Temporal Progression of Recognition Memory Impairment, Astrogliosis, and Cholinergic Dysfunction in the Streptozotocin Rat Model of Sporadic Alzheimer’s Disease
Abstract
1. Introduction
2. Results
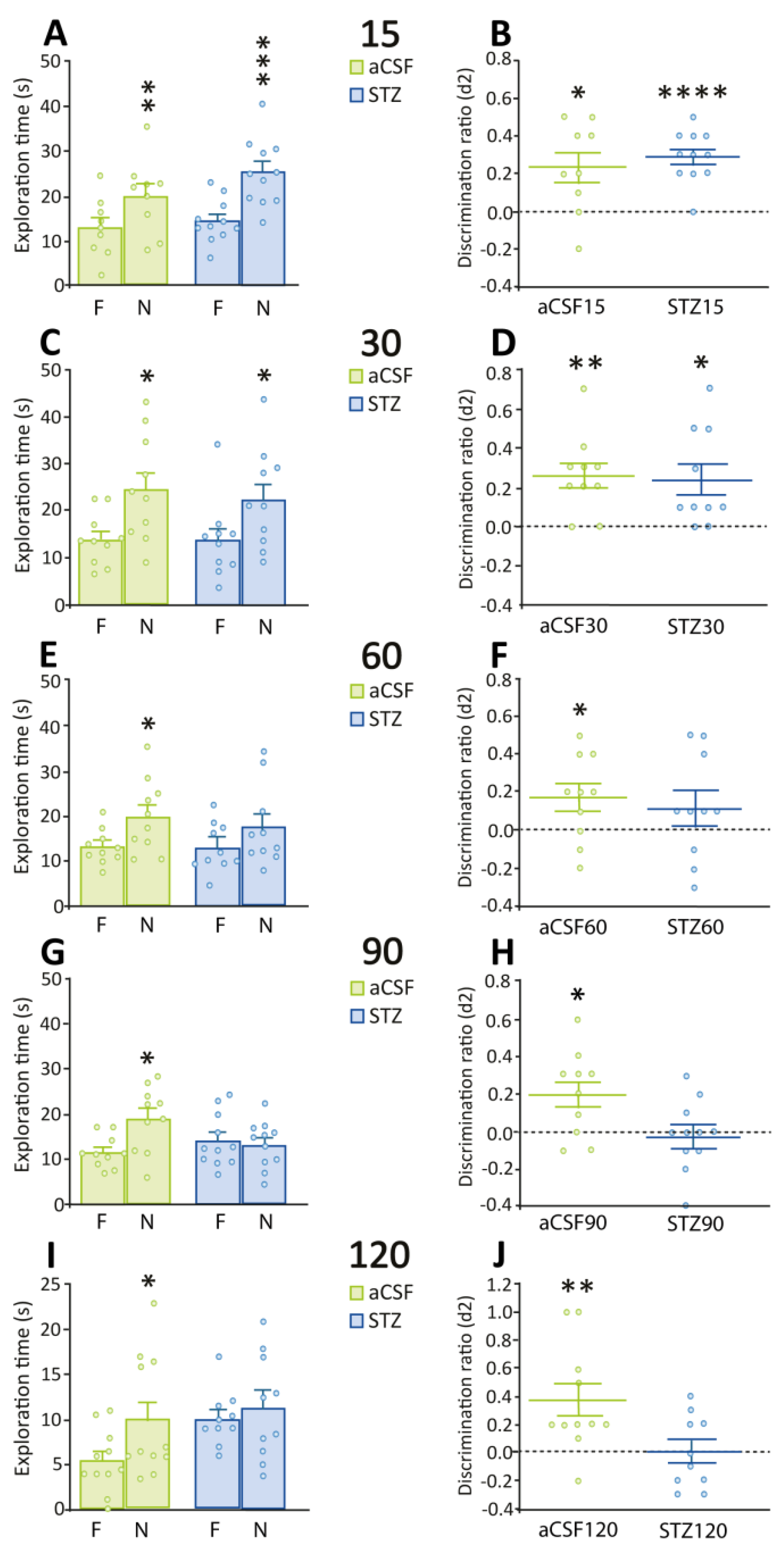
2.1. Deficits in Recognition Memory Are Seen After 60 Days of STZ Administration
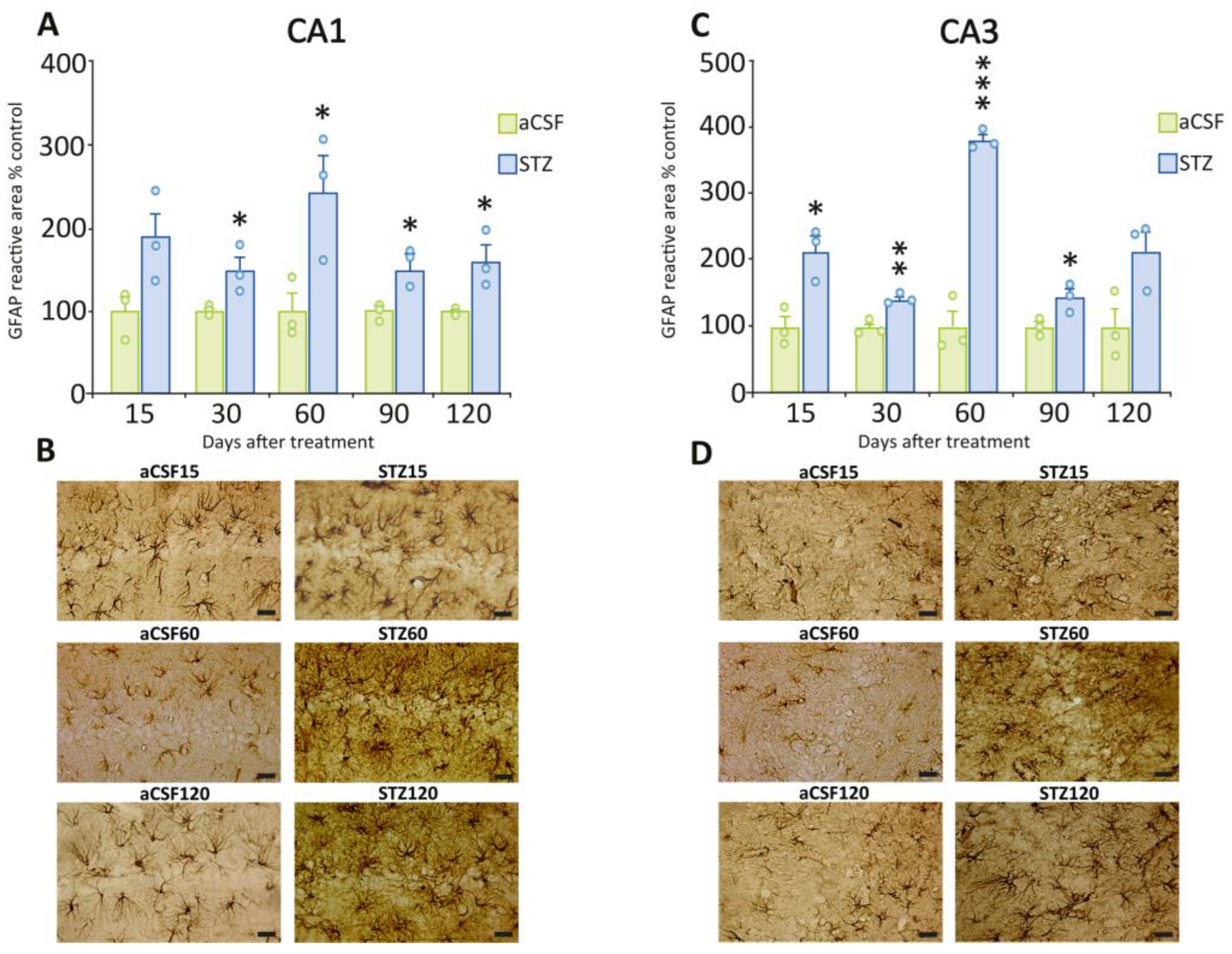
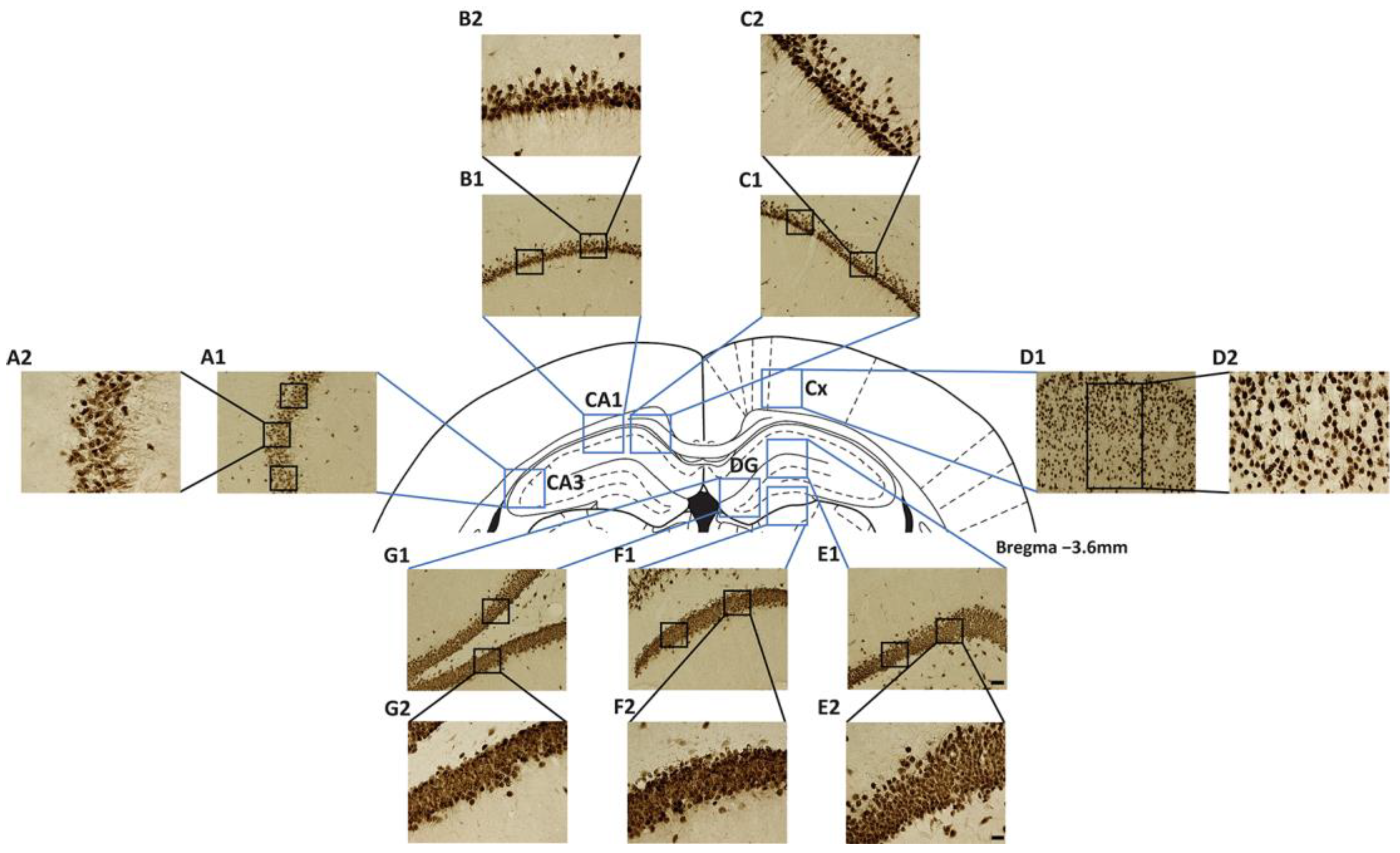
2.2. Early Onset of Astrogliosis in Hippocampal Subfields CA1 and CA3
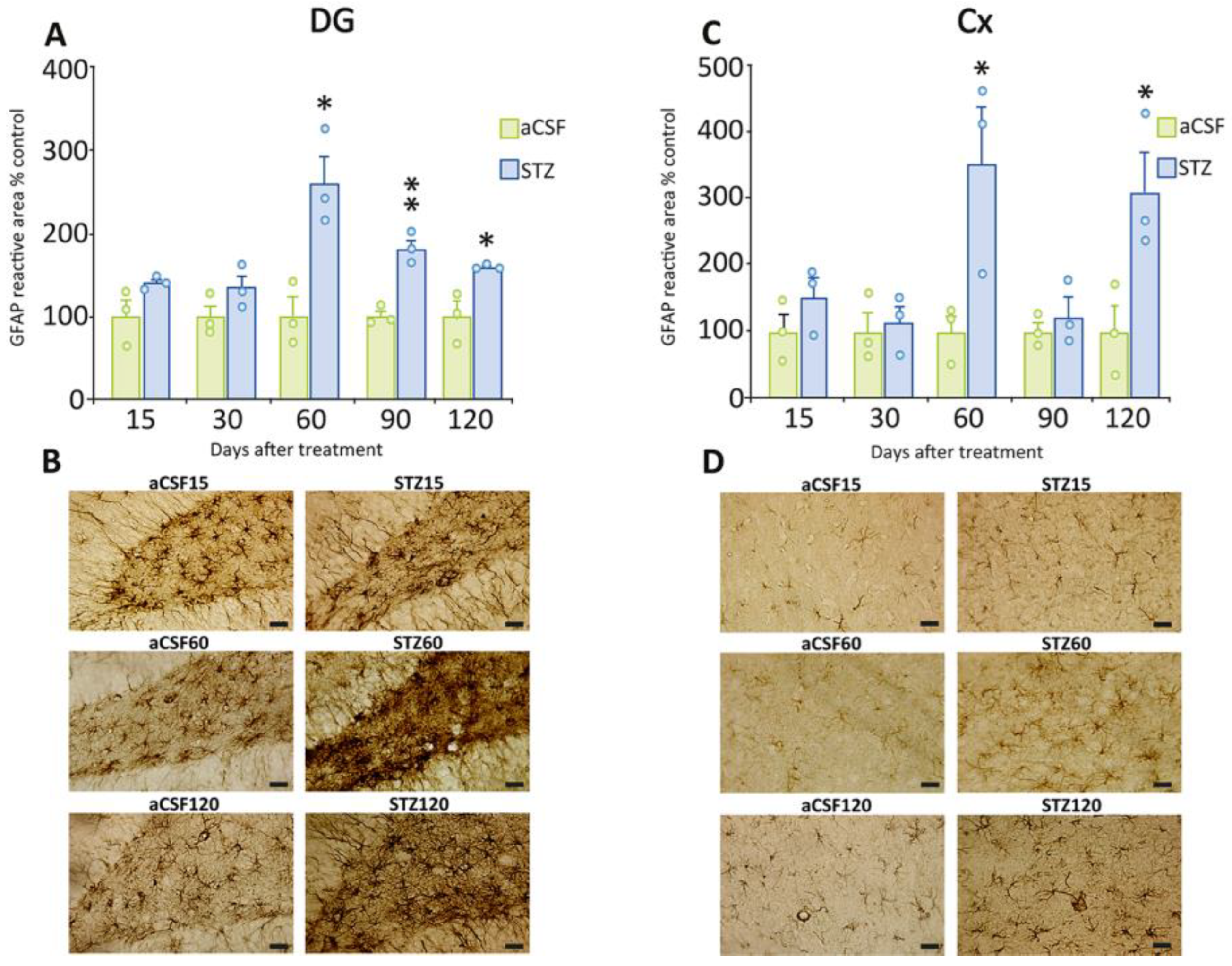
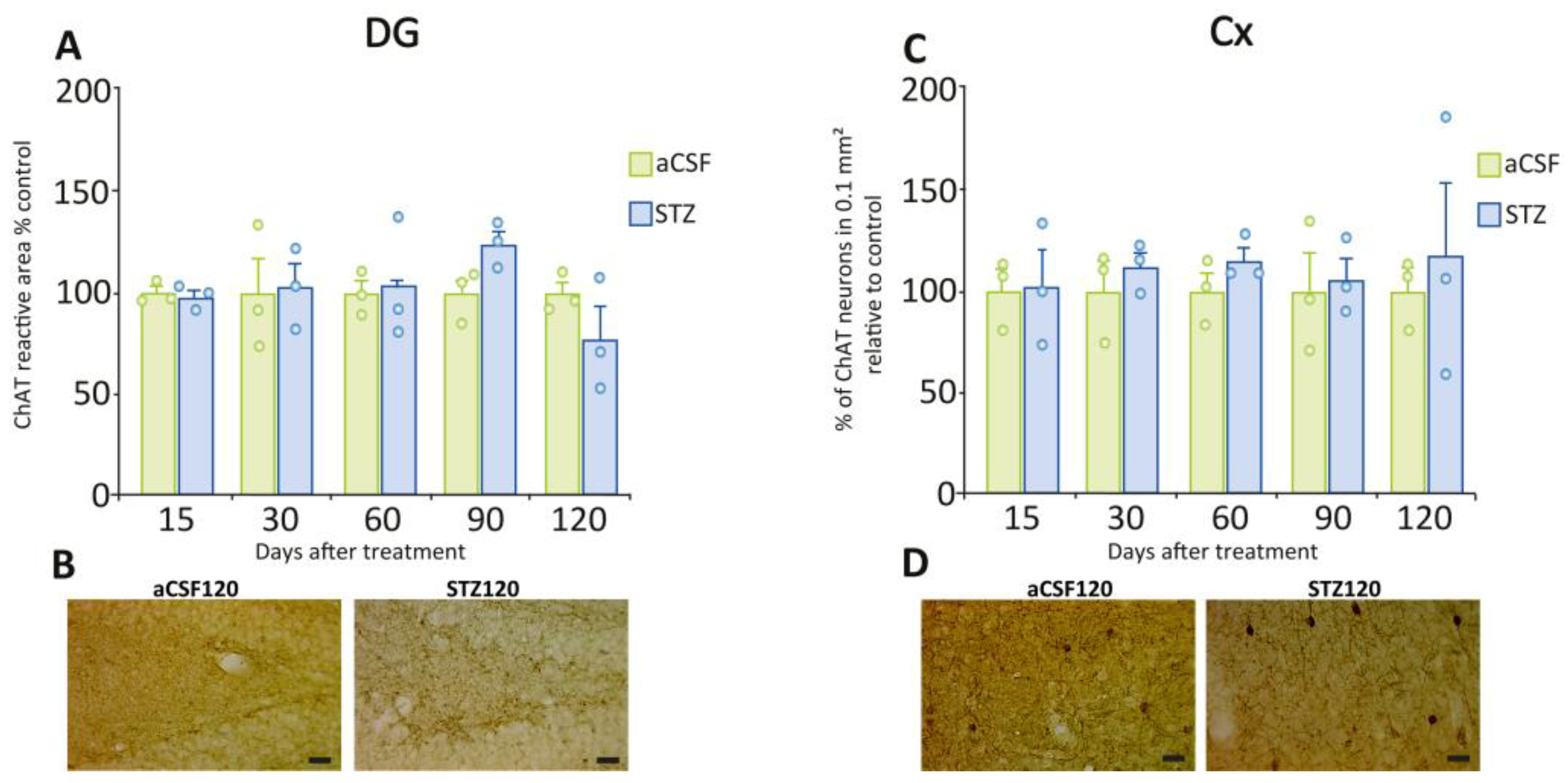
2.3. Delayed Astrogliosis in the Dentate Gyrus and Cerebral Cortex
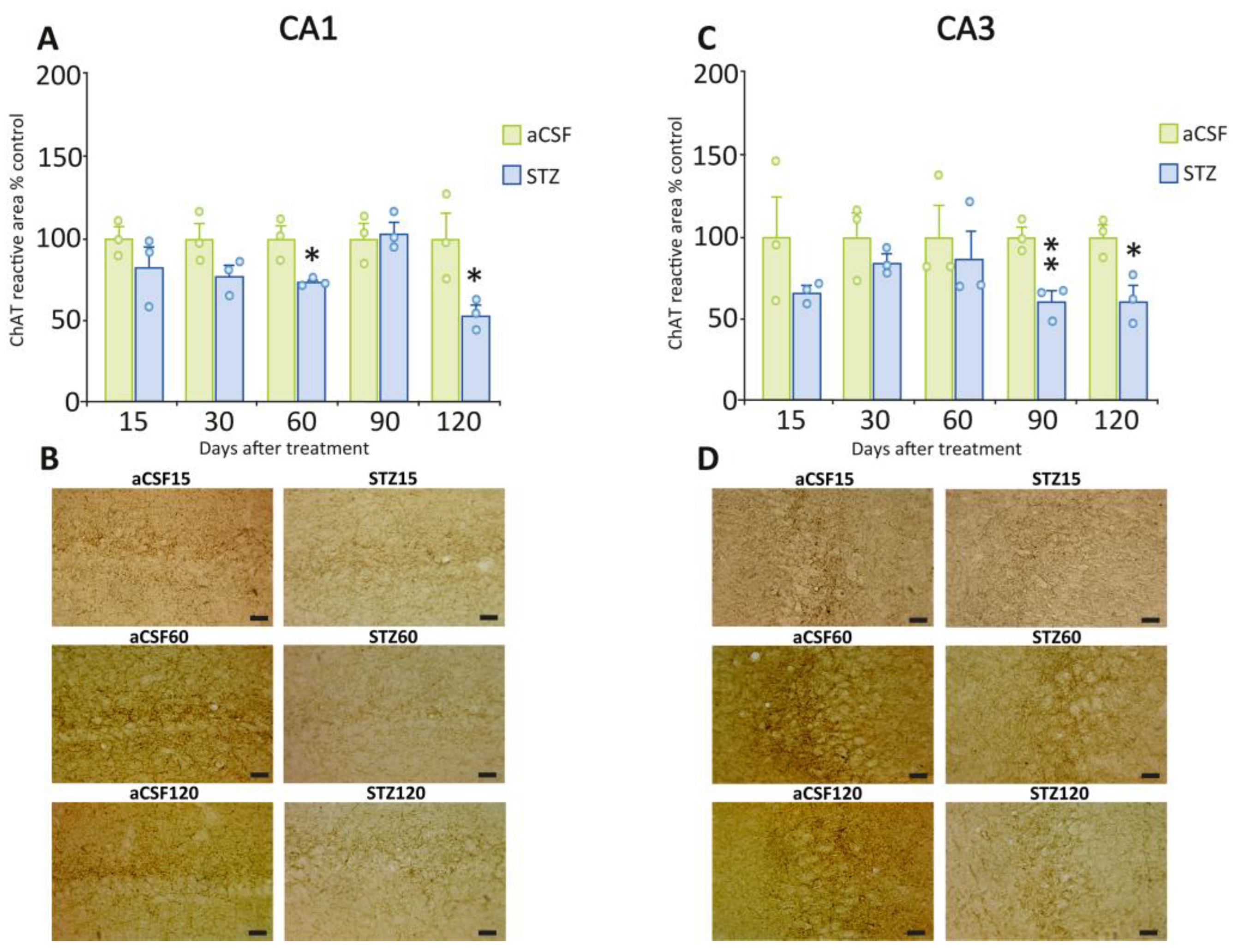
2.4. The Cholinergic System in the Hippocampus Was Significantly Affected by STZ
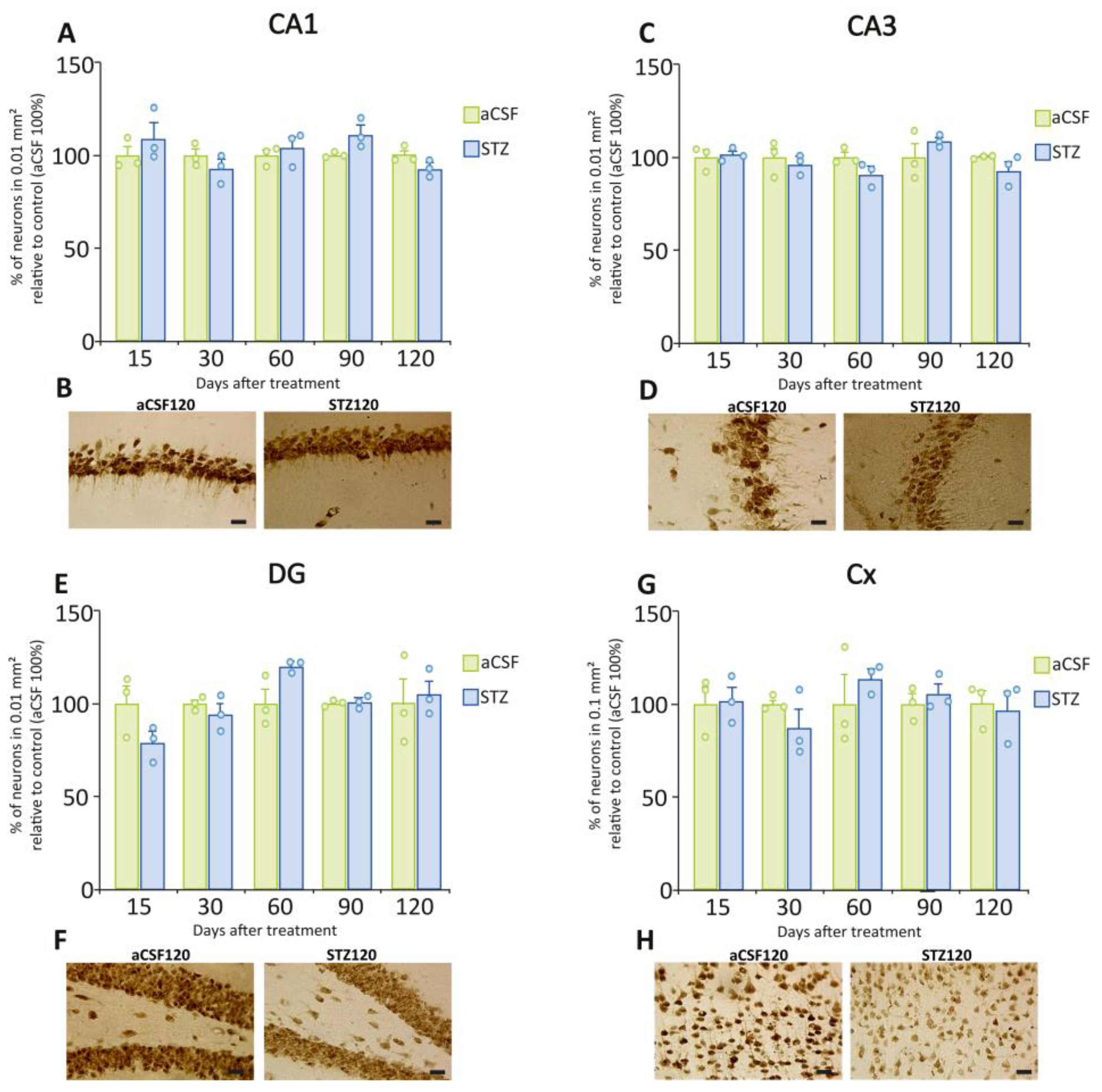
2.5. STZ Administration Did Not Induce Neuronal Loss
3. Discussion
3.1. Behavioral Studies
3.2. Immunohistochemical Studies
3.3. Limitations of the Study
4. Materials and Methods
4.1. Subjects
4.2. Drugs
4.3. Surgical Procedures
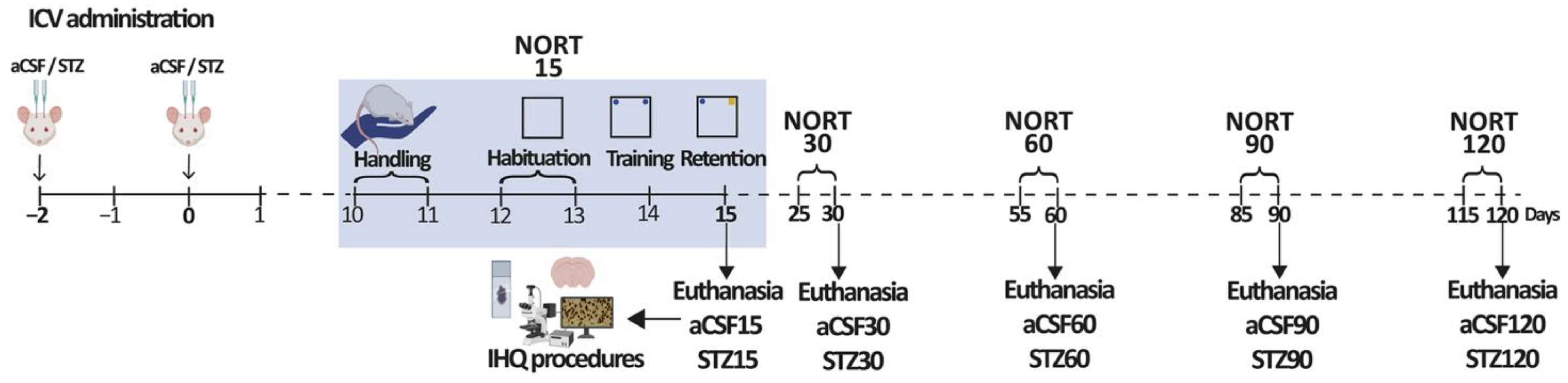
4.4. Experimental Design
4.5. Novel Object Recognition Test
4.6. Procedures
4.7. Brain Fixation and Histological Procedures
4.8. Immunohistochemical Procedures
4.9. Quantitative Analyses of IHC Assays
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s Disease |
| SAD | Sporadic Alzheimer’s Disease |
| FAD | Familial Alzheimer’s Disease |
| EOAD | Early-Onset Alzheimer’s Disease |
| LOAD | Late-Onset Alzheimer’s Disease |
| Aβ | Amyloid-Beta |
| APP | Amyloid Precursor Protein |
| PSEN1/PSEN2 | Presenilin 1/Presenilin 2 |
| APOE | Apolipoprotein E |
| STZ | Streptozotocin |
| ICV | Intracerebroventricular |
| aCSF | Artificial Cerebrospinal Fluid |
| CA1/CA3 | Cornu Ammonis 1/3 |
| DG | Dentate Gyrus |
| Cx | Cortex |
| GFAP | Glial Fibrillary Acidic Protein |
| ChAT | Choline Acetyltransferase |
| NeuN | Hexaribonucleotide Binding Protein-3 |
| NOR/NORT | Novel Object Recognition Test |
| PBS | Phosphate Buffered Saline |
| PFA | Paraformaldehyde |
| ABC | Avidin-Biotin Complex |
| DAB | Diaminobenzidine |
| ROI | Region of Interest |
| RT | Room temperature |
| SEM | Standard Error of the Mean |
| i.p. | Intraperitoneal |
| ON | Overnight |
References
- World Health Organization. Dementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 12 September 2025).
- Wimo, A.; Seeher, K.; Cataldi, R.; Cyhlarova, E.; Dielemann, J.L.; Frisell, O.; Guerchet, M.; Jönsson, L.; Malaha, A.K.; Nichols, E.; et al. The worldwide costs of dementia in 2019. Alzheimers Dement. 2023, 19, 2865–2873. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Vilmenay, K.; Poon, A.N.; Ayanian, S.; Aitken, C.F.; Chan, K.Y. Systematic Review Estimating the Burden of Dementia in the Latin America and Caribbean Region: A Bayesian Approach. Front. Neurol. 2021, 12, 628520. [Google Scholar] [CrossRef] [PubMed]
