A Hydrogel Culture System Regulates Human Adipocyte Function
Abstract
1. Introduction
2. Results
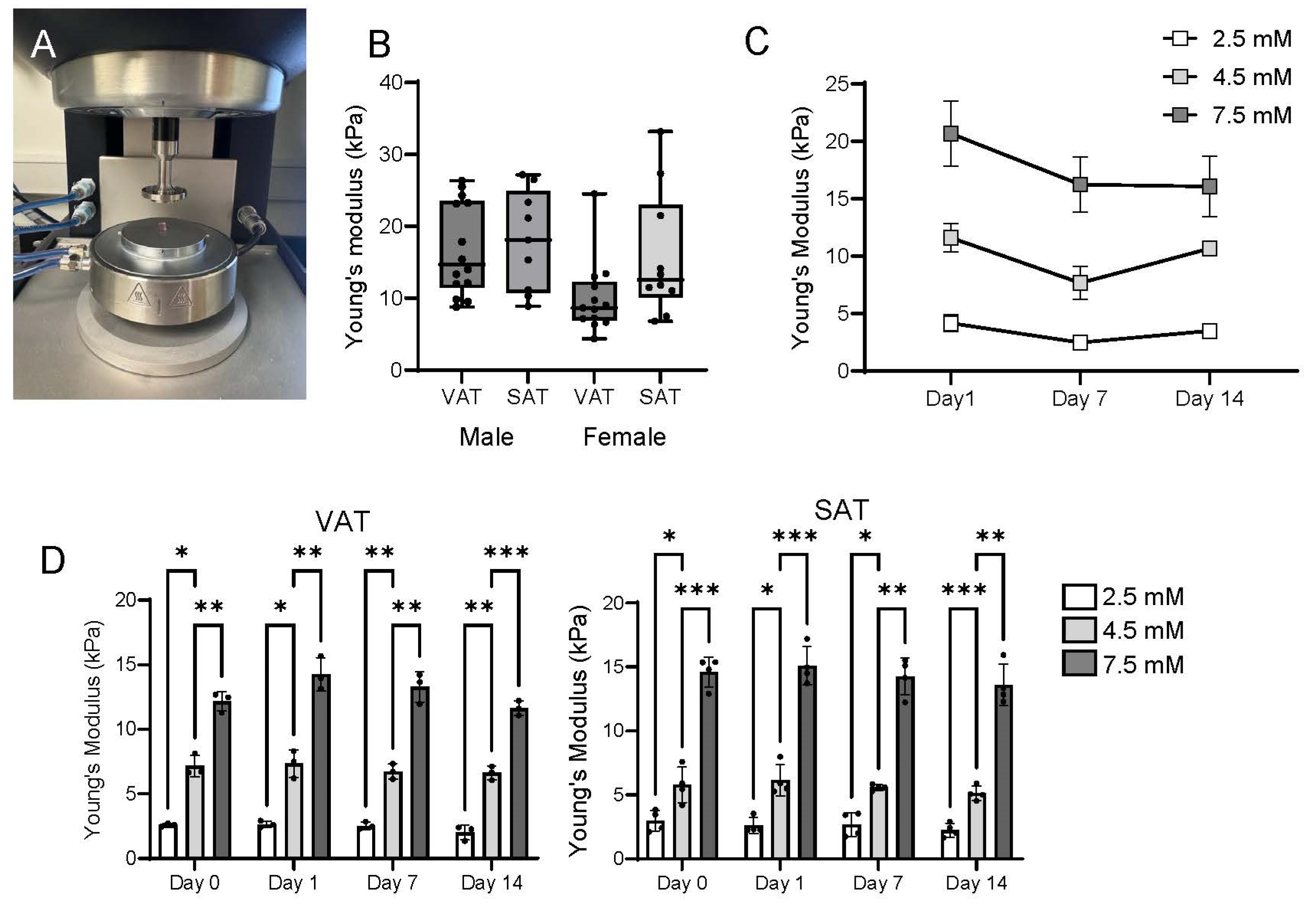
2.1. Rheologic Properties of TRUE7 Hydrogels Approximate Native Human Adipose Tissue
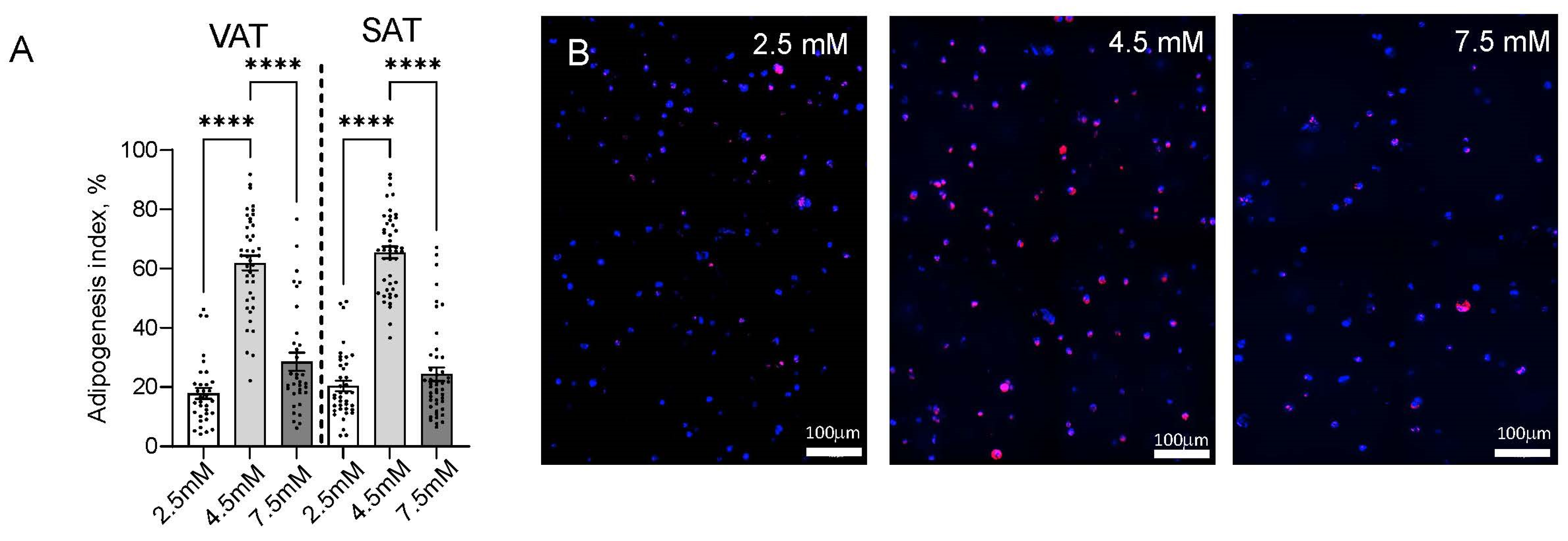
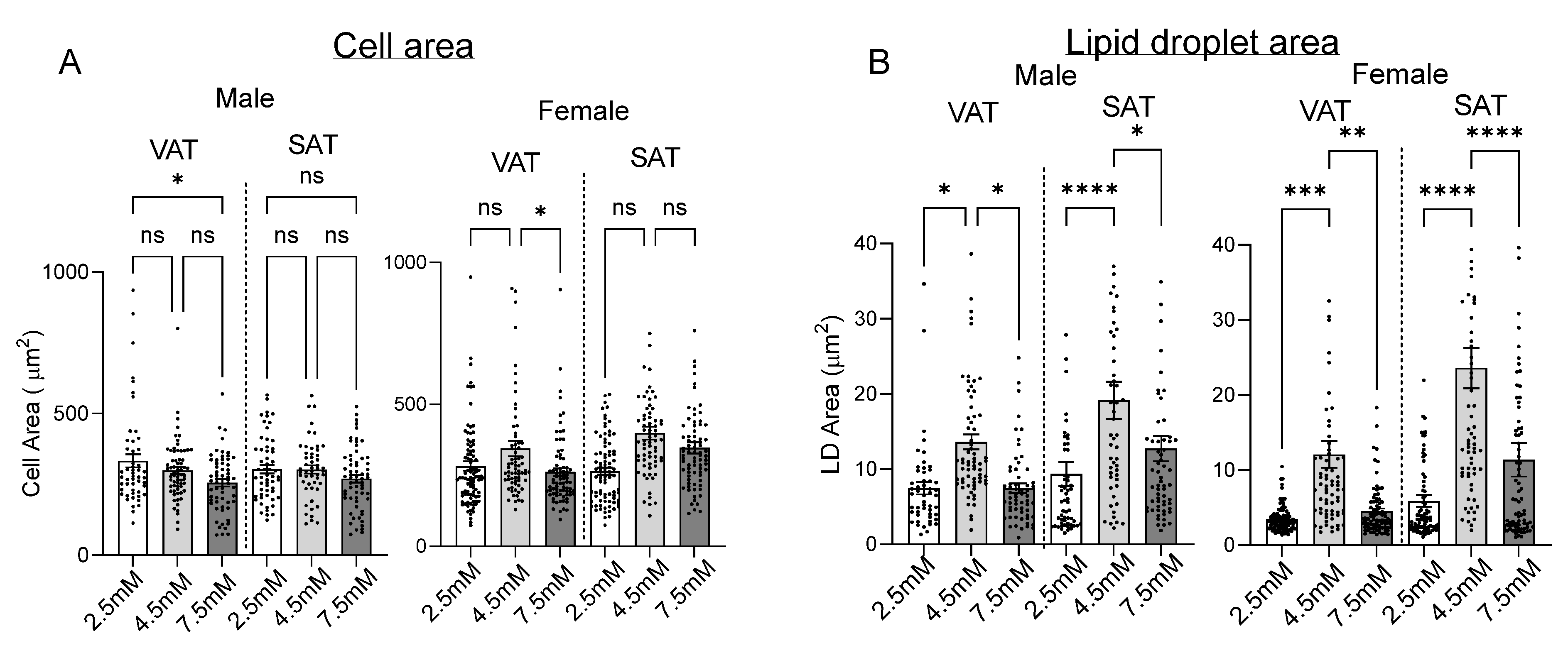
2.2. Matrix Stiffness Regulates Adipogenesis and Adipocyte Lipid Storage
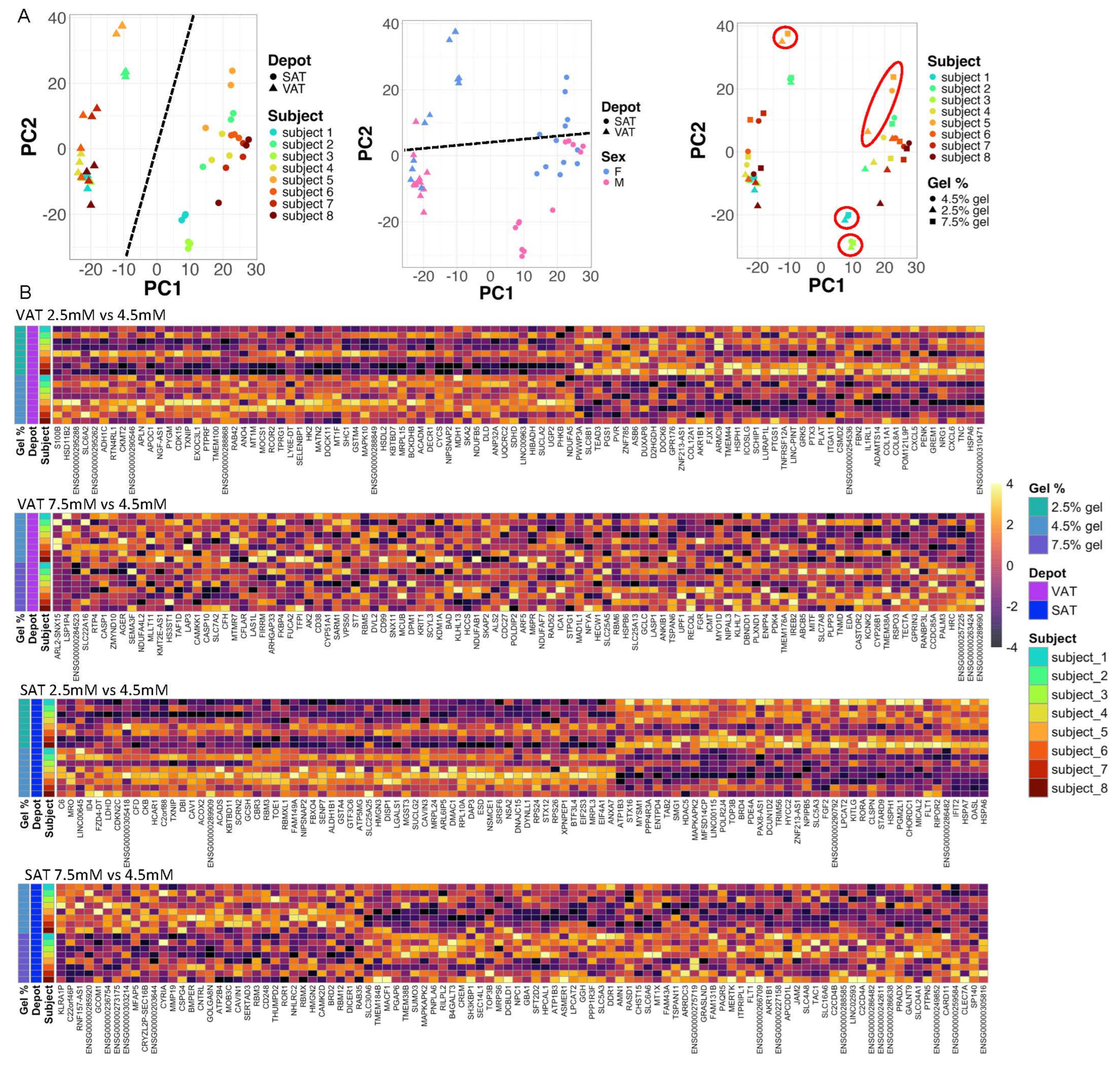
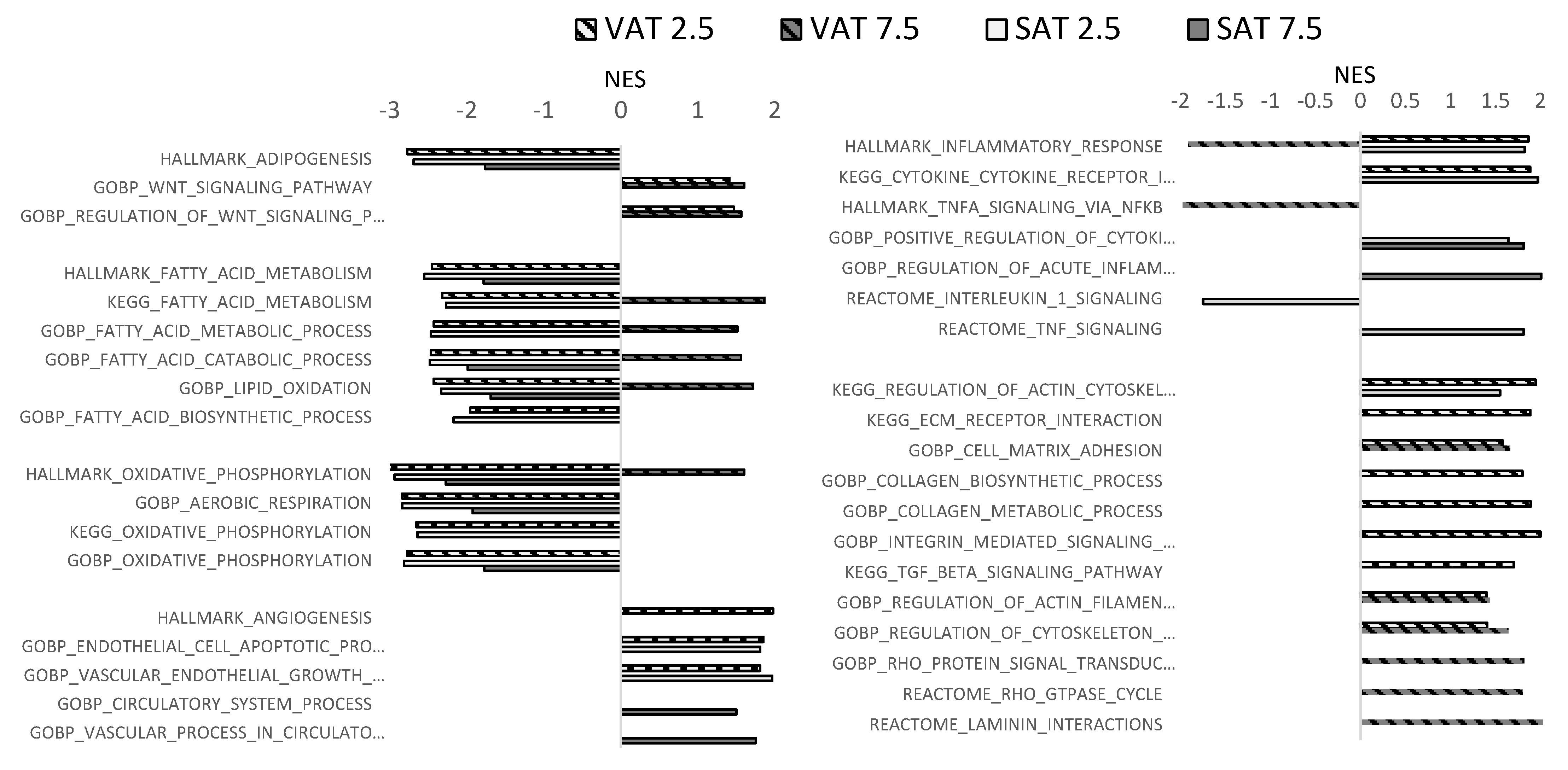
2.3. Matrix Stiffness Regulates Adipocyte Transcriptional Programs
3. Discussion
4. Materials and Methods
4.1. Human Subjects
4.2. Adipose Tissue Stromal Cell (ASC) Isolation
4.3. Hydrogel Culture
4.4. Rheology
4.5. Confocal Microscopy and Digital Analysis
4.6. RNA Sequencing (RNASeq)
4.7. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASC | Adipose tissue stromal cells |
| DEG | Differentially expressed genes |
| ECM | Extracellular matrix |
| GO | Gene ontogeny |
| GSEA | Gene set enrichment analysis |
| LD | Lipid droplet |
| MMP | matrix metalloprotease |
| NES | Normalized enrichment score |
| NDM | Non-diabetic |
| PCA | Principal component analysis |
| RNASeq | RNA sequencing |
| SAT | Subcutaneous adipose tissue |
| VAT | Visceral adipose tissue |
| μM | Micrometer |
References
- Guglielmi, V.; Cardellini, M.; Cinti, F.; Corgosinho, F.; Cardolini, I.; D’Adamo, M.; Zingaretti, M.C.; Bellia, A.; Lauro, D.; Gentileschi, P.; et al. Omental adipose tissue fibrosis and insulin resistance in severe obesity. Nutr. Diabetes 2015, 5, e175. [Google Scholar] [CrossRef] [PubMed]
