Potential Role of Circulating miR-103a, miR-145 and miR-191 as Diagnostic Biomarkers for Ulcerative Colitis and Crohn’s Disease
Abstract
1. Introduction
2. Results
2.1. Characteristics of Subjects Enrolled in the Study
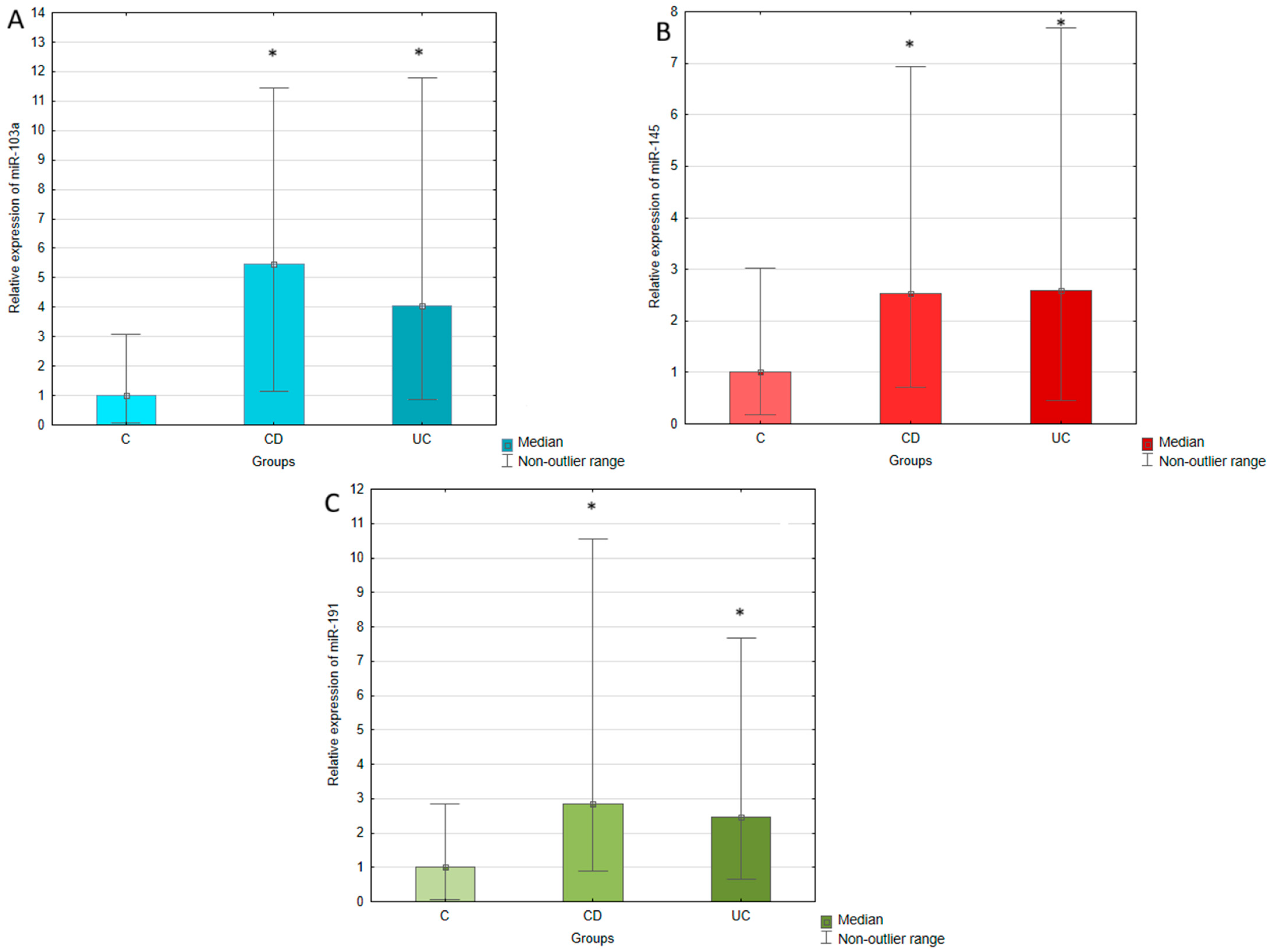
2.2. Circulating Expression of miR-103a, miR-145,miR-191 in Patients with Ulcerative Colitis, Crohn’s Disease, and Healthy Individuals
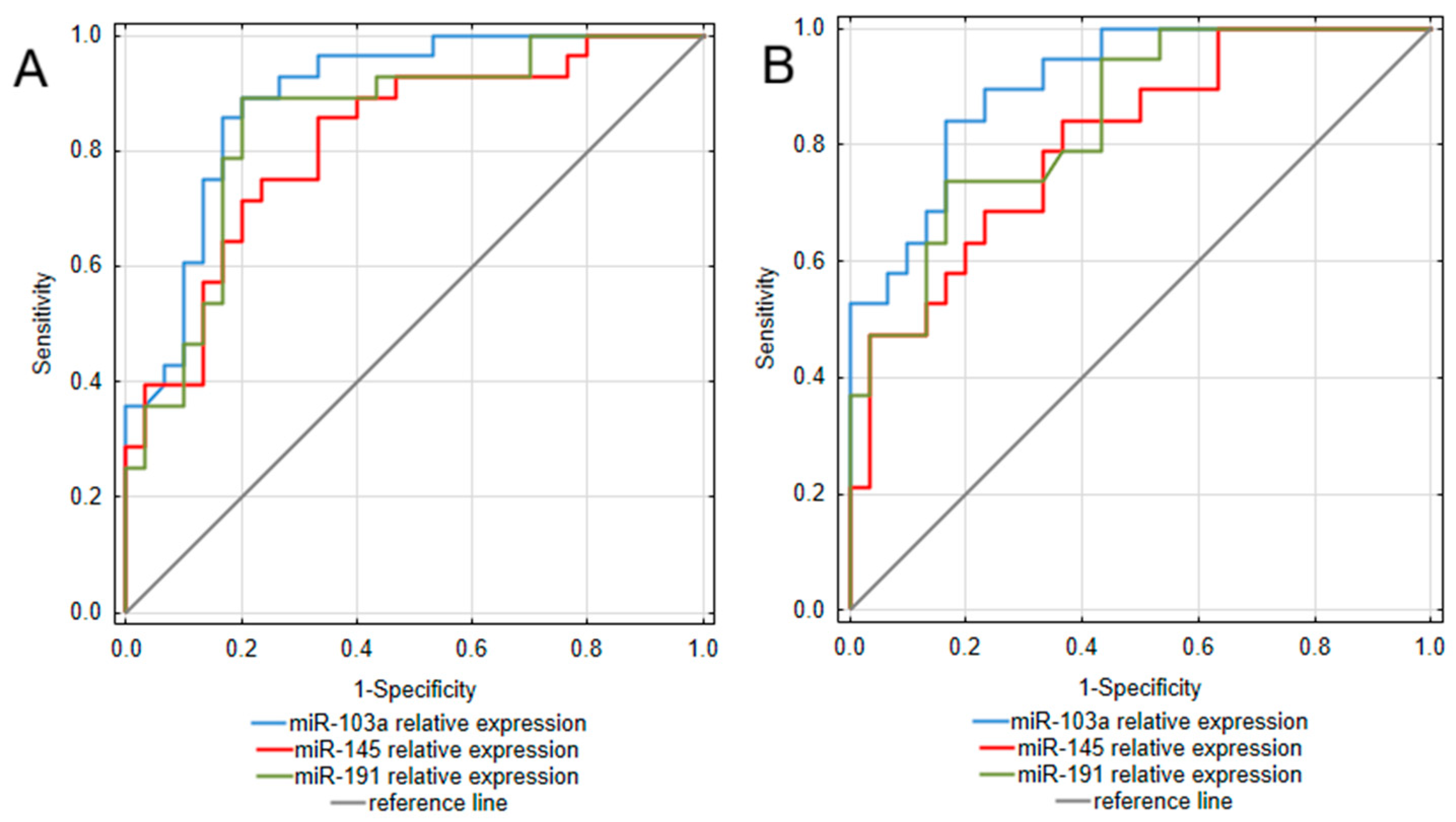
2.3. Circulating miR-103a, miR-145, miR-191 Expression, as Potential Biomarkers of Ulcerative Colitis and Crohn’s Disease
2.4. Correlation of miR-103a, miR-145, and miR-191 Expression with Clinical Characteristics
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Methods
4.3. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramos, G.P.; Papadakis, K.A. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin. Proc. 2019, 94, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Flynn, S.; Eisenstein, S. Inflammatory Bowel Disease Presentation and Diagnosis. Surg. Clin. N. Am. 2019, 99, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Le Berre, C.; Honap, S.; Peyrin-Biroulet, L. Ulcerative colitis. Lancet 2023, 402, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Dolinger, M.; Torres, J.; Vermeire, S. Crohn’s disease. Lancet 2024, 403, 1177–1191. [Google Scholar] [CrossRef]
- Le Berre, C.; Ananthakrishnan, A.N.; Danese, S.; Singh, S.; Peyrin-Biroulet, L. Ulcerative Colitis and Crohn’s Disease Have Similar Burden and Goals for Treatment. Clin. Gastroenterol. Hepatol. 2020, 18, 14–23. [Google Scholar] [CrossRef]
- Khaki-Khatibi, F.; Qujeq, D.; Kashifard, M.; Moein, S.; Maniati, M.; Vaghari-Tabari, M. Calprotectin in inflammatory bowel disease. Clin. Chim. Acta 2020, 510, 556–565. [Google Scholar] [CrossRef]
- Norouzinia, M.; Chaleshi, V.; Alizadeh, A.H.M.; Zali, M.R. Biomarkers in inflammatory bowel diseases: Insight into diagnosis, prognosis and treatment. Gastroenterol. Hepatol. Bed Bench 2017, 10, 155–167. [Google Scholar] [PubMed] [PubMed Central]
- Saliminejad, K.; Khorram Khorshid, H.R.; Soleymani Fard, S.; Ghaffari, S.H. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J. Cell. Physiol. 2019, 234, 5451–5465. [Google Scholar] [CrossRef]
