Association of Hemoglobin and Myoglobin into Supramolecular Complexes: Significance for Life and Practice
Abstract
1. Introduction
2. Protein Self-Association as a Cellular Response to Stress
2.1. Liquid–Liquid Phase Separation in the Cytoplasm
2.2. Self-Association as a Molecular Adaptation Strategy
3. Factors of Spontaneous Protein Self-Organization
3.1. Amyloidogenic Aggregation of Proteins
- (1)
- The ability to undergo conformational transition into a β-sheet-rich structure;
- (2)
- Insolubility and resistance to proteolysis, heat, and extreme pH;
- (3)
- Specific binding with diagnostic dyes such as Congo red and thioflavin T.
3.2. Mechanisms Initiating Amyloidogenesis
3.3. Aggregation of Hemoglobin and Myoglobin: From Physiological Regulation to Pathological Amyloidogenesis
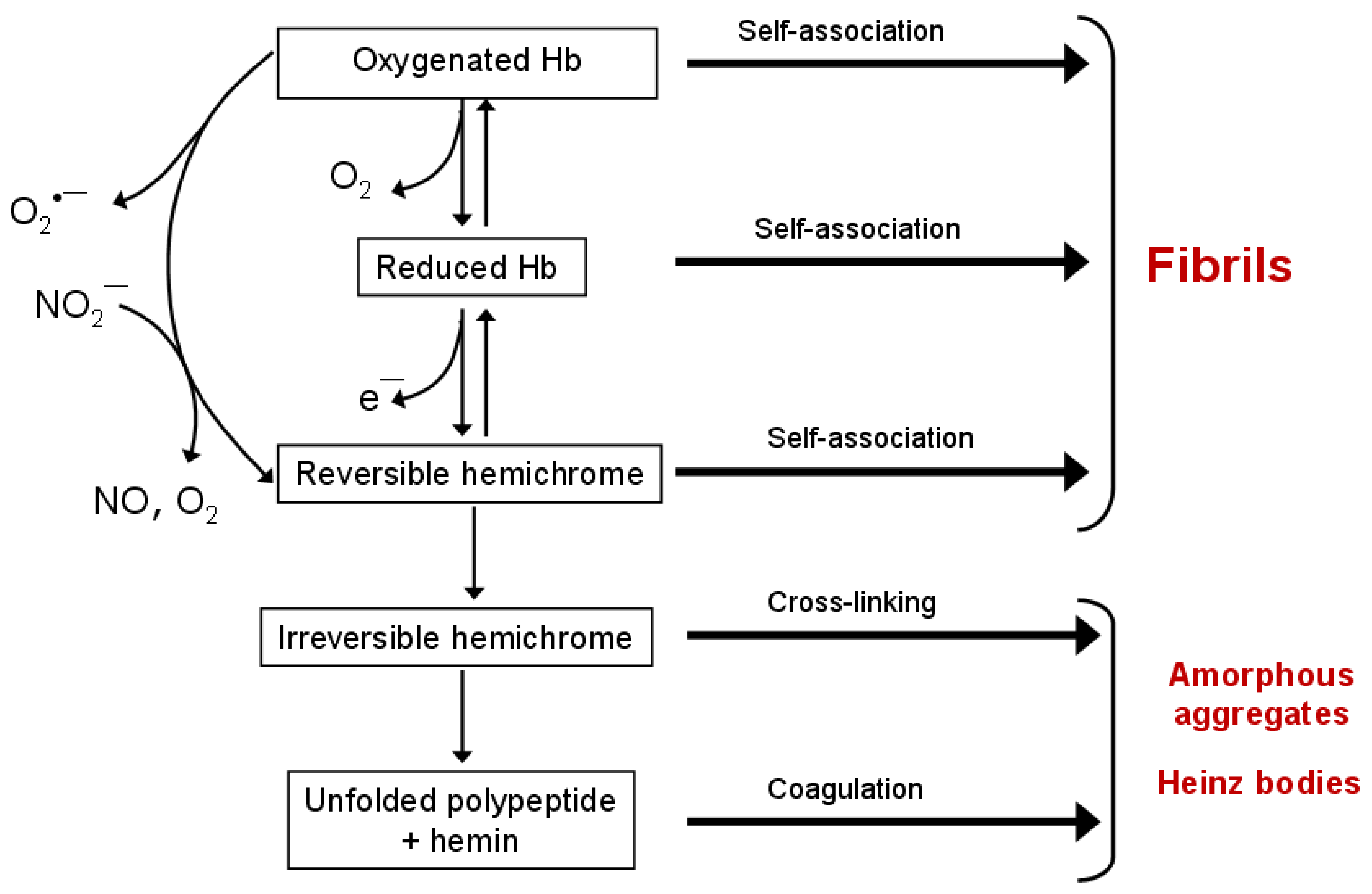
3.3.1. Factors Promoting Hb and Mb Aggregation
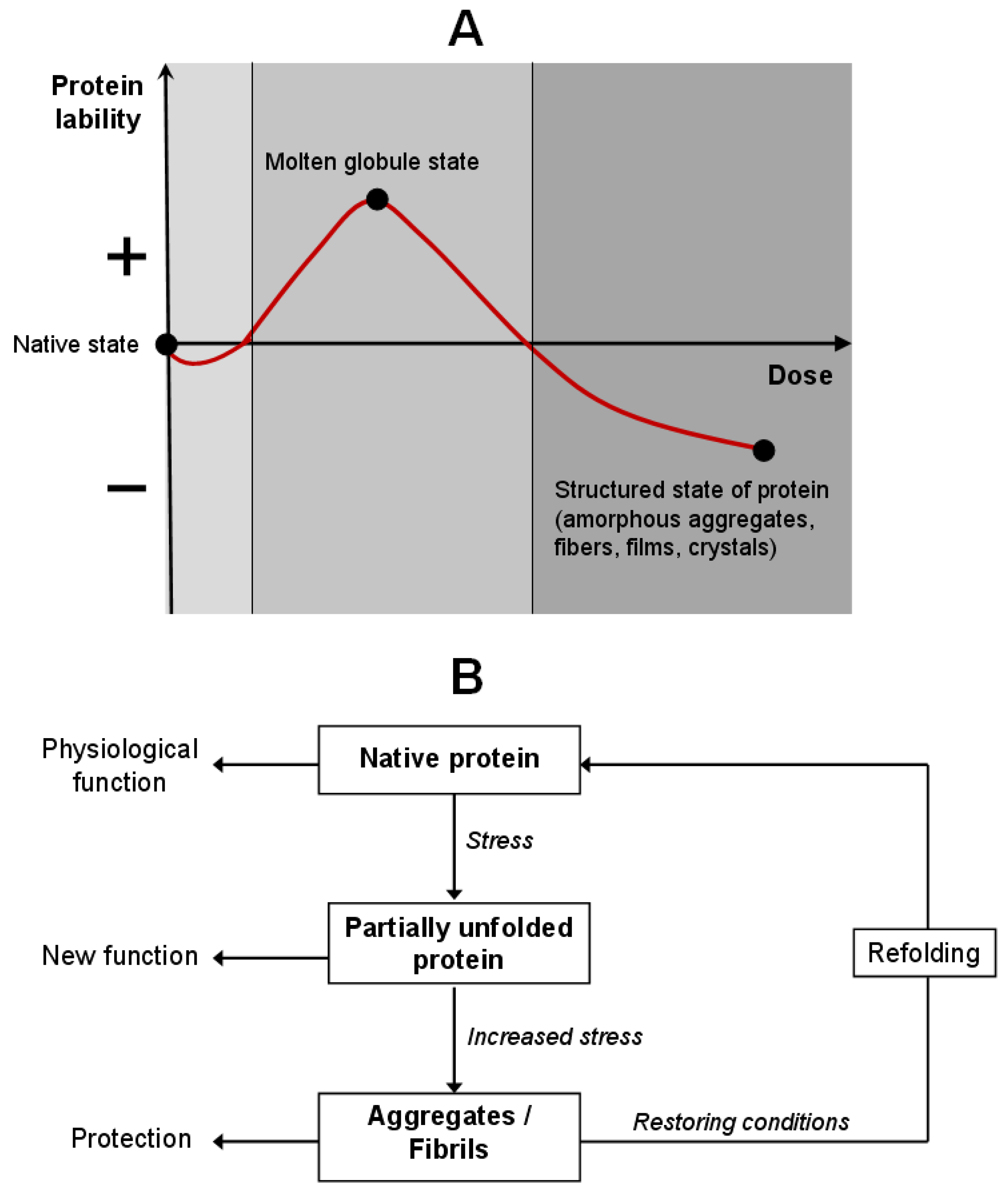
3.3.2. Intermediate States: Hemichrome and Molten Globule
4. Polymerization of Hemoglobin and Myoglobin via Intermolecular Covalent Cross-Linking
4.1. Aggregation via Disulfide Bonds and Metal Ions
4.2. Hb Aggregation via Chemical Cross-Linking Agents
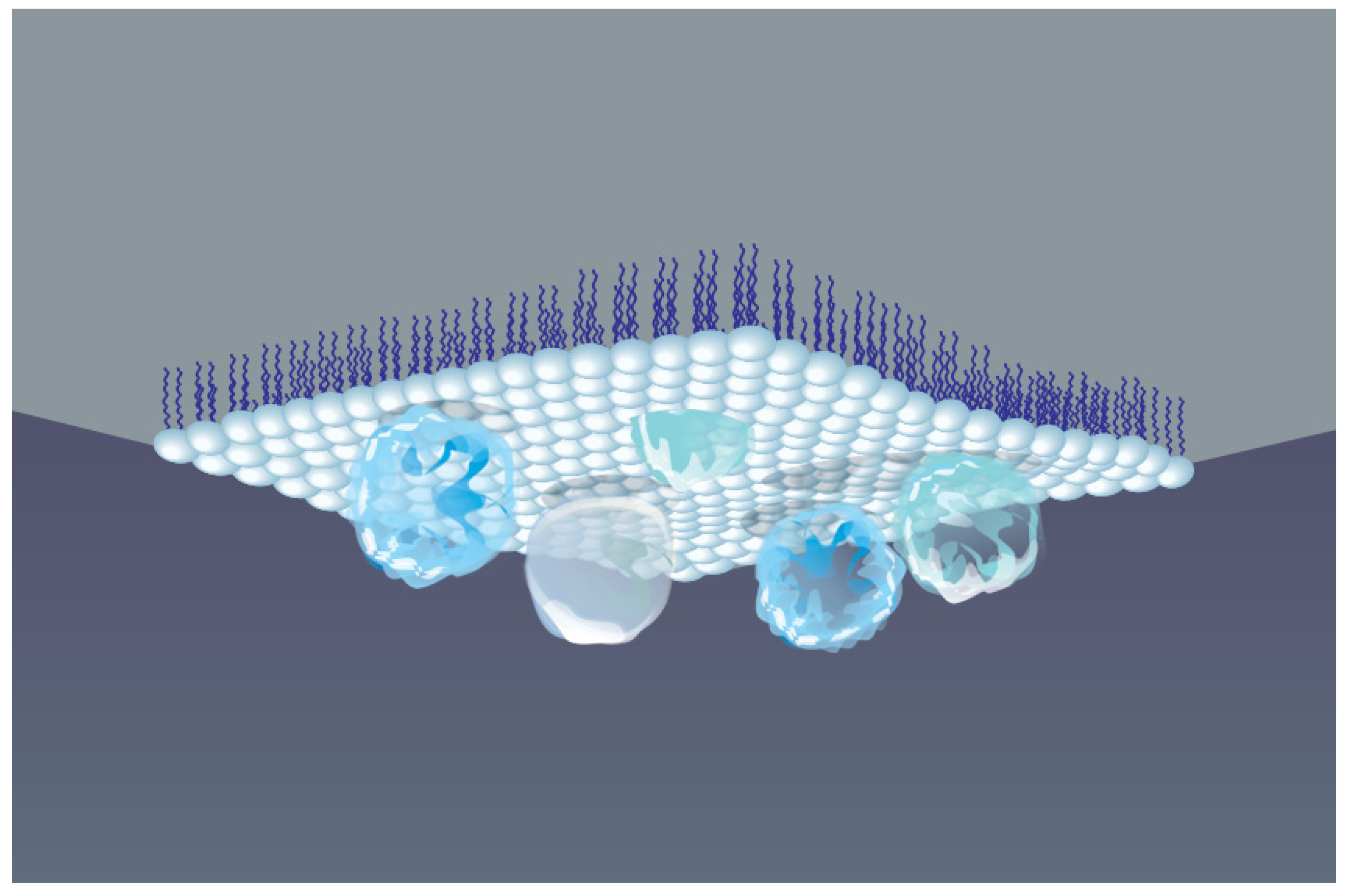
5. Association of Hemoglobin and Myoglobin at the Air–Water Interface
5.1. Protein Adsorption and Conformational Changes
- Unfolding (denaturation) of the native structure, exposing buried hydrophobic domains;
- Alteration of secondary structure: reduction in α-helix content and an increase in β-sheet elements;
- Enhanced aggregation due to local concentration effects and a decrease in electrostatic repulsion.
- Concentration effect: Local enrichment of protein at the interface;
5.2. Hemoglobin Behavior at AWI
6. Functional Nanomaterials Based on Hemoglobin and Myoglobin Fibrils
6.1. Applications of Hb/Mb Fibrils in Nanobiotechnology
6.2. ROS-Modulating Activity of Hb and Mb: Application in Biotechnology
electron donor*: e.g., TMB or ABTS
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Munder, M.C.; Midtvedt, D.; Franzmann, T.; Nüske, E.; Otto, O.; Herbig, M.; Ulbricht, E.; Müller, P.; Taubenberger, A.; Maharana, S.; et al. A pH-driven transition of the cytoplasm from a fluid- to a solid-like state promotes entry into dormancy. eLife 2016, 5, e09347. [Google Scholar] [CrossRef] [PubMed]
- Romero-Perez, P.S.; Dorone, Y.; Flores, E.; Sukenik, S.; Boeynaems, S. When Phased without Water: Biophysics of Cellular Desiccation, from Biomolecules to Condensates. Chem. Rev. 2023, 123, 9010–9035. [Google Scholar] [CrossRef] [PubMed]
- Marijan, D.; Tse, R.; Elliott, K.; Chandhok, S.; Luo, M.; Lacroix, E.; Audas, T.E. Stress-Specific Aggregation of Proteins in the Amyloid Bodies. FEBS Lett. 2019, 593, 3162–3172. [Google Scholar] [CrossRef] [PubMed]
- Campos, L.A.; Sharma, R.; Alvira, S.; Ruiz, F.M.; Ibarra-Molero, B.; Sadqi, M.; Alfonso, C.; Rivas, G.; Sanchez-Ruiz, J.M.; Garrido, A.R.; et al. Engineering protein assemblies with allosteric control via monomer fold-switching. Nat. Commun. 2019, 10, 5703. [Google Scholar] [CrossRef]
- Sharma, P.R. Red Cell Indices. In Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990; ISBN 0-409-90077-X. [Google Scholar]
- Giardine, B.; van Baal, S.; Kaimakis, P.; Riemer, C.; Miller, W.; Samara, M.; Kollia, P.; Anagnou, N.P.; Chui, D.H.K.; Wajcman, H.; et al. HbVar Database of Human Hemoglobin Variants and Thalassemia Mutations: 2007 Update. Hum. Mutat. 2007, 28, 206. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology Modelling of Protein Structures and Complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Novikova, N.N.; Kovalchuk, M.V.; Yurieva, E.A.; Konovalov, O.V.; Stepina, N.D.; Rogachev, A.V.; Yalovega, G.E.; Kosmachevskaya, O.V.; Topunov, A.F.; Yakunin, S.N. The enhancement of metal-binding properties in hemoglobin: The role of mild damaging factors. J. Phys. Chem. B 2019, 123, 8370–8377. [Google Scholar] [CrossRef]
- Kosmachevskaya, O.V.; Topunov, A.F. Nonenzymatic reactions in metabolism: Their role in evolution and adaptation. Appl. Biochem. Microbiol. 2021, 57, 543–555. [Google Scholar] [CrossRef]
- Kosmachevskaya, O.V.; Nasybullina, E.I.; Blindar, V.N.; Topunov, A.F. Binding of erythrocyte hemoglobin to the membrane to realize signal-regulatory function. Appl. Biochem. Microbiol. 2019, 55, 83–98. [Google Scholar] [CrossRef]
- Shin, Y.; Brangwynne, C.P. Liquid Phase Condensation in Cell Physiology and Disease. Science 2017, 357, eaaf4382. [Google Scholar] [CrossRef]
- Rabouille, C.; Alberti, S. Cell Adaptation upon Stress: The Emerging Role of Membrane-less Compartments. Curr. Opin. Cell Biol. 2017, 47, 34–42. [Google Scholar] [CrossRef]
- van Tartwijk, F.W.; Kaminski, C.F. Protein Condensation, Cellular Organization, and Spatiotemporal Regulation of Cytoplasmic Properties. Adv. Biol. 2022, 6, 2101328. [Google Scholar] [CrossRef] [PubMed]
- Minsky, A.; Shimoni, E.; Frenkiel-Krispin, D. Stress, order and survival. Nat. Rev. Mol. Cell Biol. 2002, 3, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Parry, B.R.; Surovtsev, I.V.; Cabeen, M.T.; O’Hern, C.S.; Dufresne, E.R.; Jacobs-Wagner, C. The bacterial cytoplasm has glass-like properties and is fluidized by metabolic activity. Cell 2014, 156, 183–194. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, J.D.; Zhao, A.; Ellington, A.D.; Marcotte, E.M. Dynamic reorganization of metabolic enzymes into intracellular bodies. Annu. Rev. Cell Dev. Biol. 2012, 28, 89–111. [Google Scholar] [CrossRef]
