Medicinal Plants for a Healthy Gut Microbiome: Scientific Insights into Modern Herbal Applications
Abstract
1. Introduction
2. The Healthy Gut Microbiome
3. Microbiome Imbalance and Disease
4. Common Medicinal Plants with Documented Microbiome Effects
4.1. Globe Artichoke (Cynara scolymus L.)
4.2. Aloe vera (Aloe vera (L.) Burm. f.)
4.3. German Chamomile (Matricaria chamomilla L.)
4.4. Pot Marigold (Calendula officinalis L.)
4.5. Ceylon Cinnamon (Cinnamomum verum J. Presl)
4.6. Dandelion (Taraxacum officinale F.H. Wigg.)
4.7. Fennel (Foeniculum vulgare Mill.)
4.8. Garlic (Allium sativum L.)
4.9. Ginger (Zingiber officinale Roscoe)
4.10. Green Tea (Camellia sinensis (L.) Kuntze)
4.11. Summary
5. Limitations and Considerations
6. Materials and Methods
7. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rithi, A.T.; Mitra, A.; Banerjee, A.; Ilanchoorian, D.; Radhakrishnan, A.K. Current Understanding of Gut Microbiome Alterations and Therapeutic Approaches for Improving Human Health. Int. J. Exp. Res. Rev. 2023, 36, 253–264. [Google Scholar]
- Bonde, A.; Daly, S.; Kirsten, J.; Kondapaneni, S.; Mellnick, V.; Menias, C.O.; Katabathina, V.S. Human gut microbiota–associated gastrointestinal malignancies: A comprehensive review. Radiographics 2021, 41, 1103–1122. [Google Scholar] [CrossRef]
- Steinert, R.E.; Rehman, A.; Sadabad, M.S.; Wittwer-Schegg, J.; Burton, J.P.; Spooren, A. Microbial micronutrient sharing, gut redox balance and keystone taxa as a basis for a new perspective to solutions targeting health from the gut. Gut Microbes 2025, 17, 2477816. [Google Scholar] [CrossRef]
- Buonocore, G. Microbiota and gut immunity in infants and young children. Glob. Pediatr. 2024, 9, 100202. [Google Scholar] [CrossRef]
- Kelly, D.; King, T.; Aminov, R. Importance of microbial colonization of the gut in early life to the development of immunity. Mutat. Res. 2007, 622, 58–69. [Google Scholar] [CrossRef]
- Bock, P.M.; Martins, A.F.; Schaan, B.D. Understanding how pre- and probiotics affect the gut microbiome and metabolic health. Am. J. Physiol. Endocrinol. Metab. 2024, 2024, E89–E102. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.L.; Chen, Y.; Shibo, H.; McDonough, C.M.; Huang, G. Gut microbiome in neuroendocrine and neuroimmune interactions: The case of genistein. Toxicol. Appl. Pharmacol. 2020, 402, 115130. [Google Scholar] [CrossRef]
- Kim, A. Dysbiosis: A review highlighting obesity and inflammatory bowel disease. J. Clin. Gastroenterol. 2015, 49, 20–24. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. An updated overview on the relationship between human gut microbiome dysbiosis and psychiatric and psychological disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2024, 128, 110861. [Google Scholar] [CrossRef]
- Mallick, K.; Khodve, G.; Ruwatia, R.; Banerjee, S. Gut microbes: Therapeutic target for neuropsychiatric disorders. J. Psychiatr. Res. 2025, 184, 27–38. [Google Scholar] [CrossRef]
- Bhattarai, S.; Janaswamy, S. The nexus of gut microbiota, diet, and health. Funct. Food Sci. 2022, 2, 47–63. [Google Scholar] [CrossRef]
- Peredo-Lovillo, A.; Romero-Luna, H.E.; Jiménez-Fernández, M. Health promoting microbial metabolites produced by gut microbiota after prebiotics metabolism. Food Res. Int. 2020, 136, 109473. [Google Scholar] [CrossRef]
- Blekhman, R.; Goodrich, J.K.; Huang, K.; Sun, Q.; Bukowski, R.; Bell, J.T.; Spector, T.D.; Keinan, A.; Ley, R.E.; Gevers, D.; et al. Host genetic variation impacts microbiome composition across human body sites. Genome Biol. 2015, 16, 191. [Google Scholar] [CrossRef] [PubMed]
- Francella, C.; Green, M.; Caspani, G.; Lai, J.K.Y.; Rilett, K.C.; Foster, J.A. Microbe–immune–stress interactions impact behaviour during postnatal development. Int. J. Mol. Sci. 2022, 23, 15064. [Google Scholar] [CrossRef]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Lakshminarayanan, B.; Stanton, C.; O’Toole, P.W.; Ross, R.P. Compositional dynamics of the human intestinal microbiota with aging: Implications for health. J. Nutr. Health Aging 2014, 18, 773–786. [Google Scholar] [CrossRef]
- Ashman, S.; Krishnamurthy, H. The gut microbiome. In Effects of Lifestyle on Men’s Health; Elsevier: Amsterdam, The Netherlands, 2019; pp. 61–98. ISBN 9780128166659. [Google Scholar]
- Yin, R.; Kuo, H.-C.; Hudlikar, R.; Sargsyan, D.; Li, S.; Wang, L.; Wu, R. Gut microbiota, dietary phytochemicals, and benefits to human health. Curr. Pharmacol. Rep. 2019, 5, 332–344. [Google Scholar] [CrossRef]
- Suchodolski, J.S.; Jergens, A.E. Recent advances and understanding of using probiotic-based interventions to restore homeostasis of the microbiome for the prevention/therapy of bacterial diseases. Microbiol. Spectr. 2016, 4, 823–841. [Google Scholar] [CrossRef]
- Jabborova, D.; Davranov, K.; Egamberdieva, D. Microorganisms for sustainability. In Medically Important Plant Biomes: Source of Secondary Metabolites; Springer Nature Singapore Pte Ltd.: Singapore, 2019; Volume 15, pp. 51–66. [Google Scholar]
- Li, X.; Liu, Y.; Liu, N.; Wu, H.; Cong, K.; Duan, L.; Chen, T.; Zhang, J. Health benefits of medicinal plant natural products via microbiota-mediated different gut axes. Pharmacol. Res. 2025, 215, 107730. [Google Scholar] [CrossRef]
- Jangra, B.; Kulshreshtha, S.; Goyal, A.; Jachak, S.M. Phytomedicine plus the role of gut microbiota in disease management: Ayurvedic perspectives on metabolic diseases and health. Phytomedicine Plus 2025, 5, 100731. [Google Scholar] [CrossRef]
- Pi, Y.; Fang, M.; Li, Y.; Cai, L.; Han, R.; Sun, W.; Jiang, X.; Chen, L.; Du, J.; Zhu, Z.; et al. Interactions between gut microbiota and natural bioactive polysaccharides in metabolic diseases: Review. Nutrients 2024, 16, 2838. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The healthy human microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef]
- Bäckhed, F.; Fraser, C.M.; Ringel, Y.; Sanders, M.E.; Sartor, R.B.; Sherman, P.M.; Versalovic, J.; Young, V.; Finlay, B.B. Defining a healthy human gut microbiome: Current concepts, future directions, and clinical applications. Cell Host Microbe 2012, 12, 611–622. [Google Scholar] [CrossRef]
- Foster, K.R.; Schluter, J.; Coyte, K.Z.; Rakoff-Nahoum, S. The evolution of the host microbiome as an ecosystem on a leash. Nature 2017, 548, 43–51. [Google Scholar] [CrossRef] [PubMed]
- McBurney, M.I.; Davis, C.; Fraser, C.M.; Schneeman, B.O.; Huttenhower, C.; Verbeke, K.; Walter, J.; Latulippe, M.E. Establishing what constitutes a healthy human gut microbiome: State of the science, regulatory considerations, and future directions. J. Nutr. 2019, 149, 1882–1895. [Google Scholar] [CrossRef]
- Politi, C.; Mobrici, M.; Parlongo, R.M.; Spoto, B.; Tripepi, G.; Pizzini, P.; Cutrupi, S.; Franco, D.; Tino, R.; Farruggio, G.; et al. Role of gut microbiota in overweight susceptibility in an adult population in Italy. Nutrients 2023, 15, 2834. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef]
- Huttenhower, C.; Gevers, D.; Knight, R.; Abubucker, S.; Badger, J.H.; Chinwalla, A.T.; Creasy, H.H.; Earl, A.M.; Fitzgerald, M.G.; Fulton, R.S.; et al. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- Marcobal, A.; Sonnenburg, J.L. Human milk oligosaccharide consumption by intestinal microbiota. Clin. Microbiol. Infect. 2012, 18, 12–15. [Google Scholar] [CrossRef]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut microbiota, intestinal permeability, and systemic inflammation: A narrative review. Intern. Emerg. Med. 2024, 19, 275–293. [Google Scholar] [CrossRef]
- Wang, G.; Huang, S.; Wang, Y.; Cai, S.; Yu, H.; Liu, H.; Zeng, X.; Zhang, G.; Qiao, S. Bridging intestinal immunity and gut microbiota by metabolites. Cell. Mol. Life Sci. 2019, 76, 3917–3937. [Google Scholar] [CrossRef]
- Lv, J.; Guo, L.; Liu, J.-J.; Zhao, H.-P.; Zhang, J.; Wang, J.-H. Alteration of the esophageal microbiota in Barrett’s esophagus and esophageal adenocarcinoma. World J. Gastroenterol. 2019, 25, 2149–2161. [Google Scholar] [CrossRef] [PubMed]
- Jacky, D.; Bibi, C.; Meng, L.M.C.; Jason, F.; Gwendoline, T.; Jeremy, L.; Wie, C.C. Effects of OsomeFood clean label plant-based meals on the gut microbiome. BMC Microbiol. 2023, 23, 88. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Strukelj, B. The influence of probiotics on the Firmicutes/Bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef] [PubMed]
- King, C.H.; Desai, H.; Sylvetsky, A.C.; LoTempio, J.; Ayanyan, S.; Carrie, J.; Crandall, K.A.; Fochtman, B.C.; Gasparyan, L.; Gulzar, N.; et al. Baseline human gut microbiota profile in healthy people and standard reporting template. PLoS ONE 2019, 14, e0206484. [Google Scholar] [CrossRef]
- Liu, Y.; Lau, H.C.H.; Yu, J. Microbial metabolites in colorectal tumorigenesis and cancer therapy. Gut Microbes 2023, 15, 2203968. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Chelakkot, C.; Choi, Y.; Kim, D.K.; Park, H.T.; Ghim, J.; Kwon, Y.; Jeon, J.; Kim, M.S.; Jee, Y.K.; Gho, Y.S.; et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp. Mol. Med. 2018, 50, e450. [Google Scholar] [CrossRef]
- Hillman, E.T.; Lu, H.; Yao, T.; Nakatsu, C.H. Microbial ecology along the gastrointestinal tract. Microbes Environ. 2017, 32, 300–313. [Google Scholar] [CrossRef]
- Neish, A.S. Microbes in gastrointestinal health and disease. Gastroenterology 2009, 136, 65–80. [Google Scholar] [CrossRef]
- Usuda, H.; Okamoto, T.; Wada, K. Leaky gut: Effect of dietary fiber and fats on microbiome and intestinal barrier. Int. J. Mol. Sci. 2021, 22, 7613. [Google Scholar] [CrossRef]
- Arumugam, P.; Saha, K.; Nighot, P. Intestinal epithelial tight junction barrier regulation by novel pathways. Inflamm. Bowel Dis. 2025, 31, 259–271. [Google Scholar] [CrossRef]
- Allam-Ndoul, B.; Castonguay-Paradis, S.; Veilleux, A. Gut microbiota and intestinal trans-epithelial permeability. Int. J. Mol. Sci. 2020, 21, 6402. [Google Scholar] [CrossRef]
- Elbadawi, M.; Ammar, R.M.; Aziz-Kalbhenn, H.; Rabini, S.; Klauck, S.M.; Dawood, M.; Saeed, M.E.M.; Kampf, C.J.; Efferth, T. Anti-inflammatory and tight junction protective activity of the herbal preparation STW 5-II on mouse intestinal organoids. Phytomedicine 2021, 88, 153589. [Google Scholar] [CrossRef]
- Xiao, H.; Kang, S. The role of the gut microbiome in energy balance with a focus on the gut-adipose tissue axis. Front. Genet. 2020, 11, 297. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.-H.; Sperandio, M.; Di Ciaula, A. Gut microbiota and short chain fatty acids: Implications in glucose homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef]
- Gibson, Y.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Kaur, A.P.; Bhardwaj, S.; Dhanjal, D.S.; Nepovimova, E.; Cruz-Martins, N.; Ku, K.; Chopra, C.; Singh, R.; Kumar, H.; Fatih, S.; et al. Plant prebiotics and their role in the amelioration of diseases. Biomolecules 2021, 11, 440. [Google Scholar] [CrossRef]
- Chaves, P.F.P.; Iacomini, M.; Cordeiro, L.M.C. Chemical characterization of fructooligosaccharides, inulin and structurally diverse polysaccharides from chamomile tea. Carbohydr. Polym. 2019, 214, 269–275. [Google Scholar] [CrossRef]
- Quezada, M.P.; Salinas, C.; Gotteland, M.; Cardemil, L. Acemannan and fructans from Aloe vera (Aloe barbadensis Miller) plants as novel prebiotics. J. Agric. Food Chem. 2017, 65, 10029–10039. [Google Scholar] [CrossRef]
- Martin, B.R.; Braun, M.M.; Wigertz, K.; Bryant, R.; Zhao, Y.; Lee, W.H.; Kempa-Steczko, A.; Weaver, C.M. Fructo-oligosaccharides and calcium absorption and retention in adolescent girls. J. Am. Coll. Nutr. 2010, 29, 382–386. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Steed, H.; Macfarlane, S. Bacterial metabolism and health-related effects of galacto-oligosaccharides and other prebiotics. J. Appl. Microbiol. 2008, 104, 305–344. [Google Scholar] [CrossRef]
- Marín-Manzano, M.C.; Abecia, L.; Hernández-Hernández, O.; Sanz, M.L.; Montilla, A.; Olano, A.; Rubio, L.A.; Moreno, F.J.; Clemente, A. Galacto-oligosaccharides derived from lactulose exert a selective stimulation on the growth of Bifidobacterium animalis in the large intestine of growing rats. J. Agric. Food Chem. 2013, 61, 7560–7567. [Google Scholar] [CrossRef]
- Nordberg Karlsson, E.; Schmitz, E.; Linares-Pastén, J.A.; Adlercreutz, P. Endo-xylanases as tools for production of substituted xylooligosaccharides with prebiotic properties. Appl. Microbiol. Biotechnol. 2018, 102, 9081–9088. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, C.; Shan, Z.; Yin, S.; Wang, Y.; Wang, C.; Liu, T.; Wang, N.; Guo, Q. In vitro fermentability of soybean oligosaccharides from wastewater of tofu production. Polymers 2022, 14, 1704. [Google Scholar] [CrossRef]
- Rini, D.M.; Xu, W.; Suzuki, T. Current research on the role of isomaltooligosaccharides in gastrointestinal health and metabolic diseases. Prev. Nutr. Food Sci. 2024, 29, 93–105. [Google Scholar] [CrossRef]
- Salinas, C.; Handford, M.; Pauly, M.; Dupree, P.; Cardemil, L. Structural modifications of fructans in Aloe barbadensis Miller (Aloe vera) grown under water stress. PLoS ONE 2016, 11, e0159819. [Google Scholar] [CrossRef]
- Zeaiter, Z.; Regonesi, M.E.; Cavini, S.; Labra, M.; Sello, G.; Di Gennaro, P. Extraction and characterization of inulin-type fructans from artichoke wastes and their effect on the growth of intestinal bacteria associated with health. BioMed Res. Int. 2019, 2019, 1083952. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Tankhaeva, L.M.; Rokhin, A.V. Glucofructans from Taraxacum officinale roots. Chem. Nat. Compd. 2009, 45, 125–127. [Google Scholar] [CrossRef]
- Mary, P.R.; Prashanth, K.V.H.; Vasu, P.; Kapoor, M. Structural diversity and prebiotic potential of short chain β-manno-oligosaccharides generated from guar gum by endo-β-mannanase (ManB-1601). Carbohydr. Res. 2019, 486, 107822. [Google Scholar] [CrossRef]
- Slavov, A.; Ognyanov, M.; Vasileva, I. Pectic polysaccharides extracted from pot marigold (Calendula officinalis) industrial waste. Food Hydrocoll. 2019, 101, 105545. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, J.; Wang, L. Role and mechanism of gut microbiota in human disease. Front. Cell. Infect. Microbiol. 2021, 11, 625913. [Google Scholar] [CrossRef] [PubMed]
- Auger, S.; Kropp, C.; Borras-Nogues, E.; Chanput, W.; Andre-Leroux, G.; Gitton-Quent, O.; Benevides, L.; Breyner, N.; Azevedo, V.; Langella, P.; et al. Intraspecific diversity of microbial anti-inflammatory molecule (MAM) from Faecalibacterium prausnitzii. Int. J. Mol. Sci. 2022, 23, 1705. [Google Scholar] [CrossRef]
- Aja, E.; Zeng, A.; Gray, W.; Connelley, K.; Chaganti, A.; Jacobs, J.P. Health effects and therapeutic potential of the gut microbe Akkermansia muciniphila. Nutrients 2025, 17, 562. [Google Scholar] [CrossRef]
- Wang, H.; Huang, X.; Tan, H.; Chen, X.; Chen, C.; Nie, S. Interaction between dietary fiber and bifidobacteria in promoting intestinal health. Food Chem. 2022, 393, 133407. [Google Scholar] [CrossRef]
- Hillman, E.T.; Kozik, A.J.; Hooker, C.A.; Burnett, J.L.; Heo, Y.; Kiesel, V.A.; Nevins, C.J.; Oshiro, J.M.K.I.; Robins, M.M.; Thakkar, R.D.; et al. Comparative genomics of the genus Roseburia reveals divergent biosynthetic pathways that may influence colonic competition among species. Microb. Genom. 2020, 6, 7–24. [Google Scholar] [CrossRef]
- Hays, K.E.; Pfaffinger, J.M.; Ryznar, R. The interplay between gut microbiota, short-chain fatty acids, and implications for host health and disease. Gut Microbes 2024, 16, 2393270. [Google Scholar] [CrossRef]
- Franzosa, E.A.; Sirota-Madi, A.; Avila-Pacheco, J.; Fornelos, N.; Haiser, H.J.; Reinker, S.; Vatanen, T.; Hall, A.B.; Mallick, H.; McIver, L.J.; et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat. Microbiol. 2019, 4, 293–305, Correction in Nat. Microbiol. 2019, 4, 898. [Google Scholar] [CrossRef]
- Abdolmaleky, H.M.; Zhou, J.-R. Gut microbiota dysbiosis, oxidative stress, inflammation, and epigenetic alterations in metabolic diseases. Antioxidants 2024, 13, 985. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Ann, C.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 6145. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Tan, J.; Taitz, J.; Nanan, R.; Grau, G.; Macia, L. Dysbiotic gut microbiota-derived metabolites and their role in non-communicable diseases. Int. J. Mol. Sci. 2023, 24, 15256. [Google Scholar] [CrossRef]
- Sun, X.; Pei, Z.; Wang, H.; Zhao, J.; Chen, W.; Lu, W. Bridging dietary polysaccharides and gut microbiome: How to achieve precision modulation for gut health promotion. Microbiol. Res. 2025, 292, 128046. [Google Scholar] [CrossRef] [PubMed]
- Elce, A.; Amato, F.; Zarrilli, F.; Calignano, A.; Troncone, R.; Castaldo, G.; Canani, R.B. Butyrate modulating effects on pro-inflammatory pathways in human intestinal epithelial cells. Benef. Microbes 2017, 8, 841–847. [Google Scholar] [CrossRef]
- McBride, D.A.; Dorn, N.C.; Yao, M.; Johnson, W.T.; Wang, W.; Bottini, N.; Shah, N.J. Short chain fatty acid mediated epigenetic modulation of inflammatory T cells in vitro. Drug Deliv. Transl. Res. 2023, 13, 1912–1924. [Google Scholar] [CrossRef]
- Harbison, J.E.; Roth-Schulze, A.J.; Giles, L.C.; Tran, C.D.; Ngui, K.M.; Penno, M.A.; Thomson, R.L.; Wentworth, J.M.; Colman, P.G.; Craig, M.E.; et al. Gut microbiome dysbiosis and increased intestinal permeability in children with islet autoimmunity and type 1 diabetes: A prospective cohort study. Pediatr. Diabetes 2019, 20, 574–583. [Google Scholar] [CrossRef]
- Shu, L.-Z.; Ding, Y.-D.; Xue, Q.-M.; Cai, W.; Deng, H. Direct and indirect effects of pathogenic bacteria on the integrity of intestinal barrier. Ther. Adv. Gastroenterol. 2023, 16, 17562848231176427. [Google Scholar] [CrossRef]
- Neurath, M.F.; Artis, D.; Becker, C. The intestinal barrier: A pivotal role in health, inflammation, and cancer. Lancet Gastroenterol. Hepatol. 2025, 10, 573–592. [Google Scholar] [CrossRef]
- Hong, M.; Han, D.H.; Hong, J.; Kim, D.J.; Suk, K.T. Are probiotics effective in targeting alcoholic liver diseases? Probiotics Antimicrob. Proteins 2019, 11, 335–347. [Google Scholar] [CrossRef]
- Potrykus, M.; Czaja-Stolc, S.; Stankiewicz, M.; Kaska, Ł.; Małgorzewicz, S. Intestinal microbiota as a contributor to chronic inflammation and its potential modifications. Nutrients 2021, 13, 3839. [Google Scholar] [CrossRef]
- Dumitru, A.; Tocia, C.; Bădescu, A.-C.; Trandafir, A.; Alexandrescu, L.; Popescu, R.; Dumitru, E.; Chisoi, A.; Manea, M.; Matei, E.; et al. Linking gut permeability to liver steatosis: Prospective cross-sectional study. Medicine 2023, 104, e42476. [Google Scholar] [CrossRef]
- Barreau, F.; Hugot, J.P. Intestinal barrier dysfunction triggered by invasive bacteria. Curr. Opin. Microbiol. 2014, 17, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.H. Basic and translational understandings of microbial recognition by toll-like receptors in the intestine. J. Neurogastroenterol. Motil. 2011, 17, 28–34. [Google Scholar] [CrossRef]
- Robinson, J.A.; Moehle, K. Structural aspects of molecular recognition in the immune system. Part II: Pattern recognition receptors. Pure Appl. Chem. 2014, 86, 1483–1538. [Google Scholar] [CrossRef]
- Iida, H.; Tohno, M.; Md Islam, A.; Sato, N.; Kobayashi, H.; Albarracin, L.; Kober, A.H.; Ikeda-Ohtsubo, W.; Suda, Y.; Aso, H.; et al. Paraimmunobiotic Bifidobacteria modulate the expression patterns of peptidoglycan recognition proteins in porcine intestinal epitheliocytes and antigen presenting cells. Cells 2019, 8, 891. [Google Scholar] [CrossRef]
- Chen, R.; Zou, J.; Chen, J.; Zhong, X.; Kang, R.; Tang, D. Pattern recognition receptors: Function, regulation and therapeutic potential. Signal Transduct. Target. Ther. 2025, 10, 216. [Google Scholar] [CrossRef]
- Zhong, J.; Kyriakis, J.M. Dissection of a signaling pathway by which pathogen-associated molecular patterns recruit the JNK and P38 MAPKs and trigger cytokine release. J. Biol. Chem. 2007, 282, 24246–24254. [Google Scholar] [CrossRef]
- Blanc, L.; Castanier, R.; Mishra, A.K.; Ray, A.; Besra, G.S.; Sutcliffe, I.; Vercellone, A.; Nigou, J. Gram-positive bacterial lipoglycans based on a glycosylated diacylglycerol lipid anchor are microbe-associated molecular patterns recognized by TLR2. PLoS ONE 2013, 8, e81593. [Google Scholar] [CrossRef]
- Moriyama, K.; Nishida, O. Targeting cytokines, pathogen-associated molecular patterns, and damage-associated molecular patterns in sepsis via blood purification. Int. J. Mol. Sci. 2021, 22, 8882. [Google Scholar] [CrossRef]
- Vitetta, L.; Oldfield, D.; Sali, A. Inflammatory bowel diseases and the efficacy of probiotics as functional foods. Front. Biosci. 2024, 16, 13. [Google Scholar] [CrossRef]
- Bajaj, A.; Markandey, M.; Kedia, S.; Ahuja, V. Gut bacteriome in inflammatory bowel disease: An update on recent advances. Indian J. Gastroenterol. 2024, 43, 103–111. [Google Scholar] [CrossRef]
- Chamorro, N.; Montero, D.A.; Gallardo, P.; Farfán, M.; Contreras, M.; la Fuente, M.D.; Dubois, K.; Hermoso, M.A.; Quera, R.; Pizarro-Guajardo, M.; et al. Landscapes and bacterial signatures of mucosa-associated intestinal microbiota in Chilean and Spanish patients with inflammatory bowel disease. Microb. Cell 2021, 8, 223–238. [Google Scholar] [CrossRef]
- Fava, F.; Danese, S. Intestinal microbiota in inflammatory bowel disease: Friend or foe? World J. Gastroenterol. 2011, 17, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, M.; Fasulo, E.; Ungaro, F.; Massimino, L.; Sinagra, E.; Danese, S.; Mandarino, F.V. Gut dysbiosis in irritable bowel syndrome: A narrative review on correlation with disease subtypes and novel therapeutic implications. Microorganisms 2023, 11, 2369. [Google Scholar] [CrossRef]
- Zhao, Y.; Zou, D.W. Gut microbiota and irritable bowel syndrome. J. Dig. Dis. 2023, 24, 312–320. [Google Scholar] [CrossRef]
- Phan, J.; Nair, D.; Jain, S.; Montagne, T.; Flores, V.; Nguyen, A.; Dietsche, S.; Gombur, S.; Cotter, P. Alterations in gut microbiome composition and function in irritable bowel syndrome and increased probiotic abundance. mSystems 2021, 6, e01215-21. [Google Scholar] [CrossRef]
- Aggeletopoulou, I.; Triantos, C. Microbiome shifts and their impact on gut physiology in irritable bowel syndrome. Int. J. Mol. Sci. 2024, 25, 12395. [Google Scholar] [CrossRef]
- Lee, K.N.; Lee, O.Y. Intestinal microbiota in pathophysiology and management of irritable bowel syndrome. World J. Gastroenterol. 2014, 20, 8886–8897. [Google Scholar] [CrossRef]
- Collins, S.M. The intestinal microbiota in the irritable bowel syndrome. In Gut Microbiome and Behavior; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 131, pp. 247–261. [Google Scholar]
- Mazidi, M.; Rezaie, P.; Pascal, A.; Ghayour, M.; Ferns, G.A. Gut microbiome and metabolic syndrome. Diabetes Metab. Syndr. Clin. Res. Rev. 2016, 10, S150–S157. [Google Scholar] [CrossRef]
- Pillai, S.S.; Gagnon, C.A.; Foster, C.; Ashraf, A.P. Exploring the gut microbiota: Key insights into its role in obesity, metabolic syndrome, and type 2 diabetes. J. Clin. Endocrinol. Metab. 2024, 109, 2709–2719. [Google Scholar] [CrossRef]
- Rajani, C.; Jia, W. Disruptions in gut microbial-host co-metabolism and the development of metabolic disorders. Clin. Sci. 2018, 132, 791–811. [Google Scholar] [CrossRef]
- Buchynskyi, M.; Kamyshna, I.; Halabitska, I.; Petakh, P.; Kunduzova, O.; Oksenych, V.; Kamyshnyi, O. Unlocking the gut-liver axis: Microbial contributions to the pathogenesis of metabolic-associated fatty liver disease. Front. Microbiol. 2025, 16, 1577724. [Google Scholar] [CrossRef]
- Lin, Y.; Bai, M.; Wang, S.; Chen, L.; Li, Z.; Li, C.; Cao, P.; Chen, Y. Lactate is a key mediator that links obesity to insulin resistance via modulating cytokine production from adipose tissue. Diabetes 2022, 71, 637–652. [Google Scholar] [CrossRef]
- AlAbduljader, H.; AlSaeed, H.; Alrabeea, A.; Sulaiman, A.; Haider, M.J.A.; Al-Mulla, F.; Ahmad, R.; Al-Rashed, F. Eicosapentaenoic acid (EPA) alleviates LPS-induced oxidative stress via the PPARα–NF-κB axis. Oxidative Med. Cell. Longev. 2025, 2025, 3509596. [Google Scholar] [CrossRef]
- Rorato, R.; Borges, B.D.C.; Uchoa, E.T.; Antunes-Rodrigues, J.; Elias, C.F.; Elias, L.L.K. LPS-induced low-grade inflammation increases hypothalamic JNK expression and causes central insulin resistance irrespective of body weight changes. Int. J. Mol. Sci. 2017, 18, 1431. [Google Scholar] [CrossRef]
- Chiang, J.Y.L.; Ferrell, J.M. Bile acid metabolism in liver pathobiology. Gene Expr. 2018, 18, 71–87. [Google Scholar] [CrossRef]
- Zhao, M.; Zhao, J.; Yang, H.; Ouyang, Z.; Lv, C.; Geng, Z.; Zhao, J. The bile acid-gut microbiota axis: A central hub for physiological regulation and a novel therapeutic target for metabolic diseases. Biomed. Pharmacother. 2025, 188, 118182. [Google Scholar] [CrossRef]
- Dong, H.; Liu, X.; Song, G.; Peng, W.; Sun, X.; Fang, W.; Qi, W. Imbalance of bile acids metabolism mediated by gut microbiota contributed to metabolic disorders in diabetic model mice. Biology 2025, 14, 291. [Google Scholar] [CrossRef]
- Molinero, N.; Ruiz, L.; Sánchez, B.; Margolles, A.; Delgado, S. Intestinal bacteria interplay with bile and cholesterol metabolism: Implications on host physiology. Front. Physiol. 2019, 10, 185. [Google Scholar] [CrossRef]
- Joyce, S.A.; Gahan, G.M. Disease-associated changes in bile acid profiles and links to altered gut microbiota. Digest. Dis. 2017, 35, 169–177. [Google Scholar] [CrossRef]
- Pathak, P.; Xie, C.; Nichols, R.G.; Ferrell, J.M.; Boehme, S.; Krausz, K.W.; Patterson, A.D.; Gonzalez, F.J.; Chiang, J.Y.L. Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatology 2018, 68, 1574–1588. [Google Scholar] [CrossRef]
- Bahitham, W.; Alghamdi, S.; Omer, I.; Alsudais, A.; Hakeem, I.; Alghamdi, A.; Abualnaja, R.; Sanai, F.M.; Rosado, A.S.; Sergi, C.M. Double trouble: How microbiome dysbiosis and mitochondrial dysfunction drive non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Biomedicines 2024, 12, 550. [Google Scholar] [CrossRef]
- Pandey, H.; Goel, P.; Srinivasan, V.M.; Tang, D.W.T.; Wong, S.H.; Lal, D. Gut microbiota in non-alcoholic fatty liver disease: Pathophysiology, diagnosis, and therapeutics. World J. Hepatol. 2025, 17, 106849. [Google Scholar] [CrossRef]
- Jayachandran, M.; Qu, S. Non-alcoholic fatty liver disease and gut microbial dysbiosis: Underlying mechanisms and gut microbiota mediated treatment strategies. Rev. Endocr. Metab. Disord. 2023, 24, 1189–1204. [Google Scholar] [CrossRef]
- Xie, C.; Halegoua-DeMarzio, D. Role of probiotics in non-alcoholic fatty liver disease: Does gut microbiota matter? Nutrients 2019, 11, 2837. [Google Scholar] [CrossRef]
- Vallianou, N.; Christodoulatos, G.S.; Karampela, I.; Tsilingiris, D.; Magkos, F.; Stratigou, T.; Kounatidis, D.; Dalamaga, M. Understanding the role of the gut microbiome and microbial metabolites in non-alcoholic fatty liver disease: Current evidence and perspectives. Biomolecules 2022, 12, 56. [Google Scholar] [CrossRef]
- Kuwahara, A.; Matsuda, K.; Kuwahara, Y.; Asano, S.; Inui, T.; Marunaka, Y. Microbiota-gut-brain axis: Enteroendocrine cells and the enteric nervous system form an interface between the microbiota and the central nervous system. Biomed. Res. 2020, 41, 199–216. [Google Scholar] [CrossRef]
- Dicks, L.M.T. Our mental health is determined by an intrinsic interplay between the central nervous system, enteric nerves, and gut microbiota. Int. J. Mol. Sci. 2024, 25, 38. [Google Scholar] [CrossRef]
