TGF-β Receptor Inhibitor SB431542 Enhanced the Sensitivity of Gastric Cancer to 5-Fluorouracil: New Combined Targeted Therapy
Abstract
1. Introduction
2. Results
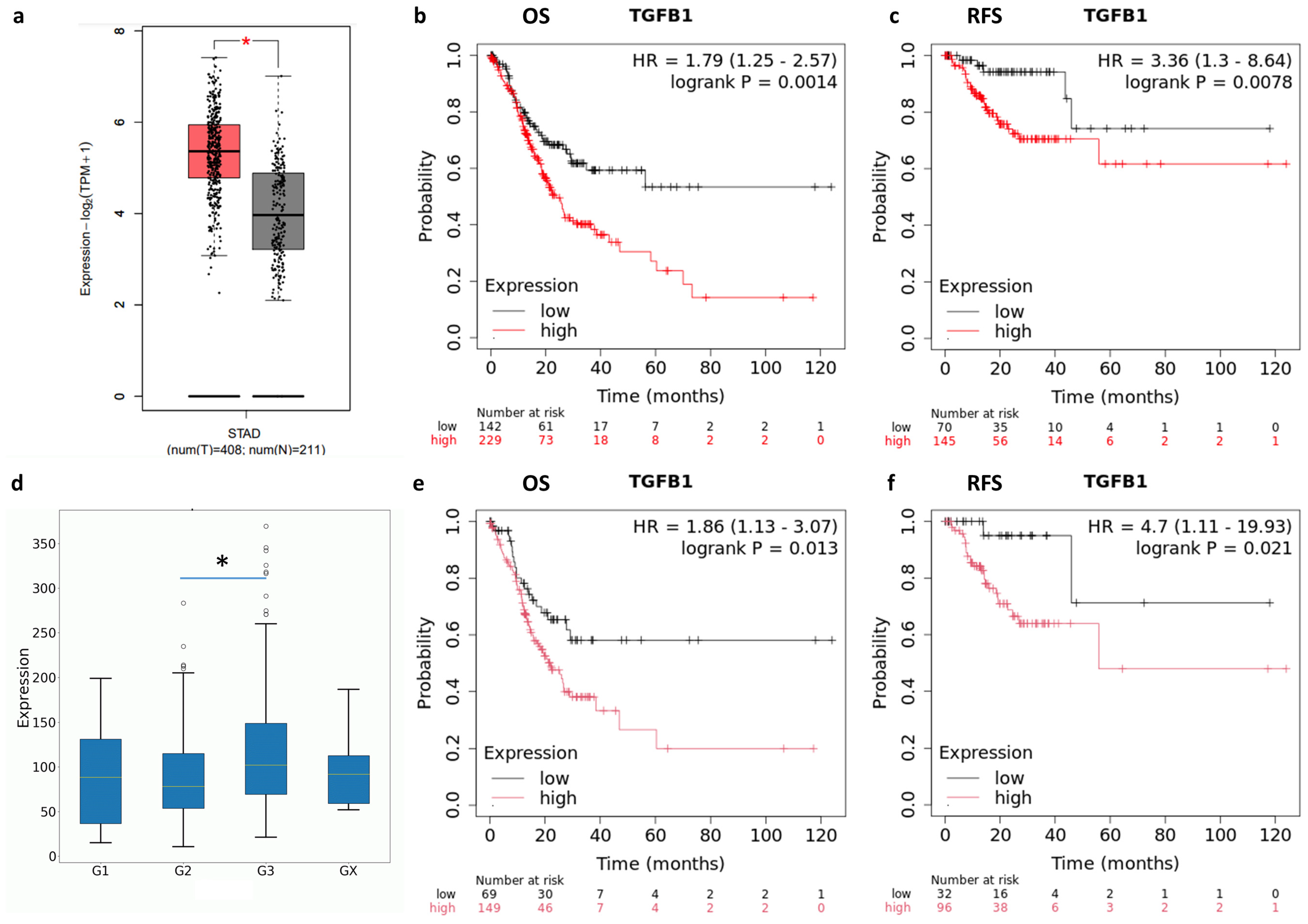
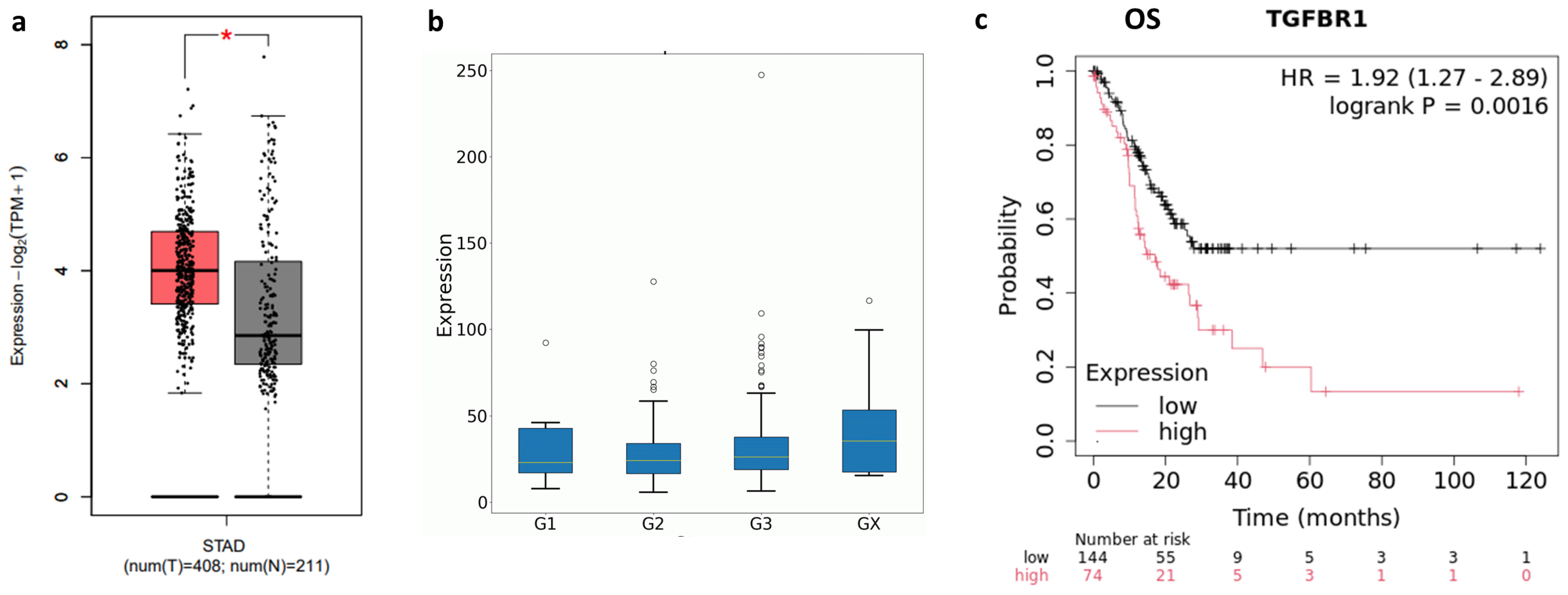
2.1. TGF-β and TGFBR1 Expression Correlate with Gastric Cancer Patients’ Prognosis
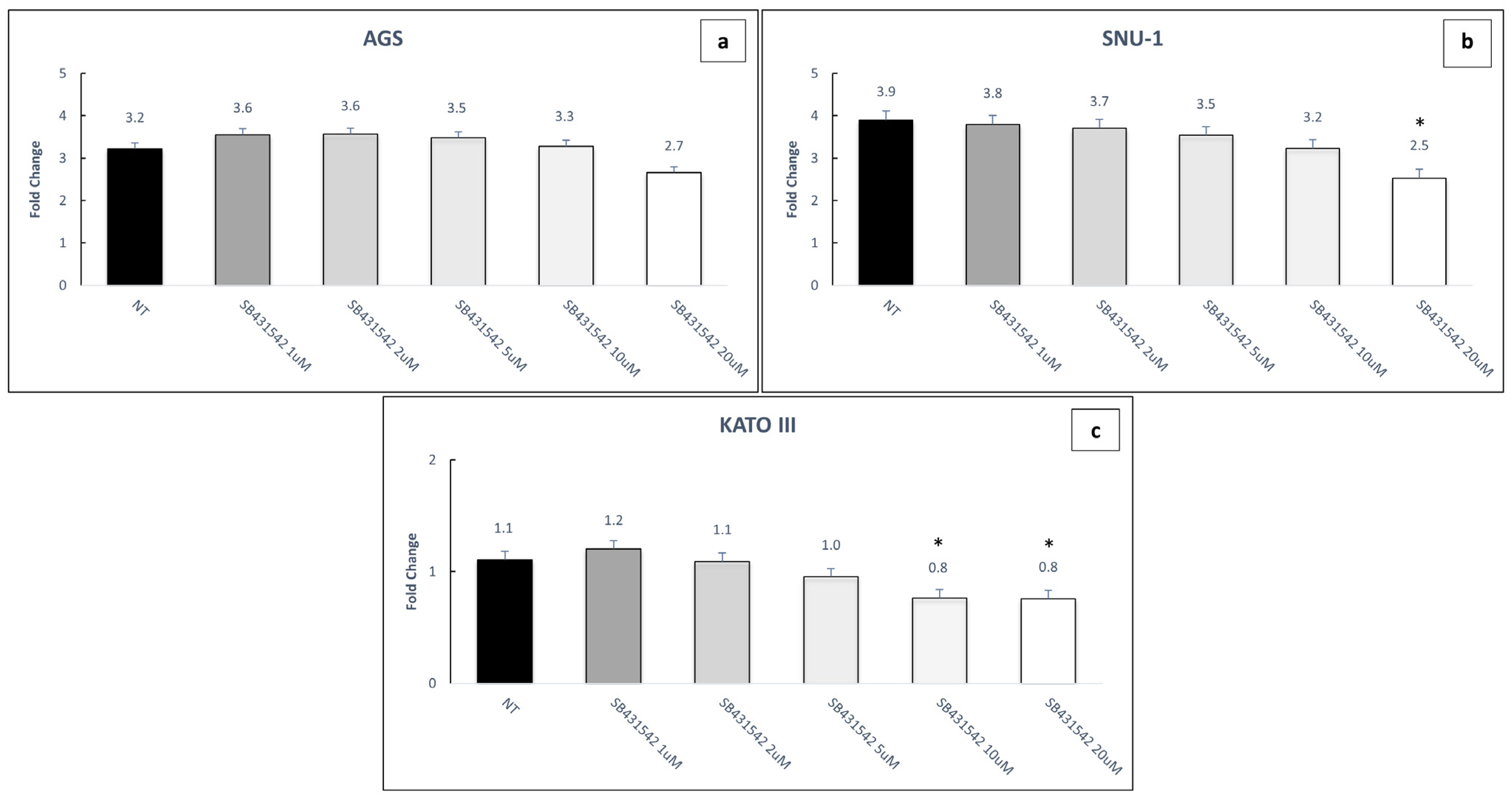
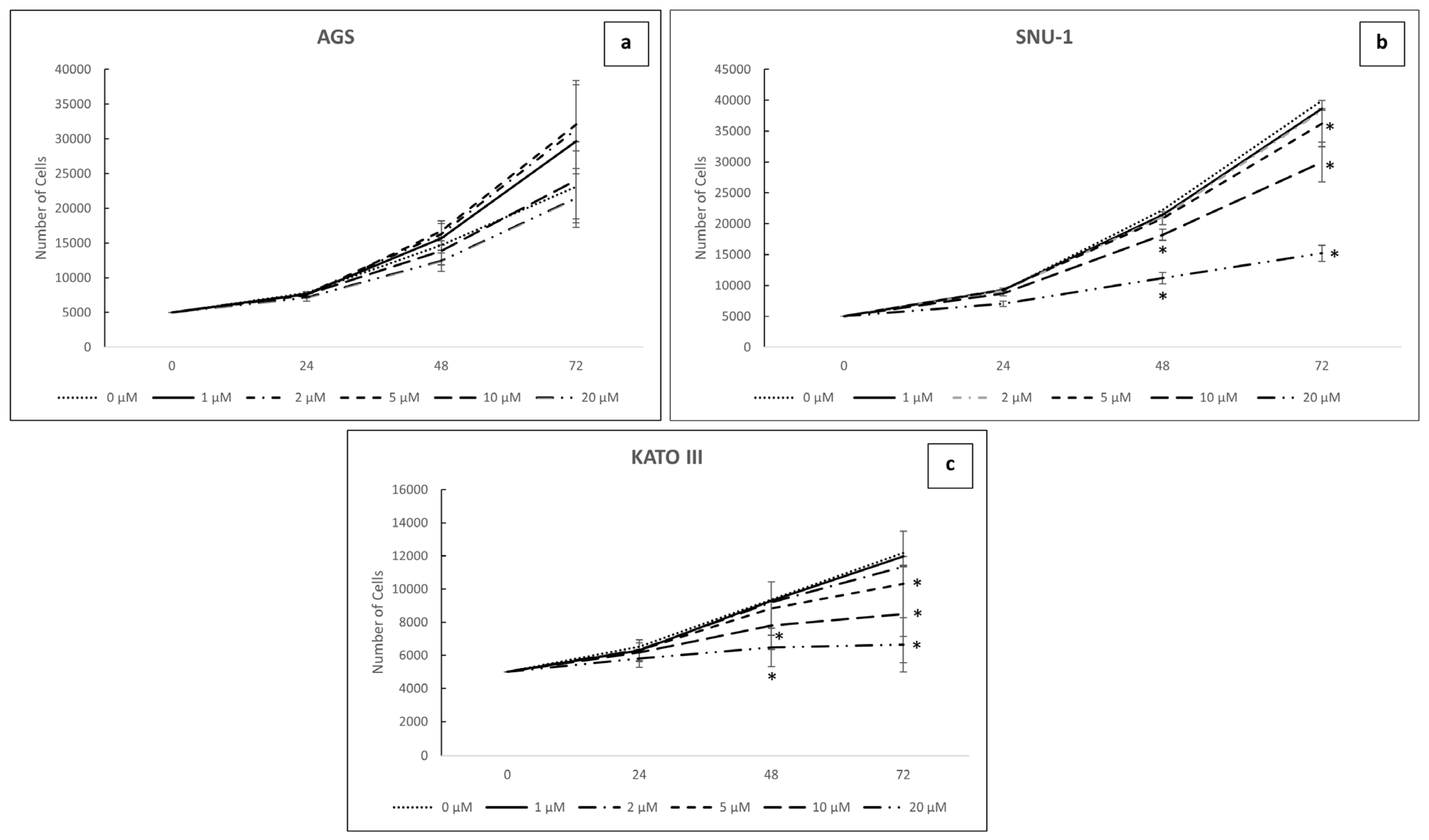
2.2. Inhibition of TGF-β Signaling Modulates Both Cell Viability and Proliferation in GC Cell Lines
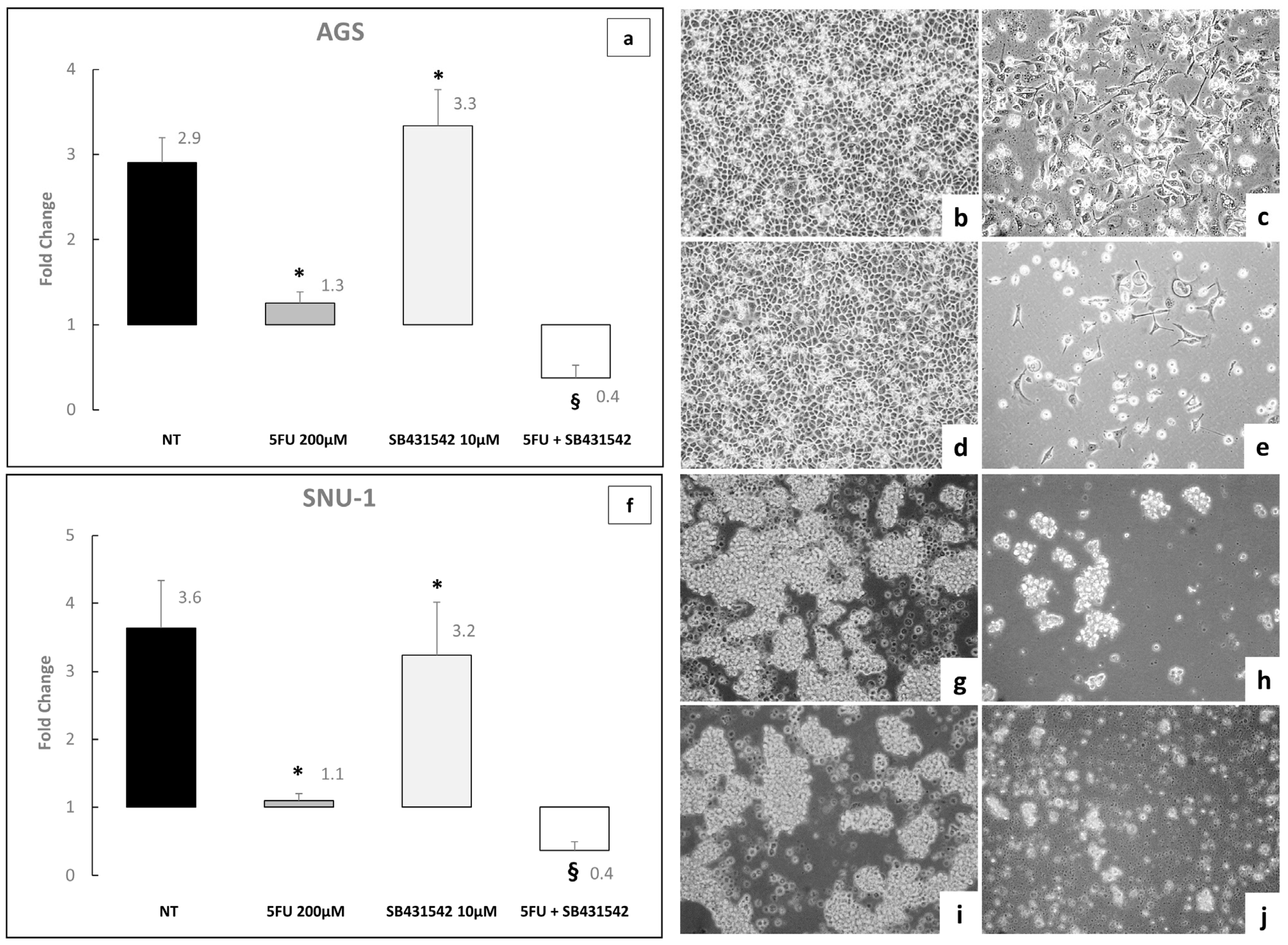
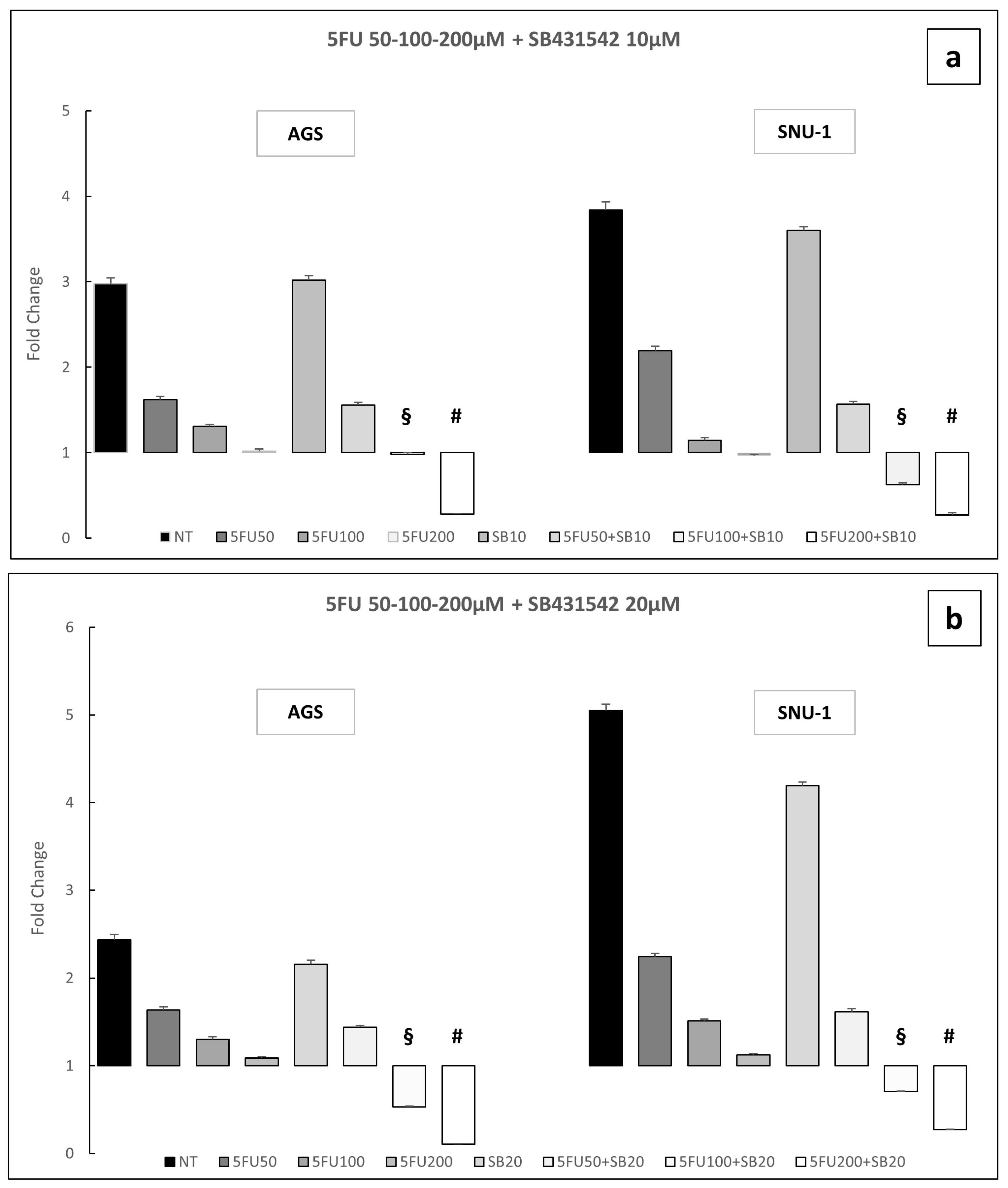
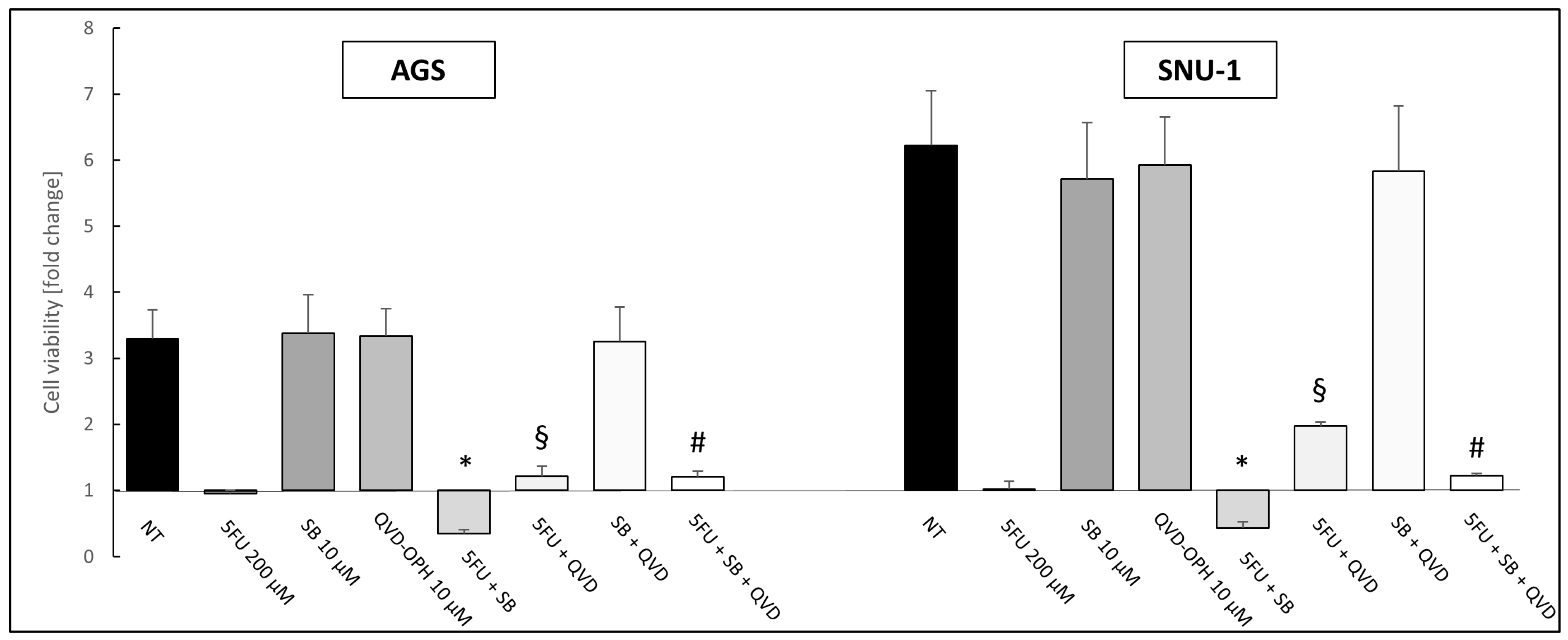
2.3. Inhibition of TGF-β Signaling Pathway Enhances the Toxic Effect of 5-Fluorouracil on AGS and SNU-1 Cell Lines
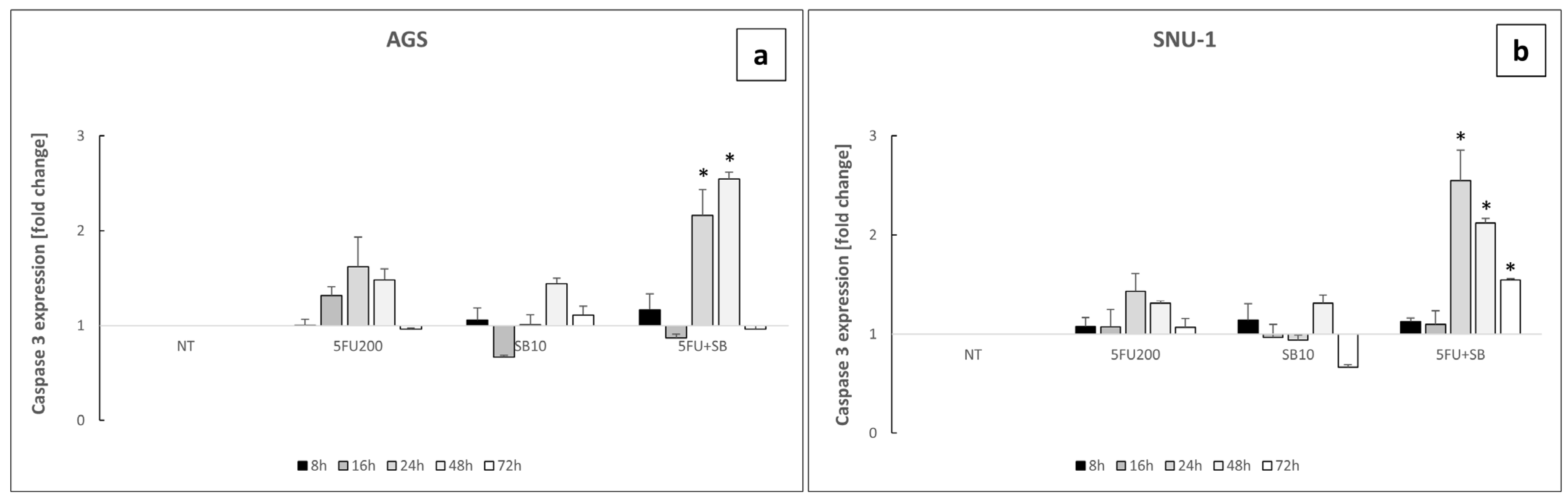
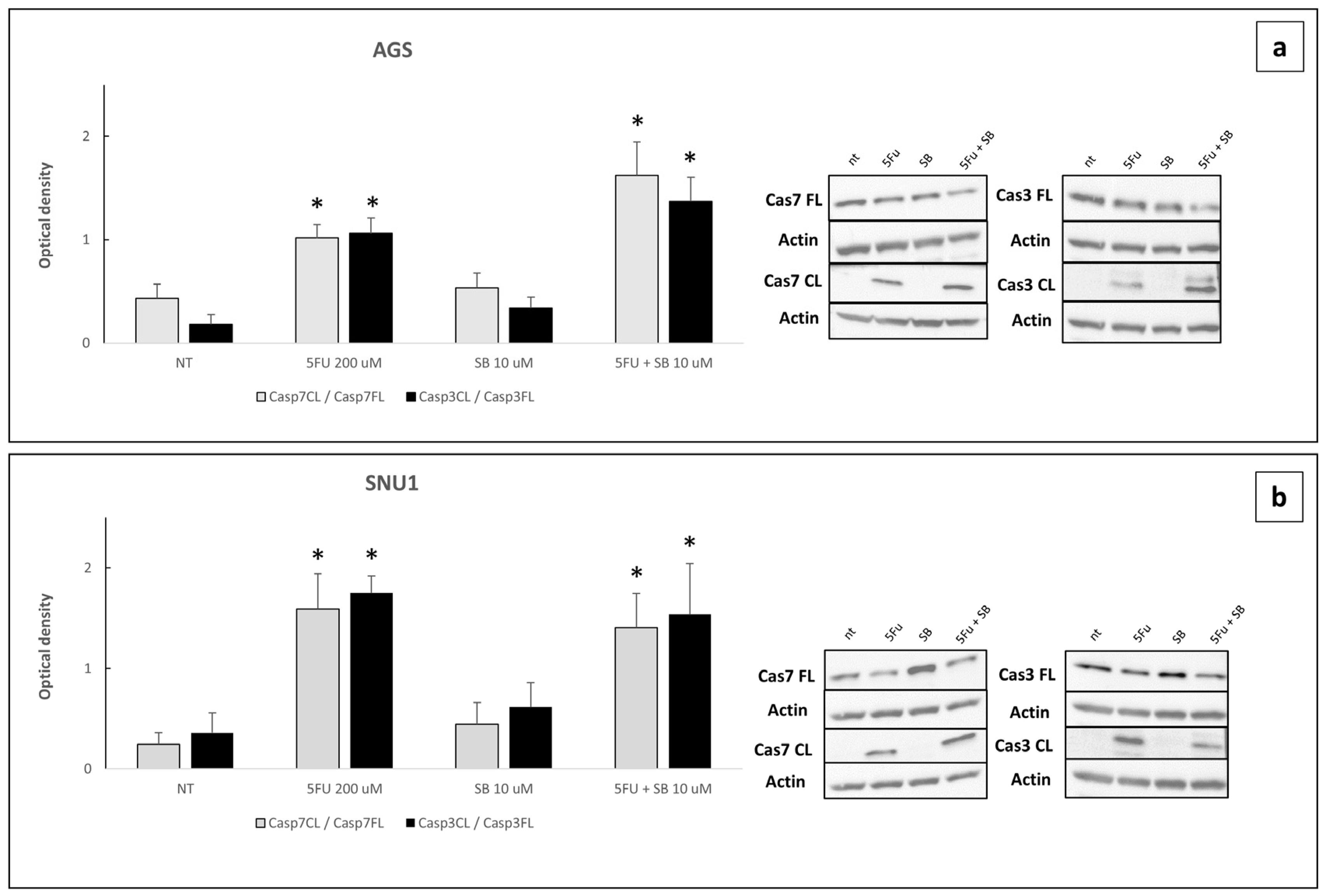
2.4. Cells Challenged with 5FU or 5FU + SB431542 Died in a Caspase-Dependent Way
2.5. FU + SB431542 Treatment Is More Effective in Affecting Clonogenicity of AGS Cells Compared to 5FU Alone
3. Discussion
4. Materials and Methods
4.1. Bioinformatics Analyses
4.2. Cell Lines
4.3. Cell Viability Experiment
4.4. Cell Growth/Proliferation Assay
4.5. Colony Assay
4.6. Caspase III–VII Assay
4.7. Western Blot Analyses
4.8. Q-VD-Oph Treatments
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5FU | 5-fluorouracil |
| GC | Gastric Cancer |
| OS | Overall Survival |
| RFS | Relapse Free Survival |
| TGF-β | Transforming Growth Factor β |
| TGFBR1 | Transforming Growth Factor β receptor 1 |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Karimi, P.; Islami, F.; Anandasabapathy, S.; Freedman, N.D.; Kamangar, F. Gastric cancer: Descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol. Biomark. Prev. 2014, 23, 700–713. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, R.; Niwa, Y.; Matsuura, T.; Maeda, O.; Ando, T.; Ohmiya, N.; Itoh, A.; Hirooka, Y.; Goto, H. Prevalence and prognosis of gastric cancer detected by screening in a large Japanese population: Data from a single institute over 30 years. J. Gastroenterol. Hepatol. 2007, 22, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Sano, T.; Coit, D.G.; Kim, H.H.; Roviello, F.; Kassab, P.; Wittekind, C.; Yamamoto, Y.; Ohashi, Y. Proposal of a new stage grouping of gastric cancer for TNM classification: International Gastric Cancer Association staging project. Gastric Cancer 2017, 20, 217–225. [Google Scholar] [CrossRef]
- Benassai, G.; Calemma, F.; Miletti, A.; Furino, E.; De Palma, G.D.; Quarto, G. Surgical treatment in late-stage gastric cancer. A retrospective analysis of 26 cases. Ann. Ital. Chir. 2021, 92, 20–27. [Google Scholar] [PubMed]
- Yamada, A.; Takeuchi, M.; Komatsu, K.; Bonate, P.L.; Poondru, S.; Yang, J. Population PK and Exposure-Response Analyses of Zolbetuximab in Patients With Locally Advanced Unresectable or Metastatic G/GEJ Adenocarcinoma. Clin. Transl. Sci. 2025, 18, e70280. [Google Scholar] [CrossRef]
- Sasako, M.; Sakuramoto, S.; Katai, H.; Kinoshita, T.; Furukawa, H.; Yamaguchi, T.; Nashimoto, A.; Fujii, M.; Nakajima, T.; Ohashi, Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J. Clin. Oncol. 2011, 29, 4387–4393. [Google Scholar] [CrossRef]
- Spiegel, D.; Palta, M.; Uronis, H. Role of Chemotherapy and Radiation Therapy in the Management of Gastric Adenocarcinoma. Surg. Clin. N. Am. 2017, 97, 421–435. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Y.; Sun, Y.; Tang, L.; Zhang, L.; Hu, Y.; He, M.; Li, Z.; Cheng, S.; Yuan, J.; et al. Predicting gastric cancer response to anti-HER2 therapy or anti-HER2 combined immunotherapy based on multi-modal data. Signal Transduct. Target. Ther. 2024, 9, 222. [Google Scholar] [CrossRef]
