Targeting Lactylation for Cancer: Mechanisms, Effects, and Therapeutic Prospects
Abstract
1. Introduction
2. Exploring Lactylation: Modification Mechanism, Key Writers, Donors and Erasers
2.1. Three Types of Lactylation Mechanisms
2.1.1. Lactylation with KATs as the Main Writers and Lactyl-CoA as the Donor
2.1.2. Lactylation Mediated by AARS, with Lactyl-AMP as the Donor
2.1.3. Non-Enzymatic Lactylation
2.2. Lactylation Erasers
2.2.1. Histone Delactylation Eraser
2.2.2. Non-Histone Delactylation Eraser
3. Lactylation Regulates Tumor Metabolic Reprogramming and Microenvironment Remodeling
3.1. Crosstalk Between Lactylation and Tumor Metabolic Reprogramming
3.1.1. Lactylation and Glycolysis
3.1.2. Lactylation and Lipid Metabolism
3.1.3. Lactylation and Mitochondrial Metabolism
3.2. Lactylation in Tumor Microenvironment Remodeling
3.2.1. Lactylation and Macrophage
3.2.2. Lactylation and T Cell
3.2.3. Lactylation and Other Immune Cells
3.2.4. Lactylation and CAFs
4. Lactylation Promotes Malignant Progression of Tumors
4.1. Digestive System Cancers
4.1.1. Colorectal Cancer
4.1.2. Liver Cancer
4.1.3. Gastric Cancer
4.1.4. Esophageal Cancer
4.2. Urinary and Reproductive System Cancers
4.2.1. Bladder Cancer
4.2.2. Prostate Cancer
4.2.3. Renal Cell Carcinoma
4.2.4. Cervical Cancer
4.2.5. Ovarian Cancer
4.2.6. Endometrial Cancer
4.3. Endocrine System Cancers
4.3.1. Breast Cancer
4.3.2. Pancreatic Cancer
4.4. Respiratory System Cancers
4.5. Other Type Cancers
5. Blocking the Lactylation: A Potential Approach to Thwart Tumors Evolution
5.1. Blocking Lactate Anabolism/Promoting Lactate Catabolism
5.2. Inhibiting Lactate Shuttle
5.3. Target Lactylation
6. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, D.; Tang, Z.Y.; Huang, H.; Zhou, G.L.; Cui, C.; Weng, Y.J.; Liu, W.C.; Kim, S.; Lee, S.; Perez-Neut, M.; et al. Metabolic regulation of gene expression by histone lactylation. Nature 2019, 574, 575–580. [Google Scholar] [CrossRef]
- Ding, T.; Yang, Y.H.; Wang, Q.C.; Wu, Y.; Han, R.; Zhang, X.T.; Kong, J.; Yang, J.T.; Liu, J.F. Global profiling of protein lactylation in Caenorhabditis elegans. Proteomics 2024, 24, e2300185. [Google Scholar] [CrossRef]
- Yang, Y.H.; Wang, Q.C.; Kong, J.; Yang, J.T.; Liu, J.F. Global profiling of lysine lactylation in human lungs. Proteomics 2023, 23, e2200437. [Google Scholar] [CrossRef]
- Hagihara, H.; Shoji, H.; Otabi, H.; Toyoda, A.; Katoh, K.; Namihira, M.; Miyakawa, T. Protein lactylation induced by neural excitation. Cell Rep. 2021, 37, 109820. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.Y.; Li, Y.X.; Duan, J.W.; Guo, S.Y.; Cai, X.N.; Zhang, X.; Long, H.; Ren, W.; Xie, Z.Y. Metabolomic, proteomic and lactylated proteomic analyses indicate lactate plays important roles in maintaining energy and C:N homeostasis in Phaeodactylum tricornutum. Biotechnol. Biofuels Bioprod. 2022, 15, 61. [Google Scholar] [CrossRef]
- Zhao, W.; Yu, H.L.; Liu, X.N.; Wang, T.T.; Yao, Y.N.; Zhou, Q.X.; Zheng, X.Z.; Tan, F. Systematic identification of the lysine lactylation in the protozoan parasite Toxoplasma gondii. Parasites Vectors 2022, 15, 180. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.H.; Su, J.; Chen, X.F.; Li, Y.J.; Xing, Z.; Guo, L.L.; Li, S.T.; Zhang, J. High-Intensity Interval Training Induces Protein Lactylation in Different Tissues of Mice with Specificity and Time Dependence. Metabolites 2023, 13, 647. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.M.; Zhang, N.; Liang, W.X. Systematic Analysis of Lysine Lactylation in the Plant Fungal Pathogen Botrytis cinerea. Front. Microbiol. 2020, 11, 594743. [Google Scholar] [CrossRef]
- Meng, X.X.; Baine, J.M.; Yan, T.C.; Wang, S. Comprehensive Analysis of Lysine Lactylation in Rice (Oryza sativa) Grains. J. Agric. Food Chem. 2021, 69, 8287–8297. [Google Scholar] [CrossRef]
- Zhang, N.W.; Jiang, N.; Yu, L.Y.; Guan, T.D.; Sang, X.Y.; Feng, Y.; Chen, R.; Chen, Q.J. Protein Lactylation Critically Regulates Energy Metabolism in the Protozoan Parasite Trypanosoma brucei. Front. Cell Dev. Biol. 2021, 9, 719720. [Google Scholar] [CrossRef]
- Yang, D.W.; Yin, J.; Shan, L.Q.; Yi, X.L.; Zhang, W.; Ding, Y.B. Identification of lysine-lactylated substrates in gastric cancer cells. iScience 2022, 25, 104630. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Gong, T.; Wu, Q.R.; Zhang, Y.X.; Zheng, X.; Li, Y.Q.; Ren, B.; Peng, X.; Zhou, X.D. Lysine lactylation regulates metabolic pathways and biofilm formation in Streptococcus mutans. Sci. Signal. 2023, 16, eadg1849. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ouyang, L.Y.; Wei, L. Novel Insight of Nitrogen Deprivation Affected Lipid Accumulation by Genome-Wide Lactylation in Nannochloropsis oceanica. J. Agric. Food Chem. 2023, 71, 10107–10123. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.B.; Li, Z.X.; Yang, J.T.; Xu, F.; Fu, X.Q.; Xu, L.P.; You, C.H.; Wang, D.J.; Su, Y.C.; Que, Y.X. Deciphering the Atlas of Post-Translational Modification in Sugarcane. J. Agric. Food Chem. 2023, 71, 10004–10017. [Google Scholar] [CrossRef]
- Yang, Z.J.; Yan, C.; Ma, J.Q.; Peng, P.P.; Ren, X.L.; Cai, S.L.; Shen, X.; Wu, Y.C.; Zhang, S.; Wang, X.Y.; et al. Lactylome analysis suggests lactylation-dependent mechanisms of metabolic adaptation in hepatocellular carcinoma. Nat. Metab. 2023, 5, 61–79. [Google Scholar] [CrossRef]
- Yao, Y.; Bade, R.; Li, G.T.; Zhang, A.Q.; Zhao, H.L.; Fan, L.F.; Zhu, R.X.; Yuan, J. Global-Scale Profiling of Differential Expressed Lysine-Lactylated Proteins in the Cerebral Endothelium of Cerebral Ischemia-Reperfusion Injury Rats. Cell. Mol. Neurobiol. 2023, 43, 1989–2004. [Google Scholar] [CrossRef]
- Dong, H.Y.; Zhang, J.J.; Zhang, H.; Han, Y.; Lu, C.C.; Chen, C.; Tan, X.X.; Wang, S.Y.; Bai, X.; Zhai, G.J.; et al. YiaC and CobB regulate lysine lactylation in Escherichia coli. Nat. Commun. 2022, 13, 6628. [Google Scholar] [CrossRef]
- Zong, Z.; Xie, F.; Wang, S.; Wu, X.; Zhang, Z.; Yang, B.; Zhou, F. Alanyl-tRNA synthetase, AARS1, is a lactate sensor and lactyltransferase that lactylates p53 and contributes to tumorigenesis. Cell 2024, 187, 2375–2392. [Google Scholar] [CrossRef]
- Gaffney, D.O.; Jennings, E.Q.; Anderson, C.C.; Marentette, J.O.; Shi, T.; Oxvig, A.M.S.; Streeter, M.D.; Johannsen, M.; Spiegel, D.A.; Chapman, E.; et al. Non-enzymatic Lysine Lactoylation of Glycolytic Enzymes. Cell Chem. Biol. 2020, 27, 206–213.e6. [Google Scholar] [CrossRef]
- Yu, J.; Chai, P.W.; Xie, M.Y.; Ge, S.F.; Ruan, J.; Fan, X.Q.; Jia, R.B. Histone lactylation drives oncogenesis by facilitating m6A reader protein YTHDF2 expression in ocular melanoma. Genome Biol. 2021, 22, 85. [Google Scholar] [CrossRef]
- Gu, X.; Zhuang, A.; Yu, J.; Yang, L.D.; Ge, S.F.; Ruan, J.; Jia, R.B.; Fan, X.Q.; Chai, P.W. Histone lactylation-boosted ALKBH3 potentiates tumor progression and diminished promyelocytic leukemia protein nuclear condensates by m1A demethylation of SP100A. Nucleic Acids Res. 2024, 52, 2273–2289. [Google Scholar] [CrossRef]
- Huang, Z.W.; Zhang, X.N.; Zhang, L.; Liu, L.L.; Zhang, J.W.; Sun, Y.X.; Xu, J.Q.; Liu, Q.T.; Long, Z.J. STAT5 promotes PD-L1 expression by facilitating histone lactylation to drive immunosuppression in acute myeloid leukemia. Signal Transduct. Target. Ther. 2023, 8, 391. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Zhang, J.; Zhou, Q.; He, X.; Zheng, Z.; Wei, Y.; Zhou, K.; Lin, Y.; Yu, H.; Zhang, H.; et al. Hypoxia induces mitochondrial protein lactylation to limit oxidative phosphorylation. Cell Res. 2024, 34, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Niu, K.; Wang, J.; Shen, W.; Jiang, R.; Liu, L.; Song, W.; Wang, X.; Zhang, X.; Zhang, R.; et al. Nucleolin lactylation contributes to intrahepatic cholangiocarcinoma pathogenesis via RNA splicing regulation of MADD. J. Hepatol. 2024, 81, 651–666. [Google Scholar] [CrossRef]
- Huang, H.; Wang, S.; Xia, H.; Zhao, X.; Chen, K.; Jin, G.; Zhou, S.; Lu, Z.; Chen, T.; Yu, H.; et al. Lactate enhances NMNAT1 lactylation to sustain nuclear NAD(+) salvage pathway and promote survival of pancreatic adenocarcinoma cells under glucose-deprived conditions. Cancer Lett. 2024, 588, 216806. [Google Scholar] [CrossRef]
- Chen, M.; Cen, K.L.; Song, Y.J.; Zhang, X.C.; Liou, Y.C.; Liu, P.; Huang, J.Y.; Ruan, J.; He, J.; Ye, W.Y.; et al. NUSAP1-LDHA-Glycolysis-Lactate feedforward loop promotes Warburg effect and metastasis in pancreatic ductal adenocarcinoma. Cancer Lett. 2023, 567, 216285. [Google Scholar] [CrossRef]
- Li, Q.; Lin, G.; Zhang, K.; Liu, X.; Li, Z.; Bing, X.; Nie, Z.; Jin, S.; Guo, J.; Min, X. Hypoxia exposure induces lactylation of Axin1 protein to promote glycolysis of esophageal carcinoma cells. Biochem. Pharmacol. 2024, 226, 116415. [Google Scholar] [CrossRef]