- Dorszewska, J.; Prendecki, M.; Oczkowska, A.; Dezor, M.; Kozubski, W. Molecular Basis of Familial and Sporadic Alzheimer’s Disease. Curr. Alzheimer Res. 2016, 13, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Giri, M.; Zhang, M.; Lü, Y. Genes associated with Alzheimer’s disease: An overview and current status. Clin. Interv. Aging 2016, 11, 665–681. [Google Scholar] [CrossRef]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Rossor, M.N.; Fox, N.C.; Freeborough, P.A.; Harvey, R.J. Clinical features of sporadic and familial Alzheimer’s disease. Neurodegeneration 1996, 5, 393–397. [Google Scholar] [CrossRef]
- Andrews, S.J.; Renton, A.E.; Fulton-Howard, B.; Podlesny-Drabiniok, A.; Marcora, E.; Goate, A.M. The complex genetic architecture of Alzheimer’s disease: Novel insights and future directions. EBioMedicine 2023, 90, 104511. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446, Correction in Lancet 2023, 402, 1132. [Google Scholar] [CrossRef]
- Lozupone, M.; Dibello, V.; Sardone, R.; Castellana, F.; Zupo, R.; Lampignano, L.; Bortone, I.; Daniele, A.; Bellomo, A.; Solfrizzi, V.; et al. The Impact of Apolipoprotein E (APOE) Epigenetics on Aging and Sporadic Alzheimer’s Disease. Biology 2023, 12, 1529. [Google Scholar] [CrossRef]
- Carter, S.F.; Herholz, K.; Rosa-Neto, P.; Pellerin, L.; Nordberg, A.; Zimmer, E.R. Astrocyte Biomarkers in Alzheimer’s Disease. Trends Mol. Med. 2019, 25, 77–95. [Google Scholar] [CrossRef]
- Eng, L.F. Glial fibrillary acidic protein (GFAP): The major protein of glial intermediate filaments in differentiated astrocytes. J. Neuroimmunol. 1985, 8, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Stogsdill, J.A.; Harwell, C.C.; Goldman, S.A. Astrocytes as master modulators of neural networks: Synaptic functions and disease-associated dysfunction of astrocytes. Ann. N. Y. Acad. Sci. 2023, 1525, 41–60. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Barres, B.A. Reactive astrocytes: Production, function, and therapeutic potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Rodríguez, J.J.; Verkhratsky, A. Neuroglia in neurodegeneration. Brain Res. Rev. 2010, 63, 189–211. [Google Scholar] [CrossRef]
- Lecanu, L.; Papadopoulos, V. Modeling Alzheimer’s disease with non-transgenic rat models. Alzheimers Res. Ther. 2013, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Woodruff-Pak, D.S. Animal models of Alzheimer’s disease: Therapeutic implications. J. Alzheimers Dis. 2008, 15, 507–521. [Google Scholar] [CrossRef]
- Lannert, H.; Hoyer, S. Intracerebroventricular administration of streptozotocin causes long-term diminutions in learning and memory abilities and in cerebral energy metabolism in adult rats. Behav. Neurosci. 1998, 112, 1199–1208. [Google Scholar] [CrossRef]
