Expression Alterations and Correlative Analysis of TPH1/hsa-miR-194-5p/NEAT1 and MAOA/hsa-miR-1276/NEAT1 Axes in Pediatric Inflammatory Bowel Disease
Abstract
1. Introduction
2. Results
2.1. Study Cohort
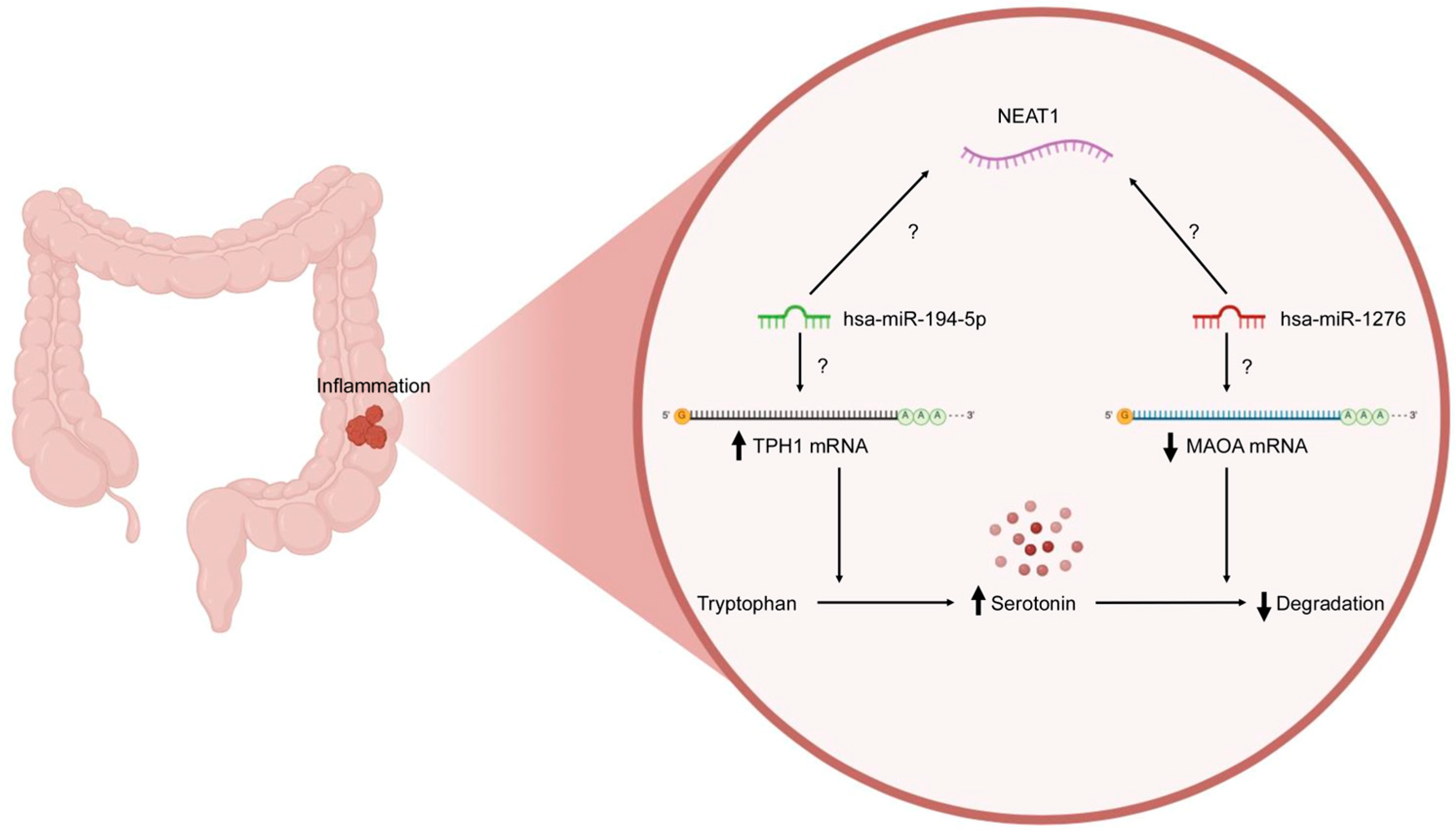
2.2. Prediction and Construction of TPH1/hsa-miR-194-5p/NEAT1 and MAOA/hsa-miR-1276/NEAT1 Axes
2.3. Functional Annotations of TPH1, MAOA, and NEAT1
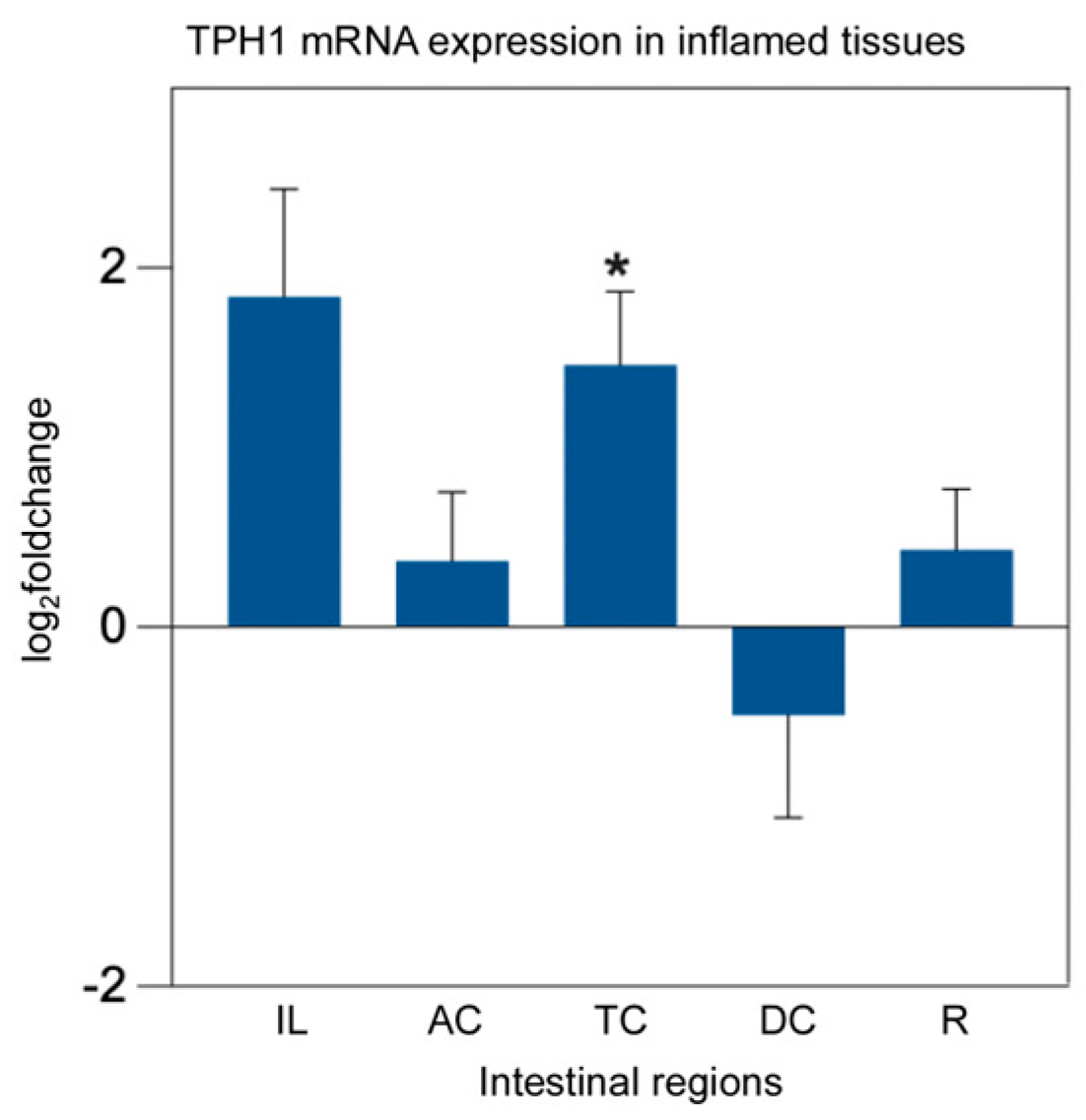
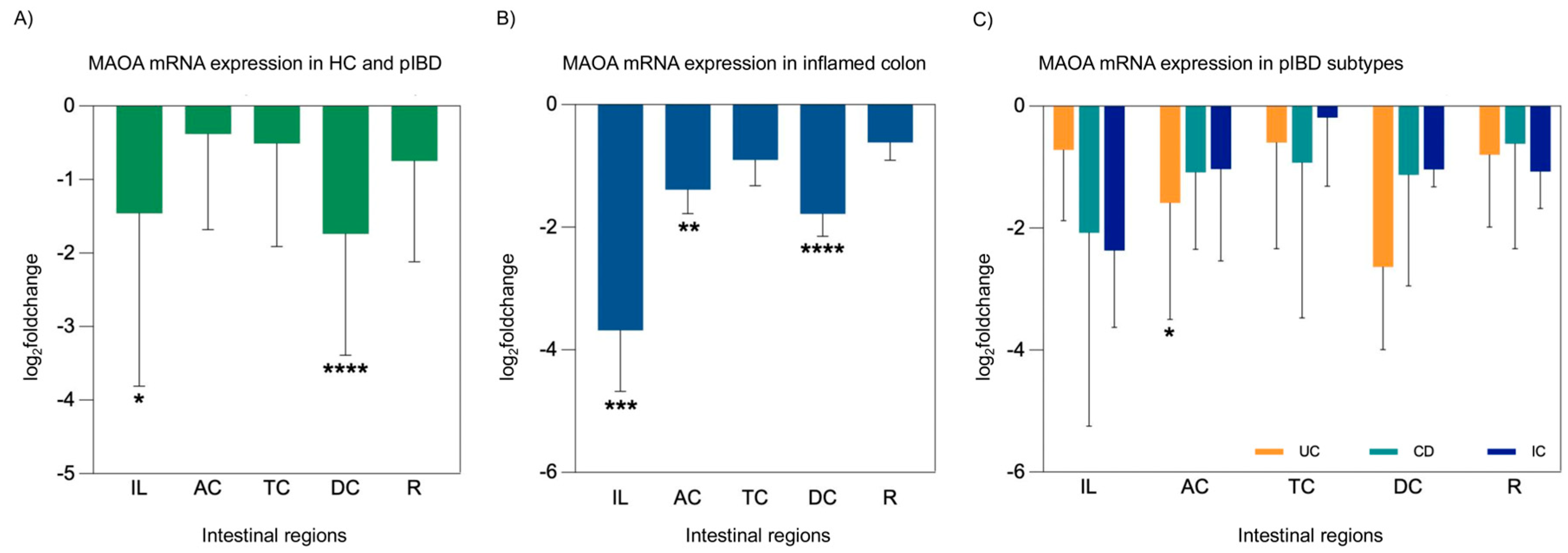
2.4. mRNA Expression Analysis of TPH1 and MAOA
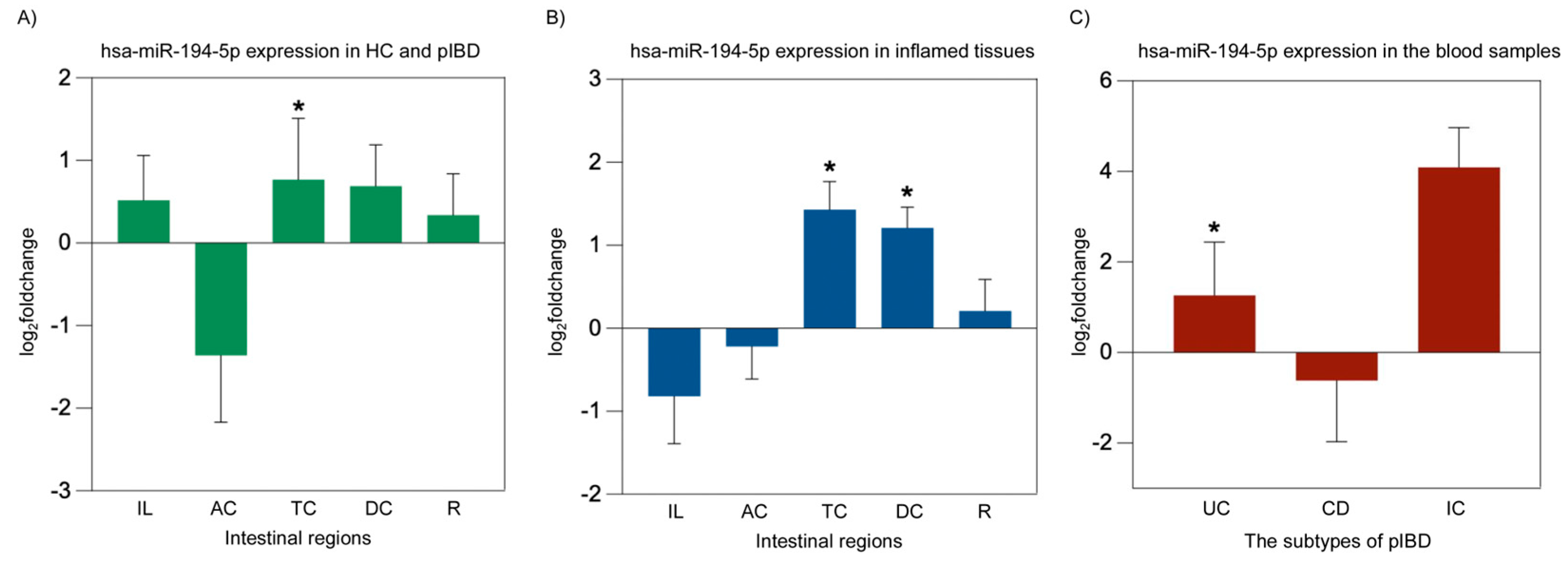
2.5. miRNA Expression Analysis of hsa-miR-194-5p and hsa-miR-1276
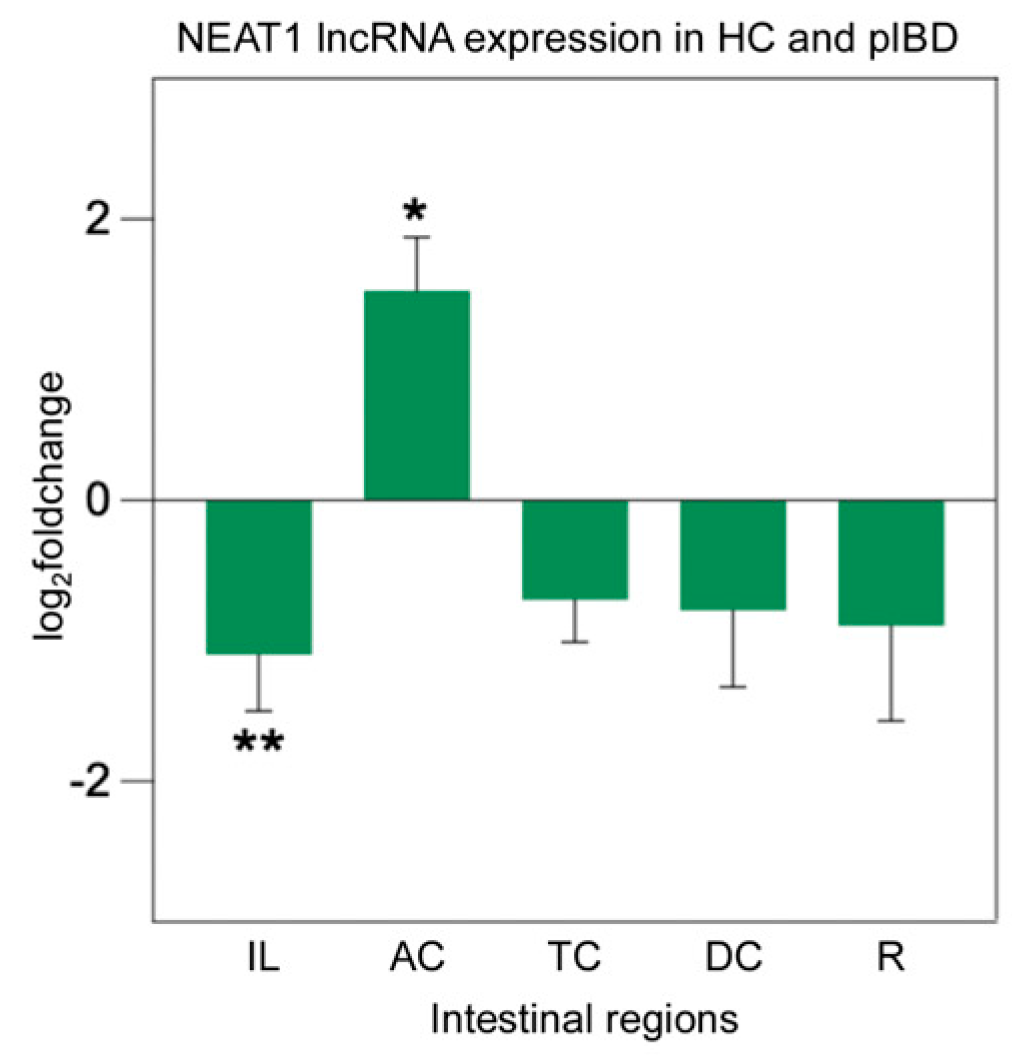
2.6. lncRNA Expression Analysis of NEAT1
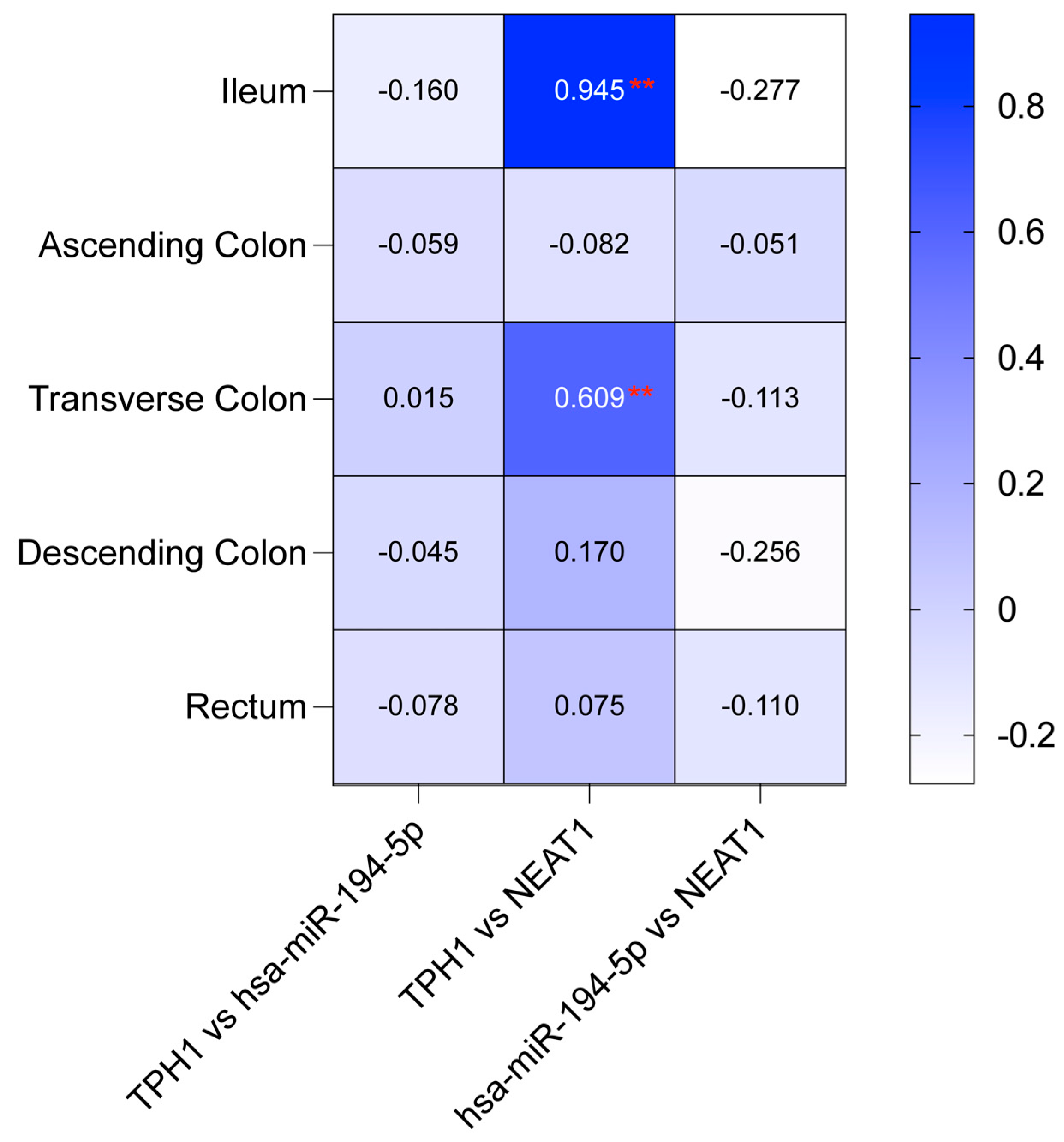
2.7. Correlation Analysis of TPH1/hsa-miR-194-5p/NEAT1 and MAOA/hsa-miR-1276/NEAT1 in IBD Patients
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. In Silico Prediction and Construction of TPH1/miR-194-5p/NEAT1 and MAOA/miR-1276/NEAT1 Regulatory Networks with Gene Ontology Analysis
4.3. Sample Collection and RNA Extraction
4.4. Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| pIBD | Pediatric inflammatory bowel disease |
| UC | Ulcerative colitis |
| CD | Crohn’s disease |
| IC | Indeterminate colitis |
| ncRNAs | Non-coding RNAs |
| miRNAs | MicroRNAs |
| lncRNAs | Long non-coding RNAs |
| TPH | Tryptophan hydroxylase |
| MAOA | Monoamine oxidase A |
| NEAT1 | Nuclear Enriched Abundant Transcript 1 |
| 5-HT | 5-hydroxytryptamine |
| TNFα | Tumor necrosis factor-alpha |
| TLR4 | Toll-like receptor 4 |
| TRAF6 | Tumor necrosis factor receptor-associated factor 6 |
| IL | Ileum |
| AC | Ascending colon |
| TC | Transverse colon |
| DC | Descending colon |
| R | Rectum |
References
- Kokkinou, E.; Soini, T.; Pandey, R.V.; van Acker, A.; Theorell, J.; Czarnewski, P.; Kvedaraite, E.; Vandamme, N.; Lourda, M.; Sorini, C.; et al. The single-cell transcriptional landscape of innate and adaptive lymphocytes in pediatric-onset colitis. Cell Rep. Med. 2023, 4, 101038. [Google Scholar] [CrossRef] [PubMed]
