Blood Derivatives in the Therapy of Ocular Surface Diseases
Abstract
1. Introduction
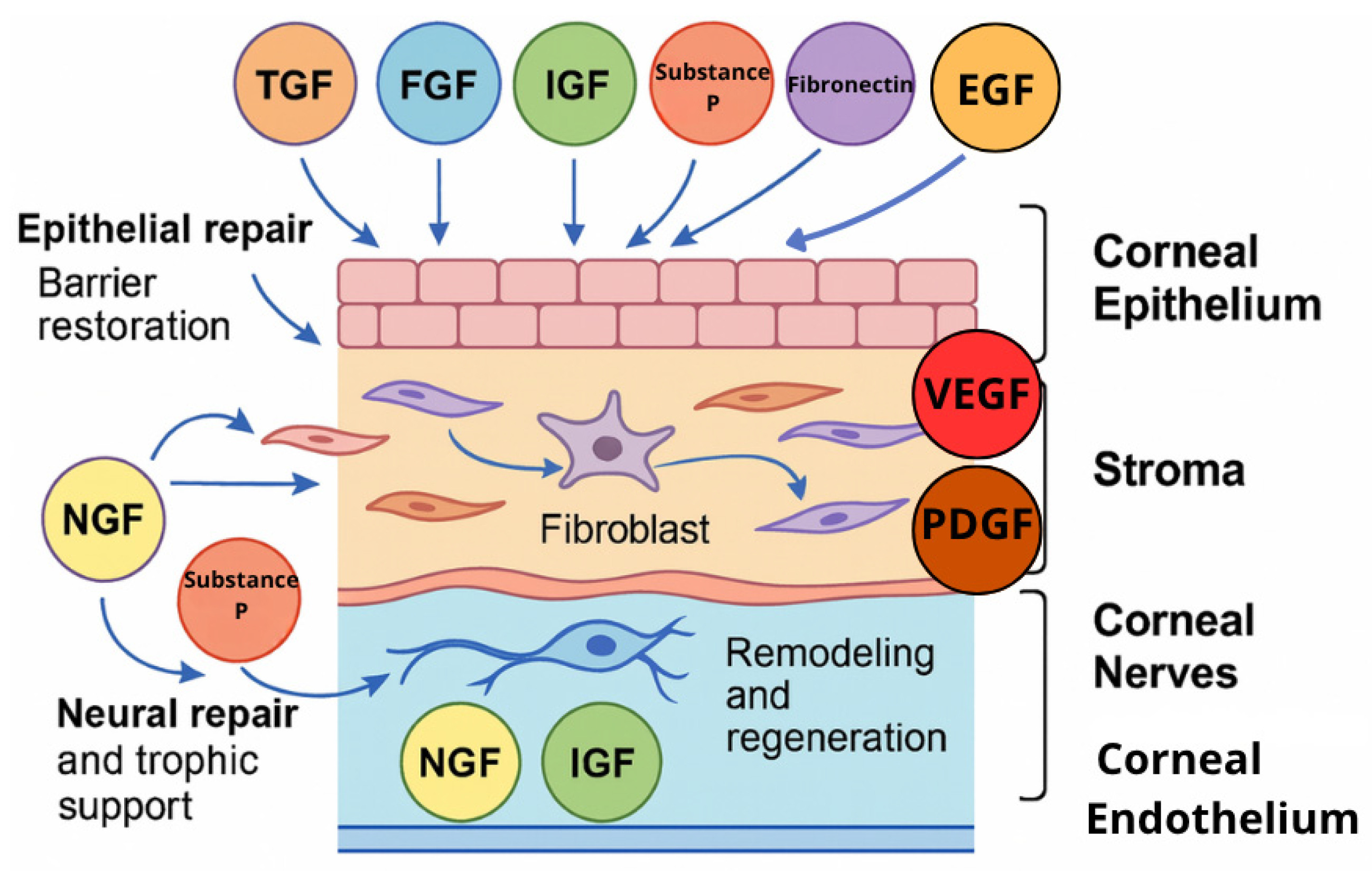
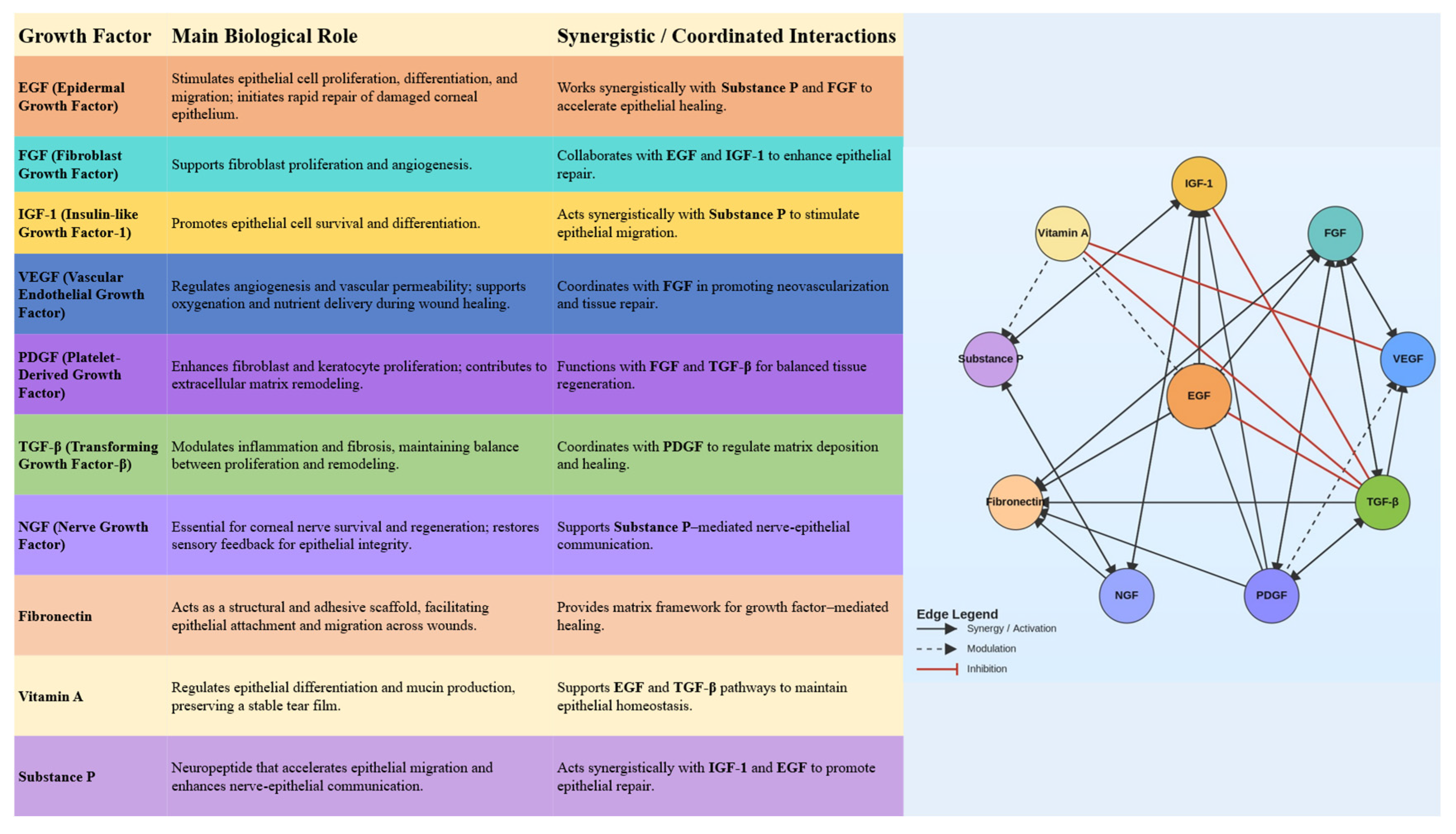
2. Molecular Basis for Blood Derivatives’ Effectiveness in Treating Ocular Surface Diseases
2.1. TGF-β
2.2. Vascular Endothelial Growth Factor
2.3. Epidermal Growth Factor
2.4. Platelet-Derived Growth Factor
2.5. Nerve Growth Factor
2.6. Insulin-like Growth Factor (IGF)
2.7. Fibroblast Growth Factor
2.8. Substance P
2.9. Vitamin A
2.10. Fibronectin
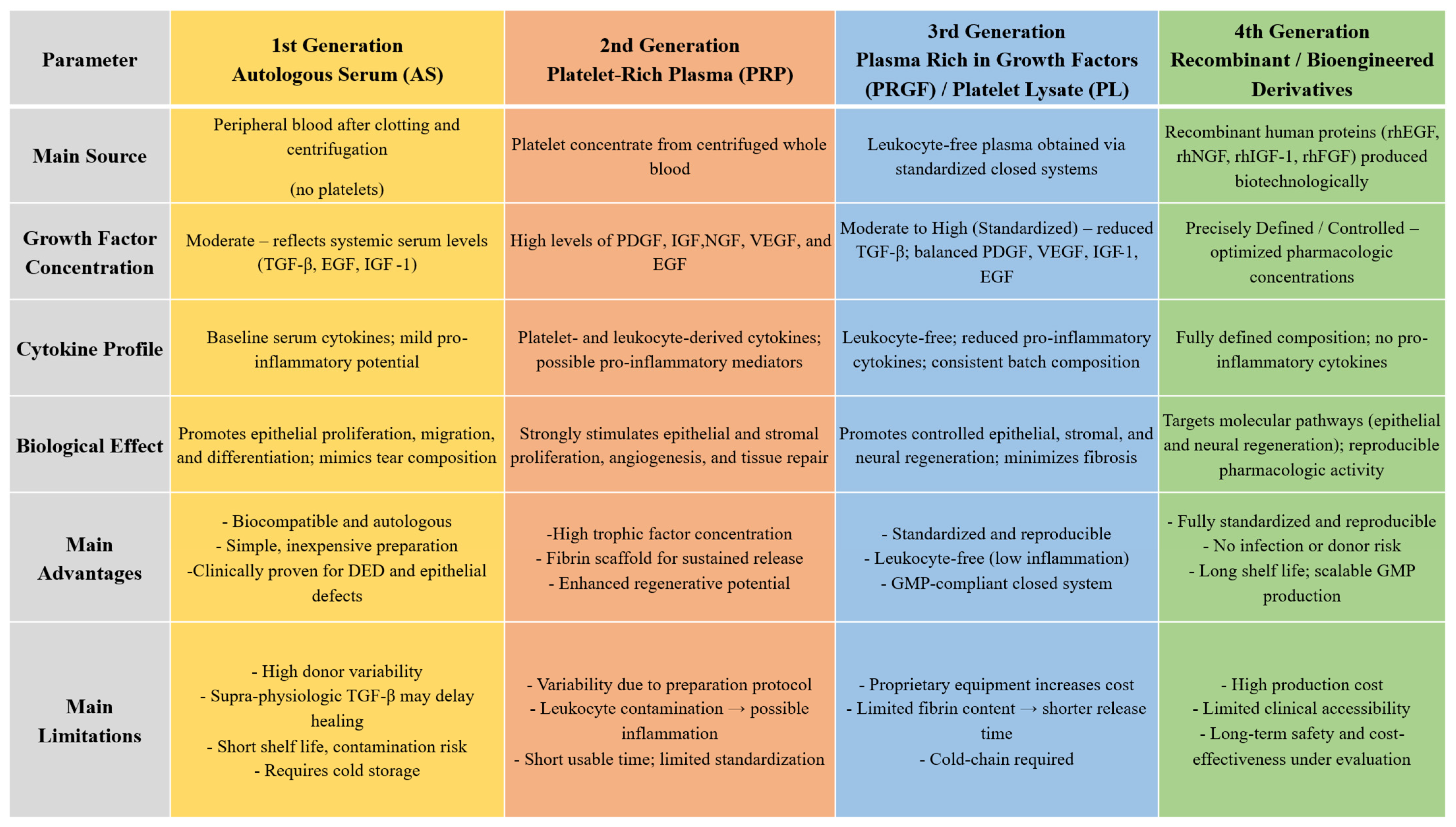
3. First-Generation Blood Derivatives: Serum-Based Therapies
3.1. Autologous Serum
| Reference | Year | Study Design | Study Group (n) | Control Group (n) | Treated Condition | Intervention | Comparator/Control | Preparation | Dosage (Times/Day) | Duration | Outcomes Measured | Outcomes Significantly Improved Compared to Control |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tananuvat et al. [52] | 2001 | Paired-eye RCT | 12 | 12 | severe DED | AS | Saline solution | 40 mL whole blood → centrifugation at 4200 rpm for 15 min → serum extraction → dilution to 20% with unpreserved normal saline solution | 6 | 2 months | Subjective symptoms, OSS, TBUT and ST | Not significant |
| Noble et al. [48] | 2004 | Crossover RCT | 16 | 16 | DED (n = 11), other ESD (n = 5) | AS | AT | Whole blood → clot at 4 °C for 2–3 days → centrifugation → dilution to 50% with sterile normal saline | Vary | 3 months | VAS, OSS, and IC | VAS, IC |
| Kojima et al. [53] | 2005 | RCT | 10 | 10 | DED | AS | AT | 40 mL whole blood → centrifugation at 1500 rpm for 5 min → dilution to 20% with saline | 6 | 2 weeks | VAS, OSS, TBUT, and ST | VAS, OSS, TBUT |
| Noda-Tsuruya et al. [65] | 2006 | RCT | 12 | 15 | DED post-LASIK | AS | AT | Not given | 5 | 12 weeks | Subjective symptoms, OSS, TBUT, and ST II | TBUT, OSS, |
| Schulze et al. [87] | 2006 | RCT | 13 | 10 | ED in diabetic patients post-pars-plana vitrectomy | AS | Hyaluronic acid | Whole blood → clot for 1 h → centrifugation at 4000 rpm for 15 min → dilution to 50% with base salt solution | hourly | until complete epithelial healing | Rate of healing of the epithelial defects | Rate of healing of the epithelial defects |
| Urzua et al. [54] | 2012 | Crossover RCT | 12 | 12 | severe DED | AS | AT | 20 mL whole blood → clot for 2 h at room temperature → centrifugation at 3500 rpm for 5 min at 4 °C → collection of supernatant → dilution at 20% with 0.9% saline solution | 4 | 2 weeks | TBUT, OSDI, OSS | OSDI |
| Celebi et al. [55] | 2014 | Crossover RCT | 20 | 20 | severe DED | AS | AT | 10 mL whole blood → clot for 2 h at room temperature → centrifugation at 4000 rpm for 10 min at 4 °C → collection of 5 mL of supernatant → dilution at 20% with 0.9% saline solution | 4 | 1 month | ST, TBUT, OSDI, OSS | TBUT, OSDI |
| Mukhopadhyay et al. [88] | 2015 | RCT | 48 (UCS), 52 (AS) | 44 | severe DED in Hansen’s disease | UCS, AS | AT | UCS: Umbilical cord blood → clot → centrifugation at 1500 rpm for 5 min → dilution to 20% with sterile saline solution AS: whole blood → centrifugation at 1500 rpm for 15 min → dilution to 20% with sterile normal saline solution | 6 | 6 weeks | McMonnies score, OSS, TBUT, ST, IC | CBS: ST, McMonnies score, TBUT, OSS and IC; AS: McMonnies score, TBUT, OSS and IC |
| Semeraro et al. [66] | 2016 | RCT | 12 | 12 | SS-related DED | AS | AT | AS: whole blood → clot for 24–48 h at 4 °C → centrifugation at 4000 rpm for 10 min → dilution to 50% with saline | 5 | 12 months | TBUT, OSDI, ST, OSS, inflammation, central corneal thickness, confocal microscopy | OSDI, number of branches, and number of beadings |
| Yılmaz et al. [67] | 2017 | Crossover RCT | 24 | 24 | DED due to systemic isotretinoin treatment | AS | AT | 20 mL whole blood → clot for 2 h at room temperature → centrifugation at 4000 rpm for 10 min at 4 °C → dilution to 40% with isotonic saline solution | Not reported | 1 month | OSDI, TBUT, and ST | TBUT, OSDI |
| Kamble et al. [76] | 2017 | RCT | 35 (UCS), 35 (AS) | 35 | ED post-keratoplasty | UCS, AS | AT | UCS: Umbilical cord blood → clot → centrifugation at 1800× g for 10 min → dilution to 20% with sterile balanced salt solution AS: whole blood → clot → centrifugation at 1800× g for 10 min → dilution to 20% with sterile balanced salt solution | 6 | until complete epithelial healing | Decrease in size of ED and rate of epithelial healing | Rate of healing of the epithelial defects, Decrease in size of the ED (UCS > AS) |
| Akcam et al. [80] | 2018 | RCT | 30 | 30 | ED post-photorefractive keratectomy | AS | AT | 20 mL whole blood → centrifugation at 1500 rpm for 10 min → dilution to 20% with artificial tears | 6 | until complete epithelial healing | Rate of healing of the epithelial defects, BCVA | Rate of healing of the epithelial defects |
| Sul et al. [79] | 2018 | RCT | 25 | 25 | ED post-pterygium surgery | AS | AT | 20 mL whole blood → clot for 1 h at room temperature → centrifugation at 3000× g for 10 min → dilution to 50% with artificial tears | 8 | until complete epithelial healing | Rate of healing of the epithelial defects, pain score, conjunctival inflammation, and recurrences | Rate of healing of the epithelial defects, pain score |
| Rodríguez Calvo-de-Mora et al. [89] | 2021 | Three-arm RCT | 21 (AS), 21 (AlS), 21 (UCS) | N/A | severe DED | AS, AlS, UCS | N/A | UCS: Umbilical cord blood → centrifugation → dilution to 20% with solution of Sterile Irrigating Solution, BSS® AS: whole blood → centrifugation → dilution to 20% with solution of Sterile Irrigating Solution, BSS® AlS: whole blood form AB-blood donors → centrifugation → dilution to 20% with solution of Sterile Irrigating Solution, BSS® | 5 | 3 months | ST, TBUT, OSS | No significant differences were found |
| Zheng et al. [56] | 2023 | RCT | 116 | 116 | DED | AS | AT | 40 mL whole blood → centrifugation at 3000 rpm for 15 min → dilution to 20% with saline solution | 4 | 12 weeks | OSDI, TBUT, ST, OSS, IC | OSDI, TBUT, ST, OSS, IC |
| Kumari et al. [59] | 2023 | RCT | 22 | 22 | moderate and severe DED | 50% AS | 20% AS | 20 mL whole blood → clot for 45 min at room temperature → centrifugation at 4000 rpm for 10 min → dilution to 20% or 50% with sterile 0.9% saline solution | 6 | 12 weeks | OSDI, TBUT, OSS, ST | in severe DED—ST, TBUT, and OSS |
| Bachtalia et al. [90] | 2025 | RCT | 10 | 10 | SS-related DED | AS | AT | whole blood → clot for 2 h at room temperature → centrifugation at (4500 rpm) 3000× g (4500 rpm) for 15 min → dilution to 50% with artificial tears | 4 | 3 months | TBUT, ST, OSS, improvement in the structure of the central corneal sub-basal nerve plexus | TBUT, ST, OSS, improvement in the structure of the central corneal sub-basal nerve plexus |
3.2. Allogeneic Serum
3.3. Umbilical Cord Serum
| Reference | Year | Study Design | Study Group (n) | Control Group (n) | Treated Condition | Intervention | Comparator/Control | Preparation | Dosage (times/Day) | Duration | Outcomes Measured | Outcomes Significantly Improved Compared to Control |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vajpayee et al. [106] | 2003 | RCT | 30 | 29 | PED | UCS | 20% AS | Umbilical cord blood → clot → centrifugation at 1500 rpm for 5 min → dilution to 20% with sterile saline solution | 6 | 3 weeks | Rate of healing of the epithelial defects | Rate of healing of the epithelial defects |
