Protein–Protein Interactions as Promising Molecular Targets for Novel Antimicrobials Aimed at Gram-Negative Bacteria
Abstract
1. Introduction
2. Targetable PPIs in Gram-Negative Bacteria
2.1. PPIs Associated with Membrane Formation and Regulation
2.1.1. BAM Complex
2.1.2. Rcs Complex
2.1.3. Lpt Complex
2.1.4. FimC-FimH
2.2. PPIs Involved in Bacterial Replication
2.2.1. FtsZ-ZipA
2.2.2. Single-Stranded DNA-Binding Protein
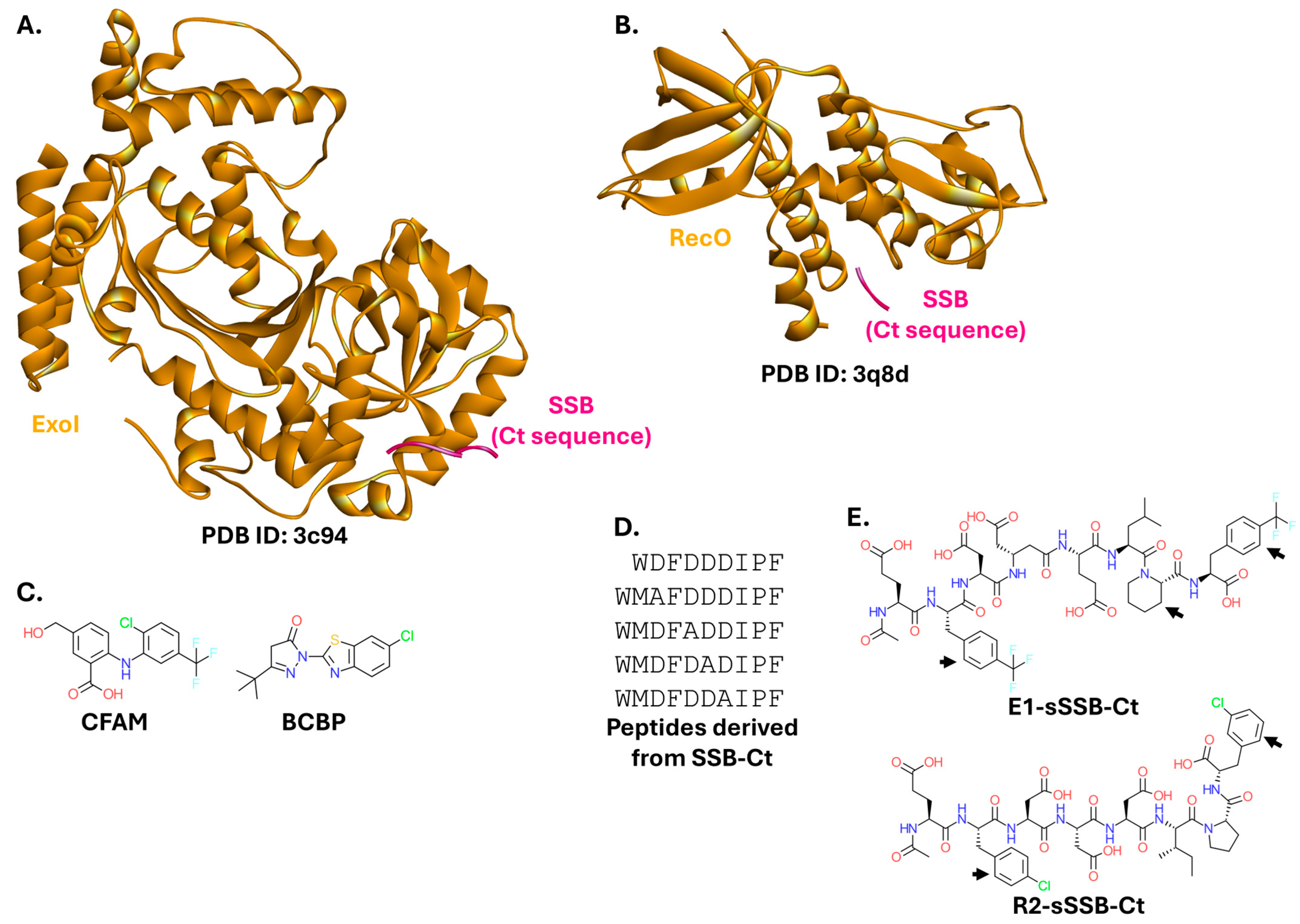
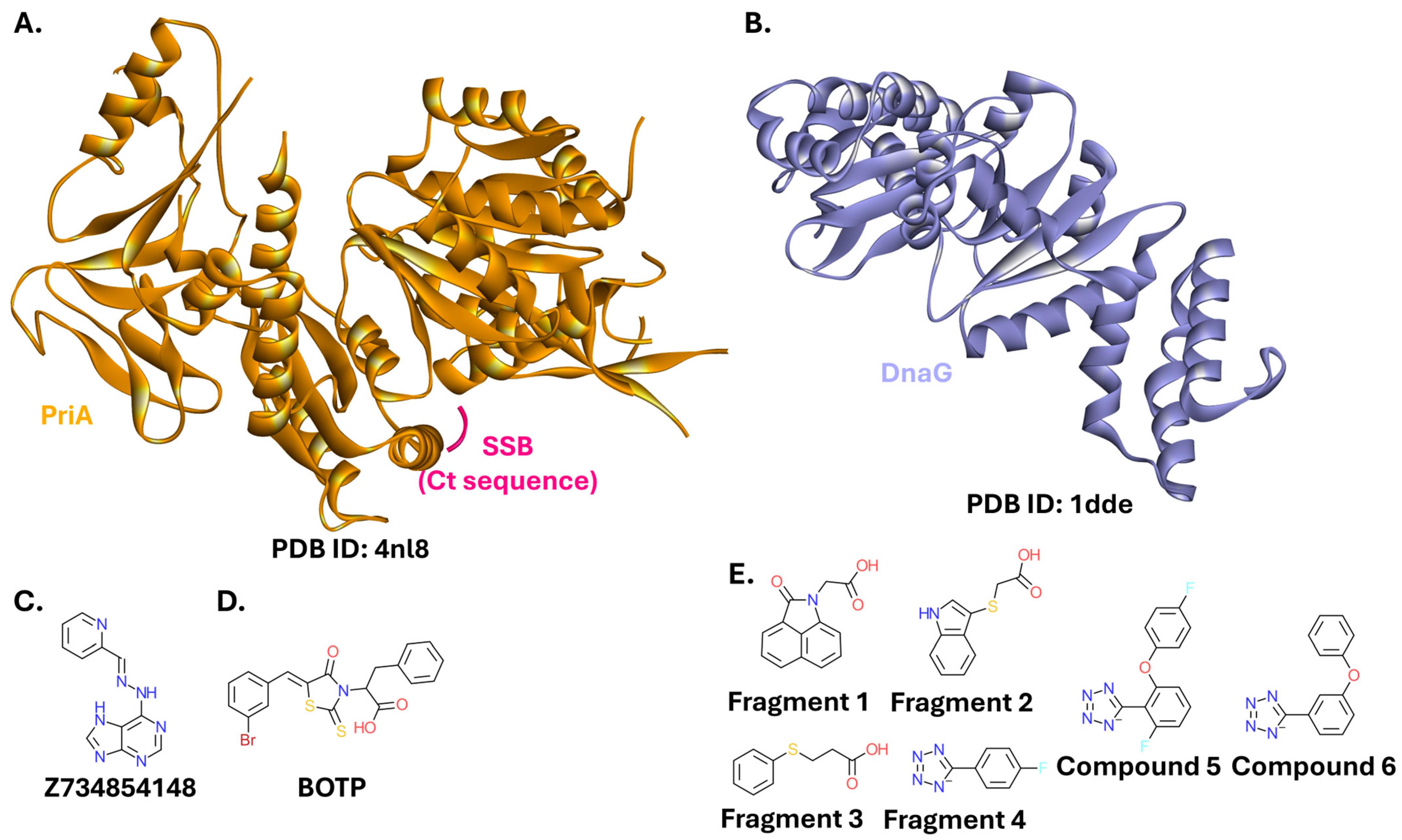
2.2.3. β-Sliding Clamp
2.3. Bacterial Transcription Machinery
2.3.1. RNA Polymerase
2.3.2. N-Utilization Substances NusB and NusE
2.4. Bacterial Translation Machinery
L10-L12 PPI
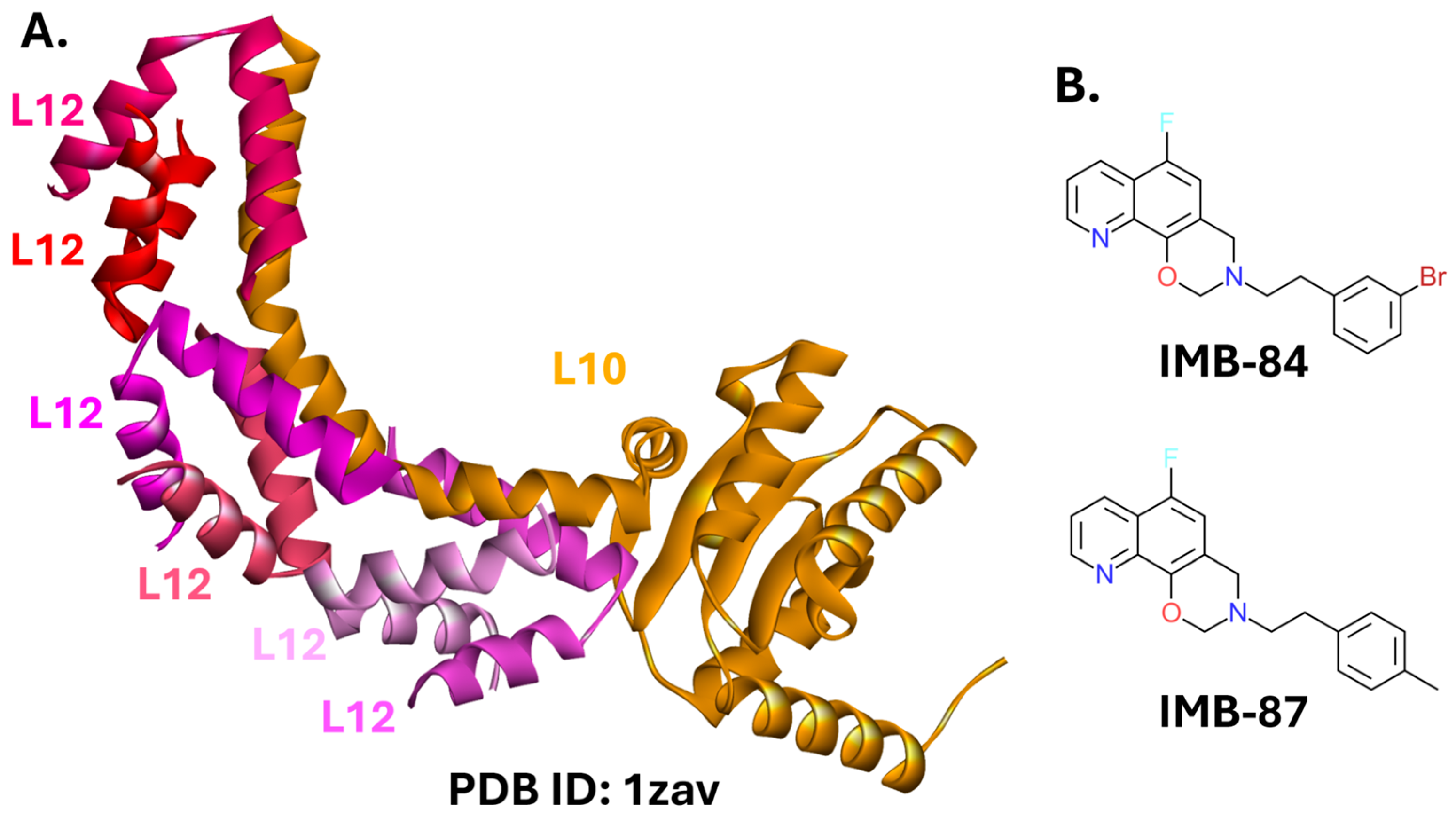
2.5. Toxin-Antitoxin Systems
2.5.1. MazEF
2.5.2. VapBC

2.5.3. PhD-Doc
2.5.4. HicAB
2.5.5. HipBA
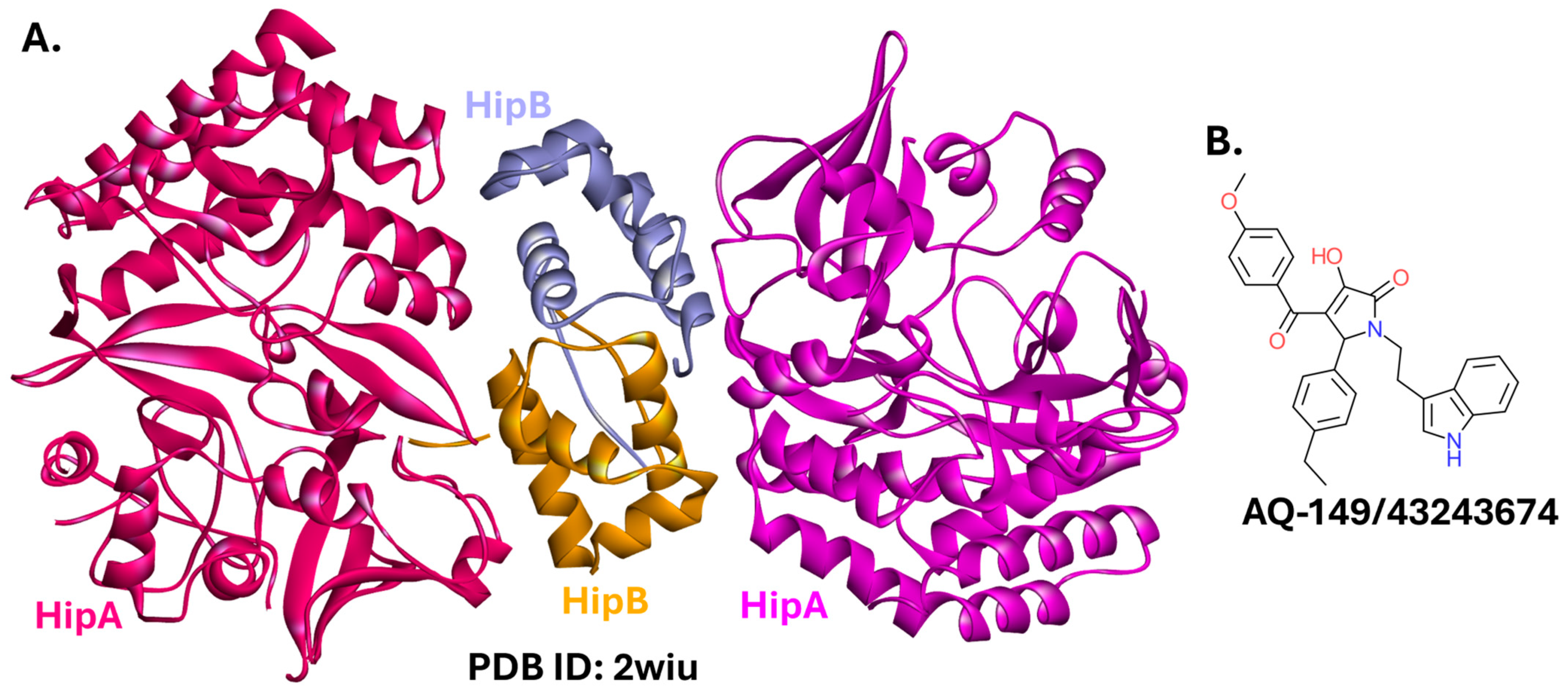
2.5.6. TplE-TplEi
3. Future Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williams, K.J. The introduction of ‘chemotherapy’ using arsphenamine—The first magic bullet. J. R. Soc. Med. 2009, 102, 343–348. [Google Scholar] [CrossRef]
- Rubin, R.P. A Brief History of Great Discoveries in Pharmacology: In Celebration of the Centennial Anniversary of the Founding of the American Society of Pharmacology and Experimental Therapeutics. Pharmacol. Rev. 2007, 59, 289–359. [Google Scholar] [CrossRef]
- Boucher, H.W.; Talbot, G.H.; Benjamin, D.K.; Bradley, J.; Guidos, R.J.; Jones, R.N.; Murray, B.E.; Bonomo, R.A.; Gilbert, D. 10 × ’20 Progress—development of new drugs active against gram-negative bacilli: An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2013, 56, 1685–1694. [Google Scholar] [CrossRef]
- Hutchings, M.; Truman, A.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef]
- Mohr, K.I. History of Antibiotics Research. Curr. Top. Microbiol. Immunol. 2016, 398, 237–272. [Google Scholar] [CrossRef]
- Fair, R.J.; Tor, Y. Antibiotics and bacterial resistance in the 21st century. Perspect. Med. Chem. 2014, 6, 25–64. [Google Scholar] [CrossRef]
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Robles Aguilar, G.; Mestrovic, T.; Smith, G.; Han, C.; et al. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef] [PubMed]
- Theuretzbacher, U. Resistance drives antibacterial drug development. Curr. Opin. Pharmacol. 2011, 11, 433–438. [Google Scholar] [CrossRef]
- Dutescu, I.A.; Hillie, S.A. Encouraging the Development of New Antibiotics: Are Financial Incentives the Right Way Forward? A Systematic Review and Case Study. Infect. Drug Resist. 2021, 14, 415–434. [Google Scholar] [CrossRef]
- World Health Organization. WHO Bacterial Priority Pathogens List, 2024-Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2024; pp. 1–72. Available online: https://www.who.int/publications/i/item/9789240093461 (accessed on 24 September 2025).
