Extracellular Matrix Remodeling in Motor Neuron Diseases
Abstract
1. Introduction
2. ECM in Amyotrophic Lateral Sclerosis
2.1. ECM and ALS Animal Models
2.2. ECM and ALS Patient-Derived Models
2.3. ECM as ALS Biomarkers
3. ECM in Spinal Muscular Atrophy
3.1. ECM and SMA Animal Models
3.2. ECM and SMA Patient-Derived Models
3.3. ECM as SMA Biomarkers
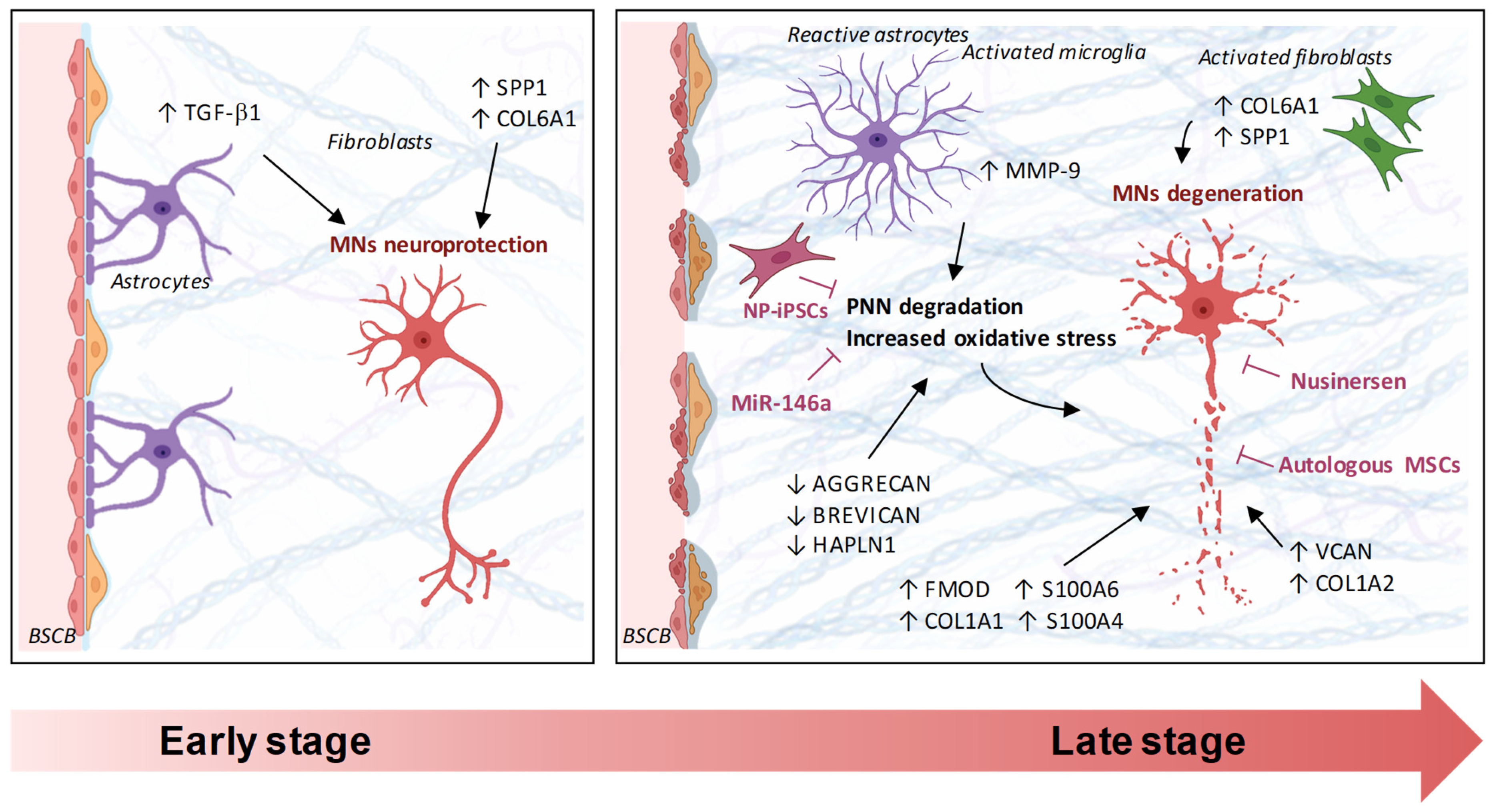
4. Integrative View and Mechanobiological Implications in MNDs
5. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| ALS | Amyotrophic lateral sclerosis |
| BSCB | Blood–spinal cord barrier |
| CNS | Central nervous system |
| CSF | Cerebrospinal fluid |
| CSPG | Chondroitin sulfate proteoglycans |
| ECM | Extracellular matrix |
| GAG | Glycosaminoglycan |
| iPSC | Induced pluripotent stem cells |
| MMP | Matrix metalloproteinase |
| MND | Motor neuron disease |
| MSCs | Mesenchymal stem cells |
| NMJ | Neuromuscular junction |
| NP | Neural progenitor cells |
| PG | Proteoglycan |
| PNNs | Perineuronal nets |
| PVMs | Perivascular macrophages |
| SMA | Spinal muscular atrophy |
| SMN | Survival motor neuron |
| ΔFRS | Delta functional rating scale |
References
- Theocharis, A.D.; Manou, D.; Karamanos, N.K. The Extracellular Matrix as a Multitasking Player in Disease. FEBS J. 2019, 286, 2830–2869. [Google Scholar] [CrossRef]
- Dzobo, K.; Dandara, C. The Extracellular Matrix: Its Composition, Function, Remodeling, and Role in Tumorigenesis. Biomimetics 2023, 8, 146. [Google Scholar] [CrossRef]
- Mouw, J.K.; Ou, G.; Weaver, V.M. Extracellular Matrix Assembly: A Multiscale Deconstruction. Nat. Rev. Mol. Cell Biol. 2014, 15, 771–785. [Google Scholar] [CrossRef]
- Nonnast, E.; Mira, E.; Mañes, S. Biomechanical Properties of Laminins and Their Impact on Cancer Progression. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2024, 1879, 189181. [Google Scholar] [CrossRef]
- Yue, B. Biology of the Extracellular Matrix: An Overview. J. Glaucoma 2014, 23, S20–S23. [Google Scholar] [CrossRef] [PubMed]
- Dalton, C.J.; Lemmon, C.A. Fibronectin: Molecular Structure, Fibrillar Structure and Mechanochemical Signaling. Cells 2021, 10, 2443. [Google Scholar] [CrossRef] [PubMed]
- Trębacz, H.; Barzycka, A. Mechanical Properties and Functions of Elastin: An Overview. Biomolecules 2023, 13, 574. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Tzanakakis, G.N.; Karamanos, N.K. Proteoglycans in Health and Disease: Novel Roles for Proteoglycans in Malignancy and Their Pharmacological Targeting. FEBS J. 2010, 277, 3904–3923. [Google Scholar] [CrossRef]
- Iozzo, R.V.; Schaefer, L. Proteoglycan Form and Function: A Comprehensive Nomenclature of Proteoglycans. Matrix Biol. 2015, 42, 11–55. [Google Scholar] [CrossRef] [PubMed]
