1. Introduction
Epilepsy is a heterogeneous neurological disease characterized by spontaneous recurrent seizures (SRS). The immediate cause of epilepsy is a pathological increase in the excitability of neuronal networks that regularly induces abnormal hyper synchronization of neuronal activity, resulting in seizures [
1].
Currently, about 1% of the world population suffers from this illness. Despite considerable efforts, the treatment of the disease largely remains symptomatic. In fact, existing antiepileptic drugs (ASMs) suppress seizures rather than modify the underlying mechanisms of the disease or provide a cure [
2]. Thus, discovering new pharmacological agents that target the fundamental mechanisms of epilepsy is a challenge in modern neuroscience.
Epileptogenesis, triggered by various disruptive events, is a multifaceted, gradual process involving molecular, cellular, and network-level changes that transform a normal brain into an epileptic one [
3]. The maladaptive regulation of neuronal excitability homeostasis has been identified as a key factor in epileptogenesis [
4]. Epileptogenesis is relatively well studied in the context of acquired temporal lobe epilepsy (TLE), which is the most common form of epilepsy. TLE is a partial syndrome in which seizure activity originates in the temporal lobe of the forebrain, often in the hippocampus.
In animal models and human studies, it has been shown that numerous changes occur in the hippocampus during TLE epileptogenesis, in particular: loss of interneurons and pyramidal neurons, inflammation, ion channel and receptor expression modification, mossy fiber rewiring, transcriptome and epigenetic changes, synaptic transmission change, epileptiform and seizure activity development [
3,
5]. Currently, no widely accepted treatment that attenuates epileptogenesis is available. Nevertheless, targeting epileptogenesis remains one of the most promising therapeutic strategies [
2]. Therefore, identification of new pharmacological agents that can inhibit, mitigate, or reverse epileptogenesis is a highly rewarding line of translational inquiry.
In our early studies, it was shown that an extract of Aquilegia vulgaris, a plant used in oriental folk medicine as an antiepileptic remedy, contains substances that interact with gamma-aminobutyric acid A (GABA-A) receptors in vitro [
6]. These compounds were found to be myo-inositol (MI) and sleep-inducing lipid oleamide. Subsequent experiments using purified commercial MI and oleamide showed that MI completely inhibited radioactive muscimol (a GABA-A receptor agonist) binding to rat brain cell membranes, while oleamide approximately doubled 3H-Flunitrezepam (a specific ligand for the GABA-A receptor benzodiazepine site) binding, suggesting that MI may be a GABA-A receptor agonist, while oleamide functions as a positive allosteric modulator of GABA-A receptors [
6]. GABA-ergic neurotransmission is the main inhibitory system in the forebrain, and its dysfunction plays a significant role in the development of epilepsy in general and ictogenesis in particular [
1]. Consistent with this idea, our experiments showed that MI pretreatment significantly decreased the severity of acute seizures induced either by pentylentetrazolium (PTZ) or by kainic acid (KA) in experimental animals [
7].
In our recent studies, using the rat kainic acid model of TLE, we identified MI as a promising antiepileptogenic drug. It has been demonstrated that MI alleviates molecular, cellular, systemic, and behavioral changes associated with epileptogenesis. Specifically, four weeks of MI treatment almost completely reversed KA-induced biochemical changes associated with epileptogenesis [
8,
9]. Furthermore, we demonstrated that in KA-treated rats, MI reduces the frequency and duration of motor SRS during and even 4 weeks after the treatment is ceased [
10].
In addition, MI counteracts epileptogenesis-induced changes in miRNA profiles, mRNA levels, and expression of the sodium-MI transporter and LRRC8A subunit of the volume-regulated anion channel in the hippocampus [
10,
11]. Notably, in the same epilepsy model, it has been shown that MI decreases the frequency and duration of electrographic (EEG) SRS in the hippocampus, alleviates spatial learning and memory deficit associated with epileptogenesis, and attenuates cell loss in the hippocampus even 4 weeks after MI treatment termination [
10]. In vivo, locally administered MI in the hippocampus suppressed evoked after-discharges in a dose- and time-dependent manner [
12].
In the CNS, which has high MI content, MI is synthesized de novo and recycled in neurons. MI functions as an essential osmolyte and a precursor of phosphoinositides, which contribute to widely used signal transduction pathways in the CNS, regulating neuronal excitability [
13]. Therefore, based on our data and the utility of MI-derived signaling pathways, we hypothesized that MI is an endogenous antiepileptogenic compound that contributes to neuronal excitability homeostasis [
9,
10].
In order to further refine the stated hypothesis, in the present study, we investigated the dose-dependent effects of MI on electrophysiological and behavioral markers of epileptogenesis in a KA-induced rat model of TLE. Thirty, sixty, and one hundred twenty mg/kg concentrations of MI were utilized in this study. The 30 mg/kg dose was previously identified as the threshold concentration for suppressing kainic acid–induced epileptogenesis in biochemical and behavioral experiments [
9]. Based on reported MI brain concentrations [
13], our calculations suggest that intraperitoneal injection of 30 mg/kg increases CNS MI levels by approximately 10–15%. Accordingly, 60 mg/kg and 120 mg/kg doses are expected to increase MI concentrations by 20–30% and up to 60%, respectively. The ‘high’ 120 mg/kg dose was chosen to remain tenfold below the known toxic concentration of intraperitoneally administered MI in rats [
14], thus ensuring non-toxic but physiologically relevant effects
We found that MI suppressed motor and electrographic SRS in a dose-dependent manner and attenuated the associated decline in spatial learning and memory. In this regard, we identified 60 mg/kg as the optimal dose. Furthermore, we have shown that KA-induced epileptogenesis and MI treatment with 60 mg/kg were associated with extensive transcriptomic and DNA methylation alterations in the hippocampus, implicating several biological pathways. Importantly, MI treatment upregulated transcripts for several ion channel subunits (including GRIK3 and GRIN3A of the kainate and NMDA glutamate receptors, and the sodium channel beta subunit SCNB4), reversing their downregulation seen during epileptogenesis.
3. Discussion
Obtained data convincingly indicate dose-dependent MI effects on KA-induced epileptogenesis, specifically on (i) reductions in electrophysiological and motor SRS and the frequency of IIS; (ii) improvement of learning and memory deficits in MWM; and (iii) selected biological pathways identified in a broad range of long-term transcriptomic and DNA methylome changes associated with epilepsy. To our knowledge, this is the first study to demonstrate long-term, large-scale transcriptomic and DNA methylome alterations following MI treatment in a kainic acid-induced status epilepticus model.