- Garcia-Seisdedos, H.; Villegas, J.A.; Levy, E.D. Infinite assembly of folded proteins in evolution, disease, and engineering. Angew. Chem. Int. Ed. 2019, 58, 5514–5531. [Google Scholar] [CrossRef]
- Petrovska, I.; Nüske, E.; Munder, M.C.; Kulasegaran, G.; Malinovska, L.; Kroschwald, S.; Richter, D.; Fahmy, K.; Gibson, K.; Verbavatz, J.-M. Filament formation by metabolic enzymes is a specific adaptation to an advanced state of cellular starvation. eLife 2014, 3, e02409. [Google Scholar] [CrossRef]
- Spinelli, S.; Marino, A.; Morabito, R.; Remigante, A. Interplay Between Metabolic Pathways and Increased Oxidative Stress in Human Red Blood Cells. Cells 2024, 13, 2026. [Google Scholar] [CrossRef]
- Englander, S.W.; Mayne, L.; Krishna, M.M.G. Protein Folding and Misfolding: Mechanism and Principles. Q. Rev. Biophys. 2008, 40, 287–326. [Google Scholar] [CrossRef]
- Uversky, V.N. Protein Intrinsic Disorder and Adaptation to Extreme Environments: Resilience of Chaos. J. Mol. Biol. 2025, 169547. [Google Scholar] [CrossRef]
- Agathokleous, E.; Calabrese, E.J. Hormesis: A General Biological Principle. Chem. Res. Toxicol. 2022, 35, 547–549. [Google Scholar] [CrossRef]
- Costantini, D. Hormesis Promotes Evolutionary Change. Dose-Response 2019, 17, 1559325819843376. [Google Scholar] [CrossRef]
- Tokuriki, N.; Tawfik, D.S. Protein Dynamism and Evolvability. Science 2009, 324, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Bychkova, V.E.; Basova, L.V.; Balobanov, V.A. How membrane surface affects protein structure. Biochemistry 2014, 79, 1483–1514. [Google Scholar] [CrossRef] [PubMed]
- Sicorello, A.; Torrassa, S.; Soldi, G.; Gianni, S.; Travaglini-Allocatelli, C.; Taddei, N.; Relini, A.; Chiti, F. Agitation and high ionic strength induce amyloidogenesis of a folded PDZ domain in native conditions. Biophys. J. 2009, 96, 2289–2298. [Google Scholar] [CrossRef] [PubMed]
- Vettore, N.; Buell, A.K. Thermodynamics of amyloid fibril formation from chemical depolymerization. Phys. Chem. Chem. Phys. 2019, 21, 26184–26194. [Google Scholar] [CrossRef]
- Boothby, T.C.; Tapia, H.; Brozena, A.H.; Piszkiewicz, S.; Smith, A.E.; Giovannini, I.; Rebecchi, L.; Pielak, G.J.; Koshland, D.; Goldstein, B. Tardigrades Use Intrinsically Disordered Proteins to Survive Desiccation. Mol. Cell 2017, 65, 975–984.e5. [Google Scholar] [CrossRef]
- Sunde, M.; Blake, C.C.F. From the globular to the fibrous state: Protein structure and structural conversion in amyloid formation. Q. Rev. Biophys. 1998, 31, 1–39. [Google Scholar] [CrossRef]
- Dobson, C.M. The structural basis of protein folding and its links with human disease. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001, 356, 133–145. [Google Scholar] [CrossRef]
- Fändrich, M.; Dobson, C.M. The behaviour of polyamino acids reveals an inverse side chain effect in amyloid structure formation. EMBO J. 2002, 21, 5682–5690. [Google Scholar] [CrossRef] [PubMed]
- Miti, T.; Mulaj, M.; Schmit, J.D.; Muschol, M. Stable, metastable, and kinetically trapped amyloid aggregate phases. Biomacromolecules 2015, 16, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Sirangelo, I.; Iannuzzi, C. Understanding the role of protein glycation in the amyloid aggregation process. Int. J. Mol. Sci. 2021, 22, 6609. [Google Scholar] [CrossRef] [PubMed]
- Hartl, F.U. Protein misfolding diseases. Annu. Rev. Biochem. 2017, 86, 21–26. [Google Scholar] [CrossRef]
- Gonzalez-Garcia, M.; Fusco, G.; De Simone, A. Membrane interactions and toxicity by misfolded protein oligomers. Front. Cell Dev. Biol. 2021, 9, 642623. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, H.; Qin, X. Amyloid Fibrils and Their Applications: Current Status and Latest Developments. Nanomaterials 2025, 15, 255. [Google Scholar] [CrossRef]
- Vekilov, P.G. Sickle-cell haemoglobin polymerization: Is it the primary pathogenic event of sickle-cell anaemia? Br. J. Haematol. 2007, 139, 173–184. [Google Scholar] [CrossRef]
- Chiti, F.; Dobson, C.M. Protein misfolding, amyloid formation, and human disease: A summary of progress over the last decade. Annu. Rev. Biochem. 2017, 86, 27–68. [Google Scholar] [CrossRef]
- Knowles, T.P.J.; Mezzenga, R. Amyloid fibrils as building blocks for natural and artificial functional materials. Adv. Mater. 2016, 28, 6546–6561. [Google Scholar] [CrossRef]
- Maji, S.K.; Perrin, M.H.; Sawaya, M.R.; Jessberger, S.; Vadodaria, K.; Rissman, R.A.; Singru, P.S.; Nilsson, K.P.R.; Simon, R.; Schubert, D.; et al. Functional Amyloids as Natural Storage of Peptide Hormones in Pituitary Secretory Granules. Science 2009, 325, 328–332. [Google Scholar] [CrossRef]
- Franco, E.M.; Alves, L.A.; Naveed, H.; Freitas, V.A.A.; Bastos, D.C.; Mattos-Graner, R.O. Amyloid Fibrils Produced by Streptococcus sanguinis Contribute to Biofilm Formation and Immune Evasion. Int. J. Mol. Sci. 2023, 24, 15686. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Wang, Y.; Yu, H.; Liu, R.; Leppert, A.; Zheng, Z.; Zhong, X.; Jin, Z.; Wang, H.; Li, X.; et al. Spider Silk Protein Forms Amyloid-Like Nanofibrils through a Non-Nucleation-Dependent Polymerization Mechanism. Small 2023, 19, e2304031. [Google Scholar] [CrossRef]
- Santos, M.R.; Henriques, B.J.; Santos, R. Amyloid-Like Structures in Marine Adhesive Proteins. Mar. Drugs 2025, 23, 363. [Google Scholar] [CrossRef]
- Liu-Smith, F.; Poe, C.; Farmer, P.J.; Meyskens, F.L., Jr. Amyloids, Melanins and Oxidative Stress in Melanomagenesis. Exp. Dermatol. 2015, 24, 171–174. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Protein glycation—Biomarkers of metabolic dysfunction and early-stage decline in health in the era of precision medicine. Redox Biol. 2021, 42, 101920. [Google Scholar] [CrossRef]