- Le Berre, C.; Honap, S.; Peyrin-Biroulet, L. Ulcerative colitis. Lancet 2023, 402, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Dolinger, M.; Torres, J.; Vermeire, S. Crohn’s disease. Lancet 2024, 403, 1177–1191. [Google Scholar] [CrossRef] [PubMed]
- Bouhuys, M.; Lexmond, W.S.; van Rheenen, P.F. Pediatric Inflammatory Bowel Disease. Pediatrics 2023, 15, e2022058037. [Google Scholar] [CrossRef]
- Klomberg, R.C.W.; Hellendoorn, A.E.; Kemos, P.; Rizopoulos, D.; Ruemmele, F.M.; Croft, N.M.; de Ridder, L.; PIBD-SETQuality Safety Registry Collaborators. Rare and severe adverse events in children with inflammatory bowel disease: Analysis of data from the PIBD-SETQuality Safety Registry. Lancet Child Adolesc. Health 2024, 8, 422–432. [Google Scholar] [CrossRef]
- Jairath, V.; Feagan, B.G. Global burden of inflammatory bowel disease. Lancet Gastroenterol. Hepatol. 2020, 5, 2–3. [Google Scholar] [CrossRef]
- Shah, P.A.; Park, C.J.; Shaughnessy, M.P.; Cowles, R.A. Serotonin as a Mitogen in the Gastrointestinal Tract: Revisiting a Familiar Molecule in a New Role. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1093–1104. [Google Scholar] [CrossRef]
- Yaghoubfar, R.; Behrouzi, A.; Ashrafian, F.; Shahryari, A.; Moradi, H.R.; Choopani, S.; Hadifar, S.; Vaziri, F.; Nojoumi, S.A.; Fateh, A.; et al. Modulation of serotonin signaling/metabolism by Akkermansia muciniphila and its extracellular vesicles through the gut-brain axis in mice. Sci. Rep. 2020, 10, 22119. [Google Scholar] [CrossRef]
- Kwon, Y.H.; Wang, H.; Denou, E.; Ghia, J.E.; Rossi, L.; Fontes, M.E.; Bernier, S.P.; Shajib, M.S.; Banskota, S.; Collins, S.M.; et al. Modulation of Gut Microbiota Composition by Serotonin Signaling Influences Intestinal Immune Response and Susceptibility to Colitis. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 709–728. [Google Scholar] [CrossRef]
- Koopman, N.; Katsavelis, D.; Hove, A.S.T.; Brul, S.; Jonge, W.J.; Seppen, J. The Multifaceted Role of Serotonin in Intestinal Homeostasis. Int. J. Mol. Sci. 2021, 22, 9487. [Google Scholar] [CrossRef]
- Renga, G.; D’Onofrio, F.; Pariano, M.; Galarini, R.; Barola, C.; Stincardini, C.; Bellet, M.M.; Ellemunter, H.; Lass-Florl, C.; Costantini, C.; et al. Bridging of host-microbiota tryptophan partitioning by the serotonin pathway in fungal pneumonia. Nat. Commun. 2023, 14, 5753, Correction in Nat. Commun. 2024, 15, 3541. https://doi.org/10.1038/s41467-024-48040-7. [Google Scholar] [CrossRef]