- de Oliveira, E.C.S.; Quaglio, A.E.V.; Grillo, T.G.; Di Stasi, L.C.; Sassaki, L.Y. MicroRNAs in inflammatory bowel disease: What do we know and what can we expect? World J. Gastroenterol. 2024, 30, 2184–2190. [Google Scholar] [CrossRef]
- de Oliveira, E.C.S.; Quaglio, A.E.V.; Magro, D.O.; Di Stasi, L.C.; Sassaki, L.Y. Intestinal Microbiota and miRNA in IBD: A Narrative Review about Discoveries and Perspectives for the Future. Int. J. Mol. Sci. 2023, 24, 7176. [Google Scholar] [CrossRef]
- Chen, L.; Lu, Q.; Deng, F.; Peng, S.; Yuan, J.; Liu, C.; Du, X. miR-103a-3p Could Attenuate Sepsis-Induced Liver Injury by Targeting HMGB1. Inflammation 2020, 43, 2075–2086. [Google Scholar] [CrossRef]
- Aebisher, D.; Bartusik-Aebisher, D.; Przygórzewska, A.; Oleś, P.; Woźnicki, P.; Kawczyk-Krupka, A. Key Interleukins in Inflammatory Bowel Disease-A Review of Recent Studies. Int. J. Mol. Sci. 2024, 26, 121. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Xu, Y.; Wen, W.; Huang, L.; Guo, Z.; Zhu, W.; Li, Y. Exosomal miR-103a-3p from Crohn’s Creeping Fat-Derived Adipose-Derived Stem Cells Contributes to Intestinal Fibrosis by Targeting TGFBR3 and Activating Fibroblasts. J. Crohn’s Colitis 2023, 17, 1291–1308. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.; Li, X.; Li, C.; Wan, L.; Shi, H.; Song, X.; Liu, X.; Chen, X.; Zhang, C.; Shan, H.; et al. TGFBR3, a potential negative regulator of TGF-β signaling, protects cardiac fibroblasts from hypoxia-induced apoptosis. J. Cell. Physiol. 2011, 226, 2586–2594. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Xiong, W.; Zhang, F.; Wang, N.; Luo, Y.; Chen, Y.; Cao, J.; Chen, Z.; Ma, C.; Chen, H. The miR-103a-3p/TGFBR3 axis regulates TGF-β-induced orbital fibroblast activation and fibrosis in thyroid-eye disease. Mol. Cell. Endocrinol. 2023, 559, 111780. [Google Scholar] [CrossRef]
- Li, R.; Liang, P.; Yuan, J.; He, F. Exosomal miR-103a-3p ameliorates lipopolysaccharide-induced immune response in BEAS-2B cells via NF-κB pathway by targeting transducin β-like 1X related protein 1. Clin. Exp. Pharmacol. Physiol. 2020, 47, 620–627. [Google Scholar] [CrossRef]