- Martin, C.R.; Osadchiy, V.; Kalani, A.; Mayer, E.A. The brain-gut-microbiome axis. Cell Mol. Gastroenterol. Hepatol. 2018, 6, 133–148. [Google Scholar] [CrossRef]
- Hou, Y.; Li, J.; Ying, S. Tryptophan metabolism and gut microbiota: A novel regulatory axis integrating the microbiome, immunity, and cancer. Metabolites 2023, 13, 1166. [Google Scholar] [CrossRef]
- Parrott, J.M.; Redus, L.; O’Connor, J.C. Kynurenine metabolic balance is disrupted in the hippocampus following peripheral lipopolysaccharide challenge. J. Neuroinflamm. 2016, 13, 124. [Google Scholar] [CrossRef] [PubMed]
- Tutakhail, A.; Boulet, L.; Khabil, S.; Nazari, Q.A.; Hamid, H.; Coudoré, F. Neuropathology of kynurenine pathway of tryptophan metabolism. Curr. Pharmacol. Rep. 2020, 6, 8–23. [Google Scholar] [CrossRef]
- Whiley, L.; Chappell, K.E.; Hondt, E.D.; Lewis, M.R.; Jiménez, B.; Snowden, S.G.; Soininen, H.; Iwona, K.; Mecocci, P.; Tsolaki, M.; et al. Metabolic phenotyping reveals a reduction in the bioavailability of serotonin and kynurenine pathway metabolites in both the urine and serum of individuals living with Alzheimer’s disease. Alzheimers Res. Ther. 2021, 13, 20. [Google Scholar] [CrossRef]
- Colle, R.; Masson, P.; Verstuyft, C.; Fève, B.; Werner, E.; Boursier-Neyret, C.; Walther, B.; David, D.J.; Boniface, B.; Falissard, B.; et al. Peripheral tryptophan, serotonin, kynurenine, and their metabolites in major depression: A case–control study. Psychiatry Clin. Neurosci. 2020, 74, 112–117. [Google Scholar] [CrossRef]
- Kearns, R. Gut–brain axis and neuroinflammation: The role of gut permeability and the kynurenine pathway in neurological disorders. Cell Mol. Neurobiol. 2024, 44, 64. [Google Scholar] [CrossRef]
- Sumi, N.; Nishioku, T.; Takata, F.; Matsumoto, J.; Watanabe, T.; Shuto, H.; Yamauchi, A.; Dohgu, S.; Kataoka, Y. Lipopolysaccharide-activated microglia induce dysfunction of the blood–brain barrier in rat microvascular endothelial cells co-cultured with microglia. Cell Mol. Neurobiol. 2010, 30, 247–253. [Google Scholar] [CrossRef]
- Mizobuchi, H.; Soma, G.-I. Low-dose lipopolysaccharide as an immune regulator for homeostasis maintenance in the central nervous system through transformation to neuroprotective microglia. Neural Regen. Res. 2021, 16, 1928–1934. [Google Scholar] [CrossRef]
- Matsumoto, J.; Dohgu, S.; Takata, F.; Nishioku, T.; Sumi, N.; Machida, T.; Takahashi, H.; Yamauchi, A.; Kataoka, Y. Lipopolysaccharide-activated microglia lower P-glycoprotein function in brain microvascular endothelial cells. Neurosci. Lett. 2012, 524, 45–48. [Google Scholar] [CrossRef]
- Wiatrak, B.; Balon, K. Protective activity of Aβ on cell cultures (PC12 and THP-1 after differentiation) preincubated with lipopolysaccharide (LPS). Mol. Neurobiol. 2021, 58, 1453–1464. [Google Scholar] [CrossRef]
- Ioghen, O.C.; Ioghen, M.R.; Popescu, B.O. Neurodegeneration: A tale of microbes and neurons. Mod. Med. 2025, 32, 7–16. [Google Scholar] [CrossRef]
- Kim, S.; Sharma, C.; Jung, U.J.; Kim, S.R. Pathophysiological role of microglial activation induced by blood-borne proteins in Alzheimer’s disease. Biomedicines 2023, 11, 1383. [Google Scholar] [CrossRef]
- Solanki, R.; Karande, A.; Ranganathan, P. Emerging role of gut microbiota dysbiosis in neuroinflammation and neurodegeneration. Front. Neurol. 2023, 14, 1149618. [Google Scholar] [CrossRef]
- Leblhuber, F.; Ehrlich, D.; Steiner, K.; Geisler, S.; Fuchs, D.; Lanser, L.; Kurz, K. The immunopathogenesis of Alzheimer’s disease is related to the composition of gut microbiota. Nutrients 2021, 13, 361. [Google Scholar] [CrossRef]
- Doroszkiewicz, J.; Groblewska, M.; Mroczko, B. The role of gut microbiota and gut–brain interplay in selected diseases of the central nervous system. Int. J. Mol. Sci. 2021, 22, 10028. [Google Scholar] [CrossRef] [PubMed]
- Amato, A.D.; Di, L.; Mannelli, C.; Lucarini, E.; Man, A.L.; Le Gall, G.; Branca, J.J.V.; Ghelardini, C.; Amedei, A.; Bertelli, E.; et al. Faecal microbiota transplant from aged donor mice affects spatial learning and memory via modulating hippocampal synaptic plasticity- and neurotransmission-related proteins in young recipients. Microbiome 2020, 8, 140. [Google Scholar] [CrossRef] [PubMed]
- Abuaish, S.; Al, N.M.; Kawther, O.; Turki, A.; Alzahrani, S.A.; Masoud, S.; Ramesa, A.; Bhat, S.; Arzoo, S.; Algahtani, N.; et al. The efficacy of fecal transplantation and Bifidobacterium supplementation in ameliorating propionic acid-induced behavioral and biochemical autistic features in juvenile male rats. J. Mol. Neurosci. 2022, 72, 372–381. [Google Scholar] [CrossRef]
- Chen, X.; Mo, X.; Zhang, Y.; He, D.; Xiao, R.; Cheng, Q.; Wang, H.; Liu, L.; Li, W.; Xie, P. A comprehensive analysis of the differential expression in the hippocampus of depression induced by gut microbiota compared to traditional stress. Gene 2024, 927, 148633. [Google Scholar] [CrossRef]
- Zhu, F.; Guo, R.; Wang, W.; Ju, Y.; Wang, Q.; Ma, Q.; Sun, Q.; Fan, Y.; Xie, Y.; Yang, Z.; et al. Transplantation of microbiota from drug-free patients with schizophrenia causes schizophrenia-like abnormal behaviors and dysregulated kynurenine metabolism in mice. Mol. Psychiatry 2020, 25, 2905–2918. [Google Scholar] [CrossRef]
- Juárez-Chairez, M.F.; Cid-Gallegos, M.S.; Jiménez-Martínez, C.; Contreras, L.F.P.; De-la-Rosa, J.J.B.-G. The role of microbiota on rheumatoid arthritis onset. Int. J. Rheum. Dis. 2024, 27, e15122. [Google Scholar] [CrossRef]
- Möller, B.; Kollert, F.; Sculean, A.; Villiger, P.M. Infectious triggers in periodontitis and the gut in rheumatoid arthritis: A complex story about association and causality. Front. Immunol. 2020, 11, 1108. [Google Scholar] [CrossRef]
- Scher, J.U.; Bretz, W.A.; Abramson, S.B. Periodontal disease and subgingival microbiota as contributors for rheumatoid arthritis pathogenesis: Modifiable risk factors? Curr. Opin. Rheumatol. 2014, 26, 424–429. [Google Scholar] [CrossRef]
- Fan, H.; Shen, R.; Yan, J.; Bai, Y.; Fu, Q.; Shi, X.; Du, G.; Wang, D. Pyroptosis: The emerging link between gut microbiota and multiple sclerosis. Drug Des. Devel. Ther. 2024, 18, 6145–6164. [Google Scholar] [CrossRef]
- Mirza, A.; Mao-Draayer, Y. The gut microbiome and microbial translocation in multiple sclerosis. Clin. Immunol. 2017, 183, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Francis, A.; Constantinescu, C.S. Gastrointestinal influences in multiple sclerosis: Focus on the role of the microbiome. Clin. Exp. Neuroimmunol. 2018, 9, 2–12. [Google Scholar] [CrossRef]
- Lin, T.-L.; Fan, Y.-H.; Chang, Y.-L.; Ho, H.J.; Wu, C.-Y.; Chen, Y.-J. Early-life infections in association with the development of atopic dermatitis in infancy and early childhood: A nationwide nested case–control study. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 615–662. [Google Scholar] [CrossRef]
- Sbihi, H.; Ct, R.; Chelsea, B.; Mandy, C.; Brett, S.B.; Turvey, S.E. Thinking bigger: How early-life environmental exposures shape the gut microbiome and influence the development of asthma and allergic disease. Allergy 2019, 74, 2103–2115. [Google Scholar] [CrossRef]
- Ng, W.Z.J.; van Hasselt, J.; Aggarwal, B.; Manoharan, A. Association between adult antibiotic use, microbial dysbiosis and atopic conditions—A systematic review. J. Asthma Allergy 2023, 16, 1115–1132. [Google Scholar] [CrossRef]
- Huang, M.-T.; Chiu, C.-J.; Tsai, C.-Y.; Lee, Y.-R.; Liu, W.-L.; Chuang, H.-L.; Huang, M.-T. Short-chain fatty acids ameliorate allergic airway inflammation via sequential induction of PMN-MDSCs and Treg cells. J. Allergy Clin. Immunol. Glob. 2023, 2, 100163. [Google Scholar] [CrossRef]
- Duan, H.; Wang, L.; Huangfu, M.; Li, H. The impact of microbiota-derived short-chain fatty acids on macrophage activities in disease: Mechanisms and therapeutic potentials. Biomed. Pharmacother. 2023, 165, 115276. [Google Scholar] [CrossRef]
- Kespohl, M.; Vachharajani, N.; Luu, M.; Harb, H.; Pautz, S.; Wolff, S.; Sillner, N.; Walker, A.; Schmitt-Kopplin, P.; Boettger, T.; et al. The microbial metabolite butyrate induces expression of Th1-associated factors in CD4+ T cells. Front. Immunol. 2017, 8, 1036. [Google Scholar] [CrossRef]
- Peng, J.; Xiao, X.; Hu, M.; Zhang, X. Interaction between gut microbiome and cardiovascular disease. Life Sci. 2018, 214, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Duttaroy, A.K. Atherosclerosis, hypertension and human blood platelet function: Role of gut microbiota and their metabolites—A review. Nutrients 2021, 13, 144. [Google Scholar] [CrossRef]
- Zhu, J.; Lyu, J.; Zhao, R.; Liu, G.; Wang, S. Gut macrobiotic and its metabolic pathways modulate cardiovascular disease. Front. Microbiol. 2023, 14, 1272479. [Google Scholar] [CrossRef] [PubMed]
- Kondapalli, N.; Katari, V.; Dalal, K.K.; Paruchuri, S.; Thodeti, C.K. Microbiota in gut-heart axis: Metabolites and mechanisms in cardiovascular disease. Compr. Physiol. Rev. 2025, 15, e70024. [Google Scholar] [CrossRef]
- de Oliveira, G.L.V. The gut microbiome in autoimmune diseases. In Microbiome and Metabolome in Diagnosis, Therapy, and Other Strategic Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 325–332. [Google Scholar]
- Garavaglia, B.; Vallino, L.; Amoruso, A.; Pane, M.; Ferraresi, A.; Isidoro, C. The role of gut microbiota, immune system, and autophagy in the pathogenesis of inflammatory bowel disease: Molecular mechanisms and therapeutic approaches. Asp. Mol. Med. 2024, 4, 100056. [Google Scholar] [CrossRef]
- Mousa, W.K.; Chehadeh, F.; Husband, S. Microbial dysbiosis in the gut drives systemic autoimmune diseases. Front. Immunol. 2022, 13, 906258. [Google Scholar] [CrossRef]
- Lucas, L.N.; Barrett, K.; Kerby, R.L.; Zhang, Q.; Cattaneo, L.E.; Stevenson, D.; Rey, F.E.; Amador-Noguez, D. Dominant bacterial phyla from the human gut show widespread ability to transform and conjugate bile acids. mSystems 2021, 6, e00805-21. [Google Scholar] [CrossRef]
- Fiorucci, S.; Carino, A.; Baldoni, M.; Santucci, L.; Costanzi, E.; Graziosi, L.; Distrutti, E.; Biagioli, M. Bile acid signaling in inflammatory bowel diseases. Dig. Dis. Sci. 2021, 66, 674–693. [Google Scholar] [CrossRef]
- Guzior, D.V.; Quinn, R.A. Review: Microbial transformations of human bile acids. Microbiome 2021, 9, 140. [Google Scholar] [CrossRef]
- Lindner, S.; Miltiadous, O.; Ramos, R.J.F.; Paredes, J.; Kousa, A.I.; Dai, A.; Fei, T.; Lauder, E.; Frame, J.; Waters, N.R.; et al. Altered microbial bile acid metabolism exacerbates T cell-driven inflammation during graft-versus-host disease. Nat. Microbiol. 2024, 9, 614–630. [Google Scholar] [CrossRef]
- Tyagi, A.; Kumar, V. The gut microbiota–bile acid axis: A crucial regulator of immune function and metabolic health. World J. Microbiol. Biotechnol. 2025, 41, 215. [Google Scholar] [CrossRef]
- Kandasamy, S.; Letchumanan, V.; Hong, K.W.; Chua, K.; Mutalib, N.A.; Lai, A.; Ng, O.; Ming, L.C.; Lim, H.X.; Thurairajasingam, S.; et al. The role of human gut microbe Ruminococcus gnavus in inflammatory diseases. Prog. Microbes Mol. Biol. 2023, 6, a0000396. [Google Scholar] [CrossRef]
- Hong, J.; Fu, T.; Liu, W.; Du, Y.; Bu, J.; Wei, G.; Yu, M.; Lin, Y.; Min, C.; Lin, D. An update on the role and potential molecules in relation to Ruminococcus gnavus in inflammatory bowel disease, obesity and diabetes mellitus. Diabetes Metab. Syndr. Obes. 2024, 17, 1235–1248. [Google Scholar] [CrossRef] [PubMed]
- Nooij, S.; Plomp, N.; Sanders, I.M.J.; Schout, L.; van der Meulen, A.E.; Terveer, E.M.; Norman, J.M.; Karcher, N.; Larralde, M.F.; Vossen, R.H.A.M.; et al. Metagenomic global survey and in-depth genomic analyses of Ruminococcus gnavus reveal differences across host lifestyle and health status. Nat. Commun. 2025, 16, 1182. [Google Scholar] [CrossRef]
- Lerminiaux, N.A.; Cameron, A.D.S. Horizontal transfer of antibiotic resistance genes in clinical environments. Can. J. Microbiol. 2019, 44, 34–44. [Google Scholar] [CrossRef]
- Schjørring, S.; Krogfelt, K.A. Assessment of bacterial antibiotic resistance transfer in the gut. Int. J. Microbiol. 2011, 2011, 312956. [Google Scholar] [CrossRef]
- de Sousa, J.M.; Lourenco, M.; Gordo, I. Horizontal gene transfer among host-associated microbes. Cell Host Microbe 2023, 31, 513–527. [Google Scholar] [CrossRef]
- Moeller, J.B.; Leonardi, I.; Schlosser, A.; Flamar, A.-L.; Bessman, N.J.; Putzel, G.G.; Thomsen, T.; Hammond, M.; Jepsen, C.S.; Skjødt, K.; et al. Modulation of the fungal mycobiome is regulated by the chitin-binding receptor FIBCD1. J. Exp. Med. 2019, 216, 2689–2700. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Y.; Yao, X.; Li, S.; Wang, G.; Huang, Y.; Yang, Y.; Zhang, A. Characterizations of the gut bacteriome, mycobiome, and virome in patients with osteoarthritis. Microbiol. Spectrum 2022, 11, e01711-22. [Google Scholar] [CrossRef]
- Kreulen, I.A.M.; de Jonge, W.J.; van den Wijngaard, R.M.; van Thiel, I.A.M. Candida spp. in human intestinal health and disease: More than a gut feeling. Mycopathologia 2023, 188, 845–862. [Google Scholar] [CrossRef]
- Di Paola, M.; Rizzetto, L.; Stefanini, I.; Vitali, F.; Massi-Benedetti, C.; Tocci, N.; Romani, L.; De Filippo, C.; Lionetti, P.; De Filippo, C.; et al. Comparative immunophenotyping of Saccharomyces cerevisiae and Candida spp. strains from Crohn’s disease patients and their interactions with the gut microbiome. J. Transl. Autoimmun. 2020, 3, 100036. [Google Scholar] [CrossRef]
- Gogokhia, L.; Round, J.L. Immune-bacteriophage interactions in inflammatory bowel diseases. Curr. Opin. Virol. 2021, 49, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Billmann-Born, S.; Lipinski, S.; Böck, J.; Till, A.; Rosenstiel, P.; Schreiber, S. The complex interplay of NOD-like receptors and the autophagy machinery in the pathophysiology of Crohn disease. Eur. J. Cell Biol. 2011, 90, 593–602. [Google Scholar] [CrossRef]
- Turpin, W.; Bedrani, L.; Espin-Garcia, O.; Xu, W.; Silverberg, M.S.; Smith, M.I.; Antonio, J.; Garay, R.; Lee, S.; Guttman, D.S.; et al. Associations of NOD2 polymorphisms with Erysipelotrichaceae in stool of healthy first-degree relatives of Crohn’s disease subjects. BMC Med. Genet. 2020, 21, 204. [Google Scholar] [CrossRef]
- Liu, H.; Gao, P.; Jia, B.; Lu, N.; Zhu, B.; Zhang, F. IBD-associated Atg16L1T300A polymorphism regulates commensal microbiota of the intestine. Front. Immunol. 2022, 12, 772189. [Google Scholar] [CrossRef]
- Plantinga, T.S.; Crisan, T.O.; Oosting, M.; van de Veerdonk, F.L.; de Jong, D.J.; Philpott, D.J.; van der Meer, J.W.M.; Girardin, S.E.; Joosten, L.A.B.; Netea, M.G. Crohn’s disease-associated ATG16L1 polymorphism modulates pro-inflammatory cytokine responses selectively upon activation of NOD2. Inflamm. Bowel Dis. 2011, 60, 1229–1235. [Google Scholar] [CrossRef]
- Ayuso, P.; Quizhpe, J.; Rosell, M.d.l.Á.; Peñalver, R.; Nieto, G. Bioactive compounds, health benefits and food applications of artichoke (Cynara scolymus L.) and artichoke by-products: A review. Appl. Sci. 2024, 14, 4940. [Google Scholar] [CrossRef]
- Ben Salem, M.; Affes, H.; Ksouda, K.; Dhouibi, R.; Sahnoun, Z.; Hammami, S.; Zeghal, K.M. Pharmacological studies of artichoke leaf extract and their health benefits. Plant Foods Hum. Nutr. 2015, 70, 441–453. [Google Scholar] [CrossRef]
- Olas, B. An overview of the versatility of the parts of the globe artichoke (Cynara scolymus L.), its by-products and dietary supplements. Nutrients 2024, 16, 599. [Google Scholar] [CrossRef]
- Lattanzio, V.; Kroon, P.A.; Linsalata, V.; Cardinali, A. Globe artichoke: A functional food and source of nutraceutical ingredients. J. Funct. Foods 2009, 1, 131–144. [Google Scholar] [CrossRef]
- Pandino, G.; Meneghini, M.; Tavazza, R.; Lombardo, S.; Mauromicale, G. Phytochemicals accumulation and antioxidant activity in callus and suspension cultures of Cynara scolymus L. Plant Cell Tissue Organ Cult. 2017, 128, 223–230. [Google Scholar] [CrossRef]
- Comino, C.; Hehn, A.; Moglia, A.; Menin, B.; Bourgaud, F.; Lanteri, S.; Portis, E. The isolation and mapping of a novel hydroxycinnamoyltransferase in the globe artichoke chlorogenic acid pathway. BMC Plant Biol. 2009, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Caponio, G.R.; Vacca, M.; Scalvenzi, L.; Annunziato, A.; Silletti, R.; Ruta, C.; Difonzo, G.; De Angelis, M.; De Mastro, G. Phenolic characterization and nutraceutical evaluation of by-products from different globe artichoke cultivars. J. Sci. Food Agric. 2025, 105, 5062–5073. [Google Scholar] [CrossRef]
- Abbas, H.; Zewail, M.; Noureldin, M.H.; Ali, M.M.; Shamaa, M.M.; Khattab, M.A.; Ibrahim, N. Neuroprotective effect of artichoke-based nanoformulation in sporadic Alzheimer’s disease mouse model: Focus on antioxidant, anti-inflammatory, and amyloidogenic pathways. Pharmaceuticals 2022, 15, 1202. [Google Scholar]
- Dabbou, S.; Dabbou, S.; Flamini, G.; Pandino, G.; Gasco, L.; Helal, A.N. Phytochemical compounds from the crop by-products of Tunisian globe artichoke cultivars Sihem Dabbou. Chem. Biodivers. 2016, 13, 1475–1483. [Google Scholar] [CrossRef]
- Zuccolo, M.; Bassoli, A.; Giorgi, A.; Giupponi, L.; Mazzini, S.; Borgonovo, G. Phytochemical profiling of residual leaves from an alpine landrace of globe artichoke (Cynara scolymus L.). Molecules 2025, 30, 2649. [Google Scholar] [CrossRef]
- Ceccarelli, N.; Curadi, M.; Picciarelli, P.; Martelloni, L.; Sbrana, C.; Giovannetti, M. Globe artichoke as a functional food. Med. J. Nutr. Metab. 2010, 3, 197–201. [Google Scholar]
- Marquesa, P.; Martob, J.; Gonçalvesb, L.M.; Pachecoc, R.; Fitase, M.; Pintob, P.; Serralheirod, M.L.M.; Ribeiro, H. Cynara scolymus L.: A promising Mediterranean extract for topical anti-aging prevention. Ind. Crops Prod. 2017, 109, 699–706. [Google Scholar] [CrossRef]
- Magielse, J.; Verlaet, A.; Breynaert, A.; Keenoy, B.M.Y.; Apers, S.; Pieters, L.; Hermans, N. Investigation of the in vivo antioxidative activity of Cynara scolymus (artichoke) leaf extract in the streptozotocin-induced diabetic rat. Mol. Nutr. Food Res. 2014, 58, 211–215. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Mauromicale, G.; Williamson, G. Phenolic acids and flavonoids in leaf and floral stem of cultivated and wild Cynara cardunculus L. genotypes. Food Chem. 2011, 126, 417–422. [Google Scholar] [CrossRef]
- Yang, M.; Ma, Y.; Wang, Z.; Khan, A.; Zhou, W.; Zhao, T.; Cao, J.; Cheng, G.; Cai, S. Phenolic constituents, antioxidant and cytoprotective activities of crude extract and fractions from cultivated artichoke inflorescence. Ind. Crops Prod. 2020, 143, 111433. [Google Scholar] [CrossRef]
- Rahimuddin, S.A.; Khoja, S.M.; Zuhaira, M.M.; Howell, N.K.; Brown, J.E. Inhibition of lipid peroxidation in UVA-treated skin fibroblasts by luteolin and its glucosides. Eur. J. Lipid Sci. Technol. 2007, 109, 647–655. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, M. A critical review of the application of chelating agents to enable Fenton and Fenton-like reactions at high pH values. J. Hazard. Mater. 2018, 15, 436–450. [Google Scholar] [CrossRef]
- Colak, E.; Ustuner, M.C.; Tekin, N.; Colak, E.; Burukoglu, D.; Degirmenci, I.; Gunes, H.V. The hepatocurative effects of Cynara scolymus L. leaf extract on carbon tetrachloride-induced oxidative stress and hepatic injury in rats. SpringerPlus 2016, 5, 216. [Google Scholar] [CrossRef]
- D’Antuono, I.; Garbetta, A.; Linsalata, V.; Minervini, F.; Cardinali, A. Polyphenols from artichoke heads (Cynara cardunculus (L.) subsp. scolymus Hayek*): In vitro bio-accessibility, intestinal uptake and bioavailability. Food Funct. 2015, 6, 1268–1277. [Google Scholar]
- Garbetta, A.; Capotorto, I.; Cardinali, A.; De Angelis, I.A.; Linsalata, V.; Pizzi, F.; Minervini, F. Antioxidant activity induced by main polyphenols present in edible artichoke heads: Influence of in vitro gastro-intestinal digestion. J. Funct. Foods 2014, 10, 456–464. [Google Scholar] [CrossRef]
- Rocchetti, G.; Giuberti, G.; Lucchini, F.; Lucini, L. Polyphenols and sesquiterpene lactones from artichoke heads: Modulation of starch digestion, gut bioaccessibility, and bioavailability following in vitro digestion and large intestine fermentation. Antioxidants 2020, 9, 306. [Google Scholar] [CrossRef]
- Williamson, G.; Clifford, M.N. Colonic metabolites of berry polyphenols: The missing link to biological activity? Br. J. Nutr. 2010, 104, S48–S66. [Google Scholar] [CrossRef]
- Breynaert, A.; Bosscher, D.; Kahnt, A.; Claeys, M.; Cos, P.; Pieters, L.; Hermans, N. Development and validation of an in vitro experimental gastrointestinal dialysis model with colon phase to study the availability and colonic metabolisation of polyphenolic compounds. Planta Med. 2015, 81, 1075–1083. [Google Scholar] [CrossRef]
- Domínguez-Fernández, M.; Ludwig, I.A.; De Peña, M.-P.; Cid, C. Bioaccessibility of Tudela artichoke (Cynara scolymus cv. Blanca de Tudela) (poly)phenols: The effects of heat treatment, simulated gastrointestinal digestion and human colonic microbiota. Food Funct. 2021, 12, 1996–2011. [Google Scholar] [CrossRef]
- Speciale, A.; Muscarà, C.; So, M.; Toscano, G.; Cimino, F.; Saija, A. In vitro protective effects of a standardized extract from Cynara cardunculus L. leaves against TNF-α-induced intestinal inflammation. Front. Pharmacol. 2022, 13, 909938. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Pautz, A.; Wollscheid, U.; Reifenberg, G.; Förstermann, U.; Li, H. Artichoke, cynarin and cyanidin downregulate the expression of inducible nitric oxide synthase in human coronary smooth muscle cells. Molecules 2014, 19, 3654–3668. [Google Scholar] [CrossRef]
- Mateus, V.; Barracosa, P.; Teixeira-Lemos, E.; Pinto, R. Effect of Cynara cardunculus L. var. altilis (DC) in inflammatory bowel disease. Appl. Sci. 2021, 11, 1629. [Google Scholar] [CrossRef]
- Pulito, C.; Mori, F.; Sacconi, A.; Casadei, L.; Ferraiuolo, M.; Valerio, M.C.; Santoro, R.; Goeman, F.; Maidecchi, A.; Mattoli, L.; et al. Cynara scolymus affects malignant pleural mesothelioma by promoting apoptosis and restraining invasion. Oncotarget 2015, 6, 18134–18150. [Google Scholar] [CrossRef]
- Kim, E.K.; Choi, E.-J. Pathological roles of MAPK signaling pathways in human diseases. BBA Mol. Basis Dis. 2010, 1802, 396–405. [Google Scholar] [CrossRef]
- Carmona-Hernandez, J.C.; Taborda-Ocampo, G.; Valdez, J.C.; Bolling, B.W.; González-Correa, C.H. Polyphenol extracts from three Colombian Passifloras (passion fruits) prevent inflammation-induced barrier dysfunction of Caco-2 cells. Molecules 2019, 24, 4614. [Google Scholar] [CrossRef]
- Arya, V.S.; Kanthlal, S.K.; Linda, G. The role of dietary polyphenols in inflammatory bowel disease: A possible clue on the molecular mechanisms involved in the prevention of immune and inflammatory reactions. J. Food Biochem. 2020, 44, e13369. [Google Scholar] [CrossRef]
- Vacca, M.; Pinto, D.; Annunziato, A.; Ressa, A.; Calasso, M.; Pontonio, E.; Celano, G.; De Angelis, M. Gluten-free bread enriched with artichoke leaf extract in vitro exerted antioxidant and anti-inflammatory properties. Antioxidants 2023, 12, 845. [Google Scholar] [CrossRef]
- Vaz, A.A.; Odriozola-Serrano, I.; Oms-Oliu, G.; Mart, O. Physicochemical properties and bioaccessibility of phenolic compounds of dietary fibre concentrates from vegetable by-products. Foods 2022, 11, 2578. [Google Scholar] [CrossRef]
- Valero, D.; Serrano, M. Bioactive compounds with health benefits of artichoke and cardoon. In Proceedings of the ISHS Acta Horticulturae 1284: X International Symposium on Artichoke, Cardoon and Their Wild Relatives, Orihuela, Spain, 12–15 March 2019; pp. 221–226. [Google Scholar]
- Xi, M.; Hou, Y.; Wang, R.; Ji, M.; Cai, Y.; Ao, J.; Shen, H.; Li, M.; Wang, J.; Luo, A. Potential application of luteolin as an active antibacterial composition in the development of hand sanitizer products. Molecules 2022, 27, 7342. [Google Scholar] [CrossRef]
- He, M.; Wu, T.; Pan, S.; Xu, X. Antimicrobial mechanism of flavonoids against Escherichia coli ATCC 25922 by model membrane study. Appl. Surf. Sci. 2014, 305, 515–521. [Google Scholar] [CrossRef]
- Vamanu, E.; Vamanu, A.; Nita, S.; Colceriu, S. Antioxidant and antimicrobial activities of ethanol extracts of Cynara scolymus (Cynarae folium, Asteraceae family). Trop. J. Pharm. Res. 2011, 10, 777–783. [Google Scholar] [CrossRef]
- Fratianni, F.; De Palma, M.; Pepe, R.; Nazzaro, F. Polyphenolic composition in different parts of some cultivars of globe artichoke (Cynara cardunculus L. var. scolymus (L.) Fiori). Food Chem. 2007, 104, 1282–1286. [Google Scholar] [CrossRef]
- Gaafar, A.A.; Salama, Z.A. Phenolic compounds from artichoke (Cynara scolymus L.) by-products and their antimicrobial activities. J. Biol. Agric. Healthc. 2013, 3, e6. [Google Scholar]
- Scavo, A.; Rial, C.; Varela, R.M.; Molinillo, J.M.G.; Mauromicale, G.; Macias, F.A. Influence of genotype and harvest time on Cynara cardunculus L. sesquiterpene lactone profile. J. Agric. Food Chem. 2019, 67, 6487–6496. [Google Scholar] [CrossRef]
- Alghazeer, R.; El-Saltani, H.; Saleh, N.A.; Al-Najjar, A.; Naili, M.B.; Hebail, F.; El-Deeb, H. Antioxidant and antimicrobial activities of Cynara scolymus L. rhizomes. Mod. Appl. Sci. 2012, 6, 54–63. [Google Scholar] [CrossRef]
- Costabile, A.; Kolida, S.; Klinder, A.; Gietl, E.; Bauerlein, M.; Frohberg, C.; Landschutze, V.; Gibson, G.R. A double-blind, placebo-controlled, cross-over study to establish the bifidogenic effect of a very-long-chain inulin extracted from globe (Cynara scolymus) in healthy human subjects. Br. J. Nutr. 2010, 104, 1007–1017. [Google Scholar] [CrossRef]
- Causey, J.L.; Feirtag, J.M.; Gahaher, D.D.; Tuqland, B.C.; Slavin, J.L. Effects of dietary inulin on serum lipids, blood glucose and the gastrointestinal environment in hypercholesterolemic men. Nutr. Res. 2000, 20, 191–201. [Google Scholar] [CrossRef]
- Davidson, M.H.; Maki, K.C. Nutritional and health benefits of inulin and oligofructose effects of dietary inulin on serum lipids. J. Nutr. 1999, 129, 1474S–1477S. [Google Scholar] [CrossRef]
- Rondanelli, M.; Riva, A.; Petrangolini, G.; Allegrini, P.; Bernardinelli, L.; Fazia, T.; Peroni, G.; Gasparri, C.; Nichetti, M.; Faliva, M.A.; et al. The metabolic effects of cynara supplementation in overweight and obese class I subjects with newly detected impaired fasting glycemia: A double-blind, placebo-controlled, randomized clinical trial. Nutrients 2020, 12, 3298. [Google Scholar] [CrossRef]
- Panahi, Y.; Kianpour, P.; Mohtashami, R.; Atkin, S.L.; Butler, A.E.; Jafari, R.; Badeli, R.; Sahebkar, A. Efficacy of artichoke leaf extract in non-alcoholic fatty liver disease: A pilot double-blind randomized controlled trial. Phytother. Res. 2018, 32, 1382–1387. [Google Scholar] [CrossRef]
- Rangboo, V.; Noroozi, M.; Zavoshy, R.; Rezadoost, S.A.; Mohammadpoorasl, A. The effect of artichoke leaf extract on alanine aminotransferase and aspartate aminotransferase in patients with nonalcoholic steatohepatitis. Int. J. Hepatol. 2016, 2016, 4030476. [Google Scholar] [CrossRef]
- Fogacci, F.; Giovannini, M.; Di Micoli, A.; Fiorini, G.; Grandi, E.; Borghi, C.; Cicero, A.F.G. A randomized, double-blind, placebo-controlled clinical trial on the effect of a dietary supplement containing dry artichoke and bergamot extracts on metabolic and vascular risk factors in individuals with suboptimal cholesterol levels. Nutrients 2024, 16, 1587. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, M.D.; Beland, F.A. An evaluation of the biological and toxicological properties of Aloe barbadensis (Miller), Aloe vera. J. Environ. Sci. Health Part C 2006, 24, 103–154. [Google Scholar] [CrossRef]