- Force, L.M.; Kocarnik, J.M.; May, M.L.; Bhangdia, K.; Crist, A.; Penberthy, L.; Pritchett, N.; Acheson, A.; Deitesfeld, L.; Aalruz, H.; et al. The global, regional, and national burden of cancer, 1990–2023, with forecasts to 2050: A systematic analysis for the Global Burden of Disease Study 2023. Lancet 2025, 406, 1565–1586. [Google Scholar] [CrossRef]
- Chen, H.; Li, Y.; Li, H.; Chen, X.; Fu, H.; Mao, D.; Chen, W.; Lan, L.; Wang, C.; Hu, K.; et al. NBS1 lactylation is required for efficient DNA repair and chemotherapy resistance. Nature 2024, 631, 663–669. [Google Scholar] [CrossRef]
- Chen, Y.P.; Wu, J.H.; Zhai, L.H.; Zhang, T.T.; Yin, H.; Gao, H.Y.; Zhao, F.; Wang, Z.; Yang, X.N.; Jin, M.P.; et al. Metabolic regulation of homologous recombination repair by MRE11 lactylation. Cell 2024, 187, 294–311.e21. [Google Scholar] [CrossRef]
- Wu, D.; Spencer, C.B.; Ortoga, L.; Zhang, H.; Miao, C. Histone lactylation-regulated METTL3 promotes ferroptosis via m6A-modification on ACSL4 in sepsis-associated lung injury. Redox Biol. 2024, 74, 103194, Erratum in: Redox Biol. 2025, 82, 103616. [Google Scholar] [CrossRef]
- Hu, X.L.; Huang, X.W.; Yang, Y.; Sun, Y.C.; Zhao, Y.H.; Zhang, Z.J.; Qiu, D.; Wu, Y.S.; Wu, G.M.; Lei, L. Dux activates metabolism-lactylation-MET network during early iPSC reprogramming with Brg1 as the histone lactylation reader. Nucleic Acids Res. 2024, 20, 5529–5548. [Google Scholar] [CrossRef]
- Dong, M.; Zhang, Y.; Chen, M.; Tan, Y.; Min, J.; He, X.; Liu, F.; Gu, J.; Jiang, H.; Zheng, L.; et al. ASF1A-dependent P300-mediated histone H3 lysine 18 lactylation promotes atherosclerosis by regulating EndMT. Acta Pharm. Sin. B 2024, 14, 3027–3048. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, S.; Wang, Z.; Han, J.; Jiang, N.; Qu, L.; Xu, K. Andrographolide regulates H3 histone lactylation by interfering with p300 to alleviate aortic valve calcification. Br. J. Pharmacol. 2024, 181, 1843–1856. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X. Virus-Induced Histone Lactylation Promotes Virus Infection in Crustacean. Adv. Sci. 2024, 11, e2401017. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Yang, K.; Wang, X.H.; Chen, L.J.; Gill, P.S.; Ha, T.; Liu, L.; Lewis, N.H.; Williams, D.L.; Li, C.F. Lactate promotes endothelial-to-mesenchymal transition via Snail1 lactylation after myocardial infarction. Sci. Adv. 2023, 9, 2401017, Erratum in: Sci. Adv. 2023, 9, eadn2108. [Google Scholar] [CrossRef]
- Huang, J.; Wang, X.; Li, N.; Fan, W.; Li, X.; Zhou, Q.; Liu, J.; Li, W.; Zhang, Z.; Liu, X.; et al. YY1 Lactylation Aggravates Autoimmune Uveitis by Enhancing Microglial Functions via Inflammatory Genes. Adv. Sci. 2024, 11, e2308031. [Google Scholar] [CrossRef]
- Wang, X.T.; Fan, W.; Li, N.; Ma, Y.; Yao, M.D.; Wang, G.Q.; He, S.Y.; Li, W.Q.; Tan, J.; Lu, Q.; et al. YY1 lactylation in microglia promotes angiogenesis through transcription activation-mediated upregulation of FGF2. Genome Biol. 2023, 24, 87. [Google Scholar] [CrossRef]
- Yang, K.; Fan, M.; Wang, X.H.; Xu, J.J.; Wang, Y.N.; Tu, F.; Gill, P.S.; Ha, T.Z.; Liu, L.; Williams, D.L.; et al. Lactate promotes macrophage HMGB1 lactylation, acetylation, and exosomal release in polymicrobial sepsis. Cell Death Differ. 2022, 29, 133–146. [Google Scholar] [CrossRef]
- Jia, M.S.; Yue, X.; Sun, W.X.; Zhou, Q.J.; Chang, C.; Gong, W.H.; Feng, J.; Li, X.; Zhan, R.A.; Mo, K.M.; et al. ULK1-mediated metabolic reprogramming regulates Vps34 lipid kinase activity by its lactylation. Sci. Adv. 2023, 9, eadg4993. [Google Scholar] [CrossRef]
- Niu, Z.; Chen, C.; Wang, S.; Lu, C.; Wu, Z.; Wang, A.; Mo, J.; Zhang, J.; Han, Y.; Yuan, Y.; et al. HBO1 catalyzes lysine lactylation and mediates histone H3K9la to regulate gene transcription. Nat. Commun. 2024, 15, 3561. [Google Scholar] [CrossRef]
- Yan, Q.; Zhou, J.; Gu, Y.; Huang, W.; Ruan, M.; Zhang, H.; Wang, T.; Wei, P.; Chen, G.; Li, W.; et al. Lactylation of NAT10 promotes N(4)-acetylcytidine modification on tRNA(Ser-CGA-1-1) to boost oncogenic DNA virus KSHV reactivation. Cell Death Differ. 2024, 31, 1362–1374. [Google Scholar] [CrossRef]
- Liu, J.R.; Du, J.K.; Li, Y.H.; Wang, F.W.; Song, D.B.; Lin, J.T.; Li, B.H.; Li, L. Catalpol induces apoptosis in breast cancer in vitro and in vivo: Involvement of mitochondria apoptosis pathway and post-translational modifications br. Toxicol. Appl. Pharmacol. 2022, 454, 116215. [Google Scholar] [CrossRef]
- Ju, J.; Zhang, H.; Lin, M.; Yan, Z.; An, L.; Cao, Z.; Geng, D.; Yue, J.; Tang, Y.; Tian, L.; et al. The alanyl-tRNA synthetase AARS1 moonlights as a lactyl-transferase to promote YAP signaling in gastric cancer. J. Clin. Investig. 2024, 134, e174587. [Google Scholar] [CrossRef]
- He, X.D.; Gong, W.; Zhang, J.N.; Nie, J.; Yao, C.F.; Guo, F.S.; Lin, Y.; Wu, X.H.; Li, F.; Li, J.; et al. Sensing and Transmitting Intracellular Amino Acid Signals through Reversible Lysine Aminoacylations. Cell Metab. 2018, 27, 151–166.e6. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Xue, M.; Thornalley, P.J. Activity, regulation, copy number and function in the glyoxalase system. Biochem. Soc. Trans. 2014, 42, 419–424. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, J.; Zhu, Z.; Mao, Q.; Xu, Z.; Singh, P.K.; Rimayi, C.C.; Moreno-Yruela, C.; Xu, S.; Li, G.; et al. Lysine L-lactylation is the dominant lactylation isomer induced by glycolysis. Nat. Chem. Biol. 2024, 21, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Haberland, M.; Montgomery, R.L.; Olson, E.N. The many roles of histone deacetylases in development and physiology: Implications for disease and therapy. Nat. Rev. Genet. 2009, 10, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Houtkooper, R.H.; Pirinen, E.; Auwerx, J. Sirtuins as regulators of metabolism and healthspan. Nat. Rev. Mol. Cell Biol. 2012, 13, 225–238. [Google Scholar] [CrossRef]
- Moreno-Yruela, C.; Zhang, D.; Wei, W.; Bæk, M.; Liu, W.C.; Gao, J.J.; Danková, D.; Nielsen, A.L.; Bolding, J.E.; Yang, L.; et al. Class I histone deacetylases (HDAC1-3) are histone lysine delactylases. Sci. Adv. 2022, 8, eabi6696. [Google Scholar] [CrossRef]
- Zhou, C.; Li, W.X.; Liang, Z.X.; Wu, X.R.; Cheng, S.J.; Peng, J.H.; Zeng, K.X.; Li, W.H.; Lan, P.; Yang, X.; et al. Mutant KRAS-activated circATXN7 fosters tumor immunoescape by sensitizing tumor-specific T cells to activation-induced cell death. Nat. Commun. 2024, 15, 499. [Google Scholar] [CrossRef]
- Yue, Q.; Wang, Z.; Shen, Y.X.; Lan, Y.F.; Zhong, X.Y.; Luo, X.; Yang, T.; Zhang, M.Q.; Zuo, B.M.; Zeng, T.C.; et al. Histone H3K9 Lactylation Confers Temozolomide Resistance in Glioblastoma via LUC7L2-Mediated MLH1 Intron Retention. Adv. Sci. 2024, 11, 2309290. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Wang, A.; Zhang, J.; Xia, M.; Jiang, Z.; Jia, B.; Lu, C.; Chen, C.; Wang, S.; Zhang, Y.; et al. Hypoxia promotes histone H3K9 lactylation to enhance LAMC2 transcription in esophageal squamous cell carcinoma. iScience 2024, 27, 110188. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Qiao, C.; Wang, J.; Zhou, Y.; Zhang, C. Histone lactylation facilitates hepatocellular carcinoma progression by upregulating endothelial cell-specific molecule 1 expression. Mol. Carcinog. 2024, 63, 2078–2089. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Huang, D.L.; Jiang, Y.; Hou, J.; Tian, M.Y.; Li, J.H.; Sun, L.; Zhang, Y.G.; Zhang, T.; Li, Z.Q.; et al. Lactate Modulates Cellular Metabolism Through Histone Lactylation-Mediated Gene Expression in Non-Small Cell Lung Cancer. Front. Oncol. 2021, 11, 647559. [Google Scholar] [CrossRef]
- Wang, J.W.; Liu, Z.; Xu, Y.Y.; Wang, Y.P.; Wang, F.; Zhang, Q.Q.; Ni, C.H.; Zhen, Y.; Xu, R.; Liu, Q.S.; et al. Enterobacterial LPS-inducible LINC00152 is regulated by histone lactylation and promotes cancer cells invasion and migration. Front. Cell. Infect. Microbiol. 2022, 12, 913815. [Google Scholar] [CrossRef]
- Cui, Z.; Li, Y.; Lin, Y.; Zheng, C.; Luo, L.; Hu, D.; Chen, Y.; Xiao, Z.; Sun, Y. Lactylproteome analysis indicates histone H4K12 lactylation as a novel biomarker in triple-negative breast cancer. Front. Endocrinol. 2024, 15, 1328679. [Google Scholar] [CrossRef]
- Wang, X.M.; Ying, T.X.; Yuan, J.M.; Wang, Y.; Su, X.Y.; Chen, S.T.; Zhao, Y.R.; Zhao, Y.Y.; Sheng, J.H.; Teng, L.S.; et al. BRAFV600E restructures cellular lactylation to promote anaplastic thyroid cancer proliferation. Endocr. Relat. Cancer 2023, 30, e220344. [Google Scholar] [CrossRef]
