Identification of Fibrillarin and Cajal Bodies Under DNA Replication Stress Conditions in Root Meristem Cells of Allium cepa
Abstract
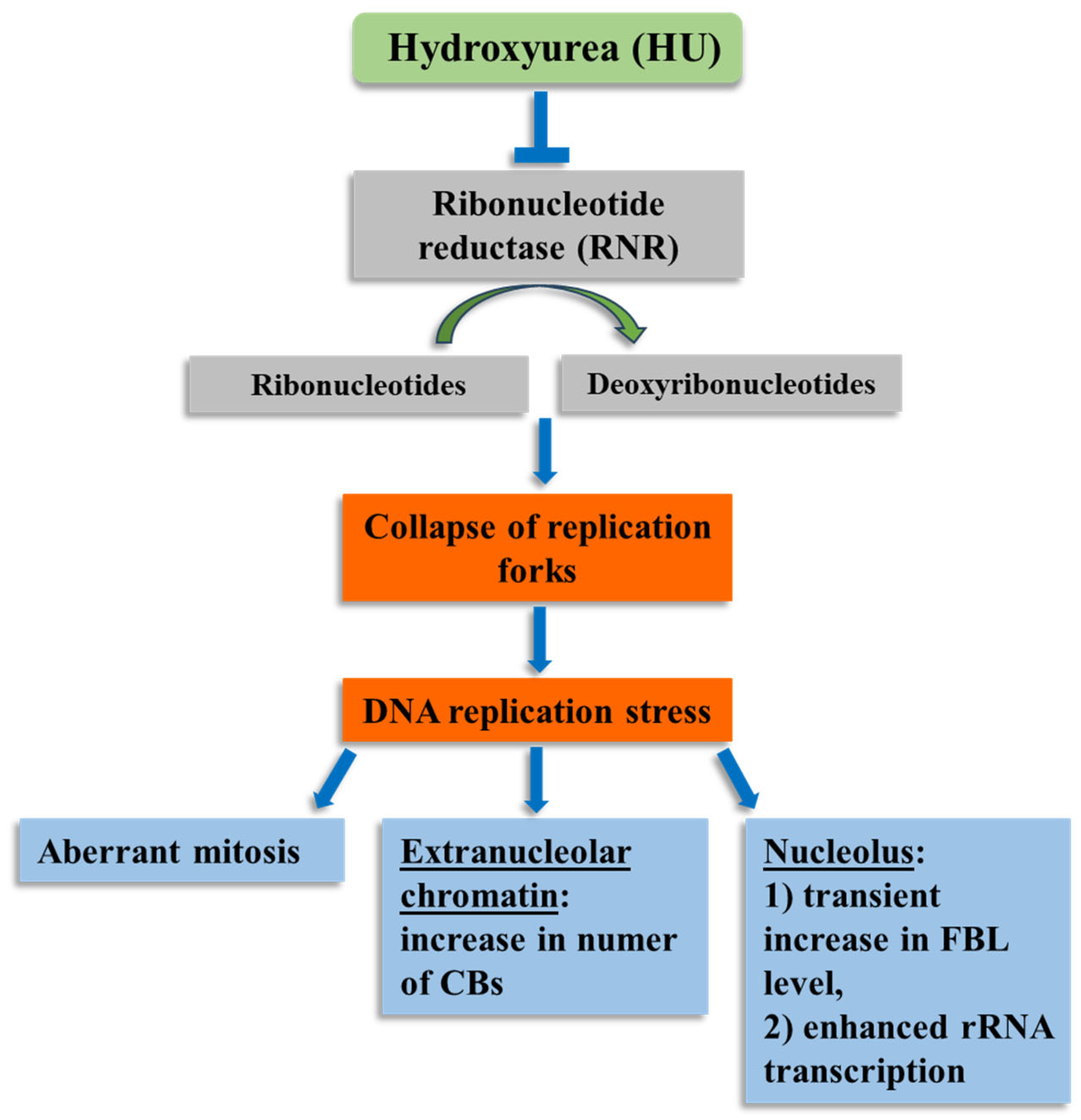
1. Introduction
2. Results
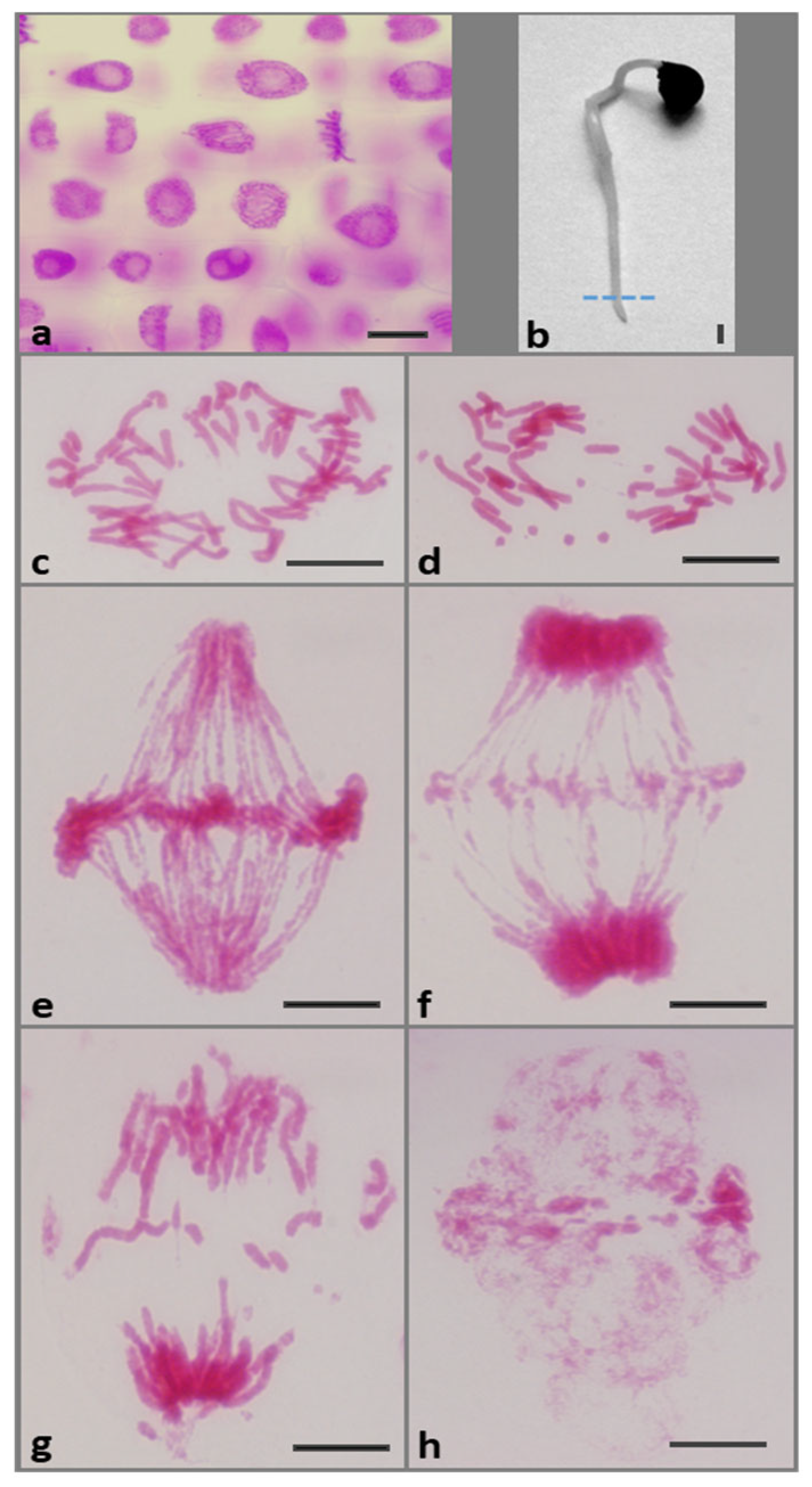
2.1. Replication Stress (RS) Generates Chromosomal Aberrations
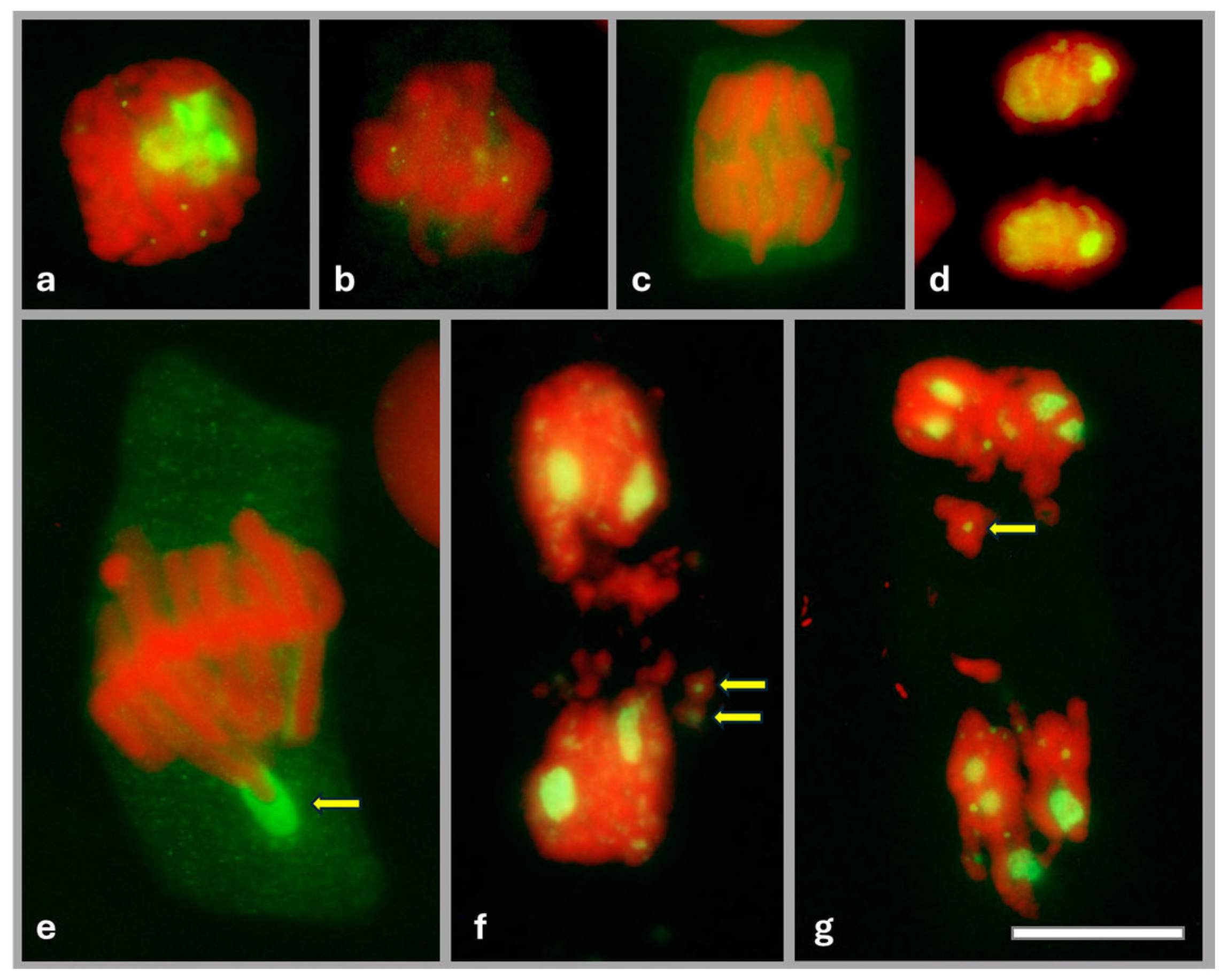
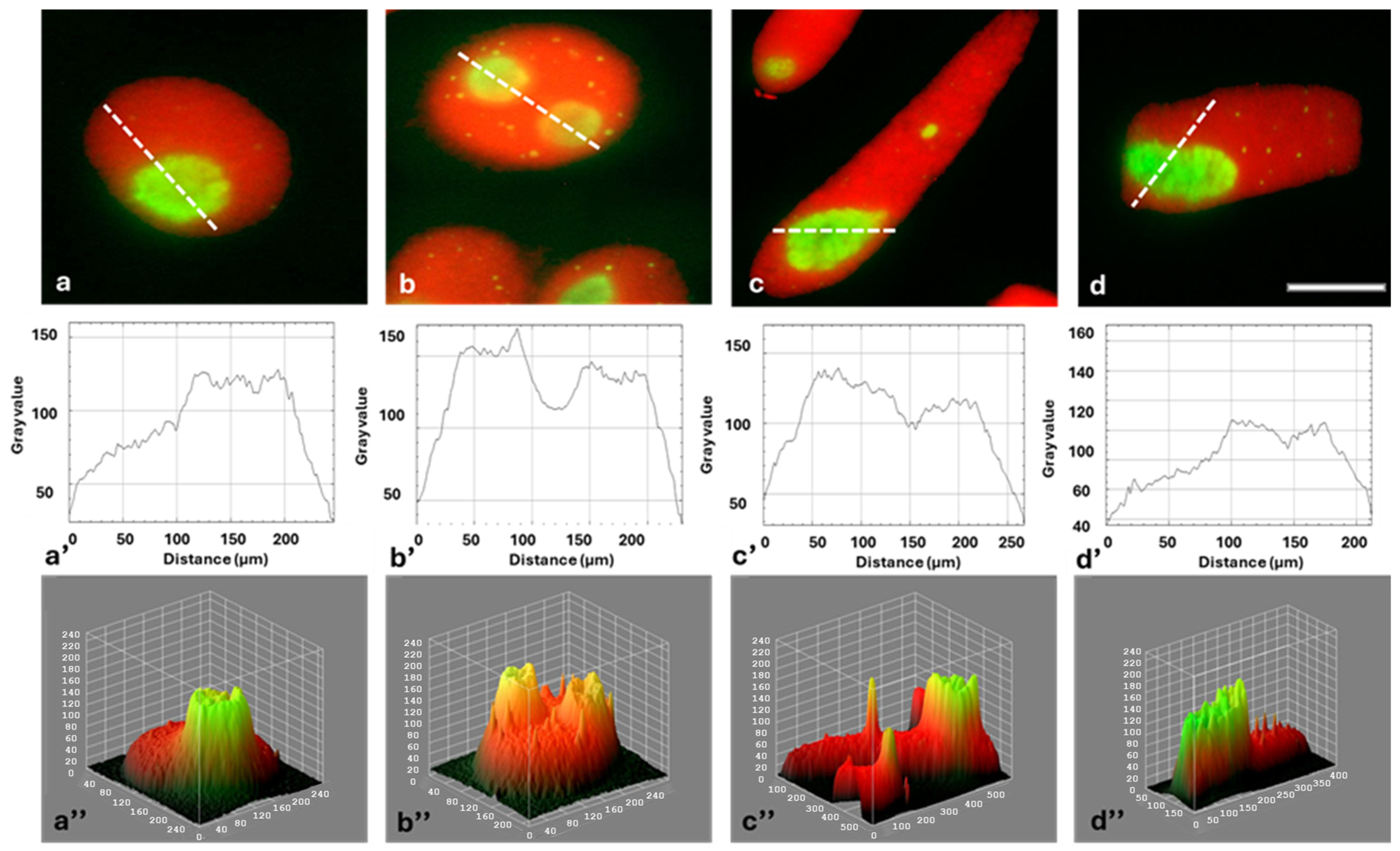
2.2. Identification of Fibrillarin (FBL) in Onion Root Meristematic Cells
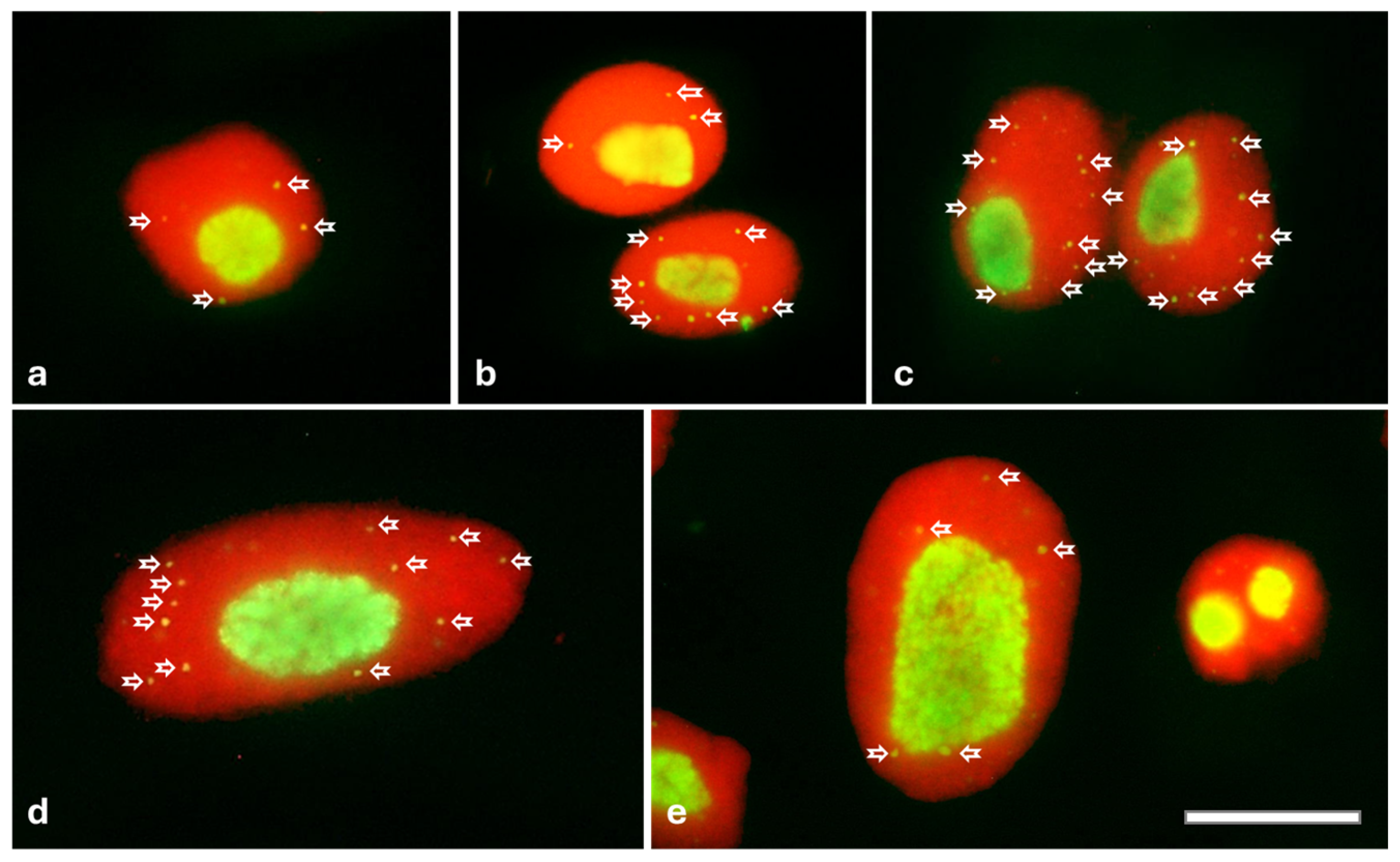
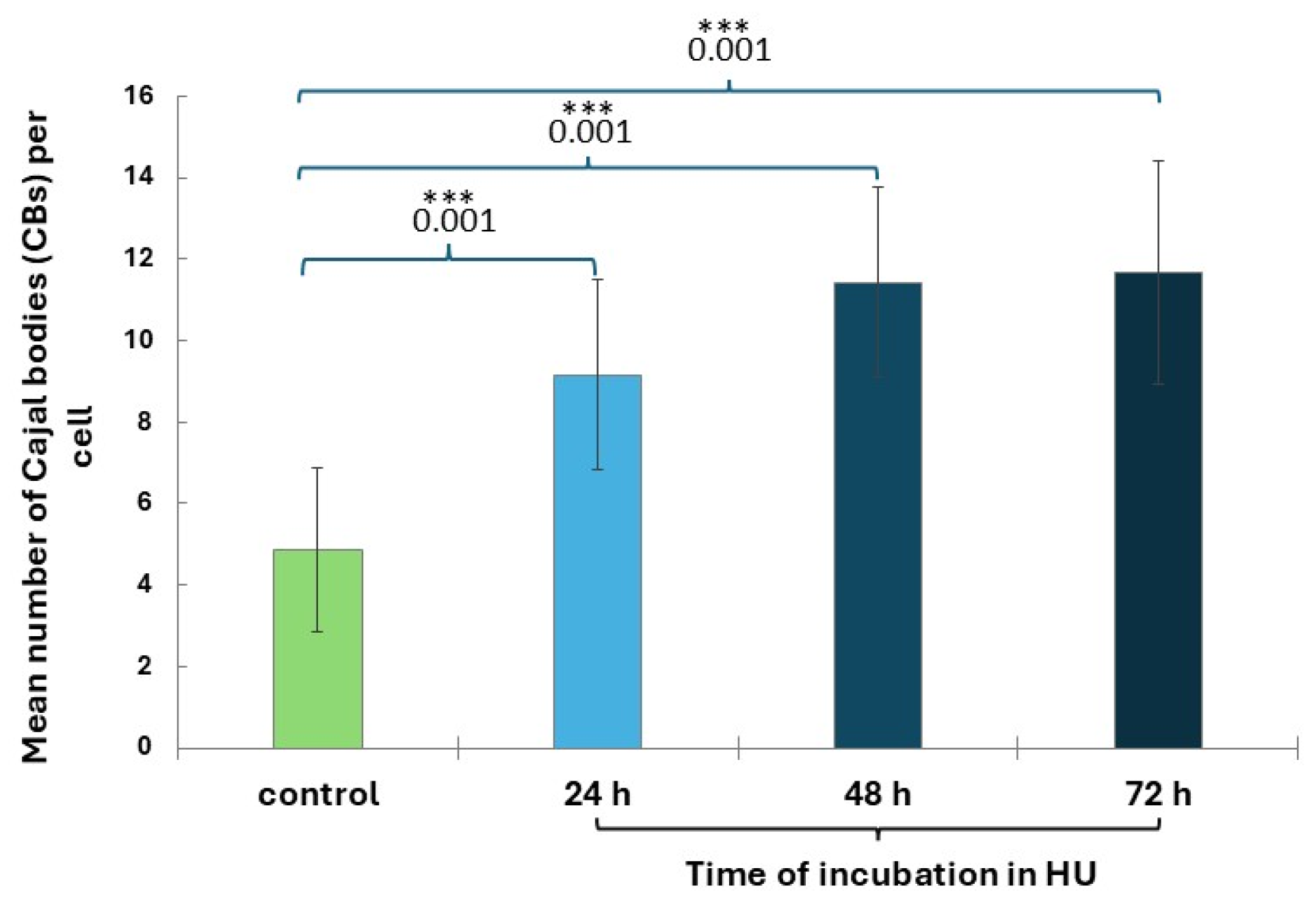
2.3. Cajal Bodies (CBs) in Root Meristem Cell Nuclei
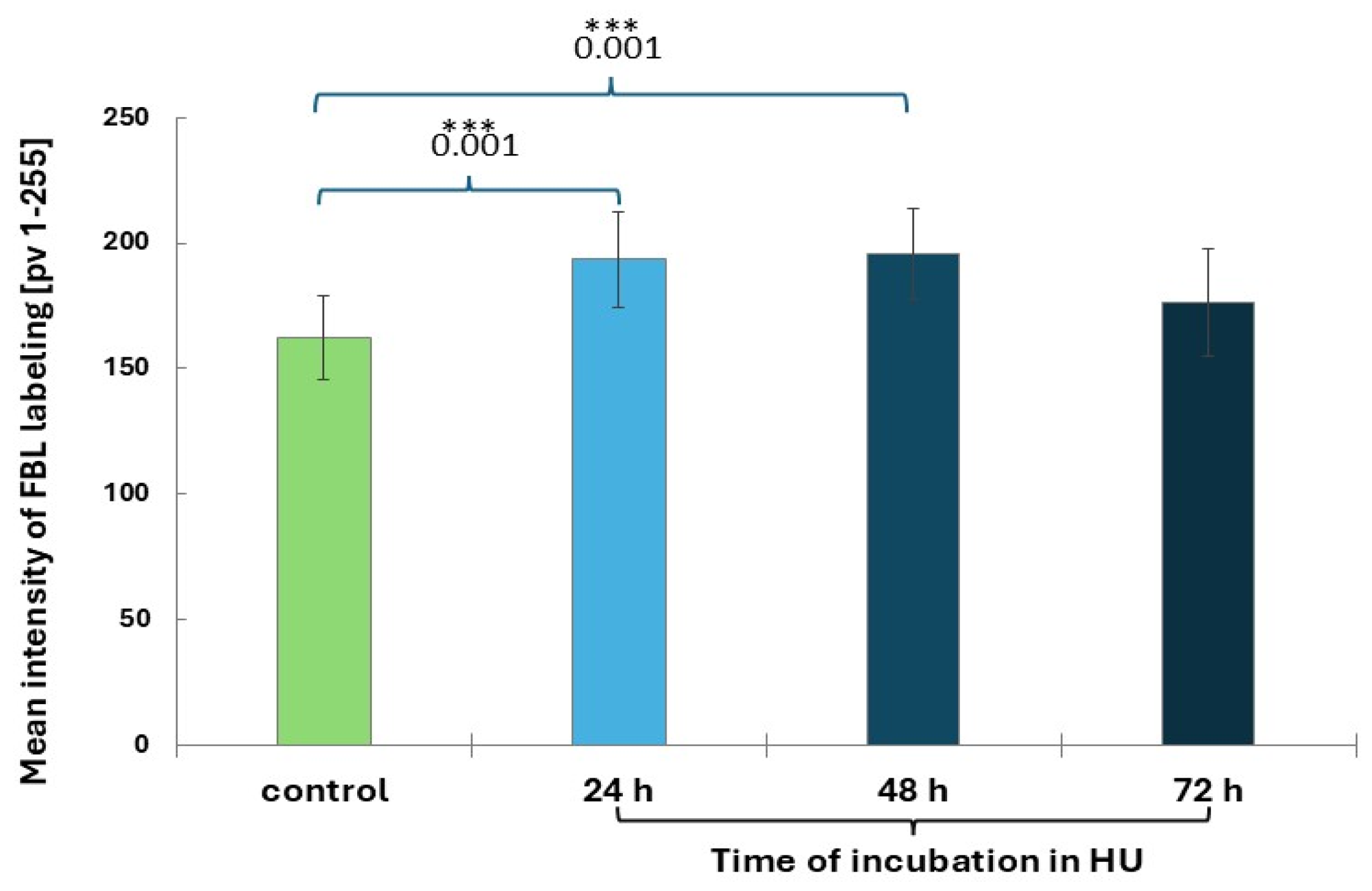
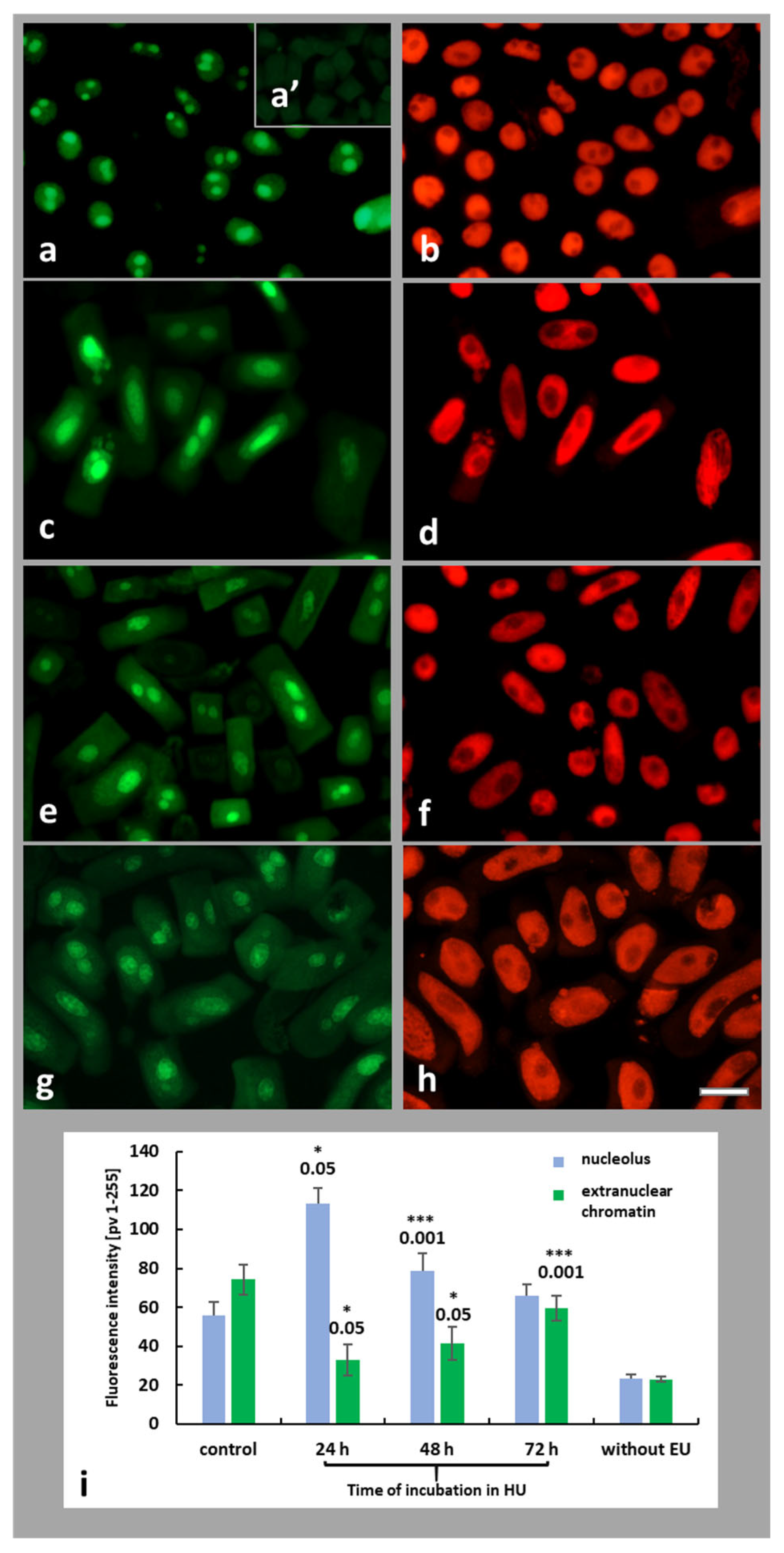
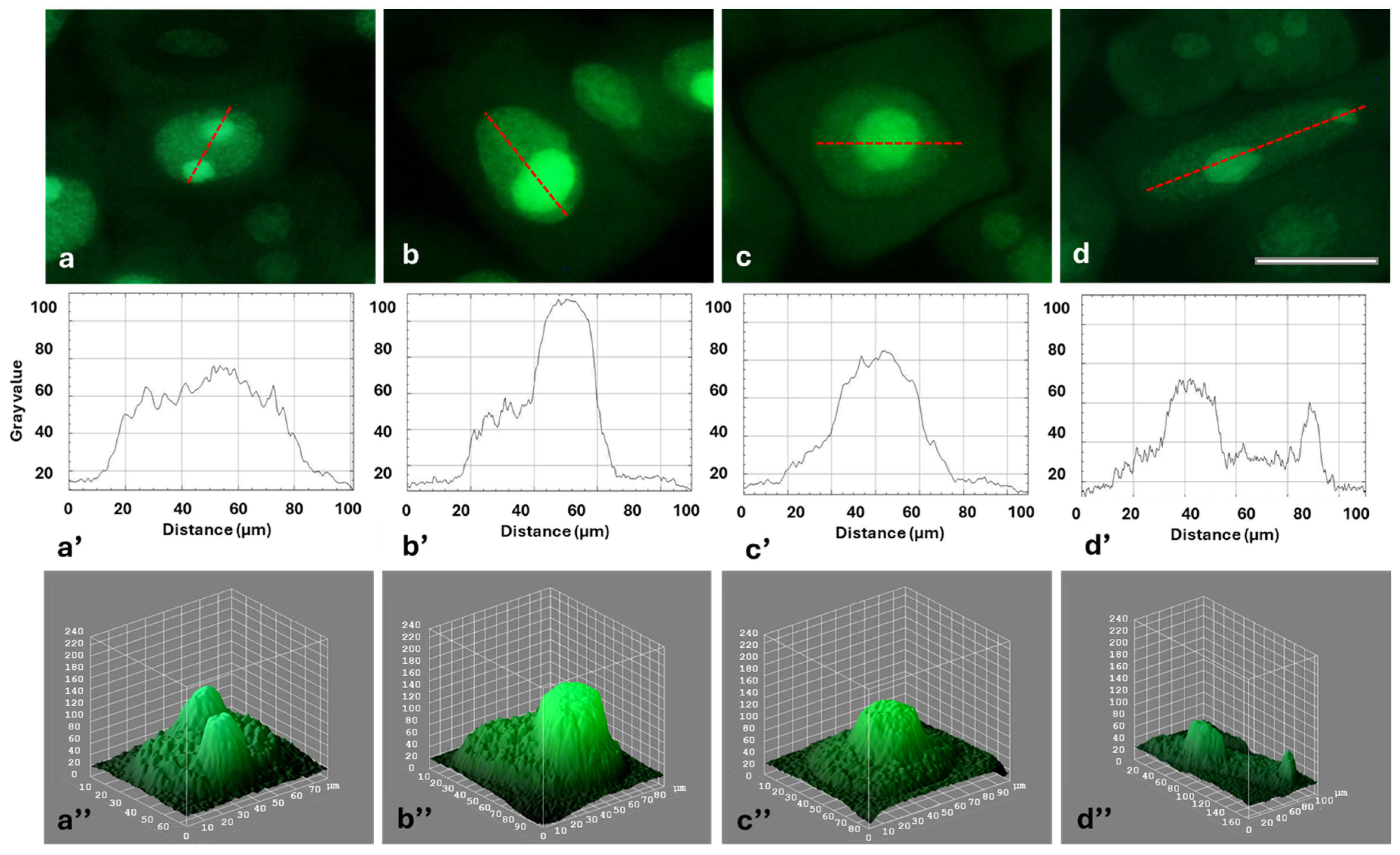
2.4. HU-Induced Changes in the Dynamics of Transcription
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. DNA Staining by the Feulgen Method
4.3. Immunocytochemical Detection of Fibrillarin (FBL)
4.4. 5-Ethynyl Uridine Incorporation
4.5. Observations and Microscope Analyses
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, Y.L.; Pasero, P. Replication stress: From chromatin to immunity and beyond. Curr. Opin. Genet. Dev. 2021, 71, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Iyer, D.R.; Rhind, N. Replication fork slowing and stalling are distinct, checkpoint-independent consequences of replicating damaged DNA. PLoS Genet. 2017, 13, e1006958. [Google Scholar] [CrossRef]
