Determination of the Number of Circulating Small Extracellular Vesicles in Pregnancy Using the Novel Marker CD9
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Characterization and Evaluation of Circulating Small EVs
2.3. The Number of Small EVs in Non-Pregnant Women and Normotensive Controls
2.4. Comparison of Number of Small EVs Between Patients with PE and Normotensive Controls
2.5. Comparison of Number of Small EVs in First Trimester Between Women Who Later Developed PE and Normotensive Controls in Prospective, Nested Case–Control Study
3. Discussion
4. Materials and Methods
4.1. Study Participants and Sample Collection
4.2. Purification of Small EVs
4.3. Western Blotting
4.4. Nanoparticle Tracking Analysis (NTA)
4.5. Transmission Electron Microscopy (TEM)
4.6. Measurement of Small-EV Numbers
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EV | Extracellular vesicle |
| EoPE | Early onset preeclampsia |
| LoPE | Late-onset preeclampsia |
| NTA | Nanoparticle tracking analysis |
| HAEC | Human aortic endothelial cell |
| HUVEC | Human umbilical vein endothelial cell |
| NIPT | Non-invasive prenatal testing |
| TEM | Transmission electron microscopy |
References
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Small EV-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Thakur, B.K.; Zhang, H.; Becker, A.; Matei, I.; Huang, Y.; Costa-Silva, B.; Zheng, Y.; Hoshino, A.; Brazier, H.; Xiang, J.; et al. Double-stranded DNA in small EVs: A novel biomarker in cancer detection. Cell Res. 2014, 24, 766–769. [Google Scholar] [CrossRef] [PubMed]
- Maas, S.L.N.; Breakefield, X.O.; Weaver, A.M. Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends Cell Biol. 2017, 27, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, A.; Sugiura, K.; Hoshino, A. Impact of small EV-mediated feto-maternal interactions on pregnancy maintenance and development of obstetric complications. J. Biochem. 2021, 169, 163–171. [Google Scholar] [CrossRef]