- Mukherjee, T.; Kumar, N.; Chawla, M.; Philpott, D.J.; Basak, S. The NF-κB signaling system in the immunopathogenesis of inflammatory bowel disease. Sci. Signal. 2024, 17, eadh1641. [Google Scholar] [CrossRef]
- Drago, L.; De La Motte, L.R.; Deflorio, L.; Sansico, D.F.; Salvatici, M.; Micaglio, E.; Biazzo, M.; Giarritiello, F. Systematic review of bidirectional interaction between gut microbiome, miRNAs, and human pathologies. Front. Microbiol. 2025, 16, 1540943. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rossi, A.F.; Cadamuro, A.C.; Biselli-Périco, J.M.; Leite, K.R.; Severino, F.E.; Reis, P.P.; Cordeiro, J.A.; Silva, A.E. Interaction between inflammatory mediators and miRNAs in Helicobacter pylori infection. Cell. Microbiol. 2016, 18, 1444–1458. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Assmann, T.S.; Cuevas-Sierra, A.; Riezu-Boj, J.I.; Milagro, F.I.; Martínez, J.A. Comprehensive Analysis Reveals Novel Interactions between Circulating MicroRNAs and Gut Microbiota Composition in Human Obesity. Int. J. Mol. Sci. 2020, 21, 9509. [Google Scholar] [CrossRef]
- Pekow, J.R.; Dougherty, U.; Mustafi, R.; Zhu, H.; Kocherginsky, M.; Rubin, D.T.; Hanauer, S.B.; Hart, J.; Chang, E.B.; Fichera, A.; et al. miR-143 and miR-145 are downregulated in ulcerative colitis: Putative regulators of inflammation and protooncogenes. Inflamm. Bowel Dis. 2012, 18, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Chen, S.; Liu, L. The landscape of miRNA-mRNA regulatory network and cellular sources in inflammatory bowel diseases: Insights from text mining and single cell RNA sequencing analysis. Front. Immunol. 2024, 15, 1454532. [Google Scholar] [CrossRef] [PubMed]
- Moparthi, L.; Koch, S. Wnt signaling in intestinal inflammation. Differentiation 2019, 108, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Chen, B.; Huang, S.; Han, J.; Zhou, G.; Xu, S.; Chen, M.; Zeng, Z.; Zhang, S. Hypermethylation of miR-145 promoter-mediated SOX9-CLDN8 pathway regulates intestinal mucosal barrier in Crohn’s disease. eBioMedicine 2022, 76, 103846. [Google Scholar] [CrossRef]