- Mazouzi, A.; Velimezi, G.; Loizou, J.I. DNA replication stress: Causes, resolution and disease. Exp. Cell Res. 2014, 329, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Nisa, M.; Eekhout, T.; Bergis, C.; Pedroza-Garcia, J.A.; He, X.; Mazubert, C.; Vercauteren, I.; Cools, T.; Brik-Chaouche, R.; Drouin-Wahbi, J.; et al. Distinctive and complementary roles of E2F transcription factors during plant replication stress responses. Mol. Plant 2023, 16, 1269–1282. [Google Scholar] [CrossRef]
- Herr, L.M.; Schaffer, E.D.; Fuchs, K.F.; Datta, A.; Brosh, R.M., Jr. Replication stress as a driver of cellular senescence and aging. Commun. Biol. 2024, 7, 616. [Google Scholar] [CrossRef]
- Khamidullina, A.I.; Abramenko, Y.E.; Bruter, A.V.; Tatarskiy, V.V. Key proteins of replication stress response and cell cycle control as cancer therapy targets. Int. J. Mol. Sci. 2024, 25, 1263. [Google Scholar] [CrossRef]
- Saxena, S.; Zou, L. Hallmarks of DNA replication stress. Mol. Cell 2022, 82, 2298–2314. [Google Scholar] [CrossRef]
- Nisa, M.U.; Huang, Y.; Benhamed, M.; Raynaud, C. The Plant DNA Damage response: Signaling pathways leading to growth inhibition and putative role in response to stress conditions. Front. Plant Sci. 2019, 10, 653. [Google Scholar] [CrossRef]
- Ge, X.Q.; Blow, J.J. Chk1 inhibits replication factory activation but allows dormant origin firing in existing factories. J. Cell Biol. 2010, 191, 1285–1297. [Google Scholar] [CrossRef]
- Herlihy, A.E.; de Bruin, R.A. The role of the transcriptional response to DNA replication stress. Genes 2017, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Alver, R.C.; Chadha, G.S.; Blow, J.J. The contribution of dormant origins to genome stability: From cell biology to human genetics. DNA Repair 2014, 19, 182–189. [Google Scholar] [CrossRef]