- Zhang, X.W.; Li, L.; Liao, M.; Liu, D.; Rehman, A.; Liu, Y.; Liu, Z.P.; Tu, P.F.; Zeng, K.W. Thermal Proteome Profiling Strategy Identifies CNPY3 as a Cellular Target of Gambogic Acid for Inducing Prostate Cancer Pyroptosis. J. Med. Chem. 2024, 67, 10005–10011. [Google Scholar] [CrossRef]
- Lei, Z.; Mozaffaritabar, S.; Kawamura, T.; Koike, A.; Kolonics, A.; Keringer, J.; Pinho, R.A.; Sun, J.Q.; Shangguan, R.A.; Radak, Z. The effects of long-term lactate and high-intensity interval training (HIIT) on brain neuroplasticity of aged mice. Heliyon 2024, 10, e24421. [Google Scholar] [CrossRef]
- Jennings, E.Q.; Ray, J.D.; Zerio, C.J.; Trujillo, M.N.; McDonald, D.M.; Chapman, E.; Spiegel, D.A.; Galligan, J.J. Sirtuin 2 Regulates Protein LactoylLys Modifications. ChemBioChem 2021, 22, 2102–2106. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.H.; Zhang, Y.; Yang, B.Y.; Sun, S.J.; Zhang, P.S.; Luo, Z.; Feng, T.T.; Cui, Z.L.; Zhu, T.; Li, Y.M.; et al. Lactylation of METTL16 promotes cuproptosis via m6A-modification on FDX1 mRNA in gastric cancer. Nat. Commun. 2023, 14, 6523. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Yao, Y.; Hu, H.B.; Wu, J.J.; Li, J.X.; Li, L.L.; Wu, J.; Sun, M.M.; Deng, Z.Y.; Zhang, Y.Y.; et al. PDHA1 hyperacetylation-mediated lactate overproduction promotes sepsis-induced acute kidney injury via Fis1 lactylation. Cell Death Dis. 2023, 14, 457. [Google Scholar] [CrossRef]
- Jin, J.; Bai, L.; Wang, D.Y.; Ding, W.; Cao, Z.X.; Yan, P.D.; Li, Y.J.; Xi, L.L.; Wang, Y.X.; Zheng, X.H.; et al. SIRT3-dependent delactylation of cyclin E2 prevents hepatocellular carcinoma growth. EMBO Rep. 2023, 24, e56052. [Google Scholar] [CrossRef]
- Gu, J.; Zhou, J.; Lu, L. Tumor metabolite lactate promotes tumorigenesis through modulating Moesin lactylation and TGF-b signaling of regulatory T cells. Ann. Oncol. 2021, 32, S1230. [Google Scholar] [CrossRef]
- Xie, B.; Zhang, M.; Li, J.; Cui, J.; Zhang, P.; Liu, F.; Wu, Y.; Deng, W.; Ma, J.; Li, X.; et al. KAT8-catalyzed lactylation promotes eEF1A2-mediated protein synthesis and colorectal carcinogenesis. Proc. Natl. Acad. Sci. USA 2024, 121, e2314128121. [Google Scholar] [CrossRef]
- Chen, B.; Deng, Y.; Hong, Y.; Fan, L.; Zhai, X.; Hu, H.; Yin, S.; Chen, Q.; Xie, X.; Ren, X.; et al. Metabolic Recoding of NSUN2-Mediated m(5)C Modification Promotes the Progression of Colorectal Cancer via the NSUN2/YBX1/m(5)C-ENO1 Positive Feedback Loop. Adv. Sci. 2024, 11, e2309840. [Google Scholar] [CrossRef]
- Liao, J.Y.; Chen, Z.Y.; Chang, R.Z.; Yuan, T.; Li, G.X.; Zhu, C.; Wen, J.Y.; Wei, Y.; Huang, Z.; Ding, Z.Y.; et al. CENPA functions as a transcriptional regulator to promote hepatocellular carcinoma progression via cooperating with YY1. Int. J. Biol. Sci. 2023, 19, 5218–5232. [Google Scholar] [CrossRef]
- Meng, Q.F.; Sun, H.H.; Zhang, Y.H.; Yang, X.Z.; Hao, S.M.; Liu, B.; Zhou, H.L.; Xu, Z.X.; Wang, Y.S. Lactylation stabilizes DCBLD1 activating the pentose phosphate pathway to promote cervical cancer progression. J. Exp. Clin. Cancer Res. 2024, 43, 36. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Zhang, B.; Lin, X.; Fu, X.; An, Y.; Zou, Y.; Wang, J.X.; Wang, Z.; Yu, T. Lactate metabolism in human health and disease. Signal Transduct. Target. Ther. 2022, 7, 305. [Google Scholar] [CrossRef]
- Li, L.; Dong, J.; Xu, C.; Wang, S. Lactate drives senescence-resistant lineages in hepatocellular carcinoma via histone H2B lactylation of NDRG1. Cancer Lett. 2025, 616, 217567. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Cheng, J.; Zhou, G.; Tao, L.; Xing, J.; Liu, L.; Chen, Z.; Song, P. Histone lactylation-driven YTHDF2 promotes non-small cell lung cancer cell glycolysis and stemness by recognizing m6A modification of SFRP2. Biochem. Pharmacol. 2025, 240, 117097. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yan, Q.; Ruan, S.; Cui, J.; Li, Z.; Zhang, Z.; Yang, J.; Fang, J.; Liu, S.; Huang, S.; et al. GCLM lactylation mediated by ACAT2 promotes ferroptosis resistance in KRAS(G12D)-mutant cancer. Cell Rep. 2025, 44, 115774. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Yi, Y.; Liu, H.; Xu, J.; Chen, S.; Wu, D.; Wang, L.; Li, F. RHOF promotes Snail1 lactylation by enhancing PKM2-mediated glycolysis to induce pancreatic cancer cell endothelial-mesenchymal transition. Cancer Metab. 2024, 12, 32. [Google Scholar] [CrossRef]
- Wan, N.; Wang, N.; Yu, S.Q.; Zhang, H.Q.; Tang, S.; Wang, D.X.; Lu, W.J.; Li, H.H.; Delafield, D.G.; Kong, Y.; et al. Cyclic immonium ion of lactyllysine reveals widespread lactylation in the human proteome. Nat. Methods 2022, 19, 854–864. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, J.; Zhang, Y.; Li, W.; Xiong, Y.; Fan, Y.; Wu, Y.; Zhao, J.; Shang, C.; Liang, H.; et al. Lactylation-Driven IGF2BP3-Mediated Serine Metabolism Reprogramming and RNA m6A-Modification Promotes Lenvatinib Resistance in HCC. Adv. Sci. 2024, 11, e2401399. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Y.; Cheng, A.; Cui, F.; Yang, Z.; Guo, J. The lactylation of glucose-6-phosphate dehydrogenase promotes malignant phenotypes in cancer cell lines. Mol. Biol. Rep. 2025, 52, 861. [Google Scholar] [CrossRef]
- Chu, Y.D.; Cheng, L.C.; Lim, S.N.; Lai, M.W.; Yeh, C.T.; Lin, W.R. Aldolase B-driven lactagenesis and CEACAM6 activation promote cell renewal and chemoresistance in colorectal cancer through the Warburg effect. Cell Death Dis. 2023, 14, 660. [Google Scholar] [CrossRef]
- Zhou, J.M.; Xu, W.Q.; Wu, Y.B.; Wang, M.; Zhang, N.; Wang, L.R.; Feng, Y.; Zhang, T.; Wang, L.; Mao, A.R. GPR37 promotes colorectal cancer liver metastases by enhancing the glycolysis and histone lactylation via Hippo pathway. Oncogene 2023, 42, 3319–3330. [Google Scholar] [CrossRef]
- Du, W.; Tan, S.; Peng, Y.; Lin, S.; Wu, Y.; Ding, K.; Chen, C.; Liu, R.; Cao, Y.; Li, Z.; et al. Histone lactylation-driven YTHDC1 promotes hepatocellular carcinoma progression via lipid metabolism remodeling. Cancer Lett. 2024, 611, 217426. [Google Scholar] [CrossRef]
- Hu, H.M.; Deng, J.L.; Pan, Y.; Wang, Z.H.; Zhang, J.; Yu Gu, X.; Fan, S.X.; Zhao, J.Z. Breviscapine targets EGFR and SRC to abrogate diabetes-driven GPX4 lactylation and ferroptosis resistance in gastric cancer. Phytomedicine 2025, 148, 157387. [Google Scholar] [CrossRef]
- Lu, R.S.; Ren, L.K.; Fei, X.B.; Liu, S.B.; Gao, Y.J.; Hou, J.Y.; Wang, C.; Liu, P.; Zhu, C.H.; Wang, X.; et al. PSMD14-Mediated LDHA Deubiquitination Upregulates ACLY Expression via H3K18 Lactylation to Promote Lipid Synthesis and Pancreatic Cancer Progression. Adv. Sci. 2025, e05762. [Google Scholar] [CrossRef] [PubMed]
- Shan, G.; Bian, Y.; Sui, Q.; Liang, J.; Ren, S.; Pan, B.; Shi, H.; Zheng, Z.; Zeng, D.; Zhu, J.; et al. Ferroptosis-induced SUMO2 lactylation counteracts ferroptosis by enhancing ACSL4 degradation in lung adenocarcinoma. Cell Discov. 2025, 11, 81. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Tang, Y.; Liu, Z.; Liu, Y.; Fu, X.; Guo, S.; Ma, J.; Ma, F.; Zhu, Z.; et al. PD-L1 delactylation-promoted nuclear translocation accelerates liver cancer growth through elevating SQLE transcription activity. Cancer Lett. 2025, 630, 217901. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Xiong, N.; Yan, R.; Li, S.T.; Liu, H.; Mao, Q.; Sun, Y.; Shen, S.; Ye, L.; Gao, P.; et al. PDHX acetylation facilitates tumor progression by disrupting PDC assembly and activating lactylation-mediated gene expression. Protein Cell 2025, 16, 49–63. [Google Scholar] [CrossRef]
- Li, C.; Ge, C.; Wang, Q.; Teng, P.; Jia, H.; Yao, S.; Huang, Z. Sirtuin 3-mediated delactylation of malic enzyme 2 disrupts redox balance and inhibits colorectal cancer growth. Cell. Oncol. 2025, 48, 979–990. [Google Scholar] [CrossRef]
- Zheng, J.; She, H.; Han, R.; Tang, J.; Dou, Y.; Lu, C.; Huang, D.; Lin, C.; Wu, D.; He, C.; et al. Dapk2 dysfunction leads to Mic60 lactylation and mitochondrial metabolic reprogramming, promoting lung cancer EGFR-TKI resistance and metastasis. Dev. Cell 2025. [Google Scholar] [CrossRef]
- Cai, J.; Song, L.; Zhang, F.; Wu, S.; Zhu, G.; Zhang, P.; Chen, S.; Du, J.; Wang, B.; Cai, Y.; et al. Targeting SRSF10 might inhibit M2 macrophage polarization and potentiate anti-PD-1 therapy in hepatocellular carcinoma. Cancer Commun. 2024, 44, 1231–1260. [Google Scholar] [CrossRef]
- Wang, L.; Li, S.S.; Luo, H.H.; Lu, Q.; Yu, S.W. PCSK9 promotes the progression and metastasis of colon cancer cells through regulation of EMT and PI3K/AKT signaling in tumor cells and phenotypic polarization of macrophages. J. Exp. Clin. Cancer Res. 2022, 41, 303. [Google Scholar] [CrossRef]
- Yang, J.; Yu, X.; Xiao, M.; Xu, H.; Tan, Z.; Lei, Y.; Guo, Y.; Wang, W.; Xu, J.; Shi, S.; et al. Histone lactylation-driven feedback loop modulates cholesterol-linked immunosuppression in pancreatic cancer. Gut 2025, 74, 1859–1872. [Google Scholar] [CrossRef]
