Multi-Modal Adsorption and Synergistic Corrosion Inhibition of a Collagen–BMIM·Br Composite on Mild Steel
Abstract
1. Introduction
2. Results and Discussion
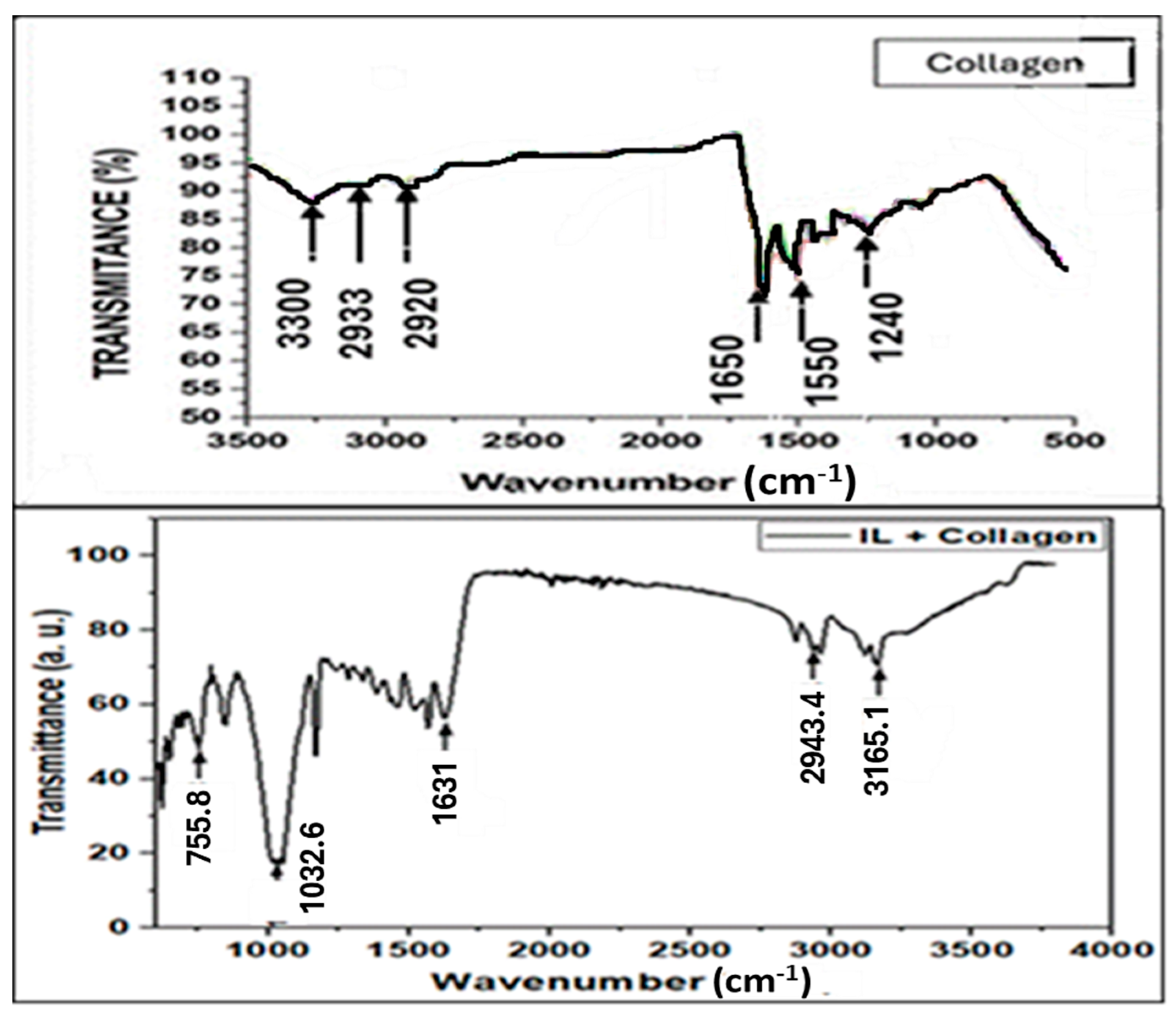
2.1. FTIR Results
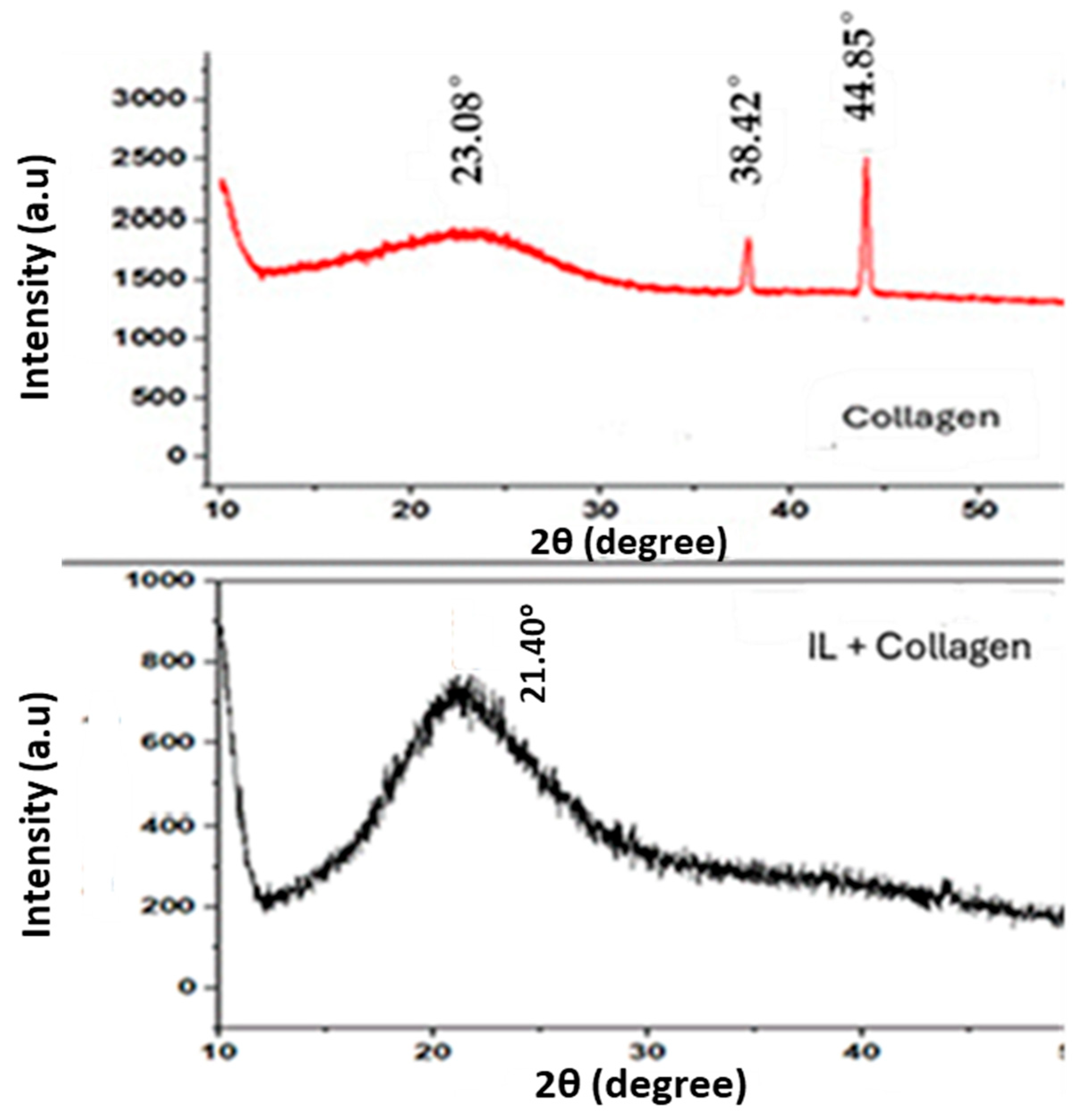
2.2. XRD Results
2.3. Weight Loss Analysis and Corrosion Rate
2.4. Inhibition Efficiency and Surface Coverage
2.5. Thermodynamics of Adsorption: Equilibrium and Energetics
2.6. Comprehensive Analysis of Adsorption Behavior Using Multiple Isotherm Models
2.6.1. Insights from Alternative Isotherm Models
- These Temkin and Frumkin isotherms models account for adsorbate-adsorbate interactions. The Temkin constant a and the Frumkin interaction parameter af are both negative and increase in magnitude with temperature. Negative values signify attractive forces between the adsorbed inhibitor molecules [13]. This suggests that as the surface coverage increases, the adsorbed collagen–IL molecules facilitate further adsorption, likely through cooperative effects such as lateral hydrogen bonding or π-π stacking, leading to the formation of a more cohesive and stable film.
- The Flory-Huggins isotherm model considers the number of adsorption sites occupied by a single adsorbate molecule. The parameter x is greater than 1 and increases with temperature (from 3.83 to 8.94), indicating that each composite molecule occupies multiple active sites on the metal surface [30]. This supports the proposed structural models where the large, flexible collagen molecule, synergistically bound with the ionic liquid, spreads across the surface, displacing multiple water molecules and effectively blocking a wide area from corrosive attack.
- The parameter El-Awady isotherm 1/y represents the number of active sites occupied by a single inhibitor molecule. Values of 1/y less than 1 (ranging from 0.267 to 0.115) suggest multi-site adsorption, where a single molecule attaches to more than one active site [32]. This finding corroborates the results from the Flory-Huggins model and reinforces the idea of a large, multi-anchoring adsorbate.
- The high R2 values for the Freundlich model, which describes adsorption on heterogeneous surfaces, indicate that the surface, while largely homogeneous for Langmuir monolayer formation, possesses some degree of heterogeneity that the composite can effectively cover.
2.6.2. Consolidated View of the Adsorption Mechanism
- Strong Monolayer Foundation: The perfect fit to the Langmuir isotherm confirms the initial formation of a well-ordered, foundational monolayer on the metal surface.
- Attractive Lateral Interactions: The analysis of the Temkin and Frumkin isotherms suggests the presence of attractive forces between the adsorbed molecules. These cooperative interactions within the film enhance its overall cohesion, stability, and compactness [13].
- Temperature-Activated Enhancement: The consistent increase in all binding constants (Kads, KT, etc.) with rising temperature underscores that the adsorption process is endothermic. This leads to the formation of a more stable and resilient protective film under thermal stress [35].
