Exploring the Mechanism of Action of Chicoric Acid Against Influenza Virus Infection Based on Network Pharmacology, Molecular Docking, and Molecular Dynamics Simulation
Abstract
1. Introduction
2. Results
2.1. Screening of Targets for Chicoric Acid Against Influenza
2.2. Venn Diagram of Chicoric Acid and Influenza Intersection Targets
2.3. Construction of Protein–Protein Interaction Networks
2.4. GO/KEGG Analysis Results
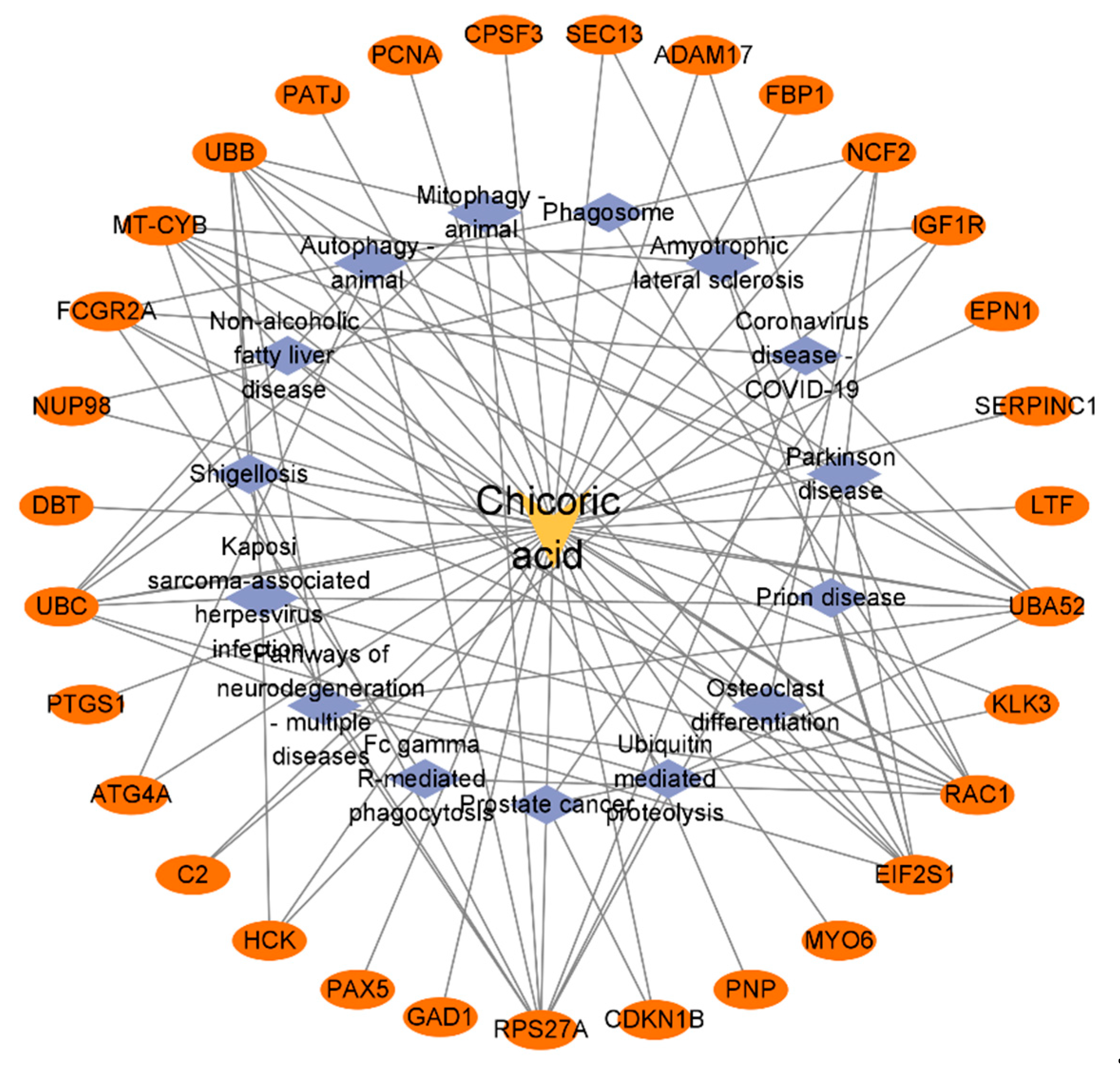
2.5. Construction of the Chicoric Acid-Target-Pathway Network
2.6. Molecular Docking Validation of Chicoric Acid
2.7. Molecular Dynamics Simulation Results
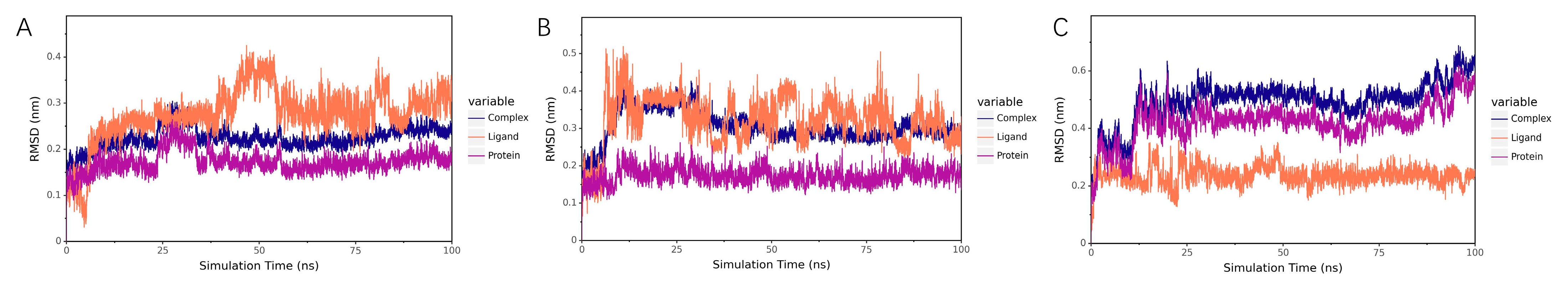
2.7.1. Stability Analysis
RMSD Analysis
Rg Analysis
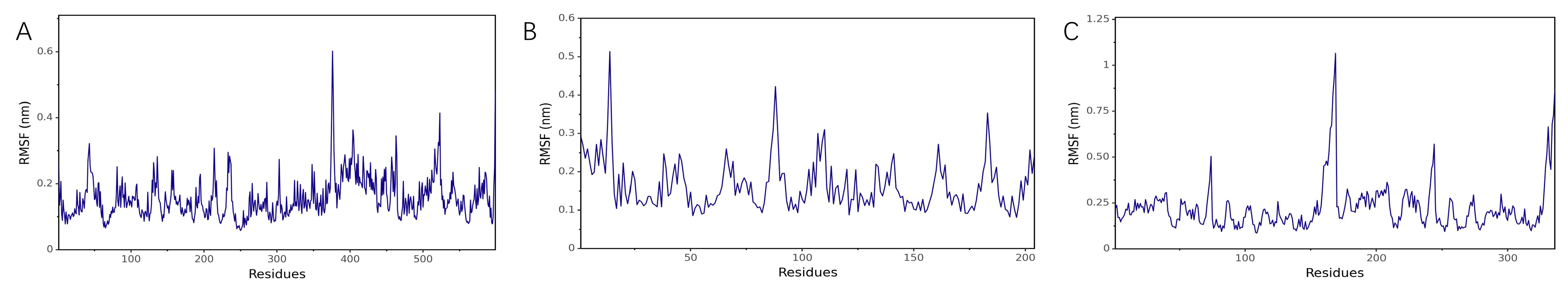
RMSF Analysis
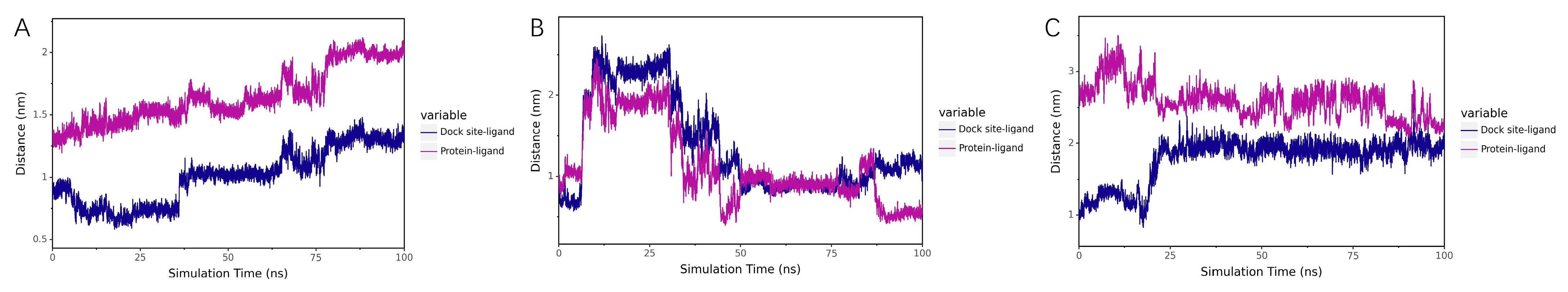
Centroid Evolution Analysis
Buried SASA Analysis
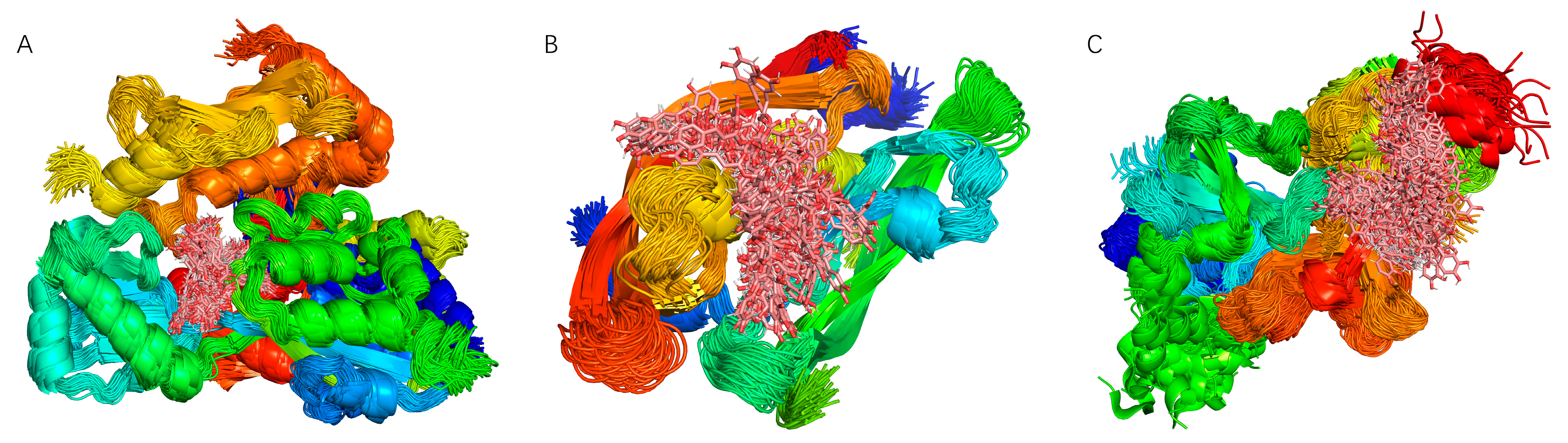
Superposition of Binding Conformations
2.7.2. Analysis of Hydrogen Bond Interactions Between the Small Molecule and Proteins
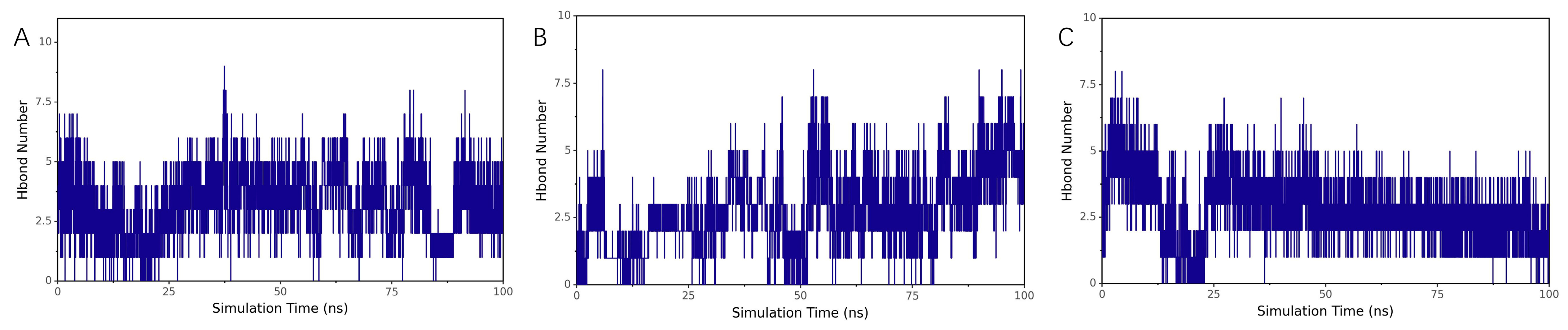
Evolution of Hydrogen Bond Numbers
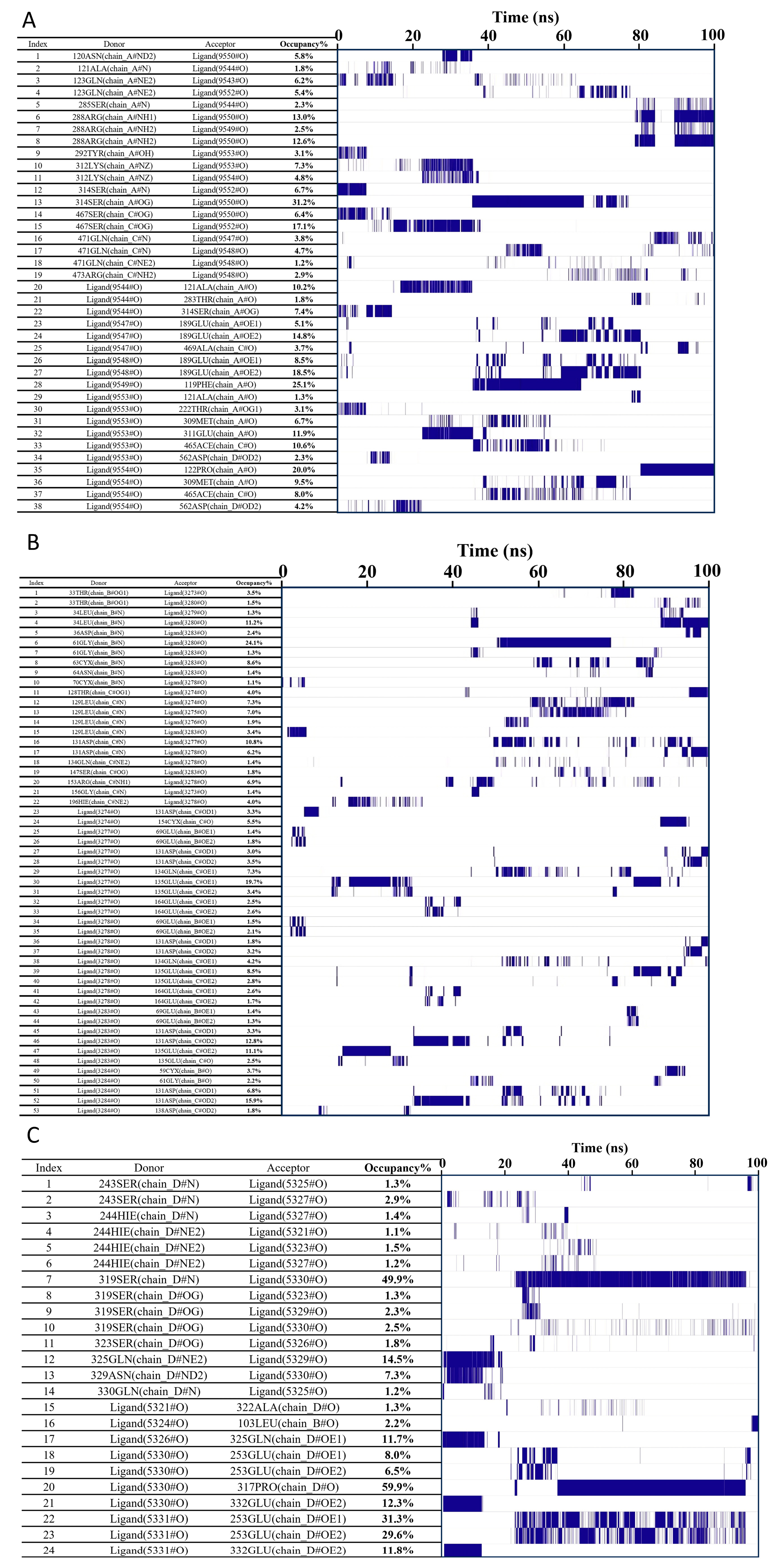
Hydrogen Bond Occupancy (Frequency) Analysis
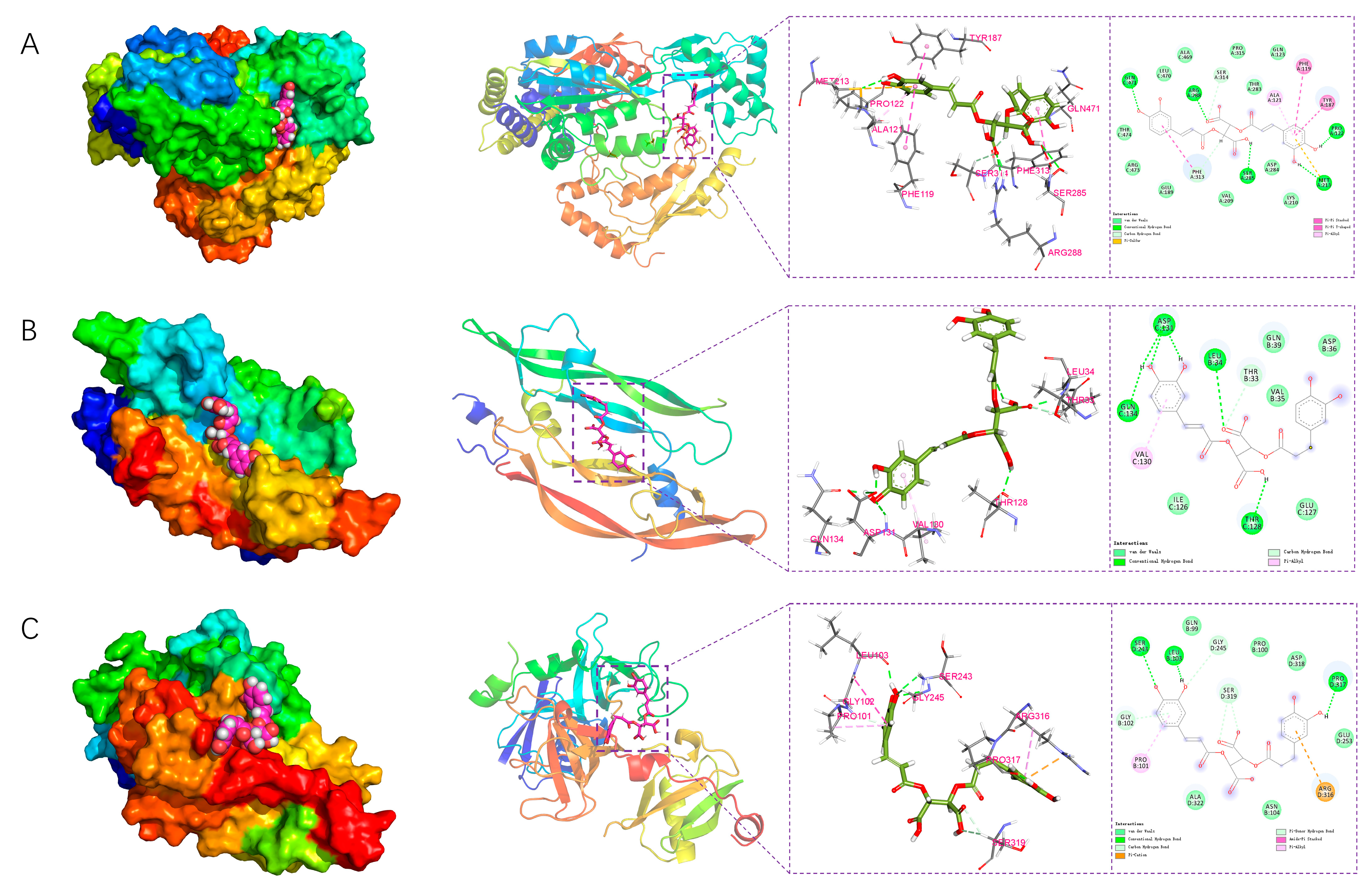
2.7.3. Analysis of Interactions Between the Small Molecule and Proteins
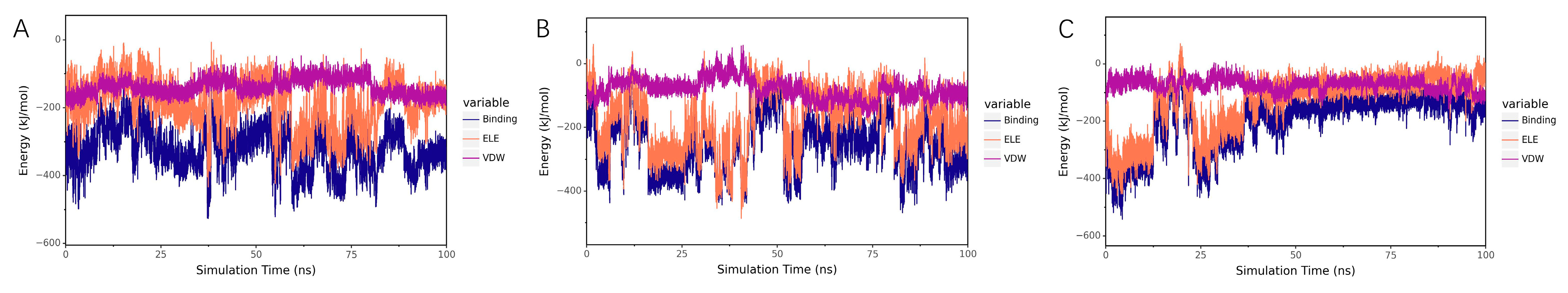
Analysis of Electrostatic and van der Waals Interactions
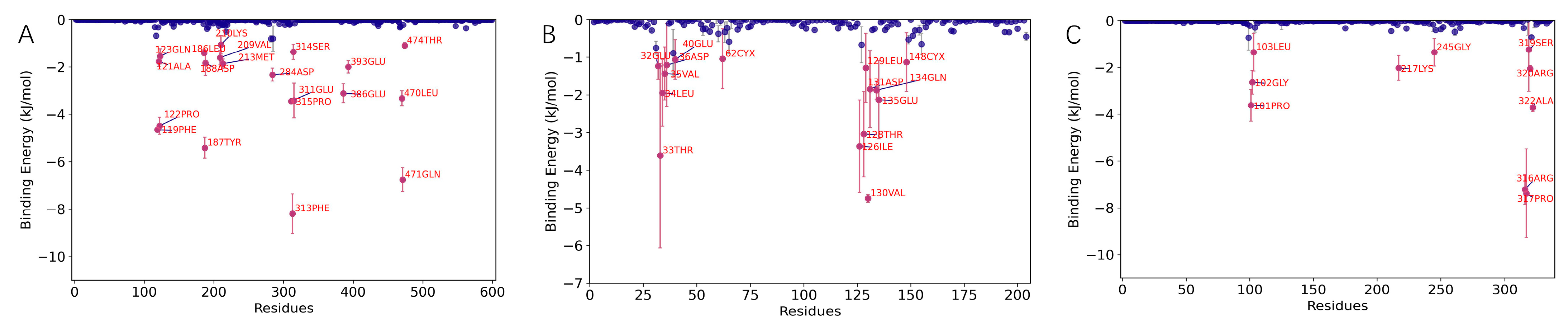
Binding Energy Analysis
Residue Contribution Analysis
Structural Analysis
3. Discussion
4. Materials and Methods
4.1. Screening of Chicoric Acid Targets
4.2. Screening of Influenza Targets
4.3. Visual Analysis of Chicoric Acid Against Influenza Virus
4.4. Construction and Analysis of the Protein–Protein Interaction Network
4.5. GO Functional Enrichment Analysis and KEGG Pathway Analysis
4.6. Construction of the Drug–Target–Pathway Network
4.7. Molecular Docking Validation of Chicoric Acid with Targets
4.8. Molecular Dynamics Simulation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Margine, I.; Krammer, F. Animal models for influenza viruses: Implications for universal vaccine development. Pathogens 2014, 3, 845–874. [Google Scholar] [CrossRef]
- Glezen, W.P. Emerging infections: Pandemic influenza. Epidemiol. Rev. 1996, 18, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Yuan, Q.; Chen, Y. Study on extraction and purification process of cichoric acid in Echinacea purpurea. Chin. Tradit. Herb. Drugs 2004, 9, 995–997. [Google Scholar]
- Hua, C.; Li, J.; Zhou, F.; Zhou, Q.; Zhang, B.; Wang, R.; Zhao, M. Process Optimization for Cichoric Acid Extraction from Cichorium lntybus L. Shoots. Food Sci. 2011, 32, 126–129. [Google Scholar]
- Huang, Q.; Feng, H.; Li, M.; Qiu, F.; Jiang, Z. Determination of Cichoric Acid in Dandelion Extract from Different Areas. Mod. Food 2021, 14, 177−178+186. [Google Scholar]
