Chemical Exchange Saturation Transfer Imaging in Neuroinflammation: Methods, Challenges, and Recommendations
Abstract
1. Introduction
2. CEST-Detectable Targets in Neuroinflammation
3. CEST Imaging
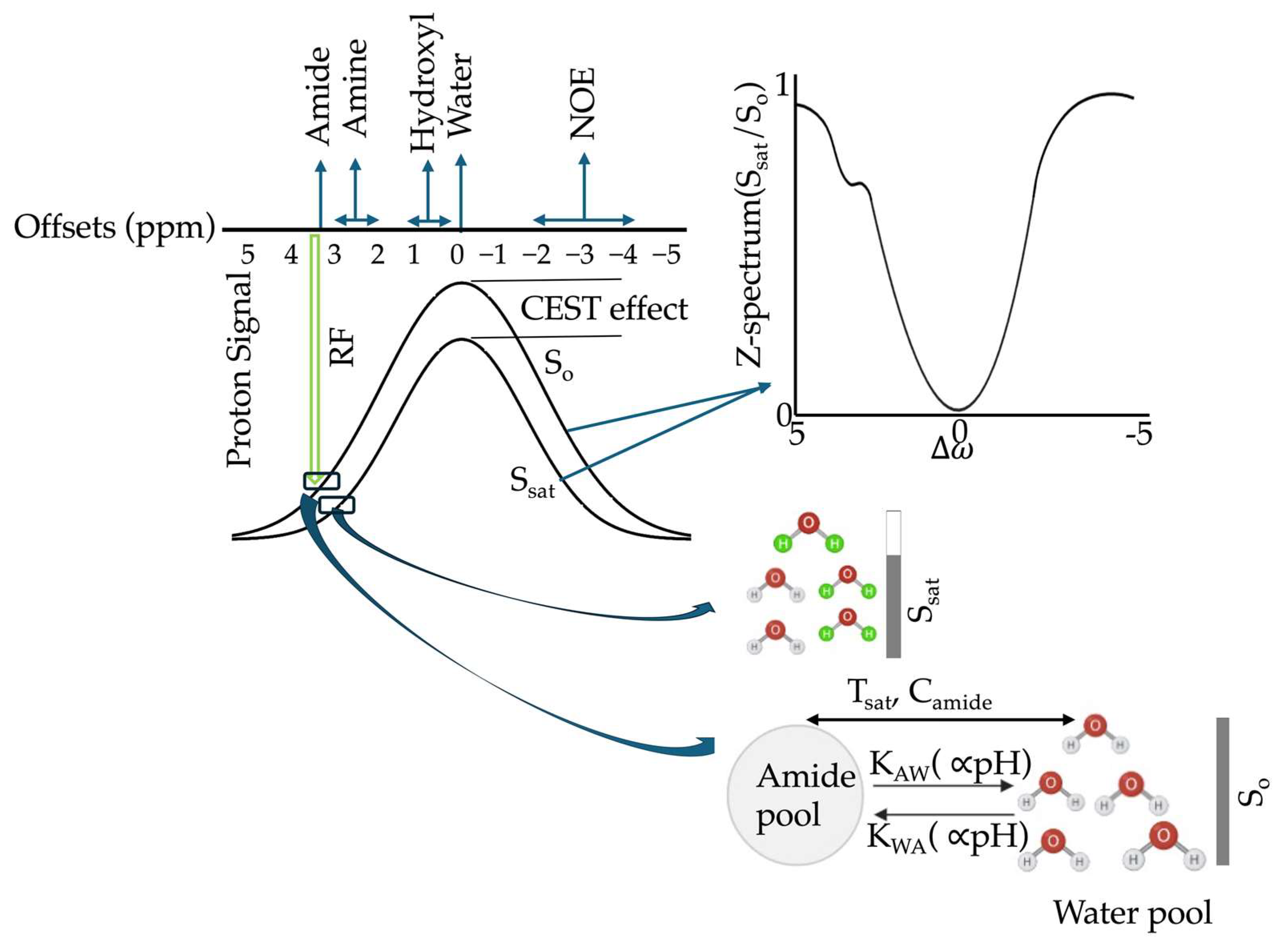
3.1. Principles and Quantification
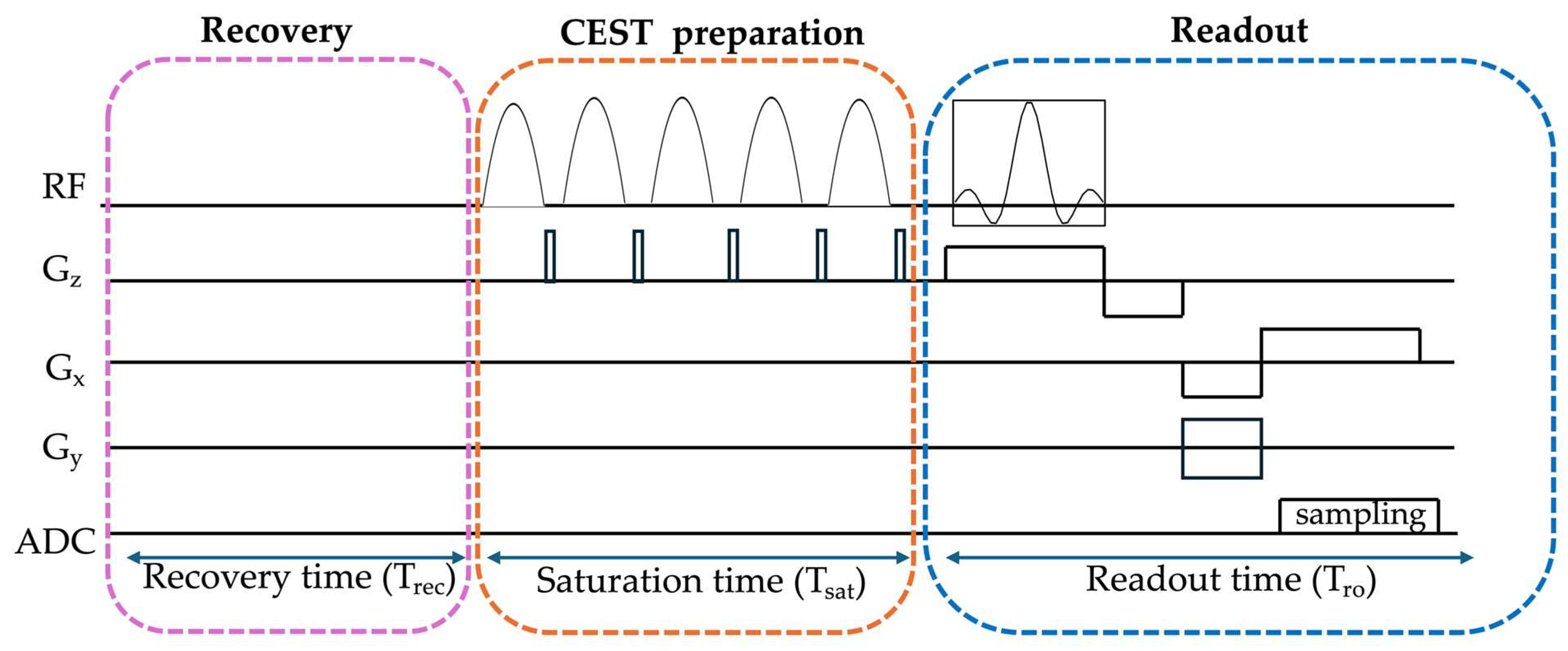
3.2. CEST Acquisition
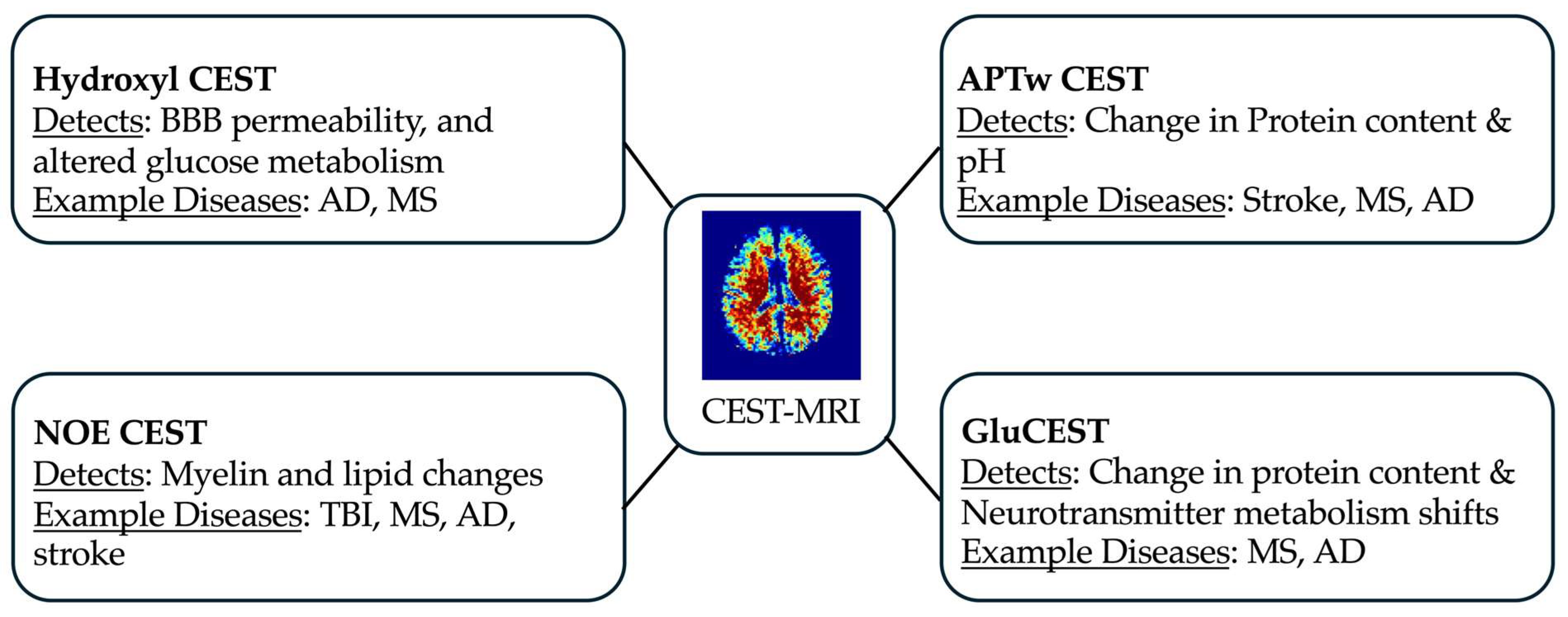
3.3. Types of CEST Imaging
3.3.1. Amide Proton Transfer-Weighted (APTw)
3.3.2. Glutamate-Weighted CEST (GluCEST)
3.3.3. Hydroxyl Proton CEST
3.3.4. Creatine CEST (CrCEST)
3.3.5. Bacterial CEST (BacCEST)
3.3.6. Cryptococcus CEST (CryptoCEST)
3.3.7. Nuclear Overhauser Enhancement (NOE) Imaging
3.4. Accelerating CEST MRI Acquisition
3.4.1. Optimizing Under-Sampling and k-Space Ordering for CEST Imaging
3.4.2. Reconstruction
3.5. CEST Post-Processing
3.5.1. Motion Correction
3.5.2. Correction
3.5.3. Correction
3.5.4. Denoising
3.5.5. Normalization Using an Unsaturated Scan
3.5.6. Contrast Generation
3.6. Artificial Intelligence (AI) Integration in CEST Imaging
3.7. Preclinical and Clinical Application of CEST to Neuroinflammation
3.7.1. Primary Neuroinflammatory Diseases
Multiple Sclerosis and Encephalitis
3.7.2. Inflammatory-Related Disorders
Alzheimer Disease
Traumatic Brain Injury (TBI)
Stroke
Spinal Cord Injury (SCI)
Sepsis-Associated Encephalopathy (SAE)
Other Neuroinflammation Applications
3.8. Potential of CEST and AI in Investigating Neuroinflammation
3.9. High Field CEST MRI for Neuroinflammation Assessment
4. Discussion
4.1. Limitations and Translational Challenges
4.2. Future Directions and Recommendations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full Form |
| 1H-MRS | Proton magnetic resonance spectroscopy |
| AD | Alzheimer’s disease |
| AI | Artificial intelligence |
| ALS | Amyotrophic lateral sclerosis |
| AMO-CEST | Attention-Based MultiOffset Deep Learning Reconstruction of Chemical Exchange Saturation Transfer |
| APP/PS1 | Amyloid precursor protein/presenilin 1 |
| APTw | Amide proton transfer-weighted |
| AREX | Apparent Exchange-dependent Relaxation |
| ARV | Antiretroviral |
| ATP | Adenosine triphosphate |
| BacCEST | Bacterial CEST |
| BBB | Blood–brain barrier |
| BM3D | Block matching combined with 3D filtering |
| bSSFP | Balanced steady-state free precession |
| CD68+ | Cluster of differentiation 68-positive |
| CEST | Chemical exchange saturation transfer |
| CEST-VN | Model-based Variational Network CEST |
| CHI3L1 | Chitinase-3-like protein-1 |
| CIRI | Cerebral ischemia–reperfusion injury |
| CNNs | Convolutional neural networks |
| CNS | Central nervous system |
| Cr | Creatine |
| CrCEST | Creatine CEST |
| CryptoCEST | Cryptococcus CEST |
| CS | Compressed sensing |
| CVAE | Conditional variational autoencoder |
| CW | Continuous-wave |
| DAMPs | Danger-associated molecular patterns |
| DCE | Dynamic contrast-enhanced |
| DECENT | Denoising CEST network |
| DeepEMR | Deep learning extrapolated semisolid magnetization transfer reference |
| DeepCEST | Deep neural network based CEST |
| DexCEST | Dextran-enhanced CEST |
| DiaCEST | Diamagnetic CEST |
| dMRI | Diffusion MRI |
| DS | Direct water saturation |
| DTI | Diffusion tensor imaging |
| EAE | Experimental autoimmune encephalomyelitis |
| EMR | Extrapolated magnetization transfer reference |
| EndoCEST | Endogenous CEST |
| EPI | Echo planar imaging |
| FLAIR | Fluid attenuated inversion recovery |
| GABA | Gamma-aminobutyric acid |
| GAGs | Glycosaminoglycans |
| GagCEST | Glycosaminoglycan CEST |
| GFAP | Glial fibrillary acidic protein |
| Glu | Glutamate |
| GluCEST | Glutamate-weighted CEST |
| GlucoCEST | Glucose CEST |
| GlycoCEST | Glycogen CEST |
| GuanCEST | guanidinium CEST |
| GRASE | Gradient and spin-echo |
| GRE | Gradient echo |
| HAND | HIV-associated neurocognitive disorder |
| HD | Huntington’s disease |
| IDH | Isocitrate dehydrogenase |
| IHC | Immunohistochemistry |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| INRESP | Implicit neural representation combined with explicit sparse prior |
| k-Z PCA | Z-spectrum principal component analysis |
| ksw | Exchange rate |
| LD | Lorentzian difference |
| LPS | Lipopolysaccharide |
| LRAZ | Low-rank approximation of the z-spectrum |
| MALDI | Matrix-assisted laser desorption/ionization |
| MI | Myo-inositol |
| MRI | Magnetic resonance imaging |
| MRS | Magnetic resonance spectroscopy |
| MTC | Magnetization transfer contrast |
| MTC-MRF | Magnetization transfer contrast MR fingerprinting |
| MTRRex | Magnetization transfer ratio with respect to exchange-dependent relaxation |
| MTRasym | Magnetization transfer ratio asymmetry |
| NAA | N-acetylaspartate |
| NODDI | Neurite orientation dispersion and density imaging |
| NOE | Nuclear Overhauser Effect |
| ParaCEST | Paramagnetic CEST |
| PBCS | Parallel blind compressed sensing |
| pCr | Phosphocreatine |
| pCW | Pulse-train |
| PD | Parkinson’s disease |
| PET | Positron emission tomography |
| PTR | Proton transfer ratio |
| QSM | Quantitative susceptibility mapping |
| RF | Radiofrequency |
| rNOE | Relayed Nuclear Overhauser Effect |
| RPCA | Robust principal component analysis |
| SAE | Sepsis-associated encephalopathy |
| SCI | Spinal cord injury |
| SENSE | Sensitivity encoding |
| sLoFNet | Single Lorentzian Fitting Network |
| SNR | Signal to noise ratio |
| SPACE | Sampling perfection with application-optimized contrasts using different flip angle evolution |
| SPECT | Single-photon emission computed tomography |
| sTREM2 | Triggering Receptor Expressed on Myloid cells 2 |
| SVM | Support vector machine |
| SWI | Susceptibility-weighted imaging |
| TBI | Traumatic Brain injury |
| Cho | Choline |
| T1w | T1-weighted |
| T2w | T2-weighted |
| TNF-α | Tumor necrosis factor-alpha |
| Tsat | Saturation time |
| Tro | Readout time |
| TSE | Turbo spin echo |
| TSPO | Translocator protein |
| UPDRS | United Parkinson’s Disease Rating Scale |
| WM | White matter |
References
- Kolliker-Frers, R.; Udovin, L.; Otero-Losada, M.; Kobiec, T.; Herrera, M.I.; Palacios, J.; Razzitte, G.; Capani, F. Neuroinflammation: An Integrating Overview of Reactive-Neuroimmune Cell Interactions in Health and Disease. Mediat. Inflamm. 2021, 2021, 9999146. [Google Scholar] [CrossRef]
- Pulli, B.; Chen, J.W. Imaging Neuroinflammation—From Bench to Bedside. J. Clin. Cell. Immunol. 2014, 5, 226. [Google Scholar] [CrossRef]
- Shi, K.; Tian, D.C.; Li, Z.G.; Ducruet, A.F.; Lawton, M.T.; Shi, F.D. Global brain inflammation in stroke. Lancet Neurol. 2019, 18, 1058–1066. [Google Scholar] [CrossRef]
- Uddin, M.N.; Tivarus, M.E.; Schifitto, G.; Rudko, D.A. Editorial: Neuroimaging of neuroinflammation in neurological disorders. Front. Neurol. 2023, 14, 1328511. [Google Scholar] [CrossRef]
- Roveta, F.; Bonino, L.; Piella, E.M.; Rainero, I.; Rubino, E. Neuroinflammatory Biomarkers in Alzheimer’s Disease: From Pathophysiology to Clinical Implications. Int. J. Mol. Sci. 2024, 25, 11941. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Liu, G. Non-Invasive Wearables in Inflammation Monitoring: From Biomarkers to Biosensors. Biosensors 2025, 15, 351. [Google Scholar] [CrossRef] [PubMed]
- Czap, A.L.; Sheth, S.A. Overview of Imaging Modalities in Stroke. Neurology 2021, 97, S42–S51. [Google Scholar] [CrossRef]
- Singh, P.; Adhikari, A.; Singh, D.; Gond, C.; Tiwari, A.K. The 18-kDa Translocator Protein PET Tracers as a Diagnostic Marker for Neuroinflammation: Development and Current Standing. ACS Omega 2022, 7, 14412–14429. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A. Positron-emission tomography (PET) and single-photon-emission computed tomography (SPECT) Diagnosis of Neurological disorders. Bangladesh J. Med. 2024, 36, 3–14. [Google Scholar] [CrossRef]