- Kosmachevskaya, O.V.; Novikova, N.N.; Topunov, A.F. Carbonyl stress in red blood cells and hemoglobin. Antioxidants 2021, 10, 253. [Google Scholar] [CrossRef]
- Lee, J.H.; Samsuzzaman, M.; Park, M.G.; Park, S.J.; Kim, S.Y. Methylglyoxal-derived hemoglobin advanced glycation end products induce apoptosis and oxidative stress in human umbilical vein endothelial cells. Int. J. Biol. Macromol. 2021, 187, 409–421. [Google Scholar] [CrossRef]
- Sun, F.; Suttapitugsakul, S.; Xiao, H.; Wu, R. Comprehensive analysis of protein glycation reveals its potential impacts on protein degradation and gene expression in human cells. J. Am. Soc. Mass Spectrom. 2019, 30, 2480–2490. [Google Scholar] [CrossRef]
- Sharma, C.; Kim, S.R. Linking oxidative stress and proteinopathy in Alzheimer’s disease. Antioxidants 2021, 10, 1231. [Google Scholar] [CrossRef] [PubMed]
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2021, 11, 194–222. [Google Scholar] [CrossRef] [PubMed]
- Kalapos, M.P. The tandem of free radicals and methylglyoxal. Chem.-Biol. Interact. 2008, 171, 251–271. [Google Scholar] [CrossRef]
- Allsop, D.; Mayes, J.; Moore, S.; Masad, A.; Tabner, B.J. Metal-dependent generation of reactive oxygen species from amyloid proteins implicated in neurodegenerative disease. Biochem. Soc. Trans. 2008, 36, 1293–1298. [Google Scholar] [CrossRef]
- Salgado, E.N.; Ambroggio, X.I.; Brodin, J.D.; Lewis, R.A.; Kuhlman, B.; Tezcan, F.A. Metal templated design of protein interfaces. Proc. Natl. Acad. Sci. USA 2010, 107, 1827–1832. [Google Scholar] [CrossRef]
- Degtyar, E.; Harrington, M.J.; Politi, Y.; Fratzl, P. The mechanical role of metal ions in biogenic protein based materials. Angew. Chem. Int. Ed. 2014, 53, 12026–12044. [Google Scholar] [CrossRef]
- Soraruf, D.; Roosen-Runge, F.; Grimaldo, M.; Zanini, F.; Schweins, R.; Seydel, T.; Zhang, F.; Roth, R.; Oettel, M.; Schreiber, F. Protein cluster formation in aqueous solution in the presence of multivalent metal ions—A light scattering study. Soft Matter 2014, 10, 894–902. [Google Scholar] [CrossRef]
- Wolf, M. Effective Interactions in Liquid-Liquid Phase Separated Protein Solutions Induced by Multivalent Ions. Ph.D. Thesis, Eberhard Karls Universität Tübingen, Tübingen, Germany, 2015. [Google Scholar] [CrossRef]
- Jayawardena, N.; Kaur, M.; Nair, S.; Malmstrom, J.; Goldstone, D.; Negron, L.; Gerrar, J.A.; Domigan, L.J. Amyloid fibrils from hemoglobin. Biomolecules 2017, 7, 37. [Google Scholar] [CrossRef]
- Fändrich, M.; Forge, V.; Buder, K.; Kittler, M.; Dobson, C.M.; Diekmann, S. Myoglobin forms amyloid fibrils by association of unfolded polypeptide segments. Proc. Natl. Acad. Sci. USA 2003, 100, 15463–15468. [Google Scholar] [CrossRef] [PubMed]
- White, J.G.; Heagan, B. Tubular polymers of normal human hemoglobin. Am. J. Pathol. 1970, 59, 101–114. [Google Scholar] [PubMed]
- Desnica, D. Self-association of hemoglobin: A dielectric dispersion study. Biopolymers 1979, 18, 1685–1694. [Google Scholar] [CrossRef]
- Brnjas-Kraljević, J.; Pifat, G.; Maricić, S. Quaternary structure, hydration, and self-association of hemoglobin. A proton magnetic relaxation study. Physiol. Chem. Phys. 1979, 11, 371–376. [Google Scholar] [PubMed]
- Minton, A.P.; Lewis, M.S. Self-association in highly concentrated solutions of myoglobin: A novel analysis of sedimentation equilibrium of highly nonideal solutions. Biophys. Chem. 1981, 14, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.S.; Riggs, A.F. Indefinite noncooperative self-association of chicken deoxy hemoglobin D. Proteins Struct. Funct. Bioinform. 2011, 79, 1499–1512. [Google Scholar] [CrossRef] [PubMed]
- Riggs, A.F. Self-association, cooperativity and supercooperativity of oxygen binding by hemoglobins. J. Exp. Biol. 1998, 201, 1073–1084. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Kang, S.; Lee, S.-G.; Jin, J.-H.; Park, J.W.; Park, S.M.; Jung, S.; Paik, S.R. Fibrillar superstructure formation of hemoglobin A and its conductive, photodynamic and photovoltaic effects. Acta Biomater. 2010, 6, 4689–4697. [Google Scholar] [CrossRef]
- Bishop, M.F.; Ferrone, F.A. The sickle-cell fiber revisited. Biomolecules 2023, 13, 413. [Google Scholar] [CrossRef]
- Borges da Silva, F.A.; Florindo, J.B.; Mattos, A.C.d.; Costa, F.F.; Lorand-Metze, I.; Metze, K. Accompanying hemoglobin polymerization in red blood cells in patients with sickle cell disease using fluorescence lifetime imaging. Int. J. Mol. Sci. 2024, 25, 12290. [Google Scholar] [CrossRef]
- Iannuzzi, C.; Vilasi, S.; Portaccio, M.; Irace, G.; Sirangelo, I. Heme binding inhibits the fibrillization of amyloidogenic apomyoglobin and determines lack of aggregate cytotoxicity. Protein Sci. 2007, 16, 507–516. [Google Scholar] [CrossRef]
- Imamura, H.; Morita, T.; Sumi, T.; Isogai, Y.; Kato, M.; Nishikawa, K. Modulation of the intermolecular interaction of myoglobin by removal of the heme. Synchrotron Radiat. 2013, 20, 919–922. [Google Scholar] [CrossRef]
- Iram, A.; Naeem, A. Detection and analysis of protofibrils and fibrils of hemoglobin: Implications for the pathogenesis and cure of heme loss related maladies. Arch. Biochem. Biophys. 2013, 533, 69–78. [Google Scholar] [CrossRef]
- Azami-Movahed, M.; Shariatizi, S.; Sabbaghian, M.; Ghasemi, A.; Ebrahim-Habibi, A.; Nemat-Gorgani, M. Heme binding site in apomyoglobin may be effectively targeted with small molecules to control aggregation. Int. J. Biochem. Cell Biol. 2013, 45, 299–307. [Google Scholar] [CrossRef]
