Nanosecond Pulsed Electric Fields (nsPEFs) for Precision Intracellular Oncotherapy: Recent Advances and Emerging Directions
Abstract
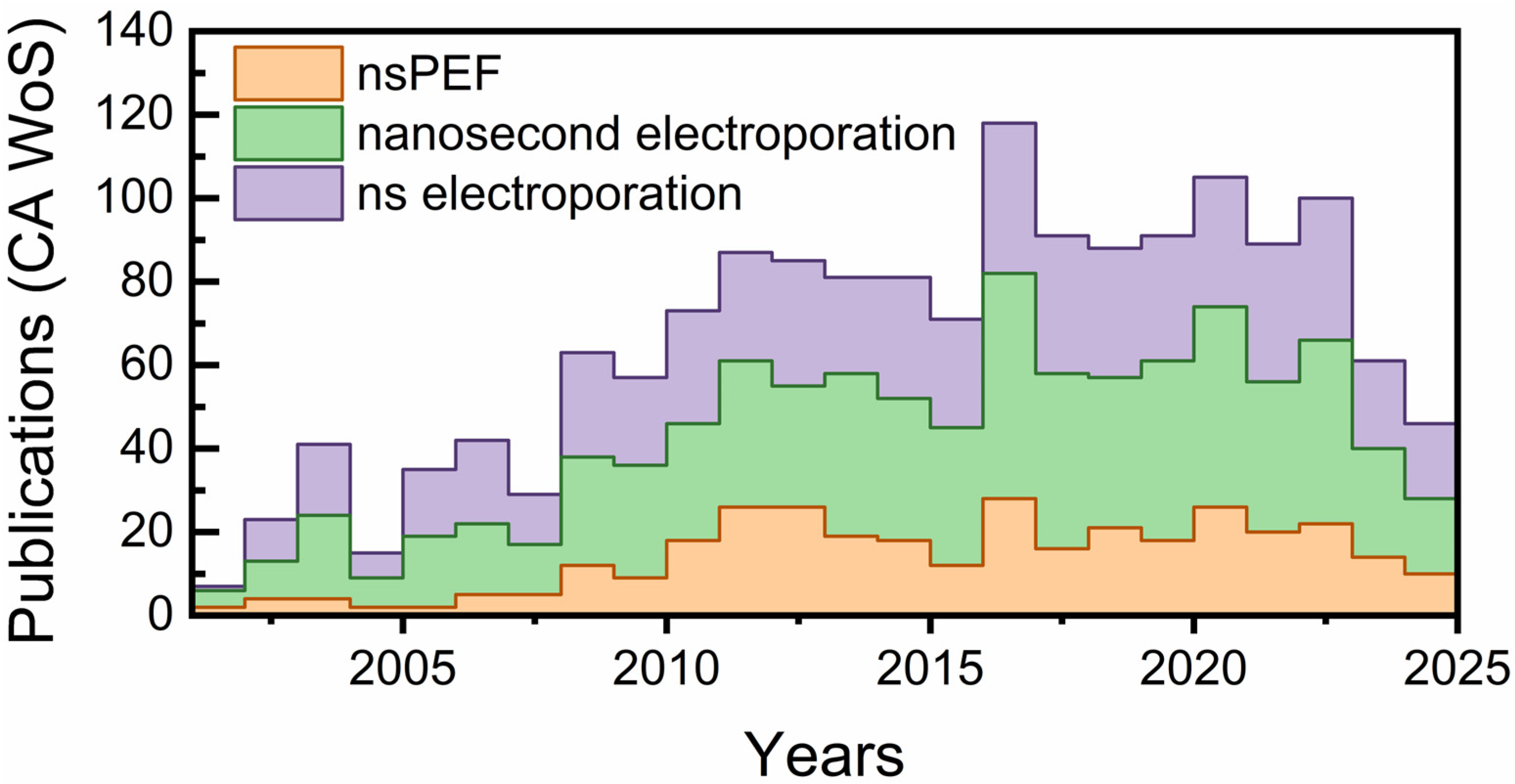
1. Introduction
1.1. Overview of nsPEF as a Transformative Oncology Tool
1.2. Significance of Intracellular Targeting in Cancer Therapy
1.3. A Broader Context: From Electroporation to Precision Bioelectric Medicine
1.4. Objectives of the Review
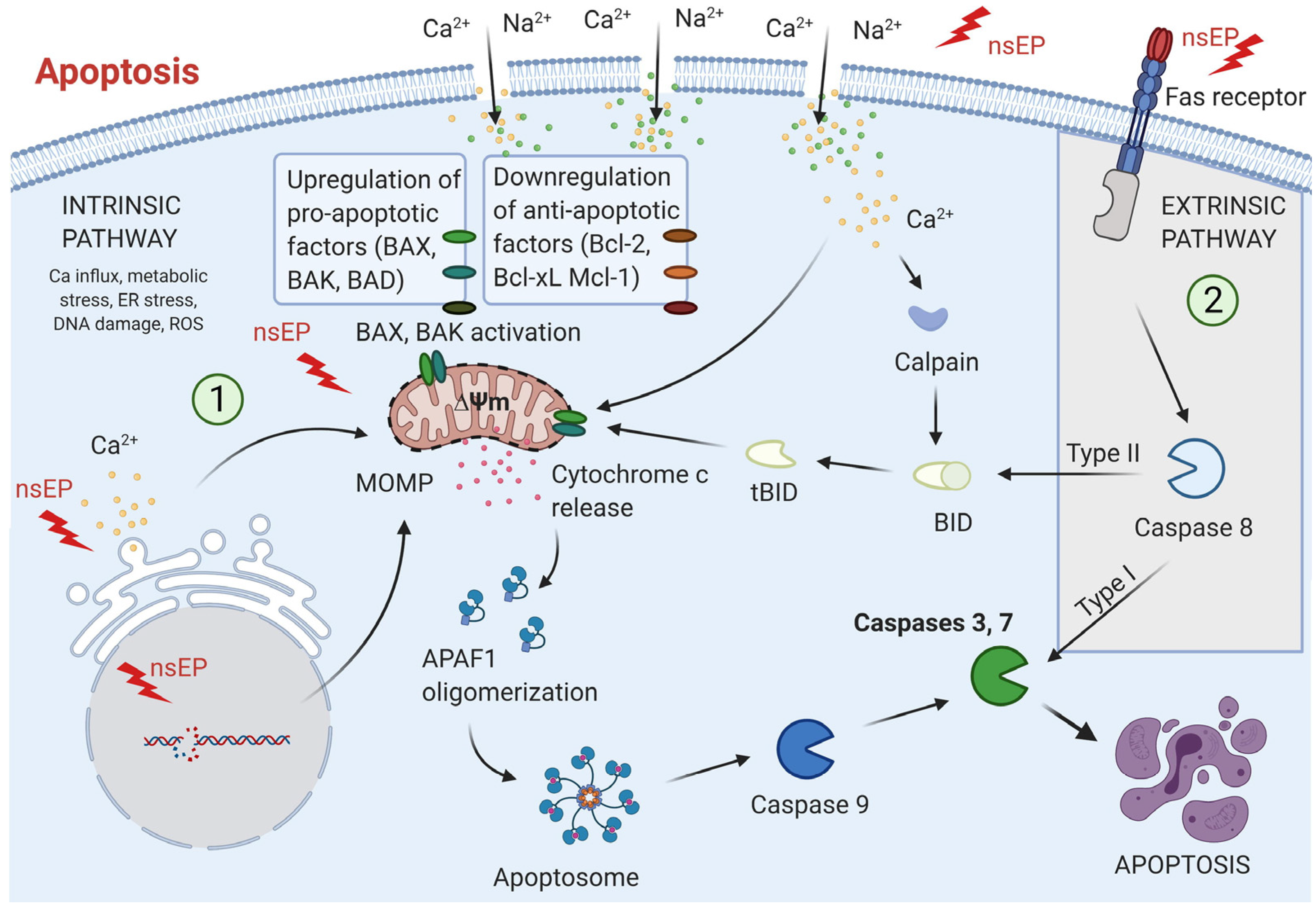
2. Intracellular Electrophysiology of nsPEF
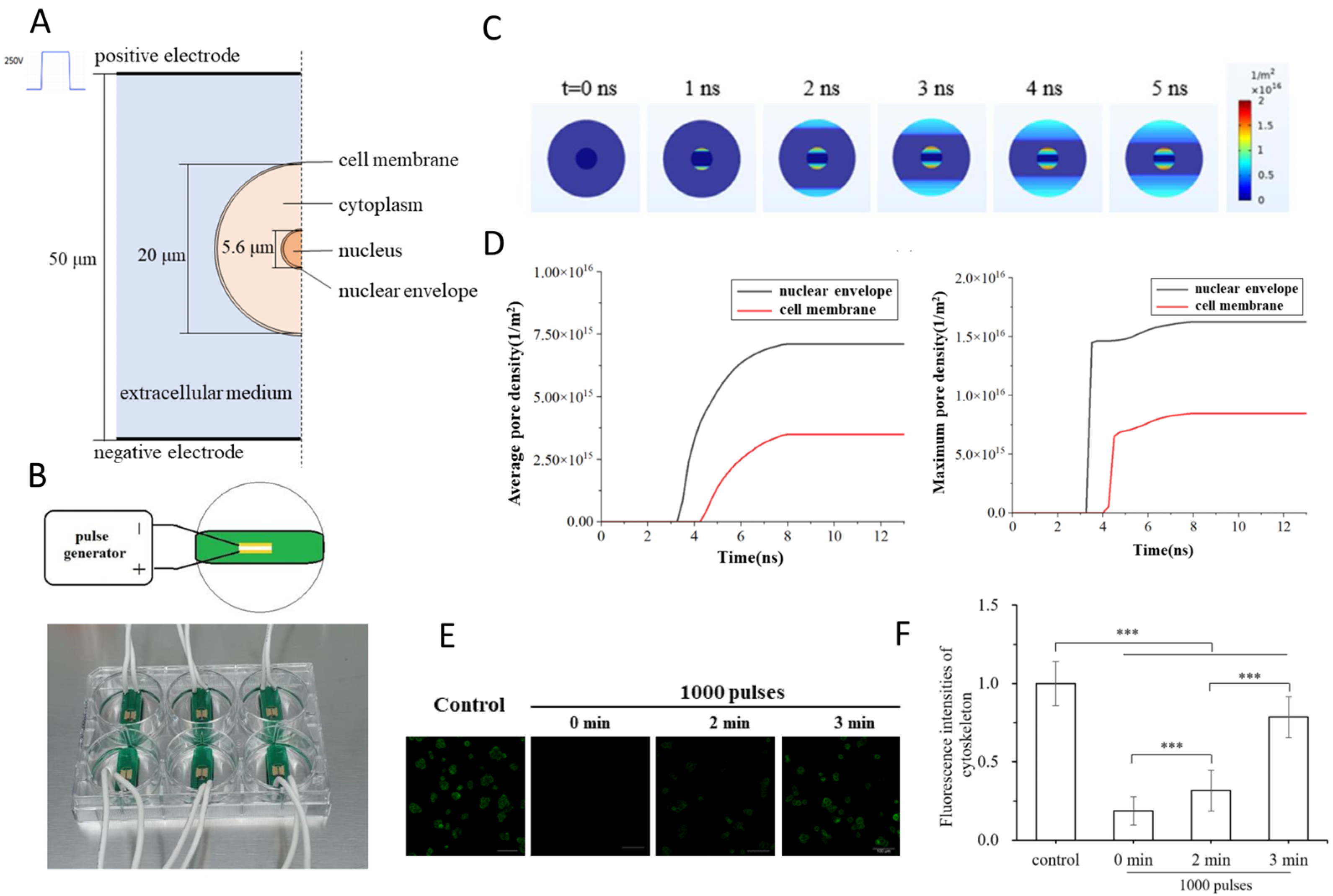
2.1. Ultrafast Electroporation and Membrane Charging
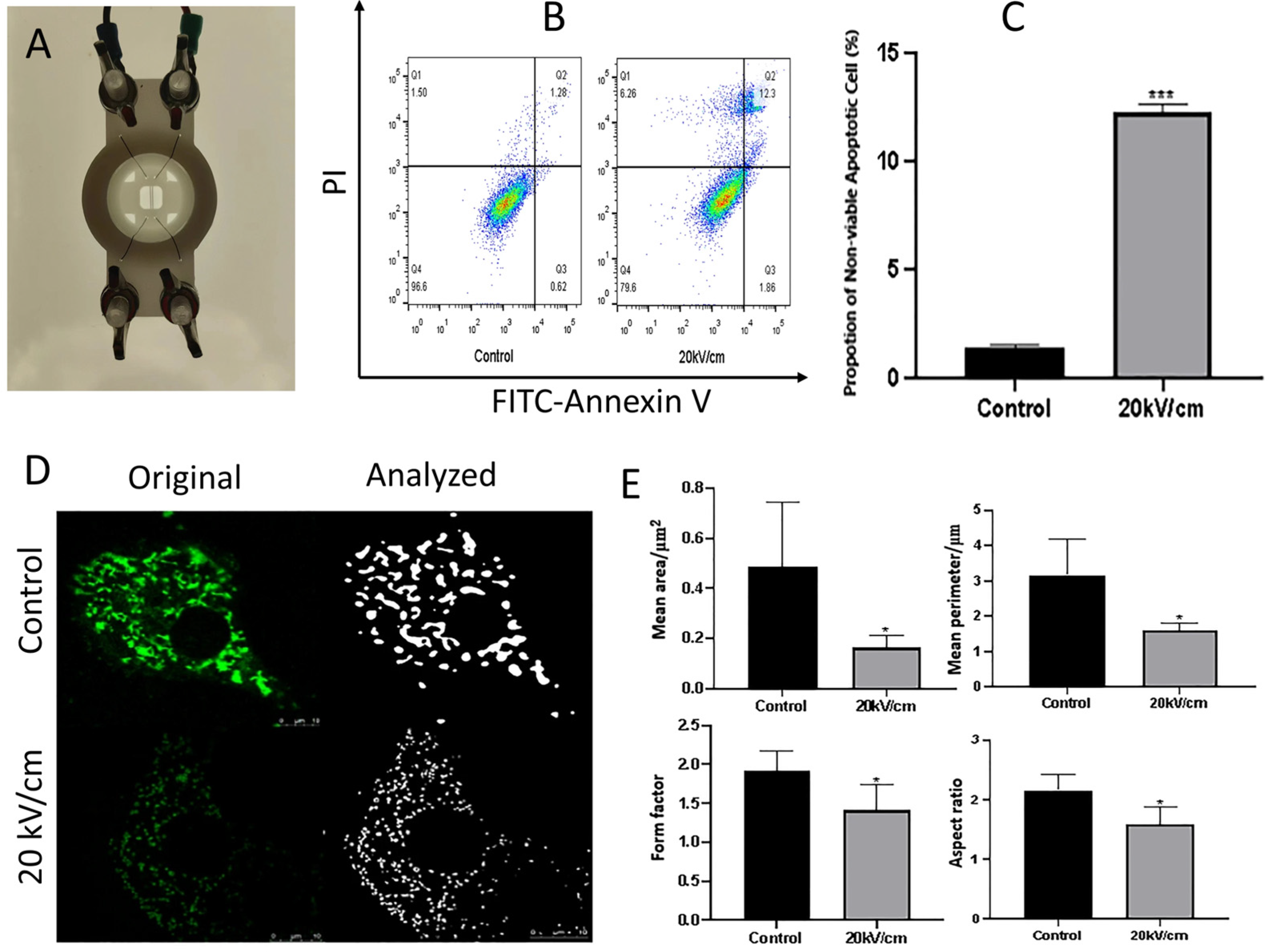
2.2. Organelle-Specific Responses: Mitochondria, Endoplasmic Reticulum, and Nucleus
2.2.1. Mitochondria
2.2.2. Endoplasmic Reticulum (ER)
2.2.3. Nucleus
2.3. Pulse Parameters for Selective Treatments
2.3.1. Pulse Duration (Width)
2.3.2. Field Strength (Amplitude)
2.3.3. Pulse Number and Repetition Rate
2.3.4. Frequency Spectrum and Rise Time
2.3.5. Tumor Selectivity and Microenvironmental Influences
3. Therapeutic Applications of nsPEF in Precision Oncology
4. Innovative Frontiers in nsPEF Therapy
4.1. Integration with Nanoparticle-Mediated Drug Delivery
4.2. Integration of nsPEFs with Drugs
| Tumor/Cell Model | Combination | nsPEF Parameters | Mechanistic Outcomes | Therapeutic Implications | Refs. |
|---|---|---|---|---|---|
| Hep3B hepatocellular carcinoma cells | Doxorubicin (0.5 µM, free drug) | 100 ns, 15 kV/cm, 400 pulses, 10 Hz | DOX uptake ↑ (delayed 40 min > immediate), Cell viability ↓, Cell cycle arrest ↑, Early apoptosis ↑, Mitochondrial swelling ↑ | Timing-dependent synergy: delayed DOX (40 min post nsPEF) most effective (SQ = 1.40 @48 h), enabling low-dose chemo with enhanced cytotoxicity | [177] |
| Murine pancreatic cancer (Panc02, C57BL/6 mice) | Neutrophil membrane-coated liposomal gemcitabine (NE/Lip-GEM) | 300 ns, 24 kV/cm, 100 pulses | NE/Lip-GEM uptake ↑ in nsPEF-treated tumors (3–3.6×), Tumor volume ↓, Ki-67 ↓, Apoptosis ↑, Cytokines (TNF-α, IL-1β, CXCL1) ↑ | nsPEFs amplify inflammatory signals → recruit NE/Lip-GEM to tumor; combination markedly enhances pancreatic tumor suppression without systemic toxicity | [179] |
| Human hepatocellular carcinoma (SMMC7721, BEL7402, HCCLM3) | Cisplatin (5 µg/mL) | 100 ns, 40 kV/cm, 12 pulses (1 pulse/min) | Membrane microdomains disrupted ↑, PI uptake ↑, Cisplatin cytotoxicity ↑, Synergy strongest within 2 h, then ↓ | nsPEFs enhance cisplatin uptake via microdomain disruption; timing critical for synergy | [180] |
| Mouse myeloma (Sp2/0) tumors in BALB/c mice | Doxorubicin (12 mg/kg, i.p.) | 3.5 kV/cm, 800 ns, 250 pulses (nano-ECT) vs. 1.4 kV/cm, 100 µs, 8 pulses | Tumor growth delay ↑, Necrosis localized ↑, PI uptake ↑, Energy delivered ↓ (15% less vs. ESOPE), Thermal effects negligible | nsPEF-based nano-ECT achieved efficacy comparable to microsecond ESOPE protocols, with better energy control and precise tumor localization | [181] |
| Human breast (MCF-7/WT, MCF-7/DX) and colon (LoVo, LoVoDX) cancer cells (DOX-sensitive & resistant) | Doxorubicin (2–50 µM) | PEF1: 10 kV/cm × 300 ns × 200; PEF2: 40 kV/cm × 20 ns × 400; PEF3: 60 kV/cm × 20 ns × 400; PEF4: 1.2 kV/cm × 100 µs × 8 (ESOPE) | Viability ↓ (greater at 72 h), IC50 ↓ markedly with PEF2/PEF3, ROS ↑, GSH ↓ in resistant cells, Confluency ↓, Mitochondrial alterations ↑ | nsPEFs potentiate DOX cytotoxicity, especially in resistant breast/colon cancer cells; ultrashort pulses (20 ns, high field) outperform ESOPE | [182] |
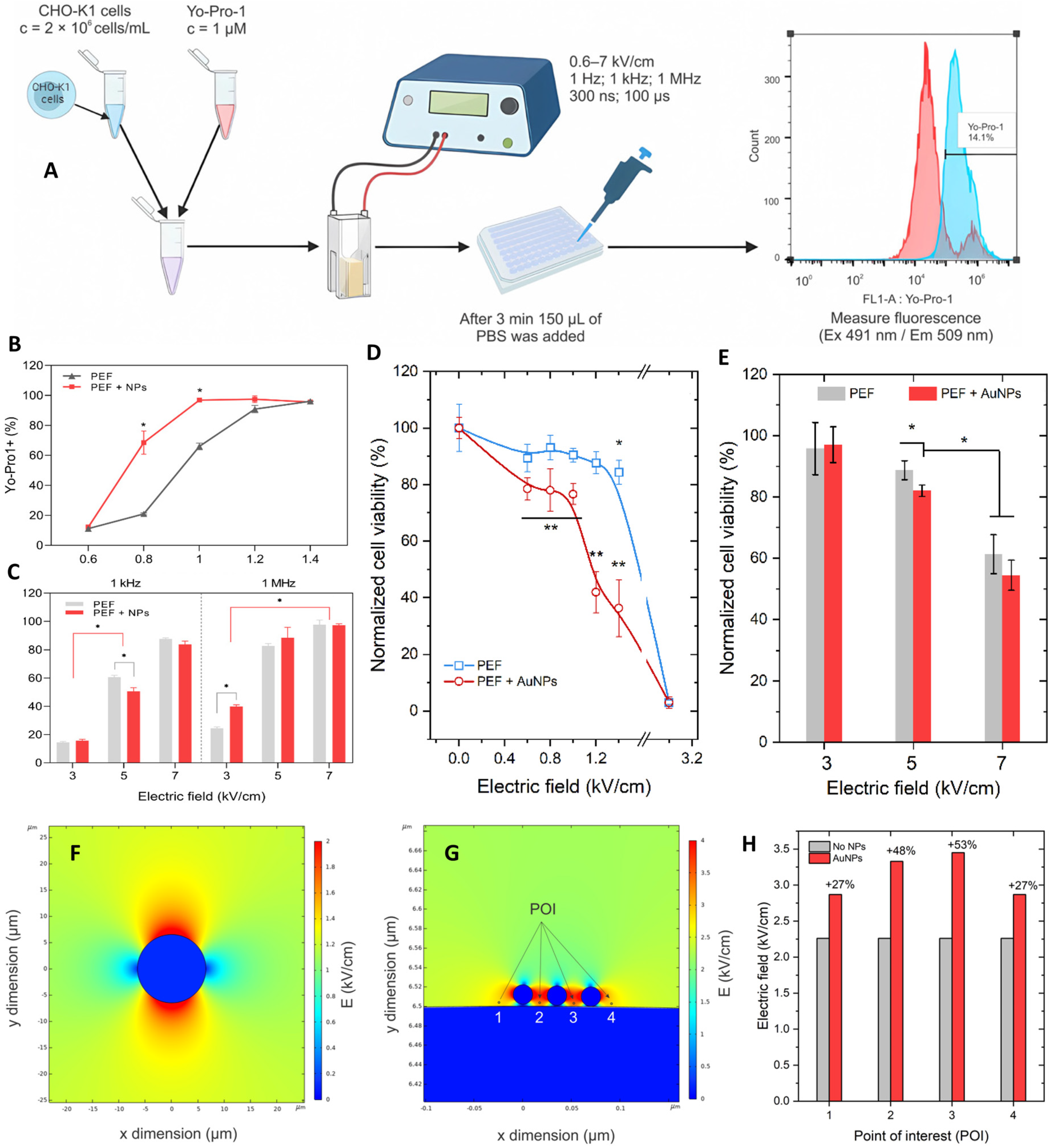
| CHO-K1 cells (DNA delivery model) | Gold nanoparticles (AuNPs; 9–22 nm, citrate-capped) + pEGFP-N1 plasmid | ESOPE: 100 µs × 8, 0.6–1.4 kV/cm; nsPEF: 300 ns × 100, 3–7 kV/cm, 1 kHz/1 MHz | Electrotransfection ↑ with AuNPs under µs pulses, GFP expression ↑ (up to 30%), ROS ↑ with µs pulses (not nsPEF), Viability ↓ at >1 kV/cm + AuNPs | AuNPs amplify µs-ECT efficiency; nsPEFs remain effective without NPs, offering lower ROS and higher viability | [174] |
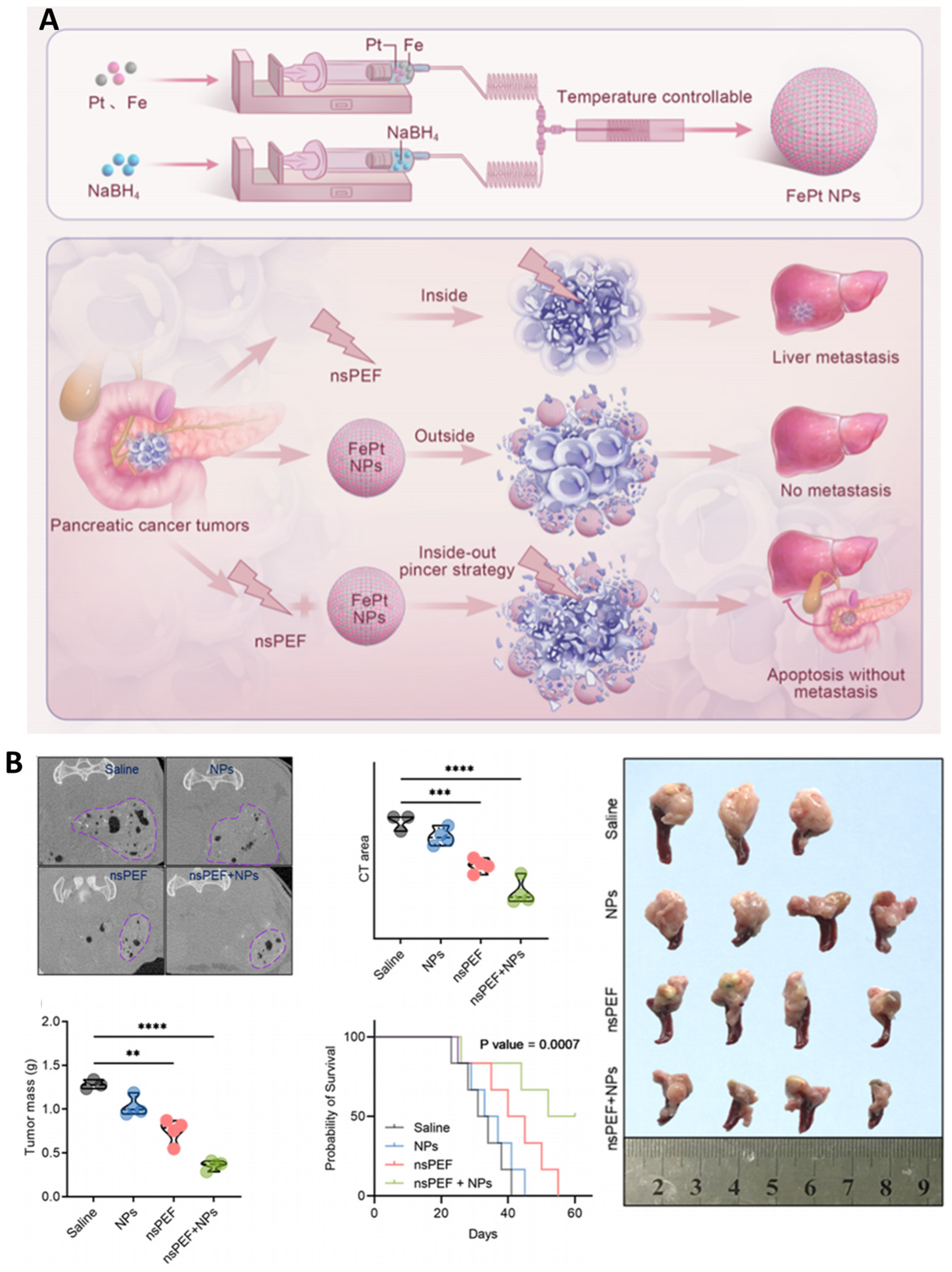
| Human pancreatic cancer (L3.6pl cells; orthotopic mouse model) | FePt nanoparticles (3–5 nm, catalytic, ROS-generating) | 300 ns, 25 kV/cm, 30 pulses (2 Hz) | Selective cytotoxicity ↑ in cancer vs. normal cells, ROS ↑ (•OH, O2•−), O2 release ↑, Apoptosis ↑, Proliferation ↓, Tumor mass ↓, CT contrast ↑, Survival ↑ (>70 days vs. 50–65 days controls) | nsPEF + FePt NPs achieve dual-strike therapy: core tumor ablation + NP uptake in periphery, preventing recurrence/metastasis; also provide CT imaging capability for theranostics | [118] |
| Murine mammary cancer (4T1) | Gold NPs (13 nm) + Bleomycin | µs: 100 µs × 8, 0.6–1.5 kV/cm; ns: 300–700 ns × 100, 6 kV/cm, 1 kHz–1 MHz | Permeabilization ↑, Cytotoxicity ↑, Resealing ↓, Strong synergy at 0.9 kV/cm (µs) and 1 MHz (ns) | AuNPs amplify fields, enabling effective BLM-ECT at lower voltages | [183] |
5. Challenges and Future Horizons
5.1. Enhancing Specificity While Minimizing Off-Target Effects
5.2. Scalability for Clinical Adoption
5.3. Synergy: Immunotherapy, Drugs, and Nanotechnology
5.4. Beyond Oncology: System Effects and Rejuvenation
5.5. A Pragmatic Roadmap
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| nsPEF | Nanosecond pulsed electric field |
| ICD | Immunogenic cell death |
| DAMPs | Danger-associated molecular patterns |
| CSC | Cancer stem cell |
| nsEP | Nanosecond pulse electroporation |
| MOMP | mitochondrial outer membrane permeabilization |
| OMM | Outer mitochondrial membrane |
| ER | Endoplasmic reticulum |
| UPR | Unfolded protein response |
| MAMs | mitochondria-associated membranes |
| TME | Tumor microenvironment |
| ICIs | Immune checkpoint inhibitors |
| ROS | Reactive oxygen species |
| NPs | Nanoparticles |
| DOX | Doxorubicin |
| AI | Artificial intelligence |
| ATP | Adenosine triphosphate |
References
- Beebe, S.J.; Sain, N.M.; Ren, W. Induction of Cell Death Mechanisms and Apoptosis by Nanosecond Pulsed Electric Fields (NsPEFs). Cells 2013, 2, 136–162. [Google Scholar] [CrossRef] [PubMed]
- Butkus, P.; Murauskas, A.; Tolvaišienė, S.; Novickij, V. Concepts and Capabilities of In-House Built Nanosecond Pulsed Electric Field (NsPEF) Generators for Electroporation: State of Art. Appl. Sci. 2020, 10, 4244. [Google Scholar] [CrossRef]
- Gianulis, E.C.; Labib, C.; Saulis, G.; Novickij, V.; Pakhomova, O.N.; Pakhomov, A.G. Selective Susceptibility to Nanosecond Pulsed Electric Field (NsPEF) across Different Human Cell Types. Cell. Mol. Life Sci. 2017, 74, 1741–1754. [Google Scholar] [CrossRef] [PubMed]
- Beebe, S.J.; Fox, P.M.; Rec, L.J.; Somers, K.; Stark, R.H.; Schoenbach, K.H. Nanosecond Pulsed Electric Field (NsPEF) Effects on Cells and Tissues: Apoptosis Induction and Tumor Growth Inhibition. IEEE Trans. Plasma Sci. 2002, 30, 286–292. [Google Scholar] [CrossRef]
- Ruiz-Fernández, A.R.; Campos, L.; Gutierrez-Maldonado, S.E.; Núñez, G.; Villanelo, F.; Perez-Acle, T. Nanosecond Pulsed Electric Field (NsPEF): Opening the Biotechnological Pandora’s Box. Int. J. Mol. Sci. 2022, 23, 6158. [Google Scholar] [CrossRef]
- Neu, W.K.; Neu, J.C. Theory of Electroporation BT-Cardiac Bioelectric Therapy: Mechanisms and Practical Implications; Efimov, I.R., Kroll, M.W., Tchou, P.J., Eds.; Springer: Boston, MA, USA, 2009; pp. 133–161. ISBN 978-0-387-79403-7. [Google Scholar]
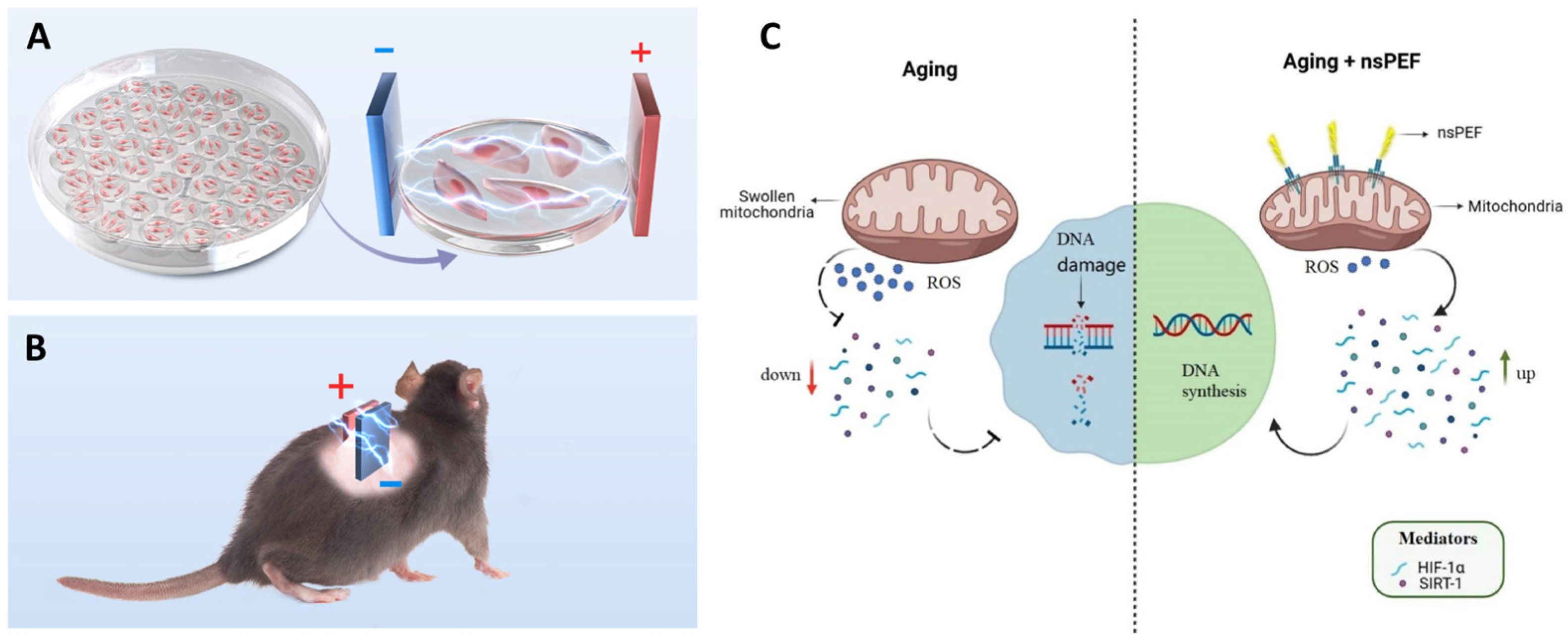
- Yin, M.; Xiao, J.; Huang, G.; Xie, H.; Liu, H.; Yuan, J.; Liu, X.; Chiarini, A.; Armato, U.; Prà, I.D.; et al. Nanosecond Pulsed Electric Field Applications Rejuvenate Aging Endothelial Cells by Rescuing Mitochondrial-to-Nuclear Retrograde Communication. Regen. Ther. 2025, 30, 207–216. [Google Scholar] [CrossRef]
- Hirata, M.; Tanioka, S.; Hamada, Y.; Oyadomari, S.; Shimomura, N. Study on Selection of Appropriate Conditions of Nanosecond Pulsed Electric Field for Activation of Unfolded Protein Response Using GFP-Expressing Cells. In Proceedings of the 2024 IEEE International Power Modulator and High Voltage Conference (IPMHVC), Indianapolis, IN, USA, 28 May–1 June 2024; pp. 1–4. [Google Scholar]
