Spatial- and Phospho-Proteomic Profiling Reveals Pancreatic and Hepatic Dysfunction in a Rat Model of Lethal Insulin Overdose
Abstract
1. Introduction
2. Results
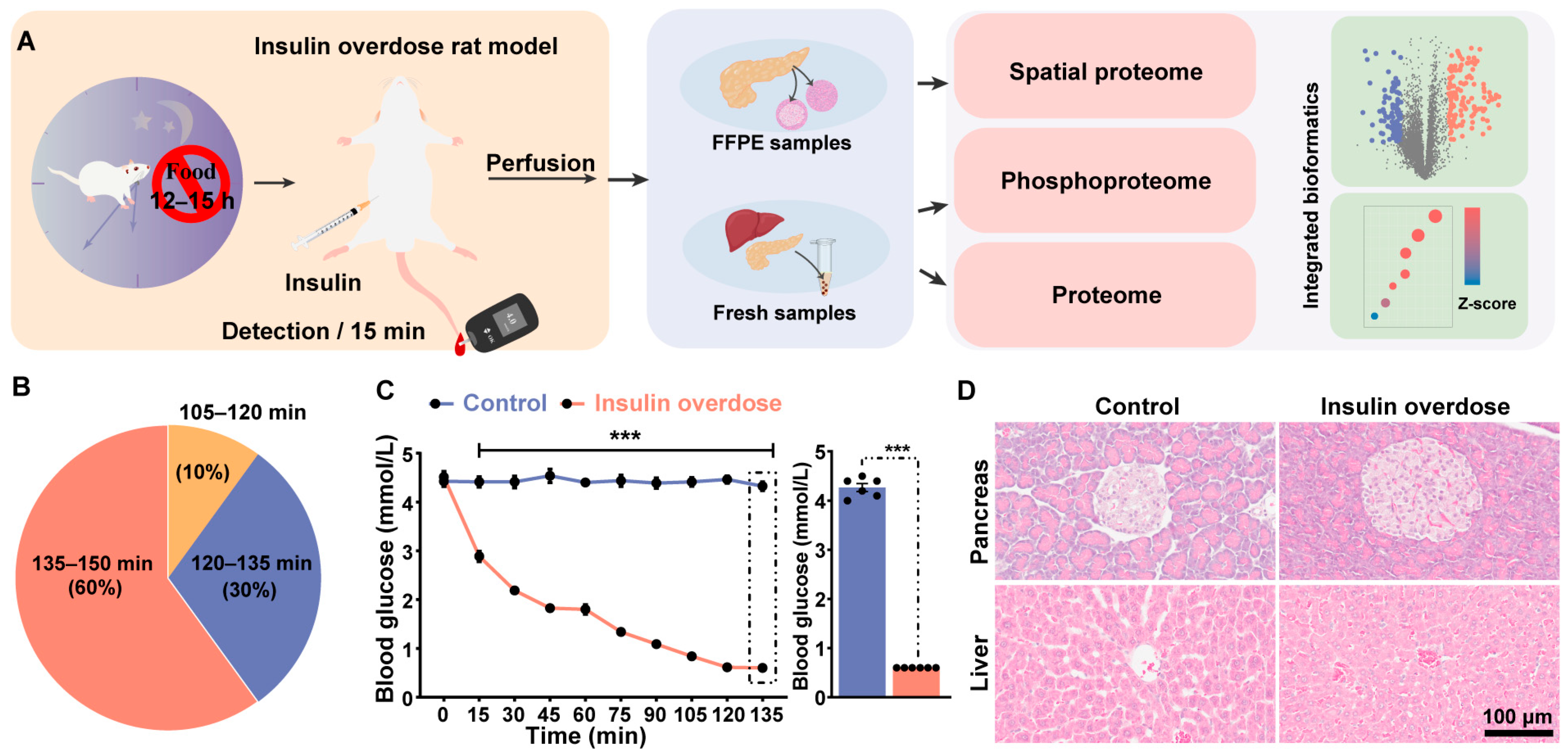
2.1. Overview of the Study and Biological Investigation
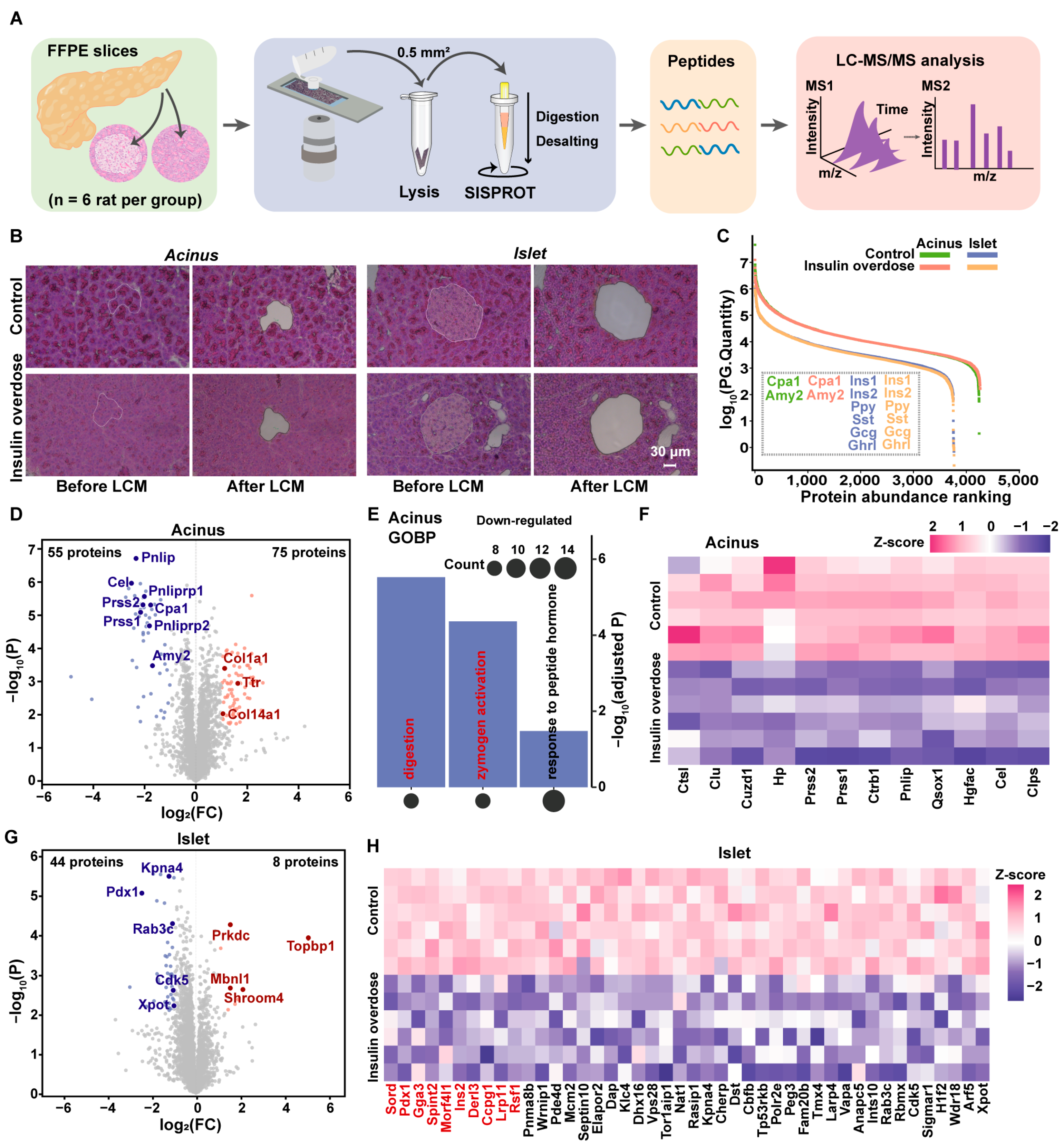
2.2. Spatial Proteome Profiling Reveals the Dysregulation of the Digestive Zymogens in Acinus and Disruption of Proteostasis in Islet
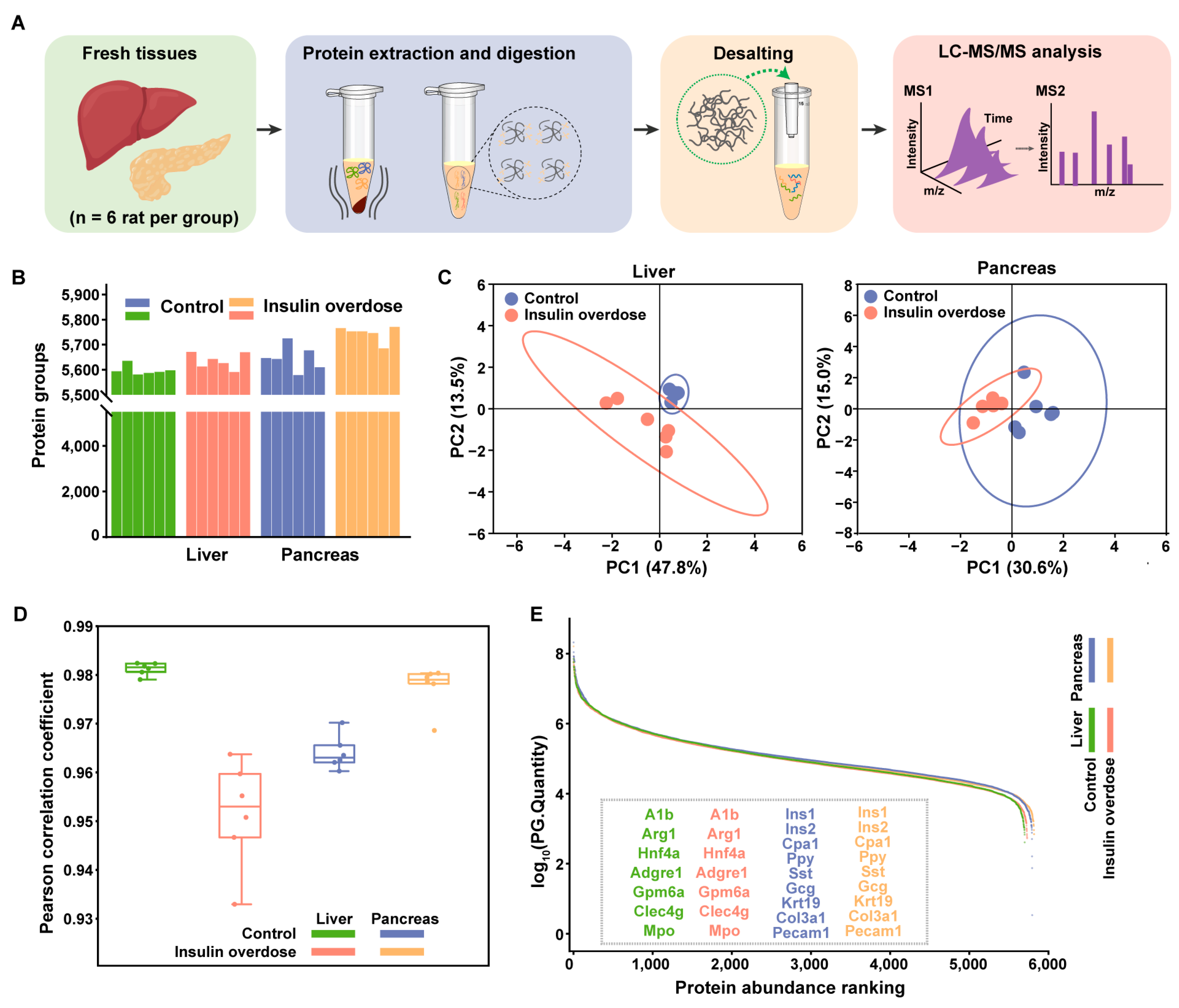
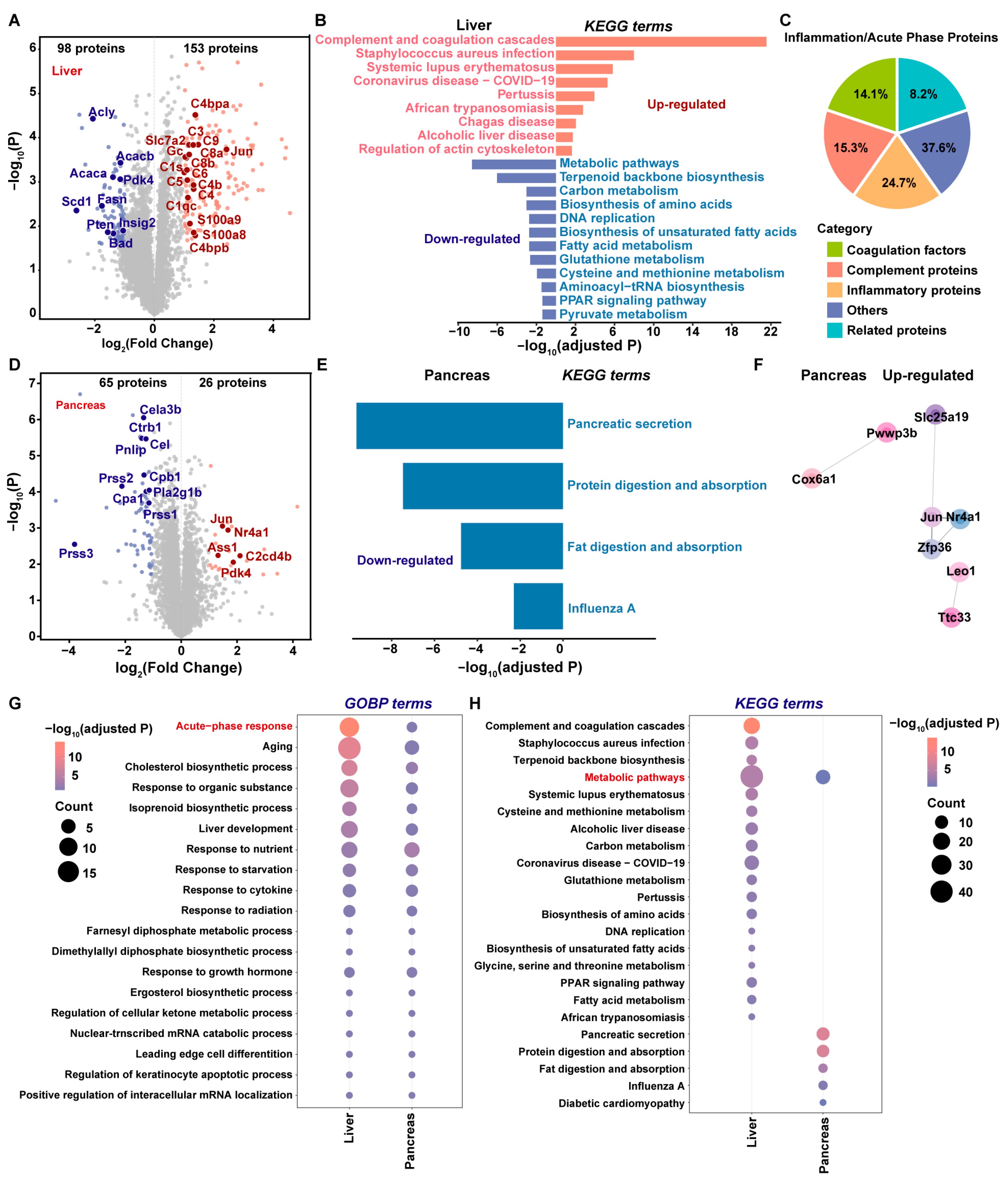
2.3. Global Proteome Profiling of Insulin-Overdose Rat Models Reveals Metabolic Disorder and the Acute Phase Response
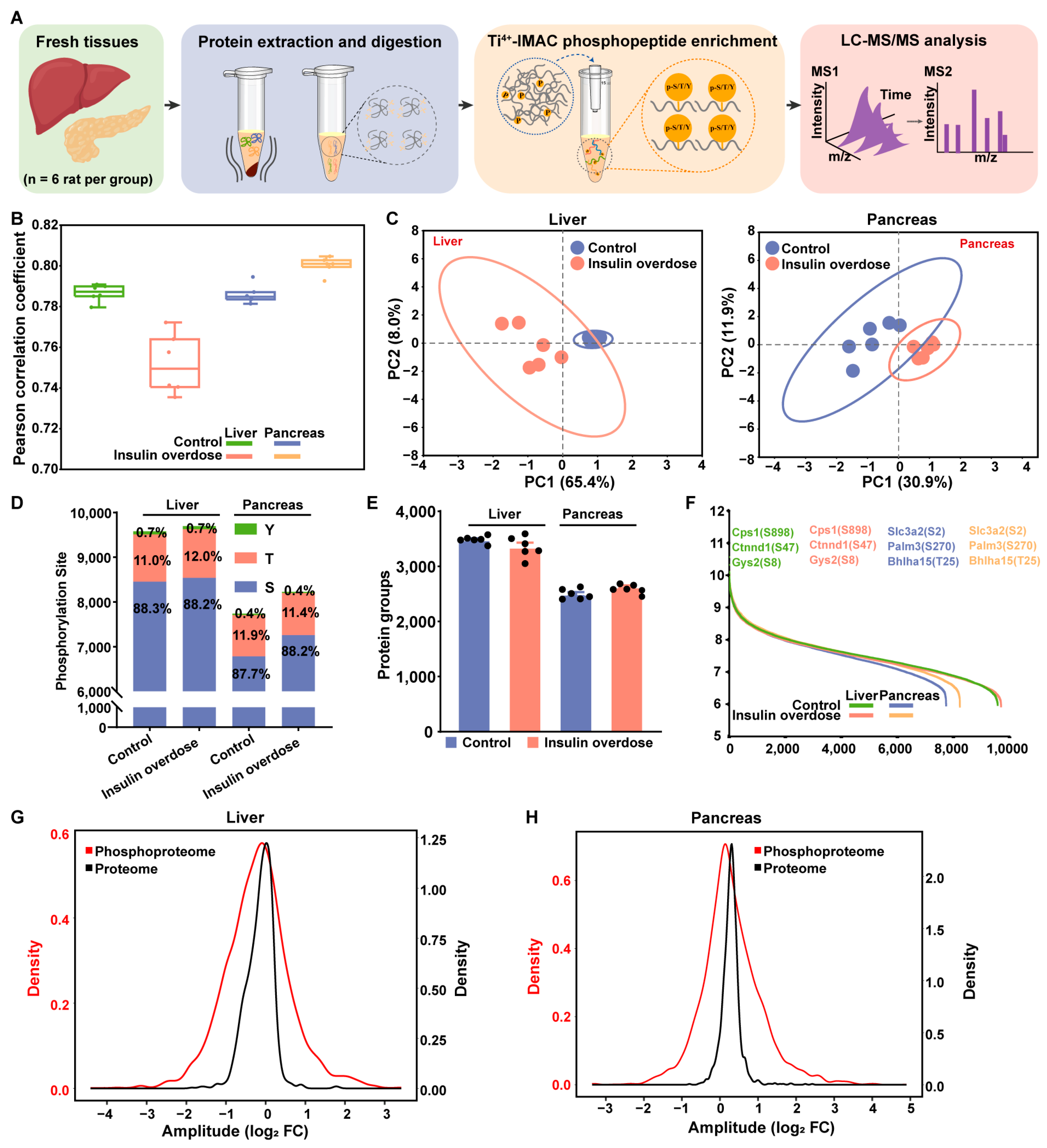
2.4. Phosphoproteome Profiling of Insulin-Overdose Rat Models Reveals Metabolic Pathway Dysregulation
3. Discussion
Limitations of the Study
4. Materials and Methods
4.1. Animals
4.2. Rat Model of Exogenous Insulin-Induced Overdose
4.3. HE Staining
4.4. Laser Microdissection
4.5. Slice Sample Processing for Protein Extraction and Digestion
4.6. Fresh Sample Processing for Protein Extraction and Digestion
4.7. Phosphopeptide Enrichment
4.8. LC-MS/MS Analysis
4.9. MS Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACN | Acetonitrile |
| AGC | Automatic gain control |
| Amy2 | Alpha-amylase |
| AP | Acute pancreatitis |
| BCA | Bicinchoninic acid |
| BP | Biological process |
| CaCl2 | Calcium chloride |
| CRP | C-reactive protein |
| DDA | Data-dependent acquisition |
| DIA | Data-independent acquisition |
| DTT | Dithiothreitol |
| FA | Formic acid |
| FDRs | False discovery rates |
| FFPE | Formalin-fixed paraffin-embedded |
| GO | Gene ontology |
| HCD | High-energy collisional dissociation |
| HE | Hematoxylin and Eosin |
| IAA | Iodoacetamide |
| IACUC | Institutional Animal Care and Use Committee |
| LCM | laser microdissection |
| LC-MS/MS | Liquid chromatography–tandem mass spectrometry |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| NaCl | Sodium chloride |
| NaF | Sodium fluoride |
| Na3VO4 | Sodium orthovanadate |
| NH4OH | Ammonium hydroxide |
| PBS | Phosphate-buffered saline |
| PCA | Principal component analysis |
| PCR | Polymerase chain reaction |