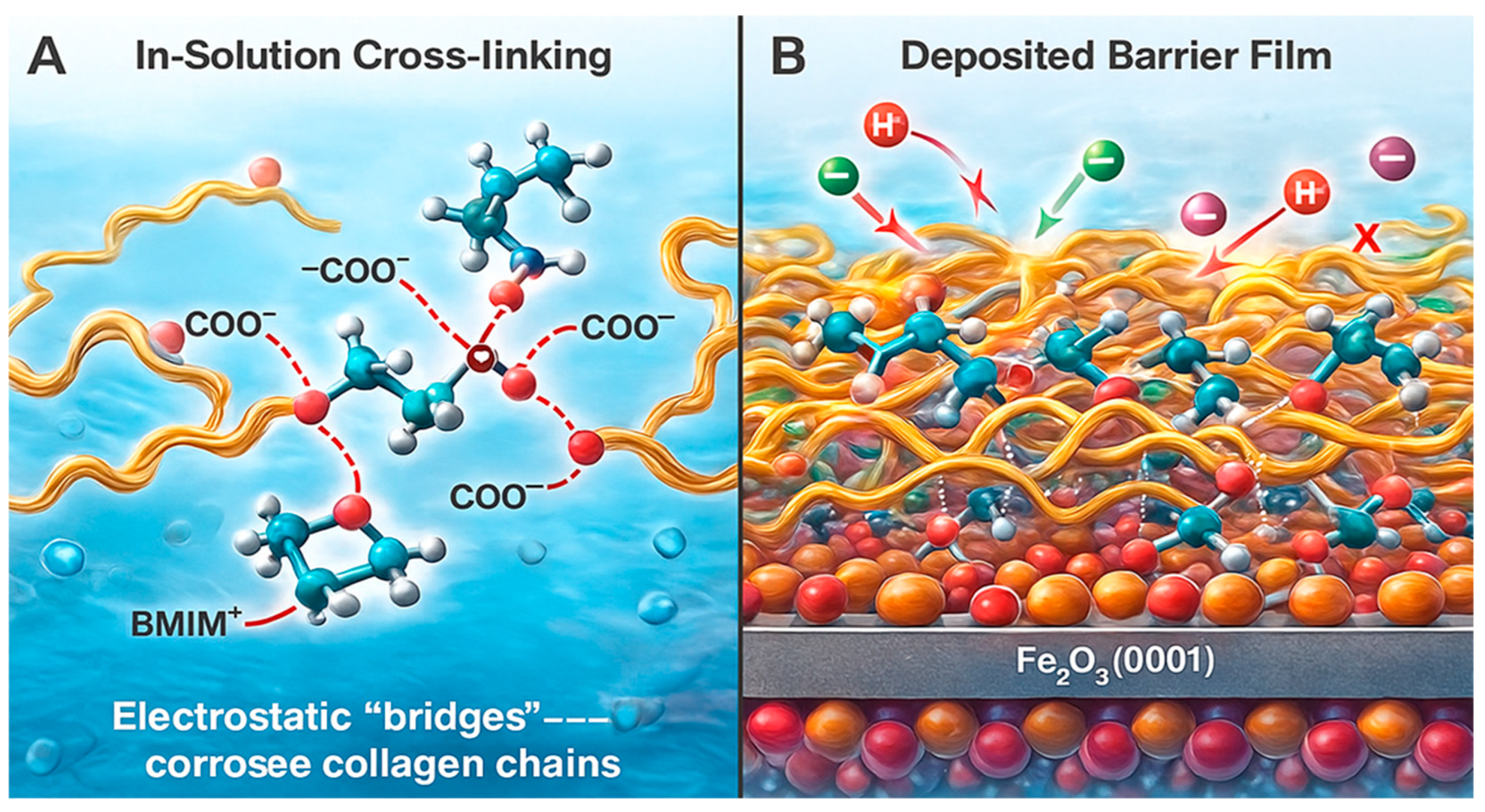
2.7. Proposed Adsorption and Interaction Mechanisms for Collagen on Oxidized Mild Steel in the Presence of BMIM·Br
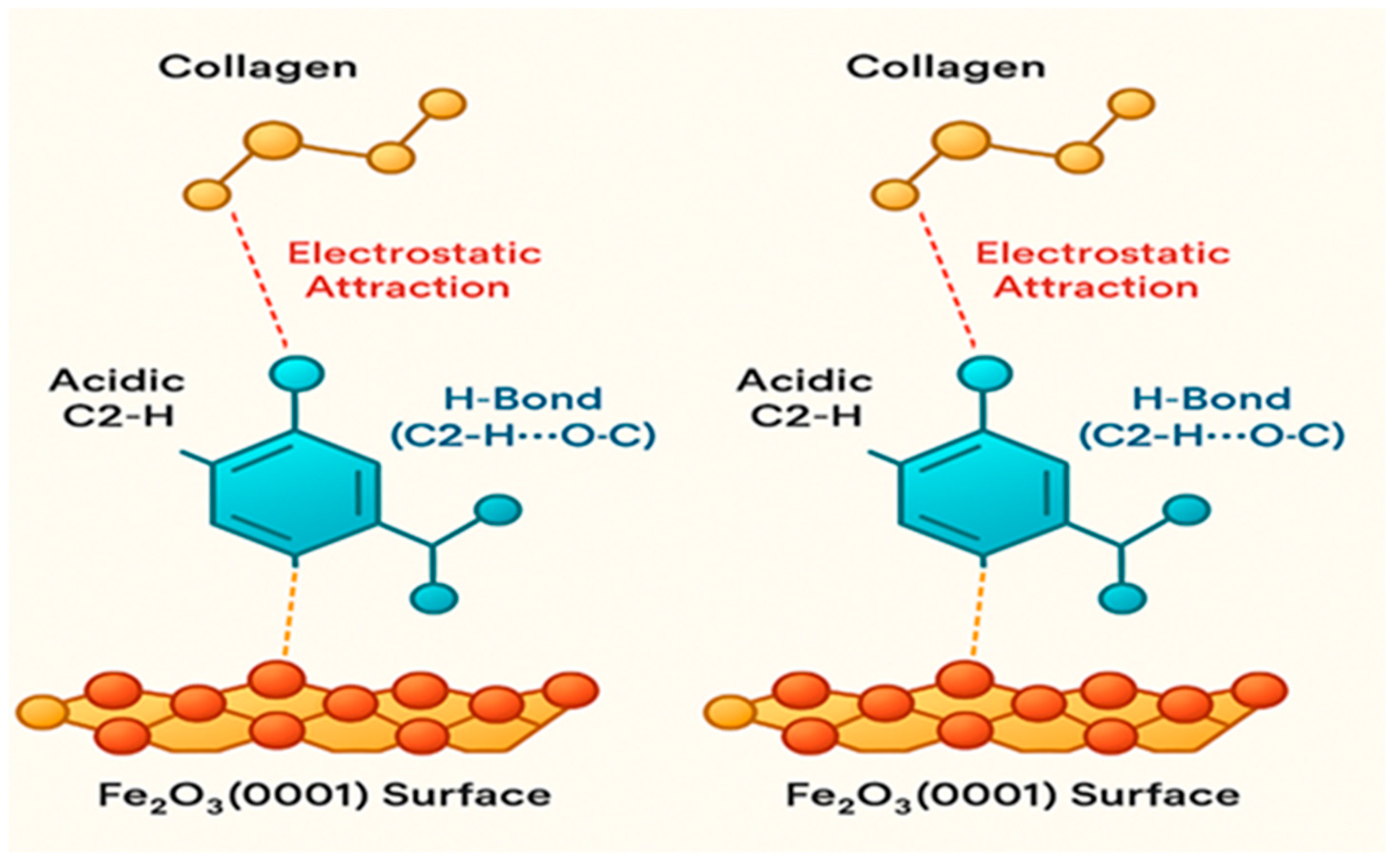
2.7.1. Model 1 (M1)—Direct Coordination
2.7.2. Model 2 (M2)—BMIM-First Monolayer
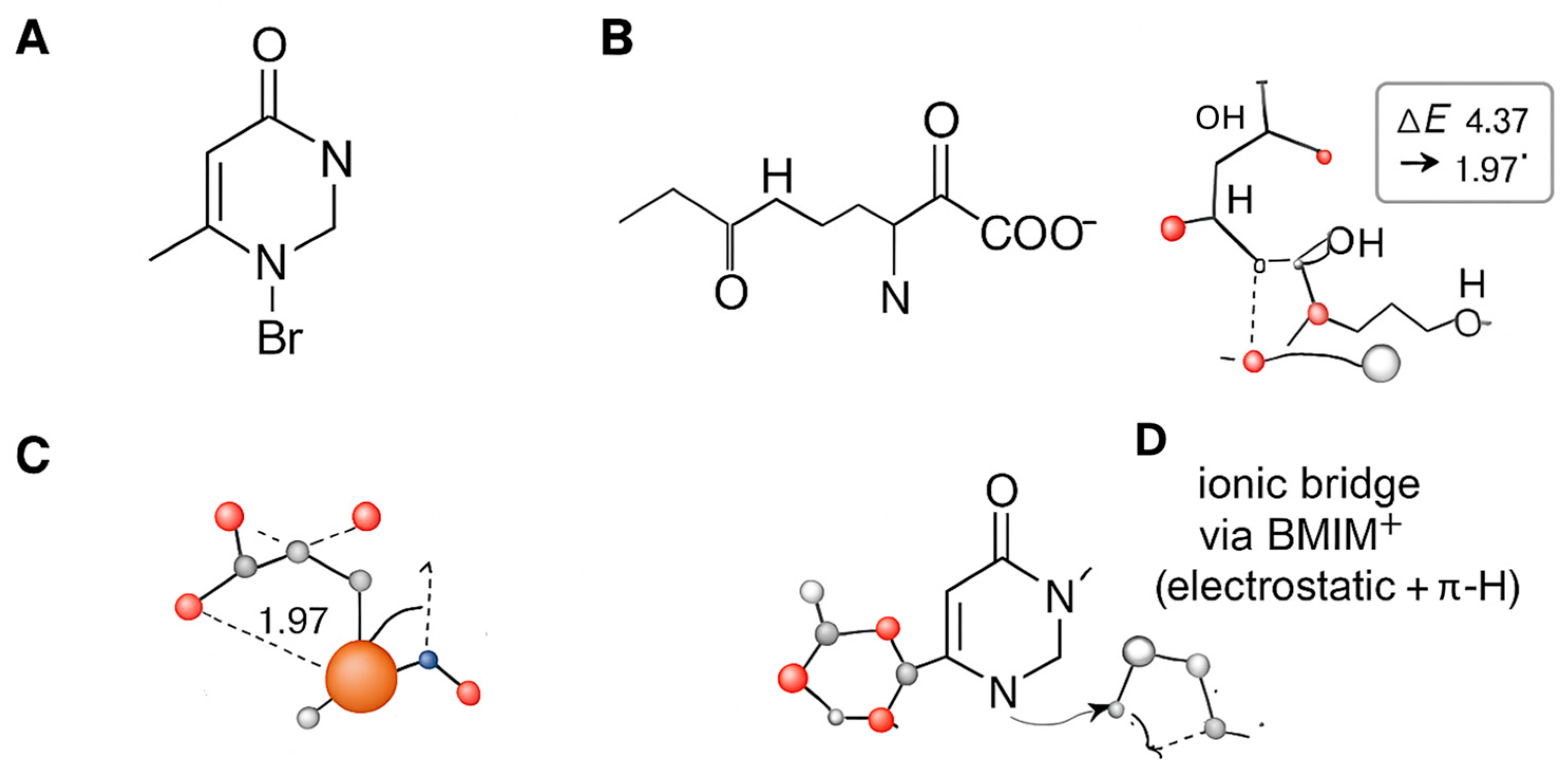
2.7.3. Model 3 (M3)—Ionic Bridge
2.7.4. Model 4 (M4)—Competitive Adsorption
2.7.5. Model 5 (M5)—Co-Accreted Polymeric Film
2.7.6. Model 6 (M6)—Surface-Induced Denaturation
2.8. Theoretical Insights via Density Functional Theory (DFT) Modeling
2.8.1. Modeling Approach and DFT Analysis of Adsorption Energies
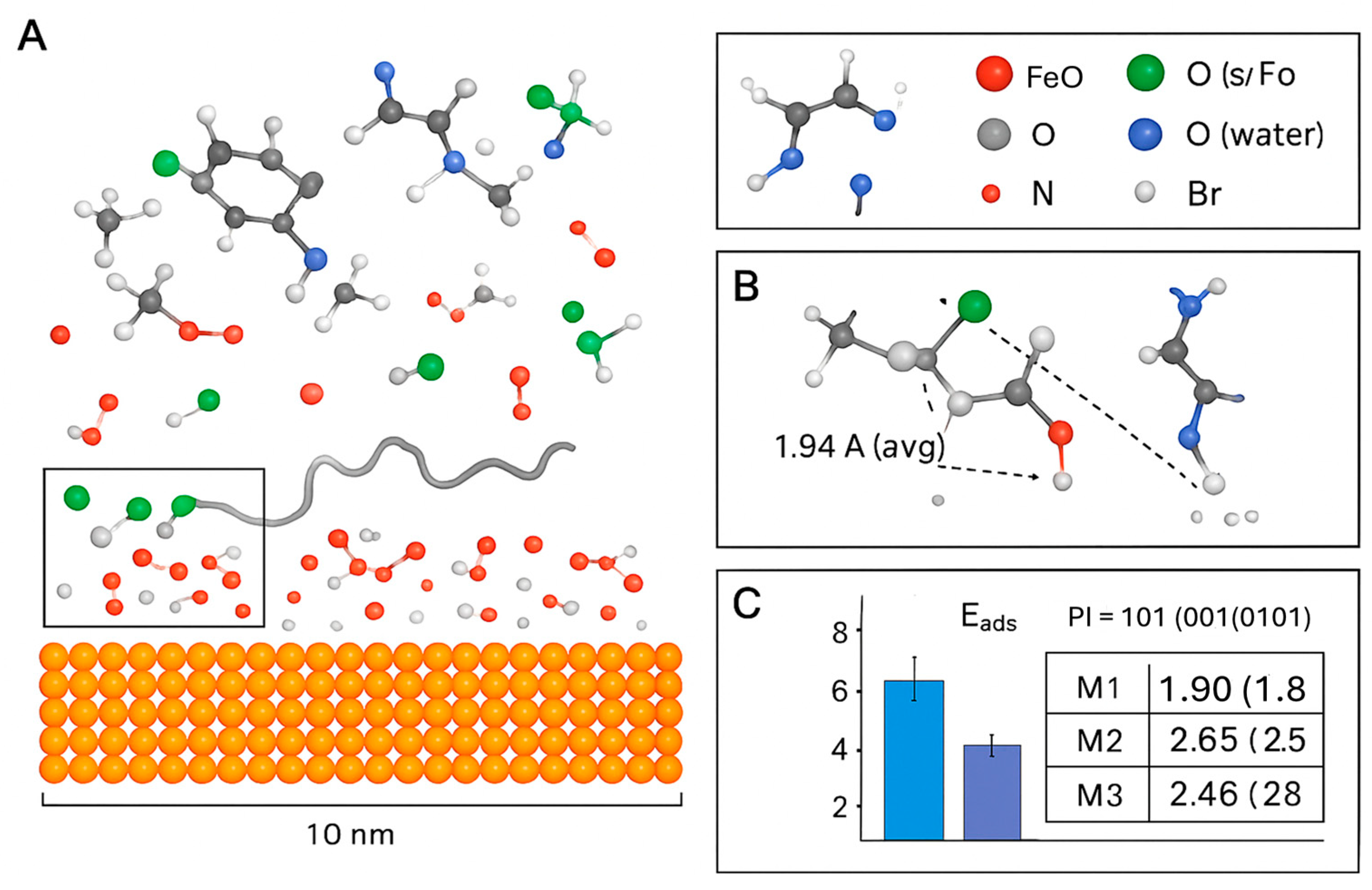
- Direct Coordination (M1) is feasible: The strong adsorption energy of −1.95 eV confirms that collagen fragments can chemisorb directly onto the oxide surface [45].
- BMIM+ adsorbs effectively (M2): The physisorption energy of −0.98 eV for BMIM+ alone validates its role as a surface-active agent.