- Agarwal, N.; Fan, A.; Huang, X.; Dehkharghani, S.; van der Kolk, A. ISMRM Clinical Focus Meeting 2023: “Imaging the Fire in the Brain”. J. Magn. Reson. Imaging 2023, 61, 1580–1596. [Google Scholar] [CrossRef]
- Plank, J.R.; Morgan, C.A.; Dell’Acqua, F.; Sundram, F.; Hoeh, N.R.; Muthukumaraswamy, S.; Lin, J.C. Mapping neuroinflammation with diffusion-weighted MRI: Randomized crossover study. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2025, 10, 944–953. [Google Scholar] [CrossRef]
- Kim, E.; Carreira Figueiredo, I.; Simmons, C.; Randall, K.; Rojo Gonzalez, L.; Wood, T.; Ranieri, B.; Sureda-Gibert, P.; Howes, O.; Pariante, C.; et al. Mapping acute neuroinflammation in vivo with diffusion-MRI in rats given a systemic lipopolysaccharide challenge. Brain Behav. Immun. 2023, 113, 289–301. [Google Scholar] [CrossRef]
- Chang, L.; Munsaka, S.M.; Kraft-Terry, S.; Ernst, T. Magnetic resonance spectroscopy to assess neuroinflammation and neuropathic pain. J. Neuroimmune Pharmacol. 2013, 8, 576–593. [Google Scholar] [CrossRef]
- Wu, B.; Warnock, G.; Zaiss, M.; Lin, C.; Chen, M.; Zhou, Z.; Mu, L.; Nanz, D.; Tuura, R.; Delso, G. An overview of CEST MRI for non-MR physicists. EJNMMI Phys. 2016, 3, 19. [Google Scholar] [CrossRef]
- Dickens, A.M.; Vainio, S.; Marjamäki, P.; Johansson, J.; Lehtiniemi, P.; Rokka, J.; Rinne, J.; Solin, O.; Haaparanta-Solin, M.; Jones, P.A. Detection of microglial activation in an acute model of neuroinflammation using PET and radiotracers 11C-(R)-PK11195 and 18F-GE-180. J. Nucl. Med. 2014, 55, 466–472. [Google Scholar] [CrossRef]
- Alam, M.M.; Lee, J.; Lee, S.-Y. Recent progress in the development of TSPO PET ligands for neuroinflammation imaging in neurological diseases. Nucl. Med. Mol. Imaging 2017, 51, 283–296. [Google Scholar] [CrossRef]
- Corcia, P.; Tauber, C.; Vercoullie, J.; Arlicot, N.; Prunier, C.; Praline, J.; Nicolas, G.; Venel, Y.; Hommet, C.; Baulieu, J.-L. Molecular imaging of microglial activation in amyotrophic lateral sclerosis. PLoS ONE 2012, 7, e52941. [Google Scholar] [CrossRef]
- Posse, S.; Otazo, R.; Dager, S.R.; Alger, J. MR spectroscopic imaging: Principles and recent advances. J. Magn. Reson. Imaging 2013, 37, 1301–1325. [Google Scholar] [CrossRef]
- van Zijl, P.C.; Yadav, N.N. Chemical exchange saturation transfer (CEST): What is in a name and what isn’t? Magn. Reson. Med. 2011, 65, 927–948. [Google Scholar] [CrossRef]
- Kortje, Z.A.; Bach, H. CEST MRI in the Management/Diagnosis of Neuroinfectious Diseases. Int. J. Mol. Sci. 2025, 26, 5650. [Google Scholar] [CrossRef]
- Stilianu, C.; Graf, C.; Huemer, M.; Diwoky, C.; Soellradl, M.; Rund, A.; Zaiss, M.; Stollberger, R. Enhanced and robust contrast in CEST MRI: Saturation pulse shape design via optimal control. Magn. Reson. Med. 2024, 92, 1867–1880. [Google Scholar] [CrossRef]
- Foo, L.S.; Harston, G.; Mehndiratta, A.; Yap, W.S.; Hum, Y.C.; Lai, K.W.; Mohamed Mukari, S.A.; Mohd Zaki, F.; Tee, Y.K. Clinical translation of amide proton transfer (APT) MRI for ischemic stroke: A systematic review (2003–2020). Quant. Imaging Med. Surg. 2021, 11, 3797–3811. [Google Scholar] [CrossRef]
- Haris, M.; Singh, A.; Cai, K.; Nath, K.; Crescenzi, R.; Kogan, F.; Hariharan, H.; Reddy, R. MICEST: A potential tool for non-invasive detection of molecular changes in Alzheimer’s disease. J. Neurosci. Methods 2012, 212, 87–93. [Google Scholar] [CrossRef]
- Jones, K.M.; Pollard, A.C.; Pagel, M.D. Clinical applications of chemical exchange saturation transfer (CEST) MRI. J. Magn. Reson. Imaging 2017, 47, 11–27. [Google Scholar] [CrossRef]
- Chen, J.; Yadav, N.N.; Stait-Gardner, T.; Gupta, A.; Price, W.S.; Zheng, G. Thiol-water proton exchange of glutathione, cysteine, and N-acetylcysteine: Implications for CEST MRI. NMR Biomed. 2020, 33, e4188. [Google Scholar] [CrossRef]
- Vinogradov, E.; Sherry, A.D.; Lenkinski, R.E. CEST: From basic principles to applications, challenges and opportunities. J. Magn. Reson. 2013, 229, 155–172. [Google Scholar] [CrossRef]
- By, S.; Barry, R.L.; Smith, A.K.; Lyttle, B.D.; Box, B.A.; Bagnato, F.R.; Pawate, S.; Smith, S.A. Amide proton transfer CEST of the cervical spinal cord in multiple sclerosis patients at 3T. Magn. Reson. Med. 2018, 79, 806–814. [Google Scholar] [CrossRef]
- Li, C.; Zhou, J.; Wang, D.; Li, X.; Jiang, S.; Zhang, Y.; Wen, Z.; Wang, G.; Yan, F.; Chen, M. Amide proton transfer imaging of Alzheimer’s disease and Parkinson’s disease. Magn. Reson. Lett. 2023, 3, 22–30. [Google Scholar] [CrossRef]
- Jia, Y.; Chen, Y.; Geng, K.; Cheng, Y.; Li, Y.; Qiu, J.; Huang, H.; Wang, R.; Zhang, Y.; Wu, R. Glutamate Chemical Exchange Saturation Transfer (GluCEST) Magnetic Resonance Imaging in Pre-clinical and Clinical Applications for Encephalitis. Front. Neurosci. 2020, 14, 750. [Google Scholar] [CrossRef]
- Jacobs, P.S.; Swain, A.; Wilson, N.; Liu, F.; Benyard, B.; Spangler, B.; Seitz, M.; Fu, A.; Nanga, R.P.R.; Elliott, M.A.; et al. Diffuse nuclear Overhauser effect MRI contrast changes detected in multiple sclerosis subjects at 7T. Brain Commun. 2025, 7, fcaf043. [Google Scholar] [CrossRef]
- van Zijl, P.C.M.; Lam, W.W.; Xu, J.; Knutsson, L.; Stanisz, G.J. Magnetization Transfer Contrast and Chemical Exchange Saturation Transfer MRI. Features and analysis of the field-dependent saturation spectrum. Neuroimage 2018, 168, 222–241. [Google Scholar] [CrossRef]
- Thomas, A.M.; Yang, E.; Smith, M.D.; Chu, C.; Calabresi, P.A.; Glunde, K.; van Zijl, P.C.M.; Bulte, J.W.M. CEST MRI and MALDI imaging reveal metabolic alterations in the cervical lymph nodes of EAE mice. J. Neuroinflamm. 2022, 19, 130. [Google Scholar] [CrossRef]
- Smith, S.A.; O’Grady, K.P.; Combes, A.J.E.; McKnight, C.D.; Bagnato, F.R. Chapter 5—Magnetization transfer and chemical exchange saturation transfer in neuroinflammation. In Advances in Magnetic Resonance Technology and Applications; Laule, C., Port, J.D., Eds.; Academic Press: Cambridge, MA, USA, 2023; Volume 9, pp. 117–142. [Google Scholar]
- Zaiss, M.; Angelovski, G.; Demetriou, E.; McMahon, M.T.; Golay, X.; Scheffler, K. QUESP and QUEST revisited—Fast and accurate quantitative CEST experiments. Magn. Reson. Med. 2018, 79, 1708–1721. [Google Scholar] [CrossRef]
- Zaiss, M.; Jin, T.; Kim, S.G.; Gochberg, D.F. Theory of chemical exchange saturation transfer MRI in the context of different magnetic fields. NMR Biomed. 2022, 35, e4789. [Google Scholar] [CrossRef]
- Zaiss, M.; Ehses, P.; Scheffler, K. Snapshot-CEST: Optimizing spiral-centric-reordered gradient echo acquisition for fast and robust 3D CEST MRI at 9.4 T. NMR Biomed. 2018, 31, e3879. [Google Scholar] [CrossRef]
- Liu, G.; Song, X.; Chan, K.W.; McMahon, M.T. Nuts and bolts of chemical exchange saturation transfer MRI. NMR Biomed. 2013, 26, 810–828. [Google Scholar] [CrossRef]
- Zaiss, M.; Schuppert, M.; Deshmane, A.; Herz, K.; Ehses, P.; Fullbier, L.; Lindig, T.; Bender, B.; Ernemann, U.; Scheffler, K. Chemical exchange saturation transfer MRI contrast in the human brain at 9.4 T. Neuroimage 2018, 179, 144–155. [Google Scholar] [CrossRef]
- Liu, G.; Gilad, A.A.; Bulte, J.W.; van Zijl, P.C.; McMahon, M.T. High-throughput screening of chemical exchange saturation transfer MR contrast agents. Contrast Media Mol. Imaging 2010, 5, 162–170. [Google Scholar] [CrossRef]