- Sun, K.; Zhang, X.; Shi, J.; Huang, J.; Wang, S.; Li, X.; Lin, H.; Zhao, D.; Ye, M.; Zhang, S.; et al. Elevated protein lactylation promotes immunosuppressive microenvironment and therapeutic resistance in pancreatic ductal adenocarcinoma. J. Clin. Investig. 2025, 135, e187024. [Google Scholar] [CrossRef]
- Pan, Z.; Chen, J.; Xu, T.; Cai, A.; Han, B.; Li, Y.; Fang, Z.; Yu, D.; Wang, S.; Zhou, J.; et al. VSIG4(+) tumor-associated macrophages mediate neutrophil infiltration and impair antigen-specific immunity in aggressive cancers through epigenetic regulation of SPP1. J. Exp. Clin. Cancer Res. CR 2025, 44, 45. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; He, J.; Zhu, J.; Pan, J.L.; Liao, W.J.; Ye, H.Y.; Wang, H.F.; Song, Y.J.; Du, Y.; Cui, B.J.; et al. Lactylation-driven METTL3-mediated RNA m6A modification promotes immunosuppression of tumor-infiltrating myeloid cells. Mol. Cell 2022, 82, 1660–1677.e10. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Yang, Y.; Jiang, F.Q.; Hu, G.; Wan, S.; Yan, W.Y.; He, X.S.; Xiao, F.; Yang, X.M.; Guo, X.; et al. Histone lactylation inhibits RARy expression in macrophages to promote colorectal tumorigenesis through activation of TRAF6-IL-6-STAT3 signaling. Cell Rep. 2024, 43, 113688. [Google Scholar] [CrossRef]
- Chaudagar, K.; Hieromnimon, H.M.; Kelley, A.; Labadie, B.; Shafran, J.; Rameshbabu, S.; Drovetsky, C.; Bynoe, K.; Solanki, A.; Markiewicz, E.; et al. Suppression of Tumor Cell Lactate-generating Signaling Pathways Eradicates Murine PTEN/p53-deficient Aggressive-variant Prostate Cancer via Macrophage Phagocytosis. Clin. Cancer Res. 2023, 29, 4930–4940. [Google Scholar] [CrossRef]
- Liu, S.; Pan, Y.; Liu, W.; Bu, X.; Shao, R.; Wang, Q.; Wu, J.; Wu, C.; Hu, W.; Xu, J.; et al. Lactylation-driven MVP upregulation boosts immunotherapy resistance by inhibiting PD-L1 degradation in hepatocellular carcinoma. J. Immunother. Cancer 2025, 13, e012230. [Google Scholar] [CrossRef]
- Hu, X.; Ouyang, W.; Chen, H.; Liu, Z.; Lai, Z.; Yao, H. Claudin-9 (CLDN9) promotes gastric cancer progression by enhancing the glycolysis pathway and facilitating PD-L1 lactylation to suppress CD8+ T cell anti-tumor immunity. Cancer Pathog. Ther. 2025, 3, 253–266. [Google Scholar] [CrossRef]
- Tong, H.; Jiang, Z.; Song, L.; Tan, K.; Yin, X.; He, C.; Huang, J.; Li, X.; Jing, X.; Yun, H.; et al. Dual impacts of serine/glycine-free diet in enhancing antitumor immunity and promoting evasion via PD-L1 lactylation. Cell Metab. 2024, 36, 2493–2510.e9. [Google Scholar] [CrossRef]
- Liang, X.; Yuan, D.; Zhao, S.; Zhou, J.; Wang, K.; Liu, X.; Liu, Y.; Li, H.; Hao, M.; Huang, W.; et al. Claudin-7 deficiency induces metabolic reprogramming of neutrophils in the colorectal cancer microenvironment. Cell Death Dis. 2025, 16, 728. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, L.; Zhang, M.; Du, Y.; Li, C.; Ren, H.; Zheng, L. H3K18 Lactylation Potentiates Immune Escape of Non-Small Cell Lung Cancer. Cancer Res. 2024, 84, 3589–3601. [Google Scholar] [CrossRef]
- Shang, X.; Cheng, B.; Zhang, C.; Zhao, C.; Wang, R.; Zhang, X.; Jiang, D.; Zhang, X.; Ma, X.; Mao, H.; et al. The LDH-H3K18La-Nur77 Axis Potentiates Immune Escape in Small Cell Lung Cancer. Adv. Sci. 2025, 12, e13608. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, D.; Wang, Y.; Liu, M.; Zhang, Y.; Feng, T.; Xiao, C.; Song, H.; Miao, R.; Xu, L.; et al. Lactylated Apolipoprotein C-II Induces Immunotherapy Resistance by Promoting Extracellular Lipolysis. Adv. Sci. 2024, 11, e2406333. [Google Scholar] [CrossRef]
- Pan, L.H.; Feng, F.; Wu, J.Q.; Fan, S.B.; Han, J.J.; Wang, S.X.; Yang, L.; Liu, W.Q.; Wang, C.L.; Xu, K. Demethylzeylasteral targets lactate by inhibiting histone lactylation to suppress the tumorigenicity of liver cancer stem cells. Pharmacol. Res. 2022, 181, 106270. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lin, J.; Yu, Z.; Li, X.; Lv, X.; Liu, P.; Sun, X.; Zhang, Z.; Gao, X.; Sun, K.; et al. Tumor-associated Schwann cell remodeling under metabolic stress via lactate sensing orchestrates pancreatic ductal adenocarcinoma development. Cell Metab. 2025, 37, 1907–1925.e14. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.K.; Yuan, X.Q.; Cai, D.Q.; Li, A.; Yang, S.J.; Yang, W.B.; Duan, J.X.; Zhuo, W.F.; Min, J.; Peng, L.; et al. Integrative analysis of lactylation-related genes and establishment of a novel prognostic signature for hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2023, 149, 11517–11530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, W.B.; Cai, X.Y.; Chen, X.Q.; Feng, Q.; Yang, Q.; Li, J.B.; Xiong, W.; Wu, T. MNDA promotes immunosuppression in microsatellite instability-high colorectal cancer by facilitating PMN-MDSC infiltration via H3K18 lactylation. J. Transl. Med. 2025, 23, 1049. [Google Scholar] [CrossRef]
- He, J.; Li, W.; Wang, S.; Lan, J.; Hong, X.; Liao, L.; Kang, D.; Wang, W.; Wang, R.; Zhang, W.; et al. Cancer associated fibroblasts-derived lactate induces oxaliplatin treatment resistance by promoting cancer stemness via ANTXR1 lactylation in colorectal cancer. Cancer Lett. 2025, 631, 217917. [Google Scholar] [CrossRef]
- Liang, L.; Yang, X.; Yao, S.; Li, X.; Wang, F. Identification of lactylation-associated fibroblast subclusters predicting prognosis and cancer immunotherapy response in colon cancer. Gene 2025, 940, 149220. [Google Scholar] [CrossRef]
- Li, P.; Yang, X.; Tang, H.; Zhou, Z.; Liu, B. Cancer-associated fibroblasts-secreted lactate promotes RNA polymerase III subunit G-mediated epithelial-mesenchymal transition in non-small cell lung cancer by increasing m6A modification of zinc finger protein 384. J. Cell Commun. Signal. 2025, 19, e70037. [Google Scholar] [CrossRef]
- Li, Z.; Liang, P.; Chen, Z.; Chen, Z.; Jin, T.; He, F.; Chen, X.; Yang, K. CAF-secreted LOX promotes PD-L1 expression via histone Lactylation and regulates tumor EMT through TGFβ/IGF1 signaling in gastric Cancer. Cell Signal 2024, 124, 111462. [Google Scholar] [CrossRef]
- Tan, J.N.; Yu, J.H.; Hou, D.; Xie, Y.Q.; Lai, D.M.; Zheng, F.; Yang, B.; Zeng, J.T.; Chen, Y.; Lu, S.H.; et al. Extracellular Vesicle-Packaged circTAX1BP1 from Cancer-Associated Fibroblasts Regulates RNA m6A Modification through Lactylation of VIRMA in Colorectal Cancer Cells. Adv. Sci. 2025, e14008. [Google Scholar] [CrossRef]
- Certo, M.; Tsai, C.H.; Pucino, V.; Ho, P.C.; Mauro, C. Lactate modulation of immune responses in inflammatory versus tumour microenvironments. Nat. Rev. Immunol. 2021, 21, 151–161. [Google Scholar] [CrossRef]
- Apostolova, P.; Pearce, E.L. Lactic acid and lactate: Revisiting the physiological roles in the tumor microenvironment. Trends Immunol. 2022, 43, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Tubbs, A.; Nussenzweig, A. Endogenous DNA Damage as a Source of Genomic Instability in Cancer. Cell 2017, 168, 644–656. [Google Scholar] [CrossRef] [PubMed]
- van den Bosch, M.; Bree, R.T.; Lowndes, N.F. The MRN complex: Coordinating and mediating the response to broken chromosomes. EMBO Rep. 2003, 4, 844–849. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Y.; Yan, F.; Zhao, G.; Wang, Y. tsRNA-08614 inhibits glycolysis and histone lactylation by ALDH1A3 to confer oxaliplatin sensitivity in colorectal cancer. Transl. Oncol. 2025, 58, 102427. [Google Scholar] [CrossRef]
- Zhu, K.; Fan, J.; Cai, H.; Zhou, C.; Gong, Z.; Li, Z.; Yu, J. The highly expressed GOLPH3 in colorectal cancer cells activates smoothened to drive glycolysis and promote cancer cell growth and radiotherapy resistance. J. Gastrointest. Oncol. 2025, 16, 415–434. [Google Scholar] [CrossRef]
- Sun, X.D.; He, L.F.; Liu, H.; Thorne, R.F.; Zeng, T.F.; Liu, L.; Zhang, B.; He, M.; Huang, Y.B.; Li, M.Y.; et al. The diapause-like colorectal cancer cells induced by SMC4 attenuation are characterized by low proliferation and chemotherapy insensitivity. Cell Metab. 2023, 35, 1563–1579.e8. [Google Scholar] [CrossRef]
- Li, W.; Zhou, C.; Yu, L.; Hou, Z.; Liu, H.; Kong, L.; Xu, Y.; He, J.; Lan, J.; Ou, Q.; et al. Tumor-derived lactate promotes resistance to bevacizumab treatment by facilitating autophagy enhancer protein RUBCNL expression through histone H3 lysine 18 lactylation (H3K18la) in colorectal cancer. Autophagy 2024, 20, 114–130. [Google Scholar] [CrossRef]
- Yang, Z.; Su, W.; Zhang, Q.; Niu, L.; Feng, B.; Zhang, Y.; Huang, F.; He, J.; Zhou, Q.; Zhou, X.; et al. Lactylation of HDAC1 Confers Resistance to Ferroptosis in Colorectal Cancer. Adv. Sci. 2025, 12, e2408845. [Google Scholar] [CrossRef]
