The Role of mRNA Alternative Processing in Mammalian Neurodevelopment
Abstract
1. Introduction
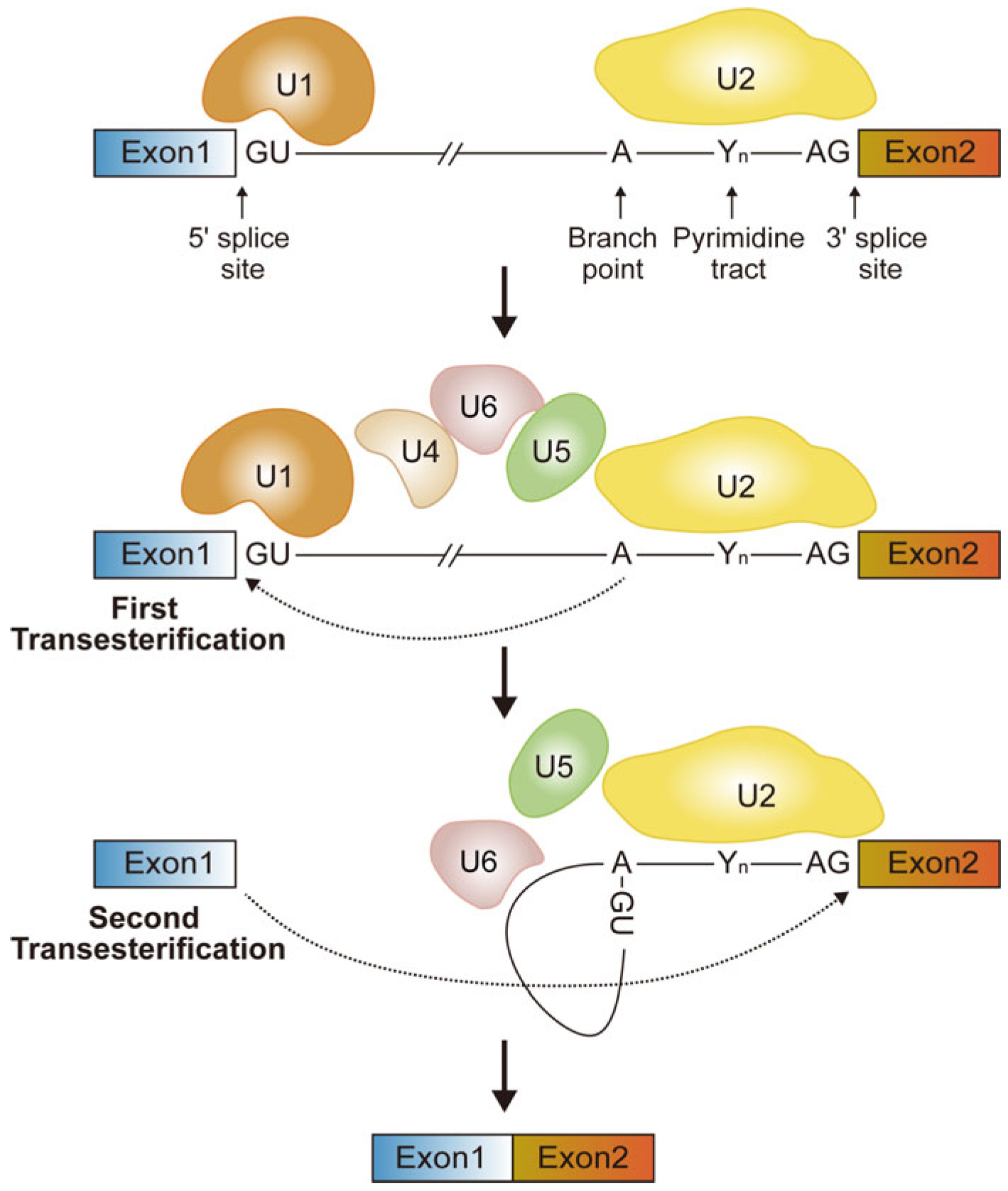
2. Alternative Splicing
2.1. The Role of Alternative Splicing in Brain Development
2.2. The Timely Expression of RBPs Regulates the AS Switch in Neural Development
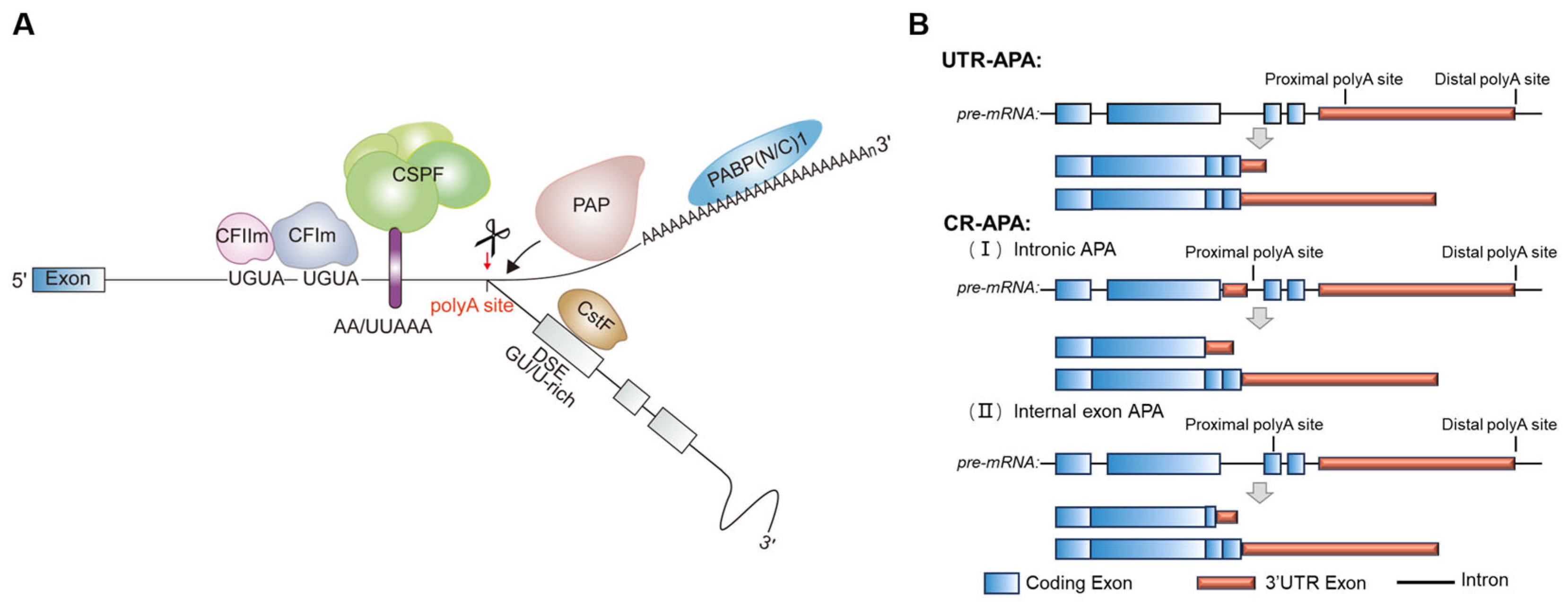
3. Alternative Polyadenylation
3.1. Unique Landscape of APA Regulation in Neural Tissue
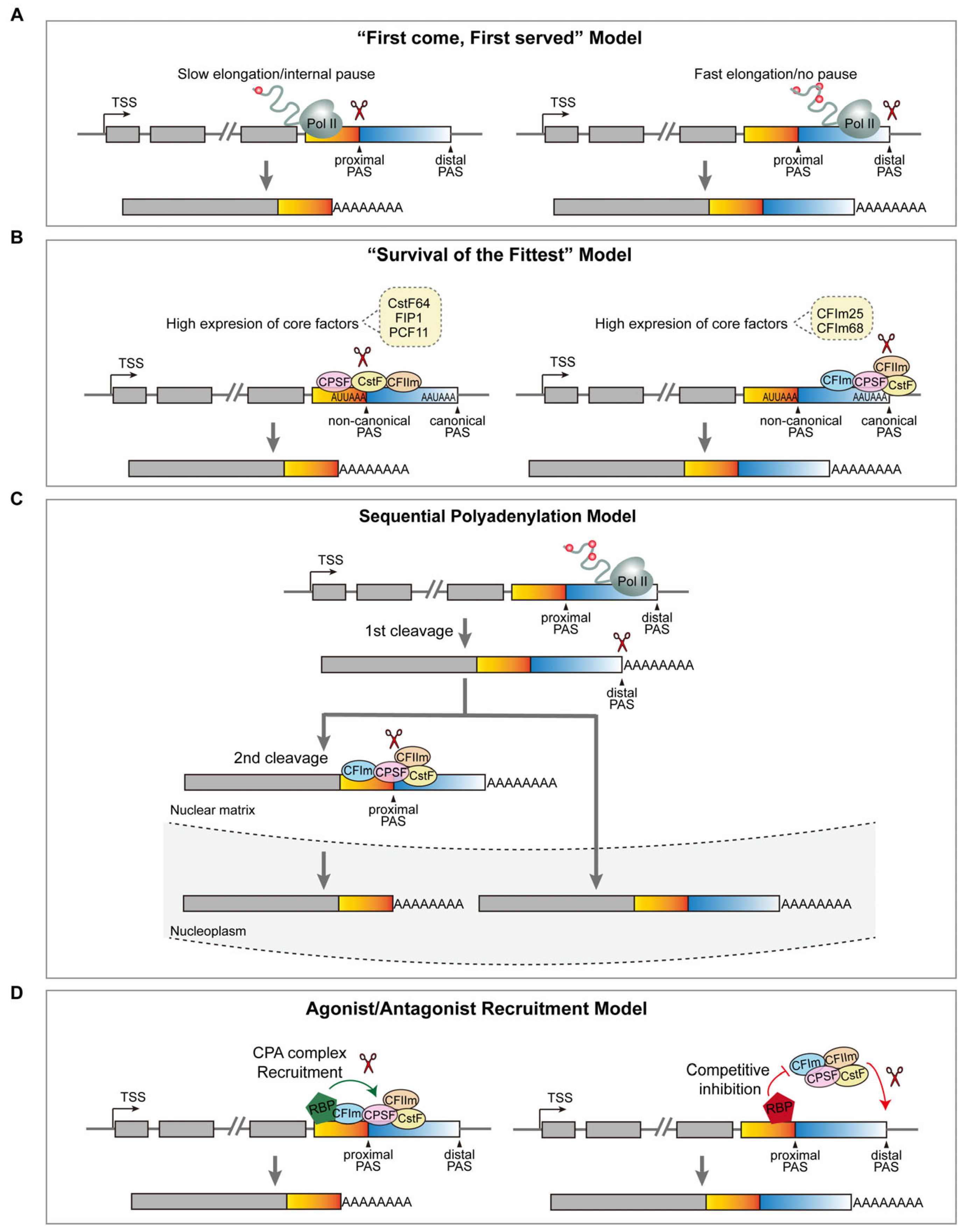
3.2. The Regulatory Mechanism of APA in Neurodevelopment
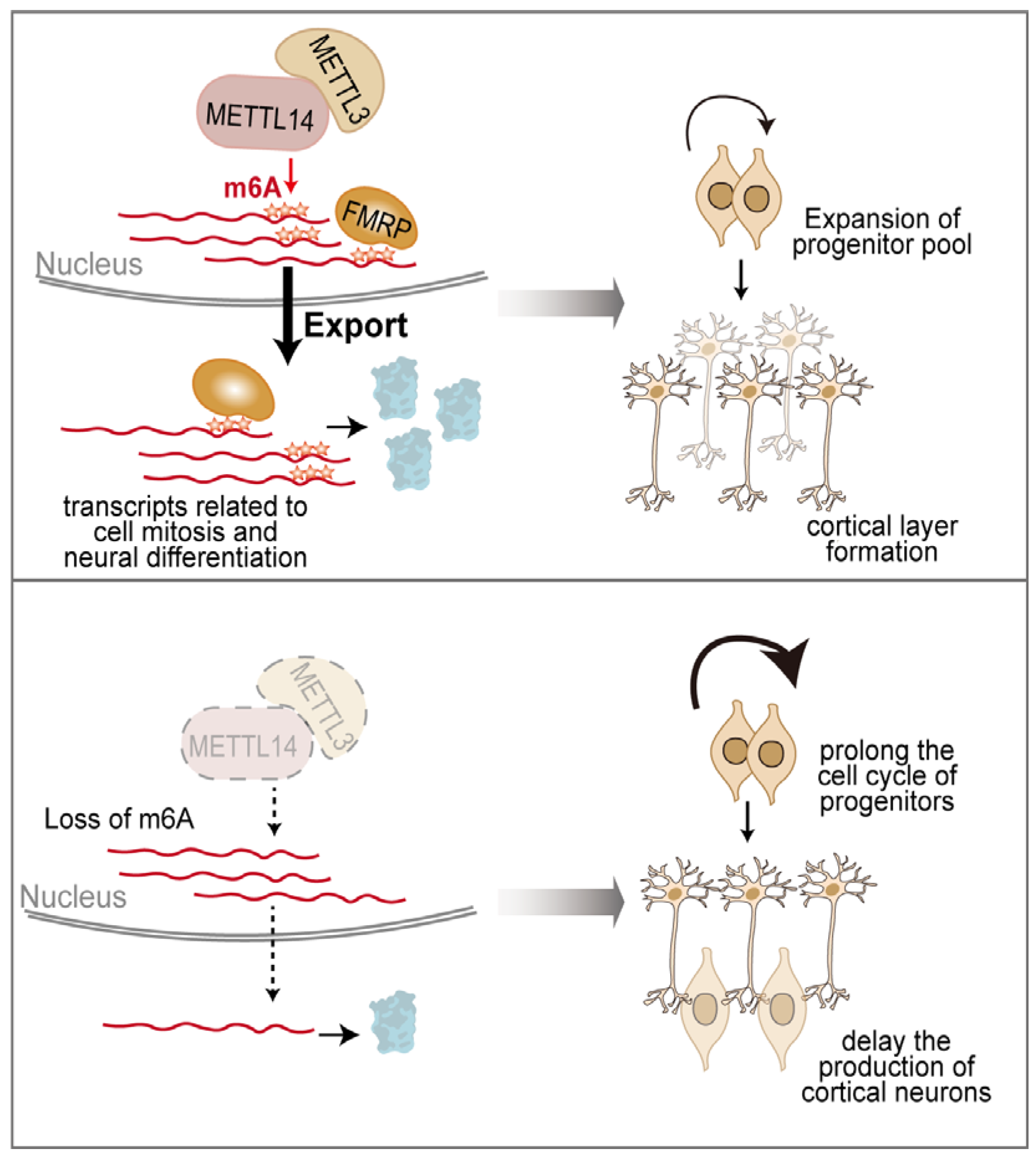
4. RNA Chemical Modifications
5. Aberrant mRNA Processing Linked to Neurodevelopmental Disorders
| Disease | Processing Type | Causative Genes | References |
|---|---|---|---|
| Autism spectrum disorder (ASD) | APA, AS, m6A, m5C | e.g., HLA-G, AFF2, PRP19, PTBP2, RBFOX1, PQBP1, CPEB4, KIAA1671, INTS1, VSIG10, TJP2, FAM167A, TMEM8A, NUP43 | [60,163,164,165,166,167,168,169,172,175,194,195,196] |
| Fragile X Syndrome (FXS) | APA, AS, m6A, m5C | e.g., FMR1, Mettl14 | [145,162,174] |
| Intellectual disability (ID) | APA, AS | e.g., PQBP1, CDK5R1 | [160,197] |
| Rett syndrome (RTT) | APA, AS | e.g., MECP2 | [180] |
| Specific Language Impairment (SLI) | APA | e.g., ARHGEF39 | [198] |
| Tourette syndrome (TS) | APA | e.g., SLITRK1 | [199] |
| Schizophrenia (SZ) | APA, m6A | e.g., RGS4, EFNB2, CPLX2 | [200,201,202,203] |
| Attention-deficit hyperactivity disorder (ADHD) | APA, m6A | e.g., CLOCK, DBH, MTHFR | [203,204,205,206,207] |
6. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A3SS | alternative 3′ splice sites |
| A5SS | alternative 5′ splice sites |
| ADAR | adenosine deaminase RNA-specific |
| ALYREF | Aly/REF export factor |
| APA | alternative polyadenylation |
| AS | alternative splicing |
| ASD | autism spectrum disorder |
| ASOs | antisense oligonucleotides |
| BAF | BRG1/BRM-associated factor |
| BDNF | brain-derived neurotrophic factor |
| CALM1 | calmodulin 1 |
| CBLB | Casitas B-lineage lymphoma proto-oncogene b |
| CDS | coding sequence |
| CFIm | cleavage factor I m |
| CFIIm | cleavage factor II m |
| CNS | central nervous system |
| CPA | cleavage and polyadenylation |
| CPEB4 | cytoplasmic polyadenylation element-binding protein 4 |
| CPSF | cleavage and polyadenylation specificity factor |
| CR-APA | coding-region APA |
| CRISPR-iPAS | CRISPR interference of polyadenylation sites |
| CstF | cleavage stimulation factor |
| CstF64τ | CstF64 paralog tau |
| DLGAP1 | DLG-associated protein 1 |
| DPF2 | double PHD fingers 2 |
| DRG | dorsal root ganglion |
| ELAVL | ELAV-like neuron-specific RNA binding protein |
| FMRP | fragile X mental retardation protein |
| FMR1 | fragile X messenger ribonucleoprotein 1 |
| m1A | N1-methyladenosine |
| m5C | 5-methylcytosine |
| m6A | N6-methyladenosine |
| m7G | N7-methylguanosine |
| MECP2 | methyl-CpG-binding protein 2 |
| MXE | mutually exclusive exons |
| NDDs | neurodevelopmental disorders |
| Nm | 2′-O-methylation |
| NOVA | neuro-oncological ventral antigens |
| NPCs | neural progenitor cells |
| NSCs | neural stem cells |
| NSPCs | neural stem/progenitor cells |
| NSUN2 | NOP2/Sun RNA methyltransferase 2 |
| NSUN4 | NOP2/Sun RNA methyltransferase 4 |
| NMD | nonsense-mediated mRNA decay |
| NRXNs | neurexins |
| PAP | poly(A) polymerase |
| PASs | polyadenylation sites |
| pCLAP | peptide Cross-Linking and Affinity Purification |
| PQBP1 | polyglutamine-binding protein 1 |
| PRP19 | pre-mRNA processing factor 19 |
| PTBP | polypyrimidine-tract-binding protein |
| RBFOX | RNA-binding fox-1 homolog |
| RI | retained introns |
| RNAP II | RNA polymerase II |
| RTT | Rett syndrome |
| ScISOr-Seq2 | Single-cell Isoform Sequencing 2 |
| SCN2A | sodium voltage-gated channel alpha subunit 2 |
| SE | skipped exons |
| SHTN1 | shootin 1 |
| SRRM4 | serine/arginine repetitive matrix 4 |
| TET | tet methylcytosine dioxygenase |
| USE | upstream sequence element |
| UTR-APA | 3′-untranslated-region APA |
References
- Nakahata, S.; Kawamoto, S. Tissue-dependent isoforms of mammalian Fox-1 homologs are associated with tissue-specific splicing activities. Nucleic Acids Res. 2005, 33, 2078–2089. [Google Scholar] [CrossRef]
- Ciolli Mattioli, C.; Rom, A.; Franke, V.; Imami, K.; Arrey, G.; Terne, M.; Woehler, A.; Akalin, A.; Ulitsky, I.; Chekulaeva, M. Alternative 3′ UTRs direct localization of functionally diverse protein isoforms in neuronal compartments. Nucleic Acids Res. 2019, 47, 2560–2573. [Google Scholar] [CrossRef]
- Chih, B.; Gollan, L.; Scheiffele, P. Alternative splicing controls selective trans-synaptic interactions of the neuroligin-neurexin complex. Neuron 2006, 51, 171–178. [Google Scholar] [CrossRef]
- Schreiner, D.; Nguyen, T.M.; Russo, G.; Heber, S.; Patrignani, A.; Ahrné, E.; Scheiffele, P. Targeted combinatorial alternative splicing generates brain region-specific repertoires of neurexins. Neuron 2014, 84, 386–398. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, T.; Yu, Z.; Tan, W.T.; Wen, M.; Shen, Y.; Lambert, F.R.P.; Huber, R.G.; Wan, Y. Genome-wide RNA structure changes during human neurogenesis modulate gene regulatory networks. Mol. Cell 2021, 81, 4942–4953.e8. [Google Scholar] [CrossRef]
- Buljan, M.; Chalancon, G.; Eustermann, S.; Wagner, G.P.; Fuxreiter, M.; Bateman, A.; Babu, M.M. Tissue-specific splicing of disordered segments that embed binding motifs rewires protein interaction networks. Mol. Cell 2012, 46, 871–883. [Google Scholar] [CrossRef]
- Vuong, J.K.; Ergin, V.; Chen, L.; Zheng, S. Multilayered regulations of alternative splicing, NMD, and protein stability control temporal induction and tissue-specific expression of TRIM46 during axon formation. Nat. Commun. 2022, 13, 2081. [Google Scholar] [CrossRef]
- Berkovits, B.D.; Mayr, C. Alternative 3′ UTRs act as scaffolds to regulate membrane protein localization. Nature 2015, 522, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Su, C.H.; Tarn, W.Y. Alternative Splicing in Neurogenesis and Brain Development. Front. Mol. Biosci. 2018, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.T.; Sandberg, R.; Luo, S.; Khrebtukova, I.; Zhang, L.; Mayr, C.; Kingsmore, S.F.; Schroth, G.P.; Burge, C.B. Alternative isoform regulation in human tissue transcriptomes. Nature 2008, 456, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Nazim, M. Post-transcriptional regulation of the transcriptional apparatus in neuronal development. Front. Mol. Neurosci. 2024, 17, 1483901. [Google Scholar] [CrossRef]
- Shepard, P.J.; Choi, E.A.; Lu, J.; Flanagan, L.A.; Hertel, K.J.; Shi, Y. Complex and dynamic landscape of RNA polyadenylation revealed by PAS-Seq. Rna 2011, 17, 761–772. [Google Scholar] [CrossRef]
- Tian, B.; Manley, J.L. Alternative polyadenylation of mRNA precursors. Nat. Rev. Mol. Cell Biol. 2017, 18, 18–30. [Google Scholar] [CrossRef]
- Agarwal, V.; Lopez-Darwin, S.; Kelley, D.R.; Shendure, J. The landscape of alternative polyadenylation in single cells of the developing mouse embryo. Nat. Commun. 2021, 12, 5101. [Google Scholar] [CrossRef]