- Hamman, J.H. Composition and applications of Aloe vera leaf gel. Molecules 2008, 13, 1599–1616. [Google Scholar] [CrossRef]
- Kumar, S.; Kalita, S.; Basumatary, I.B.; Kumar, S.; Ray, S.; Mukherjee, A. Recent advances in therapeutic and biological activities of Aloe vera. Biocatal. Agric. Biotechnol. 2024, 57, 103084. [Google Scholar] [CrossRef]
- Catalano, A.; Ceramella, J.; Iacopetta, D.; Marra, M.; Conforti, F.; Lupi, F.R.; Gabriele, D.; Borges, F.; Sinicropi, M.S. Aloe vera—An extensive review focused on recent studies. Foods 2024, 13, 2155. [Google Scholar] [CrossRef]
- Sánchez, M.; González-Burgos, E.; Iglesias, I.; Gómez-Serranillos, M.P. Pharmacological update properties of Aloe vera and its major active constituents. Molecules 2020, 25, 1324. [Google Scholar] [CrossRef]
- Eshun, K.; He, Q. Aloe vera: A valuable ingredient for the food, pharmaceutical and cosmetic industries—A review. Crit. Rev. Food Sci. Nutr. 2010, 44, 91–96. [Google Scholar] [CrossRef]
- Surjushe, A.; Vasani, R.; Saple, D.G. Aloe vera: A short review. Indian J. Dermatol. 2008, 53, 163–166. [Google Scholar] [CrossRef]
- Liu, C.; Du, P.; Guo, Y.; Xie, Y.; Yu, H.; Yao, W.; Cheng, Y.; Qian, H. Extraction, characterization of aloe polysaccharides and the in-depth analysis of its prebiotic effects on mice gut microbiota. Carbohydr. Polym. 2021, 261, 117874. [Google Scholar] [CrossRef]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Filipic, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Kuhnle, G.G.; et al. Safety of hydroxyanthracene derivatives for use in food. Food Saf. J. 2018, 16, 5090. [Google Scholar]
- Femenia, A.; Sanchez, E.S.; Simal, S.; Rossello, C. Compositional features of polysaccharides from Aloe vera (Aloe barbadensis Miller) plant tissues. Carbohydr. Polym. 1999, 39, 109–117. [Google Scholar] [CrossRef]
- Choi, S.-W.; Son, B.-W.; Son, Y.-S.; Park, Y.-I.; Lee, S.-K.; Chung, M.-H. The wound-healing effect of a glycoprotein fraction isolated from Aloe vera. Br. J. Dermatol. 2001, 145, 535–545. [Google Scholar] [CrossRef]
- Pop, R.M.; Puia, I.C.; Puia, A.; Chedea, V.S.; Levai, A.M.; Bocsan, I.C.; Buzoianu, A.D. Pot Aloe vera gel—A natural source of antioxidants. Not. Bot. Horti Agrobot. Cluj Napoca 2022, 50, 12732. [Google Scholar] [CrossRef]
- Chiodelli, G.; Pellizzoni, M.; Ruzickova, G.; Lucini, L. Effect of different Aloe fractions on the growth of lactic acid bacteria. J. Food Sci. 2016, 82, 219–224. [Google Scholar] [CrossRef]
- Walia, R.; Chaudhuri, S.R.; Dey, P. Reciprocal interaction between gut microbiota and aloe-emodin results in altered microbiome composition and metabolism of aloe-emodin. Food Biosci. 2025, 70, 107061. [Google Scholar] [CrossRef]
- Lai, Q.; Wang, H.; Guo, X.; Abbasi, A.M.; Wang, T.; Li, T.; Fu, X.; Li, J.; Liu, R.H. Comparison of phytochemical profiles, antioxidant and cellular antioxidant activities of seven cultivars of Aloe. Int. J. Food Sci. Technol. 2016, 51, 1489–1494. [Google Scholar] [CrossRef]
- Ozsoy, N.; Candoken, E.; Akev, N. Implications for degenerative disorders. Oxid. Med. Cell Longev. 2009, 2, 99–106. [Google Scholar] [CrossRef]
- Atun, S.; Aznam, N.; Arianingrum, R.; Azeeza, S.N.; Sangal, A. Antioxidant activity of Aloe vera and prediction of interaction mechanisms on ROS1 kinase and collagenase receptors. Molekul 2024, 19, 560–570. [Google Scholar] [CrossRef]
- Debnath, T.; Ghosh, M.; Lee, Y.M.; Deb, N.C.; Lee, K.; Lim, B.O. Identification of phenolic constituents and antioxidant activity of Aloe barbadensis flower extracts. Food Agric. Immunol. 2018, 29, 27–38. [Google Scholar] [CrossRef]
- Gabriel, N.N.; Qiang, J.; Ma, X.Y.; He, J.; Xu, P.; Liu, K. Dietary Aloe vera improves plasma lipid profile, antioxidant, and hepatoprotective enzyme activities in GIFT-tilapia (Oreochromis niloticus) after Streptococcus iniae challenge. Fish Physiol. Biochem. 2015, 41, 1321–1332. [Google Scholar] [CrossRef]
- Kaithwas, G.; Singh, P.; Bhatia, D. Evaluation of in vitro and in vivo antioxidant potential of polysaccharides from Aloe vera (Aloe barbadensis Miller) gel. Drug Chem. Toxicol. 2014, 37, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Caruso, F.; Pedersen, J.Z.; Incerpi, S.; Belli, S.; Sakib, R.; Rossi, M. Interaction between vitamins C and E when scavenging the superoxide radical shown by hydrodynamic voltammetry and DFT. Biophysica 2024, 4, 310–326. [Google Scholar] [CrossRef]
- Vinson, J.A.; Kharrat, H.A.; Andreoli, L. Effect of Aloe vera preparations on the human bioavailability of vitamins C and E. Phytomedicine 2005, 12, 760–765. [Google Scholar] [CrossRef]
- Abubakar, A.M.; Dibal, N.I.; Attah, M.O.O.; Chiroma, S.M. Exploring the antioxidant effects of Aloe vera: Potential role in controlling liver function and lipid profile in high-fat and fructose diet (HFFD) fed mice. Pharmacol. Res. Mod. Chin. Med. 2022, 4, 100150. [Google Scholar] [CrossRef]
- Matei, C.E.; Visan, A.I.; Cristescu, R. Aloe vera polysaccharides as therapeutic agents: Benefits versus side effects in biomedical applications. Polysaccharides 2025, 6, 36. [Google Scholar] [CrossRef]
- Jales, S.T.L.; Barbosa, R.D.M.; De Albuquerque, A.C.; Duarte, L.H.V.; Silva, G.R.; Meirelles, L.M.A.; Silva, T.M.S.; Alves, A.F.; Viseras, C.; Raffin, F.N.; et al. Development and characterization of Aloe vera mucilaginous-based hydrogels for psoriasis treatment. J. Compos. Sci. 2022, 6, 231. [Google Scholar] [CrossRef]
- Yazdani, N.; Hossini, S.E.; Edalatmanesh, M.A. Anti-inflammatory effect of Aloe vera extract on inflammatory cytokines of rats fed with a high-fat diet (HFD). Jundishapur J. Nat. Pharm. Prod. 2022, 17, e114323. [Google Scholar] [CrossRef]
- Li, L.; Xu, W.; Luo, Y.; Lao, C.; Tong, X.; Du, J.; Huang, B.; Li, D.; Chen, J.; Ye, H.; et al. Aloe polymeric acemannan inhibits the cytokine storm in mouse pneumonia models by modulating macrophage metabolism. Carbohydr. Polym. 2022, 297, 120032. [Google Scholar] [CrossRef]
- Sadgrove, N.J.; Simmonds, M.S.J. Pharmacodynamics of Aloe vera and acemannan in therapeutic applications for skin, digestion, and immunomodulation. Phytother. Res. 2021, 35, 6572–6584. [Google Scholar] [CrossRef]
- Zhou, H.; Peng, K.; Wang, J.; Wang, Y.; Wang, J.; Sun, S.; Shi, M.; Chen, J.; Ji, F.; Wang, X. Aloe-derived vesicles enable macrophage reprogramming to regulate the inflammatory immune environment. Front. Bioeng. Biotechnol. 2023, 11, 1339941. [Google Scholar] [CrossRef] [PubMed]
- Akinloye, O.A.; Akinloye, D.I.; Onigbinde, S.B.; Metibemu, D.S. Phytosterols demonstrate selective inhibition of COX-2: In vivo and in silico studies of Nicotiana tabacum. Bioorg. Chem. 2020, 102, 104037. [Google Scholar] [CrossRef]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Viljoen, A. Health benefits of chromones: Common ingredients of our daily diet. Phytochem. Rev. 2020, 19, 761–785. [Google Scholar] [CrossRef]
- Nazeam, J.A.; Gad, H.A.; El-Hefnawy, H.M.; Singab, A.-N.B. Chromatographic separation and detection methods of Aloe arborescens Miller constituents: A systematic review. J. Chromatogr. B 2017, 1058, 57–67. [Google Scholar] [CrossRef]
- Aldayel, T.S.; Grace, M.H.; Lila, M.A.; Yahya, M.A.; Omar, U.M.; Alshammary, G. LC-MS characterization of bioactive metabolites from two Yemeni Aloe spp. with antioxidant and antidiabetic properties. Arab. J. Chem. 2020, 13, 5040–5049. [Google Scholar] [CrossRef]
- Zhong, J.; Wan, J.; Ding, W.; Wu, X. One-step separation and purification of two chromones and one pyrone from Aloe barbadensis Miller: A comparison between reversed-phase flash chromatography and high-speed counter current chromatography. Phytochem. Anal. 2014, 25, 282–288. [Google Scholar] [CrossRef]
- Budai, M.M.; Varga, A.; Milesz, S.; József, T.; Benk, S. Aloe vera downregulates LPS-induced inflammatory cytokine production and expression of NLRP3 inflammasome in human macrophages. Mol. Immunol. 2013, 56, 471–479. [Google Scholar] [CrossRef]
- Wang, F.; Liu, J.; An, Q.; Wang, Y.; Yang, Y.; Huo, T.; Yang, S.; Ju, R.; Quan, Q. Aloe extracts inhibit skin inflammatory responses by regulating NF-κB, ERK, and JNK signaling pathways in an LPS-induced RAW264.7 macrophages model. Clin. Cosmet. Investig. Dermatol. 2023, 16, 267–278. [Google Scholar] [CrossRef]
- Luo, X.; Id, H.Z.; Wei, X.; Shi, M.; Fan, P.; Xie, W.; Zhang, Y.; Xu, N. Aloin suppresses lipopolysaccharide-induced inflammatory response and apoptosis by inhibiting the activation of NF-κB. Molecules 2018, 23, 517. [Google Scholar] [CrossRef]
- Keshavarzi, Z.; Hadjzadeh, M.-A.-R.; Nazari, M.; Arezumand, R. Protective effect of Aloe vera gel in ulcerative colitis: The role of inflammatory and anti-inflammatory factors. Sci. J. Kurd. Univ. Med. Sci. 2021, 26, 69–79. [Google Scholar] [CrossRef]
- Li, C.-Y.; Suzuki, K.; Hung, Y.-L.; Yang, M.-S.; Yo, C.-P.; Lin, S.-P.; Hou, Y.-C.; Fang, S.-H. Aloe metabolites prevent LPS-induced sepsis and inflammatory response by inhibiting mitogen-activated protein kinase activation. Am. J. Chin. Med. 2017, 45, 847–861. [Google Scholar] [CrossRef]
- Habeeb, F.; Stables, G.; Bradbury, F.; Nong, S.; Cameron, P.; Plevin, R.; Ferro, V.A. The inner gel component of Aloe vera suppresses bacterial-induced pro-inflammatory cytokines from human immune cells. Methods 2007, 42, 388–393. [Google Scholar] [CrossRef]
- Yang, D.; Ge, T.; Zhou, J.; Li, H.; Zhang, Y. Aloe-emodin alleviates inflammatory bowel disease in mice by modulating intestinal microbiome homeostasis via the IL-4/IL-13 axis. Heliyon 2024, 10, e34932. [Google Scholar] [CrossRef]
- Radha, M.H.; Laxmipriya, N.P. Evaluation of biological properties and clinical effectiveness of Aloe vera: A systematic review. J. Tradit. Complement. Med. 2015, 5, 21–26. [Google Scholar] [CrossRef]
- Nejatzadeh-Barandozi, F. Antibacterial activities and antioxidant capacity of Aloe vera. Org. Med. Chem. Lett. 2013, 3, 5. [Google Scholar] [CrossRef]
- Cellini, L.; Di Bartolomeo, S.; Di Campli, E.; Genovese, S.; Locatelli, M.; Di Giulio, M. In vitro activity of Aloe vera inner gel against Helicobacter pylori strains. Lett. Appl. Microbiol. 2013, 59, 43–48. [Google Scholar] [CrossRef]
- Song, D.; Wang, X.; Ma, Y.; Liu, N.-N.; Wang, H. Beneficial insights into postbiotics against colorectal cancer. Front. Nutr. 2023, 10, 1111872. [Google Scholar] [CrossRef]
- Minjares-Fuentes, R.; Femenia, A.; Comas-Serra, F.; Rossello, C.; Rodríguez-Gonzalez, V.M.; Gonzalez-Laredo, R.F.; Gallegos-Infante, J.A.; Medina-Torres, L. Effect of different drying procedures on physicochemical properties and flow behavior of Aloe vera (Aloe barbadensis Miller) gel. LWT Food Sci. Technol. 2016, 74, 378–386. [Google Scholar] [CrossRef]
- Zheng, Y.; Qin, C.; Wen, M.; Zhang, L.; Wang, W. The effects of food nutrients and bioactive compounds on the gut microbiota: A comprehensive review. Foods 2024, 13, 1345. [Google Scholar] [CrossRef]
- Rahmiatiningrum, N.; Sukardi; Warkoyo. Study of physical characteristic, water vapor transmission rate and inhibition zones of edible films from Aloe vera (Aloe barbadensis) incorporated with yellow sweet potato starch and glycerol. Food Technol. Halal Sci. J. 2019, 2, 195. [Google Scholar] [CrossRef]
- Maan, A.A.; Nazir, A.; Khan, M.K.I.; Ahmad, T.; Zia, R.; Murid, M.; Abrar, M. The therapeutic properties and applications of Aloe vera: A review. J. Herb. Med. 2018, 12, 1–10. [Google Scholar] [CrossRef]
- Gao, Y.; Kuok, K.I.; Jin, Y.; Wang, R. Biomedical applications of Aloe vera. Crit. Rev. Food Sci. Nutr. 2019, 59, S244–S256. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.W.; Chun, J.; Park, S.; Lee, H.J.; Im, J.P.; Kim, J.S. Aloe vera Is Effective and Safe in Short-term Treatment of Irritable Bowel Syndrome: A Systematic Review and Meta-analysis. J. Neurogastroenterol. Motil. 2018, 24, 528–535. [Google Scholar] [CrossRef]
- Ahluwalia, B.; Magnusson, M.K.; Böhn, L.; Störsrud, S.; Larsson, F.; Öhman, L.; Simrén, M. Aloe barbadensis Mill. extract improves symptoms in IBS patients with diarrhoea: Post hoc analysis of two randomized double-blind controlled studies. Ther. Adv. Gastroenterol. 2021, 14, 17562848211048133. [Google Scholar] [CrossRef]
- McKay, D.L.; Blumberg, J.B. A review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.). Phytother. Res. 2006, 20, 519–530. [Google Scholar] [CrossRef]
- Srivastava, J.K.; Shankar, E.; Gupta, S. Chamomile: A herbal medicine of the past with bright future. Mol. Med. Rep. 2010, 3, 895–901. [Google Scholar] [CrossRef]
- El Mihyaoui, A.; da Silva, J.C.G.E.; Charfi, S.; Castillo, M.E.C.; Lamarti, H.; Arnao, M.B. Chamomile (Matricaria chamomilla L.): A review of ethnomedicinal use, phytochemistry and pharmacological uses. Life 2022, 12, 479. [Google Scholar] [CrossRef]
- European Medicines Agency. Herbal Medicine: Summary for the Public Matricaria Flower; European Medicines Agency: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Sorme, F.M.; Tabarrai, M.; Alimadady, H.; Rahimi, R.; Sepidarkish, M.; Karimi, M. Efficacy of Matricaria chamomilla L. in infantile colic: A double blind, placebo controlled randomized trial. J. Pharm. Res. Int. 2020, 31, 1–11. [Google Scholar] [CrossRef]
- Sah, A.; Naseef, P.P.; Kuruniyan, M.S.; Jain, G.K.; Zakir, F.; Aggarwal, G. A comprehensive study of therapeutic applications of chamomile. Pharmaceuticals 2022, 15, 1284. [Google Scholar] [CrossRef]
- El Joumaa, M.M.; Borjac, J.M. Matricaria chamomilla: A valuable insight into recent advances in medicinal uses and pharmacological activities. Phytochem. Rev. 2022, 21, 1913–1940. [Google Scholar] [CrossRef]
- Sepp, J.; Koshovyi, O.; Jakstas, V.; Žvikas, V.; Botsula, I.; Kireyev, I.; Tsemenko, K.; Kukhtenko, O.; Kogermann, K.; Heinämäki, J.; et al. Phytochemical, technological, and pharmacological study on the galenic dry extracts prepared from German chamomile (Matricaria chamomilla L.) flowers. Plants 2024, 13, 350. [Google Scholar] [CrossRef]
- Avonto, C.; Wang, M.; Chittiboyina, A.G.; Avula, B.; Zhao, J.; Khan, I.A. Hydroxylated bisabolol oxides: Evidence for secondary oxidative metabolism in Matricaria chamomilla. J. Nat. Prod. 2013, 76, 1848–1853. [Google Scholar] [CrossRef]
- Alzahrani, M.S.H. The effect of different levels of Matricaria chamomilla on functional status of the liver in rats injected with carbon tetrachloride. Pak. J. Zool. 2020, 52, 2111–2119. [Google Scholar] [CrossRef]
- Borsato, A.V.; Doni-Filho, L.; Rakocevic, M.; Cocco, L.C.; Paglia, E.C. Chamomile essential oils extracted from flower heads and recovered water during drying process. J. Food Process Preserv. 2009, 33, 500–512. [Google Scholar] [CrossRef]
- Eddin, L.B.; Jha, N.K.; Goyal, S.N.; Agrawal, Y.O.; Subramanya, S.B.; Bastaki, S.M.A.; Ojha, S. Health benefits, pharmacological effects, molecular mechanisms, and therapeutic potential of α-bisabolol. Nutrients 2022, 14, 1370. [Google Scholar] [CrossRef]
- Draca, N.; Aladic, K.; Banozic, M.; Subaric, D.; Jokic, S.; Nemet, I. Chamomile waste: A comprehensive insight on phytochemical and safety profile, extraction techniques and potential application. Biocatal. Agric. Biotechnol. 2025, 63, 103468. [Google Scholar] [CrossRef]
- Chauhan, R.; Singh, S.; Kumar, V.; Kumar, A.; Kumari, A.; Rathore, S.; Kumar, R.; Singh, S. A comprehensive review on biology, genetic improvement, agro and process technology of German chamomile (Matricaria chamomilla L.). Plants 2022, 11, 29. [Google Scholar] [CrossRef]
- Singh, A.; Kaur, P.; Kumar, M.; Shafi, S.; Kumar, P.; Tiwari, A.; Tiwari, V.; Kumar, N.; Thirunavukkarasu, V.; Kumari, S.; et al. The role of phytochemicals in modulating the gut microbiota: Implications for health and disease. Med. Microecol. 2025, 24, 100125. [Google Scholar] [CrossRef]
- Martel, J.; Ojcius, D.M.; Ko, Y.-F.; Young, J.D. Phytochemicals as prebiotics and biological stress inducers. Trends Biochem. Sci. 2020, 45, 462–471. [Google Scholar] [CrossRef]
- Noshad, M.; Behbahani, B.A.; Mehrnia, M.A. Evaluation of antioxidant activity, total phenol and flavonoid and antibacterial activity of German chamomile. J. Food Sci. Technol. 2024, 21, 139–151. [Google Scholar]
- Stanojevic, L.P.; Marjanovic-Balaban, Z.R.; Kalaba, V.D.; Stanojevic, S.; Cvetkovic, D.J. Chemical composition, antioxidant and antimicrobial activity of chamomile flowers essential oil (Matricaria chamomilla L.). J. Essent. Oil Bear. Plants 2017, 19, 2017–2028. [Google Scholar] [CrossRef]
- Fejer, J.; Salamon, I. Breeding of German chamomile, Matricaria recutita L., with a high content of α-bisabolol. Acta Hortic. 2016, 1125, 287–292. [Google Scholar]
- Srivastava, J.K.; Gupta, S. Chamomile: A herbal agent for treatment of diseases of the elderly. In Foods and Dietary Supplements in the Prevention and Treatment of Disease; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 171–183. [Google Scholar]
- Rehmat, S.; Khera, R.A.; Hanif, M.A.; Ayub, M.A.; Zubair, M. Chamomilla. In Medicinal Plants of South Asia; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; pp. 101–112. [Google Scholar]
- Hernández-Ceruelos, A.; Madrigal-Santillán, E.; Morales-González, J.A.; Chamorro-Cevallos, G.; Cassani-Galindo, M.; Madrigal-Bujaidar, E. Antigenotoxic effect of Chamomilla recutita (L.) Rauschert essential oil in mouse spermatogonial cells, and determination of its antioxidant capacity in vitro. Int. J. Mol. Sci. 2010, 11, 3793–3802. [Google Scholar] [CrossRef] [PubMed]
- Cvetanovic, A.; Svarc-Gajic, J.; Zekovic, Z.; Savic, S.; Vulic, J.; Maskovic, P.; Cetkovic, G. Comparative analysis of antioxidant, antimicrobiological and cytotoxic activities of native and fermented chamomile ligulate flower extracts. Planta 2015, 242, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Palma, R.; Mendonça, P.; Cervantes, R.; Pratas, A.; Malh, B.; Marques-Ramos, A. Effects of apigenin on gastric cancer cells. Biomed. Pharmacother. 2024, 172, 116251. [Google Scholar] [CrossRef]
- Bhaskaran, N.; Shukla, S.; Kanwal, R.; Srivastava, J.K.; Gupta, S. Induction of heme oxygenase-1 by chamomile protects murine macrophages against oxidative stress. Life Sci. 2012, 90, 1027–1033. [Google Scholar] [CrossRef]
- Bhaskaran, N.; Srivastava, J.K.; Shukla, S.; Gupta, S. Chamomile confers protection against hydrogen peroxide-induced toxicity through activation of Nrf2-mediated defense response. Phytother. Res. 2013, 27, 118–125. [Google Scholar] [CrossRef]
- Bhaskaran, N.; Shukla, S.; Srivastava, J.K.; Gupta, S. Chamomile, an anti-inflammatory agent inhibits inducible nitric oxide synthase expression by blocking RelA/P65 activity. Phytother. Res. 2013, 27, 118–125. [Google Scholar] [CrossRef]
- Lairikyengbam, D.; Wetterauer, B.; Schmiech, M.; Jahraus, B.; Kirchgessner, H.; Niesler, B.; Balta, E.; Samstag, Y. Comparative analysis of whole plant, flower and root extracts of Chamomilla recutita L. and characteristic pure compounds reveals differential anti-inflammatory effects on human T cells. Front. Immunol. 2024, 15, 1388962. [Google Scholar] [CrossRef]
- Kim, S.; Jung, E.; Kim, J.; Park, Y.; Lee, J.; Park, D. Inhibitory effects of (-)-α-bisabolol on LPS-induced inflammatory response in RAW264.7 macrophages. Food Chem. Toxicol. 2011, 49, 2580–2585. [Google Scholar] [CrossRef]
- Ramazani, E.; Akaberi, M.; Ahmad, S.; Tayarani-Najaran, Z. Pharmacological and biological effects of alpha-bisabolol: An updated review of the molecular mechanisms. Life Sci. 2022, 304, 120728. [Google Scholar] [CrossRef]
- Arunachalam, S.; Nagoor Meeran, M.F.; Azimullah, S.; Jha, N.K.; Saraswathiamma, D.; Subramanya, S.; Albawardi, A.; Ojha, S. α-Bisabolol attenuates doxorubicin induced renal toxicity by modulating NF-κB/MAPK signaling and caspase-dependent apoptosis in rats. Int. J. Mol. Sci. 2022, 23, 10528. [Google Scholar] [CrossRef]
- Venkataraman, B.; Almarzooqi, S.; Raj, V.; Dudeja, P.K.; Bhongade, B.A.; Patil, R.B.; Ojha, S.K.; Attoub, S.; Adrian, T.E.; Subramanya, S.B. α-Bisabolol mitigates colon inflammation by stimulating colon PPAR-γ transcription factor: In vivo and in vitro study. PPAR Res. 2022, 13, 5498115. [Google Scholar] [CrossRef]
- Srivastava, J.K.; Pandey, M.; Gupta, S. Chamomile, a novel and selective COX-2 inhibitor with anti-inflammatory activity. Life Sci. 2009, 85, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Zargaran, A.; Borhani-Haghighi, A.; Faridi, P.; Daneshamouz, S. Potential effect and mechanism of action of topical chamomile (Matricaria chamomilla L.) oil on migraine headache: A medical hypothesis. Med. Hypotheses 2014, 83, 566–569. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; He, J.; He, D. Chamazulene reverses osteoarthritic inflammation through regulation of matrix metalloproteinases (MMPs) and NF-κB pathway in in-vitro and in-vivo models. Biosci. Biotechnol. Biochem. 2020, 84, 402–410. [Google Scholar] [CrossRef]
- Kolanos, R.; Stice, S.A. German chamomile. In Nutraceuticals; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 757–772. [Google Scholar]
- Mehmood, M.H.; Munir, S.; Khalid, U.A.; Asrar, M.; Gilani, A.H. Antidiarrhoeal, antisecretory and antispasmodic activities of Matricaria chamomilla are mediated predominantly through K⁺-channels activation. BMC Complement. Altern. Med. 2015, 15, 75. [Google Scholar] [CrossRef]
- Petrulova, V.; Vilkova, M.; Kovalikova, Z.; Sajko, M.; Repcak, M. Ethylene induction of non-enzymatic metabolic antioxidants in Matricaria chamomilla. Molecules 2020, 25, 5720. [Google Scholar] [CrossRef]
- Kimura, R.; Schwartz, J.; Bennett-Guerrero, E. A narrative review on the potential therapeutic benefits of chamomile in the acute care setting. J. Herb. Med. 2023, 41, 100714. [Google Scholar] [CrossRef]
- Vissiennon, C.; Goos, K.H.; Arnhold, J.; Nieber, K. Mechanisms on spasmolytic and anti-inflammatory effects of a herbal medicinal product consisting of myrrh, chamomile flower, and coffee charcoal. Wien. Med. Wochenschr. 2017, 167, 169–176. [Google Scholar] [CrossRef]
- Jabria, M.-A.; Aissani, N.; Tounsic, H.; Saklya, M.; Marzoukib, L.; Sebai, H. Protective effect of chamomile (Matricaria recutita L.) decoction extract against alcohol-induced injury in rat gastric mucosa. Pathophysiology 2017, 24, 1–8. [Google Scholar] [CrossRef]
- Tinazzi, M.; Sacilotto, A.; Cocetta, V.; Giacomini, I.; Raso, F.; Bulferi, G.; De Togni, H.; Lanza, R.; Consolo, P.; Berretta, M.; et al. Bowel inflammation and nutrient supplementation: Effects of a fixed combination of probiotics, vitamins, and herbal extracts in an in vitro model of intestinal epithelial barrier dysfunction. Yale J. Biol. Med. 2024, 97, 297–308. [Google Scholar] [CrossRef]
- Sebai, H.; Jabri, M.; Souli, A.; Rtibi, K.; Selmi, S.; Tebourbi, O.; El-Benna, J.; Sakly, M. Antidiarrheal and antioxidant activities of chamomile (Matricaria recutita L.) decoction extract in rats. J. Ethnopharmacol. 2014, 152, 327–332. [Google Scholar] [CrossRef]
- Aleman, R.S.; Moncada, M.; Aryana, K.J. Leaky gut and the ingredients that help treat it: A review. Molecules 2023, 28, 619. [Google Scholar] [CrossRef] [PubMed]
- Schoultz, I.; Keita, A.V. The intestinal barrier and current techniques for the assessment of gut permeability. Cells 2020, 9, 1909. [Google Scholar] [CrossRef]
- Ganzera, M.; Schneider, P.; Stuppner, H. Inhibitory effects of the essential oil of chamomile (Matricaria recutita L.) and its major constituents on human cytochrome P450 enzymes. Life Sci. 2006, 78, 856–861. [Google Scholar] [CrossRef]
- Vora, J.; Srivastava, A.; Modi, H. Antibacterial and antioxidant strategies for acne treatment through plant extracts. Inform. Med. Unlocked 2017, 13, 128–132. [Google Scholar] [CrossRef]
- Cvetanović, A.; Švarc-Gajić, J.; Zeković, Z.; Jerković, J.; Zengin, G.; Gašić, U.; Tešić, Ž.; Mašković, P.; Soares, C.; Fatima Barroso, M.; et al. The influence of the extraction temperature on polyphenolic profiles and bioactivity of chamomile (Matricaria chamomilla L.) subcritical water extracts. Food Chem. 2019, 271, 328–337. [Google Scholar] [CrossRef]
- Abdalla, R.M.; Abdelgadir, A.E. Antibacterial activity and phytochemical constituents of Cinnamomum verum and Matricaria chamomilla from Sudan. Bio Bull. 2016, 2, 8–12. [Google Scholar]
- Abdi, P.; Ourtakand, M.M.; Jahromy, S.H. The effect of Matricaria chamomilla alcoholic extract on phenotype detection of efflux pumps of methicillin resistant Staphylococcus aureus (MRSA) isolated from skin lesions. Iran. J. Med. Microbiol. 2019, 13, 220–231. [Google Scholar] [CrossRef]
- Azari, A.A.; Danesh, A. Antibacterial effect of Matricaria chamomilla alcoholic extract against drug-resistant isolates of Staphylococcus aureus and Pseudomonas aeruginosa. Infect. Epidemiol. Microbiol. 2021, 7, 29–35. [Google Scholar] [CrossRef]
- Sadat, S.S.; Azari, A.A.; Mazandarani, M. Evaluation of the antibacterial activity of ethanolic extract of Matricaria chamomilla, Malva sylvestris, and Capsella bursa-pastoris against methicillin-resistant Staphylococcus aureus. J. Med. Microbiol. Infect. Dis. 2022, 8, 127–131. [Google Scholar] [CrossRef]
- Bayoub, K.; Baibai, T.; Mountassif, D.; Retmane, A. Antibacterial activities of the crude ethanol extracts of medicinal plants against Listeria monocytogenes and some other pathogenic strains. Int. J. Med. Plants Res. 2010, 9, 4251–4258. [Google Scholar]
- Boudieb, K.; Ait Slimane-Ait Kaki, S.; Oulebsir-Mohandkaci, H.; Bennacer, A. Phytochemical characterization and antimicrobial potentialities of two medicinal plants, Chamaemelum nobile (L.) All and Matricaria chamomilla (L.). Int. J. Innov. Approaches Sci. Res. 2018, 2, 126–139. [Google Scholar] [CrossRef]
- Dai, Y.; Li, Y.; Wang, Q.; Niu, F.; Li, K.; Wang, Y.; Wang, J.; Zhou, C.; Gao, L. Chamomile: A review of its traditional uses, chemical constituents, pharmacological activities and quality control studies. Molecules 2023, 28, 133. [Google Scholar] [CrossRef]
- Das, S.; Horváth, B.; Šafranko, S.; Jokic, S.; Széchenyi, A.; Koszegi, T. Antimicrobial activity of chamomile essential oil: Effect of different formulations. Molecules 2019, 24, 4321. [Google Scholar] [CrossRef]
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.H.; Khalel, K.I. Antioxidant and antimicrobial activities of essential oil and extracts of fennel (Foeniculum vulgare L.) and chamomile (Matricaria chamomilla L.). Ind. Crops Prod. 2013, 44, 437–445. [Google Scholar] [CrossRef]
- Akram, W.; Ahmed, S.; Rihan, M.; Arora, S.; Ahmad, S.; Ahmad, F.; Haque, S.; Vashishth, R. An updated comprehensive review of the therapeutic properties of chamomile (Matricaria chamomilla L.). Int. J. Food Prop. 2024, 27, 133–164. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Wang, M.; Firrman, J.; Zhang, L.; Arango-Argoty, G.; Liu, L.; Xiao, W.; Yam, K. Apigenin impacts the growth of the gut microbiota and alters the gene expression of Enterococcus. Molecules 2017, 22, 1292. [Google Scholar] [CrossRef]
- Aguilar, A.; Benslaiman, B.; Serra, J. Effect of Iberogast (STW5) on tolerance to colonic gas in patients with irritable bowel syndrome: A randomized, double-blind, placebo-controlled clinical trial. Neurogastroenterol. Motil. 2024, 36, e14765. [Google Scholar] [CrossRef]
- Michael, R.; Bettina, V.; Eckehard, L. Functional gastrointestinal disorders in children: Effectivity, safety, and tolerability of the herbal preparation STW-5 (Iberogast®) in general practice. Complement. Ther. Med. 2022, 71, 102873. [Google Scholar] [CrossRef] [PubMed]
- Calendula Flower; European Medicines Agency: Amsterdam, The Netherlands, 2018; pp. 1–2.