- Heo, H.Y.; Zhang, Y.; Jiang, S.; Zhou, J. Influences of experimental parameters on chemical exchange saturation transfer (CEST) metrics of brain tumors using animal models at 4.7T. Magn. Reson. Med. 2019, 81, 316–330. [Google Scholar] [CrossRef]
- Zhou, J.; Heo, H.Y.; Knutsson, L.; van Zijl, P.C.M.; Jiang, S. APT-weighted MRI: Techniques, current neuro applications, and challenging issues. J. Magn. Reson. Imaging 2019, 50, 347–364. [Google Scholar] [CrossRef]
- Liu, C.; Li, Z.; Chen, Z.; Zhao, B.; Zheng, Z.; Song, X. Highly-accelerated CEST MRI using frequency-offset-dependent k-space sampling and deep-learning reconstruction. Magn. Reson. Med. 2024, 92, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Cohen, O.; Yu, V.Y.; Tringale, K.R.; Young, R.J.; Perlman, O.; Farrar, C.T.; Otazo, R. CEST MR fingerprinting (CEST-MRF) for brain tumor quantification using EPI readout and deep learning reconstruction. Magn. Reson. Med. 2023, 89, 233–249. [Google Scholar] [CrossRef] [PubMed]
- Perlman, O.; Farrar, C.T.; Heo, H.Y. MR fingerprinting for semisolid magnetization transfer and chemical exchange saturation transfer quantification. NMR Biomed. 2023, 36, e4710. [Google Scholar] [CrossRef]
- Sled, J.G. Modelling and interpretation of magnetization transfer imaging in the brain. Neuroimage 2018, 182, 128–135. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Y.; Chen, Y.; Bie, C.; Liang, Y.; He, X.; Song, X. Voxel-wise Optimization of Pseudo Voigt Profile (VOPVP) for Z-spectra fitting in chemical exchange saturation transfer (CEST) MRI. Quant. Imaging Med. Surg. 2019, 9, 1714–1730. [Google Scholar] [CrossRef]
- Sun, P.Z. Demonstration of accurate multi-pool chemical exchange saturation transfer MRI quantification—Quasi-steady-state reconstruction empowered quantitative CEST analysis. J. Magn. Reson. 2023, 348, 107379. [Google Scholar] [CrossRef]
- Saiyisan, A.; Zeng, S.; Zhang, H.; Wang, Z.; Wang, J.; Cai, P.; Huang, J. Chemical exchange saturation transfer MRI for neurodegenerative diseases: An update on clinical and preclinical studies. Neural Regen. Res. 2026, 21, 553–568. [Google Scholar] [CrossRef]
- Zhang, Y.; Zu, T.; Liu, R.; Zhou, J. Acquisition sequences and reconstruction methods for fast chemical exchange saturation transfer imaging. NMR Biomed. 2023, 36, e4699. [Google Scholar] [CrossRef]
- Longo, D.L.; Carella, A.; Corrado, A.; Pirotta, E.; Mohanta, Z.; Singh, A.; Stabinska, J.; Liu, G.; McMahon, M.T. A snapshot of the vast array of diamagnetic CEST MRI contrast agents. NMR Biomed. 2023, 36, e4715. [Google Scholar] [CrossRef] [PubMed]
- Hancu, I.; Dixon, W.T.; Woods, M.; Vinogradov, E.; Sherry, A.D.; Lenkinski, R.E. CEST and PARACEST MR contrast agents. Acta Radiol. 2010, 51, 910–923. [Google Scholar] [CrossRef]
- Mamoune, K.E.; Barantin, L.; Adriaensen, H.; Tillet, Y. Application of Chemical Exchange Saturation Transfer (CEST) in neuroimaging. J. Chem. Neuroanat. 2021, 114, 101944. [Google Scholar] [CrossRef]
- Aime, S.; Delli Castelli, D.; Terreno, E. Supramolecular adducts between poly-L-arginine and [TmIIIdotp]: A route to sensitivity-enhanced magnetic resonance imaging-chemical exchange saturation transfer agents. Angew. Chem. Int. Ed. Engl. 2003, 42, 4527–4529. [Google Scholar] [CrossRef] [PubMed]
- Lindeman, L.R.; Jones, K.M.; High, R.A.; Howison, C.M.; Shubitz, L.F.; Pagel, M.D. Differentiating lung cancer and infection based on measurements of extracellular pH with acidoCEST MRI. Sci. Rep. 2019, 9, 13002. [Google Scholar] [CrossRef]
- Desmond, K.L.; Stanisz, G.J. Understanding quantitative pulsed CEST in the presence of MT. Magn. Reson. Med. 2012, 67, 979–990. [Google Scholar] [CrossRef]
- Chan, K.W.; Bulte, J.W.; McMahon, M.T. Diamagnetic chemical exchange saturation transfer (diaCEST) liposomes: Physicochemical properties and imaging applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 111–124. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, H.; Lee, D.H.; Yu, J.; Cheng, T.; Hong, M.; Jiang, S.; Fan, H.; Huang, X.; Zhou, J.; et al. Using functional and molecular MRI techniques to detect neuroinflammation and neuroprotection after traumatic brain injury. Brain Behav. Immun. 2017, 64, 344–353. [Google Scholar] [CrossRef]
- Kogan, F.; Singh, A.; Debrosse, C.; Haris, M.; Cai, K.; Nanga, R.P.; Elliott, M.; Hariharan, H.; Reddy, R. Imaging of glutamate in the spinal cord using GluCEST. Neuroimage 2013, 77, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Zou, C.; Li, Y.; Jiang, Z.; Tang, X.; Song, X. A Brief History and Future Prospects of CEST MRI in Clinical Non-Brain Tumor Imaging. Int. J. Mol. Sci. 2021, 22, 11559. [Google Scholar] [CrossRef]
- Wen, L.; Regatte, R.R.; Gil, N.; Alexej, J. Assessment of glycosaminoglycan concentration in vivo by chemical exchange-dependent saturation transfer (gagCEST). Proc. Natl. Acad. Sci. USA 2007, 105, 2266–2270. [Google Scholar]
- Chan, K.W.; McMahon, M.T.; Kato, Y.; Liu, G.; Bulte, J.W.; Bhujwalla, Z.M.; Artemov, D.; van Zijl, P.C. Natural D-glucose as a biodegradable MRI contrast agent for detecting cancer. Magn. Reson. Med. 2012, 68, 1764–1773. [Google Scholar] [CrossRef]
- Armbruster, R.R.; Kumar, D.; Benyard, B.; Jacobs, P.; Khandavilli, A.; Liu, F.; Nanga, R.P.R.; McCormack, S.; Cappola, A.R.; Wilson, N.; et al. Personalized and muscle-specific OXPHOS measurement with integrated CrCEST MRI and proton MR spectroscopy. Nat. Commun. 2024, 15, 5387. [Google Scholar] [CrossRef]
- Kogan, F.; Hariharan, H.; Reddy, R. Chemical Exchange Saturation Transfer (CEST) Imaging: Description of Technique and Potential Clinical Applications. Curr. Radiol. Rep. 2013, 1, 102–114. [Google Scholar] [CrossRef]
- Klemmensen, M.M.; Borrowman, S.H.; Pearce, C.; Pyles, B.; Chandra, B. Mitochondrial dysfunction in neurodegenerative disorders. Neurotherapeutics 2024, 21, e00292. [Google Scholar] [CrossRef]
- Lazzarino, G.; Amorini, A.M.; Eikelenboom, M.J.; Killestein, J.; Belli, A.; Di Pietro, V.; Tavazzi, B.; Barkhof, F.; Polman, C.H.; Uitdehaag, B.M.; et al. Cerebrospinal fluid ATP metabolites in multiple sclerosis. Mult. Scler. 2010, 16, 549–554. [Google Scholar] [CrossRef]
- Liu, J.; Bai, R.; Li, Y.; Staedtke, V.; Zhang, S.; van Zijl, P.C.M.; Liu, G. MRI detection of bacterial brain abscesses and monitoring of antibiotic treatment using bacCEST. Magn. Reson. Med. 2018, 80, 662–671. [Google Scholar] [CrossRef]
- Vanherp, L.; Poelmans, J.; Weerasekera, A.; Hillen, A.; Croitor-Sava, A.R.; Sorrell, T.C.; Lagrou, K.; Vande Velde, G.; Himmelreich, U. Trehalose as quantitative biomarker for in vivo diagnosis and treatment follow-up in cryptococcomas. Transl. Res. 2021, 230, 111–122. [Google Scholar] [CrossRef]
- Vanherp, L.; Govaerts, K.; Riva, M.; Poelmans, J.; Coosemans, A.; Lagrou, K.; Gsell, W.; Vande Velde, G.; Himmelreich, U. CryptoCEST: A promising tool for spatially resolved identification of fungal brain lesions and their differentiation from brain tumors with MRI. Neuroimage Clin. 2021, 31, 102737. [Google Scholar] [CrossRef]
- Maziarz, E.K.; Perfect, J.R. Cryptococcosis. Infect. Dis. Clin. N. Am. 2016, 30, 179–206. [Google Scholar] [CrossRef]
- Lu, J.; Zhou, J.; Cai, C.; Cai, S.; Chen, Z. Observation of true and pseudo NOE signals using CEST-MRI and CEST-MRS sequences with and without lipid suppression. Magn. Reson. Med. 2015, 73, 1615–1622. [Google Scholar] [CrossRef]
- Zhou, Y.; Bie, C.; van Zijl, P.C.M.; Yadav, N.N. The relayed nuclear Overhauser effect in magnetization transfer and chemical exchange saturation transfer MRI. NMR Biomed. 2023, 36, e4778. [Google Scholar] [CrossRef]