- Cama, J.; Henney, A.M.; Winterhalter, M. Breaching the Barrier: Quantifying Antibiotic Permeability across Gram-negative Bacterial Membranes. J. Mol. Biol. 2019, 431, 3531–3546. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 464–473. [Google Scholar] [CrossRef]
- Hwang, H.; Vreven, T.; Janin, J.; Weng, Z. Protein-protein docking benchmark version 4.0. Proteins 2010, 78, 3111–3114. [Google Scholar] [CrossRef]
- Fuller, J.C.; Burgoyne, N.J.; Jackson, R.M. Predicting druggable binding sites at the protein-protein interface. Drug Discov. Today 2009, 14, 155–161. [Google Scholar] [CrossRef]
- Arkin, M.R.; Tang, Y.; Wells, J.A. Small-molecule inhibitors of protein-protein interactions: Progressing toward the reality. Chem. Biol. 2014, 21, 1102–1114. [Google Scholar] [CrossRef]
- Basse, M.J.; Betzi, S.; Bourgeas, R.; Bouzidi, S.; Chetrit, B.; Hamon, V.; Morelli, X.; Roche, P. 2P2Idb: A structural database dedicated to orthosteric modulation of protein-protein interactions. Nucleic Acids Res. 2013, 41, D824–D827. [Google Scholar] [CrossRef]
- Nada, H.; Choi, Y.; Kim, S.; Jeong, K.S.; Meanwell, N.A.; Lee, K. New insights into protein–protein interaction modulators in drug discovery and therapeutic advance. Signal Transduct. Target. Ther. 2024, 9, 341. [Google Scholar] [CrossRef]
- Nevola, L.; Giralt, E. Modulating protein–protein interactions: The potential of peptides. Chem. Commun. 2015, 51, 3302–3315. [Google Scholar] [CrossRef]
- Petta, I.; Lievens, S.; Libert, C.; Tavernier, J.; De Bosscher, K. Modulation of Protein-Protein Interactions for the Development of Novel Therapeutics. Mol. Ther. 2016, 24, 707–718. [Google Scholar] [CrossRef]
- Shin, W.H.; Kumazawa, K.; Imai, K.; Hirokawa, T.; Kihara, D. Current Challenges and Opportunities in Designing Protein-Protein Interaction Targeted Drugs. Adv. Appl. Bioinform. Chem. 2020, 13, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Camps-Fajol, C.; Cavero, D.; Minguillón, J.; Surrallés, J. Targeting protein-protein interactions in drug discovery: Modulators approved or in clinical trials for cancer treatment. Pharmacol. Res. 2025, 211, 107544. [Google Scholar] [CrossRef]
- Afonso, A.L.; Cavaleiro, C.T.; Castanho, M.A.R.B.; Neves, V.; Cavaco, M. The Potential of Peptide-Based Inhibitors in Disrupting Protein–Protein Interactions for Targeted Cancer Therapy. Int. J. Mol. Sci. 2025, 26, 3117. [Google Scholar] [CrossRef]
- Pelay-Gimeno, M.; Glas, A.; Koch, O.; Grossmann, T.N. Structure-Based Design of Inhibitors of Protein–Protein Interactions: Mimicking Peptide Binding Epitopes. Angew. Chem. Int. Ed. Engl. 2015, 54, 8896–8927. [Google Scholar] [CrossRef]
- Carro, L. Protein–protein interactions in bacteria: A promising and challenging avenue towards the discovery of new antibiotics. Beilstein J. Org. Chem. 2018, 14, 2881–2896. [Google Scholar] [CrossRef]
- Cossar, P.J.; Lewis, P.J.; McCluskey, A. Protein-protein interactions as antibiotic targets: A medicinal chemistry perspective. Med. Res. Rev. 2020, 40, 469–494. [Google Scholar] [CrossRef]
- Akhter, Y.; Hussain, R. Protein-protein complexes as targets for drug discovery against infectious diseases. Adv. Prot. Chem. Struct. Biol. 2020, 121, 237–251. [Google Scholar] [CrossRef]
- Kahan, R.; Worm, D.J.; De Castro, G.V.; Ng, S.; Barnard, A. Modulators of protein–protein interactions as antimicrobial agents. RSC Chem. Biol. 2021, 2, 387–409. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Malinverni, J.; Ruiz, N.; Kim, S.; Silhavy, T.J.; Kahne, D. Identification of a Multicomponent Complex Required for Outer Membrane Biogenesis in Escherichia coli. Cell 2005, 121, 235–245. [Google Scholar] [CrossRef]
- Bryant, J.A.; Staunton, K.A.; Doherty, H.M.; Alao, M.B.; Ma, X.; Morcinek-Orłowska, J.; Goodall, E.C.A.; Gray, J.; Milner, M.; Cole, J.A.; et al. Bam complex associated proteins in Escherichia coli are functionally linked to peptidoglycan biosynthesis, membrane fluidity and DNA replication. eLife 2024, 13. [Google Scholar] [CrossRef]
- Pfanner, N.; Wiedemann, N.; Meisinger, C.; Lithgow, T. Assembling the mitochondrial outer membrane. Nat. Struct. Mol. Biol. 2004, 11, 1044–1048. [Google Scholar] [CrossRef]
- Jiang, J.-H.; Tong, J.; Tan, K.S.; Gabriel, K. From Evolution to Pathogenesis: The Link Between β-Barrel Assembly Machineries in the Outer Membrane of Mitochondria and Gram-Negative Bacteria. Int. J. Mol. Sci. 2012, 13, 8038–8050. [Google Scholar] [CrossRef]
- Diederichs, K.A.; Ni, X.; Rollauer, S.E.; Botos, I.; Tan, X.; King, M.S.; Kunji, E.R.S.; Jiang, J.; Buchanan, S.K. Structural insight into mitochondrial β-barrel outer membrane protein biogenesis. Nat. Commun. 2020, 11, 3290. [Google Scholar] [CrossRef]
- Lundquist, K.; Billings, E.; Bi, M.; Wellnitz, J.; Noinaj, N. The assembly of β-barrel membrane proteins by BAM and SAM. Mol. Microbiol. 2021, 115, 425–435. [Google Scholar] [CrossRef]
- Diederichs, K.A.; Buchanan, S.K.; Botos, I. Building Better Barrels—β-barrel Biogenesis and Insertion in Bacteria and Mitochondria. J. Mol. Biol. 2021, 433, 166894. [Google Scholar] [CrossRef] [PubMed]
- Cottom, C.O.; Stephenson, R.; Wilson, L.; Noinaj, N. Targeting BAM for Novel Therapeutics against Pathogenic Gram-Negative Bacteria. Antibiotics 2023, 12, 679. [Google Scholar] [CrossRef]
- Gu, Y.; Li, H.; Dong, H.; Zeng, Y.; Zhang, Z.; Paterson, N.G.; Stansfeld, P.J.; Wang, Z.; Zhang, Y.; Wang, W.; et al. Structural basis of outer membrane protein insertion by the BAM complex. Nature 2016, 531, 64–69. [Google Scholar] [CrossRef]
- Steenhuis, M.; Abdallah, A.M.; de Munnik, S.M.; Kuhne, S.; Sterk, G.J.; van den Berg van Saparoea, B.; Westerhausen, S.; Wagner, S.; van der Wel, N.N.; Wijtmans, M.; et al. Inhibition of autotransporter biogenesis by small molecules. Mol. Microbiol. 2019, 112, 81–98. [Google Scholar] [CrossRef]
- Steenhuis, M.; Corona, F.; Ten Hagen-Jongman, C.M.; Vollmer, W.; Lambin, D.; Selhorst, P.; Klaassen, H.; Versele, M.; Chaltin, P.; Luirink, J. Combining Cell Envelope Stress Reporter Assays in a Screening Approach to Identify BAM Complex Inhibitors. ACS Infect. Dis. 2021, 7, 2250–2263. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, X.; Zhang, J.; Lin, Y.; You, X.; Chen, M.; Wang, Y.; Zhu, N.; Si, S. Identification of a Compound That Inhibits the Growth of Gram-Negative Bacteria by Blocking BamA–BamD Interaction. Front. Microbiol. 2020, 11, 526727. [Google Scholar] [CrossRef]
- Psonis, J.J.; Chahales, P.; Henderson, N.S.; Rigel, N.W.; Hoffman, P.S.; Thanassi, D.G. The small molecule nitazoxanide selectively disrupts BAM-mediated folding of the outer membrane usher protein. J. Biol. Chem. 2019, 294, 14357–14369. [Google Scholar] [CrossRef]
- Hagan, C.L.; Wzorek, J.S.; Kahne, D.; Sauer, R.T. Inhibition of the β-barrel assembly machine by a peptide that binds BamD. Proc. Natl. Acad. Sci. USA 2015, 112, 2011–2016. [Google Scholar] [CrossRef]
- Imai, Y.; Meyer, K.J.; Iinishi, A.; Favre-Godal, Q.; Green, R.; Manuse, S.; Caboni, M.; Mori, M.; Niles, S.; Ghiglieri, M.; et al. A new antibiotic selectively kills Gram-negative pathogens. Nature 2019, 576, 459–464, Erratum in Nature 2020, 580, E3. https://doi.org/10.1038/s41586-020-2063-9. [Google Scholar] [CrossRef]
- Kaur, H.; Jakob, R.P.; Marzinek, J.K.; Green, R.; Imai, Y.; Reddy Bolla, J.; Agustoni, E.; Robinson, C.V.; Bond, P.J.; Lewis, K.; et al. The antibiotic darobactin mimics a β-strand to inhibit outer membrane insertase. Nature 2021, 593, 125–129. [Google Scholar] [CrossRef]
- Haysom, S.F.; Machin, J.; Whitehouse, J.M.; Horne, J.E.; Fenn, K.; Ma, Y.; El Mkami, H.; Böhringer, N.; Schäberle, T.F.; Ranson, N.E.; et al. Darobactin B Stabilises a Lateral-Closed Conformation of the BAM Complex in E. coli Cells. Angew. Chem. Int. Ed. Engl. 2023, 62, e202218783. [Google Scholar] [CrossRef]
- Miller, R.D.; Iinishi, A.; Modaresi, S.M.; Yoo, B.K.; Curtis, T.D.; Lariviere, P.J.; Liang, L.; Son, S.; Nicolau, S.; Bargabos, R.; et al. Computational identification of a systemic antibiotic for Gram-negative bacteria. Nat. Microbiol. 2022, 7, 1661–1672. [Google Scholar] [CrossRef]
- Xu, Q.; Guo, M.; Yu, F. β-Barrel Assembly Machinery (BAM) Complex as Novel Antibacterial Drug Target. Molecules 2023, 28, 3758. [Google Scholar] [CrossRef]
- Meng, J.; Young, G.; Chen, J. The Rcs System in Enterobacteriaceae: Envelope Stress Responses and Virulence Regulation. Front. Microbiol. 2021, 12, 627104. [Google Scholar] [CrossRef]
- Rodríguez, L.; Peñalver, M.; Casino, P.; Portillo, F.G.D. Evolutionary analysis in Enterobacterales of the Rcs-repressor protein IgaA unveils two cytoplasmic small β-barrel domains central for function. bioRxiv 2022, 19. [Google Scholar] [CrossRef]
- Goulian, M. Two-component signaling circuit structure and properties. Curr. Opin. Microbiol. 2010, 13, 184–189. [Google Scholar] [CrossRef]
- Jacob-Dubuisson, F.; Mechaly, A.; Betton, J.M.; Antoine, R. Structural insights into the signalling mechanisms of two-component systems. Nat. Rev. Microbiol. 2018, 16, 585–593. [Google Scholar] [CrossRef]
- Cho, S.H.; Dekoninck, K.; Collet, J.F. Envelope-Stress Sensing Mechanism of Rcs and Cpx Signaling Pathways in Gram-Negative Bacteria. J. Microbiol. 2023, 61, 317–329. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, Y.; Zhang, W.; Mu, W. Rcs signal transduction system in Escherichia coli: Composition, related functions, regulatory mechanism, and applications. Microbiol. Res. 2024, 285, 127783. [Google Scholar] [CrossRef]
- Wall, E.; Majdalani, N.; Gottesman, S. The Complex Rcs Regulatory Cascade. Annu. Rev. Microbiol. 2018, 72, 111–139. [Google Scholar] [CrossRef]
- Cho, S.H.; Szewczyk, J.; Pesavento, C.; Zietek, M.; Banzhaf, M.; Roszczenko, P.; Asmar, A.; Laloux, G.; Hov, A.K.; Leverrier, P.; et al. Detecting envelope stress by monitoring β-barrel assembly. Cell 2014, 159, 1652–1664. [Google Scholar] [CrossRef]
- Hussein, N.A.; Cho, S.H.; Laloux, G.; Siam, R.; Collet, J.F. Distinct domains of Escherichia coli IgaA connect envelope stress sensing and down-regulation of the Rcs phosphorelay across subcellular compartments. PLoS Genet. 2018, 14, e1007398. [Google Scholar] [CrossRef]
- Cano, D.A.; Domínguez-Bernal, G.; Tierrez, A.; Portillo, F.G.-D.; Casadesús, J. Regulation of capsule synthesis and cell motility in Salmonella enterica by the essential gene igaA. Genetics 2002, 162, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Konovalova, A.; Perlman, D.H.; Cowles, C.E.; Silhavy, T.J. Transmembrane domain of surface-exposed outer membrane lipoprotein RcsF is threaded through the lumen of β-barrel proteins. Proc. Natl. Acad. Sci. USA 2014, 111, E4350–E4358. [Google Scholar] [CrossRef]
- Rodríguez-Alonso, R.; Létoquart, J.; Nguyen, V.S.; Louis, G.; Calabrese, A.N.; Iorga, B.I.; Radford, S.E.; Cho, S.H.; Remaut, H.; Collet, J.F. Structural insight into the formation of lipoprotein-β-barrel complexes. Nat. Chem. Biol. 2020, 16, 1019–1025. [Google Scholar] [CrossRef]
- Watanabe, N.; Savchenko, A. Molecular insights into the initiation step of the Rcs signaling pathway. Structure 2024, 32, 1381–1393.e4. [Google Scholar] [CrossRef]
- TagElDein, M.A.; Mohamed, N.G.; Shahein, Y.E.; Ziko, L.; Hussein, N.A. Altering Escherichia coli envelope integrity by mimicking the lipoprotein RcsF. Arch. Microbiol. 2023, 206, 12. [Google Scholar] [CrossRef]
- Okuda, S.; Sherman, D.J.; Silhavy, T.J.; Ruiz, N.; Kahne, D. Lipopolysaccharide transport and assembly at the outer membrane: The PEZ model. Nat. Rev. Microbiol. 2016, 14, 337–345. [Google Scholar] [CrossRef]