- Azami-Movahed, M.; Meratan, A.A.; Ghasemi, A.; Ebrahim-Habibi, A.; Nemat-Gorgani, M. Acetylation of lysine residues in apomyoglobin: Structural changes, amyloid fibrillation, and role of surface charge. Int. J. Biol. Macromol. 2018, 107, 626–634. [Google Scholar] [CrossRef]
- Banerjee, S.; Maity, S.; Chakraborti, A.S. Methylglyoxal-induced modification causes aggregation of myoglobin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 155, 1–10. [Google Scholar] [CrossRef]
- Banerjee, S. Long-term incubation of myoglobin with glyoxal induces amyloid like aggregation of the heme protein: Implications of advanced glycation end products in protein conformational disorders. J. Mol. Liq. 2021, 326, 115256. [Google Scholar] [CrossRef]
- Iram, A.; Alam, T.; Khan, J.M.; Khan, T.A.; Khan, R.H.; Naeem, A. Molten globule of hemoglobin proceeds into aggregates and advanced glycated end products. PLoS ONE 2013, 8, e72075. [Google Scholar] [CrossRef] [PubMed]
- Hrynets, Y.; Ndagijimana, M.; Betti, M. Rapid myoglobin aggregation through glucosamine-induced α-dicarbonyl formation. PLoS ONE 2015, 10, e0139022. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, A.; Basu, S.; Singha, S.; Patra, H.K. Inner-view of nanomaterial incited protein conformational changes: Insights into designable interaction. Research 2018, 2018, 9712832. [Google Scholar] [CrossRef]
- Bhattacharya, J.; Choudhuri, U.; Siwach, O.; Sen, P.; Dasgupta, A.K. Interaction of hemoglobin and copper nanoparticles: Implications in hemoglobinopathy. Nanomed. Nanotechnol. Biol. Med. 2006, 2, 191–199. [Google Scholar] [CrossRef]
- Jennings, P.A.; Wright, P.E. Formation of a molten globule intermediate early in the kinetic folding pathway of apomyoglobin. Science 1993, 262, 892–896. [Google Scholar] [CrossRef]
- Culbertson, D.S.; Olson, J.S. Folding and assembly of myoglobins and hemoglobins. In Protein Folding and Metal Ions: Mechanisms, Biology, Disease; Wittung-Stafshede, P., Gomes, C.M., Eds.; Taylor and Francis, Inc.: Boca Raton, FL, USA, 2010; pp. 97–122. [Google Scholar]
- Vergara, A.; Vitagliano, L.; Verde, C.; di Prisco, G.; Mazzarella, L. Spectroscopic and crystallographic characterization of bis-histidyl adducts in tetrameric hemoglobins. Methods Enzymol. 2008, 436, 425–444. [Google Scholar] [CrossRef]
- Samuel, P.P.; White, M.A.; Ou, W.C.; Case, D.A.; Phillips, G.N.; Olson, J.S. The interplay between molten globules and heme disassociation defines human hemoglobin disassembly. Biophys. J. 2020, 118, 1381–1400. [Google Scholar] [CrossRef]
- Giles, M.M.; Watts, A.B.; Giles, G.I.; Fry, F.H.; Littlechild, J.A.; Jacob, C. Metal and Redox Modulation of Cysteine Protein Function. Chem. Biol. 2003, 10, 677–693. [Google Scholar] [CrossRef]
- Chung, H.S.; Wang, S.B.; Venkatraman, V.; Murray, C.I.; Van Eyk, J.E. Cysteine oxidative posttranslational modifications: Emerging regulation in the cardiovascular system. Circ. Res. 2013, 112, 382–392. [Google Scholar] [CrossRef]
- Petersen, A.G.; Petersen, S.V.; Frische, S.; Drakulic, S.; Golas, M.M.; Sander, B.; Fago, A. Hemoglobin polymerization via disulfide bond formation in the hypoxia-tolerant turtle Trachemys scripta: Implications for antioxidant defense and O2 transport. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R84–R93. [Google Scholar] [CrossRef]
- Fago, A. New insights into survival strategies to oxygen deprivation in anoxia- tolerant vertebrates. Acta Physiol. 2022, 235, e13841. [Google Scholar] [CrossRef]
- Baudin-Creuza, V.; Fablet, C.; Zal, F.; Green, B.N.; Promé, D.; Marden, M.C.; Pagnier, J.; Wajcman, H. Hemoglobin Porto Alegre forms a tetramer of tetramers superstructure. Protein Sci. 2002, 11, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, J. Polymeric Hemoglobins of the house mouse (Mus musculus L.): Isolation of cysteinyl peptides. Biochem. Genet. 1969, 3, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Witting, P.K.; Douglas, D.J.; Mauk, A.G. Reaction of human myoglobin and H2O2: Involvement of a thiyl radical produced at cysteine 110. J. Biol. Chem. 2000, 275, 20391–20398. [Google Scholar] [CrossRef] [PubMed]
- Kosmachevskaya, O.V.; Novikova, N.N.; Yakunin, S.N.; Topunov, A.F. Formation of supplementary metal-binding centers in proteins under stress conditions. Biochemistry 2024, 89 (Suppl. S1), S180–S204. [Google Scholar] [CrossRef]
- Shumaev, K.B.; Kosmachevskaya, O.V.; Timoshin, A.A.; Vanin, A.F.; Topunov, A.F. Dinitrosyl iron complexes bound with haemoglobin as markers of oxidative stress. Methods Enzymol. 2008, 436, 445–461. [Google Scholar] [CrossRef]
- Khromova, V.S.; Myshkin, A.E. Coagulation of Zinc-modified Hemoglobin. Russ. J. Gen. Chem. 2002, 72, 1645–1649. [Google Scholar] [CrossRef]
- Son, S.Y.; Yoon, H.C. Zinc ion-mediated concentration of glycated hemoglobin for electrochemical biosensing. BioChip J. 2009, 3, 164–170. [Google Scholar]
- Kozlova, E.; Chernysh, A.; Moroz, V.; Sergunova, V.; Gudkova, O.; Fedorova, M.; Kuzovlev, A. Opposite effects of electroporation of red blood cell membranes under the influence of zinc ions. Acta Bioeng. Biomech. 2012, 14, 3–13. [Google Scholar]
- Oohora, K.; Onoda, A.; Kitagishi, H.; Yamaguchi, H.; Haradac, A.; Hayashi, T.A. Chemically-controlled supramolecular protein-polymer formed by a myoglobin-based self-assembly system. Chem. Sci. 2011, 2, 1033–1038. [Google Scholar] [CrossRef]
- Bobofchak, K.M.; Mito, T.; Texel, S.J.; Bellelli, A.; Nemoto, M.; Traystman, R.J.; Koehler, R.C.; Brinigar, W.S.; Fronticelli, C. A recombinant polymeric hemoglobin with conformational, functional, and physiological characteristics of an in vivo O2 transporter. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H549–H561. [Google Scholar] [CrossRef]