- Kumar, D.; Benyard, B.; Soni, N.D.; Swain, A.; Wilson, N.; Reddy, R. Feasibility of transient nuclear Overhauser effect imaging in brain at 7 T. Magn. Reson. Med. 2023, 89, 1357–1367. [Google Scholar] [CrossRef]
- Zhou, J.; Lal, B.; Wilson, D.A.; Laterra, J.; van Zijl, P.C. Amide proton transfer (APT) contrast for imaging of brain tumors. Magn. Reson. Med. 2003, 50, 1120–1126. [Google Scholar] [CrossRef]
- Zhou, J.; Payen, J.-F.; Wilson, D.A.; Traystman, R.J.; van Zijl, P.C.M. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat. Med. 2003, 9, 1085–1090. [Google Scholar] [CrossRef]
- Mueller, S.; Stirnberg, R.; Akbey, S.; Ehses, P.; Scheffler, K.; Stocker, T.; Zaiss, M. Whole brain snapshot CEST at 3T using 3D-EPI: Aiming for speed, volume, and homogeneity. Magn. Reson. Med. 2020, 84, 2469–2483. [Google Scholar] [CrossRef]
- Zhao, X.; Wen, Z.; Zhang, G.; Huang, F.; Lu, S.; Wang, X.; Hu, S.; Chen, M.; Zhou, J. Three-dimensional turbo-spin-echo amide proton transfer MR imaging at 3-Tesla and its application to high-grade human brain tumors. Mol. Imaging Biol. 2013, 15, 114–122. [Google Scholar] [CrossRef]
- Dixon, W.T.; Hancu, I.; Ratnakar, S.J.; Sherry, A.D.; Lenkinski, R.E.; Alsop, D.C. A multislice gradient echo pulse sequence for CEST imaging. Magn. Reson. Med. 2010, 63, 253–256. [Google Scholar] [CrossRef]
- Sedykh, M.; Liebig, P.; Herz, K.; Fabian, M.S.; Mennecke, A.; Weinmuller, S.; Schmidt, M.; Dorfler, A.; Zaiss, M. Snapshot CEST++: Advancing rapid whole-brain APTw-CEST MRI at 3 T. NMR Biomed. 2023, 36, e4955. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Jones, C.K.; van Zijl, P.C.; Barker, P.B.; Zhou, J. Fast 3D chemical exchange saturation transfer (CEST) imaging of the human brain. Magn. Reson. Med. 2010, 64, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yong, X.; Liu, R.; Tang, J.; Jiang, H.; Fu, C.; Wei, R.; Hsu, Y.C.; Sun, Y.; Luo, B.; et al. Whole-brain chemical exchange saturation transfer imaging with optimized turbo spin echo readout. Magn. Reson. Med. 2020, 84, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Pang, Q.; Wang, Z.; Li, G.; Fu, C.; Cao, M.; Wang, X.; Jiang, D.; She, D.; Song, Y.; et al. Three-Dimensional Single-Shot CEST Imaging at 3T Based on True FISP Readout. NMR Biomed. 2025, 38, e70109. [Google Scholar] [CrossRef]
- Jeevika; Kulkarni, S.; Jamal, F.; Chaudhary, S.R. Balanced steady-state free precession MRI: History and evolution. Eur. J. Radiol. 2025, 190, 112251. [Google Scholar] [CrossRef] [PubMed]
- Heo, H.Y.; Zhang, Y.; Lee, D.H.; Jiang, S.; Zhao, X.; Zhou, J. Accelerating chemical exchange saturation transfer (CEST) MRI by combining compressed sensing and sensitivity encoding techniques. Magn. Reson. Med. 2017, 77, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Cheema, K.; Han, P.; Lee, H.L.; Xie, Y.; Christodoulou, A.G.; Li, D. Accelerated CEST imaging through deep learning quantification from reduced frequency offsets. Magn. Reson. Med. 2024, 93, 301–310. [Google Scholar] [CrossRef]
- Kwiatkowski, G.; Kozerke, S. Accelerating CEST MRI in the mouse brain at 9.4 T by exploiting sparsity in the Z-spectrum domain. NMR Biomed. 2020, 33, e4360. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, A.; Samaraweera, C.; Uberti, M.; Bade, A.N.; Liu, Y.; Peng, D. Advancing In Vivo Molecular Bioimaging With Optimal Frequency Offset Selection and Deep Learning Reconstruction for CEST MRI. IEEE Access 2025, 13, 89967–89982. [Google Scholar] [CrossRef]
- Mahmud, S.Z.; Singh, M.; van Zijl, P.; Heo, H.Y. Fast and motion-robust saturation transfer MRI with inherent B0 correction using rosette trajectories and compressed sensing. Magn. Reson. Med. 2024, 92, 2535–2545. [Google Scholar] [CrossRef]
- Dopfert, J.; Zaiss, M.; Witte, C.; Schroder, L. Ultrafast CEST imaging. J. Magn. Reson. 2014, 243, 47–53. [Google Scholar] [CrossRef]
- Sui, R.; Chen, L.; Li, Y.; Huang, J.; Chan, K.W.Y.; Xu, X.; van Zijl, P.C.M.; Xu, J. Whole-brain amide CEST imaging at 3T with a steady-state radial MRI acquisition. Magn. Reson. Med. 2021, 86, 893–906. [Google Scholar] [CrossRef]
- Hamilton, J.; Franson, D.; Seiberlich, N. Recent advances in parallel imaging for MRI. Prog. Nucl. Magn. Reson. Spectrosc. 2017, 101, 71–95. [Google Scholar] [CrossRef]
- Deshmane, A.; Gulani, V.; Griswold, M.A.; Seiberlich, N. Parallel MR imaging. J. Magn. Reson. Imaging 2012, 36, 55–72. [Google Scholar] [CrossRef]
- Lustig, M.; Donoho, D.L.; Santos, J.M.; Pauly, J.M. Compressed Sensing MRI. IEEE Signal Process. Mag. 2008, 25, 72–82. [Google Scholar] [CrossRef]
- She, H.; Greer, J.S.; Zhang, S.; Li, B.; Keupp, J.; Madhuranthakam, A.J.; Dimitrov, I.E.; Lenkinski, R.E.; Vinogradov, E. Accelerating chemical exchange saturation transfer MRI with parallel blind compressed sensing. Magn. Reson. Med. 2019, 81, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Togao, O.; Tokunaga, C.; Oga, M.; Kikuchi, K.; Yamashita, K.; Yamamoto, H.; Yoneyama, M.; Kobayashi, K.; Kato, T.; et al. Grading of gliomas using 3D CEST imaging with compressed sensing and sensitivity encoding. Eur. J. Radiol. 2023, 158, 110654. [Google Scholar] [CrossRef]
- Wech, T.; Kostler, H. Robust motion correction in CEST imaging exploiting low-rank approximation of the z-spectrum. Magn. Reson. Med. 2018, 80, 1979–1988. [Google Scholar] [CrossRef]
- Han, P.; Cheema, K.; Lee, H.L.; Zhou, Z.; Cao, T.; Ma, S.; Wang, N.; Han, H.; Christodoulou, A.G.; Li, D. Whole-brain steady-state CEST at 3 T using MR Multitasking. Magn. Reson. Med. 2022, 87, 2363–2371. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, J.; Yang, Y.; Chen, H.; Zhou, Y.; Lin, L.; Wei, Z.; Xu, J.; Chen, Z.; Chen, L. Boosting quantification accuracy of chemical exchange saturation transfer MRI with a spatial-spectral redundancy-based denoising method. NMR Biomed. 2024, 37, e5027. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Cheema, K.; Xie, Y.; Ruan, D.; Li, D. Accelerating CEST MRI With Deep Learning-Based Frequency Selection and Parameter Estimation. NMR Biomed. 2025, 38, e70068. [Google Scholar] [CrossRef]
- Simegn, G.L.; Sun, P.Z.; Zhou, J.; Kim, M.; Reddy, R.; Zu, Z.; Zaiss, M.; Yadav, N.N.; Edden, R.A.E.; van Zijl, P.C.M.; et al. Motion and magnetic field inhomogeneity correction techniques for chemical exchange saturation transfer (CEST) MRI: A contemporary review. NMR Biomed. 2025, 38, e5294. [Google Scholar] [CrossRef]
- Bie, C.; Liang, Y.; Zhang, L.; Zhao, Y.; Chen, Y.; Zhang, X.; He, X.; Song, X. Motion correction of chemical exchange saturation transfer MRI series using robust principal component analysis (RPCA) and PCA. Quant. Imaging Med. Surg. 2019, 9, 1697–1713. [Google Scholar] [CrossRef]
- Kim, M.; Gillen, J.; Landman, B.A.; Zhou, J.; van Zijl, P.C. Water saturation shift referencing (WASSR) for chemical exchange saturation transfer (CEST) experiments. Magn. Reson. Med. 2009, 61, 1441–1450. [Google Scholar] [CrossRef]
- Papageorgakis, C.; Firippi, E.; Gy, B.; Boutelier, T.; Khormi, I.; Al-Iedani, O.; Lechner-Scott, J.; Ramadan, S.; Liebig, P.; Schuenke, P.; et al. Fast WASABI post-processing: Access to rapid B0 and B1 correction in clinical routine for CEST MRI. Magn. Reson. Imaging 2023, 102, 203–211. [Google Scholar] [CrossRef]
- Schuenke, P.; Windschuh, J.; Roeloffs, V.; Ladd, M.E.; Bachert, P.; Zaiss, M. Simultaneous mapping of water shift and B1 (WASABI)-Application to field-Inhomogeneity correction of CEST MRI data. Magn. Reson. Med. 2017, 77, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Jia, G.; Flanigan, D.; Zhou, J.; Knopp, M.V. Chemical exchange saturation transfer MR imaging of articular cartilage glycosaminoglycans at 3 T: Accuracy of B0 Field Inhomogeneity corrections with gradient echo method. Magn. Reson. Imaging 2014, 32, 41–47. [Google Scholar] [CrossRef]