- Wang, S.; van Schooten, F.J.; Jin, H.; Jonkers, D.; Godschalk, R. The Involvement of Intestinal Tryptophan Metabolism in Inflammatory Bowel Disease Identified by a Meta-Analysis of the Transcriptome and a Systematic Review of the Metabolome. Nutrients 2023, 15, 2886. [Google Scholar] [CrossRef]
- Coates, M.D.; Mahoney, C.R.; Linden, D.R.; Sampson, J.E.; Chen, J.; Blaszyk, H.; Crowell, M.D.; Sharkey, K.A.; Gershon, M.D.; Mawe, G.M.; et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology 2004, 126, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Coates, M.D.; Tekin, I.; Vrana, K.E.; Mawe, G.M. Review article: The many potential roles of intestinal serotonin (5-hydroxytryptamine, 5-HT) signalling in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2017, 46, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Ghia, J.E.; Li, N.; Wang, H.; Collins, M.; Deng, Y.; El-Sharkawy, R.T.; Cote, F.; Mallet, J.; Khan, W.I. Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology 2009, 137, 1649–1660. [Google Scholar] [CrossRef]
- Linden, D.R.; Chen, J.X.; Gershon, M.D.; Sharkey, K.A.; Mawe, G.M. Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, G207–G216. [Google Scholar] [CrossRef]
- Manocha, M.; Khan, W.I. Serotonin and GI Disorders: An Update on Clinical and Experimental Studies. Clin. Transl. Gastroenterol. 2012, 3, e13. [Google Scholar] [CrossRef] [PubMed]
- Winkle, M.; El-Daly, S.M.; Fabbri, M.; Calin, G.A. Noncoding RNA therapeutics—Challenges and potential solutions. Nat. Rev. Drug Discov. 2021, 20, 629–651. [Google Scholar] [CrossRef]
- Loganathan, T.; Doss, C.G. Non-coding RNAs in human health and disease: Potential function as biomarkers and therapeutic targets. Funct. Integr. Genom. 2023, 23, 33. [Google Scholar] [CrossRef]
- Nemeth, K.; Bayraktar, R.; Ferracin, M.; Calin, G.A. Non-coding RNAs in disease: From mechanisms to therapeutics. Nat. Rev. Genet. 2024, 25, 211–232. [Google Scholar] [CrossRef]