- Zhang, N.; Jia, Y.; Chen, G.; Cabrales, P.; Palmer, A.F. Biophysical properties and oxygenation potential of high-molecular-weight glutaraldehyde-polymerized human hemoglobins maintained in the tense and relaxed quaternary states. Tissue Eng. Part A 2011, 17, 927–940. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, S.L.; Pepper, D.S. Hemoglobin polymerization. Methods Enzymol. 1994, 231, 287–308. [Google Scholar] [CrossRef] [PubMed]
- Bu, F.; Wang, H.; Zhu, X. Studies on the resuscitative efficacy of polymerized bovine hemoglobin. Artif. Cells Blood Substit. Immobil. Biotechnol. 2000, 28, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Benesch, R.E. Bis(pyridoxal) polyphosphates as specific intramolecular cross-linking agents for hemoglobin. Methods Enzymol. 1994, 231, 267–274. [Google Scholar] [CrossRef]
- Eike, J.H.; Palmer, A.F. Oxidized mono-, di-, tri-, and polysaccharides as potential hemoglobin cross-linking reagents for the synthesis of high oxygen affinity artificial blood substitutes. Biotechnol. Prog. 2004, 20, 953–962. [Google Scholar] [CrossRef]
- Chang, W.H.; Chang, Y.; Chen, Y.C.; Sung, H.W. Hemoglobin polymerized with a naturally occurring crosslinking agent as a blood substitute: In vitro and in vivo studies. Artif. Cells Blood Substit. Immobil. Biotechnol. 2004, 32, 243–262. [Google Scholar] [CrossRef]
- Han, F.; Shen, Q.; Zheng, W.; Zuo, J.; Zhu, X.; Li, J.; Peng, C.; Li, B.; Chen, Y. The Conformational Changes of Bovine Serum Albumin at the Air/Water Interface: HDX-MS and Interfacial Rheology Analysis. Foods 2023, 12, 1601. [Google Scholar] [CrossRef]
- Xiao, H.; Huang, L.; Zhang, W.; Yin, Z. Damage of proteins at the air/water interface: Surface tension characterizes globulin interface stability. Int. J. Pharm. 2020, 584, 119445. [Google Scholar] [CrossRef] [PubMed]
- Lad, M.D.; Birembaut, F.; Matthew, J.M.; Frazier, R.A.; Green, R.J. The adsorbed conformation of globular proteins at the air/water interface. Phys. Chem. Chem. Phys. 2006, 8, 2179–2186. [Google Scholar] [CrossRef] [PubMed]
- Damodaran, S. In situ measurement of conformational changes in proteins at liquid interfaces by circular dichroism spectroscopy. Anal. Bioanal. Chem. 2003, 376, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Jean, L.; Lee, C.F.; Vaux, D.J. Enrichment of Amyloidogenesis at an Air-Water Interface. Biophys. J. 2012, 102, 1154–1162. [Google Scholar] [CrossRef]
- Trigg, B.J.; Lee, C.F.; Vaux, D.J.; Jean, L. The air–water interface determines the outcome of seeding during amyloidogenesis. Biochem. J. 2013, 456, 67–80. [Google Scholar] [CrossRef]
- Wang, L.; Bäcklund, F.G.; Yuan, Y.; Nagamani, S.; Hanczyc, P.; Sznitko, L.; Solin, N. Air−Water Interface Assembly of Protein Nanofibrils Promoted by Hydrophobic Additives. ACS Sustain. Chem. Eng. 2021, 9, 9289–9299. [Google Scholar] [CrossRef]
- Maltseva, D.; Chatterjee, S.; Yu, C.-C.; Brzezinski, M.; Nagata, Y.; Gonella, G.; Murthy, A.C.; Stachowiak, J.C.; Fawzi, N.L.; Parekh, S.H.; et al. Fibril formation and ordering of disordered FUS LC driven by hydrophobic interactions. Nat. Chem. 2023, 15, 1146–1154. [Google Scholar] [CrossRef]
- Hirsch, R.E.; Elbaum, D.; Brody, S.S.; Nagel, R.L. Oxyhemoglobin A and oxyhemoglobin S films at an air-water interface: Absorption spectra studies. J. Colloid Interface Sci. 1980, 78, 212–216. [Google Scholar] [CrossRef]
- Elbaum, D.; Harrington, J.; Roth, E.F.; Nagel, R.L. Surface activity of hemoglobin S and other human hemoglobin variants. Biochim. Biophys. Acta (BBA)-Protein Struct. 1976, 427, 57–69. [Google Scholar] [CrossRef]
- Devineau, S.; Inoue, K.-I.; Kusaka, R.; Urashima, S.-h.; Nihonyanagi, S.; Baigl, D.; Tsuneshige, A.; Tahara, T. Change of the isoelectric point of hemoglobin at the air/water interface probed by the orientational flip-flop of water molecules. Phys. Chem. Chem. Phys. 2017, 19, 10292–10300. [Google Scholar] [CrossRef]
- D’Imprima, E.; Floris, D.; Joppe, M.; Sánchez, R.; Grininger, M.; Kühlbrandt, W. Protein denaturation at the air-water interface and how to prevent it. eLife 2019, 8, e42747. [Google Scholar] [CrossRef] [PubMed]
- Imahori, K. The structure of surface-denatured protein. IV. The molecular weight, surface area and shape of the surface-denatured hemoglobin molecule. Bull. Chem. Soc. Jpn. 1952, 25, 121–123. [Google Scholar] [CrossRef]
- Chaudhary, S.; Kaur, H.; Kaur, H.; Rana, B.; Tomar, D.; Jena, K.C. Probing the bovine hemoglobin adsorption process and its influence on interfacial water structure at the air-water interface. Appl. Spectrosc. 2021, 75, 1497–1509. [Google Scholar] [CrossRef]
- Gidalevitz, D.; Huang, Z.; Rice, S.A. Protein folding at the air—water interface studied with x-ray reflectivity. Proc. Natl. Acad. Sci. USA 1999, 96, 2608–2611. [Google Scholar] [CrossRef] [PubMed]
- Sen, P.; Yamaguchi, S.; Tahara, T. New insight into the surface denaturation of proteins: Electronic sum frequency generation study of cytochrome c at water interfaces. J. Phys. Chem. B 2008, 112, 12473–12475. [Google Scholar] [CrossRef]
- Pal, P.; Mahato, M.; Kamilya, T.; Talapatra, G.B. Interaction of glucose with hemoglobin: A study in aqueous solution and at the air-water interface using the Langmuir-Blodgett technique. Phys. Chem. Chem. Phys. 2011, 13, 9385–9396. [Google Scholar] [CrossRef]
- Mahato, M.; Pal, P.; Kamilya, T.; Sarkar, R.; Chaudhuri, A.; Talapatra, G.B. Influence of KCl on the interfacial activity and conformation of hemoglobin studied by Langmuir-Blodgett technique. Phys. Chem. Chem. Phys. 2010, 12, 12997–13006. [Google Scholar] [CrossRef]