- Henegar, C.; Tordjman, J.; Achard, V.; Lacasa, D.; Cremer, I.; Guerre-Millo, M.; Poitou, C.; Basdevant, A.; Stich, V.; Viguerie, N.; et al. Adipose tissue transcriptomic signature highlights the pathological relevance of extracellular matrix in human obesity. Genome Biol. 2008, 9, R14. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Muise, E.S.; Iyengar, P.; Wang, Z.V.; Chandalia, M.; Abate, N.; Zhang, B.B.; Bonaldo, P.; Chua, S.; Scherer, P.E. Metabolic dysregulation and adipose tissue fibrosis: Role of collagen VI. Mol. Cell. Biol. 2009, 29, 1575–1591. [Google Scholar] [CrossRef] [PubMed]
- Spencer, M.; Yao-Borengasser, A.; Unal, R.; Rasouli, N.; Gurley, C.M.; Zhu, B.; Peterson, C.A.; Kern, P.A. Adipose tissue macrophages in insulin-resistant subjects are associated with collagen VI and fibrosis and demonstrate alternative activation. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E1016–E1027. [Google Scholar] [CrossRef] [PubMed]
- Vila, I.K.; Badin, P.M.; Marques, M.A.; Monbrun, L.; Lefort, C.; Mir, L.; Louche, K.; Bourlier, V.; Roussel, B.; Gui, P.; et al. Immune cell Toll-like receptor 4 mediates the development of obesity- and endotoxemia-associated adipose tissue fibrosis. Cell Rep. 2014, 7, 1116–1129. [Google Scholar] [CrossRef] [PubMed]
- Divoux, A.; Tordjman, J.; Lacasa, D.; Veyrie, N.; Hugol, D.; Aissat, A.; Basdevant, A.; Guerre-Millo, M.; Poitou, C.; Zucker, J.D.; et al. Fibrosis in human adipose tissue: Composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes 2010, 59, 2817–2825. [Google Scholar] [CrossRef] [PubMed]