| PFA | Paraformaldehyde |
| PPI | Protein-protein interaction |
| Prss1 | Serine protease 1 |
| Prss2 | Anionic trypsin-2 |
| RT | Room temperature |
| SD | Sprague-Dawley |
| SISPROT | Simple and Integrated Spintip-based Proteomics Technology |
| T1DM | Type 1 diabetes mellitus |
| Ti4+-IMAC | Ti4+-immobilized metal affinity chromatography |
| TFA | Trifluoroacetic acid |
References
- Fralick, M.; Zinman, B. The discovery of insulin in Toronto: Beginning a 100 year journey of research and clinical achievement. Diabetologia 2021, 64, 947–953, Erratum in Diabetologia 2021, 64, 1454. [Google Scholar] [CrossRef]
- Sims, E.K.; Carr, A.L.J.; Oram, R.A.; DiMeglio, L.A.; Evans-Molina, C. 100 years of insulin: Celebrating the past, present and future of diabetes therapy. Nat. Med. 2021, 27, 1154–1164. [Google Scholar] [CrossRef]
- Birkinshaw, V.J.; Gurd, M.R.; Randall, S.S.; Curry, A.S.; Price, D.E.; Wright, P.H. Investigations in a case of murder by insulin poisoning. Br. Med. J. 1958, 2, 463–468. [Google Scholar] [CrossRef]
- Deng, J.; Zhao, F.; Yu, X.; Li, D.; Zhao, Y. Identification of the Protective Role of DJ-1 in Hypoglycemic Astrocyte Injury Using Proteomics. J. Proteome Res. 2015, 14, 2839–2848. [Google Scholar] [CrossRef]
- Labay, L.M.; Bitting, C.P.; Legg, K.M.; Logan, B.K. The Determination of Insulin Overdose in Postmortem Investigations. Acad. Forensic Pathol. 2016, 6, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Tong, F.; Wu, R.; Huang, W.; Yang, Y.; Zhang, L.; Zhang, B.; Chen, X.; Tang, X.; Zhou, Y. Forensic aspects of homicides by insulin overdose. Forensic Sci. Int. 2017, 278, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Bugelli, V.; Campobasso, C.P.; Angelino, A.; Focardi, M.; Pinchi, V. CLEIA of humor vitreous in a case of suicidal insulin overdose. Leg. Med. 2019, 40, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Tong, F.; Yang, Y.; Liang, Y.; Lopsong, T.Z.; Liu, Y.L.; Zhao, S.Q.; He, G.L.; Zhou, Y.W. Advances in Neuropathologic Research of Hypoglycemic Brain Damage Caused by Insulin Overdose. Fa Yi Xue Za Zhi 2020, 36, 99–103. [Google Scholar] [CrossRef]
