The Fer Tyrosine Kinase Mediates EGFR Activation in Sperm Capacitation
Abstract
1. Introduction
2. Results
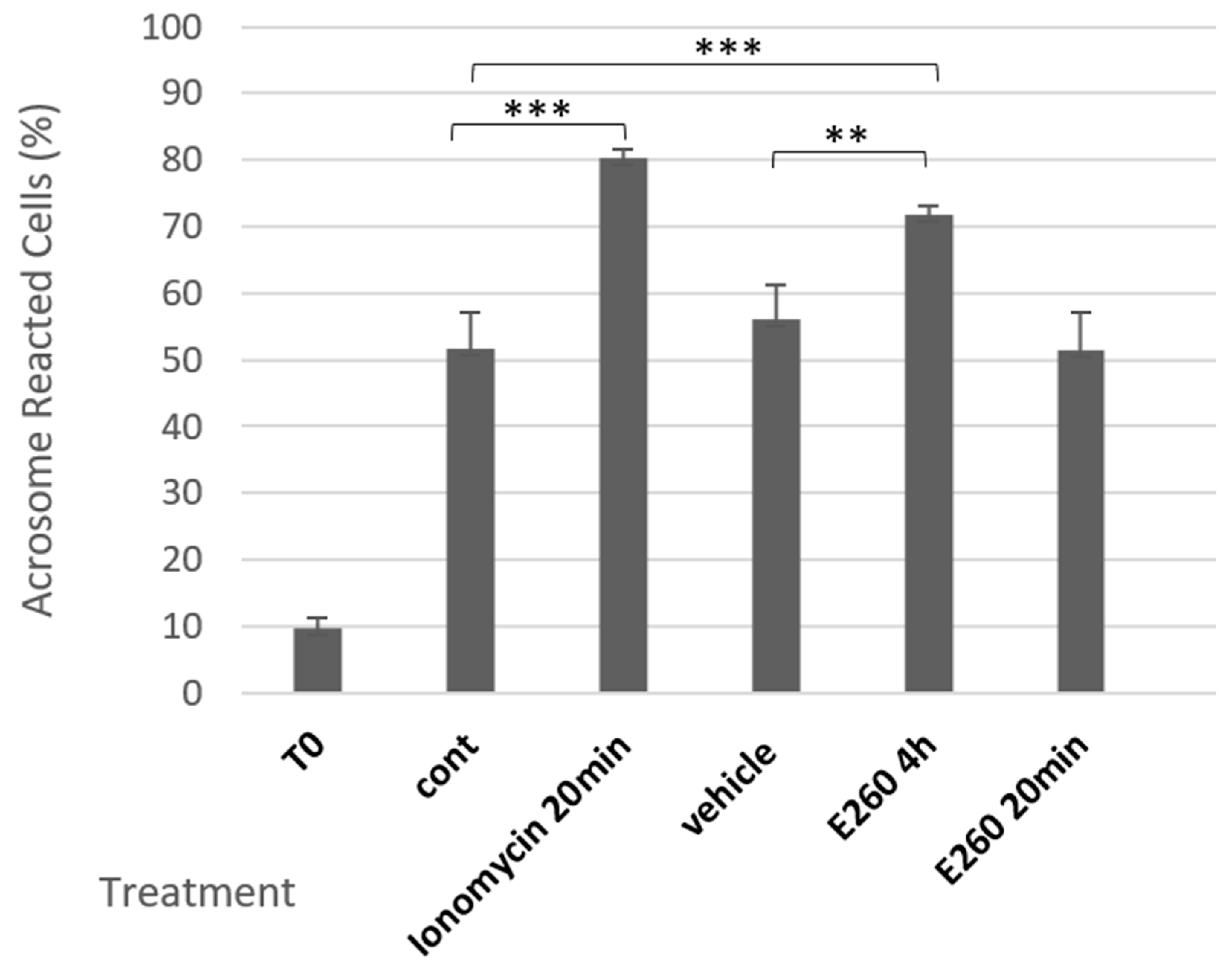
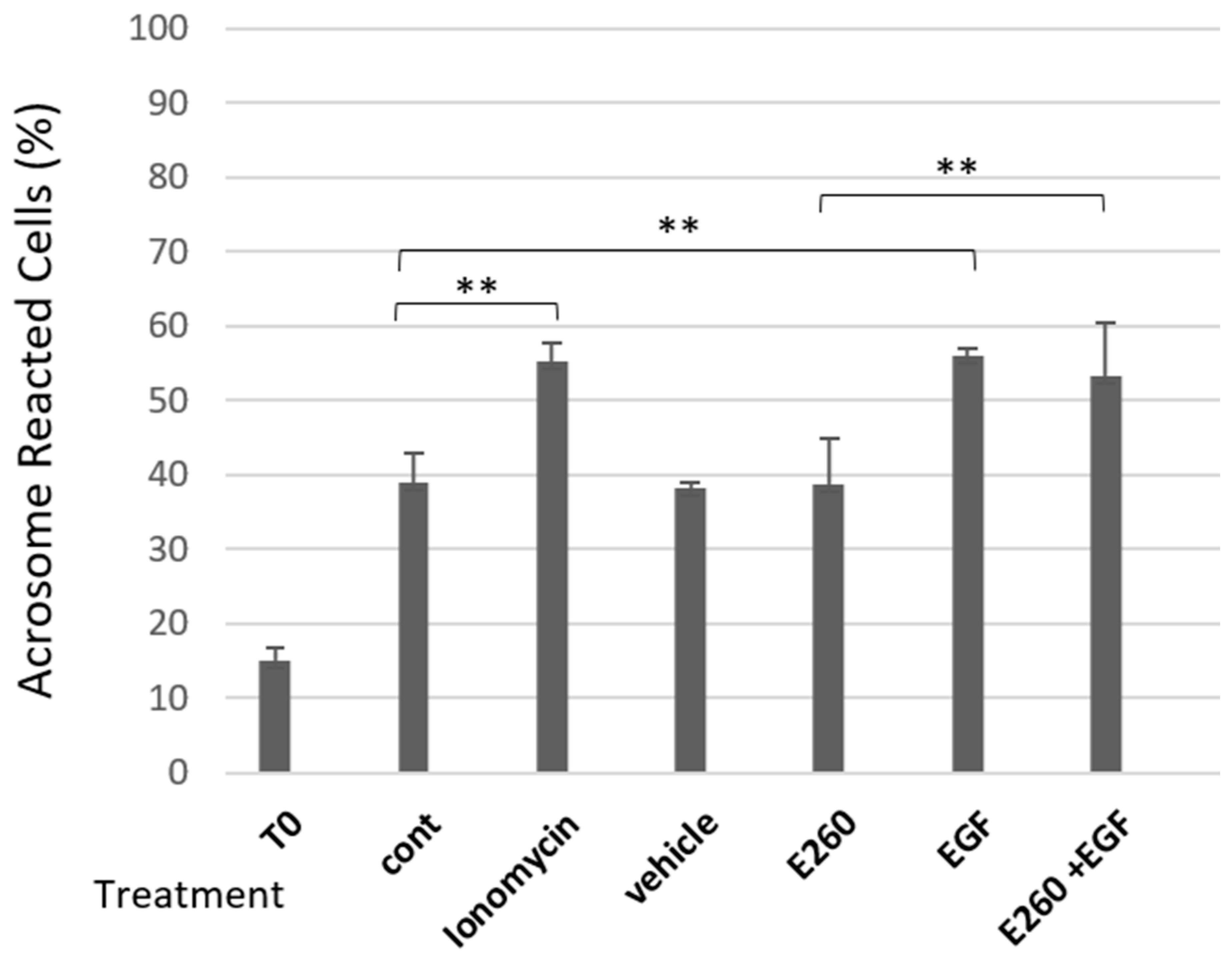
2.1. Fer Inhibition Enhances Spontaneous but Not Induced Acrosomal Reaction
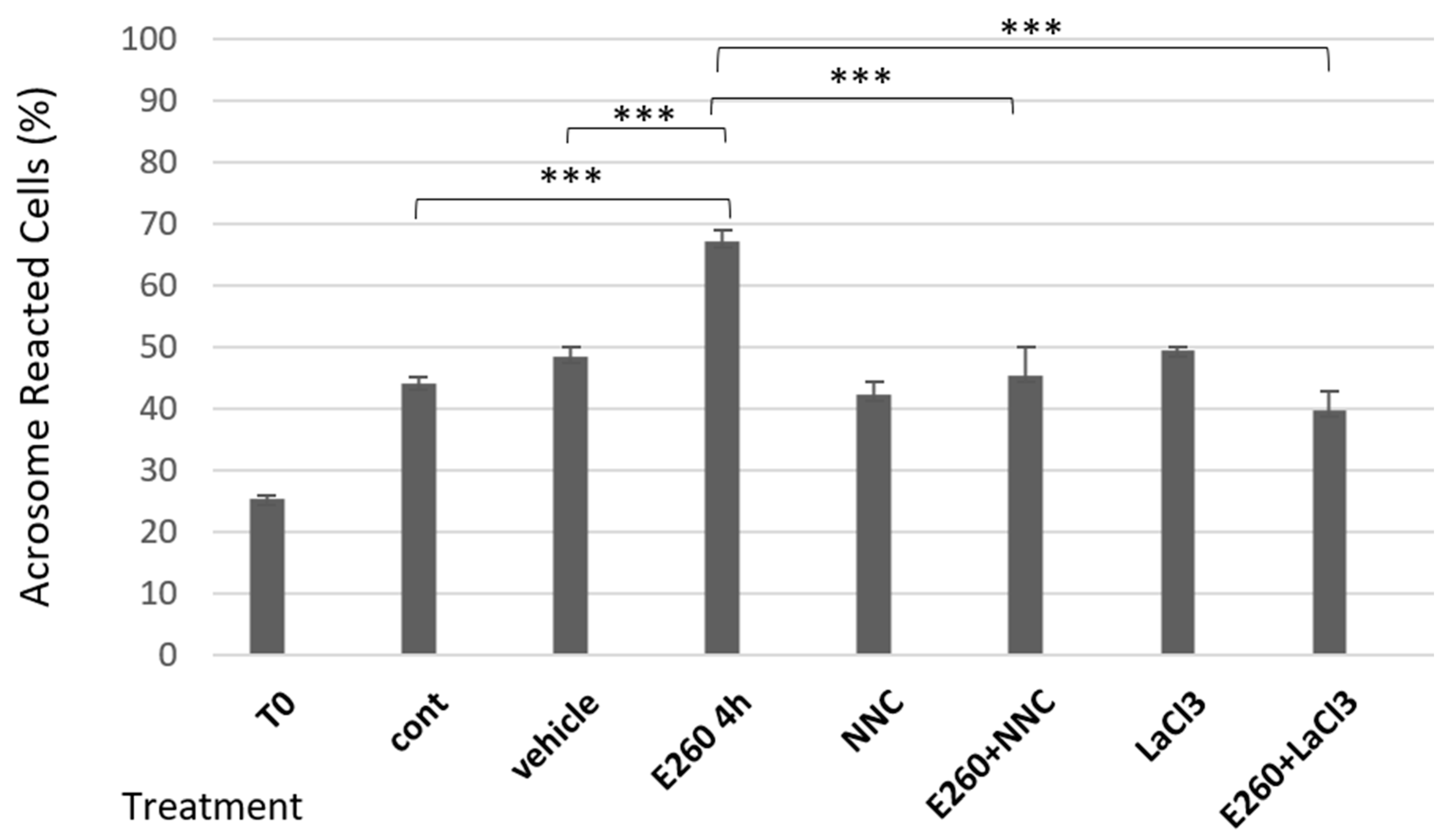
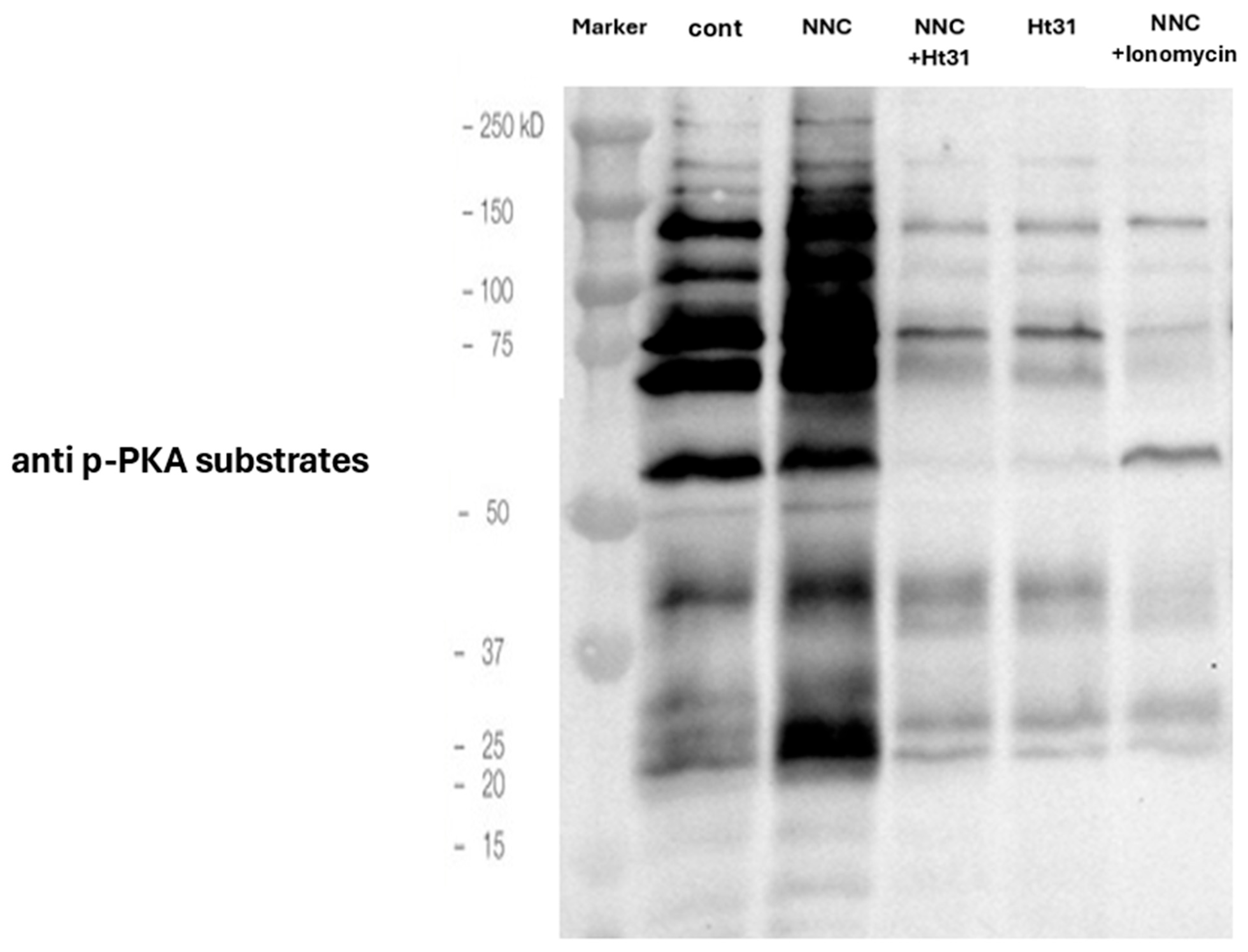
2.2. Involvement of Calcium Channels in the Spontaneous Acrosomal Reaction Induced by Fer Inhibition
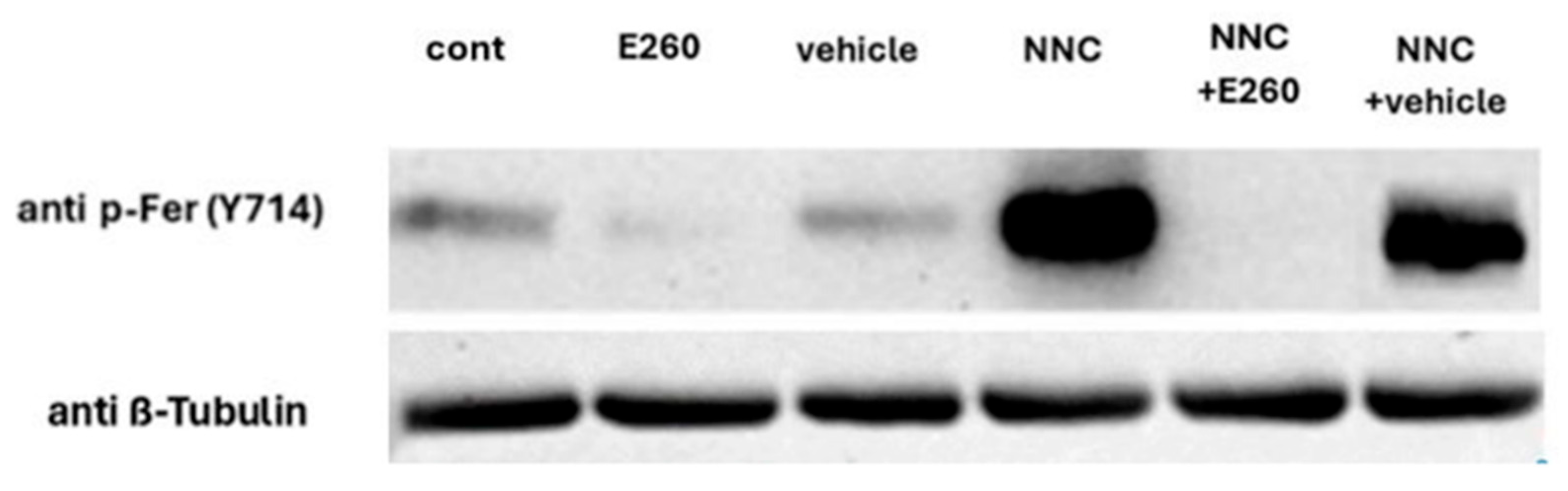
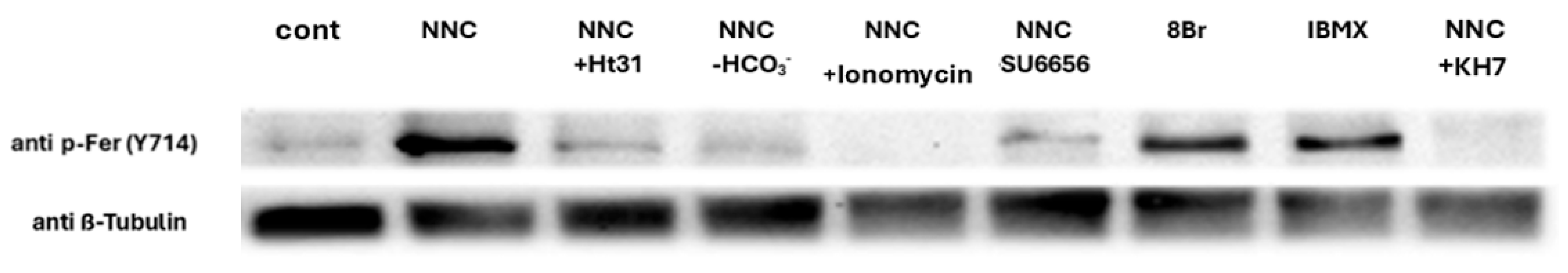
2.3. CatSper Inhibition Enhances Fer Phosphorylation/Activation
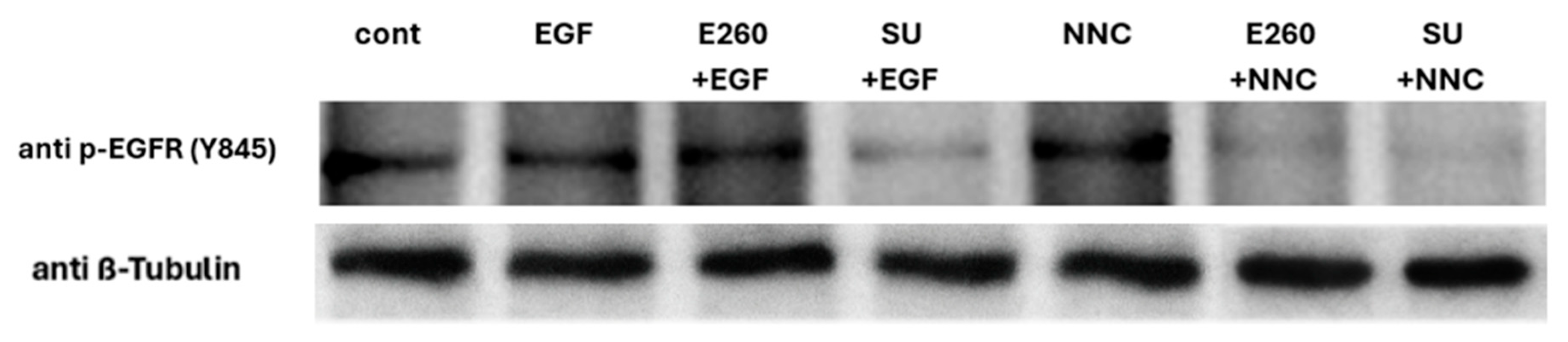
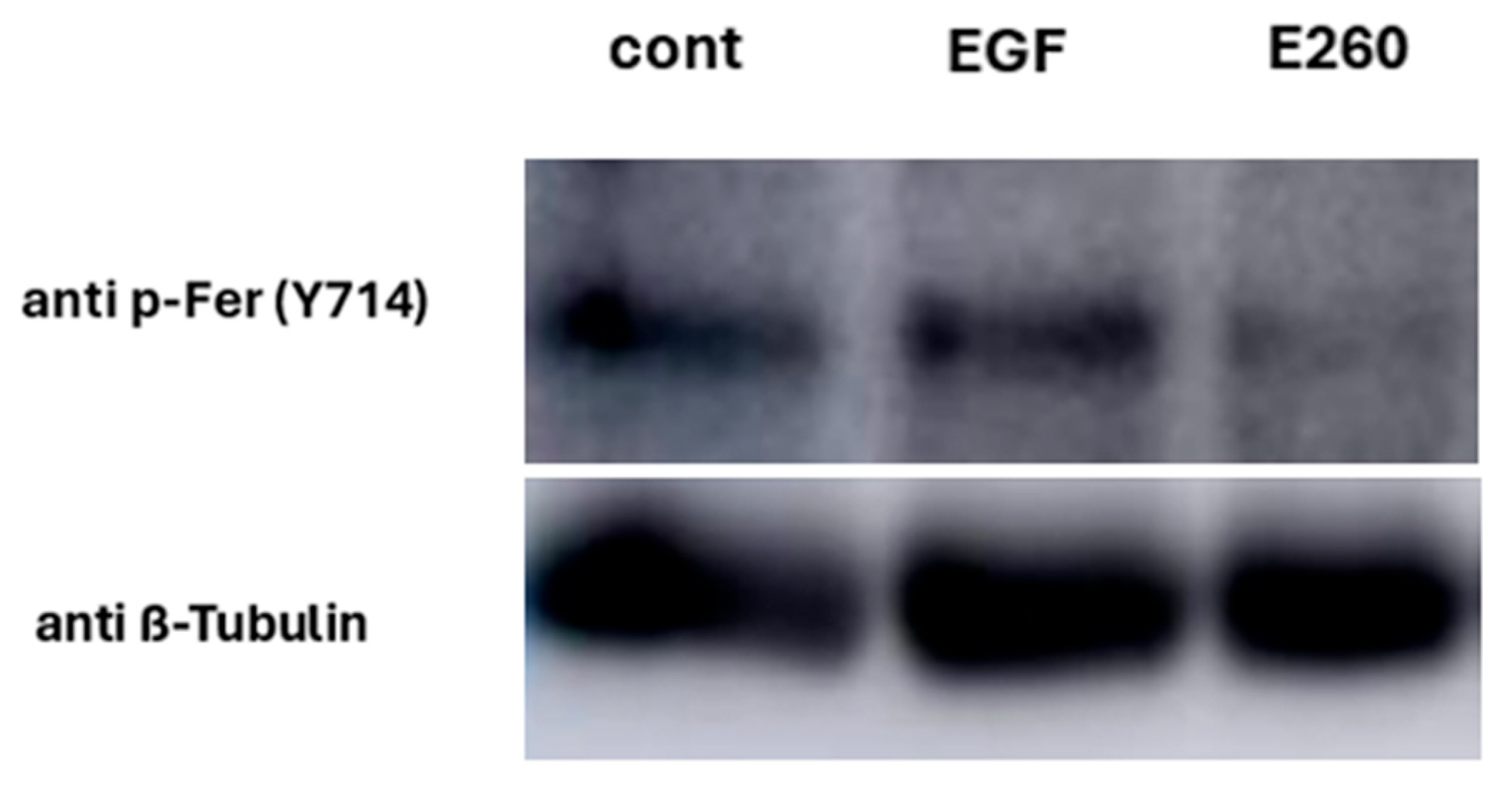
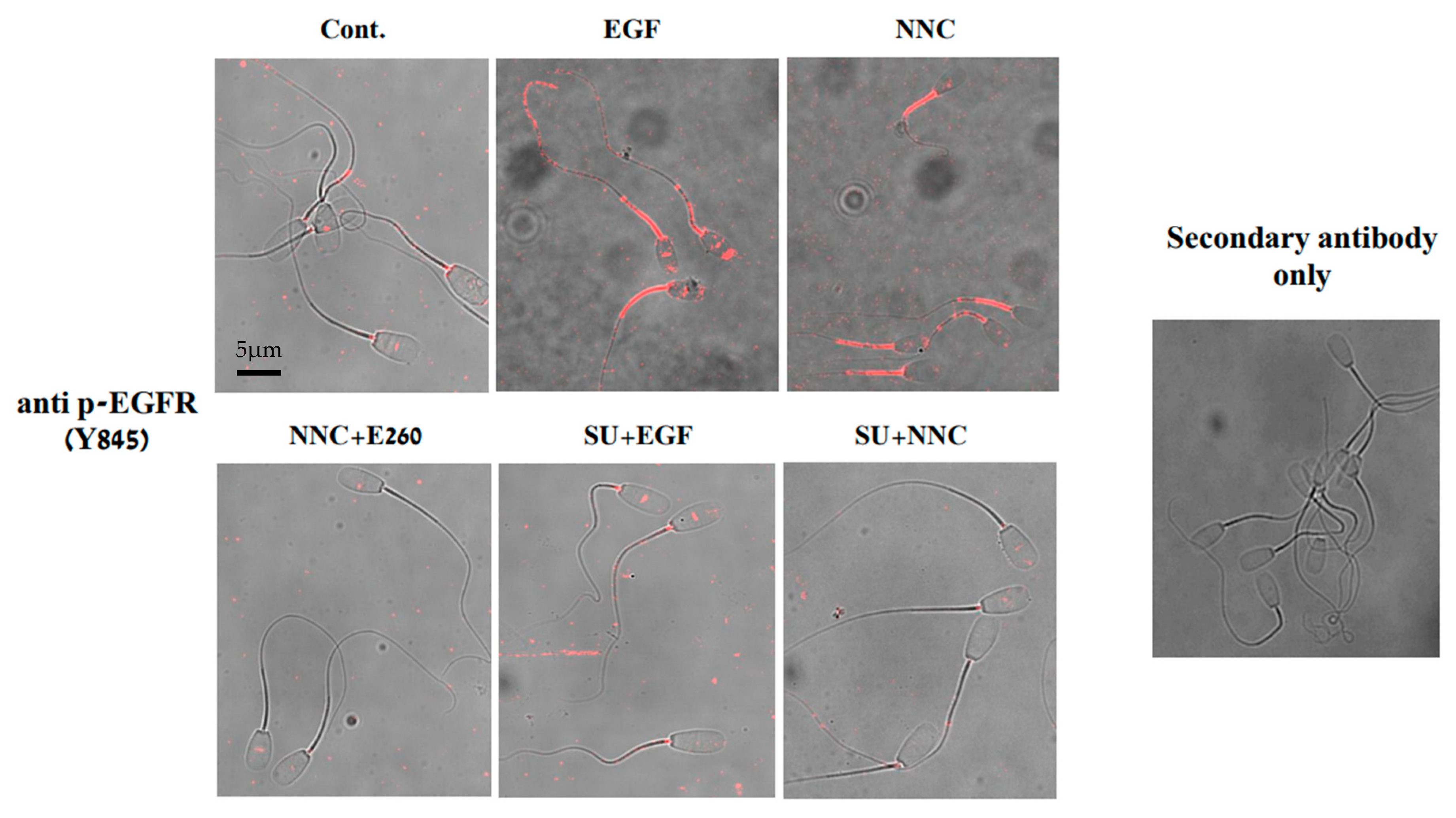
2.4. Studying the Regulatory Role of Fer in EGFR Activity
2.5. Inhibition of Fer Reduces IVF Rate
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Sperm Preparation
4.3. Assessment of Acrosome Reaction
4.4. Immunoblot Analysis
4.5. IVF Determination
4.6. Immunocytochemical Staining
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yanagimachi, R. Fertility of mammalian spermatozoa: Its development and relativity. Zygote 1994, 2, 371–372. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, F.; Zitranski, N.; Borth, H.; Buech, T.; Gudermann, T.; Boekhoff, I. CaMKIIalpha interacts with multi-PDZ domain protein MUPP1 in spermatozoa and prevents spontaneous acrosomal exocytosis. J. Cell Sci. 2009, 122 Pt 24, 4547–4557. [Google Scholar] [CrossRef]
- Breitbart, H.; Grinshtein, E. Mechanisms That Protect Mammalian Sperm from the Spontaneous Acrosome Reaction. Int. J. Mol. Sci. 2023, 24, 17005. [Google Scholar] [CrossRef]
- Sanchez-Cardenas, C.; Servin-Vences, M.R.; Jose, O.; Trevino, C.L.; Hernandez-Cruz, A.; Darszon, A. Acrosome reaction and Ca2+ imaging in single human spermatozoa: New regulatory roles of [Ca2+]i. Biol. Reprod. 2014, 91, 67. [Google Scholar] [CrossRef]
- Mata-Martinez, E.; Darszon, A.; Trevino, C.L. pH-dependent Ca2+ oscillations prevent untimely acrosome reaction in human sperm. Biochem. Biophys. Res. Commun. 2018, 497, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Mata-Martinez, E.; Sanchez-Cardenas, C.; Chavez, J.C.; Guerrero, A.; Trevino, C.L.; Corkidi, G.; Montoya, F.; Hernandez-Herrera, P.; Buffone, M.G.; Balestrini, P.A.; et al. Role of calcium oscillations in sperm physiology. Biosystems 2021, 209, 104524. [Google Scholar] [CrossRef]