- Sommerkamp, P.; Cabezas-Wallscheid, N.; Trumpp, A. Alternative Polyadenylation in Stem Cell Self-Renewal and Differentiation. Trends Mol. Med. 2021, 27, 660–672. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Yue, M.; Wang, J.; Kumar, S.; Wechsler-Reya, R.J.; Zhang, Z.; Ogawa, Y.; Kellis, M.; Duester, G.; et al. N(6)-methyladenosine RNA modification regulates embryonic neural stem cell self-renewal through histone modifications. Nat. Neurosci. 2018, 21, 195–206. [Google Scholar] [CrossRef]
- Yu, J.; Chen, M.; Huang, H.; Zhu, J.; Song, H.; Zhu, J.; Park, J.; Ji, S.J. Dynamic m6A modification regulates local translation of mRNA in axons. Nucleic Acids Res. 2018, 46, 1412–1423. [Google Scholar] [CrossRef]
- Johnson, Z.; Xu, X.; Lin, Y.; Xie, H. Dynamics of RNA m(5)C modification during brain development. Genomics 2023, 115, 110604. [Google Scholar] [CrossRef] [PubMed]
- Black, D.L. Mechanisms of alternative pre-messenger RNA splicing. Annu. Rev. Biochem. 2003, 72, 291–336. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, M.E.; Charenton, C.; Nagai, K. RNA Splicing by the Spliceosome. Annu. Rev. Biochem. 2020, 89, 359–388. [Google Scholar] [CrossRef] [PubMed]
- Blencowe, B.J. Alternative splicing: New insights from global analyses. Cell 2006, 126, 37–47. [Google Scholar] [CrossRef]
- Yeo, G.; Holste, D.; Kreiman, G.; Burge, C.B. Variation in alternative splicing across human tissues. Genome Biol. 2004, 5, R74. [Google Scholar] [CrossRef] [PubMed]
- Tilgner, H.; Jahanbani, F.; Blauwkamp, T.; Moshrefi, A.; Jaeger, E.; Chen, F.; Harel, I.; Bustamante, C.D.; Rasmussen, M.; Snyder, M.P. Comprehensive transcriptome analysis using synthetic long-read sequencing reveals molecular co-association of distant splicing events. Nat. Biotechnol. 2015, 33, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Mazin, P.; Xiong, J.; Liu, X.; Yan, Z.; Zhang, X.; Li, M.; He, L.; Somel, M.; Yuan, Y.; Phoebe Chen, Y.P.; et al. Widespread splicing changes in human brain development and aging. Mol. Syst. Biol. 2013, 9, 633. [Google Scholar] [CrossRef] [PubMed]
- Weyn-Vanhentenryck, S.M.; Feng, H.; Ustianenko, D.; Duffié, R.; Yan, Q.; Jacko, M.; Martinez, J.C.; Goodwin, M.; Zhang, X.; Hengst, U.; et al. Precise temporal regulation of alternative splicing during neural development. Nat. Commun. 2018, 9, 2189. [Google Scholar] [CrossRef]
- Belchikov, N.; Hsu, J.; Li, X.J.; Jarroux, J.; Hu, W.; Joglekar, A.; Tilgner, H.U. Understanding isoform expression by pairing long-read sequencing with single-cell and spatial transcriptomics. Genome Res. 2024, 34, 1735–1746. [Google Scholar] [CrossRef]
- Song, Y.; Botvinnik, O.B.; Lovci, M.T.; Kakaradov, B.; Liu, P.; Xu, J.L.; Yeo, G.W. Single-Cell Alternative Splicing Analysis with Expedition Reveals Splicing Dynamics during Neuron Differentiation. Mol. Cell 2017, 67, 148–161.e5. [Google Scholar] [CrossRef]
- Hardwick, S.A.; Hu, W.; Joglekar, A.; Fan, L.; Collier, P.G.; Foord, C.; Balacco, J.; Lanjewar, S.; Sampson, M.M.; Koopmans, F.; et al. Single-nuclei isoform RNA sequencing unlocks barcoded exon connectivity in frozen brain tissue. Nat. Biotechnol. 2022, 40, 1082–1092. [Google Scholar] [CrossRef]
- Joglekar, A.; Hu, W.; Zhang, B.; Narykov, O.; Diekhans, M.; Marrocco, J.; Balacco, J.; Ndhlovu, L.C.; Milner, T.A.; Fedrigo, O.; et al. Single-cell long-read sequencing-based mapping reveals specialized splicing patterns in developing and adult mouse and human brain. Nat. Neurosci. 2024, 27, 1051–1063. [Google Scholar] [CrossRef]
- Verdi, J.M.; Bashirullah, A.; Goldhawk, D.E.; Kubu, C.J.; Jamali, M.; Meakin, S.O.; Lipshitz, H.D. Distinct human NUMB isoforms regulate differentiation vs. proliferation in the neuronal lineage. Proc. Natl. Acad. Sci. USA 1999, 96, 10472–10476. [Google Scholar] [CrossRef]
- Dho, S.E.; Othman, K.; Zhang, Y.; McGlade, C.J. NUMB alternative splicing and isoform-specific functions in development and disease. J. Biol. Chem. 2025, 301, 108215. [Google Scholar] [CrossRef] [PubMed]
- Kubo, Y.; Baba, K.; Toriyama, M.; Minegishi, T.; Sugiura, T.; Kozawa, S.; Ikeda, K.; Inagaki, N. Shootin1-cortactin interaction mediates signal-force transduction for axon outgrowth. J. Cell Biol. 2015, 210, 663–676. [Google Scholar] [CrossRef]
- Toriyama, M.; Shimada, T.; Kim, K.B.; Mitsuba, M.; Nomura, E.; Katsuta, K.; Sakumura, Y.; Roepstorff, P.; Inagaki, N. Shootin1: A protein involved in the organization of an asymmetric signal for neuronal polarization. J. Cell Biol. 2006, 175, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ergin, V.; Lin, L.; Stork, C.; Chen, L.; Zheng, S. Axonogenesis Is Coordinated by Neuron-Specific Alternative Splicing Programming and Splicing Regulator PTBP2. Neuron 2019, 101, 690–706.e10. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, B.; Ushkaryov, Y.A.; Südhof, T.C. Cartography of neurexins: More than 1000 isoforms generated by alternative splicing and expressed in distinct subsets of neurons. Neuron 1995, 14, 497–507. [Google Scholar] [CrossRef]
- Siddiqui, T.J.; Pancaroglu, R.; Kang, Y.; Rooyakkers, A.; Craig, A.M. LRRTMs and neuroligins bind neurexins with a differential code to cooperate in glutamate synapse development. J. Neurosci. 2010, 30, 7495–7506. [Google Scholar] [CrossRef]
- Uemura, T.; Lee, S.J.; Yasumura, M.; Takeuchi, T.; Yoshida, T.; Ra, M.; Taguchi, R.; Sakimura, K.; Mishina, M. Trans-synaptic interaction of GluRdelta2 and Neurexin through Cbln1 mediates synapse formation in the cerebellum. Cell 2010, 141, 1068–1079. [Google Scholar] [CrossRef]
- Matsuda, K.; Yuzaki, M. Cbln family proteins promote synapse formation by regulating distinct neurexin signaling pathways in various brain regions. Eur. J. Neurosci. 2011, 33, 1447–1461. [Google Scholar] [CrossRef]
- Yasumura, M.; Yoshida, T.; Lee, S.J.; Uemura, T.; Joo, J.Y.; Mishina, M. Glutamate receptor δ1 induces preferentially inhibitory presynaptic differentiation of cortical neurons by interacting with neurexins through cerebellin precursor protein subtypes. J. Neurochem. 2012, 121, 705–716. [Google Scholar] [CrossRef]
- Aoto, J.; Martinelli, D.C.; Malenka, R.C.; Tabuchi, K.; Südhof, T.C. Presynaptic neurexin-3 alternative splicing trans-synaptically controls postsynaptic AMPA receptor trafficking. Cell 2013, 154, 75–88. [Google Scholar] [CrossRef]
- Ule, J.; Ule, A.; Spencer, J.; Williams, A.; Hu, J.S.; Cline, M.; Wang, H.; Clark, T.; Fraser, C.; Ruggiu, M.; et al. Nova regulates brain-specific splicing to shape the synapse. Nat. Genet. 2005, 37, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Iijima, T.; Iijima, Y.; Witte, H.; Scheiffele, P. Neuronal cell type-specific alternative splicing is regulated by the KH domain protein SLM1. J. Cell Biol. 2014, 204, 331–342. [Google Scholar] [CrossRef]
- Okano, H.J.; Darnell, R.B. A hierarchy of Hu RNA binding proteins in developing and adult neurons. J. Neurosci. 1997, 17, 3024–3037. [Google Scholar] [CrossRef]
- Charizanis, K.; Lee, K.Y.; Batra, R.; Goodwin, M.; Zhang, C.; Yuan, Y.; Shiue, L.; Cline, M.; Scotti, M.M.; Xia, G.; et al. Muscleblind-like 2-mediated alternative splicing in the developing brain and dysregulation in myotonic dystrophy. Neuron 2012, 75, 437–450. [Google Scholar] [CrossRef]