- Kulbacka, J.; Rembiałkowska, N.; Szewczyk, A.; Rossowska, J.; Drąg-Zalesińska, M.; Kulbacki, M.; Choromańska, A. Nanosecond PEF Induces Oxidative Stress and Apoptosis via Proteasomal Activity Inhibition in Gastric Adenocarcinoma Cells with Drug Resistance. Int. J. Mol. Sci. 2022, 23, 12943. [Google Scholar] [CrossRef]
- Ford, W.E.; Ren, W.; Blackmore, P.F.; Schoenbach, K.H.; Beebe, S.J. Nanosecond Pulsed Electric Fields Stimulate Apoptosis without Release of Pro-Apoptotic Factors from Mitochondria in B16f10 Melanoma. Arch. Biochem. Biophys. 2010, 497, 82–89. [Google Scholar] [CrossRef]
- Awasthi, K.; Wu, T.-E.; Hsu, H.-Y.; Ohta, N. Application of Nanosecond Pulsed Electric Field and Autofluorescence Lifetime Microscopy of FAD in Lung Cells. J. Phys. Chem. B 2023, 127, 5566–5575. [Google Scholar] [CrossRef]
- Taibi, A.; Perrin, M.-L.; Albouys, J.; Jacques, J.; Yardin, C.; Durand-Fontanier, S.; Bardet, S.M. 10 Ns PEFs Induce a Histological Response Linked to Cell Death and Cytotoxic T-Lymphocytes in an Immunocompetent Mouse Model of Peritoneal Metastasis. Clin. Transl. Oncol. 2021, 23, 1220–1237. [Google Scholar] [CrossRef]
- Sowa, P.W.; Novickij, V.; Kiełbik, A.; Kollotzek, F.; Heinzmann, D.; Borst, O.; Gawaz, M.P. Fractionation of Nanosecond Pulsed Electric Fields Lowers Lethal Dose by Enhancing Cardiomyocyte Membrane Permeability. Hear. Rhythm 2025, 22, e697–e709. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, X.; Zheng, S. Immune Response Triggered by the Ablation of Hepatocellular Carcinoma with Nanosecond Pulsed Electric Field. Front. Med. 2021, 15, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Fernández, A.R.; Rosemblatt, M.; Perez-Acle, T. Nanosecond Pulsed Electric Field (NsPEF) and Vaccines: A Novel Technique for the Inactivation of SARS-CoV-2 and Other Viruses? Ann. Med. 2022, 54, 1749–1756. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Pakhomova, O.N.; Pakhomov, A.G.; Weygandt, S.; Bulysheva, A.A.; Murray, L.E.; Mollica, P.A.; Muratori, C. Mechanisms and Immunogenicity of NsPEF-Induced Cell Death in B16F10 Melanoma Tumors. Sci. Rep. 2019, 9, 431. [Google Scholar] [CrossRef]
- Muratori, C.; Pakhomov, A.G.; Gianulis, E.; Meads, J.; Casciola, M.; Mollica, P.A.; Pakhomova, O.N. Activation of the Phospholipid Scramblase TMEM16F by Nanosecond Pulsed Electric Fields (NsPEF) Facilitates Its Diverse Cytophysiological Effects. J. Biol. Chem. 2017, 292, 19381–19391. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, Z.; Dong, Y.; Alhaskawi, A.; Tu, T.; Hasan Abdullah Ezzi, S.; Goutham Kota, V.; Hasan Abdulla Hasan Abdulla, M.; Li, P.; Wu, B.; et al. New Advances in Treatment of Skin Malignant Tumors with Nanosecond Pulsed Electric Field: A Literature Review. Bioelectrochemistry 2023, 150, 108366. [Google Scholar] [CrossRef]
- Beebe, S.J.; Schoenbach, K.H. Nanosecond Pulsed Electric Fields: A New Stimulus to Activate Intracellular Signaling. BioMed Res. Int. 2005, 2005, 301289. [Google Scholar] [CrossRef]
- Huang, H.; Huang, F.; Liang, X.; Fu, Y.; Cheng, Z.; Huang, Y.; Chen, Z.; Duan, Y.; Chen, Y. Afatinib Reverses EMT via Inhibiting CD44-Stat3 Axis to Promote Radiosensitivity in Nasopharyngeal Carcinoma. Pharmaceuticals 2023, 16, 37. [Google Scholar] [CrossRef]
- Oshin, E.A.; Minhas, Z.; Biancatelli, R.M.L.C.; Catravas, J.D.; Heller, R.; Guo, S.; Jiang, C. Synergistic Effects of Nanosecond Pulsed Plasma and Electric Field on Inactivation of Pancreatic Cancer Cells in Vitro. Sci. Rep. 2024, 14, 885. [Google Scholar] [CrossRef]
- Casciati, A.; Taddei, A.R.; Rampazzo, E.; Persano, L.; Viola, G.; Cani, A.; Bresolin, S.; Cesi, V.; Antonelli, F.; Mancuso, M.; et al. Involvement of Mitochondria in the Selective Response to Microsecond Pulsed Electric Fields on Healthy and Cancer Stem Cells in the Brain. Int. J. Mol. Sci. 2024, 25, 2233. [Google Scholar] [CrossRef]
- Orlacchio, R.; Kolosnjaj-Tabi, J.; Mattei, N.; Lévêque, P.; Rols, M.P.; Arnaud-Cormos, D.; Golzio, M. Effects of Nanosecond Pulsed Electric Field (NsPEF) on a Multicellular Spheroid Tumor Model: Influence of Pulse Duration, Pulse Repetition Rate, Absorbed Energy, and Temperature. Int. J. Mol. Sci. 2023, 24, 14999. [Google Scholar] [CrossRef]
- Li, K.; Fan, L.; Lin, J.; Heng, B.C.; Deng, Z.; Zheng, Q.; Zhang, J.; Jiang, Y.; Ge, Z. Nanosecond Pulsed Electric Fields Prime Mesenchymal Stem Cells to Peptide Ghrelin and Enhance Chondrogenesis and Osteochondral Defect Repair in Vivo. Sci. China Life Sci. 2022, 65, 927–939. [Google Scholar] [CrossRef]
- Batista Napotnik, T.; Reberšek, M.; Vernier, P.T.; Mali, B.; Miklavčič, D. Effects of High Voltage Nanosecond Electric Pulses on Eukaryotic Cells (in Vitro): A Systematic Review. Bioelectrochemistry 2016, 110, 1–12. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, H.; Fu, M.; Xu, H.; Huang, H.; Zhong, M.; Zhang, M.; Hua, W.; Lv, K.; Zhu, G. TMEM64 Aggravates the Malignant Phenotype of Glioma by Activating the Wnt/β-Catenin Signaling Pathway. Int. J. Biol. Macromol. 2024, 260, 129332. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Fu, M.; Ge, C.; Zhou, H.; Huang, H.; Zhong, M.; Zhang, M.; Xu, H.; Zhu, G.; Hua, W.; et al. M6A-Mediated Upregulation of LncRNA CHASERR Promotes the Progression of Glioma by Modulating the MiR-6893-3p/TRIM14 Axis. Mol. Neurobiol. 2024, 61, 5418–5440. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, H.; Miyake, M.; Hirai, H.; Teranishi, K.; Shimomura, N.; Oyadomari, S. Effects on Endoplasmic Reticulum Stress Response of Applying Nanosecond Pulsed Electric Fields. In Proceedings of the 2015 IEEE Pulsed Power Conference (PPC), Austin, TX, USA, 31 May–4 June 2015; pp. 1–4. [Google Scholar]
- Rossi, A.; Pakhomova, N.O.; Mollica, P.A.; Casciola, M.; Mangalanathan, U.; Pakhomov, G.A.; Muratori, C. Nanosecond Pulsed Electric Fields Induce Endoplasmic Reticulum Stress Accompanied by Immunogenic Cell Death in Murine Models of Lymphoma and Colorectal Cancer. Cancers 2019, 11, 2034. [Google Scholar] [CrossRef] [PubMed]
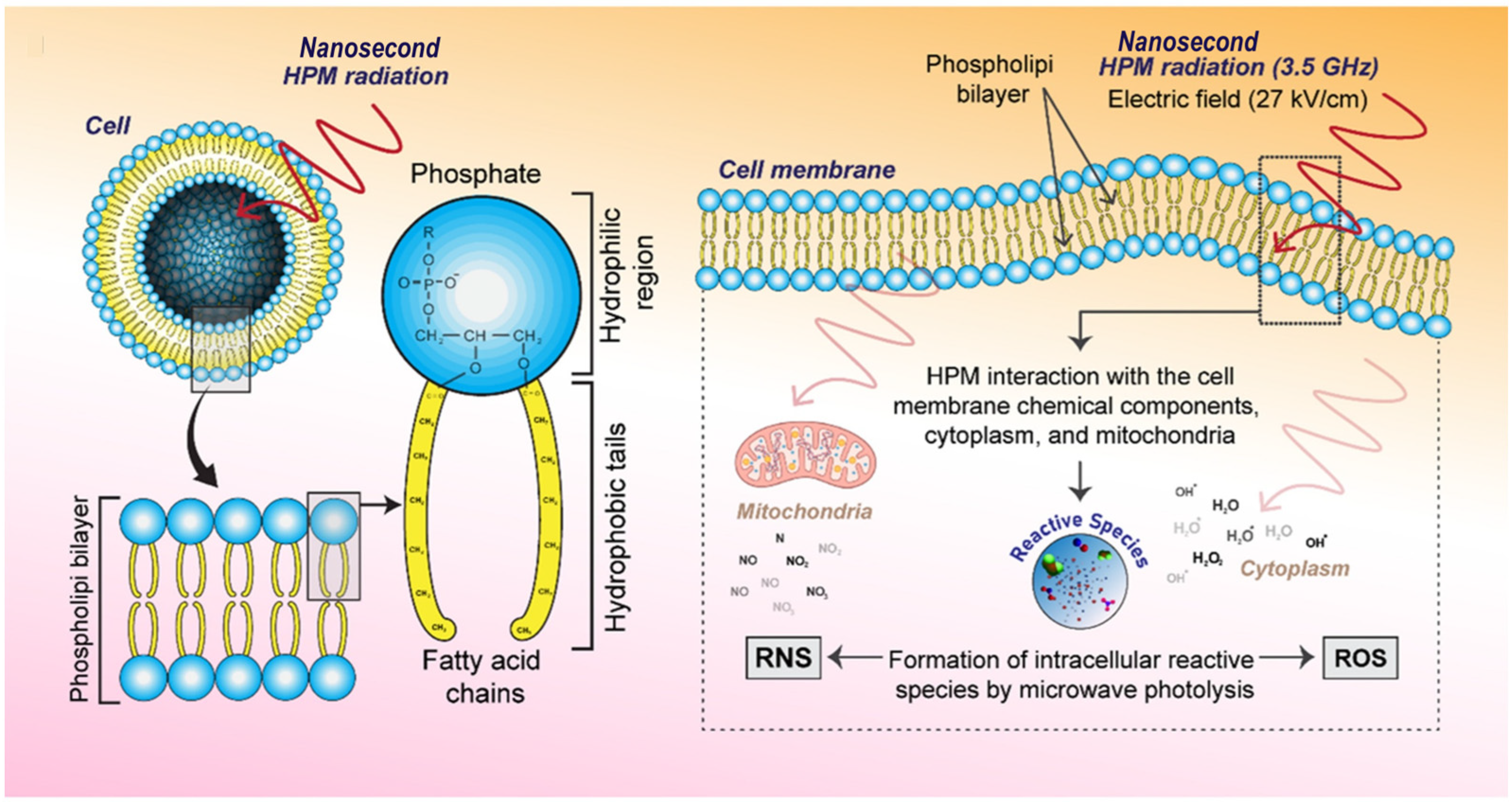
- Rana, J.N.; Mumtaz, S.; Choi, E.H.; Han, I. ROS Production in Response to High-Power Microwave Pulses Induces P53 Activation and DNA Damage in Brain Cells: Radiosensitivity and Biological Dosimetry Evaluation. Front. Cell Dev. Biol. 2023, 11, 1067861. [Google Scholar] [CrossRef]
- Rana, J.N.; Mumtaz, S.; Han, I.; Choi, E.H. Formation of Reactive Species via High Power Microwave Induced DNA Damage and Promoted Intrinsic Pathway-Mediated Apoptosis in Lung Cancer Cells: An in Vitro Investigation. Fundam. Res. 2024, 4, 1542–1556. [Google Scholar] [CrossRef]
- Baker, C.; Willis, A.; Milestone, W.; Baker, M.; Garner, A.L.; Joshi, R.P. Numerical Assessments of Geometry, Proximity and Multi-Electrode Effects on Electroporation in Mitochondria and the Endoplasmic Reticulum to Nanosecond Electric Pulses. Sci. Rep. 2024, 14, 23854. [Google Scholar] [CrossRef]
- Zhuang, J.; Shi, F.; Guo, J. Biomedical Applications of Pulsed Discharge and Pulsed Electric Field BT-Pulsed Discharge Plasmas: Characteristics and Applications; Shao, T., Zhang, C., Eds.; Springer Nature: Singapore, 2023; pp. 737–760. ISBN 978-981-99-1141-7. [Google Scholar]
- Zhang, Z.; He, D.; Gong, W.; Xu, Z.; Wang, H.; Li, Q. Simulation and Mechanism Study of Cavity Discharge Under Pulsed Electric Field. In Proceedings of the 2024 IEEE 5th International Conference on Dielectrics (ICD), Toulouse, France, 30 June–4 July 2024; pp. 1–4. [Google Scholar]
- Go, E.-J.; Yang, D.; Ryu, W.; Chon, H.J.; Kim, C.; Park, K.-S.; Kim, D.-H.; Han, D.K.; Park, W. Optimal Voltage and Electrical Pulse Conditions for Electrical Ablation to Induce Immunogenic Cell Death (ICD). J. Ind. Eng. Chem. 2021, 94, 225–232. [Google Scholar] [CrossRef]
- Wang, Z.-B.; Zhang, X.; Fang, C.; Liu, X.-T.; Liao, Q.-J.; Wu, N.; Wang, J. Immunotherapy and the Ovarian Cancer Microenvironment: Exploring Potential Strategies for Enhanced Treatment Efficacy. Immunology 2024, 173, 14–32. [Google Scholar] [CrossRef]
- Huang, L.; Tan, J.; Lin, P.; Chen, Z.; Huang, Q.; Yao, H.; Jiang, L.; Long, B.; Long, Y. Autoimmune Encephalitis Followed by Hemophagocytic Lymph Histiocytosis: A Case Report. Front. Immunol. 2024, 15, 1383255. [Google Scholar] [CrossRef] [PubMed]