- Itoh, T.; Hasegawa, J.; Tsujita, K.; Kanaho, Y.; Takenawa, T. The tyrosine kinase Fer is a downstream target of the PLD-PA pathway that regulates cell migration. Sci. Signal. 2009, 2, ra52. [Google Scholar] [CrossRef]
- Grinshtain, E.; Shpungin, S.; Baum, M.; Nir, U.; Breitbart, H. The Fer tyrosine kinase protects sperm from spontaneous acrosome reaction. Dev. Biol. 2022, 487, 24–33. [Google Scholar] [CrossRef]
- Elkis, Y.; Cohen, M.; Yaffe, E.; Satmary-Tusk, S.; Feldman, T.; Hikri, E.; Nyska, A.; Feiglin, A.; Ofran, Y.; Shpungin, S.; et al. A novel Fer/FerT targeting compound selectively evokes metabolic stress and necrotic death in malignant cells. Nat. Commun. 2017, 8, 940. [Google Scholar] [CrossRef]
- Schrier, I.; Slotki-Itzchakov, O.; Elkis, Y.; Most-Menachem, N.; Adato, O.; Fitoussi-Allouche, D.; Shpungin, S.; Unger, R.; Nir, U. Fer governs mTORC1 regulating pathways and sustains viability of pancreatic ductal adenocarcinoma cells. Front. Oncol. 2024, 14, 1427029. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Han, Y.; Lu, T.; Yuan, D.; Lu, K.; Cai, Y.; Zhou, X.; Wang, X. Exosome-transported FER inhibitor suppresses progression of diffuse large B-cell lymphoma via regulating AJUBA/Hippo axis. npj Precis. Oncol. 2025, 9, 258. [Google Scholar] [CrossRef]