- Kim, B.G.; Li, C.; Qiao, W.; Mamura, M.; Kasprzak, B.; Anver, M.; Wolfraim, L.; Hong, S.; Mushinski, E.; Potter, M.; et al. Smad4 signalling in T cells is required for suppression of gastrointestinal cancer. Nature 2006, 441, 1015–1019, Erratum in Nature 2006, 444, 780. https://doi.org/10.1038/nature05421. [Google Scholar] [CrossRef]
- Batlle, E.; Massagué, J. Transforming Growth Factor-β Signaling in Immunity and Cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Zhou, Y.; Bai, X.; Che, W.; Zhang, W.; Zhang, D.; Chen, Q.; Duan, W.; Nie, G.; Hou, Y. The VEGFA-Induced MAPK-AKT/PTEN/TGFβ Signal Pathway Enhances Progression and MDR in Gastric Cancer. Genes 2024, 15, 1266. [Google Scholar] [CrossRef] [PubMed]
- Vander Ark, A.; Cao, J.; Li, X. TGF-β receptors: In and beyond TGF-β signaling. Cell Signal 2018, 52, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhou, L.; Lin, S.; Li, Y.; Xiao, S.; Liu, J.; Li, Z.; Cui, Y.; Zhang, J. Metabolic profiles of gastric cancer cell lines with different degrees of differentiation. Int. J. Clin. Exp. Pathol. 2018, 11, 869–875. [Google Scholar]
- Mayer, B.; Klement, G.; Kaneko, M.; Man, S.; Jothy, S.; Rak, J.; Kerbel, R.S. Multicellular gastric cancer spheroids recapitulate growth pattern and differentiation phenotype of human gastric carcinomas. Gastroenterology 2001, 121, 839–852. [Google Scholar] [CrossRef]
- Shah, S.; Pocard, M.; Mirshahi, M. Targeting the differentiation of gastric cancer cells (KATO-III) downregulates epithelial-mesenchymal and cancer stem cell markers. Oncol. Rep. 2019, 42, 670–678. [Google Scholar] [CrossRef]