| Sharma et al. [113] | 2011 | RCT | 12 | 10 (AT), 11 (AS) | acute ocular chemical burns (AOCB) | UCS | AT, 20% AS | UCS: Umbilical cord blood → clot → centrifugation at 1800× g for 10 min → dilution to 20% with sterile balanced salt solution AS: whole blood → clot → centrifugation at 1800× g for 10 min → dilution to 20% with sterile balanced salt solution | 10 | 3 months | Pain score, size, and area of epithelial defect, extent of limbal ischemia, corneal clarity, and symblepharon formation | Pain score, rate of healing of the epithelial defect, limbal ischemia, corenal clarity, vascularization |
| Mukhopadhyay et al. [88] | 2015 | RCT | 48 (UCS), 52 (AS) | 44 | severe DED in Hansen’s disease | UCS, AS | AT | UCS: Umbilical cord blood → clot → centrifugation at 1500 rpm for 5 min → dilution to 20% with sterile saline solution AS: whole blood → centrifugation at 1500 rpm for 15 min → dilution to 20% with sterile normal saline solution | 6 | 6 weeks | McMonnies score, OSS, TBUT, ST, IC | CBS: ST, McMonnies score, TBUT, OSS and IC; AS: McMonnies score, TBUT, OSS and IC |
| Sharma et al. [114] | 2016 | RCT | 15 (UCS), 15 (AMT) | 15 | AOCB | Standard medical therapy + UCS/AMT | Standard medical therapy alone | UCS: Umbilical cord blood → clot → centrifugation at 1800× g for 10 min → dilution to 20% with sterile balanced salt solution | 10 | 3 months | Corneal clarity, rate of healing of the epithelial defects, pain score, BCVA, symblepharon, tear film status, and lid abnormalities | UCS: corneal clarity, pain score |
| Kamble et al. [76] | 2017 | RCT | 35 (UCS), 35 (AS) | 35 | ED post-keratoplasty | UCS, AS | AT | UCS: Umbilical cord blood → clot → centrifugation at 1800× g for 10 min → dilution to 20% with sterile balanced salt solution AS: whole blood → clot → centrifugation at 1800× g for 10 min → dilution to 20% with sterile balanced salt solution | 6 | until complete epithelial healing | Decrease in size of ED and rate of epithelial healing | Rate of healing of the epithelial defects, Decrease in size of the ED (UCS > AS) |
| Campos et al. [111] | 2019 | Crossover RCT | 31 | 29 | severe DED, associated with PED | UCS | 20% AS | UCS: Umbilical cord blood → centrifugation at 2800× g for 10 min → dilution to 20% with sterile phosphate-buffered saline AS: whole blood → centrifugation at 2800× g for 10 min → dilution to 20% with sterile phosphate-buffered saline | 8 | 1 month | OSDI, OSS, TBUT, ST, | OSDI subset A score, OSS |
| Moradian et al. [105] | 2020 | RCT | 40 | 40 | ED in diabetic patients post-vitrectomy | UCS | AT | Umbilical cord blood → centrifugation at 1500 rpm for 5 min → dilution to 20% with preservative-free artificial tears | 6 | 12 days | Rate of healing of the epithelial defects | Rate of healing of the epithelial defects |
| Rodríguez Calvo-de-Mora et al. [89] | 2021 | Three-arm RCT | 21 (AS), 21 (AlS), 21 (UCS) | N/A | severe DED | AS, AlS, UCS | N/A | UCS: Umbilical cord blood → centrifugation → dilution to 20% with solution of Sterile Irrigating Solution, BSS® AS: whole blood → centrifugation → dilution to 20% with solution of Sterile Irrigating Solution, BSS® AlS: whole blood form AB blood donors → centrifugation → dilution to 20% with solution of Sterile Irrigating Solution, BSS® | 5 | 3 months | ST, TBUT, OSS | No significant differences were found |
| Kumar et al. [112] | 2023 | RCT | 20 (DED), 21 (AOCB), 20 (OA) | 20 (DED), 21 (AOCB), 20 (OA) | DED, AOCB, OA | UCS | AS | Not given | Not reported | 3 months | BCVA, eye sensation score (ESS), OSDI, TBUT, ST, OSS, epithelial defect, limbal ischemia, corneal clarity, and improvement in grade of severity | BCVA, eye sensation score, OSDI, ST, TBUT, and OSS (DED), BCVA, reepithelialization, reduction in limbal ischemia, and corneal clarity (ACB), improvement in ye sensation score (OA) |
4. Second-Generation Blood Derivatives—Platelet-Rich Plasma
| Reference | Year | Study Design | Study Group (n) | Control Group (n) | Treated Condition | Intervention | Comparator/Control | Preparation | Dosage (times/Day) | Duration | Outcomes Measured | Outcomes Significantly Improved Compared to Control |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Panda et al. [121] | 2012 | RCT | 10 | 10 | AOCB | PRP | AT | Not given | 10 | 3 months | Corneal clarity, rate of healing of the epithelial defects, BCVA | Corneal clarity, rate of healing of the epithelial defects |
| Javaloy et al. [122] | 2013 | RCT | 54 | 54 | corneal sensitivity post-LASIK | PRP | Saline solution | Not given | 6 | 3 months | OSS, corneal sensitivity, confocal microscopy of sub-basal nerve plexus | OSS |
| García-Conca et al. [124] | 2019 | RCT | 44 | 39 | DED | PRP | AT | 40 mL whole blood → blood processing with RegenKit® Ophthalmology PRPTM Preparation kit (Regen Lab USA LLC, New York, NY, USA) → centrifugation at 1500× g for 5 min → resuspension of the cell pellet in the supernatant | 6 | 1 month | OSDI, OSS, TBUT, ST, and tear osmolarity | OSDI score, BCVA, hyperaemia, ST, TBUT, OSS, tear osmolarity |
| Kamiya et al. [125] | 2021 | RCT | 5 | 5 | ED post-phototherapeutic keratectomy | PRP | AT | 30–32 mL whole blood with ACD-A as anticoagulant→ injection into a TriCell PRP Kit → centrifugation at 3200 rpm for 4 min → mixing plasma and buffy coat → centrifugation at 3300 rpm for 3 min → sewparation of PRP and platelet-poor plasma | 6 | 2 weeks | Rate of healing of the epithelial defects, decrease in size of the ED, subjective symptoms | Rate of healing of the epithelial defects, Decrease in size of the ED |
| Metheetrairut et al. [8] | 2022 | RCT | 10 | 10 | DED | PRP | 20% AS | AS: 35 mL whole blood → clot for 2 h at 31 °C → centrifugation at 3000× g for 30 min at 4 °C → dilution to 20% with balanced salt solution PRP: 35 mL whole blood with sodium citrate → centrifugation at 2200× g for 10 min at 22 ± 2 °C → dilution to 20% with balanced salt solution | 10 | 1 month | ST, BCVA, OSDI, TBUT, OSS | ST and BCVA |
| Kang et al. [126] | 2023 | RCT | 14 | 16 | SS-related DED | PRP | 20% AS | AS: 24 mL whole blood → clot for 2 h at room temperature → centrifugation at 3500 rpm for 15 min → dilution to 20% with 0.1% sodium hyaluronate preservative-free eye drops PRP: 22 mL whole blood with 3.2% sodium citrate → injection into a PRS Bio Kit → centrifugation at 3000× g for 3 min → mixing plasma and buffy coat → dilution to 20% with 0.1% sodium hyaluronate preservative-free eye drops | 6 | 12 weeks | OSS, ST, TBUT, OSDI, IC metaplasia grade and goblet cell density grade | No significant differences were found |
| Jongkhajornpong et al. [10] | 2024 | RCT | 48 | 48 | DED | PRP | 100% AS | AS: 50 mL whole blood → clot for 2 h at room temperature → centrifugation at 3000× g for 15 min PRP: 36 mL whole blood with 3.2% sodium citrate → centrifugation at 350× g for 10 min at 20 °C | 8 | 4 weeks | OSDI, OSS, TBUT, ST, meibum quality and expressibility | No significant differences were found |
| Sachan et al. [129] | 2025 | RCT | 20 | 20 | DED | PRP | AT | 10 mL whole blood with sodium citrate → centrifugation at 2400 rpm → separation of upper 2/3 → centrifugation at 3600 rpm → extraction of PRP and buffy coat → dilution to 20% with balanced salt solution | Not reported | 3 months | OSDI, tear meniscus height, TBUT, ST, OSS, IC, BCVA | OSDI, tear meniscus height, TBUT, ST, OSS, IC |
5. Third-Generation Blood Derivatives—Platelet Lysate and Plasma Rich in Growth Factors
5.1. Plasma Rich in Growth Factors
5.2. Platelet Lysate
6. Fourth-Generation Blood Derivatives—Recombinant and Bioengineered Alternatives
7. Finger-Prick Autologous Blood
8. Blood-Derived Therapies in Clinical Guidelines for Ocular Surface Diseases
9. Current Limitations and Future Directions
10. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gipson, I.K. The ocular surface: The challenge to enable and protect vision: The Friedenwald lecture. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4390–4398. [Google Scholar] [CrossRef]
- Klenkler, B.; Sheardown, H.; Jones, L. Growth factors in the tear film: Role in tissue maintenance, wound healing, and ocular pathology. Ocul. Surf. 2007, 5, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Imanishi, J.; Kamiyama, K.; Iguchi, I.; Kita, M.; Sotozono, C.; Kinoshita, S. Growth factors: Importance in wound healing and maintenance of transparency of the cornea. Prog. Retin. Eye Res. 2000, 19, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Nakagawa, S.; Nishida, T. Stimulatory effects of fibronectin and EGF on migration of corneal epithelial cells. Investig. Ophthalmol. Vis. Sci. 1987, 28, 205–211. [Google Scholar]
- Sommer, A. Effects of vitamin A deficiency on the ocular surface. Ophthalmology 1983, 90, 592–600. [Google Scholar] [CrossRef]
- Mendes, B.B.; Gómez-Florit, M.; Babo, P.S.; Domingues, R.M.; Reis, R.L.; Gomes, M.E. Blood derivatives awaken in regenerative medicine strategies to modulate wound healing. Adv. Drug Deliv. Rev. 2018, 129, 376–393. [Google Scholar] [CrossRef]
- Jongkhajornpong, P.; Anothaisintawee, T.; Lekhanont, K.; Numthavaj, P.; McKay, G.; Attia, J.; Thakkinstian, A. Short-term Efficacy and Safety of Biological Tear Substitutes and Topical Secretagogues for Dry Eye Disease: A Systematic Review and Network Meta-analysis. Cornea 2022, 41, 1137–1149. [Google Scholar] [CrossRef]
- Metheetrairut, C.; Ngowyutagon, P.; Tunganuntarat, A.; Khowawisetsut, L.; Kittisares, K.; Prabhasawat, P. Comparison of epitheliotrophic factors in platelet-rich plasma versus autologous serum and their treatment efficacy in dry eye disease. Sci. Rep. 2022, 12, 8906. [Google Scholar] [CrossRef] [PubMed]
- Quinto, G.G.; Campos, M.; Behrens, A. Autologous serum for ocular surface diseases. Arq. Bras. Oftalmol. 2008, 71 (Suppl. 6), 47–54. [Google Scholar] [CrossRef]
- Jongkhajornpong, P.; Lekhanont, K.; Rattanasiri, S.; Pisitkun, P.; Thakkinstian, A. Comparison of Corneal Epitheliotrophic Factors of Undiluted Autologous Platelet-Rich Plasma and Autologous Serum Eye Drops for Dry Eye Disease. Ophthalmol. Ther. 2025, 14, 363–377. [Google Scholar] [CrossRef]
- Pan, Q.; Angelina, A.; Marrone, M.; Stark, W.J.; Akpek, E.K. Autologous serum eye drops for dry eye. Cochrane Database Syst. Rev. 2017, 2, CD009327. [Google Scholar] [CrossRef]
- Hachana, S.; Larrivée, B. TGF-β Superfamily Signaling in the Eye: Implications for Ocular Pathologies. Cells 2022, 11, 2336. [Google Scholar] [CrossRef]
- Ogata, F.T.; Verma, S.; Coulson-Thomas, V.J.; Gesteira, T.F. TGF-β-Based Therapies for Treating Ocular Surface Disorders. Cells 2024, 13, 1105. [Google Scholar] [CrossRef]
- Tandon, A.; Tovey, J.C.; Sharma, A.; Gupta, R.; Mohan, R.R. Role of transforming growth factor Beta in corneal function, biology and pathology. Curr. Mol. Med. 2010, 10, 565–578. [Google Scholar]
- de Oliveira, R.C.; Wilson, S.E. Fibrocytes, Wound Healing, and Corneal Fibrosis. Investig. Ophthalmol. Vis. Sci. 2020, 61, 28. [Google Scholar] [CrossRef]
- Guo, X.; Hutcheon, A.E.K.; Tran, J.A.; Zieske, J.D. TGF-β-target genes are differentially regulated in corneal epithelial cells and fibroblasts. New Front. Ophthalmol. 2017, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef]
- Goswami, A.G.; Basu, S.; Huda, F.; Pant, J.; Ghosh Kar, A.; Banerjee, T.; Shukla, V.K. An appraisal of vascular endothelial growth factor (VEGF): The dynamic molecule of wound healing and its current clinical applications. Growth Factors 2022, 40, 73–88. [Google Scholar] [CrossRef]
- Di, G.; Zhao, X.; Qi, X.; Zhang, S.; Feng, L.; Shi, W.; Zhou, Q. VEGF-B promotes recovery of corneal innervations and trophic functions in diabetic mice. Sci. Rep. 2017, 7, 40582. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Chen, R.; Sun, G.; Liu, X.; Lin, X.; He, C.; Xing, L.; Liu, L.; Jensen, L.D.; Kumar, A.; et al. VEGF-B prevents excessive angiogenesis by inhibiting FGF2/FGFR1 pathway. Signal Transduct. Target. Ther. 2023, 8, 305. [Google Scholar] [CrossRef]
- Ghafar, N.A.; Jalil, N.A.A.; Kamarudin, T.A. Wound healing of the corneal epithelium: A review. Asian Biomed. (Res. Rev. News) 2021, 15, 199–212. [Google Scholar] [CrossRef]
- Peterson, J.L.; Ceresa, B.P. Epidermal Growth Factor Receptor Expression in the Corneal Epithelium. Cells 2021, 10, 2409. [Google Scholar] [CrossRef]
- Liu, C.Y.; Kao, W.W. Corneal Epithelial Wound Healing. Prog. Mol. Biol. Transl. Sci. 2015, 134, 61–71. [Google Scholar]
- Papadopoulos, N.; Lennartsson, J. The PDGF/PDGFR pathway as a drug target. Mol. Asp. Med. 2018, 62, 75–88. [Google Scholar] [CrossRef]
- Gallego-Muñoz, P.; Ibares-Frías, L.; Garrote, J.A.; Valsero-Blanco, M.C.; Cantalapiedra-Rodríguez, R.; Merayo-Lloves, J.; Carmen Martínez-García, M. Human corneal fibroblast migration and extracellular matrix synthesis during stromal repair: Role played by platelet-derived growth factor-BB, basic fibroblast growth factor, and transforming growth factor-β1. J. Tissue Eng. Regen. Med. 2018, 12, e737–e746. [Google Scholar] [CrossRef]
- Wilson, S.E. Corneal wound healing. Exp. Eye Res. 2020, 197, 108089. [Google Scholar] [CrossRef]
- Rocco, M.L.; Soligo, M.; Manni, L.; Aloe, L. Nerve Growth Factor: Early Studies and Recent Clinical Trials. Curr. Neuropharmacol. 2018, 16, 1455–1465. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, H.; Huang, S. Role of NGF and its receptors in wound healing (Review). Exp. Ther. Med. 2021, 21, 599. [Google Scholar] [CrossRef]
- Annunziata, M.; Granata, R.; Ghigo, E. The IGF system. Acta Diabetol. 2011, 48, 1–9. [Google Scholar] [CrossRef]
- Stuard, W.L.; Titone, R.; Robertson, D.M. The IGF/Insulin-IGFBP Axis in Corneal Development, Wound Healing, and Disease. Front. Endocrinol. 2020, 11, 24. [Google Scholar] [CrossRef]
- Xie, Y.; Su, N.; Yang, J.; Tan, Q.; Huang, S.; Jin, M.; Ni, Z.; Zhang, B.; Zhang, D.; Luo, F.; et al. FGF/FGFR signaling in health and disease. Signal Transduct. Target. Ther. 2020, 5, 181. [Google Scholar] [CrossRef]
- Farooq, M.; Khan, A.W.; Kim, M.S.; Choi, S. The Role of Fibroblast Growth Factor (FGF) Signaling in Tissue Repair and Regeneration. Cells 2021, 10, 3242. [Google Scholar] [CrossRef]
- Ljubimov, A.V.; Saghizadeh, M. Progress in corneal wound healing. Prog. Retin. Eye Res. 2015, 49, 17–45. [Google Scholar] [CrossRef]
- Bignami, F.; Rama, P.; Ferrari, G. Substance P and its Inhibition in Ocular Inflammation. Curr. Drug Targets 2016, 17, 1265–1274. [Google Scholar] [CrossRef]
- Kopel, J.; Keshvani, C.; Mitchell, K.; Reid, T. The Activity of Substance P (SP) on the Corneal Epithelium. J. Clin. Transl. Ophthalmol. 2023, 1, 35–51. [Google Scholar] [CrossRef]
- Yang, L.; Sui, W.; Li, Y.; Qi, X.; Wang, Y.; Zhou, Q.; Gao, H. Substance P Inhibits Hyperosmotic Stress-Induced Apoptosis in Corneal Epithelial Cells through the Mechanism of Akt Activation and Reactive Oxygen Species Scavenging via the Neurokinin-1 Receptor. PLoS ONE 2016, 11, e0149865. [Google Scholar] [CrossRef]
- Słoniecka, M.; Le Roux, S.; Zhou, Q.; Danielson, P. Substance P Enhances Keratocyte Migration and Neutrophil Recruitment through Interleukin-8. Mol. Pharmacol. 2016, 89, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Di, G.; Song, P.; Han, W.; Chen, P.; Wang, Y. Role of vitamin A on the ocular surface. Exp. Eye Res. 2025, 250, 110179. [Google Scholar] [CrossRef] [PubMed]
- Zinder, R.; Cooley, R.; Vlad, L.G.; Molnar, J.A. Vitamin A and Wound Healing. Nutr. Clin. Pract. 2019, 34, 839–849. [Google Scholar] [CrossRef]