- Keszthelyi, A.; Minchell, N.E.; Baxter, J. The causes and consequences of topological stress during DNA replication. Genes 2016, 7, 134. [Google Scholar] [CrossRef]
- Zeman, M.K.; Cimprich, K. The causes and consequences of replication stress. Nat. Cell Biol. 2013, 16, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, S.; Méndez, J. DNA replication stress: From molecular mechanisms to human disease. Chromosoma 2017, 126, 1–15. [Google Scholar] [CrossRef]
- Wozniak, K.J.; Simmons, L.A. Hydroxyurea induces a stress response that alters DNA replication and nucleotide metabolism in Bacillus subtilis. J. Bacteriol. 2021, 203, e0017121. [Google Scholar] [CrossRef]
- Zimanyi, C.M.; Chen, P.Y.; Kang, G.; Funk, M.A.; Drennan, C.L. Molecular basis for allosteric specificity regulation in class Ia ribonucleotide reductase from Escherichia coli. eLife 2016, 5, e07141. [Google Scholar] [CrossRef] [PubMed]
- Żabka, A.; Polit, J.T.; Maszewski, J. Inter- and intrachromosomal asynchrony of cell division cycle events in root meristem cells of Allium cepa: Possible connection with gradient of cyclin B-like proteins. Plant Cell Rep. 2010, 29, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Żabka, A.; Polit, J.T.; Maszewski, J. DNA replication stress induces deregulation of the cell cycle events in root meristems of Allium cepa. Ann. Bot. 2012, 110, 1581–1591. [Google Scholar] [CrossRef]
- Żabka, A.; Trzaskoma, P.; Maszewski, J. Dissimilar effects of β-lapachone- and hydroxyurea-induced DNA replication stress in root meristem cells of Allium cepa. Plant Physiol. Biochem. 2013, 73, 282–293. [Google Scholar] [CrossRef]