- Deng, Z.; Fan, T.; Xiao, C.; Tian, H.; Zheng, Y.; Li, C.; He, J. TGF-β signaling in health, disease, and therapeutics. Signal Transduct. Target. Ther. 2024, 9, 61. [Google Scholar] [CrossRef]
- Lan, X.Q.; Deng, C.J.; Wang, Q.Q.; Zhao, L.M.; Jiao, B.W.; Xiang, Y. The role of TGF-beta signaling in muscle atrophy, sarcopenia and cancer cachexia. Gen. Comp. Endocrinol. 2024, 353, 114513. [Google Scholar] [CrossRef]
- Tian, M.; Schiemann, W.P. The TGF-beta paradox in human cancer: An update. Future Oncol. 2009, 5, 259–271. [Google Scholar] [CrossRef]
- Zou, A.; Lambert, D.; Yeh, H.; Yasukawa, K.; Behbod, F.; Fan, F.; Cheng, N. Elevated CXCL1 expression in breast cancer stroma predicts poor prognosis and is inversely associated with expression of TGF-beta signaling proteins. BMC Cancer 2014, 14, 781. [Google Scholar] [CrossRef]
- Han, S.; Wang, Y.; Shi, X.; Zong, L.; Liu, L.; Zhang, J.; Qian, Q.; Jin, J.; Ma, Y.; Cui, B.; et al. Negative roles of B7-H3 and B7-H4 in the microenvironment of cervical cancer. Exp. Cell Res. 2018, 371, 222–230. [Google Scholar] [CrossRef]
- Newsted, D.; Banerjee, S.; Watt, K.; Nersesian, S.; Truesdell, P.; Blazer, L.L.; Cardarelli, L.; Adams, J.J.; Sidhu, S.S.; Craig, A.W. Blockade of TGF-beta signaling with novel synthetic antibodies limits immune exclusion and improves chemotherapy response in metastatic ovarian cancer models. Oncoimmunology 2018, 8, e1539613. [Google Scholar] [CrossRef]