- Zhang, X.; Shi, B.; Li, Y.; Li, Y.; Ye, W. Chemical constituents in aerial parts of Pulsatilla chinensis. Chin. Tradit. Herb. Drugs 2008, 5, 651–653. [Google Scholar]
- Wang, H.; Liu, W.; Lu, X.; Chen, S.; Ai, T. Determination of Cichoric Acid in Echinacea purpuea. China J. Chin. Mater. Med. 2002, 27, 418–420. [Google Scholar]
- Xiao, H.; Yang, S.; Wang, X.; Wang, J.; Song, Y. Effect of Chicoric Acid on Oxidative Protein Damage. Food Sci. 2018, 39, 119–124. [Google Scholar]
- Meng, C.; Fu, H.; Zhou, L.; lei, Y.; Wang, Y. Studies on the Antioxidative Activity and Inhibitory Kinetics on Tyrosinase-catalyzing Reaction of Cichoric Acid. J. Chin. Inst. Food Sci. Technol. 2017, 17, 53–59. [Google Scholar]
- Wang, Y.; Gao, S.; Li, L.; Zhang, L.; Sun, Y.; Bo, F. Research advances on bioactivity and pharmacological effects of chicoric acid. Chin. J. New Drug 2020, 29, 1729–1733. [Google Scholar]
- Wu, C.; Li, J.; Cong, X.; Gao, Y.; Yu, F.; Lv, Y.; Yang, M.; Li, J.; Feng, S.; Li, F. Antiviral and Antibacterial Effect of Chicoric Acid: A Review. Chin. J. Exp. Tradit. Med. Formulae 2022, 28, 220–228. [Google Scholar]
- Healy, E.F.; Sanders, J.; King, P.J.; Robinson, W.E., Jr. A docking study of L-chicoric acid with HIV-1 integrase. J. Mol. Graph. Model. 2009, 27, 584–589. [Google Scholar] [CrossRef]
- Nobela, O.; Renslow, R.S.; Thomas, D.G.; Colby, S.M.; Sitha, S.; Njobeh, P.B.; du Preez, L.; Tugizimana, F.; Madala, N.E. Efficient discrimination of natural stereoisomers of chicoric acid, an HIV-1 integrase inhibitor. J. Photochem. Photobiol. B Biol. 2018, 189, 258–266. [Google Scholar] [CrossRef]
- Verma, D.; Mitra, D.; Paul, M.; Chaudhary, P.; Kamboj, A.; Thatoi, H.; Janmeda, P.; Jain, D.; Panneerselvam, P.; Shrivastav, R.; et al. Potential inhibitors of SARS-CoV-2 (COVID 19) proteases PLpro and Mpro/3CLpro: Molecular docking and simulation studies of three pertinent medicinal plant natural components. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100038. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, T.M.; Kwaśniewski, M.; Ożarowski, M. In silico studies of selected xanthophylls as potential candidates against SARS-CoV-2 targeting main protease (Mpro) and papain-like protease (PLpro). Herba Pol. 2021, 67, 1–8. [Google Scholar] [CrossRef]
- Abd El-Aziz, N.M.; Khalifa, I.; Darwish, A.M.; Badr, A.N.; Aljumayi, H.; Hafez, E.-S.; Shehata, M.G. Docking analysis of some bioactive compounds from traditional plants against SARS-CoV-2 target proteins. Molecules 2022, 27, 2662. [Google Scholar] [CrossRef] [PubMed]
- Šilhár, P.; Čapková, K.; Salzameda, N.T.; Barbieri, J.T.; Hixon, M.S.; Janda, K.D. Botulinum neurotoxin A protease: Discovery of natural product exosite inhibitors. J. Am. Chem. Soc. 2010, 132, 2868–2869. [Google Scholar] [CrossRef]
- Meeprasert, A.; Rungrotmongkol, T.; Suan Li, M.; Hannongbua, S. In silico screening for potent inhibitors against the NS3/4A protease of hepatitis C virus. Curr. Pharm. Des. 2014, 20, 3465–3477. [Google Scholar] [CrossRef]