- Knezovic, A.; Salkovic-Petrisic, M. Cholinergic neurotransmission in the brain of streptozotocin-induced rat model of sporadic Alzheimer’s disease: Long-term follow-up. J. Neural Transm. 2025, 132, 1461–1477. [Google Scholar] [CrossRef]
- Knezovic, A.; Osmanovic-Barilar, J.; Curlin, M.; Hof, P.R.; Simic, G.; Riederer, P.; Salkovic-Petrisic, M. Staging of cognitive deficits and neuropathological and ultrastructural changes in streptozotocin-induced rat model of Alzheimer’s disease. J. Neural Transm. 2015, 122, 577–592. [Google Scholar] [CrossRef]
- Kamat, P.K. Streptozotocin induced Alzheimer’s disease like changes and the underlying neural degeneration and regeneration mechanism. Neural Regen. Res. 2015, 10, 1050–1052. [Google Scholar] [CrossRef]
- Salkovic-Petrisic, M.; Hoyer, S. Central insulin resistance as a trigger for sporadic Alzheimer-like pathology: An experimental approach. J. Neural Transm. 2007, 72, 217–233. [Google Scholar] [CrossRef]
- Salkovic-Petrisic, M.; Knezovic, A.; Hoyer, S.; Riederer, P. What have we learned from the streptozotocin-induced animal model of sporadic Alzheimer’s disease, about the therapeutic strategies in Alzheimer’s research. J. Neural Transm. 2013, 120, 233–252. [Google Scholar] [CrossRef]
- Ghafarimoghadam, M.; Mashayekh, R.; Gholami, M.; Fereydani, P.; Shelley-Tremblay, J.; Kandezi, N.; Sabouri, E.; Motaghinejad, M. A review of behavioral methods for the evaluation of cognitive performance in animal models: Current techniques and links to human cognition. Physiol. Behav. 2022, 244, 113652. [Google Scholar] [CrossRef]
- Ren, H.; Tang, L.; Yuan, Z.; Liu, Y.; Zhou, X.; Xiao, X.; Wu, X.; Chen, W.; Chen, Y.; Wang, H.; et al. Combined administration of catalpol, puerarin, gastrodin, and borneol modulates the Tlr4/Myd88/NF-κB signaling pathway and alleviates microglia inflammation in Alzheimer’s disease. Front. Pharmacol. 2024, 15, 1492237. [Google Scholar] [CrossRef] [PubMed]
- Biasibetti, R.; Almeida Dos Santos, J.P.; Rodrigues, L.; Wartchow, K.M.; Suardi, L.Z.; Nardin, P.; Selistre, N.G.; Vázquez, D.; Gonçalves, C.A. Hippocampal changes in STZ-model of Alzheimer’s disease are dependent on sex. Behav. Brain Res. 2017, 316, 205–214. [Google Scholar] [CrossRef]
- Rostami, F.; Javan, M.; Moghimi, A.; Haddad-Mashadrizeh, A.; Fereidoni, M. Streptozotocin-induced hippocampal astrogliosis and insulin signaling malfunction as experimental scales for subclinical sporadic Alzheimer model. Life Sci. 2017, 188, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Knezovic, A.; Loncar, A.; Homolak, J.; Smailovic, U.; Osmanovic Barilar, J.; Ganoci, L.; Bozina, N.; Riederer, P.; Salkovic-Petrisic, M. Rat brain glucose transporter-2, insulin receptor and glial expression are acute targets of intracerebroventricular streptozotocin: Risk factors for sporadic Alzheimer’s disease? J. Neural Transm. 