- Kim, E.C.; Kim, T.K.; Park, S.H.; Kim, M.S. The wound healing effects of vitamin A eye drops after a corneal alkali burn in rats. Acta Ophthalmol. 2012, 90, e540–e546. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.E.; Gisbert, S.; Palazón, A.; Alio, J.L. Quantification of Growth Factors and Fibronectin in Diverse Preparations of Platelet-Rich Plasma for the Treatment of Ocular Surface Disorders (E-PRP). Transl. Vis. Sci. Technol. 2020, 9, 22. [Google Scholar] [CrossRef]
- Liu, Y.; Yanai, R.; Lu, Y.; Kimura, K.; Nishida, T. Promotion by fibronectin of collagen gel contraction mediated by human corneal fibroblasts. Exp. Eye Res. 2006, 83, 1196–1204. [Google Scholar] [CrossRef]
- Ralph, R.A.; Doane, M.G.; Dohlman, C.H. Clinical experience with a mobile ocular perfusion pump. Arch. Ophthalmol. 1975, 93, 1039–1043. [Google Scholar] [CrossRef]
- Fox, R.I.; Chan, R.; Michelson, J.B.; Belmont, J.B.; Michelson, P.E. Beneficial effect of artificial tears made with autologous serum in patients with keratoconjunctivitis sicca. Arthritis Rheum. 1984, 27, 459–461. [Google Scholar] [CrossRef]
- Tsubota, K.; Goto, E.; Fujita, H.; Ono, M.; Inoue, H.; Saito, I.; Shimmura, S. Treatment of dry eye by autologous serum application in Sjögren’s syndrome. Br. J. Ophthalmol. 1999, 83, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Tsubota, K.; Goto, E.; Shimmura, S.; Shimazaki, J. Treatment of persistent corneal epithelial defect by autologous serum application. Ophthalmology 1999, 106, 1984–1989. [Google Scholar] [CrossRef]
- Geerling, G.; Maclennan, S.; Hartwig, D. Autologous serum eye drops for ocular surface disorders. Br. J. Ophthalmol. 2004, 88, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Noble, B.A.; Loh, R.S.; MacLennan, S.; Pesudovs, K.; Reynolds, A.; Bridges, L.R.; Burr, J.; Stewart, O.; Quereshi, S. Comparison of autologous serum eye drops with conventional therapy in a randomised controlled crossover trial for ocular surface disease. Br. J. Ophthalmol. 2004, 88, 647–652. [Google Scholar] [CrossRef]
- Wróbel-Dudzińska, D.; Przekora, A.; Kazimierczak, P.; Ćwiklińska-Haszcz, A.; Kosior-Jarecka, E.; Żarnowski, T. The Comparison between the Composition of 100% Autologous Serum and 100% Platelet-Rich Plasma Eye Drops and Their Impact on the Treatment Effectiveness of Dry Eye Disease in Primary Sjogren Syndrome. J. Clin. Med. 2023, 12, 3126. [Google Scholar] [CrossRef] [PubMed]
- Vazirani, J.; Sridhar, U.; Gokhale, N.; Doddigarla, V.R.; Sharma, S.; Basu, S. Autologous serum eye drops in dry eye disease: Preferred practice pattern guidelines. Indian J. Ophthalmol. 2023, 71, 1357–1363. [Google Scholar] [CrossRef]
- Liu, L.; Hartwig, D.; Harloff, S.; Herminghaus, P.; Wedel, T.; Geerling, G. An optimised protocol for the production of autologous serum eyedrops. Graefes Arch. Clin. Exp. Ophthalmol. 2005, 243, 706–714. [Google Scholar] [CrossRef]
- Tananuvat, N.; Daniell, M.; Sullivan, L.J.; Yi, Q.; McKelvie, P.; McCarty, D.J.; Taylor, H.R. Controlled study of the use of autologous serum in dry eye patients. Cornea 2001, 20, 802–806. [Google Scholar] [CrossRef]
- Kojima, T.; Ishida, R.; Dogru, M.; Goto, E.; Matsumoto, Y.; Kaido, M.; Tsubota, K. The effect of autologous serum eyedrops in the treatment of severe dry eye disease: A prospective randomized case-control study. Am. J. Ophthalmol. 2005, 139, 242–246. [Google Scholar] [CrossRef]
- Urzua, C.A.; Vasquez, D.H.; Huidobro, A.; Hernandez, H.; Alfaro, J. Randomized double-blind clinical trial of autologous serum versus artificial tears in dry eye syndrome. Curr. Eye Res. 2012, 37, 684–688. [Google Scholar] [CrossRef]
- Celebi, A.R.; Ulusoy, C.; Mirza, G.E. The efficacy of autologous serum eye drops for severe dry eye syndrome: A randomized double-blind crossover study. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Zhu, S.Q. Randomized controlled trial on the efficacy and safety of autologous serum eye drops in dry eye syndrome. World J. Clin. Cases 2023, 11, 6774–6781. [Google Scholar] [CrossRef]
- Kojima, T.; Higuchi, A.; Goto, E.; Matsumoto, Y.; Dogru, M.; Tsubota, K. Autologous serum eye drops for thetreatment of dry eye diseases. Cornea 2008, 27 (Suppl. 1), S25–S30. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.K.; Huang, W.; Kim, G.Y.; Lim, B.S. Comparison of autologous serum eye drops with different diluents. Curr. Eye Res. 2013, 38, 9–17. [Google Scholar] [CrossRef]
- Kumari, N.; Kusumesh, R.; Kumari, R.; Sinha, B.P.; Singh, V. Comparative evaluation of effectiveness of twenty versus fifty percent autologous serum eye drops in treatment of dry eye. Indian J. Ophthalmol. 2023, 71, 1603–1607. [Google Scholar] [CrossRef]
- Marks, D.C.; van der Meer, P.F. Biomedical Excellence for Safer Transfusion (BEST) Collaborative. Serum eye drops: A survey of international production methods. Vox Sang. 2017, 112, 310–317. [Google Scholar] [CrossRef] [PubMed]
- López-García, J.S.; García-Lozano, I.; Rivas, L.; Ramírez, N.; Méndez, M.T.; Raposo, R. Stability of Growth Factors in Autologous Serum Eyedrops After Long-Term Storage. Curr. Eye Res. 2016, 41, 292–298. [Google Scholar] [CrossRef]
- Jirsova, K.; Levova, K.; Kalousova, M.; Fales, I.; Frankova, V.; Vesela, V.; Zima, T.; Utheim, T.P.; Bednar, J. Time and Temperature Stability of TGF-β1, EGF and IGF-1 in 20% and 100% Human Serum. Folia Biol. 2022, 68, 45–49. [Google Scholar] [CrossRef]
- López-García, J.S.; García-Lozano, I.; Rivas, L.; Viso-Garrote, M.; Raposo, R.; Méndez, M.T. Lyophilized Autologous Serum Eyedrops: Experimental and Comparative Study. Am. J. Ophthalmol. 2020, 213, 260–266. [Google Scholar] [CrossRef]
- Hartwig, D.; Herminghaus, P.; Wedel, T.; Liu, L.; Schlenke, P.; Dibbelt, L.; Geerling, G. Topical treatment of ocular surface defects: Comparison of the epitheliotrophic capacity of fresh frozen plasma and serum on corneal epithelial cells in an in vitro cell culture model. Transfus. Med. 2005, 15, 107–113. [Google Scholar] [CrossRef]
- Noda-Tsuruya, T.; Asano-Kato, N.; Toda, I.; Tsubota, K. Autologous serum eye drops for dry eye after LASIK. J. Refract. Surg. 2006, 22, 61–66. [Google Scholar] [CrossRef]
- Semeraro, F.; Forbice, E.; Nascimbeni, G.; Taglietti, M.; Romano, V.; Guerra, G.; Costagliola, C. Effect of Autologous Serum Eye Drops in Patients with Sjögren Syndrome-related Dry Eye: Clinical and In Vivo Confocal Microscopy Evaluation of the Ocular Surface. Vivo 2016, 30, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, U.; Küçük, E.; Koç, Ç.; Gökler, E. Comparison of Autologous Serum Versus Preservative Free Artificial Tear in Patients with Dry Eyes Due to Systemic Isotretinoin Therapy. Curr. Eye Res. 2017, 42, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M.; Cruciani, M.; Mengoli, C.; Marano, G.; Capuzzo, E.; Pati, I.; Masiello, F.; Veropalumbo, E.; Pupella, S.; Vaglio, S.; et al. Serum eye drops for the treatment of ocular surface diseases: A systematic review and meta-analysis. Blood Transfus. 2019, 17, 200–209. [Google Scholar]
- Wang, L.; Cao, K.; Wei, Z.; Baudouin, C.; Labbé, A.; Liang, Q. Autologous Serum Eye Drops versus Artificial Tear Drops for Dry Eye Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Ophthalmic Res. 2020, 63, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Quan, N.G.; Leslie, L.; Li, T. Autologous Serum Eye Drops for Dry Eye: Systematic Review. Optom. Vis. Sci. 2023, 100, 564–571. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, N.; Ge, Z.; Li, F. Blood component therapy for dry eye disease: A systematic review and network meta-analysis. Front. Med. 2024, 11, 1500160. [Google Scholar] [CrossRef]