- O’Mara, M.; Zhang, S.; Knaus, U.G. Spatiotemporal H(2)O(2) flashes coordinate actin cytoskeletal remodeling and regulate cell migration and wound healing. Nat. Commun. 2025, 16, 6868. [Google Scholar] [CrossRef]
- Ren, D.; Xia, J. Calcium signaling through CatSper channels in mammalian fertilization. Physiology 2010, 25, 165–175. [Google Scholar] [CrossRef]
- Biscardi, J.S.; Maa, M.C.; Tice, D.A.; Cox, M.E.; Leu, T.H.; Parsons, S.J. c-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J. Biol. Chem. 1999, 274, 8335–8343. [Google Scholar] [CrossRef]
- Etkovitz, N.; Tirosh, Y.; Chazan, R.; Jaldety, Y.; Daniel, L.; Rubinstein, S.; Breitbart, H. Bovine sperm acrosome reaction induced by G-protein-coupled receptor agonists is mediated by epidermal growth factor receptor transactivation. Dev. Biol. 2009, 334, 447–457. [Google Scholar] [CrossRef]
- Yaffe, E.; Hikri, E.; Elkis, Y.; Cohen, O.; Segal, A.; Makovski, A.; Varvak, A.; Shpungin, S.; Nir, U. Oncogenic properties of a spermatogenic meiotic variant of fer kinase expressed in somatic cells. Cancer Res. 2014, 74, 6474–6485. [Google Scholar] [CrossRef] [PubMed]