2017, 124, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Liu, S.; Sun, H.M.; Ma, M.D.; Gao, Y.J.; Qi, C.C.; Xia, Q.R.; Ge, J.F. Bilateral intracerebroventricular injection of streptozotocin induces AD-like behavioral impairments and neuropathological features in mice: Involved with the fundamental role of neuroinflammation. Biomed. Pharmacother. 2022, 153, 113375. [Google Scholar] [CrossRef]
- Luo, H.; Xiang, Y.; Qu, X.; Liu, H.; Liu, C.; Li, G.; Han, L.; Qin, X. Apelin-13 Suppresses Neuroinflammation Against Cognitive Deficit in a Streptozotocin-Induced Rat Model of Alzheimer’s Disease Through Activation of BDNF-TrkB Signaling Pathway. Front. Pharmacol. 2019, 10, 395. [Google Scholar] [CrossRef]
- Prickaerts, J.; Fahrig, T.; Blokland, A. Cognitive performance and biochemical markers in septum, hippocampus and striatum of rats after an i.c.v. injection of streptozotocin: A correlation analysis. Behav. Brain Res. 1999, 102, 73–88. [Google Scholar] [CrossRef]
- Rinne, J.O.; Kaasinen, V.; Järvenpää, T.; Någren, K.; Roivainen, A.; Yu, M.; Oikonen, V.; Kurki, T. Brain acetylcholinesterase activity in mild cognitive impairment and early Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2003, 74, 113–115. [Google Scholar] [CrossRef]
- Strada, O.; Hirsch, E.C.; Javoy-Agid, F.; Lehéricy, S.; Ruberg, M.; Hauw, J.J.; Agid, Y. Does loss of nerve growth factor receptors precede loss of cholinergic neurons in Alzheimer’s disease? An autoradiographic study in the human striatum and basal forebrain. J. Neurosci. 1992, 12, 4766–4774. [Google Scholar] [CrossRef]
- Akhtar, A.; Bishnoi, M.; Sah, S.P. Sodium orthovanadate improves learning and memory in intracerebroventricular-streptozotocin rat model of Alzheimer’s disease through modulation of brain insulin resistance induced tau pathology. Brain Res. Bull. 2020, 164, 83–97. [Google Scholar] [CrossRef]
- Zidan, E.F.; El-Mezayen, N.S.; Elrewini, S.H.; Afify, E.A.; Ali, M.A. Memantine/Rosuvastatin Therapy Abrogates Cognitive and Hippocampal Injury in an Experimental Model of Alzheimer’s Disease in Rats: Role of TGF-β1/Smad Signaling Pathway and Amyloid-β Clearance. J. Neuroimmune Pharmacol. 2024, 20, 4. [Google Scholar] [CrossRef] [PubMed]
- Osmanovic Barilar, J.; Knezovic, A.; Grünblatt, E.; Riederer, P.; Salkovic-Petrisic, M. Nine-month follow-up of the insulin receptor signalling cascade in the brain of streptozotocin rat model of sporadic Alzheimer’s disease. J. Neural Transm. 2015, 122, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.S.L.; Tureck, L.V.; Souza, L.C.; Mello-Hortega, J.V.; Piumbini, A.L.; Teixeira, M.D.; Furtado-Alle, L.; Vital, M.A.B.F.; Souza, R.L.R. Animal model of Alzheimer’s disease induced by streptozotocin: New insights about cholinergic pathway. Brain Res. 2023, 1799, 148175. [Google Scholar] [CrossRef]