- Żabka, A.; Trzaskoma, P.; Winnicki, K.; Polit, J.T.; Chmielnicka, A.; Maszewski, J. The biphasic interphase-mitotic polarity of cell nuclei induced under DNA replication stress seems to be correlated with Pin2 localization in root meristems of Allium cepa. J. Plant Physiol. 2015, 174, 62–70. [Google Scholar] [CrossRef]
- Żabka, A.; Winnicki, K.; Polit, J.T.; Bernasinska, J.; Maszewski, J. Localization sites of nuclear envelope SUN2-like proteins in root meristem cells of Allium cepa under hydroxyurea-induced DNA replication stress. Acta Physiol. Plant. 2015, 37, 163. [Google Scholar] [CrossRef]
- Żabka, A.; Gocek, N.; Polit, J.T.; Maszewski, J. Oxidative replication stress induced by long-term exposure to hydroxyurea in root meristem cells of Vicia faba. Plant Cell Rep. 2024, 43, 87. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.T.; Rao, P.N. Mammalian cell fusion: Induction of premature chromosome condensation in interphase nuclei. Nature 1970, 226, 717–722. [Google Scholar] [CrossRef]
- Gotoh, E.; Durante, M. Chromosome condensation outside of mitosis: Mechanisms and new tools. J. Cell Physiol. 2006, 209, 297–304. [Google Scholar] [CrossRef]
- Himanen, K.; Vuylsteke, M.; Vanneste, S.; Vercruysse, S.; Boucheron, E.; Alard, P.; Chriqui, D.; Van Montagu, M.; Inzé, D.; Beeckman, T. Transcript profiling of early lateral root initiation. Proc. Natl. Acad. Sci. USA 2004, 101, 5146–5151. [Google Scholar] [CrossRef] [PubMed]