- Lackey, D.E.; Burk, D.H.; Ali, M.R.; Mostaedi, R.; Smith, W.H.; Park, J.; Scherer, P.E.; Seay, S.A.; McCoin, C.S.; Bonaldo, P.; et al. Contributions of adipose tissue architectural and tensile properties toward defining healthy and unhealthy obesity. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E233–E246. [Google Scholar] [CrossRef] [PubMed]
- Muir, L.A.; Neeley, C.K.; Meyer, K.A.; Baker, N.A.; Brosius, A.M.; Washabaugh, A.R.; Varban, O.A.; Finks, J.F.; Zamarron, B.F.; Flesher, C.G.; et al. Adipose tissue fibrosis, hypertrophy, and hyperplasia: Correlations with diabetes in human obesity. Obesity 2016, 24, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Soták, M.; Rajan, M.R.; Clark, M.; Biörserud, C.; Wallenius, V.; Hagberg, C.E.; Börgeson, E. Healthy Subcutaneous and Omental Adipose Tissue Is Associated with High Expression of Extracellular Matrix Components. Int. J. Mol. Sci. 2022, 23, 520. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Juliar, B.A.; Strieder-Barboza, C.; Karmakar, M.; Flesher, C.G.; Baker, N.A.; Varban, O.A.; Lumeng, C.N.; Putnam, A.J.; O’Rourke, R.W. Viscoelastic characterization of diabetic and non-diabetic human adipose tissue. Biorheology 2020, 57, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.A.; Muir, L.A.; Washabaugh, A.R.; Neeley, C.K.; Chen, S.Y.; Flesher, C.G.; Vorwald, J.; Finks, J.F.; Ghaferi, A.A.; Mulholland, M.W.; et al. Diabetes-Specific Regulation of Adipocyte Metabolism by the Adipose Tissue Extracellular Matrix. J. Clin. Endocrinol. Metab. 2017, 102, 1032–1043. [Google Scholar] [CrossRef] [PubMed]