- Martorana, A.M.; Moura, E.C.C.M.; Sperandeo, P.; Di Vincenzo, F.; Liang, X.; Toone, E.; Zhou, P.; Polissi, A. Degradation of Components of the Lpt Transenvelope Machinery Reveals LPS-Dependent Lpt Complex Stability in Escherichia coli. Front. Mol. Biosci. 2021, 8, 758228. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, C.; Qiao, S.; Yu, S.; Chen, L.; Kim, S.; Wang, K.; Zheng, J.; Zhang, Y.; Wu, F.; et al. Surface lipoprotein sorting by crosstalk between Lpt and Lol pathways in gram-negative bacteria. Nat. Commun. 2025, 16, 4357. [Google Scholar] [CrossRef]
- Owens, T.W.; Taylor, R.J.; Pahil, K.S.; Bertani, B.R.; Ruiz, N.; Kruse, A.C.; Kahne, D. Structural basis of unidirectional export of lipopolysaccharide to the cell surface. Nature 2019, 567, 550–553. [Google Scholar] [CrossRef]
- Suits, M.D.L.; Sperandeo, P.; Dehò, G.; Polissi, A.; Jia, Z. Novel structure of the conserved gram-negative lipopolysaccharide transport protein A and mutagenesis analysis. J. Mol. Biol. 2008, 380, 476–488. [Google Scholar] [CrossRef]
- Botos, I.; Majdalani, N.; Mayclin, S.J.; Gehret McCarthy, J.; Lundquist, K.; Wojtowicz, D.; Barnard, T.J.; Gumbart, J.C.; Buchanan, S.K. Structural and Functional Characterization of the LPS Transporter LptDE from Gram-Negative Pathogens. Structure 2016, 24, 965–976. [Google Scholar] [CrossRef]
- Fehlbaum, P.; Bulet, P.; Chernysh, S.; Briand, J.P.; Roussel, J.P.; Letellier, L.; Hetru, C.; Hoffmann, J.A. Structure-activity analysis of thanatin, a 21-residue inducible insect defense peptide with sequence homology to frog skin antimicrobial peptides. Proc. Natl. Acad. Sci. USA 1996, 3, 1221–1225. [Google Scholar] [CrossRef]
- Vetterli, S.U.; Zerbe, K.; Müller, M.; Urfer, M.; Mondal, M.; Wang, S.Y.; Moehle, K.; Zerbe, O.; Vitale, A.; Pessi, G.; et al. Thanatin targets the intermembrane protein complex required for lipopolysaccharide transport in Escherichia coli. Sci. Adv. 2018, 4, eaau2634. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, Q.; Xu, T.; Malakar, P.K.; Zhu, Y.; Jing Liu, J.; Zhao, Y.; Zhang, Z. Thanatin: A Promising Antimicrobial Peptide Targeting the Achilles’ Heel of Multidrug-Resistant Bacteria. Int. J. Mol. Sci. 2024, 25, 9496. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Wang, W.; Zhang, J.; Lin, Y.; Hong, B.; You, X.; Song, D.; Wang, Y.; Jiang, J.; et al. Identification of an anti-Gram-negative bacteria agent disrupting the interaction between lipopolysaccharide transporters LptA and LptC. Int. J. Antimicrob. Agents 2019, 53, 442–448. [Google Scholar] [CrossRef]
- Dai, X.; Yuan, M.; Lu, Y.; Zhu, X.; Liu, C.; Zheng, Y.; Si, S.; Yuan, L.; Zhang, J.; Li, Y. Identification of a Small Molecule That Inhibits the Interaction of LPS Transporters LptA and LptC. Antibiotics 2022, 11, 1385. [Google Scholar] [CrossRef]
- Ren, Y.; Dong, W.; Li, Y.; Cao, W.; Xiao, Z.; Zhou, Y.; Teng, Y.; You, X.; Yang, X.; Huang, H.; et al. The Prediction of LptA and LptC Protein–Protein Interactions and Virtual Screening for Potential Inhibitors. Molecules 2024, 29, 1827. [Google Scholar] [CrossRef]
- Vetterli, S.U.; Moehle, K.; Robinson, J.A. Synthesis and antimicrobial activity against Pseudomonas aeruginosa of macrocyclic β-hairpin peptidomimetic antibiotics containing N-methylated amino acids. Bioorganic Med. Chem. 2016, 24, 6332–6339. [Google Scholar] [CrossRef] [PubMed]
- Andolina, G.; Bencze, L.C.; Zerbe, K.; Müller, M.; Steinmann, J.; Kocherla, H.; Mondal, M.; Sobek, J.; Moehle, K.; Malojčić, G.; et al. A Peptidomimetic Antibiotic Interacts with the Periplasmic Domain of LptD from Pseudomonas aeruginosa. ACS Chem. Biol. 2018, 13, 666–675. [Google Scholar] [CrossRef]
- Sabnis, A.; Edwards, A.M. Lipopolysaccharide as an antibiotic target. Biochim. Biophys. Acta Mol. Cell Res. 2023, 1870, 119507. [Google Scholar] [CrossRef]
- Wei, X.; Zhou, D.; Xu, C.; Chen, P.; Chen, S.; Cheng, Z.; Jin, Y.; Jin, S.; Wu, W. Murepavadin Enhances the Killing Efficacy of Ciprofloxacin against Pseudomonas aeruginosa by Inhibiting Drug Efflux. Antibiotics 2024, 13, 810. [Google Scholar] [CrossRef]
- Hernández-García, M.; Barbero-Herranz, R.; Bastón-Paz, N.; Díez-Aguilar, M.; López-Collazo, E.; Márquez-Garrido, F.J.; Hernández-Pérez, J.M.; Baquero, F.; Ekkelenkamp, M.B.; Fluit, A.C.; et al. Unravelling the mechanisms causing murepavadin resistance in Pseudomonas aeruginosa: Lipopolysaccharide alterations and its consequences. Front. Cell Infect. Microbiol. 2024, 14, 1446626. [Google Scholar] [CrossRef]
- Zampaloni, C.; Mattei, P.; Bleicher, K.; Winther, L.; Thäte, C.; Bucher, C.; Adam, J.M.; Alanine, A.; Amrein, K.E.; Baidin, V.; et al. A novel antibiotic class targeting the lipopolysaccharide transporter. Nature 2024, 625, 566–571, Erratum in Nature 2024, 631, E17. [Google Scholar] [CrossRef]
- Pahil, K.S.; Gilman, M.S.A.; Baidin, V.; Clairfeuille, T.; Mattei, P.; Bieniossek, C.; Dey, F.; Muri, D.; Baettig, R.; Lobritz, M.; et al. A new antibiotic traps lipopolysaccharide in its intermembrane transporter. Nature 2024, 625, 572–577, Erratum in Nature 2024, 625, E27 and Nature 2024, 631, E18. [Google Scholar] [CrossRef]
- Yoon, Y.; Song, S. Structural Insights into the Lipopolysaccharide Transport (Lpt) System as a Novel Antibiotic Target. J. Microbiol. 2024, 62, 261–275. [Google Scholar] [CrossRef]
- Hahn, E.; Wild, P.; Hermanns, U.; Sebbel, P.; Glockshuber, R.; Häner, M.; Taschner, N.; Burkhard, P.; Aebi, U.; Müller, S.A. Exploring the 3D Molecular Architecture of Escherichia coli Type 1 Pili. J. Mol. Biol. 2002, 323, 845–857. [Google Scholar] [CrossRef]
- Sauer, M.M.; Jakob, R.P.; Eras, J.; Baday, S.; Eriş, D.; Navarra, G.; Bernèche, S.; Ernst, B.; Maier, T.; Glockshuber, R. Catch-bond mechanism of the bacterial adhesin FimH. Nat. Commun. 2016, 7, 10738. [Google Scholar] [CrossRef]
- Mydock-McGrane, L.K.; Hannan, T.J.; Janetka, J.W. Rational design strategies for FimH antagonists: New drugs on the horizon for urinary tract infection and Crohn’s disease. Expert Opin. Drug Discov. 2017, 12, 711–731. [Google Scholar] [CrossRef]
- Sarshar, M.; Behzadi, P.; Ambrosi, C.; Zagaglia, C.; Palamara, A.T.; Scribano, D. FimH and Anti-Adhesive Therapeutics: A Disarming Strategy Against Uropathogens. Antibiotics 2020, 9, 397. [Google Scholar] [CrossRef]
- Lopatto, E.D.B.; Santiago-Borges, J.M.; Sanick, D.A.; Malladi, S.K.; Azimzadeh, P.N.; Timm, M.W.; Fox, I.F.; Schmitz, A.J.; Turner, J.S.; Ahmed, S.M.S.; et al. Monoclonal antibodies targeting the FimH adhesin protect against uropathogenic E. coli UTI. Sci. Adv. 2025, 11, eadw0698. [Google Scholar] [CrossRef] [PubMed]
- Hermanns, U.; Sebbel, P.; Eggli, V.; Glockshuber, R. Characterization of FimC, a periplasmic assembly factor for biogenesis of type 1 pili in Escherichia coli. Biochemistry 2000, 39, 11564–11570. [Google Scholar] [CrossRef]
- Zavialov, A.V.; Tischenko, V.M.; Fooks, L.J.; Brandsdal, B.O.; Aqvist, J.; Zav’yalov, V.P.; Macintyre, S.; Knight, S.D. Resolving the energy paradox of chaperone/usher-mediated fibre assembly. Biochem. J. 2005, 389, 685–694. [Google Scholar] [CrossRef]
- Alonso-Caballero, A.; Schönfelder, J.; Poly, S.; Corsetti, F.; De Sancho, D.; Artacho, E.; Perez-Jimenez, R. Mechanical architecture and folding of E. coli type 1 pilus domains. Nat. Commun. 2018, 9, 2758. [Google Scholar] [CrossRef]
- Choudhury, D.; Thompson, A.; Stojanoff, V.; Langermann, S.; Pinkner, J.; Hultgren, S.J.; Knight, S.D. X-ray structure of the FimC-FimH chaperone-adhesin complex from uropathogenic Escherichia coli. Science 1999, 285, 1061–1066. [Google Scholar] [CrossRef]
- Sauer, F.G.; Fütterer, K.; Pinkner, J.S.; Dodson, K.W.; Hultgren, S.J.; Waksman, G. Structural basis of chaperone function and pilus biogenesis. Science 1999, 285, 1058–1061. [Google Scholar] [CrossRef]
- Larsson, A.; Johansson, S.M.C.; Pinkner, J.S.; Hultgren, S.J.; Almqvist, F.; Kihlberg, J.; Linusson, A. Multivariate design, synthesis, and biological evaluation of peptide inhibitors of FimC/FimH protein-protein interactions in uropathogenic Escherichia coli. J. Med. Chem. 2005, 48, 935–945. [Google Scholar] [CrossRef]
- Cameron, T.A.; Margolin, W. Insights into the assembly and regulation of the bacterial divisome. Nat. Rev. Microbiol. 2023, 22, 33–45. [Google Scholar] [CrossRef]
- Pradhan, P.; Margolin, W.; Beuria, T.K. Targeting the Achilles Heel of FtsZ: The Interdomain Cleft. Front. Microbiol. 2021, 12, 732796. [Google Scholar] [CrossRef]
- Mosyak, L.; Zhang, Y.; Glasfeld, E.; Haney, S.; Stahl, M.; Seehra, J.; Somers, W.S. The bacterial cell-division protein ZipA and its interaction with an FtsZ fragment revealed by X-ray crystallography. EMBO J. 2000, 19, 3179–3191. [Google Scholar] [CrossRef]
- Hernández-Rocamora, V.M.; Reija, B.; García, C.; Natale, P.; Alfonso, C.; Minton, A.P.; Zorrilla, S.; Rivas, G.; Vicente, M. Dynamic interaction of the Escherichia coli cell division ZipA and FtsZ proteins evidenced in nanodiscs. J. Biol. Chem. 2012, 287, 30097–30104. [Google Scholar] [CrossRef]
- Cabré, E.J.; Sánchez-Gorostiaga, A.; Carrara, P.; Ropero, N.; Casanova, M.; Palacios, P.; Stano, P.; Jiménez, M.; Rivas, G.; Vicente, M. Bacterial division proteins FtsZ and ZipA induce vesicle shrinkage and cell membrane invagination. J. Biol. Chem. 2013, 288, 26625–26634. [Google Scholar] [CrossRef]
- Kenny, C.H.; Ding, W.; Kelleher, K.; Benard, S.; Dushin, E.G.; Sutherland, A.G.; Mosyak, L.; Kriz, R.; Ellestad, G. Development of a fluorescence polarization assay to screen for inhibitors of the FtsZ/ZipA interaction. Anal. Biochem. 2003, 323, 224–233. [Google Scholar] [CrossRef]
- Rush, T.S.; Grant, J.A.; Mosyak, L.; Nicholls, A. A shape-based 3-D scaffold hopping method and its application to a bacterial protein-protein interaction. J. Med. Chem. 2005, 48, 1489–1495. [Google Scholar] [CrossRef]
- Sutherland, A.G.; Alvarez, J.; Ding, W.; Foreman, K.W.; Kenny, C.H.; Labthavikul, P.; Mosyak, L.; Petersen, P.J.; Rush, T.S.; Ruzin, A.; et al. Structure-based design of carboxybiphenylindole inhibitors of the ZipA–FtsZ interaction. Org. Biomol. Chem. 2003, 1, 4138–4140. [Google Scholar] [CrossRef]
- Nogales, E.; Downing, K.H.; Amos, L.A.; Löwe, J. Tubulin and FtsZ form a distinct family of GTPases. Nat. Struct. Biol. 1998, 5, 451–458. [Google Scholar] [CrossRef]
- Kaur, S.; Modi, N.H.; Panda, D.; Roy, N. Probing the binding site of curcumin in Escherichia coli and Bacillus subtilis FtsZ—A structural insight to unveil antibacterial activity of curcumin. Eur. J. Med. Chem. 2010, 45, 4209–4214. [Google Scholar] [CrossRef] [PubMed]
- Beuria, T.K.; Santra, M.K.; Panda, D. Sanguinarine Blocks Cytokinesis in Bacteria by Inhibiting FtsZ Assembly and Bundling. Biochemistry 2005, 44, 16584–16593. [Google Scholar] [CrossRef]
- Pitts, B.J.R.; Meyerson, L.R. Inhibition of Na,K-ATPase activity and ouabain binding by sanguinarine. Drug Dev. Res. 1981, 1, 43–49. [Google Scholar] [CrossRef]
- Wolff, J.; Knipling, L. Antimicrotubule properties of benzophenanthridine alkaloids. Biochemistry 1993, 32, 13334–13339. [Google Scholar] [CrossRef]
- Sciò, P.; Scoffone, V.C.; Parisi, A.; Bufano, M.; Caneva, M.; Trespidi, G.; Irudal, S.; Barbieri, G.; Cariani, L.; Orena, B.S.; et al. Identification of a New FtsZ Inhibitor by Virtual Screening, Mechanistic Insights, and Structure–Activity Relationship Analyses. ACS Infect. Dis. 2025, 11, 998–1007. [Google Scholar] [CrossRef]
- Ma, Y.; Kong, Y.; Ding, H.; Guo, T.; Chen, W.; Xue, J.; Dong, E.; Ma, S. Design, Synthesis, and Bioactivity Evaluation of Novel 1-Methyl-2-phenylpyridin-1-ium Derivatives as Broad-Spectrum FtsZ Inhibitors. J. Agric. Food Chem. 2025, 73, 4958–4975. [Google Scholar] [CrossRef]