- Mitchell, M.D.; Peiris, H.N.; Kobayashi, M.; Koh, Y.Q.; Duncombe, G.; Illanes, S.E.; Rice, G.E.; Salomon, C. Placental small EVs in normal and complicated pregnancy. Am. J. Obstet. Gynecol. 2015, 213, 173–181. [Google Scholar] [CrossRef]
- Sun, Q.; Chang, H.; Wang, H.; Zheng, L.; Weng, Y.; Zheng, D.; Zheng, D. Regulatory roles of extracellular vesicles in pregnancy complications. J. Adv. Res. 2025, in press. [Google Scholar] [CrossRef]
- Yu, Y.; Min, Z.; Zhou, Z.; Linhong, M.; Tao, R.; Yan, L.; Song, H. Hypoxia-induced small EVs promote hepatocellular carcinoma proliferation and metastasis via miR-1273f transfer. Exp. Cell Res. 2019, 385, 111649. [Google Scholar] [CrossRef]
- Huang, C.; Fisher, K.P.; Hammer, S.S.; Navitskaya, S.; Blanchard, G.J.; Busik, J.V. Plasma Small EVs Contribute to Microvascular Damage in Diabetic Retinopathy by Activating the Classical Complement Pathway. Diabetes 2018, 67, 1639–1649. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Kano, M.; Akutsu, Y.; Hanari, N.; Hoshino, I.; Murakami, K.; Usui, A.; Suito, H.; Takahashi, I.; Otsuka, R.; et al. Quantification of plasma exosome is a potential prognostic marker for esophageal squamous cell carcinoma. Oncol. Rep. 2016, 36, 2535–2543. [Google Scholar] [CrossRef]
- Silva, J.; Garcia, V.; Rodriguez, M.; Compte, M.; Cisneros, E.; Veguillas, P.; Garcia, J.M.; Dominguez, G.; Campos-Martin, Y.; Cuevas, J.; et al. Analysis of Small EV Release and Its Prognostic Value in Human Colorectal Cancer. Genes Chromosomes Cancer 2012, 51, 409–418. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, J.K.; Lee, E.Y.; Lee, E.B.; Song, Y.W. Circulating exosomes from patients with systemic lupus erythematosus induce a proinflammatory immune response. Arthritis Res. Ther. 2016, 18, 264, Correction in Arthritis Res. Ther. 2020, 22, 109.. [Google Scholar]