- Kodiyan, J.; Amber, K.T. A review of the use of topical calendula in the prevention and treatment of radiotherapy-induced skin reactions. Antioxidants 2015, 4, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Ejiohuo, O.; Folami, S.; Maigoro, A.Y. Calendula in modern medicine: Advancements in wound healing and drug delivery applications. Eur. J. Med. Chem. Rep. 2024, 12, 100199. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, S.; Li, T.; Li, N.; Han, D.; Zhang, B.; Xu, Z.Z.; Zhang, S.; Pang, J.; Wang, S.; et al. Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis. Microbiome 2021, 9, 184. [Google Scholar] [CrossRef]
- Babaee, N.; Moslemi, D.; Khalilpour, M.; Vejdani, F.; Moghadamnia, Y.; Bijani, A.; Baradaran, M.; Kazemi, M.T.; Khalilpour, A.; Pouramir, M.; et al. Antioxidant capacity of Calendula officinalis flowers extract and prevention of radiation-induced oropharyngeal mucositis in patients with head and neck cancers: A randomized controlled clinical study. DARU J. Pharm. Sci. 2013, 21, 18. [Google Scholar] [CrossRef]
- Khairnar, M.S.; Pawar, B.; Marawar, P.P.; Mani, A. Evaluation of Calendula officinalis as an anti-plaque and anti-gingivitis agent. J. Indian Soc. Periodontol. 2013, 17, 741–747. [Google Scholar] [CrossRef]
- Efstratiou, E.; Hussain, A.I.; Nigam, P.S.; Moore, J.E.; Ayub, M.A.; Rao, J.R. Antimicrobial activity of Calendula officinalis petal extracts against fungi, as well as Gram-negative and Gram-positive clinical pathogens. Complement. Ther. Clin. Pract. 2012, 18, 173–176. [Google Scholar] [CrossRef]
- da Silva, L.M.; Araújo, A.C.; Bunn, V.S.; Mariano, L.N.B.; Somensi, L.B.; Boeing, T.; Klein, L.C.; de Andrade, S.F. Calendula officinalis L. inflorescences extract: In vivo evaluation of its gastric ulcer healing potential. Biomed. Biopharm. Res. 2020, 17, 75–89. [Google Scholar] [CrossRef]
- Kaur, J.; Sidhu, S.; Chopra, K.; Khan, M.U. Calendula officinalis ameliorates L-arginine-induced acute necrotizing pancreatitis in rats. Pharm. Biol. 2016, 54, 2951–2959. [Google Scholar] [CrossRef]
- Ak, G.; Zengin, G.; Sinan, K.I.; Mahomoodally, M.F.; Yılmaz, M.A.; Gallo, M.; Montesano, D. A comparative bio-evaluation and chemical profiles of Calendula officinalis L. extracts prepared via different extraction techniques. Appl. Sci. 2020, 10, 5920. [Google Scholar] [CrossRef]
- Raal, A.; Orav, A.; Nesterovitsch, J.; Maidla, K. Analysis of carotenoids, flavonoids and essential oil of Calendula officinalis cultivars growing in Estonia. Nat. Prod. Commun. 2016, 11, 1157–1160. [Google Scholar] [CrossRef]
- Khalid, K.A.; El-Ghorab, A.H. The effect of presowing low temperature on essential oil content and chemical composition of Calendula officinalis. J. Essent. Oil Bear. Plants 2013, 9, 37–41. [Google Scholar]
- Ak, G.; Zengin, G.; Ceylan, R.; Fawzi, M.; Jugreet, S.; Mollica, A.; Stefanucci, A. Chemical composition and biological activities of essential oils from Calendula officinalis L. flowers and leaves. Flavour Fragr. J. 2021, 36, 554–563. [Google Scholar] [CrossRef]
- Chamansara, R.; Rashidfarokhi, R.; Sakallı, E.A.; Öztinen, N.; Koşar, M. Comparison of commercial Calendula officinalis L. samples with pharmacopeial drug: Antiradical activities and chemical profiles. J. Res. Pharm. 2022, 26, 809–819. [Google Scholar] [CrossRef]
- Poljšak, N.; Glavač, N.K. Analytical evaluation and antioxidant activity of selected vegetable oils to support evidence-based use in dermal products. Nat. Prod. Commun. 2024, 19, 1934578X241281245. [Google Scholar] [CrossRef]
- Salama, A.B.; Sabry, R.M. Production potential of pot marigold (Calendula officinalis) as a dual-purpose crop. Sarhad J. Agric. 2023, 39, 298–307. [Google Scholar] [CrossRef]
- Rigane, G.; Ben Younes, S.; Ghazghazi, H.; Ben Salem, R. Investigation into the biological activities and chemical composition of Calendula officinalis L. growing in Tunisia. Int. Food Res. J. 2013, 20, 3001–3007. [Google Scholar]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K. Isorhamnetin and quercetin derivatives as anti-acetylcholinesterase principles of marigold (Calendula officinalis) flowers and preparations. Int. J. Mol. Sci. 2017, 18, 1685. [Google Scholar] [CrossRef]
- Manivannan, A.; Narasegowda, S.; Prakash, T. Comparative study on color coordinates, phenolics, flavonoids, carotenoids, and antioxidant potential of marigold (Tagetes sp.) with diverse colored petals. J. Food Meas. Charact. 2021, 15, 4343–4353. [Google Scholar] [CrossRef]
- Soliman, D.M.; Elkaramany, M.F.; El-Sayed, I.M. Using hydrogel polymers to mitigate the negative impact of salinity stress on Calendula officinalis plants. Egypt. J. Chem. 2025, 67, 57–77. [Google Scholar] [CrossRef]
- Neves, A.R.; Faria, R.; Biswas, S.; Costa, D. Plant polysaccharides in formulation coating. In Plant Polysaccharides as Pharmaceutical Excipients; Elsevier Inc.: Amsterdam, The Netherlands, 2023; pp. 391–413. [Google Scholar]
- Cetkovic, G.S.; Djilas, S.M.; Canadanovic-Brunet, J.M.; Tumbas, V.T. Antioxidant properties of marigold extracts. Food Res. Int. 2004, 37, 643–650. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, R.; Finiuk, N.; Stoika, R.; Lin, H.; Wang, X.; Jin, M. Active compounds from Calendula officinalis flowers act via PI3K and ERK signaling pathways to offer neuroprotective effects against Parkinson’s disease. Food Sci. Nutr. 2024, 12, 450–458. [Google Scholar] [CrossRef]
- Cruceriu, D.; Diaconeasa, Z.; Socaci, S.; Socaciu, C.; Rakosy-Tican, E.; Balacescu, O. Biochemical profile, selective cytotoxicity and molecular effects of Calendula officinalis extracts on breast cancer cell lines. Not. Bot. Horti Agrobot. Cluj Napoca 2020, 48, 24–39. [Google Scholar] [CrossRef]
- Golubova, D.; Salmon, M.; Su, H.; Tansley, C.; Kaithakottil, G.; Linsmith, G.; Schudoma, C.; Swarbreck, D.; O’Connell, M.; Patron, N. Biosynthesis and bioactivity of anti-inflammatory triterpenoids in Calendula officinalis. Nat. Commun. 2025, 16, 6941. [Google Scholar] [CrossRef]
- Shivasharan, B.D.; Nagakannan, P.; Thippeswamy, B.S.; Veerapur, V.P.; Bansal, P.; Unnikrishnan, M.K. Protective effect of Calendula officinalis Linn. flowers against 3-nitropropionic acid induced experimental Huntington’s disease in rats. Drug Chem. Toxicol. 2013, 36, 466–473. [Google Scholar] [CrossRef]
- Shivasharan, B.D.; Nagakannan, P.; Thippeswamy, B.S.; Veerapur, V.P. Protective effect of Calendula officinalis L. flowers against monosodium glutamate induced oxidative stress and excitotoxic brain damage in rats. Indian J. Clin. Biochem. 2013, 28, 292–298. [Google Scholar] [CrossRef]
- Dinda, M.; Dasgupta, U.; Singh, N.; Bhattacharyya, D.; Karmakar, P. PI3K-mediated proliferation of fibroblasts by Calendula officinalis tincture: Implication in wound healing. Phytother. Res. 2015, 29, 607–616. [Google Scholar] [CrossRef]
- Colombo, E.; Sangiovanni, E.; Ambrosio, M.D.; Bosisio, E.; Ciocarlan, A.; Fumagalli, M.; Guerriero, A.; Harghel, P.; Agli, M.D. A bio-guided fractionation to assess the inhibitory activity of Calendula officinalis L. on the NF-ΚB driven transcription in human gastric epithelial cells. Evid.-Based Complement. Altern. Med. 2015, 2015, 727342. [Google Scholar] [CrossRef]
- Kadowaki, W.; Miyata, R.; Mizuno, S.; Fujinami, M.; Sato, Y.; Kumazawa, S. Prenylated acetophenones from the roots of Calendula officinalis and their anti-inflammatory activity. Biosci. Biotechnol. Biochem. 2015, 87, 683–687. [Google Scholar] [CrossRef]
- Caamal-Herrera, I.O.; Muñoz-Rodríguez, D.; Madera-Santana, T.; Azamar-Barrios, J.A. Identification of volatile compounds in hydro-alcoholic extracts of Calendula officinalis L. flowers and Mimosae tenuiflorae bark using GC/MS. Int. J. Appl. Res. Nat. Prod. 2016, 9, 20–30. [Google Scholar]
- Nicolaus, C.; Junghanns, S.; Hartmann, A.; Murillo, R.; Ganzera, M. In vitro studies to evaluate the wound healing properties of Calendula officinalis extracts. J. Ethnopharmacol. 2017, 196, 94–103. [Google Scholar] [CrossRef]
- Zournatzis, I.; Liakos, V.; Papadopoulos, S.; Wogiatzi, E. Calendula officinalis—A comprehensive review. Pharmacol. Res. Nat. Prod. 2025, 6, 100140. [Google Scholar] [CrossRef]
- Shafeie, N.; Naini, A.T.; Jahromi, H.K. Comparison of different concentrations of Calendula officinalis gel on cutaneous wound healing. Biomed. Pharmacol. J. 2015, 8, 979–992. [Google Scholar] [CrossRef]
- Okuma, C.H.; Andrade, T.A.M.; Caetano, G.F.; Finci, L.I.; Maciel, N.R.; Topan, J.F.; Cefali, L.C.; Polizello, A.C.M.; Carlo, T.; Rogerio, A.P.; et al. Development of lamellar gel phase emulsion containing marigold oil (Calendula officinalis) as a potential modern wound dressing. Eur. J. Pharm. Sci. 2015, 71, 62–72. [Google Scholar] [CrossRef]
- Sapkota, B.; Kunwar, P. A review on traditional uses, phytochemistry and pharmacological activities of Calendula officinalis Linn. Nat. Prod. Commun. 2024, 19, 1934578X241259021. [Google Scholar] [CrossRef]
- Karnwal, A. In vitro antibacterial activity of Hibiscus rosa-sinensis, Chrysanthemum indicum, and Calendula officinalis flower extracts against Gram-negative and Gram-positive food poisoning bacteria. Adv. Tradit. Med. 2021, 22, 607–619. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Ricardo, C.C.; Alves, M.J.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Edible flowers as sources of phenolic compounds with bioactive potential. Food Res. Int. 2017, 105, 580–588. [Google Scholar] [CrossRef]
- Cwikla, C.; Schmidt, K.; Matthias, A.; Bone, K.M.; Lehmann, R.; Tiralongo, E. Investigations into the antibacterial activities of phytotherapeutics against Helicobacter pylori and Campylobacter jejuni. Phytother. Res. 2010, 24, 649–656. [Google Scholar] [CrossRef]
- Darekar, D.P.; Hate, M.S. Phytochemical screening of Calendula officinalis (Linn.) using gas-chromatography-mass spectroscopy with potential antibacterial activity. J. Sci. Res. 2021, 65, 6–12. [Google Scholar]
- Shahen, Z.; Mahmud, S.; Sohana, S.N.; Rony, M.H.; Imran, A.S.; Maruf, A.A.; Azim, A.A.; Islam, M.; Islam, R.; Uddin, E.; et al. Effect of antibiotic susceptibility and inhibitory activity for the control of growth and survival of microorganisms of extracts of Calendula officinalis. Eur. J. Med. Health Sci. 2019, 1, 1–9. [Google Scholar] [CrossRef]
- Khouchlaa, A.; El Baaboua, A.; El Moudden, H.; Lakhdar, F.; Bakrim, S.; El Menyiy, N.; Belmehdi, O.; Harhar, H.; El Omari, N.; Balahbib, A.; et al. Traditional uses, bioactive compounds, and pharmacological investigations of Calendula arvensis L.: A comprehensive review. Adv. Pharmacol. Pharm. Sci. 2023, 2023, 2482544. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Koprowska, K.; Gottschling, A.; Janda-Milczarek, K. Edible flowers as a source of dietary fibre (total, insoluble and soluble) as a potential athlete’s dietary supplement. Nutrients 2022, 14, 2470. [Google Scholar] [CrossRef]
- Givol, O.; Kornhaber, R.; Visentin, D.; Cleary, M.; Haik, J.; Harats, M. A systematic review of Calendula officinalis extract for wound healing. Wound Repair Regen. 2019, 27, 548–561. [Google Scholar] [CrossRef]
- Pazhohideh, Z.; Mohammadi, S.; Bahrami, N.; Mojab, F.; Abedi, P.; Maraghi, E. The effect of Calendula officinalis versus metronidazole on bacterial vaginosis in women: A double-blind randomized controlled trial. J. Adv. Pharm. Technol. Res. 2018, 9, 15–19. [Google Scholar] [CrossRef]
- Tedeschi, C.; Benvenuti, C. Comparison of vaginal gel isoflavones versus no topical treatment in vaginal dystrophy: Results of a preliminary prospective study. Gynecol. Endocrinol. 2012, 28, 652–654. [Google Scholar] [CrossRef]
- Geekiyanage, S.; Azad, R.; Ranawaka, R.A.A.K.; Maddumage, R.P. Cinnamon: Botany, cultivars, and genetic diversity. In Cinnamon: Production, Processing, and Functional Properties; Academic Press: Cambridge, MA, USA, 2025; pp. 23–49. [Google Scholar]
- Bhagya Chandrasekara, C.H.W.M.R.; Naranpanawa, D.N.U.; Bandusekara, B.S.; Pushpakumara, D.K.N.G.; Wijesundera, D.S.A.; Bandaranayake, P.C.G. Universal barcoding regions, rbcL, matK and trnH-psbA do not discriminate Cinnamomum species in Sri Lanka. PLoS ONE 2021, 16, e0245592. [Google Scholar] [CrossRef]
- Anderson, R.A.; Zhan, Z.; Luo, R.; Guo, X.; Guo, Q.; Zhou, J.; Kong, J.; Davis, P.A.; Stoecker, B.J. Cinnamon extract lowers glucose, insulin and cholesterol in people with elevated serum glucose. J. Tradit. Complement. Med. 2016, 6, 332–336. [Google Scholar] [CrossRef]
- Beejmohun, V.; Peytavy-Izard, M.; Mignon, C.; Muscente-Paque, D.; Deplanque, X.; Ripoll, C.; Chapal, N. Acute effect of Ceylon cinnamon extract on postprandial glycemia: Alpha-amylase inhibition, starch tolerance test in rats, and randomized crossover clinical trial in healthy volunteers. BMC Complement. Altern. Med. 2014, 14, 351. [Google Scholar] [CrossRef]
- Solomon, T.P.J.; Blannin, A.K. Effects of short-term cinnamon ingestion on in vivo glucose tolerance. Diabetes Obes. Metab. 2007, 9, 895–901. [Google Scholar] [CrossRef]
- Beji, R.S.; Khemir, S.; Wannes, W.A.; Ayari, K.; Ksouri, R. Antidiabetic, antihyperlipidemic and antioxidant influences of the spice cinnamon (Cinnamomum zeylanicumon) in experimental rats. Braz. J. Pharm. Sci. 2018, 54, e17576. [Google Scholar] [CrossRef]
- Spence, C. Cinnamon: The historic spice, medicinal uses, and flavour chemistry. Int. J. Gastron. Food Sci. 2024, 35, 100858. [Google Scholar] [CrossRef]
- Renda, K.C.; Jeewanthi, A.M.D.S.; Weddagala, T. Value creation and food products of cinnamon. In Cinnamon; Springer Nature Switzerland AG: Cham, Switzerland, 2020; pp. 363–376. [Google Scholar]
- Ballin, N.Z.; Sørensen, A.T. Coumarin content in cinnamon containing food products on the Danish market. Food Control 2014, 38, 198–203. [Google Scholar] [CrossRef]
- Shinjyo, N.; Waddell, G.; Green, J. A tale of two cinnamons: A comparative review of the clinical evidence of Cinnamomum verum and C. cassia as diabetes interventions. J. Herb. Med. 2020, 21, 100342, Correction in J. Herb. Med. 2021, 28, 100383. [Google Scholar] [CrossRef]
- Mancak, M.; Çalişkan, U.K. Are cinnamon derivatives effective and safe for diabetes? Turk. J. Med. Sci. 2025, 55, 313–327. [Google Scholar] [CrossRef]
- Kevser, K.; Hamza, A.; Bulent, B. Cinnamon novel formulations and encapsulation: Chemistry and functionality. In Cinnamon: Technology, Processing, and Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2025; pp. 401–425. [Google Scholar]
- Barbarossa, A.; Sblano, S.; Rosato, A.; Carrieri, A.; Corbo, F.; Clodoveo, M.L.; Fracchiolla, G.; Carocci, A. Synergistic action of Cinnamomum verum essential oil with sertraline. Antibiotics 2022, 11, 1617. [Google Scholar] [CrossRef]
- Abeysekera, W.P.K.M.; Premakumara, G.A.S.; Ratnasooriya, W.D.; Abeysekera, W.K.S.M. Anti-inflammatory, cytotoxicity and antilipidemic properties: Novel bioactivities of true cinnamon (Cinnamomum zeylanicum Blume) leaf. BMC Complement. Med. Ther. 2022, 22, 259. [Google Scholar] [CrossRef]
- Senevirathne, B.S.; Jayasinghe, M.A.; Pavalakumar, D.; Siriwardhana, C.G. Ceylon cinnamon: A versatile ingredient for futuristic diabetes management. J. Future Foods 2022, 2, 125–142. [Google Scholar] [CrossRef]
- Lipan, L.; Cano-Lamadrid, M.; Issa-Issa, H.; Munoz, C.; Hernandez, F.; Carbonell-Barrachina, A.; Sendra, E. Multivariate analysis of chemical markers to distinguish “Ceylon” and “Cassia” cinnamon in the Spanish market. Food Chem. X 2025, 28, 102484. [Google Scholar] [CrossRef]
- Singh, N.; Singh, A.; Nandal, A.; Kumar, S.; Singh, S.; Ahmad, S.; Narasimhan, B. Phytochemical and pharmacological review of Cinnamomum verum J. Presl—A versatile spice used in food and nutrition. Food Chem. 2021, 338, 127773. [Google Scholar] [CrossRef]
- Sharma, V.; Devkota, L.; Kishore, N.; Dhital, S. Understanding the interplay between dietary fiber, polyphenols, and digestive enzymes. Food Hydrocoll. 2025, 166, 111310. [Google Scholar] [CrossRef]
- Nemzer, B.V.; Al-Taher, F.; Kalita, D.; Yashin, A.Y.; Yashin, Y.I. Health-improving effects of polyphenols on the human intestinal microbiota: A review. Int. J. Mol. Sci. 2025, 26, 1335. [Google Scholar] [CrossRef]
- Cheng, B.; Feng, H.; Li, C.; Jia, F.; Zhang, X. The mutual effect of dietary fiber and polyphenol on gut microbiota: Implications for metabolic and microbial modulation and associated health benefits. Carbohydr. Polym. 2025, 358, 123541. [Google Scholar] [CrossRef]
- Mathew, S.; Abraham, T.E. Studies on the antioxidant activities of cinnamon (Cinnamomum verum) bark extracts, through various in vitro models. Food Chem. 2006, 94, 520–528. [Google Scholar] [CrossRef]
- Beniaich, G.; Zouirech, O.; Allali, A.; Bouslamti, M.; Maliki, I.; El Moussaoui, A.; Chebaibi, M.; Nafidi, H.A.; Bin Jardan, Y.A.; Bourhia, M.; et al. Chemical characterization, antioxidant, insecticidal and anti-cholinesterase activity of essential oils extracted from Cinnamomum verum L. Separations 2023, 10, 348. [Google Scholar] [CrossRef]
- Farias, A.P.P.; Monteiro, O.d.S.; da Silva, J.K.R.; Figueiredo, P.L.B.; Maia, A.A.C.R.; Monteiro, I.N.; Maia, J.G.S. Chemical composition and biological activities of two chemotype oils from Cinnamomum verum J. Presl growing in North Brazil. J. Food Sci. Technol. 2020, 57, 3176–3183. [Google Scholar] [CrossRef]
- Simsek, U.G.; Ciftci, M.; Dogan, G.; Ozcelik, M. Antioxidant activity of cinnamon bark oil (Cinnamomum zeylanicum L.) in Japanese quails under thermo neutral and heat stressed conditions. Kafkas Univ. Vet. Fak. Derg. 2013, 19, 889–894. [Google Scholar]
- Khalisyaseen, O.; Mohammed, M.T. Analytics detection of phytochemical compounds in Cinnamomum zeylanicum bark extract. Egypt J. Chem. 2023, 66, 265–273. [Google Scholar]
- Yadav, N.; Saini, N.; Rathi, N.; Chauhan, S.; Sharma, P.K.; Sangwan, N.S. Health-promoting effects of cinnamon. In Cinnamon: Production, Processing, and Functional Properties; Academic Press: Cambridge, MA, USA, 2025; pp. 219–241. [Google Scholar]
- Pangaribowo, D.A.; Nugraha, F.A.S.; Puspaningtyas, A.R.; Sary, I.P. Structure modification of cinnamic acid to (E)-1-(3,4-dihydroisoquinoline-2(1H)-yl)-3-phenylprop-2-en-1-one and antioxidant activity test by DPPH method. Borneo J. Pharm. 2024, 7, 254–263. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, J.Y. Intestinal anti-inflammatory effects of cinnamon extracts in a co-culture model of intestinal epithelial Caco-2 cells and RAW264.7 macrophages. Appl. Biol. Chem. 2017, 60, 553–561. [Google Scholar] [CrossRef]
- Schink, A.; Naumoska, K.; Kitanovski, Z.; Thines, E.; Pöschl, U.; Lucas, K. Anti-inflammatory effects of cinnamon extract and identification of active compounds influencing the TLR2 and TLR4 signaling pathways. Food Funct. 2018, 9, 5950. [Google Scholar] [CrossRef] [PubMed]
- Youn, H.S.; Lee, J.K.; Choi, Y.J.; Saitoh, S.I.; Miyake, K.; Hwang, D.H.; Lee, J.Y. Cinnamaldehyde suppresses Toll-like receptor 4 activation mediated through the inhibition of receptor oligomerization. Biochem. Pharmacol. 2008, 75, 494–502. [Google Scholar] [CrossRef]
- Kim, N.-Y.; Kim, S.; Park, H.-M.; Lim, C.-M.; Kim, J.; Park, J.-Y.; Jeon, K.-B.; Poudel, A.; Lee, H.P.; Oh, S.-R.; et al. Cinnamomum verum extract inhibits NOX2/ROS and PKCδ/JNK/AP-1/NF-ΚB pathway-mediated inflammatory response in PMA-stimulated THP-1 monocytes. Phytomedicine 2023, 112, 154685. [Google Scholar] [CrossRef]
- Tung, Y.-T.; Chua, M.-T.; Wang, S.-Y.; Chang, S.-T. Anti-inflammation activities of essential oil and its constituents from indigenous cinnamon (Cinnamomum osmophloeum) twigs. Bioresour. Technol. 2008, 99, 3908–3913. [Google Scholar] [CrossRef]
- Vasconcelos, N.G.; Croda, J.; Simionatto, S. Antibacterial mechanisms of cinnamon and its constituents: A review. Microb. Pathog. 2018, 120, 198–203. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.-Z.; Brooks, J.D.; Corke, H. Antibacterial properties and major bioactive components of cinnamon stick (Cinnamomum burmannii): Activity against foodborne pathogenic bacteria. J. Agric. Food Chem. 2007, 55, 5484–5490. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef]