- Zha, B.; Zhang, C.; Wu, C. The Stiffness of Extracellular Matrix in Regulating Cellular Metabolism. Am. J. Physiol. Cell Physiol. 2025, 329, C298–C306. [Google Scholar] [CrossRef]
- Zhao, T.; Huang, Y.; Zhu, J.; Qin, Y.; Wu, H.; Yu, J.; Zhai, Q.; Li, S.; Qin, X.; Wang, D.; et al. Extracellular Matrix Signaling Cues: Biological Functions, Diseases, and Therapeutic Targets. MedComm 2025, 6, e70281. [Google Scholar] [CrossRef]
- Melrose, J.; Hayes, A.J.; Bix, G. The CNS/PNS Extracellular Matrix Provides Instructive Guidance Cues to Neural Cells and Neuroregulatory Proteins in Neural Development and Repair. Int. J. Mol. Sci. 2021, 22, 5583. [Google Scholar] [CrossRef] [PubMed]
- Chelyshev, Y.A.; Kabdesh, I.M.; Mukhamedshina, Y.O. Extracellular Matrix in Neural Plasticity and Regeneration. Cell. Mol. Neurobiol. 2020, 42, 647–664. [Google Scholar] [CrossRef]
- Pyka, M.; Wetzel, C.; Aguado, A.; Geissler, M.; Hatt, H.; Faissner, A. Chondroitin Sulfate Proteoglycans Regulate Astrocyte-Dependent Synaptogenesis and Modulate Synaptic Activity in Primary Embryonic Hippocampal Neurons. Eur. J. Neurosci. 2011, 33, 2187–2202. [Google Scholar] [CrossRef] [PubMed]
- Frischknecht, R.; Gundelfinger, E.D. The Brain’s Extracellular Matrix and Its Role in Synaptic Plasticity. Adv. Exp. Med. Biol. 2012, 970, 153–171. [Google Scholar] [CrossRef]
- Auer, S.; Schicht, M.; Hoffmann, L.; Budday, S.; Frischknecht, R.; Blümcke, I.; Paulsen, F. The Role of Perineuronal Nets in Physiology and Disease: Insights from Recent Studies. Cells 2025, 14, 321. [Google Scholar] [CrossRef] [PubMed]
- Ortega, J.A.; Soares de Aguiar, G.P.; Chandravanshi, P.; Levy, N.; Engel, E.; Álvarez, Z. Exploring the Properties and Potential of the Neural Extracellular Matrix for Next-Generation Regenerative Therapies. WIREs Nanomed. Nanobiotechnol. 2024, 16, e1962. [Google Scholar] [CrossRef]
- Ferrer-Ferrer, M.; Dityatev, A. Shaping Synapses by the Neural Extracellular Matrix. Front. Neuroanat. 2018, 12, 40. [Google Scholar] [CrossRef]
- Höhn, L.; Hußler, W.; Richter, A.; Smalla, K.-H.; Birkl-Toeglhofer, A.-M.; Birkl, C.; Vielhaber, S.; Leber, S.L.; Gundelfinger, E.D.; Haybaeck, J.; et al. Extracellular Matrix Changes in Subcellular Brain Fractions and Cerebrospinal Fluid of Alzheimer’s Disease Patients. Int. J. Mol. Sci. 2023, 24, 5532. [Google Scholar] [CrossRef]
- Yang, Q.; Yan, C.; Sun, Y.; Xie, Z.; Yang, L.; Jiang, M.; Ni, J.; Chen, B.; Xu, S.; Yuan, Z.; et al. Extracellular Matrix Remodeling Alleviates Memory Deficits in Alzheimer’s Disease by Enhancing the Astrocytic Autophagy-Lysosome Pathway. Adv. Sci. 2024, 11, 2400480. [Google Scholar] [CrossRef]