- Synergy in the Ionic Bridge (M3) is confirmed: The most significant finding is the dramatically increased adsorption energy for the M3 configuration (−2.65 eV). This value is more negative than the sum of M1 and M2, providing clear evidence of a synergistic effect where the BMIM+ cation acts as a bridge, enhancing the binding of collagen to the surface [46]. The computed charge transfer from the inhibitor molecules to the metal surface also increases in this bridged configuration, indicating a more effective barrier to charge transfer during corrosion [38].
- Enhanced Reactivity: The HOMO-LUMO gap (ΔE) narrows for the adsorbed species, with the smallest gap observed for the M3 complex (4.37 eV). A smaller ΔE generally indicates higher chemical reactivity and better electron-donating ability, correlating with the superior inhibition performance of the composite [39,40].
2.8.2. Molecular Dynamics (MD) Simulations: A Complementary Tool
- Dramatic Increase in Contact Points: The simulation quantitatively shows that the composite system establishes over 45% more contact points with the metal surface compared to collagen alone. This is visualized by the dense network of molecules in direct contact with the surface in Figure 8, creating a comprehensive barrier.
- Co-Accreted Polymeric Film (M5): The simulation captures the spontaneous formation of a cross-linked network, where BMIM+ cations dynamically bridge between anionic sites on different collagen chains. This validates the “Co-Accreted Polymeric Film” mechanism (M5), leading to the formation of a thick, cohesive layer that significantly hinders the diffusion of corrosive species [22].
- Surface-Induced Spreading (M6): The collagen fragment is observed to unfold and spread across the surface, maximizing its contact area, which is consistent with the proposed M6 mechanism [41].