- Mahato, M.; Pal, P.; Kamilya, T.; Sarkar, R.; Talapatra, G.B. pH induced structural modulation and interfacial activity of hemoglobin at the air/water interface. J. Phys. Chem. B 2010, 114, 495–502. [Google Scholar] [CrossRef]
- Sasso, L.; Gerrard, J.A. Self-Assembled Biological Nanofibers for Biosensor Applications. In Micro and Nanofabrication Using Self-Assembled Biological Nanostructures, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 1–20. [Google Scholar]
- Bolisetty, S.; Adamcik, J.; Heier, J.; Mezzenga, R. Amyloid directed synthesis of titanium dioxide nanowires and their applications in hybrid photovoltaic devices. Adv. Funct. Mater. 2012, 22, 3424–3428. [Google Scholar] [CrossRef]
- Rao, S.P.; Meade, S.J.; Healy, J.P.; Sutton, K.H.; Larsen, N.G.; Staiger, M.P.; Gerrard, J.A. Amyloid fibrils as functionalizable components of nanocomposite materials. Biotechnol. Prog. 2012, 28, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Knowles, T.P.J.; Oppenheim, T.W.; Buell, A.K.; Chirgadze, D.Y.; Welland, M.E. Nanostructured films from hierarchical self-assembly of amyloidogenic proteins. Nat. Nanotechnol. 2010, 5, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.; Kang, S.; Koo, H.J.; Lee, J.H.; Lee, Y.S.; Paik, S.R. Nanoporous protein matrix made of amyloid fibrils of β2-microglobulin. Biotechnol. Prog. 2010, 26, 1759–1764. [Google Scholar] [CrossRef] [PubMed]
- Bhak, G.; Lee, S.; Park, J.W.; Cho, S.; Paik, S.R. Amyloid hydrogel derived from curly protein fibrils of alpha-synuclein. Biomaterials 2010, 31, 5986–5995. [Google Scholar] [CrossRef]
- Nystrom, G.; Fernandez-Ronco, M.P.; Bolisetty, S.; Mazzotti, M.; Mezzenga, R. Amyloid templated gold aerogels. Adv. Mater. 2016, 28, 472–478. [Google Scholar] [CrossRef]
- Casey, C.; Sleator, R.D. Prions: Structure, function, evolution, and disease. Arch. Microbiol. 2024, 207, 1. [Google Scholar] [CrossRef]
- Trusova, V.M. Protein fibrillar nanopolymers: Molecular-level insights into their structural, physical and mechanical properties. Biophys. Rev. Lett. 2015, 10, 135–156. [Google Scholar] [CrossRef]
- Hong, S.; Choi, D.W.; Kim, H.N.; Park, C.G.; Lee, W.; Park, H.H. Protein-based nanoparticles as drug delivery systems. Pharmaceutics 2020, 12, 604. [Google Scholar] [CrossRef]
- Aljabali, A.A.A.; Rezigue, M.; Alsharedeh, R.H.; Obeid, M.A.; Mishra, V.; Serrano-Aroca, Á.; El-Tanani, M.; Tambuwala, M.M. Protein-based nanomaterials: A new tool for targeted drug delivery. Ther. Deliv. 2022, 13, 321–338. [Google Scholar] [CrossRef]
- Mahato, M.; Pal, P.; Tah, B.; Talapatra, G.B. Hemoglobin–phospholipid interaction and biocomposite formation at air/water interface. Colloids Surf. A Physicochem. Eng. Asp. 2012, 414, 375–383. [Google Scholar] [CrossRef]
- Furuno, T. Atomic force microscopy study on the unfolding of globular proteins in the Langmuir films. Thin Solid Films 2014, 552, 170–179. [Google Scholar] [CrossRef]
- Kamilya, T.; Pal, P.; Talapatra, G.B. Interaction of ovalbumin with phospholipids Langmuir–Blodgett film. J. Phys. Chem. B 2007, 111, 1199–1205. [Google Scholar] [CrossRef]
- Liao, Z.; Lampe, J.W.; Ayyaswamy, P.S.; Eckmann, D.M.; Dmochowski, I.J. Protein assembly at the air-water interface studied by fluorescence microscopy. Langmuir 2011, 27, 12775–12781. [Google Scholar] [CrossRef]
- Ariga, K.; Yamauchi, Y.; Mori, T.; Hill, J.P. 25th anniversary article: What can be done with the Langmuir-Blodgett method? Recent developments and its critical role in materials science. Adv. Mater. 2013, 25, 6477–6512. [Google Scholar] [CrossRef]
- Hussain, S.A.; Dey, B.; Bhattacharjee, D.; Mehta, N. Unique supramolecular assembly through Langmuir—Blodgett (LB) technique. Heliyon 2018, 4, e01038. [Google Scholar] [CrossRef]
- Gu, W.; Li, Q.; Wang, R.; Zhang, L.; Liu, Z.; Jiao, T. Recent Progress in the Applications of Langmuir–Blodgett Film Technology. Nanomaterials 2024, 14, 1039. [Google Scholar] [CrossRef]
- Zhavnerko, G.; Marletta, G. Developing Langmuir–Blodgett strategies towards practical devices. Mater. Sci. Eng. B. 2010, 169, 43–48. [Google Scholar] [CrossRef]
- Maheshkumar, J.; Adhathathreyan, A. Langmuir and Langmuir–Blodgett films of capsules of haemoglobin at air/water and solid/air interfaces. J. Chem. Sci. 2013, 125, 219–227. [Google Scholar] [CrossRef]
- Jiang, Y.; Wu, X.; Wang, X.; Kong, B.; Wang, J.; Zhang, H. Living Hydrogel with Blood Derived Elements for Wound Healing. Mater. Today Bio 2025, 33, 102002. [Google Scholar] [CrossRef]
- Jiang, S.; Zheng, Y.; Xia, H.; Liu, Z.; Rao, S.; Wang, Y.; Sun, H.; Lu, X.; Xie, C. Oxygen-Releasing Hydrogels for Tissue Regeneration. Adv. NanoBiomed Res. 2024, 4, 2300133. [Google Scholar] [CrossRef]
- Gondim, A.C.S.; Guimarães, W.G.; Sousa, E.H.S. Heme-Based Gas Sensors in Nature and Their Chemical and Biotechnological Applications. BioChem 2022, 2, 43–63. [Google Scholar] [CrossRef]
- Altinkaynak, C.; Turk, M.; Ekremoğlu, M.; Özdemir, N. Peroxidase-Like Activity of Hemoglobin-Based Hybrid Materials against Different Substrates and Their Enhanced Application for H2O2 Detection. Bull. Chem. Soc. Ethiop. 2021, 35, 537–550. [Google Scholar] [CrossRef]
- Sutherland, K.; Miller, C.; Bassett, A.; Cannon, J.; Cattron, E.; Escobedo, E.; Judge, K.; Hannesson, M.; Johansen, J.; Scott, D. Substrate Inhibition in Myoglobin and Hemoglobin: Kinetic Insights into Pseudo-Peroxidase Activity. Appl. Chem. 2025, 5, 23. [Google Scholar] [CrossRef]