Dose-Dependence of MI effects
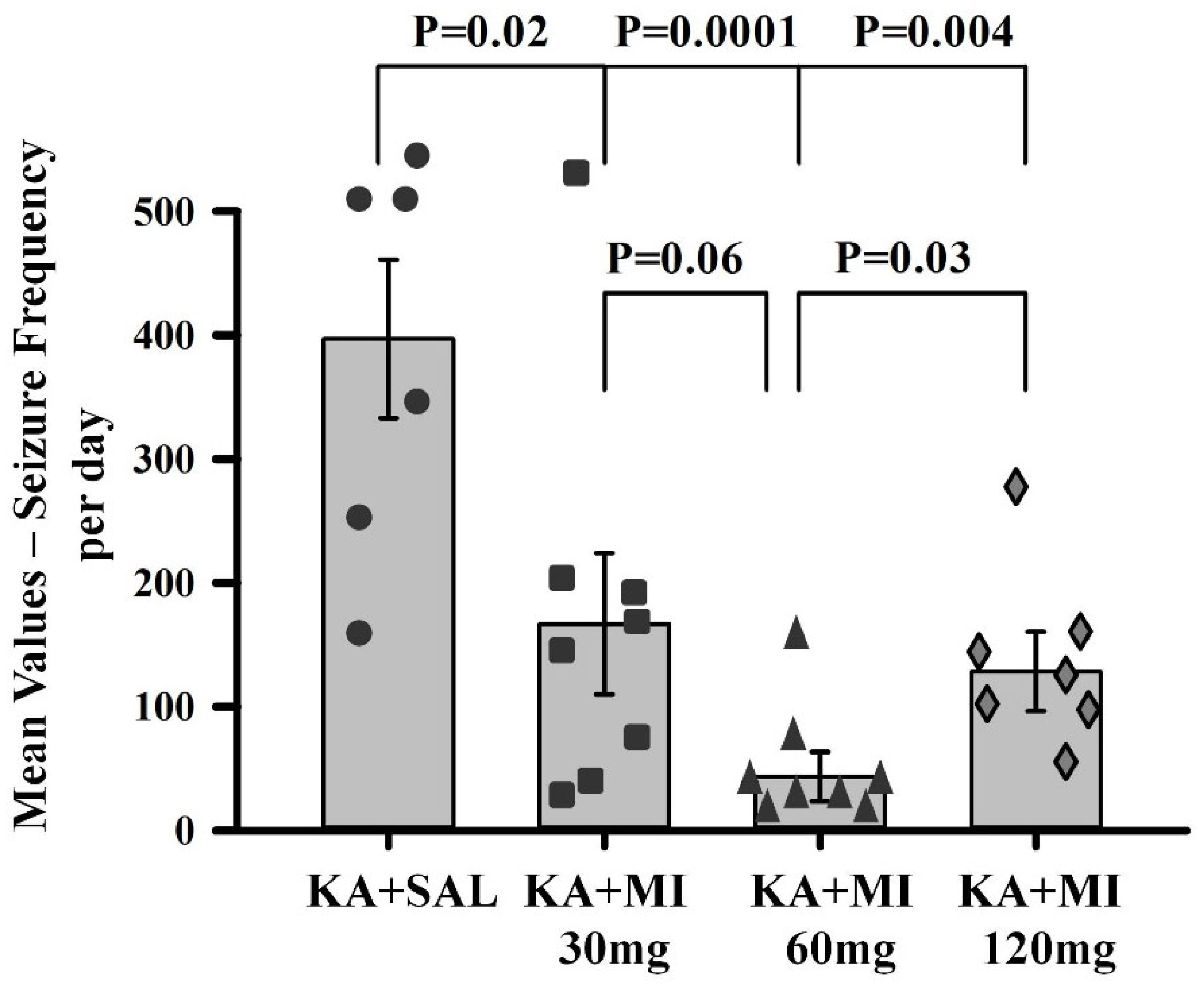
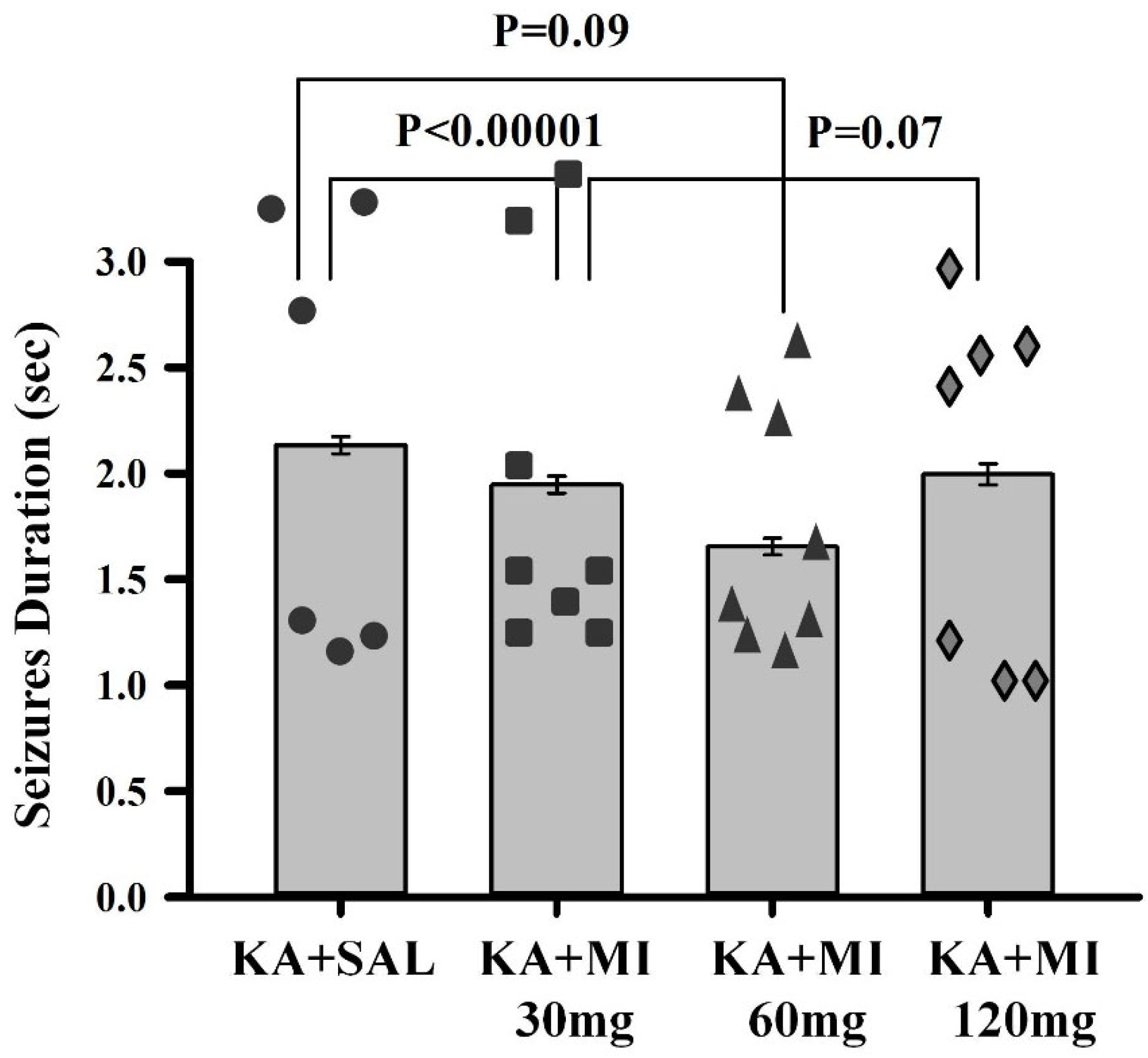
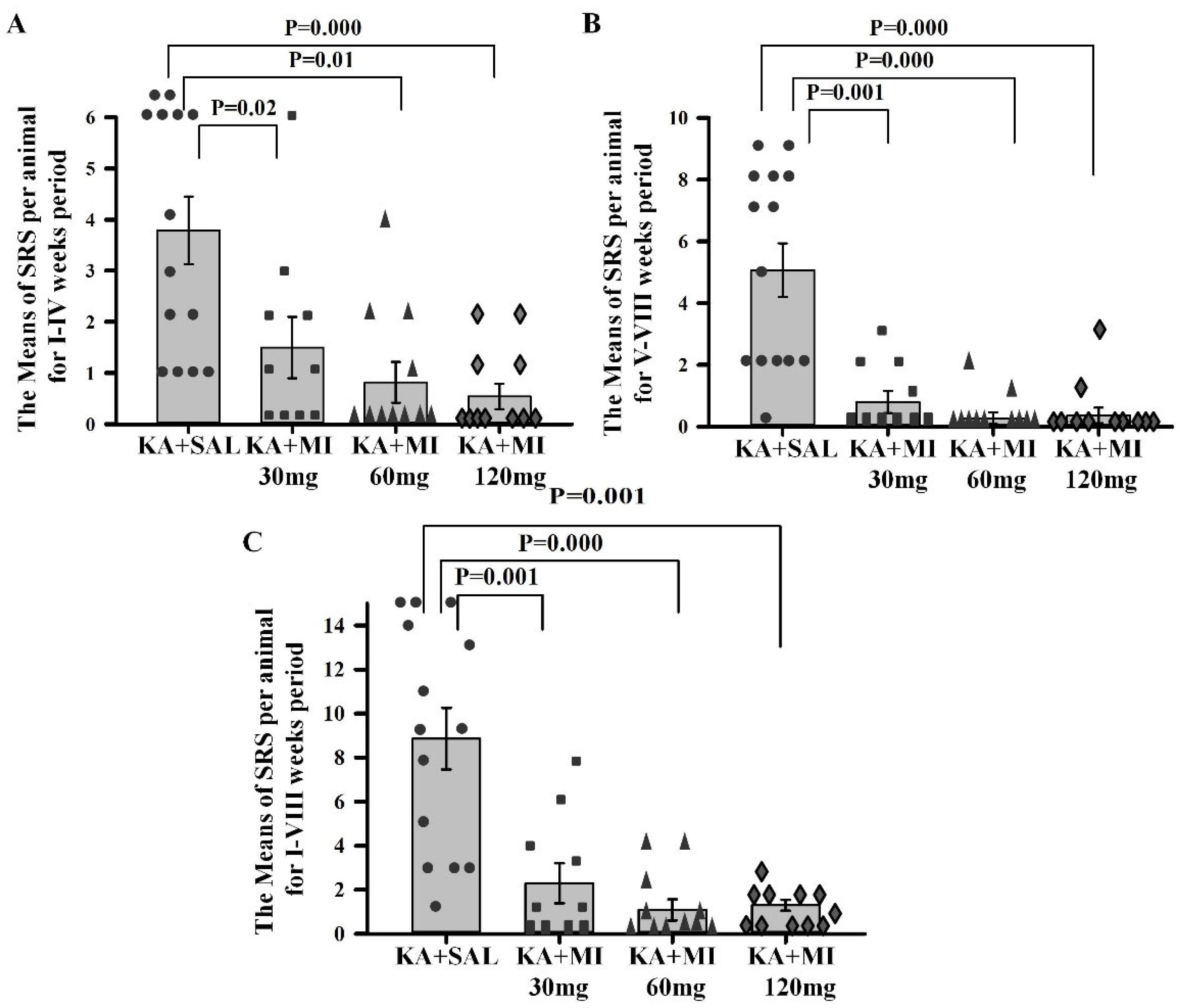
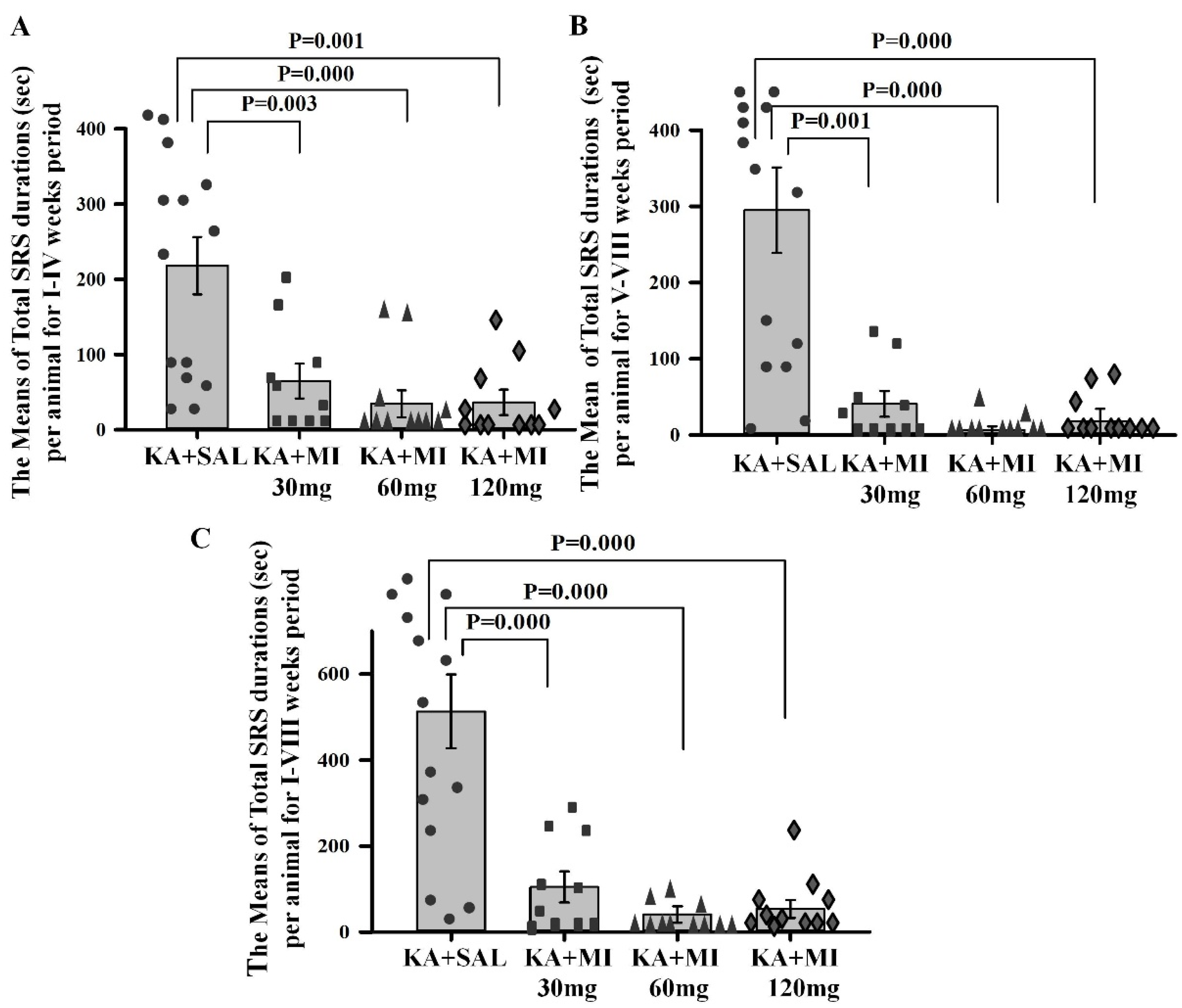
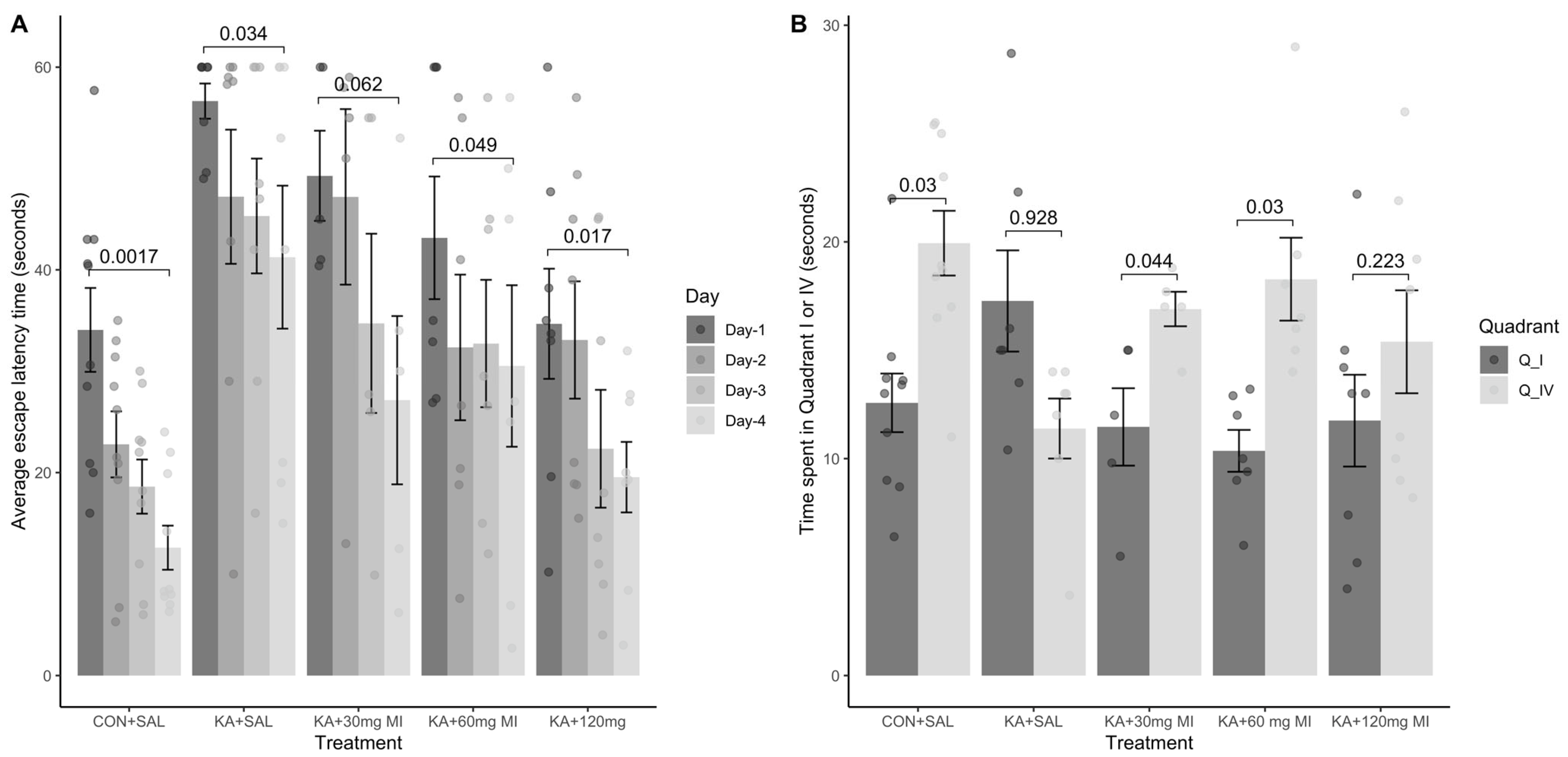
We tested the effects of different MI concentrations (30 mg/kg, 60 mg/kg, and 120 mg/kg) on epileptogenesis in the KA epilepsy model in rats. We found that MI had long-lasting, dose-dependent effects on motor and electrographic manifestations of epileptogenesis. The 60 mg/kg dose was identified as the most effective, while the lower 30 mg/kg and higher 120 mg/kg concentrations displayed less efficacy. Notably, in MWM spatial learning and memory task experiments, analysis of learning dynamics indicated that the 120 mg/kg MI dose displayed the highest efficacy in mitigating the decline of spatial learning, while other KA-treated groups also displayed spatial learning, albeit to a lesser degree. Interestingly, in the spatial memory test, the 30 mg/kg and 60 mg/kg MI doses significantly alleviated spatial memory deficits, whereas the KA+MI 120 mg and KA+SAL groups did not show spatial memory. This apparent inconsistency between learning and memory tasks is probably related to the learning paradigm, which required four training trials daily over four days, while spatial memory was tested in a single trial on the fifth day.