- Poblador Rodriguez, E.; Moser, P.; Dymerska, B.; Robinson, S.; Schmitt, B.; van der Kouwe, A.; Gruber, S.; Trattnig, S.; Bogner, W. A comparison of static and dynamic ∆B0 mapping methods for correction of CEST MRI in the presence of temporal B0 field variations. Magn. Reson. Med. 2019, 82, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Windschuh, J.; Zaiss, M.; Ehses, P.; Lee, J.S.; Jerschow, A.; Regatte, R.R. Assessment of frequency drift on CEST MRI and dynamic correction: Application to gagCEST at 7 T. Magn. Reson. Med. 2019, 81, 573–582. [Google Scholar] [CrossRef]
- Wu, Q.; Gong, P.; Liu, S.; Li, Y.; Liang, D.; Zheng, H.; Wu, Y. B1 inhomogeneity corrected CEST MRI based on direct saturation removed omega plot model at 5T. Magn. Reson. Med. 2024, 92, 532–542. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Q.; Wu, Y. A CVAE-based generative model for generalized B1 inhomogeneity corrected chemical exchange saturation transfer MRI at 5 T. Neuroimage 2025, 312, 121202. [Google Scholar] [CrossRef]
- Goldenberg, J.M.; Berthusen, A.J.; Cárdenas-Rodríguez, J.; Pagel, M.D. Differentiation of myositis-induced models of bacterial infection and inflammation with T2-weighted, CEST, and DCE-MRI. Tomography 2019, 5, 283–291. [Google Scholar] [CrossRef]
- Romdhane, F.; Villano, D.; Irrera, P.; Consolino, L.; Longo, D.L. Evaluation of a similarity anisotropic diffusion denoising approach for improving in vivo CEST-MRI tumor pH imaging. Magn. Reson. Med. 2021, 85, 3479–3496. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, I.Y.; Lu, D.; Manderville, E.; Lo, E.H.; Zheng, H.; Sun, P.Z. pH-sensitive amide proton transfer effect dominates the magnetization transfer asymmetry contrast during acute ischemia-quantification of multipool contribution to in vivo CEST MRI. Magn. Reson. Med. 2018, 79, 1602–1608. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.Q.; Hum, Y.C.; Lai, K.W.; Yap, W.-S.; Zhang, Y.; Heo, H.-Y.; Tee, Y.K. Artificial intelligence in chemical exchange saturation transfer magnetic resonance imaging. Artif. Intell. Rev. 2025, 58, 210. [Google Scholar] [CrossRef]
- Faiyaz, A.; Doyley, M.M.; Schifitto, G.; Uddin, M.N. Artificial intelligence for diffusion MRI-based tissue microstructure estimation in the human brain: An overview. Front. Neurol. 2023, 14, 1168833. [Google Scholar] [CrossRef]
- Zaiss, M.; Deshmane, A.; Schuppert, M.; Herz, K.; Glang, F.; Ehses, P.; Lindig, T.; Bender, B.; Ernemann, U.; Scheffler, K. DeepCEST: 9.4 T Chemical exchange saturation transfer MRI contrast predicted from 3 T data—A proof of concept study. Magn. Reson. Med. 2019, 81, 3901–3914. [Google Scholar] [CrossRef]
- Glang, F.; Deshmane, A.; Prokudin, S.; Martin, F.; Herz, K.; Lindig, T.; Bender, B.; Scheffler, K.; Zaiss, M. DeepCEST 3T: Robust MRI parameter determination and uncertainty quantification with neural networks-application to CEST imaging of the human brain at 3T. Magn. Reson. Med. 2020, 84, 450–466. [Google Scholar] [CrossRef]
- Guo, C.; Wu, J.; Rosenberg, J.T.; Roussel, T.; Cai, S.; Cai, C. Fast chemical exchange saturation transfer imaging based on PROPELLER acquisition and deep neural network reconstruction. Magn. Reson. Med. 2020, 84, 3192–3205. [Google Scholar] [CrossRef]
- Xu, J.; Zu, T.; Hsu, Y.C.; Wang, X.; Chan, K.W.; Zhang, Y. Accelerating CEST imaging using a model-based deep neural network with synthetic training data. Magn. Reson. Med. 2024, 91, 583–599. [Google Scholar] [CrossRef]
- Xiao, G.; Zhang, X.; Yang, G.; Jia, Y.; Yan, G.; Wu, R. Deep learning to reconstruct quasi-steady-state chemical exchange saturation transfer from a non-steady-state experiment. NMR Biomed. 2023, 36, e4940. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Zhang, X.; Tang, H.; Huang, W.; Chen, Y.; Zhuang, C.; Chen, B.; Yang, L.; Chen, Y.; Yan, G.; et al. Deep learning for dense Z-spectra reconstruction from CEST images at sparse frequency offsets. Front. Neurosci. 2023, 17, 1323131. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Shen, D.; Chan, K.W.Y.; Huang, J. Attention-Based MultiOffset Deep Learning Reconstruction of Chemical Exchange Saturation Transfer (AMO-CEST) MRI. IEEE J. Biomed. Health Inform. 2024, 28, 4636–4647. [Google Scholar] [CrossRef]
- Liu, B.; She, H.; Du, Y.P. Scan-Specific Unsupervised Highly Accelerated Non-Cartesian CEST Imaging Using Implicit Neural Representation and Explicit Sparse Prior. IEEE Trans. Biomed. Eng. 2024, 71, 3032–3045. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, X.; Lin, L.; Cai, S.; Cai, C.; Chen, Z.; Xu, J.; Chen, L. Learned spatiotemporal correlation priors for CEST image denoising using incorporated global-spectral convolution neural network. Magn. Reson. Med. 2023, 90, 2071–2088. [Google Scholar] [CrossRef]
- Radke, K.L.; Kamp, B.; Adriaenssens, V.; Stabinska, J.; Gallinnis, P.; Wittsack, H.J.; Antoch, G.; Muller-Lutz, A. Deep Learning-Based Denoising of CEST MR Data: A Feasibility Study on Applying Synthetic Phantoms in Medical Imaging. Diagnostics 2023, 13, 3326. [Google Scholar] [CrossRef]
- Kurmi, Y.; Viswanathan, M.; Zu, Z. Enhancing SNR in CEST imaging: A deep learning approach with a denoising convolutional autoencoder. Magn. Reson. Med. 2024, 92, 2404–2419. [Google Scholar] [CrossRef]
- Kim, B.; Schar, M.; Park, H.; Heo, H.Y. A deep learning approach for magnetization transfer contrast MR fingerprinting and chemical exchange saturation transfer imaging. Neuroimage 2020, 221, 117165. [Google Scholar] [CrossRef]
- Perlman, O.; Zhu, B.; Zaiss, M.; Rosen, M.S.; Farrar, C.T. An end-to-end AI-based framework for automated discovery of rapid CEST/MT MRI acquisition protocols and molecular parameter quantification (AutoCEST). Magn. Reson. Med. 2022, 87, 2792–2810. [Google Scholar] [CrossRef] [PubMed]
- Perlman, O.; Ito, H.; Herz, K.; Shono, N.; Nakashima, H.; Zaiss, M.; Chiocca, E.A.; Cohen, O.; Rosen, M.S.; Farrar, C.T. Quantitative imaging of apoptosis following oncolytic virotherapy by magnetic resonance fingerprinting aided by deep learning. Nat. Biomed. Eng. 2022, 6, 648–657. [Google Scholar] [CrossRef]
- Mohammed Ali, S.; Yadav, N.N.; Wirestam, R.; Singh, M.; Heo, H.Y.; van Zijl, P.C.; Knutsson, L. Deep learning-based Lorentzian fitting of water saturation shift referencing spectra in MRI. Magn. Reson. Med. 2023, 90, 1610–1624. [Google Scholar] [CrossRef] [PubMed]
- Heo, H.Y.; Singh, M.; Yedavalli, V.; Jiang, S.; Zhou, J. CEST and nuclear Overhauser enhancement imaging with deep learning-extrapolated semisolid magnetization transfer reference: Scan-rescan reproducibility and reliability studies. Magn. Reson. Med. 2024, 91, 1002–1015. [Google Scholar] [CrossRef]
- Duhme, C.; Lippe, C.; Hoerr, V.; Jiang, X. Transformer-Based Parameter Fitting of Models Derived from Bloch-McConnell Equations for CEST MRI Analysis. In Machine Learning in Medical Imaging; Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2025; pp. 108–116. [Google Scholar]