- Navid, A.; Ahmad, S.; Sajjad, R.; Raza, S.; Azam, S.S. Structure Based in Silico Screening Revealed a Potent Acinetobacter Baumannii Ftsz Inhibitor from Asinex Antibacterial Library. IEEE/ACM Trans. Comput. Biol. Bioinform. 2022, 19, 3008–3018. [Google Scholar] [CrossRef] [PubMed]
- Woods, C.J.; Malaisree, M.; Hannongbua, S.; Mulholland, A.J. A water-swap reaction coordinate for the calculation of absolute protein-ligand binding free energies. J. Chem. Phys. 2011, 134, 054114. [Google Scholar] [CrossRef]
- Woods, C.J.; Malaisree, M.; Michel, J.; Long, B.; McIntosh-Smith, S.; Mulholland, A.J. Rapid decomposition and visualisation of protein-ligand binding free energies by residue and by water. Faraday Discuss. 2014, 169, 477–499. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Saha, R.; Koley, T.; Naz, F.; Sharma, S.; Khan, M.I.; Kumar, M.; Kaur, P.; Ethayathulla, A.S. Structure-based identification of potential natural compound inhibitors targeting bacterial cytoskeleton protein FtsZ from Acinetobacter baumannii by computational studies. J. Biomol. Struct. Dyn. 2025, 43, 4578–4590. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Anbarasu, A. Unveiling Berberine analogues as potential inhibitors of Escherichia coli FtsZ through machine learning molecular docking and molecular dynamics approach. Sci. Rep. 2025, 15, 14668. [Google Scholar] [CrossRef]
- Kumar, H.; Manoharan, A.; Anbarasu, A.; Ramaiah, S. Computational study of the piperidine and FtsZ interaction in Salmonella Typhi: Implications for disrupting cell division machinery. J. Biomol. Struct. Dyn. 2025, 43, 5886–5899. [Google Scholar] [CrossRef]
- Ray, S.; Dhaked, H.P.S.; Panda, D. Antimicrobial peptide CRAMP (16-33) stalls bacterial cytokinesis by inhibiting FtsZ assembly. Biochemistry 2014, 53, 6426–6429. [Google Scholar] [CrossRef]
- Paradis-Bleau, C.; Sanschagrin, F.; Levesque, R.C. Identification of Pseudomonas aeruginosa FtsZ peptide inhibitors as a tool for development of novel antimicrobials. J. Antimicrob. Chemother. 2004, 54, 278–280. [Google Scholar] [CrossRef] [PubMed]
- Du, R.L.; Hung, C.H.; Leung, A.S.L.; Ding, K.; Kong, W.P.; Wang, Y.; Liang, Z.G.; Chan, P.H.; Wong, K.Y. Development of Broad-Spectrum Antimicrobial Peptides through the Conjugation of FtsZ-Binding and Cell-Penetrating Peptides. ACS Infect. Dis. 2025, 11, 2190–2204. [Google Scholar] [CrossRef]
- Han, H.; Wang, Z.; Li, T.; Teng, D.; Mao, R.; Hao, Y.; Yang, N.; Wang, X.; Wang, J. Recent progress of bacterial FtsZ inhibitors with a focus on peptides. FEBS J. 2021, 288, 1091–1106. [Google Scholar] [CrossRef] [PubMed]
- Awuni, E. An overview of drugging the bacterial cytoskeleton, rod, and divisome systems. SLAS Discov. Adv. Sci. Drug Discov. 2025, 35, 100261. [Google Scholar] [CrossRef]
- Awuni, E. Status of Targeting MreB for the Development of Antibiotics. Front. Chem. 2020, 7, 884. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Y.; Song, Z.; Zhu, C.; Tang, Y.; Peng, J.; Liu, P. MreB: Unraveling the molecular mechanisms of bacterial shape, division, and environmental adaptation. Cell Commun. Signal. 2025, 23, 377. [Google Scholar] [CrossRef]
- Bertonha, A.F.; Silva, C.C.L.; Shirakawa, K.T.; Trindade, D.M.; Dessen, A. Penicillin-binding protein (PBP) inhibitor development: A 10-year chemical perspective. Exp. Biol. Med. 2023, 248, 1657–1670. [Google Scholar] [CrossRef]
- Costes, A.; Lecointe, F.; McGovern, S.; Quevillon-Cheruel, S.; Polard, P. The C-Terminal Domain of the Bacterial SSB Protein Acts as a DNA Maintenance Hub at Active Chromosome Replication Forks. PLOS Genet. 2010, 6, e1001238. [Google Scholar] [CrossRef]
- Marceau, A.H. Functions of single-strand DNA-binding proteins in DNA replication, recombination, and repair. Methods Mol. Biol. 2012, 922, 1–21. [Google Scholar] [CrossRef]
- Bianco, P.R. The mechanism of action of the SSB interactome reveals it is the first OB-fold family of genome guardians in prokaryotes. Protein Sci. 2021, 30, 1757–1775. [Google Scholar] [CrossRef]
- Krejci, L.; Sung, P. RPA Not that Different from SSB. Structure 2002, 10, 601–602. [Google Scholar] [CrossRef][Green Version]
- Madru, C.; Martínez-Carranza, M.; Laurent, S.; Alberti, A.C.; Chevreuil, M.; Raynal, B.; Haouz, A.; Le Meur, R.A.; Delarue, M.; Henneke, G.; et al. DNA-binding mechanism and evolution of replication protein A. Nat. Commun. 2023, 14, 2326. [Google Scholar] [CrossRef]
- Brutlag, D.; Kornberg, A. Enzymatic Synthesis of Deoxyribonucleic Acid: X.X.X.V.I. A proofreading function for the 3′ → 5′ exonuclease activity in deoxyribonucleic acid polymerases. J. Biol. Chem. 1972, 247, 241–248. [Google Scholar] [CrossRef]
- Thomas, K.R.; Olivera, B.M. Processivity of DNA exonucleases. J. Biol. Chem. 1978, 253, 424–429. [Google Scholar] [CrossRef]
- Lu, D.; Myers, A.R.; George, N.P.; Keck, J.L. Mechanism of Exonuclease I stimulation by the single-stranded DNA-binding protein. Nucleic Acids Res. 2011, 39, 6536. [Google Scholar] [CrossRef][Green Version]
- Lu, D.; Keck, J.L. Structural basis of Escherichia coli single-stranded DNA-binding protein stimulation of exonuclease I. Proc. Natl. Acad Sci. USA 2008, 105, 9169–9174. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Bernstein, D.A.; Satyshur, K.A.; Keck, J.L. Small-molecule tools for dissecting the roles of SSB/protein interactions in genome maintenance. Proc. Natl. Acad Sci. USA 2009, 107, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Marceau, A.H.; Bernstein, D.A.; Walsh, B.W.; Shapiro, W.; Simmons, L.A.; Keck, J.L. Protein Interactions in Genome Maintenance as Novel Antibacterial Targets. PLoS ONE 2013, 8, e58765. [Google Scholar] [CrossRef]
- Lu, D.; Windsor, M.A.; Gellman, S.H.; Keck, J.L. Peptide inhibitors identify roles for SSB C-terminal residues in SSB/exonuclease I complex formation. Biochemistry 2009, 48, 6764–6771. [Google Scholar] [CrossRef][Green Version]
- Tököli, A.; Bodnár, B.; Bogár, F.; Paragi, G.; Hetényi, A.; Bartus, E.; Wéber, E.; Hegedüs, Z.; Szabó, Z.; Kecskeméti, G.; et al. Structural Adaptation of the Single-Stranded DNA-Binding Protein C-Terminal to DNA Metabolizing Partners Guides Inhibitor Design. Pharmaceutics 2023, 15, 1032. [Google Scholar] [CrossRef] [PubMed]
- Ryzhikov, M.; Koroleva, O.; Postnov, D.; Tran, A.; Korolev, S. Mechanism of RecO recruitment to DNA by single-stranded DNA binding protein. Nucleic Acids Res. 2011, 39, 6305–6314. [Google Scholar] [CrossRef]
- Sandler, S.J.; Leroux, M.; Windgassen, T.A.; Keck, J.L. Escherichia coli K-12 has two distinguishable PriA-PriB replication restart pathways. Mol. Microbiol. 2021, 116, 1140–1150. [Google Scholar] [CrossRef]
- Duckworth, A.T.; Ducos, P.L.; McMillan, S.D.; Satyshur, K.A.; Blumenthal, K.H.; Deorio, H.R.; Larson, J.A.; Sandler, S.J.; Grant, T.; Keck, J.L. Replication fork binding triggers structural changes in the PriA helicase that govern DNA replication restart in E. coli. Nat. Commun. 2023, 14, 2725. [Google Scholar] [CrossRef]
- Voter, A.F.; Killoran, M.P.; Ananiev, G.E.; Wildman, S.A.; Hoffmann, F.M.; Keck, J.L. A High-Throughput Screening Strategy to Identify Inhibitors of SSB Protein-Protein Interactions in an Academic Screening Facility. SLAS Discov. 2018, 23, 94–101. [Google Scholar] [CrossRef]
- Alnammi, M.; Liu, S.; Ericksen, S.S.; Ananiev, G.E.; Voter, A.F.; Guo, S.; Keck, J.L.; Hoffmann, F.M.; Wildman, S.A.; Gitter, A. Evaluating Scalable Supervised Learning for Synthesize-on-Demand Chemical Libraries. J. Chem. Inf. Model. 2023, 63, 5513–5528. [Google Scholar] [CrossRef]
- Bogutzki, A.; Naue, N.; Litz, L.; Pich, A.; Curth, U. E. coli primase and DNA polymerase III holoenzyme are able to bind concurrently to a primed template during DNA replication. Sci. Rep. 2019, 9, 14460. [Google Scholar] [CrossRef]
- Chilingaryan, Z.; Headey, S.J.; Lo, A.T.Y.; Xu, Z.Q.; Otting, G.; Dixon, N.E.; Scanlon, M.J.; Oakley, A.J. Fragment-Based Discovery of Inhibitors of the Bacterial DnaG-SSB Interaction. Antibiotics 2018, 7, 14. [Google Scholar] [CrossRef]
- Bhattacharyya, B.; George, N.P.; Thurmes, T.M.; Zhou, R.; Jani, N.; Wessel, S.R.; Sandler, S.J.; Ha, T.; Keck, J.L. Structural mechanisms of PriA-mediated DNA replication restart. Proc. Natl. Acad. Sci. USA 2013, 111, 1373–1378. [Google Scholar] [CrossRef]
- Keck, J.L.; Roche, D.D.; Lynch, A.S.; Berger, J.M. Structure of the RNApolymerase domain of, E. coli primase. Science 2000, 287, 2482–2486. [Google Scholar] [CrossRef]
- Glanzer, J.G.; Endres, J.L.; Byrne, B.M.; Liu, S.; Bayles, K.W.; Oakley, G.G. Identification of inhibitors for single-stranded DNA-binding proteins in eubacteria. J. Antimicrob. Chemother. 2016, 71, 3432–3440. [Google Scholar] [CrossRef]
- Ilic, S.; Cohen, S.; Singh, M.; Tam, B.; Dayan, A.; Akabayov, B. DnaG Primase—A Target for the Development of Novel Antibacterial Agents. Antibiotics 2018, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Yao, N.Y.; O’Donnell, M. DNA Replication: How Does a Sliding Clamp Slide? Curr. Biol. 2017, 27, R174–R176. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, S.; Søgaard, C.K.; Olsen, J.G.; Otterlei, M.; Kragelund, B.B. The bacterial DNA sliding clamp, β-clamp: Structure, interactions, dynamics and drug discovery. Cell. Mol. Life Sci. 2024, 81, 245. [Google Scholar] [CrossRef]
- Altieri, A.S.; Kelman, Z. DNA sliding clamps as therapeutic targets. Front. Mol. Biosci. 2018, 5, 383372. [Google Scholar] [CrossRef]
- Georgescu, R.E.; Yurieva, O.; Kim, S.S.; Kuriyan, J.; Kong, X.P.; O’Donnell, M. Structure of a small-molecule inhibitor of a DNA polymerase sliding clamp. Proc. Natl. Acad. Sci. USA 2008, 105, 11116–11121. [Google Scholar] [CrossRef]
- Wijffels, G.; Johnson, W.M.; Oakley, A.J.; Turner, K.; Epa, V.C.; Briscoe, S.J.; Polley, M.; Liepa, A.J.; Hofmann, A.; Buchardt, J.; et al. Binding inhibitors of the bacterial sliding clamp by design. J. Med. Chem. 2011, 54, 4831–4838. [Google Scholar] [CrossRef]
- Yin, Z.; Whittell, L.R.; Wang, Y.; Jergic, S.; Liu, M.; Harry, E.J.; Dixon, N.E.; Beck, J.L.; Kelso, M.J.; Oakley, A.J. Discovery of lead compounds targeting the bacterial sliding clamp using a fragment-based approach. J. Med. Chem. 2014, 57, 2799–2806. [Google Scholar] [CrossRef]
- Yin, Z.; Whittell, L.R.; Wang, Y.; Jergic, S.; Ma, C.; Lewis, P.J.; Dixon, N.E.; Beck, J.L.; Kelso, M.J.; Oakley, A.J. Bacterial Sliding Clamp Inhibitors that Mimic the Sequential Binding Mechanism of Endogenous Linear Motifs. J. Med. Chem. 2015, 58, 4693–4702. [Google Scholar] [CrossRef]
- Caputo, A.; Elisi, G.M.; Levati, E.; Barotti, G.; Sartini, S.; Wagner, J.; Burnouf, D.Y.; Ottonello, S.; Rivara, S.; Montanini, B. Small molecules targeting the eubacterial β-sliding clamp discovered by combined in silico and in vitro screening approaches. J. Enzym. Inhib. Med. Chem. 2025, 40, 2440861. [Google Scholar] [CrossRef]
- André, C.; Martiel, I.; Wolff, P.; Landolfo, M.; Lorber, B.; da Veiga, C.S.; Dejaegere, A.; Dumas, P.; Guichard, G.; Oliéric, V.; et al. Interaction of a Model Peptide on Gram Negative and Gram Positive Bacterial Sliding Clamps. ACS Infect. Dis. 2019, 5, 1022–1034. [Google Scholar] [CrossRef]
- Wolff, P.; Oliéric, V.; Briand, J.P.; Chaloin, O.; Dejaegere, A.; Dumas, P.; Ennifar, E.; Guichard, G.; Wagner, J.; Burnouf, D.Y. Structure-based design of short peptide ligands binding onto the E. coli processivity ring. J. Med. Chem. 2011, 54, 4627–4637. [Google Scholar] [CrossRef]