- Qu, S.; Feng, B.; Xing, M.; Qiu, Y.; Ma, L.; Yang, Z.; Ji, Y.; Huang, F.; Wang, Y.; Zhou, J.; et al. PRMT5 K240lac confers ferroptosis resistance via ALKBH5/SLC7A11 axis in colorectal cancer. Oncogene 2025, 44, 2814–2830. [Google Scholar] [CrossRef]
- Deng, J.; Li, Y.; Yin, L.; Liu, S.; Li, Y.; Liao, W.; Mu, L.; Luo, X.; Qin, J. Histone lactylation enhances GCLC expression and thus promotes chemoresistance of colorectal cancer stem cells through inhibiting ferroptosis. Cell Death Dis. 2025, 16, 193. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Peng, J.; Wu, D.; Xie, Q.; Hou, Y.; Li, L.; Zhang, X.; Liang, Y.; Feng, J.; Chen, J.; et al. Histone lactylation-boosted AURKB facilitates colorectal cancer progression by inhibiting HNRNPM-mediated PSAT1 mRNA degradation. J. Exp. Clin. Cancer Res. CR 2025, 44, 233. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Qian, J.; Wang, Y.; Zhu, Y.; Chen, Y.; Shen, J.; Jiang, J.; Cao, Y.; Wang, N.; Huang, X.; et al. Platelet-derived exosomal LINC00183 facilitate colorectal cancer malignant progression driven by histone lactylation through stabilizing ENO1. Cell Death Dis. 2025, 16, 593. [Google Scholar] [CrossRef]
- Huang, J.; Xie, H.; Li, J.; Huang, X.; Cai, Y.; Yang, R.; Yang, D.; Bao, W.; Zhou, Y.; Li, T.; et al. Histone lactylation drives liver cancer metastasis by facilitating NSF1-mediated ferroptosis resistance after microwave ablation. Redox Biol. 2025, 81, 103553. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.Y.; Li, L.Q.; Wang, S.S.; Wang, Z.J.; Qu, L.H.; Wang, C.L.; Xu, K. Royal jelly acid suppresses hepatocellular carcinoma tumorigenicity by inhibiting H3 histone lactylation at H3K9la and H3K14la sites. Phytomedicine 2023, 118, 154940. [Google Scholar] [CrossRef]
- Huimin, W.; Xin, W.; Shan, Y.; Junwang, Z.; Jing, W.; Yuan, W.; Qingtong, L.; Xiaohui, L.; Jia, Y.; Lili, Y. Lactate promotes the epithelial-mesenchymal transition of liver cancer cells via TWIST1 lactylation. Exp. Cell Res. 2025, 447, 114474. [Google Scholar] [CrossRef]
- Yan, J.; Zhou, Y.; Xu, J.; Dong, Y.; Yang, X.; Yang, X.; Wu, A.; Chang, S.; Wang, Y.; Zhang, Q.; et al. Delactylation diminished the growth inhibitory role of CA3 by restoring DUOX2 expression in hepatocellular carcinoma. Exp. Cell Res. 2025, 444, 114392. [Google Scholar] [CrossRef]
- Fan, M.; Liu, J.S.; Wei, X.L.; Nie, Y.; Liu, H.L. Histone Lactylation-Driven Ubiquitin-Specific Protease 34 Promotes Cisplatin Resistance in Hepatocellular Carcinoma. Gastroenterol. Res. 2025, 18, 23–30. [Google Scholar] [CrossRef]
- Wu, Z.; Peng, Y.; Chen, W.; Xia, F.; Song, T.; Ke, Q. Lactylation-driven transcriptional activation of FBXO33 promotes gallbladder cancer metastasis by regulating p53 polyubiquitination. Cell Death Dis. 2025, 16, 144. [Google Scholar] [CrossRef] [PubMed]
- Tsukihara, S.; Akiyama, Y.; Shimada, S.; Hatano, M.; Igarashi, Y.; Taniai, T.; Tanji, Y.; Kodera, K.; Yasukawa, K.; Umeura, K.; et al. Delactylase effects of SIRT1 on a positive feedback loop involving the H19-glycolysis-histone lactylation in gastric cancer. Oncogene 2025, 44, 724–738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Xiang, H.; Peng, Q.; Ma, L.; Weng, C.; Liu, G.; Lu, L. METTL14 attenuates cancer stemness by suppressing ATF5/WDR74/β-catenin axis in gastric cancer. Cancer Sci. 2025, 116, 112–127. [Google Scholar] [CrossRef]
- Yang, H.; Yang, S.F.; He, J.X.; Li, W.Q.; Zhang, A.G.; Li, N.N.; Zhou, G.K.; Sun, B.S. Glucose transporter 3 (GLUT3) promotes lactylation modifications by regulating lactate dehydrogenase A (LDHA) in gastric cancer. Cancer Cell Int. 2023, 23, 303. [Google Scholar] [CrossRef]
- Zhao, X.; Li, M.; Fu, Y.; Chen, C.; Chen, Y.; Xu, L.; Bao, L.; Ma, Z.; Xu, J.; Zhou, S.; et al. PSMD14-mediated PFKFB2 deubiquitination activates H3K27 lactylation to drive cancer stemness in gastric adenocarcinoma. Cell Death Differ. 2025, 1–18. [Google Scholar] [CrossRef]
- Xie, B.; Lin, J.T.; Chen, X.W.; Zhou, X.J.; Zhang, Y.; Fan, M.J.; Xiang, J.Y.; He, N.; Hu, Z.H.; Wang, F.F. CircXRN2 suppresses tumor progression driven by histone lactylation through activating the Hippo pathway in human bladder cancer. Mol. Cancer 2023, 22, 151. [Google Scholar] [CrossRef]
- Li, F.; Zhang, H.H.; Huang, Y.; Li, D.Q.; Zheng, Z.S.; Xie, K.F.; Cao, C.; Wang, Q.; Zhao, X.L.; Huang, Z.H.; et al. Single-cell transcriptome analysis reveals the association between histone lactylation and cisplatin resistance in bladder cancer. Drug Resist. Update 2024, 73, 101059. [Google Scholar] [CrossRef]
- Xing, Z.; Yang, T.; Li, X.; Xu, H.; Hong, Y.; Shao, S.; Li, T.; Ye, L.; Li, Y.; Jin, X.; et al. High-glucose-associated YTHDC1 lactylation reduces the sensitivity of bladder cancer to enfortumab vedotin therapy. Cell Rep. 2025, 44, 115545. [Google Scholar] [CrossRef]
- Jin, H.; Wu, P.; Lv, C.; Zhang, S.; Zhang, Y.; Li, C.; Gao, R.; Shan, G.; Bi, H.; Chang, H.; et al. Mannose inhibits PKM2 lactylation to induce pyroptosis in bladder cancer and activate antitumor immune responses. Commun. Biol. 2025, 8, 689. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, Q.; Yang, Q.; Zhou, Y.; Wang, J. Hypoxia-induced PYCR1 regulates glycolysis and histone lactylation to promote bladder cancer progression and metastasis via SLC6A14/Glutamine metabolism. Cancer Biol. Ther. 2025, 26, 2546219. [Google Scholar] [CrossRef]
- Deng, X.; Huang, Y.; Zhang, J.; Chen, Y.; Jiang, F.; Zhang, Z.; Li, T.; Hou, L.; Tan, W.; Li, F. Histone lactylation regulates PRKN-Mediated mitophagy to promote M2 Macrophage polarization in bladder cancer. Int. Immunopharmacol. 2025, 148, 114119. [Google Scholar] [CrossRef]
- Ji, F.H.; Qian, Y.H.; Guo, X.C.; Liao, H.H.; Huang, J.C.; Xu, Z.H.; Yu, M.M.; Wu, Y.Y.; Bao, J.W.; Chen, H.J.; et al. Targeting RPS6KC1 to overcome enzalutamide resistance in prostate cancer. Biomark. Res. 2025, 13, 109. [Google Scholar] [CrossRef]
- Chaudagar, K.; Hieromnimon, H.M.; Khurana, R.; Labadie, B.; Hirz, T.; Mei, S.L.; Hasan, R.; Shafran, J.; Kelley, A.; Apostolov, E.; et al. Reversal of Lactate and PD-1-mediated Macrophage Immunosuppression Controls Growth of PTEN/p53-deficient Prostate Cancer. Clin. Cancer Res. 2023, 29, 1952–1968. [Google Scholar] [CrossRef]
- He, Y.M.; Ji, Z.Z.; Gong, Y.M.; Fan, L.C.; Xu, P.H.; Chen, X.Y.; Miao, J.J.; Zhang, K.; Zhang, W.T.; Ma, P.F.; et al. Numb/Parkin-directed mitochondrial fitness governs cancer cell fate via metabolic regulation of histone lactylation. Cell Rep. 2023, 42, 112033. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Du, G.; Chen, X.; Wang, J.; Liu, K.; Zhao, H.; Cheng, C.; He, Y.; Jing, N.; Xu, P.; et al. Zeb1-controlled metabolic plasticity enables remodeling of chromatin accessibility in the development of neuroendocrine prostate cancer. Cell Death Differ. 2024, 31, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.W.; Yang, Z.H.; Yu, Y.; Zhang, P. HIF1α lactylation enhances KIAA1199 transcription to promote angiogenesis and vasculogenic mimicry in prostate cancer. Int. J. Biol. Macromol. 2022, 222, 2225–2243. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Tang, Y.; Yang, H.; Zheng, J. YTHDC1 lactylation regulates its phase separation to enhance target mRNA stability and promote RCC progression. Mol. Cell 2025, 85, 2733–2748.e7. [Google Scholar] [CrossRef]
- Liu, R.; Zou, Z.H.; Chen, L.W.; Feng, Y.F.; Ye, J.H.; Deng, Y.L.; Zhu, X.J.; Zhang, Y.X.; Lin, J.D.; Cai, S.H.; et al. FKBP10 promotes clear cell renal cell carcinoma progression and regulates sensitivity to the HIF2α blockade by facilitating LDHA phosphorylation. Cell Death Dis. 2024, 15, 64. [Google Scholar] [CrossRef]
- Tang, Y.; Dai, C.; Yang, H.; Yang, X.; Chen, Z.; Dou, J.; Zhang, L.; Bai, T.; Zheng, J. KAT8-mediated MDH2 lactylation promotes renal cancer progression by enhancing mitochondrial function and stress resistance. Int. J. Biol. Macromol. 2025, 322, 146571. [Google Scholar] [CrossRef]
- Yang, J.F.; Luo, L.; Zhao, C.Y.; Li, X.Y.; Wang, Z.M.; Zeng, Z.W.; Yang, X.; Zheng, X.B.; Jie, H.Q.; Kang, L.; et al. A Positive Feedback Loop between Inactive VHL-Triggered Histone Lactylation and PDGFRβ Signaling Drives Clear Cell Renal Cell Carcinoma Progression. Int. J. Biol. Sci. 2022, 18, 3470–3483. [Google Scholar] [CrossRef]