- Anastasiadou, E.; Jacob, L.S.; Slack, F.J. Non-coding RNA networks in cancer. Nat. Rev. Cancer 2018, 18, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Chini, A.; Guha, P.; Malladi, V.S.; Guo, Z.; Mandal, S.S. Novel long non-coding RNAs associated with inflammation and macrophage activation in human. Sci. Rep. 2023, 13, 4036. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Wang, W.; Xing, Y.; Wang, T.; Xu, X.; Wang, J. NF-kappaB target microRNAs and their target genes in TNFalpha-stimulated HeLa cells. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2014, 1839, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Liu, C.; Zou, X.; Wu, W.; Zhang, C.; Yuan, D. MiRNA-194 Regulates Palmitic Acid-Induced Toll-Like Receptor 4 Inflammatory Responses in THP-1 Cells. Nutrients 2015, 7, 3483–3496. [Google Scholar] [CrossRef]
- Zhang, P.; Cao, L.; Zhou, R.; Yang, X.; Wu, M. The lncRNA Neat1 promotes activation of inflammasomes in macrophages. Nat. Commun. 2019, 10, 1495. [Google Scholar] [CrossRef]
- Lin, X.; Lu, Y.; Zhang, C.; Cui, Q.; Tang, Y.D.; Ji, X.; Cui, C. LncRNADisease v3.0: An updated database of long non-coding RNA-associated diseases. Nucleic Acids Res. 2024, 52, D1365–D1369. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X. miRDB: An online database for prediction of functional microRNA targets. Nucleic Acids Res. 2020, 48, D127–D131. [Google Scholar] [CrossRef]
- Liu, W.; Wang, X. Prediction of functional microRNA targets by integrative modeling of microRNA binding and target expression data. Genome Biol. 2019, 20, 18. [Google Scholar] [CrossRef]
- Li, J.H.; Liu, S.; Zhou, H.; Qu, L.H.; Yang, J.H. starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014, 42, D92–D97. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Shajib, M.S.; Chauhan, U.; Adeeb, S.; Chetty, Y.; Armstrong, D.; Halder, S.L.S.; Marshall, J.K.; Khan, W.I. Characterization of Serotonin Signaling Components in Patients with Inflammatory Bowel Disease. J. Can. Assoc. Gastroenterol. 2019, 2, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Akopian, A.A.; Arutiunian, M.V.; Agavelian, A.M. The role of monoamine oxidase in large intestine pathology. Vopr. Meditsinskoi Khimii 1994, 40, 54–57. [Google Scholar]