It is important to note that in the present study, electrographic spontaneous recurrent seizures (SRS), interictal spikes (IIS), and epileptiform activity were significantly suppressed by the 60 mg/kg dose of MI, whereas the higher 120 mg/kg dose was less effective. Although the difference in suppression of motor SRS between doses did not reach statistical significance, a clear numerical trend favored the 60 mg/kg dose. This pattern was further supported by performance in the spatial memory task, where the 60 mg/kg dose also showed greater efficacy. These findings collectively suggest that the pathological hyperexcitability of the hippocampus during epileptogenesis is selectively and effectively mitigated by an MI dose near 60 mg/kg. Doses above this threshold appear to lose efficacy or even exert disruptive effects, as previously reported by Williams and Jope [
14].
Interestingly, in the spatial learning task (as opposed to memory retention), the 120 mg/kg dose demonstrated high effectiveness. This apparent discrepancy may be explained by either (i) task-specific mechanisms of learning versus memory or (ii) the possibility that the optimal dose of MI for learning lies between 60 and 120 mg/kg, with efficacy tapering off at the upper end of this range.
It is noteworthy that MI exhibited a non-linear, inverted U-shaped dose–response relationship, with lower doses producing stronger antiepileptogenic effects than the highest dose (120 mg/kg). Such non-linear responses are common in biological systems, particularly in CNS receptor pharmacology [
18]. Mechanistically, this may reflect the activation of multiple signaling pathways with differing sensitivity to MI concentration, resulting in selective engagement of distinct transduction mechanisms at different dose levels.
Data obtained in the current study agree with our previous report that, under a similar experimental paradigm, the MI threshold concentration of 30 mg/kg suppresses motor and electrographic SRS, molecular changes, as well as spatial learning and memory deficit comorbidities associated with TLE epileptogenesis [
10]. Importantly, the 60 mg/kg MI dose not only selectively and effectively suppressed the electrographic and motor SRS frequency, but also significantly decreased the frequency of IIS—an effect not observed after treatment with the 30 mg/kg dose of MI [
10]. IIS represents an electrophysiological marker of excessive increase in forebrain excitability in general and hippocampus in particular [