- Strieder-Barboza, C.; Baker, N.A.; Flesher, C.G.; Karmakar, M.; Neeley, C.K.; Polsinelli, D.; Dimick, J.B.; Finks, J.F.; Ghaferi, A.A.; Varban, O.A.; et al. Advanced glycation end-products regulate extracellular matrix-adipocyte metabolic crosstalk in diabetes. Sci. Rep. 2019, 9, 19748. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Qiao, Y.; Quan, S.; Yang, C.; Li, J. Relationship of matrix stiffness and cell morphology in regulation of osteogenesis and adipogenesis of BMSCs. Mol. Biol. Rep. 2022, 49, 2677–2685. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Chu, J.S.; Tsou, A.D.; Diop, R.; Tang, Z.; Wang, A.; Li, S. The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-beta. Biomaterials 2011, 32, 3921–3930. [Google Scholar] [CrossRef] [PubMed]
- Takata, K.; Goto, T.; Kuroda, M.; Kimura, Y.; Harada, I.; Ueda, K.; Kawada, T.; Kioka, N. Stiffness of the extracellular matrix regulates differentiation into beige adipocytes. Biochem. Biophys. Res. Commun. 2020, 532, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Young, D.A.; Choi, Y.S.; Engler, A.J.; Christman, K.L. Stimulation of adipogenesis of adult adipose-derived stem cells using substrates that mimic the stiffness of adipose tissue. Biomaterials 2013, 34, 8581–8588. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lin, S.; Shao, X.; Shi, S.; Zhang, Q.; Xue, C.; Lin, Y.; Zhu, B.; Cai, X. Regulating osteogenesis and adipogenesis in adipose-derived stem cells by controlling underlying substrate stiffness. J. Cell. Physiol. 2018, 233, 3418–3428. [Google Scholar] [CrossRef] [PubMed]
- Ky, A.; McCoy, A.J.; Flesher, C.G.; Friend, N.E.; Li, J.; Akinleye, K.; Patsalis, C.; Lumeng, C.N.; Putnam, A.J.; O’Rourke, R.W. Matrix density regulates adipocyte phenotype. Adipocyte 2023, 12, 2268261. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.L.; Jin, G.Z. YAP and ECM Stiffness: Key Drivers of Adipocyte Differentiation and Lipid Accumulation. Cells 2024, 13, 1905. [Google Scholar] [CrossRef] [PubMed]