- Angelova, H.; Pechlivanova, D.; Krumova, E.; Miteva-Staleva, J.; Kostadinova, N.; Dzhambazova, E.; Landzhov, B. Moderate protective effect of Kyotorphin against the late consequences of intracerebroventricular streptozotocin model of Alzheimer’s disease. Amino Acids 2019, 51, 1501–1513. [Google Scholar] [CrossRef]
- Afshar, S.; Shahidi, S.; Rohani, A.H.; Komaki, A.; Asl, S.S. The effect of NAD-299 and TCB-2 on learning and memory, hippocampal BDNF levels and amyloid plaques in Streptozotocin-induced memory deficits in male rats. Psychopharmacology 2018, 235, 2809–2822. [Google Scholar] [CrossRef]
- Dalli, T.; Beker, M.; Terzioglu-Usak, S.; Akbas, F.; Elibol, B. Thymoquinone activates MAPK pathway in hippocampus of streptozotocin-treated rat model. Biomed. Pharmacother. 2018, 99, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Salkovic-Petrisic, M.; Tribl, F.; Schmidt, M.; Hoyer, S.; Riederer, P. Alzheimer-like changes in protein kinase B and glycogen synthase kinase-3 in rat frontal cortex and hippocampus after damage to the insulin signalling pathway. J. Neurochem. 2006, 96, 1005–1015. [Google Scholar] [CrossRef]
- Izquierdo, I.; Medina, J.H. Memory formation: The sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiol. Learn. Mem. 1997, 68, 285–316. [Google Scholar] [CrossRef]
- Vazdarjanova, A.; McGaugh, J.L. Basolateral amygdala is involved in modulating consolidation of memory for classical fear conditioning. J. Neurosci. 1999, 19, 6615–6622. [Google Scholar] [CrossRef]
- Rai, S.; Kamat, P.K.; Nath, C.; Shukla, R. Glial activation and post-synaptic neurotoxicity: The key events in Streptozotocin (ICV) induced memory impairment in rats. Pharmacol. Biochem. Behav. 2014, 117, 104–117. [Google Scholar] [CrossRef]
- Bezerra, J.R.; de Souza Nascimento, T.; Tavares, J.; de Aguiar, M.S.S.; Maia, M.V.V.; de Andrade, G.M. Neuroprotective Effect of Chlorogenic Acid in an Animal Model of Sporadic Alzheimer’s Disease Induced by Streptozotocin. Mol. Neurobiol. 2025, 62, 674–692. [Google Scholar] [CrossRef]
- Yuliani, T.; Lobentanzer, S.; Klein, J. Central cholinergic function and metabolic changes in streptozotocin-induced rat brain injury. J. Neurochem. 2021, 158, 1307–1319. [Google Scholar] [CrossRef]
- Zappa Villar, M.F.; López Hanotte, J.; Falomir Lockhart, E.; Trípodi, L.S.; Morel, G.R.; Reggiani, P.C. Intracerebroventricular streptozotocin induces impaired Barnes maze spatial memory and reduces astrocyte branching in the CA1 and CA3 hippocampal regions. J. Neural Transm. 2018, 125, 1787–1803. [Google Scholar] [CrossRef]