- Spellman, R.; Llorian, M.; Smith, C.W. Crossregulation and functional redundancy between the splicing regulator PTB and its paralogs nPTB and ROD1. Mol. Cell 2007, 27, 420–434. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Qian, H.; Xue, Y.; Fu, X.D. PTB/nPTB: Master regulators of neuronal fate in mammals. Biophys. Rep. 2018, 4, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Llorian, M.; Schwartz, S.; Clark, T.A.; Hollander, D.; Tan, L.Y.; Spellman, R.; Gordon, A.; Schweitzer, A.C.; de la Grange, P.; Ast, G.; et al. Position-dependent alternative splicing activity revealed by global profiling of alternative splicing events regulated by PTB. Nat. Struct. Mol. Biol. 2010, 17, 1114–1123. [Google Scholar] [CrossRef]
- Nazim, M.; Lin, C.H.; Feng, A.C.; Xiao, W.; Yeom, K.H.; Li, M.; Daly, A.E.; Tan, X.; Vu, H.; Ernst, J.; et al. Alternative splicing of a chromatin modifier alters the transcriptional regulatory programs of stem cell maintenance and neuronal differentiation. Cell Stem Cell 2024, 31, 754–771.e6. [Google Scholar] [CrossRef]
- Shibasaki, T.; Tokunaga, A.; Sakamoto, R.; Sagara, H.; Noguchi, S.; Sasaoka, T.; Yoshida, N. PTB deficiency causes the loss of adherens junctions in the dorsal telencephalon and leads to lethal hydrocephalus. Cereb. Cortex 2013, 23, 1824–1835. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, M.H.; Wu, X.; Kodani, A.; Fan, J.; Doan, R.; Ozawa, M.; Ma, J.; Yoshida, N.; Reiter, J.F.; et al. Cell-Type-Specific Alternative Splicing Governs Cell Fate in the Developing Cerebral Cortex. Cell 2016, 166, 1147–1162.e15. [Google Scholar] [CrossRef]
- Licatalosi, D.D.; Yano, M.; Fak, J.J.; Mele, A.; Grabinski, S.E.; Zhang, C.; Darnell, R.B. Ptbp2 represses adult-specific splicing to regulate the generation of neuronal precursors in the embryonic brain. Genes. Dev. 2012, 26, 1626–1642. [Google Scholar] [CrossRef] [PubMed]
- Makeyev, E.V.; Zhang, J.; Carrasco, M.A.; Maniatis, T. The MicroRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol. Cell 2007, 27, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Boutz, P.L.; Stoilov, P.; Li, Q.; Lin, C.H.; Chawla, G.; Ostrow, K.; Shiue, L.; Ares, M., Jr.; Black, D.L. A post-transcriptional regulatory switch in polypyrimidine tract-binding proteins reprograms alternative splicing in developing neurons. Genes. Dev. 2007, 21, 1636–1652. [Google Scholar] [CrossRef] [PubMed]
- Calarco, J.A.; Superina, S.; O’Hanlon, D.; Gabut, M.; Raj, B.; Pan, Q.; Skalska, U.; Clarke, L.; Gelinas, D.; van der Kooy, D.; et al. Regulation of vertebrate nervous system alternative splicing and development by an SR-related protein. Cell 2009, 138, 898–910. [Google Scholar] [CrossRef]
- Raj, B.; Irimia, M.; Braunschweig, U.; Sterne-Weiler, T.; O’Hanlon, D.; Lin, Z.Y.; Chen, G.I.; Easton, L.E.; Ule, J.; Gingras, A.C.; et al. A global regulatory mechanism for activating an exon network required for neurogenesis. Mol. Cell 2014, 56, 90–103. [Google Scholar] [CrossRef]
- Irimia, M.; Weatheritt, R.J.; Ellis, J.D.; Parikshak, N.N.; Gonatopoulos-Pournatzis, T.; Babor, M.; Quesnel-Vallières, M.; Tapial, J.; Raj, B.; O’Hanlon, D.; et al. A highly conserved program of neuronal microexons is misregulated in autistic brains. Cell 2014, 159, 1511–1523. [Google Scholar] [CrossRef]
- Quesnel-Vallières, M.; Irimia, M.; Cordes, S.P.; Blencowe, B.J. Essential roles for the splicing regulator nSR100/SRRM4 during nervous system development. Genes. Dev. 2015, 29, 746–759. [Google Scholar] [CrossRef]
- Conboy, J.G. Developmental regulation of RNA processing by Rbfox proteins. Wiley Interdiscip. Rev. RNA 2017, 8, 10.1002/wrna.1398. [Google Scholar] [CrossRef]
- Damianov, A.; Black, D.L. Autoregulation of Fox protein expression to produce dominant negative splicing factors. RNA 2010, 16, 405–416. [Google Scholar] [CrossRef]
- Weyn-Vanhentenryck, S.M.; Mele, A.; Yan, Q.; Sun, S.; Farny, N.; Zhang, Z.; Xue, C.; Herre, M.; Silver, P.A.; Zhang, M.Q.; et al. HITS-CLIP and integrative modeling define the Rbfox splicing-regulatory network linked to brain development and autism. Cell Rep. 2014, 6, 1139–1152. [Google Scholar] [CrossRef]
- Kim, K.K.; Nam, J.; Mukouyama, Y.S.; Kawamoto, S. Rbfox3-regulated alternative splicing of Numb promotes neuronal differentiation during development. J. Cell Biol. 2013, 200, 443–458. [Google Scholar] [CrossRef]
- Gehman, L.T.; Meera, P.; Stoilov, P.; Shiue, L.; O’Brien, J.E.; Meisler, M.H.; Ares, M., Jr.; Otis, T.S.; Black, D.L. The splicing regulator Rbfox2 is required for both cerebellar development and mature motor function. Genes. Dev. 2012, 26, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Gehman, L.T.; Stoilov, P.; Maguire, J.; Damianov, A.; Lin, C.H.; Shiue, L.; Ares, M., Jr.; Mody, I.; Black, D.L. The splicing regulator Rbfox1 (A2BP1) controls neuronal excitation in the mammalian brain. Nat. Genet. 2011, 43, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Jacko, M.; Weyn-Vanhentenryck, S.M.; Smerdon, J.W.; Yan, R.; Feng, H.; Williams, D.J.; Pai, J.; Xu, K.; Wichterle, H.; Zhang, C. Rbfox Splicing Factors Promote Neuronal Maturation and Axon Initial Segment Assembly. Neuron 2018, 97, 853–868.e6. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Y.; Yin, G.L.; Darnell, R.B. The neuronal RNA-binding protein Nova-2 is implicated as the autoantigen targeted in POMA patients with dementia. Proc. Natl. Acad. Sci. USA 1998, 95, 13254–13259. [Google Scholar] [CrossRef]
- Jelen, N.; Ule, J.; Zivin, M. Cholinergic regulation of striatal Nova mRNAs. Neuroscience 2010, 169, 619–627. [Google Scholar] [CrossRef]
- Lewis, H.A.; Chen, H.; Edo, C.; Buckanovich, R.J.; Yang, Y.Y.; Musunuru, K.; Zhong, R.; Darnell, R.B.; Burley, S.K. Crystal structures of Nova-1 and Nova-2 K-homology RNA-binding domains. Structure 1999, 7, 191–203. [Google Scholar] [CrossRef]
- Buckanovich, R.J.; Darnell, R.B. The neuronal RNA binding protein Nova-1 recognizes specific RNA targets in vitro and in vivo. Mol. Cell Biol. 1997, 17, 3194–3201. [Google Scholar] [CrossRef]
- Ule, J.; Stefani, G.; Mele, A.; Ruggiu, M.; Wang, X.; Taneri, B.; Gaasterland, T.; Blencowe, B.J.; Darnell, R.B. An RNA map predicting Nova-dependent splicing regulation. Nature 2006, 444, 580–586. [Google Scholar] [CrossRef]
- Piton, A. NOVA1/2 genes and alternative splicing in neurodevelopment. Curr. Opin. Genet. Dev. 2025, 93, 102373. [Google Scholar] [CrossRef]
- Saito, Y.; Miranda-Rottmann, S.; Ruggiu, M.; Park, C.Y.; Fak, J.J.; Zhong, R.; Duncan, J.S.; Fabella, B.A.; Junge, H.J.; Chen, Z.; et al. NOVA2-mediated RNA regulation is required for axonal pathfinding during development. eLife 2016, 5, e14371. [Google Scholar] [CrossRef]
- Mattioli, F.; Hayot, G.; Drouot, N.; Isidor, B.; Courraud, J.; Hinckelmann, M.V.; Mau-Them, F.T.; Sellier, C.; Goldman, A.; Telegrafi, A.; et al. De Novo Frameshift Variants in the Neuronal Splicing Factor NOVA2 Result in a Common C-Terminal Extension and Cause a Severe Form of Neurodevelopmental Disorder. Am. J. Hum. Genet. 2020, 106, 438–452. [Google Scholar] [CrossRef]
- Rogalska, M.E.; Mancini, E.; Bonnal, S.; Gohr, A.; Dunyak, B.M.; Arecco, N.; Smith, P.G.; Vaillancourt, F.H.; Valcárcel, J. Transcriptome-wide splicing network reveals specialized regulatory functions of the core spliceosome. Science 2024, 386, 551–560. [Google Scholar] [CrossRef]