- Gill, A.O.; Holley, R.A. Mechanisms of bactericidal action of cinnamaldehyde against Listeria monocytogenes and of eugenol against L. monocytogenes and Lactobacillus sakei. Appl. Environ. Microbiol. 2004, 70, 5750–5755. [Google Scholar] [CrossRef]
- Anne, M.; Amalaradjou, R.; Venkitanarayanan, K. Effect of trans-cinnamaldehyde on reducing resistance to environmental stresses in Cronobacter sakazakii. Foodborne Pathog. Dis. 2015, 8, 403–409. [Google Scholar]
- Usta, J.; Kreydiyyeh, S.; Barnabe, P.; Bou-Moughlabay, Y.; Nakkash-Chmaisse, H. Comparative study on the effect of cinnamon and clove extracts and their main components on different types of ATPases. Hum. Exp. Toxicol. 2003, 22, 355–362. [Google Scholar] [CrossRef]
- Gill, A.O.; Holley, R.A. Inhibition of membrane-bound ATPases of Escherichia coli and Listeria monocytogenes by plant oil aromatics. Int. J. Food Microbiol. 2006, 111, 170–174. [Google Scholar] [CrossRef]
- Domadia, P.; Swarup, S.; Bhunia, A.; Sivaraman, J.; Dasgupta, D. Inhibition of bacterial cell division protein FtsZ by cinnamaldehyde. Biochem. Pharmacol. 2007, 74, 831–840. [Google Scholar] [CrossRef]
- Vollmer, W. The prokaryotic cytoskeleton: A putative target for inhibitors and antibiotics? Appl. Microbiol. Biotechnol. 2006, 73, 37–47. [Google Scholar] [CrossRef]
- Jia, P.; Xue, Y.J.; Duan, X.J.; Shao, S.H. Effect of cinnamaldehyde on biofilm formation and SarA expression by methicillin-resistant Staphylococcus aureus. Lett. Appl. Microbiol. 2011, 53, 409–416. [Google Scholar] [CrossRef]
- Kavanaugh, N.L.; Ribbeck, K. Selected antimicrobial essential oils eradicate Pseudomonas spp. and Staphylococcus aureus biofilm. Appl. Environ. Microbiol. 2012, 78, 4057–4061. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, W.; Zhang, W.; Yang, A.; Liu, Y.; Jiang, Y.A.N.; Huang, S.; Su, J. Inhibitory effects of citral, cinnamaldehyde, and tea polyphenols on mixed biofilm formation by foodborne Staphylococcus aureus and Salmonella enteritidis. J. Food Prot. 2014, 77, 927–933. [Google Scholar] [CrossRef]
- Brackman, G.; Hillaert, U.; Van Calenbergh, S.; Nelis, H.J.; Coenye, T. Use of quorum sensing inhibitors to interfere with biofilm formation and development in Burkholderia multivorans and Burkholderia cenocepacia. Res. Microbiol. 2009, 160, 144–151. [Google Scholar] [CrossRef]
- Niu, C.; Gilbert, E.S. Colorimetric method for identifying plant essential oil components that affect biofilm formation and structure. Appl. Environ. Microbiol. 2004, 70, 6951–6956. [Google Scholar] [CrossRef]
- Upadhyay, A.; Upadhyaya, I.; Kollanoor-Johny, A.; Venkitanarayanan, K. Antibiofilm effect of plant derived antimicrobials on Listeria monocytogenes. Food Microbiol. 2013, 36, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.F.; dos Santos, A.R.; Trevisan, D.A.C.; Ribeiro, A.B.; Campanerut-Sa, P.A.Z.; Kukolj, C.; de Souza, E.M.; Cardoso, R.F.; Svidzinski, T.I.E.; de Abreu Filho, B.A.; et al. Cinnamaldehyde induces changes in the protein profile of Salmonella Typhimurium biofilm. Res. Microbiol. 2018, 169, 33–43. [Google Scholar] [CrossRef]
- Brackman, G.; Celen, S.; Hillaert, U.; Van Calenbergh, S.; Cos, P.; Maes, L.; Nelis, H.J.; Coenye, T. Structure-activity relationship of cinnamaldehyde analogs as inhibitors of AI-2 based quorum sensing and their effect on virulence of Vibrio spp. PLoS ONE 2011, 6, e16084. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, L. Antibiofilm effect and mechanism of protocatechuic aldehyde against Vibrio parahaemolyticus. Front. Microbiol. 2022, 13, 1060506. [Google Scholar] [CrossRef]
- Smith, C.K.; Moore, C.A.; Elahi, E.N.; Smart, A.T.S.; Hotchkiss, S.A.M. Human skin absorption and metabolism of the contact allergens, cinnamic aldehyde, and cinnamic alcohol. Toxicol. Appl. Pharmacol. 2000, 168, 189–199. [Google Scholar] [CrossRef]
- Yuan, J.H.; Dieter, M.P.; Buctter, J.R.; Jameson, C.W. Toxicokinetics of cinnamaldehyde in F344 rats. Food Chem. Toxicol. 1992, 30, 997–1004. [Google Scholar] [CrossRef]
- Lu, Q.-Y.; Summanen, P.H.; Lee, R.-P.; Huang, J.; Henning, S.M.; Heber, D.; Finegold, S.M.; Li, Z. Prebiotic potential and chemical composition of seven culinary spice extracts. J. Food Sci. 2017, 82, 1807–1813. [Google Scholar] [CrossRef]
- Sutherland, J.; Miles, M.; Hedderley, D.; Li, J.; Devoy, S.; Sutton, K.; Lauren, D. In vitro effects of food extracts on selected probiotic and pathogenic bacteria. Int. J. Food Sci. Nutr. 2009, 60, 717–727. [Google Scholar] [CrossRef]
- Khare, P.; Jagtap, S.; Jain, Y.; Baboota, R.K.; Mangal, P.; Boparai, R.K.; Bhutani, K.K.; Sharma, S.S.; Premkumar, L.S.; Kondepudi, K.K.; et al. Cinnamaldehyde supplementation prevents fasting-induced hyperphagia, lipid accumulation, and inflammation in high-fat diet-fed mice. BioFactors 2016, 42, 201–211. [Google Scholar] [CrossRef]
- Gruenwald, J.; Freder, J.; Armbruester, N. Cinnamon and health. Crit. Rev. Food Sci. Nutr. 2010, 50, 822–834. [Google Scholar] [CrossRef]
- Singletary, K. Cinnamon: Update of potential health benefits. Nutr. Today 2019, 54, 42–52. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, Y.D.; Kim, M.S.; Kim, K.T.; Kim, J.Y. Cinnamon (Cinnamomum cassia) water extract improves diarrhea symptoms by changing the gut environment: A randomized controlled trial. Food Funct. 2023, 14, 1520–1529. [Google Scholar] [CrossRef]
- Moridpour, A.H.; Kavyani, Z.; Khosravi, S.; Farmani, E.; Daneshvar, M.; Musazadeh, V.; Faghfouri, A.H. The effect of cinnamon supplementation on glycemic control in patients with type 2 diabetes mellitus: An updated systematic review and dose-response meta-analysis of randomized controlled trials. Phytother. Res. 2024, 38, 117–130. [Google Scholar] [CrossRef]
- Rachid, A.P.; Moncada, M.; de Mesquita, M.F.; Brito, J.; Silva, M.L.; Bernardo, M.A. Effect of aqueous cinnamon extract on the postprandial glycemia levels in patients with type 2 diabetes mellitus: A randomized controlled trial. Nutrients 2022, 14, 1576. [Google Scholar] [CrossRef]
- Schutz, K.; Carle, R.; Schieber, A. Taraxacum—A review on its phytochemical and pharmacological profile. J. Ethnopharmacol. 2006, 107, 313–323. [Google Scholar] [CrossRef]
- Di Napoli, A.; Zucchetti, P. A comprehensive review of the benefits of Taraxacum officinale on human health. Bull. Natl. Res. Cent. 2021, 45, 110. [Google Scholar] [CrossRef]
- Pfingstgraf, I.O.; Taulescu, M.; Pop, R.M.; Or, R.; Vlase, L.; Uifalean, A.; Todea, D.; Alexescu, T.; Toma, C.; Parvu, A.E. Protective effects of Taraxacum officinale L. (dandelion) root extract in experimental acute on chronic liver failure. Antioxidants 2021, 10, 504. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Sun-waterhouse, D. The potential of dandelion in the fight against gastrointestinal diseases: A review. J. Ethnopharmacol. 2022, 293, 115272. [Google Scholar] [CrossRef]
- Lim, T.K. Taraxacum officinale. In Edible Medicinal and Non-Medicinal Plants; Springer: Dordrecht, The Netherlands, 2014; pp. 516–536. [Google Scholar]
- Yan, Q.; Xing, Q.; Liu, Z.; Zou, Y.; Liu, X.; Xia, H. The phytochemical and pharmacological profile of dandelion. Biomed. Pharmacother. 2024, 179, 117334. [Google Scholar] [CrossRef]
- Tsai, W.-C.; Chang, H.-C.; Tseng, Y.-H.; Yin, H.-Y.; Liao, J.-W.; Chandra, D.; Wen, H.-W. Toxicity evaluation of water extract of tissue-cultured Taraxacum formosanum by acute, subacute administration, and Ames test. Electron. J. Biotechnol. 2020, 45, 38–45. [Google Scholar] [CrossRef]
- Ključevšek, T.; Kref, S. Allergic potential of medicinal plants from the Asteraceae family. Health Sci. Rep. 2025, 8, e70398. [Google Scholar] [CrossRef] [PubMed]
- Jedrejek, D.; Lis, B.; Rolnik, A.; Stochmal, A.; Olas, B. Comparative phytochemical, cytotoxicity, antioxidant and haemostatic studies of Taraxacum officinale root preparations. Food Chem. Toxicol. 2019, 126, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, S.; Adil, S.; El-Hack, M.E.A.B.D.; Alagawany, M.; Farag, M.R. Beneficial uses of dandelion herb (Taraxacum officinale) in poultry nutrition. Worlds Poult. Sci. J. 2017, 73, 591–602. [Google Scholar] [CrossRef]
- Tekam, I.; Dubey, S.; Mahajan, S.; Sheikh, S.; Singh, S.M.; Kewat, A. Quantitative and qualitative assessment of phytochemicals in dandelion (Taraxacum officinale) leaves. J. Exp. Zool. India 2024, 27, 699–701. [Google Scholar] [CrossRef]
- Xue, Y.; Zhang, S.; Du, M.; Zhu, M. Dandelion extract suppresses reactive oxidative species and inflammasome in intestinal epithelial cells. J. Funct. Foods 2017, 29, 10–18. [Google Scholar] [CrossRef]
- Brezo-Borjan, T.; Švarc-Gajić, J.V. Subcritical water extraction of dandelion (Taraxacum officinale L.) flowers: Influence of temperature on polyphenols content and antioxidant activity. Food Feed Res. 2024, 51, 219–222. [Google Scholar] [CrossRef]
- Kour, J.; Sharma, R.; Nayik, G.A.; Ramaiyan, B.; Sofi, S.A.; Alam, M.S.; Anand, N. Dandelion. In Antioxidants in Vegetables and Nuts—Properties and Health Benefit; Springer Nature Singapore Pte Ltd.: Singapore, 2020; pp. 237–248. [Google Scholar]
- Biel, W.; Jaroszewska, A.; Łysoń, E.; Telesiński, A. The chemical composition and antioxidant properties of common dandelion leaves compared with sea buckthorn. Can. J. Plant Sci. 2017, 97, 1165–1174. [Google Scholar] [CrossRef]
- Colle, D.; Arantes, L.P.; Rauber, R.; de Mattos, S.E.C.; Rocha, J.B.T.; Nogueira, C.W.; Soares, F.A.A. Antioxidant properties of Taraxacum officinale fruit extract are involved in the protective effect against cellular death induced by sodium nitroprusside in brain of rats. Pharm. Biol. 2012, 50, 883–891. [Google Scholar] [CrossRef]
- Epure, A.; Pârvu, A.E.; Vlase, L.V.; Benedec, D.; Hanganu, D.; Oniga, O.; Vlase, A.-M.; Ielciu, I.; Toiu, A.; Oniga, I. New approaches on the anti-inflammatory and cardioprotective properties of Taraxacum officinale tincture. Pharmaceuticals 2023, 16, 358. [Google Scholar] [CrossRef]
- Epure, A.; Pârvu, A.; Vlase, L.; Benedec, D.; Hanganu, D.; Vlase, A.M.; Oniga, I. Polyphenolic compounds, antioxidant activity and nephroprotective properties of Romanian Taraxacum officinale. Farmacia 2022, 70, 47–53. [Google Scholar] [CrossRef]
- Jalili, C.; Abbasi, A.; Rahmani-Kukia, N.; Andarzi, S.; Kakebaraie, S.; Zamir, T. The relationship between aflatoxin B1 with the induction of extrinsic/intrinsic pathways of apoptosis and the protective role of taraxasterol in TM3 Leydig cell line. Ecotoxicol. Environ. Saf. 2024, 276, 116316. [Google Scholar] [CrossRef]
- Ge, B.; Sang, R.; Wang, W.; Yan, K.; Yu, Y.; Kong, L.; Yu, M.; Liu, X.; Zhang, X. Protection of taraxasterol against acetaminophen-induced liver injury elucidated through network pharmacology and in vitro and in vivo experiments. Phytomedicine 2023, 116, 154872. [Google Scholar] [CrossRef]
- Korbášová, M.; Tomenendálová, J.; Chloupek, J. Anti-tumour effect of combinations of three acids isolated from Taraxacum officinale. Acta Vet. Brno 2022, 91, 77–85. [Google Scholar] [CrossRef]
- Liu, B.; He, Z.; Wang, J.; Xin, Z.; Wang, J.; Li, F.; Fu, Y. Taraxasterol inhibits LPS-induced inflammatory response in BV2 microglia cells by activating LXRα. Front. Pharmacol. 2018, 9, 278. [Google Scholar] [CrossRef]
- Jin, L.; Li, M.; Kai, G.; He, B.; Li, L.; Liu, Y.; Zhang, J.; Wang, B.; Sun, C. Taraxasterol acetate from Taraxacum officinale ameliorates dextran sulfate sodium–induced ulcerative colitis in mice in association with changes of the metabolism and structure of gut microbiota. Food Front. 2025, 6, 891–908. [Google Scholar] [CrossRef]
- Park, C.M.; Cho, C.W.; Song, Y.S. TOP 1 and 2, polysaccharides from Taraxacum officinale, inhibit NF-κB-mediated inflammation and accelerate Nrf2-induced antioxidative potential through the modulation of PI3K-Akt signaling pathway in RAW 264.7 cells. Food Chem. Toxicol. 2014, 66, 56–64. [Google Scholar] [CrossRef]
- Molan, A.L.; Flanagan, J.; Wei, W.; Moughan, P.J. Selenium-containing green tea has higher antioxidant and prebiotic activities than regular green tea. Food Chem. 2009, 114, 829–835. [Google Scholar] [CrossRef]
- Díaz, K.; Espinoza, L.; Madrid, A.; Pizarro, L.; Chamy, R. Isolation and identification of compounds from bioactive extracts of Taraxacum officinale Weber ex F. H. Wigg. (dandelion) as a potential source of antibacterial agents. Evid.-Based Complement. Altern. Med. 2018, 2018, 2706417. [Google Scholar] [CrossRef]
- Ionescu, D.; Predan, G.; Rizea, G.D.; Mihele, D.; Dune, A.; Ivopol, G.; Ioniţă, C. Antimicrobial activity of some hydroalcoholic extracts of artichoke (Cynara scolymus), burdock (Arctium lappa) and dandelion (Taraxacum officinale). For. Wood Ind. Agric. Food Eng. 2013, 6, 113–120. [Google Scholar]
- Cao, Z.; Ding, Y.; Liu, Z.; Liu, M.; Wu, H.; Zhao, J.; Dong, X.; Shang, H. Extraction condition optimization and prebiotic potential of dandelion (Taraxacum mongolicum Hand.-Mazz.) polysaccharides. Ind. Crops Prod. 2023, 194, 130118. [Google Scholar] [CrossRef]
- Salvatore, S.; Ruffolo, A.F.; Stabile, G.; Casiraghi, A.; Zito, G.; De Seta, F. A randomized controlled trial comparing a new D-mannose-based dietary supplement to placebo for the treatment of uncomplicated Escherichia coli urinary tract infections. Eur. Urol. Focus 2023, 9, 654–659. [Google Scholar] [CrossRef]
- Jin, X.; Xiao, J.; Lu, C.; Ma, W.; Fan, Y.; Xue, X.; Xia, Y.; Chen, N.; Liu, J.; Pei, X. Breastmilk microbiome changes associated with lactational mastitis and treatment with dandelion extract. Front. Microbiol. 2023, 14, 1247868. [Google Scholar] [CrossRef]
- Badgujar, S.B.; Patel, V.V.; Bandivdekar, A.H. Foeniculum vulgare Mill: A review of its botany, phytochemistry, pharmacology, contemporary application, and toxicology. BioMed Res. Int. 2014, 2014, 842674. [Google Scholar] [CrossRef] [PubMed]
- Rather, M.A.; Dar, B.A.; Sofi, S.N.; Bhat, B.A.; Qurishi, M.A. Foeniculum vulgare: A comprehensive review of its traditional use, phytochemistry, pharmacology, and safety. Arab. J. Chem. 2016, 9, S1574–S1583. [Google Scholar] [CrossRef]
- Gori, L.; Gallo, E.; Mascherini, V.; Mugelli, A.; Vannacci, A.; Firenzuoli, F. Can estragole in fennel seed decoctions really be considered a danger for human health? A fennel safety update. Evid.-Based Complement. Altern. Med. 2012, 2012, 860542. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, R.; Barreira, J.C.M.; Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Effects of oral dosage form and storage period on the antioxidant properties of four species used in traditional herbal medicine. Phytother. Res. 2011, 25, 484–492. [Google Scholar] [CrossRef]
- Noreen, S.; Tufail, T.; Badar, H.; Ain, U.; Godswill, C.A. Pharmacological, nutraceutical, functional and therapeutic properties of fennel (Foeniculum vulgare). Int. J. Food Prop. 2023, 26, 915–927. [Google Scholar] [CrossRef]
- Cioanca, O.; Hancianu, M.; Mircea, C.; Trifan, A.; Hritcu, L. Essential oils from Apiaceae as valuable resources in neurological disorders: Foeniculum vulgare Aetheroleum. Ind. Crops Prod. 2016, 88, 51–57. [Google Scholar] [CrossRef]
- Faudale, M.A.; Viladomat, F.R.; Jaume, B.; Poli, F.; Codina, C. Antioxidant activity and phenolic composition of wild, edible, and medicinal fennel from different Mediterranean countries. J. Agric. Food Chem. 2008, 56, 1912–1920. [Google Scholar] [CrossRef]
- Shahrahmani, H.; Ghazanfarpour, M.; Shahrahmani, N.; Abdi, F.; Sewell, R.D.E.; Rafieian-Kopae, M. Effect of fennel on primary dysmenorrhea: A systematic review and meta-analysis. J. Integr. Complement. Med. 2021, 18, 261–269. [Google Scholar] [CrossRef]
- Di Napoli, M.; Castagliuolo, G.; Badalamenti, N.; Bruno, M.; Varcamonti, M.; Zanfardino, A. Antimicrobial, antibiofilm, and antioxidant properties of essential oil of Foeniculum vulgare Mill. leaves. Plants 2022, 11, 3573. [Google Scholar] [CrossRef]
- Seid, M.; Dekebo, A.; Babu, N. Phytochemical investigation and antimicrobial evaluation of Foeniculum vulgare leaves extract ingredient of Ethiopian local liquor. J. Pharm. Nutr. Sci. 2018, 8, 20–28. [Google Scholar] [CrossRef]
- Rahimi, R.; Ardekani, M.R.S. Medicinal properties of Foeniculum vulgare Mill. in traditional Iranian medicine and modern phytotherapy. Chin. J. Integr. Med. 2013, 19, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Bampidis, V.; Azimonti, G.; Bastos, M.d.L.; Christensen, H.; Fašmon Durjava, M.; Kouba, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; Mayo, B.; et al. Safety and efficacy of a feed additive consisting of a tincture derived from the fruit of Foeniculum vulgare Mill. ssp. vulgare var. dulce (sweet fennel tincture) for use in all animal species (FEFANA Asbl). EFSA J. 2023, 21, e07693. [Google Scholar]
- Subehan, S.F.H.Z.; Kadota, S.; Tezuka, Y. Inhibition on human liver cytochrome P450 3A4 by constituents of fennel (Foeniculum vulgare): Identification and characterization of a mechanism-based inactivator. J. Agric. Food Chem. 2007, 55, 10162–10167. [Google Scholar] [CrossRef]
- Subehan; Usia, T.; Iwata, H.; Kadota, S.; Tezuka, Y. Mechanism-based inhibition of CYP3A4 and CYP2D6 by Indonesian medicinal plants. J. Ethnopharmacol. 2006, 105, 449–455. [Google Scholar] [CrossRef]
- Salama, Y.; Al-Maharik, N. Micromeria fruticosa and Foeniculum vulgare essential oils inhibit melanoma cell growth and migration by targeting MMP9 and NF-κB signaling. Chem. Biol. Technol. Agric. 2024, 11, 6. [Google Scholar] [CrossRef]
- Javed, R.; Hanif, M.A.; Ayub, M.A.; Rehman, R. Fennel. In Medicinal Plants of South Asia; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; pp. 241–256. [Google Scholar]
- Chang, S.; Nafchi, A.M.; Karim, A.A. Chemical composition, antioxidant activity and antimicrobial properties of three selected varieties of Iranian fennel seeds. J. Essent. Oil Res. 2016, 28, 357–363. [Google Scholar] [CrossRef]
- Diao, W.-R.; Hu, Q.-P.; Zhang, H.; Xu, J.-G. Chemical composition, antibacterial activity and mechanism of action. Food Control 2014, 35, 109–116. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, X.I.; Devshilt, I.; Yun, Q.I. Fennel main constituent, trans-anethole treatment against LPS-induced acute lung injury by regulation of Th17/Treg function. Mol. Med. Rep. 2018, 18, 1369–1376. [Google Scholar] [CrossRef]
- Ncube, N.H.; Gupta, J. Foeniculum vulgare (fennel): A comprehensive review of its anti-diabetic properties. Asian Pac. J. Trop. Biomed. 2025, 15, 85–97. [Google Scholar] [CrossRef]
- Crescenzi, M.A.; Gallart-Ayala, H.; Stellato, C.; Popolo, A.; Ivanisevic, J.; Piacente, S.; Montoro, P. A targeted mass spectrometric approach to evaluate the anti-inflammatory activity of the major metabolites of Foeniculum vulgare Mill. waste in human bronchial epithelium. Molecules 2025, 30, 1407. [Google Scholar] [CrossRef]
- Saddiqi, H.A.; Iqbal, Z. Usage and significance of fennel (Foeniculum vulgare Mill.) seeds in Eastern medicine. In Nuts and Seeds in Health and Disease Prevention; Elsevier Inc.: Amsterdam, The Netherlands, 2011; pp. 461–467. [Google Scholar]
- Sayah, K.; El Omari, N.; Kharbach, M.; Bouyahya, A.; Kamal, R.; Marmouzi, I.; Cherrah, Y.; Faouzi, E.M.A. Comparative study of leaf and rootstock aqueous extracts of Foeniculum vulgare on chemical profile and in vitro antioxidant and antihyperglycemic activities. Adv. Pharmacol. Pharm. Sci. 2020, 2020, 8852570. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, D.U.; Kim, Y.S.; Kim, H.P. 5-Lipoxygenase inhibition of the fructus of Foeniculum vulgare and its constituents. Biomol. Ther. 2012, 20, 113–117. [Google Scholar] [CrossRef]
- Mechraoui, I.; Mahfoudi, R.; Djeridane, A.; Yilmaz, M.A.; Yousfi, M. Comparative chemical profiling and antioxidant properties of essential oils extracted from Foeniculum vulgare subsp. piperitum. Biocatal. Agric. Biotechnol. 2024, 60, 103306. [Google Scholar]
- Shahat, A.A.; Ibrahim, A.Y.; Hendawy, S.F.; Omer, E.A.; Hammouda, F.M.; Abdel-Rahman, F.H.; Saleh, M.A. Chemical composition, antimicrobial and antioxidant activities of essential oils from organically cultivated fennel cultivars. Molecules 2011, 16, 1366–1377. [Google Scholar] [CrossRef]