- Salomon, C.; Torres, M.J.; Kobayashi, M.; Scholz-Romero, K.; Sobrevia, L.; Dobierzewska, A.; Illanes, S.E.; Mitchell, M.D.; Rice, G.E. A Gestational Profile of Placental Small EVs in Maternal Plasma and Their Effects on Endothelial Cell Migration. PLoS ONE 2014, 9, e98667. [Google Scholar] [CrossRef] [PubMed]
- Arias, M.; Monteiro, L.J.; Acuna-Gallardo, S.; Varas-Godoy, M.; Rice, G.E.; Monckeberg, M.; Diaz, P.; Illanes, S.E. Extracellular vesicle concentration in maternal plasma as an early marker of gestational diabetes. Rev. Med. Chil. 2019, 147, 1503–1509. [Google Scholar] [CrossRef]
- Salomon, C.; Guanzou, D.; Scholz-Romero, K.; Longo, S.; Correa, P.; Illanes, S.E.; Rice, G.E. Placental Exosomes as Early Biomarker of Preeclampsia: Potential Role of Exosomal MicroRNAs Across Gestation. J. Clin. Endocrinol. Metab. 2017, 102, 3182–3194. [Google Scholar] [CrossRef] [PubMed]
- Pillay, P.; Maharaj, N.; Moodley, J.; Mackraj, I. Placental small EVs and pre-eclampsia: Maternal circulating levels in normal pregnancies and, early and late onset pre-eclamptic pregnancies. Placenta 2016, 46, 18–25. [Google Scholar] [CrossRef]
- Murugesan, S.; Hussey, H.; Saravanakumar, L.; Sinkey, R.G.; Sturdivant, A.B.; Powell, M.F.; Berkowitz, D.E. Extracellular Vesicles From Women With Severe Preeclampsia Impair Vascular Endothelial Function. Anesth. Analg. 2022, 134, 713–723. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, P.; Han, C.; Li, J.; Liu, L.; Zhao, Z.; Gao, Y.; Qin, Y.; Xu, Q.; Yan, Y.; et al. Association of placenta-derived extracellular vesicles with pre-eclampsia and associated hypercoagulability: A clinical observational study. BJOG Int. J. Obstet. Gynaecol. 2021, 128, 1037–1046. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, R.; Zhang, J.; Wang, P.; Wang, Z.; Gao, J.; Liu, X. Role of Extracellular Vesicles in Placental Inflammation and Local Immune Balance. Mediat. Inflamm. 2021, 2021, 5558048. [Google Scholar] [CrossRef]