- Gilbert, P.E.; Brushfield, A.M. The role of the CA3 hippocampal subregion in spatial memory: A process oriented behavioral assessment. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 774–781. [Google Scholar] [CrossRef]
- Emsley, J.G.; Macklis, J.D. Astroglial heterogeneity closely reflects the neuronal-defined anatomy of the adult murine CNS. Neuron Glia Biol. 2006, 2, 175–186. [Google Scholar] [CrossRef]
- Qin, H.; Zhou, L.; Haque, F.T.; Martin-Jimenez, C.; Trang, A.; Benveniste, E.N.; Wang, Q. Diverse signaling mechanisms and heterogeneity of astrocyte reactivity in Alzheimer’s disease. J. Neurochem. 2024, 168, 3536–3557. [Google Scholar] [CrossRef]
- Nairuz, T.; Heo, J.C.; Lee, J.H. Differential Glial Response and Neurodegenerative Patterns in CA1, CA3, and DG Hippocampal Regions of 5XFAD Mice. Int. J. Mol. Sci. 2024, 25, 12156. [Google Scholar] [CrossRef]
- Zimmer, T.S.; Orr, A.L.; Orr, A.G. Astrocytes in selective vulnerability to neurodegenerative disease. Trends Neurosci. 2024, 47, 289–302. [Google Scholar] [CrossRef]
- Gáll, Z.; Boros, B.; Kelemen, K.; Urkon, M.; Zolcseak, I.; Márton, K.; Kolcsar, M. Melatonin improves cognitive dysfunction and decreases gliosis in the streptozotocin-induced rat model of sporadic Alzheimer’s disease. Front. Pharmacol. 2024, 15, 1447757. [Google Scholar] [CrossRef]
- Clarke, L.E.; Liddelow, S.A.; Chakraborty, C.; Münch, A.E.; Heiman, M.; Barres, B.A. Normal aging induces A1-like astrocyte reactivity. Proc. Natl. Acad. Sci. USA 2018, 115, E1896–E1905. [Google Scholar] [CrossRef]
- Gamage, R.; Wagnon, I.; Rossetti, I.; Childs, R.; Niedermayer, G.; Chesworth, R.; Gyengesi, E. Cholinergic Modulation of Glial Function During Aging and Chronic Neuroinflammation. Front. Cell Neurosci. 2020, 14, 577912. [Google Scholar] [CrossRef]
- Ortinski, P.I.; Dong, J.; Mungenast, A.; Yue, C.; Takano, H.; Watson, D.J.; Haydon, P.G.; Coulter, D.A. Selective induction of astrocytic gliosis generates deficits in neuronal inhibition. Nat. Neurosci. 2010, 13, 584–591. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Astrocyte Reactivity: Subtypes, States, and Functions in CNS Innate Immunity. Trends Immunol. 2020, 41, 758–770. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Zorec, R.; Rodríguez, J.J.; Parpura, V. Astroglia dynamics in ageing and Alzheimer’s disease. Curr. Opin. Pharmacol. 2016, 26, 74–79. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Butt, A.; Li, B.; Illes, P.; Zorec, R.; Semyanov, A.; Tang, Y.; Sofroniew, M.V. Astrocytes in human central nervous system diseases: A frontier for new therapies. Signal Transduct. Target. Ther. 2023, 8, 396. [Google Scholar] [CrossRef]