- Guo, Y.; Hui, C.; Xiong, W.; Zhang, W. Advances in antiviral therapy targeting to hepatitis C virus NS3/4A protease. Chin. J. Clin. Infect. Dis. 2014, 7, 89–93. [Google Scholar]
- Huang, C.; Niu, Y.; Nan, Y.; Yuan, L.; Dou, H.L.; Jiang, W.J. Exploring the Mechanism of Cepharanthine in the Treatment of COVID-19 Based on Network Pharmacology and Molecular Docking Technology. J. Ningxia Med. Univ. 2023, 45, 531–540. [Google Scholar]
- Jin, Y.; Zu, M.; Hong, Y.; Li, L. Research on the mechanism of anti-influenza effect of Moslae Herba based on network pharmacology. Mil. Med. Sci. 2022, 46, 691–698. [Google Scholar]
- Li, Z.; Qian, R.; Wang, Y. Study on mechanism of modified Fuzilizhong decoction in treating bovine diarrhea based on network pharmacology. Heilongjiang Anim. Sci. Vet. Med. 2021, 15, 75−84+153. [Google Scholar]
- Xu, X.; Dong, Y.; Liu, M.; Li, Y.; Zhang, Y. Clinical significance of UbA52 level in the urine of patients with type 2 diabetes mellitus and diabetic kidney disease. Nefrología 2021, 41, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Oshima, S.; Maeyashiki, C.; Nibe, Y.; Otsubo, K.; Matsuzawa, Y.; Nemoto, Y.; Nagaishi, T.; Okamoto, R.; Tsuchiya, K.; et al. The ubiquitin hybrid gene UBA52 regulates ubiquitination of ribosome and sustains embryonic development. Sci. Rep. 2016, 6, 36780. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Q.; Liu, T.; Chang, G.; Sun, Z.; Gao, Z.; Wang, F.; Zhou, H.; Liu, R.; Zheng, M.; et al. Host interaction analysis of PA-N155 and PA-N182 in chicken cells reveals an essential role of UBA52 for replication of H5N1 avian influenza virus. Front. Microbiol. 2018, 9, 936. [Google Scholar] [CrossRef]
- Crinelli, R.; Bianchi, M.; Radici, L.; Carloni, E.; Giacomini, E.; Magnani, M. Molecular dissection of the human ubiquitin C promoter reveals heat shock element architectures with activating and repressive functions. PLoS ONE 2015, 10, e0136882. [Google Scholar] [CrossRef]
- Vihervaara, A.; Sergelius, C.; Vasara, J.; Blom, M.A.; Elsing, A.N.; Roos-Mattjus, P.; Sistonen, L. Transcriptional response to stress in the dynamic chromatin environment of cycling and mitotic cells. Proc. Natl. Acad. Sci. USA 2013, 110, E3388–E3397. [Google Scholar] [CrossRef]
- Bianchi, M.; Crinelli, R.; Arbore, V.; Magnani, M. Induction of ubiquitin C (UBC) gene transcription is mediated by HSF 1: Role of proteotoxic and oxidative stress. FEBS Open Bio 2018, 8, 1471–1485. [Google Scholar] [CrossRef]
- Kong, X. To Study the Mechanism of Hematopoietivvell Kinase (HCK) Regulate NLRP3INFLAMMASOME Activation. Master’s Thesis, LanZhou University, Lanzhou, China, 2017. [Google Scholar]
- Luo, S. Construction of Kinases Gene Co-Expression Network in Glioma Andregulation of Tumor-Associated Macrophages by Identifiedimmune-Associated Kinase Gene HCK. Master’s Thesis, China Medical University, Shenyang, China, 2023. [Google Scholar]