- Giri, P.M.; Banerjee, A.; Ghosal, A.; Layek, B. Neuroinflammation in Neurodegenerative Disorders: Current Knowledge and Therapeutic Implications. Int. J. Mol. Sci. 2024, 25, 3995. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.M.; Liu, N.; Qin, Z.H.; Wang, Y. Mitochondrial-derived damage-associated molecular patterns amplify neuroinflammation in neurodegenerative diseases. Acta Pharmacol. Sin. 2022, 43, 2439–2447. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Ren, C.; Yao, R.Q.; Zhang, H.; Feng, Y.W.; Yao, Y.M. Sepsis-associated encephalopathy: A vicious cycle of immunosuppression. J. Neuroinflamm. 2020, 17, 14. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Banks, W.A. Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav. Immun. 2015, 45, 1–12. [Google Scholar] [CrossRef]
- Arroyo, D.S.; Soria, J.A.; Gaviglio, E.A.; Rodriguez-Galan, M.C.; Iribarren, P. Toll-like receptors are key players in neurodegeneration. Int. Immunopharmacol. 2011, 11, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Yanez Lopez, M.; Pardon, M.C.; Baiker, K.; Prior, M.; Yuchun, D.; Agostini, A.; Bai, L.; Auer, D.P.; Faas, H.M. Myoinositol CEST signal in animals with increased Iba-1 levels in response to an inflammatory challenge-Preliminary findings. PLoS ONE 2019, 14, e0212002. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, X.; Liu, S.; Xin, L.; Xuan, W.; Zhuang, C.; Chen, Y.; Chen, B.; Zheng, X.; Wu, R.; et al. CEST imaging combined with 1H-MRS reveal the neuroprotective effects of riluzole by improving neurotransmitter imbalances in Alzheimer’s disease mice. Alzheimer’s Res. Ther. 2025, 17, 20. [Google Scholar] [CrossRef]
- Yanez Lopez, M.P.; Pardon, M.-C.; Prior, M.; Morris, B.; Auer, D.P.; Faas, H. Monitoring neuroinflammation in vivo with CEST imaging. In Proceedings of the International Workshop on Chemical Exchange Saturation Transfer (CEST), Torino, Italy, May 2014. [Google Scholar]
- Huang, J.; Lai, J.H.C.; Tse, K.H.; Cheng, G.W.Y.; Liu, Y.; Chen, Z.; Han, X.; Chen, L.; Xu, J.; Chan, K.W.Y. Deep neural network based CEST and AREX processing: Application in imaging a model of Alzheimer’s disease at 3 T. Magn. Reson. Med. 2022, 87, 1529–1545. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; van Zijl, P.C.M.; Wei, Z.; Lu, H.; Duan, W.; Wong, P.C.; Li, T.; Xu, J. Early detection of Alzheimer’s disease using creatine chemical exchange saturation transfer magnetic resonance imaging. Neuroimage 2021, 236, 118071. [Google Scholar] [CrossRef]
- Ries, M.; Watts, H.; Mota, B.C.; Lopez, M.Y.; Donat, C.K.; Baxan, N.; Pickering, J.A.; Chau, T.W.; Semmler, A.; Gurung, B.; et al. Annexin A1 restores cerebrovascular integrity concomitant with reduced amyloid-beta and tau pathology. Brain 2021, 144, 1526–1541. [Google Scholar] [CrossRef]
- Machhi, J.; Yeapuri, P.; Lu, Y.; Foster, E.; Chikhale, R.; Herskovitz, J.; Namminga, K.L.; Olson, K.E.; Abdelmoaty, M.M.; Gao, J.; et al. CD4+ effector T cells accelerate Alzheimer’s disease in mice. J. Neuroinflamm. 2021, 18, 272. [Google Scholar] [CrossRef]
- Liu, T.; Chen, Y.; Thomas, A.M.; Song, X. CEST MRI with distribution-based analysis for assessment of early stage disease activity in a mouse model of multiple sclerosis: An initial study. NMR Biomed. 2019, 32, e4139. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, J.; Thomas, A.; Liu, G. Dextran-enhanced CEST MRI reveals the size effect of BBB dysfunction associated with neuroinflammation. Veins Lymphat. 2022, 11. [Google Scholar] [CrossRef]
- O’Grady, K.P.; Satish, S.; Owen, Q.R.; Box, B.A.; Bagnato, F.; Combes, A.J.E.; Cook, S.R.; Westervelt, H.J.; Feiler, H.R.; Lawless, R.D.; et al. Relaxation-Compensated Chemical Exchange Saturation Transfer MRI in the Brain at 7T: Application in Relapsing-Remitting Multiple Sclerosis. Front. Neurol. 2022, 13, 764690. [Google Scholar] [CrossRef]
- Thomas, A.M.; Xu, J.; Calabresi, P.A.; van Zijl, P.C.M.; Bulte, J.W.M. Monitoring diffuse injury during disease progression in experimental autoimmune encephalomyelitis with on resonance variable delay multiple pulse (onVDMP) CEST MRI. Neuroimage 2020, 204, 116245. [Google Scholar] [CrossRef]
- Lee, D.W.; Heo, H.; Woo, D.C.; Kim, J.K.; Lee, D.H. Amide Proton Transfer-weighted 7-T MRI Contrast of Myelination after Cuprizone Administration. Radiology 2021, 299, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Huang, J.; Lai, J.H.C.; Tse, K.H.; Xu, J.; Chan, K.W.Y. Chemical exchange saturation transfer MRI detects myelin changes in cuprizone mouse model at 3T. NMR Biomed. 2023, 36, e4937. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Shen, Z.; Chen, Y.; Dai, Z.; Zhang, X.; Mao, Y.; Zhang, B.; Zeng, H.; Chen, P.; Wu, R. Mapping the Changes of Glutamate Using Glutamate Chemical Exchange Saturation Transfer (GluCEST) Technique in a Traumatic Brain Injury Model: A Longitudinal Pilot Study. ACS Chem. Neurosci. 2019, 10, 649–657. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, W.; Jiang, S.; Zhang, Y.; Heo, H.Y.; Wang, X.; Peng, Y.; Wang, J.; Zhou, J. Amide proton transfer-weighted MRI detection of traumatic brain injury in rats. J. Cereb. Blood Flow Metab. 2017, 37, 3422–3432. [Google Scholar] [CrossRef]
- Lee, D.W.; Kwon, J.I.; Heo, H.; Woo, C.W.; Yu, N.H.; Kim, K.W.; Woo, D.C. Cerebral Glutamate Alterations Using Chemical Exchange Saturation Transfer Imaging in a Rat Model of Lipopolysaccharide-Induced Sepsis. Metabolites 2023, 13, 636. [Google Scholar] [CrossRef]
- Lee, D.; Ryu, H.; Chae, Y.J.; Binjaffar, H.; Woo, C.W.; Woo, D.C.; Lee, D.W. Amide Proton Transfer-Weighted MR Imaging and Signal Variations in a Rat Model of Lipopolysaccharide-Induced Sepsis-Associated Encephalopathy. Metabolites 2025, 15, 465. [Google Scholar] [CrossRef]
- Liu, H.; Jablonska, A.; Li, Y.; Cao, S.; Liu, D.; Chen, H.; Van Zijl, P.C.; Bulte, J.W.; Janowski, M.; Walczak, P.; et al. Label-free CEST MRI Detection of Citicoline-Liposome Drug Delivery in Ischemic Stroke. Theranostics 2016, 6, 1588–1600. [Google Scholar] [CrossRef]
- Li, Z.; Gong, P.; Zhang, M.; Li, C.; Xiao, P.; Yu, M.; Wang, X.; An, L.; Bi, F.; Song, X.; et al. Multi-parametric MRI assessment of melatonin regulating the polarization of microglia in rats after cerebral ischemia/reperfusion injury. Brain Res. Bull. 2023, 204, 110807. [Google Scholar] [CrossRef]
- Mu, C.; Reed, J.L.; Wang, F.; Yan, X.; Lu, M.; Gore, J.C.; Chen, L.M. Validation of qMT and CEST MRI as Biomarkers of Response to Treatment After Lumbar Spinal Cord Injury in Rats. NMR Biomed. 2025, 38, e70015. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.; Reed, J.L.; Wang, F.; Tantawy, M.N.; Gore, J.C.; Chen, L.M. Spatiotemporal Dynamics of Neuroinflammation Relate to Behavioral Recovery in Rats with Spinal Cord Injury. Mol. Imaging Biol. 2024, 26, 240–252. [Google Scholar] [CrossRef]
- Wang, F.; Gore, J.C.; Chen, L.M. Early Detection of Neuroinflammation and White Matter Damage Following Dorsal Spinal Nerve Root Sectioning in a Nonhuman Primate Model. bioRxiv 2025. [Google Scholar] [CrossRef]
- Gauthier, G.C.; Summerlin, M.; Sajja, B.R.; Uberti, M.G.; Foster, E.G.; Kumar, M.; Thiele, M.; Gorantla, S.; Bade, A.N.; Liu, Y. CEST MRI Affirms HIV-1-Associated Neurometabolic Impairments in a Humanized Mouse Model. Res. Sq. 2025. [Google Scholar] [CrossRef]
- Bade, A.N.; Gendelman, H.E.; McMillan, J.; Liu, Y. Chemical exchange saturation transfer for detection of antiretroviral drugs in brain tissue. AIDS 2021, 35, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, M.; Du, H.; Xu, D.; Wang, J.; Ren, Q.; Wang, R.; Gong, H.; Liu, Y.; Qi, K.; et al. The alteration of glutamate involved in the brain of Parkinson’s disease patients using glutamate chemical exchange saturation transfer (GluCEST). Behav. Brain Res. 2025, 483, 115484. [Google Scholar] [CrossRef]
