MA, Sánchez-Martín G, Sánchez MP, Serratosa JM. Adeno-Associated Virus-Based Gene Therapy for Lafora Disease in Epm2b-Deficient Mice. International Journal of Molecular Sciences. 2025; 26(24):11930. https://doi.org/10.3390/ijms262411930
Chicago/Turabian StyleZafra-Puerta, Luis, Nerea Iglesias-Cabeza, Pascual Sanz, María Adelaida García-Gimeno, Gema Sánchez-Martín, Marina P. Sánchez, and José M. Serratosa. 2025. "Adeno-Associated Virus-Based Gene Therapy for Lafora Disease in Epm2b-Deficient Mice" International Journal of Molecular Sciences 26, no. 24: 11930. https://doi.org/10.3390/ijms262411930
APA StyleZafra-Puerta, L., Iglesias-Cabeza, N., Sanz, P., García-Gimeno, M. A., Sánchez-Martín, G., Sánchez, M. P., & Serratosa, J. M. (2025). Adeno-Associated Virus-Based Gene Therapy for Lafora Disease in Epm2b-Deficient Mice. International Journal of Molecular Sciences, 26(24), 11930. https://doi.org/10.3390/ijms262411930