- Khetan, S.; Guvendiren, M.; Legant, W.R.; Cohen, D.M.; Chen, C.S.; Burdick, J.A. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat. Mater. 2013, 12, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Di Caprio, N.; Bellas, E. Collagen Stiffness and Architecture Regulate Fibrotic Gene Expression in Engineered Adipose Tissue. Adv. Biosyst. 2020, 4, e1900286. [Google Scholar] [CrossRef] [PubMed]
- Alkhouli, N.; Mansfield, J.; Green, E.; Bell, J.; Knight, B.; Liversedge, N.; Tham, J.C.; Welbourn, R.; Shore, A.C.; Kos, K.; et al. The mechanical properties of human adipose tissues and their relationships to the structure and composition of the extracellular matrix. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E1427–E1435. [Google Scholar] [CrossRef] [PubMed]
- Feig, V.R.; Tran, H.; Lee, M.; Bao, Z. Mechanically tunable conductive interpenetrating network hydrogels that mimic the elastic moduli of biological tissue. Nat. Commun. 2018, 9, 2740. [Google Scholar] [CrossRef] [PubMed]
- Fontanella, C.G.; Toniolo, I.; Foletto, M.; Prevedello, L.; Carniel, E.L. Mechanical Behavior of Subcutaneous and Visceral Abdominal Adipose Tissue in Patients with Obesity. Processes 2022, 10, 1798. [Google Scholar] [CrossRef]