- Lu, Y.L.; Liu, Y.; McCoy, M.J.; Yoo, A.S. MiR-124 synergism with ELAVL3 enhances target gene expression to promote neuronal maturity. Proc. Natl. Acad. Sci. USA 2021, 118, e2015454118. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.C.; Lu, Y.H.; Liu, Y.R.; Lin, Y.J. RBM4a-regulated splicing cascade modulates the differentiation and metabolic activities of brown adipocytes. Sci. Rep. 2016, 6, 20665. [Google Scholar] [CrossRef]
- Su, C.H.; Hung, K.Y.; Hung, S.C.; Tarn, W.Y. RBM4 Regulates Neuronal Differentiation of Mesenchymal Stem Cells by Modulating Alternative Splicing of Pyruvate Kinase M. Mol. Cell Biol. 2017, 37, e00466-16. [Google Scholar] [CrossRef]
- Baralle, F.E.; Giudice, J. Alternative splicing as a regulator of development and tissue identity. Nat. Rev. Mol. Cell Biol. 2017, 18, 437–451. [Google Scholar] [CrossRef]
- Cereda, M.; Pozzoli, U.; Rot, G.; Juvan, P.; Schweitzer, A.; Clark, T.; Ule, J. RNAmotifs: Prediction of multivalent RNA motifs that control alternative splicing. Genome Biol. 2014, 15, R20. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.I.; Sanchez-Pulido, L.; Haerty, W.; Ponting, C.P. RBFOX and PTBP1 proteins regulate the alternative splicing of micro-exons in human brain transcripts. Genome Res. 2015, 25, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Frias, M.A.; Mele, A.; Ruggiu, M.; Eom, T.; Marney, C.B.; Wang, H.; Licatalosi, D.D.; Fak, J.J.; Darnell, R.B. Integrative modeling defines the Nova splicing-regulatory network and its combinatorial controls. Science 2010, 329, 439–443. [Google Scholar] [CrossRef]
- Derti, A.; Garrett-Engele, P.; Macisaac, K.D.; Stevens, R.C.; Sriram, S.; Chen, R.; Rohl, C.A.; Johnson, J.M.; Babak, T. A quantitative atlas of polyadenylation in five mammals. Genome Res. 2012, 22, 1173–1183. [Google Scholar] [CrossRef]
- Hoque, M.; Ji, Z.; Zheng, D.; Luo, W.; Li, W.; You, B.; Park, J.Y.; Yehia, G.; Tian, B. Analysis of alternative cleavage and polyadenylation by 3′ region extraction and deep sequencing. Nat. Methods 2013, 10, 133–139. [Google Scholar] [CrossRef]
- Di Giammartino, D.C.; Nishida, K.; Manley, J.L. Mechanisms and consequences of alternative polyadenylation. Mol. Cell 2011, 43, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Mayr, C. Regulation by 3′-Untranslated Regions. Annu. Rev. Genet. 2017, 51, 171–194. [Google Scholar] [CrossRef]
- Wang, R.; Zheng, D.; Wei, L.; Ding, Q.; Tian, B. Regulation of Intronic Polyadenylation by PCF11 Impacts mRNA Expression of Long Genes. Cell Rep. 2019, 26, 2766–2778.e6. [Google Scholar] [CrossRef]
- Tian, B.; Pan, Z.; Lee, J.Y. Widespread mRNA polyadenylation events in introns indicate dynamic interplay between polyadenylation and splicing. Genome Res. 2007, 17, 156–165. [Google Scholar] [CrossRef]
- Zheng, D.; Wang, R.; Ding, Q.; Wang, T.; Xie, B.; Wei, L.; Zhong, Z.; Tian, B. Cellular stress alters 3′UTR landscape through alternative polyadenylation and isoform-specific degradation. Nat. Commun. 2018, 9, 2268. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Yuan, S.; Wang, Y.; Fu, Y.; Ge, Y.; Ge, Y.; Lan, X.; Feng, Y.; Qiu, F.; Li, P.; et al. The role of alternative polyadenylation in the antiviral innate immune response. Nat. Commun. 2017, 8, 14605. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Feng, W.; Xu, S.; He, B. Extensive Involvement of Alternative Polyadenylation in Single-Nucleus Neurons. Genes 2020, 11, 709. [Google Scholar] [CrossRef]
- Gruber, A.J.; Zavolan, M. Alternative cleavage and polyadenylation in health and disease. Nat. Rev. Genet. 2019, 20, 599–614. [Google Scholar] [CrossRef]
- Zhang, H.; Lee, J.Y.; Tian, B. Biased alternative polyadenylation in human tissues. Genome Biol. 2005, 6, R100. [Google Scholar] [CrossRef]
- Sandberg, R.; Neilson, J.R.; Sarma, A.; Sharp, P.A.; Burge, C.B. Proliferating cells express mRNAs with shortened 3′ untranslated regions and fewer microRNA target sites. Science 2008, 320, 1643–1647. [Google Scholar] [CrossRef]
- Gautheret, D.; Poirot, O.; Lopez, F.; Audic, S.; Claverie, J.M. Alternate polyadenylation in human mRNAs: A large-scale analysis by EST clustering. Genome Res. 1998, 8, 524–530. [Google Scholar] [CrossRef]
- Liu, D.; Brockman, J.M.; Dass, B.; Hutchins, L.N.; Singh, P.; McCarrey, J.R.; MacDonald, C.C.; Graber, J.H. Systematic variation in mRNA 3′-processing signals during mouse spermatogenesis. Nucleic Acids Res. 2007, 35, 234–246. [Google Scholar] [CrossRef]
- Miura, P.; Shenker, S.; Andreu-Agullo, C.; Westholm, J.O.; Lai, E.C. Widespread and extensive lengthening of 3′ UTRs in the mammalian brain. Genome Res. 2013, 23, 812–825. [Google Scholar] [CrossRef]
- Lianoglou, S.; Garg, V.; Yang, J.L.; Leslie, C.S.; Mayr, C. Ubiquitously transcribed genes use alternative polyadenylation to achieve tissue-specific expression. Genes. Dev. 2013, 27, 2380–2396. [Google Scholar] [CrossRef] [PubMed]
- Smibert, P.; Miura, P.; Westholm, J.O.; Shenker, S.; May, G.; Duff, M.O.; Zhang, D.; Eads, B.D.; Carlson, J.; Brown, J.B.; et al. Global patterns of tissue-specific alternative polyadenylation in Drosophila. Cell Rep. 2012, 1, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Lackford, B.; Yao, C.; Charles, G.M.; Weng, L.; Zheng, X.; Choi, E.A.; Xie, X.; Wan, J.; Xing, Y.; Freudenberg, J.M.; et al. Fip1 regulates mRNA alternative polyadenylation to promote stem cell self-renewal. Embo J. 2014, 33, 878–889. [Google Scholar] [CrossRef]
- Gallicchio, L.; Olivares, G.H.; Berry, C.W.; Fuller, M.T. Regulation and function of alternative polyadenylation in development and differentiation. RNA Biol. 2023, 20, 908–925. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, E.; Thomaidou, D. Post-transcriptional mechanisms controlling neurogenesis and direct neuronal reprogramming. Neural Regen. Res. 2024, 19, 1929–1939. [Google Scholar] [CrossRef]
- Lau, A.G.; Irier, H.A.; Gu, J.; Tian, D.; Ku, L.; Liu, G.; Xia, M.; Fritsch, B.; Zheng, J.Q.; Dingledine, R.; et al. Distinct 3′UTRs differentially regulate activity-dependent translation of brain-derived neurotrophic factor (BDNF). Proc. Natl. Acad. Sci. USA 2010, 107, 15945–15950. [Google Scholar] [CrossRef]
- Andreassi, C.; Riccio, A. To localize or not to localize: mRNA fate is in 3′UTR ends. Trends Cell Biol. 2009, 19, 465–474. [Google Scholar] [CrossRef]
- Yudin, D.; Hanz, S.; Yoo, S.; Iavnilovitch, E.; Willis, D.; Gradus, T.; Vuppalanchi, D.; Segal-Ruder, Y.; Ben-Yaakov, K.; Hieda, M.; et al. Localized regulation of axonal RanGTPase controls retrograde injury signaling in peripheral nerve. Neuron 2008, 59, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Bae, B.; Gruner, H.N.; Lynch, M.; Feng, T.; So, K.; Oliver, D.; Mastick, G.S.; Yan, W.; Pieraut, S.; Miura, P. Elimination of Calm1 long 3′-UTR mRNA isoform by CRISPR-Cas9 gene editing impairs dorsal root ganglion development and hippocampal neuron activation in mice. Rna 2020, 26, 1414–1430. [Google Scholar] [CrossRef]
- Tushev, G.; Glock, C.; Heumüller, M.; Biever, A.; Jovanovic, M.; Schuman, E.M. Alternative 3′ UTRs Modify the Localization, Regulatory Potential, Stability, and Plasticity of mRNAs in Neuronal Compartments. Neuron 2018, 98, 495–511.e6. [Google Scholar] [CrossRef]