- Chung, J.; Sun, D.; Hitchens, T.K.; Modo, M.; Bandos, A.; Mettenburg, J.; Wang, P.; Jin, T. Dual contrast CEST MRI for pH-weighted imaging in stroke. Magn. Reson. Med. 2024, 91, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Wu, Y.; Cheung, J.S.; Igarashi, T.; Wu, L.; Zhang, X.; Sun, P.Z. Mapping tissue pH in an experimental model of acute stroke—Determination of graded regional tissue pH changes with non-invasive quantitative amide proton transfer MRI. Neuroimage 2019, 191, 610–617. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, P.Z. Demonstration of pH imaging in acute stroke with endogenous ratiometric chemical exchange saturation transfer magnetic resonance imaging at 2 ppm. NMR Biomed. 2023, 36, e4850. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ju, L.; Qiao, G.; Liang, Y.; Wu, Y.; Chu, C.; Rogers, J.; Li, Y.; Cao, S.; Dawson, V.L.; et al. Elucidating metabolite and pH variations in stroke through guanidino, amine and amide CEST MRI: A comparative multi-field study at 9.4T and 3T. Neuroimage 2025, 305, 120993. [Google Scholar] [CrossRef]
- Msayib, Y.; Harston, G.W.J.; Ray, K.J.; Larkin, J.R.; Sutherland, B.A.; Sheerin, F.; Blockley, N.P.; Okell, T.W.; Jezzard, P.; Baldwin, A.; et al. Quantitative chemical exchange saturation transfer imaging of nuclear overhauser effects in acute ischemic stroke. Magn. Reson. Med. 2022, 88, 341–356. [Google Scholar] [CrossRef]
- Heo, H.Y.; Tee, Y.K.; Harston, G.; Leigh, R.; Chappell, M.A. Amide proton transfer imaging in stroke. NMR Biomed. 2023, 36, e4734. [Google Scholar] [CrossRef]
- Solomou, G.; Young, A.M.H.; Bulstrode, H. Microglia and macrophages in glioblastoma: Landscapes and treatment directions. Mol. Oncol. 2024, 18, 2906–2926. [Google Scholar] [CrossRef]
- Huang, J.; Chen, Z.; Park, S.W.; Lai, J.H.C.; Chan, K.W.Y. Molecular Imaging of Brain Tumors and Drug Delivery Using CEST MRI: Promises and Challenges. Pharmaceutics 2022, 14, 451. [Google Scholar] [CrossRef]
- Warnert, E.A.H.; Wood, T.C.; Incekara, F.; Barker, G.J.; Vincent, A.J.P.; Schouten, J.; Kros, J.M.; van den Bent, M.; Smits, M.; Tamames, J.A.H. Mapping tumour heterogeneity with pulsed 3D CEST MRI in non-enhancing glioma at 3 T. MAGMA 2022, 35, 53–62. [Google Scholar] [CrossRef]
- Xu, Z.; Ke, C.; Liu, J.; Xu, S.; Han, L.; Yang, Y.; Qian, L.; Liu, X.; Zheng, H.; Lv, X.; et al. Diagnostic performance between MR amide proton transfer (APT) and diffusion kurtosis imaging (DKI) in glioma grading and IDH mutation status prediction at 3 T. Eur. J. Radiol. 2021, 134, 109466. [Google Scholar] [CrossRef] [PubMed]
- Field, S.E.; Curle, A.J.; Barker, R.A. Inflammation and Huntington’s disease—A neglected therapeutic target? Expert Opin. Investig. Drugs 2024, 33, 451–467. [Google Scholar] [CrossRef] [PubMed]
- Blusch, A.; Bjorkqvist, M. Neuroinflammation in Huntington’s disease: Causes, consequences, and treatment strategies. J. Huntingt. Dis. 2025, 14, 258–269. [Google Scholar] [CrossRef]
- Pepin, J.; de Longprez, L.; Trovero, F.; Brouillet, E.; Valette, J.; Flament, J. Complementarity of gluCEST and 1H-MRS for the study of mouse models of Huntington’s disease. NMR Biomed. 2020, 33, e4301. [Google Scholar] [CrossRef]
- Perot, J.B.; Celestine, M.; Palombo, M.; Dhenain, M.; Humbert, S.; Brouillet, E.; Flament, J. Longitudinal multimodal MRI characterization of a knock-in mouse model of Huntington’s disease reveals early gray and white matter alterations. Hum. Mol. Genet. 2022, 31, 3581–3596. [Google Scholar] [CrossRef] [PubMed]
- Sartoretti, E.; Sartoretti, T.; Wyss, M.; Reischauer, C.; van Smoorenburg, L.; Binkert, C.A.; Sartoretti-Schefer, S.; Mannil, M. Amide proton transfer weighted (APTw) imaging based radiomics allows for the differentiation of gliomas from metastases. Sci. Rep. 2021, 11, 5506. [Google Scholar] [CrossRef]
- Wu, M.; Jiang, T.; Guo, M.; Duan, Y.; Zhuo, Z.; Weng, J.; Xie, C.; Sun, J.; Li, J.; Cheng, D. Amide proton transfer-weighted imaging and derived radiomics in the classification of adult-type diffuse gliomas. Eur. Radiol. 2024, 34, 2986–2996. [Google Scholar] [CrossRef] [PubMed]
- Bie, C.; Li, Y.; Zhou, Y.; Bhujwalla, Z.M.; Song, X.; Liu, G.; van Zijl, P.C.M.; Yadav, N.N. Deep learning-based classification of preclinical breast cancer tumor models using chemical exchange saturation transfer magnetic resonance imaging. NMR Biomed. 2022, 35, e4626. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Yuan, W.; Jia, Z.; Chen, J.; Li, L.; Yan, Z.; Liao, Y.; Mao, L.; Hu, S.; Liu, X. Preoperative MR radiomics based on high-resolution T2-weighted images and amide proton transfer-weighted imaging for predicting lymph node metastasis in rectal adenocarcinoma. Abdom. Radiol. 2023, 48, 458–470. [Google Scholar] [CrossRef]
- Zhuo, Z.; Qu, L.; Zhang, P.; Duan, Y.; Cheng, D.; Xu, X.; Sun, T.; Ding, J.; Xie, C.; Liu, X.; et al. Prediction of H3K27M-mutant brainstem glioma by amide proton transfer-weighted imaging and its derived radiomics. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4426–4436. [Google Scholar] [CrossRef]
- Hagiwara, A.; Tatekawa, H.; Yao, J.; Raymond, C.; Everson, R.; Patel, K.; Mareninov, S.; Yong, W.H.; Salamon, N.; Pope, W.B.; et al. Visualization of tumor heterogeneity and prediction of isocitrate dehydrogenase mutation status for human gliomas using multiparametric physiologic and metabolic MRI. Sci. Rep. 2022, 12, 1078. [Google Scholar] [CrossRef]
- Yuan, Y.; Yu, Y.; Chang, J.; Chu, Y.H.; Yu, W.; Hsu, Y.C.; Patrick, L.A.; Liu, M.; Yue, Q. Convolutional neural network to predict IDH mutation status in glioma from chemical exchange saturation transfer imaging at 7 Tesla. Front. Oncol. 2023, 13, 1134626. [Google Scholar] [CrossRef]
- Chu, Z.; Qu, Y.; Zhong, T.; Liang, S.; Wen, Z.; Zhang, Y. A Dual-Aware deep learning framework for identification of glioma isocitrate dehydrogenase genotype using magnetic resonance amide proton transfer modalities. Nan Fang Yi Ke Da Xue Xue Bao J. South. Med. Univ. 2023, 43, 1379–1387. [Google Scholar]
- Paprottka, K.J.; Kleiner, S.; Preibisch, C.; Kofler, F.; Schmidt-Graf, F.; Delbridge, C.; Bernhardt, D.; Combs, S.E.; Gempt, J.; Meyer, B.; et al. Fully automated analysis combining [18F]-FET-PET and multiparametric MRI including DSC perfusion and APTw imaging: A promising tool for objective evaluation of glioma progression. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4445–4455. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Unberath, M.; Heo, H.Y.; Eberhart, C.G.; Lim, M.; Blakeley, J.O.; Jiang, S. Learning-based analysis of amide proton transfer-weighted MRI to identify true progression in glioma patients. Neuroimage Clin. 2022, 35, 103121. [Google Scholar] [CrossRef]
- Mahmud, S.Z.; Heo, H.Y. When CEST meets diffusion: Multi-echo diffusion-encoded CEST (dCEST) MRI to measure intracellular and extracellular CEST signal distributions. Magn. Reson. Med. 2025, 94, 982–992. [Google Scholar] [CrossRef]
- Weninger, L.; Na, C.H.; Jutten, K.; Merhof, D. Analyzing the effects of free water modeling by deep learning on diffusion MRI structural connectivity estimates in glioma patients. PLoS ONE 2020, 15, e0239475. [Google Scholar] [CrossRef] [PubMed]
- Platt, T.; Ladd, M.E.; Paech, D. 7 Tesla and Beyond: Advanced Methods and Clinical Applications in Magnetic Resonance Imaging. Investig. Radiol. 2021, 56, 705–725. [Google Scholar] [CrossRef] [PubMed]
- Walker-Samuel, S.; Ramasawmy, R.; Torrealdea, F.; Rega, M.; Rajkumar, V.; Johnson, S.P.; Richardson, S.; Goncalves, M.; Parkes, H.G.; Arstad, E.; et al. In vivo imaging of glucose uptake and metabolism in tumors. Nat. Med. 2013, 19, 1067–1072. [Google Scholar] [CrossRef]