- Tillmon, H.; Soteros, B.M.; Shen, L.; Cong, Q.; Wollet, M.; General, J.; Chin, H.; Lee, J.B.; Carreno, F.R.; Morilak, D.A.; et al. Complement and microglia activation mediate stress-induced synapse loss in layer 2/3 of the medial prefrontal cortex in male mice. Nat. Commun. 2024, 15, 9803. [Google Scholar] [CrossRef]
- Wu, Y.; Eisel, U.L.M. Microglia-Astrocyte Communication in Alzheimer’s Disease. J. Alzheimers Dis. 2023, 95, 785–803. [Google Scholar] [CrossRef]
- Li, S.; Chen, Y.; Chen, G. Cognitive disorders: Potential astrocyte-based mechanism. Brain Res. Bull. 2025, 220, 111181. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, D.; Wang, X. The emerging roles of ferroptosis in cells of the central nervous system. Front. Neurosci. 2022, 16, 1032140. [Google Scholar] [CrossRef]
- Maimaiti, Y.; Su, T.; Zhang, Z.; Ma, L.; Zhang, Y.; Xu, H. NOX4-mediated astrocyte ferroptosis in Alzheimer’s disease. Cell Biosci. 2024, 14, 88. [Google Scholar] [CrossRef]
- Park, M.W.; Cha, H.W.; Kim, J.; Kim, J.H.; Yang, H.; Yoon, S.; Boonpraman, N.; Yi, S.S.; Yoo, I.D.; Moon, J.S. NOX4 promotes ferroptosis of astrocytes by oxidative stress-induced lipid peroxidation via the impairment of mitochondrial metabolism in Alzheimer’s diseases. Redox Biol. 2021, 41, 101947. [Google Scholar] [CrossRef]
- Çarçak, N.; Onat, F.; Sitnikova, E. Astrocytes as a target for therapeutic strategies in epilepsy: Current insights. Front. Mol. Neurosci. 2023, 16, 1183775. [Google Scholar] [CrossRef]
- Won, W.; Bhalla, M.; Lee, J.H.; Lee, C.J. Astrocytes as Key Regulators of Neural Signaling in Health and Disease. Annu. Rev. Neurosci. 2025, 48, 251–276. [Google Scholar] [CrossRef]
- Bartus, R.T.; Dean, R.L., 3rd; Beer, B.; Lippa, A.S. The cholinergic hypothesis of geriatric memory dysfunction. Science 1982, 217, 408–414. [Google Scholar] [CrossRef]
- Davies, P.; Maloney, A.J. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet 1976, 2, 1403. [Google Scholar] [CrossRef]
- Ballinger, E.C.; Ananth, M.; Talmage, D.A.; Role, L.W. Basal Forebrain Cholinergic Circuits and Signaling in Cognition and Cognitive Decline. Neuron 2016, 91, 1199–1218. [Google Scholar] [CrossRef]
- Schliebs, R.; Arendt, T. The cholinergic system in aging and neuronal degeneration. Behav. Brain Res. 2011, 221, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guo, Z.; Mao, Y.F.; Zheng, T.; Zhang, B. Intranasal Insulin Ameliorates Cerebral Hypometabolism, Neuronal Loss, and Astrogliosis in Streptozotocin-Induced Alzheimer’s Rat Model. Neurotox. Res. 2018, 33, 716–724. [Google Scholar] [CrossRef]