- Lendvai, D.; Morawski, M.; Négyessy, L.; Gáti, G.; Jäger, C.; Baksa, G.; Glasz, T.; Attems, J.; Tanila, H.; Arendt, T.; et al. Neurochemical Mapping of the Human Hippocampus Reveals Perisynaptic Matrix around Functional Synapses in Alzheimer’s Disease. Acta Neuropathol. 2013, 125, 215–229. [Google Scholar] [CrossRef]
- Rosh, I.; Tripathi, U.; Hussein, Y.; Rike, W.A.; Djamus, J.; Shklyar, B.; Manole, A.; Houlden, H.; Winkler, J.; Gage, F.H.; et al. Synaptic Dysfunction and Extracellular Matrix Dysregulation in Dopaminergic Neurons from Sporadic and E326K-GBA1 Parkinson’s Disease Patients. npj Park. Dis. 2024, 10, 38. [Google Scholar] [CrossRef]
- Freitas, A.; Aroso, M.; Barros, A.; Fernández, M.; Conde-Sousa, E.; Leite, M.; Carvalho, E.D.; Ribeiro, C.C.; Ferreira, R.; Pêgo, A.P.; et al. Characterization of the Striatal Extracellular Matrix in a Mouse Model of Parkinson’s Disease. Antioxidants 2021, 10, 1095. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, S.; Yong, V.W. The Extracellular Matrix as Modifier of Neuroinflammation and Remyelination in Multiple Sclerosis. Brain 2021, 144, 1958–1973. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, E.L.; Jain, R.W.; Ghorbani, S.; Gorter, R.P.; D’Mello, C.; Yong, V.W. Uncovering Novel Extracellular Matrix Transcriptome Alterations in Lesions of Multiple Sclerosis. Int. J. Mol. Sci. 2024, 25, 1240. [Google Scholar] [CrossRef] [PubMed]
- Shijo, T.; Warita, H.; Suzuki, N.; Kitajima, Y.; Ikeda, K.; Akiyama, T.; Ono, H.; Mitsuzawa, S.; Nishiyama, A.; Izumi, R.; et al. Aberrant Astrocytic Expression of Chondroitin Sulfate Proteoglycan Receptors in a Rat Model of Amyotrophic Lateral Sclerosis. J. Neurosci. Res. 2018, 96, 222–233. [Google Scholar] [CrossRef]
- Chen, L.; Wang, N.; Zhang, Y.; Li, D.; He, C.; Li, Z.; Zhang, J.; Guo, Y. Proteomics Analysis Indicates the Involvement of Immunity and Inflammation in the Onset Stage of SOD1-G93A Mouse Model of ALS. J. Proteom. 2023, 272, 104776. [Google Scholar] [CrossRef]
- Rossi, S.; Milani, M.; Della Valle, I.; Apolloni, S. Transcriptomic Profiling of Symptomatic and End-Stage SOD1-G93A Transgenic Mice Reveals Extracellular Matrix Components as Key Players in ALS Pathogenesis. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2025, 1871, 167707. [Google Scholar] [CrossRef]
- Cheung, S.W.; Willis, E.F.; Simmons, D.G.; Bellingham, M.C.; Noakes, P.G. Phagocytosis of Aggrecan-Positive Perineuronal Nets Surrounding Motor Neurons by Reactive Microglia Expressing MMP-9 in TDP-43Q331K ALS Model Mice. Neurobiol. Dis. 2024, 200, 106614. [Google Scholar] [CrossRef]