- Karimi, N.; Dalirfardouel, R.; Dias, T.; Lotcall, J.; Lasser, C. Tetraspanins distinguish separate extracellular vesicle subpopulations in human serum and plasma– Contributions of platelet extracellular vesicles in plasma samples. J. Extracell. Vesicles 2022, 11, e12213. [Google Scholar] [CrossRef]
- Hoshino, A.; Kim, H.S.; Bojmar, L.; Gyan, K.E.; Cioffi, M.; Hernandez, J.; Zambirinis, C.P.; Rodrigues, G.; Molina, H.; Heissel, S.; et al. Extracellular Vesicle and Particle Biomarkers Define Multiple Human Cancers. Cell 2020, 182, 1044–1061. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andraintsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Kilinc, S.; Paisner, R.; Camarda, R.; Gupta, S.; Momcilovic, O.; Kohnz, R.A.; Avsaroglu, B.; L’Etoile, N.D.; Perera, R.M.; Nomura, D.K.; et al. Oncogene Regulated Release of Extracellular Vesicles. Dev. Cell 2021, 56, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Bebelman, M.P.; Smit, M.J.; Pegtel, D.M.; Baglio, S.R. Biogenesis and function of extracellular vesicles in cancer. Pharmacol. Ther. 2018, 188, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wang, K.; Zhu, C.; Fan, L.; Zhu, Y.; Wang, J.F.; Zhao, Y.; Zhu, C.; Zhang, W.; Yang, F.; et al. Tumor-derived extracellular vesicles as a biomarker for breast cancer diagnosis and metastasis monitoring. iScience 2024, 27, 109506. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.H.; Quan, Y.H.; Rho, J.; Hong, S.; Park, Y.; Choi, Y.; Park, J.; Yong, H.S.; Han, K.N.; Choi, Y.H.; et al. Levels of Extracellular Vesicles in Pulmonary and Peripheral Blood Correlate with Stages of Lung Cancer Patients. World J. Surg. 2020, 44, 3522–3529. [Google Scholar] [CrossRef]