- Mederle, A.L.; Andrei, D.; Ghenciu, L.A.; Stoicescu, E.R.; Iacob, R.; Haţegan, O.A. Comparative Efficacy of Platelet-Rich Plasma, Autologous Serum, and Artificial Tears in Dry Eye Disease: A Systematic Review and Meta-Analysis. Biomedicines 2025, 13, 2316. [Google Scholar] [CrossRef] [PubMed]
- Jeng, B.H.; Dupps, W.J., Jr. Autologous serum 50% eyedrops in the treatment of persistent corneal epithelial defects. Cornea 2009, 28, 1104–1108. [Google Scholar] [CrossRef]
- Lekhanont, K.; Jongkhajornpong, P.; Choubtum, L.; Chuckpaiwong, V. Topical 100% serum eye drops for treating corneal epithelial defect after ocular surgery. BioMed Res. Int. 2013, 2013, 521315, Erratum in BioMed Res. Int. 2014, 2014, 595973. [Google Scholar] [CrossRef] [PubMed]
- Lekhanont, K.; Jongkhajornpong, P.; Anothaisintawee, T.; Chuckpaiwong, V. Undiluted Serum Eye Drops for the Treatment of Persistent Corneal Epitheilal Defects. Sci. Rep. 2016, 6, 38143. [Google Scholar] [CrossRef]
- Kamble, N.; Sharma, N.; Maharana, P.K.; Bandivadekar, P.; Nagpal, R.; Agarwal, T.; Velpandian, T.; Mittal, S.; Vajpayee, R.B. Evaluation of the Role of Umbilical Cord Serum and Autologous Serum Therapy in Reepithelialization After Keratoplasty: A Randomized Controlled Clinical Trial. Eye Contact Lens 2017, 43, 324–329. [Google Scholar] [CrossRef]
- Lee, Y.K.; Lin, Y.C.; Tsai, S.H.; Chen, W.L.; Chen, Y.M. Therapeutic outcomes of combined topical autologous serum eye drops with silicone-hydrogel soft contact lenses in the treatment of corneal persistent epithelial defects: A preliminary study. Contact Lens Anterior Eye 2016, 39, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Wang, W.Y.; Lin, Y.C.; Tsai, S.H.; Lou, Y.T. Use of Autologous Serum Eye Drops with Contact Lenses in the Treatment of Chemical Burn-Induced Bilateral Corneal Persistent Epithelial Defects. BioMed Res. Int. 2022, 2022, 6600788. [Google Scholar] [CrossRef]
- Sul, S.; Korkmaz, S.; Alacamli, G.; Ozyol, P.; Ozyol, E. Application of autologous serum eye drops after pterygium surgery: A prospective study. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 1939–1943. [Google Scholar] [CrossRef]
- Akcam, H.T.; Unlu, M.; Karaca, E.E.; Yazici, H.; Aydin, B.; Hondur, A.M. Autologous serum eye-drops and enhanced epithelial healing time after photorefractive keratectomy. Clin. Exp. Optom. 2018, 101, 34–37. [Google Scholar] [CrossRef]
- Kirgiz, A.; Akdemir, M.O.; Yilmaz, A.; Kaldirim, H.; Atalay, K.; Asik Nacaroglu, S. The Use of Autologous Serum Eye Drops after Epithelium-off Corneal Collagen Crosslinking. Optom. Vis. Sci. 2020, 97, 300–304. [Google Scholar] [CrossRef]
- Yeh, S.I.; Chu, T.W.; Cheng, H.C.; Wu, C.H.; Tsao, Y.P. The Use of Autologous Serum to Reverse Severe Contact Lens-induced Limbal Stem Cell Deficiency. Cornea 2020, 39, 736–741. [Google Scholar] [CrossRef]
- Roumeau, S.; Dutheil, F.; Sapin, V.; Baker, J.S.; Watson, S.L.; Pereira, B.; Chiambaretta, F.; Navel, V. Efficacy of treatments for neurotrophic keratopathy: A systematic review and meta-analysis. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 2623–2637. [Google Scholar] [CrossRef] [PubMed]
- Welder, J.D.; Bakhtiari, P.; Djalilian, A.R. Limbitis secondary to autologous serum eye drops in a patient with atopic keratoconjunctivitis. Case Rep. Ophthalmol. Med. 2011, 2011, 576521. [Google Scholar] [CrossRef]
- McDonnell, P.J.; Schanzlin, D.J.; Rao, N.A. Immunoglobulin deposition in the cornea after application of autologous serum. Arch. Ophthalmol. 1988, 106, 1423–1425. [Google Scholar] [CrossRef]
- Sanz-Marco, E.; Lopez-Prats, M.J.; Garcia-Delpech, S.; Udaondo, P.; Diaz-Llopis, M. Fulminant bilateral Haemophilus influenzae keratitis in a patient with hypovitaminosis A treated with contaminated autologous serum. Clin. Ophthalmol. 2011, 5, 71–73. [Google Scholar] [CrossRef]
- Schulze, S.D.; Sekundo, W.; Kroll, P. Autologous serum for the treatment of corneal epithelial abrasions in diabetic patients undergoing vitrectomy. Am. J. Ophthalmol. 2006, 142, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Sen, S.; Datta, H. Comparative role of 20% cord blood serum and 20% autologous serum in dry eye associated with Hansen’s disease: A tear proteomic study. Br. J. Ophthalmol. 2015, 99, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Calvo-de-Mora, M.; Domínguez-Ruiz, C.; Barrero-Sojo, F.; Rodríguez-Moreno, G.; Antúnez Rodríguez, C.; Ponce Verdugo, L.; Hernández Lamas, M.D.C.; Hernández-Guijarro, L.; Villalvilla Castillo, J.; Fernández-Baca Casares, I.; et al. Autologous versus allogeneic versus umbilical cord sera for the treatment of severe dry eye disease: A double-blind randomized clinical trial. Acta Ophthalmol. 2022, 100, e396–e408. [Google Scholar] [CrossRef]
- Bachtalia, K.; Plakitsi, A.; Voudouri, A.; Terzidou, C.; Dalianis, G.; Kopsinis, G.; Palioura, S. The Effect of Autologous Serum Tears 50% on the Ocular Surface of Patients With Severe Dry Eye Disease due to Sjogren Syndrome: A Prospective, Double-Blind, Randomized, Controlled, Contralateral Eye Study. Cornea 2025, 44, 856–865. [Google Scholar] [CrossRef]
- Chmielewska, K.; Janus, J.; Mikołowska, A.; Wrzodak, K.; Stącel, M.; Antoniewicz-Papis, J. Correlation between serum cytokine levels and the effect of allogeneic serum-based eye drops. Transfus. Apher. Sci. 2024, 63, 103912. [Google Scholar] [CrossRef]
- Ripa, M.; Jabbehdari, S.; Yazdanpanah, G.; Lukacs, E.; Karcher, B.; Iqbal, O.; Bouchard, C. The Role of Multisystem Disease in Composition of Autologous Serum tears and ocular surface symptom improvement. Ocul. Surf. 2020, 18, 499–504. [Google Scholar] [CrossRef]
- Kang, N.H.; Lee, S.; Jun, R.M. Comparison of epitheliotrophic factors in autologous serum eyedrops from sera of chronic renal failure patients vs. normal controls. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 1705–1712. [Google Scholar] [CrossRef] [PubMed]
- Harloff, S.; Hartwig, D.; Kasper, K.; Wedel, T.; Müller, M.; Geerling, G. Epitheliotrophic capacity of serum eye drops from healthy donors versus serum from immunosuppressed patients with rheumatoid arthritis. Klin. Monbl. Augenheilkd. 2008, 225, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Harritshøj, L.H.; Nielsen, C.; Ullum, H.; Hansen, M.B.; Julian, H.O. Ready-made allogeneic ABO-specific serum eye drops: Production from regular male blood donors, clinical routine, safety and efficacy. Acta Ophthalmol. 2014, 92, 783–786. [Google Scholar] [CrossRef] [PubMed]
- van der Meer, P.F.; Verbakel, S.K.; Honohan, Á.; Lorinser, J.; Thurlings, R.M.; Jacobs, J.F.M.; de Korte, D.; Eggink, C.A. Allogeneic and autologous serum eye drops: A pilot double-blind randomized crossover trial. Acta Ophthalmol. 2021, 99, 837–842. [Google Scholar] [CrossRef]
- Gabriel, C.; Marks, D.C.; Henschler, R.; Schallmoser, K.; Burnouf, T.; Koh, M.B.C. Eye drops of human origin-Current status and future needs: Report on the workshop organized by the ISBT Working Party for Cellular Therapies. Vox Sang. 2023, 118, 301–309. [Google Scholar] [CrossRef]
- Voß, S.; Behrmann, T.; Reichl, S. Development of In Vitro Dry Eye Models to Study Proliferative and Anti-Inflammatory Effects of Allogeneic Serum Eye Drops. Int. J. Mol. Sci. 2023, 24, 1567. [Google Scholar] [CrossRef]
- Chiang, C.C.; Chen, W.L.; Lin, J.M.; Tsai, Y.Y. Allogeneic serum eye drops for the treatment of persistent corneal epithelial defect. Eye 2009, 23, 290–293. [Google Scholar] [CrossRef]
- Na, K.S.; Kim, M.S. Allogeneic serum eye drops for the treatment of dry eye patients with chronic graft-versus-host disease. J. Ocul. Pharmacol. Ther. 2012, 28, 479–483. [Google Scholar] [CrossRef]