- Alvau, A.; Battistone, M.A.; Gervasi, M.G.; Navarrete, F.A.; Xu, X.; Sanchez-Cardenas, C.; De la Vega-Beltran, J.L.; Da Ros, V.G.; Greer, P.A.; Darszon, A.; et al. The tyrosine kinase FER is responsible for the capacitation-associated increase in tyrosine phosphorylation in murine sperm. Development 2016, 143, 2325–2333. [Google Scholar] [CrossRef] [PubMed]
- Wiser, A.; Sachar, S.; Ghetler, Y.; Shulman, A.; Breitbart, H. Assessment of sperm hyperactivated motility and acrosome reaction can discriminate the use of spermatozoa for conventional in vitro fertilisation or intracytoplasmic sperm injection: Preliminary results. Andrologia 2014, 46, 313–315. [Google Scholar] [CrossRef]
- Visconti, P.E. Understanding the molecular basis of sperm capacitation through kinase design. Proc. Natl. Acad. Sci. USA 2009, 106, 667–668. [Google Scholar] [CrossRef] [PubMed]
- Visconti, P.E.; Bailey, J.L.; Moore, G.D.; Pan, D.; Olds-Clarke, P.; Kopf, G.S. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development 1995, 121, 1129–1137. [Google Scholar] [CrossRef]