- Quan, Q.; Zhong, F.; Wang, X.; Chen, K.; Guo, L. PAR2 Inhibition Enhanced the Sensitivity of Colorectal Cancer Cells to 5-FU and Reduced EMT Signaling. Oncol. Res. 2019, 27, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Hashemzehi, M.; Yavari, N.; Rahmani, F.; Asgharzadeh, F.; Soleimani, A.; Shakour, N.; Avan, A.; Hadizadeh, F.; Fakhraie, M.; Marjaneh, R.M.; et al. Inhibition of transforming growth factor-beta by Tranilast reduces tumor growth and ameliorates fibrosis in colorectal cancer. EXCLI J. 2021, 20, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.H.; Filippi, C.; Sun, S.; Lehec, S.; Dhawan, A.; Hughes, R.D. Hypoxic preconditioning potentiates the trophic effects of mesenchymal stem cells on co-cultured human primary hepatocytes. Stem Cell Res. Ther. 2015, 6, 237. [Google Scholar] [CrossRef]
- Meng, X.M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-beta: The master regulator of fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.L.; Li, Z.; Lv, C.M.; Wang, W. MiR-187 influences cisplatin-resistance of gastric cancer cells through regulating the TGF-beta/Smad signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9907–9914. [Google Scholar] [CrossRef]
- Beckwith, M.; Ruscetti, F.W.; Sing, G.K.; Urba, W.J.; Longo, D.L. Anti-IgM induces transforming growth factor-beta sensitivity in a human B-lymphoma cell line: Inhibition of growth is associated with a downregulation of mutant p53. Blood 1995, 85, 2461–2470. [Google Scholar] [CrossRef]
- Colak, S.; Ten Dijke, P. Targeting TGF-beta Signaling in Cancer. Trends Cancer 2017, 3, 56–71. [Google Scholar] [CrossRef]
- Hussain, S.M.; Kansal, R.G.; Alvarez, M.A.; Hollingsworth, T.J.; Elahi, A.; Miranda-Carboni, G.; Hendrick, L.E.; Pingili, A.K.; Albritton, L.M.; Dickson, P.V.; et al. Role of TGF-beta in pancreatic ductal adenocarcinoma progression and PD-L1 expression. Cell Oncol. 2021, 44, 673–687. [Google Scholar] [CrossRef]