- Sun, Y.; Hamilton, K.; Tong, L. Recent molecular insights into canonical pre-mRNA 3′-end processing. Transcription 2020, 11, 83–96. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.; Shi, Y.; Walz, T.; Tong, L. Structural Insights into the Human Pre-mRNA 3′-End Processing Machinery. Mol. Cell 2020, 77, 800–809.e6. [Google Scholar] [CrossRef]
- Shi, Y.; Manley, J.L. The end of the message: Multiple protein-RNA interactions define the mRNA polyadenylation site. Genes. Dev. 2015, 29, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Danckwardt, S.; Hentze, M.W.; Kulozik, A.E. 3′ end mRNA processing: Molecular mechanisms and implications for health and disease. Embo j 2008, 27, 482–498. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y. Alternative polyadenylation: New insights from global analyses. RNA 2012, 18, 2105–2117. [Google Scholar] [CrossRef]
- Pinto, P.A.; Henriques, T.; Freitas, M.O.; Martins, T.; Domingues, R.G.; Wyrzykowska, P.S.; Coelho, P.A.; Carmo, A.M.; Sunkel, C.E.; Proudfoot, N.J.; et al. RNA polymerase II kinetics in polo polyadenylation signal selection. Embo J. 2011, 30, 2431–2444. [Google Scholar] [CrossRef]
- Peterson, M.L.; Bertolino, S.; Davis, F. An RNA polymerase pause site is associated with the immunoglobulin mus poly(A) site. Mol. Cell Biol. 2002, 22, 5606–5615. [Google Scholar] [CrossRef]
- Skourti-Stathaki, K.; Proudfoot, N.J.; Gromak, N. Human senataxin resolves RNA/DNA hybrids formed at transcriptional pause sites to promote Xrn2-dependent termination. Mol. Cell 2011, 42, 794–805. [Google Scholar] [CrossRef]
- Yao, C.; Biesinger, J.; Wan, J.; Weng, L.; Xing, Y.; Xie, X.; Shi, Y. Transcriptome-wide analyses of CstF64-RNA interactions in global regulation of mRNA alternative polyadenylation. Proc. Natl. Acad. Sci. USA 2012, 109, 18773–18778. [Google Scholar] [CrossRef]
- Yao, C.; Choi, E.A.; Weng, L.; Xie, X.; Wan, J.; Xing, Y.; Moresco, J.J.; Tu, P.G.; Yates, J.R., 3rd; Shi, Y. Overlapping and distinct functions of CstF64 and CstF64τ in mammalian mRNA 3′ processing. RNA 2013, 19, 1781–1790. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, X.; Forouzmand, E.; Jeong, J.; Qiao, F.; Sowd, G.A.; Engelman, A.N.; Xie, X.; Hertel, K.J.; Shi, Y. Molecular Mechanisms for CFIm-Mediated Regulation of mRNA Alternative Polyadenylation. Mol. Cell 2018, 69, 62–74.e4. [Google Scholar] [CrossRef]
- Brumbaugh, J.; Di Stefano, B.; Wang, X.; Borkent, M.; Forouzmand, E.; Clowers, K.J.; Ji, F.; Schwarz, B.A.; Kalocsay, M.; Elledge, S.J.; et al. Nudt21 Controls Cell Fate by Connecting Alternative Polyadenylation to Chromatin Signaling. Cell 2018, 172, 106–120.e21. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Yang, Y.; Li, G.; Huang, L.; Wen, M.; Ruan, W.; Guo, X.; Zhang, C.; Zuo, X.; Luo, D.; et al. Alternative polyadenylation by sequential activation of distal and proximal PolyA sites. Nat. Struct. Mol. Biol. 2022, 29, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Mitschka, S.; Mayr, C. Context-specific regulation and function of mRNA alternative polyadenylation. Nat. Rev. Mol. Cell Biol. 2022, 23, 779–796. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Tian, B. RNA-binding proteins in regulation of alternative cleavage and polyadenylation. Adv. Exp. Med. Biol. 2014, 825, 97–127. [Google Scholar] [CrossRef]
- Ogorodnikov, A.; Levin, M.; Tattikota, S.; Tokalov, S.; Hoque, M.; Scherzinger, D.; Marini, F.; Poetsch, A.; Binder, H.; Macher-Göppinger, S.; et al. Transcriptome 3′end organization by PCF11 links alternative polyadenylation to formation and neuronal differentiation of neuroblastoma. Nat. Commun. 2018, 9, 5331. [Google Scholar] [CrossRef]
- La Rosa, P.; Bielli, P.; Compagnucci, C.; Cesari, E.; Volpe, E.; Farioli Vecchioli, S.; Sette, C. Sam68 promotes self-renewal and glycolytic metabolism in mouse neural progenitor cells by modulating Aldh1a3 pre-mRNA 3′-end processing. eLife 2016, 5, e20750. [Google Scholar] [CrossRef]
- Hilgers, V.; Lemke, S.B.; Levine, M. ELAV mediates 3′ UTR extension in the Drosophila nervous system. Genes. Dev. 2012, 26, 2259–2264. [Google Scholar] [CrossRef]
- Akamatsu, W.; Fujihara, H.; Mitsuhashi, T.; Yano, M.; Shibata, S.; Hayakawa, Y.; Okano, H.J.; Sakakibara, S.; Takano, H.; Takano, T.; et al. The RNA-binding protein HuD regulates neuronal cell identity and maturation. Proc. Natl. Acad. Sci. USA 2005, 102, 4625–4630. [Google Scholar] [CrossRef]
- Liu, X.; Xie, H.; Liu, W.; Zuo, J.; Li, S.; Tian, Y.; Zhao, J.; Bai, M.; Li, J.; Bao, L.; et al. Dynamic regulation of alternative polyadenylation by PQBP1 during neurogenesis. Cell Rep. 2024, 43, 114525. [Google Scholar] [CrossRef]
- Wang, M.K.; Gao, C.C.; Yang, Y.G. Emerging Roles of RNA Methylation in Development. Acc. Chem. Res. 2023, 56, 3417–3427. [Google Scholar] [CrossRef]
- Wiener, D.; Schwartz, S. The epitranscriptome beyond m(6)A. Nat. Rev. Genet. 2021, 22, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Wei, J.; He, C. Where, When, and How: Context-Dependent Functions of RNA Methylation Writers, Readers, and Erasers. Mol. Cell 2019, 74, 640–650. [Google Scholar] [CrossRef]
- Desrosiers, R.; Friderici, K.; Rottman, F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc. Natl. Acad. Sci. USA 1974, 71, 3971–3975. [Google Scholar] [CrossRef] [PubMed]
- Dominissini, D.; Moshitch-Moshkovitz, S.; Schwartz, S.; Salmon-Divon, M.; Ungar, L.; Osenberg, S.; Cesarkas, K.; Jacob-Hirsch, J.; Amariglio, N.; Kupiec, M.; et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 2012, 485, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.M.; Gershowitz, A.; Moss, B. Methylated nucleotides block 5′ terminus of HeLa cell messenger RNA. Cell 1975, 4, 379–386. [Google Scholar] [CrossRef]
- Li, X.; Xiong, X.; Wang, K.; Wang, L.; Shu, X.; Ma, S.; Yi, C. Transcriptome-wide mapping reveals reversible and dynamic N(1)-methyladenosine methylome. Nat. Chem. Biol. 2016, 12, 311–316. [Google Scholar] [CrossRef]
- Li, Y.; Yi, Y.; Gao, X.; Wang, X.; Zhao, D.; Wang, R.; Zhang, L.S.; Gao, B.; Zhang, Y.; Zhang, L.; et al. 2′-O-methylation at internal sites on mRNA promotes mRNA stability. Mol. Cell 2024, 84, 2320–2336.e6. [Google Scholar] [CrossRef]
- Zhao, B.S.; He, C. Pseudouridine in a new era of RNA modifications. Cell Res. 2015, 25, 153–154. [Google Scholar] [CrossRef]
- Lin, T.Y.; Mehta, R.; Glatt, S. Pseudouridines in RNAs: Switching atoms means shifting paradigms. FEBS Lett. 2021, 595, 2310–2322. [Google Scholar] [CrossRef]
- Arango, D.; Sturgill, D.; Alhusaini, N.; Dillman, A.A.; Sweet, T.J.; Hanson, G.; Hosogane, M.; Sinclair, W.R.; Nanan, K.K.; Mandler, M.D.; et al. Acetylation of Cytidine in mRNA Promotes Translation Efficiency. Cell 2018, 175, 1872–1886.e24. [Google Scholar] [CrossRef]
- Meyer, K.D.; Saletore, Y.; Zumbo, P.; Elemento, O.; Mason, C.E.; Jaffrey, S.R. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell 2012, 149, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Hess, M.E.; Hess, S.; Meyer, K.D.; Verhagen, L.A.; Koch, L.; Brönneke, H.S.; Dietrich, M.O.; Jordan, S.D.; Saletore, Y.; Elemento, O.; et al. The fat mass and obesity associated gene (Fto) regulates activity of the dopaminergic midbrain circuitry. Nat. Neurosci. 2013, 16, 1042–1048. [Google Scholar] [CrossRef]