- Majid, M.A.; Ullah, H.; Alshehri, A.M.; Tabassum, R.; Aleem, A.; Khan, A.R.; Batool, Z.; Nazir, A.; Bibi, I. Development of Novel Polymer Haemoglobin Based Particles as an Antioxidant, Antibacterial and an Oxygen Carrier Agents. Sci. Rep. 2024, 14, 3031. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, A.; Tian, J.; Yang, Q.; Chen, Y.; Muhammad, A.; Chew, K.-H.; Li, Z.; Lei, Z.; Yang, P.; Ren, H. Hemoglobin-Mediated Synthesis of Iron-Hybridized Gold Nanoclusters for Colorimetric Biosensing and Antibacterial Wound Therapy. Chem. Eng. J. 2025, 525, 170293. [Google Scholar] [CrossRef]
- Penjweini, R.; Andreoni, A.; Rosales, T.; Kim, J.; Brenner, M.D.; Sackett, D.L.; Chung, J.H.; Knutson, J.R. Intracellular Oxygen Mapping Using a Myoglobin-mCherry Probe with Fluorescence Lifetime Imaging. J. Biomed. Opt. 2018, 23, 107001. [Google Scholar] [CrossRef]
- Adepu, K.K.; Anishkin, A.; Adams, S.H.; Chintapalli, S.V. A Versatile Delivery Vehicle for Cellular Oxygen and Fuels or Metabolic Sensor? A Review and Perspective on the Functions of Myoglobin. Physiol. Rev. 2024, 104, 1611–1642. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, H.; Hu, N. Fabrication of Electroactive Layer-by-Layer Films of Myoglobin with Gold Nanoparticles of Different Sizes. J. Phys. Chem. B 2006, 110, 2171–2179. [Google Scholar] [CrossRef]
- Alsenaidy, M.A. Aggregation and Conformational Stability Evaluation of Myoglobin in the Presence of Ionic Surfactant. Saudi Pharm. J. 2018, 26, 515–519. [Google Scholar] [CrossRef]
- Kosmachevskaya, O.V.; Topunov, A.F. Alternate and Additional Functions of Erythrocyte Hemoglobin. Biochemistry 2018, 83, 1575–1593. [Google Scholar] [CrossRef]
- Reeder, B.J. Redox and Peroxidase Activities of the Hemoglobin Superfamily: Relevance to Health and Disease. Antioxid. Redox Signal. 2017, 26, 734–753. [Google Scholar] [CrossRef] [PubMed]
- Nasybullina, E.I.; Kosmachevskaya, O.V.; Topunov, A.F. Glycation of Leghemoglobin by Methylglyoxal in Comparison with Other Hemoglobins and the Influence on Peroxidase Activity. Appl. Biochem. Microbiol. 2025, 61, 824–833. [Google Scholar] [CrossRef]
- Tuttle, C.; Hannesson, M.; Henrichsen, A.; Hainsworth, L.; Condie, C.; Whitesides, A.; Oren, A.; Tanner, S.; Terry, B.; Cannon, J.; et al. A Case for Myoglobin − Macromolecular Rate Theory Applied to Pseudo Peroxidase Kinetics. Biochem. Biophys. Mol. Biol. 2025, 13, e19205. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhang, Y.; Zhang, X.; Zhou, X.; Teng, X.; Yan, M.; Bi, H. Horseradish Peroxidase-Immobilized Magnetic Mesoporous Silica Nanoparticles as a Potential Candidate to Eliminate Intracellular Reactive Oxygen Species. Nanoscale 2015, 7, 2941–2950. [Google Scholar] [CrossRef]
- Ye, Y.; Zou, J.; Wu, W.; Wang, Z.; Wen, S.; Liang, Z.; Liu, S.; Lin, Y.; Chen, X.; Luo, T.; et al. Advanced Nanozymes Possess Peroxidase-like Catalytic Activities in Biomedical and Antibacterial Fields: Review and Progress. Nanoscale 2024, 16, 3324–3346. [Google Scholar] [CrossRef]
- Reeder, B.J.; Wilson, M.T. Hemoglobin and Myoglobin Associated Oxidative Stress: From Molecular Mechanisms to Disease States. Curr. Med. Chem. 2005, 12, 2741–2751. [Google Scholar] [CrossRef]
- Sil, R.; Chakraborti, A.S. Major Heme Proteins Hemoglobin and Myoglobin with Respect to Their Roles in Oxidative Stress—A Brief Review. Front. Chem. 2025, 13, 1543455. [Google Scholar] [CrossRef]
- Rerkshanandana, P.; Zhao, X.; Xiong, Y.; Chen, Y.; Steffen, A.; Chaiwaree, S.; Kloypan, C.; Pruss, A.; Georgieva, R.; Bäumler, H. Hemoglobin in Submicron Particles (HbMPs) Is Stabilized against Oxidation. Antioxidants 2024, 13, 1477. [Google Scholar] [CrossRef]
- Lehn, J.-M. Supramolecular Chemistry: Concepts and Perspectives; Wiley-VCH: Weinheim, Germany, 1995. [Google Scholar]
- Bozza, M.T.; Jeney, V. Pro-Inflammatory Actions of Heme and Other Hemoglobin-Derived DAMPs. Front. Immunol. 2020, 11, 1323. [Google Scholar] [CrossRef]
- Fu, S.; Zhu, X.; Huang, F.; Chen, X. Anti-PEG Antibodies and Their Biological Impact on PEGylated Drugs: Challenges and Strategies for Optimization. Pharmaceutics 2025, 17, 1074. [Google Scholar] [CrossRef]
- Saita, Y.; Hattori, K.; Hokari, A.; Ohyama, T.; Inoue, J.; Nishimura, T.; Nemoto, S.; Aoyagi, S. Plasma Myoglobin Indicates Muscle Damage Associated with Acceleration/Deceleration during Football. J. Sports Med. Phys. Fit. 2023, 63, 1337–1342. [Google Scholar] [CrossRef]
- Alanazi, J.; Aleanizy, F.S.; Alqahtani, F.Y. Nanotechnology Approaches for Mitigating Biologic Immunogenicity: A Literature Review. Pharmaceutics 2025, 17, 888. [Google Scholar] [CrossRef]
| Hemoglobin/Myoglobin | Mutation | Type of Supramolecular Structure |
|---|---|---|
| HbS | β6 Glu → Val | Forms ordered polymeric filaments resembling amyloid fibrils upon deoxygenation |
| HbC | β6 Glu → Lys | Prone to intracellular crystallization in homozygous (HbCC) or HbSC compound heterozygotes. |
| Hb Olympia | β20 Val → Met | Self-assembles into supramolecular forms. |
| Hb Korle Bu | β73 Asp → Asn | Under oxidative stress (e.g., during infection), it forms intracellular inclusions resembling ordered aggregates. |
| Hb Zurich | β63 His → Arg | Disruption of heme–protein interaction triggers aggregation into Heinz bodies. |
| Hb Philly | α35 Tyr → Phe | Mutations at the α1β1-interface induce unusual polymerization or precipitation under specific conditions. |
| Hb Machida | β6 Glu → Gln | Increased surface hydrophobicity reduces the solubility of oxyHb, promoting aggregation. |
| Hb Rainier | β145 Tyr → Cys | Oligomerization/aggregation via intermolecular disulfide bonds. |