- Cheung, S.W.; Bhavnani, E.; Simmons, D.G.; Bellingham, M.C.; Noakes, P.G. Perineuronal Nets Are Phagocytosed by MMP-9 Expressing Microglia and Astrocytes in the SOD1G93A ALS Mouse Model. Neuropathol. Appl. Neurobiol. 2024, 50, e12982. [Google Scholar] [CrossRef]
- Forostyak, S.; Forostyak, O.; Kwok, J.C.F.; Romanyuk, N.; Rehorova, M.; Kriska, J.; Dayanithi, G.; Raha-Chowdhury, R.; Jendelova, P.; Anderova, M.; et al. Transplantation of Neural Precursors Derived from Induced Pluripotent Cells Preserve Perineuronal Nets and Stimulate Neural Plasticity in ALS Rats. Int. J. Mol. Sci. 2020, 21, 9593. [Google Scholar] [CrossRef] [PubMed]
- Swindell, W.R.; Bojanowski, K.; Kindy, M.S.; Chau, R.M.W.; Ko, D. GM604 Regulates Developmental Neurogenesis Pathways and the Expression of Genes Associated with Amyotrophic Lateral Sclerosis. Transl. Neurodegener. 2018, 7, 30. [Google Scholar] [CrossRef]
- Adachi, K.; Miyata, K.; Chida, Y.; Hirose, M.; Morisaki, Y.; Yamanaka, K.; Misawa, H. Depletion of Perivascular Macrophages Delays ALS Disease Progression by Ameliorating Blood-Spinal Cord Barrier Impairment in SOD1G93A Mice. Front. Cell Neurosci. 2023, 17, 1291673. [Google Scholar] [CrossRef]
- Wong, C.-O.; Venkatachalam, K. Motor Neurons from ALS Patients with Mutations in C9ORF72 and SOD1 Exhibit Distinct Transcriptional Landscapes. Hum. Mol. Genet. 2019, 28, 2799–2810. [Google Scholar] [CrossRef]
- Lin, J.; Huang, P.; Chen, W.; Ye, C.; Su, H.; Yao, X. Key Molecules and Pathways Underlying Sporadic Amyotrophic Lateral Sclerosis: Integrated Analysis on Gene Expression Profiles of Motor Neurons. Front. Genet. 2020, 11, 578143. [Google Scholar] [CrossRef] [PubMed]
- Swindell, W.R. Meta-Analysis of Differential Gene Expression in Lower Motor Neurons Isolated by Laser Capture Microdissection from Post-Mortem ALS Spinal Cords. Front. Genet. 2024, 15, 1385114. [Google Scholar] [CrossRef]
- Ziff, O.J.; Clarke, B.E.; Taha, D.M.; Crerar, H.; Luscombe, N.M.; Patani, R. Meta-Analysis of Human and Mouse ALS Astrocytes Reveals Multi-Omic Signatures of Inflammatory Reactive States. Genome Res. 2022, 32, 71–84. [Google Scholar] [CrossRef]
- Caldi Gomes, L.; Hänzelmann, S.; Hausmann, F.; Khatri, R.; Oller, S.; Parvaz, M.; Tzeplaeff, L.; Pasetto, L.; Gebelin, M.; Ebbing, M.; et al. Multiomic ALS Signatures Highlight Subclusters and Sex Differences Suggesting the MAPK Pathway as Therapeutic Target. Nat. Commun. 2024, 15, 4893. [Google Scholar] [CrossRef]
- Reis, A.L.G.; Maximino, J.R.; Lage, L.A.D.P.C.; Gomes, H.R.; Pereira, J.; Brofman, P.R.S.; Senegaglia, A.C.; Rebelatto, C.L.K.; Daga, D.R.; Paiva, W.S.; et al. Proteomic Analysis of Cerebrospinal Fluid of Amyotrophic Lateral Sclerosis Patients in the Presence of Autologous Bone Marrow Derived Mesenchymal Stem Cells. Stem Cell Res. Ther. 2024, 15, 301. [Google Scholar] [CrossRef]