- Hao, Y.; Baker, D.; Ten Dijke, P. TGF-beta-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis. Int. J. Mol. Sci. 2019, 20, 2767. [Google Scholar] [CrossRef] [PubMed]
- Siegel, P.M.; Massague, J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat. Rev. Cancer 2003, 3, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Moraveji, S.F.; Esfandiari, F.; Taleahmad, S.; Nikeghbalian, S.; Sayahpour, F.A.; Masoudi, N.S.; Shahverdi, A.; Baharvand, H. Suppression of transforming growth factor-beta signaling enhances spermatogonial proliferation and spermatogenesis recovery following chemotherapy. Hum. Reprod. 2019, 34, 2430–2442. [Google Scholar] [CrossRef]
- Alyoussef, A. Blocking TGF-β type 1 receptor partially reversed skin tissue damage in experimentally induced atopic dermatitis in mice. Cytokine 2018, 106, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Ferrão, P.M.; Nisimura, L.M.; Moreira, O.C.; Land, M.G.; Pereira, M.C.; de Mendonça-Lima, L.; Araujo-Jorge, T.C.; Waghabi, M.C.; Garzoni, L.R. Inhibition of TGF-β pathway reverts extracellular matrix remodeling in T. cruzi-infected cardiac spheroids. Exp Cell Res. 2018, 362, 260–267. [Google Scholar] [CrossRef]
- Ilson, D.H. Advances in the treatment of gastric cancer. Curr. Opin. Gastroenterol. 2018, 34, 465–468. [Google Scholar] [CrossRef]
- de Sa, C.E.C.; de Iracema Gomes Cubero, D.; da Costa, R.E.A.R.; di Pardo, R.; Cruz, F.J.S.M.; de Melo Sette, C.V.; da Silva, E.A.; del Giglio, A. Induction Therapy with Cisplatin, 5-Fluorouracil, Leucovorin, and Paclitaxel in Patients with Head and Neck Cancer. In Vivo 2023, 37, 1339–1345. [Google Scholar] [CrossRef]