- Huber, B.; Borchers, K.; Tovar, G.E.; Kluger, P.J. Methacrylated gelatin and mature adipocytes are promising components for adipose tissue engineering. J. Biomater. Appl. 2016, 30, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Wenderott, J.K.; Flesher, C.G.; Baker, N.A.; Neeley, C.K.; Varban, O.A.; Lumeng, C.N.; Muhammad, L.N.; Yeh, C.; Green, P.F.; O’Rourke, R.W. Elucidating nanoscale mechanical properties of diabetic human adipose tissue using atomic force microscopy. Sci. Rep. 2020, 10, 20423. [Google Scholar] [CrossRef] [PubMed]
- Kilian, K.A.; Bugarija, B.; Lahn, B.T.; Mrksich, M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl. Acad. Sci. USA 2010, 107, 4872–4877. [Google Scholar] [CrossRef] [PubMed]
- Hogrebe, N.J.; Gooch, K.J. Direct influence of culture dimensionality on human mesenchymal stem cell differentiation at various matrix stiffnesses using a fibrous self-assembling peptide hydrogel. J. Biomed. Mater. Res. A 2016, 104, 2356–2368. [Google Scholar] [CrossRef] [PubMed]
- Friend, N.E.; McCoy, A.J.; Stegemann, J.P.; Putnam, A.J. A combination of matrix stiffness and degradability dictate microvascular network assembly and remodeling in cell-laden poly (ethylene glycol) hydrogels. Biomaterials 2023, 295, 122050. [Google Scholar] [CrossRef] [PubMed]
- Hunt, N.C.; Smith, A.M.; Gbureck, U.; Shelton, R.M.; Grover, L.M. Encapsulation of fibroblasts causes accelerated alginate hydrogel degradation. Acta Biomater. 2010, 6, 3649–3656. [Google Scholar] [CrossRef] [PubMed]
- Kesselman, D.; Kossover, O.; Mironi-Harpaz, I.; Seliktar, D. Time-dependent cellular morphogenesis and matrix stiffening in proteolytically responsive hydrogels. Acta Biomater. 2013, 9, 7630–7639. [Google Scholar] [CrossRef] [PubMed]