1]. Thus, our data strongly indicate that the pathological increase in hippocampal excitability during epileptogenesis is mitigated selectively and effectively by 60 mg/kg MI. This is consistent with our hypothesis that MI helps maintain neuronal excitability within a normal range. Furthermore, it has been suggested that disruption of pyramidal neuron firing homeostasis and, consequently, excessive increase in excitability disrupts the specificity of long-term potentiation (LTP)—the proposed cellular mechanism of learning—and corrupts existing memory engrams, thus undermining declarative learning and memory [
19]. The described disruption of LTP and the established synaptic weights are likely to contribute to the comorbidity of spatial memory and learning deficits associated with TLE. Thus, MI-induced mitigation of spatial learning and memory deficits observed in our experiments is consistent with the limited increase in hippocampal excitability caused by MI treatment during epileptogenesis.
Transcriptomic changes
Both KA+SAL and KA+MI treatments were associated with massive transcriptomic changes, not only compared with the CON+SAL group, but also between KA-treated groups. Not surprisingly, KA+MI treatment induced a larger number of gene expression changes compared with controls than did KA+SAL treatment, leading to significant transcriptomic differences between the KA+SAL and KA+MI groups. At least part of these differences should be responsible for the attenuation of KA-induced epilepsy in the KA+MI group.
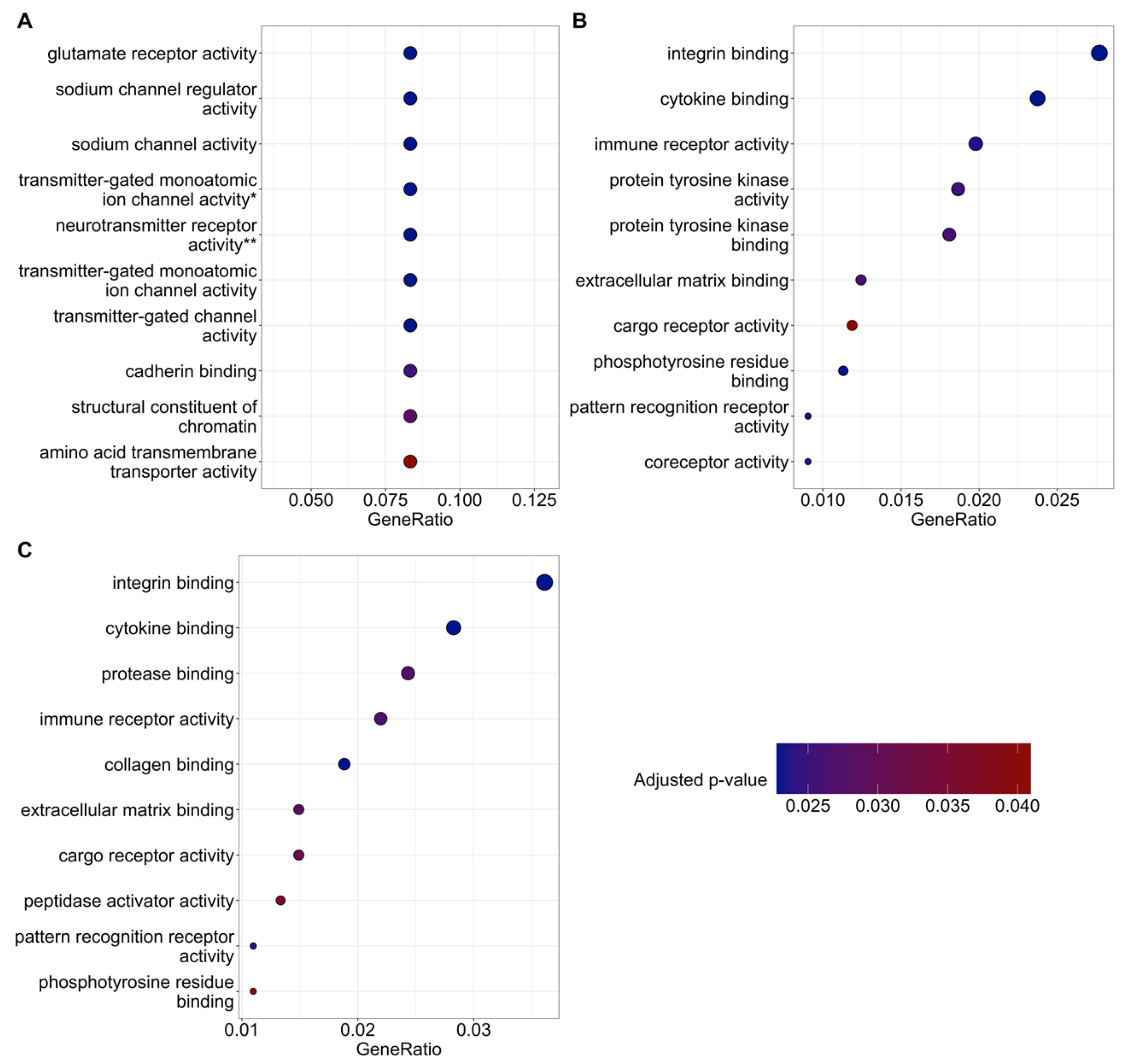
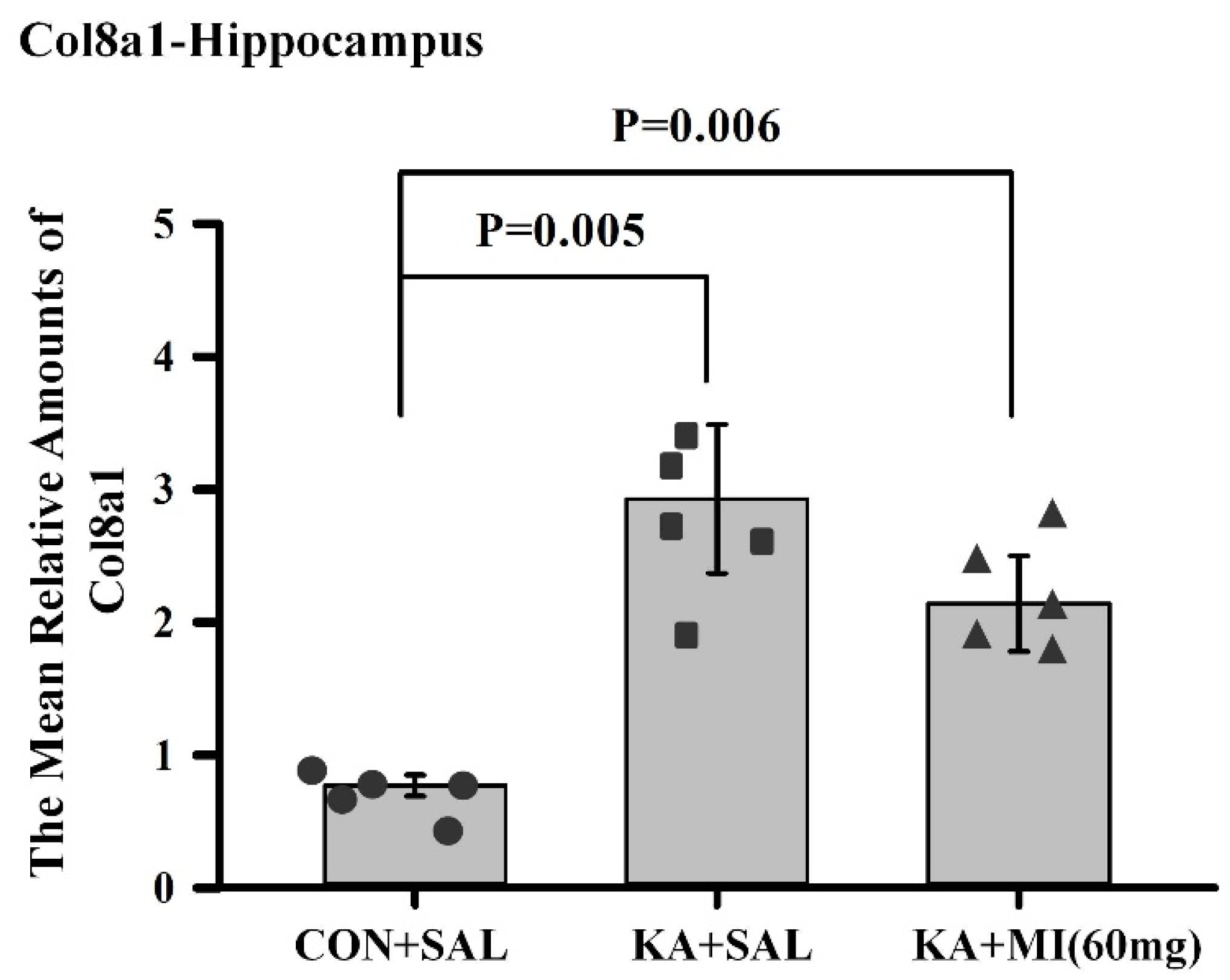
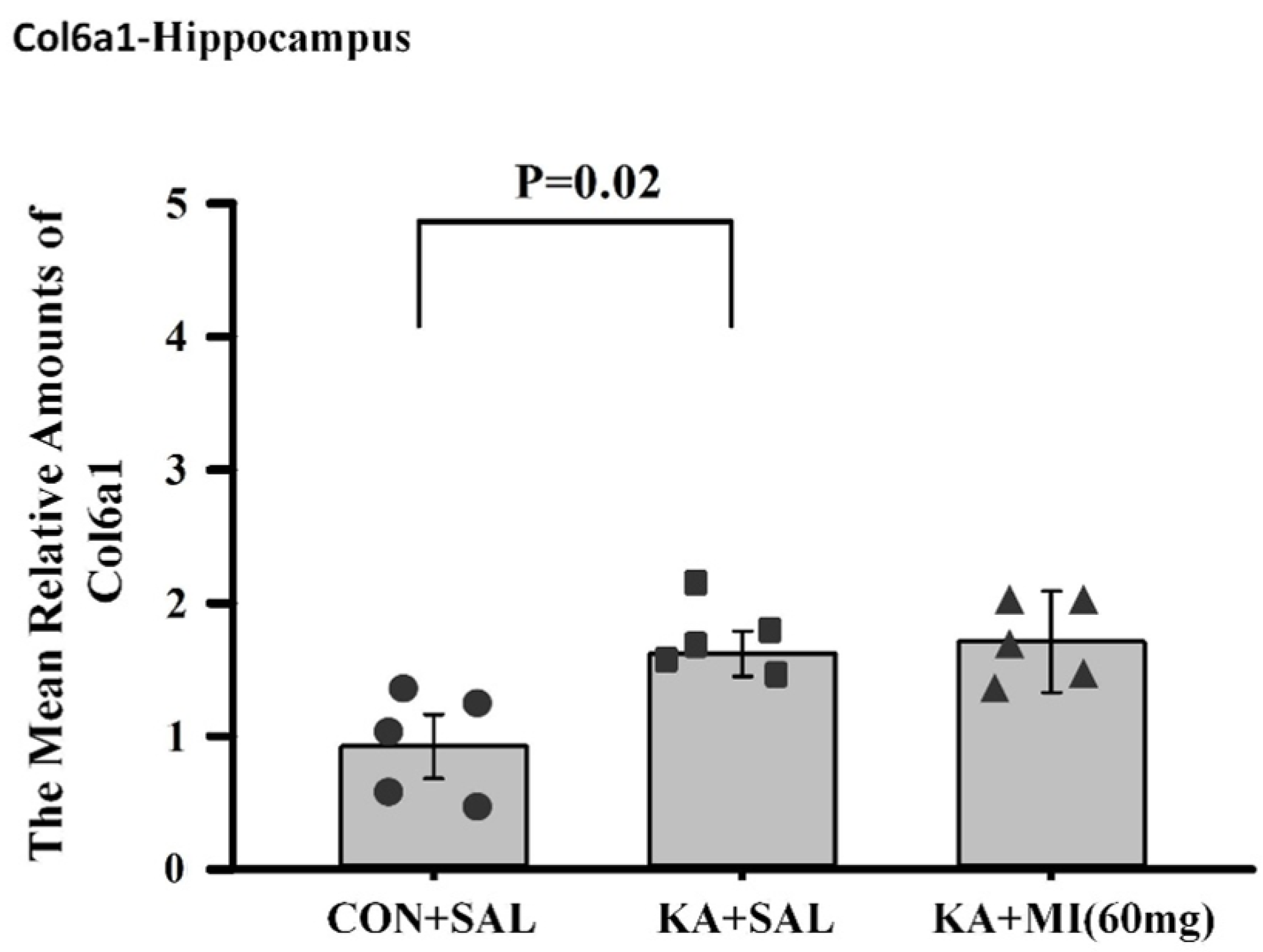
There are several genes and corresponding biological pathways commonly differentially expressed in KA-treated groups relative to CON+SAL. These pathways include integrin binding, growth factor binding, transmembrane protein kinase activity, immune receptor activity, and extracellular matrix binding. The KA+MI transcriptome compared with CON+SAL also revealed differential expression of collagen binding activity—an upregulated pathway closely related to extracellular matrix binding. These pathways could be related either to epileptogenesis or to compensatory changes against it. Taking into account that epileptogenesis was clearly reduced in the MI-treated group, we suggest that increased amounts of various collagens are linked to compensatory changes against epilepsy. This suggestion is supported by recent data from Ramos-Moreno et al. [
20], which showed that upregulation of Col6a1 after seizures leads to decreased glutamatergic transmission and reduced network excitability.