- Dimitrakopoulos, F.; Kottorou, A.E.; Rodgers, K.; Sherwood, J.T.; Koliou, G.; Lee, B.; Yang, A.; Brahmer, J.R.; Baylin, S.B.; Yang, S.C.; et al. Clinical Significance of Plasma CD9-Positive Exosomes in HIV Seronegative and Seropositive Lung Cancer Patients. Cancers 2021, 13, 5193. [Google Scholar] [CrossRef]
- Mitchell, P.J.; Welton, J.; Staffurth, J.; Court, J.; Mason, M.D.; Tabi, Z.; Clayton, A. Can urinary exosomes act as treatment response markers in prostate cancer? J. Transl. Med. 2009, 7, 4. [Google Scholar] [CrossRef]
- Logozzi, M.; Mizzoni, D.; Raimo, R.D.; Giuliani, A.; Maggi, M.; Sciarra, A.; Fais, S. Plasmatic Exosome Number and Size Distinguish Prostate Cancer Patients From Healthy Individuals: A Prospective Clinical Study. Front. Oncol. 2021, 11, 727317. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Vizio, D.D.; Driedonks, T.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404, Correction in J. Extracell. Vesicles 2024, 13, e12451.. [Google Scholar] [CrossRef]
- Bowman-Gibson, S.; Chandiramani, C.; Stone, M.L.; Waker, C.A.; Maxwell, R.A.; Dhanraj, D.N.; Brown, T.L. Streamlined Analysis of Maternal Plasma Indicates Small Extracellular Vesicles are Significantly Elevated in Early-Onset Preeclampsia. Reprod. Sci. 2024, 31, 2771–2782. [Google Scholar] [CrossRef]
- Truong, G.; Guanzon, D.; Kinhal, V.; Elfeky, O.; Lai, A.; Longo, S.; Nuzhat, Z.; Palma, C.; Scholz-Romero, K.; Menon, R.; et al. Oxygen tension regulates the miRNA profile and bioactivity of exosomes released from extravillous trophoblast cells—Liquid biopsies for monitoring complications of pregnancy. PLoS ONE 2017, 12, e0174514. [Google Scholar] [CrossRef]