- Craig, A.W.; Zirngibl, R.; Williams, K.; Cole, L.A.; Greer, P.A. Mice devoid of fer protein-tyrosine kinase activity are viable and fertile but display reduced cortactin phosphorylation. Mol. Cell Biol. 2001, 21, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.; Wong, T.W. Growth factor-dependent phosphorylation of the actin-binding protein cortactin is mediated by the cytoplasmic tyrosine kinase FER. J. Biol. Chem. 1998, 273, 23542–23548. [Google Scholar] [CrossRef]
- Uruno, T.; Liu, J.; Zhang, P.; Fan, Y.; Egile, C.; Li, R.; Mueller, S.C.; Zhan, X. Activation of Arp2/3 complex-mediated actin polymerization by cortactin. Nat. Cell Biol. 2001, 3, 259–266. [Google Scholar] [CrossRef]
- Weaver, A.M.; Karginov, A.V.; Kinley, A.W.; Weed, S.A.; Li, Y.; Parsons, J.T.; Cooper, J.A. Cortactin promotes and stabilizes Arp2/3-induced actin filament network formation. Curr. Biol. 2001, 11, 370–374. [Google Scholar] [CrossRef]
- Alekhina, O.; Burstein, E.; Billadeau, D.D. Cellular functions of WASP family proteins at a glance. J. Cell Sci. 2017, 130, 2235–2241. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D. Regulation of actin filament assembly by Arp2/3 complex and formins. Annu. Rev. Biophys. Biomol. Struct. 2007, 36, 451–477. [Google Scholar] [CrossRef] [PubMed]