- Huang, C.; Xue, L.; Lin, X.; Shen, Y.; Wang, X. Histone Lactylation-Driven GPD2 Mediates M2 Macrophage Polarization to Promote Malignant Transformation of Cervical Cancer Progression. DNA Cell Biol. 2024, 43, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Zhai, G.; Niu, Z.; Jiang, Z.; Zhao, F.; Wang, S.; Chen, C.; Zheng, W.; Wang, A.; Zang, Y.; Han, Y.; et al. DPF2 reads histone lactylation to drive transcription and tumorigenesis. Proc. Natl. Acad. Sci. USA 2024, 121, e2421496121. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.; You, Y.; Wei, L.; Li, Q.; Sun, H.; Sun, M.; Li, X.; Yang, S.; Zeng, T.; Zhang, L.; et al. ICAT drives lactylation of tumor-associated macrophages via the c-Myc-ENO1 axis to promote cervical cancer progression. Free Radic. Biol. Med. 2025, 241, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Wang, S.; Huang, Z.; Wang, T.; Yu, Y. H3K18la Facilitates TRA2A-Mediated Alternative Splicing of STIL, Suppressing Ferroptosis and Cisplatin Treatment Sensitivity in Ovarian Cancer. Cancer Res. Treat. 2025. [Google Scholar] [CrossRef]
- Lu, B.; Chen, S.; Guan, X.; Chen, X.; Du, Y.; Yuan, J.; Wang, J.; Wu, Q.; Zhou, L.; Huang, X.; et al. Lactate accumulation induces H4K12la to activate super-enhancer-driven RAD23A expression and promote niraparib resistance in ovarian cancer. Mol. Cancer 2025, 24, 83. [Google Scholar] [CrossRef]
- Zheng, C.; Tan, H.; Niu, G.; Huang, X.; Lu, J.; Chen, S.; Li, H.; Zhu, J.; Zhou, Z.; Xu, M.; et al. ACAT1-Mediated ME2 Acetylation Drives Chemoresistance in Ovarian Cancer by Linking Glutaminolysis to Lactate Production. Adv. Sci. 2025, 12, e2416467. [Google Scholar] [CrossRef]
- Hu, X.; Huang, Z.; Li, L. LDHB Mediates Histone Lactylation to Activate PD-L1 and Promote Ovarian Cancer Immune Escape. Cancer Investig. 2025, 43, 70–79. [Google Scholar] [CrossRef]
- Mi, J.; Zhao, L.; Shen, Y.; Mo, S.; Kuang, Y. PFKP Lactylation Promotes the Ovarian Cancer Progression Through Targeting PTEN. Biochem. Genet. 2024, 63, 5294–5311. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Ma, R.; Chen, Y.; Wang, J.; Zhang, L.; Wang, B.; Zhang, Z.; Huang, L.; Zhang, H.; et al. Cold atmospheric plasma drives USP49/HDAC3 axis mediated ferroptosis as a novel therapeutic strategy in endometrial cancer via reinforcing lactylation dependent p53 expression. J. Transl. Med. 2025, 23, 442. [Google Scholar] [CrossRef]
- Wei, S.; Zhang, J.; Zhao, R.; Shi, R.; An, L.; Yu, Z.; Zhang, Q.; Zhang, J.; Yao, Y.; Li, H.; et al. Histone lactylation promotes malignant progression by facilitating USP39 expression to target PI3K/AKT/HIF-1α signal pathway in endometrial carcinoma. Cell Death Discov. 2024, 10, 121. [Google Scholar] [CrossRef]
- Li, J.; Chen, Z.; Jin, M.; Gu, X.; Wang, Y.; Huang, G.; Zhao, W.; Lu, C. Histone H4K12 lactylation promotes malignancy progression in triple-negative breast cancer through SLFN5 downregulation. Cell Signal 2024, 124, 111468. [Google Scholar] [CrossRef]
- Pandkar, M.R.; Samaiya, A.; Shukla, S. Oncometabolite lactate enhances breast cancer progression by orchestrating histone lactylation-dependent c-Myc expression. Transl. Oncol. 2023, 37, 101758. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Huo, D. Understanding genetic architecture of breast cancer: How can proteome-wide association studies contribute? Br. J. Cancer 2024, 131, 1869–1870. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Xu, S.; Ping, J.; Jia, G.; Dou, Y.; Henry, J.E.; Zhang, B.; Guo, X.; Cote, M.L.; Cai, Q.; et al. A proteome-wide association study identifies putative causal proteins for breast cancer risk. Br. J. Cancer 2024, 131, 1796–1804. [Google Scholar] [CrossRef] [PubMed]
- Navarro, G.; Gómez-Autet, M.; Morales, P.; Rebassa, J.B.; Llinas Del Torrent, C.; Jagerovic, N.; Pardo, L.; Franco, R. Homodimerization of CB(2) cannabinoid receptor triggered by a bivalent ligand enhances cellular signaling. Pharmacol. Res. 2024, 208, 107363. [Google Scholar] [CrossRef]
- He, X.; Li, Y.; Li, J.; Li, Y.; Chen, S.; Yan, X.; Xie, Z.; Du, J.; Chen, G.; Song, J.; et al. HDAC2-Mediated METTL3 Delactylation Promotes DNA Damage Repair and Chemotherapy Resistance in Triple-Negative Breast Cancer. Adv. Sci. 2025, 12, e2413121. [Google Scholar] [CrossRef]
- Li, J.; Feng, L.; Zhang, L.; Zhang, W.; Zhang, Y.; Liu, X.; Cai, Q.; Zhao, W.; Huang, G.; Lu, C. Saikosaponin D Mitigates Radioresistance in Triple-Negative Breast Cancer by Inducing MRE11 De-Lactylation via HIF1α/HDAC5 Pathway. Theranostics 2025, 15, 8935–8951. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Ma, L.; Zhao, S.; Hui, X.; Xiong, W.; Cheng, S.; Zhang, Y. ZMIZ1 lactylation induces tamoxifen resistance in breast cancer through increasing transcriptional activity of Nanog to impact cell stemness and cholesterol uptake. Cell Biol. Toxicol. 2025, 41, 117. [Google Scholar] [CrossRef]
- Zhu, S.; Sun, C.; Cai, Z.; Wu, J.; Han, X.; Wang, J.; Wang, C. Multifunctional nanoparticle-mediated targeting of metabolic reprogramming and DNA damage response pathways to treat drug-resistant triple-negative breast cancer. J. Control. Release 2025, 381, 113601. [Google Scholar] [CrossRef]
- Yu, H.; Zhou, Q.; Chen, Y.; Zhong, C.; Fu, T.; Xiong, X.; Zhu, F.; Wang, L.; Sun, Y. Neddylation inhibitor MLN4924 enhances H3K18 lactylation via binding to LDH and downregulates ITGB4 to block metastasis. J. Biol. Chem. 2025, 301, 110575. [Google Scholar] [CrossRef]
- Zheng, B.; Pan, Y.; Qian, F.; Liu, D.; Ye, D.; Yu, B.; Zhong, S.; Zheng, W.; Wang, X.; Zhou, B.; et al. High Sugar Induced RCC2 Lactylation Drives Breast Cancer Tumorigenicity Through Upregulating MAD2L1. Adv. Sci. 2025, 12, e2415530. [Google Scholar] [CrossRef]
- Zhang, K.; Guo, L.; Li, X.; Hu, Y.; Luo, N. Cancer-associated fibroblasts promote doxorubicin resistance in triple-negative breast cancer through enhancing ZFP64 histone lactylation to regulate ferroptosis. J. Transl. Med. 2025, 23, 247. [Google Scholar] [CrossRef]
- Cui, Z.; Zheng, C.; Lin, Y.; Xiao, Z.; Li, Y.; Peng, W.; He, S.; Li, A.; Wu, X.; Chen, Y.; et al. Lactate Dehydrogenase C4 Accelerates Triple-Negative Breast Cancer Progression by Promoting Acetyl-CoA Acyltransferase 2 Lactylation to Increase Free Fatty Acid Accumulation. Adv. Sci. 2025, 12, e11849. [Google Scholar] [CrossRef]
- Xu, Y.; Meng, W.; Dai, Y.; Xu, L.; Ding, N.; Zhang, J.; Zhuang, X. Anaerobic metabolism promotes breast cancer survival via Histone-3 Lysine-18 lactylation mediating PPARD axis. Cell Death Discov. 2025, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Qiu, S.; Yang, X.; Wu, Y.; Yao, X.; Hu, H.; Luo, J.; Wijaya, C.S.; Ma, L.; Long, X.; et al. Hypoxia-Induced PRMT1 Lactylation Drives Vimentin Arginine Asymmetric Dimethylation in Tumor Metastasis. Adv. Sci. 2025, 12, e09861. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Yang, X.; Xu, C.; Song, Q.; Zhao, H.; Sun, T.; Liu, J.; Zhang, Y.; Sun, G.; Xue, Y.; et al. SIRT4 Promotes Pancreatic Cancer Stemness by Enhancing Histone Lactylation and Epigenetic Reprogramming Stimulated by Calcium Signaling. Adv. Sci. 2025, 12, 2412553. [Google Scholar] [CrossRef]
- Li, F.; Si, W.; Xia, L.; Yin, D.; Wei, T.; Tao, M.; Cui, X.; Yang, J.; Hong, T.; Wei, R. Positive feedback regulation between glycolysis and histone lactylation drives oncogenesis in pancreatic ductal adenocarcinoma. Mol. Cancer 2024, 23, 90. [Google Scholar] [CrossRef]
- Ding, F.; Guo, Y.; Zhang, H.; Zhong, Y.; Zhang, D.; Huang, Q.; Zheng, Z.; Liu, G.; Zhang, X.; Weng, S. High glucose-induced mitochondrial fission drives pancreatic cancer progression through the H3K18la/TTK/BUB1B signal pathway. Cell Signal 2025, 135, 112027. [Google Scholar] [CrossRef]
- Li, T.; Hu, C.; Huang, T.; Zhou, Y.; Tian, Q.; Chen, H.; He, R.; Yuan, Y.; Jiang, Y.; Jiang, H.; et al. Cancer-Associated Fibroblasts Foster a High-Lactate Microenvironment to Drive Perineural Invasion in Pancreatic Cancer. Cancer Res. 2025, 85, 2199–2217. [Google Scholar] [CrossRef]
- Liu, M.; Gu, L.; Zhang, Y.; Li, Y.; Zhang, L.; Xin, Y.; Wang, Y.; Xu, Z.X. LKB1 inhibits telomerase activity resulting in cellular senescence through histone lactylation in lung adenocarcinoma. Cancer Lett. 2024, 595, 217025. [Google Scholar] [CrossRef]
- Chang, Z. NCAPD3 contributes to lung cancer progression through modulated lactate-induced histone lactylation and MEK/ERK/LDHA axis. Cancer Cell Int. 2025, 25, 189. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Duan, X.; Gu, G.; Zhu, Q.; Shu, J.; Fei, F. LDHA-Mediated H3K18 Lactylation Promotes the Glycolysis in Non-Small Cell Lung Cancer Through Targeting PTEN. Biochem. Genet. 2025. [Google Scholar] [CrossRef]
- Wu, S.; Liu, M.; Wang, X.; Wang, S. The histone lactylation of AIM2 influences the suppression of ferroptosis by ACSL4 through STAT5B and promotes the progression of lung cancer. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2025, 39, e70308. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; He, Q.; Gao, Y.; He, X.; Luo, H.; Shao, L.; Dong, J.; Li, F. SLC4A7 suppresses lung adenocarcinoma oncogenesis by reducing lactate transport and protein lactylation. Int. J. Oncol. 2025, 66, 33. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Tang, Y.; Wang, S.; Huang, Y.; Chi, Q.; Xu, K.; Xue, L. Natural product fargesin interferes with H3 histone lactylation via targeting PKM2 to inhibit non-small cell lung cancer tumorigenesis. Biofactors 2024, 50, 592–607. [Google Scholar] [CrossRef]