- Zhang, C.; Qu, X.H. Effects of Hck Gene on Proliferation and Invasion of Lung Adenocarcinoma Cells via EGFR Pathway. China Contin. Med. Educ. 2020, 12, 162–166. [Google Scholar]
- Roskoski, R., Jr. Src protein-tyrosine kinase structure, mechanism, and small molecule inhibitors. Pharmacol. Res. 2015, 94, 9–25. [Google Scholar] [CrossRef]
- Weisberg, E.; Parent, A.; Yang, P.L.; Sattler, M.; Liu, Q.; Liu, Q.; Wang, J.; Meng, C.; Buhrlage, S.J.; Gray, N.; et al. Repurposing of kinase inhibitors for treatment of COVID-19. Pharm. Res. 2020, 37, 167. [Google Scholar] [CrossRef]
- Hershko, D.D.; Shapira, M. Prognostic role of p27Kip1 deregulation in colorectal cancer. Cancer 2006, 107, 668–675. [Google Scholar] [CrossRef]
- He, W.; Wang, X.; Chen, L.; Guan, X. A crosstalk imbalance between p27kip1 and its interacting molecules enhances breast carcinogenesis. Cancer Biother. Radiopharm. 2012, 27, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, R.; Kan, X.; Zhang, J.; Sun, W.; Goltzman, D.; Miao, D. A Sonic Hedgehog-Gli-Bmi1 signaling pathway plays a critical role in p27 deficiency induced bone anabolism. Int. J. Biol. Sci. 2022, 18, 956–969. [Google Scholar] [CrossRef] [PubMed]
- Park, B.M.; Roh, J.-i.; Lee, J.; Lee, H.-W. Generation of knockout mouse models of cyclin-dependent kinase inhibitors by engineered nuclease-mediated genome editing. Lab. Anim. Res. 2018, 34, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Wurz, K.; Garcia, R.L.; Goff, B.A.; Mitchell, P.S.; Lee, J.H.; Tewari, M.; Swisher, E.M. MiR-221 and MiR-222 alterations in sporadic ovarian carcinoma: Relationship to CDKN1B, CDKNIC and overall survival. Genes Chromosomes Cancer 2010, 49, 577–584. [Google Scholar] [CrossRef]
- Sharma, S.S.; Pledger, W.J. The non-canonical functions of p27Kip1 in normal and tumor biology. Cell Cycle 2016, 15, 1189–1201. [Google Scholar] [CrossRef]
- Tian, Y.-F.; Chen, T.-J.; Lin, C.-Y.; Chen, L.-T.; Lin, L.-C.; Hsing, C.-H.; Lee, S.-W.; Sheu, M.-J.; Lee, H.-H.; Shiue, Y.-L.; et al. SKP2 overexpression is associated with a poor prognosis of rectal cancer treated with chemoradiotherapy and represents a therapeutic target with high potential. Tumor Biol. 2013, 34, 1107–1117. [Google Scholar] [CrossRef]
- Zhong, S.; Li, Y.-G.; Ji, D.-F.; Lin, T.-B.; Lv, Z.-Q. Protocatechualdehyde induces S-phase arrest and apoptosis by stimulating the p27KIP1-Cyclin A/D1-CDK2 and mitochondrial apoptotic pathways in HT-29 cells. Molecules 2016, 21, 934. [Google Scholar] [CrossRef]
- Luo, J.; Zhao, H.; Chen, L.; Liu, M. Multifaceted functions of RPS27a: An unconventional ribosomal protein. J. Cell. Physiol. 2023, 238, 485–497. [Google Scholar] [CrossRef]
- Warner, J.R.; McIntosh, K.B. How common are extraribosomal functions of ribosomal proteins? Mol. Cell 2009, 34, 3–11. [Google Scholar] [CrossRef]
- Wool, I.G. Extraribosomal functions of ribosomal proteins. Trends Biochem. Sci. 1996, 21, 164–165. [Google Scholar] [CrossRef]