- Milioto, C.; Carcolé, M.; Giblin, A.; Coneys, R.; Attrebi, O.; Ahmed, M.; Harris, S.S.; Lee, B.I.; Yang, M.; Ellingford, R.A.; et al. PolyGR and polyPR Knock-in Mice Reveal a Conserved Neuroprotective Extracellular Matrix Signature in C9orf72 ALS/FTD Neurons. Nat. Neurosci. 2024, 27, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Månberg, A.; Skene, N.; Sanders, F.; Trusohamn, M.; Remnestål, J.; Szczepińska, A.; Aksoylu, I.S.; Lönnerberg, P.; Ebarasi, L.; Wouters, S.; et al. Altered Perivascular Fibroblast Activity Precedes ALS Disease Onset. Nat. Med. 2021, 27, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Holdom, C.J.; Ngo, S.T.; McCombe, P.A.; Henderson, R.D.; Steyn, F.J. Low Plasma Hyaluronan Is Associated with Faster Functional Decline in Patients with Amyotrophic Lateral Sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2022, 23, 42–48. [Google Scholar] [CrossRef]
- Hußler, W.; Höhn, L.; Stolz, C.; Vielhaber, S.; Garz, C.; Schmitt, F.C.; Gundelfinger, E.D.; Schreiber, S.; Seidenbecher, C.I. Brevican and Neurocan Cleavage Products in the Cerebrospinal Fluid—Differential Occurrence in ALS, Epilepsy and Small Vessel Disease. Front. Cell Neurosci. 2022, 16, 838432. [Google Scholar] [CrossRef]
- Sánchez-Torres, J.L.; Yescas-Gómez, P.; Torres-Romero, J.; Espinosa, O.R.; Canovas, L.L.; Tecalco-Cruz, Á.C.; Ponce-Regalado, M.D.; Alvarez-Sánchez, M.E. Matrix Metalloproteinases Deregulation in Amyotrophic Lateral Sclerosis. J. Neurol. Sci. 2020, 419, 117175. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Huang, X.; Huang, M.; Liu, C.; Xu, J. Mendelian Randomization of Plasma Proteomics Identifies Novel ALS-Associated Proteins and Their GO Enrichment and KEGG Pathway Analyses. BMC Neurol. 2025, 25, 82. [Google Scholar] [CrossRef]
- Murray, L.M.; Lee, S.; Bäumer, D.; Parson, S.H.; Talbot, K.; Gillingwater, T.H. Pre-Symptomatic Development of Lower Motor Neuron Connectivity in a Mouse Model of Severe Spinal Muscular Atrophy. Human. Mol. Genet. 2010, 19, 420–433. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.H.; Brandt, K.; Claus, P.; Jung, K. Comparative Meta-Analysis of Transcriptomic Studies in Spinal Muscular Atrophy: Comparison between Tissues and Mouse Models. BMC Med. Genom. 2024, 17, 266. [Google Scholar] [CrossRef]
- Oprişoreanu, A.-M.; Smith, H.L.; Arya, S.; Webster, R.; Zhong, Z.; Eaton-Hart, C.; Wehner, D.; Cardozo, M.J.; Becker, T.; Talbot, K.; et al. Interaction of Axonal Chondrolectin with Collagen XIXa1 Is Necessary for Precise Neuromuscular Junction Formation. Cell Rep. 2019, 29, 1082–1098.e10. [Google Scholar] [CrossRef]