- Compain, G.; Monsarrat, C.; Blagojevic, J.; Brillet, K.; Dumas, P.; Hammann, P.; Kuhn, L.; Martiel, I.; Engilberge, S.; Oliéric, V.; et al. Peptide-Based Covalent Inhibitors Bearing Mild Electrophiles to Target a Conserved His Residue of the Bacterial Sliding Clamp. JACS Au 2024, 4, 432–440. [Google Scholar] [CrossRef]
- Kling, A.; Lukat, P.; Almeida, D.V.; Bauer, A.; Fontaine, E.; Sordello, S.; Zaburannyi, N.; Herrmann, J.; Wenzel, S.C.; König, C.; et al. Targeting DnaN for tuberculosis therapy using novel griselimycins. Science 2015, 348, 1106–1112. [Google Scholar] [CrossRef]
- Fenwick, M.K.; Pierce, P.G.; Abendroth, J.; Barrett, K.F.; Barrett, L.K.; Bowatte, K.; Choi, R.; Chun, I.; Conrady, D.G.; Craig, J.K.; et al. Exquisite selectivity of griselimycin extends to beta subunit of DNA polymerases from Gram-negative bacterial pathogens. Commun. Biol. 2024, 7, 1622. [Google Scholar] [CrossRef]
- Murakami, K.S. Structural biology of bacterial RNA polymerase. Biomolecules 2015, 5, 848–864. [Google Scholar] [CrossRef]
- Lee, J.; Borukhov, S. Bacterial RNA Polymerase-DNA Interaction-The Driving Force of Gene Expression and the Target for Drug Action. Front. Mol. Biosci. 2016, 3, 73. [Google Scholar] [CrossRef]
- Werner, F.; Grohmann, D. Evolution of multisubunit RNA polymerases in the three domains of life. Nat. Rev. Microbiol. 2011, 9, 85–98. [Google Scholar] [CrossRef]
- Kirsch, S.H.; Haeckl, F.P.J.; Müller, R. Beyond the approved: Target sites and inhibitors of bacterial RNA polymerase from bacteria and fungi. Nat. Prod. Rep. 2022, 39, 1226–1263. [Google Scholar] [CrossRef]
- Vassylyev, D.G.; Sekine, S.; Laptenko, O.; Lee, J.; Vassylyeva, M.N.; Borukhov, S.; Yokoyama, S. Crystal structure of a bacterial RNA polymerase holoenzyme at 2.6 Å resolution. Nature 2002, 417, 712–719. [Google Scholar] [CrossRef]
- Johnston, E.B.; Lewis, P.J.; Griffith, R. The interaction of Bacillus subtilis sigmaA with RNA polymerase. Protein Sci. 2009, 18, 2287–2297. [Google Scholar] [CrossRef]
- Hudson, B.P.; Quispe, J.; Lara-González, S.; Kim, Y.; Berman, H.M.; Arnold, E.; Ebright, R.H.; Lawson, C.L. Three-dimensional EM structure of an intact activator-dependent transcription initiation complex. Proc. Natl. Acad. Sci. USA 2009, 106, 19830–19835. [Google Scholar] [CrossRef]
- Hinsberger, S.; Hüsecken, K.; Groh, M.; Negri, M.; Haupenthal, J.; Hartmann, R.W. Discovery of novel bacterial RNA polymerase inhibitors: Pharmacophore-based virtual screening and hit optimization. J. Med. Chem. 2013, 56, 8332–8338. [Google Scholar] [CrossRef]
- Ma, C.; Yang, X.; Kandemir, H.; Mielczarek, M.; Johnston, E.B.; Griffith, R.; Kumar, N.; Lewis, P.J. Inhibitors of bacterial transcription initiation complex formation. ACS Chem. Biol. 2013, 8, 1972–1980. [Google Scholar] [CrossRef]
- Mielczarek, M.; Thomas, R.V.; Ma, C.; Kandemir, H.; Yang, X.; Bhadbhade, M.; Black, D.S.; Griffith, R.; Lewis, P.J.; Kumar, N. Synthesis and biological activity of novel mono-indole and mono-benzofuran inhibitors of bacterial transcription initiation complex formation. Bioorg. Med. Chem. 2015, 23, 1763–1775. [Google Scholar] [CrossRef]
- Ma, C.; Yang, X.; Lewis, P.J. Bacterial Transcription Inhibitor of RNA Polymerase Holoenzyme Formation by Structure-Based Drug Design: From in Silico Screening to Validation. ACS Infect. Dis. 2016, 2, 39–46. [Google Scholar] [CrossRef]
- Sartini, S.; Levati, E.; Maccesi, M.; Guerra, M.; Spadoni, G.; Bach, S.; Benincasa, M.; Scocchi, M.; Ottonello, S.; Rivara, S.; et al. New Antimicrobials Targeting Bacterial RNA Polymerase Holoenzyme Assembly Identified with an in Vivo BRET-Based Discovery Platform. ACS Chem. Biol. 2019, 14, 1727–1736. [Google Scholar] [CrossRef]
- Caputo, A.; Sartini, S.; Levati, E.; Minato, I.; Elisi, G.M.; Di Stasi, A.; Guillou, C.; Goekjian, P.G.; Garcia, P.; Gueyrard, D.; et al. An Optimized Workflow for the Discovery of New Antimicrobial Compounds Targeting Bacterial RNA Polymerase Complex Formation. Antibiotics 2022, 11, 1449. [Google Scholar] [CrossRef]
- Hüsecken, K.; Negri, M.; Fruth, M.; Boettcher, S.; Hartmann, R.W.; Haupenthal, J. Peptide-based investigation of the Escherichia coli RNA polymerase σ(70):core interface as target site. ACS Chem. Biol. 2013, 8, 758–766. [Google Scholar] [CrossRef]
- Kamal, A.A.M.; Haupenthal, J.; Habib, M.; Hartmann, R.W.; Empting, M. Hit evaluation of an α-helical peptide: Ala-scan, truncation and sidechain-to-sidechain macrocyclization of an RNA polymerase inhibitor. Biol. Chem. 2019, 400, 333–342. [Google Scholar] [CrossRef]
- Zheng, Y.; Kan, C.H.; Tsang, T.F.; Liu, Y.; Liu, T.; Tsang, M.W.; Lam, L.Y.; Yang, X.; Ma, C. Discovery of Inhibitors Targeting Protein-Protein Interaction between Bacterial RNA Polymerase and NusG as Novel Antimicrobials. J. Med. Chem. 2024, 67, 16556–16575. [Google Scholar] [CrossRef]
- Ye, J.; Kan, C.H.; Yang, X.; Ma, C. Inhibition of bacterial RNA polymerase function and protein-protein interactions: A promising approach for next-generation antibacterial therapeutics. RSC Med. Chem. 2024, 15, 1471–1487. [Google Scholar] [CrossRef]
- Arnvig, K.B.; Zeng, S.; Quan, S.; Papageorge, A.; Zhang, N.; Villapakkam, A.C.; Squires, C.L. Evolutionary comparison of ribosomal operon antitermination function. J. Bacteriol. 2008, 190, 7251–7257. [Google Scholar] [CrossRef]
- Bubunenko, M.; Court, D.L.; Al Refaii, A.; Saxena, S.; Korepanov, A.; Friedman, D.I.; Gottesman, M.E.; Alix, J.H. Nus transcription elongation factors and RNase III modulate small ribosome subunit biogenesis in Escherichia coli. Mol. Microbiol. 2013, 87, 382–393. [Google Scholar] [CrossRef]
- Baniulyte, G.; Singh, N.; Benoit, C.; Johnson, R.; Ferguson, R.; Paramo, M.; Stringer, A.M.; Scott, A.; Lapierre, P.; Wade, J.T. Identification of regulatory targets for the bacterial Nus factor complex. Nat. Commun. 2017, 8, 2027. [Google Scholar] [CrossRef]
- Greive, S.J.; Lins, A.F.; Von Hippel, P.H. Assembly of an RNA-Protein Complex: Binding of NusB and NusE (S10) proteins to boxA RNA nucleates the formation of the antitermination complex involved in controlling rRNA transcription in Escherichia coli. J. Biol. Chem. 2005, 280, 36397–36408. [Google Scholar] [CrossRef]
- Burmann, B.M.; Schweimer, K.; Luo, X.; Wahl, M.C.; Stitt, B.L.; Gottesman, M.E.; Rösch, P. A NusE:NusG complex links transcription and translation. Science 2010, 328, 501–504. [Google Scholar] [CrossRef]
- Cossar, P.J.; Ma, C.; Gordon, C.P.; Ambrus, J.I.; Lewis, P.J.; McCluskey, A. Identification and validation of small molecule modulators of the NusB-NusE interaction. Bioorg. Med. Chem. Lett. 2017, 27, 162–167. [Google Scholar] [CrossRef]
- Cossar, P.J.; Abdel-Hamid, M.K.; Ma, C.; Sakoff, J.A.; Trinh, T.N.; Gordon, C.P.; Lewis, P.J.; McCluskey, A. Small-Molecule Inhibitors of the NusB-NusE Protein-Protein Interaction with Antibiotic Activity. ACS Omega 2017, 2, 3839–3857. [Google Scholar] [CrossRef]
- Luo, X.; Hsiao, H.H.; Bubunenko, M.; Weber, G.; Court, D.L.; Gottesman, M.E.; Urlaub, H.; Wahl, M.C. Structural and functional analysis of the E. coli NusB-S10 transcription antitermination complex. Mol. Cell 2008, 32, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Luo, M.J.; Yeung, A.C.M.; Lewis, P.J.; Chan, P.K.S.; Ip, M.; Ma, C. First-In-Class Inhibitor of Ribosomal RNA Synthesis with Antimicrobial Activity against Staphylococcus aureus. Biochemistry 2017, 56, 5049–5052. [Google Scholar] [CrossRef]
- Qiu, Y.; Chan, S.T.; Lin, L.; Shek, T.L.; Tsang, T.F.; Barua, N.; Zhang, Y.; Ip, M.; Chan, P.K.S.; Blanchard, N.; et al. Design, synthesis and biological evaluation of antimicrobial diarylimine and –amine compounds targeting the interaction between the bacterial NusB and NusE proteins. Eur. J. Med. Chem. 2019, 178, 214–231. [Google Scholar] [CrossRef]
- Qiu, Y.; Chan, S.T.; Lin, L.; Shek, T.L.; Tsang, T.F.; Zhang, Y.; Ip, M.; Chan, P.K.S.; Blanchard, N.; Hanquet, G.; et al. Nusbiarylins, a new class of antimicrobial agents: Rational design of bacterial transcription inhibitors targeting the interaction between the NusB and NusE proteins. Bioorg. Chem. 2019, 92, 103203. [Google Scholar] [CrossRef]
- Chu, A.J.; Qiu, Y.; Harper, R.; Lin, L.; Ma, C.; Yang, X. Nusbiarylins Inhibit Transcription and Target Virulence Factors in Bacterial Pathogen Staphylococcus aureus. Int. J. Mol. Sci. 2020, 21, 5772. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Chu, A.J.; Tsang, T.F.; Zheng, Y.; Lam, N.M.; Li, K.S.L.; Ip, M.; Yang, X.; Ma, C. Synthesis and biological evaluation of nusbiarylin derivatives as bacterial rRNA synthesis inhibitor with potent antimicrobial activity against MRSA and VRSA. Bioorg. Chem. 2022, 124, 105863. [Google Scholar] [CrossRef]
- Ye, J.; Yang, X.; Ma, C. Ligand-Based Drug Design of Novel Antimicrobials against Staphylococcus aureus by Targeting Bacterial Transcription. Int. J. Mol. Sci. 2023, 24, 339. [Google Scholar] [CrossRef]
- Savelsbergh, A.; Mohr, D.; Wilden, B.; Wintermeyer, W.; Rodnina, M.V. Stimulation of the GTPase activity of translation elongation factor G by ribosomal protein L7/12. J. Biol. Chem. 2000, 275, 890–894. [Google Scholar] [CrossRef]
- Burma, D.P. Conformational change of 23S RNA in 50S ribosome is responsible for translocation in protein synthesis. J. Biosci. 1984, 6, 419–430. [Google Scholar] [CrossRef]
- Hagiya, A.; Naganuma, T.; Maki, Y.; Ohta, J.; Tohkairin, Y.; Shimizu, T.; Nomura, T.; Hachimori, A.; Uchiumi, T. A mode of assembly of P0, P1, and P2 proteins at the GTPase-associated center in animal ribosome: In vitro analyses with P0 truncation mutants. J. Biol. Chem. 2005, 280, 39193–39199. [Google Scholar] [CrossRef]
- Wang, W.; Liu, C.; Zhu, N.; Lin, Y.; Jiang, J.; Wang, Y.; Li, Y.; Si, S. Identification of anti-Gram-negative bacteria agents targeting the interaction between ribosomal proteins L12 and L10. Acta Pharm. Sin. B 2018, 8, 772–783. [Google Scholar] [CrossRef]
- Diaconu, M.; Kothe, U.; Schlünzen, F.; Fischer, N.; Harms, J.M.; Tonevitsky, A.G.; Stark, H.; Rodnina, M.V.; Wahl, M.C. Structural basis for the function of the ribosomal L7/12 stalk in factor binding and GTPase activation. Cell 2005, 121, 991–1004. [Google Scholar] [CrossRef]
- Jurėnas, D.; Fraikin, N.; Goormaghtigh, F.; Van Melderen, L. Biology and evolution of bacterial toxin-antitoxin systems. Nat. Rev. Microbiol. 2022, 20, 335–350. [Google Scholar] [CrossRef]
- Równicki, M.; Lasek, R.; Trylska, J.; Bartosik, D. Targeting Type II Toxin-Antitoxin Systems as Antibacterial Strategies. Toxins 2020, 12, 568. [Google Scholar] [CrossRef]
- Pizzolato-Cezar, L.R.; Spira, B.; Machini, M.T. Bacterial toxin-antitoxin systems: Novel insights on toxin activation across populations and experimental shortcomings. Curr. Res. Microb. Sci. 2023, 5, 100204. [Google Scholar] [CrossRef]
- Równicki, M.; Pieńko, T.; Czarnecki, J.; Kolanowska, M.; Bartosik, D.; Trylska, J. Artificial activation of Escherichia coli mazEF and hipBA toxin–antitoxin systems by antisense peptide nucleic acids as an antibacterial strategy. Front. Microbiol. 2018, 9, 424809. [Google Scholar] [CrossRef]
- Kumar, S.; Kolodkin-Gal, I.; Engelberg-Kulka, H. Novel quorum-sensing peptides mediating interspecies bacterial cell death. mBio 2013, 4, 10–1128. [Google Scholar] [CrossRef]
- Nikolic, N. Autoregulation of bacterial gene expression: Lessons from the MazEF toxin–antitoxin system. Curr. Genet. 2019, 65, 133–138. [Google Scholar] [CrossRef]
- Yan, Z.; Li, G.; Gao, Y.; Zhai, W.; Qi, Y.; Zhai, M. The extracellular death factor (EDF) protects Escherichia coli by scavenging hydroxyl radicals induced by bactericidal antibiotics. Springerplus 2015, 4, 182. [Google Scholar] [CrossRef][Green Version]