- Cathcart, M.K.; Bhattacharjee, A. Monoamine oxidase A (MAO-A): A signature marker of alternatively activated monocytes/macrophages. Inflamm. Cell Signal. 2014, 1, e161. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Tang, A.; Wang, X.; Chen, X.; Zhao, L.; Xiao, Z.; Shen, S. Inhibition of lncRNA NEAT1 suppresses the inflammatory response in IBD by modulating the intestinal epithelial barrier and by exosome-mediated polarization of macrophages. Int. J. Mol. Med. 2018, 42, 2903–2913. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Liu, R.; Wu, X.; Ma, K.; Luo, W.; Nie, K.; Zhang, C.; Meng, X.; Tong, T.; Chen, X.; et al. LncRNA NEAT1 mediates intestinal inflammation by regulating TNFRSF1B. Ann. Transl. Med. 2021, 9, 773. [Google Scholar] [CrossRef]
- Jagt, J.Z.; van Rheenen, P.F.; Thoma, S.M.A.; Gower, J.; Reimering-Hartgerink, P.B.; van der Wielen, H.; van Steenbergen, E.J.; Goutbeek, A.M.; van Dijk-Lokkart, E.M.; Vlietstra, S.; et al. The top 10 research priorities for inflammatory bowel disease in children and young adults: Results of a James Lind Alliance Priority Setting Partnership. Lancet Gastroenterol. Hepatol. 2023, 8, 690–691. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef]
- Grondin, J.A.; Khan, W.I. Emerging Roles of Gut Serotonin in Regulation of Immune Response, Microbiota Composition and Intestinal Inflammation. J. Can. Assoc. Gastroenterol. 2024, 7, 88–96. [Google Scholar] [CrossRef]
- Hatamnejad, M.R.; Baradaran Ghavami, S.; Shirvani, M.; Asghari Ahmadabad, M.; Shahrokh, S.; Farmani, M.; Sherkat, G.; Asadzadeh Aghdaei, H.; Zali, M.R. Selective serotonin reuptake inhibitors and inflammatory bowel disease; Beneficial or malpractice. Front. Immunol. 2022, 13, 980189. [Google Scholar] [CrossRef]
- Ba, D.M.; Yadav, S.; Liu, G.; Leslie, D.L.; Vrana, K.E.; Coates, M.D. Clinical outcomes associated with antidepressant use in inflammatory bowel disease patients and a matched control cohort. Sci. Rep. 2024, 14, 1060. [Google Scholar] [CrossRef]
- Haq, S.; Wang, H.; Grondin, J.; Banskota, S.; Marshall, J.K.; Khan, I.I.; Chauhan, U.; Cote, F.; Kwon, Y.H.; Philpott, D.; et al. Disruption of autophagy by increased 5-HT alters gut microbiota and enhances susceptibility to experimental colitis and Crohn’s disease. Sci. Adv. 2021, 7, eabi6442. [Google Scholar] [CrossRef]