- Vanneste, S.; De Rybel, B.; Beemster, G.T.; Ljung, K.; De Smet, I.; Van Isterdael, G.; Naudts, M.; Iida, R.; Gruissem, W.; Tasaka, M.; et al. Cell cycle progression in the pericycle is not sufficient for SOLITARY ROOT/IAA14-mediated lateral root initiation in Arabidopsis thaliana. Plant Cell 2005, 17, 3035–3050. [Google Scholar] [CrossRef]
- Jiao, L.; Liu, Y.; Yu, X.Y.; Pan, X.; Zhang, Y.; Tu, J.; Song, Y.H.; Li, Y. Ribosome biogenesis in disease: New players and therapeutic targets. Signal Transduct. Target Ther. 2023, 8, 15. [Google Scholar]
- Maehama, T.; Nishio, M.; Otani, J.; Mak, T.W.; Suzuki, A. Nucleolar stress: Molecular mechanisms and related human diseases. Cancer Sci. 2023, 114, 2078–2086. [Google Scholar] [CrossRef]
- Rakitina, D.V.; Taliansky, M.; Brown, J.W.; Kalinina, N.O. Two RNA-binding sites in plant fibrillarin provide interactions with various RNA substrates. Nucleic Acids Res. 2011, 39, 8869–8880. [Google Scholar] [CrossRef]
- Amin, M.A.; Matsunaga, S.; Ma, N.; Takata, H.; Yokoyama, M.; Uchiyama, S.; Fukui, K. Fibrillarin, a nucleolar protein, is required for normal nuclear morphology and cellular growth in HeLa cells. Biochem. Biophys. Res. Commun. 2007, 360, 320–326. [Google Scholar] [CrossRef]
- Rodriguez-Corona, U.; Pereira-Santana, A.; Sobol, M.; Rodriguez-Zapata, L.C.; Hozak, P.; Castano, E. Novel ribonuclease activity differs between fibrillarins from Arabidopsis thaliana. Front. Plant Sci. 2017, 8, 1878. [Google Scholar] [CrossRef] [PubMed]
- Ochs, R.L.; Lischwe, M.A.; Spohn, W.H.; Busch, H. Fibrillarin: A new protein of the nucleolus identified by autoimmune sera. Biol. Cell 1985, 54, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Cerdido, A.; Medina, F.J. Subnucleolar location of fibrillarin and variation in its levels during the cell cycle and during differentiation of plant cells. Chromosoma 1995, 103, 625–634. [Google Scholar] [CrossRef]