- Chang, M.; Lv, H.; Zhang, W.; Ma, C.; He, X.; Zhao, S.; Zhang, Z.W.; Zeng, Y.X.; Song, S.; Niu, Y.; et al. Region-specific RNA m(6)A methylation represents a new layer of control in the gene regulatory network in the mouse brain. Open Biol. 2017, 7, 170166. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Chang, M.; Lv, H.; Zhang, Z.W.; Zhang, W.; He, X.; Wu, G.; Zhao, S.; Zhang, Y.; Wang, D.; et al. RNA m(6)A methylation participates in regulation of postnatal development of the mouse cerebellum. Genome Biol. 2018, 19, 68. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.J.; Ringeling, F.R.; Vissers, C.; Jacob, F.; Pokrass, M.; Jimenez-Cyrus, D.; Su, Y.; Kim, N.S.; Zhu, Y.; Zheng, L.; et al. Temporal Control of Mammalian Cortical Neurogenesis by m(6)A Methylation. Cell 2017, 171, 877–889.e17. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.C.; Huang, C.; Shen, H.; Sun, B.; Cheng, X.; Zhang, Y.J.; Yang, Y.G.; Shu, Q.; Yang, Y.; et al. m(6)A Regulates Neurogenesis and Neuronal Development by Modulating Histone Methyltransferase Ezh2. Genom. Proteom. Bioinform. 2019, 17, 154–168. [Google Scholar] [CrossRef]
- Wu, M.; Wu, X.; Sun, H.; Wang, W.; Zhang, L.; Liu, X.; Zhang, Y.; Zhang, X.; Liu, J.; Shen, B.; et al. VIRMA-mediated m(6)A modification regulates forebrain formation through modulating ribosome biogenesis. Sci. Adv. 2025, 11, eadq9643. [Google Scholar] [CrossRef]
- Li, M.; Zhao, X.; Wang, W.; Shi, H.; Pan, Q.; Lu, Z.; Perez, S.P.; Suganthan, R.; He, C.; Bjørås, M.; et al. Ythdf2-mediated m(6)A mRNA clearance modulates neural development in mice. Genome Biol. 2018, 19, 69. [Google Scholar] [CrossRef]
- Edens, B.M.; Vissers, C.; Su, J.; Arumugam, S.; Xu, Z.; Shi, H.; Miller, N.; Rojas Ringeling, F.; Ming, G.L.; He, C.; et al. FMRP Modulates Neural Differentiation through m(6)A-Dependent mRNA Nuclear Export. Cell Rep. 2019, 28, 845–854.e5. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, X.; Weng, Y.L.; Lu, Z.; Liu, Y.; Lu, Z.; Li, J.; Hao, P.; Zhang, Y.; Zhang, F.; et al. m(6)A facilitates hippocampus-dependent learning and memory through YTHDF1. Nature 2018, 563, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Malovic, E.; Pandey, S.C. N(6)-methyladenosine (m(6)A) epitranscriptomics in synaptic plasticity and behaviors. Neuropsychopharmacology 2023, 48, 221–222. [Google Scholar] [CrossRef] [PubMed]
- Livneh, I.; Moshitch-Moshkovitz, S.; Amariglio, N.; Rechavi, G.; Dominissini, D. The m(6)A epitranscriptome: Transcriptome plasticity in brain development and function. Nat. Rev. Neurosci. 2020, 21, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Liao, Y.; Chen, J.; Chen, A.; Wu, S.; Huang, Y.; Qian, H.; Gao, F.; Wu, G.; Chen, Y.; et al. N6-methyladenosine demethylase FTO regulates synaptic and cognitive impairment by destabilizing PTEN mRNA in hypoxic-ischemic neonatal rats. Cell Death Dis. 2023, 14, 820. [Google Scholar] [CrossRef] [PubMed]
- Flores, J.V.; Cordero-Espinoza, L.; Oeztuerk-Winder, F.; Andersson-Rolf, A.; Selmi, T.; Blanco, S.; Tailor, J.; Dietmann, S.; Frye, M. Cytosine-5 RNA Methylation Regulates Neural Stem Cell Differentiation and Motility. Stem Cell Rep. 2017, 8, 112–124. [Google Scholar] [CrossRef]
- Xu, X.; Johnson, Z.; Wang, A.; Padget, R.L.; Smyth, J.W.; Xie, H. Folate regulates RNA m(5)C modification and translation in neural stem cells. BMC Biol. 2022, 20, 261. [Google Scholar] [CrossRef] [PubMed]
- Blanco, S.; Dietmann, S.; Flores, J.V.; Hussain, S.; Kutter, C.; Humphreys, P.; Lukk, M.; Lombard, P.; Treps, L.; Popis, M.; et al. Aberrant methylation of tRNAs links cellular stress to neuro-developmental disorders. Embo J. 2014, 33, 2020–2039. [Google Scholar] [CrossRef]
- Li, Q.; Liu, H.; Li, L.; Guo, H.; Xie, Z.; Kong, X.; Xu, J.; Zhang, J.; Chen, Y.; Zhang, Z.; et al. Mettl1-mediated internal m(7)G methylation of Sptbn2 mRNA elicits neurogenesis and anti-alzheimer’s disease. Cell Biosci. 2023, 13, 183. [Google Scholar] [CrossRef]
- Orji, O.C.; Stones, J.; Rajani, S.; Markus, R.; Öz, M.D.; Knight, H.M. Global Co-regulatory Cross Talk Between m(6)A and m(5)C RNA Methylation Systems Coordinate Cellular Responses and Brain Disease Pathways. Mol. Neurobiol. 2025, 62, 5006–5021. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Y.; Jin, M.; Wang, J. The crosstalk between m(6)A RNA methylation and other epigenetic regulators: A novel perspective in epigenetic remodeling. Theranostics 2021, 11, 4549–4566. [Google Scholar] [CrossRef]
- Park, C.W.; Lee, S.M.; Yoon, K.J. Epitranscriptomic regulation of transcriptome plasticity in development and diseases of the brain. BMB Rep. 2020, 53, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Hamada, N.; Ito, H.; Nishijo, T.; Iwamoto, I.; Morishita, R.; Tabata, H.; Momiyama, T.; Nagata, K. Essential role of the nuclear isoform of RBFOX1, a candidate gene for autism spectrum disorders, in the brain development. Sci. Rep. 2016, 6, 30805. [Google Scholar] [CrossRef]
- O’Brien, J.E.; Drews, V.L.; Jones, J.M.; Dugas, J.C.; Barres, B.A.; Meisler, M.H. Rbfox proteins regulate alternative splicing of neuronal sodium channel SCN8A. Mol. Cell Neurosci. 2012, 49, 120–126. [Google Scholar] [CrossRef]
- Fogel, B.L.; Wexler, E.; Wahnich, A.; Friedrich, T.; Vijayendran, C.; Gao, F.; Parikshak, N.; Konopka, G.; Geschwind, D.H. RBFOX1 regulates both splicing and transcriptional networks in human neuronal development. Hum. Mol. Genet. 2012, 21, 4171–4186. [Google Scholar] [CrossRef]
- Wang, Q.; Moore, M.J.; Adelmant, G.; Marto, J.A.; Silver, P.A. PQBP1, a factor linked to intellectual disability, affects alternative splicing associated with neurite outgrowth. Genes. Dev. 2013, 27, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, T.; Shirane, M.; Nakayama, K.I. SRRM4-dependent neuron-specific alternative splicing of protrudin transcripts regulates neurite outgrowth. Sci. Rep. 2017, 7, 41130. [Google Scholar] [CrossRef]
- Suhl, J.A.; Muddashetty, R.S.; Anderson, B.R.; Ifrim, M.F.; Visootsak, J.; Bassell, G.J.; Warren, S.T. A 3′ untranslated region variant in FMR1 eliminates neuronal activity-dependent translation of FMRP by disrupting binding of the RNA-binding protein HuR. Proc. Natl. Acad. Sci. USA 2015, 112, E6553–E6561. [Google Scholar] [CrossRef] [PubMed]
- Sebat, J.; Lakshmi, B.; Malhotra, D.; Troge, J.; Lese-Martin, C.; Walsh, T.; Yamrom, B.; Yoon, S.; Krasnitz, A.; Kendall, J.; et al. Strong association of de novo copy number mutations with autism. Science 2007, 316, 445–449. [Google Scholar] [CrossRef]
- Martin, C.L.; Duvall, J.A.; Ilkin, Y.; Simon, J.S.; Arreaza, M.G.; Wilkes, K.; Alvarez-Retuerto, A.; Whichello, A.; Powell, C.M.; Rao, K.; et al. Cytogenetic and molecular characterization of A2BP1/FOX1 as a candidate gene for autism. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2007, 144b, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Grove, J.; Ripke, S.; Als, T.D.; Mattheisen, M.; Walters, R.K.; Won, H.; Pallesen, J.; Agerbo, E.; Andreassen, O.A.; Anney, R.; et al. Identification of common genetic risk variants for autism spectrum disorder. Nat. Genet. 2019, 51, 431–444. [Google Scholar] [CrossRef]