- Manzella, C.R.; Jayawardena, D.; Pagani, W.; Li, Y.; Alrefai, W.A.; Bauer, J.; Jung, B.; Weber, C.R.; Gill, R.K. Serum Serotonin Differentiates Between Disease Activity States in Crohn’s Patients. Inflamm. Bowel Dis. 2020, 26, 1607–1618. [Google Scholar] [CrossRef]
- Sanidad, K.Z.; Rager, S.L.; Carrow, H.C.; Ananthanarayanan, A.; Callaghan, R.; Hart, L.R.; Li, T.; Ravisankar, P.; Brown, J.A.; Amir, M.; et al. Gut bacteria-derived serotonin promotes immune tolerance in early life. Sci. Immunol. 2024, 9, eadj4775. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Wang, H.; Terc, J.D.; Zambrowicz, B.; Yang, Q.M.; Khan, W.I. Blocking peripheral serotonin synthesis by telotristat etiprate (LX1032/LX1606) reduces severity of both chemical- and infection-induced intestinal inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G455–G465. [Google Scholar] [CrossRef] [PubMed]
- Margolis, K.G.; Stevanovic, K.; Li, Z.; Yang, Q.M.; Oravecz, T.; Zambrowicz, B.; Jhaver, K.G.; Diacou, A.; Gershon, M.D. Pharmacological reduction of mucosal but not neuronal serotonin opposes inflammation in mouse intestine. Gut 2014, 63, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Minderhoud, I.M.; Oldenburg, B.; Schipper, M.E.; ter Linde, J.J.; Samsom, M. Serotonin synthesis and uptake in symptomatic patients with Crohn’s disease in remission. Clin. Gastroenterol. Hepatol. 2007, 5, 714–720. [Google Scholar] [CrossRef]
- Jorandli, J.W.; Thorsvik, S.; Skovdahl, H.K.; Kornfeld, B.; Saeterstad, S.; Gustafsson, B.I.; Sandvik, A.K.; van Beelen Granlund, A. The serotonin reuptake transporter is reduced in the epithelium of active Crohn’s disease and ulcerative colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 319, G761–G768. [Google Scholar] [CrossRef]
- Bai, J.; Wang, Y.; Li, F.; Wu, Y.; Chen, J.; Li, M.; Wang, X.; Lv, B. Research advancements and perspectives of inflammatory bowel disease: A comprehensive review. Sci. Prog. 2024, 107, 368504241253709. [Google Scholar] [CrossRef]
- Heydari, R.; Karimi, P.; Meyfour, A. Long non-coding RNAs as pathophysiological regulators, therapeutic targets and novel extracellular vesicle biomarkers for the diagnosis of inflammatory bowel disease. Biomed. Pharmacother. 2024, 176, 116868. [Google Scholar] [CrossRef]