- Rice, G.E.; Scholz-Romero, K.; Sweeney, E.; Peiris, H.; Kobayashi, M.; Duncombe, G.; Mitchell, M.D.; Salomon, C. The Effect of Glucose on the Release and Bioactivity of Exosomes From First Trimester Trophoblast Cells. J. Clin. Endocrinol. Metab. 2015, 100, E1280–E1288. [Google Scholar] [CrossRef]
- Shen, L.; Li, Y.; Li, R.; Diao, Z.; Yany, M.; Wu, M.; Sun, H.; Yan, G.; Hu, Y. Placenta-associated serum exosomal miR-155 derived from patients with preeclampsia inhibits eNOS expression in human umbilical vein endothelial cells. Int. J. Mol. Med. 2018, 41, 1731–1739. [Google Scholar] [CrossRef]
- Chang, X.; Yao, J.; He, Q.; Liu, M.; Duan, T.; Wang, K. Exosomes from women with preeclampsia induced vascular dysfunction by delivering sFlt (soluble fms-like tyrosine kinase)-1 and sEng (soluble endoglin) to endothelial cells. Hypertension 2018, 72, 1381–1390. [Google Scholar] [CrossRef]
- Venturella, M.; Criscuoli, M.; Carraro, F.; Naldini, A.; Zocco, D. Interplay between Hypoxia and Extracellular Vesicles in Cancer and Inflammation. Biology 2021, 10, 606. [Google Scholar] [CrossRef]
- Zeisler, H.; Llurba, E.; Chantraine, F.; Vatish, M.; Staff, A.C.; Sennström, M.; Olovsson, M.; Brennecke, S.P.; Stepan, H.; Allegranza, D.; et al. Predictive Value of the sFlt-1:PlGF Ratio in Women with Suspected Preeclampsia. N. Engl. J. Med. 2016, 374, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Matsubara, K.; Nakamoto, O.; Ushijima, J.; Ohkuchi, A.; Koide, K.; Makino, S.; Mimura, K.; Morikawa, M.; Naruse, K.; et al. Outline of the new definition and classification of “Hypertensive Disorders of Pregnancy (HDP)”; a revised JSSHP statement of 2005. Hypertens Res. Pregnancy 2018, 6, 33–37. [Google Scholar] [CrossRef]
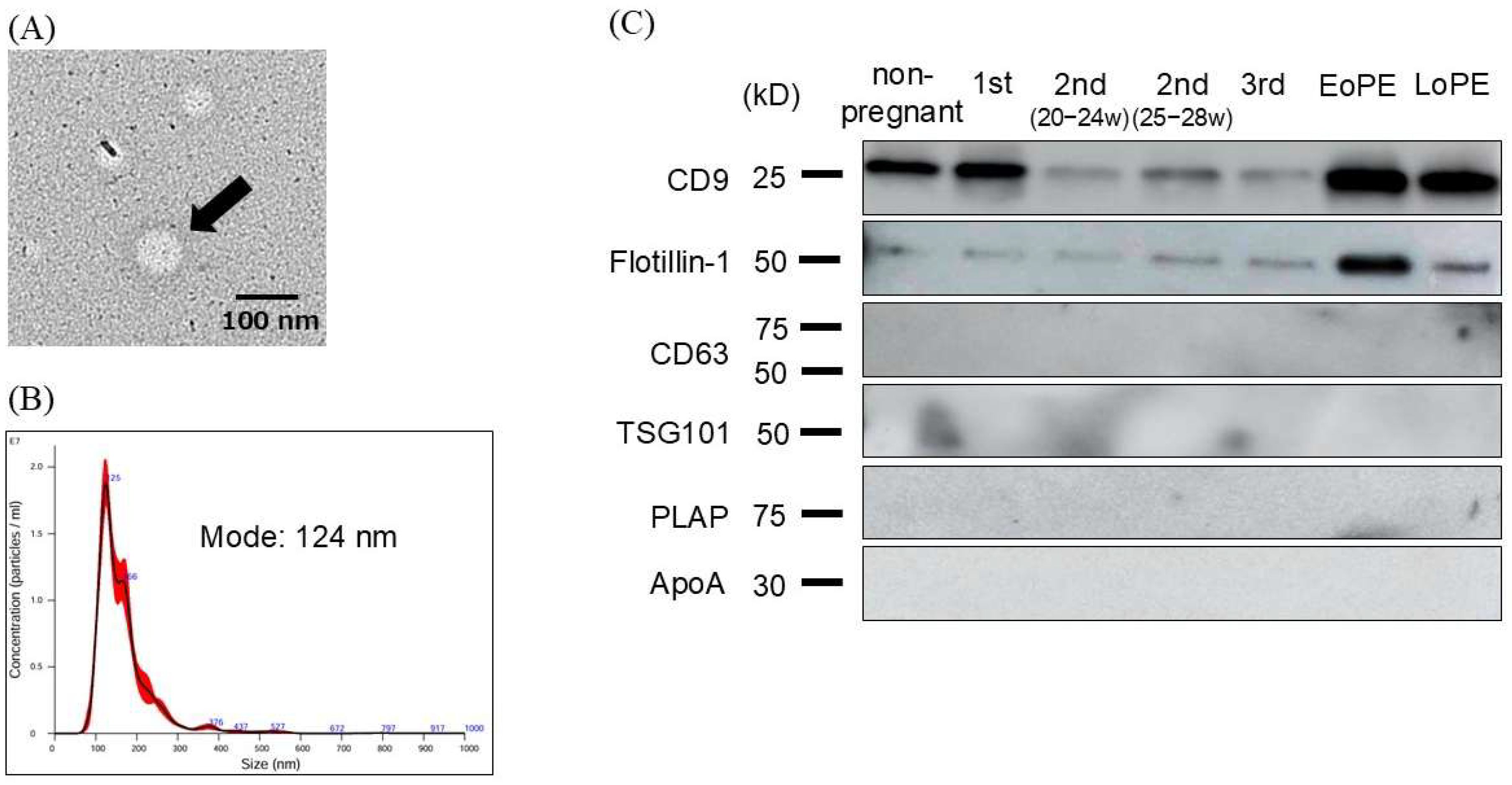
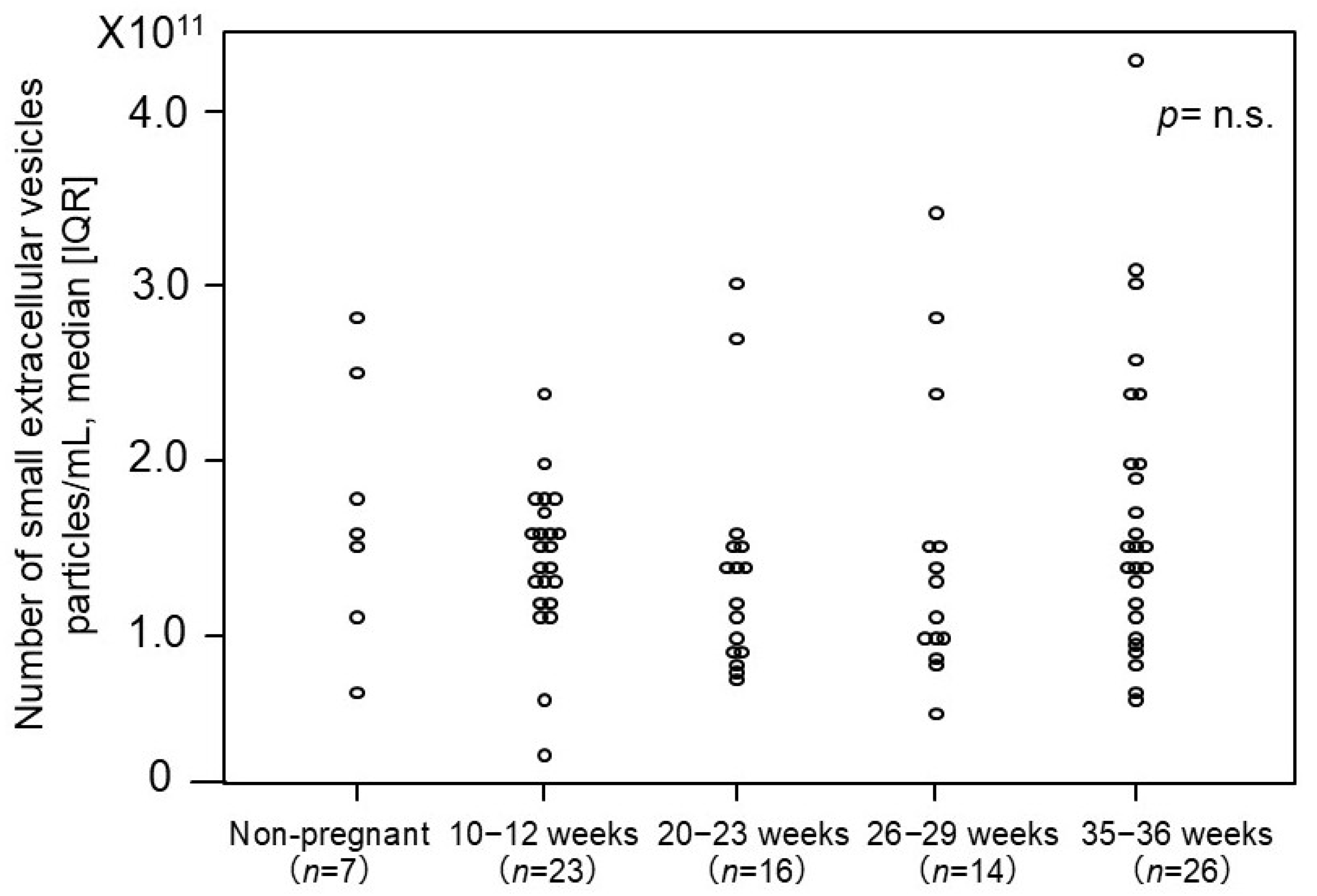
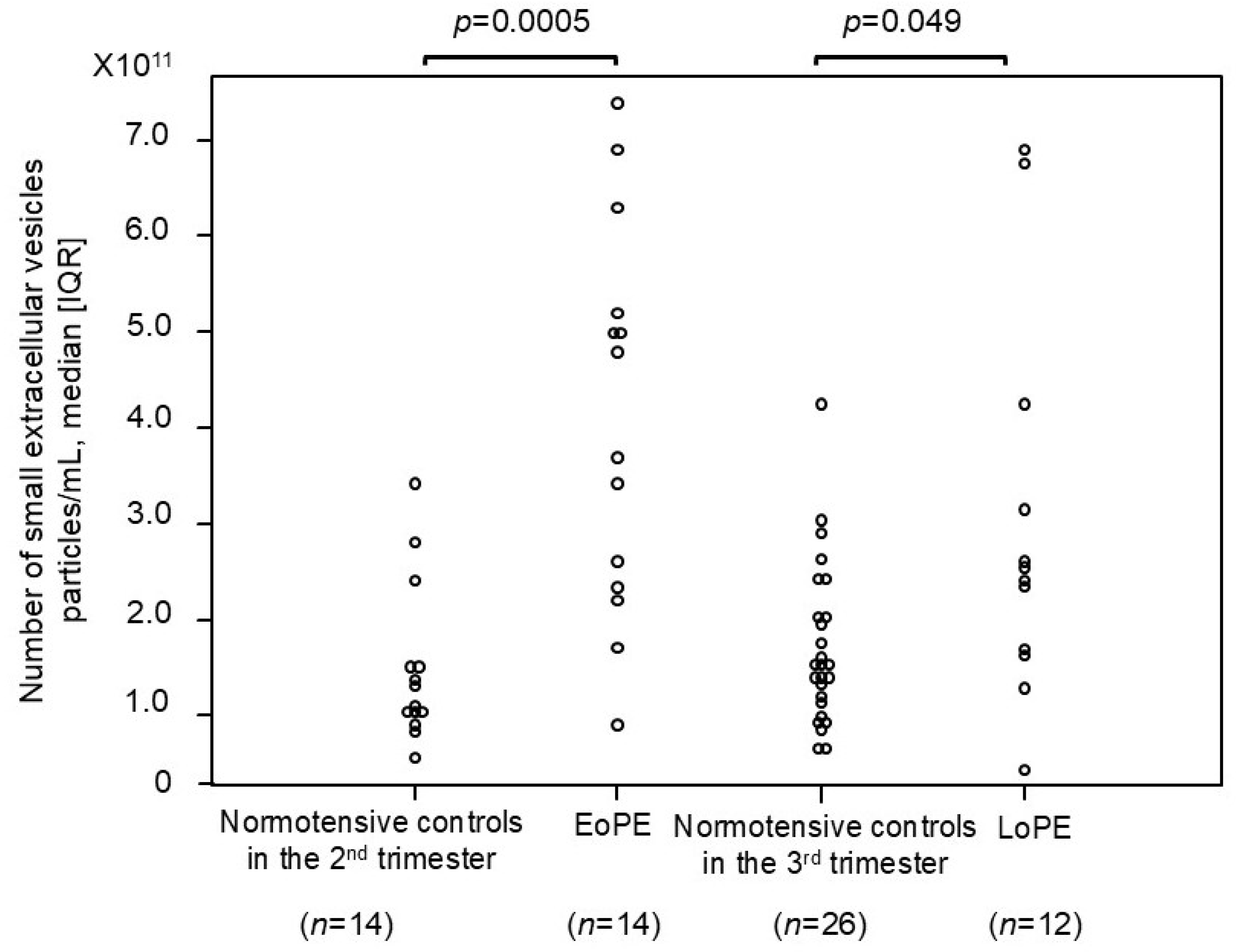
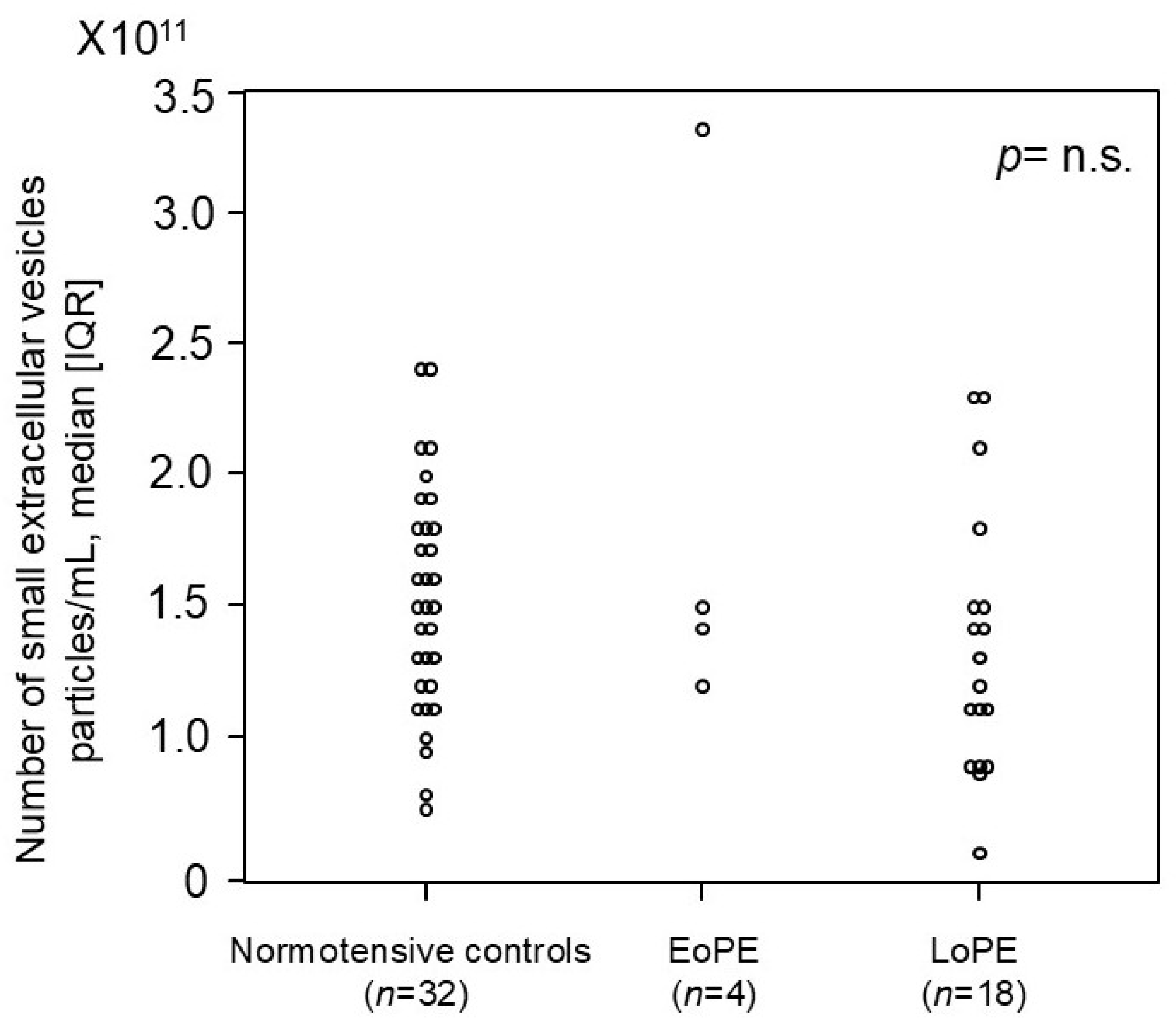