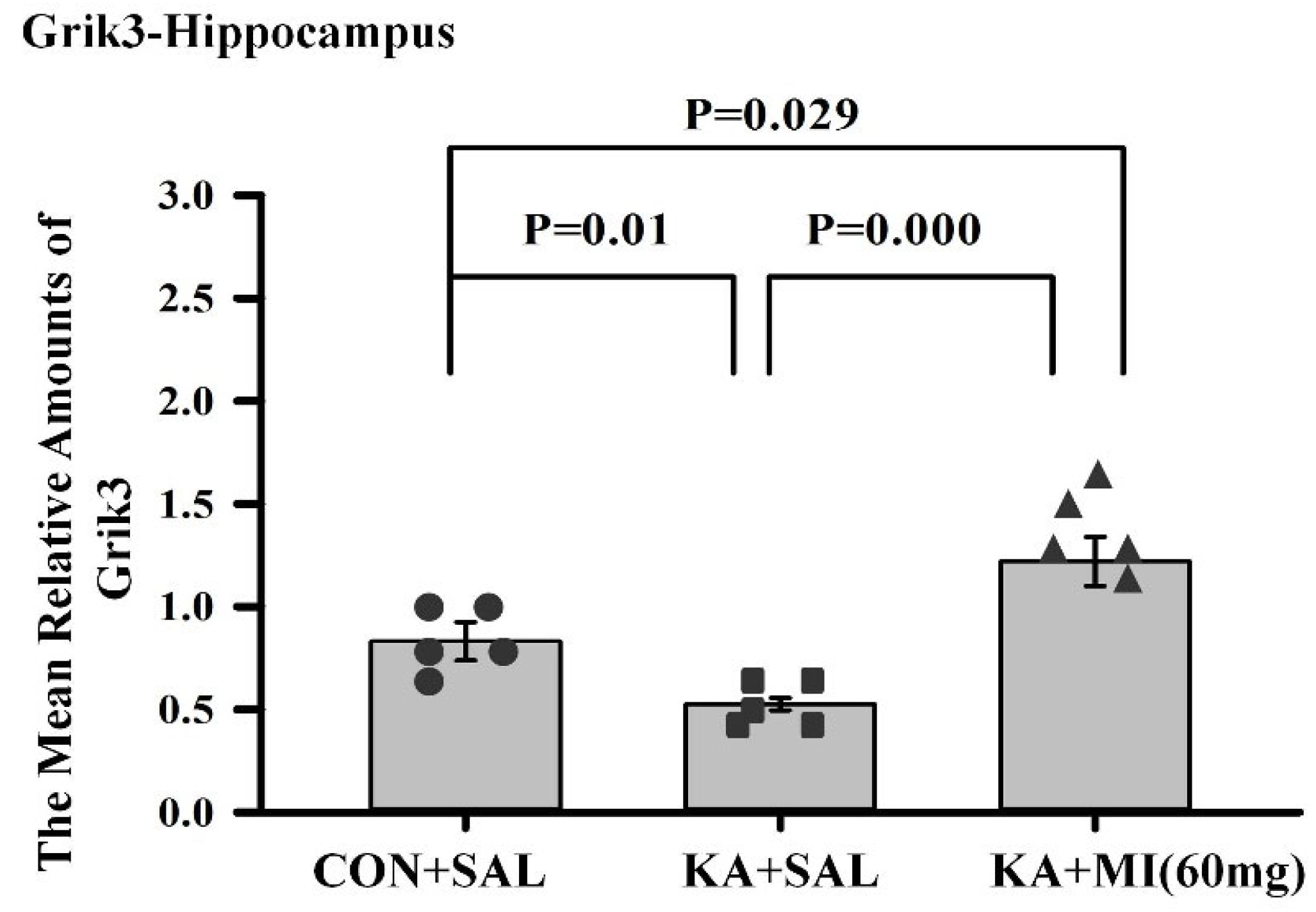
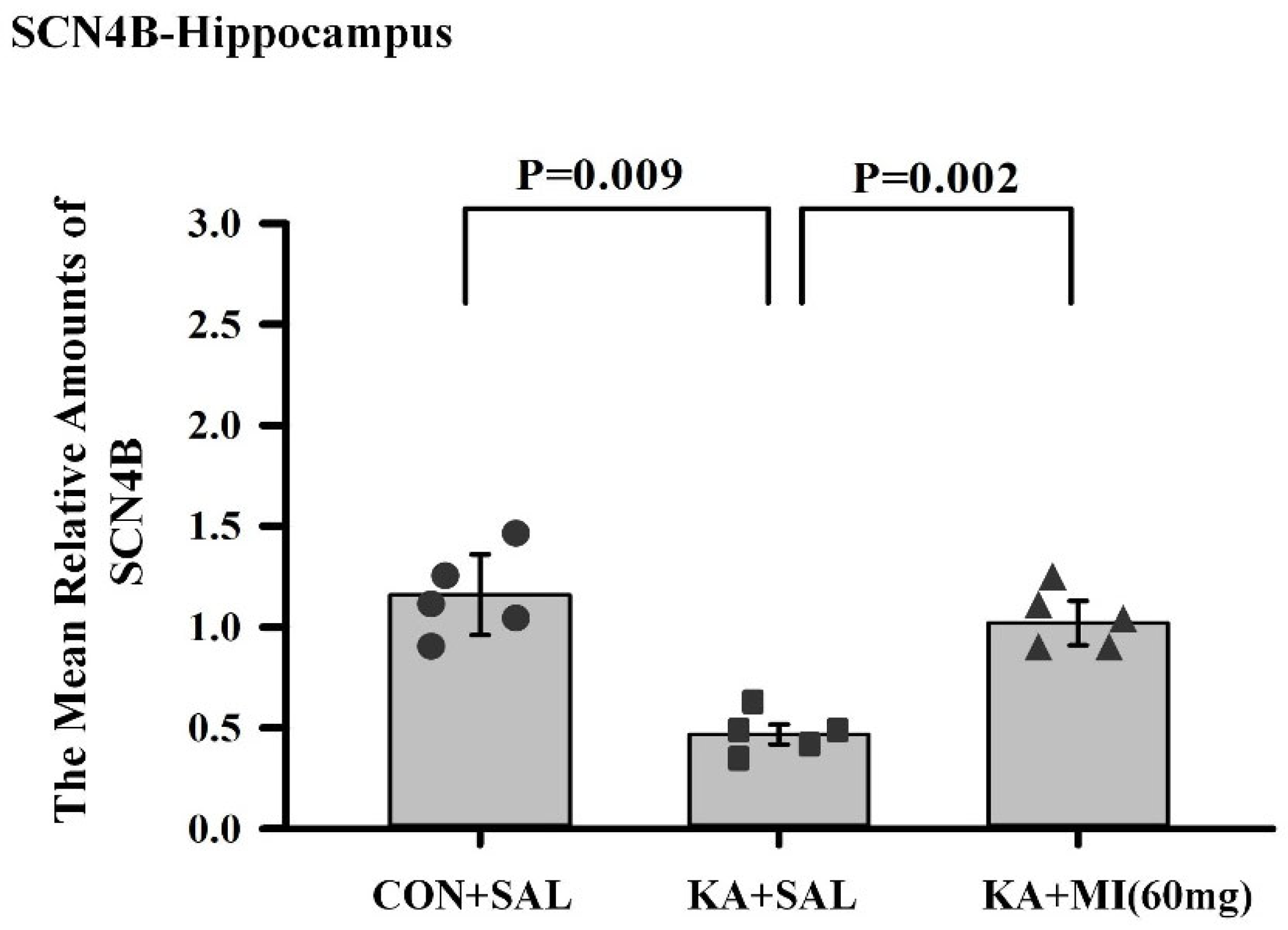
The most intriguing aspect of comparative RNA-SEQ data concerns the differences in sodium channel subunits and glutamate ionotropic receptor subunits. Namely, in the hippocampus of the KA+MI group, upregulation of the GRIK3 glutamate receptor subunit was observed, and its level was significantly higher compared with both KA+SAL and CON+SAL groups. In the case of SCNB4 and GRIN3A, MI treatment prevented their KA-induced downregulation, and expression remained at control levels. How can this apparent paradox—higher levels of excitatory proteins alongside markedly decreased seizure activity—be explained? We speculate that these changes are cell-type specific and propose the following scenario for MI-targeted action:
We propose that the described ion channel subunit changes primarily occur in hippocampal pyramidal neurons. It has been demonstrated in TLE rat models that during epileptogenesis and chronic epilepsy, there is downregulation of hyperpolarization-activated, cyclic nucleotide-gated (HCN) ion channels in the CA1 pyramidal neuron apical dendrite [
21]. Indeed, under our experimental design, in KA+SAL animals compared with CON+SAL, there is a significant downregulation of HCN1 gene expression (see
Supplementary File S5). Decreased conductance increases excitability of pyramidal neurons by raising input resistance of the apical dendrite, enhancing temporal summation of excitatory synaptic potentials in the dendrite, and promoting their spread from dendrite to soma [
21]. We speculate that the upregulation of GRIK3 kainate glutamate receptor subunits in KA+MI hippocampi occurs preferentially in the CA1 pyramidal neuron apical dendrites, and thus compensates for HCN1 channel downregulation. Layer V pyramidal apical dendrites have a high density of kainate receptors, mainly extrasynaptic, activated by ambient or spillover glutamate [
22]. We propose that MI treatment specifically upregulates extrasynaptic kainate receptors in these dendrites, increasing resting “leak” conductance, lowering input resistance, and decreasing both the membrane and space constants. These homeostatic effects likely lower CA1 pyramidal neuron excitability and reduce the propensity for epileptiform activity. From this follows that downregulation of GRIK3 in KA+SAL is a pro-epileptic change, increasing CA1 excitability.