- Hu, Y.; Lu, Y.; Fang, Y.; Zhang, Q.; Zheng, Z.; Zheng, X.; Ye, X.; Chen, Y.; Ding, J.; Yang, J. Role of long non-coding RNA in inflammatory bowel disease. Front. Immunol. 2024, 15, 1406538. [Google Scholar] [CrossRef]
- Jiang, F.; Wu, M.; Li, R. The significance of long non-coding RNAs in the pathogenesis, diagnosis and treatment of inflammatory bowel disease. Precis. Clin. Med. 2023, 6, pbad031. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, H.; Fu, J.; Fan, L.; Lu, S.; Zhang, H.; Liu, Z. Knockdown of long non-coding RNA NEAT1 relieves inflammation of ulcerative colitis by regulating the miR-603/FGF9 pathway. Exp. Ther. Med. 2022, 23, 131. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, Z.; Liu, K.; Yang, X.; Zou, H.; Zhou, J.; Miao, X.; Chen, W.; Xiong, L.; Wen, Y. Neat1-miRNA204-5p-PI3K-AKT axis as a potential mechanism for photodynamic therapy treated colitis in mice. Photodiagn. Photodyn. Ther. 2018, 24, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.; Liu, Y.; Zhong, J.; Shen, Y. Inhibition of LncRNA-NEAT1 alleviates intestinal epithelial cells (IECs) dysfunction in ulcerative colitis by maintaining the homeostasis of the glucose metabolism through the miR-410-3p-LDHA axis. Bioengineered 2022, 13, 8961–8971. [Google Scholar] [CrossRef]
- Levine, A.; Koletzko, S.; Turner, D.; Escher, J.C.; Cucchiara, S.; de Ridder, L.; Kolho, K.L.; Veres, G.; Russell, R.K.; Paerregaard, A.; et al. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 795–806. [Google Scholar] [CrossRef]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A free online platform for data visualization and graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef]
- Chomczynski, P.; Sacchi, N. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: Twenty-something years on. Nat. Protoc. 2006, 1, 581–585. [Google Scholar] [CrossRef]
- Huang, Z.; Fasco, M.J.; Kaminsky, L.S. Optimization of Dnase I removal of contaminating DNA from RNA for use in quantitative RNA-PCR. Biotechniques 1996, 20 , 1012–1014, 1016, 1018–1020. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Removal of small fragments of nucleic Acid from preparations of plasmid DNA by precipitation with lithium chloride. CSH Protoc. 2006, 2006, pdb-prot3918. [Google Scholar] [CrossRef]