- Tanori, M.; Casciati, A.; Zambotti, A.; Pinto, R.; Gianlorenzi, I.; Pannicelli, A.; Giardullo, P.; Benassi, B.; Marino, C.; Mancuso, M.; et al. Microsecond Pulsed Electric Fields: An Effective Way to Selectively Target and Radiosensitize Medulloblastoma Cancer Stem Cells. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 1495–1507. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Wang, Z.; Zhou, H.; Li, P.; Lu, H.; Tu, T. Low-Intensity Nanosecond Pulsed Electric Field Accelerates Osteogenic Transformation of Human Dermal Fibroblasts by Enhancing Cell Pluripotency. Cell. Reprogram. 2023, 25, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Chen, S.; Alfadhl, Y.; Chen, X.; Sun, L.; Yu, L.; Zhou, J. Pulse Width and Intensity Effects of Pulsed Electric Fields on Cancerous and Normal Skin Cells. Sci. Rep. 2022, 12, 18039. [Google Scholar] [CrossRef]
- Batista Napotnik, T.; Polajžer, T.; Miklavčič, D. Cell Death Due to Electroporation–A Review. Bioelectrochemistry 2021, 141, 107871. [Google Scholar] [CrossRef]
- Campelo, S.N.; Huang, P.-H.; Buie, C.R.; Davalos, R.V. Recent Advancements in Electroporation Technologies: From Bench to Clinic. Annu. Rev. Biomed. Eng. 2023, 25, 77–100. [Google Scholar] [CrossRef]
- Choi, S.-E.; Khoo, H.; Hur, S.C. Recent Advances in Microscale Electroporation. Chem. Rev. 2022, 122, 11247–11286. [Google Scholar] [CrossRef]
- Qian, K.; Yao, C.; Wang, Y.; Yang, Q.; Xiang, S.; Pei, Q.; Zhu, T.; Liu, H.; Dong, S. Potential of Ultrashort Pulsed Electric Fields to Disrupt Dense Structure in Glioma Tumors. IEEE Trans. Biomed. Eng. 2025, 72, 3233–3243. [Google Scholar] [CrossRef]
- Ren, W.; Beebe, S.J. An Apoptosis Targeted Stimulus with Nanosecond Pulsed Electric Fields (NsPEFs) in E4 Squamous Cell Carcinoma. Apoptosis 2011, 16, 382–393. [Google Scholar] [CrossRef]
- Estlack, L.E.; Roth, C.C.; Thompson, G.L.; Lambert, W.A.; Ibey, B.L. Nanosecond Pulsed Electric Fields Modulate the Expression of Fas/CD95 Death Receptor Pathway Regulators in U937 and Jurkat Cells. Apoptosis 2014, 19, 1755–1768. [Google Scholar] [CrossRef]
- Lei, Y.; Dong, S.; Liang, R.; Xiang, S.; Huang, Q.; Ma, J.; Kou, H.; Yu, L.; Yao, C. Parallel Resonant Magnetic Field Generator for Biomedical Applications. IEEE Trans. Biomed. Circuits Syst. 2025, 19, 496–510. [Google Scholar] [CrossRef]
- Radzevičiūtė, E.; Malyško-Ptašinskė, V.; Novickij, J.; Novickij, V.; Girkontaitė, I. Transfection by Electroporation of Cancer and Primary Cells Using Nanosecond and Microsecond Electric Fields. Pharmaceutics 2022, 14, 1239. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Xu, J.; Liu, Q.; Wu, X.; Zhang, Q.; Tang, J. Single-Cell Electroporation with High-Frequency Nanosecond Pulse Bursts: Simulation Considering the Irreversible Electroporation Effect and Experimental Validation. Bioelectrochemistry 2021, 140, 107822. [Google Scholar] [CrossRef] [PubMed]
- Fesmire, C.C.; Williamson, R.H.; Petrella, R.A.; Kaufman, J.D.; Topasna, N.; Sano, M.B. Integrated Time Nanosecond Pulse Irreversible Electroporation (INSPIRE): Assessment of Dose, Temperature, and Voltage on Experimental and Clinical Treatment Outcomes. IEEE Trans. Biomed. Eng. 2024, 71, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Chiang, C.; Wang, X.; Bertani, P.; Ma, Y.; Cheng, J.; Talesara, V.; Lee, L.J.; Lu, W. Cell Membrane Damage and Cargo Delivery in Nano-Electroporation. Nanoscale 2023, 15, 4080–4089. [Google Scholar] [CrossRef]
- Sözer, E.B.; Pakhomov, A.G.; Semenov, I.; Casciola, M.; Kim, V.; Vernier, P.T.; Zemlin, C.W. Analysis of Electrostimulation and Electroporation by High Repetition Rate Bursts of Nanosecond Stimuli. Bioelectrochemistry 2021, 140, 107811. [Google Scholar] [CrossRef]
- Milestone, W.; Hu, Q.; Loveless, A.M.; Garner, A.L.; Joshi, R.P. Modeling Coupled Single Cell Electroporation and Thermal Effects from Nanosecond Electric Pulse Trains. J. Appl. Phys. 2022, 132, 94701. [Google Scholar] [CrossRef]
- Marszalek, P.; Liu, D.S.; Tsong, T.Y. Schwan Equation and Transmembrane Potential Induced by Alternating Electric Field. Biophys. J. 1990, 58, 1053–1058. [Google Scholar] [CrossRef]
- Mercadal, B.; Vernier, P.T.; Ivorra, A. Dependence of Electroporation Detection Threshold on Cell Radius: An Explanation to Observations Non Compatible with Schwan’s Equation Model. J. Membr. Biol. 2016, 249, 663–676. [Google Scholar] [CrossRef]
- Muratori, C.; Silkuniene, G.; Mollica, P.A.; Pakhomov, A.G.; Pakhomova, O.N. The Role of ESCRT-III and Annexin V in the Repair of Cell Membrane Permeabilization by the Nanosecond Pulsed Electric Field. Bioelectrochemistry 2021, 140, 107837. [Google Scholar] [CrossRef]
- Lee, D.; Naikar, J.S.; Chan, S.S.Y.; Meivita, M.P.; Li, L.; Tan, Y.S.; Bajalovic, N.; Loke, D.K. Ultralong Recovery Time in Nanosecond Electroporation Systems Enabled by Orientational-Disordering Processes. Nanoscale 2022, 14, 7934–7942. [Google Scholar] [CrossRef] [PubMed]
- Gudvangen, E.; Kim, V.; Novickij, V.; Battista, F.; Pakhomov, A.G. Electroporation and Cell Killing by Milli- to Nanosecond Pulses and Avoiding Neuromuscular Stimulation in Cancer Ablation. Sci. Rep. 2022, 12, 1763. [Google Scholar] [CrossRef] [PubMed]
- Vižintin, A.; Marković, S.; Ščančar, J.; Miklavčič, D. Electroporation with Nanosecond Pulses and Bleomycin or Cisplatin Results in Efficient Cell Kill and Low Metal Release from Electrodes. Bioelectrochemistry 2021, 140, 107798. [Google Scholar] [CrossRef] [PubMed]
- Sundararajan, R. Nanosecond Electroporation: Another Look. Mol. Biotechnol. 2009, 41, 69–82. [Google Scholar] [CrossRef]
- Reberšek, M.; Miklavčič, D. Advantages and Disadvantages of Different Concepts of Electroporation Pulse Generation. Automatika 2011, 52, 12–19. [Google Scholar] [CrossRef]
- Pakhomov, A.G.; Pakhomova, O.N. The Interplay of Excitation and Electroporation in Nanosecond Pulse Stimulation. Bioelectrochemistry 2020, 136, 107598. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, T.; Shen, H.; Zhou, X.; Han, Q.; Li, L.; Zhang, L.; Wang, C.; Dong, X. Tumor Microenvironment-Responsive MnOx-Mesoporous Carbon Nanoparticles for Enhanced Chemodynamic Synergistic Antitumor Therapy. ACS Appl. Nano Mater. 2025, 8, 2763–2773. [Google Scholar] [CrossRef]
- Pakhomov, A.G.; Grigoryev, S.; Semenov, I.; Casciola, M.; Jiang, C.; Xiao, S. The Second Phase of Bipolar, Nanosecond-Range Electric Pulses Determines the Electroporation Efficiency. Bioelectrochemistry 2018, 122, 123–133. [Google Scholar] [CrossRef]
- Li, J.; He, H.-G.; Guan, C.; Ding, Y.; Hu, X. Dynamic Joint Prediction Model of Severe Radiation-Induced Oral Mucositis among Nasopharyngeal Carcinoma: A Prospective Longitudinal Study. Radiother. Oncol. 2025, 209, 110993. [Google Scholar] [CrossRef]
- Chen, Y.; Deng, Y.; Li, Y.; Qin, Y.; Zhou, Z.; Yang, H.; Sun, Y. Oxygen-Independent Radiodynamic Therapy: Radiation-Boosted Chemodynamics for Reprogramming the Tumor Immune Environment and Enhancing Antitumor Immune Response. ACS Appl. Mater. Interfaces 2024, 16, 21546–21556. [Google Scholar] [CrossRef]
- Feng, C.; Wang, Y.; Xu, J.; Zheng, Y.; Zhou, W.; Wang, Y.; Luo, C. Precisely Tailoring Molecular Structure of Doxorubicin Prodrugs to Enable Stable Nanoassembly, Rapid Activation, and Potent Antitumor Effect. Pharmaceutics 2024, 16, 1582. [Google Scholar] [CrossRef]
- Beebe, S.J.; Chen, Y.-J.; Sain, N.M.; Schoenbach, K.H.; Xiao, S. Transient Features in Nanosecond Pulsed Electric Fields Differentially Modulate Mitochondria and Viability. PLoS ONE 2012, 7, e51349. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Zhang, M.; Chen, R.; Teng, P.; Dai, X.; Wu, B.; Hong, L.; Ma, L.; Liu, L.; Wu, S.; et al. Microsecond Pulsed Electric Fields Induce Myocardial Ablation by Secondary Mitochondrial Damage and Cell Death Mechanisms. Sci. Rep. 2025, 15, 10132. [Google Scholar] [CrossRef] [PubMed]
- Shang, T.; Sun, G.; Shen, S.; Zhang, Y.; Han, X.; Ding, W. Simulation of the Effect of Nanosecond Pulsed Electric Field on Mitochondria. In The Proceedings of the 17th Annual Conference of China Electrotechnical Society; Xie, K., Hu, J., Yang, Q., Li, J., Eds.; Springer Nature: Singapore, 2023; pp. 406–414. [Google Scholar]
- Awasthi, K.; Huang, W.-C.; Wei, C.-Y.; Hsu, H.-Y.; Ohta, N. Unveiling the Susceptibility of Nanosecond Pulsed Electric Field on Intracellular Function in Breast Cancerous and Normal Cells Using Fluorescence Imaging. Biosens. Bioelectron. 2025, 272, 117129. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.; Gao, S.; Jiang, Z.; Ding, Q.; Cheng, Z. Recent Advances in Mitochondria-Targeting Theranostic Agents. Exploration 2024, 4, 20230063. [Google Scholar] [CrossRef]