- Bäumer, D.; Lee, S.; Nicholson, G.; Davies, J.L.; Parkinson, N.J.; Murray, L.M.; Gillingwater, T.H.; Ansorge, O.; Davies, K.E.; Talbot, K. Alternative Splicing Events Are a Late Feature of Pathology in a Mouse Model of Spinal Muscular Atrophy. PLoS Genet. 2009, 5, e1000773. [Google Scholar] [CrossRef]
- Zhang, Z.; Lotti, F.; Dittmar, K.; Younis, I.; Wan, L.; Kasim, M.; Dreyfuss, G. SMN Deficiency Causes Tissue-Specific Perturbations in the Repertoire of snRNAs and Widespread Defects in Splicing. Cell 2008, 133, 585–600. [Google Scholar] [CrossRef]
- Sleigh, J.N.; Barreiro-Iglesias, A.; Oliver, P.L.; Biba, A.; Becker, T.; Davies, K.E.; Becker, C.G.; Talbot, K. Chondrolectin Affects Cell Survival and Neuronal Outgrowth in in Vitro and in Vivo Models of Spinal Muscular Atrophy. Human. Mol. Genet. 2014, 23, 855–869. [Google Scholar] [CrossRef]
- Martínez-Silva, M.D.L.; Imhoff-Manuel, R.D.; Sharma, A.; Heckman, C.; Shneider, N.A.; Roselli, F.; Zytnicki, D.; Manuel, M. Hypoexcitability Precedes Denervation in the Large Fast-Contracting Motor Units in Two Unrelated Mouse Models of ALS. eLife 2018, 7, e30955. [Google Scholar] [CrossRef]
- Wootz, H.; Enjin, A.; Wallén-Mackenzie, Å.; Lindholm, D.; Kullander, K. Reduced VGLUT2 Expression Increases Motor Neuron Viability in Sod1G93A Mice. Neurobiol. Dis. 2010, 37, 58–66. [Google Scholar] [CrossRef]
- Calvo, A.C.; Cibreiro, G.A.; Merino, P.T.; Roy, J.F.; Galiana, A.; Rufián, A.J.; Cano, J.M.; Martín, M.A.; Moreno, L.; Larrodé, P.; et al. Collagen XIX Alpha 1 Improves Prognosis in Amyotrophic Lateral Sclerosis. Aging Dis. 2019, 10, 278–292. [Google Scholar] [CrossRef]
- Hunter, G.; Aghamaleky Sarvestany, A.; Roche, S.L.; Symes, R.C.; Gillingwater, T.H. SMN-Dependent Intrinsic Defects in Schwann Cells in Mouse Models of Spinal Muscular Atrophy. Human. Mol. Genet. 2014, 23, 2235–2250. [Google Scholar] [CrossRef]
- Welby, E.; Rehborg, R.J.; Harmelink, M.; Ebert, A.D. Assessment of Cerebral Spinal Fluid Biomarkers and microRNA-Mediated Disease Mechanisms in Spinal Muscular Atrophy Patient Samples. Human. Mol. Genet. 2022, 31, 1830–1843. [Google Scholar] [CrossRef] [PubMed]
- Sison, S.L.; Patitucci, T.N.; Seminary, E.R.; Villalon, E.; Lorson, C.L.; Ebert, A.D. Astrocyte-Produced miR-146a as a Mediator of Motor Neuron Loss in Spinal Muscular Atrophy. Human. Mol. Genet. 2017, 26, 3409–3420. [Google Scholar] [CrossRef]
- Dayangac-Erden, D.; Gur-Dedeoglu, B.; Eskici, F.N.; Oztemur-Islakoglu, Y.; Erdem-Ozdamar, S. Do Perineuronal Net Elements Contribute to Pathophysiology of Spinal Muscular Atrophy? In Vitro and Transcriptomics Insights. OMICS J. Integr. Biol. 2018, 22, 598–606. [Google Scholar] [CrossRef]