- Lomas, R.J.; Chandrasekar, A.; Macdonald-Wallis, C.; Kaye, S.; Rauz, S.; Figueiredo, F.C. Patient-reported outcome measures for a large cohort of serum eye drops recipients in the UK. Eye 2021, 35, 3425–3432. [Google Scholar] [CrossRef] [PubMed]
- Maharajan, N.; Cho, G.W.; Choi, J.H.; Jang, C.H. Regenerative Therapy Using Umbilical Cord Serum. Vivo 2021, 35, 699–705. [Google Scholar] [CrossRef]
- Yoon, K.C.; You, I.C.; Im, S.K.; Jeong, T.S.; Park, Y.G.; Choi, J. Application of umbilical cord serum eyedrops for the treatment of neurotrophic keratitis. Ophthalmology 2007, 114, 1637–1642. [Google Scholar] [CrossRef]
- Yoon, K.C.; Im, S.K.; Park, Y.G.; Jung, Y.D.; Yang, S.Y.; Choi, J. Application of umbilical cord serum eyedrops for the treatment of dry eye syndrome. Cornea 2006, 25, 268–272. [Google Scholar] [CrossRef]
- Moradian, S.; Ebrahimi, M.; Kanaani, A.; Faramarzi, A.; Safi, S. Topical Umbilical Cord Serum for Corneal Epithelial Defects after Diabetic Vitrectomy. J. Ophthalmic Vis. Res. 2020, 15, 160–165. [Google Scholar] [CrossRef]
- Vajpayee, R.B.; Mukerji, N.; Tandon, R.; Sharma, N.; Pandey, R.M.; Biswas, N.R.; Malhotra, N.; Melki, S.A. Evaluation of umbilical cord serum therapy for persistent corneal epithelial defects. Br. J. Ophthalmol. 2003, 87, 1312–1316. [Google Scholar] [CrossRef]
- Yoon, K.C.; Heo, H.; Jeong, I.Y.; Park, Y.G. Therapeutic effect of umbilical cord serum eyedrops for persistent corneal epithelial defect. Korean J. Ophthalmol. 2005, 19, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.C.; Jeong, I.Y.; Im, S.K.; Park, Y.G.; Kim, H.J.; Choi, J. Therapeutic effect of umbilical cord serum eyedrops for the treatment of dry eye associated with graft-versus-host disease. Bone Marrow Transplant. 2007, 39, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Susiyanti, M.; Kurnia, D.A.; Fasha, I.; Irawati, Y.; Rachmadi, L.; Liem, I.K.; Artini, W. Treatment of Severe Dry Eye in Stevens-Johnson Syndrome with Umbilical Cord Serum Eye Drops. Clin. Ophthalmol. 2022, 16, 4089–4095. [Google Scholar] [CrossRef]
- Giannaccare, G.; Pellegrini, M.; Bernabei, F.; Moscardelli, F.; Buzzi, M.; Versura, P.; Campos, E.C. In Vivo Confocal Microscopy Automated Morphometric Analysis of Corneal Subbasal Nerve Plexus in Patients With Dry Eye Treated With Different Sources of Homologous Serum Eye Drops. Cornea 2019, 38, 1412–1417. [Google Scholar] [CrossRef]
- Campos, E.; Versura, P.; Buzzi, M.; Fontana, L.; Giannaccare, G.; Pellegrini, M.; Lanconelli, N.; Brancaleoni, A.; Moscardelli, F.; Sebastiani, S.; et al. Blood derived treatment from two allogeneic sources for severe dry eye associated to keratopathy: A multicentre randomised cross over clinical trial. Br. J. Ophthalmol. 2020, 104, 1142–1147. [Google Scholar] [CrossRef]
- Kumar, A.; Chaurasiya, D.; Sultan, S.; Soni, D.; Kubrey, S.; Singh, P.; Verma, S.; Mohan, R.R.; Sharma, B. Therapeutic Profile of Human Umbilical Cord Blood Serum and Autologous Serum Therapies in Treatment of Ocular Surface Disorders: A Pilot Study. J. Ocul. Pharmacol. Ther. 2023, 39, 36–47. [Google Scholar] [CrossRef]
- Sharma, N.; Goel, M.; Velpandian, T.; Titiyal, J.S.; Tandon, R.; Vajpayee, R.B. Evaluation of umbilical cord serum therapy in acute ocular chemical burns. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1087–1092. [Google Scholar] [CrossRef]
- Sharma, N.; Singh, D.; Maharana, P.K.; Kriplani, A.; Velpandian, T.; Pandey, R.M.; Vajpayee, R.B. Comparison of Amniotic Membrane Transplantation and Umbilical Cord Serum in Acute Ocular Chemical Burns: A Randomized Controlled Trial. Am. J. Ophthalmol. 2016, 168, 157–163. [Google Scholar] [CrossRef]
- Marx, R.E. Platelet-rich plasma (PRP): What is PRP and what is not PRP? Implant Dent. 2001, 10, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Dhurat, R.; Sukesh, M. Principles and Methods of Preparation of Platelet-Rich Plasma: A Review and Author’s Perspective. J. Cutan. Aesthet. Surg. 2014, 7, 189–197. [Google Scholar] [CrossRef]
- Marx, R.E. Platelet-rich plasma: Evidence to support its use. J. Oral Maxillofac. Surg. 2004, 62, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Muruzabal, F.; Tayebba, A.; Riestra, A.; Perez, V.L.; Merayo-Lloves, J.; Orive, G. Autologous serum and plasma rich in growth factors in ophthalmology: Preclinical and clinical studies. Acta Ophthalmol. 2015, 93, e605–e614. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.; Grimalt, R. A Review of Platelet-Rich Plasma: History, Biology, Mechanism of Action, and Classification. Skin Appendage Disord. 2018, 4, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Alio, J.L.; Abad, M.; Artola, A.; Rodriguez-Prats, J.L.; Pastor, S.; Ruiz-Colecha, J. Use of autologous platelet-rich plasma in the treatment of dormant corneal ulcers. Ophthalmology 2007, 114, 1286–1293.e1. [Google Scholar] [CrossRef]
- Panda, A.; Jain, M.; Vanathi, M.; Velpandian, T.; Khokhar, S.; Dada, T. Topical autologous platelet-rich plasma eyedrops for acute corneal chemical injury. Cornea 2012, 31, 989–993. [Google Scholar] [CrossRef]
- Javaloy, J.; Alió, J.L.; Rodriguez, A.E.; Vega, A.; Muñoz, G. Effect of platelet-rich plasma in nerve regeneration after LASIK. J. Refract. Surg. 2013, 29, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Avila, M.Y.; Igua, A.M.; Mora, A.M. Randomised, prospective clinical trial of platelet-rich plasma injection in the management of severe dry eye. Br. J. Ophthalmol. 2019, 103, 648–653. [Google Scholar] [CrossRef]
- García-Conca, V.; Abad-Collado, M.; Hueso-Abancens, J.R.; Mengual-Verdú, E.; Piñero, D.P.; Aguirre-Balsalobre, F.; Molina, J.C. Efficacy and safety of treatment of hyposecretory dry eye with platelet-rich plasma. Acta Ophthalmol. 2019, 97, e170–e178. [Google Scholar] [CrossRef]
- Kamiya, K.; Takahashi, M.; Shoji, N. Effect of Platelet-Rich Plasma on Corneal Epithelial Healing after Phototherapeutic Keratectomy: An Intraindividual Contralateral Randomized Study. BioMed Res. Int. 2021, 2021, 5752248. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Lee, J.H.; Hwang, J.; Chung, S.H. Efficacy and safety of platelet-rich plasma and autologous-serum eye drops for dry eye in primary Sjögren’s syndrome: A randomized trial. Sci. Rep. 2023, 13, 19279. [Google Scholar] [CrossRef]
- Freire, V.; Andollo, N.; Etxebarria, J.; Durán, J.A.; Morales, M.C. In vitro effects of three blood derivatives on human corneal epithelial cells. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5571–5578. [Google Scholar] [CrossRef]
- Kwaku Akowuah, P.; Junior Obinwanne, C.; Owusu, E.; Kyeremeh, S.; Bonsu, K.; Karikari, L.A.A.; Akyaa Akomeah, F.; Kyei Nkansah, E.; Kobia-Acquah, E. Platelet-rich plasma for treating dry eye disease—A systematic review and meta-analysis. Cont. Lens Anterior Eye 2024, 47, 102091. [Google Scholar] [CrossRef] [PubMed]
- Sachan, S.; Dwivedi, K.; Singh, S.P.; Kumar, S.; Singh, V.K. Comparison of 20% Autologous Platelet-Rich Plasma Versus Conventional Treatment in Moderate to Severe Dry Eye Patients. Turk. J. Ophthalmol. 2025, 55, 112–119. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Allam, I.Y.; Shaheen, M.S.; Lazreg, S.; Doheim, M.F. Lacrimal gland injection of platelet rich plasma for treatment of severe dry eye: A comparative clinical study. BMC Ophthalmol. 2022, 22, 343. [Google Scholar] [CrossRef]
- López-Plandolit, S.; Morales, M.C.; Freire, V.; Etxebarría, J.; Durán, J.A. Plasma rich in growth factors as a therapeutic agent for persistent corneal epithelial defects. Cornea 2010, 29, 843–848. [Google Scholar] [CrossRef]