- Kumar, S.; Engelberg-Kulka, H. Quorum sensing peptides mediating interspecies bacterial cell death as a novel class of antimicrobial agents. Curr. Opin. Microbiol. 2014, 21, 22–27. [Google Scholar] [CrossRef]
- Jin, C.; Kang, S.M.; Kim, D.H.; Lee, Y.; Lee, B.J. Discovery of Antimicrobial Agents Based on Structural and Functional Study of the Klebsiella pneumoniae MazEF Toxin–Antitoxin System. Antibiotics 2024, 13, 398. [Google Scholar] [CrossRef]
- Floris, M.; Masciocchi, J.; Fanton, M.; Moro, S. Swimming into peptidomimetic chemical space using pepMMsMIMIC. Nucleic Acids Res. 2011, 39, W261–W269. [Google Scholar] [CrossRef][Green Version]
- Farhadi, T.; Hashemian, S.M.R.; Farhadi, Z. In Silico Designing of Peptidomimetics Enhancing Endoribonucleolytic Activities of Acinetobacter MazF Toxin as the Novel Anti-bacterial Candidates. Int. J. Pept. Res. Ther. 2020, 26, 1061–1071. [Google Scholar] [CrossRef]
- Zhu, L.; Sharp, J.D.; Kobayashi, H.; Woychik, N.A.; Inouye, M. Noncognate Mycobacterium tuberculosis Toxin-Antitoxins Can Physically and Functionally Interact. J. Biol. Chem. 2010, 285, 39732–39738. [Google Scholar] [CrossRef]
- Winther, K.S.; Gerdes, K. Enteric virulence associated protein VapC inhibits translation by cleavage of initiator tRNA. Proc. Natl. Acad. Sci. USA 2011, 108, 7403–7407. [Google Scholar] [CrossRef]
- Dienemann, C.; Bøggild, A.; Winther, K.S.; Gerdes, K.; Brodersen, D.E. Crystal structure of the VapBC toxin-antitoxin complex from Shigella flexneri reveals a hetero-octameric DNA-binding assembly. Mol. Biol. 2011, 414, 713–722. [Google Scholar] [CrossRef]
- Lopes, A.P.Y.; Lopes, L.M.; Fraga, T.R.; Chura-Chambi, R.M.; Sanson, A.L.; Cheng, E.; Nakajima, E.; Morganti, L.; Martins, E.A.L. VapC from the leptospiral VapBC toxin-antitoxin module displays ribonuclease activity on the initiator tRNA. PLoS ONE 2014, 9, e101678. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.G.; Lee, S.J.; Chae, S.; Lee, K.Y.; Kim, J.H.; Lee, B.J. Structural and functional studies of the Mycobacterium tuberculosis VapBC30 toxin-antitoxin system: Implications for the design of novel antimicrobial peptides. Nucleic Acids Res. 2015, 43, 7624–7637. [Google Scholar] [CrossRef]
- Kang, S.M.; Kim, D.H.; Lee, K.Y.; Park, S.J.; Yoon, H.J.; Lee, S.J.; Im, H.; Lee, B.J. Functional details of the Mycobacterium tuberculosis VapBC26 toxin-antitoxin system based on a structural study: Insights into unique binding and antibiotic peptides. Nucleic Acids Res. 2017, 45, 8564–8580. [Google Scholar] [CrossRef]
- Deep, A.; Tiwari, P.; Agarwal, S.; Kaundal, S.; Kidwai, S.; Singh, R.; Thakur, K.G. Structural, functional and biological insights into the role of Mycobacterium tuberculosis VapBC11 toxin–antitoxin system: Targeting a tRNase to tackle mycobacterial adaptation. Nucleic Acids Res. 2018, 46, 11639–11655. [Google Scholar] [CrossRef]
- Sundar, S.; Rajan, M.P.; Piramanayagam, S. In Silico Derived Peptides for Inhibiting the Toxin–Antitoxin Systems of Mycobacterium tuberculosis: Basis for Developing Peptide-Based Therapeutics. Int. J. Pept. Res. Ther. 2019, 25, 1467–1475. [Google Scholar] [CrossRef]
- Kang, S.M.; Moon, H.; Han, S.W.; Kim, B.W.; Kim, D.H.; Kim, B.M.; Lee, B.J. Toxin-Activating Stapled Peptides Discovered by Structural Analysis Were Identified as New Therapeutic Candidates That Trigger Antibacterial Activity against Mycobacterium tuberculosis in the Mycobacterium smegmatis Model. Microorganisms 2021, 9, 568. [Google Scholar] [CrossRef]
- Kang, S.M.; Jin, C.; Kim, D.H.; Lee, Y.; Lee, B.J. Structural and Functional Study of the Klebsiella pneumoniae VapBC Toxin-Antitoxin System, including the Development of an Inhibitor That Activates VapC. J. Med. Chem. 2020, 63, 13669–13679. [Google Scholar] [CrossRef]
- Park, D.W.; Yoon, H.J.; Lee, K.Y.; Park, S.J.; Cheon, S.H.; Lee, H.H.; Lee, S.J.; Lee, B.J. Crystal structure of proteolyzed VapBC and DNA-bound VapBC from Salmonella enterica Typhimurium LT2 and VapC as a putative Ca2+ -dependent ribonuclease. FASEB J. 2020, 34, 3051–3068. [Google Scholar] [CrossRef]
- Sun, H.; Coussens, N.P.; Danchik, C.; Wachsmuth, L.M.; Henderson, M.J.; Patnaik, S.; Hall, M.D.; Molinaro, A.L.; Daines, D.A.; Shen, M. Discovery of Small-Molecule VapC1 Nuclease Inhibitors by Virtual Screening and Scaffold Hopping from an Atomic Structure Revealing Protein-Protein Interactions with a Native VapB1 Inhibitor. J. Chem. Inf. Model. 2022, 62, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Van Eldere, J.; Slack, M.P.E.; Ladhani, S.; Cripps, A.W. Non-typeable Haemophilus influenzae, an under-recognised pathogen. Lancet Infect. Dis. 2014, 14, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- Leibovitz, E.; Greenberg, D.; Piglansky, L.; Raiz, S.; Porat, N.; Press, J.; Leiberman, A.; Dagan, R. Recurrent acute otitis media occurring within one month from completion of antibiotic therapy: Relationship to the original pathogen. Pediatr. Infect. Dis. J. 2003, 22, 209–215. [Google Scholar] [CrossRef]
- Fisher, R.A.; Gollan, B.; Helaine, S. Persistent bacterial infections and persister cells. Nat. Rev. Microbiol. 2017, 15, 453–464. [Google Scholar] [CrossRef]
- Ren, D.; Walker, A.N.; Daines, D.A. Toxin-antitoxin loci vapBC-1 and vapXD contribute to survival and virulence in nontypeable Haemophilus influenzae. BMC Microbiol. 2012, 12, 263. [Google Scholar] [CrossRef]
- Molinaro, A.L.; Kashipathy, M.M.; Lovell, S.; Battaile, K.P.; Coussens, N.P.; Shen, M.; Daines, D.A. Crystal Structure of VapBC-1 from Nontypeable Haemophilus influenzae and the Effect of PIN Domain Mutations on Survival during Infection. J. Bacteriol. 2019, 201, 10–1128. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Y.; Inouye, M.; Woychik, N.A. Bacterial addiction module toxin Doc inhibits translation elongation through its association with the 30S ribosomal subunit. Proc. Natl. Acad. Sci. USA 2008, 105, 5885–5890. [Google Scholar] [CrossRef]
- Garcia-Pino, A.; Christensen-Dalsgaard, M.; Wyns, L.; Yarmolinsky, M.; Magnuson, R.D.; Gerdes, K.; Loris, R. Doc of prophage P1 is inhibited by its antitoxin partner Phd through fold complementation. J. Biol. Chem. 2008, 283, 30821–30827. [Google Scholar] [CrossRef]
- Castro-Roa, D.; Garcia-Pino, A.; De Gieter, S.; Van Nuland, N.A.J.; Loris, R.; Zenkin, N. The Fic protein Doc uses an inverted substrate to phosphorylate and inactivate EF-Tu. Nat. Chem. Biol. 2013, 9, 811–817. [Google Scholar] [CrossRef]
- Garcia-Pino, A.; Balasubramanian, S.; Wyns, L.; Gazit, E.; De Greve, H.; Magnuson, R.D.; Charlier, D.; van Nuland, N.A.J.; Loris, R. Allostery and intrinsic disorder mediate transcription regulation by conditional cooperativity. Cell 2010, 142, 101–111. [Google Scholar] [CrossRef]
- Helaine, S.; Cheverton, A.M.; Watson, K.G.; Faure, L.M.; Matthews, S.A.; Holden, D.W. Internalization of Salmonella by macrophages induces formation of nonreplicating persisters. Science 2014, 343, 204–208. [Google Scholar] [CrossRef]
- De Castro, G.V.; Worm, D.J.; Grabe, G.J.; Rowan, F.C.; Haggerty, L.; De La Lastra, A.L.; Popescu, O.; Helaine, S.; Barnard, A. Characterization of the Key Determinants of Phd Antitoxin Mediated Doc Toxin Inactivation in Salmonella. ACS Chem. Biol. 2022, 17, 1598–1606. [Google Scholar] [CrossRef]
- Worm, D.J.; Grabe, G.J.; de Castro, G.V.; Rabinovich, S.; Warm, I.; Isherwood, K.; Helaine, S.; Barnard, A. Stapled Phd Peptides Inhibit Doc Toxin Induced Growth Arrest in Salmonella. ACS Chem. Biol. 2023, 18, 2485–2494. [Google Scholar] [CrossRef]
- Turnbull, K.J.; Gerdes, K. HicA toxin of Escherichia coli derepresses hicAB transcription to selectively produce HicB antitoxin. Mol. Microbiol. 2017, 104, 781–792. [Google Scholar] [CrossRef]
- Jørgensen, M.G.; Pandey, D.P.; Jaskolska, M.; Gerdes, K. HicA of Escherichia coli defines a novel family of translation-independent mRNA interferases in bacteria and archaea. J. Bacteriol. 2009, 191, 1191–1199. [Google Scholar] [CrossRef]
- Manav, M.C.; Turnbull, K.J.; Jurėnas, D.; Garcia-Pino, A.; Gerdes, K.; Brodersen, D.E. The E. coli HicB Antitoxin Contains a Structurally Stable Helix-Turn-Helix DNA Binding Domain. Structure 2019, 27, 1675–1685.e3. [Google Scholar] [CrossRef]
- Gerdes, K. Diverse genetic contexts of HicA toxin domains propose a role in anti-phage defense. mBio 2024, 15, e0329323. [Google Scholar] [CrossRef]
- Kim, D.H.; Kang, S.M.; Park, S.J.; Jin, C.; Yoon, H.J.; Lee, B.J. Functional insights into the Streptococcus pneumoniae HicBA toxin–antitoxin system based on a structural study. Nucleic Acids Res. 2018, 46, 6371–6386. [Google Scholar] [CrossRef]
- Li, G.; Shen, M.; Lu, S.; Le, S.; Tan, Y.; Wang, J.; Zhao, X.; Shen, W.; Guo, K.; Yang, Y.; et al. Identification and Characterization of the HicAB Toxin-Antitoxin System in the Opportunistic Pathogen Pseudomonas aeruginosa. Toxins 2016, 8, 113. [Google Scholar] [CrossRef]
- Bagabas, S.S.; Trujillo-Mendoza, J.; Stocks, M.J.; Turner, D.P.J.; Oldfield, N.J. The Characterization of a Gonococcal HicAB Toxin–Antitoxin System Capable of Causing Bacteriostatic Growth Arrest. Microorganisms 2025, 13, 1619. [Google Scholar] [CrossRef]
- Encina-Robles, J.; Pérez-Villalobos, V.; Bustamante, P. The HicAB System: Characteristics and Biological Roles of an Underappreciated Toxin-Antitoxin System. Int. J. Mol. Sci. 2024, 25, 12165. [Google Scholar] [CrossRef]
- Germain, E.; Castro-Roa, D.; Zenkin, N.; Gerdes, K. Molecular mechanism of bacterial persistence by HipA. Mol. Cell 2013, 52, 248–254. [Google Scholar] [CrossRef]
- Kaspy, I.; Rotem, E.; Weiss, N.; Ronin, I.; Balaban, N.Q.; Glaser, G. HipA-mediated antibiotic persistence via phosphorylation of the glutamyl-tRNA-synthetase. Nat. Commun. 2013, 4, 3001. [Google Scholar] [CrossRef]
- Schumacher, M.A.; Piro, K.M.; Xu, W.; Hansen, S.; Lewis, K.; Brennan, R.G. Molecular mechanisms of HipA-mediated multidrug tolerance and its neutralization by HipB. Science 2009, 323, 396–401. [Google Scholar] [CrossRef]
- Hansen, S.; Vulić, M.; Min, J.; Yen, T.J.; Schumacher, M.A.; Brennan, R.G.; Lewis, K. Regulation of the Escherichia coli HipBA toxin-antitoxin system by proteolysis. PLoS ONE 2012, 7, e39185, Erratum in PLoS ONE 2012, 7. https://doi.org/10.1371/annotation/e608601c-eadd-4c11-adb2-7b605aba9c44. [Google Scholar] [CrossRef]
- Li, T.; Yin, N.; Liu, H.; Pei, J.; Lai, L. Novel Inhibitors of Toxin HipA Reduce Multidrug Tolerant Persisters. ACS Med. Chem. Lett. 2016, 7, 449–453. [Google Scholar] [CrossRef]
- Evdokimov, A.; Voznesensky, I.; Fennell, K.; Anderson, M.; Smith, J.F.; Fisher, D.A. New kinase regulation mechanism found in HipBA: A bacterial persistence switch. Acta Crystallogr. D Struct. Biol. 2009, 65, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, R.E.; Gallegos-Monterrosa, R.; Coulthurst, S.J. Type VI secretion system effector proteins: Effective weapons for bacterial competitiveness. Cell. Microbiol. 2020, 22, e13241. [Google Scholar] [CrossRef]
- Jurėnas, D.; Journet, L. Activity, delivery, and diversity of Type VI secretion effectors. Mol. Microbiol. 2021, 115, 383–394. [Google Scholar] [CrossRef]
- Jiang, F.; Wang, X.; Wang, B.; Chen, L.; Zhao, Z.; Waterfield, N.R.; Yang, G.; Jin, Q. The Pseudomonas aeruginosa Type VI Secretion PGAP1-like Effector Induces Host Autophagy by Activating Endoplasmic Reticulum Stress. Cell Rep. 2016, 16, 1502–1509. [Google Scholar] [CrossRef]
- Unni, R.; Pintor, K.L.; Diepold, A.; Unterweger, D. Presence and absence of type VI secretion systems in bacteria. Microbiology 2022, 168, 001151. [Google Scholar] [CrossRef]