- Zhou, J.; Zaiss, M.; Knutsson, L.; Sun, P.Z.; Ahn, S.S.; Aime, S.; Bachert, P.; Blakeley, J.O.; Cai, K.; Chappell, M.A.; et al. Review and consensus recommendations on clinical APT-weighted imaging approaches at 3T: Application to brain tumors. Magn. Reson. Med. 2022, 88, 546–574. [Google Scholar] [CrossRef]

| Diseases | CEST Parameters | Blood/Tissue Biomarkers | Saturation Parameters | Clinical/Preclinical | Reference |
|---|---|---|---|---|---|
| AD | Hydroxyl protons at 0.6 ppm (↑) | Iba1+ (strong response) | = 0.9 μT, Tsat = 1.6 s Offsets: ±4 ppm (0.2 ppm steps) | Preclinical | [137] |
| AD | Glu (3.0 ppm) ↓, (2.75 ppm) ↓; Glu and GABA levels (↑) after riluzole | 1H-MRS: Glu, GABA (↑) after riluzole; IHC: Aβ, tau, GFAP (↓) after riluzole Nissl: neuronal survival (↑) | = 4 μT; 2 s CW; GRE readout; Offsets: ±5 ppm (0.1 ppm steps) | Preclinical | [138] |
| AD | Hydroxyl protons 0.4–0.8 ppm (↑) | Inferred neuroinflammation due to elevated MI in prior 1H-MRS studies | = 0.9 μT; Tsat = 1.6 s; Offsets: ±4 ppm | Preclinical | [139] |
| AD | APTw at 3.5 ppm (↓); MT contrast (↓); rNOE unchanged; | 6E10 IHC: Aβ plaque (↑) | = 0.6 μT; CW; Tsat = 3 s; Offsets: ±20 ppm | Preclinical | [140] |
| AD | Cr at 2 ppm (↓) | 1H-MRS and 31P-MRS: Cr/PCr (no change); GFAP and IBA1 activation (stronger in APP vs. Tau) | = 2 μT; Tsat = 1 s | Preclinical | [141] |
| AD | MI at 0.6 ppm (↑) | 1H-MRS: MI/Cr ↑ 51%; GFAP IHC: astrocyte proliferation | = 75 Hz; Tsat = 5 s; Offsets: 0–2 ppm (step = 0.1 ppm) | Preclinical | [23] |
| AD | GlucoCEST (0.8–2.2 ppm): ↑ before treatment, ↓ after hrANXA1 treatment, | BBB via Evans blue; hrANXA1: ↓ TNFα, IFNγ; ↑ IL-10; ↓ CD3+ T-cell, Aβ40, and p-tau, and ↑ IDE, neprilysin | = 1.6 µT; Tsat = 3 s; Offsets: ±3.2 ppm (17 offsets) | Preclinical | [142] |
| AD | 2DG-CEST (0.8–2.2 ppm); ↓ %ΔMTR in APP/PS1; ↓ Aβ-Th1 (87%) and Aβ-Th17 (67%) groups | ↑ IFNγ, TNFα, and IL-17; Iba1+ microglia activation; ↑ iNOS, ↓ Arg1 (M1 shift); ↓ Foxp3, Il10, Il13 (anti-inflammatory genes) | = 3 µT; Tsat = 1 s Offsets: −1600 to 1600 Hz in steps of 80 Hz. | Preclinical | [143] |
| MS | Hydroxyls (1 ppm ↑); Amines (2 ppm ↑) | IBA1 immunofluorescence (↑); Gd-enhanced T1w MRI confirmation | = 1 μT; Tsat = 2.5 s; Offsets: ±4 ppm; (0.25 ppm steps) | Preclinical | [144] |
| MS | CEST signals at 1.6, 3.2, and 5.2 ppm (↑) | Flow cytometry: CD11b, CD86, IL-17a (↑); MALDI: ↑ alanine, lactate, malate, phenylalanine | = 1 μT; Tsat = 3 s Offsets: 0.4–6.0 ppm (0.4 ppm steps) | Preclinical | [32] |
| MS | DexCEST at +1.0 ppm (OH ↑) | Gd-MRI (structural); Fluorescence (Dex3-FITC, Dex40-TRITC): BBB permeability | = 1.8 μT; Tsat = 3 s; Offsets: ±3 ppm; (31 offsets, 0.2 ppm steps) | Preclinical | [145] |
| MS | Glu at 3.0 ppm (↑) | ↑ Glutamate in WM lesions: correlated with EDSS and cognitive decline | = 7 T; Brms = 1.97 μT; Tsat = 670 ms pulse train; Offsets: ±5 ppm (43 offsets) | Clinical | [146] |
| MS | onVDMP: ↓ signal in corpus callosum, hypothalamus, and 3rd ventricle (early EAE). APTw: No significant change | GFAP (↑), CD11B/CD68 (↑); astrogliosis without demyelination | = 11.7 T, = 46.8 μT, 32 pulses; APTw CEST: = 1 μT, Tsat = 3 s; Offsets: ±8 ppm (0.4 ppm steps) | Preclinical | [147] |
| MS | APTw at 3.5 ppm ↑ in DEM; ↓ in REM | TEM and Black-Gold II staining: showing DEM and REM | = 2.3 mT Tsat = 5 s; Offsets: ±6 ppm (0.5 ppm steps) | Preclinical | [148] |
| MS | rNOE at −3.5 ppm (↓ in DEM, recovered in REM); Amide at +3.5 ppm (↓ in DEM, remained low in REM) | FluoroMyelin and MBP: ↑ week 8, and ↓ week 14 | = 0.8 μT; Tsat = 3 s; Offsets: ±13 ppm; | Preclinical | [149] |
| TBI | Glu at 3.0ppm (↑) | IL-6 and TNF-α (↑) | = 5.9 μT; Tsat = 2 s; Offsets: ±5 ppm | Preclinical | [150] |
| TBI | Amide at 3.5 ppm (↑ day 3–7); ↓ with pinocembrin—↓ inflammation | Iba1 and GFAP IHC; Cresyl violet (neuron survival) | = 1.3 μT; Tsat = 4 s; Offsets: ±3.5 ppm | Preclinical | [57] |
| TBI | Amide at 3.5 ppm (↓ 1–6 h; ↑ 2–3 days peri-lesional) | Iba1+, GFAP+ glial activation (↑ 3 days) | = 1.3 μT; Tsat = 4 s; Offset: ±3.5 ppm | Preclinical | [151] |
| Encephalitis | Glu at 3.0ppm (↑; ↓ after treatment) | Preclinical: S. aureus–induced microgliosis & astrogliosis; Clinical: ↑ CSF white blood cells | = 7 T; = 3.6 µT, Tsat = 2 s; = 3 T; = 5.9 µT; Tsat = 2 s Offsets for both: ±5 ppm (0.2 ppm steps) | Both | [29] |
| SAE | Glu at 3.0ppm (↑) | 1H-MRS: Glu (↑); Iba-1, NeuN, DAPI | = 3.6 μT; Tsat = 1 s; Offsets: ±6 ppm (0.5 ppm steps) | Preclinical | [152] |
| SAE | Amide at 3.5 ppm (↑) hippocampus) | Neuroinflammation in LPS-induced SAE | = 2.3 μT; Tsat = 5 s; Offsets: ±6 ppm (0.5 ppm steps) | Preclinical | [153] |
| Ischemic Stroke | OH at +1.0 ppm and NH2 at +2.0 ppm from CDPC (↑ after injection) | ↑ Fluorescence and T2w (after liposomal CDPC delivery); | = 11.7 T; = 2.7 µT (in vivo) Tsat = 3 s; Offsets: ±4 ppm (0.2 ppm steps) | Preclinical | [154] |
| Ischemic stroke | Amide at 3.5 ppm (↓) CIRI and ↑ Melatonin); Guanidium at 2.0 ppm (↓ CIRI and ↑ Melatonin) | ↓ IL-1β; ↑ Arg1, CD206 (M2 polarization); H&E, TTC, TUNEL, and NeuN —reduced damage | = 7 T; = 1 µT Offsets: ±10 ppm; 51 offsets | Preclinical | [155] |
| SCI | Amide at 3.5 ppm (↑ Week 1); ↓ after riluzole | Iba-1 (↓ after riluzole), GFAP (no change); LFB (↑ after riluzole) & BBB score (↑ after riluzole) | = 1 μT Tsat = 2.0 s; Offsets: ±5 ppm; 33 offsets | Preclinical | [156] |
| Traumatic SCI | Amide at +3.5 ppm (↓); NOE at −1.6 ppm (↓); (Week 1 post-injury) | PET-TSPO (↑); Iba-1 and GFAP (↑) | = 9.4 T; CW Tsat = 2.0 s; Offsets: ±5 ppm; | Preclinical | [157] |
| Spinal Dorsal Nerve Root | Amide at 3.5 ppm (↑) NOE at −1.6 ppm (↓) | MRI (FA ↓, RD ↑, Pool Size Ratio ↓; | = 1.0 µT; CW Tsat = 5s; Offset: ±5 ppm (0.2 ppm steps) | Preclinical | [158] |
| HAND | Glu at 3 ppm (↓ in cortex, hippocampus, cortex at 12 WPI); Cr at 2 ppm (↓ in cortex and hippocampus at 6–12 WPI); NOE at −3.5 ppm (↑ in cortex and thalamus at 6 and 12 WPI) | IHC: p24, Iba-1, GFAP, HLA-DR activation (↑) | = 7 T; = 2 µT; Tsat = 2 s; Offsets: ±5 ppm 51 offsets (0.1–0.2 ppm steps) | Preclinical | [159] |
| HIV | Hydroxyl and amine of Lamivudine (3TC) and Emtricitabine (FTC) (↑) | Plasma & brain t 3TC/FTC via UPLC–MS/MS | = 2 μT; Tsat = 1 s; Offsets: ±5 ppm (0.2 ppm steps) | Preclinical | [160] |
| PD | Glu at 3.0ppm (↑) | Neuroinflammation inferred from astrocytic glutamate dysregulation | = 3 T; = 3 μT; Offsets: ±6 ppm 54 offsets | Clinical | [161] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mensah, E.A.; Faiyaz, A.; Schifitto, G.; Uddin, M.N. Chemical Exchange Saturation Transfer Imaging in Neuroinflammation: Methods, Challenges, and Recommendations. Int. J. Mol. Sci. 2025, 26, 11059. https://doi.org/10.3390/ijms262211059
Mensah EA, Faiyaz A, Schifitto G, Uddin MN. Chemical Exchange Saturation Transfer Imaging in Neuroinflammation: Methods, Challenges, and Recommendations. International Journal of Molecular Sciences. 2025; 26(22):11059. https://doi.org/10.3390/ijms262211059
Chicago/Turabian StyleMensah, Emmanuel A., Abrar Faiyaz, Giovanni Schifitto, and Md Nasir Uddin. 2025. "Chemical Exchange Saturation Transfer Imaging in Neuroinflammation: Methods, Challenges, and Recommendations" International Journal of Molecular Sciences 26, no. 22: 11059. https://doi.org/10.3390/ijms262211059
APA StyleMensah, E. A., Faiyaz, A., Schifitto, G., & Uddin, M. N. (2025). Chemical Exchange Saturation Transfer Imaging in Neuroinflammation: Methods, Challenges, and Recommendations. International Journal of Molecular Sciences, 26(22), 11059. https://doi.org/10.3390/ijms262211059