- Tokarz, V.L.; MacDonald, P.E.; Klip, A. The cell biology of systemic insulin function. J. Cell Biol. 2018, 217, 2273–2289. [Google Scholar] [CrossRef]
- Zhou, Q.; Melton, D.A. Pancreas regeneration. Nature 2018, 557, 351–358, Erratum in Nature 2018, 560, E34. [Google Scholar] [CrossRef]
- Li, Y.; Tang, Y.; Shi, S.; Gao, S.; Wang, Y.; Xiao, D.; Chen, T.; He, Q.; Zhang, J.; Lin, Y. Tetrahedral Framework Nucleic Acids Ameliorate Insulin Resistance in Type 2 Diabetes Mellitus via the PI3K/Akt Pathway. ACS Appl. Mater. Interfaces 2021, 13, 40354–40364. [Google Scholar] [CrossRef]
- Arya, V.B.; Mohammed, Z.; Blankenstein, O.; De Lonlay, P.; Hussain, K. Hyperinsulinaemic hypoglycaemia. Horm. Metab. Res. 2014, 46, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Kreipe, L.; Vernay, M.C.; Oppliger, A.; Wellnitz, O.; Bruckmaier, R.M.; van Dorland, H.A. Induced hypoglycemia for 48 hours indicates differential glucose and insulin effects on liver metabolism in dairy cows. J. Dairy Sci. 2011, 94, 5435–5448. [Google Scholar] [CrossRef]
- Li, Z.; Tremmel, D.M.; Ma, F.; Yu, Q.; Ma, M.; Delafield, D.G.; Shi, Y.; Wang, B.; Mitchell, S.A.; Feeney, A.K.; et al. Proteome-wide and matrisome-specific alterations during human pancreas development and maturation. Nat. Commun. 2021, 12, 1020. [Google Scholar] [CrossRef]
- Cao, L.; Huang, C.; Cui Zhou, D.; Hu, Y.; Lih, T.M.; Savage, S.R.; Krug, K.; Clark, D.J.; Schnaubelt, M.; Chen, L.; et al. Proteogenomic characterization of pancreatic ductal adenocarcinoma. Cell 2021, 184, 5031–5052.e26. [Google Scholar] [CrossRef]
- Zhang, L.; Lanzoni, G.; Battarra, M.; Inverardi, L.; Zhang, Q. Proteomic profiling of human islets collected from frozen pancreata using laser capture microdissection. J. Proteom. 2017, 150, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Bo, T.; Gao, L.; Yao, Z.; Shao, S.; Wang, X.; Proud, C.G.; Zhao, J. Hepatic selective insulin resistance at the intersection of insulin signaling and metabolic dysfunction-associated steatotic liver disease. Cell Metab. 2024, 36, 947–968. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.N.; Chang, B.S.; Kim, J.H. MicroRNA dysregulation in liver and pancreas of CMP-Neu5Ac hydroxylase null mice disrupts insulin/PI3K-AKT signaling. BioMed Res. Int. 2014, 2014, 236385. [Google Scholar] [CrossRef]