| Hb Pôrto Alegre | β9 Ser → Cys | |
| Mb V68N | Val68 → Asn | Loss of heme → instability → forms amyloid-like fibrils in vitro. |
| Mb H64Q | His64 → Gln | Reduced O2 affinity and protein instability → enhanced aggregation propensity. |
| Oxidative Modification | Chemical Changes | Mechanism of Aggregation | Biological Consequences | References |
|---|---|---|---|---|
| Oxidation of Met, His, Trp | Methionine sulfoxides; oxidized His and Trp derivatives | Conformational changes, protein destabilization | Loss of O2 binding; predisposition to degradation and aggregation | [82] |
| Oxidation of heme iron | Formation of methemoglobin/metmyoglobin | Intermolecular disulfide cross-linking | Aggregation, loss of solubility | [82,83] |
| Oxidation of cysteine | Disulfide bond formation; sulfinic/sulfonic acid derivatives | Covalent cross-linking of protein molecules | Formation of stable, degradation-resistant aggregates | [26,27,28,50,51] |
| Oxidation of tyrosine | Tyrosyl radical formation; dityrosine cross-links | Increased hydrophobicity, protein unfolding | Severe oxidative damage; aggregation and proteasome overload | [50,51] |
| Carbonylation | Addition of carbonyl groups to Lys, Arg, Thr via lipid peroxidation products (MDA, 4-HNE) | Structural disruption, reduced stability | Destabilization of globular fold, increased aggregation propensity | [50,51,52] |
| Heme destruction | Release of heme and Fe2+/Fe3+ | Hemin induces aggregation; Fe2+ enhances oxidative stress via Fenton reaction | Amplification of oxidative damage to proteins and lipids | [53,54,55] |
| Denaturation and unfolding | Loss of tertiary/secondary structure | Exposure of hydrophobic regions → hydrophobic interactions | Self-assembly into insoluble aggregates | [11,12,13] |
| Formation of heme–globin complexes | Binding of free heme outside the heme pocket | Conformational changes, self-assembly | Precursors to Heinz body formation | [83] |
| Protein | Biotechnological Tool | Protein Properties Underlying the Principle of Action | Application Areas | Source |
|---|---|---|---|---|
| Hb | Oxygen-releasing hydrogels | The natural ability of hemoglobin to bind oxygen at high concentrations and release it at low concentrations, using techniques such as altering the hydrogel microenvironment (e.g., pH or temperature) to control the rate of release. | Wound healing, tissue engineering, treatment of ischemic conditions, therapeutic delivery, and biosensing. | [143,144] |
| Hb/Mb | Highly sensitive electrochemical biosensors O2, CO and NO | The natural ability of heme iron to bind small gaseous molecules (O2, CO, NO) with high affinity | Biosensing of small gaseous molecules (O2, CO, NO) | [145] |
| Hb/Mb | Nanozyme-based materials for H2O2 detection | Hb and Mb intrinsically possess pseudo-peroxidase activity due to their heme iron center. | Highly sensitive and selective biosensors for H2O2 in various fields: clinical diagnostics, environmental monitoring, and food analysis. | [146,147] |
| Hb/Mb | Nanocarriers with antibacterial activity | Broad antibacterial activity of the globin part. Heme proteins can enhance peroxidase-like activity, catalyzing the decomposition of H2O2 to generate ROS. | Destruction of bacteria, including multidrug-resistant strains. Treatment of bacterial septic arthritis, healing of infected wounds. | [148,149] |
| Mb | Oxygen-sensitive optical biosensor | This approach leverages myoglobin’s natural oxygen-binding function, using changes in its color or fluorescence to indicate oxygen levels. | Monitoring tissue oxygenation, detecting cardiac biomarkers. | [150,151] |
| Mb | Conductive biointerfaces for nanowires | The ability of the heme group to undergo reversible FeIII/FeII oxidation-reduction reactions makes direct electron transfer between the protein’s redox center and the electrode surface possible. | Highly sensitive biosensors for medical diagnostics, such as for detecting Mb, a biomarker for acute myocardial infarction. | [152] |
| Mb | Model system for screening aggregation inhibitors | Mb readily forms amyloid fibrils under specific conditions, and its aggregation can be monitored using spectroscopic techniques like circular dichroism and fluorescence. | Study of the influence of different factors (pH, temperature, surfactants) on protein stability and aggregation propensity, and testing of inhibitory compounds. | [153] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosmachevskaya, O.V.; Novikova, N.N.; Topunov, A.F. Association of Hemoglobin and Myoglobin into Supramolecular Complexes: Significance for Life and Practice. Int. J. Mol. Sci. 2025, 26, 11700. https://doi.org/10.3390/ijms262311700
Kosmachevskaya OV, Novikova NN, Topunov AF. Association of Hemoglobin and Myoglobin into Supramolecular Complexes: Significance for Life and Practice. International Journal of Molecular Sciences. 2025; 26(23):11700. https://doi.org/10.3390/ijms262311700
Chicago/Turabian StyleKosmachevskaya, Olga V., Natalia N. Novikova, and Alexey F. Topunov. 2025. "Association of Hemoglobin and Myoglobin into Supramolecular Complexes: Significance for Life and Practice" International Journal of Molecular Sciences 26, no. 23: 11700. https://doi.org/10.3390/ijms262311700
APA StyleKosmachevskaya, O. V., Novikova, N. N., & Topunov, A. F. (2025). Association of Hemoglobin and Myoglobin into Supramolecular Complexes: Significance for Life and Practice. International Journal of Molecular Sciences, 26(23), 11700. https://doi.org/10.3390/ijms262311700