- Matt, P.; van Zwieten-Boot, B.; Calvo Rojas, G.; Ter Hofstede, H.; Garcia-Carbonero, R.; Camarero, J.; Abadie, E.; Pignatti, F. The European Medicines Agency review of Tegafur/Gimeracil/Oteracil (Teysuno™) for the treatment of advanced gastric cancer when given in combination with cisplatin: Summary of the Scientific Assessment of the Committee for medicinal products for human use (CHMP). Oncologist 2011, 16, 1451–1457. [Google Scholar] [CrossRef]
- Lavitrano, M.; Ianzano, L.; Bonomo, S.; Cialdella, A.; Cerrito, M.G.; Pisano, F.; Missaglia, C.; Giovannoni, R.; Romano, G.; McLean, C.M.; et al. BTK inhibitors synergise with 5-FU to treat drug-resistant TP53-null colon cancers. J Pathol. 2020, 250, 134–147. [Google Scholar] [CrossRef]
- Teplitsky, J.E.; Vinokurtseva, A.; Armstrong, J.J.; Denstedt, J.; Liu, H.; Hutnik, C.M.L. ALK5 Inhibition of Subconjunctival Scarring From Glaucoma Surgery: Effects of SB-431542 Compared to Mitomycin C in Human Tenon’s Capsule Fibroblasts. Transl. Vis. Sci. Technol. 2023, 12, 31. [Google Scholar] [CrossRef]
- Maeda, S.; Hayashi, M.; Komiya, S.; Imamura, T.; Miyazono, K. Endogenous TGF-beta signaling suppresses maturation of osteoblastic mesenchymal cells. EMBO J. 2004, 23, 552–563. [Google Scholar] [CrossRef]
- Wang, X.; Eichhorn, P.J.A.; Thiery, J.P. TGF-beta, EMT, and resistance to anti-cancer treatment. Semin. Cancer Biol. 2023, 97, 1–11. [Google Scholar] [CrossRef]
- Killinger, M.; Veselá, B.; Procházková, M.; Matalová, E.; Klepárník, K. A single-cell analytical approach to quantify activated caspase-3/7 during osteoblast proliferation, differentiation, and apoptosis. Anal. Bioanal. Chem. 2021, 413, 5085–5093. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef] [PubMed]