- Redin, C.; Gerard, B.; Lauer, J.; Herenger, Y.; Muller, J.; Quartier, A.; Masurel-Paulet, A.; Willems, M.; Lesca, G.; El-Chehadeh, S.; et al. Efficient strategy for the molecular diagnosis of intellectual disability using targeted high-throughput sequencing. J. Med. Genet. 2014, 51, 724–736. [Google Scholar] [CrossRef]
- Li, D.; Wang, Q.; Bayat, A.; Battig, M.R.; Zhou, Y.; Bosch, D.G.; van Haaften, G.; Granger, L.; Petersen, A.K.; Pérez-Jurado, L.A.; et al. Spliceosome malfunction causes neurodevelopmental disorders with overlapping features. J. Clin. Investig. 2024, 134, e171235. [Google Scholar] [CrossRef]
- Parras, A.; Anta, H.; Santos-Galindo, M.; Swarup, V.; Elorza, A.; Nieto-González, J.L.; Picó, S.; Hernández, I.H.; Díaz-Hernández, J.I.; Belloc, E.; et al. Autism-like phenotype and risk gene mRNA deadenylation by CPEB4 mis-splicing. Nature 2018, 560, 441–446. [Google Scholar] [CrossRef]
- Garcia-Cabau, C.; Bartomeu, A.; Tesei, G.; Cheung, K.C.; Pose-Utrilla, J.; Picó, S.; Balaceanu, A.; Duran-Arqué, B.; Fernández-Alfara, M.; Martín, J.; et al. Mis-splicing of a neuronal microexon promotes CPEB4 aggregation in ASD. Nature 2025, 637, 496–503. [Google Scholar] [CrossRef]
- Dominguez-Alonso, S.; Tubío-Fungueiriño, M.; González-Peñas, J.; Fernández-Prieto, M.; Parellada, M.; Arango, C.; Carracedo, A.; Rodriguez-Fontenla, C. Alternative splicing analysis in a Spanish ASD (Autism Spectrum Disorders) cohort: In silico prediction and characterization. Sci. Rep. 2025, 15, 10730. [Google Scholar] [CrossRef] [PubMed]
- Wanke, K.A.; Devanna, P.; Vernes, S.C. Understanding Neurodevelopmental Disorders: The Promise of Regulatory Variation in the 3′UTRome. Biol. Psychiatry 2018, 83, 548–557. [Google Scholar] [CrossRef]
- Jan, S.M.; Fahira, A.; Hassan, E.S.G.; Abdelhameed, A.S.; Wei, D.; Wadood, A. Integrative approaches to m6A and m5C RNA modifications in autism spectrum disorder revealing potential causal variants. Mamm. Genome 2025, 36, 280–292. [Google Scholar] [CrossRef]
- Santoro, M.R.; Bray, S.M.; Warren, S.T. Molecular mechanisms of fragile X syndrome: A twenty-year perspective. Annu. Rev. Pathol. 2012, 7, 219–245. [Google Scholar] [CrossRef]
- Collins, S.C.; Bray, S.M.; Suhl, J.A.; Cutler, D.J.; Coffee, B.; Zwick, M.E.; Warren, S.T. Identification of novel FMR1 variants by massively parallel sequencing in developmentally delayed males. Am. J. Med. Genet. A 2010, 152a, 2512–2520. [Google Scholar] [CrossRef]
- Tran, S.S.; Jun, H.I.; Bahn, J.H.; Azghadi, A.; Ramaswami, G.; Van Nostrand, E.L.; Nguyen, T.B.; Hsiao, Y.E.; Lee, C.; Pratt, G.A.; et al. Widespread RNA editing dysregulation in brains from autistic individuals. Nat. Neurosci. 2019, 22, 25–36. [Google Scholar] [CrossRef]
- Hagberg, B.; Aicardi, J.; Dias, K.; Ramos, O. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett’s syndrome: Report of 35 cases. Ann. Neurol. 1983, 14, 471–479. [Google Scholar] [CrossRef]
- Rodrigues, D.C.; Kim, D.S.; Yang, G.; Zaslavsky, K.; Ha, K.C.; Mok, R.S.; Ross, P.J.; Zhao, M.; Piekna, A.; Wei, W.; et al. MECP2 Is Post-transcriptionally Regulated during Human Neurodevelopment by Combinatorial Action of RNA-Binding Proteins and miRNAs. Cell Rep. 2016, 17, 720–734. [Google Scholar] [CrossRef] [PubMed]
- Kriaucionis, S.; Bird, A. The major form of MeCP2 has a novel N-terminus generated by alternative splicing. Nucleic Acids Res. 2004, 32, 1818–1823. [Google Scholar] [CrossRef] [PubMed]
- Amir, R.E.; Van den Veyver, I.B.; Wan, M.; Tran, C.Q.; Francke, U.; Zoghbi, H.Y. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 1999, 23, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, A.M.; Oliveira, G.; Katz, C.; Feng, J.; Yan, J.; Yang, C.; Marques, C.; Ataíde, A.; Miguel, T.S.; Borges, L.; et al. MECP2 coding sequence and 3′UTR variation in 172 unrelated autistic patients. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2007, 144b, 475–483. [Google Scholar] [CrossRef]
- Choi, G.; Lee, S.; Yoo, S.; Do, J.T. MECP2 Dysfunction in Rett Syndrome: Molecular Mechanisms, Multisystem Pathology, and Emerging Therapeutic Strategies. Int. J. Mol. Sci. 2025, 26, 8277. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, O.; Yasui, D.H. The Molecular Functions of MeCP2 in Rett Syndrome Pathology. Front. Genet. 2021, 12, 624290. [Google Scholar] [CrossRef]
- Li, R.; Dong, Q.; Yuan, X.; Zeng, X.; Gao, Y.; Chiao, C.; Li, H.; Zhao, X.; Keles, S.; Wang, Z.; et al. Misregulation of Alternative Splicing in a Mouse Model of Rett Syndrome. PLoS Genet. 2016, 12, e1006129. [Google Scholar] [CrossRef]
- Osenberg, S.; Karten, A.; Sun, J.; Li, J.; Charkowick, S.; Felice, C.A.; Kritzer, M.; Nguyen, M.V.C.; Yu, P.; Ballas, N. Activity-dependent aberrations in gene expression and alternative splicing in a mouse model of Rett syndrome. Proc. Natl. Acad. Sci. USA 2018, 115, E5363–E5372. [Google Scholar] [CrossRef]
- Jiang, Y.; Fu, X.; Zhang, Y.; Wang, S.F.; Zhu, H.; Wang, W.K.; Zhang, L.; Wu, P.; Wong, C.C.L.; Li, J.; et al. Rett syndrome linked to defects in forming the MeCP2/Rbfox/LASR complex in mouse models. Nat. Commun. 2021, 12, 5767. [Google Scholar] [CrossRef]
- Greene, D.; Thys, C.; Berry, I.R.; Jarvis, J.; Ortibus, E.; Mumford, A.D.; Freson, K.; Turro, E. Mutations in the U4 snRNA gene RNU4-2 cause one of the most prevalent monogenic neurodevelopmental disorders. Nat. Med. 2024, 30, 2165–2169. [Google Scholar] [CrossRef]
- Burns, V.F.; Radford, E.J. ReNU syndrome – a newly discovered prevalent neurodevelopmental disorder. Trends Genet. 2024, 40, 914–916. [Google Scholar] [CrossRef] [PubMed]
- Danovi, S. RNU4-2 variants cause neurodevelopmental disorders. Nat. Genet. 2024, 56, 1541. [Google Scholar] [CrossRef]
- Chen, Y.; Dawes, R.; Kim, H.C.; Ljungdahl, A.; Stenton, S.L.; Walker, S.; Lord, J.; Lemire, G.; Martin-Geary, A.C.; Ganesh, V.S.; et al. De novo variants in the RNU4-2 snRNA cause a frequent neurodevelopmental syndrome. Nature 2024, 632, 832–840. [Google Scholar] [CrossRef]
- Kuroda, Y.; Nagai, K.; Kawai, Y.; Naruto, T.; Saijou, H.; Morikawa, S.; Goto, T.; Sato, M.; Kurosawa, K. Genotype-phenotype correlations and phenotypic expansion in a case series of ReNU syndrome associated with RNU4-2 variants. J. Med Genet. 2025, 62, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Barbour, K.; Friedman, J.; Bird, L.M.; Del Campo, M.; Wigby, K.; Jones, M.; Chong, A.; Grinspan, Z.M. The Prevalence of RNU4-2-Associated Autosomal Dominant Intellectual Disability Syndrome. Pediatr. Neurol. 2024, 164, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Peñafiel-Sam, J.; Valenzuela, I.; Peris, P. Severe Osteoporosis in an Adult Subject with RNU4-2 Gene Mutation. Calcif. Tissue Int. 2025, 116, 40. [Google Scholar] [CrossRef]
- Rosenblum, J.; Beysen, D.; Jansen, A.C.; De Rademaeker, M.; Reyniers, E.; Janssens, K.; Meuwissen, M. RNU4-2-Related Neurodevelopmental Disorder Is Associated With a Recognisable Facial Gestalt. Clin. Genet. 2024, 107, 104–112. [Google Scholar] [CrossRef]
- Rousseau, P.; Le Discorde, M.; Mouillot, G.; Marcou, C.; Carosella, E.D.; Moreau, P. The 14 bp deletion-insertion polymorphism in the 3′ UT region of the HLA-G gene influences HLA-G mRNA stability. Hum. Immunol. 2003, 64, 1005–1010. [Google Scholar] [CrossRef]