- Snaar, S.; Wiesmeijer, K.; Jochemsen, A.G.; Tanke, H.J.; Dirks, R.W. Mutational analysis of fibrillarin and its mobility in living human cells. J. Cell Biol. 2000, 151, 653–662. [Google Scholar] [CrossRef]
- Boudonck, K.; Dolan, L.; Shaw, P.J. The movement of coiled bodies visualized in living plant cells by the green fluorescent protein. Mol. Biol. Cell 1999, 10, 2297–2307. [Google Scholar] [CrossRef]
- Love, A.J.; Yu, C.; Petukhova, N.V.; Kalinina, N.O.; Chen, J.; Taliansky, M.E. Cajal bodies and their role in plant stress and disease responses. RNA Biol. 2017, 4, 779–790. [Google Scholar] [CrossRef]
- Morris, G.E. The Cajal body. Biochim. Biophys. Acta 2008, 1783, 2108–2115. [Google Scholar] [CrossRef]
- Collier, S.; Pendle, A.; Boudonck, K.; van Rij, T.; Dolan, L.; Shaw, P. A distant coilin homologue is required for the formation of cajal bodies in Arabidopsis. Mol. Biol. Cell 2006, 17, 2942–2951. [Google Scholar] [CrossRef]
- Walker, M.; Tian, L.; Matera, A. Reduced viability, fertility and fecundity in mice lacking the cajal body marker protein, coilin. PLoS ONE 2009, 4, e6171. [Google Scholar] [CrossRef] [PubMed]
- Strzelecka, M.; Trowitzsch, S.; Weber, G.; Luhrmann, R.; Oates, A.C.; Neugebauer, K.M. Coilin-dependent snRNP assembly is essential for zebrafish embryogenesis. Nat. Struct. Mol. Biol. 2010, 17, 403–409. [Google Scholar] [CrossRef]