- Faravelli, I.; Gagliardi, D.; Abati, E.; Meneri, M.; Ongaro, J.; Magri, F.; Parente, V.; Petrozzi, L.; Ricci, G.; Farè, F.; et al. Multi-Omics Profiling of CSF from Spinal Muscular Atrophy Type 3 Patients after Nusinersen Treatment: A 2-Year Follow-up Multicenter Retrospective Study. Cell. Mol. Life Sci. 2023, 80, 241. [Google Scholar] [CrossRef] [PubMed]
- Kessler, T.; Latzer, P.; Schmid, D.; Warnken, U.; Saffari, A.; Ziegler, A.; Kollmer, J.; Möhlenbruch, M.; Ulfert, C.; Herweh, C.; et al. Cerebrospinal Fluid Proteomic Profiling in Nusinersen-treated Patients with Spinal Muscular Atrophy. J. Neurochem. 2020, 153, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Rocha, D.N.; Carvalho, E.D.; Relvas, J.B.; Oliveira, M.J.; Pêgo, A.P. Mechanotransduction: Exploring New Therapeutic Avenues in Central Nervous System Pathology. Front. Neurosci. 2022, 16, 861613. [Google Scholar] [CrossRef]
- Di, X.; Gao, X.; Peng, L.; Ai, J.; Jin, X.; Qi, S.; Li, H.; Wang, K.; Luo, D. Cellular Mechanotransduction in Health and Diseases: From Molecular Mechanism to Therapeutic Targets. Signal Transduct. Target. Ther. 2023, 8, 282. [Google Scholar] [CrossRef] [PubMed]
- Tewari, B.P.; Woo, A.M.; Prim, C.E.; Chaunsali, L.; Patel, D.C.; Kimbrough, I.F.; Engel, K.; Browning, J.L.; Campbell, S.L.; Sontheimer, H. Astrocytes Require Perineuronal Nets to Maintain Synaptic Homeostasis in Mice. Nat. Neurosci. 2024, 27, 1475–1488. [Google Scholar] [CrossRef] [PubMed]
- Tewari, B.P.; Chaunsali, L.; Prim, C.E.; Sontheimer, H. A Glial Perspective on the Extracellular Matrix and Perineuronal Net Remodeling in the Central Nervous System. Front. Cell. Neurosci. 2022, 16, 1022754. [Google Scholar] [CrossRef] [PubMed]
- Pillai, E.K.; Franze, K. Mechanics in the Nervous System: From Development to Disease. Neuron 2024, 112, 342–361. [Google Scholar] [CrossRef]
- Procès, A.; Luciano, M.; Kalukula, Y.; Ris, L.; Gabriele, S. Multiscale Mechanobiology in Brain Physiology and Diseases. Front. Cell Dev. Biol. 2022, 10, 823857. [Google Scholar] [CrossRef]
- Pagnamenta, A.T.; Kaiyrzhanov, R.; Zou, Y.; Da’as, S.I.; Maroofian, R.; Donkervoort, S.; Dominik, N.; Lauffer, M.; Ferla, M.P.; Orioli, A.; et al. An Ancestral 10-Bp Repeat Expansion in VWA1 Causes Recessive Hereditary Motor Neuropathy. Brain 2021, 144, 584–600. [Google Scholar] [CrossRef]
- Deschauer, M.; Hengel, H.; Rupprich, K.; Kreiß, M.; Schlotter-Weigel, B.; Grimmel, M.; Admard, J.; Schneider, I.; Alhaddad, B.; Gazou, A.; et al. Bi-Allelic Truncating Mutations in VWA1 Cause Neuromyopathy. Brain 2021, 144, 574–583. [Google Scholar] [CrossRef]