- Sánchez-Avila, R.M.; Merayo-Lloves, J.; Riestra, A.C.; Fernandez, M.L.; Matilla, M.; Muruzabal, F. Plasma rich in growth factors for the treatment of dry eye after refractive surgery. J. Refract. Surg. 2018, 34, 222–229. [Google Scholar]
- Mora, A.M.; Córdoba, C.M.; Jimenez-Mora, M.A.; Padilla-Pantoja, F.D. Sustained long-term benefits of autologous subconjunctival platelet-rich plasma injections for severe dry eye disease. Regen. Med. 2025, 20, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Telandro, A.; Barguigua, A.; Baba, M.; Körber, N. Evaluation of the Use of Highly Concentrated Autologous Platelet-Rich Plasma and Platelet-Rich Fibrin Membrane to Improve the Outcome in the Management of Severe Dry Eye Disease, Corneal Neurotrophic Ulcer and Corneal Burn. Cureus 2024, 16, e51794. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anitua, E.; Muruzabal, F.; de la Fuente, M.; Riestra, A.; Merayo-Lloves, J.; Orive, G. PRGF exerts more potent proliferative and anti-inflammatory effects than autologous serum on a cell culture inflammatory model. Exp. Eye Res. 2016, 151, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; de la Fuente, M.; Sánchez-Ávila, R.M.; de la Sen-Corcuera, B.; Merayo-Lloves, J.; Muruzábal, F. Beneficial Effects of Plasma Rich in Growth Factors (PRGF) Versus Autologous Serum and Topical Insulin in Ocular Surface Cells. Curr. Eye Res. 2023, 48, 456–464. [Google Scholar] [CrossRef]
- Anitua, E.; de la Fuente, M.; Muruzabal, F.; Riestra, A.; Merayo-Lloves, J.; Orive, G. Plasma rich in growth factors (PRGF) eye drops stimulates scarless regeneration compared to autologous serum in the ocular surface stromal fibroblasts. Exp. Eye Res. 2015, 135, 118–126. [Google Scholar] [CrossRef]
- López-Plandolit, S.; Morales, M.C.; Freire, V.; Grau, A.E.; Durán, J.A. Efficacy of plasma rich in growth factors for the treatment of dry eye. Cornea 2011, 30, 1312–1317. [Google Scholar] [CrossRef]
- Merayo-Lloves, J.; Sanchez-Avila, R.M.; Riestra, A.C.; Anitua, E.; Begoña, L.; Orive, G.; Fernandez-Vega, L. Safety and Efficacy of Autologous Plasma Rich in Growth Factors Eye Drops for the Treatment of Evaporative Dry Eye. Ophthalmic Res. 2016, 56, 68–73. [Google Scholar] [CrossRef]
- Lozano-Sanroma, J.; Barros, A.; Alcalde, I.; Alvarado-Villacorta, R.; Sánchez-Ávila, R.M.; Queiruga-Piñeiro, J.; Cueto, L.F.; Anitua, E.; Merayo-Lloves, J. Efficacy and Safety of Plasma Rich in Growth Factor in Patients with Congenital Aniridia and Dry Eye Disease. Diseases 2024, 12, 76. [Google Scholar] [CrossRef]
- Sanchez-Avila, R.M.; Merayo-Lloves, J.; Fernandez, M.L.; Rodriguez-Gutierrez, L.A.; Jurado, N.; Muruzabal, F.; Orive, G.; Anitua, E. Plasma Rich in Growth Factors for the Treatment of Dry Eye after LASIK Surgery. Ophthalmic Res. 2018, 60, 80–86. [Google Scholar] [CrossRef]
- Sanchez-Avila, R.M.; Merayo-Lloves, J.; Riestra, A.C.; Anitua, E.; Muruzabal, F.; Orive, G.; Fernández-Vega, L. The Effect of Immunologically Safe Plasma Rich in Growth Factor Eye Drops in Patients with Sjögren Syndrome. J. Ocul. Pharmacol. Ther. 2017, 33, 391–399. [Google Scholar] [CrossRef]
- Sanchez-Avila, R.M.; Merayo-Lloves, J.; Muruzabal, F.; Orive, G.; Anitua, E. Plasma rich in growth factors for the treatment of dry eye from patients with graft versus host diseases. Eur. J. Ophthalmol. 2020, 30, 94–103. [Google Scholar] [CrossRef]
- Sanchez-Avila, R.M.; Merayo-Lloves, J.; Riestra, A.C.; Fernandez-Vega Cueto, L.; Anitua, E.; Begoña, L.; Muruzabal, F.; Orive, G. Treatment of patients with neurotrophic keratitis stages 2 and 3 with plasma rich in growth factors (PRGF-Endoret) eye-drops. Int. Ophthalmol. 2018, 38, 1193–1204. [Google Scholar] [CrossRef]
- Wang, M.; Yennam, S.; Pflugfelder, S. Initial experiences using plasma rich in growth factors to treat keratoneuralgia. Front. Med. 2022, 9, 946828. [Google Scholar] [CrossRef]
- de la Sen-Corcuera, B.; Montero-Iruzubieta, J.; Sánchez-Ávila, R.M.; Orive, G.; Anitua, E.; Caro-Magdaleno, M.; Merayo-Lloves, J. Plasma Rich in Growth Factors for the Treatment of Cicatrizing Conjunctivitis. Clin. Ophthalmol. 2020, 14, 1619–1627. [Google Scholar] [CrossRef]
- Sánchez-Avila, R.M.; Merayo-Lloves, J.; Fernández, M.L.; Rodríguez-Gutiérrez, L.A.; Rodríguez-Calvo, P.P.; Fernández-Vega Cueto, A.; Muruzabal, F.; Orive, G.; Anitua, E. Plasma rich in growth factors eye drops to treat secondary ocular surface disorders in patients with glaucoma. Int. Med. Case Rep. J. 2018, 11, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Soifer, M.; Tovar, A.; Wang, M.; Mousa, H.M.; Yennam, S.; Sabater, A.L.; Pflugfelder, S.C.; Perez, V.L. A multicenter report of the use of plasma rich in growth factors (PRGF) for the treatment of patients with ocular surface diseases in North America. Ocul. Surf. 2022, 25, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; de la Fuente, M.; Muruzábal, F.; Merayo-Lloves, J. Short- and Long-Term Stability of Plasma Rich in Growth Factors Eye Drops. Cornea 2021, 40, 107–112. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Hodge, C.; Hoque, M.; Petsoglou, C.; Sutton, G. Human Platelets and Derived Products in Treating Ocular Surface Diseases—A Systematic Review. Clin. Ophthalmol. 2020, 14, 3195–3210. [Google Scholar] [CrossRef]
- Zhang, J.; Crimmins, D.; Faed, J.M.; Flanagan, P.; McGhee, C.N.J.; Patel, D.V. Characteristics of Platelet Lysate Compared to Autologous and Allogeneic Serum Eye Drops. Transl. Vis. Sci. Technol. 2020, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.J.; Sun, Y.C.; Christopher, K.; Pai, A.S.; Lu, C.J.; Hu, F.R.; Lin, S.Y.; Chen, W.L. Comparison of corneal epitheliotrophic capacities among human platelet lysates and other blood derivatives. PLoS ONE 2017, 12, e0171008. [Google Scholar] [CrossRef]
- Petsoglou, C.; Wen, L.; Hoque, M.; Zhu, M.; Valtink, M.; Sutton, G.; You, J. Effects of human platelet lysate on the growth of cultured human corneal endothelial cells. Exp. Eye Res. 2021, 208, 108613. [Google Scholar] [CrossRef]
- Widyaningrum, R.; Burnouf, T.; Nebie, O.; Delila, L.; Wang, T.J. A purified human platelet pellet lysate rich in neurotrophic factors and antioxidants repairs and protects corneal endothelial cells from oxidative stress. Biomed. Pharmacother. 2021, 142, 112046. [Google Scholar] [CrossRef]
- Pezzotta, S.; Del Fante, C.; Scudeller, L.; Rossi, G.C.; Perotti, C.; Bianchi, P.E.; Antoniazzi, E. Long-term safety and efficacy of autologous platelet lysate drops for treatment of ocular GvHD. Bone Marrow Transplant. 2017, 52, 101–106. [Google Scholar] [CrossRef]
- Zallio, F.; Mazzucco, L.; Monaco, F.; Astori, M.R.; Passera, R.; Drago, G.; Tamiazzo, S.; Rapetti, M.; Dolcino, D.; Guaschino, R.; et al. A Single-Center Pilot Prospective Study of Topical Application of Platelet-Derived Eye Drops for Patients with Ocular Chronic Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2016, 22, 1664–1670. [Google Scholar] [CrossRef]
- Pezzotta, S.; Del Fante, C.; Scudeller, L.; Cervio, M.; Antoniazzi, E.R.; Perotti, C. Autologous platelet lysate for treatment of refractory ocular GVHD. Bone Marrow Transplant. 2012, 47, 1558–1563. [Google Scholar] [CrossRef] [PubMed]
- Sugar, A.; Hussain, M.; Chamberlain, W.; Dana, R.; Kelly, D.P.; Ta, C.; Irvine, J.; Daluvoy, M.; Perez, V.; Olson, J.; et al. A Randomized Trial of Topical Fibrinogen-Depleted Human Platelet Lysate Treatment of Dry Eye Secondary to Chronic Graft-versus-Host Disease. Ophthalmol. Sci. 2022, 2, 100176. [Google Scholar] [CrossRef]
- Fea, A.M.; Aragno, V.; Testa, V.; Machetta, F.; Parisi, S.; D’Antico, S.; Spinetta, R.; Fusaro, E.; Grignolo, F.M. The Effect of Autologous Platelet Lysate Eye Drops: An In Vivo Confocal Microscopy Study. BioMed Res. Int. 2016, 2016, 8406832. [Google Scholar] [CrossRef] [PubMed]