- Welch, M.D.; Mullins, R.D. Cellular control of actin nucleation. Annu. Rev. Cell Dev. Biol. 2002, 18, 247–288. [Google Scholar] [CrossRef]
- Miyata, H.; Oura, S.; Morohoshi, A.; Shimada, K.; Mashiko, D.; Oyama, Y.; Kaneda, Y.; Matsumura, T.; Abbasi, F.; Ikawa, M. SPATA33 localizes calcineurin to the mitochondria and regulates sperm motility in mice. Proc. Natl. Acad. Sci. USA 2021, 118, e2106673118. [Google Scholar] [CrossRef]
- Breitbart, H.; Etkovitz, N. Role and regulation of EGFR in actin remodeling in sperm capacitation and the acrosome reaction. Asian J. Androl. 2011, 13, 106–110. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Itach, S.B.; Finklestein, M.; Etkovitz, N.; Breitbart, H. Hyper-activated motility in sperm capacitation is mediated by phospholipase D-dependent actin polymerization. Dev. Biol. 2012, 362, 154–161. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.V.; Dogan, S.; Belser, L.E.; Kaya, A.; Topper, E.; Moura, A.; Thibaudeau, G.; Memili, E. Molecular morphology and function of bull spermatozoa linked to histones and associated with fertility. Reproduction 2013, 146, 263–272. [Google Scholar] [CrossRef] [PubMed]