- Paraskevi, A.; Theodoropoulos, G.; Papaconstantinou, I.; Mantzaris, G.; Nikiteas, N.; Gazouli, M. Circulating MicroRNA in inflammatory bowel disease. J. Crohn’s Colitis 2012, 6, 900–904. [Google Scholar] [CrossRef]
- Gu, Y.; Ampofo, E.; Menger, M.D.; Laschke, M.W. miR-191 suppresses angiogenesis by activation of NF-κB signaling. FASEB J. 2017, 31, 3321–3333. [Google Scholar] [CrossRef]
- He, Y.; Cui, Y.; Wang, W.; Gu, J.; Guo, S.; Ma, K.; Luo, X. Hypomethylation of the hsa-miR-191 locus causes high expression of hsa-mir-191 and promotes the epithelial-to-mesenchymal transition in hepatocellular carcinoma. Neoplasia 2011, 13, 841–853. [Google Scholar] [CrossRef]
- de Bruyn, M.; Vandooren, J.; Ugarte-Berzal, E.; Arijs, I.; Vermeire, S.; Opdenakker, G. The molecular biology of matrix metalloproteinases and tissue inhibitors of metalloproteinases in inflammatory bowel diseases. Crit. Rev. Biochem. Mol. Biol. 2016, 51, 295–358. [Google Scholar] [CrossRef]
- Derkacz, A.; Olczyk, P.; Olczyk, K.; Komosinska-Vassev, K. The Role of Extracellular Matrix Components in Inflammatory Bowel Diseases. J. Clin. Med. 2021, 10, 1122. [Google Scholar] [CrossRef]
- Abu-Freha, N.; Cohen, B.; Gordon, M.; Weissmann, S.; Kestenbaum, E.H.; Vosko, S.; Abu-Tailakh, M.; Ben-Shoshan, L.; Cohen, D.L.; Shirin, H. Colorectal cancer among inflammatory bowel disease patients: Risk factors and prevalence compared to the general population. Front. Med. 2023, 10, 1225616. [Google Scholar] [CrossRef]
- Saberinia, A.; Alinezhad, A.; Jafari, F.; Soltany, S.; Akhavan Sigari, R. Oncogenic miRNAs and target therapies in colorectal cancer. Clin. Chim. Acta 2020, 508, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Fasihi, A.M.; Soltani, B.; Atashi, A.; Nasiri, S. Introduction of hsa-miR-103a and hsa-miR-1827 and hsa-miR-137 as new regulators of Wnt signaling pathway and their relation to colorectal carcinoma. J. Cell. Biochem. 2018, 119, 5104–5117. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Wei, B.; Chen, Z.; Wang, J.; Zhao, L.; Peng, X.; Liu, K.; Lai, Y.; Ni, L. Identification of a four-microRNA panel in serum as promising biomarker for colorectal carcinoma detection. Biomark. Med. 2020, 14, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Mozammel, N.; Amini, M.; Baradaran, B.; Mahdavi, S.Z.B.; Hosseini, S.S.; Mokhtarzadeh, A. The function of miR-145 in colorectal cancer progression; an updated review on related signaling pathways. Pathol. Res. Pract. 2023, 242, 154290. [Google Scholar] [CrossRef]
- Nagpal, N.; Kulshreshtha, R. miR-191: An emerging player in disease biology. Front. Genet. 2014, 23, 99. [Google Scholar] [CrossRef]
- Zhang, X.F.; Li, K.K.; Gao, L.; Li, S.Z.; Chen, K.; Zhang, J.B.; Wang, D.; Tu, R.F.; Zhang, J.X.; Tao, K.X.; et al. miR-191 promotes tumorigenesis of human colorectal cancer through targeting C/EBPβ. Oncotarget 2015, 6, 4144–4158. [Google Scholar] [CrossRef]
| Parameter | UC | CD | C |
|---|---|---|---|
| N | 28 | 19 | 30 |
| Sex (females/males) | 16/12 | 7/12 | 19/11 |
| Age [years] | 41.4 ± 13.9 | 34.9 ± 11.3 | 40.6 ± 11.4 |
| Disease duration [years] | 7.9 ± 6.0 | 8.1 ± 4.8 | - |
| Treatment | |||
| Conventional anti-inflammatory treatment | 6 | 3 | - |
| Biological treatment | 17 | 12 | |
| Small-molecule drugs | 5 | 4 | |
| Disease activity: | |||
| Indices values | pMayo: 0 (0–3) | CDAI: 57.0 (39.0–125.5) | - |
| Clinical remission | 17 | 15 | |
| Mild | 5 | 0 | |
| Moderate | 4 | 4 | |
| Severe | 2 | 0 | |
| CRP [mg/L] | 2.70 (1–5.40) | 2.27 (0.94–3.57) | - |
| RBC [×106/μL] | 4.4 (4.02–5.10) | 4.7 ± 0.50 | - |
| HGB [g/dL] | 13.57 ± 1.91 | 14.35 ± 1.55 | - |
| MCV [fL] | 90.2 (85.8–95.4) | 91.85 ± 6.73 | - |
| PLT [×103/μL] | 294.5 ± 82.4 | 261.1 ±86.8 | - |
| WBC [×103/μL] | 7.01 ± 2.33 | 5.82 ± 1.80 | - |
| Neutrophils [×103/μL] | 4.03 ± 1.75 | 3.71 ± 1.17 | - |
| Lymphocytes [×103/μL] | 2.10 ± 0.84 | 1.39 ± 0.56 | - |
| Monocytes [×103/μL] | 0.57 ± 0.18 | 0.55 ± 0.22 | - |
| Eozynophils [×103/μL] | 0.15 (0.11–0.22) | 0.07 (0.05–0.11) | - |
| Basophils [×103/μL] | 0.04 (0.03–0.05) | 0.02 (0.02–0.03) | - |
| ALT [U/L] | 25.34 ± 10.23 | 21.04 ± 14.86 | - |
| AST [U/L] | 23.68 ± 7.32 | 22.73 ± 8.64 | - |
| miRNA | UC | CD | ||
|---|---|---|---|---|
| Fold Change | p Value | Fold Change | p Value | |