- Warriner, D.; Debono, M.; Gandhi, R.A.; Chong, E.; Creagh, F. Acute hepatic injury following treatment of a long-acting insulin analogue overdose necessitating urgent insulin depot excision. Diabet. Med. 2012, 29, 232–235. [Google Scholar] [CrossRef]
- Bian, C.; He, X.; Wang, Q.; Zheng, Z.; Zhang, Y.; Xiong, H.; Li, Y.; Zhao, M.; Li, J. Biochemical Toxicological Study of Insulin Overdose in Rats: A Forensic Perspective. Toxics 2023, 12, 17. [Google Scholar] [CrossRef]
- Ilic, S.; Brcic, I.; Mester, M.; Filipovic, M.; Sever, M.; Klicek, R.; Barisic, I.; Radic, B.; Zoricic, Z.; Bilic, V.; et al. Over-dose insulin and stable gastric pentadecapeptide BPC 157. Attenuated gastric ulcers, seizures, brain lesions, hepatomegaly, fatty liver, breakdown of liver glycogen, profound hypoglycemia and calcification in rats. J. Physiol. Pharmacol. 2009, 60 (Suppl. S7), 107–114. [Google Scholar]
- Jensen, V.F.H.; Molck, A.M.; Nowak, J.; Fels, J.J.; Lykkesfeldt, J.; Bogh, I.B. Prolonged insulin-induced hypoglycaemia reduces ss-cell activity rather than number in pancreatic islets in non-diabetic rats. Sci. Rep. 2022, 12, 14113. [Google Scholar] [CrossRef]
- Mao, Y.; Chen, Y.; Li, Y.; Ma, L.; Wang, X.; Wang, Q.; He, A.; Liu, X.; Dong, T.; Gao, W.; et al. Deep spatial proteomics reveals region-specific features of severe COVID-19-related pulmonary injury. Cell Rep. 2024, 43, 113689. [Google Scholar] [CrossRef]
- Chen, W.; Wang, S.; Adhikari, S.; Deng, Z.; Wang, L.; Chen, L.; Ke, M.; Yang, P.; Tian, R. Simple and Integrated Spintip-Based Technology Applied for Deep Proteome Profiling. Anal. Chem. 2016, 88, 4864–4871. [Google Scholar] [CrossRef]
- Huang, P.; Kong, Q.; Gao, W.; Chu, B.; Li, H.; Mao, Y.; Cai, Z.; Xu, R.; Tian, R. Spatial proteome profiling by immunohistochemistry-based laser capture microdissection and data-independent acquisition proteomics. Anal. Chim. Acta 2020, 1127, 140–148. [Google Scholar] [CrossRef]