- Győrffy, B. Integrated analysis of public datasets for the discovery and validation of survival-associated genes in solid tumors. Innovation 2024, 5, 100625. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Liu, X.; Cho, M.; Li, Y.; Tran, D.H.; Wang, X. Pan-cancer discovery of somatic mutations from RNA sequencing data. Commun. Biol. 2024, 7, 619. [Google Scholar] [CrossRef]
- Inman, G.J.; Nicolás, F.J.; Callahan, J.F.; Harling, J.D.; Gaster, L.M.; Reith, A.D.; Laping, N.J.; Hill, C.S. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol. Pharmacol. 2002, 62, 65–74. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonomo, S.; Giovannoni, R.; Lavitrano, M.; Cadamuro, M.; Conconi, D. TGF-β Receptor Inhibitor SB431542 Enhanced the Sensitivity of Gastric Cancer to 5-Fluorouracil: New Combined Targeted Therapy. Int. J. Mol. Sci. 2025, 26, 11250. https://doi.org/10.3390/ijms262311250
Bonomo S, Giovannoni R, Lavitrano M, Cadamuro M, Conconi D. TGF-β Receptor Inhibitor SB431542 Enhanced the Sensitivity of Gastric Cancer to 5-Fluorouracil: New Combined Targeted Therapy. International Journal of Molecular Sciences. 2025; 26(23):11250. https://doi.org/10.3390/ijms262311250
Chicago/Turabian StyleBonomo, Sara, Roberto Giovannoni, Marialuisa Lavitrano, Massimiliano Cadamuro, and Donatella Conconi. 2025. "TGF-β Receptor Inhibitor SB431542 Enhanced the Sensitivity of Gastric Cancer to 5-Fluorouracil: New Combined Targeted Therapy" International Journal of Molecular Sciences 26, no. 23: 11250. https://doi.org/10.3390/ijms262311250
APA StyleBonomo, S., Giovannoni, R., Lavitrano, M., Cadamuro, M., & Conconi, D. (2025). TGF-β Receptor Inhibitor SB431542 Enhanced the Sensitivity of Gastric Cancer to 5-Fluorouracil: New Combined Targeted Therapy. International Journal of Molecular Sciences, 26(23), 11250. https://doi.org/10.3390/ijms262311250