| Treatment | Total Number of Oocytes | Number of 2-Cell Embryos | Fertilization Rate (%) | Inhibition (%) |
|---|---|---|---|---|
| Control | 98 | 78 | 80 ± 1.7 | - |
| Vehicle | 108 | 96 | 88 ± 2.0 | - |
| E260 | 121 | 27 | 22 ± 2.4 | 75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yemini-Talbi, O.; Nir, U.; Breitbart, H. The Fer Tyrosine Kinase Mediates EGFR Activation in Sperm Capacitation. Int. J. Mol. Sci. 2025, 26, 11218. https://doi.org/10.3390/ijms262211218
Yemini-Talbi O, Nir U, Breitbart H. The Fer Tyrosine Kinase Mediates EGFR Activation in Sperm Capacitation. International Journal of Molecular Sciences. 2025; 26(22):11218. https://doi.org/10.3390/ijms262211218
Chicago/Turabian StyleYemini-Talbi, Odeya, Uri Nir, and Haim Breitbart. 2025. "The Fer Tyrosine Kinase Mediates EGFR Activation in Sperm Capacitation" International Journal of Molecular Sciences 26, no. 22: 11218. https://doi.org/10.3390/ijms262211218
APA StyleYemini-Talbi, O., Nir, U., & Breitbart, H. (2025). The Fer Tyrosine Kinase Mediates EGFR Activation in Sperm Capacitation. International Journal of Molecular Sciences, 26(22), 11218. https://doi.org/10.3390/ijms262211218


_Kim.png)