| Normotensive Controls 2nd Trimester (n = 14) | EoPE (n = 14) | p-Value | Normotensive Controls 3rd Trimester (n=26) | LoPE (n = 12) | p-Value | |
|---|---|---|---|---|---|---|
| Maternal age (years) | 32.5 [30.3–34.0] | 36.0 [31.5–39.5] | 0.16 | 34.0 [31.5–38.5] | 35.0 [32.5–36.8] | 0.98 |
| BMI (kg/m2) | 20.4 [19.0–23.2] | 22.6 [20.2–23.7] | 0.19 | 23.2 [21.4–27.2] | 24.5 [20.8–26.7] | 0.65 |
| Gestational age at blood collection (weeks) | 26.9 [26.7–27.3] | 31.4 [28.6–33.3] | 0.003 | 36.9 [36.5–37.1] | 36.6 [35.7–38.5] | 0.94 |
| Systolic blood pressure (mmHg) | 116 [108–123] | 165 [161–176] | <0.001 | 117 [106–126] | 154 [151–170] | <0.001 |
| Diastolic blood pressure (mmHg) | 72 [62–79] | 100 [89.5–108.5] | <0.001 | 71 [64–84] | 98 [90–102] | <0.001 |
| Proteinuria (g/day) | – | 3.0 [1.6–5.8] | NA | – | 1.2 [0.9–1.6] | NA |
| Serum creatinine (mg/dL) | 0.49 [0.39–0.52] | 0.70 [0.50–0.79] | 0.03 | 0.55 [0.45–0.67] | 0.61 [0.55–0.68] | 0.31 |
| Gestational age at onset (weeks) | – | 31.7 [28.7–33.0] | NA | – | 36.1 [35.1–37.5] | NA |
| Gestational age at delivery (weeks) | 39.3 [38.1–40.6] | 33.6 [33.0–34.3] | <0.001 | 39.6 [39.1–40.8] | 38.0 [36.8–38.5] | 0.004 |
| Birth weight (g) | 2994 [2842–3156] | 1750 [1292–1904] | <0.001 | 3156 [2839–3414] | 2813 [2299–2988] | 0.03 |
| Normotensive Controls (n = 32) | EoPE (n = 4) | LoPE (n = 18) | p-Value | |
|---|---|---|---|---|
| Maternal age (years) | 35.5 [30.8–39.0] | 38.5 [36.8–39.2] | 35.5 [32.3–37.0] | 0.45 |
| BMI (kg/m2) | 21.7 [19.6–23.9] | 21.5 [20.7–24.2] | 22.2 [19.7–25.3] | 0.9 |
| Gestational age at blood collection (weeks) | 10.7 [10.3–11.4] | 11.4 [10.6–12.1] | 11.4 [10.7–12.0] | 0.27 |
| Systolic blood pressure (mmHg) | 116 [107–125] | 118 [113–122] | 116 [107–125] | 0.98 |
| Diastolic blood pressure (mmHg) | 66 [57–73] | 81 [70–84] | 70 [63–76] | 0.15 |
| Proteinuria (g/day) | – | 2.7 [1.8–4.5] | 0.7 [0.4–2.1] | 0.15 |