- Blevins, G.T., Jr.; Huang, H.S.; Tangoku, A.; McKay, D.W.; Rayford, P.L. Estrogens influence cholecystokinin stimulated pancreatic amylase release and acinar cell membrane cholecystokinin receptors in rat. Life Sci. 1991, 48, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, R.; Contreras, H.; Weidemann, S.; Gorbokon, N.; Menz, A.; Buscheck, F.; Luebke, A.M.; Kluth, M.; Hube-Magg, C.; Hinsch, A.; et al. Carboxypeptidase A1 (CPA1) Immunohistochemistry Is Highly Sensitive and Specific for Acinar Cell Carcinoma (ACC) of the Pancreas. Am. J. Surg. Pathol. 2022, 46, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Venis, S.M.; Moon, H.R.; Yang, Y.; Utturkar, S.M.; Konieczny, S.F.; Han, B. Engineering of a functional pancreatic acinus with reprogrammed cancer cells by induced PTF1a expression. Lab Chip 2021, 21, 3675–3685. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Imasaka, M.; Iwama, H.; Nishiura, H.; Ohmuraya, M. Double deficiency of cathepsin B and L in the mouse pancreas alters trypsin activity without affecting acute pancreatitis severity. Pancreatology 2022, 22, 880–886. [Google Scholar] [CrossRef]
- Modenbach, J.M.; Moller, C.; Asgarbeik, S.; Geist, N.; Rimkus, N.; Dorr, M.; Wolfgramm, H.; Steil, L.; Susemihl, A.; Graf, L.; et al. Biochemical analyses of cystatin-C dimers and cathepsin-B reveals a trypsin-driven feedback mechanism in acute pancreatitis. Nat. Commun. 2025, 16, 1702. [Google Scholar] [CrossRef]
- Wartmann, T.; Mayerle, J.; Kahne, T.; Sahin-Toth, M.; Ruthenburger, M.; Matthias, R.; Kruse, A.; Reinheckel, T.; Peters, C.; Weiss, F.U.; et al. Cathepsin L inactivates human trypsinogen, whereas cathepsin L-deletion reduces the severity of pancreatitis in mice. Gastroenterology 2010, 138, 726–737. [Google Scholar] [CrossRef] [PubMed]
- Matamala, N.; Martinez, M.T.; Lara, B.; Perez, L.; Vazquez, I.; Jimenez, A.; Barquin, M.; Ferrarotti, I.; Blanco, I.; Janciauskiene, S.; et al. Alternative transcripts of the SERPINA1 gene in alpha-1 antitrypsin deficiency. J. Transl. Med. 2015, 13, 211. [Google Scholar] [CrossRef] [PubMed]