- Dai, J.; Lu, X.; Zhang, C.; Qu, T.; Li, W.; Su, J.; Guo, R.; Yin, D.; Wu, P.; Han, L.; et al. NNMT promotes acquired EGFR-TKI resistance by forming EGR1 and lactate-mediated double positive feedback loops in non-small cell lung cancer. Mol. Cancer 2025, 24, 79. [Google Scholar] [CrossRef]
- Yan, F.; Teng, Y.; Li, X.; Zhong, Y.; Li, C.; Yan, F.; He, X. Hypoxia promotes non-small cell lung cancer cell stemness, migration, and invasion via promoting glycolysis by lactylation of SOX9. Cancer Biol. Ther. 2024, 25, 2304161. [Google Scholar] [CrossRef]
- Zhang, R.; Li, L.; Yu, J. Lactate-induced IGF1R protein lactylation promotes proliferation and metabolic reprogramming of lung cancer cells. Open Life Sci. 2024, 19, 20220874. [Google Scholar] [CrossRef]
- Li, L.L.; Li, Z.W.; Meng, X.Q.; Wang, X.Y.; Song, D.; Liu, Y.X.; Xu, T.Y.; Qin, J.; Sun, N.; Tian, K.F.; et al. Histone lactylation-derived LINC01127 promotes the self-renewal of glioblastoma stem cells via the cis-regulating the MAP4K4 to activate JNK pathway. Cancer Lett. 2023, 579, 216467. [Google Scholar] [CrossRef]
- De Leo, A.; Ugolini, A.; Yu, X.; Scirocchi, F.; Scocozza, D.; Peixoto, B.; Pace, A.; D’Angelo, L.; Liu, J.K.C.; Etame, A.B.; et al. Glucose-driven histone lactylation promotes the immunosuppressive activity of monocyte-derived macrophages in glioblastoma. Immunity 2024, 57, 1105–1123.e8. [Google Scholar] [CrossRef]
- Sun, T.; Liu, B.; Li, Y.Y.; Wu, J.; Cao, Y.F.; Yang, S.Y.; Tan, H.L.; Cai, L.Z.; Zhang, S.Q.; Qi, X.Y.; et al. Oxamate enhances the efficacy of CAR-T therapy against glioblastoma via suppressing ectonucleotidases and CCR8 lactylation. J. Exp. Clin. Cancer Res. 2023, 42, 253. [Google Scholar] [CrossRef]
- Wang, X.B.; Shi, Y.Q.; Shi, H.; Liu, X.Y.; Liao, A.J.; Liu, Z.G.; Orlowski, R.Z.; Zhang, R.; Wang, H.H. MUC20 regulated by extrachromosomal circular DNA attenuates proteasome inhibitor resistance of multiple myeloma by modulating cuproptosis. J. Exp. Clin. Cancer Res. 2024, 43, 68. [Google Scholar] [CrossRef]
- Sun, Y. Tumor microenvironment and cancer therapy resistance. Cancer Lett. 2016, 380, 205–215. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, Y.; Wu, C.; Shi, J. Engineering lactate-modulating nanomedicines for cancer therapy. Chem. Soc. Rev. 2023, 52, 973–1000. [Google Scholar] [CrossRef] [PubMed]
- Hensley, C.T.; Faubert, B.; Yuan, Q.; Lev-Cohain, N.; Jin, E.; Kim, J.; Jiang, L.; Ko, B.; Skelton, R.; Loudat, L.; et al. Metabolic Heterogeneity in Human Lung Tumors. Cell 2016, 164, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. The Science and Translation of Lactate Shuttle Theory. Cell Metab. 2018, 27, 757–785. [Google Scholar] [CrossRef]
- Faubert, B.; Li, K.Y.; Cai, L.; Hensley, C.T.; Kim, J.; Zacharias, L.G.; Yang, C.D.; Do, Q.N.; Doucette, S.; Burguete, D.; et al. Lactate Metabolism in Human Lung Tumors. Cell 2017, 171, 358–371. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Chandel, N.S. Waste Not, Want Not: Lactate Oxidation Fuels the TCA Cycle. Cell Metab. 2017, 26, 803–804. [Google Scholar] [CrossRef]
- Hay, N. Reprogramming glucose metabolism in cancer: Can it be exploited for cancer therapy? Nat. Rev. Cancer 2016, 16, 635–649. [Google Scholar] [CrossRef]
- Barron, C.C.; Bilan, P.J.; Tsakiridis, T.; Tsiani, E. Facilitative glucose transporters: Implications for cancer detection, prognosis and treatment. Metab. Clin. Exp. 2016, 65, 124–139. [Google Scholar] [CrossRef]
- Doherty, J.R.; Cleveland, J.L. Targeting lactate metabolism for cancer therapeutics. J. Clin. Investig. 2013, 123, 3685–3692. [Google Scholar] [CrossRef]
- Verdura, S.; Cuyàs, E.; Ruiz-Torres, V.; Micol, V.; Joven, J.; Bosch-Barrera, J.; Menendez, J.A. Lung Cancer Management with Silibinin: A Historical and Translational Perspective. Pharmaceuticals 2021, 14, 559. [Google Scholar] [CrossRef]
- Spagnuolo, C.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Sureda, A.; Nabavi, S.F.; Devi, K.P.; Loizzo, M.R.; Tundis, R.; et al. Genistein and cancer: Current status, challenges, and future directions. Adv. Nutr. 2015, 6, 408–419. [Google Scholar] [CrossRef]
- Pattni, B.S.; Jhaveri, A.; Dutta, I.; Baleja, J.D.; Degterev, A.; Torchilin, V. Targeting energy metabolism of cancer cells: Combined administration of NCL-240 and 2-DG. Int. J. Pharm. 2017, 532, 149–156. [Google Scholar] [CrossRef]
- Gao, F.; Tang, Y.; Liu, W.L.; Zou, M.Z.; Huang, C.; Liu, C.J.; Zhang, X.Z. Intra/Extracellular Lactic Acid Exhaustion for Synergistic Metabolic Therapy and Immunotherapy of Tumors. Adv. Mater. 2019, 31, e1904639. [Google Scholar] [CrossRef] [PubMed]
- Zang, S.; Huang, K.; Li, J.; Ren, K.; Li, T.; He, X.; Tao, Y.; He, J.; Dong, Z.; Li, M.; et al. Metabolic reprogramming by dual-targeting biomimetic nanoparticles for enhanced tumor chemo-immunotherapy. Acta Biomater. 2022, 148, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Huang, Z.; Su, J.; Li, J.; Zhao, S.; Wu, L.; Zhang, J.; He, Y.; Zhang, G.; Tao, J.; et al. Benserazide is a novel inhibitor targeting PKM2 for melanoma treatment. Int. J. Cancer 2020, 147, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Rani, R.; Kumar, V. Recent Update on Human Lactate Dehydrogenase Enzyme 5 (hLDH5) Inhibitors: A Promising Approach for Cancer Chemotherapy. J. Med. Chem. 2016, 59, 487–496. [Google Scholar] [CrossRef]
- Sharma, D.; Singh, M.; Rani, R. Role of LDH in tumor glycolysis: Regulation of LDHA by small molecules for cancer therapeutics. Semin. Cancer Biol. 2022, 87, 184–195. [Google Scholar] [CrossRef]
- Tseng, S.J.; Kempson, I.M.; Huang, K.Y.; Li, H.J.; Fa, Y.C.; Ho, Y.C.; Liao, Z.X.; Yang, P.C. Targeting Tumor Microenvironment by Bioreduction-Activated Nanoparticles for Light-Triggered Virotherapy. ACS Nano 2018, 12, 9894–9902. [Google Scholar] [CrossRef]
- Tian, Z.; Yang, K.; Yao, T.; Li, X.; Ma, Y.; Qu, C.; Qu, X.; Xu, Y.; Guo, Y.; Qu, Y. Catalytically Selective Chemotherapy from Tumor-Metabolic Generated Lactic Acid. Small 2019, 15, e1903746. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Wang, Z.; Zheng, H.; Wang, L.; Xu, S.; Liu, X.; Li, W.; Pan, Y.; Wang, W.; Cai, X.; et al. Two-Dimensional Tin Selenide (SnSe) Nanosheets Capable of Mimicking Key Dehydrogenases in Cellular Metabolism. Angew. Chem. 2020, 59, 3618–3623. [Google Scholar] [CrossRef] [PubMed]
- Futagi, Y.; Kobayashi, M.; Narumi, K.; Furugen, A.; Iseki, K. Identification of a selective inhibitor of human monocarboxylate transporter 4. Biochem. Biophys. Res. Commun. 2018, 495, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Lin, C.; He, Y.; Lu, L.; Xu, K.; Tao, B.; Xia, Z.; Zeng, R.; Mao, Y.; Luo, Z.; et al. Engineering of Cascade-Responsive Nanoplatform to Inhibit Lactate Efflux for Enhanced Tumor Chemo-Immunotherapy. ACS Nano 2020, 14, 14164–14180. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.; Shi, L.; Hu, Y.; Song, G.; Cai, K.; Li, M.; Luo, Z. Tumor-Targeted Disruption of Lactate Transport with Reactivity-Reversible Nanocatalysts to Amplify Oxidative Damage. Small 2021, 17, e2100130. [Google Scholar] [CrossRef]
- Wang, H.; Yang, C.; Doherty, J.R.; Roush, W.R.; Cleveland, J.L.; Bannister, T.D. Synthesis and structure-activity relationships of pteridine dione and trione monocarboxylate transporter 1 inhibitors. J. Med. Chem. 2014, 57, 7317–7324. [Google Scholar] [CrossRef]
- Quanz, M.; Bender, E.; Kopitz, C.; Grünewald, S.; Schlicker, A.; Schwede, W.; Eheim, A.; Toschi, L.; Neuhaus, R.; Richter, C.; et al. Preclinical Efficacy of the Novel Monocarboxylate Transporter 1 Inhibitor BAY-8002 and Associated Markers of Resistance. Mol. Cancer Ther. 2018, 17, 2285–2296. [Google Scholar] [CrossRef]
- Gurrapu, S.; Jonnalagadda, S.K.; Alam, M.A.; Ronayne, C.T.; Nelson, G.L.; Solano, L.N.; Lueth, E.A.; Drewes, L.R.; Mereddy, V.R. Coumarin carboxylic acids as monocarboxylate transporter 1 inhibitors: In vitro and in vivo studies as potential anticancer agents. Bioorganic Med. Chem. Lett. 2016, 26, 3282–3286. [Google Scholar] [CrossRef]
- Tateishi, H.; Tsuji, A.B.; Kato, K.; Sudo, H.; Sugyo, A.; Hanakawa, T.; Zhang, M.R.; Saga, T.; Arano, Y.; Higashi, T. Synthesis and evaluation of (11)C-labeled coumarin analog as an imaging probe for detecting monocarboxylate transporters expression. Bioorganic Med. Chem. Lett. 2017, 27, 4893–4897. [Google Scholar] [CrossRef]