- Wang, H.; Yu, J.; Zhang, L.; Xiong, Y.; Chen, S.; Xing, H.; Tian, Z.; Tang, K.; Wei, H.; Rao, Q.; et al. RPS27a promotes proliferation, regulates cell cycle progression and inhibits apoptosis of leukemia cells. Biochem. Biophys. Res. Commun. 2014, 446, 1204–1210. [Google Scholar] [CrossRef]
- Sun, X.-X.; DeVine, T.; Challagundla, K.B.; Dai, M.-S. Interplay between ribosomal protein S27a and MDM2 protein in p53 activation in response to ribosomal stress. J. Biol. Chem. 2011, 286, 22730–22741. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Sharp, M.; Walker, R.; Brammar, W.; Varley, J. Differential expression of translation-associated genes in benign and malignant human breast tumours. Br. J. Cancer 1992, 65, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.; Mafune, K.; Yow, H.; Rivers, E.N.; Ravikumar, T.; Steele, G.D., Jr.; Chen, L.B. Ubiquitin-ribosomal protein S27a gene overexpressed in human colorectal carcinoma is an early growth response gene. Cancer Res. 1993, 53, 1916–1920. [Google Scholar] [PubMed]
- Kanayama, H.; Tanaka, K.; Aki, M.; Kagawa, S.; Miyaji, H.; Satoh, M.; Okada, F.; Sato, S.; Shimbara, N.; Ichihara, A. Changes in expressions of proteasome and ubiquitin genes in human renal cancer cells. Cancer Res. 1991, 51, 6677–6685. [Google Scholar]
- Fatima, G.; Mathan, G.; Kumar, V. The HBx protein of hepatitis B virus regulates the expression, intracellular distribution and functions of ribosomal protein S27a. J. Gen. Virol. 2012, 93, 706–715. [Google Scholar] [CrossRef]
- Contin, R.; Arnoldi, F.; Mano, M.; Burrone, O. Rotavirus replication requires a functional proteasome for effective assembly of viroplasms. J. Virol. 2011, 85, 2781–2792. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, M.; Dou, J.; Wang, X.; Dai, X.; Li, X. Effects of Astragalus membranaceus (Fisch.) Bunge.on Piglets Flora Imbalance Diarrhea Based on Network Pharmacology and Molecular Docking. China Anim. Husb. Vet. Med. 2022, 49, 3200–3211. [Google Scholar]
- Rong, Q.; Liu, J.; Cheng, Y.; Wang, Y.; Gong, L.; Sun, F. Exploring the Molecular Mechanism of Mulberry leaf and Eucommia ulmoides Against Infectious Bursal Disease of Poultry Based on Network Pharmacology. China Anim. Husb. Vet. Med. 2024, 51, 392–406. [Google Scholar]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ouyang, S.; Yu, B.; Liu, Y.; Huang, K.; Gong, J.; Zheng, S.; Li, Z.; Li, H.; Jiang, H. PharmMapper server: A web server for potential drug target identification using pharmacophore mapping approach. Nucleic Acids Res. 2010, 38, W609–W614. [Google Scholar] [CrossRef] [PubMed]
- Abugessaisa, I.; Kasukawa, T. Practical Guide to Life Science Databases; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Online Mendelian Inheritance in Man, OMIM. McKusick-Nathans Institute of Genetic Medicine, Johns Hopkins University, 2020. Huntington Disease; HD. Available online: https://omim.org/ (accessed on 1 May 2024).