- Zeybek, A.; Oz, N.; Kalemci, S.; Edgunlu, T.; Kiziltug, M.T.; Tosun, K.; Tunc, M.; Tekin, L.; Erdal, M.E. Diagnostic Value of MiR-125b as a Potential Biomarker for Stage I Lung Adenocarcinoma. Curr. Mol. Med. 2019, 19, 216–227. [Google Scholar] [CrossRef]
- Zeybek, A.; Oz, N.; Kalemci, S.; Tosun, K.; Edgunlu, T.G.; Kiziltug, M.T.; Tekin, L.; Erdal, M.E. The role of Wnt pathway antagonists in early-stage lung adenocarcinoma. Mol. Biol. Rep. 2022, 49, 9–17. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
| Healthy Controls | Patients with pIBD | |
|---|---|---|
| Participants | 22 | 22 |
| Mean age in years | 13.09 ± 4.13 | 14.45 ± 2.75 |
| % Female | 40.9 | 45.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiziltug, M.T.; Erdal, M.E.; Tasdelen, B.; Tuncel, F.; Usta, Y. Expression Alterations and Correlative Analysis of TPH1/hsa-miR-194-5p/NEAT1 and MAOA/hsa-miR-1276/NEAT1 Axes in Pediatric Inflammatory Bowel Disease. Int. J. Mol. Sci. 2025, 26, 11923. https://doi.org/10.3390/ijms262411923
Kiziltug MT, Erdal ME, Tasdelen B, Tuncel F, Usta Y. Expression Alterations and Correlative Analysis of TPH1/hsa-miR-194-5p/NEAT1 and MAOA/hsa-miR-1276/NEAT1 Axes in Pediatric Inflammatory Bowel Disease. International Journal of Molecular Sciences. 2025; 26(24):11923. https://doi.org/10.3390/ijms262411923
Chicago/Turabian StyleKiziltug, Mehmet Tughan, Mehmet Emin Erdal, Bahar Tasdelen, Ferah Tuncel, and Yusuf Usta. 2025. "Expression Alterations and Correlative Analysis of TPH1/hsa-miR-194-5p/NEAT1 and MAOA/hsa-miR-1276/NEAT1 Axes in Pediatric Inflammatory Bowel Disease" International Journal of Molecular Sciences 26, no. 24: 11923. https://doi.org/10.3390/ijms262411923
APA StyleKiziltug, M. T., Erdal, M. E., Tasdelen, B., Tuncel, F., & Usta, Y. (2025). Expression Alterations and Correlative Analysis of TPH1/hsa-miR-194-5p/NEAT1 and MAOA/hsa-miR-1276/NEAT1 Axes in Pediatric Inflammatory Bowel Disease. International Journal of Molecular Sciences, 26(24), 11923. https://doi.org/10.3390/ijms262411923