- Guerini, F.R.; Bolognesi, E.; Chiappedi, M.; Ghezzo, A.; Canevini, M.P.; Mensi, M.M.; Vignoli, A.; Agliardi, C.; Zanette, M.; Clerici, M. An HLA-G(∗)14bp insertion/deletion polymorphism associates with the development of autistic spectrum disorders. Brain Behav. Immun. 2015, 44, 207–212. [Google Scholar] [CrossRef]
- Mondal, K.; Ramachandran, D.; Patel, V.C.; Hagen, K.R.; Bose, P.; Cutler, D.J.; Zwick, M.E. Excess variants in AFF2 detected by massively parallel sequencing of males with autism spectrum disorder. Hum. Mol. Genet. 2012, 21, 4356–4364. [Google Scholar] [CrossRef] [PubMed]
- Moncini, S.; Castronovo, P.; Murgia, A.; Russo, S.; Bedeschi, M.F.; Lunghi, M.; Selicorni, A.; Bonati, M.T.; Riva, P.; Venturin, M. Functional characterization of CDK5 and CDK5R1 mutations identified in patients with non-syndromic intellectual disability. J. Hum. Genet. 2016, 61, 283–293. [Google Scholar] [CrossRef]
- Devanna, P.; Chen, X.S.; Ho, J.; Gajewski, D.; Smith, S.D.; Gialluisi, A.; Francks, C.; Fisher, S.E.; Newbury, D.F.; Vernes, S.C. Next-gen sequencing identifies non-coding variation disrupting miRNA-binding sites in neurological disorders. Mol. Psychiatry 2018, 23, 1375–1384. [Google Scholar] [CrossRef]
- Abelson, J.F.; Kwan, K.Y.; O’Roak, B.J.; Baek, D.Y.; Stillman, A.A.; Morgan, T.M.; Mathews, C.A.; Pauls, D.L.; Rasin, M.R.; Gunel, M.; et al. Sequence variants in SLITRK1 are associated with Tourette’s syndrome. Science 2005, 310, 317–320. [Google Scholar] [CrossRef]
- Gong, Y.; Wu, C.N.; Xu, J.; Feng, G.; Xing, Q.H.; Fu, W.; Li, C.; He, L.; Zhao, X.Z. Polymorphisms in microRNA target sites influence susceptibility to schizophrenia by altering the binding of miRNAs to their targets. Eur. Neuropsychopharmacol. 2013, 23, 1182–1189. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, R.; Nie, F.; Wang, X.; Jiang, C.; Liu, M.; Valenzuela, R.K.; Liu, W.; Shi, Y.; Ma, J. MicroRNA-137 Inhibits EFNB2 Expression Affected by a Genetic Variant and Is Expressed Aberrantly in Peripheral Blood of Schizophrenia Patients. EBioMedicine 2016, 12, 133–142. [Google Scholar] [CrossRef]
- Begemann, M.; Grube, S.; Papiol, S.; Malzahn, D.; Krampe, H.; Ribbe, K.; Friedrichs, H.; Radyushkin, K.A.; El-Kordi, A.; Benseler, F.; et al. Modification of cognitive performance in schizophrenia by complexin 2 gene polymorphisms. Arch. Gen. Psychiatry 2010, 67, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Liufu, C.; Luo, L.; Pang, T.; Zheng, H.; Yang, L.; Lu, L.; Chang, S. Integration of multi-omics summary data reveals the role of N6-methyladenosine in neuropsychiatric disorders. Mol. Psychiatry 2024, 29, 3141–3150. [Google Scholar] [CrossRef]
- Xu, X.; Breen, G.; Chen, C.K.; Huang, Y.S.; Wu, Y.Y.; Asherson, P. Association study between a polymorphism at the 3′-untranslated region of CLOCK gene and attention deficit hyperactivity disorder. Behav. Brain Funct. 2010, 6, 48. [Google Scholar] [CrossRef]
- Kissling, C.; Retz, W.; Wiemann, S.; Coogan, A.N.; Clement, R.M.; Hünnerkopf, R.; Conner, A.C.; Freitag, C.M.; Rösler, M.; Thome, J. A polymorphism at the 3′-untranslated region of the CLOCK gene is associated with adult attention-deficit hyperactivity disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2008, 147, 333–338. [Google Scholar] [CrossRef]
- Tong, J.; McKinley, L.A.; Cummins, T.D.; Johnson, B.; Matthews, N.; Vance, A.; Heussler, H.; Gill, M.; Kent, L.; Bellgrove, M.A.; et al. Identification and functional characterisation of a novel dopamine beta hydroxylase gene variant associated with attention deficit hyperactivity disorder. World J. Biol. Psychiatry 2015, 16, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Spellicy, C.J.; Northrup, H.; Fletcher, J.M.; Cirino, P.T.; Dennis, M.; Morrison, A.C.; Martinez, C.A.; Au, K.S. Folate metabolism gene 5,10-methylenetetrahydrofolate reductase (MTHFR) is associated with ADHD in myelomeningocele patients. PLoS ONE 2012, 7, e51330. [Google Scholar] [CrossRef] [PubMed]
- Fernando, M.B.; Fan, Y.; Zhang, Y.; Tokolyi, A.; Murphy, A.N.; Kammourh, S.; Deans, P.J.M.; Ghorbani, S.; Onatzevitch, R.; Pero, A.; et al. Phenotypic complexities of rare heterozygous neurexin-1 deletions. Nature 2025, 642, 710–720. [Google Scholar] [CrossRef]
- Havens, M.A.; Hastings, M.L. Splice-switching antisense oligonucleotides as therapeutic drugs. Nucleic Acids Res. 2016, 44, 6549–6563. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.F.; Krainer, A.R.; Cleveland, D.W. Antisense Oligonucleotide Therapies for Neurodegenerative Diseases. Annu. Rev. Neurosci. 2019, 42, 385–406. [Google Scholar] [CrossRef]
- Dutertre, M.; Chakrama, F.Z.; Combe, E.; Desmet, F.O.; Mortada, H.; Polay Espinoza, M.; Gratadou, L.; Auboeuf, D. A recently evolved class of alternative 3′-terminal exons involved in cell cycle regulation by topoisomerase inhibitors. Nat. Commun. 2014, 5, 3395. [Google Scholar] [CrossRef]
- Araki, S.; Nakayama, Y.; Sano, O.; Nakao, S.; Shimizu-Ogasawara, M.; Toyoshiba, H.; Nakanishi, A.; Aparicio, S. Decoding Transcriptome Dynamics of Genome-Encoded Polyadenylation and Autoregulation with Small-Molecule Modulators of Alternative Polyadenylation. Cell Chem. Biol. 2018, 25, 1470–1484.e5. [Google Scholar] [CrossRef]
- Mullari, M.; Lyon, D.; Jensen, L.J.; Nielsen, M.L. Specifying RNA-Binding Regions in Proteins by Peptide Cross-Linking and Affinity Purification. J. Proteome Res. 2017, 16, 2762–2772. [Google Scholar] [CrossRef]
- Mullari, M.; Fossat, N.; Skotte, N.H.; Asenjo-Martinez, A.; Humphreys, D.T.; Bukh, J.; Kirkeby, A.; Scheel, T.K.H.; Nielsen, M.L. Characterising the RNA-binding protein atlas of the mammalian brain uncovers RBM5 misregulation in mouse models of Huntington’s disease. Nature Communications 2023, 14, 4348. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Zhang, B.; He, Y.; Sun, Z.; Li, J.; Li, Y.; Yi, H.; Zhao, Y.; Zou, X.; Li, Y.; et al. CRISPR-iPAS: A novel dCAS13-based method for alternative polyadenylation interference. Nucleic Acids Res. 2022, 50, e26. [Google Scholar] [CrossRef] [PubMed]
- Kaida, D.; Berg, M.G.; Younis, I.; Kasim, M.; Singh, L.N.; Wan, L.; Dreyfuss, G. U1 snRNP protects pre-mRNAs from premature cleavage and polyadenylation. Nature 2010, 468, 664–668. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zuo, J.; Zhang, G.; Han, X.; Tian, Y. The Role of mRNA Alternative Processing in Mammalian Neurodevelopment. Int. J. Mol. Sci. 2025, 26, 11075. https://doi.org/10.3390/ijms262211075
Liu X, Zuo J, Zhang G, Han X, Tian Y. The Role of mRNA Alternative Processing in Mammalian Neurodevelopment. International Journal of Molecular Sciences. 2025; 26(22):11075. https://doi.org/10.3390/ijms262211075
Chicago/Turabian StyleLiu, Xian, Jian Zuo, Guicheng Zhang, Xiaoyu Han, and Yao Tian. 2025. "The Role of mRNA Alternative Processing in Mammalian Neurodevelopment" International Journal of Molecular Sciences 26, no. 22: 11075. https://doi.org/10.3390/ijms262211075
APA StyleLiu, X., Zuo, J., Zhang, G., Han, X., & Tian, Y. (2025). The Role of mRNA Alternative Processing in Mammalian Neurodevelopment. International Journal of Molecular Sciences, 26(22), 11075. https://doi.org/10.3390/ijms262211075