2.8.3. Consolidated Theoretical Insight
2.8.4. Bridging Macroscopic Thermodynamics with Molecular-Level Simulations
Spontaneous, Strong Adsorption (ΔG°ads and Kads) Is Explained by Multi-Point Attachment
Endothermic Adsorption (ΔH°ads > 0) Is Driven by Competitive Water Displacement
Large Positive Entropy Change (ΔS°ads > 0) Is Visualized as the Release of Ordered Water
Exceptional Thermal Stability Is Corroborated by the Dense, Cross-Linked Film
The “Ionic Bridge” (M3) Is the Molecular Culprit for Synergy and Mixed Adsorption Mode
3. Materials and Methods
3.1. Materials and Sample Preparation
3.2. Preparation of Inhibitor Solutions
3.3. Characterization Techniques
3.3.1. Fourier Transform Infrared (FTIR) Spectroscopy
3.3.2. X-Ray Diffraction (XRD)
3.4. Corrosion Inhibition Studies
Weight Loss Method
3.5. Adsoption Studies
3.5.1. Langmuir Isotherm
3.5.2. The Freundlich Isotherm
3.5.3. Temkin Isotherm
3.5.4. Frumkin Isotherm
3.5.5. Flory–Huggins Isotherm
3.5.6. El-Awady Isotherm
3.5.7. Weight Loss and Thermodynamic Studies for Composites
3.6. Computational Methods
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verma, C.; Ebenso, E.E.; Quraishi, M.A. Ionic liquids as green and sustainable corrosion inhibitors for metals and alloys: An overview. J. Mol. Liq. 2017, 233, 403–414. [Google Scholar] [CrossRef]
- Saji, V.S. A review on recent patents in corrosion inhibitors. Recent Pat. Corros. Sci. 2010, 3, 6–12. [Google Scholar] [CrossRef]
- Fouda, A.S.; El-Desoky, H.S.; Abdel-Galeil, M.M.; Shalabi, K. Amide Compounds as Corrosion Inhibitors for Carbon Steel in Acidic Environment. Prot. Met. Phys. Chem. Surf. 2022, 58, 151–167. [Google Scholar] [CrossRef]
- Atta, A.M.; El-Mahdy, G.A.; Al-Lohedan, H.A.; Ezzat, A.O. A new green ionic liquid-based corrosion inhibitor for steel in acidic environments. Molecules 2015, 20, 11131–11153. [Google Scholar] [CrossRef]
- Verma, C.; Ebenso, E.E.; Quraishi, M.A. Ionic liquids as green corrosion inhibitors for industrial metals and alloys. Green Chem. 2017, 19, 4048–4058. [Google Scholar]
- Megawati, M.; Yosef, K. Imidazolium-based ionic liquids as corrosion inhibitors for mild steel in acidic medium: Thermodynamic and adsorption studies. J. Mol. Liq. 2022, 345, 117827. [Google Scholar]
- Chen, L.; Lu, D.; Zhang, Y. Organic Compounds as Corrosion Inhibitors for Carbon Steel in HCl Solution: A Comprehensive Review. Materials 2022, 15, 2023. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Singh, V.K.; Quraishi, M.A. Effect of fruit extracts of some environmentally benign green corrosion inhibitors on the corrosion of mild steel in hydrochloric acid solution. J. Mater. Environ. Sci. 2010, 1, 162–174. [Google Scholar]
- Hunt, P.A.; Kirchner, B.; Welton, T. Characterising the Electronic Structure of Ionic Liquids: An Examination of the 1-Butyl-3-Methylimidazolium Chloride Ion Pair. J. Am. Chem. Soc. 2006, 128, 14047–14061. [Google Scholar] [CrossRef] [PubMed]
- Gioia, C.; Ricci, A.; Bernardi, L.; Bouzakri, S.; Thakur, V.K. Ionic liquids as versatile tools in the design of collagen-based materials: A review. ACS Sustain. Chem. Eng. 2022, 10, 13188–13208. [Google Scholar]
- Umoren, S.A.; Solomon, M.M. Synergistic corrosion inhibition effect of metal cations and mixtures of organic compounds: A review. J. Mol. Liq. 2017, 248, 246–273. [Google Scholar] [CrossRef]
- Shahryari, Z.; Ghorbani, M.; Ehsani, A.; Hadi, M.; Javidparvar, A.A.; Ramezanzadeh, B. A combined experimental and theoretical study of the green corrosion inhibition of mild steel in HCl solution by aqueous Citrullus lanatus fruit (CLF) extract. J. Mol. Liq. 2021, 334, 116487. [Google Scholar]
- Alreface, S.H.; Rhee, K.Y.; Verma, C. Challenges and advantages of using ionic liquids as green corrosion inhibitors: The state-of-the-art. J. Mol. Liq. 2022, 345, 117803. [Google Scholar]
- Dhongde, V.; Wadhwani, P.; Sahu, S.K.; Rajendran, S.; Kadu, K.; Bhanvase, B.A. A comprehensive review on the recent developments in polymer-functionalized graphene oxide and its coatings for corrosion protection. Prog. Org. Coat. 2024, 189, 108305. [Google Scholar]
- Hegazy, M.A.; Abdallah, M.; Alfakeer, M. Corrosion inhibition performance of a novel cationic surfactant for carbon steel in HCl solution: Combining experimental and computational studies. J. Mol. Liq. 2021, 323, 114610. [Google Scholar]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Silver, F.H.; Bradica, G. Mechanobiology of force transduction in dermal tissue. Skin Res. Technol. 2002, 8, 3–23. [Google Scholar] [CrossRef]
- Kong, J.; Yu, S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef]
- Benedetto, A.; Ballone, P. Room temperature ionic liquids meet biomolecules: A microscopic view of structure and dynamics. ACS Sustain. Chem. Eng. 2016, 4, 392–412. [Google Scholar] [CrossRef]
- Weerachanchai, P.; Lee, J.M. Effect of water on the corrosion of carbon steel in 1-butyl-3-methylimidazolium bromide ionic liquid. Corros. Sci. 2014, 82, 122–132. [Google Scholar]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef]