| Serum creatinine (mg/dL) | 0.50 [0.45–0.55] | 0.75 [0.65–0.82] | 0.63 [0.60–0.69] | <0.001 a |
| Gestational age at onset (weeks) | – | 30.2 [29.5–31.2] | 37.5 [36.5–38.9] | 0.002 |
| Gestational age at delivery (weeks) | 39.0 [38.0–40.0] | 31.5 [31.0–32.6] | 38.7 [36.8–40.0] | 0.002 b,c |
| Birth weight (g) | 3091 [2891–3290] | 1280 [1118–1482] | 2802 [2620–2920] | <0.001 a,b,c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narumi, R.; Suzuki, H.; Ogoyama, M.; Saga, Y.; Tozawa, S.; Noguchi, S.; Ohkuchi, A.; Takizawa, T.; Fujiwara, H.; Takahashi, H. Determination of the Number of Circulating Small Extracellular Vesicles in Pregnancy Using the Novel Marker CD9. Int. J. Mol. Sci. 2025, 26, 10906. https://doi.org/10.3390/ijms262210906
Narumi R, Suzuki H, Ogoyama M, Saga Y, Tozawa S, Noguchi S, Ohkuchi A, Takizawa T, Fujiwara H, Takahashi H. Determination of the Number of Circulating Small Extracellular Vesicles in Pregnancy Using the Novel Marker CD9. International Journal of Molecular Sciences. 2025; 26(22):10906. https://doi.org/10.3390/ijms262210906
Chicago/Turabian StyleNarumi, Risa, Hirotada Suzuki, Manabu Ogoyama, Yasushi Saga, Shohei Tozawa, Syunya Noguchi, Akihide Ohkuchi, Toshihiro Takizawa, Hiroyuki Fujiwara, and Hironori Takahashi. 2025. "Determination of the Number of Circulating Small Extracellular Vesicles in Pregnancy Using the Novel Marker CD9" International Journal of Molecular Sciences 26, no. 22: 10906. https://doi.org/10.3390/ijms262210906
APA StyleNarumi, R., Suzuki, H., Ogoyama, M., Saga, Y., Tozawa, S., Noguchi, S., Ohkuchi, A., Takizawa, T., Fujiwara, H., & Takahashi, H. (2025). Determination of the Number of Circulating Small Extracellular Vesicles in Pregnancy Using the Novel Marker CD9. International Journal of Molecular Sciences, 26(22), 10906. https://doi.org/10.3390/ijms262210906