- Singh, R.P.; Kumari, K. Bacterial type VI secretion system (T6SS): An evolved molecular weapon with diverse functionality. Biotechnol. Lett. 2023, 45, 309–331. [Google Scholar] [CrossRef]
- Lu, D.; Zheng, Y.; Liao, N.; Wei, L.; Xu, B.; Liu, X.; Liu, J. The structural basis of the Tle4-Tli4 complex reveals the self-protection mechanism of H2-T6SS in Pseudomonas aeruginosa. Acta Crystallogr. D Struct. Biol. 2014, 70, 3233–3243. [Google Scholar] [CrossRef]
- Gao, X.; Mu, Z.; Qin, B.; Sun, Y.; Cui, S. Structure-based prototype peptides targeting the Pseudomonas aeruginosa type VI secretion system effector as a novel antibacterial strategy. Front. Cell. Infect. Microbiol. 2017, 7, 292590. [Google Scholar] [CrossRef]
- Abola, E.; Kuhn, P.; Earnest, T.; Stevens, R.C. Automation of X-ray crystallography. Nat. Struct. Biol. 2000, 7, 973–977. [Google Scholar] [CrossRef]
- Muchmore, S.W.; Olson, J.; Jones, R.; Pan, J.; Blum, M.; Greer, J.; Merrick, S.M.; Magdalinos, P.; Nienaber, V.L. Automated Crystal Mounting and Data Collection for Protein Crystallography. Structure 2000, 8, R243–R246. [Google Scholar] [CrossRef]
- Bard, J.; Ercolani, K.; Svenson, K.; Olland, A.; Somers, W. Automated systems for protein crystallization. Methods 2004, 34, 329–347. [Google Scholar] [CrossRef]
- Stewart, P.S.; Mueller-Dieckmann, J. Automation in biological crystallization. Acta Crystallogr. F Struct. Biol. Commun. 2014, 70, 686–696. [Google Scholar] [CrossRef]
- Weber, P.; Pissis, C.; Navaza, R.; Mechaly, A.E.; Saul, F.; Alzari, P.M.; Haouz, A. High-Throughput Crystallization Pipeline at the Crystallography Core Facility of the Institut Pasteur. Molecules 2019, 24, 4451. [Google Scholar] [CrossRef] [PubMed]
- Matsugaki, N.; Senda, T. Advances in macromolecular crystallography at the Photon Factory: Automation from crystallization to structural determination. J. Synchrotron Radiat. 2025, 32, 567–576. [Google Scholar] [CrossRef]
- Stephens, D.C.; Crabtree, A.; Beasley, H.K.; Garza-Lopez, E.; Mungai, M.; Vang, L.; Neikirk, K.; Vue, Z.; Vue, N.; Marshall, A.G.; et al. In the Age of Machine Learning Cryo-EM Research is Still Necessary: A Path toward Precision Medicine. Adv. Biol. 2023, 7, 2300122. [Google Scholar] [CrossRef]
- Nogales, E.; Scheres, S.H.W. Cryo-EM: A Unique Tool for the Visualization of Macromolecular Complexity. Mol. Cell 2015, 58, 677–689. [Google Scholar] [CrossRef]
- Casañal, A.; Shakeel, S.; Passmore, L.A. Interpretation of medium resolution cryoEM maps of multi-protein complexes. Curr. Opin. Struct. Biol. 2019, 58, 166–174. [Google Scholar] [CrossRef]
- Malhotra, S.; Joseph, A.P.; Thiyagalingam, J.; Topf, M. Assessment of protein–protein interfaces in cryo-EM derived assemblies. Nat. Commun. 2021, 12, 3399. [Google Scholar] [CrossRef]
- Larsen, E.K.; Olivieri, C.; Walker, C.; Manu, V.S.; Gao, J.; Bernlohr, D.A.; Tonelli, M.; Markley, J.L.; Veglia, G. Probing Protein-Protein Interactions Using Asymmetric Labeling and Carbonyl-Carbon Selective Heteronuclear NMR Spectroscopy. Molecules 2018, 23, 1937. [Google Scholar] [CrossRef]
- Purslow, J.A.; Khatiwada, B.; Bayro, M.J.; Venditti, V. NMR Methods for Structural Characterization of Protein-Protein Complexes. Front. Mol. Biosci. 2020, 7, 9. [Google Scholar] [CrossRef]
- Clabbers, M.T.B.; Shiriaeva, A.; Gonen, T. MicroED: Conception, practice and future opportunities. IUCrJ 2022, 9, 169–179. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Evans, R.; O’Neill, M.; Pritzel, A.; Antropova, N.; Senior, A.; Green, T.; Žídek, A.; Bates, R.; Blackwell, S.; Yim, J.; et al. Protein complex prediction with AlphaFold-Multimer. bioRxiv 2021. [Google Scholar] [CrossRef]
- Bryant, P.; Pozzati, G.; Elofsson, A. Improved prediction of protein-protein interactions using AlphaFold2. Nat. Commun. 2022, 13, 1265, Erratum in Nat. Commun. 2022, 13, 1694. https://doi.org/10.1038/s41467-022-29480-5. [Google Scholar] [CrossRef]
- Johansson-Åkhe, I.; Wallner, B. Improving peptide-protein docking with AlphaFold-Multimer using forced sampling. Front. Bioinform. 2022, 2, 959160. [Google Scholar] [CrossRef] [PubMed]
- Wallner, B. AFsample: Improving multimer prediction with AlphaFold using massive sampling. Bioinformatics 2023, 39, btad573. [Google Scholar] [CrossRef]
- Chen, B.; Xie, Z.; Qiu, J.; Ye, Z.; Xu, J.; Tang, J. Improved the heterodimer protein complex prediction with protein language models. Briefings Bioinform. 2023, 24, bbad221. [Google Scholar] [CrossRef]
- Mirabello, C.; Wallner, B.; Nystedt, B.; Azinas, S.; Carroni, M. Unmasking AlphaFold to integrate experiments and predictions in multimeric complexes. Nat. Commun. 2024, 15, 8724. [Google Scholar] [CrossRef]
- Abulude, I.J.; Luna, I.C.R.; Varela, A.S.; Camilli, A.; Kadouri, D.E.; Guo, X. Using AlphaFold-Multimer to study novel protein-protein interactions of predation essential hypothetical proteins in Bdellovibrio. Front. Bioinform. 2025, 5, 1566486. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Krishna, R.; Wang, J.; Ahern, W.; Sturmfels, P.; Venkatesh, P.; Kalvet, I.; Lee, G.R.; Morey-Burrows, F.S.; Anishchenko, I.; Humphreys, I.R.; et al. Generalized biomolecular modeling and design with RoseTTAFold All-Atom. Science 2024, 384, eadl2528. [Google Scholar] [CrossRef]
- Gainza, P.; Bunker, R.D.; Townson, S.A.; Castle, J.C. Machine learning to predict de novo protein–protein interactions. Trends Biotechnol. 2025. [Google Scholar] [CrossRef]
- Passaro, S.; Corso, G.; Wohlwend, J.; Reveiz, M.; Thaler, S.; Somnath, V.R.; Getz, N.; Portnoi, T.; Roy, J.; Stark, H.; et al. Boltz-2: Towards Accurate and Efficient Binding Affinity Prediction. bioRxiv 2025. [Google Scholar] [CrossRef]
- Clackson, T.; Wells, J.A. A hot spot of binding energy in a hormone-receptor interface. Science 1995, 267, 383–386. [Google Scholar] [CrossRef]
- Gohlke, H.; Kiel, C.; Case, D.A. Insights into protein-protein binding by binding free energy calculation and free energy decomposition for the Ras-Raf and Ras-RalGDS complexes. J. Mol. Biol. 2003, 330, 891–913. [Google Scholar] [CrossRef]
- Schmidtke, P.; Bidon-chanal, A.; Luque, F.J.; Barril, X. MDpocket: Open-source cavity detection and characterization on molecular dynamics trajectories. Bioinformatics 2011, 27, 3276–3285. [Google Scholar] [CrossRef]
- Durrant, J.D.; McCammon, J.A. Molecular dynamics simulations and drug discovery. BMC Biol. 2011, 9, 71. [Google Scholar] [CrossRef]
- Moreira, I.S.; Fernandes, P.A.; Ramos, M.J. Computational alanine scanning mutagenesis—An improved methodological approach. J. Comput. Chem. 2007, 28, 644–654. [Google Scholar] [CrossRef]
- Martins, S.A.; Perez, M.A.S.; Moreira, I.S.; Sousa, S.F.; Ramos, M.J.; Fernandes, P.A. Computational Alanine Scanning Mutagenesis: MM-PBSA vs TI. J. Chem. Theory Comput. 2013, 9, 1311–1319. [Google Scholar] [CrossRef]
- Simões, I.C.M.; Costa, I.P.D.; Coimbra, J.T.S.; Ramos, M.J.; Fernandes, P.A. New Parameters for Higher Accuracy in the Computation of Binding Free Energy Differences upon Alanine Scanning Mutagenesis on Protein-Protein Interfaces. J. Chem. Inf. Model. 2017, 57, 60–72. [Google Scholar] [CrossRef]
- Bergey, C.M.; Watkins, A.M.; Arora, P.S. HippDB: A database of readily targeted helical protein-protein interactions. Bioinformatics 2013, 29, 2806–2807. [Google Scholar] [CrossRef]
- Sukhwal, A.; Sowdhamini, R. Oligomerisation status and evolutionary conservation of interfaces of protein structural domain superfamilies. Mol. Biosyst. 2013, 9, 1652–1661. [Google Scholar] [CrossRef]
- Sumbalova, L.; Stourac, J.; Martinek, T.; Bednar, D.; Damborsky, J. HotSpot Wizard 3.0: Web server for automated design of mutations and smart libraries based on sequence input information. Nucleic Acids Res. 2018, 46, W356–W362. [Google Scholar] [CrossRef]
- Barlow, K.A.; Ó Conchúir, S.; Thompson, S.; Suresh, P.; Lucas, J.E.; Heinonen, M.; Kortemme, T. Flex ddG: Rosetta Ensemble-Based Estimation of Changes in Protein-Protein Binding Affinity upon Mutation. J. Phys. Chem. B 2018, 122, 5389–5399. [Google Scholar] [CrossRef]
- Zhou, Y.; Myung, Y.; Rodrigues, C.H.M.; Ascher, D.B. DDMut-PPI: Predicting effects of mutations on protein–protein interactions using graph-based deep learning. Nucleic Acids Res. 2024, 52, W207–W214. [Google Scholar] [CrossRef]
- Chen, Y.C.; Sargsyan, K.; Wright, J.D.; Chen, Y.-H.; Huang, Y.S.; Lim, C. PPI-hotspotID for detecting protein–protein interaction hot spots from the free protein structure. eLife 2024, 13, RP96643. [Google Scholar] [CrossRef]
- Detering, C.; Claussen, H.; Gastreich, M.; Lemmen, C.; Hurst, W.J. KnowledgeSpace—A publicly available virtual chemistry space. J. Cheminf. 2010, 2, O9. [Google Scholar] [CrossRef]
- Warr, W.A. Report on an NIH Workshop on Ultralarge Chemistry Databases. ChemRxiv 2021. [Google Scholar] [CrossRef]
- Cieślak, M.; Danel, T.; Krzysztyńska-Kuleta, O.; Kalinowska-Tłuścik, J. Machine learning accelerates pharmacophore-based virtual screening of MAO inhibitors. Sci. Rep. 2024, 14, 8228. [Google Scholar] [CrossRef]
- Gentile, F.; Agrawal, V.; Hsing, M.; Ton, A.T.; Ban, F.; Norinder, U.; Gleave, M.E.; Cherkasov, A. Deep Docking: A Deep Learning Platform for Augmentation of Structure Based Drug Discovery. ACS Central Sci. 2020, 6, 939–949. [Google Scholar] [CrossRef]
- Gentile, F.; Yaacoub, J.C.; Gleave, J.; Fernandez, M.; Ton, A.T.; Ban, F.; Stern, A.; Cherkasov, A. Artificial intelligence-enabled virtual screening of ultra-large chemical libraries with deep docking. Nat. Protoc. 2022, 17, 672–697. [Google Scholar] [CrossRef]
- Sadybekov, A.A.; Sadybekov, A.V.; Liu, Y.; Iliopoulos-Tsoutsouvas, C.; Huang, X.P.; Pickett, J.; Houser, B.; Patel, N.; Tran, N.K.; Tong, F.; et al. Synthon-based ligand discovery in virtual libraries of over 11 billion compounds. Nature 2021, 601, 452–459. [Google Scholar] [CrossRef]
- Beroza, P.; Crawford, J.J.; Ganichkin, O.; Gendelev, L.; Harris, S.F.; Klein, R.; Miu, A.; Steinbacher, S.; Klingler, F.M.; Lemmen, C. Chemical space docking enables large-scale structure-based virtual screening to discover ROCK1 kinase inhibitors. Nat. Commun. 2022, 13, 6447. [Google Scholar] [CrossRef]
- Podobnik, M.; Kraševec, N.; Zavec, A.B.; Naneh, O.; Flašker, A.; Caserman, S.; Hodnik, V.; Anderluh, G. How to Study Protein-protein Interactions. Acta Chim. Slov. 2016, 63, 424–439. [Google Scholar] [CrossRef]
- Zhou, M.; Li, Q.; Wang, R. Current Experimental Methods for Characterizing Protein-Protein Interactions. ChemMedChem 2016, 11, 738–756. [Google Scholar] [CrossRef]
- Akbarzadeh, S.; Coşkun, Ö.; Günçer, B. Studying protein-protein interactions: Latest and most popular approaches. J. Struct. Biol. 2024, 216, 108118. [Google Scholar] [CrossRef]
- Hong, S.H.; Nguyen, T.; Ongkingco, J.F.; Nazzaro, A.; Arora, P.S. From Concepts to Inhibitors: A Blueprint for Targeting Protein-Protein Interactions. Chem. Rev. 2025, 125, 6819–6869. [Google Scholar] [CrossRef]
- Yellaboina, S.; Goyal, K.; Mande, S.C. Inferring genome-wide functional linkages in E. coli by combining improved genome context methods: Comparison with high-throughput experimental data. Genome Res. 2007, 17, 527–535. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rajagopala, S.V.; Sikorski, P.; Kumar, A.; Mosca, R.; Vlasblom, J.; Arnold, R.; Franca-Koh, J.; Pakala, S.B.; Phanse, S.; Ceol, A.; et al. The binary protein-protein interaction landscape of Escherichia coli. Nat. Biotechnol. 2014, 32, 285–290. [Google Scholar] [CrossRef]
- Cherrak, Y.; Filella-Merce, I.; Schmidt, V.; Byrne, D.; Sgoluppi, V.; Chaiaheloudjou, R.; Betzi, S.; Morelli, X.; Nilges, M.; Pellarin, R.; et al. Inhibiting Type VI Secretion System Activity with a Biomimetic Peptide Designed to Target the Baseplate Wedge Complex. mBio 2021, 12, e0134821. [Google Scholar] [CrossRef]