- Szatmary, P.; Grammatikopoulos, T.; Cai, W.; Huang, W.; Mukherjee, R.; Halloran, C.; Beyer, G.; Sutton, R. Acute Pancreatitis: Diagnosis and Treatment. Drugs 2022, 82, 1251–1276. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, K.; Polonsky, K.S. Pdx1 and other factors that regulate pancreatic beta-cell survival. Diabetes Obes. Metab. 2009, 11 (Suppl. S4), 30–37. [Google Scholar] [CrossRef]
- Jayaram, B.; Syed, I.; Kyathanahalli, C.N.; Rhodes, C.J.; Kowluru, A. Arf nucleotide binding site opener [ARNO] promotes sequential activation of Arf6, Cdc42 and Rac1 and insulin secretion in INS 832/13 beta-cells and rat islets. Biochem. Pharmacol. 2011, 81, 1016–1027. [Google Scholar] [CrossRef]
- Roy, G.; Ordonez, A.; Binns, D.D.; Rodrigues-Dos-Santos, K.; Kwakye, M.B.; King, G.C.; Kuntz, R.L.; Mukherjee, N.; Templin, A.T.; Tan, Z.; et al. VDAC1 is a target for pharmacologically induced insulin hypersecretion in beta cells. Cell Rep. 2025, 44, 115834. [Google Scholar] [CrossRef]
- Liang, Y.; Kaneko, K.; Xin, B.; Lee, J.; Sun, X.; Zhang, K.; Feng, G.S. Temporal analyses of postnatal liver development and maturation by single-cell transcriptomics. Dev. Cell 2022, 57, 398–414.e5. [Google Scholar] [CrossRef]
- Jani, P.K.; Petkau, G.; Kawano, Y.; Klemm, U.; Guerra, G.M.; Heinz, G.A.; Heinrich, F.; Durek, P.; Mashreghi, M.F.; Melchers, F. The miR-221/222 cluster regulates hematopoietic stem cell quiescence and multipotency by suppressing both Fos/AP-1/IEG pathway activation and stress-like differentiation to granulocytes. PLoS Biol. 2023, 21, e3002015. [Google Scholar] [CrossRef]
- Canzoneri, R.; Naipauer, J.; Stedile, M.; Rodriguez Pena, A.; Lacunza, E.; Gandini, N.A.; Curino, A.C.; Facchinetti, M.M.; Coso, O.A.; Kordon, E.; et al. Identification of an AP1-ZFP36 Regulatory Network Associated with Breast Cancer Prognosis. J. Mammary Gland Biol. Neoplasia 2020, 25, 163–172. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Q.; Lin, Y.; Lin, Z.; Zhong, G.; Chen, L. Transcriptome profiling and weighted gene co-expression network analysis reveal changes of hub genes and molecular pathways in rat lungs following deep hypothermic circulatory arrest. PLoS ONE 2025, 20, e0328887. [Google Scholar] [CrossRef]