- López Hanotte, J.; Peralta, F.; Reggiani, P.C.; Zappa Villar, M.F. Investigating the Impact of Intracerebroventricular Streptozotocin on Female Rats with and without Ovaries: Implications for Alzheimer’s Disease. Neurochem. Res. 2024, 49, 2785–2802. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Nitsch, R.; Hoyer, S. Local action of the diabetogenic drug, streptozotocin, on glucose and energy metabolism in rat brain cortex. Neurosci. Lett. 1991, 128, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Galeano, P.; Martino Adami, P.V.; Do Carmo, S.; Blanco, E.; Rotondaro, C.; Capani, F.; Castaño, E.M.; Cuello, A.C.; Morelli, L. Longitudinal analysis of the behavioral phenotype in a novel transgenic rat model of early stages of Alzheimer’s disease. Front. Behav. Neurosci. 2014, 8, 321. [Google Scholar] [CrossRef]
- Ennaceur, A.; Delacour, J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav. Brain Res. 1988, 31, 47–59. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 5th ed.; Academic Press: San Diego, CA, USA, 2005. [Google Scholar]
- McGregor, R.; Shan, L.; Wu, M.F.; Siegel, J.M. Diurnal fluctuation in the number of hypocretin/orexin and histamine producing: Implication for understanding and treating neuronal loss. PLoS ONE 2017, 12, e0178573. [Google Scholar] [CrossRef]
- Niño-Rivero, S.; Torterolo, P.; Lagos, P. Melanin-concentrating hormone receptor-1 is located in primary cilia of the dorsal raphe neurons. J. Chem. Neuroanat. 2019, 98, 55–62. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niño-Rivero, S.; Cabral, R.; Fleitas, J.; Alcalde-Ahlig, L.; Castaño, E.M.; Morelli, L.; McGregor, R.; Galeano, P.; Lagos, P. Temporal Progression of Recognition Memory Impairment, Astrogliosis, and Cholinergic Dysfunction in the Streptozotocin Rat Model of Sporadic Alzheimer’s Disease. Int. J. Mol. Sci. 2025, 26, 10944. https://doi.org/10.3390/ijms262210944
Niño-Rivero S, Cabral R, Fleitas J, Alcalde-Ahlig L, Castaño EM, Morelli L, McGregor R, Galeano P, Lagos P. Temporal Progression of Recognition Memory Impairment, Astrogliosis, and Cholinergic Dysfunction in the Streptozotocin Rat Model of Sporadic Alzheimer’s Disease. International Journal of Molecular Sciences. 2025; 26(22):10944. https://doi.org/10.3390/ijms262210944
Chicago/Turabian StyleNiño-Rivero, Sofía, Rossana Cabral, Jazmín Fleitas, Lucía Alcalde-Ahlig, Eduardo M. Castaño, Laura Morelli, Ronald McGregor, Pablo Galeano, and Patricia Lagos. 2025. "Temporal Progression of Recognition Memory Impairment, Astrogliosis, and Cholinergic Dysfunction in the Streptozotocin Rat Model of Sporadic Alzheimer’s Disease" International Journal of Molecular Sciences 26, no. 22: 10944. https://doi.org/10.3390/ijms262210944
APA StyleNiño-Rivero, S., Cabral, R., Fleitas, J., Alcalde-Ahlig, L., Castaño, E. M., Morelli, L., McGregor, R., Galeano, P., & Lagos, P. (2025). Temporal Progression of Recognition Memory Impairment, Astrogliosis, and Cholinergic Dysfunction in the Streptozotocin Rat Model of Sporadic Alzheimer’s Disease. International Journal of Molecular Sciences, 26(22), 10944. https://doi.org/10.3390/ijms262210944