- Asadipour, K.; Zhou, C.; Yi, V.; Beebe, S.J.; Xiao, S. Ultra-Low Intensity Post-Pulse Affects Cellular Responses Caused by Nanosecond Pulsed Electric Fields. Bioengineering 2023, 10, 1069. [Google Scholar] [CrossRef]
- Kasprzycka, W.; Trębińska-Stryjewska, A.; Lewandowski, R.B.; Stępińska, M.; Osuchowska, P.N.; Dobrzyńska, M.; Achour, Y.; Osuchowski, Ł.P.; Starzyński, J.; Mierczyk, Z.; et al. Nanosecond Pulsed Electric Field Only Transiently Affects the Cellular and Molecular Processes of Leydig Cells. Int. J. Mol. Sci. 2021, 22, 11236. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, L.; Li, Y.; He, D.; Zheng, L. Chrysophanol Localizes in Mitochondria to Promote Cell Death through Upregulation of Mitochondrial Cyclophilin D in HepG2 Cells. Chin. Herb. Med. 2021, 13, 221–227. [Google Scholar] [CrossRef]
- Zhang, R.; Shan, H.; Li, Y.; Ma, Y.; Liu, S.; Liu, X.; Yang, X.; Zhang, J.; Zhang, M. Cyclophilin D Contributes to Airway Epithelial Mitochondrial Damage in Chronic Obstructive Pulmonary Disease. Lung 2023, 201, 287–295. [Google Scholar] [CrossRef]
- He, X.; Jiang, Z.; Akakuru, O.U.; Li, J.; Wu, A. Nanoscale Covalent Organic Frameworks: From Controlled Synthesis to Cancer Therapy. Chem. Commun. 2021, 57, 12417–12435. [Google Scholar] [CrossRef]
- Wu, S.A.; Li, Z.J.; Qi, L. Endoplasmic Reticulum (ER) Protein Degradation by ER-Associated Degradation and ER-Phagy. Trends Cell Biol. 2025, 35, 576–591. [Google Scholar] [CrossRef]
- Hirata, M.; Tanioka, S.; Hamada, Y.; Oyadomari, S.; Shimomura, N. Study of Appropriate Condition of Nanosecond Pulsed Electric Fields for Induction of Unfolded Protein Response Using GFP-Expressing Cell. In Proceedings of the 2023 IEEE Pulsed Power Conference (PPC), San Antonio, TX, USA, 25–29 June 2023; pp. 1–5. [Google Scholar]
- Yao, C.; Ma, X.; Qian, K.; Wang, Y.; Dong, S. Simulation and Experimental Study on the Responses of Subcellular Structures in Tumor Cells Induced by 5 Ns Pulsed Electric Fields. Appl. Sci. 2023, 13, 8142. [Google Scholar] [CrossRef]
- Cantu, J.C.; Tolstykh, G.P.; Tarango, M.; Beier, H.T.; Ibey, B.L. Caveolin-1 Is Involved in Regulating the Biological Response of Cells to Nanosecond Pulsed Electric Fields. J. Membr. Biol. 2021, 254, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-J.; Xiong, Z.-A.; Zhang, M.; Yao, C.-G.; Zhao, Z.-Y.; Hua, Y.-Y.; Zhou, W. Picosecond Pulsed Electric Fields Induce Apoptosis in HeLa Cells via the Endoplasmic Reticulum Stress and Caspase-Dependent Signaling Pathways. Int. J. Oncol. 2013, 42, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Furumoto, Y.; Sato, D.; Teranishi, K.; Shimomura, N.; Hamada, Y.; Miyake, M.; Oyadomari, S. Activation of Endoplasmic Reticulum Stress Response by Applying of Nanosecond Pulsed Electric Fields for Medical Application. In Proceedings of the 2018 IEEE International Power Modulator and High Voltage Conference (IPMHVC), Jackson, WY, USA, 3–7 June 2018; pp. 456–460. [Google Scholar]
- Jing, R.; Jiang, Z.; Tang, X. Advances in Millimeter-Wave Treatment and Its Biological Effects Development. Int. J. Mol. Sci. 2024, 25, 8638. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Luo, Z.; Ovcjak, A.; Alanazi, R.; Bao, M.-H.; Feng, Z.-P.; Sun, H.-S. Role of TRPM2 in Brain Tumours and Potential as a Drug Target. Acta Pharmacol. Sin. 2022, 43, 759–770. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, W.; Liu, H.; Dong, S.; Zhou, Q.; Yao, C. Augmenting the Electrosensitivity of Tumor Cells by Combining Nanosecond and Microsecond Pulsed Electric Fields. In Proceedings of the 2024 3rd International Conference on Health Big Data and Intelligent Healthcare (ICHIH), Zhuhai, China, 13–15 December 2024; pp. 199–204. [Google Scholar]
- Yang, Q.; Kajimoto, S.; Kobayashi, Y.; Hiramatsu, H.; Nakabayashi, T. Regulation of Cell Volume by Nanosecond Pulsed Electric Fields. J. Phys. Chem. B 2021, 125, 10692–10700. [Google Scholar] [CrossRef]
- Li, K.; Ning, T.; Wang, H.; Jiang, Y.; Zhang, J.; Ge, Z. Nanosecond Pulsed Electric Fields Enhance Mesenchymal Stem Cells Differentiation via DNMT1-Regulated OCT4/NANOG Gene Expression. Stem Cell Res. Ther. 2020, 11, 308. [Google Scholar] [CrossRef]
- Chang, A.-Y.; Liu, X.; Tian, H.; Hua, L.; Yang, Z.; Wang, S. Microfluidic Electroporation Coupling Pulses of Nanoseconds and Milliseconds to Facilitate Rapid Uptake and Enhanced Expression of DNA in Cell Therapy. Sci. Rep. 2020, 10, 6061. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Song, J.; Liu, X.; Liu, S.; Yang, N.; Wang, L.; Liu, Y.; Zhao, Y.; Zhou, W.; et al. Tumor Cell-Targeting and Tumor Microenvironment–Responsive Nanoplatforms for the Multimodal Imaging-Guided Photodynamic/Photothermal/Chemodynamic Treatment of Cervical Cancer. Int. J. Nanomed. 2024, 19, 5837–5858. [Google Scholar] [CrossRef]
- Zhao, Y. Enhancing Panvascular Medicine: Unveiling the Nexus of Pan-Cardio-Oncology and Expanding Therapeutic Frontiers. Sci. Bull. 2025, 70, 798–800. [Google Scholar]
- Hamada, Y.; Furumoto, Y.; Izutani, A.; Taniuchi, S.; Miyake, M.; Oyadomari, M.; Teranishi, K.; Shimomura, N.; Oyadomari, S. Nanosecond Pulsed Electric Fields Induce the Integrated Stress Response via Reactive Oxygen Species-Mediated Heme-Regulated Inhibitor (HRI) Activation. PLoS ONE 2020, 15, e0229948. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, F.; Gusbeth, C.; Frey, W.; Maisch, J.; Nick, P. Nanosecond Pulsed Electrical Fields Enhance Product Recovery in Plant Cell Fermentation. Protoplasma 2020, 257, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Chafai, D.E.; Vostárek, F.; Dráberová, E.; Havelka, D.; Arnaud-Cormos, D.; Leveque, P.; Janáček, J.; Kubínová, L.; Cifra, M.; Dráber, P. Microtubule Cytoskeleton Remodeling by Nanosecond Pulsed Electric Fields. Adv. Biosyst. 2020, 4, 2000070. [Google Scholar] [CrossRef] [PubMed]
- You, W.; Zhou, Z.; Li, Z.; Yan, J.; Wang, Y. From Foe to Friend: Rewiring Oncogenic Pathways through Artificial Selenoprotein to Combat Immune-Resistant Tumor. J. Pharm. Anal. 2025, 101322. [Google Scholar] [CrossRef]
- Du, J.; Meng, X.; Yang, M.; Chen, G.; Li, J.; Zhu, Z.; Wu, X.; Hu, W.; Tian, M.; Li, T.; et al. NGR-Modified CAF-Derived Exos Targeting Tumor Vasculature to Induce Ferroptosis and Overcome Chemoresistance in Osteosarcoma. Adv. Sci. 2025, 12, 2410918. [Google Scholar] [CrossRef]
- Ninagawa, Y.; Sugiura, R.; Kato, E.; Wada, K.; Yagi, I.; Uchida, S. Effects of Pulse Width and Electrical Energy of Low-Voltage Nanosecond Pulsed Electric Fields on Mitochondria in Cancer Cells. IEEJ Trans. Electr. Electron. Eng. 2024, 19, 1140–1146. [Google Scholar] [CrossRef]
- Camera, F.; Colantoni, E.; Garcia-Sanchez, T.; Benassi, B.; Consales, C.; Muscat, A.; Vallet, L.; Mir, L.M.; Andre, F.; Merla, C. In Vitro Imaging and Molecular Characterization of Ca2+ Flux Modulation by Nanosecond Pulsed Electric Fields. Int. J. Mol. Sci. 2023, 24, 15616. [Google Scholar] [CrossRef]
- Awasthi, K.; Chang, F.-L.; Wu, T.-E.; Hsu, H.-Y.; Ohta, N. Modulation of Calcium Signaling by Nanosecond Electric Pulses and Cell Death through Apoptosis in A549 Lung Cancerous Cells. Sens. Actuators B Chem. 2022, 369, 132348. [Google Scholar] [CrossRef]
- Ruiz-Fernández, A.R.; Campos, L.; Villanelo, F.; Gutiérrez-Maldonado, S.E.; Perez-Acle, T. Exploring the Conformational Changes Induced by Nanosecond Pulsed Electric Fields on the Voltage Sensing Domain of a Ca2+ Channel. Membranes 2021, 11, 473. [Google Scholar] [CrossRef]
- Joshi, R. Intra-Cellular Calcium Release Dynamics Due to Nanosecond Electric Pulsing BT-Ultrashort Electric Pulse Effects in Biology and Medicine; Beebe, S.J., Joshi, R., Schoenbach, K.H., Xiao, S., Eds.; Springer: Singapore, 2021; pp. 143–157. ISBN 978-981-10-5113-5. [Google Scholar]
- Liu, Z.; Zou, Y.; Sun, Y.; Chen, X.; Chen, X.; Ren, Z. Effects of Nanosecond Pulsed Electric Fields in Cell Vitality, Apoptosis, and Proliferation of TPC-1 Cells. Anal. Cell. Pathol. 2021, 2021, 9913716. [Google Scholar] [CrossRef] [PubMed]