- Lafarga, M.; Berciano, M.T.; Garcia-Segura, L.M.; Andres, M.A.; Carmo-Fonseca, M. Acute osmotic/stress stimuli induce a transient decrease of transcriptional activity in the neurosecretory neurons of supraoptic nuclei. J. Neurocytol. 1998, 27, 205–217. [Google Scholar] [CrossRef]
- Cioce, M.; Lamond, A.I. Cajal bodies: A long history of discovery. Annu. Rev. Cell Dev. Biol. 2005, 21, 105–131. [Google Scholar] [CrossRef] [PubMed]
- Sleeman, J.E.; Ajuh, P.; Lamond, A.I. snRNP protein expression enhances the formation of Cajal bodies containing p80-coilin and SMN. J. Cell Sci. 2001, 114, 4407–4419. [Google Scholar] [CrossRef] [PubMed]
- Lemm, I.; Girard, C.; Kuhn, A.N.; Watkins, N.J.; Schneider, M.; Bordonné, R.; Lührmann, R. Ongoing U snRNP biogenesis is required for the integrity of Cajal bodies. Mol. Biol. Cell 2006, 17, 3221–3231. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, Z.; Gu, Y.; Kwairanga, S.H.; Bailey, L.J.; Khan, A.; Nasser, M.; Aljarrah, D.; Arber, C.; Wray, S.; Serpell, L.C.; et al. Mutant MAPT induces rDNA transcriptional hyperactivation and nucleolar stress in cellular models. Res. Sq. 2025. [Google Scholar] [CrossRef]
- Kobayashi, T. A new role of the rDNA and nucleolus in the nucleus-rDNA instability maintains genome integrity. Bioessays 2008, 30, 267–272. [Google Scholar] [CrossRef]
- Salim, D.; Bradford, W.D.; Freeland, A.; Cady, G.; Wang, J.; Pruitt, S.C.; Gerton, J.L. DNA replication stress restricts ribosomal DNA copy number. PLoS Genet. 2017, 13, e1007006. [Google Scholar] [CrossRef]