- Li, L.; Yue, T.; Feng, J.; Zhang, Y.; Hou, J.; Wang, Y. Recent progress in lactate oxidase-based drug delivery systems for enhanced cancer therapy. Nanoscale 2024, 16, 8739–8758. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Huang, S.; Li, M.; Chen, J.; Pei, D.; Tang, Z.; Guo, B. Bacteria-responsive programmed self-activating antibacterial hydrogel to remodel regeneration microenvironment for infected wound healing. Natl. Sci. Rev. 2024, 11, nwae044. [Google Scholar] [CrossRef]
- Jiang, S.; Chen, X.; Lin, J.; Huang, P. Lactate-Oxidase-Instructed Cancer Diagnosis and Therapy. Adv. Mater. 2023, 35, e2207951. [Google Scholar] [CrossRef]
- Urbańska, K.; Orzechowski, A. Unappreciated Role of LDHA and LDHB to Control Apoptosis and Autophagy in Tumor Cells. Int. J. Mol. Sci. 2019, 20, 2085. [Google Scholar] [CrossRef]
- Cheng, Q.; Shi, X.L.; Li, Q.L.; Wang, L.; Wang, Z. Current Advances on Nanomaterials Interfering with Lactate Metabolism for Tumor Therapy. Adv. Sci. 2024, 11, e2305662. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.W.; Wang, J.W.; Wang, X.N.; Fan, J.X.; Liu, X.H.; Li, B.; Han, Z.Y.; Cheng, S.X.; Zhang, X.Z. Inhibition of Tumor Progression through the Coupling of Bacterial Respiration with Tumor Metabolism. Angew. Chem. 2020, 59, 21562–21570. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cheng, L.; Ma, S.; Ding, L.; Zhang, W.; Xu, Z.; Li, D.; Gao, L. Self-Assembled Multiple-Enzyme Composites for Enhanced Synergistic Cancer Starving-Catalytic Therapy. ACS Appl. Mater. Interfaces 2020, 12, 20191–20201. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, L.; Morandi, A.; Giannoni, E.; Chiarugi, P. Lactate: A Metabolic Driver in the Tumour Landscape. Trends Biochem. Sci. 2019, 44, 153–166. [Google Scholar] [CrossRef]
- Lipstein, M.R.; Pal, I.; Bates, S.E.; Deng, C. Metabolic symbiosis in cancer and its therapeutic implication. Semin. Oncol. 2017, 44, 233–234. [Google Scholar] [CrossRef]
- Ullah, M.S.; Davies, A.J.; Halestrap, A.P. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1alpha-dependent mechanism. J. Biol. Chem. 2006, 281, 9030–9037. [Google Scholar] [CrossRef]
- Payen, V.L.; Mina, E.; Van Hée, V.F.; Porporato, P.E.; Sonveaux, P. Monocarboxylate transporters in cancer. Mol. Metab. 2020, 33, 48–66. [Google Scholar] [CrossRef]
- Ma, X.M.; Geng, K.; Wang, P.; Jiang, Z.Z.; Law, B.Y.K.; Xu, Y. MCT4-dependent lactate transport: A novel mechanism for cardiac energy metabolism injury and inflammation in type 2 diabetes mellitus. Cardiovasc. Diabetol. 2024, 23, 96. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Huang, X.; Liang, C.Q.; Zhang, P. Evodiamine impairs HIF1A histone lactylation to inhibit Sema3A-mediated angiogenesis and PD-L1 by inducing ferroptosis in prostate cancer. Eur. J. Pharmacol. 2023, 957, 176007. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, H.; Dong, M.; Min, J.; He, X.; Tan, Y.; Liu, F.; Chen, M.; Chen, X.; Yin, Q.; et al. Macrophage MCT4 inhibition activates reparative genes and protects from atherosclerosis by histone H3 lysine 18 lactylation. Cell Rep. 2024, 43, 114180. [Google Scholar] [CrossRef]
- Wang, Z.H.; Zhang, P.; Peng, W.B.; Ye, L.L.; Xiang, X.; Wei, X.S.; Niu, Y.R.; Zhang, S.Y.; Xue, Q.Q.; Wang, H.L.; et al. Altered phenotypic and metabolic characteristics of FOXP3+CD3+CD56+ natural killer T (NKT)-like cells in human malignant pleural effusion. OncoImmunology 2023, 12, 2160558. [Google Scholar] [CrossRef]
- Wang, N.X.; Wang, W.W.; Wang, X.Q.; Mang, G.; Chen, J.F.; Yan, X.Y.; Tong, Z.H.; Yang, Q.N.; Wang, M.D.; Chen, L.Q.; et al. Histone Lactylation Boosts Reparative Gene Activation Post-Myocardial Infarction. Circ. Res. 2022, 131, 893–908. [Google Scholar] [CrossRef]
- Wang, P.; Xie, D.; Xiao, T.; Cheng, C.; Wang, D.; Sun, J.; Wu, M.; Yang, Y.; Zhang, A.; Liu, Q. H3K18 lactylation promotes the progression of arsenite-related idiopathic pulmonary fibrosis via YTHDF1/m6A/NREP. J. Hazard. Mater. 2024, 461, 132582. [Google Scholar] [CrossRef]
- Gao, X.; Pang, C.; Fan, Z.; Wang, Y.; Duan, Y.; Zhan, H. Regulation of newly identified lysine lactylation in cancer. Cancer Lett. 2024, 587, 216680. [Google Scholar] [CrossRef]
- Cheng, X.L.; Wang, K.; Zhao, Y.; Wang, K. Research progress on post-translational modification of proteins and cardiovascular diseases. Cell Death Discov. 2023, 9, 275. [Google Scholar] [CrossRef]
- Vaughan, R.A.; Gannon, N.P.; Garcia-Smith, R.; Licon-Munoz, Y.; Barberena, M.A.; Bisoffi, M.; Trujillo, K.A. β-alanine suppresses malignant breast epithelial cell aggressiveness through alterations in metabolism and cellular acidity in vitro. Mol. Cancer 2014, 13, 14. [Google Scholar] [CrossRef]
- Guertin, D.A.; Wellen, K.E. Acetyl-CoA metabolism in cancer. Nat. Rev. Cancer 2023, 23, 156–172. [Google Scholar] [CrossRef]
- Yegutkin, G.G.; Boison, D. ATP and Adenosine Metabolism in Cancer: Exploitation for Therapeutic Gain. Pharmacol. Rev. 2022, 74, 797–822. [Google Scholar] [CrossRef] [PubMed]
- Niu, B.; Liao, K.; Zhou, Y.; Wen, T.; Quan, G.; Pan, X.; Wu, C. Application of glutathione depletion in cancer therapy: Enhanced ROS-based therapy, ferroptosis, and chemotherapy. Biomaterials 2021, 277, 121110. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Chen, X.; Wang, H.G.; Gu, X.Q.; Yuan, Y.; Zhang, Z.X. Global profiling of protein lysine lactylation and potential target modified protein analysis in hepatocellular carcinoma. Proteomics 2023, 23, e2200432. [Google Scholar] [CrossRef] [PubMed]

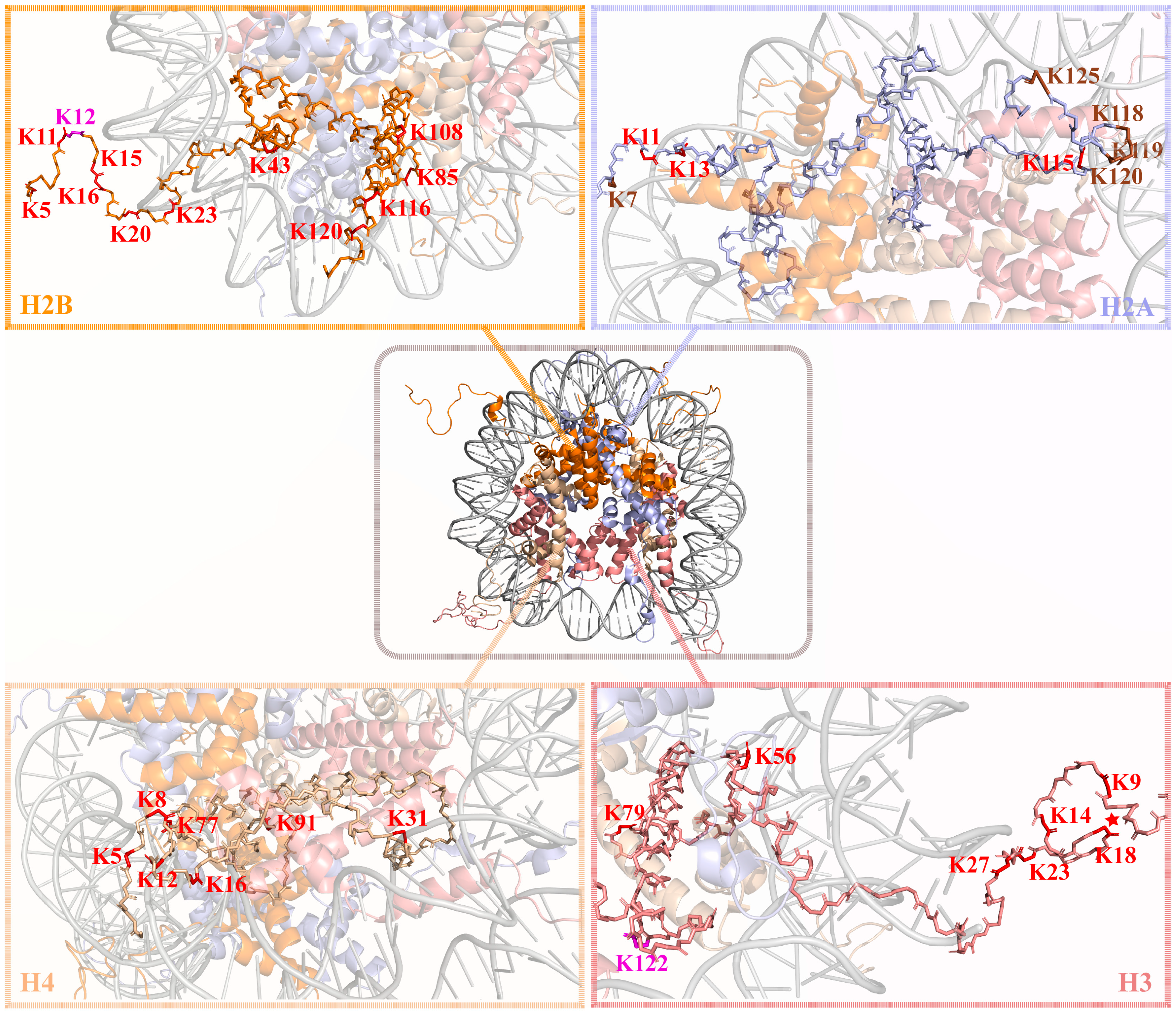


| Site | Upregulated Genes | Effect | Refs |
|---|---|---|---|
| H3K18 | YTHDF2, ALKBH3, circATXN7 | Promote tumor proliferation, migration, invasion, metastasis, chemotherapy resistance | [20,21,51] |
| H3K9 | LUC7L2, LAMC2, ESM1 | Regulates tumor development | [52,53,54] |
| H4K8 | HK1, IDH3G, LINC00152 | Regulates cellular metabolism, invasion and migration | [55,56] |
| H4K12 | CCNB1, ABCs | Promotes proliferation and chemotherapy resistance | [57,58] |
| Name | Lactylation Site | Effects | Refs |
|---|---|---|---|
| MOESIN | K72 | Strengthen their own typical function | [65] |
| MRE11 | K673 | [30] | |
| eEF1A2 | K408 | [66] | |
| NCL | K477 | [24] | |
| YAP | K90 | [44] | |
| TEAD | K108 | [44] | |
| NSUN2 | K356 | [67] | |
| NBS1 | K388 | [29] | |
| METTL16 | K229 | [62] | |
| VPS34 | K356/K781 | [40] | |
| CCNE2 | K348 | [64] | |
| CENPA | K124 | [68] | |
| DCBLD1 | K172 | Maintain its own stability | [69] |
| NUSAP1 | K34 | Inhibit self-degradation | [26] |
| AXIN1 | K147 | Promote ubiquitination | [27] |
| NMNAT1 | K128 | Enhance enzyme activity | [25] |
| Strategy | Target | Inhibitor | Refs |
|---|---|---|---|
| Block lactate anabolism | GLUT1 | Silibinin (C25H22O10) | [202] |
| Genistein (C15H10O5) | [203] | ||
| HK2 | 2-DG (C6H12O5) | [204] | |
| PFK | 3PO (C12H8N2O) | [205] | |
| PFK15 (C17H12N2O) | [206] | ||
| PKM2 | Benserazide (C10H14N2O4) | [207] | |
| LDHA | Oxamate (C2H2NO3−) | [208,209] | |
| GNE-140 (C25H23ClN2O3S2) | |||
| PSTMB (C13H9F3Se) | |||
| Promote lactate catabolism | LOX | Natural lactate oxidase (LOX) | [210,211] |
| LDH | Selenide nanosheets | [212] | |
| Inhibit lactate Shuttle | MCT4 | Bindarit (C19H20N2O3) and its derivatives | [213] |
| Nanomaterial-based inhibitors | [214,215] | ||
| MCT1 | Novel substituted pteridine-derived inhibitors | [216] | |
| BAY-8002 (C20H14ClNO5S) | [217] | ||
| Coumarin carboxylic acids (C10H6O4) | [218,219] | ||
| Target lactylation | AARS1 | β-alanine | [18] |
| MRE11 | Cell-penetrating peptide | [30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, D.; Li, D.; Sun, Y.; Shi, J.; Zhang, S.; Wang, C. Targeting Lactylation for Cancer: Mechanisms, Effects, and Therapeutic Prospects. Int. J. Mol. Sci. 2025, 26, 11278. https://doi.org/10.3390/ijms262311278
Chang D, Li D, Sun Y, Shi J, Zhang S, Wang C. Targeting Lactylation for Cancer: Mechanisms, Effects, and Therapeutic Prospects. International Journal of Molecular Sciences. 2025; 26(23):11278. https://doi.org/10.3390/ijms262311278
Chicago/Turabian StyleChang, Dong, Daolong Li, Yuxi Sun, Jiekang Shi, Shengping Zhang, and Chuangui Wang. 2025. "Targeting Lactylation for Cancer: Mechanisms, Effects, and Therapeutic Prospects" International Journal of Molecular Sciences 26, no. 23: 11278. https://doi.org/10.3390/ijms262311278
APA StyleChang, D., Li, D., Sun, Y., Shi, J., Zhang, S., & Wang, C. (2025). Targeting Lactylation for Cancer: Mechanisms, Effects, and Therapeutic Prospects. International Journal of Molecular Sciences, 26(23), 11278. https://doi.org/10.3390/ijms262311278