- Kim, V.; Gudvangen, E.; Kondratiev, O.; Redondo, L.; Xiao, S.; Pakhomov, A.G. Peculiarities of Neurostimulation by Intense Nanosecond Pulsed Electric Fields: How to Avoid Firing in Peripheral Nerve Fibers. Int. J. Mol. Sci. 2021, 22, 7051. [Google Scholar] [CrossRef] [PubMed]
- Haberkorn, I.; Siegenthaler, L.; Buchmann, L.; Neutsch, L.; Mathys, A. Enhancing Single-Cell Bioconversion Efficiency by Harnessing Nanosecond Pulsed Electric Field Processing. Biotechnol. Adv. 2021, 53, 107780. [Google Scholar] [CrossRef]
- Adamovich, I.V.; Butterworth, T.; Orriere, T.; Pai, D.Z.; Lacoste, D.A.; Cha, M.S. Nanosecond Second Harmonic Generation for Electric Field Measurements with Temporal Resolution Shorter than Laser Pulse Duration. J. Phys. D Appl. Phys. 2020, 53, 145201. [Google Scholar] [CrossRef]
- Hruza, G.J.; Zelickson, B.D.; Selim, M.M.; Rohrer, T.E.; Newman, J.; Park, H.; Jauregui, L.; Nuccitelli, R.; Knape, W.A.; Ebbers, E.; et al. Safety and Efficacy of Nanosecond Pulsed Electric Field Treatment of Seborrheic Keratoses. Dermatol. Surg. 2020, 46, 1183–1189. [Google Scholar] [CrossRef]
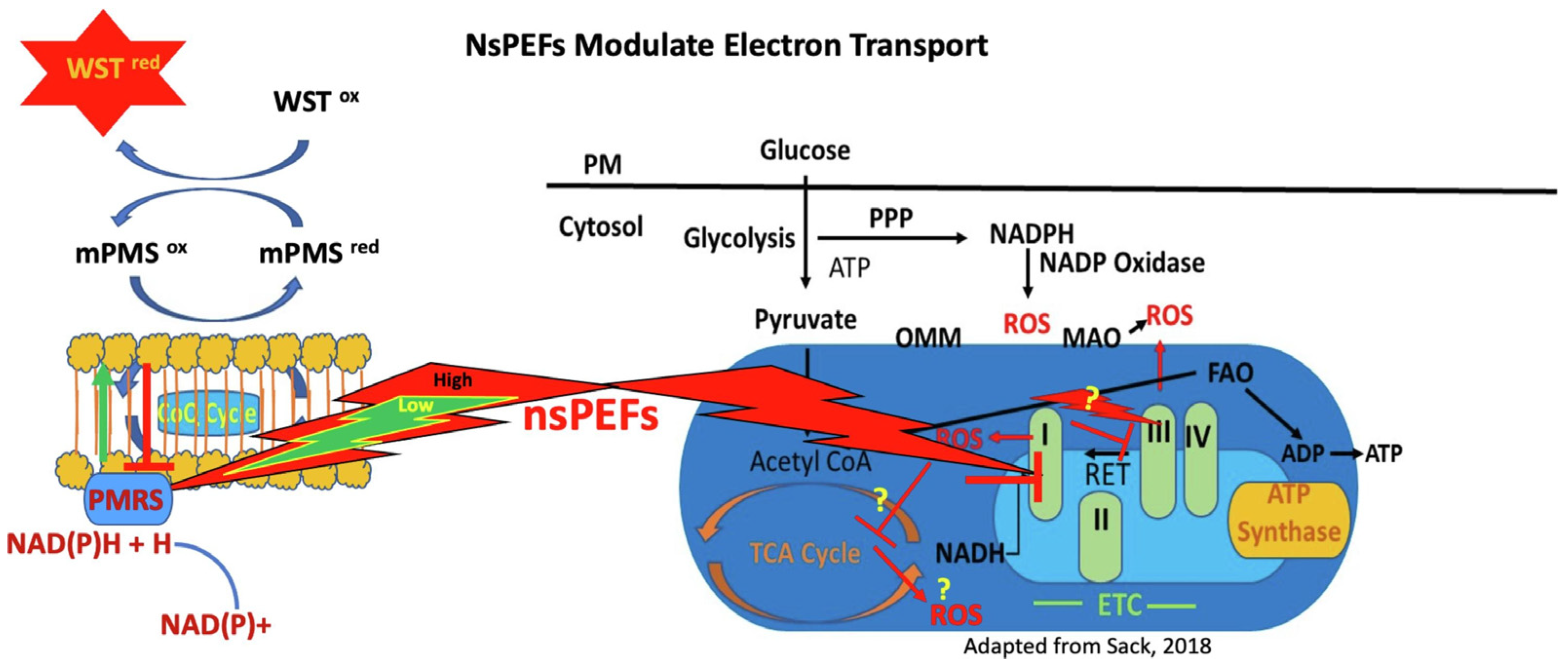
- Asadipour, K.; Hani, M.B.; Potter, L.; Ruedlinger, B.L.; Lai, N.; Beebe, S.J. Nanosecond Pulsed Electric Fields (NsPEFs) Modulate Electron Transport in the Plasma Membrane and the Mitochondria. Bioelectrochemistry 2024, 155, 108568. [Google Scholar] [CrossRef]
- Nanajian, A.; Scott, M.; Burcus, N.I.; Ruedlinger, B.L.; Oshin, E.A.; Beebe, S.J.; Guo, S. Nano-Pulse Treatment Overcomes the Immunosuppressive Tumor Microenvironment to Elicit In Situ Vaccination Protection against Breast Cancer. Vaccines 2024, 12, 633. [Google Scholar] [CrossRef]
- Nies, M.; Watanabe, K.; Kawamura, I.; Wang, B.J.; Litt, J.; Turovskiy, R.; Danitz, D.J.; Uecker, D.R.; Linder, K.E.; Maejima, Y.; et al. Ablating Myocardium Using Nanosecond Pulsed Electric Fields: Preclinical Assessment of Feasibility, Safety, and Durability. Circ. Arrhythmia Electrophysiol. 2024, 17, e012854. [Google Scholar] [CrossRef]
- Chittams-Miles, A.E.; Areej, M.; Purcell, E.B.; Claudia, M. Nanosecond Pulsed Electric Fields Increase Antibiotic Susceptibility in Methicillin-Resistant Staphylococcus Aureus. Microbiol. Spectr. 2023, 12, e0299223. [Google Scholar] [CrossRef]
- Florkowski, M.; Kuniewski, M. Effects of Nanosecond Impulse and Step Excitation in Pulsed Electro Acoustic Measurements on Signals for Space Charge Determination in High-Voltage Electrical Insulation. Measurement 2023, 211, 112677. [Google Scholar] [CrossRef]
- Alderman, D.; Tremble, C.; Singleton, D.; Sanders, J.; Jiang, C. Effects of Pulse Rise Time and Repetition Frequency on Nanosecond Pulsed Plasma Ignition for Combustion. Plasma Res. Express 2021, 3, 14001. [Google Scholar] [CrossRef]
- Liu, H.; Shi, F.; Tang, X.; Zheng, S.; Kolb, J.; Yao, C. Application of Bioimpedance Spectroscopy to Characterize Chemoresistant Tumor Cell Selectivity of Nanosecond Pulse Stimulation. Bioelectrochemistry 2020, 135, 107570. [Google Scholar] [CrossRef]
- Liu, H.; Yao, C.; Zhao, Y.; Chen, X.; Dong, S.; Wang, L.; Davalos, R.V. In Vitro Experimental and Numerical Studies on the Preferential Ablation of Chemo-Resistant Tumor Cells Induced by High-Voltage Nanosecond Pulsed Electric Fields. IEEE Trans. Biomed. Eng. 2021, 68, 2400–2411. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, T.; Xie, L.; Wang, H.; Zhao, J.; Xu, L.; Fang, C. Effect of Pulsed Field Ablation on Solid Tumor Cells and Microenvironment. Front. Oncol. 2022, 12, 899722. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Chen, T.; Wu, Q.; Zhou, L.; Zhou, W.; Wu, L.; Wang, S.; Lu, J.; Wang, W.; Li, D.; et al. Blocking Exposed PD-L1 Elicited by Nanosecond Pulsed Electric Field Reverses Dysfunction of CD8+ T Cells in Liver Cancer. Cancer Lett. 2020, 495, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xu, M.; Sun, R.; Zhao, J.; Zhao, Q.; Wang, Y.; Tian, G.; Jiang, T. Single-Cell Analysis Reveals Nanosecond Pulsed Electric Field Ablation Induced Myeloid Cells Remodeling in Pancreatic Cancer. Bioelectrochemistry 2022, 148, 108266. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhu, Y.; Cheng, P.; Dai, G.; Jin, L.; Wang, Q.; Chen, X.; Cai, C. Anticancer Therapy Using FePt Nanoparticles Combined with Nanosecond Pulsed Electric Fields. ACS Appl. Nano Mater. 2025, 8, 38. [Google Scholar] [CrossRef]
- Zou, Y.; Sun, Y.; Chen, X.; Hong, L.; Dong, G.; Bai, X.; Wang, H.; Rao, B.; Ren, Z.; Yu, Z. Nanosecond Pulse Effectively Ablated Hepatocellular Carcinoma with Alterations in the Gut Microbiome and Serum Metabolites. Front. Pharmacol. 2023, 14, 1163628. [Google Scholar] [CrossRef]
- Nuccitelli, R.; McDaniel, A. Nano-Pulse Stimulation Therapy in Oncology. Bioelectricity 2024, 6, 72–79. [Google Scholar] [CrossRef]
- Ibrahimi, N.; Vallet, L.; Andre, F.M.; Rivaletto, M.; Novac, B.M.; Mir, L.M.; Pécastaing, L. An Overview of Subnanosecond Pulsed Electric Field Biological Effects: Toward Contactless Technologies for Cancer Treatment. Bioelectricity 2023, 5, 76–98. [Google Scholar] [CrossRef]
- Xu, Z.; Pan, C.; Chen, L.; Qian, J.; Chen, X.; Zhou, L.; Zheng, S. Nanosecond Pulsed Electric Field Induces an Antitumor Effect in Triple-Negative Breast Cancer via CXCL9 Axis Dependence in Mice. Cancers 2023, 15, 2076. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Zhao, Y.; Fang, F.; Song, J.; Chen, H.; Xu, Y.; Wang, Z.; Li, F. Reactive Oxygen and Nitrogen Species Release of Single Pancreatic Cancer Cells Subjected to Pulsed Electric Field Ablation: Concentration and Dynamics. Anal. Chem. 2025, 97, 16194–16202. [Google Scholar] [CrossRef] [PubMed]
- Sauer, N.; Szlasa, W.; Szewczyk, A.; Novickij, V.; Saczko, J.; Baczyńska, D.; Daczewska, M.; Kulbacka, J. Effects of Nanosecond Pulsed Electric Field on Immune Checkpoint Receptors in Melanoma Cells. Pharmaceuticals 2023, 16, 1362. [Google Scholar] [CrossRef]
- Liu, Q.; Dong, W.; Liu, R.; Xu, L.; Ran, L.; Xie, Z.; Lei, S.; Su, X.; Yue, Z.; Xiong, D.; et al. Chromatin Landscape Alteration Uncovers Multiple Transcriptional Circuits during Memory CD8+ T-Cell Differentiation. Protein Cell 2025, 16, 575–601. [Google Scholar] [CrossRef]
- Liu, Z.; Li, X.; Gao, Y.; Liu, J.; Feng, Y.; Liu, Y.; Wang, J.; Wang, C.; Wang, D.; He, J.; et al. Epigenetic Reprogramming of Runx3 Reinforces CD8+ T-Cell Function and Improves the Clinical Response to Immunotherapy. Mol. Cancer 2023, 22, 84. [Google Scholar] [CrossRef]
- Luo, Z.; Guo, F.; Xiang, S.; Dong, S.; Yao, C.; Liu, H. Nanosecond Pulsed Bipolar Cancellation of the Killing Effect on Glioblastoma. IEEE Trans. Biomed. Eng. 2025, 72, 2138–2146. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, W.; Xu, D.; Dong, G.; Ren, Z.; Aji, T.; Ji, J.; Zhao, Q.; Pan, J.; Chen, X.; et al. Nanosecond Pulsed Electric Field Ablation as First-Line Curative Therapy for Hepatocellular Carcinoma in High-Risk Locations a Prospective Multicenter. Int. J. Surg. 2025, 111, 3289–3298. [Google Scholar] [CrossRef]
- He, L.; Yang, H.; Tang, J.; Liu, Z.; Chen, Y.; Lu, B.; He, H.; Tang, S.; Sun, Y.; Liu, F.; et al. Intestinal Probiotics E. Coli Nissle 1917 as a Targeted Vehicle for Delivery of P53 and Tum-5 to Solid Tumors for Cancer Therapy. J. Biol. Eng. 2019, 13, 58. [Google Scholar] [CrossRef]
- Ma, W.; Liu, H.; Xu, F.; Xu, L.; Zhang, C.; Lou, W.; Xie, L.; Jiang, T. High-Voltage Electrical Pulses for Pancreatic Cancer Treatment in the Era of Precision Medicine. Int. J. Surg. 2025. [Google Scholar] [CrossRef]
- Xu, L.; Wang, E.; Kang, Y.; Fu, D.; Luo, L.; Quan, Y.; Xi, Y.; Huang, J.; Cui, X.; Zeng, J.; et al. Schottky Nanodiodes Array Enabled Triboelectric Nanosecond Pulse Generator for Ultralow-Cost Tumor Therapy. Device 2025, 3, 100721. [Google Scholar] [CrossRef]
- Vallin, J.R.; Azarin, S.M. Leveraging the Immunological Impacts of Irreversible Electroporation as a New Frontier for Cancer Therapy. Annu. Rev. Chem. Biomol. Eng. 2025, 16, 169–193. [Google Scholar] [CrossRef] [PubMed]
- Yousfi, N.; Merbahi, N.; Bouajila, J.; Taillandier, P.; Debouba, M. Microbial Fermentation Assisted by Pulsed Electric Fields, Magnetic Fields and Cold Atmospheric Plasma: State of the Art. Fermentation 2025, 11, 417. [Google Scholar] [CrossRef]