- Herring, J.A.; Crabtree, J.E.; Hill, J.T.; Tessem, J.S. Loss of glucose-stimulated beta-cell Nr4a1 expression impairs insulin secretion and glucose homeostasis. Am. J. Physiol. Cell Physiol. 2024, 327, C1111–C1124. [Google Scholar] [CrossRef]
- Alf, M.F.; Duarte, J.M.; Schibli, R.; Gruetter, R.; Kramer, S.D. Brain glucose transport and phosphorylation under acute insulin-induced hypoglycemia in mice: An 18F-FDG PET study. J. Nucl. Med. 2013, 54, 2153–2160. [Google Scholar] [CrossRef]
- Tape, C.J.; Worboys, J.D.; Sinclair, J.; Gourlay, R.; Vogt, J.; McMahon, K.M.; Trost, M.; Lauffenburger, D.A.; Lamont, D.J.; Jorgensen, C. Reproducible automated phosphopeptide enrichment using magnetic TiO2 and Ti-IMAC. Anal. Chem. 2014, 86, 10296–10302. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.R.; Bae, S.H.; Wales, T.E.; Engen, J.R.; Lee, J.; Jang, H.; Park, S. The serine phosphorylations in the IRS-1 PIR domain abrogate IRS-1 and IR interaction. Proc. Natl. Acad. Sci. USA 2024, 121, e2401716121. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Deng, J.; Zhu, C.; Ma, X.; Jiang, M.; Fan, D. Ginsenoside F4 Alleviates Skeletal Muscle Insulin Resistance by Regulating PTP1B in Type II Diabetes Mellitus. J. Agric. Food Chem. 2023, 71, 14263–14275. [Google Scholar] [CrossRef]
- Martinez Baez, A.; Ayala, G.; Pedroza-Saavedra, A.; Gonzalez-Sanchez, H.M.; Chihu Amparan, L. Phosphorylation Codes in IRS-1 and IRS-2 Are Associated with the Activation/Inhibition of Insulin Canonical Signaling Pathways. Curr. Issues Mol. Biol. 2024, 46, 634–649. [Google Scholar] [CrossRef]
- Burchfield, J.G.; Diaz-Vegas, A.; James, D.E. The insulin signalling network. Nat. Metab. 2025, 7, 1745–1764. [Google Scholar] [CrossRef] [PubMed]
- Neukamm, S.S.; Toth, R.; Morrice, N.; Campbell, D.G.; Mackintosh, C.; Lehmann, R.; Haering, H.U.; Schleicher, E.D.; Weigert, C. Identification of the amino acids 300–600 of IRS-2 as 14-3-3 binding region with the importance of IGF-1/insulin-regulated phosphorylation of Ser-573. PLoS ONE 2012, 7, e43296. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, X.; Li, Y.; Mao, Y.; Su, Y.; Mao, Y.; Yang, Y.; Gao, W.; Fu, C.; Chen, W.; et al. Multimodal single cell-resolved spatial proteomics reveal pancreatic tumor heterogeneity. Nat. Commun. 2024, 15, 10100. [Google Scholar] [CrossRef]