- Amberger, J.S.; Bocchini, C.A.; Scott, A.F.; Hamosh, A. OMIM.org: Leveraging knowledge across phenotype–gene relationships. Nucleic Acids Res. 2019, 47, D1038–D1043. [Google Scholar] [CrossRef]
- Chen, H.; Boutros, P.C. VennDiagram: A package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinform. 2011, 12, 35. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Forster, A.; van Lenthe, E.; Spadetto, E.; Visscher, L. Two-component GW calculations: Cubic scaling implementation and comparison of vertex-corrected and partially self-consistent GW variants. J. Chem. Theory Comput. 2023, 19, 5958–5976. [Google Scholar] [CrossRef]
- Berendsen, H.J.; van der Spoel, D.; van Drunen, R. GROMACS: A message-passing parallel molecular dynamics implementation. Comput. Phys. Commun. 1995, 91, 43–56. [Google Scholar] [CrossRef]
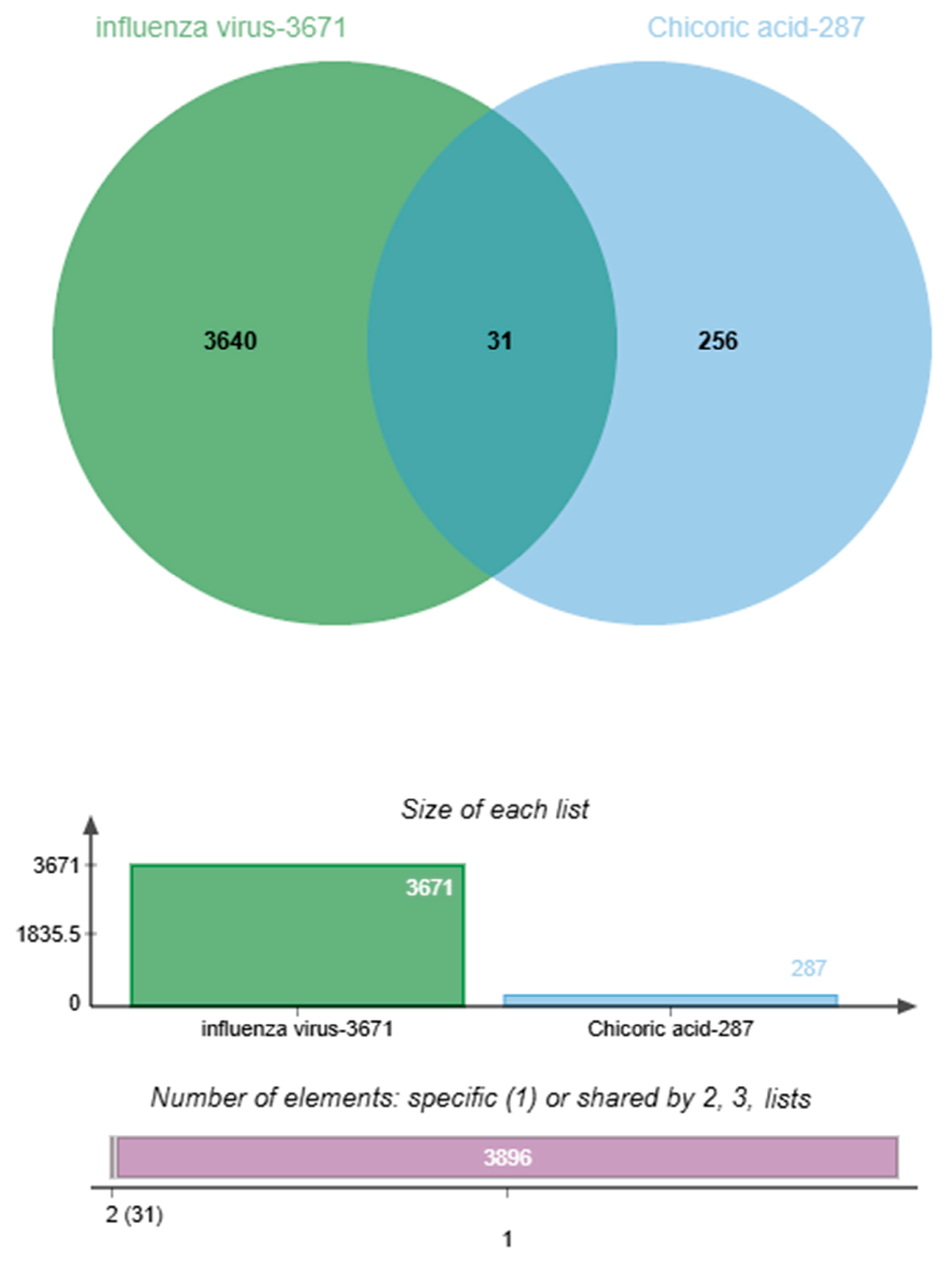
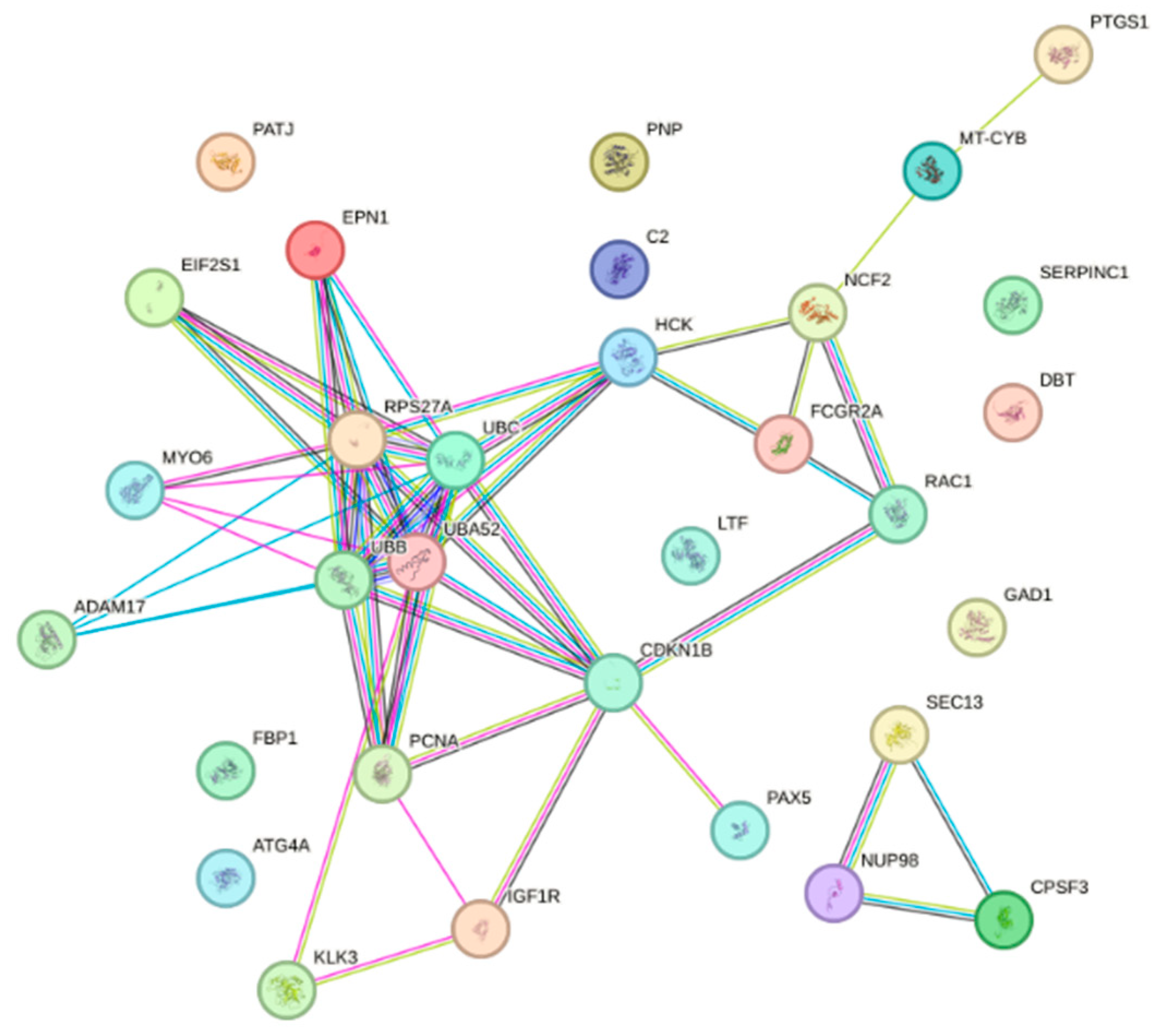

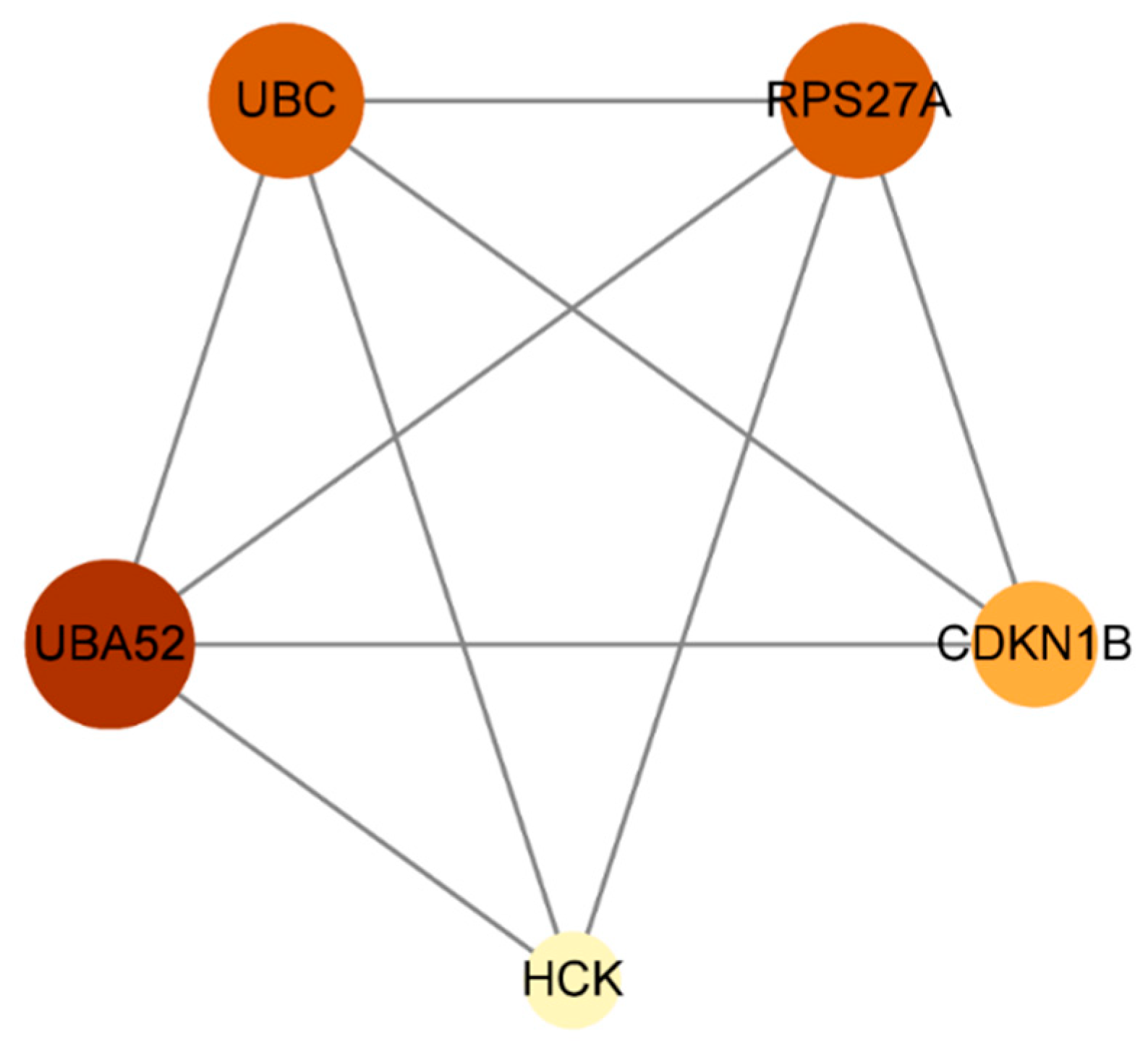

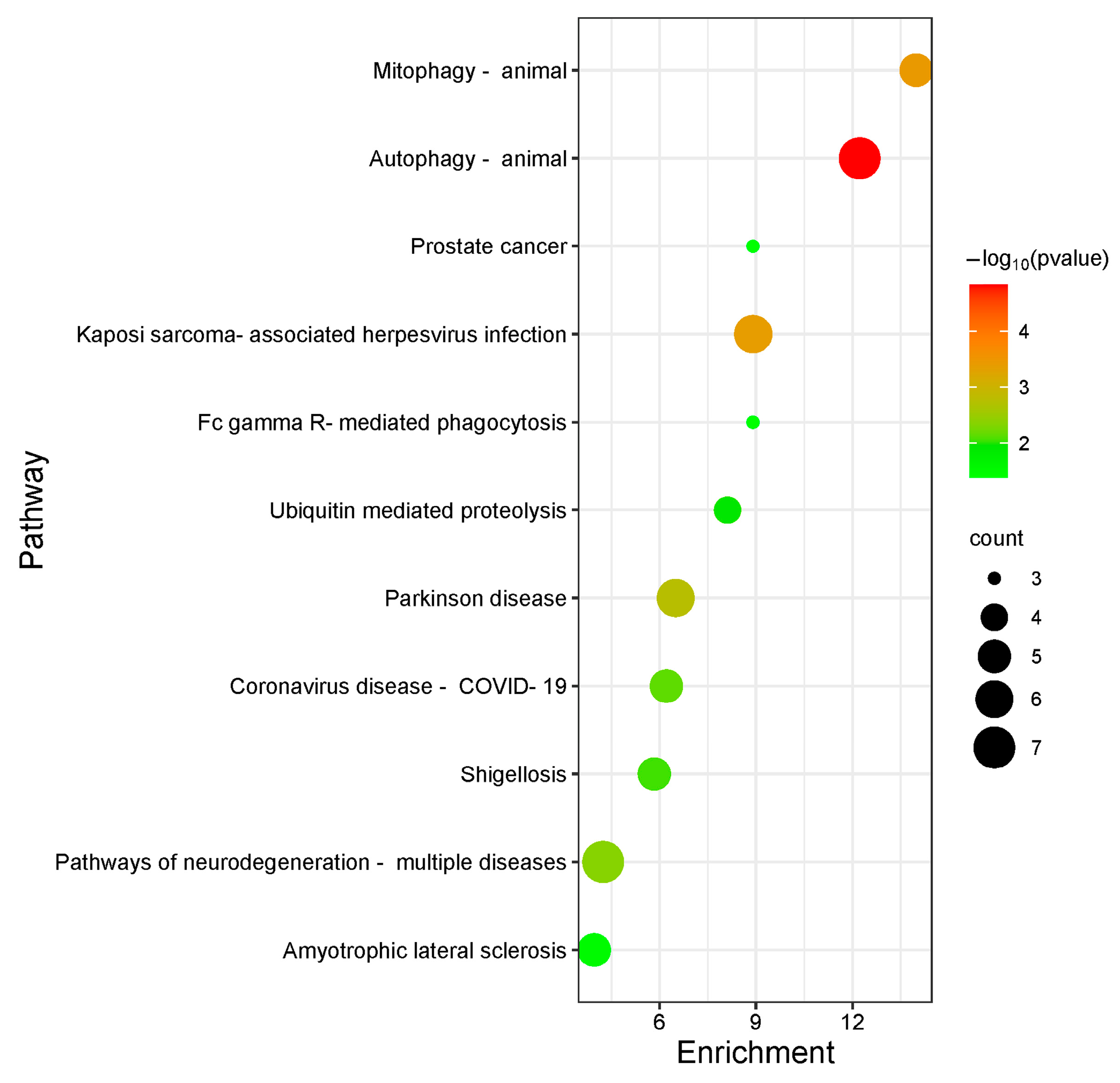



| Serial Number | Uniport ID | Gene | Name |
|---|---|---|---|
| 1 | Q9Y6I3 | EPN1 | Epsin-1 |
| 2 | Q29122 | MYO6 | Unconventional myosin-VI |
| 3 | P52948 | NUP98 | Nuclear pore complex protein Nup98-Nup96 |
| 4 | P12004 | PCNA | Proliferating Cell Nuclear Antigen |
| 5 | P63000 | RAC1 | Rac Family Small GTPase 1 |
| 6 | P02788 | LTF | Lactotransferrin |
| 7 | P55735 | SEC13 | Protein SEC13 homolog |
| 8 | P35997 | RPS27A | Small ribosomal subunit protein eS27A |
| 9 | P62987 | UBA52 | Ubiquitin–ribosomal protein eL40 fusion protein |
| 10 | P0CG47 | UBB | Polyubiquitin-B |
| 11 | P0CG48 | UBC | Polyubiquitin-C |