- Varghese, F.; Philpott, J.M.; Neuber, J.U.; Hargrave, B.; Zemlin, C.W. Surgical Ablation of Cardiac Tissue with Nanosecond Pulsed Electric Fields in Swine. Cardiovasc. Eng. Technol. 2023, 14, 52–59. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zhu, J.; Feng, Y.; Liang, F.; You, K.; Chai, H.; Sui, Z.; Hao, H.; Li, G.; Zhao, J.; et al. Neuromorphic-Enabled Video-Activated Cell Sorting. Nat. Commun. 2024, 15, 10792. [Google Scholar] [CrossRef]
- Xiao, Y.; Yu, J.; Huang, Q.; Xiao, W. Immunomodulatory Impacts of Thermal and Pulsed Field Ablation Therapy on Hepatocellular Carcinoma Associated with Viral Hepatitis. Die Radiol. 2025, 1–9. [Google Scholar] [CrossRef]
- Steelman, Z.A.; Coker, Z.N.; Kiester, A.; Noojin, G.; Ibey, B.L.; Bixler, J.N. Quantitative Phase Microscopy Monitors Subcellular Dynamics in Single Cells Exposed to Nanosecond Pulsed Electric Fields. J. Biophotonics 2021, 14, e202100125. [Google Scholar] [CrossRef]
- Rana, J.N.; Gul, K.; Mumtaz, S. Isorhamnetin: Reviewing Recent Developments in Anticancer Mechanisms and Nanoformulation-Driven Delivery. Int. J. Mol. Sci. 2025, 26, 7381. [Google Scholar] [CrossRef]
- Rana, J.N.; Mumtaz, S. Prunin: An Emerging Anticancer Flavonoid. Int. J. Mol. Sci. 2025, 26, 2678. [Google Scholar] [CrossRef]
- Bystrov, D.A.; Volegova, D.D.; Korsakova, S.A.; Salmina, A.B.; Yurchenko, S.O. Electric Field-Induced Effects in Eukaryotic Cells: Current Progress and Limitations. Tissue Eng. Part B Rev. 2025. [Google Scholar] [CrossRef]
- Li, Q.-G.; Liu, Z.-G.; Dong, G.; Sun, Y.; Zou, Y.-W.; Chen, X.-L.; Wu, B.; Chen, X.-H.; Ren, Z.-G. Nanosecond Pulsed Electric Field Ablates Rabbit VX2 Liver Tumors in a Non-Thermal Manner. PLoS ONE 2023, 18, e0273754. [Google Scholar] [CrossRef]
- Haberkorn, I.; Buchmann, L.; Häusermann, I.; Mathys, A. Nanosecond Pulsed Electric Field Processing of Microalgae Based Biorefineries Governs Growth Promotion or Selective Inactivation Based on Underlying Microbial Ecosystems. Bioresour. Technol. 2021, 319, 124173. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.H.; Schoenbach, K.H.; Beebe, S.J. Nanosecond Pulsed Electric Fields (NsPEF) Induce Direct Electric Field Effects and Biological Effects on Human Colon Carcinoma Cells. DNA Cell Biol. 2005, 24, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Stacey, M.; Fox, P.; Buescher, S.; Kolb, J. Nanosecond Pulsed Electric Field Induced Cytoskeleton, Nuclear Membrane and Telomere Damage Adversely Impact Cell Survival. Bioelectrochemistry 2011, 82, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Fan, A.; Liu, G.; Wu, X. Nanosecond Pulse Electric Field Treatment Initiates Mitochondrial Apoptosis Pathway by Inducing Mitochondrial Morphological Changes in Myocardial Cells. J. Interv. Card. Electrophysiol. 2024. [Google Scholar] [CrossRef]
- Liu, J.; Hong, M.; Li, Y.; Chen, D.; Wu, Y.; Hu, Y. Programmed Cell Death Tunes Tumor Immunity. Front. Immunol. 2022, 13, 847345. [Google Scholar] [CrossRef]
- Kari, S.; Subramanian, K.; Altomonte, I.A.; Murugesan, A.; Yli-Harja, O.; Kandhavelu, M. Programmed Cell Death Detection Methods: A Systematic Review and a Categorical Comparison. Apoptosis 2022, 27, 482–508. [Google Scholar] [CrossRef]
- Szlasa, W.; Michel, O.; Sauer, N.; Novickij, V.; Lewandowski, D.; Kasperkiewicz, P.; Tarek, M.; Saczko, J.; Kulbacka, J. Nanosecond Pulsed Electric Field Suppresses Growth and Reduces Multi-Drug Resistance Effect in Pancreatic Cancer. Sci. Rep. 2023, 13, 351. [Google Scholar] [CrossRef]
- Zhang, J.; Ghasemi, N.; Zare, F.; Duley, J.A.; Cowley, D.M.; Shaw, P.N.; Koorts, P.; Bansal, N. Nanosecond Pulsed Electric Field Treatment of Human Milk: Effects on Microbiological Inactivation, Whey Proteome and Bioactive Protein. Food Chem. 2023, 406, 135073. [Google Scholar] [CrossRef]
- Kulbacka, J.; Rembiałkowska, N.; Szewczyk, A.; Moreira, H.; Szyjka, A.; Girkontaitė, I.; Grela, K.P.; Novickij, V. The Impact of Extracellular Ca2+ and Nanosecond Electric Pulses on Sensitive and Drug-Resistant Human Breast and Colon Cancer Cells. Cancers 2021, 13, 3216. [Google Scholar] [CrossRef]
- Akter, K.; Gul, K.; Mumtaz, S. Revisiting Curcumin in Cancer Therapy: Recent Insights into Molecular Mechanisms, Nanoformulations, and Synergistic Combinations. Curr. Issues Mol. Biol. 2025, 47, 716. [Google Scholar] [CrossRef]
- Beebe, S.J. Considering Effects of Nanosecond Pulsed Electric Fields on Proteins. Bioelectrochemistry 2015, 103, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Lu, Z.; Liu, H.; Huang, Q.; Zheng, X.; Li, X.; Zhou, Y. Anti-Tumor Effects of Nanosecond Pulsed Electric Fields in a Murine Model of Pancreatic Cancer. Bioelectrochemistry 2025, 161, 108803. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.Y.; Zhou, X.; Yang, J.; Zhou, Y.; He, B.; Huang, W.T.; Wang, Y.; Guo, Z. Migration Inhibition and Selective Cytotoxicity of Cobalt Hydroxide Nanosheets on Different Cancer Cell Lines. New J. Chem. 2022, 46, 10289–10298. [Google Scholar] [CrossRef]
- Yin, S.; Chen, X.; Hu, C.; Zhang, X.; Hu, Z.; Yu, J.; Feng, X.; Jiang, K.; Ye, S.; Shen, K.; et al. Nanosecond Pulsed Electric Field (NsPEF) Treatment for Hepatocellular Carcinoma: A Novel Locoregional Ablation Decreasing Lung Metastasis. Cancer Lett. 2014, 346, 285–291. [Google Scholar] [CrossRef]
- Breton, M.; Mir, L.M. Microsecond and Nanosecond Electric Pulses in Cancer Treatments. Bioelectromagnetics 2012, 33, 106–123. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Y.; Guo, J.; Chen, Q.; Zhang, J.; Fang, J. Nanosecond Pulsed Electric Fields as a Novel Drug Free Therapy for Breast Cancer: An in Vivo Study. Cancer Lett. 2014, 343, 268–274. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, Y.; Yao, C.; Schmelz, E.M.; Davalos, R.V. Differential Effects of Nanosecond Pulsed Electric Fields on Cells Representing Progressive Ovarian Cancer. Bioelectrochemistry 2021, 142, 107942. [Google Scholar] [CrossRef]
- Ren, Z.; Chen, X.; Cui, G.; Yin, S.; Chen, L.; Jiang, J.; Hu, Z.; Xie, H.; Zheng, S.; Zhou, L. Nanosecond Pulsed Electric Field Inhibits Cancer Growth Followed by Alteration in Expressions of NF-ΚB and Wnt/β-Catenin Signaling Molecules. PLoS ONE 2013, 8, e74322. [Google Scholar] [CrossRef]
- Miao, X.; Yin, S.; Shao, Z.; Zhang, Y.; Chen, X. Nanosecond Pulsed Electric Field Inhibits Proliferation and Induces Apoptosis in Human Osteosarcoma. J. Orthop. Surg. Res. 2015, 10, 104. [Google Scholar] [CrossRef]
- Xiang, X.-W.; Liu, H.-T.; Liu, W.; Yan, Z.-Y.; Zeng, Y.-L.; Wang, Y.-J.; Liu, J.; Chen, Y.-C.; Yu, S.-X.; Zhu, C.-H.; et al. Revolutionizing Wound Healing: Ultrashort Pulse Electric Fields in Seconds for Highly Aligned Extracellular Matrix and Efficient Cell Migration. Chem. Eng. J. 2023, 471, 144267. [Google Scholar] [CrossRef]
- Tu, T.; Ouyang, C.; Li, P.; Ni, Z.; Wang, Z.; Lai, J.; Chen, X.; Liu, Z.; Lu, H. Enhancing Fibroblast-Based Bone Regeneration by Harnessing Nanosecond Pulsed Electric Field. Bioelectrochemistry 2026, 167, 109089. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhu, Y.; Chen, Q.; Shi, S.; Huang, Q.; Chen, X.; Li, X.; Cheng, P.; Wu, H.; Hu, S. Nanosecond Pulsed Electric Field-Empowered Physical–Chemical Cascade Ferroptosis Therapy for Triple-Negative Breast Cancer. J. Mater. Chem. B 2025, 13, 10512–10524. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, K.; Li, S.-P.; Zhu, C.-Y.; Hsu, H.-Y.; Ohta, N. Fluorescence Microscopic Approach for Detection of Two Different Modes of Breast Cancer Cell Death Induced by Nanosecond Pulsed Electric Field. Sens. Actuators B Chem. 2023, 378, 133199. [Google Scholar] [CrossRef]
- Qian, J.; Ding, L.; Wu, Q.; Yu, X.; Li, Q.; Gu, Y.; Wang, S.; Mao, J.; Liu, X.; Li, B.; et al. Nanosecond Pulsed Electric Field Stimulates CD103+ DC Accumulation in Tumor Microenvironment via NK-CD103+ DC Crosstalk. Cancer Lett. 2024, 593, 216514. [Google Scholar] [CrossRef]
- Rembiałkowska, N.; Kucharczyk, J.; Radzevičiūtė-Valčiukė, E.; Novickij, V.; Tonci, M.; Dündar, A.; Kulbacka, J.; Szlasa, W. Enhancing Lung Cancer Growth Inhibition with Calcium Ions: Role of Mid- and High-Frequency Electric Field Pulses. Biomed. Pharmacother. 2024, 181, 117691. [Google Scholar] [CrossRef]
- Liu, J.; Fang, C.; Jin, X.; Tian, G.; Sun, Z.; Hong, L.; Pan, J.; Chen, X.; Zhao, J.; Cao, H.; et al. Nanosecond Pulsed Electric Field Ablation-Induced Modulation of Sphingolipid Metabolism Is Associated with Ly6c2+ Mononuclear Phagocyte Differentiation in Liver Cancer. Mol. Oncol. 2023, 17, 1093–1111. [Google Scholar] [CrossRef]