- Kong, Q.; Ke, M.; Weng, Y.; Qin, Y.; He, A.; Li, P.; Cai, Z.; Tian, R. Dynamic Phosphotyrosine-Dependent Signaling Profiling in Living Cells by Two-Dimensional Proximity Proteomics. J. Proteome Res. 2022, 21, 2727–2735. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chen, T.; Wu, S.; Yang, C.; Bai, M.; Shu, K.; Li, K.; Zhang, G.; Jin, Z.; He, F.; et al. iProX: An integrated proteome resource. Nucleic Acids Res. 2019, 47, D1211–D1217. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Ma, J.; Liu, Y.; Chen, Z.; Xiao, N.; Lu, Y.; Fu, Y.; Yang, C.; Li, M.; Wu, S.; et al. iProX in 2021: Connecting proteomics data sharing with big data. Nucleic Acids Res. 2022, 50, D1522–D1527. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Li, S.; Kong, Q.; He, A.; Ke, M.; Yu, Z.; Wang, Y.; Long, X.; Yuan, Y.; Tian, R.; et al. Spatial- and Phospho-Proteomic Profiling Reveals Pancreatic and Hepatic Dysfunction in a Rat Model of Lethal Insulin Overdose. Int. J. Mol. Sci. 2025, 26, 11018. https://doi.org/10.3390/ijms262211018
Zhang J, Li S, Kong Q, He A, Ke M, Yu Z, Wang Y, Long X, Yuan Y, Tian R, et al. Spatial- and Phospho-Proteomic Profiling Reveals Pancreatic and Hepatic Dysfunction in a Rat Model of Lethal Insulin Overdose. International Journal of Molecular Sciences. 2025; 26(22):11018. https://doi.org/10.3390/ijms262211018
Chicago/Turabian StyleZhang, Jiaxin, Shiyi Li, Qian Kong, An He, Mi Ke, Zhonghao Yu, Yuxuan Wang, Xiao Long, Yuhao Yuan, Ruijun Tian, and et al. 2025. "Spatial- and Phospho-Proteomic Profiling Reveals Pancreatic and Hepatic Dysfunction in a Rat Model of Lethal Insulin Overdose" International Journal of Molecular Sciences 26, no. 22: 11018. https://doi.org/10.3390/ijms262211018
APA StyleZhang, J., Li, S., Kong, Q., He, A., Ke, M., Yu, Z., Wang, Y., Long, X., Yuan, Y., Tian, R., & Zhou, Y. (2025). Spatial- and Phospho-Proteomic Profiling Reveals Pancreatic and Hepatic Dysfunction in a Rat Model of Lethal Insulin Overdose. International Journal of Molecular Sciences, 26(22), 11018. https://doi.org/10.3390/ijms262211018