| miR-103a | 4.0 | 0.00000 | 5.5 | 0.00000 |
| miR-145 | 2.6 | 0.00004 | 2.5 | 0.00037 |
| miR-191 | 2.5 | 0.00001 | 2.8 | 0.00006 |
| Analyzed miRNA | Analyzed Groups | AUC (95% CI) | Youden Index | Cut-Off [Relative Expression] | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|---|
| miR-103a | UC | 0.893 (0.811–0.975) | 0.69 | 25.02 | 89.3 | 80.0 | 80.6 | 88.9 |
| CD | 0.905 (0.825–0.985) | 0.68 | 26.54 | 84.2 | 83.3 | 76.2 | 89.3 | |
| miR-145 | UC | 0.815 (0.706–0.925) | 0.52 | 5.88 | 85.7 | 66.7 | 70.6 | 83.3 |
| CD | 0.805 (0.683–0.928) | 0.48 | 5.06 | 84.2 | 63.3 | 59.3 | 90.5 | |
| miR-191 | UC | 0.848 (0.745–0.95) | 0.69 | 25.11 | 89.3 | 80.0 | 80.6 | 88.9 |
| CD | 0.843 (0.735–0.951) | 0.57 | 36.5 | 73.7 | 83.3 | 73.7 | 83.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Górecka, A.; Kruszniewska-Rajs, C.; Gola, J.; Romańczyk, T.; Romańczyk, M.; Komosinska-Vassev, K. Potential Role of Circulating miR-103a, miR-145 and miR-191 as Diagnostic Biomarkers for Ulcerative Colitis and Crohn’s Disease. Int. J. Mol. Sci. 2025, 26, 11927. https://doi.org/10.3390/ijms262411927
Górecka A, Kruszniewska-Rajs C, Gola J, Romańczyk T, Romańczyk M, Komosinska-Vassev K. Potential Role of Circulating miR-103a, miR-145 and miR-191 as Diagnostic Biomarkers for Ulcerative Colitis and Crohn’s Disease. International Journal of Molecular Sciences. 2025; 26(24):11927. https://doi.org/10.3390/ijms262411927
Chicago/Turabian StyleGórecka, Aleksandra, Celina Kruszniewska-Rajs, Joanna Gola, Tomasz Romańczyk, Marcin Romańczyk, and Katarzyna Komosinska-Vassev. 2025. "Potential Role of Circulating miR-103a, miR-145 and miR-191 as Diagnostic Biomarkers for Ulcerative Colitis and Crohn’s Disease" International Journal of Molecular Sciences 26, no. 24: 11927. https://doi.org/10.3390/ijms262411927
APA StyleGórecka, A., Kruszniewska-Rajs, C., Gola, J., Romańczyk, T., Romańczyk, M., & Komosinska-Vassev, K. (2025). Potential Role of Circulating miR-103a, miR-145 and miR-191 as Diagnostic Biomarkers for Ulcerative Colitis and Crohn’s Disease. International Journal of Molecular Sciences, 26(24), 11927. https://doi.org/10.3390/ijms262411927