- Altintas, M.M.; Azad, A.; Nayer, B.; Contreras, G.; Zaias, J.; Faul, C.; Reiser, J.; Nayer, A. Mast cells, macrophages, and crown-like structures distinguish subcutaneous from visceral fat in mice. J. Lipid Res. 2011, 52, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Michaud, A.; Drolet, R.; Noël, S.; Paris, G.; Tchernof, A. Visceral fat accumulation is an indicator of adipose tissue macrophage infiltration in women. Metabolism 2012, 61, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.A.; Muir, L.A.; Lumeng, C.N.; O’Rourke, R.W. Differentiation and Metabolic Interrogation of Human Adipocytes. Methods Mol. Biol. 2017, 1566, 61–76. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Pachter, L.; Salzberg, S.L. TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics 2009, 25, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013, 14, R36. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Korotkevich, G.; Sukhov, V.; Budin, N.; Shpak, B.; Artyomov, M.N.; Sergushichev, A. Fast gene set enrichment analysis. bioRxiv 2016. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Milacic, M.; Beavers, D.; Conley, P.; Gong, C.; Gillespie, M.; Griss, J.; Haw, R.; Jassal, B.; Matthews, L.; May, B.; et al. The Reactome Pathway Knowledgebase 2024. Nucleic Acids Res. 2024, 52, D672–D678. [Google Scholar] [CrossRef] [PubMed]
- Liberzon, A.; Birger, C.; Thorvaldsdóttir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Gene Ontology Consortium; Aleksander, S.A.; Balhoff, J.; Carbon, S.; Cherry, J.M.; Drabkin, H.J.; Ebert, D.; Feuermann, M.; Gaudet, P.; Harris, N.L.; et al. The Gene Ontology knowledgebase in 2023. Genetics 2023, 224, iyad031. [Google Scholar] [CrossRef] [PubMed]