- Dongare, V.; Kulkarni, C.; Kondawar, M.; Magdum, C.; Haldavnekar, V.; Arvindekar, A. Inhibition of aldose reductase and anti-cataract action of trans-anethole isolated from Foeniculum vulgare Mill. fruits. Food Chem. 2012, 132, 385–390. [Google Scholar] [CrossRef]
- Sharopov, F.; Valiev, A.; Satyal, P.; Gulmurodov, I.; Yusufi, S.; Setzer, W.N.; Wink, M. Cytotoxicity of the essential oil of fennel (Foeniculum vulgare) from Tajikistan. Foods 2017, 6, 73. [Google Scholar] [CrossRef]
- Mohamed, M.E.; Kandeel, M.; El-Lateef, H.M.A.; El-Beltagi, H.S.; Younis, N.S. The protective effect of anethole against renal ischemia/reperfusion: The role of the TLR2,4/MYD88/NF-κB pathway. Antioxidants 2022, 11, 535. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Wojciuk, B.; Wojciechowska-Koszko, I.; Łopusiewicz, Ł.; Grygorcewicz, B.; Pruss, A.; Sienkiewicz, M.; Fijałkowski, K.; Kowalczyk, E.; Dołęgowska, B. Innate immune response against Staphylococcus aureus preincubated with subinhibitory concentration of trans-anethole. Int. J. Mol. Sci. 2020, 21, 4178. [Google Scholar] [CrossRef]
- Korinek, M.; Handoussa, H.; Tsai, Y.-H.; Chen, Y.-Y.; Chen, M.-H.; Chiou, Z.-W.; Fang, Y.; Chang, F.-R.; Yen, C.-H.; Hsieh, C.-F.; et al. Anti-inflammatory and antimicrobial volatile oils: Fennel and cumin inhibit neutrophilic inflammation via regulating calcium and MAPKs. Front. Pharmacol. 2021, 12, 674095. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, S.K. Fennel and fennel seed. In Handbook of Herbs and Spices; Woodhead Publishing Limited: Cambridge, UK, 2012; Volume 2, pp. 275–302. [Google Scholar]
- Syed, F.Q.; Mirza, M.B.; Elkady, A.I.; Hakeem, K.R.; Alkarim, S. An insight of multitudinous and inveterate pharmacological applications of Foeniculum vulgare (fennel). In Plant and Human Health, Volume 3: Pharmacology and Therapeutic Uses; Springer International Publishing: Cham, Switzerland, 2019; Volume 3, pp. 231–254. [Google Scholar]
- Ignjatović, M.G.; Kitić, D.; Radenković, M.; Kostić, M.; Milutinović, M.; Ranković, G.N.; Branković, S. The effect of the aqueous and methanol fennel stem extracts (Foeniculum vulgare Miller) on isolated rat ileum contractility. Vojnosanit. Pregl. 2018, 75, 809–814. [Google Scholar] [CrossRef]
- Al Akeel, R.; Mateen, A.; Syed, R.; Alyousef, A.A.; Shaik, M.R. Screening, purification and characterization of anionic antimicrobial proteins from Foeniculum vulgare. Molecules 2017, 22, 602. [Google Scholar] [CrossRef]
- Moumen, B.E.; Bouzoubaa, A.; Drioiche, A.; Eddahmouny, M.; Al Kamaly, O.; Shahat, A.A.; Touijer, H.; Hadi, N.; Kharchouf, S.; Cherrat, A.; et al. Unveiling the chemical composition, antioxidant, and antimicrobial potentials of Foeniculum vulgare Mill: A combined in vitro and in silico approach. Int. J. Mol. Sci. 2025, 26, 4499. [Google Scholar] [CrossRef]
- Patil, S.S.; Pawar, M.M.; Patel, M.P.; Patel, S.S.; Modi, C.P.; Patel, J.R. Effect of fennel (Foeniculum vulgare) seed supplementation on growth performance, haemato-biochemical parameters and faecal microbiota of Mehsana goat kids. Indian J. Vet. Sci. Biotechnol. 2024, 20, 16–20. [Google Scholar]
- Pawar, M.; Patil, S.; Gami, Y.M.; Patel, S.S.; Raval, S.H.; Modi, C.P.; Patel, J.R. Effect of dietary addition of fennel (Foeniculum vulgare) seed on growth performance, haemato-biochemical profile and faecal microbiota of Kankrej calves. Int. J. Bio-Resour. Stress Manag. 2024, 15, 01–07. [Google Scholar] [CrossRef]
- Rafieian, F.; Amani, R.; Rezaei, A.; Karaça, A.C.; Jafari, S.M. Exploring fennel (Foeniculum vulgare): Composition, functional properties, potential health benefits, and safety. Crit. Rev. Food Sci. Nutr. 2024, 64, 6924–6941. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Scribano, M.L.; Kohn, A.; Caporaso, N.; Festi, D.; Campanale, M.C.; Di Rienzo, T.; Guarino, M.; Taddia, M.; et al. Curcumin and fennel essential oil improve symptoms and quality of life in patients with irritable bowel syndrome. J. Gastrointest. Liver Dis. 2016, 25, 151–157. [Google Scholar] [CrossRef]
- Di Ciaula, A.; Portincasa, P.; Maes, N.; Albert, A. Efficacy of bio-optimized extracts of turmeric and essential fennel oil on the quality of life in patients with irritable bowel syndrome. Ann. Gastroenterol. 2018, 31, 685–691. [Google Scholar] [CrossRef]
- Bayan, L.; Koulivand, P.H.; Gorji, A. Garlic: A review of potential therapeutic effects. Avicenna J. Phytomed. 2014, 4, 1–14. [Google Scholar]
- Banerjee, S.K.; Mukherjee, P.K.; Maulik, S.K. Garlic as an antioxidant: The good, the bad and the ugly. Phytother. Res. 2003, 17, 97–106. [Google Scholar] [CrossRef]
- Amagase, H. Significance of garlic and its constituents in cancer and cardiovascular disease: Clarifying the real bioactive constituents of garlic. J. Nutr. 2006, 136, 716S–725S. [Google Scholar] [CrossRef]
- Iciek, M.; Kwiecien, I.; Włodek, L. Biological properties of garlic and garlic-derived organosulfur compounds. Environ. Mol. Mutagen. 2009, 50, 247–265. [Google Scholar] [CrossRef]
- Ried, K. Garlic lowers blood pressure in hypertensive subjects, improves arterial stiffness and gut microbiota: A review and meta-analysis. Exp. Ther. Med. 2020, 19, 1472–1478. [Google Scholar] [CrossRef] [PubMed]
- Stevinson, C.; Pittler, M.H.; Ernst, E. Garlic for treating hypercholesterolemia. Ann. Intern. Med. 2000, 133, 420–429. [Google Scholar] [CrossRef]
- Sobenin, I.A.; Myasoedova, V.A.; Iltchuk, M.I.; Dong-wei, Z.; Orekhov, A.N. Therapeutic effects of garlic in cardiovascular atherosclerotic disease. Chin. J. Nat. Med. 2019, 17, 721–728. [Google Scholar] [CrossRef]
- Najafi, N.; Masoumi, S.J. The effect of garlic (Allium sativum) supplementation in patients with type 2 diabetes mellitus: A systematic review. Int. J. Nutr. Sci. 2018, 3, 7–11. [Google Scholar]
- Zugaro, S.; Benedetti, E.; Caioni, G. Garlic (Allium sativum L.) as an ally in the treatment of inflammatory bowel diseases. Curr. Issues Mol. Biol. 2023, 45, 685–698. [Google Scholar] [CrossRef]
- Borrelli, F.; Capasso, R.; Izzo, A.A. Garlic (Allium sativum L.): Adverse effects and drug interactions in humans. Mol. Nutr. Food Res. 2007, 51, 1386–1397. [Google Scholar] [CrossRef]
- Macan, H.; Uykimpang, R.; Alconcel, M.; Takasu, J.; Razon, R.; Amagase, H.; Niihara, Y. Aged garlic extract may be safe for patients on warfarin therapy. J. Nutr. 2018, 136, 793–795. [Google Scholar] [CrossRef]
- Rana, S.V.; Pal, R.; Vaiphei, K.; Sharma, S.K.; Ola, R.P. Garlic in health and disease. Nutr. Res. Rev. 2011, 24, 60–71. [Google Scholar] [CrossRef]
- Yun, H.-M.; Ok, J.; Park, K.-R.; Kil, C.; Jeong, H.-S.; Bae, S.; Tae, J. Potential therapeutic effects of functionally active compounds isolated from garlic. Pharmacol. Ther. 2014, 142, 183–195. [Google Scholar] [CrossRef]
- Melguizo-Rodríguez, L.; García-Recio, E.; Ruiz, C.; de Luna-Bertos, E.; Illescas-Montes, R.; Costela-Ruiz, V.J. Biological properties and therapeutic applications of garlic and its components. Food Funct. 2022, 13, 2415. [Google Scholar] [CrossRef]
- Bhatwalkar, S.B.; Mondal, R.; Babu, S.N.K.; Adam, J.K.; Govender, P.; Anupam, R. Antibacterial properties of organosulfur compounds of garlic (Allium sativum). Front. Microbiol. 2021, 12, 613077. [Google Scholar] [CrossRef]
- Rahman, M.S. Allicin and other functional active components in garlic: Health benefits and bioavailability. Int. J. Food Prop. 2007, 10, 245–268. [Google Scholar] [CrossRef]
- Oyaluna, Z.E.; Abolaji, A.O.; Bodede, O.; Olanlokun, J.O.; Prinsloo, G.; Steenkamp, P.; Babalola, C.P. Chemical analysis of alliin-rich Allium sativum (garlic) extract and its safety evaluation in Drosophila melanogaster. Toxicol. Rep. 2024, 13, 101760. [Google Scholar] [CrossRef]
- Hirata, Y.; Nagase, H.; Satoh, K.; Takemori, H.; Furuta, K.; Kamatari, Y.O. Antiferroptotic properties of allicin and related organosulfur compounds—Diallyl disulfide and diallyl trisulfide—From garlic. Food Chem. Toxicol. 2025, 195, 115124. [Google Scholar] [CrossRef] [PubMed]
- Thakur, P.; Dhiman, A.; Kumar, S.; Suhag, R. Garlic (Allium sativum L.): A review on bio-functionality, allicin’s potency and drying methodologies. S. Afr. J. Bot. 2024, 171, 129–146. [Google Scholar] [CrossRef]
- De Greef, D.; Barton, E.M.; Sandberg, E.N.; Croley, C.R.; Pumarol, J.; Lok, T.; Das, N.; Bishayee, A. Anticancer potential of garlic and its bioactive constituents: A systematic and comprehensive review. Semin. Cancer Biol. 2021, 73, 219–264. [Google Scholar] [CrossRef]
- Xie, C.; Gao, W.; Liang, X.; Chye, F.Y. Effects of garlic-derived fructan and oligofructose mixtures on intestinal health and constipation relief in mice. J. Sci. Food Agric. 2024, 104, 7476–7487. [Google Scholar] [CrossRef]
- Rahim, M.A.; Saeed, F.; Khalid, W.; Anjum, F.M. Functional and nutraceutical properties of fructo-oligosaccharides derivatives: A review. Int. J. Food Prop. 2021, 24, 1588–1602. [Google Scholar] [CrossRef]
- Muir, J.G.; Shepherd, S.J.; Rosella, O.; Rose, M.; Barrett, J.S.; Gibson, P.R. Fructan and free fructose content of common Australian vegetables and fruit. J. Agric. Food Chem. 2007, 55, 6619–6627. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Huang, X.; Zeng, Y.; Wu, X.; Peng, X. Study on prebiotic effectiveness of neutral garlic fructan in vitro. Food Sci. Hum. Wellness 2013, 2, 119–123. [Google Scholar] [CrossRef]
- Ha, J.; Kim, J.; Kim, S.; Lee, K.J.; Shin, H. Garlic-induced enhancement of Bifidobacterium: Enterotype-specific modulation of gut microbiota and probiotic populations. Microorganisms 2024, 12, 1971. [Google Scholar] [CrossRef]
- Capasso, A. Antioxidant action and therapeutic efficacy of Allium sativum L. Molecules 2013, 18, 690–700. [Google Scholar] [CrossRef]
- Miroddi, M.; Calapai, F.; Calapai, G. Potential beneficial effects of garlic in oncohematology. Mini-Rev. Med. Chem. 2011, 11, 461–472. [Google Scholar] [CrossRef]
- Thomson, M.; Ali, M. Garlic (Allium sativum): A review of its potential use as an anti-cancer agent. Curr. Cancer Drug Targets 2003, 3, 67–81. [Google Scholar] [CrossRef]
- Bedouhene, S.; Senani, N.; Rekeb, T.; Chabane, M.D.; Dermeche, S.; Messaoudi, D. Dual protection of aqueous garlic extract biomolecules against hemolysis and its oxidation products in preventing inflammation. Cell. Mol. Biol. 2024, 70, 29–37. [Google Scholar] [CrossRef]
- Binduga, U.E.; Kopeć, A.; Skoczylas, J.; Szychowski, K.A. Comparison of the cytotoxic mechanisms of different garlic (Allium sativum L.) cultivars with the crucial involvement of peroxisome proliferator-activated receptor gamma. Int. J. Mol. Sci. 2025, 26, 10387. [Google Scholar] [CrossRef]
- Gorrepati, K.; Krishna, R.; Singh, S.; Shirsat, D.V.; Soumia, P.S.; Mahajan, V. Harnessing the nutraceutical and therapeutic potential of Allium spp.: Current insights and future directions. Front. Nutr. 2024, 11, 1497953. [Google Scholar] [CrossRef]
- Hitchcock, J.K.; Mkwanazi, N.; Barnett, C.; Graham, L.M.; Katz, A.; Hunter, R.; Schäfer, G.; Kaschula, C.H. The garlic compound Z-ajoene, S-thiolates COX2 and STAT3 and dampens the inflammatory response in RAW264.7 macrophages. Mol. Nutr. Food Res. 2020, 65, 2000854. [Google Scholar] [CrossRef]
- Jamel, D.S.; Malik, S.T.A.; Hassan, A.K.; Ayesh, A.A. Biological functions of garlic (Allium sativum L.) and its active compounds against pathogens. Plant Protection 2025, 9, 193–201. [Google Scholar] [CrossRef]
- Quintero-Fabián, S.; Ortuño-Sahagún, D.; Vázquez-Carrera, M.; López-Roa, R.I. Alliin, a garlic (Allium sativum) compound, prevents LPS-induced inflammation in 3T3-L1 adipocytes. Mediat. Inflamm. 2013, 2013, 381815. [Google Scholar] [CrossRef]
- Sadeghi, M.; Miroliaei, M.; Fateminasab, F.; Moradi, M. Screening cyclooxygenase 2 inhibitors from Allium sativum L. compounds: In silico approach. J. Mol. Model. 2022, 28, 24. [Google Scholar] [CrossRef]
- Jikah, A.N.; Edo, G.I. Mechanisms of action by sulphur compounds in Allium sativum: A review. Pharmacol. Res. Mod. Chin. Med. 2023, 9, 100323. [Google Scholar] [CrossRef]
- Shang, A.; Cao, S.-Y.; Xu, X.-Y.; Gan, R.-Y.; Tang, G.-Y.; Corke, H.; Mavumengwana, V.; Li, H.-B. Bioactive compounds and biological functions of garlic (Allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef]
- Nakamoto, M.; Kunimura, K.; Suzuki, J.-I.; Kodera, Y. Antimicrobial properties of hydrophobic compounds in garlic: Allicin, vinyldithiin, ajoene and diallyl polysulfides (review). Exp. Ther. Med. 2020, 19, 1550–1553. [Google Scholar] [CrossRef]
- Bastaki, S.M.A.; Ojha, S.; Kalasz, H.; Adeghate, E. Chemical constituents and medicinal properties of Allium species. Mol. Cell. Biochem. 2021, 476, 4301–4321. [Google Scholar] [CrossRef]
- Huang, L.; Liu, Z.; Wang, J.; Fu, J.; Jia, Y.; Ji, L.; Wang, T. Bioactivity and health effects of garlic essential oil: A review. Food Sci. Nutr. 2023, 11, 2450–2470. [Google Scholar] [CrossRef]
- Bonilla-Luque, O.M.; Nunes, B.; Ezzaky, Y.; Possas, A.; Achemchem, F.; Cadavez, V.; Gonzales-Barron, Ú.; Valero, A. Meta-analysis of antimicrobial activity of Allium, Ocimum, and Thymus spp. confirms their promising application for increasing food safety. Food Res. Int. 2024, 188, 114408. [Google Scholar] [CrossRef]
- García, M.T.; Garcia-Vargas, J.M.; Fernández, L.A.G.; Cuevas, P.; Gracia, I. Garlic extracts: Effect of pH on inhibition of Helicobacter pylori. Life 2023, 13, 1434. [Google Scholar] [CrossRef]
- Huang, G.; Khan, R.; Zheng, Y.; Lee, P.-C.; Li, Q.; Khan, I. Exploring the role of gut microbiota in advancing personalized medicine. Front. Microbiol. 2023, 14, 1274925. [Google Scholar] [CrossRef]
- Tang, Y.; Li, F.; Gu, D.; Wang, W.; Huang, J.; Jiao, X. Antimicrobial effect and the mechanism of diallyl trisulfide against Campylobacter jejuni. Antibiotics 2021, 10, 246. [Google Scholar] [CrossRef] [PubMed]
- Cady, N.C.; Mckean, K.A.; Behnke, J.; Kubec, R.; Mosier, A.P.; Kasper, S.H.; Burz, D.S.; Musah, R.A. Inhibition of biofilm formation, quorum sensing and infection in Pseudomonas aeruginosa by natural products-inspired organosulfur compounds. PLoS ONE 2012, 7, e38492. [Google Scholar] [CrossRef]
- Chen, K.; Xie, K.; Liu, Z.; Nakasone, Y.; Sakao, K.; Hossain, A.; Hou, D.-X. Preventive effects and mechanisms of garlic on dyslipidemia and gut microbiome dysbiosis. Nutrients 2019, 11, 1225. [Google Scholar] [CrossRef]
- Li, W.-Q.; Zhang, J.-Y.; Ma, J.-L.; Li, Z.-X.; Zhang, L.; Zhang, Y.; Guo, Y.; Zhou, T.; Li, J.-Y.; Shen, L.; et al. Effects of Helicobacter pylori treatment and vitamin and garlic supplementation on gastric cancer incidence and mortality: Follow-up of a randomized intervention trial. BMJ 2019, 366, l5016. [Google Scholar] [CrossRef]
- Rahmatinia, E.; Amidi, B.; Naderi, N.; Ahmadipour, S.; Ahmadvand, H.; Pahlevan-Fallahy, M.T.; Ghorbanzadeh, V.; Nazari, A. Randomized, double-blind clinical trial evaluating the impact of freeze-dried garlic extract capsules on blood pressure, lipid profile, and nitric oxide levels in individuals at risk for hypertension. Horm. Mol. Biol. Clin. Investig. 2024, 45, 139–147. [Google Scholar] [CrossRef]
- Fu, Z.; Lv, J.; Gao, X.; Zheng, H.; Shi, S.; Xu, X.; Zhang, B.; Wu, H.; Song, Q. Effects of garlic supplementation on components of metabolic syndrome: A systematic review, meta-analysis, and meta-regression of randomized controlled trials. BMC Complement. Med. Ther. 2023, 23, 260. [Google Scholar] [CrossRef]
- Valls, R.M.; Companys, J.; Calderón-Pérez, L.; Salamanca, P.; Pla-Pagà, L.; Sandoval-Ramírez, B.A.; Bueno, A.; Puzo, J.; Crescenti, A.; Del Bas, J.M.; et al. Effects of an optimized aged garlic extract on cardiovascular disease risk factors in moderate hypercholesterolemic subjects: A randomized, crossover, double-blind, sustained and controlled study. Nutrients 2022, 14, 405. [Google Scholar] [CrossRef]
- Gupta, S.K.; Sharma, A. Medicinal properties of Zingiber officinale Roscoe—A review. IOSR J. Pharm. Biol. Sci. 2014, 9, 124–129. [Google Scholar] [CrossRef]
- Riaz, M.; Rehman, U.; Akram, M.; Naveed, A. Zingiber officinale Roscoe (Pharmacological activity). J. Med. Plants Res. 2011, 5, 344–348. [Google Scholar]
- Lete, I.; Allué, J. The effectiveness of ginger in the prevention of nausea and vomiting during pregnancy and chemotherapy. Integr. Med. Insights 2016, 11, 11–17. [Google Scholar] [CrossRef]
- Bhatt, N.; Waly, M.I.; Ali, A. Ginger: A functional herb. Food Med. 2013, 1, 51–71. [Google Scholar]
- Garza-Cadena, C.; Ortega-Rivera, D.M.; Machorro-García, G.; Gonzalez-Zermeno, E.M.; Homma-Duenas, D.; Plata-Gryl, M.; Castro-Munoz, R. A comprehensive review on ginger (Zingiber officinale) as a potential source of nutraceuticals for food formulations: Towards the polishing of gingerol and other present biomolecules. Food Chem. 2023, 413, 135629. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Pharmacological uses and health benefits of ginger (Zingiber officinale) in traditional Asian and ancient Chinese medicine, and modern practice. Not Sci. Biol. 2019, 11, 309–319. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Clinical aspects and health benefits of ginger (Zingiber officinale) in both traditional Chinese medicine and modern industry. Acta Agric. Scand. B Soil Plant Sci. 2019, 69, 546–556. [Google Scholar] [CrossRef]
- Unuofin, J.O.; Masuku, N.P.; Paimo, O.K.; Lebelo, S.L. Ginger from farmyard to town: Nutritional and pharmacological applications. Front. Pharmacol. 2021, 12, 779352. [Google Scholar] [CrossRef]
- Chauhan, N. Pharmacological aspects of 6-gingerol: A review. Agric. Sci. Digest 2022, 42, 528–533. [Google Scholar] [CrossRef]
- Naghsh, F. Nano drug delivery study of anticancer properties on ginger using QM/MM methods. Orient. J. Chem. 2015, 31, 465–478. [Google Scholar] [CrossRef]
- Arcusa, R.; Villaño, D.; Marhuenda, J.; Cano, M.; Cerdà, B.; Zafrilla, P. Potential role of ginger (Zingiber officinale Roscoe) in the prevention of neurodegenerative diseases. Front. Nutr. 2022, 9, 809621. [Google Scholar] [CrossRef]
- Pazmandi, K.; Szöllosi, A.G.; Fekete, T. The “root” causes behind the anti-inflammatory actions of ginger compounds in immune cells. Front. Immunol. 2024, 15, 1400956. [Google Scholar] [CrossRef]
- Kiyama, R. Nutritional implications of ginger: Chemistry, biological activities and signaling pathways. J. Nutr. Biochem. 2020, 86, 108486. [Google Scholar] [CrossRef] [PubMed]
- Crichton, M.; Davidson, A.R.; Innerarity, C.; Marx, W.; Lohning, A.; Isenring, E.; Marshall, S. Orally consumed ginger and human health: An umbrella review. Am. J. Clin. Nutr. 2022, 115, 1511–1527. [Google Scholar] [CrossRef] [PubMed]
- AlAskar, A.; Shaheen, N.A.; Khan, A.H.; AlGhasham, N.; Mendoza, M.A.; Matar, D.B.; Gmati, G.; AlJeraisy, M.; AlSuhaibani, A. Effect of daily ginger consumption on platelet aggregation. J. Herb. Med. 2020, 20, 100316. [Google Scholar] [CrossRef]
- Ishfaq, M.; Hu, W.; Hu, Z.; Guan, Y.; Zhang, R. A review of nutritional implications of bioactive compounds of ginger (Zingiber officinale Roscoe), their biological activities and nano-formulations. Ital. J. Food Sci. 2022, 34, 1–12. [Google Scholar] [CrossRef]
- Edo, G.I.; Igbuku, U.A.; Makia, R.S.; Isoje, E.F.; Gaaz, T.S.; Yousif, E.; Jikah, A.N.; Zainulabdeen, K.; Akpoghelie, P.O.; Opiti, R.A.; et al. Phytochemical profile, therapeutic potentials, nutritional composition, and food applications of ginger: A comprehensive review. Discov. Food 2025, 5, 25. [Google Scholar] [CrossRef]
- Ersedo, T.L.; Teka, T.A.; Forsido, S.F.; Dessalegn, E.; Adebo, J.A.; Tamiru, M.; Astatkie, T. Food flavor enhancement, preservation, and bio-functionality of ginger (Zingiber officinale): A review. Int. J. Food Prop. 2023, 26, 928–951. [Google Scholar] [CrossRef]
- Ahmad, I.; Irm, M.; Ahmed, I.; Haoran, Y.; Taj, S.; Bhat, T.A.; Khan, S.K.; Puswal, S.M.; Khalil, H.S.; Sopjani, M.; et al. Role of ginger in fish nutrition with special emphasis on growth, health, gut and liver morphology. J. World Aquac. Soc. 2024, 55, e13101. [Google Scholar] [CrossRef]
- Segneanu, A.-E.; Bradu, I.A.; Vlase, G.; Vlase, T.; Bejenaru, C.; Bejenaru, L.E.; Mogo, G.D.; Ciocîlteu, M.V.; Herea, D.-D.; Boia, E.R. Design and evaluation of a Zingiber officinale–kaolinite–maltodextrin delivery system: Antioxidant, antimicrobial, and cytotoxic activity assessment. Pharmaceutics 2025, 17, 751. [Google Scholar] [CrossRef]