| 12 | P12318 | FCGR2A | Fc Gamma Receptor IIa |
| 13 | P05979 | PTGS1 | Prostaglandin G/H synthase 1 |
| 14 | Q9UKF6 | CPSF3 | Cleavage And Polyadenylation Specific Factor 3 |
| 15 | P46527 | CDKN1B | Cyclin-Dependent Kinase Inhibitor 1B |
| 16 | P78536 | ADAM17 | ADAM Metallopeptidase Domain 17 |
| 17 | P06681 | C2 | Complement C2 |
| 18 | P19878 | NCF2 | Neutrophil cytosol factor 2 |
| 19 | P00156 | MT-CYB | Cytochrome b |
| 20 | P00491 | PNP | Purine nucleoside phosphorylase |
| 21 | P01008 | SERPINC1 | Antithrombin-III |
| 22 | P08631 | HCK | Tyrosine–protein kinase HCK |
| 23 | P08069 | IGF1R | Insulin-like growth factor 1 receptor |
| 24 | Q02548 | PAX5 | Paired box protein Pax-5 |
| 25 | Q8WYN0 | ATG4A | Cysteine protease ATG4A |
| 26 | P07288 | KLK3 | Prostate-specific antigen |
| 27 | Q99259 | GAD1 | Glutamate decarboxylase 1 |
| 28 | Q8NI35 | PATJ | InaD-like protein |
| 29 | Q5T0N5 | FBP1 | Formin-binding protein 1-like |
| 30 | P05198 | EIF2S1 | Eukaryotic Translation Initiation Factor 2 Subunit Alpha |
| 31 | P11181 | DBT | Lipoamide acyltransferase component of branched-chain alpha-keto acid dehydrogenase complex, mitochondrial |
| Gene | PDB ID | Free Energy of Binding/(kJ/mol) |
|---|---|---|
| UBC | 6ULH | −21.57 |
| HCK | 6Z3F | −20.69 |
| UBA52 | 7OWC | −19.31 |
| RPS27A | 6SQR | −13.69 |
| CDKN1B | 6P8G | −12.46 |
| Complex | ΔEvdw | ΔEele | ΔEpol | ΔEnonpol | ΔEMMPBSA | −TΔS | ΔGbind * |
|---|---|---|---|---|---|---|---|
| (a) | |||||||
| Protein–Ligand | −181.42 ± 2.57 | −72.79 ± 8.44 | 233.84 ± 10.26 | −25.33 ± 0.13 | −45.70 ± 1.28 | 27.76 ± 1.34 | −17.94 ± 2.57 |