- Yun, S.H.; Mansurov, V.; Yang, L.; Yoon, J.; Leblanc, N.; Craviso, G.L.; Zaklit, J. Modulating Ca2+ Influx into Adrenal Chromaffin Cells with Short-Duration Nanosecond Electric Pulses. Biophys. J. 2024, 123, 2537–2556. [Google Scholar] [CrossRef]
- Balevičiūtė, A.; Radzevičiūtė, E.; Želvys, A.; Malyško-Ptašinskė, V.; Novickij, J.; Zinkevičienė, A.; Kašėta, V.; Novickij, V.; Girkontaitė, I. High-Frequency Nanosecond Bleomycin Electrochemotherapy and Its Effects on Changes in the Immune System and Survival. Cancers 2022, 14, 6254. [Google Scholar] [CrossRef]
- Franklin, S.; Beier, H.T.; Ibey, B.L.; Nash, K. External Stimulation by Nanosecond Pulsed Electric Fields to Enhance Cellular Uptake of Nanoparticles. In Proceedings of the SPIE-The International Society for Optical Engineering, San Francisco, CA, USA, 13 March 2015; Volume 9326, p. 932610. [Google Scholar]
- Kulbacka, J.; Pucek, A.; Wilk, K.A.; Dubińska-Magiera, M.; Rossowska, J.; Kulbacki, M.; Kotulska, M. The Effect of Millisecond Pulsed Electric Fields (MsPEF) on Intracellular Drug Transport with Negatively Charged Large Nanocarriers Made of Solid Lipid Nanoparticles (SLN): In Vitro Study. J. Membr. Biol. 2016, 249, 645–661. [Google Scholar] [CrossRef]
- Souiade, L.; Domingo-Diez, J.; Alcaide, C.; Gámez, B.; Gámez, L.; Ramos, M.; Serrano Olmedo, J.J. Improving the Efficacy of Magnetic Nanoparticle-Mediated Hyperthermia Using Trapezoidal Pulsed Electromagnetic Fields as an In Vitro Anticancer Treatment in Melanoma and Glioblastoma Multiforme Cell Lines. Int. J. Mol. Sci. 2023, 24, 15933. [Google Scholar] [CrossRef]
- Ahmadi Kamalabadi, M.; Neshastehriz, A.; Ghaznavi, H.; Amini, S.M. Folate Functionalized Gold-Coated Magnetic Nanoparticles Effect in Combined Electroporation and Radiation Treatment of HPV-Positive Oropharyngeal Cancer. Med. Oncol. 2022, 39, 196. [Google Scholar] [CrossRef] [PubMed]
- Radzevičiūtė-Valčiukė, E.; Gečaitė, J.; Želvys, A.; Zinkevičienė, A.; Žalnėravičius, R.; Malyško-Ptašinskė, V.; Nemeikaitė-Čenienė, A.; Kašėta, V.; German, N.; Novickij, J.; et al. Improving NonViral Gene Delivery Using MHz Bursts of Nanosecond Pulses and Gold Nanoparticles for Electric Field Amplification. Pharmaceutics 2023, 15, 1178. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Xiang, J.; Zhuo, Y.; Pei, K. Molecular Dynamics Study of Protein-Mediated Electroporation of Kv Channels Induced by NsPEFs: Advantages of Bipolar Pulses. Biomacromolecules 2025, 26, 1002–1011. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, S.; Konishi, D.; Uto, Y.; Shimomura, N. Effects of Nanosecond Pulsed Electric Fields Application on Cancer Cell and Combination of Anticancer Drug. Electr. Eng. Jpn. 2022, 215, e23376. [Google Scholar] [CrossRef]
- Ma, R.; Wang, Y.; Wang, Z.; Yin, S.; Liu, Z.; Yan, K. Enhanced Cellular Doxorubicin Uptake via Delayed Exposure Following Nanosecond Pulsed Electric Field Treatment: An In Vitro Study. Pharmaceutics 2024, 16, 851. [Google Scholar] [CrossRef]
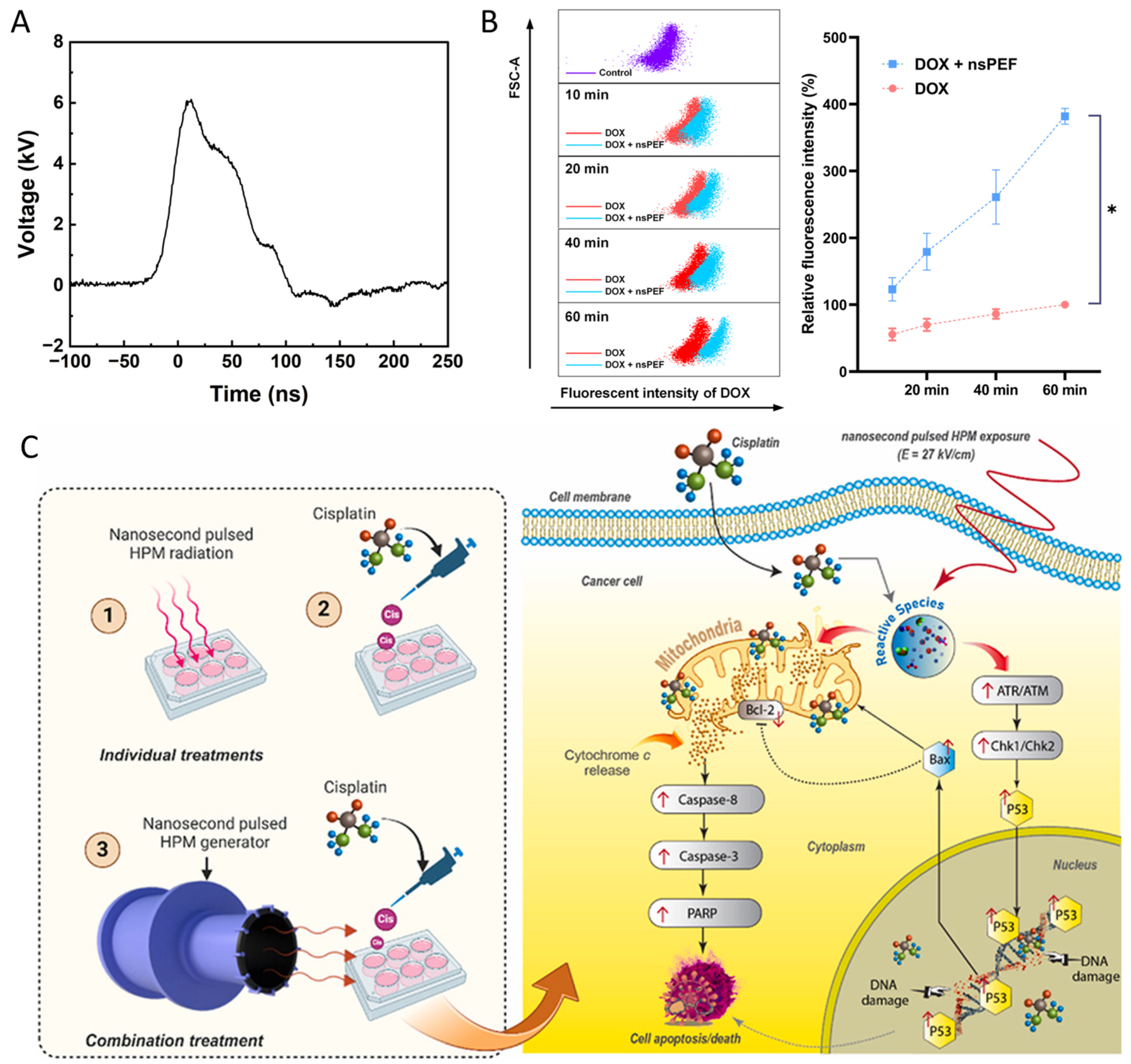
- Rana, J.N.; Mumtaz, S.; Han, I.; Choi, E.H. Harnessing the Synergy of Nanosecond High-Power Microwave Pulses and Cisplatin to Increase the Induction of Apoptosis in Cancer Cells through the Activation of ATR/ATM and Intrinsic Pathways. Free Radic. Biol. Med. 2024, 225, 221–235. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, H.; Xu, D.; Sun, R.; Fang, C.; Zhao, Q.; He, C.; Pan, Y.; Xu, F.; Jiang, T. Neutrophil Membrane-Coated Nanoparticles for Enhanced Nanosecond Pulsed Electric Field Treatment of Pancreatic Cancer. Int. J. Hyperth. 2022, 39, 1026–1035. [Google Scholar] [CrossRef]
- Yin, S.; Chen, X.; Xie, H.; Zhou, L.; Guo, D.; Xu, Y.; Wu, L.; Zheng, S. Nanosecond Pulsed Electric Field (NsPEF) Enhance Cytotoxicity of Cisplatin to Hepatocellular Cells by Microdomain Disruption on Plasma Membrane. Exp. Cell Res. 2016, 346, 233–240. [Google Scholar] [CrossRef]
- Novickij, V.; Malyško, V.; Želvys, A.; Balevičiūtė, A.; Zinkevičienė, A.; Novickij, J.; Girkontaitė, I. Electrochemotherapy Using Doxorubicin and Nanosecond Electric Field Pulses: A Pilot in Vivo Study. Molecules 2020, 25, 4601. [Google Scholar] [CrossRef]
- Rembiałkowska, N.; Novickij, V.; Baczyńska, D.; Dubińska-Magiera, M.; Saczko, J.; Rudno-Rudzińska, J.; Maciejewska, M.; Kulbacka, J. Micro- and Nanosecond Pulses Used in Doxorubicin Electrochemotherapy in Human Breast and Colon Cancer Cells with Drug Resistance. Molecules 2022, 27, 2052. [Google Scholar] [CrossRef] [PubMed]
- Lekešytė, B.; Mickevičiūtė, E.; Malakauskaitė, P.; Szewczyk, A.; Radzevičiūtė-Valčiukė, E.; Malyško-Ptašinskė, V.; Želvys, A.; German, N.; Ramanavičienė, A.; Kulbacka, J.; et al. Application of Gold Nanoparticles for Improvement of Electroporation-Assisted Drug Delivery and Bleomycin Electrochemotherapy. Pharmaceutics 2024, 16, 1278. [Google Scholar] [CrossRef] [PubMed]
- Nuccitelli, R.; Tran, K.; Sheikh, S.; Athos, B.; Kreis, M.; Nuccitelli, P. Optimized Nanosecond Pulsed Electric Field Therapy Can Cause Murine Malignant Melanomas to Self-Destruct with a Single Treatment. Int. J. Cancer 2010, 127, 1727–1736. [Google Scholar] [CrossRef] [PubMed]
- de Assis, T.A.; Dall’Agnol, F.F.; Forbes, R.G. Field Emitter Electrostatics: A Review with Special Emphasis on Modern High-Precision Finite-Element Modelling. J. Phys. Condens. Matter 2022, 34, 493001. [Google Scholar] [CrossRef]
- Kulbacka, J.; Rembiałkowska, N.; Radzevičiūtė-Valčiukė, E.; Szewczyk, A.; Novickij, V. Cardiomyocytes Permeabilization and Electrotransfection by Unipolar and Bipolar Asymmetric Electric Field Pulses. Bioelectricity 2024, 6, 91–96. [Google Scholar] [CrossRef]
- Shu, T.; Ding, L.; Fang, Z.; Yu, S.; Chen, L.; Moser, M.A.J.; Zhang, W.; Qin, Z.; Zhang, B. Lethal Electric Field Thresholds for Cerebral Cells With Irreversible Electroporation and H-FIRE Protocols: An In Vitro Three-Dimensional Cell Model Study. J. Biomech. Eng. 2022, 144, 101010. [Google Scholar] [CrossRef]
- Kos, B.; Mattison, L.; Ramirez, D.; Cindrič, H.; Sigg, D.C.; Iaizzo, P.A.; Stewart, M.T.; Miklavčič, D. Determination of Lethal Electric Field Threshold for Pulsed Field Ablation in Ex Vivo Perfused Porcine and Human Hearts. Front. Cardiovasc. Med. 2023, 10, 1160231. [Google Scholar] [CrossRef]
- Lv, Y.; Liu, H.; Feng, Z.; Zhang, J.; Chen, G.; Yao, C. The Enlargement of Ablation Area by Electrolytic Irreversible Electroporation (E-IRE) Using Pulsed Field with Bias DC Field. Ann. Biomed. Eng. 2022, 50, 1964–1973. [Google Scholar] [CrossRef]
- Semenov, I.; Xiao, S.; Pakhomov, A.G. Primary Pathways of Intracellular Ca2+ Mobilization by Nanosecond Pulsed Electric Field. Biochim. Biophys. Acta-Biomembr. 2013, 1828, 981–989. [Google Scholar] [CrossRef]
- Sanders, J.M.; Kuthi, A.; Vernier, P.T.; Wu, Y.-H.; Jiang, C.; Gundersen, M.A. Scalable, Compact, Nanosecond Pulse Generator with a High Repetition Rate for Biomedical Applications Requiring Intense Electric Fields. In Proceedings of the 2009 IEEE Pulsed Power Conference, Washington, DC, USA, 28 June–2 July 2009; pp. 1418–1421. [Google Scholar]
- Riaz, K.; Leung, S.-F.; Fan, Z.; Lee, Y.-K. Electric Field Enhanced 3D Scalable Low-Voltage Nano-Spike Electroporation System. Sens. Actuators A Phys. 2017, 255, 10–20. [Google Scholar] [CrossRef]
- Vanneman, M.; Dranoff, G. Combining Immunotherapy and Targeted Therapies in Cancer Treatment. Nat. Rev. Cancer 2012, 12, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Vizintin, A.; Markovic, S.; Scancar, J.; Kladnik, J.; Turel, I.; Miklavcic, D. Nanosecond Electric Pulses Are Equally Effective in Electrochemotherapy with Cisplatin as Microsecond Pulses. Radiol. Oncol. 2022, 56, 326. [Google Scholar] [CrossRef]
- McBride, S.; Avazzadeh, S.; Wheatley, A.M.; O’Brien, B.; Coffey, K.; Elahi, A.; O’Halloran, M.; Quinlan, L.R. Ablation Modalities for Therapeutic Intervention in Arrhythmia-Related Cardiovascular Disease: Focus on Electroporation. J. Clin. Med. 2021, 10, 2657. [Google Scholar] [CrossRef]
- Rembiałkowska, N.; Novickij, V.; Radzevičiūtė-Valčiukė, E.; Mickevičiūtė, E.; Gajewska-Naryniecka, A.; Kulbacka, J. Susceptibility of Various Human Cancer Cell Lines to Nanosecond and Microsecond Range Electrochemotherapy: Feasibility of Multi-Drug Cocktails. Int. J. Pharm. 2023, 646, 123485. [Google Scholar] [CrossRef]