| Subject Demographics | Male | Female |
|---|---|---|
| n | 6 | 8 |
| Age (mean, S.D.) | 41 (8) | 42 (11) |
| BMI (mean, S.D.) | 42 (5) | 41 (3) |
| HbA1c (mean, S.D.) | 5.3 (0.6) | 5.3 (0.2) |
| Hypertension (n) | 2 | 1 |
| Sleep apnea (n) | 4 | 2 |
| Hyperlipidemia (n) | 3 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, J.J.; Li, J.; Liu, J.Y.-C.; Patsalis, C.; Wu, P.; Kawase, Y.H.; Ky, A.; Pasternak, C.; Mason, D.D.; Bozadjieva-Kramer, N.; et al. A Hydrogel Culture System Regulates Human Adipocyte Function. Int. J. Mol. Sci. 2025, 26, 10865. https://doi.org/10.3390/ijms262210865
Kwon JJ, Li J, Liu JY-C, Patsalis C, Wu P, Kawase YH, Ky A, Pasternak C, Mason DD, Bozadjieva-Kramer N, et al. A Hydrogel Culture System Regulates Human Adipocyte Function. International Journal of Molecular Sciences. 2025; 26(22):10865. https://doi.org/10.3390/ijms262210865
Chicago/Turabian StyleKwon, Jason Junhyoung, Jie Li, Joshua Yu-Chung Liu, Christopher Patsalis, Peizi Wu, Yoshiki H. Kawase, Alexander Ky, Carter Pasternak, Damian D. Mason, Nadejda Bozadjieva-Kramer, and et al. 2025. "A Hydrogel Culture System Regulates Human Adipocyte Function" International Journal of Molecular Sciences 26, no. 22: 10865. https://doi.org/10.3390/ijms262210865
APA StyleKwon, J. J., Li, J., Liu, J. Y.-C., Patsalis, C., Wu, P., Kawase, Y. H., Ky, A., Pasternak, C., Mason, D. D., Bozadjieva-Kramer, N., Loebel, C., Lumeng, C. N., & O’Rourke, R. W. (2025). A Hydrogel Culture System Regulates Human Adipocyte Function. International Journal of Molecular Sciences, 26(22), 10865. https://doi.org/10.3390/ijms262210865