| (b) | |||||||
| Protein–Ligand | −105.75 ± 3.19 | −99.40 ± 5.31 | 213.29 ± 4.44 | −19.17 ± 0.25 | −11.03 ± 5.74 | 33.40 ± 3.58 | 22.37 ± 4.23 |
| (c) | |||||||
| Protein–Ligand | −159.84 ± 2.73 | −18.89 ± 7.83 | 60.75 ± 8.12 | −21.47 ± 0.09 | −139.44 ± 2.56 | 19.92 ± 2.25 | −119.52 ± 1.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, W.; Ye, F.; Hou, Z.; Pang, Q. Exploring the Mechanism of Action of Chicoric Acid Against Influenza Virus Infection Based on Network Pharmacology, Molecular Docking, and Molecular Dynamics Simulation. Int. J. Mol. Sci. 2025, 26, 10884. https://doi.org/10.3390/ijms262210884
Guo W, Ye F, Hou Z, Pang Q. Exploring the Mechanism of Action of Chicoric Acid Against Influenza Virus Infection Based on Network Pharmacology, Molecular Docking, and Molecular Dynamics Simulation. International Journal of Molecular Sciences. 2025; 26(22):10884. https://doi.org/10.3390/ijms262210884
Chicago/Turabian StyleGuo, Weijun, Fuhao Ye, Zengyao Hou, and Quanhai Pang. 2025. "Exploring the Mechanism of Action of Chicoric Acid Against Influenza Virus Infection Based on Network Pharmacology, Molecular Docking, and Molecular Dynamics Simulation" International Journal of Molecular Sciences 26, no. 22: 10884. https://doi.org/10.3390/ijms262210884
APA StyleGuo, W., Ye, F., Hou, Z., & Pang, Q. (2025). Exploring the Mechanism of Action of Chicoric Acid Against Influenza Virus Infection Based on Network Pharmacology, Molecular Docking, and Molecular Dynamics Simulation. International Journal of Molecular Sciences, 26(22), 10884. https://doi.org/10.3390/ijms262210884