- Kawamura, K.; Qi, F.; Meng, Q.; Hayashi, I.; Kobayashi, J. Nucleolar protein nucleolin functions in replication stress-induced DNA damage responses. J. Radiat. Res. 2019, 60, 281–288. [Google Scholar] [CrossRef]
- Żabka, A.; Winnicki, K.; Polit, J.T.; Bernasinska-Słomczewska, J.; Maszewski, J. 5-Aminouracil and other inhibitors of DNA replication induce biphasic interphase-mitotic cells in apical root meristems of Allium cepa. Plant Cell Rep. 2020, 39, 1013–1028. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żabka, A.; Gocek-Szczurtek, N.; Wróblewski, M.; Polit, J.T. Identification of Fibrillarin and Cajal Bodies Under DNA Replication Stress Conditions in Root Meristem Cells of Allium cepa. Int. J. Mol. Sci. 2025, 26, 11321. https://doi.org/10.3390/ijms262311321
Żabka A, Gocek-Szczurtek N, Wróblewski M, Polit JT. Identification of Fibrillarin and Cajal Bodies Under DNA Replication Stress Conditions in Root Meristem Cells of Allium cepa. International Journal of Molecular Sciences. 2025; 26(23):11321. https://doi.org/10.3390/ijms262311321
Chicago/Turabian StyleŻabka, Aneta, Natalia Gocek-Szczurtek, Mateusz Wróblewski, and Justyna Teresa Polit. 2025. "Identification of Fibrillarin and Cajal Bodies Under DNA Replication Stress Conditions in Root Meristem Cells of Allium cepa" International Journal of Molecular Sciences 26, no. 23: 11321. https://doi.org/10.3390/ijms262311321
APA StyleŻabka, A., Gocek-Szczurtek, N., Wróblewski, M., & Polit, J. T. (2025). Identification of Fibrillarin and Cajal Bodies Under DNA Replication Stress Conditions in Root Meristem Cells of Allium cepa. International Journal of Molecular Sciences, 26(23), 11321. https://doi.org/10.3390/ijms262311321