- Sankaranarayanan, K.; Sivasallam, K.; Dharmalingam, S. A review on the interaction of ionic liquids with natural macromolecules: Design, characterization, and applications. J. Mol. Liq. 2022, 367, 120447. [Google Scholar]
- Kiefer, J.; Fries, J.; Leipertz, A. Experimental vibrational study of imidazolium-based ionic liquids: Raman and infrared spectra of 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide and 1-ethyl-3-methylimidazolium ethyl-sulfate. Appl. Spectrosc. 2007, 61, 1306–1311. [Google Scholar] [CrossRef]
- Sionkowska, A.; Skopinska-Wisniewska, J.; Gawron, M.; Kolodziejczak, A.; Jankowska, A. Characterization of collagen and its blends with hyaluronic acid and chitosan in infrared and Raman spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 153, 757–763. [Google Scholar]
- Wang, H.; Gurau, G.; Rogers, R.D. Ionic liquid processing of cellulose. Chem. Soc. Rev. 2012, 41, 1519–1537. [Google Scholar] [CrossRef]
- Khandelwal, P.; Singh, D.K. Correlation between structural and mechanical properties of collagen-based scaffolds: A review. J. Biomater. Sci. Polym. Ed. 2021, 32, 1862–1887. [Google Scholar]
- Davis, J.R. Corrosion: Understanding the Basics; ASM International: Materials Park, OH, USA, 2000. [Google Scholar]
- Ansari, K.R.; Quraishi, M.A. Experimental and theoretical investigation of polymeric compounds as corrosion inhibitors for mild steel in acid media. J. Taiwan Inst. Chem. Eng. 2014, 45, 1125–1135. [Google Scholar]
- Alfakeer, M.; Abdallah, M.; Fawzy, A. Corrosion inhibition effect of expired ampicillin and flucloxacillin drugs for mild steel in aqueous acidic medium. Int. J. Electrochem. Sci. 2020, 15, 3283–3297. [Google Scholar] [CrossRef]
- Verma, C.; Ebenso, E.E.; Quraishi, M.A. Corrosion inhibitors for ferrous and non-ferrous metals and alloys in ionic sodium chloride solutions: A review. J. Mol. Liq. 2018, 248, 927–942. [Google Scholar] [CrossRef]
- Chugh, B.; Thakur, S.; Pani, B.; Singh, A.K.; Lgaz, H. Evaluation of a novel cationic surfactant based on schiff base as a corrosion inhibitor for carbon steel in 1 M HCl solution: Experimental and theoretical studies. J. Mol. Liq. 2022, 366, 120293. [Google Scholar]
- Dwivedi, M.; Singh, A.; Kumar, A. A comprehensive review on the application of biopolymer-ionic liquid composites as corrosion inhibitors. J. Mol. Liq. 2023, 391, 123232. [Google Scholar]
- Umoren, S.A.; Solomon, M.M. Effect of halide ions on the corrosion inhibition efficiency of different organic species—A review. J. Ind. Eng. Chem. 2015, 21, 81–100. [Google Scholar] [CrossRef]
- Verma, C.; Ebenso, E.E.; Quraishi, M.A. Molecular structural aspects of organic corrosion inhibitors: Influence of –CN and –NO2 substituents on designing of potential corrosion inhibitors for aqueous media. J. Mol. Liq. 2020, 316, 113874. [Google Scholar] [CrossRef]
- Zarrouk, A.; El Ouali, I.; Bouachrine, M.; Warad, I.; Hammouti, B. Temperature effect on the corrosion inhibition of carbon steel in acidic media by an aromatic hydrazide: Experimental and theoretical studies. Int. J. Electrochem. Sci. 2020, 15, 569–589. [Google Scholar]
- Hegazy, M.A.; Abdallah, M. Comprehensive adsorption and corrosion inhibition studies of two novel cationic surfactants on carbon steel in hydrochloric acid. J. Mol. Liq. 2021, 322, 114555. [Google Scholar]
- Singh, A.K.; Quraishi, M.A. Effect of Cefazolin on the corrosion of mild steel in HCl solution. Corros. Sci. 2010, 52, 152–160. [Google Scholar] [CrossRef]
- Şahin, M.; Bilgiç, S.; Yılmaz, H. The inhibition effects of some cyclic nitrogen compounds on the corrosion of the steel in NaCl mediums. Appl. Surf. Sci. 2002, 195, 1–7. [Google Scholar] [CrossRef]
- Obot, I.B.; Obi-Egbedi, N.O. Theoretical study of benzimidazole and its derivatives and their potential activity as corrosion inhibitors. Corros. Sci. 2010, 52, 657–660. [Google Scholar] [CrossRef]
- Lgaz, H.; Lee, H.-S.; Masroor, S.; Bhat, A.R.; Shubhalaxmi; Chung, I.-M.; Salghi, R.; Ouhssine, M. Corrosion inhibition of mild steel in hydrochloric acid medium by newly synthesized hydrazone derivatives: Experimental and computational studies. J. Mol. Liq. 2019, 280, 240–252. [Google Scholar]
- Leon-Lopez, A.; Morales-Penaloza, A.; Martinez-Juarez, V.M.; Vargas-Torres, A.; Zeugolis, D.I.; Aguirre-Alvarez, G. Hydrolyzed collagen—Sources and applications. Molecules 2019, 24, 4031. [Google Scholar] [CrossRef]
- Kaya, S.; Tüzün, B. DFT and MD simulation of some amino acids as green corrosion inhibitors for carbon steel in acidic medium. J. Indian Chem. Soc. 2023, 100, 100836. [Google Scholar]
- Verma, C.; Lgaz, H.; Verma, D.K.; Ebenso, E.E.; Bahadur, I.; Quraishi, M.A. Molecular dynamics and Monte Carlo simulations as powerful tools for study of interfacial adsorption behaviour of corrosion inhibitors in aqueous phase: A review. J. Mol. Liq. 2018, 260, 99–120. [Google Scholar]
- Obot, I.B.; Kaya, S. Density Functional Theory (DFT) modelling and Monte Carlo simulation assessment of inhibition performance of some carbohydrazide derivatives on steel corrosion. J. Taiwan Inst. Chem. Eng. 2021, 124, 391–398. [Google Scholar]
- ASTM G1-03; Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens. ASTM International: West Conshohocken, PA, USA, 2017.