GRIN3A NMDA receptor subunits are unconventional because they render NMDA receptors less calcium permeable, making synapses less prone to LTP [
23]. We speculate that GRIN3A downregulation in KA+SAL pyramidal neurons is a pro-epileptic change that enhances potentiation of excitatory synapses. In KA+MI, GRIN3A remains at control levels, contributing to normal excitability.
SCNB4, the sodium channel β4 subunit, is expressed in hippocampal pyramidal neurons [
24]. Its co-expression with sodium channel α-subunits shifts activation to more negative potentials [
24]. We propose that downregulation of SCNB4 in KA+SAL is pro-epileptic, shifting activation positively, facilitating action potential backpropagation, and increasing excitability [
25]. Restoration of SCNB4 in KA+MI likely contributes to the recovery of normal excitability.
RNA-SEQ; Overlap with previous data
Our earlier experiments (30 mg/kg MI) showed similar gene expression trends for SLC5A3 (sodium-myo-inositol transporter), GFAP (glial fibrillary acidic protein), and LRRC8A (volume-regulated anion channel subunit [
10,
11]. The current results confirm and extend these observations.
Methylome changes
Our data for the first time: (i) convincingly indicate long-term DNA methylation alterations in hippocampi of KA+SAL and KA+MI groups; (ii) provide a detailed map of altered methylation sites. Importantly, all differentially methylated sites were located in promoters or first exons.
KA+MI and KA+SAL groups both diverged from CON+SAL. Five genes were hypermethylated in both KA groups (Dnajc18, Hdac7, LOC102548917, Ubxn4, Rab6b). Only two genes (Them4, Ptges3l) were commonly hypomethylated. MI also induced specific methylation changes relative to KA+SAL. Given that samples were collected four weeks after MI cessation and eight weeks after SE induction, these changes are long-lasting and likely contribute to MI’s benefits. However, the overlap with RNA-SEQ was small. Genes differentially expressed and methylated included: KA+MI vs. CON+SAL (Zfp717, Ptges3l, Hdac7); KA+SAL vs. CON+SAL (Smurf2, Arhgap24, Hdac7). None showed strict inverse methylation–expression correlation, consistent with evidence that disease-associated hypermethylation can both up- and down-regulate expression [
26].
Of note, HDAC7 was upregulated in both KA groups at RNA and protein levels, with hypermethylation also detected. HDACs generally repress transcription via histone deacetylation [
27] but also mediate non-canonical functions, e.g., neuroprotection [
28]. HDAC7 downregulation is linked to depressive behavior [
29]. Our earlier work showed MI mitigates apoptosis after SE [
30], potentially via HDAC7.
LncRNAs and KA-induced SE
Among differentially expressed transcripts (KA+SAL and KA+MI vs. CON+SAL), 15 were lncRNAs (
Supplementary Tables S4 and S5). ENSRNOG00000064277 was upregulated only in the hippocampus, enriched in the cytoplasm, not the nucleus. LncRNAs regulate chromatin, methylation, R-loops, and splicing [
31] but also act in cytoplasm—for instance, glycoLINC scaffolds glycolytic metabolons [
32]. LncRNAs are increasingly implicated in epilepsy [
33,
34]. Thus, lncRNAs identified here merit further study as therapeutic targets.
It should be emphasized that the observed transcriptomic and DNA methylation alterations following MI and kainic acid treatment are associative and may not directly account for the behavioral or electrographic antiepileptogenic effects.
4. Materials and Methods
4.1. Animals
Animals were housed individually and maintained under a 12 h light/12 h dark cycle and had free access to food and water. Experimental design was approved by the Bioethics Committee of the I. Beritashvili Centre of Experimental Biomedicine (Protocol N03/1 November 2019). We monitored health parameters—body weight and mortality of the animals. These parameters stayed normal across animal groups and were not different between the groups.
4.2. KA-Induced SE
Male Wistar rats, 2.5–3 months of age, received a single intraperitoneal (IP) injection of kainic acid (KA; 10 mg/kg, Abcam, Cambridge, UK, Cat. No. 120100) dissolved in saline. After injection, each animal was placed in an individual plastic cage for observation for 4 h. Seizures were scored according to a modified Racine scale (0–6) [
35,
36].
The seizure intensity (scores) and durations on video recordings were measured by two coauthors (not aware of the injection status), substituting for each other for a weekly period. Thus, the data regarding motor seizures were obtained in a blind manner.
For further experiments, only animals that exhibited a 4–6 score SRS and with a total duration of at least 60 min during the 4 h observation period were selected. This selection was used to ensure induction of epileptogenesis and, consequently, the development of SRS (i.e., epilepsy). In each series of experiments, selected KA-treated rats were injected with different doses of MI, 30 mg/kg, 60 mg/kg, or 120 mg/kg, and designated as KA+MI30, KA+MI60, and KA+MI120 groups, respectively. One KA-treated group received intraperitoneal (IP) saline (0.9% NaCl sterile solution, 1 mL/kg) instead of MI and was designated as KA+SAL.
IP injections (twice daily) of MI or saline started 4 h following KA treatment and continued for 28 days after the start of the experiment. The control group of animals (CON+SAL), healthy intact animals, received, at exactly the same time schedule, an intraperitoneal injection of only saline (0.9% NaCl solution, 1 mL/kg) twice daily for 28 days. To observe long-term effects of MI, after termination of MI or saline injections, animals were maintained in cages without treatment for an additional 4 weeks. The animals were continuously video-monitored (24 h) in their cages. At the end of 8 weeks from the injection of KA, EEG was recorded in KA-treated animals. Finally, after the termination of the EEG sessions, the animals were tested in the Morris Water Maze (MWM).
The most effective MI dose identified in the behavioral and electrographic experiments was used in epigenetic and transcriptome studies (
Figure 16).
4.3. Video-Monitoring
Animals were housed individually and maintained under a 12 h light/12 h dark cycle. Animal behavior was monitored continuously (24/7) by infrared video cameras (IRIP66 HIK VISION, Hangzhou, China) as described in our previous paper [
10]. Only seizures of grades 4–6 were identified and evaluated, since lower seizure grades can easily be confused with normal behavior.
The number of animals for motor seizure analyses in different KA-treated groups was as follows: KA+SAL n = 14, KA+MI (30 mg) n = 10, KA+MI (60 mg) n = 11, and KA+MI (120 mg) n = 11.
4.4. Surgery and EEG Recording
At 28 days after termination of treatment with different doses of MI or with saline, a steel bipolar electrode was implanted in the right dorsal hippocampus of KA-injected animals under ketamine/xylazine anesthesia using the rat brain atlas [
37]. The following coordinates were selected for the placement of the electrode in the right hippocampus: 4.5 mm caudally from the bregma, 2.8 mm laterally from the midline, and 3 mm ventral to the skull surface. The animals were allowed to recover for 2 days after surgery, and hippocampal EEG was recorded for the following 3 days, 3 h per day.
Recording periods were alternated in the morning and afternoon between KA+SAL and KA+MI rats to ensure that each group was recorded for equivalent periods of the day.
An EEG was recorded with an amplifier (Pinnacle Technology, Ottawa Lake, WI, USA), low-pass filtered at 50 Hz, and acquired with the software Sirenia version 2.2.7 (Pinnacle Technology, Ottawa Lake, WI, USA). The duration, frequency, and number of electrographic SRS were analyzed.
The SRS activity was identified as high-frequency spike-and-wave activity with the amplitude exceeding the background activity at least twice. Additionally, the number of interictal spikes was evaluated. Interictal spikes were identified as brief, sharp negative or negative-positive deflections with a duration < 1 s and an amplitude at least twice that of the background.