- Yimingjiang, M.; Tuergan, T.; Chen, X.; Wen, H.; Shao, Y.; Zhang, R.; Aihaiti, K.; Xue, J.; Aji, T.; Zhang, W. Comparative Analysis of Immunoactivation by Nanosecond Pulsed Electric Fields and PD-1 Blockade in Murine Hepatocellular Carcinoma. Anal. Cell. Pathol. 2020, 2020, 9582731. [Google Scholar] [CrossRef] [PubMed]
| Tumor Model | Pulse Parameters | Primary Outcomes | Therapeutic Implications | Ref. |
|---|---|---|---|---|
| Human breast cancer cells (MDA-MB-231) | 5 ns, 70–100 kV/cm, 2000–10,000 pulses, 100 Hz | Nuclear pores ↑, ER Ca2+ release ↑, Mitochondrial depolarization ↑, Cytoskeleton integrity ↓ | Apoptosis ↑, ICD ↑, Therapy resistance ↓ | [80] |
| Rat myocardial cells (H9C2) | 100 ns, 20 kV/cm, 80 pulses | Mitochondrial membrane potential ↓, Mitochondrial area ↓, Perimeter ↓, Apoptosis ↑ | Initiates mitochondrial apoptosis pathway ↑, Non-thermal ablation potential ↑, Tissue selectivity ↑ | [145] |
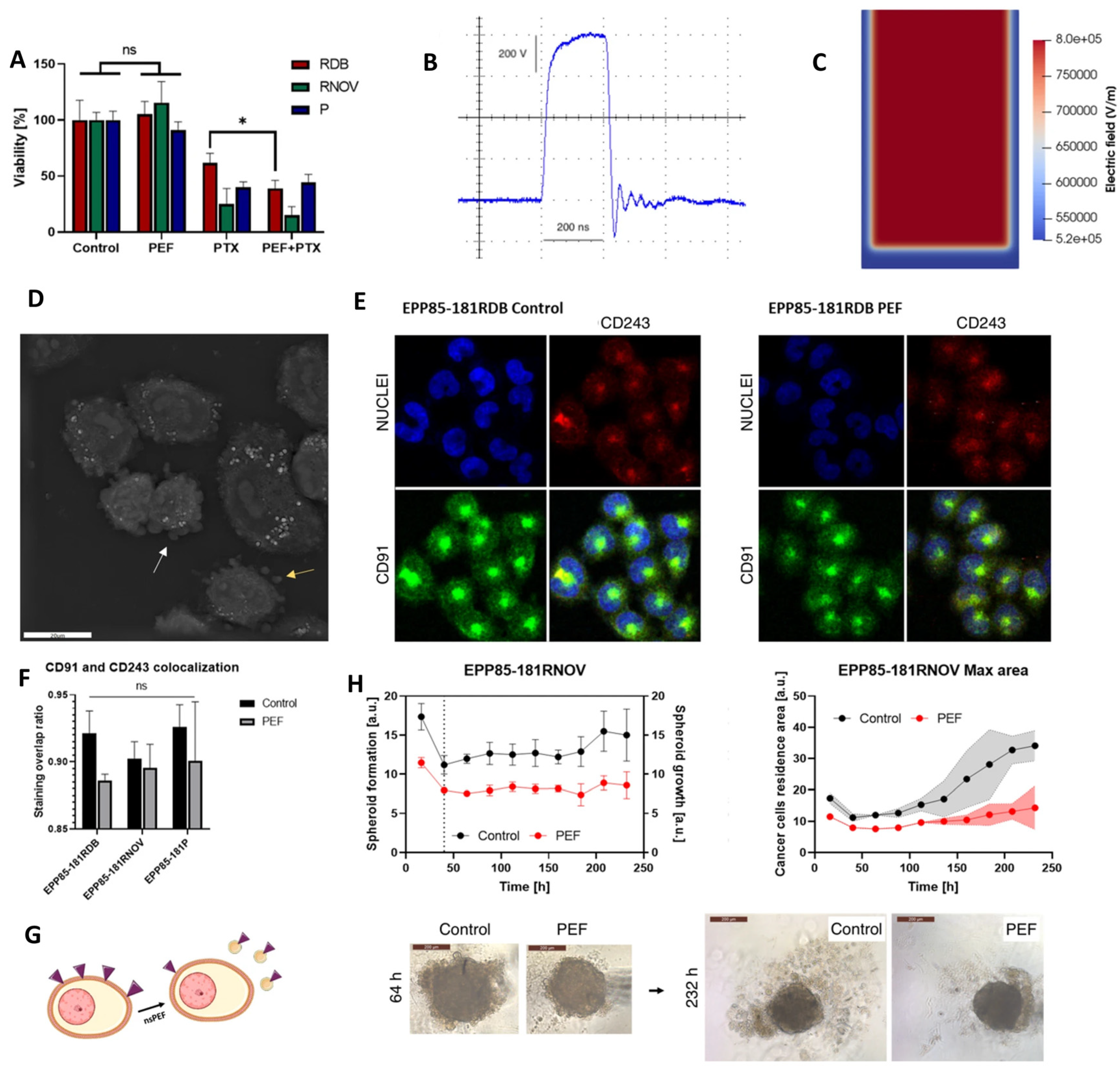
| Human pancreatic cancer cells (EPP85-181RDB, EPP85-181RNOV, EPP85-181P) | 200 ns, 8 kV/cm, 100 pulses, 10 kHz | MDR protein (P-gp, LRP) expression ↓, Microvesicle release ↑, Actin remodeling ↑/↓ (cell line-dependent), Spheroid growth ↓, Cadherin expression ↓ | MDR ↓, Paclitaxel sensitivity ↑ (transient), Tumor growth ↓, Cell adhesion ↑ | [148] |
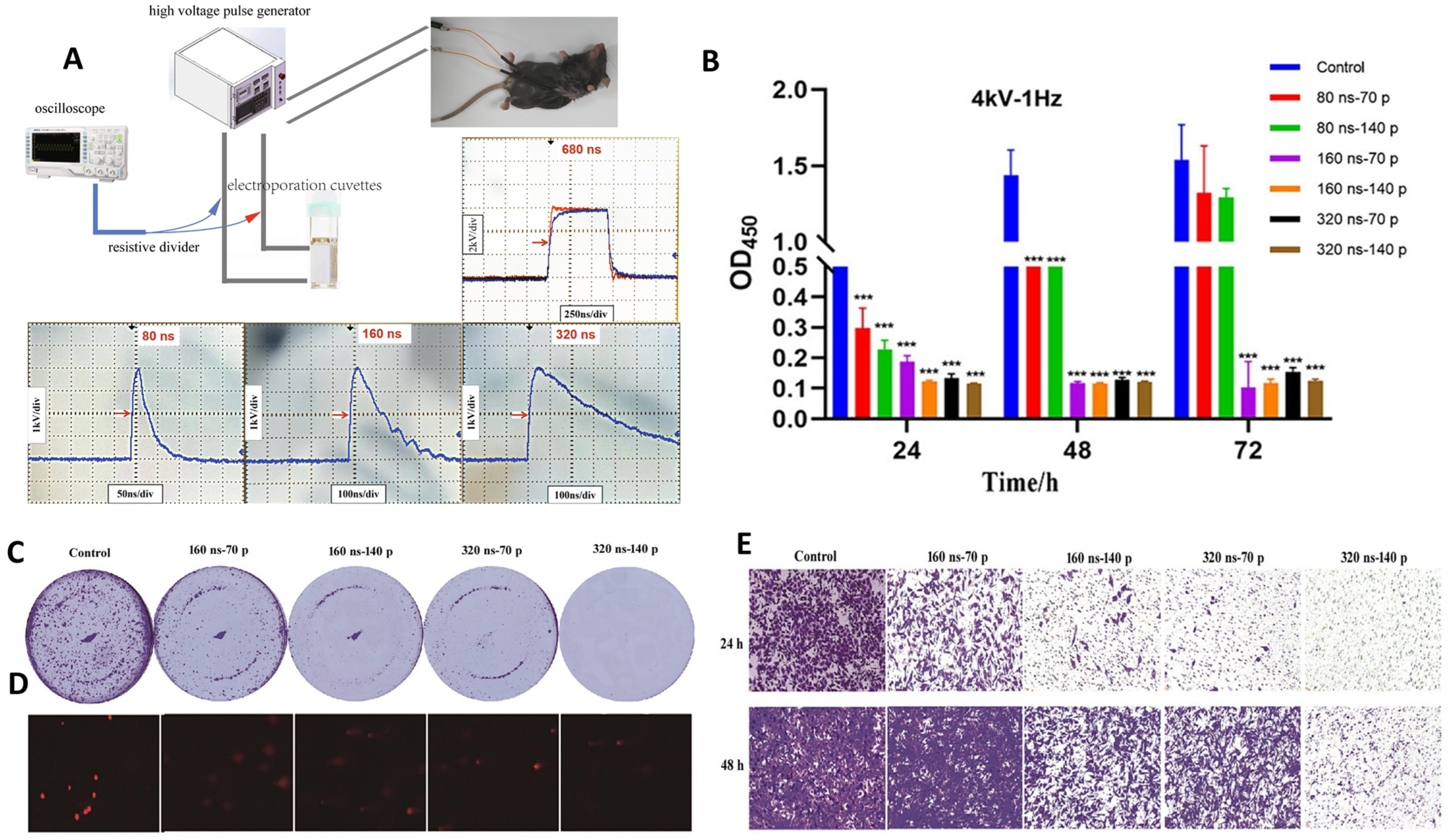
| Murine pancreatic adenocarcinoma (Panc02 cells, C57BL/6J mice) | 80–320 ns (cells), 160 ns/4 kV/70–280 pulses/1 Hz (in vitro); 680 ns/6 kV/400 pulses/1 Hz (in vivo) | Cell viability ↓, Colony formation ↓, Migration ↓, DNA damage ↑, Tumor growth ↓, Gene expression changes ↑ (CDK1, CENPA, UBE2C, etc.), ceRNA network altered | Tumor suppression ↑, Metastasis ↓, No organ toxicity, Prognostic genes identified for pancreatic cancer | [153] |
| Breast cancer cells (MCF-7, MDA-MB-231) | 50 ns, 20 kV/cm, 2 kHz, 120 s | MCF-7: Apoptosis ↑ (Annexin V ↑, caspase-3/7 ↑, ΔΨm ↓, ROS ↑, NADH lifetime ↑). MDA-MB-231: Necroptosis ↑ (plasma membrane rupture ↑, nuclear swelling ↑, caspase-independent, NADH lifetime ↓, Ca2+ dysregulation ↑) | Subtype-specific responses: apoptosis in hormone-positive cells ↑, necroptosis in triple-negative cells ↑, therapy resistance ↓ | [164] |
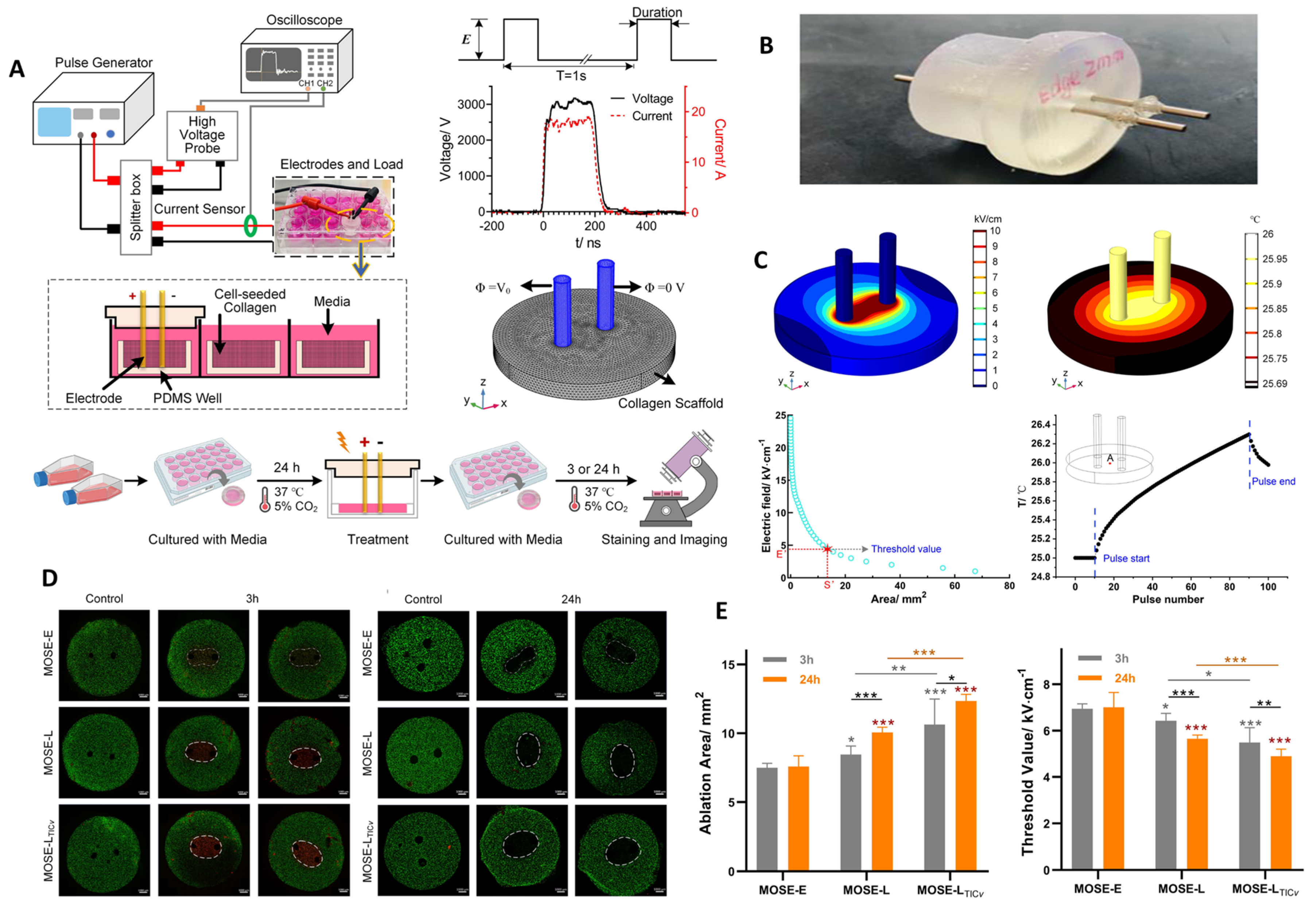
| Mouse ovarian surface epithelial cells (MOSE-E: benign, MOSE-L: malignant, MOSE-LTICv: highly aggressive) | 200 ns, 15 kV/cm, 80 pulses, 1 Hz, 3D collagen scaffolds ± Nocodazole | Ablation area ↑ with malignancy stage (MOSE-LTICv > MOSE-L > MOSE-E), Lethal threshold ↓ with malignancy stage, Ablation area stable in benign cells but ↑ in malignant/aggressive cells over 24 h, Thermal rise negligible (<1.5 °C) | Selective ablation of late-stage/aggressive cells ↑, Tumor recurrence ↓, Benign tissue sparing ↑, Combination with Nocodazole further ↑ ablation | [158] |
| H9c2 cardiac myoblasts (non-cancer) and 4T1-luc breast cancer cells | 60 ns, 40 kV/cm, 1 Hz, 1–75 pulses (charging effect up to 0.02 Vs/cm) | Biphasic modulation of plasma tPMET ↑ (low) ↓ (high), ETS I ↓, H9c2: mROS ↑ cROS ↓, 4T1: cROS ↑ mROS ↑ | Cancer adaptation via glycolysis ↑, Normal cell stress ↑, Redox selectivity exploitable | [107] |
| Human hepatocellular carcinoma (HepG2) cells | 300 ns, 10–30 kV/cm, 1 Hz, 200 pulses | Cell viability ↓ (dose-dependent), ROS ↑, ΔΨm ↓, Apoptosis ↑ (Annexin V, caspase-3/7), Necrosis ↑ at higher fields | nsPEFs trigger apoptosis at moderate fields and necrosis at high fields; potential for dose-controlled ablation in liver cancer | [165] |
| Human melanoma (C32, A375) | 200 ns, 100 pulses, 4–16 kV/cm, 10 kHz | Viability ↓ (C32: to 51%, A375: to 66% at 16 kV/cm), Membrane permeabilization ↑, Morphological changes (contraction, vesiculation, lipid redistribution ↑), PD-1 expression ↑, MHC-II expression ↑, Cytokine secretion: TNF-α ↑, IL-6 ↑/↓ (cell line dependent), IL-1β ↑ | nsPEFs modulate immune checkpoint receptors and cytokines, enhancing antitumor immunity and supporting synergy with checkpoint inhibitors (PD-1/PD-L1 axis). | [124] |
| Human lung carcinoma (A549, 2D & 3D spheroids) | 200 ns, 100 pulses, 4–16 kV/cm, 10 kHz | Viability ↓, Membrane permeability ↑, PD-1 ↑, MHC-II ↑, Cytokines (TNF-α ↑, IL-1β ↑, IL-6 variable) | nsPEFs enhance immunogenicity and checkpoint pathways → synergy with PD-1/PD-L1 blockade | [166] |
| CT-26 murine colorectal carcinoma (cells & peritoneal metastasis mice) | 10 ns, 1–1000 pulses, 10–200 Hz | Cell death ↑, Membrane permeability ↑, Ca2+ influx ↑, Mitochondrial swelling ↑, Tumor regression ↑, CD8+ T-cell infiltration ↑ | nsPEFs induce cytotoxicity + immune activation → potential for CRC peritoneal metastasis therapy | [12] |
| Human umbilical vein endothelial cells (HUVECs, D-galactose-induced senescence) & aged rodents (skin) | 3 kV/cm, 3 min/day × 14 days (in vitro & in vivo) | SA-β-Gal ↓, ROS ↓, Proliferation (EdU+) ↑, ΔΨm ↑, HIF-1α ↑, SIRT1 ↑, Angiogenesis ↑ in aged skin | nsPEFs rejuvenate aging ECs by restoring mitochondrial–nuclear retrograde communication and promoting vascular regeneration | [7] |
| Liver cancer patients (clinical, n = 15) & mouse HCC model | 300 ns, 30 kV, 800–3200 pulses, 2–3 electrodes | Local RFS (12 mo) ↑ (86.7%), Overall RFS ↑ (60%), ALT/AST transient ↑, Sphingolipid metabolism ↑ in Ly6c2+ MNPs, CD8+ T-cell activation ↑ | nsPEFs safe & effective; efficacy linked to sphingolipid-driven MNP differentiation → durable antitumor immunity | [167] |
| Human lung cancer (A549) | 50 or 100 ns, 20 kV/cm, 2 kHz, microelectrode chip | Apoptosis ↑ (blebbing, PS externalization, caspase-3/7 ↑), Intracellular Ca2+ ↑ (ER release, CRAC entry), ROS (O2−) ↑, NADH lifetime ↑, ΔΨm ↓ | nsPEFs induce Ca2+-dependent mitochondrial apoptosis, linking ER release, ROS generation, and ΔΨm collapse in lung cancer | [99] |
| Bovine adrenal chromaffin cells (neuroendocrine model) | Single pulses, 3–50 ns; E-field 1.1–13.4 MV/m | ↑ Ca2+ influx with increasing pulse duration (≥11 ns), shift from VGCC-mediated to mixed VGCC + non-VGCC pathways; short pulses (≤5 ns) mimic physiological nAChR activation | Precise tuning of Ca2+ influx by ultrashort pulses suggests potential for neuromodulation and electrostimulation therapies without membrane damage | [168] |
| Gastric adenocarcinoma (EPG85-257P sensitive, EPG85-257RDB resistant) | 10 ns, 200 pulses, 12.5–50 kV/cm, 1 kHz | Viability ↓ with dose, Ca2+ amplified cytotoxicity; Membrane permeabilization ↑; F-actin reorganization; ROS ↑, GSH/GSSG ↓; Proteasomal activity ↓; Apoptosis ↑ (comet assay, AIF nuclear localization) | nsPEFs + Ca2+ overcome drug resistance by inducing oxidative stress, cytoskeletal disruption, proteasomal inhibition, and apoptosis | [9] |
| Melanoma (A375, C32) & Colon cancer (LoVo, LoVoDX) | 10–300 ns, 100–400 pulses, 5–10 kV/cm | Viability ↓ (dose- and Ca2+-dependent), Membrane permeabilization ↑, ROS ↑, GSH ↓, Apoptosis ↑, Necrosis ↑ at higher doses, Ca2+ influx sustained | nsPEFs + Ca2+ induce strong cytotoxicity and oxidative stress, effective against resistant LoVoDX cells; supports Ca-electrochemotherapy development | [169] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gul, K.; Mumtaz, S. Nanosecond Pulsed Electric Fields (nsPEFs) for Precision Intracellular Oncotherapy: Recent Advances and Emerging Directions. Int. J. Mol. Sci. 2025, 26, 11268. https://doi.org/10.3390/ijms262311268
Gul K, Mumtaz S. Nanosecond Pulsed Electric Fields (nsPEFs) for Precision Intracellular Oncotherapy: Recent Advances and Emerging Directions. International Journal of Molecular Sciences. 2025; 26(23):11268. https://doi.org/10.3390/ijms262311268
Chicago/Turabian StyleGul, Kainat, and Sohail Mumtaz. 2025. "Nanosecond Pulsed Electric Fields (nsPEFs) for Precision Intracellular Oncotherapy: Recent Advances and Emerging Directions" International Journal of Molecular Sciences 26, no. 23: 11268. https://doi.org/10.3390/ijms262311268
APA StyleGul, K., & Mumtaz, S. (2025). Nanosecond Pulsed Electric Fields (nsPEFs) for Precision Intracellular Oncotherapy: Recent Advances and Emerging Directions. International Journal of Molecular Sciences, 26(23), 11268. https://doi.org/10.3390/ijms262311268