- Nickerson, N.N.; Jao, C.C.; Xu, Y.; Quinn, J.; Skippington, E.; Alexander, M.K.; Miu, A.; Skelton, N.; Hankins, J.V.; Lopez, M.S.; et al. A Novel Inhibitor of the LolCDE ABC Transporter Essential for Lipoprotein Trafficking in Gram-Negative Bacteria. Antimicrob. Agents Chemother. 2018, 62, e02151-17. [Google Scholar] [CrossRef]
- Yang, Q.; Cai, Y.; Wang, Z.; Guo, S.; Qiu, S.; Zhang, A. Lipoprotein transport system Lol may be a selective target for Gram-negative bacteria. Front. Cell. Infect. Microbiol. 2024, 14, 1463316. [Google Scholar] [CrossRef]
- Song, S.; Shim, S.Y. Advances in Small Molecule Inhibitors Targeting the Bacterial Lipoprotein Transport System (Lol) in Gram-Negative Bacteria. Chem. Asian J. 2025, 20, e00350. [Google Scholar] [CrossRef]
- Zielenkiewicz, U.; Kowalewska, M.; Kaczor, C.; Cegłowski, P. In vivo interactions between toxin-antitoxin proteins epsilon and zeta of streptococcal plasmid pSM19035 in Saccharomyces cerevisiae. J. Bacteriol. 2009, 191, 3677–3684. [Google Scholar] [CrossRef]
- Lioy, V.S.; Rey, O.; Balsa, D.; Pellicer, T.; Alonso, J.C. A toxin–antitoxin module as a target for antimicrobial development. Plasmid 2010, 63, 31–39. [Google Scholar] [CrossRef]
- Rocker, A.; Peschke, M.; Kittilä, T.; Sakson, R.; Brieke, C.; Meinhart, A. The ng_ζ1 toxin of the gonococcal epsilon/zeta toxin/antitoxin system drains precursors for cell wall synthesis. Nat. Commun. 2018, 9, 1686. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhou, K.; Liu, P.; Dong, Y.; Gao, Z.; Zhang, J.; Liu, Q. Structural insight into the E. coli HigBA complex. Biochem. Biophys. Res. Commun. 2016, 478, 1521–1527. [Google Scholar] [CrossRef]
- Kang, S.M.; Jin, C.; Kim, D.H.; Park, S.J.; Han, S.W.; Lee, B.J. Structure-based design of peptides that trigger Streptococcus pneumoniae cell death. FEBS J. 2021, 288, 1546–1564. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Z.; Liu, G.; Geng, Z.; Dong, Y.; Zhang, H. Structural Insights into the Transcriptional Regulation of HigBA Toxin–Antitoxin System by Antitoxin HigA in Pseudomonas aeruginosa. Front. Microbiol. 2020, 10, 493058. [Google Scholar] [CrossRef]
- Cherny, I.; Overgaard, M.; Borch, J.; Bram, Y.; Gerdes, K.; Gazit, E. Structural and thermodynamic characterization of the Escherichia coli RelBE toxin-antitoxin system: Indication for a functional role of differential stability. Biochemistry 2007, 46, 12152–12163. [Google Scholar] [CrossRef]
- Dawson, C.C.; Cummings, J.E.; Starkey, J.M.; Slayden, R.A. Discovery of a novel type IIb RelBE toxin-antitoxin system in Mycobacterium tuberculosis defined by co-regulation with an antisense RNA. Mol. Microbiol. 2022, 117, 1419–1433. [Google Scholar] [CrossRef] [PubMed]
- Duperray, M.; François, J.M.; Capp, J.P. Tuning the expression of the bacterial relBE toxin–antitoxin system in Saccharomyces cerevisiae allows characterizing the subsequent growth inhibition. FEMS Yeast Res. 2023, 23, foad009. [Google Scholar] [CrossRef]
- ElBanna, S.A.; Moneib, N.A.; Aziz, R.K.; Samir, R. Genomics-guided identification of a conserved CptBA-like toxin-antitoxin system in Acinetobacter baumannii. J. Adv. Res. 2020, 30, 159–170. [Google Scholar] [CrossRef]
- Górska, A.; Sloderbach, A.; Marszałł, M.P. Siderophore–drug complexes: Potential medicinal applications of the ‘Trojan horse’ strategy. Trends Pharmacol. Sci. 2014, 35, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Sanabria-Ríos, D.J.; Rivera-Torres, Y.; Rosario, J.; Gutierrez, R.; Torres-García, Y.; Montano, N.; Ortíz-Soto, G.; Ríos-Olivares, E.; Rodríguez, J.W.; Carballeira, N.M. Chemical conjugation of 2-hexadecynoic acid to C5-curcumin enhances its antibacterial activity against multi-drug resistant bacteria. Bioorg. Med. Chem. Lett. 2015, 25, 5067–5071. [Google Scholar] [CrossRef]
- Lee, H.; Lim, S.I.; Shin, S.H.; Lim, Y.; Koh, J.W.; Yang, S. Conjugation of Cell-Penetrating Peptides to Antimicrobial Peptides Enhances Antibacterial Activity. ACS Omega 2019, 4, 15694–15701. [Google Scholar] [CrossRef]
- Dassonville-Klimpt, A.; Sonnet, P. Advances in ‘Trojan horse’ strategies in antibiotic delivery systems. Futur. Med. Chem. 2020, 12, 983–986. [Google Scholar] [CrossRef]
- Pieńko, T.; Czarnecki, J.; Równicki, M.; Wojciechowska, M.; Wierzba, A.J.; Gryko, D.; Bartosik, D.; Trylska, J. Vitamin B12-peptide nucleic acids use the BtuB receptor to pass through the Escherichia coli outer membrane. Biophys. J. 2021, 120, 725–737. [Google Scholar] [CrossRef]
- Wierzba, A.J.; Wojciechowska, M.; Trylska, J.; Gryko, D. Vitamin B12—Peptide Nucleic Acid Conjugates. Methods Mol. Biol. 2021, 2355, 65–82. [Google Scholar] [CrossRef]
- Kravchenko, S.V.; Domnin, P.A.; Grishin, S.Y.; Vershinin, N.A.; Gurina, E.V.; Zakharova, A.A.; Azev, V.N.; Mustaeva, L.G.; Gorbunova, E.Y.; Kobyakova, M.I.; et al. Enhancing the Antimicrobial Properties of Peptides through Cell-Penetrating Peptide Conjugation: A Comprehensive Assessment. Int. J. Mol. Sci. 2023, 24, 16723. [Google Scholar] [CrossRef]
- Tsylents, U.; Siekierska, I.; Trylska, J. Peptide nucleic acid conjugates and their antimicrobial applications—A mini-review. Eur. Biophys. J. 2023, 52, 533–544. [Google Scholar] [CrossRef]
- Macyszyn, J.; Burmistrz, M.; Mieczkowski, A.; Wojciechowska, M.; Trylska, J. Conjugates of Aminoglycosides with Stapled Peptides as a Way to Target Antibiotic-Resistant Bacteria. ACS Omega 2023, 8, 19047–19056. [Google Scholar] [CrossRef]
- Tsylents, U.; Burmistrz, M.; Wojciechowska, M.; Stępień, J.; Maj, P.; Trylska, J. Iron uptake pathway of Escherichia coli as an entry route for peptide nucleic acids conjugated with a siderophore mimic. Front. Microbiol. 2024, 15, 1331021. [Google Scholar] [CrossRef]
- Siekierska, I.; Burmistrz, M.; Trylska, J. Evaluating delivery of peptide nucleic acids to Gram-negative bacteria using differently linked membrane-active peptides and their stapled analogs. Bioorg. Med. Chem. Lett. 2024, 114, 129993. [Google Scholar] [CrossRef]
- Luo, S.; Li, X.R.; Gong, X.T.; Kulikovsky, A.; Qu, F.; Beis, K.; Severinov, K.; Dubiley, S.; Feng, X.; Dong, S.H.; et al. Trojan horse peptide conjugates remodel the activity spectrum of clinical antibiotics. Proc. Natl. Acad. Sci. USA 2024, 122, e2319483121. [Google Scholar] [CrossRef]
- Pals, M.J.; Wijnberg, L.; Yildiz, Ç.; Velema, W.A. Catechol-Siderophore Mimics Convey Nucleic Acid Therapeutics into Bacteria. Angew. Chem. Int. Ed. Engl. 2024, 63, e202402405. [Google Scholar] [CrossRef]
- Li, X.; Dong, S.; Pan, Q.; Liu, N.; Zhang, Y. Antibiotic conjugates: Using molecular Trojan Horses to overcome drug resistance. Biomed. Pharmacother. 2025, 186, 118007. [Google Scholar] [CrossRef]
- Tsylents, U.; Maj, P.; Wdowiak, M.; Stadnicki, J.; Mieczkowski, A.; Wojciechowska, M.; Trylska, J. Modulating backbone flexibility in hydroxamate siderophores for improved iron chelation and peptide nucleic acid (PNA) delivery into bacteria. bioRxiv 2025. [Google Scholar] [CrossRef]
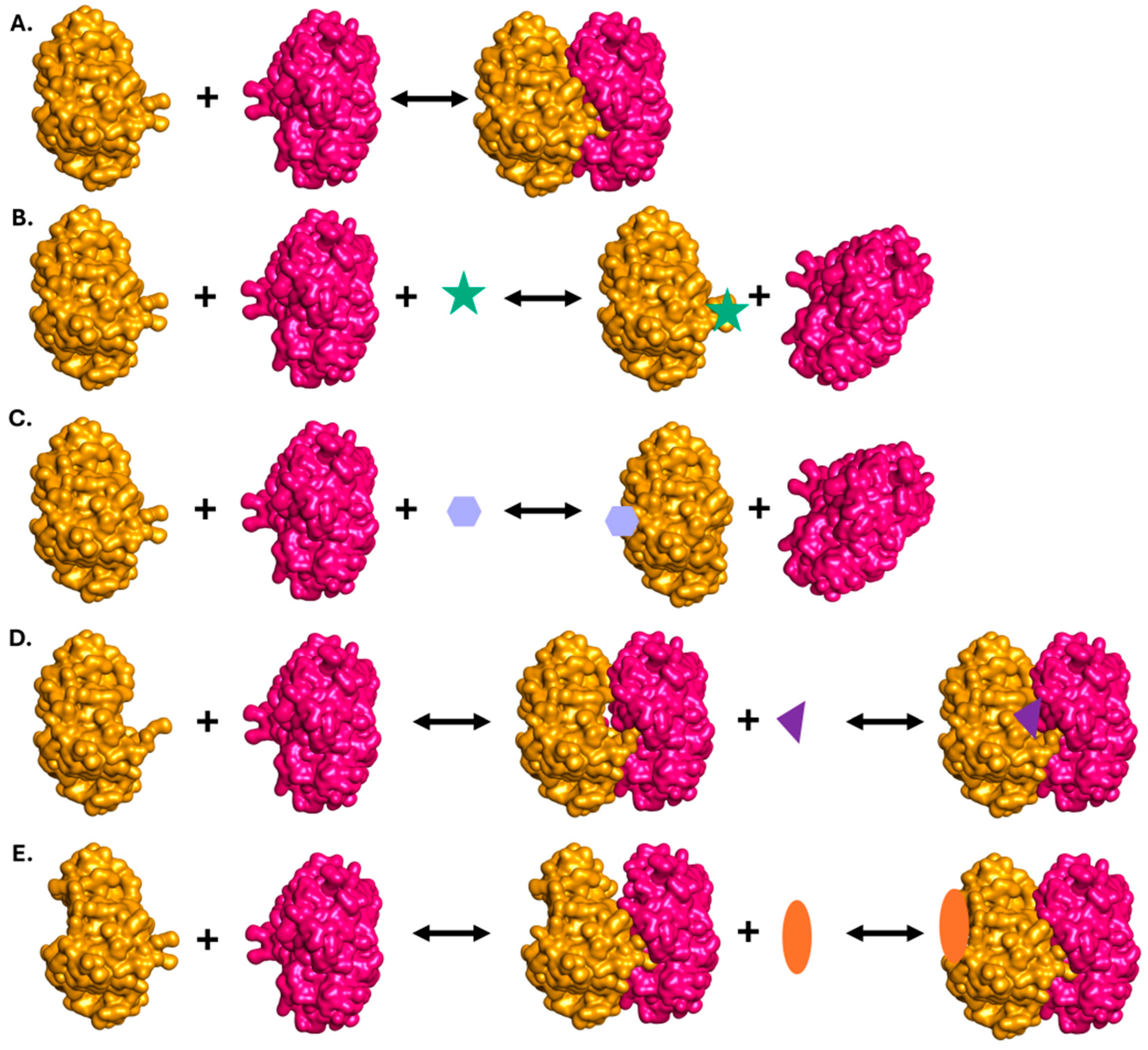

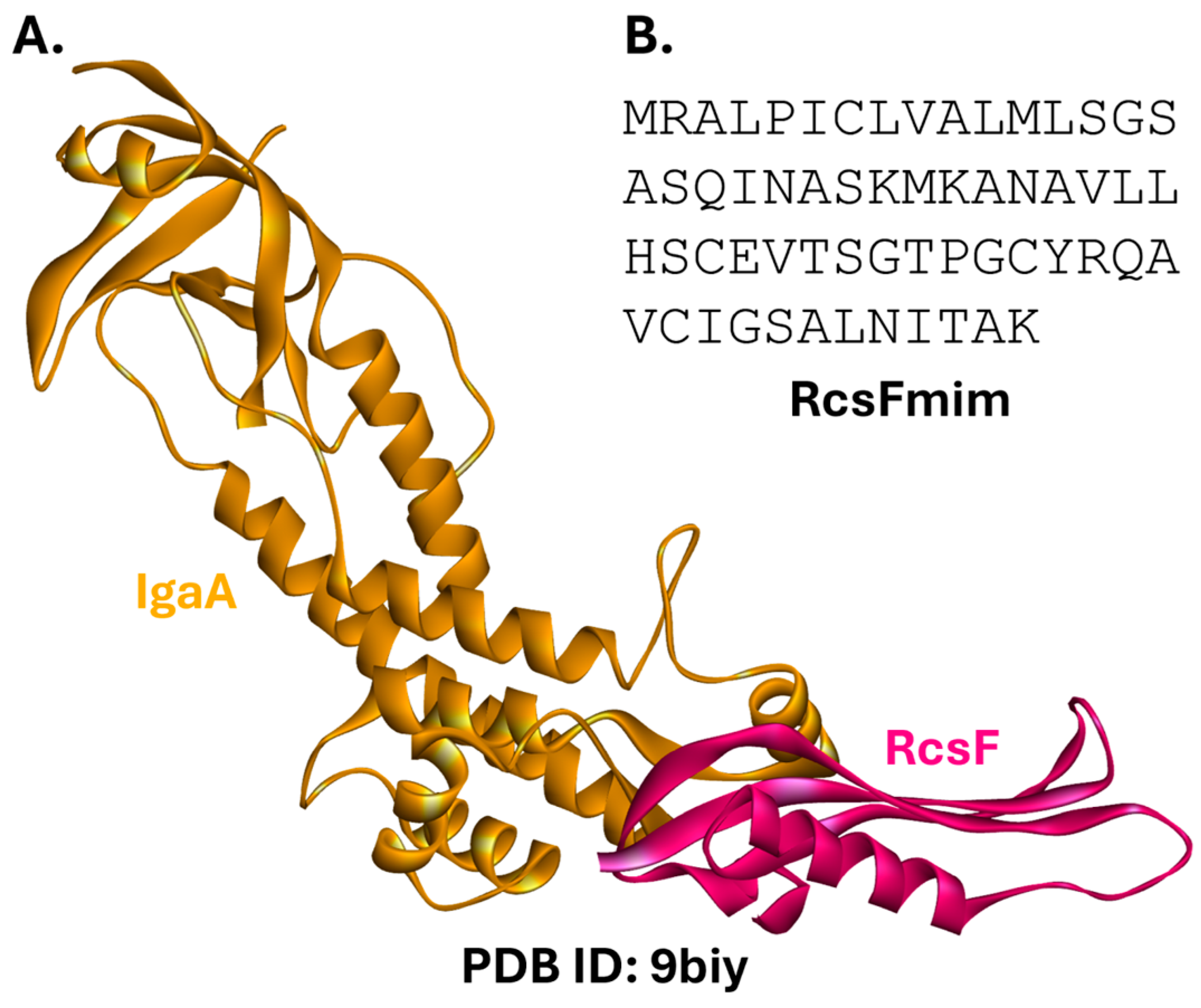

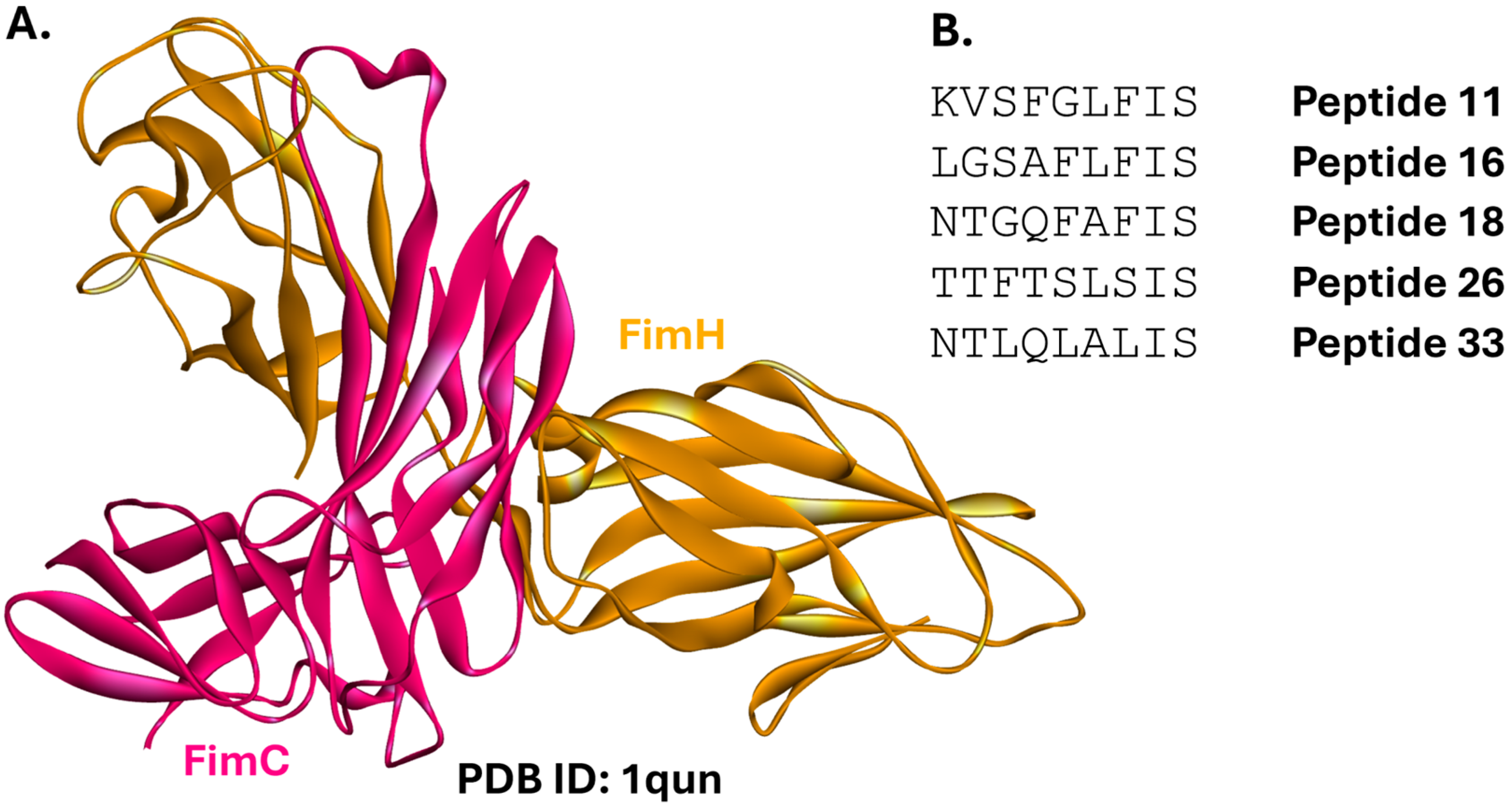
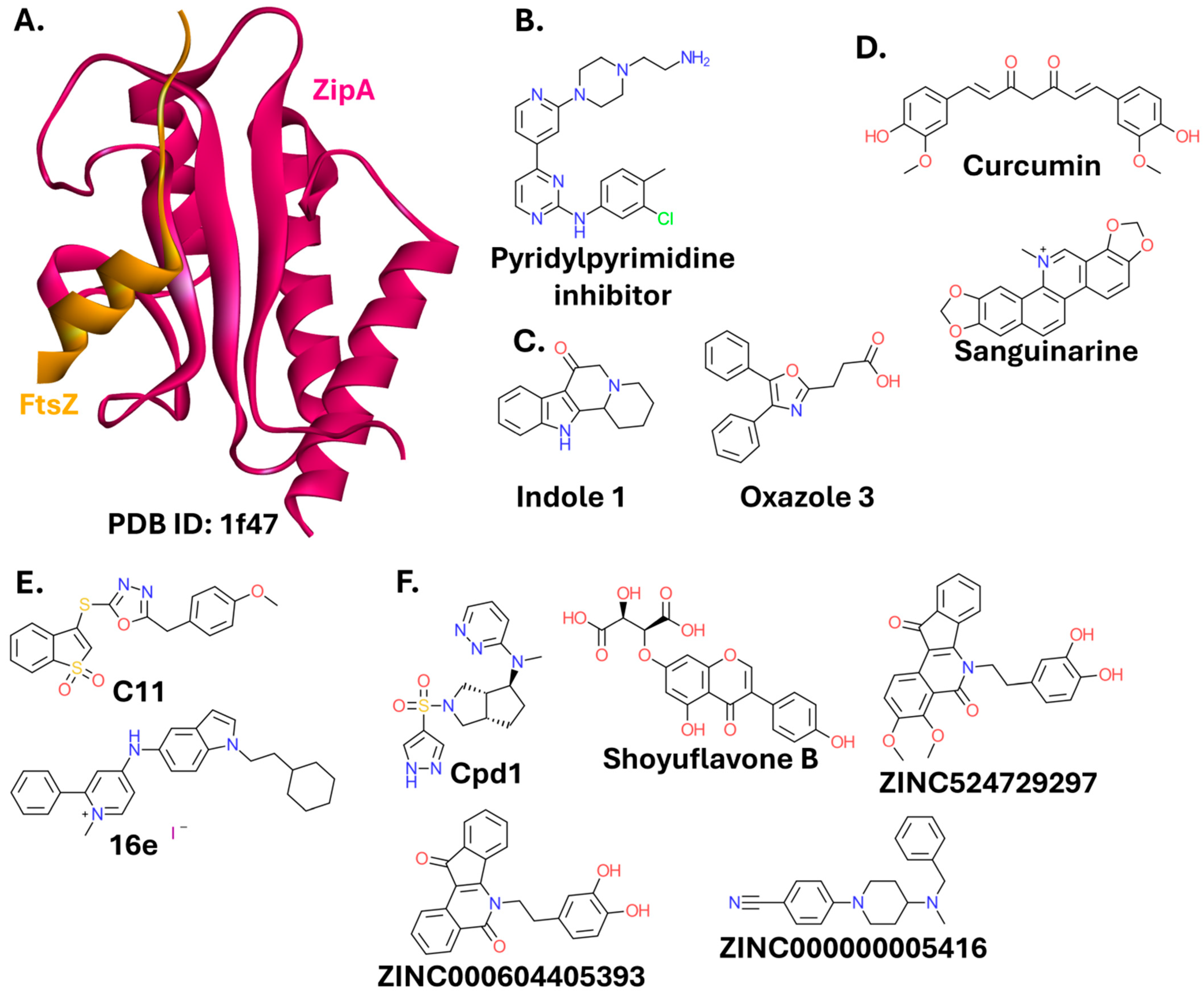
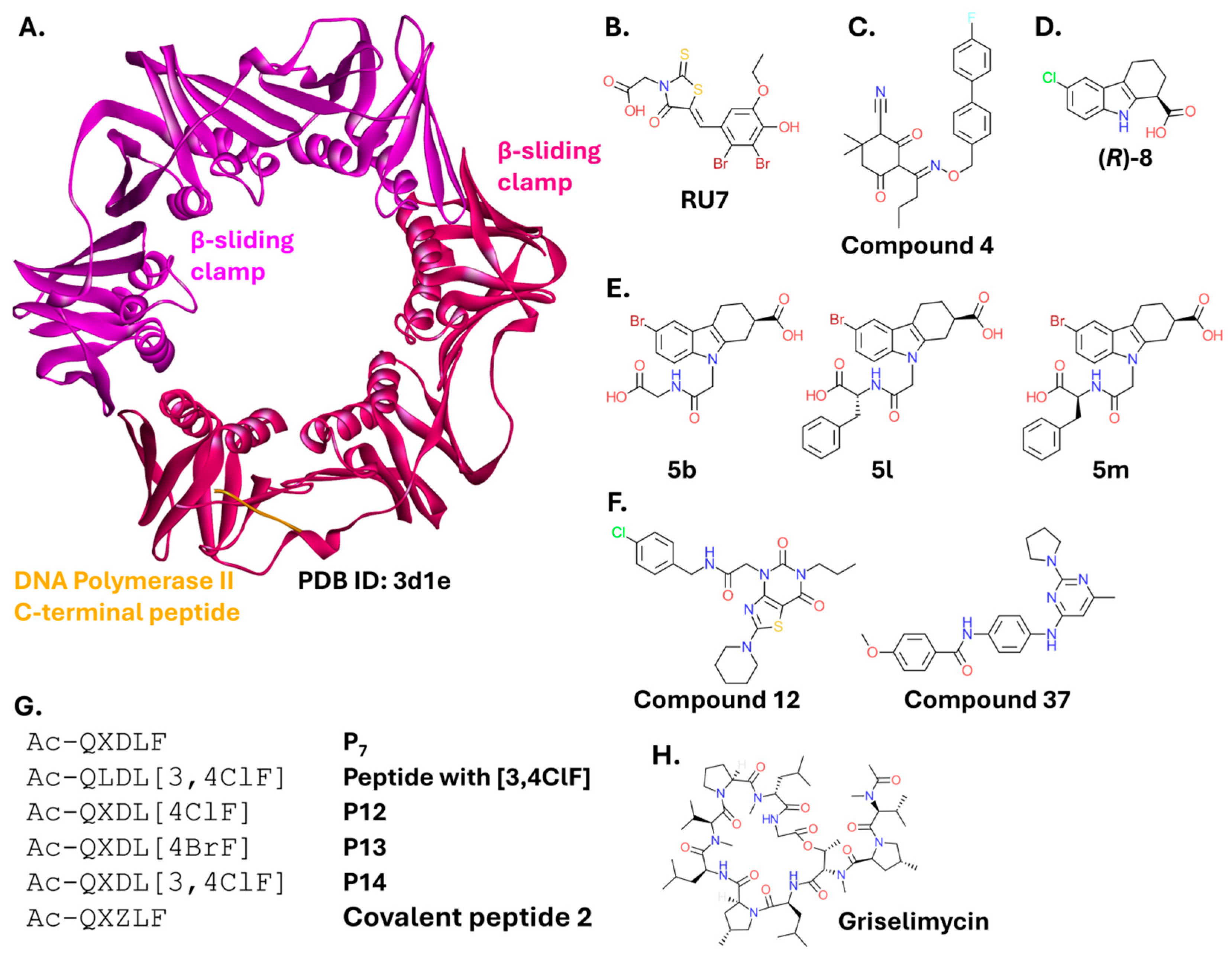
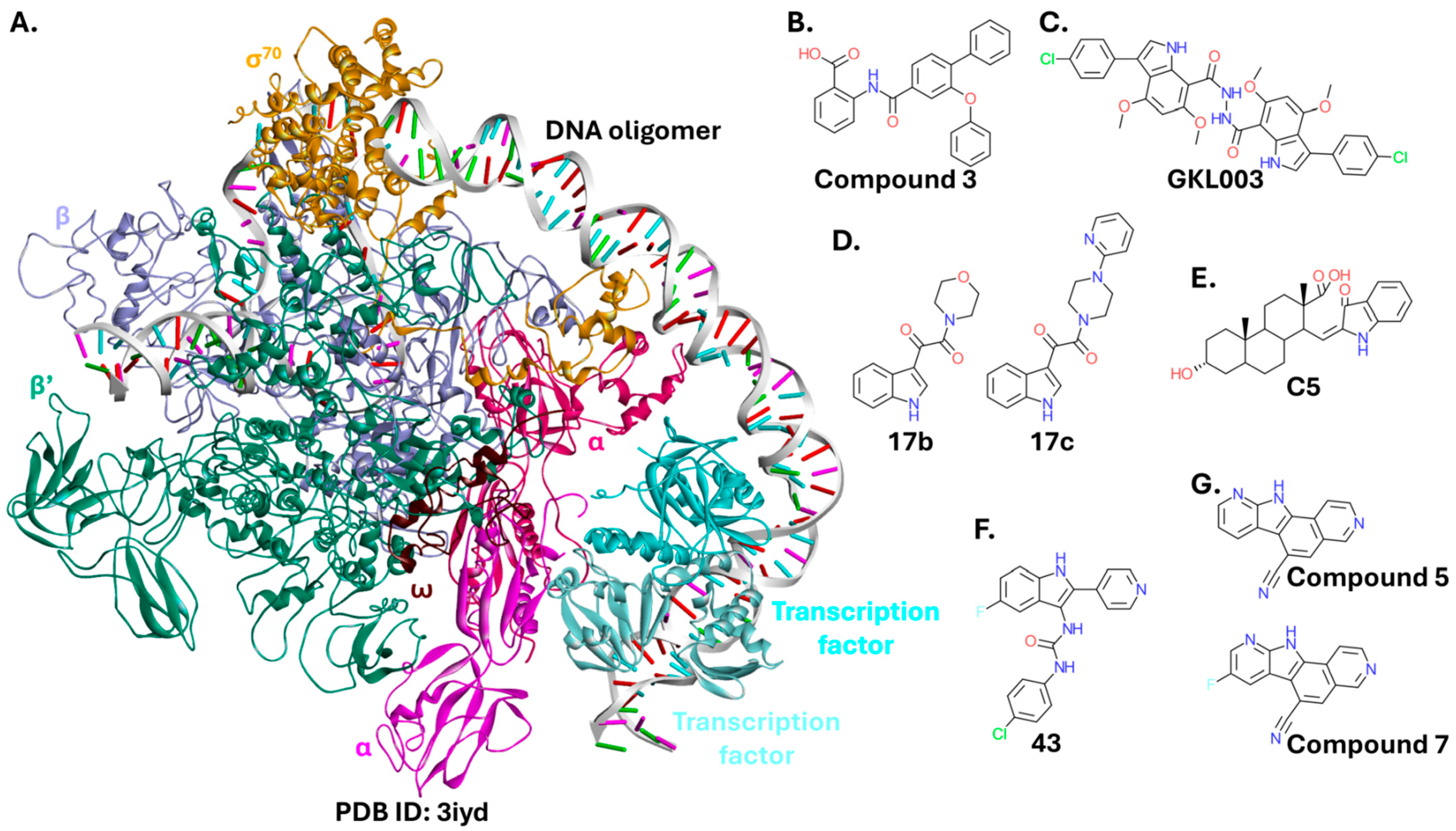


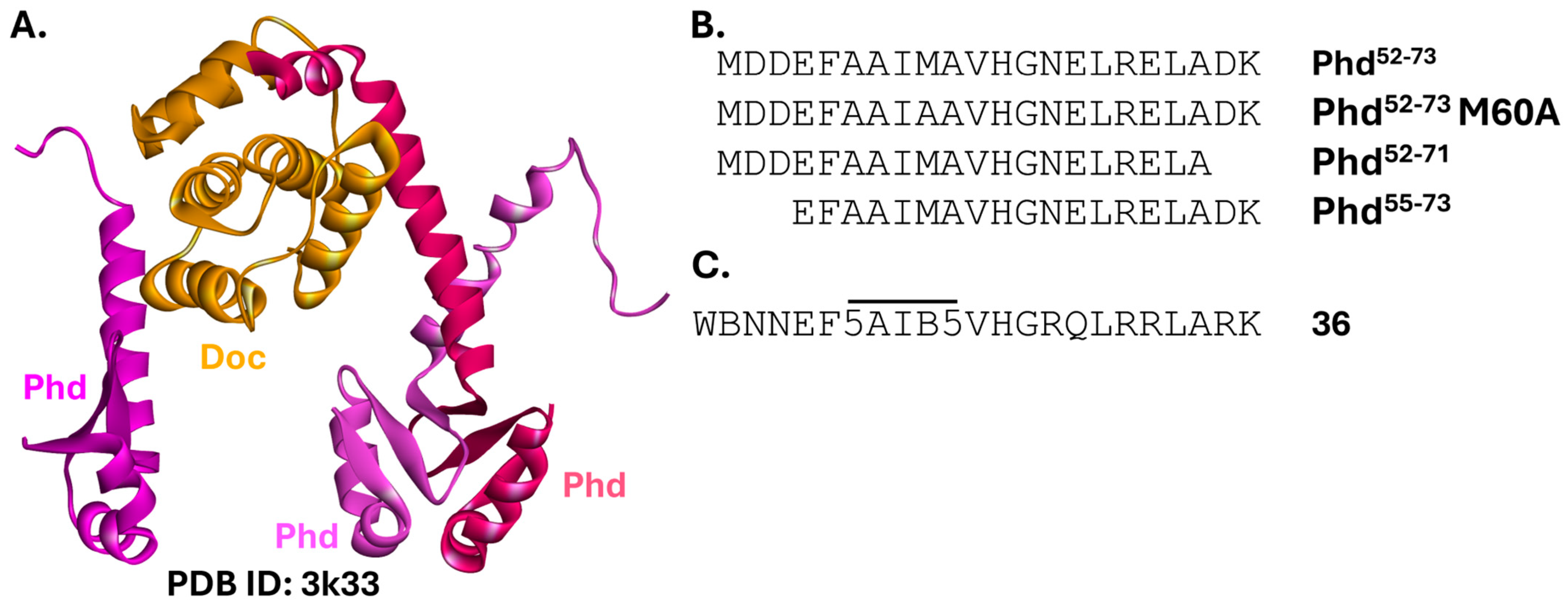
| PPI Complex | Molecular Target | Compound Type | Remarks | References |
|---|---|---|---|---|
| BAM complex | Within BAM complex | Small molecule | Exact mechanism unclear | [37] |
| [38] | ||||
| BamA | Small molecule | [39] | ||
| Within BAM complex | Small molecule | Inhibition depends on BamA, BamB, BamD, and BamE | [40] | |
| BamD | Peptide | [41] | ||
| BamA | Peptide | Binds at the BamA barrel domain | [42,43,44] | |
| [45] | ||||
| Rcs | IgaA | Peptide | BamA as a secondary target | [60] |
| Lpt | LptA | Peptide | [67,68,69] | |
| Small molecule | [70] | |||
| LptA and LptC | Small molecule | [71] | ||
| [72] | ||||
| LptD | Peptidomimetic | In clinical trials | [73,74,75] | |
| LptF | Peptidomimetic | In clinical trials | [78,79] | |
| FimC-FimH | FimH | Peptide | [91] | |
| FtsZ-ZipA | ZipA | Small molecule | Toxic to eukaryotic cells | [97,98] |
| [99] | ||||
| FtsZ oligomer | FtsZ | Small molecule | [101] | |
| Toxic to eukaryotic cells | [102] | |||
| [105] | ||||
| [106] | ||||
| [107] | ||||
| [110] | ||||
| [111] | ||||
| [112] | ||||
| Peptide | [113] | |||
| [114] | ||||
| Modified peptide | [115] | |||
| SSB and partner proteins | ExoI | Small molecule | [130] | |
| Peptide | [132] | |||
| Modified peptide | [133] | |||
| RecO | Modified peptide | [133] | ||
| PriA | Small molecule | Structures not disclosed | [137] | |
| [138] | ||||
| [130] | ||||
| DnaG | Fragment/small molecule | [140] | ||
| β-sliding clamp and partner proteins | β-sliding clamp | Small molecule | [148] | |
| Small molecule and modified peptide | [149] | |||
| Fragment | [150] | |||
| Small molecule | [151] | |||
| [152] | ||||
| Peptides | [153] | |||
| Modified peptide | [154] | |||
| Covalent inhibitors | [155] | |||
| [157] | ||||
| RNAP core-σ70 | Within the complex | Small molecule | Effective on E. coli with efflux system deficiency | [165] |
| β’ | Small molecule | [166] | ||
| β’ or σ factor | Small molecule | Improved permeability | [167] | |
| β’ | Small molecule | [168] | ||
| Require cell permeabilizer | [169] | |||
| [170] | ||||
| Peptide | Do not affect bacterial growth | [171] | ||
| Modified peptide | Loss of inhibitory activity | [172] | ||
| RNAP-NusG | β’ | Small molecule | [173] | |
| NusB-NusE | NusB | Small molecule | [180] | |
| Peptide | ||||
| Small molecule | [181] | |||
| Nusbiarylins | [183] | |||
| [184] | ||||
| [185] | ||||
| [187] | ||||
| L10-L12 | L10 and L12 | Small molecule | Capable of binding to either PPI partner | [192] |
| MazE-MazF | MazF | Peptide | Effective only under stress conditions | [198,200,201] |
| Small molecule | [202] | |||
| Peptide | [202] | |||
| Modified peptide | [204] | |||
| VapB-VapC | VapC | Peptide | [214] | |
| Small molecule | [214] | |||
| VapB1-VapC1 | VapC1 | Small molecule | Toxin inhibitor to combat persisters | [216] |
| PhD-Doc | Doc | Peptide | Toxin inhibitor to combat persisters | [227] |
| Modified peptide | [228] | |||
| HicA-HicB | HicB | Peptide | [233] | |
| HipB-HipA | HipA | Small molecule | Not targeting PPI directly. Toxin inhibitor to combat persisters | [241] |
| TplE-TplEi | TplEi | Peptide | [249] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maj, P.; Trylska, J. Protein–Protein Interactions as Promising Molecular Targets for Novel Antimicrobials Aimed at Gram-Negative Bacteria. Int. J. Mol. Sci. 2025, 26, 10861. https://doi.org/10.3390/ijms262210861
Maj P, Trylska J. Protein–Protein Interactions as Promising Molecular Targets for Novel Antimicrobials Aimed at Gram-Negative Bacteria. International Journal of Molecular Sciences. 2025; 26(22):10861. https://doi.org/10.3390/ijms262210861
Chicago/Turabian StyleMaj, Piotr, and Joanna Trylska. 2025. "Protein–Protein Interactions as Promising Molecular Targets for Novel Antimicrobials Aimed at Gram-Negative Bacteria" International Journal of Molecular Sciences 26, no. 22: 10861. https://doi.org/10.3390/ijms262210861
APA StyleMaj, P., & Trylska, J. (2025). Protein–Protein Interactions as Promising Molecular Targets for Novel Antimicrobials Aimed at Gram-Negative Bacteria. International Journal of Molecular Sciences, 26(22), 10861. https://doi.org/10.3390/ijms262210861