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Cullity, B.D.; Stock, S.R. Elements of X-Ray Diffraction, 3rd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2001. [Google Scholar]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B. A detailed electrochemical/theoretical exploration of the aqueous Chinese gooseberry fruit shell extract as a green and cheap corrosion inhibitor for mild steel in acidic solution. J. Mol. Liq. 2019, 282, 366–384. [Google Scholar] [CrossRef]
- Ebenso, E.E.; Verma, C.; Olasunkanmi, L.O.; Akpan, E.D.; Quraishi, M.A. Natural Polysaccharides as Corrosion Inhibitors. In Green Corrosion Chemistry and Engineering: Opportunities and Challenges; Sharma, S.K., Ed.; Wiley-VCH: Hoboken, NJ, USA, 2021; pp. 59–89. [Google Scholar]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, G.; Shi, B. Thermal properties of collagen and its application in biomaterials. J. Therm. Anal. Calorim. 2016, 124, 1099–1106. [Google Scholar]
- Migahed, M.A.; Al-Sabagh, A.M.; Nasser, N.M.; Khamis, E.A.; El-moneim, A.A. Synthesis and evaluation of some cationic surfactants as corrosion inhibitors for carbon steel in acidic media: Experimental and theoretical studies. J. Mol. Liq. 2020, 318, 114315. [Google Scholar]
- Hegazy, M.A. A novel Schiff base-based cationic gemini surfactants: Synthesis and effect on corrosion inhibition of carbon steel in hydrochloric acid solution. Corros. Sci. 2009, 51, 2610–2618. [Google Scholar] [CrossRef]
- Gece, G. The use of quantum chemical methods in corrosion inhibitor studies. Corros. Sci. 2008, 50, 2981–2992. [Google Scholar] [CrossRef]



| Conc. (g/L) | 30 °C | 40 °C | 50 °C | 60 °C |
|---|---|---|---|---|
| 1.0 | 0.0291 ± 0.0002 | 0.0406 ± 0.0003 | 0.0529 ± 0.0004 | 0.0572 ± 0.0005 |
| 1.5 | 0.0260 ± 0.0002 | 0.0397 ± 0.0003 | 0.0491 ± 0.0003 | 0.0551 ± 0.0004 |
| 2.0 | 0.0246 ± 0.0002 | 0.0373 ± 0.0003 | 0.0483 ± 0.0003 | 0.0529 ± 0.0004 |
| 2.5 | 0.0228 ± 0.0002 | 0.0351 ± 0.0003 | 0.0454 ± 0.0003 | 0.0519 ± 0.0004 |
| Conc. (g/L) | 30 °C | 40 °C | 50 °C | 60 °C |
|---|---|---|---|---|
| 1.0 | 95.63 | 95.48 | 95.42 | 95.40 |
| 1.5 | 96.09 | 95.58 | 95.75 | 95.57 |
| 2.0 | 96.30 | 95.84 | 95.82 | 95.75 |
| 2.5 | 96.57 | 96.09 | 96.07 | 95.83 |
| Inhibitor System | Acid Environment | Temperature (°C) | Maximum Inhibition Efficiency (%) | Reference |
|---|---|---|---|---|
| Gelatin | 1 M HCl | 30 | ~90 | [10] |
| Chitosan–Ionic Liquid Composite | 1 M HCl | 30 | ~94 | [12] |
| Lignin | 1 M HCl | 25 | ~92 | [13] |
| Collagen–BMIM·Br (This work) | 1.5 M HCl | 30–60 | >96.5 (at 30 °C); >95.8 (at 60 °C) | – |
| Temperature (K) | Kads (L/g) | ΔG°ads (kJ/mol) | ΔH°ads (kJ/mol) | ΔS°ads (J/mol·K) |
|---|---|---|---|---|
| 303 | 18.03 | −17.38 | ||
| 313 | 18.44 | −18.01 | +12.85 | +66.4 |
| 323 | 18.75 | −18.63 | ||
| 333 | 19.28 | −19.29 |
| Model | Parameter (s) | MS (303 K) | MS (313 K) | MS (323 K) | MS (333 K) |
|---|---|---|---|---|---|
| Langmuir | Kads (M−1) | 57.78 | 76.35 | 84.71 | 117.71 |
| ΔG°ads (kJ·mol−1) | −19.53 | −20.89 | −21.86 | −23.47 | |
| R2 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | |
| Temkin | a (J·mol−1) | 99.99 | 151.43 | 150.71 | 206.83 |
| log KT | 41.53 | 62.75 | 62.46 | 85.69 | |
| R2 | 0.9930 | 0.9080 | 0.9600 | 0.9917 | |
| Freundlich | 1/n | 96.05 | 145.8 | 144.1 | 197.6 |
| KF (M−1) | 0.956 | 0.954 | 0.954 | 0.954 | |
| R2 | 0.9929 | 0.9084 | 0.9601 | 0.9917 | |
| Frumkin | af (dimensionless) | −36.30 | −56.35 | −60.07 | −90.62 |
| log Kads | 31.49 | 47.99 | 51.10 | 76.40 | |
| R2 | 0.9873 | 0.8671 | 0.9425 | 0.9893 | |
| Flory-Huggins | x (sites) | 3.83 | 5.71 | 6.10 | 8.94 |
| log Kads | 5.18 | 7.60 | 8.15 | 11.92 | |
| R2 | 0.9925 | 0.8979 | 0.9566 | 0.9908 | |
| El-Awady | 1/y (sites per mol.) | 0.267 | 0.163 | 0.163 | 0.115 |
| K′ (M−1) | 21.90 | 20.77 | 20.87 | 20.70 | |
| R2 | 0.9927 | 0.8996 | 0.9573 | 0.9910 |
| Model Configuration | Description | Eads (eV) | Eads (kJ/mol) | EHOMO (eV) | ELUMO (eV) | ΔE (eV) |
|---|---|---|---|---|---|---|
| Isolated GPH | Collagen Tripeptide | -- | -- | −6.12 | −1.05 | 5.07 |
| Isolated BMIM+ | Ionic Liquid Cation | -- | -- | −7.85 | −0.38 | 7.47 |
| M1: Direct Coordination | GPH on Fe2O3 | −1.95 | −188 | −5.88 | −1.21 | 4.67 |
| M2: BMIM-First Layer | BMIM+ on Fe2O3 | −0.98 | −95 | −7.72 | −0.45 | 7.27 |
| M3: Ionic Bridge | GPH+BMIM+ on Fe2O3 | −2.65 | −256 | −5.65 | −1.28 | 4.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klink, M.J. Multi-Modal Adsorption and Synergistic Corrosion Inhibition of a Collagen–BMIM·Br Composite on Mild Steel. Int. J. Mol. Sci. 2025, 26, 11355. https://doi.org/10.3390/ijms262311355
Klink MJ. Multi-Modal Adsorption and Synergistic Corrosion Inhibition of a Collagen–BMIM·Br Composite on Mild Steel. International Journal of Molecular Sciences. 2025; 26(23):11355. https://doi.org/10.3390/ijms262311355
Chicago/Turabian StyleKlink, Michael John. 2025. "Multi-Modal Adsorption and Synergistic Corrosion Inhibition of a Collagen–BMIM·Br Composite on Mild Steel" International Journal of Molecular Sciences 26, no. 23: 11355. https://doi.org/10.3390/ijms262311355
APA StyleKlink, M. J. (2025). Multi-Modal Adsorption and Synergistic Corrosion Inhibition of a Collagen–BMIM·Br Composite on Mild Steel. International Journal of Molecular Sciences, 26(23), 11355. https://doi.org/10.3390/ijms262311355