- Okafor, I.A.; Okafor, U.S. The methanolic extract of Zingiber officinale causes hypoglycemia and proinflammatory response in the rat pancreas. Physiol. Pharmacol. 2022, 26, 433–439. [Google Scholar] [CrossRef]
- Dugasania, S.; Pichikac, M.R.; Nadarajahc, V.D.; Balijepallic, M.K.; Tandraa, S.; Korlakunta, J.N. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol swarnalatha. J. Ethnopharmacol. 2010, 127, 515–520. [Google Scholar] [CrossRef]
- Kuo, P.-C.; Damu, A.G.; Cherng, C.-Y.; Jeng, J.-F.; Teng, C.-M.; Lee, E.-J.; Wu, T.-S. Isolation of a natural antioxidant, dehydrozingerone from Zingiber officinale and synthesis of its analogues for recognition of effective antioxidant and antityrosinase agents. Arch. Pharm. Res. 2005, 28, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Saiah, W.; Halzoune, H.; Djaziri, R.; Tabani, K.; Koceir, E.A.; Omari, N. Antioxidant and gastroprotective actions of butanol fraction of Zingiber officinale against diclofenac sodium-induced gastric damage in rats. J. Food Biochem. 2017, 42, e12456. [Google Scholar]
- Kartini, S.; Abu Bakar, M.F.; Abu Bakar, F.I.; Hendrika, Y.; Juariah, S.; Endrini, S. Antioxidant and antidiabetic activities of Zingiber officinale var. rubrum extracted with natural deep eutectic solvents. Food Res. 2024, 8, 5479. [Google Scholar] [CrossRef]
- Dineshbabu, J.; Periakaruppan, R.; Parameshwaran, K.; Rajalakshmi, M.; Thabhassum, S.S.; Sathana, B.S. Extraction and characterization of Zingiber officinale–based essential oil and an assessment of its antioxidant, antibacterial, and antibiofilm activities. Biomass Convers. Biorefin. 2023, 15, 27249–27256. [Google Scholar] [CrossRef]
- Mao, Q.-Q.; Xu, X.-Y.; Cao, S.-Y.; Gan, R.-Y.; Li, H.-B. Bioactive compounds and bioactivities of ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef]
- Ezzata, S.M.; Ezzata, M.I.; Okbaa, M.M.; Menzec, E.T.; Abdel-Naim, A.B. The hidden mechanism beyond ginger (Zingiber officinale Rosc.) potent in vivo and in vitro anti-inflammatory activity. J. Ethnopharmacol. 2018, 214, 113–123. [Google Scholar] [CrossRef]
- Si, Y.; Xia, G.; Niu, H.; Fang, H.; Cheng, Y. Six isomers of diphenylheptane dimers from Zingiber officinale peel exert renal protection activities through anti-fibrosis and anti-inflammatory effects. Bioorg. Chem. 2025, 159, 108394. [Google Scholar] [CrossRef]
- Walstab, J.; Kruger, D.; Stark, T.; Hofmann, T.; Demir, I.E.; Ceyhan, G.O.; Feistel, B.; Schemann, M.; Niesler, B. Ginger and its pungent constituents non-competitively inhibit activation of human recombinant and native 5-HT3 receptors of enteric neurons. Neurogastroenterol. Motil. 2013, 25, 439-e302. [Google Scholar] [CrossRef]
- Chatturong, U.; Kajsongkram, T.; Tunsophon, S.; Chanasong, R.; Chootip, K. Ginger extract and [6]-gingerol inhibit contraction of rat entire small intestine. J. Evid. Based Integr. Med. 2018, 23, 2515690X18774273. [Google Scholar] [CrossRef]
- Iwami, M.; Shiina, T.; Hirayama, H. Inhibitory effects of zingerone, a pungent component of Zingiber officinale Roscoe, on colonic motility in rats. J. Nat. Med. 2011, 65, 89–94. [Google Scholar] [CrossRef]
- Iwami, M.; Shiina, T.; Hirayama, H.; Shimizu, Y. Intraluminal administration of zingerol, a non-pungent analogue of zingerone, inhibits colonic motility in rats. Biomed. Res. 2011, 32, 181–185. [Google Scholar] [CrossRef]
- Chuah, S.-K.; Wu, K.-L.; Tai, W.-C.; Changchien, C.-S. The effects of ginger on gallbladder motility in healthy male humans. J. Neurogastroenterol. Motil. 2011, 17, 411–415. [Google Scholar] [CrossRef]
- Hu, M.-L.; Rayner, C.K.; Wu, K.-L.; Chuah, S.-K.; Tai, W.-C.; Chou, Y.-P.; Chiu, Y.-C.; Chiu, K.-W.; Hu, T.-H. Effect of ginger on gastric motility and symptoms of functional dyspepsia. World J. Gastroenterol. 2011, 17, 105–110. [Google Scholar] [CrossRef]
- Syed, Z.A.; Fahim, A.; Safdar, M.; Imtiaz, R. Role of ginger in management of nausea among patients receiving chemotherapy. Pak. J. Med. Sci. 2024, 40, 2036. [Google Scholar] [CrossRef] [PubMed]
- Beristain-Bauza, S.D.C.; Hernández-Carranza, P.; Cid-Pérez, T.S.; Ávila-Sosa, R.; Ruiz-López, I.I.; Ochoa-Velasco, C.E. Antimicrobial activity of ginger (Zingiber officinale) and its application in food products. Food Rev. Int. 2019, 35, 407–426. [Google Scholar] [CrossRef]
- Elfaky, M.A.; Okairy, H.M.; Abdallah, H.M.; Koshak, A.E.; Mohamed, G.A.; Ibrahim, S.R.M.; Alzain, A.A.; Hegazy, W.A.H.; El-Sayed, K.; Seleem, N.M. Assessing the antibacterial potential of 6-gingerol: Combined experimental and computational approaches. Saudi Pharm. J. 2024, 32, 102041. [Google Scholar] [CrossRef]
- Babaeekhou, L.; Ghane, M. Antimicrobial activity of ginger on cariogenic bacteria: Molecular networking and molecular docking analyses. J. Biomol. Struct. Dyn. 2021, 39, 2164–2175. [Google Scholar] [CrossRef]
- Attari, V.E.; Somi, M.H.; Jafarabadi, M.A.; Ostadrahimi, A.; Moaddab, S.Y.; Lotfi, N. The gastro-protective effect of ginger (Zingiber officinale Roscoe) in Helicobacter pylori positive functional dyspepsia. Adv. Pharm. Bull. 2019, 9, 321–324. [Google Scholar] [CrossRef]
- Ivashkin, V.T.; Kudryavtseva, A.V.; Krasnov, G.S.; Poluektov, Y.M.; Morozova, M.A.; Shifrin, O.S.; Beniashvili, A.G.; Mamieva, Z.A.; Kovaleva, A.L.; Ulyanin, A.I.; et al. Efficacy and safety of a food supplement with standardized menthol, limonene, and gingerol content in patients with irritable bowel syndrome: A double-blind, randomized, placebo-controlled trial. PLoS ONE 2022, 17, e0263880. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, Y.; Li, P.; Chen, X.; Liu, F.; Hou, Q. Ginger relieves intestinal hypersensitivity of diarrhea predominant irritable bowel syndrome by inhibiting proinflammatory reaction. BMC Complement. Med. Ther. 2020, 20, 279. [Google Scholar] [CrossRef]
- Van Tilburg, M.A.L.; Palsson, O.S.; Ringel, Y.; Whitehead, W.E. Is ginger effective for the treatment of irritable bowel syndrome? A double-blind randomized controlled pilot trial. Complement. Ther. Med. 2014, 22, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Shivashankara, A.R.; Rao, S.; George, T.; Abraham, S.; Colin, M.D.; Palatty, P.L.; Baliga, M.S. Tea (Camellia sinensis L. Kuntze) as hepatoprotective agent: A revisit. In Dietary Interventions in Liver Disease: Foods, Nutrients, and Dietary Supplements; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 183–192. [Google Scholar]
- Shivashankara, A.R.; Kumar, A.; Ravi, R.; Simon, P.; Rai, P.; Francis, A.; Baliga, M.S. Use of tea (Camellia sinensis [L.] Kuntze) as a hepatoprotective agent in geriatric conditions. In Foods and Dietary Supplements in the Prevention and Treatment of Disease in Older Adults; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 99–104. [Google Scholar]
- Samanta, S. Potential bioactive components and health promotional benefits of tea (Camellia sinensis). J. Am. Coll. Nutr. 2020, 41, 65–69. [Google Scholar] [CrossRef]
- Zhang, L.; Ho, C.T.; Zhou, J.; Santos, J.S.; Armstrong, L.; Granato, D. Chemistry and biological activities of processed Camellia sinensis teas: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1474–1495. [Google Scholar] [CrossRef]
- Ruchika, A.S. An update on disease preventing potential of green tea in comparison with some tisanes. S. Afr. J. Bot. 2022, 144, 92–96. [Google Scholar] [CrossRef]
- Hu, J.; Webster, D.; Cao, J.; Shao, A. The safety of green tea and green tea extract consumption in adults—Results of a systematic review. Regul. Toxicol. Pharmacol. 2018, 95, 412–433. [Google Scholar] [CrossRef]
- Jochmann, N.; Baumann, G.; Stangl, V. Green tea and cardiovascular disease: From molecular targets towards human health. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 758–765. [Google Scholar] [CrossRef]
- Park, J.-H.; Bae, J.-H.; Im, S.-S.; Song, D.-K. Green tea and type 2 diabetes. Integr. Med. Res. 2014, 3, 4–10. [Google Scholar] [CrossRef]
- Pervin, M.; Unno, K.; Ohishi, T.; Tanabe, H.; Miyoshi, N.; Nakamura, Y. Beneficial effects of green tea catechins on neurodegenerative diseases. Molecules 2018, 23, 1297. [Google Scholar] [CrossRef]
- Yang, C.S.; Wang, X. Green tea and cancer prevention. Nutr. Cancer 2010, 62, 931–937. [Google Scholar] [CrossRef]
- Westerterp-Plantenga, M.S. Green tea catechins, caffeine and body-weight regulation. Physiol. Behav. 2010, 100, 42–46. [Google Scholar] [CrossRef]
- Koo, M.W.L.; Cho, C.H. Pharmacological effects of green tea on the gastrointestinal system. Eur. J. Pharmacol. 2004, 500, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Sarma, D.N.; Barrett, M.L.; Chavez, M.L.; Gardiner, P.; Ko, R.; Mahady, G.B.; Marles, R.J.; Pellicore, L.S.; Giancaspro, G.I.; Dog, T.L. Safety of green tea extracts: A systematic review by the US Pharmacopeia. Drug Saf. 2008, 31, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Capasso, L.; De Masi, L.; Sirignano, C.; Maresca, V.; Nebbioso, A.; Rigano, D.; Bontempo, P. Epigallocatechin gallate (EGCG): Pharmacological properties, biological activities and therapeutic potential. Molecules 2025, 30, 654. [Google Scholar] [CrossRef]
- Joseph, S.; Nallaswamy, D.; Rajeshkumar, S.; Dathan, P.; Ismail, S.; Jacob, J.; Rasheed, N. A glimpse through the origin, composition and biomedical applications of green tea and its polyphenols: A review. Plant Sci. Today 2024, 11, 330–341. [Google Scholar] [CrossRef]
- Dong, X.W. Epigallocatechin-gallate: Unraveling its protective mechanisms and therapeutic potential. Cell Biochem. Funct. 2025, 43, e70056. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Multitargeted therapy of cancer by green tea polyphenols. Cancer Lett. 2008, 269, 269–280. [Google Scholar] [CrossRef]
- Rashidinejad, A.; Boostani, S.; Babazadeh, A.; Rehman, A.; Rezaei, A.; Akbari-Alavijeh, S. Opportunities and challenges for the nanodelivery of green tea catechins in functional foods. Food Res. Int. 2021, 142, 110186. [Google Scholar] [CrossRef]
- Nagle, D.G.; Ferreira, D.; Zhou, Y.-D. Epigallocatechin-3-gallate (EGCG): Chemical and biomedical perspectives. Phytochemistry 2006, 67, 1849–1855. [Google Scholar] [CrossRef]
- Dang, S.; Gupta, S.; Bansal, R.; Ali, J.; Gabrani, R. Nano-encapsulation of a natural polyphenol, green tea catechins: Way to preserve its antioxidative potential. In Free Radicals in Human Health and Disease; Springer: New Delhi, India, 2015; pp. 397–415. [Google Scholar]
- Mehmood, S.; Maqsood, M.; Mahtab, N.; Issa, M.; Sahar, A.; Zaib, S.; Gul, S. Epigallocatechin gallate: Phytochemistry, bioavailability, utilization challenges, and strategies. J. Food Biochem. 2022, 46, e1418. [Google Scholar] [CrossRef]
- da Silva Pinto, M. Tea: A new perspective on health benefit. Food Res. Int. 2013, 53, 558–567. [Google Scholar] [CrossRef]
- Luo, Q.; Luo, L.; Zhao, J.; Wang, Y.; Luo, H. Biological potential and mechanisms of tea’s bioactive compounds: An updated review. J. Adv. Res. 2024, 65, 345–363. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Bibi, J.; Ali, A.; Suheryani, I.; Kakar, I.; Ali, S.; Fangfang, X.; Ali, S.; Yunjuan, L.; Ullah, M. Pharmacological values and therapeutic properties of black tea (Camellia sinensis): A comprehensive overview. Biomed. Pharmacother. 2018, 100, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Aristizabal, L.S.; Ortiz, A.; Ospina-Ocampo, L.F. Evaluation of the antioxidant capacity and characterization of phenolic compounds obtained from tea (Camellia sinensis) for products of different brands sold in Colombia. Vitae Nat. Prod. 2015, 24, 132–145. [Google Scholar]
- Peluso, I.; Serafini, M. Antioxidant and pharmacological properties of tea: From dietary modulation of oxidative stress to pharmacological mechanisms. Br. J. Pharmacol. 2017, 174, 1195–1208. [Google Scholar] [CrossRef]
- Puligundla, P.; Mok, C.; Ko, S.; Liang, J.; Recharla, N. Nanotechnological approaches to enhance the bioavailability and therapeutic efficacy of green tea polyphenols. J. Funct. Foods 2017, 34, 139–151. [Google Scholar] [CrossRef]
- Boudou, F.; Belakredar, A.; Keziz, A.; Alsaeedi, H.; Cornu, D.; Bechelany, M.; Barhoum, A. Camellia sinensis phytochemical profiling, drug-likeness, and antibacterial activity against Gram-positive and Gram-negative bacteria: In vitro and in silico insights. Front. Chem. 2025, 13, 1555574. [Google Scholar] [CrossRef]
- Yasmeen, H.; Hasnain, S. In vitro antioxidant effect of Camellia sinensis on human cell cultures. Pak. J. Pharm. Sci. 2015, 28, 1573–1581. [Google Scholar]
- Manhas, S.; Devi, A.; Khan, Z.A. Unveiling the multifaceted health benefits of Kangra green tea leaves: Antioxidant, antidiabetic and antibacterial properties. Ann. Biol. 2025, 41, 15–20. [Google Scholar]
- Truong, V.-L.; Jeong, W.-S. Cellular defensive mechanisms of tea polyphenols: Structure-activity relationship. Int. J. Mol. Sci. 2021, 22, 9109. [Google Scholar] [CrossRef]
- Chen, G.; Chen, R.; Chen, D.; Ye, H.; Hu, B.; Zeng, X.; Liu, Z. Tea polysaccharides as potential therapeutic options for metabolic diseases. J. Agric. Food Chem. 2018, 67, 5350–5360. [Google Scholar] [CrossRef]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial properties of green tea catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef]
- Farhan, M. Green tea catechins: Nature’s way of preventing and treating cancer. Int. J. Mol. Sci. 2022, 23, 10713. [Google Scholar] [CrossRef]
- Yang, W.S.; Ko, J.; Kim, E.; Kim, J.H.; Park, J.G.; Sung, N.Y.; Kim, H.G.; Yang, S.; Rho, H.S.; Hong, Y.D.; et al. 21-O-Angeloyltheasapogenol E3, a novel triterpenoid saponin from the seeds of tea plants, inhibits macrophage-mediated inflammatory responses in a NF-κB-dependent manner. Mediat. Inflamm. 2014, 2014, 658351. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sun, W.; Jin, L. Caffeic acid alleviates inflammatory response in rheumatoid arthritis fibroblast-like synoviocytes by inhibiting phosphorylation of IκB kinase α/β and IκBα. Int. Immunopharmacol. 2017, 48, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.M.; Kim, I.T.; Park, Y.M.; Ha, J.; Choi, J.W.; Park, H.J.; Lee, Y.S.; Lee, K.T. Anti-inflammatory effect of caffeic acid methyl ester and its mode of action through the inhibition of prostaglandin E2, nitric oxide and tumor necrosis factor-α production. Biochem. Pharmacol. 2004, 68, 2327–2336. [Google Scholar] [CrossRef]
- Genaro-Mattos, T.C.; Maurício, Â.Q.; Rettori, D.; Alonso, A.; Hermes-Lima, M. Antioxidant activity of caffeic acid against iron-induced free radical generation: A chemical approach. PLoS ONE 2015, 10, e0129963. [Google Scholar]
- Mawarti, H.; Nugraha, J.; Purwanto, D.A.; Soeroso, J. Identifying and revealing active compound from green tea (Camellia sinensis) for curing systemic lupus erythematosus by acting as CASPASE 1 inhibitor. Medico Legal Update 2020, 20, 457–463. [Google Scholar]
- Rosenthal, R.; Waesch, A.; Masete, K.V.; Massarani, A.S.; Schulzke, J.; Hering, N.A. The green tea component (−)-epigallocatechin-3-gallate protects against cytokine-induced epithelial barrier damage in intestinal epithelial cells. Front. Pharmacol. 2025, 16, 1559812. [Google Scholar] [CrossRef]
- Choi, K.; Park, S.; Kwon, Y.; Lee, J.; Kwon, O.; Yeon, J. Green tea extract and Piper retrofractum attenuate deoxycholic acid-induced damage and enhance the tight junction barrier: An analysis in a Caco-2 cell culture model and a DSS co-induced mouse model. Food Biosci. 2023, 52, 102416. [Google Scholar] [CrossRef]
- Truong, V.-L.; Jeong, W.-S. Antioxidant and anti-inflammatory roles of tea polyphenols in inflammatory bowel diseases. Food Sci. Human Wellness 2022, 11, 502–511. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Navajas-Porras, B.; López-Maldonado, A.; Pastoriza, S.; Rufián-Henare, J.Á. Green tea and its relation to human gut microbiome. Molecules 2021, 26, 3907. [Google Scholar] [CrossRef]
- Andrade, E.D.S.; Santos, R.A.; Guillermo, L.V.C.; Miyoshi, N.; Ferraz da Costa, D.C. Immunomodulatory effects of green tea catechins and their ring fission metabolites in a tumor microenvironment perspective. Molecules 2024, 29, 4575. [Google Scholar] [CrossRef]
- Wang, M.; Li, J.; Hu, T.; Zhao, H. Metabolic fate of tea polyphenols and their crosstalk with gut microbiota. Food Sci. Human Wellness 2022, 11, 455–466. [Google Scholar] [CrossRef]
- Reygaert, W.C. Green tea catechins: Their use in treating and preventing infectious diseases. BioMed Res. Int. 2018, 2018, 9105261. [Google Scholar] [CrossRef]
- Afifah, S.H.; Apriliana, E.; Setiawan, G.; Berawi, K.N. Antibacterial activity of green tea epigallocatechin gallate (EGCG) on Gram positive and Gram negative bacteria. Medula 2024, 14, 2330–2335. [Google Scholar]
- Reygaert, W.C. The antimicrobial possibilities of green tea. Front. Microbiol. 2014, 5, 434. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, L.; Luo, Y.; Zhang, J.; Wang, X.; Sun, K.; Zeng, L. The prebiotic properties of green and dark tea contribute to the protective effects in chemical-induced colitis in mice: A fecal microbiota transplantation study. J. Agric. Food Chem. 2020, 68, 6368–6380. [Google Scholar] [CrossRef]
- Axling, U.; Olsson, C.; Xu, J.; Fernandez, C.; Larsson, S.; Ström, K.; Ahrné, S.; Holm, C.; Molin, G.; Berger, K. Green tea powder and Lactobacillus plantarum affect gut microbiota, lipid metabolism and inflammation in high-fat fed C57BL/6J mice. Nutr. Metab. 2012, 9, 105. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Wu, Z.; Zhang, P.; Zhang, X. Tea polyphenols: A natural antioxidant regulates gut flora to protect the intestinal mucosa and prevent chronic diseases. Antioxidants 2022, 11, 253. [Google Scholar] [CrossRef]
- Choi, S.R.; Lee, H.; Singh, D.; Cho, D.; Chung, J.-O.; Roh, J.-H.; Kim, W.-G.; Lee, C.H. Bidirectional interactions between green tea (GT) polyphenols and human gut bacteria. J. Microbiol. Biotechnol. 2023, 33, 1317–1328. [Google Scholar] [CrossRef]
- Khairudin, M.A.S.; Jalil, A.M.M.; Hussin, N. Effects of polyphenols in tea (Camellia sinensis sp.) on the modulation of gut microbiota in human trials and animal studies. Gastroenterol. Insights 2021, 12, 202–216. [Google Scholar] [CrossRef]
- Asbaghi, O.; Rezaei Kelishadi, M.; Larky, D.A.; Bagheri, R.; Amirani, N.; Goudarzi, K.; Kargar, F.; Ghanavati, M.; Zamani, M. The effects of green tea extract supplementation on body composition, obesity-related hormones and oxidative stress markers: A grade-assessed systematic review and dose-response meta-analysis of randomised controlled trials. Br. J. Nutr. 2024, 131, 1125–1157. [Google Scholar] [CrossRef]
- Colonetti, L.; Grande, A.J.; Toreti, I.R.; Ceretta, L.B.; da Rosa, M.I.; Colonetti, T. Green tea promotes weight loss in women with polycystic ovary syndrome: Systematic review and meta-analysis. Nutr. Res. 2022, 104, 1–9. [Google Scholar] [CrossRef]
- Holtmann, G.; Adam, B.; Haag, S.; Collet, W.; Grünewald, E.; Windeck, T. Efficacy of artichoke leaf extract in the treatment of patients with functional dyspepsia: A six-week placebo-controlled, double-blind, multicentre trial. Aliment. Pharmacol. Ther. 2003, 18, 1099–1105. [Google Scholar] [CrossRef]
- European Medicines Agency. European Union Herbal Monograph on Cynara cardunculus L. (Syn. Cynara scolymus L.), Folium; European Medicines Agency: Amsterdam, The Netherlands, 2018; p. 1. [Google Scholar]
- Shallan, M.A.; Ali, M.A.; Meshrf, W.A.; Marrez, D.A. In vitro antimicrobial, antioxidant and anticancer activities of globe artichoke (Cynara cardunculus var. scolymus L.) bracts and receptacles ethanolic extract. Biocatal. Agric. Biotechnol. 2020, 29, 101774. [Google Scholar]
- European Medicines Agency. European Union Herbal Monograph on Aloe barbadensis Mill. and on Aloe (Various Species, Mainly Aloe ferox Mill. and its Hybrids), Folii Succus Siccatus; European Medicines Agency: London, UK, 2016. [Google Scholar]
- Langmead, L.; Feakins, R.M.; Goldthorpe, S.; Holt, H.; Tsironi, E.; De Silva, A.; Jewell, D.P.; Rampton, D.S. Randomized, double-blind, placebo-controlled trial of oral aloe vera gel for active ulcerative colitis. Aliment. Pharmacol. Ther. 2004, 19, 739–747. [Google Scholar] [CrossRef]