In different KA-treated groups, the number of animals used for EEG analyses was as follows: KA+SAL n = 6, KA+MI (30 mg) n = 8, KA+MI (60 mg) n = 8, and KA+MI (120 mg) n = 7.
4.5. Morris Water Maze (MWM)
MWM test [
38] was used to study spatial learning and memory in the following groups of animals: KA+SAL, KA+MI 30 mg, KA+MI 60 mg, KA+MI 120 mg, and CON+SAL. The MWM test was conducted on the same animals used in electrophysiological studies (see above). Animal training/testing was carried out as described in our previous paper [
10], with a 4-day learning session and spatial memory testing on the 5th day. The number of animals in different KA-treated and control groups used for the MWM test was as follows: CON+SAL n = 10, KA+SAL n = 7, KA+MI (30 mg) n = 5, KA+MI (60 mg) n = 7, and KA+MI (120 mg) n = 8. The training protocol for different groups of animals was identical.
4.6. Molecular Biological Studies
The optimal dose of MI identified in behavioral and electrographic experiments was utilized in molecular biology studies. To this end, transcriptomics (RNA-seq) and methylome (reduced representation bisulfite sequencing, RRBS-seq) experiments were performed on rats’ hippocampus with the design described above (
Figure 16). Each experiment included CON+SAL, KA+SAL, and KA+MI (60 mg/kg) groups of rats, each group containing 4 animals. At the end of eight weeks from KA injection, rats were decapitated, and the hippocampus was removed from each brain and immediately frozen on dry ice. The hippocampus samples were shipped on dry ice to the commercial service provider Diagenode, for carrying out both types of experiments (see subsections below for details).
The validation of RNA-seq results was carried out using real-time polymerase chain reaction (RT-PCR) assay and Western immunoblotting on hippocampus and neocortex samples. Each group of animals contained 5 rats.
4.6.1. RNA-SEQ
The RNA-SEQ experiment on 12 hippocampal samples (4 samples from each CON+SAL, KA+SAL and KA+MI (60 mg/kg) groups) was performed by RNA-SEQ services (Diagenode, Seraing, Belgium, Cat# G02030000). RNAs were extracted using the RNeasy Mini Kit (Qiagen, Venlo, The Netherlands, #74104). RNA was quantified using the Qubit™ RNA BR Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA, Q10210) and further assessed for integrity using the RNA 6000 Pico Kit (5067-1513, Agilent, Santa Clara, CA, USA) on a 2100 Bioanalyzer system (Agilent, Santa Clara, CA, USA).
The 12 RNA samples were processed together, and library preparation was performed with 500 ng of input RNA using: NEBNext
® rRNA Depletion Kit (Human/Mouse/Rat) (NEB #E6310), followed by NEBNext Ultra II Directional RNA Library Prep Kit for Illumina (NEB #E7760) and NEBNext
® Multiplex Oligos for Illumina
® Index Primers Set 1 (NEB #E6440). The other details of the experiments were analogous to those described in our previous publication [
39]. Illumina sequencing was applied with paired-end 50 bp reads, generating on average 50 M raw reads/sample (Diagenode G02030003).
4.6.2. Reduced-Representation Bisulfite Sequencing (RRBS)
The RRBS experiment was conducted on 12 hippocampal samples (4 samples from each of CON+SAL, KA+SAL, and KA+MI groups). DNA Methylation Profiling (RRBS Service, Diagenode Cat# G02020000) was performed for this purpose. Genomic DNA quality control, RNase treatment, RRBS library preparation, library quality control, and deep sequencing were conducted according to methods described in our previous paper [
39] at Diagenode, and details are provided in
Supplementary File S21.
4.6.3. RT-PCR
RNA Isolation. RNA was isolated separately from the hippocampus and neocortex using the NucleoSpin RNA Total RNA Isolation Kit (Macherey-Nagel, Düren, Germany, Cat. No. 740955.50).
The concentration of RNA was measured by absorbance at wavelengths 280/260 nm on a Nanodrop.
mRNA Measurement. Complementary DNA (cDNA) from the RNA fractions of hippocampus and neocortex was synthesized using the High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific, Cat. No. 4368814). Relative cDNA copy number was determined by real-time PCR using the Step One Real-Time PCR System (Applied Biosystems, Waltham, MA, USA) with the SYBR Green detection method and was normalized to beta-actin. The sequences of primers for selected genes are provided in
Supplementary File S22 (Supplementary Table S21).
4.6.4. Electrophoresis and Immunoblotting
Protein Determination, Electrophoresis, and Western Immunoblotting were carried out according to previously described methods [
9]. After the electrophoresis, the proteins were transferred onto nitrocellulose membranes (0.45 microns) and stained with Ponceau S solution (0.1%), digitalized, and uniform gel loading and transfer were confirmed with Lab Works 4.0 (UVP). HDAC-7 anti-HDAC7 primary antibody (ABIN7263279, Antibodies Online) was used for the detection of the target protein with established immunochemical procedures [
9].
The optical densities of the bands corresponding to HDAC-7 were measured using Lab Works 4.0 (UVP). The autoradiographs were calibrated by including in each gel 4 standards of homogenate protein fraction (15, 30, 45, and 60 μg of corresponding total protein) obtained from the control rats. The optical density of each sample band was divided by the optical density of the band for the 30 μg protein standard to give the “relative amount of protein”, and data obtained in such a way was used for statistical analysis. Data were not normalized to actin or any housekeeping protein in brain tissue samples, because such proteins cannot be guaranteed to remain unchanged by KA-induced SE. We controlled gel loading by Ponceau S staining, Lab Works 4.0 (UVP) analysis, and calibration with protein standards
4.7. Statistical Methods
4.7.1. Electrographic SRS Frequency, Electrographic SRS Duration, Motor SRS Frequency, and Motor SRS Duration
A one-way analysis of variance (ANOVA) and Tukey’s HSD (honestly significant difference) test were used to assess differences among groups for electrographic SRS frequency and duration. For motor SRS frequency and duration, one-way ANOVA and two-sample t-tests were applied.
4.7.2. Interictal Spikes
The Shapiro–Wilk test was used to test the hypothesis of normal distribution. The Kruskal–Wallis test, as well as the Wilcoxon rank-sum test with the Holm correction, were used to assess the significance of differences in a non-parametric way.
4.7.3. Spatial Learning and Spatial Memory Task
A two-way analysis of variance (ANOVA) and Tukey’s HSD test were used to assess differences across two factors. Finally, two-sample t-tests were used to assess differences in escape latent period on the 4th day between the control (CON+SAL) and the other groups.
4.7.4. Bioinformatics Analysis
Preprocessing of RNA-seq data
The raw fastq files were analyzed with the nf-core/rnaseq 3.11.2 pipeline [
40], with default parameters and using the mRatBN7.2.110 Ensembl genome reference [
41]. This pipeline first performs quality control with FastQC, followed by adapter and quality trimming with Trim Galore, and then quantifies gene expression count matrices with STAR [
42] and Salmon [
43].
Preprocessing of methylation (bisulfite) sequencing data
Methyl-seq data were analyzed with the nf-core/methylseq 2.6.0 pipeline [
40], using default settings, and the same genome reference as for the RNA-seq data (mRatBN7.2.110). This pipeline uses FastQC and Trim Galore! for quality control and read trimming, followed by alignment and methylation level quantification using the Bismark suite [
44].
Analysis of RNA-seq data
For each contrast, the DESeq2 R package, (version 1.44) [
45] was used to identify differentially expressed genes from the count matrix. In short, DESeq2 fits a generalized linear model for each gene, which includes a term representing the contrast of interest. The statistical significance of this term was used to assess whether the gene is differentially expressed. Furthermore, DESeq2 uses a shrinkage approach to produce robust estimates of log fold change and variances.
Enrichment of differentially expressed genes in Gene Ontology [
46] biological processes were performed with a hypergeometric test, as implemented in the package clusterProfiler (version 4.12) [
47], considering genes with adjusted
p-value ≤ 0.05 as differentially expressed.
Analysis of methylation (bisulfite) sequencing data
For each contrast, differentially methylated sites were identified with the limma R package, (version3.60) [
48]. In a similar fashion to DESeq2, limma fits a linear model for each methylation site, including a term representing the contrast of interest. The statistical significance of this term was used to assess whether the site is differentially methylated. A shrinkage approach was applied to produce robust variance estimates. Enrichment analysis was performed as described for the RNA-seq data.
All
p-values were adjusted for multiple testing with the false discovery rate (FDR) approach [
49] in both analyses.