- Geremicca, W.; Fonte, C.; Vecchio, S. Blood components for topical use in tissue regeneration: Evaluation of corneal lesions treated with platelet lysate and considerations on repair mechanisms. Blood Transfus. 2010, 8, 107–112. [Google Scholar]
- Abu-Ameerh, M.A.; Jafar, H.D.; Hasan, M.H.; Al Bdour, M.D.; Msallam, M.; Ababneh, O.H.; Alhattab, D.M.; Al-Kurdi, B.; Awidi, A.A.; Awidi, A.S. Platelet lysate promotes re-epithelialization of persistent epithelial defects: A pilot study. Int. Ophthalmol. 2019, 39, 1483–1490. [Google Scholar] [CrossRef]
- Mallis, P.; Michalopoulos, E.; Sarri, E.F.; Papadopoulou, E.; Theodoropoulou, V.; Katsimpoulas, M.; Stavropoulos-Giokas, C. Evaluation of the Regenerative Potential of Platelet-Lysate and Platelet-Poor Plasma Derived from the Cord Blood Units in Corneal Wound Healing Applications: An In Vitro Comparative Study on Corneal Epithelial Cells. Curr. Issues Mol. Biol. 2022, 44, 4415–4438. [Google Scholar] [CrossRef]
- Samarkanova, D.; Martin, S.; Bisbe, L.; Puig, J.; Calatayud-Pinuaga, M.; Rodriguez, L.; Azqueta, C.; Coll, R.; Casaroli-Marano, R.; Madrigal, A.; et al. Clinical evaluation of allogeneic eye drops from cord blood platelet lysate. Blood Transfus. 2021, 19, 347–356. [Google Scholar]
- Dai, X.; Tunc, U.; Zhu, X.; Karakus, S. Effect of Topical Recombinant Human Nerve Growth Factor on Corneal Epithelial Regeneration in Refractory Epithelial Keratopathy. Ocul. Immunol. Inflamm. 2024, 32, 2074–2080. [Google Scholar] [CrossRef]
- Li, J.L.; Zhao, J.; Guo, Z.F.; Xiao, C.; Liu, X. Efficacy of recombinant human epidermal growth factor plus sodium hyaluronate eye drops in diabetic dry eye post-cataract surgery. World J. Diabetes 2024, 15, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Tauber, J.; Laurie, G.W.; Parsons, E.C.; Odrich, M.G.; Lacripep Study Group; Lacripep Study Group. Lacripep for the Treatment of Primary Sjögren-Associated Ocular Surface Disease: Results of the First-In-Human Study. Cornea 2023, 42, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, J.; Yuan, J.; Pan, Z.; Siri, G.; Hauswirth, S.G.; Mantelli, F.; Shi, W. Cenegermin for the Treatment of Moderate or Severe Neurotrophic Keratopathy: Results from a Prospective, Phase IV, Open-Label Study in China. Ophthalmol. Ther. 2025, 14, 3021–3033. [Google Scholar] [CrossRef]
- Pflugfelder, S.C.; Massaro-Giordano, M.; Perez, V.L.; Hamrah, P.; Deng, S.X.; Espandar, L.; Foster, C.S.; Affeldt, J.; Seedor, J.A.; Afshari, N.A.; et al. Topical Recombinant Human Nerve Growth Factor (Cenegermin) for Neurotrophic Keratopathy: A Multicenter Randomized Vehicle-Controlled Pivotal Trial. Ophthalmology 2020, 127, 14–26. [Google Scholar] [CrossRef]
- Bruscolini, A.; Marenco, M.; Albanese, G.M.; Lambiase, A.; Sacchetti, M. Long-term clinical efficacy of topical treatment with recombinant human nerve growth factor in neurotrophic keratopathy: A novel cure for a rare degenerative corneal disease? Orphanet J. Rare Dis. 2022, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Brazzell, R.K.; Stern, M.E.; Aquavella, J.V.; Beuerman, R.W.; Baird, L. Human recombinant epidermal growth factor in experimental corneal wound healing. Investig. Ophthalmol. Vis. Sci. 1991, 32, 336–340. [Google Scholar]
- Yoo, H.; Yoon, S.; Jang, I.-J.; Yu, K.-S.; Hyon, J.Y.; Hwang, J.; Hwang, I.; Sunwoo, J.; Chung, J.-Y. Safety, Tolerability, and Serum/Tear Pharmacokinetics of Human Recombinant Epidermal Growth Factor Eyedrops in Healthy Subjects. Pharmaceuticals 2022, 15, 1312. [Google Scholar] [CrossRef] [PubMed]
- Partal, A.; Scott, E. Low-cost protocol for the production of autologous serum eye drops by blood collection and processing centres for the treatment of ocular surface diseases. Transfus. Med. 2011, 21, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Than, J.; Balal, S.; Wawrzynski, J.; Nesaratnam, N.; Saleh, G.M.; Moore, J.; Patel, A.; Shah, S.; Sharma, B.; Kumar, B.; et al. Fingerprick autologous blood: A novel treatment for dry eye syndrome. Eye 2017, 31, 1655–1663. [Google Scholar] [CrossRef]
- Balal, S.; Nitiahpapand, R.; Hassan, A.; Than, J.; Patel, A.; Kumar, B.; Sharma, A. Finger-Prick Autologous Blood in the Treatment of Persistent Corneal Epithelial Defects. Cornea 2020, 39, 594–597. [Google Scholar] [CrossRef]
- Hassan, A.; Balal, S.; Cook, E.; Dehbi, H.M.; Pardhan, S.; Bourne, R.; Ahmad, S.; Sharma, A. Finger-Prick Autologous Blood (FAB) Eye Drops for Dry Eye Disease: Single Masked Multi-Centre Randomised Controlled Trial. Clin. Ophthalmol. 2022, 16, 3973–3979. [Google Scholar] [CrossRef]
- Jones, L.; Downie, L.E.; Korb, D.; Benitez-Del-Castillo, J.M.; Dana, R.; Deng, S.X.; Dong, P.N.; Geerling, G.; Hida, R.Y.; Liu, Y.; et al. TFOS DEWS II Management and Therapy Report. Ocul. Surf. 2017, 15, 575–628. [Google Scholar] [CrossRef]
- Jones, L.; Craig, J.P.; Markoulli, M.; Karpecki, P.; Akpek, E.K.; Basu, S.; Bitton, E.; Chen, W.; Dhaliwal, D.K.; Dogru, M.; et al. TFOS DEWS III: Management and Therapy. Am. J. Ophthalmol. 2025, 279, 289–386. [Google Scholar] [CrossRef] [PubMed]


| Reference | Year | Study Design | Study Group (n) | Control Group (n) | Treated Condition | Intervention | Comparator/Control | Preparation | Dosage (Times/Day) | Duration | Outcomes Measured | Outcomes Significantly Improved Compared to Control |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| van der Meer et al. [96] | 2021 | Crossover RCT | 15 | 15 | severe DED | AlS | 50% AS | AlS: whole blood from never-transfused male repeat donors with blood group AB → clot for 6–24 h at room temperature → centrifugation with an Accumulated Centrifugal Effect (ACE) of 9 × 107→ extraction of serum → centrifugation at 6580 g for 7 min → dilution to 50% with saline AS: whole blood → clot for 6–24 h at room temperature → centrifugation with an Accumulated Centrifugal Effect (ACE) of 9 × 107 → extraction of serum → centrifugation at 6580× g for 7 min → dilution to 50% with saline | 6 | 1 month | OSDI, TBUT, BCVA, OSS, ST | No significant differences were found |
| Rodríguez Calvo-de-Mora et al. [89] | 2021 | Three-arm RCT | 21 (AS), 21 (AlS), 21 (UCS) | N/A | severe DED | AS, AlS, UCS | N/A | UCS: Umbilical cord blood → centrifugation → dilution to 20% with solution of Sterile Irrigating Solution, BSS® AS: whole blood → centrifugation → dilution to 20% with solution of Sterile Irrigating Solution, BSS® AlS: whole blood form AB-blood donors → centrifugation → dilution to 20% with solution of Sterile Irrigating Solution, BSS® | 5 | 3 months | ST, TBUT, OSS | No significant differences were found |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stępień, P.; Żarnowski, T.; Wróbel-Dudzińska, D. Blood Derivatives in the Therapy of Ocular Surface Diseases. Int. J. Mol. Sci. 2025, 26, 11097. https://doi.org/10.3390/ijms262211097
Stępień P, Żarnowski T, Wróbel-Dudzińska D. Blood Derivatives in the Therapy of Ocular Surface Diseases. International Journal of Molecular Sciences. 2025; 26(22):11097. https://doi.org/10.3390/ijms262211097
Chicago/Turabian StyleStępień, Piotr, Tomasz Żarnowski, and Dominika Wróbel-Dudzińska. 2025. "Blood Derivatives in the Therapy of Ocular Surface Diseases" International Journal of Molecular Sciences 26, no. 22: 11097. https://doi.org/10.3390/ijms262211097
APA StyleStępień, P., Żarnowski, T., & Wróbel-Dudzińska, D. (2025). Blood Derivatives in the Therapy of Ocular Surface Diseases. International Journal of Molecular Sciences, 26(22), 11097. https://doi.org/10.3390/ijms262211097