- Nagy, S.; Pagnamenta, A.T.; Cali, E.; Braakman, H.M.H.; Wijntjes, J.; Kusters, B.; Gotkine, M.; Elpeleg, O.; Meiner, V.; Lenberg, J.; et al. Autosomal Recessive VWA1-Related Disorder: Comprehensive Analysis of Phenotypic Variability and Genetic Mutations. Brain Commun. 2024, 6, fcae377. [Google Scholar] [CrossRef]
- Allen, J.M.; Zamurs, L.; Brachvogel, B.; Schlötzer-Schrehardt, U.; Hansen, U.; Lamandé, S.R.; Rowley, L.; Fitzgerald, J.; Bateman, J.F. Mice Lacking the Extracellular Matrix Protein WARP Develop Normally but Have Compromised Peripheral Nerve Structure and Function. J. Biol. Chem. 2009, 284, 12020–12030. [Google Scholar] [CrossRef]
- Zeng, C.-W. Multipotent Mesenchymal Stem Cell-Based Therapies for Spinal Cord Injury: Current Progress and Future Prospects. Biology 2023, 12, 653. [Google Scholar] [CrossRef] [PubMed]
| Disease | Model/Source | ECM Molecules | Function | Refs. |
|---|---|---|---|---|
| ALS | SOD1-G93A mouse | Fn1, Fga, Col1a1, Fmod | Structural/Adhesion | [27,28] |
| ALS | TDP-43 Q331K mouse | MMP-9 ↑; PNN degradation | Synaptic ECM | [29,30] |
| ALS | C9ORF72 KI mouse | Col6a1 ↑ | Structural/Protective | [40] |
| ALS | iPSC motor neurons (C9, sALS) | COL6A1, SPP1 | Structural/Signaling | [34,35] |
| ALS | LCM MNs (post-mortem) | ECM remodeling genes | Structural/Signaling | [36] |
| ALS | Patient CSF (MSC-treated) | APOA1, APP, C4A, FGA, FGG | Repair pathways | [39] |
| ALS | Plasma/serum | SPP1, hyaluronan | Biomarkers | [41,42] |
| SMA | Severe mouse | ECM-related genes | Structural/Signaling | [46,47] |
| SMA | NMJ (zebrafish/mouse) | Chodl-Col19a1 axis | Synaptic ECM | [48,49,50,51] |
| SMA | iPSC motor neurons | TNC ↓; THBS2 ↓ | Synaptic ECM | [56] |
| SMA | Patient fibroblasts/CSF | HAPLN1 ↓; VCAN ↑; COL1A2 ↑ | Structural/Signaling | [58,59,60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apolloni, S.; Tortoriello, S.; Milani, M.; Rossi, S. Extracellular Matrix Remodeling in Motor Neuron Diseases. Int. J. Mol. Sci. 2025, 26, 11376. https://doi.org/10.3390/ijms262311376
Apolloni S, Tortoriello S, Milani M, Rossi S. Extracellular Matrix Remodeling in Motor Neuron Diseases. International Journal of Molecular Sciences. 2025; 26(23):11376. https://doi.org/10.3390/ijms262311376
Chicago/Turabian StyleApolloni, Savina, Silvia Tortoriello, Martina Milani, and Simona Rossi. 2025. "Extracellular Matrix Remodeling in Motor Neuron Diseases" International Journal of Molecular Sciences 26, no. 23: 11376. https://doi.org/10.3390/ijms262311376
APA StyleApolloni, S., Tortoriello, S., Milani, M., & Rossi, S. (2025). Extracellular Matrix Remodeling in Motor Neuron Diseases. International Journal of Molecular Sciences, 26(23), 11376. https://doi.org/10.3390/ijms262311376