- Davis, K.; Philpott, S.; Kumar, D.; Mendall, M. Randomised double-blind placebo-controlled trial of aloe vera for irritable bowel syndrome. Int. J. Clin. Pract. 2006, 60, 1080–1086. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, A.K.; Gupta, A.; Bishayee, A.; Pandey, A.K. Therapeutic potential of aloe vera—A miracle gift of nature. Phytomedicine 2019, 60, 152996. [Google Scholar] [CrossRef]
- European Medicines Agency. European Union Herbal Monograph on Matricaria recutita L., Flos; European Medicines Agency: London, UK, 2015. [Google Scholar]
- Amsterdam, J.D.; Li, Y.; Soeller, I.; Rockwell, K.; Mao, J.J.; Shults, J. A randomized, double-blind, placebo-controlled trial of oral Matricaria recutita (chamomile) extract therapy for generalized anxiety disorder. J. Clin. Psychopharmacol. 2009, 29, 378–382. [Google Scholar] [CrossRef]
- European Medicines Agency. Assessment Report on Calendula officinalis L., Flos; European Medicines Agency: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Ghafarifarsani, H.; Hoseinifar, S.H.; Molayemraftar, T.; Raeeszadeh, M.; Van Doan, H. Pot marigold (Calendula officinalis) powder in rainbow trout (Oncorhynchus mykiss) feed: Effects on growth, immunity, and Yersinia ruckeri resistance. Aquac. Nutr. 2023, 2023, 7785722. [Google Scholar] [CrossRef]
- European Medicines Agency. Community Herbal Monograph on Cinnamomum verum J.S. Presl, Cortex; European Medicines Agency: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Allen, R.W.; Schwartzman, E.; Baker, W.L.; Coleman, C.I.; Phung, O.J. Cinnamon use in type 2 diabetes: An updated systematic review and meta-analysis. Ann. Fam. Med. 2013, 11, 452–459. [Google Scholar] [CrossRef]
- European Medicines Agency. Community Herbal Monograph on Taraxacum officinale ex Wigg., Radix cum Herba; European Medicines Agency: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Tanasa (Acretei), M.-V.; Negreanu-Pirjol, T.; Olariu, L.; Negreanu-Pirjol, B.-S.; Lepadatu, A.-C.; Anghel (Cireasa), L.; Rosoiu, N. Bioactive compounds from vegetal organs of Taraxacum species (dandelion) with biomedical applications: A review. Int. J. Mol. Sci. 2025, 26, 450. [Google Scholar] [CrossRef]
- European Medicines Agency. European Union Herbal Monograph on Foeniculum vulgare subsp. Vulgare var. Dulce (Mill.) Batt. & Trab., Fructus; European Medicines Agency: Amsterdam, The Netherlands, 2024; pp. 1–10. [Google Scholar]
- Soukaina, B.; Oumaima, A.; Radia, E.; Yassine, E.B.M.; Ezzahra, E.K.; Khiraoui, F.; Khadija, E.; Abdelhalem, M.; Laarbi, O.M. Assessing acute and subacute toxicity and phytochemical screening of the methanolic extract of Foeniculum vulgare in Wistar rats. J. Appl. Toxicol. 2025. [Google Scholar] [CrossRef]
- Poojar, B.; Ommurugan, B.; Adiga, S.; Thomas, H. Evaluation of antiurolithiatic property of ethanolic extract of fennel seeds in male Wistar albino rats. Asian J. Pharm. Clin. Res. 2017, 10, 313–316. [Google Scholar] [CrossRef]
- Ried, K.; Toben, C.; Fakler, P. Effect of garlic on serum lipids: An updated meta-analysis. Nutr. Rev. 2013, 71, 282–299. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. European Union Herbal Monograph on Zingiber officinale, Rhizoma; European Medicines Agency: Amsterdam, The Netherlands, 2025. [Google Scholar]
- Viljoen, E.; Visser, J.; Koen, N.; Musekiwa, A. A systematic review and meta-analysis of the effect and safety of ginger in the treatment of pregnancy-associated nausea and vomiting. Nutr. J. 2014, 13, 20. [Google Scholar] [CrossRef]
- Yang, C.S.; Wang, Z.-Y. Tea and cancer. J. Natl. Cancer Inst. 1993, 85, 1038–1049. [Google Scholar] [CrossRef]
- Phung, O.J.; Baker, W.L.; Matthews, L.J.; Lanosa, M.; Thorne, A.; Coleman, C.I. Effect of green tea catechins with or without caffeine on anthropometric measures: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2010, 91, 73–81. [Google Scholar] [CrossRef]
- Bond, T.; Derbyshire, E. Tea compounds and the gut microbiome: Findings from trials and mechanistic studies. Nutrients 2019, 11, 2364. [Google Scholar] [CrossRef]
- Agrawal, V.; Amresh, G.; Road, F.; Chaturvedi, S. Improvement in bioavailability of class-III drug: Phytolipid delivery system. Int. J. Pharm. Pharm. Sci. 2012, 4, 37–42. [Google Scholar]
- Katoa, L.S.; Lelisa, C.A.; da Silva, B.D.; Galvana, D.; Conte-Junior, C.A. Micro- and nanoencapsulation of natural phytochemicals: Challenges and recent perspectives for the food and nutraceuticals industry applications. In Advances in Food and Nutrition Research; Elsevier Inc.: Amsterdam, The Netherlands, 2023; Volume 104, pp. 77–137. [Google Scholar]
- Ohnishi, K.; Irie, K.; Murakami, A. Modulation of protein quality control systems as novel mechanisms underlying functionality of food phytochemicals. Food Funct. 2013, 3, 400–415. [Google Scholar] [CrossRef]
- Todd, E.; Elnour, R.; Simpson, R.; Castaneda, M.; Shanahan, E.R. Munching microbes: Diet-microbiome interactions shape gut health and cancer outcomes. Microbiol. Aust. 2021, 42, 60–64. [Google Scholar] [CrossRef]
- Speckmann, B.; Ehring, E.; Hu, J.; Mateos, A.R. Exploring substrate–microbe interactions: A metabiotic approach toward developing targeted synbiotic compositions. Gut Microbes 2024, 16, 2305716. [Google Scholar] [CrossRef]
- Bayram, B.; González-Sarrías, A.; Istas, G.; Garcia-Aloy, M.; Morand, C.; Tuohy, K.; García-Villalba, R.; Mena, P. Breakthroughs in the health effects of plant food bioactives: A perspective on microbiomics, nutri(epi)genomics, and metabolomics. J. Agric. Food Chem. 2018, 66, 10686–10692. [Google Scholar] [CrossRef]
- Liu, Z.; De Bruijn, W.J.C.; Bruins, M.E.; Vincken, J.P. Reciprocal interactions between epigallocatechin-3-gallate (EGCG) and human gut microbiota in vitro. J. Agric. Food Chem. 2020, 68, 9804–9815. [Google Scholar] [CrossRef]
- Balaj, G.; Tamanai-Shacoori, Z.; Olivier-Jimenez, D.; Sauvager, A.; Faustin, M.; Bousarghin, L.; David-Le Gall, S.; Guyot, S.; Nebija, D.; Tomasi, S.; et al. An insight into an intriguing oxidative biotransformation pathway of 5-O-caffeoylquinic acid by a gut bacterium. Food Funct. 2022, 13, 6195–6204. [Google Scholar] [CrossRef]
- Takagaki, A.; Nanjo, F. Metabolism of (-)-epigallocatechin gallate by rat intestinal flora. J. Agric. Food Chem. 2010, 58, 1313–1321. [Google Scholar] [CrossRef]
- Takagaki, A.; Yoshioka, Y.; Yamashita, Y.; Nagano, T.; Ikeda, M.; Hara-Terawaki, A.; Seto, R.; Ashida, H. Effects of microbial metabolites of (-)-epigallocatechin gallate on glucose uptake in L6 skeletal muscle cells and glucose tolerance in ICR mice. Biol. Pharm. Bull. 2019, 42, 212–221. [Google Scholar]
- Chen, K.; Nakasone, Y.; Yi, S.; Ibrahim, H.R.; Sakao, K.; Hossain, M.A.; Hou, D.X. Natural garlic organosulfur compounds prevent metabolic disorder of lipid and glucose by increasing gut commensal Bacteroides acidifaciens. J. Agric. Food Chem. 2022, 70, 5829–5837. [Google Scholar] [CrossRef]
- Chauhan, A.; Dewali, S.; Pathak, V.M.; Bisht, S.S.; Chauhan, R.; Kaur, D.; Tuli, H.S.; Haque, S.; Ahmad, F. Therapeutic role of allicin in gastrointestinal cancers: Mechanisms and safety aspects. Discover Oncol. 2025, 16, 1731. [Google Scholar] [CrossRef]
- Holgado, F.; Campos-Monfort, G.; de las Heras, C.; Rupérez, P. Assessment of the prebiotic potential of globe artichoke by-product through in vitro fermentation by human faecal microbiota. Bioact. Carbohydr. Diet. Fibre 2022, 28, 100328. [Google Scholar] [CrossRef]
- Holgado, F.; Campos-Monfort, G.; de las Heras, C.; Rupérez, P. In vitro fermentability of globe artichoke by-product by Lactobacillus acidophilus and Bifidobacterium bifidum. Bioact. Carbohydr. Diet. Fibre 2021, 26, 100286. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Ghyselinck, J.; Marzorati, M.; Villar, A.; Zangara, A.; Smidt, C.R.; Risco, E. In vitro evaluation of prebiotic properties of a commercial artichoke inflorescence extract revealed bifidogenic effects. Nutrients 2020, 12, 1552. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Jiang, T.; He, D. Advances in the implications of the gut microbiota on the treatment efficacy of disease-modifying anti-rheumatic drugs in rheumatoid arthritis. Front. Immunol. 2023, 14, 1189036. [Google Scholar] [CrossRef]
- Verma, T.; Aggarwal, A.; Dey, P.; Chauhan, A.K.; Rashid, S.; Chen, K.-T.; Sharma, R. Medicinal and therapeutic properties of garlic, garlic essential oil, and garlic-based snack food: An updated review. Front. Nutr. 2023, 10, 1120377. [Google Scholar] [CrossRef]
- Ovesná, J.; Kucera, L.; Hornícková, J.; Svobodová, L.; Stavelíková, H.; Velísek, J.; Milella, L. Diversity of S-alk(en)yl cysteine sulphoxide content within a collection of garlic (Allium sativum L.) and its association with the morphological and genetic background assessed by AFLP. Sci. Hortic. 2011, 129, 541–547. [Google Scholar] [CrossRef]
- Shishikura, Y.; Khokhar, S. Factors affecting the levels of catechins and caffeine in tea beverage: Estimated daily intakes and antioxidant activity. J. Sci. Food Agric. 2005, 85, 2125–2133. [Google Scholar] [CrossRef]
- Honow, R.; Gu, K.-L.R.; Hesse, A.; Siener, R. Oxalate content of green tea of different origin, quality, preparation and time of harvest. Urol. Res. 2010, 38, 377–381. [Google Scholar] [CrossRef]
- Mamani, C.; Stadler, T.; Alberto, A.; Barbosa, L.C.A.; Eliana, M.; Queiroz, L.R. Determination of maleic hydrazide residues in garlic bulbs by HPLC. Talanta 2012, 89, 369–376. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Chanda, S.; Udayabanu, M.; Singh, M.; Agarwal, S. Anti-inflammatory and anti-arthritic potential of standardized extract of Clerodendrum serratum (L.) Moon. Front. Pharmacol. 2021, 12, 629607. [Google Scholar] [CrossRef]
- Ali, S.; Galgut, J.; Choudhary, R. On the novel action of melanolysis by a leaf extract of Aloe vera and its active ingredient aloin, potent skin depigmenting agents. Planta Med. 2012, 78, 767–777. [Google Scholar] [CrossRef]
- Jangra, A.; Sharma, G.; Sihag, S.; Chhokar, V. The dark side of miracle plant—Aloe vera: A review. Mol. Biol. Rep. 2022, 49, 5029–5040. [Google Scholar] [CrossRef]
- Abid, A.; Javed, M.; Zafar, S.; Hamdani, S.A.Z.; Shah, S.H.B.U.; Abid, J.; Ahmad, A.M.R. The Green Healer: An Updated Review on the Phytochemical Profile and Therapeutic Potential of Aloe Vera. Front. Nutr. 2025, 12, 1689700. [Google Scholar] [CrossRef]
- Szymczak, J.; Grygiel-Górniak, B.; Cielecka-Piontek, J. Zingiber Officinale Roscoe: The Antiarthritic Potential of a Popular Spice—Preclinical and Clinical Evidence. Nutrients 2024, 16, 741. [Google Scholar] [CrossRef] [PubMed]
- Nikkhah Bodagh, M.; Maleki, I.; Hekmatdoost, A. Ginger in Gastrointestinal Disorders: A Systematic Review of Clinical Trials. Food Sci. Nutr. 2019, 7, 96–108. [Google Scholar] [CrossRef]
- Ali, A.; Gilani, A.H. Medicinal Value of Ginger with Focus on Its Use in Nausea and Vomiting of Pregnancy. Int. J. Food Prop. 2007, 10, 269–278. [Google Scholar] [CrossRef]
- Abdul Rani, A.N.; Gaurav, A.; Lee, V.S.; Mad Nasir, N.; Md Zain, S.; Patil, V.M.; Lee, M.T. Insights into Biological Activities Profile of Gingerols and Shogaols for Potential Pharmacological Applications. Arch. Pharm. Res. 2025, 48, 638–675. [Google Scholar] [CrossRef]
- del Bosque-Plata, L.; Gragnoli, C. Cinnamon Treatment Shows Promise for Glycemic Control but May Cause Adverse Effects in Some People. Clin. Nutr. Open Sci. 2025, 59, 184–188. [Google Scholar] [CrossRef]
- Medagama, A.B. The Glycaemic Outcomes of Cinnamon, a Review of the Experimental Evidence and Clinical Trials. Nutr. J. 2015, 14, 108. [Google Scholar] [CrossRef]
- Zorzi, K.G.; Carvalko, E.L.S.; Lino, G.; Lino von Poser, G.; Teixeira, H.F. On the Use of Nanotechnology-Based Strategies for Association of Complex Matrices from Plant Extracts. Rev. Bras. Farmacogn. 2015, 25, 426–436. [Google Scholar] [CrossRef]
- Kolberg, M.; Paur, I.; Balstad, T.R.; Pedersen, S.; Jacobs, D.R.; Blomhoff, R. Plant Extracts of Spices and Coffee Synergistically Dampen Nuclear Factor–ΚB in U937 Cells. Nutr. Res. 2013, 33, 817–830. [Google Scholar] [CrossRef]
- Kumar, N.; Kulsoom, M.; Shukla, V.; Kumar, D.; Priyanka; Kumar, S.; Tiwari, J.; Dwivedi, N. Profiling of Heavy Metal and Pesticide Residues in Medicinal Plants. Environ. Sci. Pollut. Res. 2018, 25, 29505–29510. [Google Scholar] [CrossRef]
- Asgari Lajayer, B.; Ghorbanpour, M.; Nikabadi, S. Heavy Metals in Contaminated Environment: Destiny of Secondary Metabolite Biosynthesis, Oxidative Status and Phytoextraction in Medicinal Plants. Ecotoxicol. Environ. Saf. 2017, 145, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Asiminicesei, D.M.; Vasilachi, I.C.; Gavrilescu, M. Heavy Metal Contamination of Medicinal Plants and Potential Implications on Human Health. Rev. Chim. 2020, 71, 16–36. [Google Scholar] [CrossRef]
- Kosalec, I.; Cvek, J.; Tomić, A.S. Contaminants of Medicinal Herbs and Herbal Products. Herb Herb. Prod. Contam. 2009, 60, 485–501. [Google Scholar] [CrossRef]
- Harris, E.S.J.; Cao, S.; Littlefield, B.A.; Craycroft, J.A.; Scholten, R.; Kaptchuk, T.; Fu, Y.; Wang, W.; Liu, Y.; Chen, H.; et al. Heavy Metal and Pesticide Content in Commonly Prescribed Individual Raw Chinese Herbal Medicines. Sci. Total Environ. 2011, 409, 4297–4305. [Google Scholar] [CrossRef]
- Jităreanu, A.; Trifan, A.; Vieriu, M.; Caba, I.-C.; Mârtu, I.; Agoroaei, L. Current Trends in Toxicity Assessment of Herbal Medicines: A Narrative Review. Processes 2023, 11, 83. [Google Scholar] [CrossRef]
- Faisal, R.; Shinwari, L.; Aziz, I.; Khalil, A.T. Therapeutic and Adverse Effects of Commonly Used Medicinal Plants: Standardization and Quality Assurance. Proc. Pak. Acad. Sci. B Life Environ. Sci. 2019, 56, 1–9. [Google Scholar]
- Moreira, D.D.L.; Teixeira, S.S.; Monteiro, M.H.D.; De-Oliveira, A.C.A.X.; Paumgartten, F.J.R. Traditional Use and Safety of Herbal Medicines. Rev. Bras. Farmacogn. 2014, 24, 248–257, Erratum in Rev. Bras. Farmacogn. 2014, 24, 502–503. [Google Scholar] [CrossRef]
- Canigueral, S.; Tschopp, R.; Ambrosetti, L.; Vignutelli, A.; Scaglione, F.; Petrini, O. The Development of Herbal Medicinal Products. Quality, Safety and Efficacy as Key Factors. Pharmaceut. Med. 2008, 22, 107–118. [Google Scholar]
- Pferschy-Wenzig, E.M.; Bauer, R. The Relevance of Pharmacognosy in Pharmacological Research on Herbal Medicinal Products. Epilepsy Behav. 2015, 52, 344–362. [Google Scholar] [CrossRef]
- Dubey, R.K.; Shukla, S. Exploring Novel Herbal Compounds and Formulations for Inflammatory Bowel Disease (IBD) Management. Chin. J. Appl. Physiol. 2023, 39, e20230003. [Google Scholar] [CrossRef]
- Dzwonkowski, M.; Bahirwani, J.; Rollins, S.; Muratore, A.; Christian, V.; Schneider, Y. Selected Use of Complementary and Alternative Medicine (CAM) Agents in IBD. Curr. Gastroenterol. Rep. 2025, 27, 1. [Google Scholar] [CrossRef]
- Shamsaddini, E.; Hasheminasab, F.S.; Raeiszadeh, M.; Haji-Maghsoudi, S.; Azizian, A.; Azimi, M. The Use of Herbal Medicine in Patients with Inflammatory Bowel Disorders in Iran: A Cross-Sectional Study. Eur. J. Integr. Med. 2024, 71, 102384. [Google Scholar] [CrossRef]
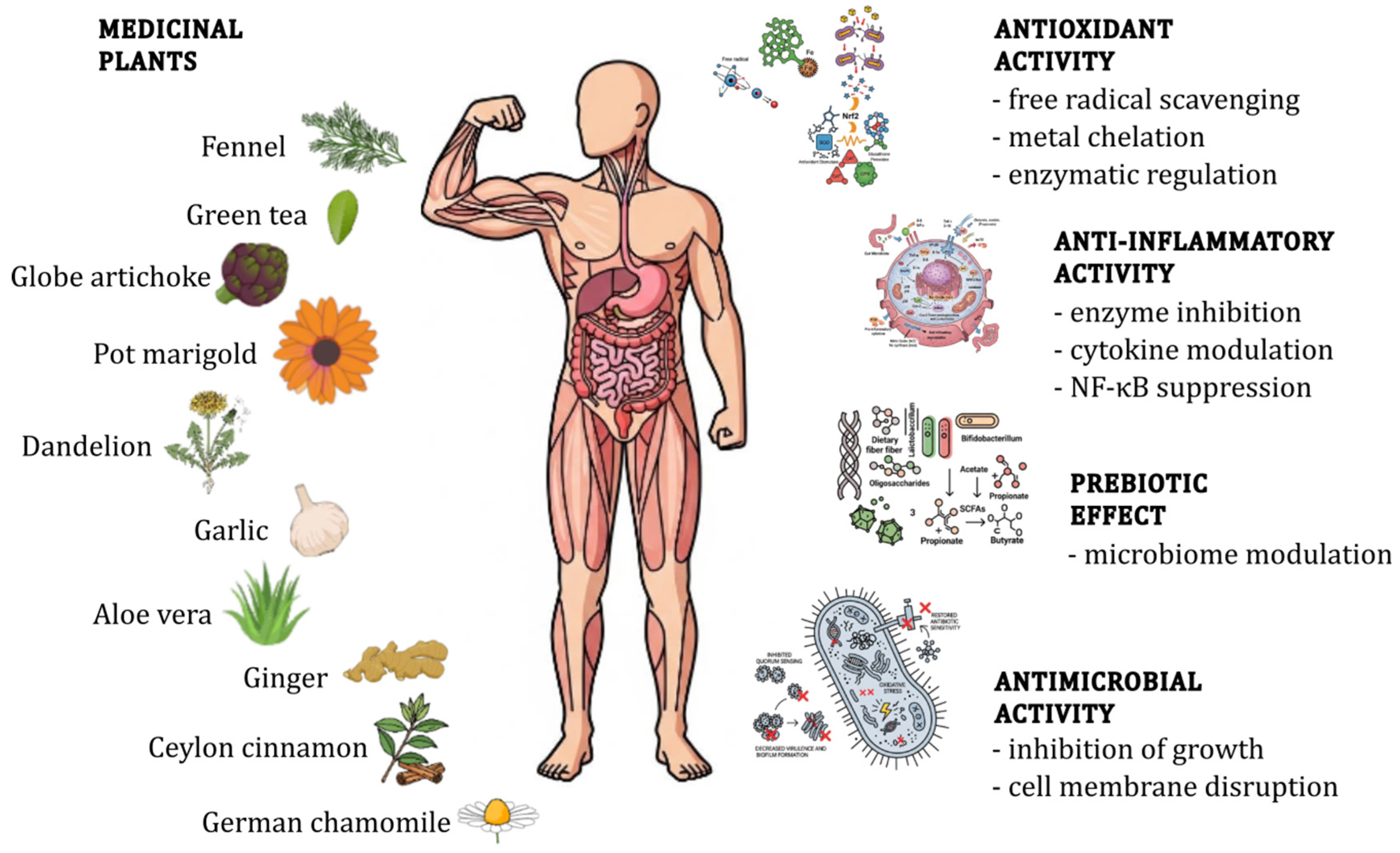
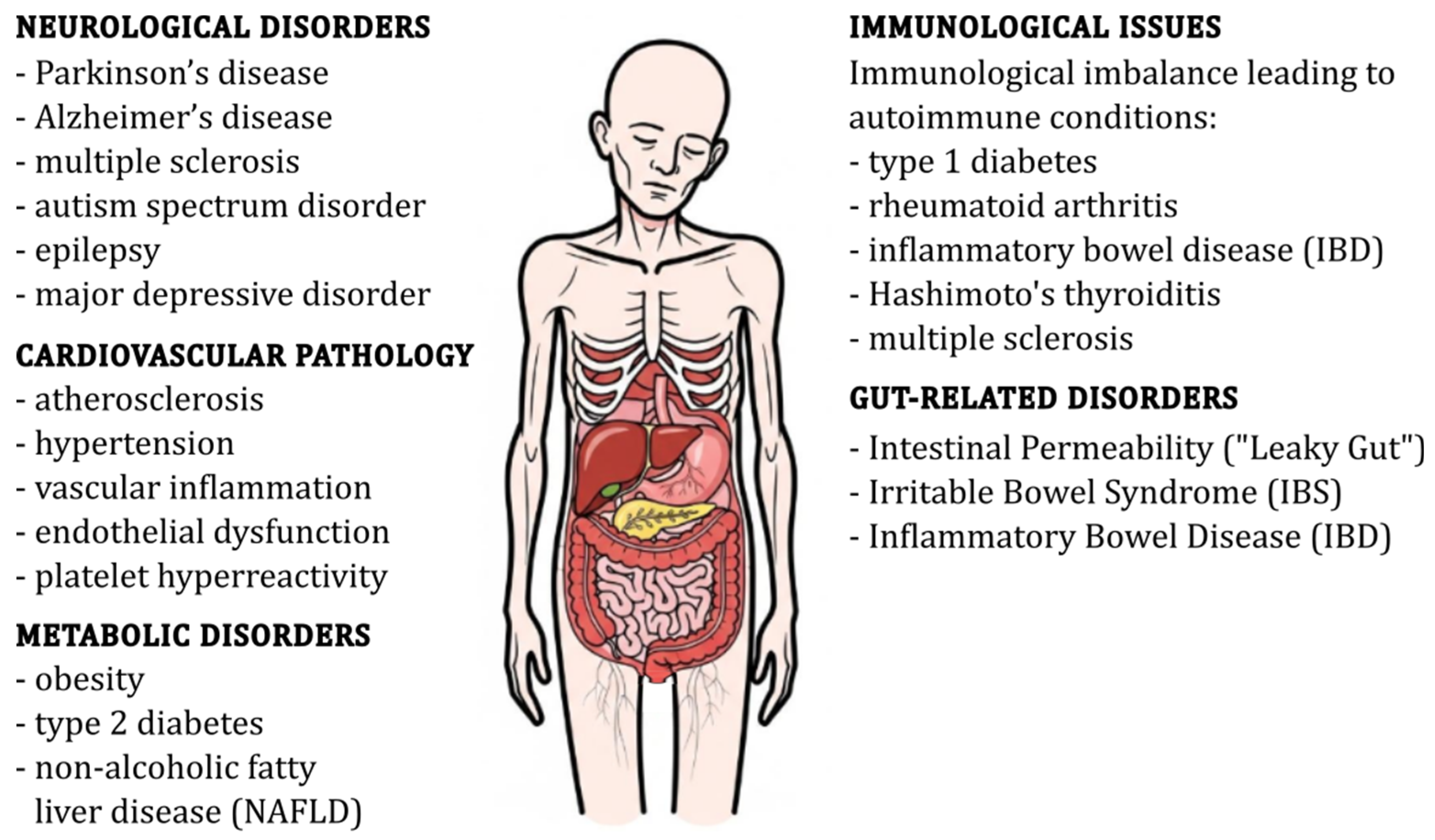
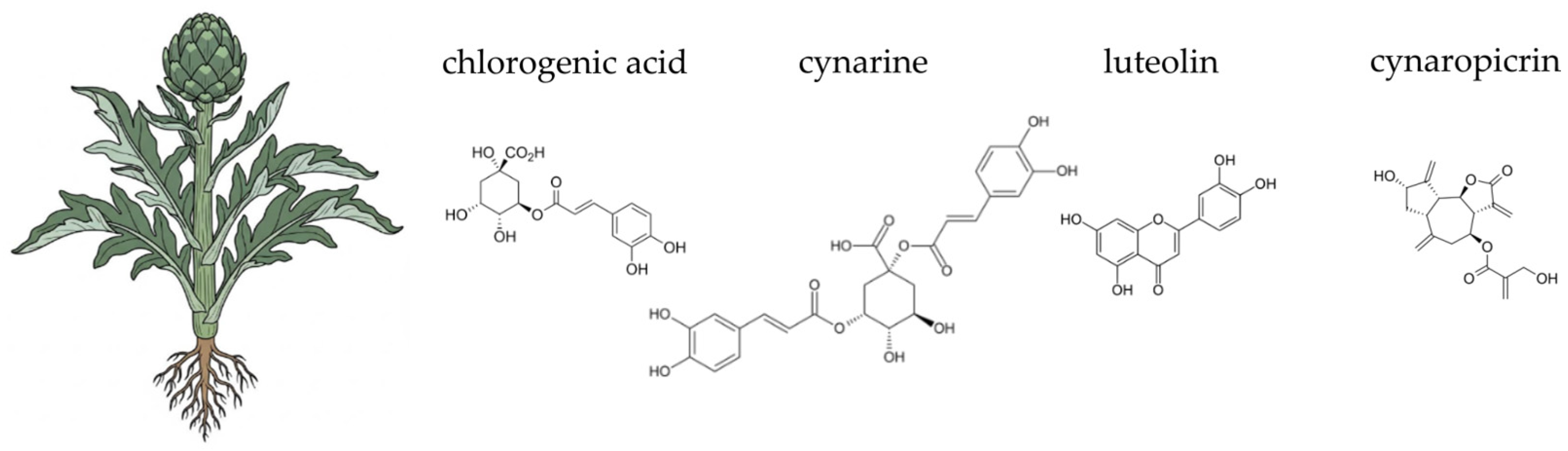
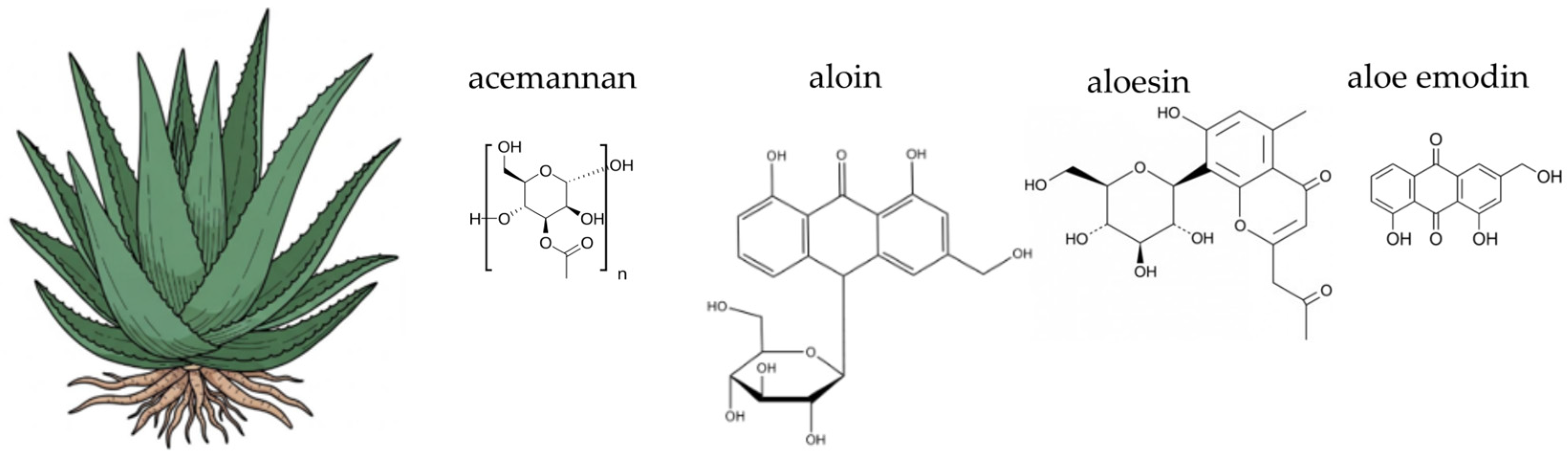
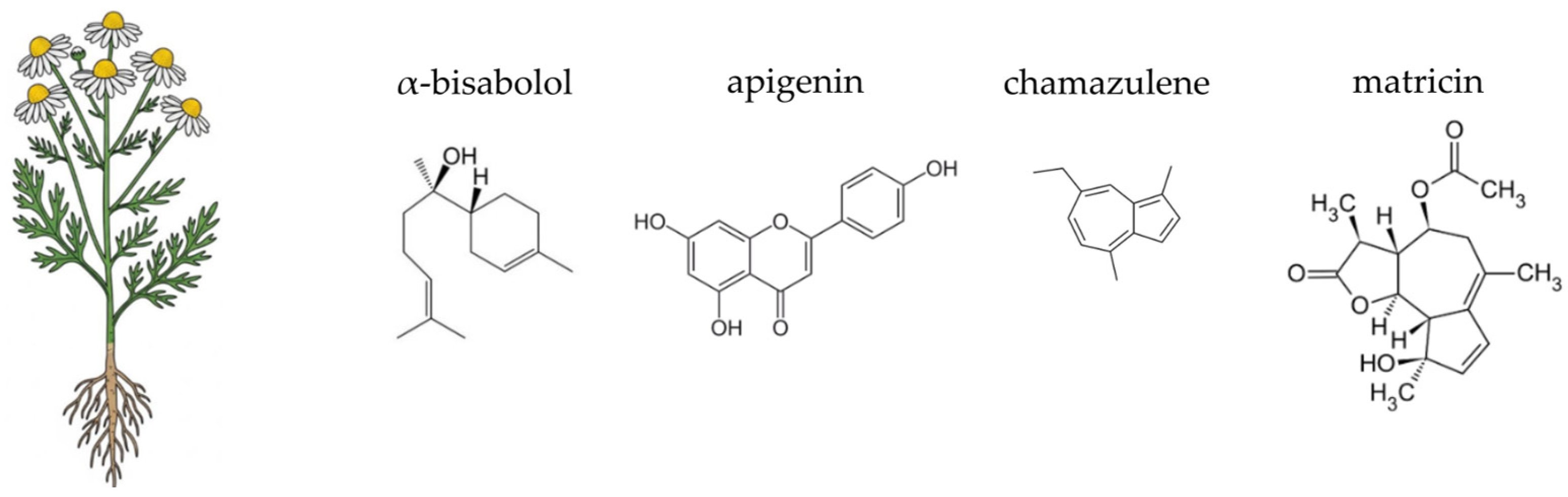
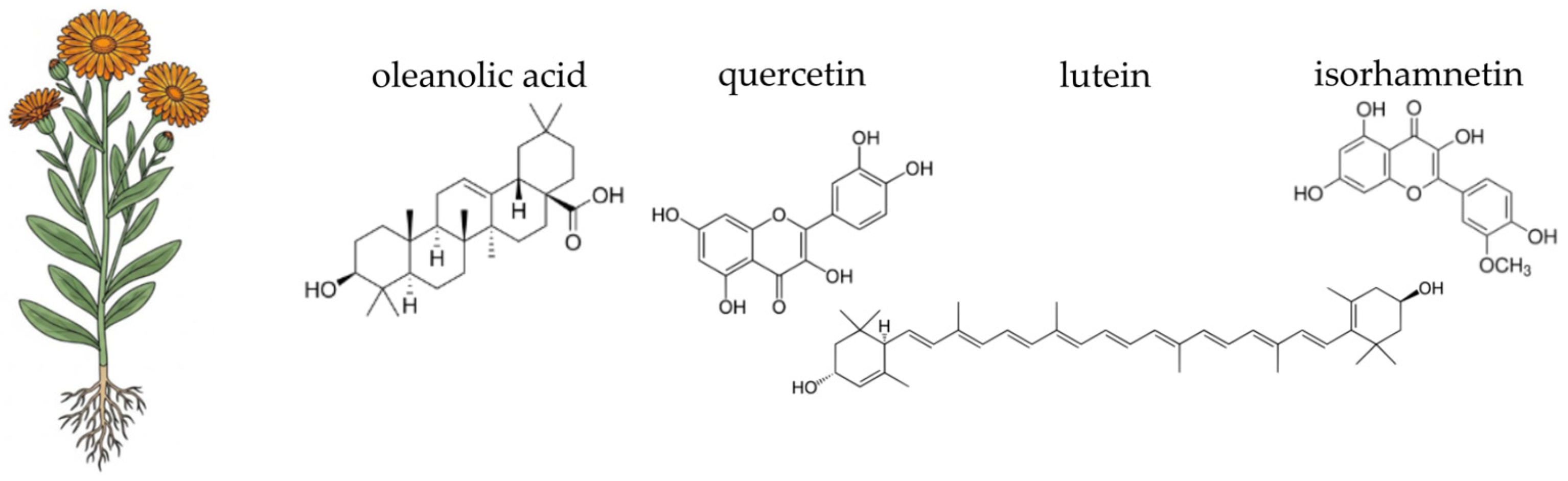

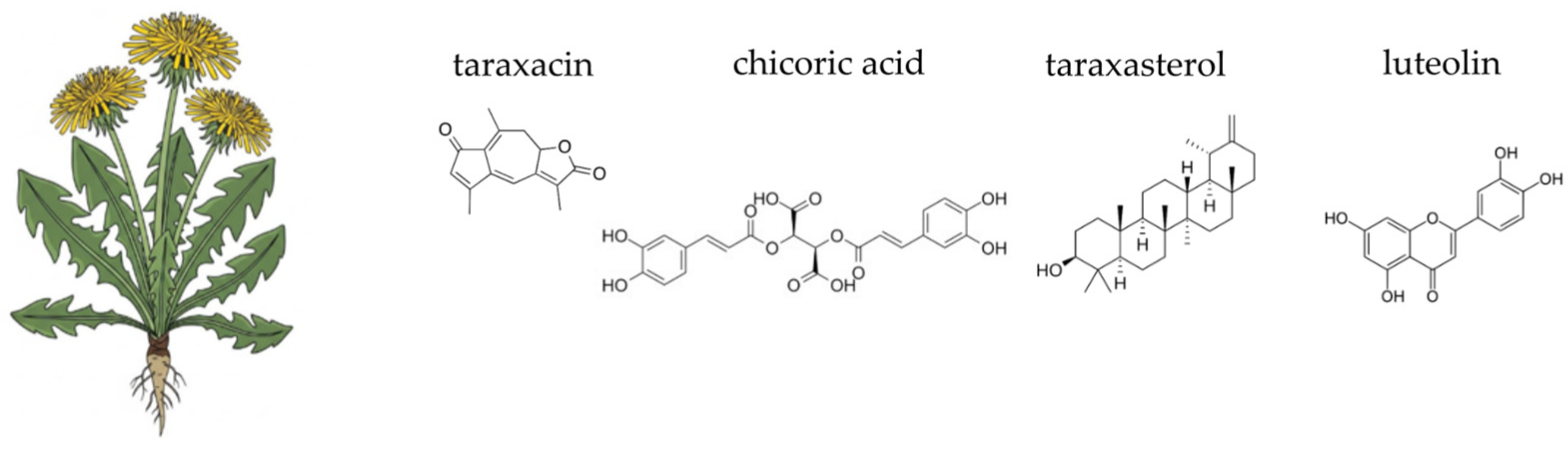
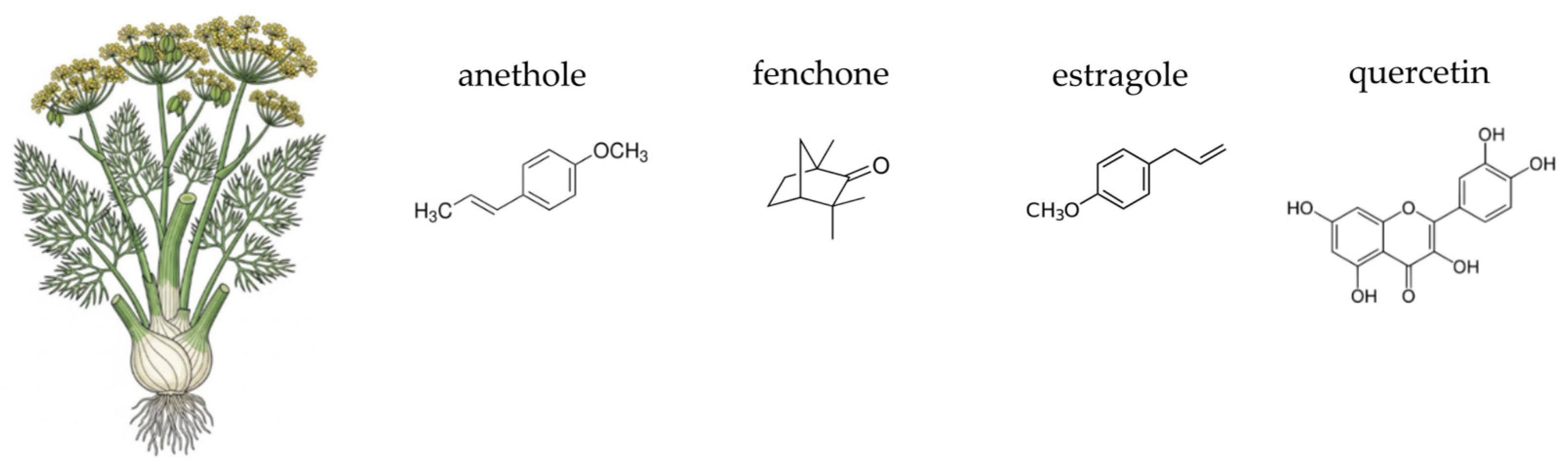
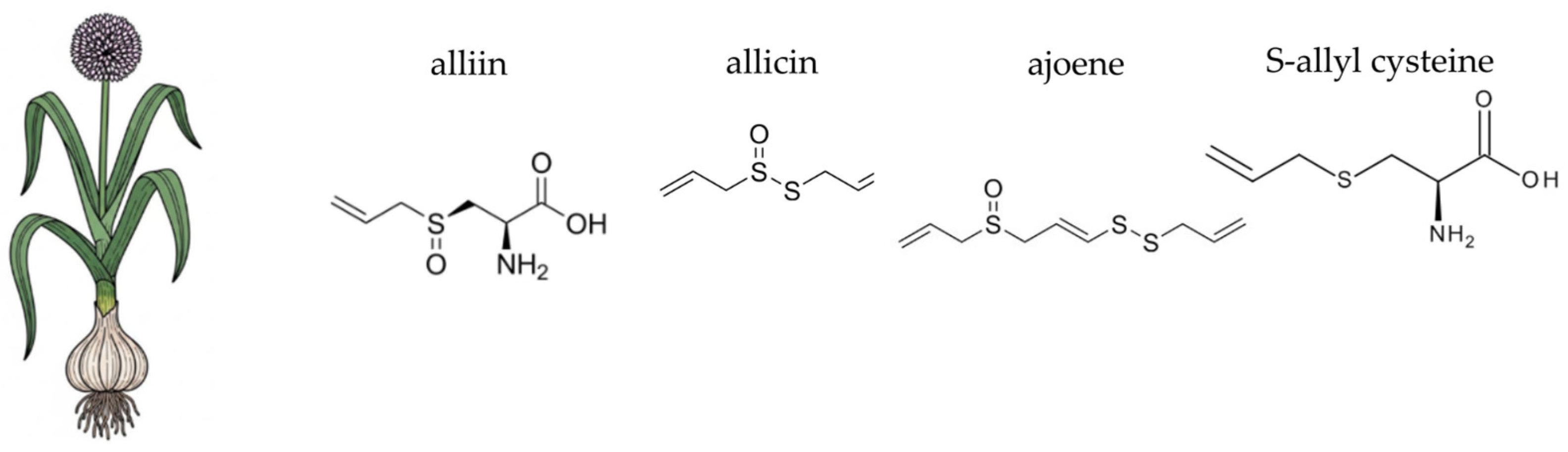
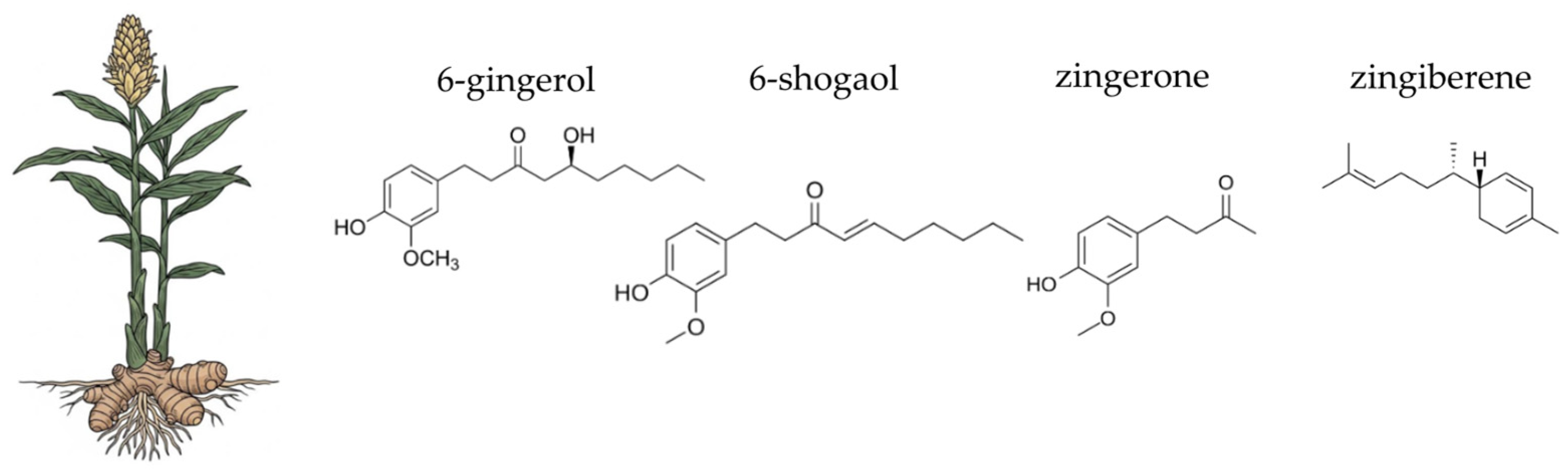
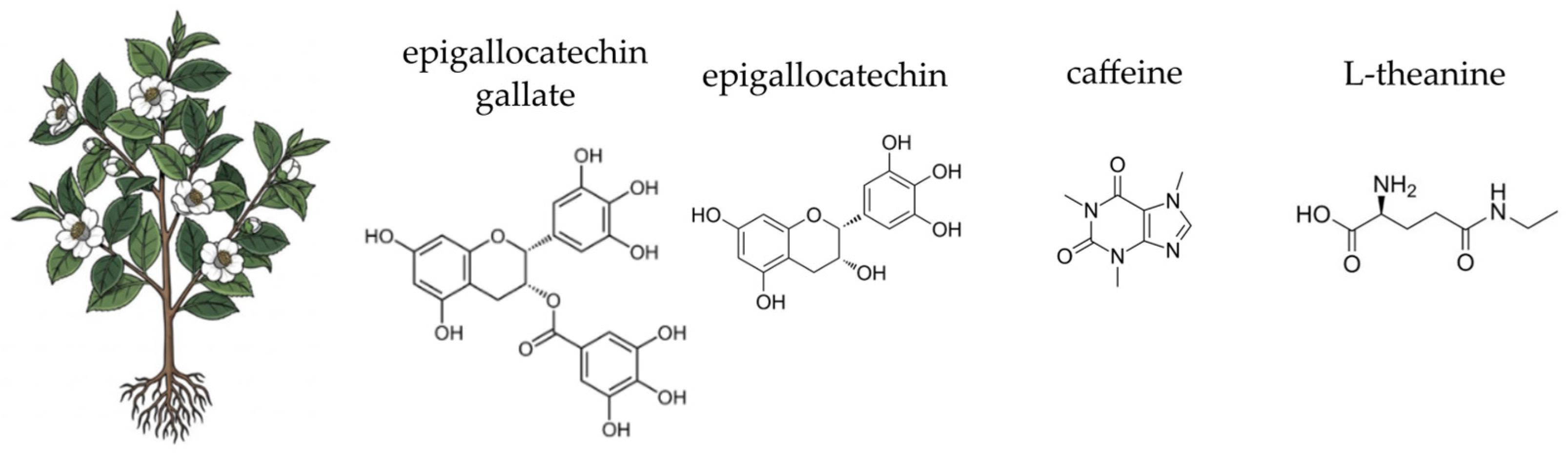
| Types of Prebiotics | Occurrence in Selected Medicinal Plants | Potential Benefits | Reference |
|---|---|---|---|
| Fructooligosaccharides (FOS) | artichoke, garlic—75% of its dry weight, dandelion, artichoke, chamomile, aloe vera | Improve calcium absorption, decrease triglycerides, improve immunity, inhibit pathogenic microorganisms, prevent cancer, and control diabetes | [52,53,54] |
| Galactooligosaccharides (GOS) | lack of this compound in plants | Increase bifidogenic activity | [55,56] |
| Xylooligosaccharides (XOS) | no | Non-carcinogenic nature, exhibit a positive effect on the intestinal flora, non-digestibility | [57] |
| Soybean oligosaccharides (SOS) | no | Increase the level of IgG, modulate body weight and the immune system | [58] |
| Isomaltooligosaccharides (IMO) | no | Improve gastrointestinal flora | [59] |
| Fructans | dandelion—40% of dry matter, artichoke, aloe vera, chamomile, garlic | Modulate gut physiology to provide protection from pathogens, improve the level of glucose, | [60,61,62] |
| Guar gum | no | Improve cholesterol, glycemia | [57,63] |
| Pectinoligosaccharides (POS) | pot marigold | Anti-inflammatory effect | [64] |
| Plant Name (Latin Name) | Key Active Compounds | Plant Part Used | Examples of Dosages | Antioxidant Activity | Anti- Inflammatory Activity | Antimicrobial Activity | Prebiotic Effect | References |
|---|---|---|---|---|---|---|---|---|
| Globe artichoke (Cynara scolymus) | cynarin, chlorogenic acid, luteolin, apigenin, fructooligosaccharides, inulin-type fructans | leaves, flower heads |
| strong | moderate | weak | very strong | [61,184,670,671,672] |
| Aloe vera (Aloe vera) | acemannan, aloin, aloesin, fructooligosaccharides, fructans | leaf pulp (gel) |
| moderate | strong | moderate | strong | [673,674,675,676] |
| German chamomile (Matricaria chamomilla) | α-bisabolol, luteolin, apigenin, quercetin, patuletin, fructooligosaccharides | flower heads |
| moderate | strong | moderate | weak | [339,677,678] |
| Pot marigold (Calendula officinalis) | α-cadinol, rutin, quercetin, luteolin, lycopene, β-carotene, acidic pectic type polysaccharides, pectinoligosaccharides | flowers |
| moderate | strong | moderate | weak | [348,679,680] |
| Ceylon cinnamon (Cinnamomum verum) | trans-cinnamaldehyde, eugenol | bark |
| very strong | strong | strong | moderate | [447,681,682] |
| Dandelion (Taraxacum officinale) | taraxacin, fructooligosaccharides, luteolin, quercetin, chlorogenic acid, caffeic acid, taraxasterol | root, leaves |
| strong | moderate | weak | very strong | [683,684] |
| Fennel (Foeniculum vulgare) | trans-anethole, fenchone, estragole, quercetin, rutin, kaempferol, dietary fibre, caffeic acid, quinic acid, chlorogenic acid, fructooligosaccharides | seeds (fruits) |
| moderate | moderate | moderate | weak | [519,685,686,687] |
| Garlic (Allium sativum) | alliin, allicin, ajoene, S-allyl cysteine, inulin, fructooligosaccharides | bulb (cloves) |
| strong | strong | very strong | strong | [444,522,567,688] |
| Ginger (Zingiber officinale) | α-tocopherol, ascorbic acid, [6]-gingerol, [6]-shogaol, zingiberene | rhizome |
| strong | very strong | moderate | moderate | [443,444,689,690] |
| Green Tea (Camellia sinensis) | epigallocatechin gallate, epicatechin, epigallocatechin, epicatechin gallate, quercetin, kaempferol, gallic acid, caffeine | leaves |
| very strong | strong | moderate | moderate | [657,691,692,693] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacyga, K.; Tabiś, A.; Pacyga, P. Medicinal Plants for a Healthy Gut Microbiome: Scientific Insights into Modern Herbal Applications. Int. J. Mol. Sci. 2025, 26, 10875. https://doi.org/10.3390/ijms262210875
Pacyga K, Tabiś A, Pacyga P. Medicinal Plants for a Healthy Gut Microbiome: Scientific Insights into Modern Herbal Applications. International Journal of Molecular Sciences. 2025; 26(22):10875. https://doi.org/10.3390/ijms262210875
Chicago/Turabian StylePacyga, Katarzyna, Aleksandra Tabiś, and Paweł Pacyga. 2025. "Medicinal Plants for a Healthy Gut Microbiome: Scientific Insights into Modern Herbal Applications" International Journal of Molecular Sciences 26, no. 22: 10875. https://doi.org/10.3390/ijms262210875
APA StylePacyga, K., Tabiś, A., & Pacyga, P. (2025). Medicinal Plants for a Healthy Gut Microbiome: Scientific Insights into Modern Herbal Applications. International Journal of Molecular Sciences, 26(22), 10875. https://doi.org/10.3390/ijms262210875





